Evaluation of left ventricular diastolic function in the intensive care unit
(CONSULTANT-LEVEL EXAMINATION)
Overview
Left ventricular (LV) diastolic dysfunction is a clinical entity that remains poorly understood and identified in the intensive care unit (ICU) setting. It is essential to clarify the difference between diastolic heart failure, LV diastolic dysfunction, and increased LV filling pressure. Diastolic heart failure is a clinical syndrome defined by the presence of symptoms of congestive heart failure in the setting of diastolic dysfunction (and preserved systolic function).1 This syndrome is also called heart failure with a normal LV ejection fraction and, in cardiology, accounts for more than 50% of all cases of acute heart failure. The proportion may be even higher in the ICU setting, although no precise statistics are available.
Diastolic dysfunction is an alteration in LV properties marked by degradation in LV relaxation or an increase in LV stiffness (or both). Some evidence indicates that diastolic dysfunction increases the risk for death in patients with septic shock.2 In addition, evaluation of diastolic function can provide the intensivist with important hemodynamic information concerning patients’ fluid responsiveness. In patients with a steep LV pressure-volume relationship, infusion of small amounts of fluid may significantly increase LV diastolic pressure and precipitate acute pulmonary edema. Diastolic dysfunction may coexist with systolic dysfunction in patients with congestive heart failure. Furthermore, in the absence of congestive heart failure, LV filling pressure or LV end-diastolic pressure is not necessarily increased in patients with diastolic (or even systolic) dysfunction. Both diastolic function and LV filling pressure can be explored with echocardiography.
Diastole is classically split into three different physiologic events: LV relaxation, LV passive filling, and left atrial (LA) contraction. Impairment in each of these events may lead to congestive heart failure. Impairment of LV relaxation is mainly due to failure of calcium recapture in the sarcoplasmic reticulum, whereas myocardial infiltration or fibrosis increases LV stiffness.3
Recently, Nagueh et al described a practical approach for classifying diastolic function with transthoracic echocardiography (TTE) that involves taking several parameters into account: mitral inflow, early septal and lateral mitral annular systolic velocity with tissue Doppler, LA volume, pulmonary venous return flow, and even the response to a Valsalva maneuver.4 In ICU patients, obtaining some of these parameters (e.g., the response to a Valsalva maneuver) can pose quite a challenge.5 Detection of other parameters (e.g., pulmonary venous flow [PVF]) may require the application of transesophageal echocardiography (TEE).
Mitral flow: Left ventricular filling
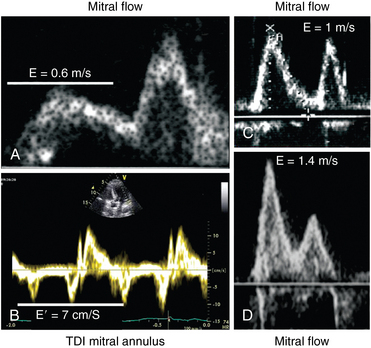
Mitral inflow can be recorded (with both TTE and TEE) by placing the pulsed wave Doppler sample volume at the tip of the mitral leaflets (Figure 32-1). Color flow imaging can help optimize alignment of the Doppler beam, particularly if the left ventricle is dilated. Two waves are described in mitral inflow: the E wave (early filling velocity), which corresponds to the early LV/LA pressure gradient and is affected mainly by LV relaxation and preload, and the A wave, which is due to atrial contraction, reflects the LA/LV pressure gradient during late diastole, and is affected by LV compliance and LA contractile function (and thus disappears in atrial fibrillation) (see Figure 32-1).
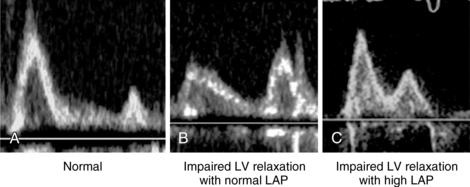
Figure 32-1 Mitral flow recorded with pulsed wave Doppler. A, Normal pattern; B, Impairment of left ventricular (LV) relaxation with normal left atrial pressure (LAP); C, Impairment of LV relaxation with high LAP.
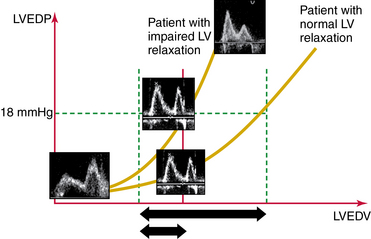
Parameters of mitral inflow include measurements of E and A peak systolic velocity, the E/A ratio, and the deceleration time (DT) of E. The E/A ratio has three main patterns. In the normal pattern, E has a higher peak velocity than A does and therefore E/A is greater than 1 (Figure 32-1A). The second pattern has a small E wave with an E/A ratio of less than 1 and increased DT. This pattern usually corresponds to impairment of LV relaxation, yet with low atrial pressure (Figure 32-1B). The third pattern consists of very high E velocity with an E/A ratio higher than 2 and a short DT. It is associated with severe impairment of LV compliance and with high LV diastolic and pulmonary arterial occlusion pressure (Figure 32-1C).6 Mitral flow is affected by many factors, such as heart rate, preload, afterload, and LA and LV contractility. Nonetheless, since many of these factors are frequently altered in critically ill patients, diastolic function should not be assessed by the mitral flow pattern alone but rather by a global interpretation of all the available information.
Pulmonary venous flow
To record PVF, the pulsed wave Doppler sample volume is placed in the pulmonary vein just distal to its entry point. Although good alignment can be achieved with TTE in some patients (Figure 32-2), measurement of PVF usually requires performance of TEE. The pulmonary venous waveform consists of a peak systolic (S) velocity, which is usually divided into two peaks, S1 and S2, and a peak diastolic (D) velocity. Following these antegrade waves is a retrograde reverse A wave (Ar) caused by atrial contraction (see Figure 32-2). Further information can be obtained by analyzing ensuing parameters such as the S/D ratio, systolic filling fraction (integral of S/[S + D]), peak Ar (reverse A) velocity in late diastole, duration of Ar velocity, and its difference from the mitral A-wave duration. The velocity of the S1 component is primarily defined by variation in LA pressure, as well as by both LA contractility and relaxation.7 The S2 component is instead related to stroke volume and propagation of the pulse wave along the branches of the pulmonary artery. D-wave velocity, on the other hand, is a function of LV filling and compliance and thus accompanies the changes observed in mitral E velocity. Pulmonary venous Ar velocity and duration are influenced by late diastolic LV pressure, atrial preload, and LA contractility. Decreasing LA compliance with increasing LA pressure decreases S velocity and increases D velocity, respectively, thereby reducing the S/D ratio to less than 1.8–10 This situation is also characterized by a drop of less than 40% in the systolic filling fraction8 and shortening of the DT of D velocity, usually less than 150 msec.
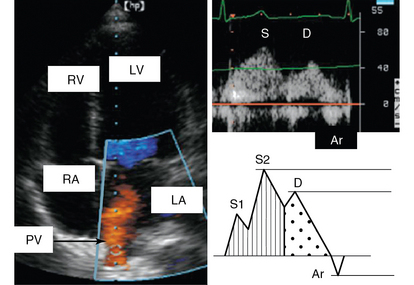
Figure 32-2 Pulmonary venous flow. Left, Color pulmonary venous flow (orange) on an apical view; right, pulmonary venous flow recorded with pulsed Doppler. Ar, Atrial reverse flow; D, diastolic flow; LA, left atrium; LV, left ventricle; PV, pulmonary vein; RA, right atrium; RV, right ventricle; S, systolic flow.
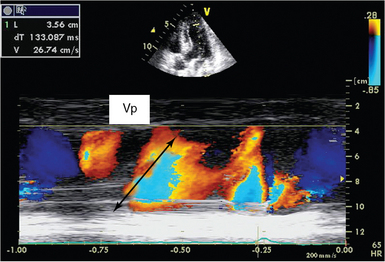
Color m-mode propagation velocity
The propagation velocity (Vp) of mitral inflow is obtained from an apical four-chamber view by placing the color Doppler box on LV inflow and adding an M-mode across it. Vp is measured on M-mode image as the slope of the red-blue transition (Figure 32-3).11 A steeper Vp of greater than 50 cm/sec is considered normal, and a flatter Vp lower than 45 cm/sec is predictive of diastolic dysfunction.4 Vp is closely related to LV relaxation and has been suggested to be independent of loading conditions, but it has poor reproducibility.
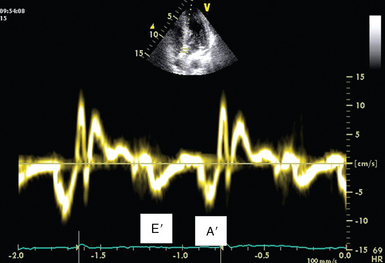
Tissue doppler mitral annulus velocity
Mitral annular velocity is measured on an apical view with tissue Doppler imaging (TDI). The sample volume should be positioned on the septal or lateral insertion sites of the mitral annulus, at the level of the insertion of the mitral leaflets, and its size should be adjusted as necessary (usually 5 to 10 mm) to cover the full range of excursion of the mitral annulus in both systole and diastole. Because mitral annular velocities have high signal amplitude, Doppler spectral gain should be set low enough to ensure a thin, clear Doppler trace. TDI waveforms can be obtained in nearly all patients (>95%) regardless of the two-dimensional image quality (Figure 32-4). Primary measurements include the peak velocities of systolic (S) and early and late diastolic excursion. Early diastolic annular velocity can be referred to as Ea, Em, E′, or e′, whereas late diastolic velocity is referred to as Aa, Am, A′, or a′. Peak E′ velocity is related to LV relaxation12 and is considered to be relatively independent of preload. An E′ value lower than 8 cm/sec at the level of the septum or lower than 10 cm/sec at the level of the lateral wall is considered indicative of impaired LV relaxation.4 An E′/A′ ratio of less than 1 is also highly suggestive of impaired LV relaxation.
Left atrial volume
LA volume is measured with apical four-chamber and two-chamber views. It is clinically important because of the significant relationship between LA remodeling and other echocardiographic indices of diastolic function. LA volume often reflects the cumulative effects of filling pressure over time. A normal-sized left atrium effectively rules out any chronic diastolic dysfunction. An LA volume greater than 34 mL/m2 is associated with diastolic dysfunction and chronic increases in LV and LA diastolic pressure; it is an independent predictor of death, heart failure, atrial fibrillation, and ischemic stroke.4,13
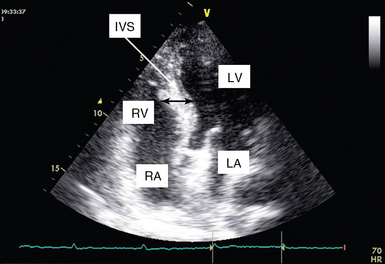
Left ventricular hypertrophy
LV hypertrophy is a main cause of diastolic dysfunction, although relaxation may also be impaired in patients with normal wall thickness. Systemic hypertension is the most common cause of LV hypertrophy, and diastolic dysfunction may be the first sign of the disease (preceding ventricular wall thickening or even a measurable rise in blood pressure).14 LV hypertrophy is best assessed by measuring LV mass. In the ICU it is rather difficult to calculate the latter, and thus LV thickness may serve as a surrogate (Figure 32-5). Nevertheless, in patients with hypovolemia and severely decreased preload, the decrease in diastolic diameter may cause the thickness of the walls to appear increased despite a normal ventricular mass (pseudohypertrophy), which is usually associated with LV obstruction.
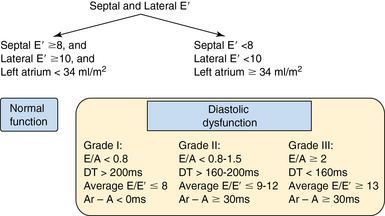
Assessment of diastolic dysfunction
Diastolic dysfunction is classified into three grades (according to Nagueh et al): mild (grade I), moderate (grade II), and severe (grade III). This classification is a strong prognosticator of all-cause mortality in the general population (Figure 32-6).15
It is essential to know the limits of each parameter. The E/A ratio appears to have better diagnostic value in patients who also have depressed systolic function than in those with normal function. The E/E′ threshold differs when E′ is recorded in the lateral or medial junction of the mitral annulus (particularly in the case of myocardial ischemia), and thus both should be recorded and averaged.
E/Vp is difficult to measure and has the lowest reproducibility of all the other Doppler indices. PVF can provide various indices but invariably requires performance of TEE. Nonetheless, E/E′ is the most feasible and easily obtained parameter and has good reproducibility. From a practical point of view, estimation of LV filling pressure in ICU patients is best achieved by evaluating all available parameters rather than focusing on only one.16
This classification does, however, have a few limitations. In older patients (>60 years) it is common to find an age-related grade I pattern (E/A ratio <1 and DT >200 msec) regardless of the actual diastolic dysfunction. Therefore a history of cardiovascular disease or the presence of LV hypertrophy is helpful in establishing a clear diagnosis. In athletes, LA size may be enlarged (≥34 mL/m2), but this is not diagnostic of diastolic dysfunction if there is no decrease in E′. Despite these limitations, the Nagueh classification provides valuable information in routine ICU practice (see Figure 32-6).
Pearls and highlights
• LV diastolic dysfunction is commonly observed in ICU patients and should be assessed by means of echocardiography, especially in patients with sepsis, respiratory failure, or shock.
• LV diastolic dysfunction is classified into three grades (according to Nagueh et al): mild (grade I), moderate (grade II), and severe (grade III). This classification is a strong prognosticator of all-cause mortality in the general population.
• The hemodynamic status of ICU patients is constantly changing (e.g., preload, afterload), and thus LV diastolic function cannot be evaluated solely by the mitral flow or pulmonary venous flow pattern.
• Normal LA size effectively rules out chronic LV diastolic dysfunction.
• Early mitral annulus velocity is the simplest and most easily applicable and reproducible Doppler parameter used (in conjunction with assessment of the mitral flow pattern and LA size) for the evaluation of LV diastolic function in ICU patients.
• The E/E′ threshold differs when E′ is recorded in the lateral or medial junction of the mitral annulus (particularly in the case of myocardial ischemia), and thus both should be recorded and averaged.
• From a practical point of view, estimation of LV filling pressure in ICU patients is best achieved by evaluating all available parameters rather than focusing on only one.
References
1. Paulus, WJ, Tschöpe, C, Sanderson, JE, et al, How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart . 2007; 28:2539–2550.
2. Landesberg, G, Gilon, D, Meroz, Y, et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012; 33:895–903.
3. Slama, M, Maizel, J. Echocardiographic measurement of ventricular function. Curr Opin Crit Care. 2006; 12:241–248.
4. Nagueh, SF, Appleton, CP, Gillebert, TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009; 22:107–133.
5. Hurrell, D, Nishimura, RA, Ilstrup, DM, Appleton, CP, Utility of preload alteration in assessment of left ventricular filling pressure by Doppler echocardiography: a simultaneous catheterization and Doppler echocardiographic study. J Am Coll Cardio. 1997; 30:459–467.
6. Appleton, CP, Hatle, LK, Popp, RL, Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardio. 1988; 12:426–440.
7. Appleton, CP, Hemodynamic determinants of Doppler pulmonary venous flow velocity components: new insights from studies in lightly sedated normal dogs. J Am Coll Cardio. 1997; 30:1562–1574.
8. Kuecherer, HF, Muhiudeen, IA, Kusumoto, FM, et al. Estimation of mean left atrial pressure from transesophageal pulsed Doppler echocardiography of pulmonary venous flow. Circulation. 1990; 82:1127–1139.
9. Ommen, SR, Nishimura, RA, Appleton, CP, et al, Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulatio. 2000; 102:1788–1794.
10. Yamamuro, A, Yoshida, K, Hozumi, T, et al. Noninvasive evaluation of pulmonary capillary wedge pressure in patients with acute myocardial infarction by deceleration time of pulmonary venous flow velocity in diastole. J Am Coll Cardiol. 1999; 34:90–94.
11. Takatsuji, H, Mikami, T, Urasawa, K, et al, A new approach for evaluation of left ventricular diastolic function: spatial and temporal analysis of left ventricular filling flow propagation by color M-mode Doppler echocardiography. J Am Coll Cardio. 1996; 27:365–371.
12. Slama, M, Ahn, J, Peltier, M, et al. Validation of echocardiographic and Doppler indexes of left ventricular relaxation in adult hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2005; 289:H1131–H1136.
13. Tsang, TS, Barnes, ME, Gersh, BJ, et al. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden,. Am J Cardiol. 2002; 90:1284–1289.
14. Dupont, S, Maizel, J, Mentaverri, R, et al. The onset of left ventricular diastolic dysfunction in SHR rats is not related to hypertrophy or hypertension. Am J Physiol Heart Circ Physiol. 2012; 302:H1524–H1532.
15. Redfield, MM, Jacobsen, SJ, Burnett, JC, Jr., et al, Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAM. 2003; 289:194–202.
16. Feissel, M, Maizel, J, Robles, G, et al. Clinical relevance of echocardiography in acute severe dyspnea. J Am Soc Echocardiogr. 2009; 22:1159–1164.