CHAPTER 311 Evaluation and Management of Spinal Axis Tumors
Metastatic
Epidemiology
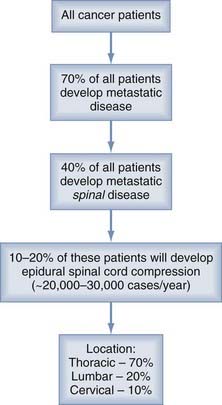
It is estimated that 1,437,180 men and women (745,180 men and 692,000 women) will be diagnosed with and 565,650 men and women will die of cancer of all sites in 2008 (http://seer.cancer.gov/csr/1975_2005/results_single/sect_01_table.01.pdf). Fifty to 70% of all cancer patients have metastases at the time of their death, and the spine is the most common osseous site.1–3 As shown in Figure 311-1, about 5% to 10% of cancer patients and up to 40% of patients with preexisting nonspinal bone metastases will develop metastatic epidural spinal cord compression from metastases.2,4–8 Of those with spinal disease, 10% to 20% will develop symptomatic spinal cord compression, resulting in 20,000 to 30,000 cases per year.9 This number is expected to increase as the baby-boomer population ages and advances in cancer treatment improve longevity, resulting in more metastatic disease.7,10,11
About 50% of spinal metastases arise from one of three primary sites—breast, lung, or prostate6,12—with additional cases from renal, gastrointestinal, thyroid, sarcoma, and the lymphoreticular malignancies, lymphoma and multiple myeloma. Metastases from prostate, breast, melanoma, and lung commonly cause spinal metastases in 90.5%, 74.3%, 54.5%, and 44.9% of patients, respectively.2 However, the risk for neurological deficits as a result of epidural spinal cord compression varies with the site of primary disease, as follows: 22% with breast cancer, 15% with lung cancer, and 10% with prostate cancer.7 Overall, 10% of patients will present with no known history of cancer, although in some of the older surgical literature, this figure was as high as 70% of the study population.11,13–16 In more than 50% of these cases, the lung is the primary source of malignancy.7,15
The thoracic spine is the most common site of disease (70%), followed by the lumbar spine (20%) and cervical spine (10%).6,7,17 Metastatic spinal disease can arise in any of three locations: the vertebral column (85%), the paravertebral region (10% to 15%), and rarely the epidural or subarachnoid and intramedullary space (<5%).6,7,17 The posterior half of the vertebral body is usually involved first, with the anterior body, lamina, and pedicles invaded later.18 Intradural metastases, including intramedullary, from non-neural primary tumors are extremely rare but have been reported.19,20 Multiple lesions at noncontiguous levels occur in 10% to 40% of cases.6,7,17,21
Presentation and Diagnosis
Spinal cord compression results from any one or a combination of four processes: direct compression from an enlarging soft tissue mass, pressure caused by fracture and retropulsion of bony fragments into the canal, severe kyphosis following vertebral collapse, and, rarely, extension of a paraspinal tumor through the intervertebral foramen. Neurological symptoms are usually gradually progressive, but alternatively may occur rapidly and present as a neurological emergency. Neurological symptoms can broadly be divided into radicular and myelopathic, the features of which depend on the level and extent of disease. Myelopathy often presents as a gait disturbance, followed by spasticity, generalized weakness, sensory loss, and autonomic dysfunction. Bowel and bladder dysfunction, which may be present on initial evaluation, is rarely unaccompanied by other symptoms. When it does appear as the only symptom, the lesion is most likely at the level of the conus medullaris. Painless urinary retention with incontinence or recurrent urinary tract infections, especially in males, strongly suggests a neurological cause. Patients who have lesions at multiple levels are more likely to be nonambulatory.22 All patients with metastatic disease should undergo a thorough baseline neurological examination. The results can be classified into well-known neurological scales such as the extended Frankel23 grading system (Table 311-1), the Eastern Cooperative Oncology Group (ECOG) performance score (Table 311-2), and the American Spinal Injury Association (ASIA) score (Table 311-3). Other authors have used a scale that focuses on gait (Table 311-4).
| GRADE | DESCRIPTION |
|---|---|
| A | No motor or sensory function |
| B | Preserved sensation only, no motor function |
| C |
From Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179.
TABLE 311-2 Eastern Cooperative Oncology Group Performance Status Grades
| GRADE | DESCRIPTION |
|---|---|
| 0 | Fully active, able to carry on all predisease performance without restriction |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature (light housework, office work) |
| 2 | Ambulatory and capable of all self-care but unable to carry out any work activities; up and about >50% of waking hours |
| 3 | Capable of only limited self-care, confined to bed or chair >50% of waking hours |
| 4 | Completely disabled; cannot carry on any self-care; totally confined to bed or chair |
| 5 | Dead |
TABLE 311-3 American Spinal Injury Association Impairment Scale
| GRADE | DESCRIPTION |
|---|---|
| A (complete) | No motor or sensory function is preserved through S4-5. |
| B (incomplete) | Sensory but no motor function is preserved below the neurological level and extends through S4-5. |
| C (incomplete) | Motor function is preserved below the neurological level, and most key muscles below the neurological level have a muscle grade <3. |
| D (incomplete) | Motor function is preserved below the neurological level, and most key muscles below the neurological level have a muscle grade ≥3. |
| E (normal) | Motor and sensory function are normal. |
| GRADE | DESCRIPTION |
|---|---|
| 1 | Normal |
| 2 | Gait with assistance |
| 3 | Paresis without gait function but still able to move legs |
| 4 | Paraplegia |
In patients with suspected epidural spinal cord compression from metastatic disease, the entire spine needs to be imaged. Magnetic resonance imaging (MRI) is the most sensitive and specific imaging modality for detecting metastatic disease. It provides excellent soft tissue, bone marrow, and neural structure visualization in the axial, coronal, and sagittal planes. Sagittal screening images can quickly evaluate the entire spine. Other modalities include plain radiographs, bone scans, and computed tomography (CT) enhanced with myelography. Radiographs are abnormal in 85% of patients with metastatic epidural compression.6 Most metastatic lesions are osteolytic, and 30% to 60% of the bone must be removed before it is visible on plain radiographs.7 Only 5% of metastases cause an osteoblastic response. Radionuclide bone scans are usually more sensitive but less specific in detecting metastatic disease than plain radiographs.5–7 Radionuclide studies identify areas of increased bone deposition. Therefore, they can easily detect osteoblastic metastases but can only detect osteolytic lesions if there is a significant amount of bone repair occurring. CT with coronal and sagittal reconstructions is more sensitive and specific than radionuclide scans.6 When combined with myelography, it provides a detailed image of both the bony and neural structures. The bony detail is especially important when planning surgical decompression. CT myelogram is useful for patients who have had spinal reconstruction with placement of metallic instrumentation, including titanium, because it is often difficult to obtain good-quality images on MRI because of artifact. However, performing a myelogram can cause neurological worsening if it is done in the presence of a high-grade block rostral to the puncture.6,7
Management
Deciding on the optimal treatment for these patients is often a difficult and complex process because of the numerous issues involved and requires input from the spine surgeon, oncologist, radiation oncologist, patient, and family members.24 With the rare exception of when the spine is the sole site of newly metastatic disease in a patient whose cancer has been successfully treated in the past, treatment is considered palliative. It is imperative that treatment be rendered as soon as possible because neurological outcome after treatment is primarily dependent on the neurological status before treatment.25,26 The primary histology and posttreatment ambulatory status are the factors that have been most consistently cited as determining survival.27–29 In one study, the median survival for breast cancer was 650 days, whereas for lung cancer it was 120 days.30 The issues that are most important to these often sick and debilitated patients are their ambulatory function, pain control, autonomic function (sexual and bowel and bladder control), overall survival, and quality of life.24
Treatment can involve chemotherapy, radiation therapy, surgery, or combination.31 Indications for surgery include radioresistant tumors (sarcoma, lung, colon, renal cell, breast), obvious spinal instability, clinically significant neural compression secondary to retropulsed bone or from spinal deformity, intractable pain unresponsive to nonoperative measures, and radiation failure (progression of deficit during treatment or spinal cord tolerance reached). Even if the patient satisfies one or more of these indications, the type and goals of surgery must be determined by the patient’s ability to tolerate the procedure (i.e., the patient’s general medical condition coupled with the complexity of the proposed operation) and, more importantly, by their estimated life expectancy. The latter is primarily based on the extent and aggressiveness of the cancer and its response to previous therapies. In general, the goals of surgery are to correct and prevent any further deformity by stabilizing the spine, decompress neural structures (spinal cord and nerves), obtain a diagnosis if the primary is unknown, and prevent local recurrence.
Harrington32 devised a five-category classification scheme for metastatic spine tumors based on bone destruction and neurological compromise: (1) no significant neurological involvement; (2) involvement of bone without collapse or instability; (3) major neurological impairment (sensory or motor) without significant involvement of bone; (4) vertebral collapse with pain resulting from mechanical causes or instability, but with no significant neurological compromise; and (5) vertebral collapse or instability combined with major neurological impairment. He mainly recommended that patients in categories 1, 2, and 3 be treated nonsurgically with chemotherapy, hormonal manipulation, or local irradiation. Patients in categories 4 or 5 require surgical intervention, but the definitive treatment option for those in category 3 were less clear.
The concept of spinal instability has been cited as a major factor in determining whether a patient is a surgical candidate. Kostuik and associates33 defined stability using a two-column concept of spinal architecture. Their anterior column consists of the entire vertebral body including the cortex, whereas the posterior column consists of pedicles, laminae, and spinous processes. The anterior column was further divided into anterior and posterior halves, as well as left and right sides, which results in four quadrants within the vertebral body. The posterior column is divided into left and right sides, for a total of six vertebral segments. The authors thought that the spine was stable if no more than two of the six segments were destroyed and unstable if three or more segments were destroyed. Tomita and colleagues34 stated that instability was present if any of the following features were present: transitional deformity, vertebral body collapse greater than 50%, three column involvement (as defined by Denis35), or involvement of the same column in two or more adjacent levels. Cybulski’s definition was simpler: pathologic fracture or evidence of bone in the spinal canal.36 It has been difficult to implement the concept of spinal instability as a factor in determining treatment because there is no agreed-on definition and the implication of such a designation is uncertain. There are certainly many patients who have been treated successfully with radiation who would have fit one definition or another of spinal instability. In fact, although the authors of the randomized controlled trial evaluating surgery compared with radiotherapy alone (see later) categorized the study patients on the presence or absence of spinal instability using the definition by Cybulski, it was neither an exclusion criteria nor a factor controlled for in the randomization process (18 of 51 patients in the radiation arm and 20 of 50 in surgery arm had an “unstable” spine).37
Most surgeons would agree that surgery should only be offered to patients with an estimated life expectancy of greater than 3 to 6 months.38 However, determining this estimate is difficult and is usually left to the expertise of the individual oncologist. In an effort to more accurately predict survival, Tokuhashi and associates proposed a preoperative prognostic scoring system in 1990 with a revised version in 1998.39,40 Their model takes into account six variables: general medical condition (Karnofsky performance score), number of extraspinal metastases, number of vertebral metastases, status of metastases to the major internal organs, primary tumor type, and presence of a neurological deficit (Table 311-5). In this revised system, total scores of 0 to 8, 9 to 11, and 12 to 15 predicted with high accuracy a life expectancy of less than 6 months, 6 months or more, and 1 year or more, respectively.39 Using this scoring system, excisional surgery (i.e., circumferential decompressive surgery with reconstruction and stabilization) was performed in those with a score greater than 12. Conservative treatment, which primarily consisted of radiation alone, was performed in patients with a score of 0 to 8. In patients with a score of 9 to 11, palliative surgery (posterior decompression alone or instrumented) was undertaken, and only in the rare patient with a single spinal lesion and no visceral metastases was excisional surgery considered. Several others, including the authors, have used this scoring system and have found it useful in making decisions regarding treatment.41–45 Another similar scoring system, using the Karnofsky score, primary tumor, and presence of visceral metastases, has shown similar abilities to stratify patient survival.46
| PROGNOSTIC FACTOR | POINTS |
|---|---|
| General Condition (Karnofsky Performance Status)% | |
| Poor (10-40) | 0 |
| Moderate (50-70) | 1 |
| Good (80-100) | 2 |
| No. of Extraspinal Bone Metastases Foci | |
| 3 or more | 0 |
| 1-2 | 1 |
| 0 | 2 |
| No. of Metastases in the Vertebral Body | |
| 3 or more | 0 |
| 2 | 1 |
| 1 | 2 |
| Metastases to the Major Internal Organs | |
| Unremovable | 0 |
| Removable | 1 |
| No metastases | 2 |
| Primary Site of the Cancer | |
| Lung, stomach | |
| Kidney, liver, uterus, other, unidentified | |
| Thyroid, prostate, breast, rectum | |
| Spinal Cord Palsy | |
| Complete | 0 |
| Incomplete | 1 |
| None | 2 |
From Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30:2186.
The scoring system by Tomita and associates used three factors: grade of malignancy, visceral metastases, and bone metastases (Table 311-6).34 For patients with a score of 2 or 3, the treatment goal is long-term local control and a wide marginal excision. For a score of 4 or 5 points, marginal or intralesional excision is recommended for middle-term local control. For a score of 6 or 7 points, the treatment goal is short-term palliation with palliative surgery; and finally, a score of 8 to 10 indicates nonoperative supportive care. The median survival times were 38.2, 21.5, 10.1, and 5.3 months, respectively. Of note, paralysis was specifically excluded as a prognostic factor because the authors believed that this did not significantly alter survival. Some authors think that this system is inadequate because it does not take into account pain and neurological function.47
TABLE 311-6 Tomita Scoring System
| PROGNOSTIC FACTOR | POINTS |
|---|---|
| Primary Tumor | |
| Slow growth (breast, prostate, thyroid) | 1 |
| Moderate growth (kidney, uterus) | 2 |
| Rapid growth (lung, liver, stomach, colon, unknown primary) | 4 |
| Visceral Metastases | |
| Treatable | 2 |
| Untreatable | 4 |
| Bone Metastases | |
| Solitary or isolated | 1 |
| Multiple | 2 |
From Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine. 2001;26:298.
North and coworkers devised a prognostic scoring system for survival and ambulatory status based on their surgical treatment of 61 patients.29 They identified risk factors for poor outcome for both outcomes after surgery and then, based on the presence or absence of these factors, calculated median survival and ambulatory rates (Tables 311-7 and 311-8). Their study highlighted the importance of tumor pathology, overall median survival of 10 months with breast cancer being 1.7 years and lung cancer 3 months, and the detrimental effect of additive risk factors. A number of other algorithms have been put forth, each with their own set of factors, but all take into consideration the degree of systemic disease, the type of cancer, and the overall prognosis of the patient.38,48,49
TABLE 311-7 Risk Factors for Poor Outcome after Decompressive Spinal Surgery
| OUTCOME | DESCRIPTION OF RISK FACTOR |
|---|---|
| Ambulation |
From North RB, LaRocca VR, Schwartz J, et al. Surgical management of spinal metastases: analysis of prognostic factors during a 10-year experience. J Neurosurg Spine. 2005;2:564.
TABLE 311-8 Prognostic Scoring System for Ambulatory Status and Survival after Decompressive Spinal Surgery
| OUTCOME | PROGNOSIS |
|---|---|
| Ambulation | |
| Breast cancer | Very low probability of losing ability to walk |
| Other tumor and 0 or 1 other risk factor | 72% likely to walk at 1.6 yr |
| Other tumor and 2 or 3 other risk factors | 50% likely to walk at 1.8-3.5 mo |
| Survival | |
| Breast cancer | 50% alive at 1.7 yr |
| Other tumor, no other risk factor | 50% alive at 8.8 mo |
| Other tumor and 1 or more risk factor | 50% alive at 4.3 mo |
From North RB, LaRocca VR, Schwartz J, et al. Surgical management of spinal metastases: analysis of prognostic factors during a 10-year experience. J Neurosurg Spine. 2005;2:564.
Medications
There is strong evidence to support the use of steroids in patients with newly diagnosed metastatic spinal disease that causes spinal cord dysfunction. Steroids have numerous theoretical benefits, including reducing vasogenic edema, protecting against lipid peroxidation and hydrolysis, enhancing blood flow, preventing ischemia and intracellular calcium accumulation, stabilizing lysosomal membranes, attenuating the inflammatory response, and supporting cellular energy metabolism.50 Dexamethasone is the most widely used steroid in patients with cancer, but methylprednisolone is more commonly used in trauma.
Loading doses range from 10 to 100 mg, followed by 4 to 24 mg 4 times a day and tapered down over several weeks.5,6,17,51–54 Larger doses are often used for those patients who present with a severe baseline or worsening neurological examination. Some physicians advocate using the trauma dose protocol in patients with rapid neurological deterioration.55 In a well-designed randomized controlled trial (RCT) that compared high-dose dexamethasone followed by radiotherapy with radiotherapy alone, 81% of patients in the treatment group were ambulatory after treatment compared with 63% in the control group.54 In another RCT, patients with a complete myelographic block who received a bolus of 100 mg followed by a standard maintenance dose had no better pain relief, ambulation, or bladder function than those who received a 10-mg bolus and the same maintenance therapy.56 It is clear, however, that higher doses are associated with more complications.57 Therefore, an appropriate regimen of dexamethasone would be an initial bolus of 10 mg followed by 16 mg/day and tapered over several weeks depending on the patient’s response to other treatments.57a Dexamethasone can often be a bridging therapy during the interval between when the patient presents and the time that a final decision is made regarding the optimal therapy (i.e., surgery versus radiation). Rarely does the patient’s neurological status decline within the first few days of presentation while taking steroids.
Steroids are not necessary for patients who present without clinical evidence of spinal cord compression. In a cohort study of 20 patients published by Maranzano and associates, all presented without neurological deficits or with only a radiculopathy.58 After radiation without steroids, all patients remained ambulatory. Finally, steroids do have an oncolytic effect on some tumors, namely lymphoma and thymoma. For patients in whom these tumors are suspected, steroids should be withheld until enough tissue is obtained to make a diagnosis.5
Bisphosphonates are a family of drugs that have shown to be effective in treating or preventing some of the complications related to osseous metastases in various cancers.59,60 There are two types of bisphosphonates: pyrophosphates (e.g., clodronate, etidronate) and aminobisphosphonates (e.g., pamidronate, zoledronic acid). These drugs work by inhibiting osteoclast activity and thus decreasing bone resorption. They also have direct tumoricidal effect. There have been a number of RCTs evaluating the use of bisphosphonates in the prevention of skeletal-related events (SREs), defined as pathologic fracture, spinal cord compression, radiation or surgery for bone metastases, or hypercalcemia. When these SREs are taken collectively, bisphosphonates have been shown to decrease the number of and time to an SRE in prostate cancer,61,62 breast cancer,63–65 multiple myeloma,64,66 lung cancer,67 and renal cell carcinoma.68 Not all bisphosphonates are equally effective. For example, Small and colleagues69 found that pamidronate did not prevent SREs, decrease analgesic use, or increase mobility in patients with metastatic prostate cancer compared with placebo. Conversely, zoledronic acid has been shown to be of benefit in prostate cancer.70
Ross and colleagues71 performed a meta-analysis of the evidence for the use of bisphosphonates in skeletal metastases from various cancers. Only randomized trials were analyzed, and the primary outcome measures were time to first SRE and reduction in skeletal morbidity assessed by pathologic fractures (vertebral, nonvertebral, combined), treatment (orthopedic surgery or radiotherapy), hypercalcemia, and spinal cord compression. Data from 18 studies were eligible for the meta-analyses, allowing the authors to analyze the effect of bisphosphonates on individual SREs rather than as a whole. They found that bisphosphonates significantly reduced the odds of suffering vertebral, nonvertebral, or combined fractures and hypercalcemia, but not spinal cord compression. Fewer patients underwent radiotherapy, but orthopedic surgery rates were not decreased. Benefits were apparent only after 6 months. Therefore, based on available data, bisphosphonates can reduce the incidence of morbidity related to skeletal metastases but should not be used with the intention of preventing spinal cord compression or as a treatment once it develops. They can be used in patients with multiple, painful spinal metastases that are not causing neurological symptoms or spinal cord compression.
Few metastatic tumors causing spinal cord compression are treated solely with chemotherapy. There are numerous reports in the literature documenting successful decompression in lymphoma,72–78 breast cancer,79 prostate cancer,80 germ cell tumors,81–84 hepatoblastoma,85 neuroblastoma,86,87 and Ewing’s sarcoma.87,88 Most of the literature is small case series and case reports and is disproportionately represented by pediatric patients.87,89,90 However, special mention needs to be given to prostate cancer. Patients with newly diagnosed, untreated prostate cancer and spinal metastases with spinal cord compression may be effectively treated with hormonal therapy followed by radiation.91,92 The lesions may be osteolytic, osteoblastic, or mixed. Typically, ketoconazole is started and gradually titrated up for the first several weeks. This effectively shuts off the production of testosterone (chemical castration). This can also be achieved by surgical castration. During this time period, the prostate-specific antigen (PSA) and testosterone levels are followed to assess for a response. If these levels decrease, ketoconazole is eventually switched over to chronic antiandrogen therapy with bicalutamide (Casodex) and goserelin acetate (Zoladex), avoiding the complications that are associated with long-term ketoconazole use. If the patient remains neurologically stable, conventional external-beam radiotherapy is initiated. However, if during the antiandrogen therapy there is no response based on the PSA or testosterone or the patient develops new or worsening neurological status, surgery should be performed, followed by radiation. Huddart and coworkers found that age younger than 65 years, no previous hormonal therapy, and a single level of compression were factors predictive of better outcome.93 Patients with no prior hormone therapy had a median survival of 627 days, but there was a 45% risk for developing a further episode of cord compression at the same or new site by 2 years.
Surgery
There has been an explosion of literature on the surgical management of spinal metastatic disease. This is due in part to the growing recognition of the beneficial effects of surgery in terms of preserving or restoring neurological function and relieving pain, coupled with the availability of surgical approaches and instrumentation that allow aggressive decompression and reconstruction of the spine. For many years, laminectomy was the only surgical option offered to patients with metastatic spine disease. In fact, “surgery” is to some extent still equated with laminectomy, contributing to the radiotherapy bias. One of the reasons that laminectomy was the dominant surgical procedure is its relative ease. It can be performed quickly with minimal intraoperative risk and does not require spinal column reconstruction or placement of internal stabilization devices. Despite its widespread use, there was no consensus among surgeons at the time regarding its effectiveness. Some thought that it was the only reasonable hope for recovering neurological deficits, whereas others found it to be of little value except for obtaining tissue to make a diagnosis and relieving pain.94,95 These surgeries were associated with significant complications, specifically wound infection or dehiscence and spinal instability. Findlay’s review of the literature found the incidence of these complications to be about 11%.96
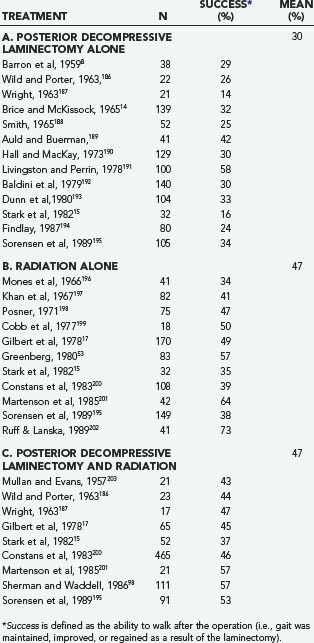
After a number of years, it became clear that laminectomy alone or in combination with radiation was no more effective in terms of preserving or restoring neurological function than radiation alone (Table 311-9). For instance, in 1978, Gilbert and colleagues17 published a single-institution, retrospective analysis of 235 patients treated with either decompressive laminectomy followed by radiation (n = 65) or radiation alone (n = 170). After treatment, 46% of those who underwent the combination treatment were ambulatory, compared with 49% of those who had radiation alone. The pretreatment neurological function was the most reliable indicator of posttreatment function. There was no significant difference in the rate of neurological recovery between the two groups. Of the 22 patients who developed rapidly progressive weakness (<48 hours), 9 underwent surgery, and 13 received radiation. None of the surgical patients improved, but 7 of the radiation patients did. The authors concluded that radiation should be the treatment of choice and that a decompressive laminectomy is indicated in only three situations: (1) to establish a diagnosis; (2) to treat a relapse if the patient is unable to undergo further radiation; and (3) if symptoms progress during radiation.
As a result, laminectomy became viewed as a procedure of minimal neurologic benefit with significant morbidity, and it was believed that radiation should assume the primary treatment role. Indiscriminate use of decompressive laminectomy was prone to failure because in most cases the tumor lies ventral to the thecal sac, making it impossible to accomplish a meaningful decompression or tumor resection without significant retraction on the thecal sac. Furthermore, a laminectomy can cause or worsen preexisting spinal instability. This can lead to progressive deformity, which in turn can result in pain and neurological compromise. Based on these data, decompressive laminectomy alone, without supplemental internal fixation, can be performed in cases in which the pathology is strictly confined to the lamina and spinous process. Others have continued to use it as a “palliative” minimal surgery for patients with a markedly attenuated survival who would not benefit from more extensive surgery.30,34,39,97
The results of decompressive laminectomy, however, do appear to be improved if internal fixation is performed as well. In a review of 134 patients treated with either a laminectomy (n = 111) or laminectomy with stabilization (n = 23), Sherman and Waddell found that the latter group had better posttreatment ambulatory status (92% versus 57%), sphincter function, and pain control and less recurrent neurological dysfunction.98 These results have been supported by other studies.55,98–104 A recent review of the literature performed by Witham and colleagues found that 64% of patients who underwent a laminectomy and stabilization had neurological improvement compared with those undergoing radiation alone (36%) or laminectomy with or without radiation (42%).105 Only anterior decompressive surgeries with instrumentation had a higher rate of neurological improvement (75%).
During the past 20 years, a new philosophy on the surgical management of metastatic spinal disease has emerged as a result of the poor results with laminectomy. One of the first reported series performing anterior spinal decompression was in 1982 by Siegal and coworkers.106 In his 1984 article, Findlay reviewed the small amount of data that existed at the time on anterior spinal surgery and found “dramatic results” with regard to neurological function, but warned that “… it is unclear as to how often such success could be achieved”.96 Since this time, there has been undisputed evidence that direct decompression of the spinal cord, usually of the ventral aspect because this is the most common site of metastatic spread, has superior neurological results, thus ushering in the new era of circumferential spinal cord decompression. The pillars of surgical management today (for those patients that are thought to benefit from aggressive surgery) are complete decompression of the spinal cord, reconstruction, and immediate stabilization of the spinal column.
A large amount of literature on circumferential spinal cord decompression has emerged during the past 20 years (Table 311-10). These publications can be grouped into four categories based on their overall focus:
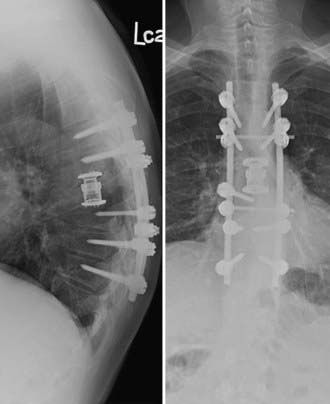
Approaches can broadly be classified as anterior (e.g., transthoracic, retroperitoneal) or posterior, including posterolateral trajectories (e.g., laminectomy, transpedicular, costotransversectomy, lateral extracavitary). The transpedicular approach in the thoracic and thoracolumbar spine has gained popularity because it allows circumferential decompression of the cord followed by reconstruction of the anterior column if needed (e.g., expandable cage, Steinmann pins with methylmethacrylate) and posterior instrumentation (Fig. 311-2). Wang and coworkers122 used this approach in 140 patients, with 96% of patients experiencing postoperative pain improvement and 75% of nonambulators regaining the ability to walk. Wound complications occurred in 16 (11.4%) patients, but unlike in other series, it was not associated with preoperative radiation. Street and colleagues used bilateral costotransversectomies to resect the diseased vertebrae and circumferentially decompress the cord.124 In our opinion, the costotransversectomy affords a wider exposure of the involved vertebra and an improved working angle (posterolateral trajectory) than the more posteriorly directed trajectory of the transpedicular approach. All patients remained neurologically stable or improved after surgery, and three of six nonambulators regained ambulatory function. Eleven patients (26%) had major complications, with nine requiring early reoperation, seven of these for wound-related complications. Chaichana and associates132 used either an anterior approach (through the vertebral body), posterior approach (through the posterior elements), or combined approach in 78 patients with the goal of completely decompressing the spinal cord. After surgical resection, 61 (78%) patients were able to ambulate at their last follow-up examination, and 12 of 23 nonambulators regained the ability to walk. Using multivariate analysis, patients who were able to walk were 2.3 times more likely to maintain this function, whereas patients who had a compressive pathologic fracture were 2.1 times less likely to walk after surgery. Furthermore, preoperative radiotherapy and symptom duration of more than 48 hours were strong negative independent predictors of regaining ambulatory function, whereas postoperative radiotherapy was a positive predictor. The authors speculated that preoperative radiation may have direct (radiation-induced myelitis) and indirect (reactive gliosis, fibrosis, compromised spinal cord blood supply) effects that may prevent neurological recovery after surgery.
Overall, the data shown in Table 311-10 suggest that neurological outcomes achieved with circumferential decompression are far superior to those achieved with decompressive laminectomy or radiation. On average, nearly 90% of patients experienced an improvement in their pain. Ambulatory function was present, on average, in 85% of the patients after surgery and was regained in 60%. Based on this literature review, a meta-analysis was conducted that confirmed the superiority in terms of maintaining and regaining ambulatory function.133 Surgical patients were 1.3 times more likely to be ambulatory after treatment and twice as likely to regain ambulatory function. Overall ambulatory success rates for surgery and radiation were 85% and 64%, respectively. Analogous to the controversy of laminectomy compared with radiation in the “old era,” it was clear that a randomized controlled trial was needed to more adequately answer the question of how circumferential surgery compared with radiation in this “new era.”
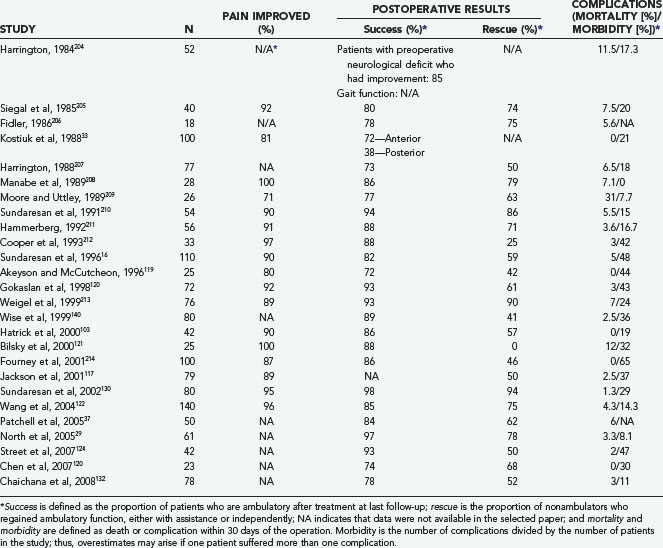
Until recently, only one randomized controlled trial had been attempted. In 1980, Young and associates134 randomized patients with a symptomatic epidural spinal lesion to receive either laminectomy followed by radiotherapy or radiotherapy alone. Although the study found no significant difference, as expected, there were too few patients enrolled, and furthermore, the major goal of the study was to demonstrate that a properly conducted RCT was feasible. In 2005, Patchell and associates37 published the results of their prospective randomized clinical trial comparing direct decompressive surgery (n = 50) followed by radiation compared to radiation only (n = 51). Both groups were treated with the same steroid radiation protocol (total dose of 30 Gy delivered over 10 fractions). Patients in the surgical arm were statistically more likely to walk after surgery (84% versus 57%), retain ambulatory function for a longer time (median, 122 versus 13 days), and have a greater chance of regaining ambulatory function when nonambulatory before treatment (62% versus 19%). Surgical patients also maintained continence, muscle strength (ASIA scores), functional ability (Frankel scores), and survival time for longer and required less narcotic and steroid use than the radiation-only patients.
This landmark study, along with subsequent ones showing a benefit to patient quality of life30,131,135 and cost-effectiveness136 with surgery, provided strong evidence for a paradigm shift in the management of metastatic epidural spinal disease. Traditional indications for surgery include radioresistant tumors (sarcoma, lung, colon, renal cell), obvious spinal instability, clinically significant neural compression secondary to retropulsed bone or from spinal deformity, intractable pain unresponsive to nonoperative measures, and radiation failure (progression of deficit during treatment or spinal cord tolerance reached). After this study, surgery should be considered the primary treatment modality in all patients with newly diagnosed metastatic disease who do not harbor any of the indications for radiotherapy (see later section).
Even with the aforementioned evidence, there are still some that feel strongly that radiation should be the primary therapy for all these patients.46,137 Some continue to suggest that patients do only “slightly better with surgery compared to radiotherapy” and that surgery should still only be performed if traditional indications (as listed previously), including paraplegia at diagnosis, are present or that most patients “… have a life span of only a few months and will not live long enough to develop disease recurrence or progression … in the irradiated spine.”12,137 Surgery suffers from the assumed disadvantage of being an invasive treatment fraught with an unacceptably high rate of complications. At the most aggressive end of the surgical spectrum, circumferential decompressive surgery, with reconstruction and stabilization, the benefits may come at the expense of a relatively high rate of complications, with one of the most common being wound breakdown.104 Using the National Inpatient Sample, Patil and colleagues analyzed more than 26,000 admissions for surgically managed spinal metastasis from 1993 to 2002.138 The overall mortality and morbidity rates were 5.6% and 21.9%, respectively. Pulmonary (6.7%) and postoperative hematomas (5.9%) were the most common complications, and complications were more likely in older patients and in patients with two or more comorbidities. One comorbidity increased the risk for in-hospital death almost fourfold. The strongest factors that have been shown to lead to wound complications include preoperative radiation, nutritional status, and amount of corticosteroid use.139–141 Thus, if surgery is being considered, ideally it should be performed soon after the diagnosis and before any radiation.
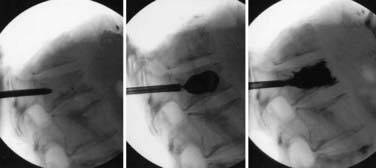
Some surgeons have begun to use minimally invasive surgical techniques in an effort to decrease the rate of complications.142–144 However, the gain made by less tissue trauma using these techniques is at a cost of more limited access and visualization required to aggressively decompress the spinal cord; thus, minimally invasive techniques have a small, selective role in these patients. Kyphoplasty and vertebroplasty are two truly minimally invasive techniques that have a proven benefit. The typical candidate for a vertebroplasty is one with a painful osteolytic metastasis or minimal pain but impending vertebral body collapse secondary to tumor infiltration.145,146 Contraindications include disruption or fragmentation of the posterior body cortex, severe loss of vertebral height, and significant epidural disease. Kyphoplasty is typically performed for patients with a significant loss of height or kyphosis (Fig. 311-3). For both of these procedures, the risk for complications is relatively small, and the improvement in pain is rapid and sustained.147,148 Newer techniques include using kyphoplasty during open procedures to strengthen and reduce a collapsed, fractured vertebral body149 and performing a percutaneous debulking before kyphoplasty balloon inflation and augmentation.150
Radiation
Indications for radiotherapy are radiosensitive tumors (lymphoma, multiple myeloma,151 small cell lung carcinoma, seminoma of testes, neuroblastoma, Ewing’s sarcoma), expected survival of less than 3 to 4 months, inability of the patient to tolerate an operation, total neurological deficit below the level of compression for more than 24 to 48 hours, and multilevel or diffuse spinal involvement. The standard radiation portal involves the diseased level with a 5-cm margin, which effectively includes two vertebral bodies above and below.152 The total radiation dose is usually 3000 to 4000 cGy and is administered over a 10- to 20-day course, with higher doses delivered in the first few days and then tapered down or the dose kept constant with each treatment (e.g., 10 fractions of 3 Gy each or 20 fractions of 2 Gy each).137,152 This is termed long-course radiotherapy. Rades and associates have shown that a 30-Gy per 10-fraction course was associated with similar outcome compared with 40 Gy per 20 fractions and was thus preferable because of less treatment time and lower costs.153
Because many patients with metastatic spinal disease have a markedly attenuated survival, short-course radiotherapy has been evaluated in the hope of decreasing patient discomfort and time spent receiving treatment. It typically comprises of 1 to 5 fractions given over 1 to 5 days (e.g., 1 or 2 fractions of 8 Gy or 5 fractions of 4 Gy each).154 Long-course therapy has been shown to have better local control and progression-free survival155 and is in general reserved for those patients who have a good survival (>6 months), whereas short-course therapy is adequate for those with poor survival (<6 months).156–158 Patients with multiple myeloma have better functional outcomes with long-course therapy, and it may be used even in the setting of spinal instability.151,159 Those with non–small cell lung cancer and bladder cancer should have short-course therapy because of their uniformly poor survival.160,161
Similar to the surgical literature, there have been attempts to predict survival in patients who received radiation so that the appropriate radiation therapy schedule can be administered. Rades and associates recently developed the first score comprising 6 factors (Table 311-11).137,162 The 6- and 12-month survival rates were 4% and 0% for patients with a total score of 20 to 25 points, 11% and 6% for 26 to 30 points, 48% and 23% for 31 to 35 points, 87% and 70% for 36 to 40 points, and 99% and 89% for 41 to 45 points. The authors concluded that patients who achieve a score of 20 to 30 points appear to be well-treated with short-course radiotherapy because of their low 6- and 12-month survival rates. A score of 31 to 35 points was considered a “gray zone,” and short-course therapy could be considered for these patients, but those who scored 36 points or more should undergo long-course therapy. The authors used the same scoring system minus the bone metastases factor to predict ambulatory status after radiation.163 Patients with a score of 28 or less should be offered short-course therapy because of their poor survival, but those with scores greater than 28 should be given long-course therapy, and in particular, patients with scores of 29 to 37 should be considered for surgery because the radiotherapy-alone results were not optimal. Patients with 37 or more points did well with radiotherapy alone.
TABLE 311-11 First Score for Predicting Survival after Radiation Therapy
| FACTOR | SCORE |
|---|---|
| Type of Primary Tumor | |
| Breast cancer | 8 |
| Prostate cancer | 7 |
| Myeloma, lymphoma | 9 |
| Lung cancer | 3 |
| Other tumors | 4 |
| Other Bone Metastases at the Time of Radiation Therapy | |
| Yes | 5 |
| No | 7 |
| Visceral Metastases at the Time of Radiation Therapy | |
| Yes | 2 |
| No | 8 |
| Interval from Tumor Diagnosis to Metastatic Spinal Cord Compression | |
| ≤15 mo | 4 |
| ≥15 mo | 7 |
| Ambulatory Status before Radiation Therapy | |
| Ambulatory | 7 |
| Nonambulatory | 3 |
| Time to Develop Motor Deficits before Radiation Therapy | |
| 1-7 days | 3 |
| 8-14 days | 6 |
| >14 days | 8 |
From Rades D, Dunst J, Schild SE. The First score predicting overall survival in patients with metastatic spinal cord compression. Cancer. 2008;112:157.
A similar scoring system was devised by van der Linden and colleagues (Table 311-12). The median overall survival was 3 months for group A (0 to 3 points), 9 months for group B (4 to 5 points), and 18.7 months for group C (6 points). The authors thought that radiation should be the primary therapy for all patients without significant neurological deficit or bony involvement without collapse or instability and that surgery should only be considered for those patients who fall into group C.
TABLE 311-12 Van der Linden’s Scoring System
| PROGNOSTIC FACTOR | POINTS |
|---|---|
| Karnofsky Performance Score | |
| 80-100 | 2 |
| 50-70 | 1 |
| 20-40 | 0 |
| Primary Tumor | |
| Breast | 3 |
| Prostate | 2 |
| Lung | 1 |
| Other | 0 |
| Visceral Metastases | |
| No | 1 |
| Yes | 0 |
From Rades D, Dunst J, Schild SE. The First score predicting overall survival in patients with metastatic spinal cord compression. Cancer. 2008;112:157.
Patients with radiosensitive tumors (breast, prostate, myeloma, lymphoma) overall have a better functional outcome than those who have more radioresistant tumors (sarcoma, lung, colon, renal cell), especially when the diagnosis of spinal cord compression is made late.164 Many patients have disease isolated to the spine, usually the vertebral body, without epidural compression. For these patients, a single dose, usually 8 Gy, provides good pain relief and is as efficacious as various fractionated regimens.165,166
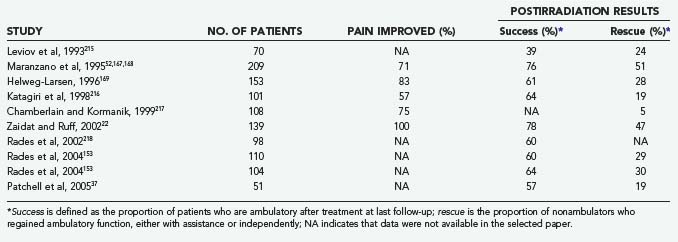
Table 311-9 depicts the results of radiotherapy during the era in which decompressive laminectomy was the predominant surgical procedure. There have been a number of reports since then, all uncontrolled cohort studies, which are shown in Table 311-13. One of the largest reports is by Maranzano and colleagues,52,167,168 who treated 209 patients with radiation (30 Gy) and steroids. Pain was present in 98% of patients before treatment, and 65% had some degree of neurological dysfunction. The average follow-up was 49 months. Pain was improved in 71% of patients, ambulatory function was improved in 36%, and bladder function was improved in 44%. Overall, 76% recovered or preserved the ability to walk. The median survival for the whole group was 6 months, with a 1-year survival rate of 28%. Favorable factors for survival included ambulatory status, both before and after treatment, and histology. Helweg-Larsen169 followed 153 patients for a median of 2.6 months. Normal gait was present in 60 (39%) patients, assisted ambulation in 19 (12%), paresis without gait function in 31 (20%), and paraplegia in 43 (29%). Neurogenic bladder was present in 57 (37%). The total radiation dose was 28 Gy, given in fractions of 4 Gy on 7 consecutive days. In total, 21 of the 74 initially nonambulatory patients (12 paraparetic, 9 paraplegic) recovered some gait function. Seven patients (2 with normal gait, 5 with assisted gait) progressed to a nonambulatory state because of treatment failure. Of those patients who presented with sphincter dysfunction, 10 (18%) regained bladder function. The median survival was 5.4 months.
Chamberlain and Kormanik reported on their 10-year experience treating 108 patients with radiation only. Seventy-seven patients had a single level involvement, whereas 31 had multiple sites. Patients received a total of 30 Gy, given in 3 Gy increments over 10 days in addition to a 10 mg bolus of dexamethasone followed by 16 mg/day. The median survival was not significantly different between those with single site disease (4.5 months) compared to multiple sites (4.0 months). Pain improved in 75% of patients. Neurologic deficit improved in 60%, stabilized in 35% and worsened in 5%. All ambulatory patients remained ambulatory after treatment. Eighty percent of non-ambulating patients regained the ability to walk (either independently or with an assistive device); only 5% of non-weight bearing patients regained weight-bearing function.217
The average pain improvement, ambulatory success, and rescue for the articles listed in Table 311-13 are 77%, 63%, and 29%, respectively. These figures are similar to those found by Falkmer and colleagues164 in their recent review article but appear to be inferior to those found in the newer surgical literature. As stated previously, the recently released results of the first well-designed RCT comparing stand-alone radiotherapy and surgery with adjuvant radiotherapy show a marked benefit for surgery.170 Thus, for patients who meet surgical criteria, the role of standard radiotherapy is as adjuvant therapy only. Conversely, there are many patients who cannot tolerate surgery or in whom surgery would be inappropriate (e.g., highly radiosensitive tumors, short life expectancy). In these patients, radiation should still serve as the primary mode of treatment.
If patients fail the first round of radiotherapy, further conventional radiotherapy is infrequently performed at the same area. Many of these patients have a very short life span and are given supportive care only, or are referred for surgical management. Nonetheless, reirradiation is an option. Rades and associates reported on 124 patients who underwent reirradiation with a total dose ranging from 77.5 to 142.6 Gy, with 92% receiving less than or equal to 120 Gy.171 Motor function did improve in 36% of patients and were stable in 50%. Posttreatment motor function was dependent on the effect of the first course of radiation, ECOG performance status, time to development of motor deficits before reirradiation, and presence of visceral metastases. No patients developed late toxicity (radiation-induced myelopathy). Nonetheless, most radiation therapists are uncomfortable reirradiating the spinal cord.
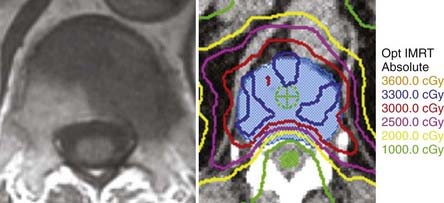
With conventional external-beam radiation, a significant amount of normal tissue, including the spinal cord, is exposed to radiation, which can lead to radiation-induced myelopathy.172–174 If radiation could be delivered to the target while decreasing the amount delivered to normal tissue, injury to the spinal cord would theoretically be reduced. Nonconventional radiotherapy, which includes stereotactic radiosurgery (SRS) and intensity-modulated radiotherapy (IMRT), is able to do just that and is typically performed with one treatment (Fig. 311-4).175–181 Yamada and colleagues treated 93 patients with 103 lesions with IMRT to doses of 18 to 24 Gy in a single fraction with the spinal cord dose limited to a maximum of 14 Gy.182 With the median follow-up of 15 months, the local control rate was 90%, and all patients with local control also reported durable symptom palliation. The median time to local failure was 9 months, and all deaths were attributable to progressive systemic disease rather than local failure. Acute toxicity was mild, and there were no instances of radiation myelopathy. Ryu and coworkers treated 49 patients with 61 lesions using radiosurgery at a single setting with doses ranging from 10 to 16 Gy.183 The median time to pain relief was 14 days; complete pain relief was achieved in 46% and partial relief in 18.9%, and overall pain control rate for 1 year was 84%. Similar symptomatic relief was achieved by Gibbs and associates in their treatment of 74 patients with 102 spinal metastases.184 In the largest series to date, Gerszten and colleagues treated 500 tumors with maximal intratumoral dose ranging 12.5 to 25 Gy.185 Long-term pain control was achieved in 86% of cases, tumor control was achieved in 90%, and 84% of patients with neurological deficits experienced some clinical improvement. Currently, the optimal patient for radiosurgery is one with a well-circumscribed tumor who has pain as the predominant symptom, lacks severe spinal cord compression or progressive neurological deficit, is a poor candidate for open surgery, and has reached the limit for external-beam radiotherapy. However, as implementation of this technology becomes more widespread within the oncology community, it is anticipated that the indications for it will broaden and may in fact be a viable alternative in many patients who are being offered one of the classic treatments—surgery or conventional radiotherapy.
Bilsky MH, Shannon FJ, Sheppard S, et al. Diagnosis and management of a metastatic tumor in the atlantoaxial spine. Spine. 2002;27:1062.
Bilsky M, Smith M. Surgical approach to epidural spinal cord compression. Hematol Oncol Clin North Am. 2006;20:1307.
Fourney DR, Abi-Said D, Lang FF, et al. Use of pedicle screw fixation in the management of malignant spinal disease: experience in 100 consecutive procedures. J Neurosurg. 2001;94(suppl 1):25.
Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32:193.
Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2008;8;:271.
Klimo PJr, Thompson CJ, Kestle JR, et al. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7:64.
Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643.
Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24:3388.
Rades D, Heidenreich F, Karstens J. Final results of a prospective study of the prognostic value of the time to develop motor deficits before irradiation in metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2002;53:975.
Rades D, Lange M, Veninga T, et al. Preliminary results of spinal cord compression recurrence evaluation (score-1) study comparing short-course versus long-course radiotherapy for local control of malignant epidural spinal cord compression. Int J Radiat Oncol Biol Phys. 2009;73:228.
Rades D, Rudat V, Veninga T, et al. Prognostic factors for functional outcome and survival after reirradiation for in-field recurrences of metastatic spinal cord compression. Cancer. 2008;113:1090-1096.
Sciubba DM, Witham TF, Gokaslan Z. Management of metastatic spine disease with spinal cord compression. Contemp Spine Surg. 2008;9:1.
Sundaresan N, Digiacinto GV, Hughes JE, et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29:645.
Sundaresan N, Rothman A, Manhart K, et al. Surgery for solitary metastases of the spine. Rationale and results of treatment. Spine. 2002;27:1802.
Tang V, Harvey D, Park Dorsay J, et al. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45:671.
Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30:2186.
Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15:1110.
Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine. 2001;26:298.
Vecht C, Haaxma-Reiche H, van Putten W, et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39:1255.
Witham TF, Khavkin YA, Gallia GL, et al. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol. 2006;2:87.
1 Bohm P, Huber J. The surgical treatment of bony metastases of the spine and limbs. J Bone Joint Surg Br. 2002;84:521.
2 Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine. 1990;15:1.
3 Harrington K. Metastatic tumors of the spine: diagnosis and treatment. J Am Acad Orthop Surg. 1993;1:76.
4 Healey JH, Brown HK. Complications of bone metastases. Cancer. 2000;88(suppl 12):2940.
5 Bilsky MH, Lis E, Raizer J, et al. The diagnosis and treatment of metastatic spinal tumor. Oncologist. 1999;4:459.
6 Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327:614.
7 Gerszten P, Welch W. Current surgical management of metastatic spinal disease. Oncology (Huntingt). 2000;14:1013.
8 Barron KD, Hirano A, Araki S. Experiences with metastatic neoplasms involving the spinal cord. Neurology. 1959;9:91.
9 Kwok Y, Tibbs PA, Patchell RA. Clinical approach to metastatic epidural spinal cord compression. Hematol Oncol Clin North Am. 2006;20:1297.
10 Schaberg J, Gainor BJ. A profile of metastatic carcinoma of the spine. Spine. 1985;10:19.
11 Lada R, Kaminski HJ, Ruff R. Metastatic spinal cord compression. In: Vecht C, editor. Neuro-Oncology Part III. Neurological Disorders in Systemic Cancer. Amsterdam: Elsevier Biomedical Publishers; 1997:167-189.
12 Yalamanchili M, Lesser GJ. Malignant spinal cord compression. Curr Treat Options Oncol. 2003;4:509.
13 Botterell EH, Fitzgerald GW. Spinal cord compression produced by extradural malignant tumours: early recognition, treatment and results. Can Med J. 1959;80:791.
14 Brice J, McKissock W. Surgical treatment of malignant extradural spinal tumours. BMJ. 1965;1:1339.
15 Stark R, Henson R, Evans S. Spinal metastases: a retrospective survey from a general hospital. Brain. 1982;105:189.
16 Sundaresan N, Steinberger AA, Moore F, et al. Indications and results of combined anterior-posterior approaches for spine surgery. J Neurosurg. 1996;85:438.
17 Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3:40.
18 Adams M, Sonntag VKH. Surgical treatment of metastatic cervical spine disease. Contemp Neurosurg. 2001;23:1.
19 Hirsh L, Thanki A, Spector H. Spinal subdural metastatic adenocarcinoma. Neurosurgery. 1982;10:621.
20 Schijns OE, Kurt E, Wessels P, et al. Intramedullary spinal cord metastasis as a first manifestation of a renal cell carcinoma: report of a case and review of the literature. Clin Neurol Neurosurg. 2000;102:249.
21 Cook AM, Lau TN, Tomlinson MJ, et al. Magnetic resonance imaging of the whole spine in suspected malignant spinal cord compression: impact on management. Clin Oncol (R Coll Radiol). 1998;10:39.
22 Zaidat O, Ruff R. Treatment of spinal epidural metastasis improves patient survival and functional state. Neurology. 2002;58:1360.
23 Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179.
24 Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “all I care about is walking and living my life.”. JAMA. 2008;299:937.
25 Poortmans P, Vulto A, Raaijmakers E. Always on a Friday? Time pattern of referral for spinal cord compression. Acta Oncol. 2001;40:88.
26 Levack P, Graham J, Collie D, et al. Don’t wait for a sensory level—listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol). 2002;14:472.
27 Rades D, Veninga T, Stalpers LJ, et al. Improved posttreatment functional outcome is associated with better survival in patients irradiated for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2007;67:1506.
28 Helweg-Larsen S, Sorensen PS, Kreiner S. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46:1163.
29 North RB, LaRocca VR, Schwartz J, et al. Surgical management of spinal metastases: analysis of prognostic factors during a 10-year experience. J Neurosurg Spine. 2005;2:564.
30 Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2008;8;:271.
31 Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol. 2008;7:459.
32 Harrington KD. Metastatic disease of the spine. J Bone Joint Surg Am. 1986;68:1110.
33 Kostuik JP, Errico TJ, Gleason TF, et al. Spinal stabilization of vertebral column tumors. Spine. 1988;13:250.
34 Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine. 2001;26:298.
35 Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817.
36 Cybulski GR. Methods of surgical stabilization for metastatic disease of the spine. Neurosurgery. 1989;25:240.
37 Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643.
38 Walker MP, Yaszemski MJ, Kim CW, et al. Metastatic disease of the spine: evaluation and treatment. Clin Orthop Relat Res. 2003;415:S165.
39 Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30:2186.
40 Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15:1110.
41 Chataigner H, Onimus M. Surgery in spinal metastasis without spinal cord compression: indications and strategy related to the risk of recurrence. Eur Spine J. 2000;9:523.
42 Enkaoua EA, Doursounian L, Chatellier G, et al. Vertebral metastases: a critical appreciation of the preoperative prognostic Tokuhashi score in a series of 71 cases. Spine. 1997;22:2293.
43 Oberndorfer S, Grisold W. The management of malignant spinal cord compression [letter]. Spine. 2000;25:653.
44 Ulmar B, Richter M, Cakir B, et al. The Tokuhashi score: significant predictive value for the life expectancy of patients with breast cancer with spinal metastases. Spine. 2005;30:2222.
45 Tang V, Harvey D, Park Dorsay J, et al. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45:671.
46 van der Linden YM, Dijkstra SP, Vonk EJ, et al. Prediction of survival in patients with metastases in the spinal column: results based on a randomized trial of radiotherapy. Cancer. 2005;103:320.
47 Bauer H, Tomita K, Kawahara N, et al. Surgical strategy for spinal metastases [letter]. Spine. 2002;27:1124.
48 Bilsky M, Smith M. Surgical approach to epidural spinal cord compression. Hematol Oncol Clin North Am. 2006;20:1307.
49 Sciubba DM, Witham TF, Gokaslan Z. Management of metastatic spine disease with spinal cord compression. Contemp Spine Surg. 2008;9:1.
50 Amar PA, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027.
51 Portenoy RK, Lipton RB, Foley KM. Back pain in the cancer patient: an algorithm for evaluation and management. Neurology. 1987;37:134.
52 Maranzano E, Latini P, Checcaglini F, et al. Radiation therapy in metastatic spinal cord compression. A prospective analysis of 105 consecutive patients. Cancer. 1991;67:1311.
53 Greenberg HS, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: results with a new treatment protocol. Ann Neurol. 1980;8:361.
54 Sorensen S, Helweg-Larsen S, Mouridsen H. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer. 1994;1:22.
55 Olerud C, Jonsson B. Surgical palliation of symptomatic spinal metastases. Acta Orthop Scand. 1996;67:513.
56 Vecht C, Haaxma-Reiche H, van Putten W, et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39:1255.
57 Heimdal K, Hirschberg H, Slettebo H, et al. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12:141.
57a Chamberlain MC, Kormanik P. Epidural spinal cord compression: a single institution’s retrospective experience. Neuro-oncol. 1999;1(2):120-123.
58 Maranzano E, Latini P, Beneventi S. Radiotherapy without steroid in selected metastatic spinal cord compression patients. A phase II trial. Am J Clin Oncol. 1996;19:179.
59 Major PP, Cook R. Efficacy of bisphosphonates in the management of skeletal complications of bone metastases and selection of clinical endpoints. Am J Clin Oncol. 2002;25:S10.
60 Coleman RE. Should bisphosphonates be the treatment of choice for metastatic bone disease? Semin Oncol. 2001;28:35.
61 Heidenreich A. Bisphosphonates in the management of metastatic prostate cancer. Oncology. 2003;65(suppl 1):5.
62 Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458.
63 Lipton A. Efficacy and safety of intravenous bisphosphonates in patients with bone metastases caused by metastatic breast cancer. Clin Breast Cancer. 2007;7(suppl 1):S14.
64 Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735.
65 Coleman RE. Efficacy of zoledronic acid and pamidronate in breast cancer patients: a comparative analysis of randomized phase III trials. Am J Clin Oncol. 2002;25:S25.
66 Ibrahim A, Scher N, Williams G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res. 2003;9:2394.
67 Rosen LS, Gordon D, Tchekmedyian S, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150.
68 Lipton A, Zheng M, Seaman J. Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinoma. Cancer. 2003;98:962.
69 Small EJ, Smith MR, Seaman JJ, et al. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21:4277.
70 Saad F. Treatment of bone complications in advanced prostate cancer: rationale for bisphosphonate use and results of a phase III trial with zoledronic acid. Semin Oncol. 2002;29:19.
71 Ross JR, Saunders Y, Edmonds PM, et al. Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ. 2003;327:469.
72 Tsukada T, Ohno T, Tsuji K, et al. Primary epidural non-Hodgkin’s lymphoma in clinical stage IEA presenting with paraplegia and showing complete recovery after combination therapy. Intern Med. 1992;31:513.
73 Acquaviva A, Marconcini S, Municchi G, et al. Non-Hodgkin lymphoma in a child presenting with acute paraplegia: a case report. Pediatr Hematol Oncol. 2003;20:245.
74 Matsubara H, Watanabe KI, Sakai H, et al. Rapid improvement of paraplegia caused by epidural involvements of Burkitt’s lymphoma with chemotherapy. Spine. 2004;29:E4.
75 Aviles A, Fernandez R, Gonzalez JL, et al. Spinal cord compression as a primary manifestation of aggressive malignant lymphomas: long-term analysis of treatments with radiotherapy, chemotherapy or combined therapy. Leuk Lymphoma. 2002;43:355.
76 McDonald AC, Nicoll JA, Rampling RP. Non-Hodgkin’s lymphoma presenting with spinal cord compression: a clinicopathological review of 25 cases. Eur J Cancer. 2000;36:207.
77 Burch PA, Grossman SA. Treatment of epidural cord compressions from Hodgkin’s disease with chemotherapy. A report of two cases and a review of the literature. Am J Med. 1988;84:555.
78 Wong ET, Portlock CS, O’Brien JP, et al. Chemosensitive epidural spinal cord disease in non-Hodgkins lymphoma. Neurology. 1996;46:1543.
79 Boogerd W, van der Sande JJ, Kroger R. Early diagnosis and treatment of spinal epidural metastasis in breast cancer: a prospective study. J Neurol Neurosurg Psychiatry. 1992;55:1188.
80 Sasagawa I, Gotoh H, Miyabayashi H, et al. Hormonal treatment of symptomatic spinal cord compression in advanced prostatic cancer. Int Urol Nephrol. 1991;23:351.
81 Pashankar FD, Steinbok P, Blair G, et al. Successful chemotherapeutic decompression of primary endodermal sinus tumor presenting with severe spinal cord compression. J Pediatr Hematol Oncol. 2001;23:170.
82 Gale J, Mead GM, Simmonds PD. Management of spinal cord and cauda equina compression secondary to epidural metastatic disease in adults with malignant germ cell tumours. Clin Oncol (R Coll Radiol). 2002;14:481.
83 Lee JK, Kim SH, Kim JH, et al. Metastatic spinal cord compression of testicular yolk sac tumor. Childs Nerv Syst. 2002;18:171.
84 Cooper K, Bajorin D, Shapiro W, et al. Decompression of epidural metastases from germ cell tumors with chemotherapy. J Neurooncol. 1990;8:275.
85 Jadhav M, Langenburg S, Fontanesi J, et al. Hepatoblastoma with spinal metastases. J Pediatr Hematol Oncol. 2000;22:524.
86 De Bernardi B, Pianca C, Pistamiglio P, et al. Neuroblastoma with symptomatic spinal cord compression at diagnosis: treatment and results with 76 cases. J Clin Oncol. 2001;19:183.
87 Hayes FA, Thompson EI, Hvizdala E, et al. Chemotherapy as an alternative to laminectomy and radiation in the management of epidural tumor. J Pediatr. 1984;104:221.
88 Sharafuddin MJ, Haddad FS, Hitchon PW, et al. Treatment options in primary Ewing’s sarcoma of the spine: report of seven cases and review of the literature. Neurosurgery. 1992;30:610.
89 Bouffet E, Marec-Berard P, Thiesse P, et al. Spinal cord compression by secondary epi- and intradural metastases in childhood. Childs Nerv Syst. 1997;13:383.
90 Pollono D, Tomarchia S, Drut R, et al. Spinal cord compression: a review of 70 pediatric patients. Pediatr Hematol Oncol. 2003;20:457.
91 Tazi H, Manunta A, Rodriguez A, et al. Spinal cord compression in metastatic prostate cancer. Eur Urol. 2003;44:527.
92 Cereceda LE, Flechon A, Droz JP. Management of vertebral metastases in prostate cancer: a retrospective analysis in 119 patients. Clin Prostate Cancer. 2003;2:34.
93 Huddart RA, Rajan B, Law M, et al. Spinal cord compression in prostate cancer: treatment outcome and prognostic factors. Radiother Oncol. 1997;44:229.
94 Black P. Spinal metastasis: current status and recommended guidelines for management. Neurosurgery. 1979;5:726.
95 Nicholls PJ, Jarecky TW. The value of posterior decompression by laminectomy for malignant tumors of the spine. Clin Orthop Relat Res. 1985;201:210.
96 Findlay GFG. Adverse effects of the management of malignant spinal cord compression. J Neurol Neurosurg Psych. 1984;47:761.
97 Schoeggl A, Reddy M, Matula C. Neurological outcome following laminectomy in spinal metastases. Spinal Cord. 2002;40:363.
98 Sherman R, Waddell J. Laminectomy for metastatic epidural spinal cord tumors. Posterior stabilization, radiotherapy, and preoperative assessment. Clin Orthop Relat Res. 1986;207:55.
99 Bauer HCF. Posterior decompression and stabilization for spinal metastases. Analysis of sixty-seven consecutive patients. J Bone Joint Surg Am. 1997;79:514.
100 Rompe JD, Hopf CG, Eysel P. Outcome after palliative posterior surgery for metastatic disease of the spine—evaluation of 106 consecutive patients after decompression and stabilisation with the Cotrel-Dubousset instrumentation. Arch Orthop Trauma Surg. 1999;119:394.
101 Kluger P, Korge A, Scharf HP. Strategy for the treatment of patients with spinal neoplasms. Spinal Cord. 1997;35:429.
102 Jonsson B, Sjostrom L, Olerud C, et al. Outcome after limited posterior surgery for thoracic and lumbar spine metastases. Eur Spine J. 1996;5:36.
103 Hatrick NC, Lucas JD, Timothy AR, et al. The surgical treatment of metastatic disease of the spine. Radiother Oncol. 2000;56:335.
104 Jansson KA, Bauer HC. Survival, complications and outcome in 282 patients operated for neurological deficit due to thoracic or lumbar spinal metastases. Eur Spine J. 2006;15:196.
105 Witham TF, Khavkin YA, Gallia GL, et al. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol. 2006;2:87.
106 Siegal T, Siegal T, Robin G, et al. Anterior decompression of the spine for metastatic epidural cord compression: a promising avenue of therapy? Ann Neurol. 1982;11:28.
107 Mazel C, Hoffmann E, Antonietti P, et al. Posterior cervicothoracic instrumentation in spine tumors. Spine. 2004;29:1246.
108 Heidecke V, Rainov NG, Burkert W. Results and outcome of neurosurgical treatment for extradural metastases in the cervical spine. Acta Neurochir (Wien). 2003;145:873.
109 Fourney DR, Abi-Said D, Rhines LD, et al. Simultaneous anterior-posterior approach to the thoracic and lumbar spine for the radical resection of tumors followed by reconstruction and stabilization. J Neurosurg. 2001;94:232.
110 Kamat A, Gilkes C, Barua NU, et al. Single-stage posterior transpedicular approach for circumferential epidural decompression and three-column stabilization using a titanium cage for upper thoracic spine neoplastic disease: a case series and technical note. Br J Neurosurg. 2008;22:92.
111 Bilsky MH, Shannon FJ, Sheppard S, et al. Diagnosis and management of a metastatic tumor in the atlantoaxial spine. Spine. 2002;27:1062.
112 Fourney DR, York JE, Cohen ZR, et al. Management of atlantoaxial metastases with posterior occipitocervical stabilization. J Neurosurg (Spine 2). 2003;98:165.
113 Ernstberger T, Kogel M, Konig F, et al. Expandable vertebral body replacement in patients with thoracolumbar spine tumors. Arch Orthop Trauma Surg. 2005;125:660.
114 Rao G, Suki D, Chakrabarti I, et al. Surgical management of primary and metastatic sarcoma of the mobile spine. J Neurosurg Spine. 2008;9:120.
115 Ogihara S, Seichi A, Hozumi T, et al. Prognostic factors for patients with spinal metastases from lung cancer. Spine. 2006;31:1585.
116 Chen YJ, Chang GC, Chen HT, et al. Surgical results of metastatic spinal cord compression secondary to non-small cell lung cancer. Spine. 2007;32:E413.
117 Jackson RJ, Gokaslan ZL, Loh SA. Metastatic renal cell carcinoma of the spine: surgical treatment and results. J Neurosurg (Spine 1). 2001;94:18.
118 Shehadi JA, Sciubba DM, Suk I, et al. Surgical treatment strategies and outcome in patients with breast cancer metastatic to the spine: a review of 87 patients. Eur Spine J. 2007;16:1179.
118a Gokaslan ZL, York JE, Walsh GL, et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998 Oct;89(4):599-609.
119 Akeyson EW, McCutcheon IE. Single-stage posterior vertebrectomy and replacement combined with posterior instrumentation for spinal metastasis. J Neurosurg. 1996;85:211.
120 Chen YJ, Hsu HC, Chen KH, et al. Transpedicular partial corpectomy without anterior vertebral reconstruction in thoracic spinal metastases. Spine. 2007;32:E623.
121 Bilsky MH, Boland P, Lis E, et al. Single-stage posterolateral transpedicle approach for spondylectomy, epidural decompression, and circumferential fusion of spinal metastases. Spine. 2000;25:2240.
122 Wang JC, Boland P, Mitra N, et al. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1;:287.
123 McLain RF, Lieberman IH. Endoscopic approaches to metastatic thoracic disease. Spine. 2000;25:1855.
124 Street J, Fisher C, Sparkes J, et al. Single-stage posterolateral vertebrectomy for the management of metastatic disease of the thoracic and lumbar spine: a prospective study of an evolving surgical technique. J Spinal Disord Tech. 2007;20:509.
125 Shaw B, Mansfield FL, Borges LF. One-stage posterolateral decompression and stabilization for primary and metastatic vertebral tumors in the thoracic and lumbar spine. J Neurosurg. 1989;70:405.
126 Chou D, Lu D, Chi J, et al. Rib-head osteotomies for posterior placement of expandable cages in the treatment of metastatic thoracic spine tumors. J Clin Neurosci. 2008;15:1043.
127 Cybulski GR, Stone JL, Opesanmi O. Spinal cord decompression via a modified costotransversectomy approach combined with posterior instrumentation for management of metastatic neoplasms of the thoracic spine. Surg Neurol. 1991;35:280.
128 Crocker M, Chitnavis B. Total thoracic vertebrectomy with anterior and posterior column reconstruction via single posterior approach. Br J Neurosurg. 2007;21:28.
129 Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochir (Wien). 1998;140:957.
130 Sundaresan N, Rothman A, Manhart K, et al. Surgery for solitary metastases of the spine. Rationale and results of treatment. Spine. 2002;27:1802.
131 Mannion RJ, Wilby M, Godward S, et al. The surgical management of metastatic spinal disease: prospective assessment and long-term follow-up. Br J Neurosurg. 2007;21:593.
132 Chaichana KL, Woodworth GF, Sciubba DM, et al. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery. 2008;62:683.
133 Klimo PJr, Thompson CJ, Kestle JR, et al. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7:64.
134 Young R, Post E, King G. Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg. 1980;53:741.
135 Falicov A, Fisher CG, Sparkes J, et al. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine. 2006;31:2849.
136 Thomas KC, Nosyk B, Fisher CG, et al. Cost-effectiveness of surgery plus radiotherapy versus radiotherapy alone for metastatic epidural spinal cord compression. Int J Radiat Oncol Biol Phys. 2006;66:1212.
137 Rades D, Dunst J, Schild SE. The First score predicting overall survival in patients with metastatic spinal cord compression. Cancer. 2008;112:157.
138 Patil CG, Lad SP, Santarelli J, et al. National inpatient complications and outcomes after surgery for spinal metastasis from 1993-2002. Cancer. 2007;110:625.
139 McPhee IB, Williams RP, Swanson CE. Factors influencing wound healing after surgery for metastatic disease of the spine. Spine. 1998;23:726.
140 Wise JJ, Fischgrund JS, Herkowitz HN, et al. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine. 1999;24:1943.
141 McDonnell MF, Glassman SD, Dimar JR, et al. Perioperative complications of anterior procedures of the spine. J Bone Joint Surg Am. 1996;78:839.
142 Deutsch H, Boco T, Lobel J. Minimally invasive transpedicular vertebrectomy for metastatic disease to the thoracic spine. J Spinal Disord Tech. 2008;21:101.
143 Huang TJ, Hsu RW, Li YY, et al. Minimal access spinal surgery (MASS) in treating thoracic spine metastasis. Spine. 2006;31:1860.
144 Singh K, Samartzis D, Vaccaro AR, et al. Current concepts in the management of metastatic spinal disease. The role of minimally-invasive approaches. J Bone Joint Surg Br. 2006;88:434.
145 Jensen ME, Kallmes DE. Percutaneous vertebroplasty in the treatment of malignant spine disease. Cancer J. 2002;8:194.
146 Weill A, Chiras J, Simon JM, et al. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199:241.
147 Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(suppl 1):23.
148 Masala S, Fiori R, Massari F, et al. Vertebroplasty and kyphoplasty: new equipment for malignant vertebral fractures treatment. J Exp Clin Cancer Res. 2003;22:75.
149 Fuentes S, Metellus P, Pech-Gourg G, et al. Open kyphoplasty for management of metastatic and severe osteoporotic spinal fracture [technical note]. J Neurosurg Spine. 2007;6:284.
150 Gerszten PC, Welch WC. Combined percutaneous transpedicular tumor debulking and kyphoplasty for pathological compression fractures [technical note]. J Neurosurg Spine. 2007;6:92.
151 Rao G, Ha CS, Chakrabarti I, et al. Multiple myeloma of the cervical spine: treatment strategies for pain and spinal instability. J Neurosurg Spine. 2006;5:140.
152 Linstadt D. Spinal cord. In: Leibel S, Phillips T, editors. Textbook of Radiation Oncology. Philadelphia: Saunders; 1998:408-411.
153 Rades D, Fehlauer F, Stalpers LJ, et al. A prospective evaluation of two radiotherapy schedules with 10 versus 20 fractions for the treatment of metastatic spinal cord compression: final results of a multicenter study. Cancer. 2004;101:2687.
154 Maranzano E, Latini P, Perrucci E, et al. Short-course radiotherapy (8 Gy × 2) in metastatic spinal cord compression: an effective and feasible treatment. Int J Radiat Oncol Biol Phys. 1997;38:1037.
155 Rades D, Lange M, Veninga T, et al. Preliminary results of spinal cord compression recurrence evaluation (score-1) study comparing short-course versus long-course radiotherapy for local control of malignant epidural spinal cord compression. Int J Radiat Oncol Biol Phys. 2009;73:228.
156 Rades D, Stalpers LJ, Veninga T, et al. Evaluation of functional outcome and local control after radiotherapy for metastatic spinal cord compression in patients with prostate cancer. J Urol. 2006;175:552.
157 Rades D, Veninga T, Stalpers LJ, et al. Prognostic factors predicting functional outcomes, recurrence-free survival, and overall survival after radiotherapy for metastatic spinal cord compression in breast cancer patients. Int J Radiat Oncol Biol Phys. 2006;64:182.
158 Rades D, Hoskin PJ, Karstens JH, et al. Radiotherapy of metastatic spinal cord compression in very elderly patients. Int J Radiat Oncol Biol Phys. 2007;67:256.
159 Rades D, Hoskin PJ, Stalpers LJ, et al. Short-course radiotherapy is not optimal for spinal cord compression due to myeloma. Int J Radiat Oncol Biol Phys. 2006;64:1452.
160 Rades D, Walz J, Schild SE, et al. Do bladder cancer patients with metastatic spinal cord compression benefit from radiotherapy alone? Urology. 2007;69:1081.
161 Rades D, Stalpers LJ, Schulte R, et al. Defining the appropriate radiotherapy regimen for metastatic spinal cord compression in non-small cell lung cancer patients. Eur J Cancer. 2006;42:1052.
162 Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24:3388.
163 Rades D, Rudat V, Veninga T, et al. A score predicting posttreatment ambulatory status in patients irradiated for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2008;72:905-908.
164 Falkmer U, Jarhult J, Wersall P, et al. A systematic overview of radiation therapy effects in skeletal metastases. Acta Oncol. 2003;42:620.
165 Jeremic B, Shibamoto Y, Acimovic L, et al. A randomized trial of three single-dose radiation therapy regimens in the treatment of metastatic bone pain. Int J Radiat Oncol Biol Phys. 1998;42:161.
166 Jeremic B. Single fraction external beam radiation therapy in the treatment of localized metastatic bone pain. A review. J Pain Symptom Manage. 2001;22:1048.
167 Latini P, Maranzano E, Ricci S, et al. Role of radiotherapy in metastatic spinal cord compression: preliminary results from a prospective trial. Radiother Oncol. 1989;15:227.
168 Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959.
169 Helweg-Larsen S. Clinical outcome in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol Scand. 1996;94:269.
170 Patchell R, Tibbs P, Regine W, et al. A randomized trial of direct decompressive surgical resection in the treatment of spinal cord compression caused by metastasis (abstract). Chicago, IL: 2003 Annual ASCO Meeting, May 31-June 3, 2003.
171 Rades D, Rudat V, Veninga T, et al. Prognostic factors for functional outcome and survival after reirradiation for in-field recurrences of metastatic spinal cord compression. Cancer. 2008;113:1090-1096.
172 Tan B, Hkor T. Radiation myelitis in carcinoma of the nasopharynx. Clin Radiol. 1969;20:329.
173 Koehler PJ, Verbiest H, Jager J, et al. Delayed radiation myelopathy: serial MR-imaging and pathology. Clin Neurol Neurosurg. 1996;98:197.
174 Wara W, Phillips T, Sheline G, et al. Radiation tolerance of the spinal cord. Cancer. 1975;35:1558.
175 Finn MA, Vrionis FD, Schmidt MH. Spinal radiosurgery for metastatic disease of the spine. Cancer Control. 2007;14:405.
176 Gerszten PC, Burton SA, Belani CP, et al. Radiosurgery for the treatment of spinal lung metastases. Cancer. 2006;107:2653.
177 Ryu S, Yin FF, Rock J, et al. Image-guided and intensity modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97:2013.
178 Ryu SI, Kim DH, Martin DP, et al. Image-guided spinal stereotactic radiosurgery. Tech Neurosurg. 2003;8:56.
179 Ryu SI, Chang SD, Kim DH, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838.
180 Gertszten P, Ozhasoglu C, Burton S, et al. Feasibility of frameless single-fraction stereotactic radiosurgery for spinal lesions. Neurosurg Focus. 2001;13(article 2):1.
181 Gerszten PC, Welch WC. CyberKnife radiosurgery for the spine. Tech Neurosurg. 2003;9:232.
182 Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484.
183 Ryu S, Jin R, Jin JY, et al. Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manage. 2008;35:292.
184 Gibbs IC, Kamnerdsupaphon P, Ryu MR, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185.
185 Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32:193.
186 Wild W, Porter R. Metastatic epidural tumor of the spine: a study of 45 cases. Arch Surg. 1963;87:825.
187 Wright R. Malignant tumors in the spinal extradural space: results of surgical treatment. Ann Surg. 1963;157:227.
188 Smith R. An evaluation of surgical treatment for spinal cord compression due to metastatic carcinoma. J Neurol Neurosurg Psych. 1965;28:152.
189 Auld AW, Buerman A. Metastatic spinal epidural tumors: an analysis of 50 cases. Arch Neurol. 1966;15:100.
190 Hall AJ, Mackay NNS. The results of laminectomy for compression of the cord or cauda equina by extradural malignant tumour. J Bone Joint Surg Br. 1973;55:497.
191 Livingston KE, Perrin RG. The neurosurgical management of spinal metastases causing cord and cauda equina compression. J Neurosurg. 1978;49:839.
192 Baldini M, Tonnarelli GP, Princi L, et al. Neurological results in spinal cord metastases. Neurochirurgia. 1979;22:159.
193 Dunn RC, Kelly WA, Wohns RNW, et al. Spinal epidural neoplasia. A 15-year review of the results of surgical therapy. J Neurosurg. 1980;52:47.
194 Findlay GFG. The role of vertebral body collapse in the management of malignant spinal cord compression. J Neurol Neurosurg Psych. 1987;50:151.
195 Sorensen PS, Brgesen SE, Rohde K, et al. Metastatic epidural spinal cord compression. Results of treatment and survival. Cancer. 1989;65:1502.
196 Mones RJ, Dozier D, Berrett A. Analysis of medical treatment of malignant extradural spinal cord tumors. Cancer. 1966;19:1842.
197 Khan FR, Glicksman AS, Chu FCH, et al. Treatment by radiotherapy of spinal cord compression due to extradural metastases. Radiology. 1967;89:495.
198 Posner JB. Spinal cord compression: a neurological emergency. Clin Bull. 1971;1:65.
199 Cobb CA, Leavens ME, Eckles N. Indications for nonoperative treatment of spinal cord compression due to breast cancer. J Neurosurg. 1977:47.
200 Constans JP, de Divitiis E, Donzelli R, et al. Spinal metastases with neurological manifestations: review of 600 cases. J Neurosurg. 1983;59:111.
201 Martenson JA, Evans RG, Lie MR, et al. Treatment outcome and complications in patients treated for malignant epidural spinal cord compression. J Neurooncol. 1985;3:77.
202 Ruff R, Lanska D. Epidural metastases in prospectively evaluated veterans with cancer and back pain. Cancer. 1989;63:2234.
203 Mullan J, Evans J. Neoplastic disease of the spinal extradural space: a review of fifty cases. Arch Surg. 1957;74:900.
204 Harrington K. Anterior cord decompression and spinal stabilization for patients with metastatic lesions of the spine. J Neurosurg. 1984;61:107.
205 Siegal T, Tiqva P, Siegal T. Vertebral body resection for epidural compression by malignant tumors. J Bone Joint Surg Am. 1985;67:375.
206 Fidler MW. Anterior decompression and stabilisation of metastatic spinal fractures. J Bone Joint Surg Br. 1986;68:83.
207 Harrington K. Anterior decompression and stabilization of the spine as a treatment for vertebral collapse and spinal cord compression from metastatic malignancy. Clin Orthop Relat Res. 1988;233:177.
208 Manabe S, Tateishi A, Abe M, et al. Surgical treatment of metastatic tumors of the spine. Spine. 1989;14:41.
209 Moore A, Uttley D. Anterior decompression and stabilization of the spine in malignant disease. Neurosurgery. 1989;24:713.
210 Sundaresan N, Digiacinto GV, Hughes JE, et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29:645.
211 Hammerberg KW. Surgical treatment of metastatic spine disease. Spine. 1992;17:1148.
212 Cooper PR, Errico TJ, Martin R, et al. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery. 1993;32:1.
213 Weigel B, Maghsudi M, Neumann C, et al. Surgical management of symptomatic spinal metastases. Postoperative outcome and quality of life. Spine. 1999;24:2240.
214 Fourney DR, Abi-Said D, Lang FF, et al. Use of pedicle screw fixation in the management of malignant spinal disease: experience in 100 consecutive procedures. J Neurosurg. 2001;94(suppl 1):25.
215 Leviov M, Dale J, Stein M, et al. The management of metastatic spinal cord compression: a radiotherapeutic success ceiling. Int J Radiat Oncol Biol Phys. 1993;27:231.
216 Katagiri H, Takahashi M, Inagaki J, et al. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys. 1998;42:1127.
217 Chamberlain MC, Kormanik P. Epidural spinal cord compression: a single institution’s retrospective experience. Neuro Oncol. 1999;1:120.
218 Rades K, Heidenreich F, Karstens J. Final results of a prospective study of the prognostic value of the time to develop motor deficits before irradiation in metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2002;53:975.