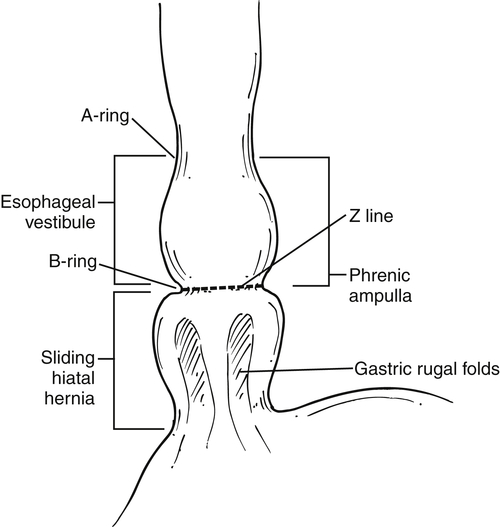
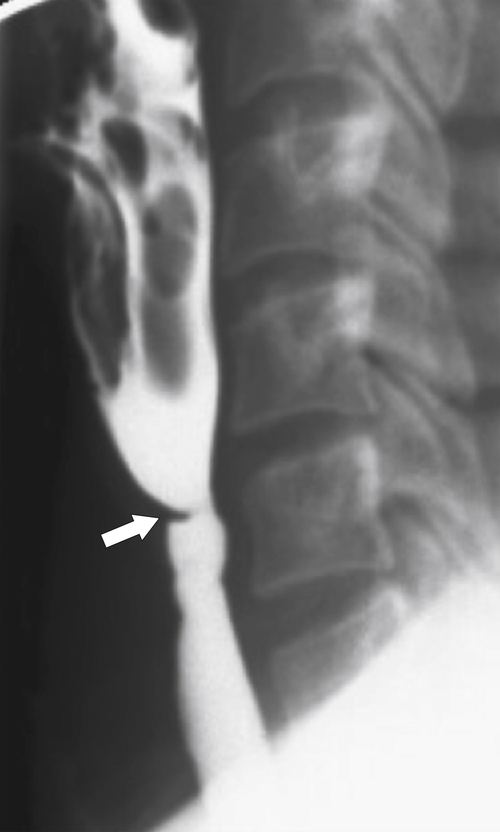
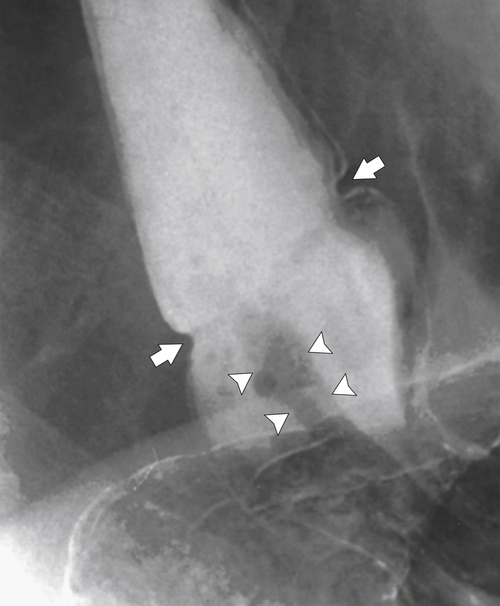
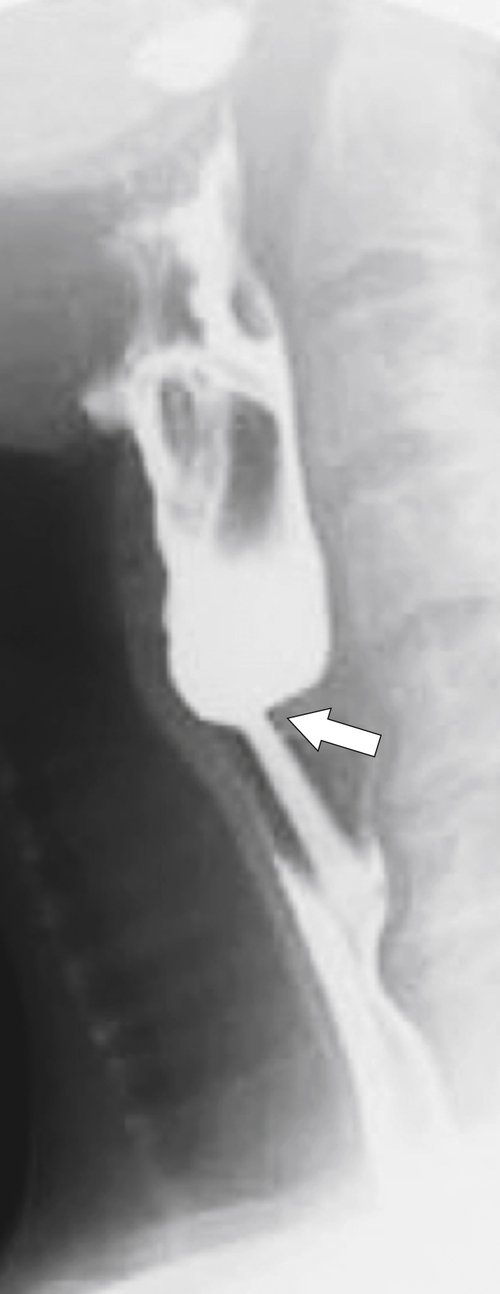
). At the upper end of the vestibule is a slight narrowing, or A-ring, caused by smooth muscle (internal esophageal sphincter), which can be normal or may cause slight dysphagia if hypertrophied. The B-ring is at the GE junction itself (at the lower end of the vestibule, also known as phrenic ampulla) and is not seen unless a hiatal hernia is present. The Z line may be seen as a slight narrowing at the lower end of the phrenic ampulla and represents the epithelial junction between the esophagus (squamous) and stomach (columnar) and will not be seen unless a hiatal hernia is present. Dysphagia will not occur unless the B ring in the lower esophagus is less than 12 to 13 mm, when it is known as a Schatzki∗ ring (see discussion later in chapter).
Figure 1-1 Schematic representation of lower esophageal anatomy (in the presence of a small hiatal hernia).
Techniques
Oral Contrast Studies
Although cross-sectional imaging techniques are critical for the evaluation of malignant esophageal disease for staging purposes, most esophageal abnormalities are too small and fine to be accurately evaluated by them. Contrast examination of the esophagus (usually with barium) is an appropriate tool for the evaluation of most esophageal disease, but the radiologist’s role has been greatly diminished since the advent and routine use of direct optical endoscopy. However, given that the barium swallow and upper gastrointestinal (UGI) studies can be exquisite tools for the assessment of both morphological (gross appearance of the pharynx, esophagus, GE junction) and functional esophageal abnormalities (pharyngeal function, esophageal dysmotility, gastroesophageal reflux disease [GERD]), radiologists should still be familiar with their use and imaging findings.
A UGI swallow examination is best performed with both single- and double-contrast techniques. Before beginning the examination, the radiologist should further question the patient about his or her symptoms and history. The information gleaned from the patient can offer clues and greater specificity about what the radiologist might expect, and the radiologist might then modify or tailor the examination accordingly. At this point, the radiologist should explain the procedure to the patient because compliance is crucial to obtain an optimal examination. For instance, after the initial ingestion of effervescent gas granules, the patient should try as best as possible to refrain from eructation, which might defeat the purpose of performing a double-contrast examination. Maximal esophageal and gastric
distention provides better images and therefore a greater ability to detect subtle disease. In the upright position, the patient then ingests the gas granules, followed by a small sip of water to aid rapid swallowing. The goal is to prevent the granules from “fizzing” in the mouth, which reduces their distensive effect in the esophagus and stomach. The patient then swallows a cup of high-density (“thick”) barium, at which point images are taken of the gas-distended esophagus and small mucosal abnormalities can be identified. If any abnormality is identified at this point, multiple tangential views should be taken to allow the radiologist to evaluate the lesion in more detail once the examination is finished. Too often, inadequate oblique and tangential views are taken, resulting in the lesion being visible in a limited plane, which may make formal diagnosis difficult or even impossible. Frequently the examination is performed in conjunction with a UGI series with gastric and duodenal evaluation, and the radiologist will need to then concentrate on these organs while they are maximally distended with air. The radiologist should return later to a final evaluation of the esophagus using a single-contrast examination with low-density (“thin”) barium, with the patient typically in the right anterior or prone oblique position. The patient takes several sips of barium, and esophageal motility and distensibility are evaluated as the radiologist observes the stripping waves of esophageal bolus propulsion. The lower esophagus is finally evaluated for hernias and the mucosal B-ring. GERD or a hiatal hernia may not initially be evident, and the patient should be asked to perform a Valsalva∗ maneuver as a provocative measure to increase intraabdominal pressure. This action may elicit either the hiatal hernia or reflux and perhaps be the answer to the patient’s symptoms. In this position, the normal longitudinal mucosal relief images are also observed, and this observation may permit variceal visualization. Finally, the stomach and duodenum should be briefly evaluated in case the patient’s symptoms are due to disease in these organs.If the patient’s symptom is upper dysphagia, then anteroposterior (AP) and lateral views of the upper esophagus are taken immediately after the ingestion of the effervescent granules. While the patient drinks the barium, the radiologist both observes and performs rapid sequence images (3 to 4 per second) because the barium usually passes through the esophagus too fast for the radiologist to time the exposure correctly. Therefore functional information (i.e., a cricopharyngeus spasm) and morphological disease can be obtained at the same time.
The use of nonionic water-soluble contrast medium, instead of barium, is warranted when there is any risk of aspiration or esophageal leak. Although barium is inert and not toxic if inhaled, it may remain within bronchi for an extended period of time. Ionic contrast medium within the bronchi is hyperosmolar and can cause pulmonary edema and generally should not be used for esophageal examination. Barium is also toxic within the mediastinum and peritoneum, hence the use of nonionic contrast medium if esophageal perforation is suspected. Water-soluble contrast medium, which is less dense than barium, may not identify small leaks. If no initial leak or aspiration is identified, it is then prudent to follow the examination with denser barium, which may identify a small, contained leak. Even if there is some leakage of barium in these circumstances, it will likely be small, given that water-soluble contrast medium failed to identify any leakage.
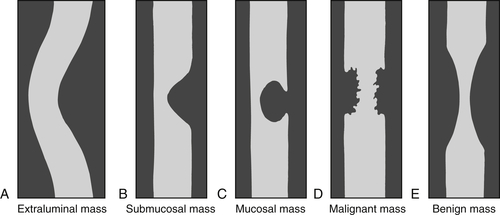
Contrast studies of the esophagus (including any viscus) are often effective at characterizing the nature of the lesion or abnormality. Extrinsic (extraluminal) masses tend to displace the bowel because of their mass effect and demonstrate shallow, or obtuse, margins on contrast studies (
Fig.1-2). Masses that originate in the submucosa (or have an intramural origin) tend to demonstrate sharper, less obtuse margins (
Fig. 1-2). Mucosal masses tend to demonstrate acute margins, sometimes pedunculated and sometimes with a stalk. Furthermore, the intraluminal contrast appearances can suggest malignancy or benignity because malignant lesions tend to demonstrate abrupt, sharp margins that are usually irregular (sometimes termed shouldering) and are often short (Fig. 1-2). Benign lesions, on the other hand, demonstrate smoother borders with little irregularity, although some larger lesions may ulcerate as they outgrow their vascular supply. These rules of thumb generally apply to the entire gastrointestinal (GI) tract.
Figure 1-2 Schematic representation of extraluminal (A), submucosal (B), mucosal (C), malignant (D), and benign (E) mass features in contrast imaging of the GI tract.
Computed Tomography
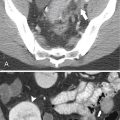
Computed tomography (CT), although still important in the evaluation of esophageal disease, is not the investigation technique of choice for most diseases unless the patient has esophageal malignancies for which it is used for staging purposes. CT can also be used to evaluate extraluminal or submucosal masses that may impinge on the esophagus because these cannot be observed directly by barium or endoscopic studies. It is also used to evaluate traumatic conditions of perforation, which are iatrogenic, traumatic, or spontaneous. Ideally the patient is asked to drink a cup of contrast material immediately before the CT to delineate the lumen.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) has even fewer applications in the esophagus given that respiratory motion artifacts are common when the chest is evaluated with MRI, although it may serve to evaluate mediastinal and paraesophageal abnormalities when CT is not indicated.
Endoscopic Ultrasound
Endoscopic ultrasound has a role in evaluation of submucosal esophageal masses but is rarely performed. Ultrasound has little use otherwise in the esophagus.
Nuclear Medicine
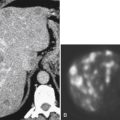
Nuclear medicine still has a role in the functional examination of esophageal motility and reflux disorder, particularly in children. The patient swallows technetium-99m (
99mTc) sulfur colloid, and multiple dynamic views are taken to assess esophageal transit time, particularly in patients with lower esophageal sphincter abnormalities. This test may be used in patients who cannot tolerate manometric endoscopic studies.
99mTc pertechnetate is also used, particularly in children. After the patient swallows the radiolabelled liquid, multiple dynamic images are obtained for the evaluation of gastroesophageal reflux disease (GERD) or delayed gastric emptying. In an adult, however, the use of positron emission tomography (PET) or PET/CT has become more widespread. The use of PET or PET/CT in the evaluation of the esophagus itself is
limited because most primary malignancies can be evaluated directly with endoscopy or by CT. However, PET or PET/CT has proved to be particularly useful in the staging and follow-up assessment of extraesophageal disease, mainly regional lymphadenopathy, which endoscopy cannot see, and CT often cannot determine whether the node is metastatic or benign, particularly if it is small.
Diverticula
Zenker Diverticulum
Zenker
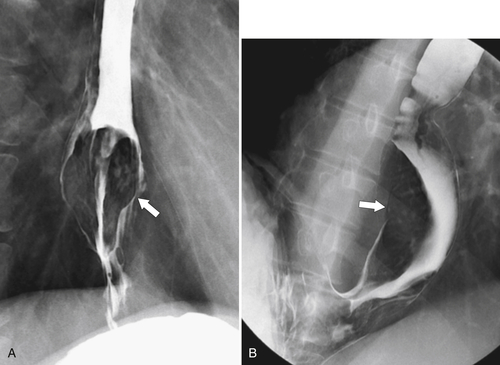
∗ diverticulum is a pulsion diverticulum, usually seen in the elderly, that is due to prolonged intraluminal pressure pushing the esophageal mucosa and submucosa through the medial defect (Killian dehiscence) between the horizontal and oblique fibers of the inferior constrictor muscle at the pharyngoesophageal junction. Patients usually have evidence of esophageal dysmotility and GERD. Patients usually present because of dysphagia, halitosis, and sometimes aspiration pneumonia as fetid food becomes trapped in the diverticulum and steadily enlarges it.
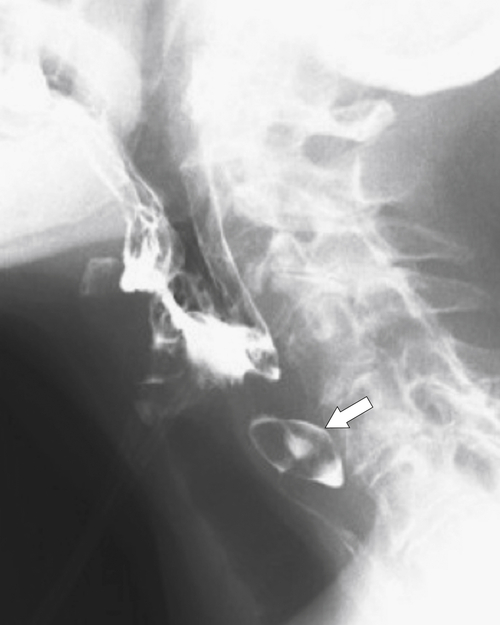
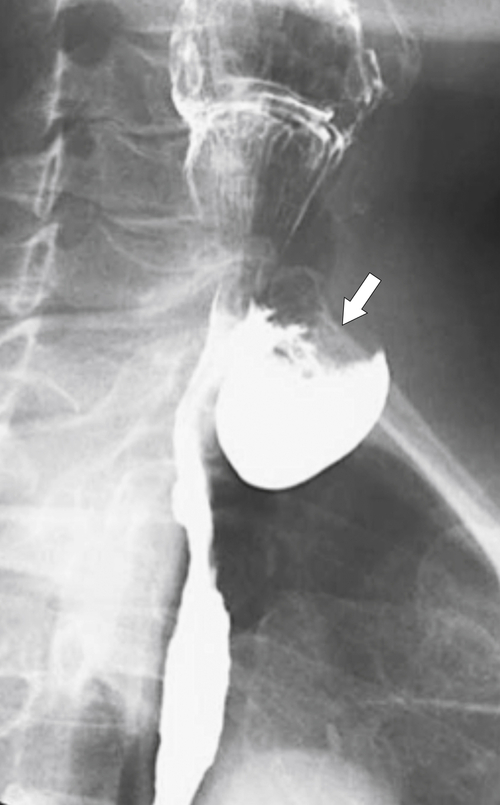
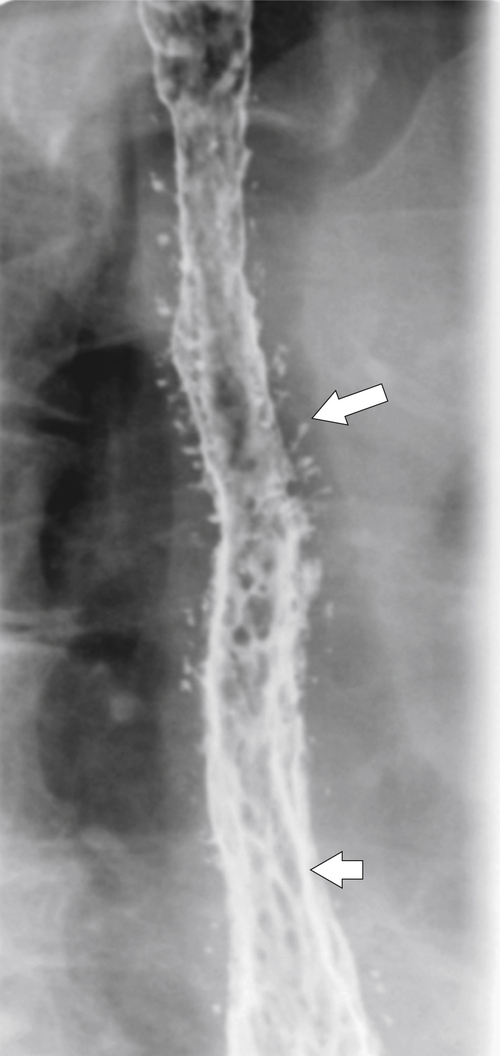
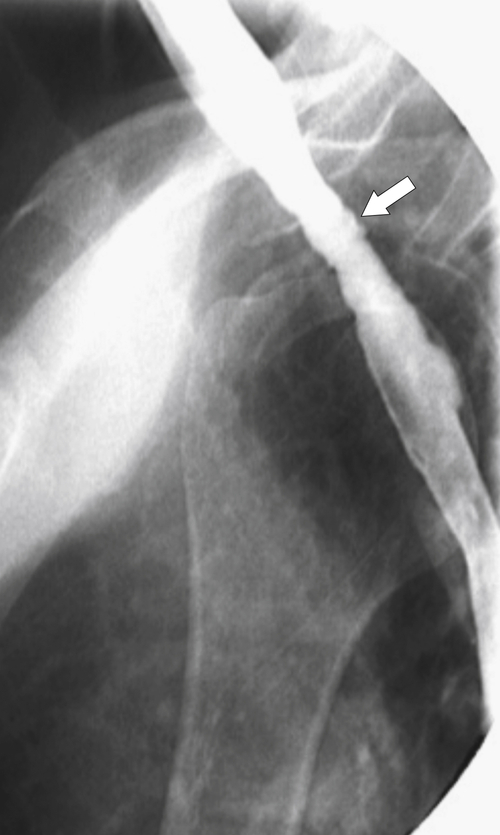
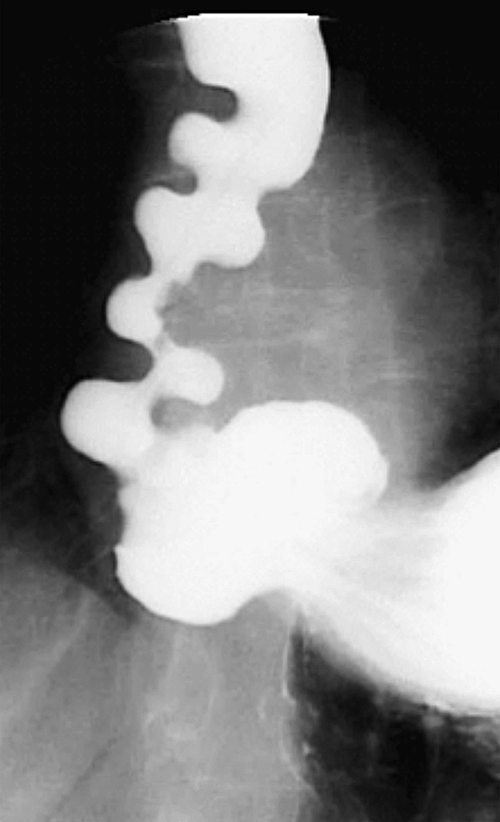
Zenker diverticulum is confirmed at barium swallow as a contrast-filled sac that is posterolateral to the esophagus just above C5-6 and the cricopharyngeus muscle. When it is small, Zenker diverticulum is usually detected best in the true lateral position as a small posterior outpouch, but as it enlarges, it is easy to identify as it extends laterally to avoid the cervical spine (
Figs. 1-3 and
1-4). The larger the diverticulum, the greater the compression on the normal esophagus, which can become narrow.
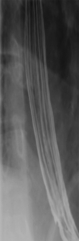
Figure 1-3 Lateral UGI swallow in a 76-year-old woman with a small Zenker diverticulum (arrow) with a peanut lodged inside.
Figure 1-4 Left posterior oblique barium swallow in a 69-year-old man with a large outpouching (arrow) from the left esophagus due to a Zenker diverticulum.
There is an increased incidence of ulceration and carcinoma developing in the diverticulum. Perforation can also occur in patients because of the inadvertent placement of endoscopic instruments or nasogastric tubes.
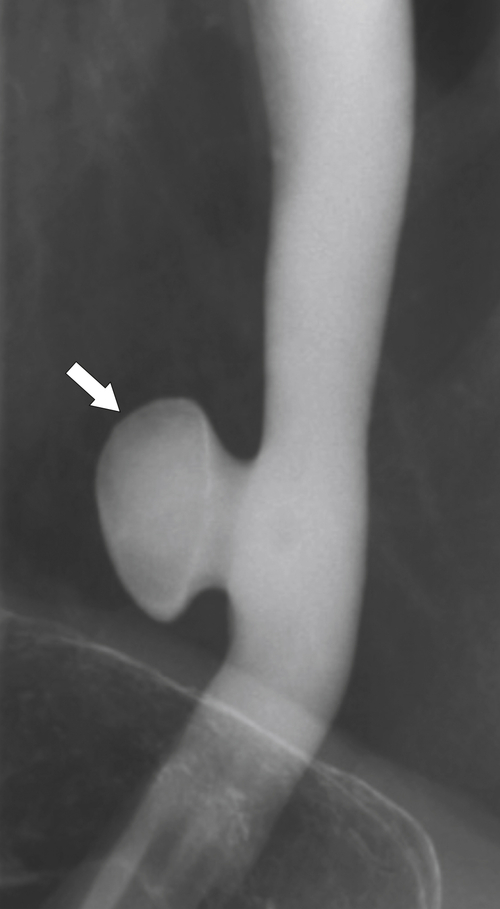
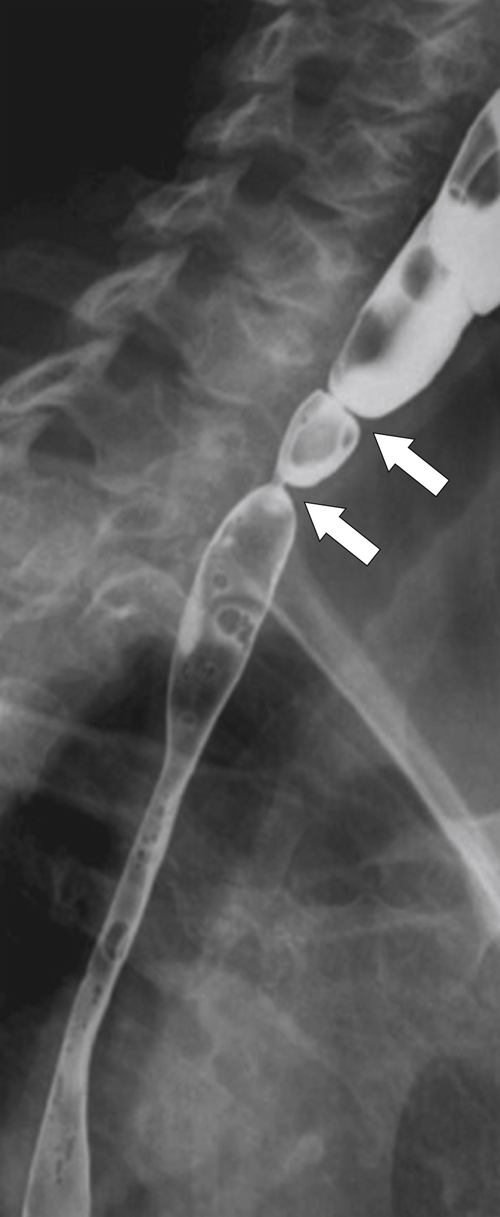
Killian-Jamieson Diverticulum
Killian-Jamieson
∗ diverticula are rare; they are observed below the level of the cricopharyngeus muscle, anterolateral to the cervical esophagus. They are also pulsion diverticula through the Killian-Jamieson space (similar to Zenker diverticula) but are much smaller than most Zenker diverticula and therefore produce symptoms and complications less commonly. They are seen as small, rounded, smooth outpouches of the lateral upper esophageal wall (
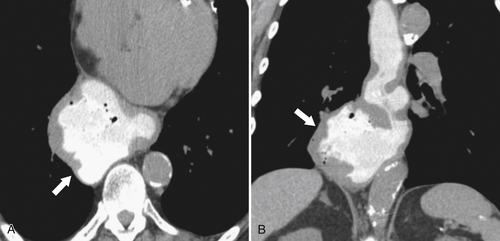
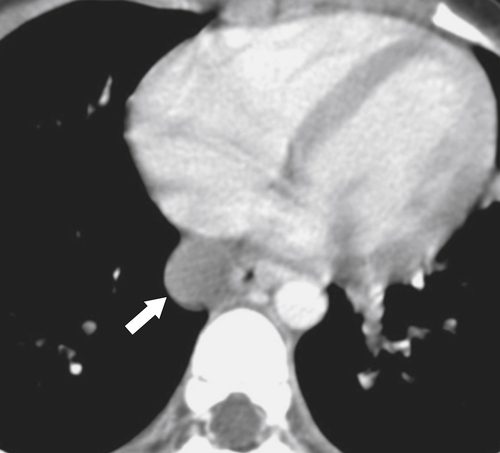
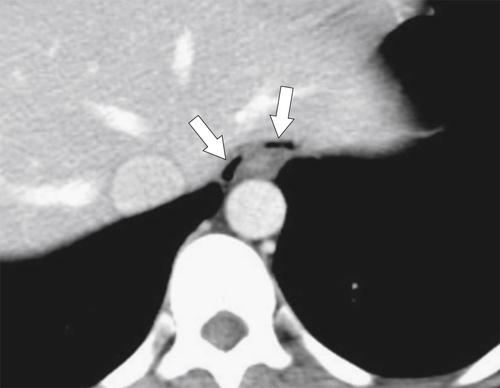
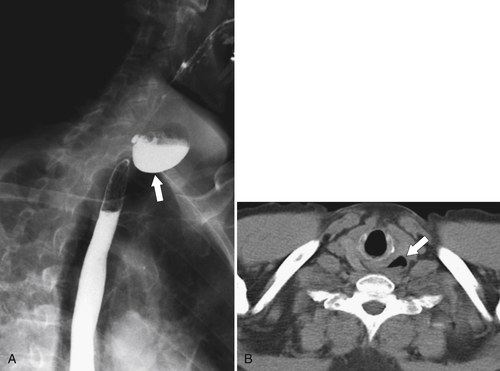
Fig. 1-5). Rarely, they can be large and sometimes confused with Zenker diverticula and can even be observed with CT (Fig. 1-6).
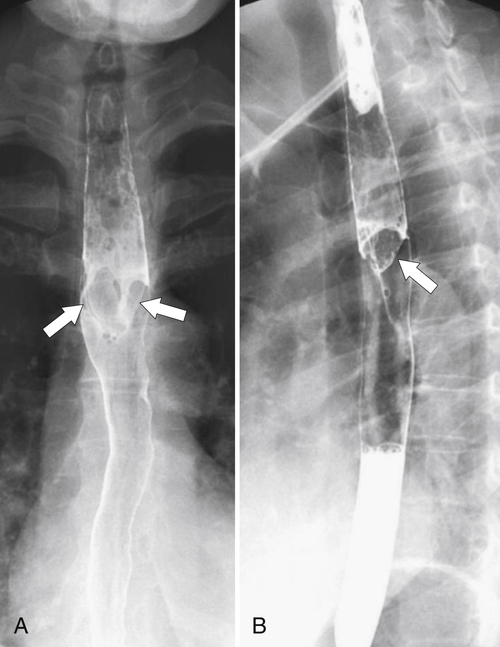
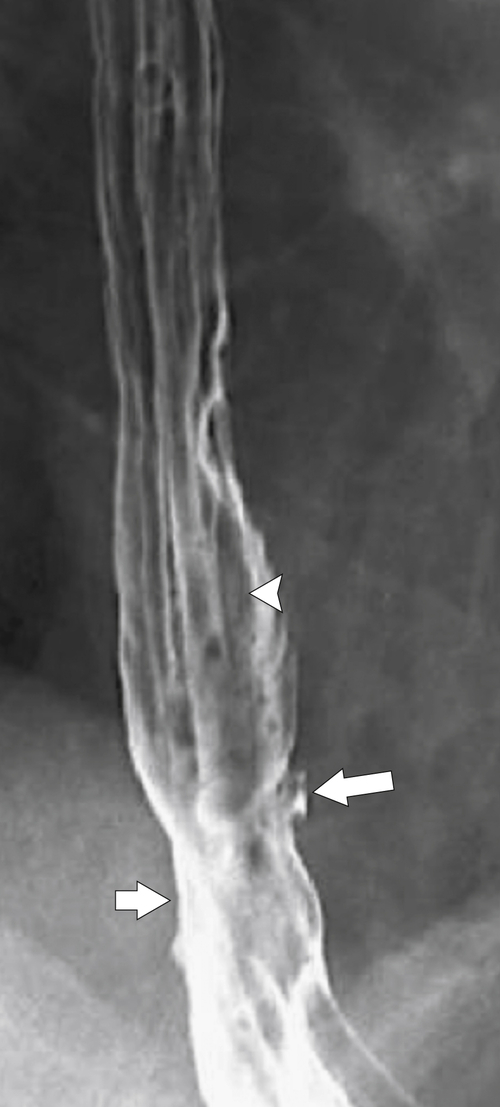
Figure 1-5 AP (A) and lateral (B) barium swallow in a 78-year-old woman with residual contrast on either side of the upper esophagus (arrows) due to a Killian-Jamieson diverticula.
Figure 1-6 UGI swallow (A) and axial noncontrast (B) CT in a 71-year-old woman with a large Killian-Jamieson diverticulum (arrows).
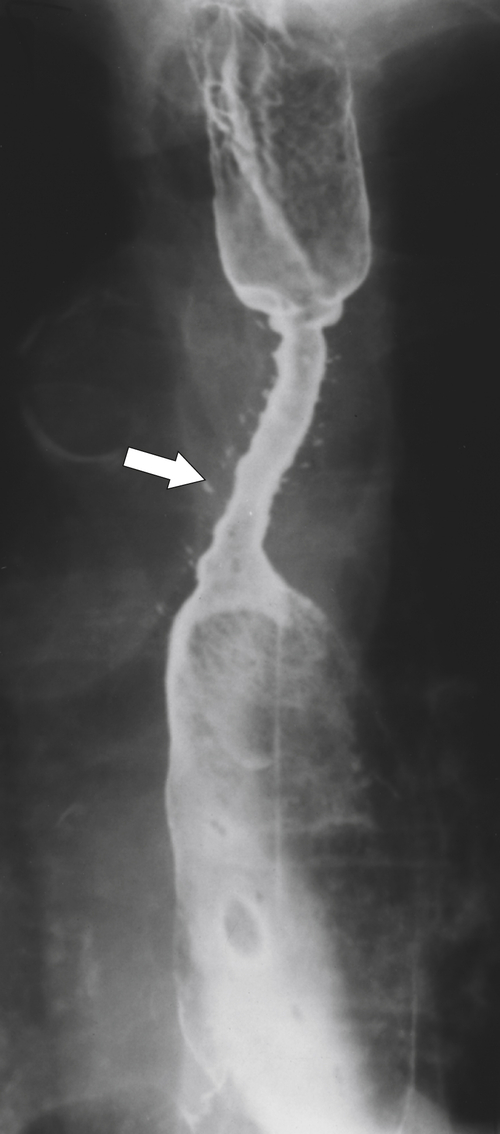
Midesophageal Diverticulum
Midesophageal diverticula are usually anterior, occurring at the level of the carina. They either are due to traction from fibrotic disease in the mediastinum (i.e., healed granulomatous disease), which retracts the whole esophagus toward the fibrotic process, or, more commonly, are due to pulsion from increased intraesophageal pressure (
Figs. 1-7 and
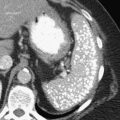
1-8). Traction diverticula in a UGI swallow are usually narrow or triangular with a pointed apex toward the mediastinal disease. Pulsion diverticula typically have a much wider neck, are larger, and fail to empty of barium easily because they have no muscular layer (Fig. 1-9). Most patients with pulsion diverticula have evidence of motility disorders.
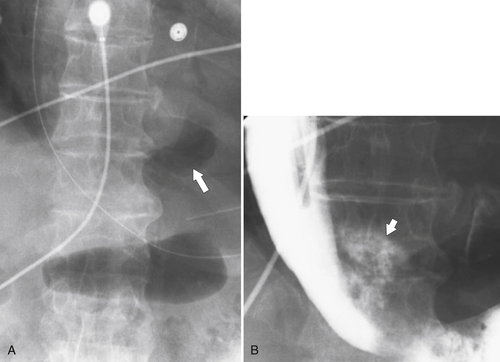
Figure 1-7 UGI swallow in a 70-year-old man with a small midesophageal traction diverticulum (large arrow) from prior tuberculous mediastinal adenopathy. There is also a tracheoesophageal fistula (small arrow).
Figure 1-8 UGI swallow in a 66-year-old man with a small midesophageal pulsion diverticulum (arrow).
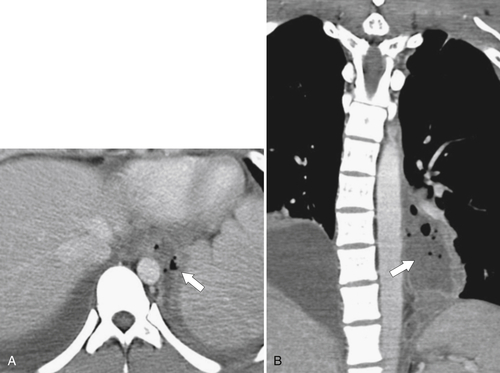
Figure 1-9 Coronal (A) and axial (B) contrast-enhanced CT in a 66-year-old man with a midesophageal pulsion diverticulum (arrow). Contrast freely refluxes through the wide-necked orifice (arrow).
Intramural Pseudodiverticula
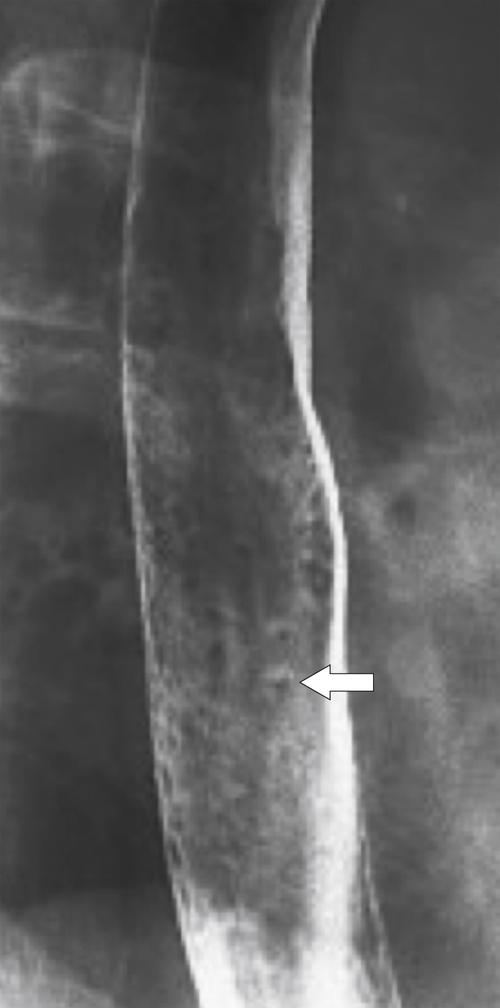
Intramural pseudodiverticula are dilated mucous glands rather than true diverticula. These are seen as single, or more usually multiple, small, flask-like outpouchings from the esophageal lumen. They are associated with GERD and secondary stricture formation. They may be missed at esophagogastroduodenoscopy (EGD) and only observed at a UGI examination as numerous highly characteristic tiny outpouches from the esophageal lumen, typically at right angles (
Fig. 1-12). When they are viewed en face, they can be mistaken for ulcer disease, but they are readily classified when viewed in the lateral plane.
Figure 1-12 Esophageal barium swallow study in a 66-year-old man with multiple pseudodiverticula (arrow) and stricture due to chronic reflux esophagitis.
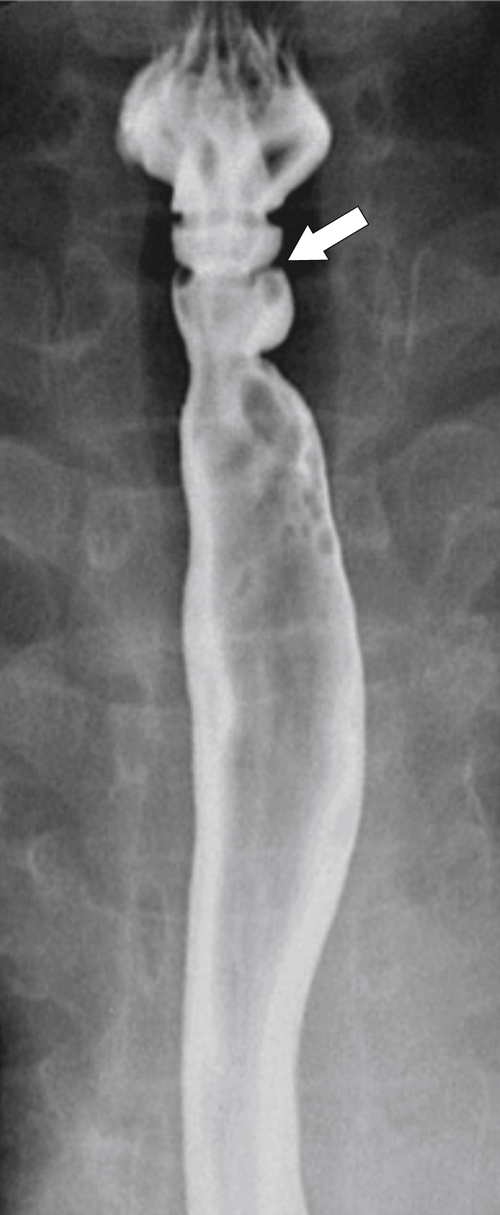
Esophageal Webs and Rings
Esophageal webs and rings are usually located in the anterior upper esophagus and result from a variety of causes, which are either idiopathic or secondary to fibrosis from pemphigoid and epidermolysis bullosa, eosinophilic esophagitis, celiac disease, graft-versus-host disease, and Plummer-Vinson
∗ syndrome (
Figs. 1-13 and
1-14). The latter is associated with iron deficiency anemia, angular stomatitis, atrophic glossitis, and dysphagia. With lateral views with a UGI examination, these webs are seen as thin (web-like) defects at right angles to the direction of the esophageal lumen, which are usually shelf-like but can be circumferential (Fig. 1-15). Many webs are asymptomatic but can cause dysphagia. Sometimes an anterior web is combined with either posterior osteophyte impression or cricopharyngeal spasm (Fig. 1-16).
Figure 1-13 UGI swallow in a 39-year-old woman with epidermolysis bullosa and several circumferential esophageal webs (arrow).
Figure 1-14 Barium swallow of the cervical esophagus in a 44-year-old woman demonstrates an anterior esophageal web (arrow).
Figure 1-15 UGI swallow of the upper esophagus in an 84-year-old woman with a circumferential web (arrow).
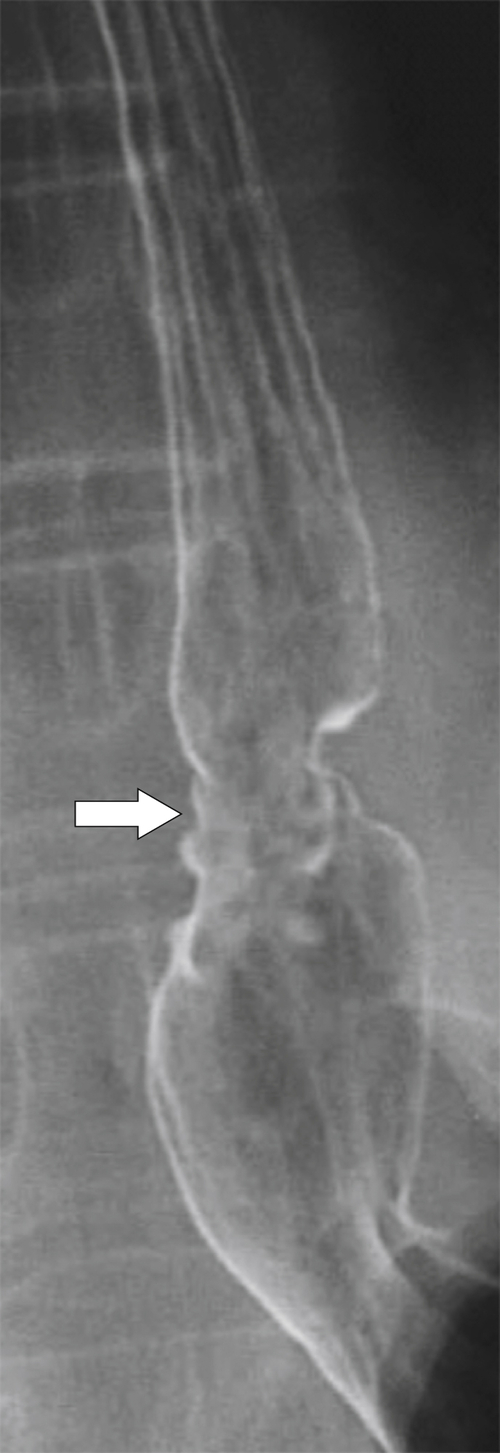
Figure 1-16 UGI of the cervical esophagus in a 60-year-old man demonstrating an anterior web (large arrow) and posterior impression (small arrow) due to cricopharyngeus spasm.
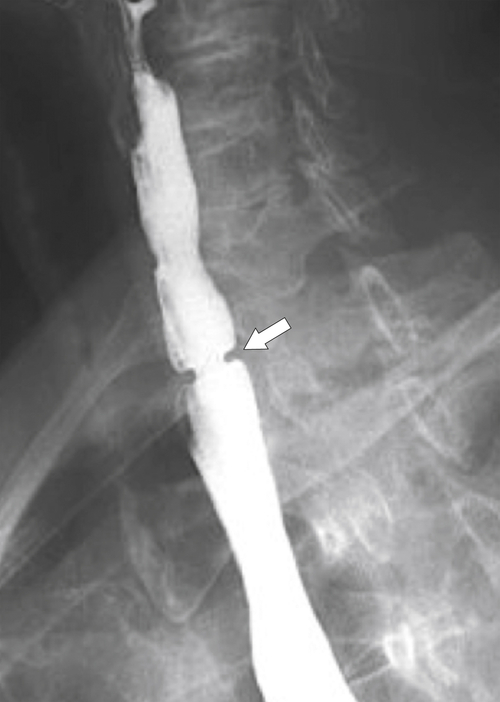
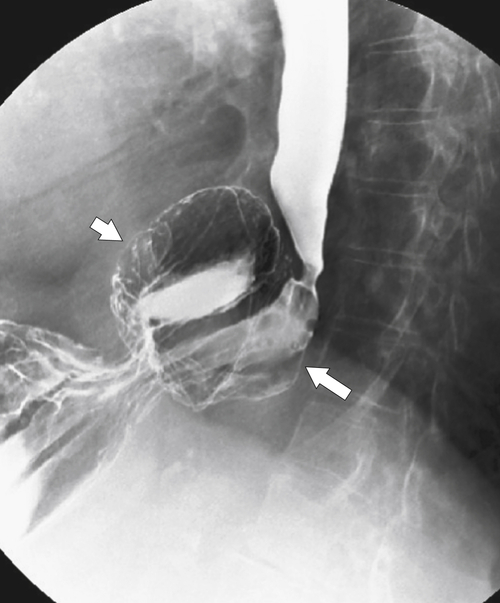
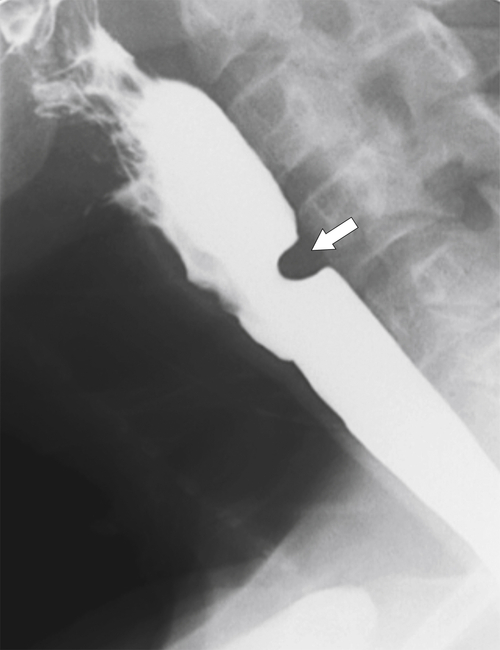
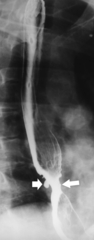
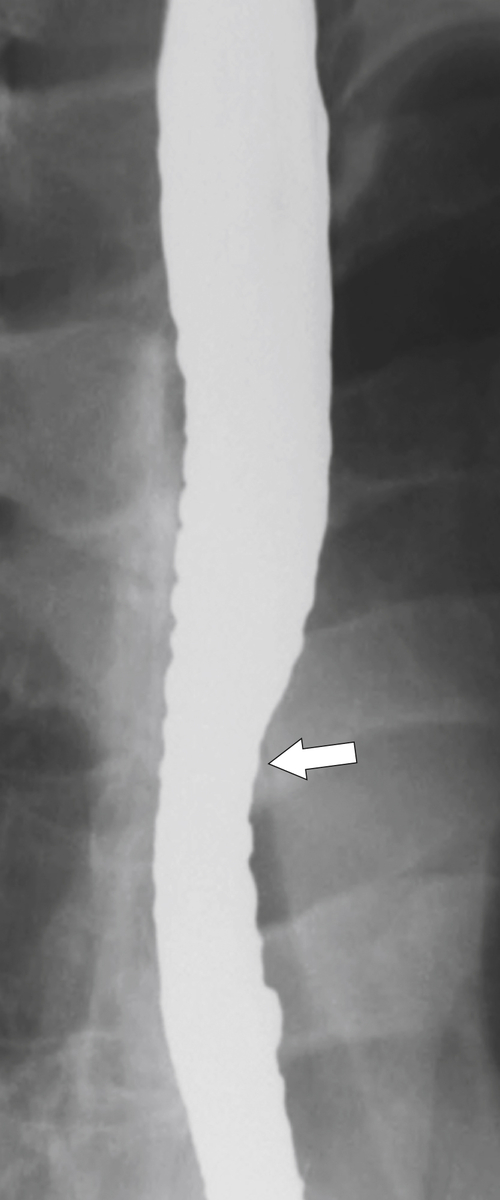
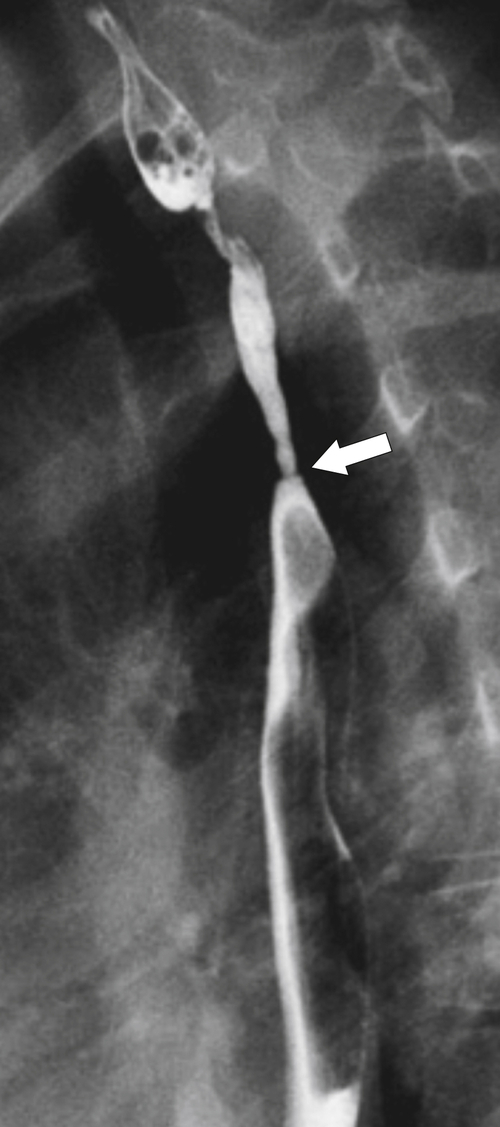
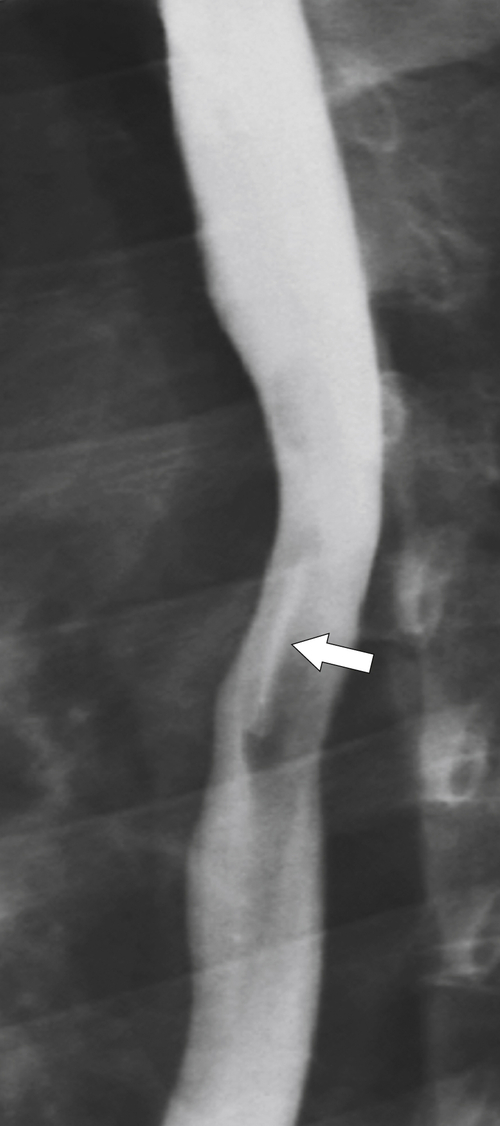
The rings are visualized as fixed and smooth associated with a small hiatal hernia below the rings during fluoroscopic observation after the patient swallows thin barium (
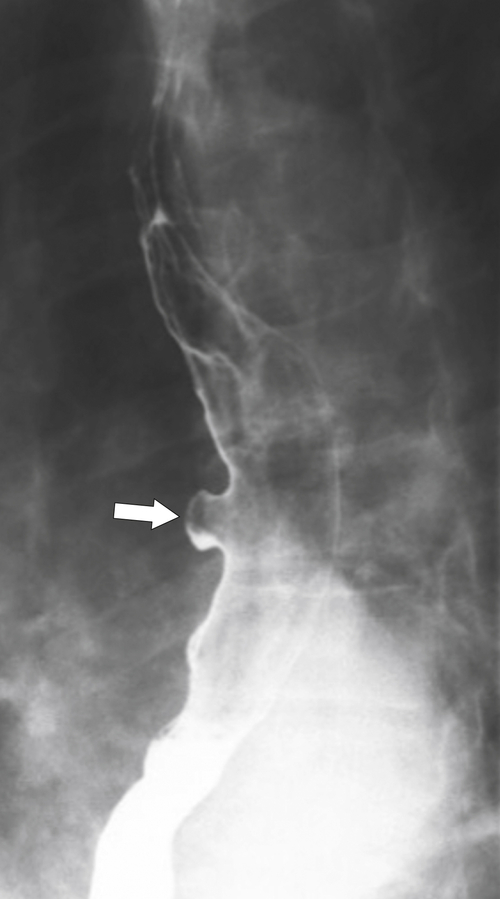
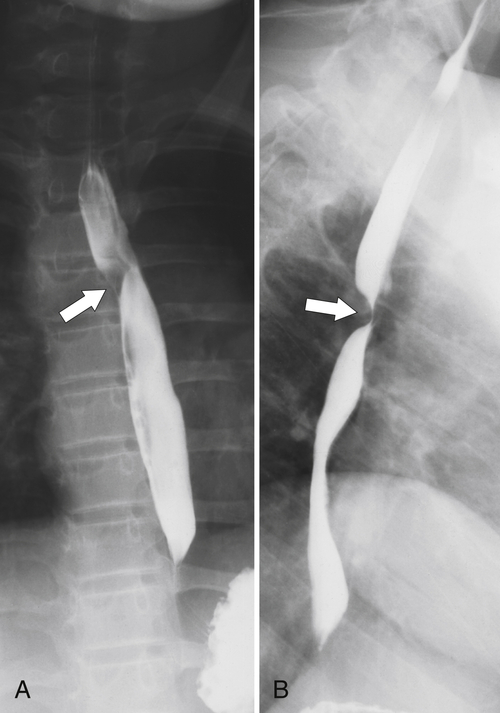
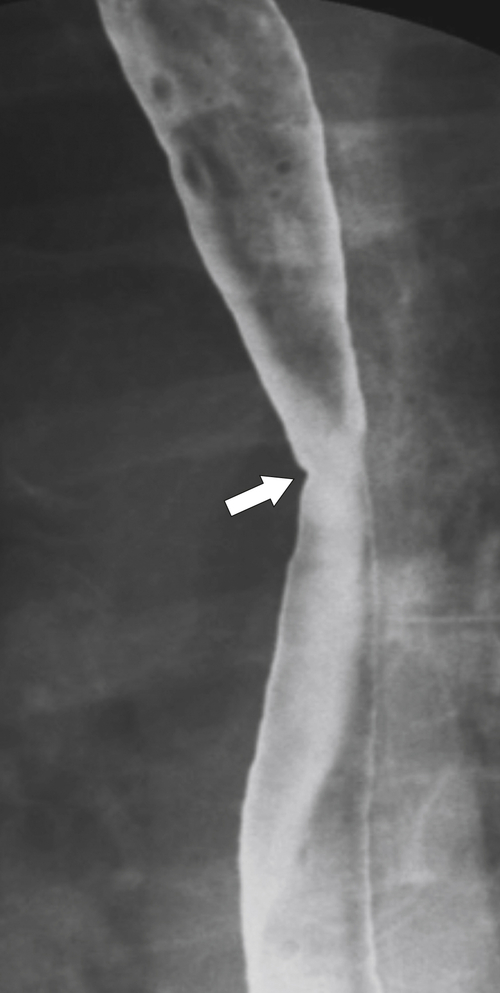
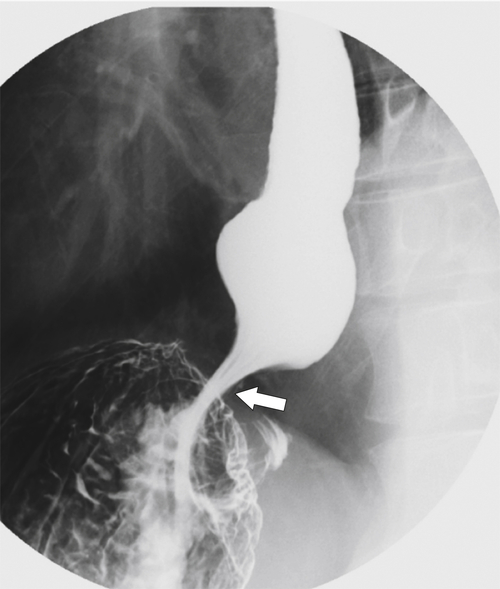
Fig. 1-17). The rings can be missed on upright swallowing studies and are best elicited with the patient in the prone oblique position, the position most likely to distend the distal esophagus. The diameter of the rings can be confirmed by the patient’s swallowing a 13-mm pill in the upright position. Narrowing to 13 mm or less is considered significant, at which point the pill will become stuck at the B-ring (Fig. 1-17).
Figure 1-17 A, Barium swallow in a 64-year-old man with a Schatzki ring (short arrow). There is a small hiatal hernia with prominent gastric folds (long arrow). B, A 13-mm pill (arrowhead) failed to pass through the lower esophageal stricture.
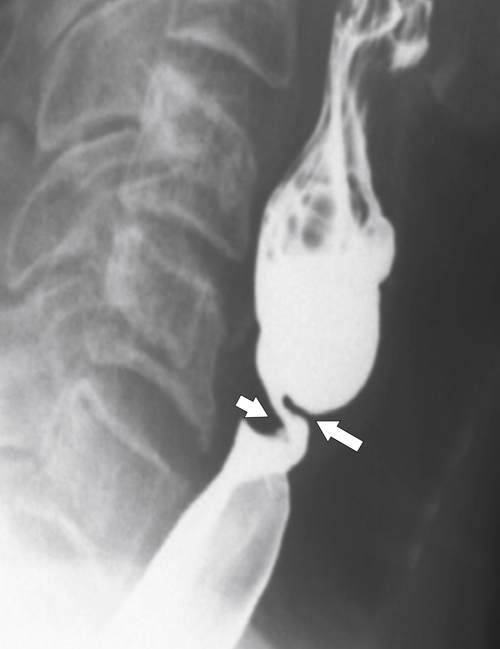
Inflammatory Esophagogastric Pseudopolyp or Fold
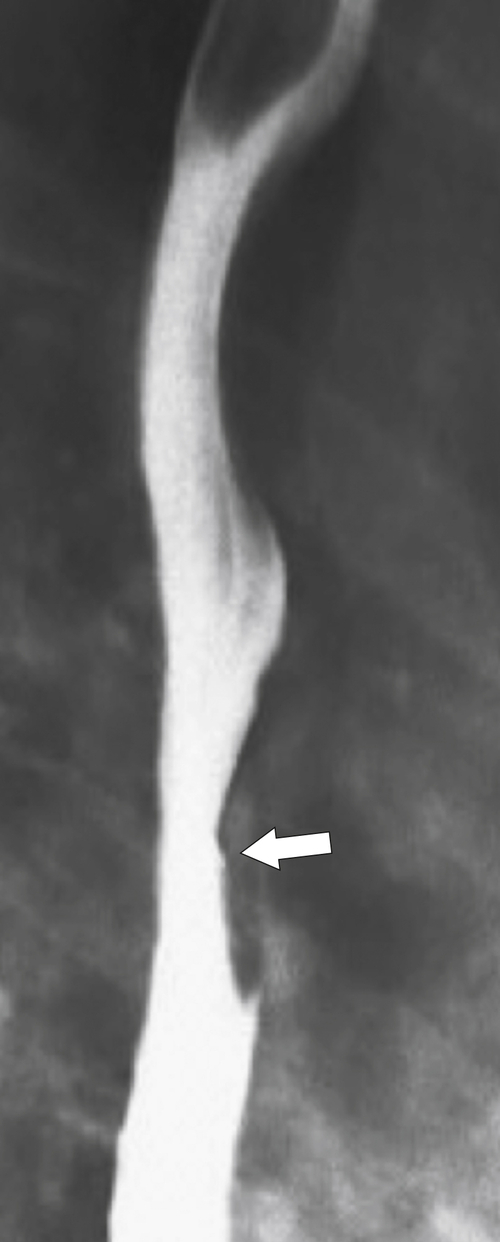
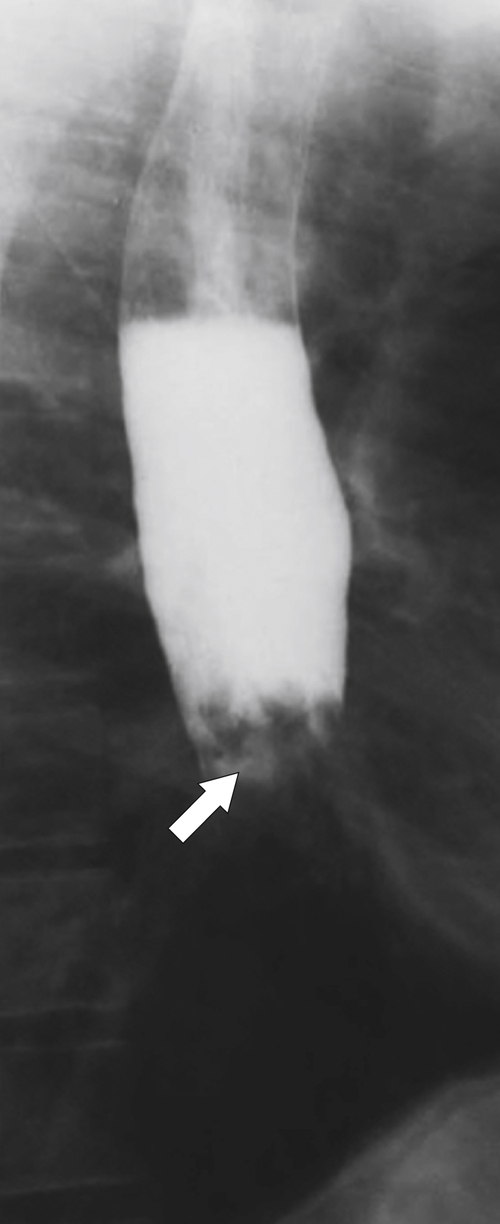
An inflammatory esophagogastric pseudopolyp or fold is an extension of a thickened gastric fold protruding up into the lower esophagus and mimics the appearance of a polyp (it is sometimes termed a
sentinel polyp) (
Fig. 1-18). GERD is usually associated. If the fold is excessively large, a biopsy is recommended to exclude adenocarcinoma at the GE junction.
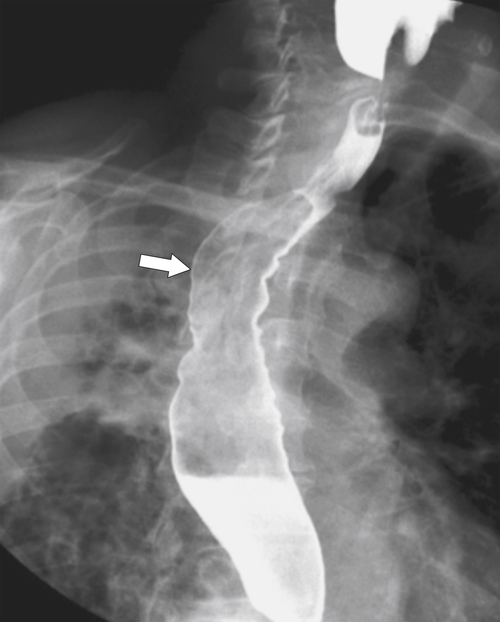
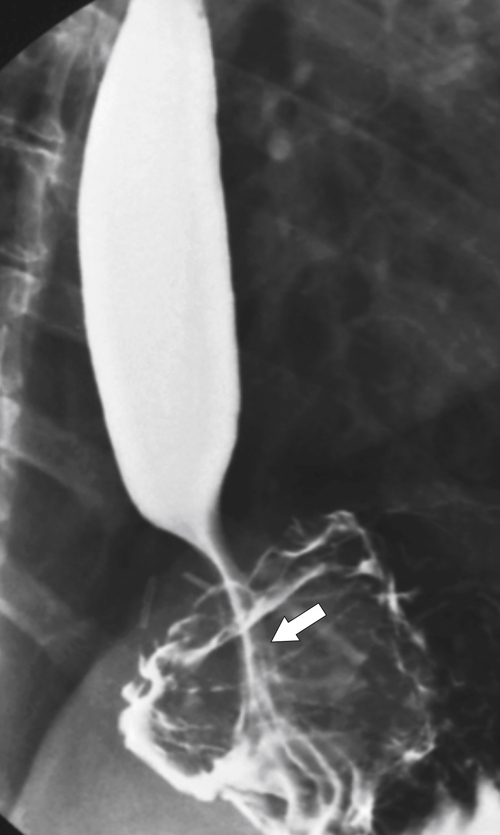
Figure 1-18 Barium swallow demonstrating mild B-ring (Schatzki) narrowing (arrows) and an inflammatory pseudopolyp (arrowheads).
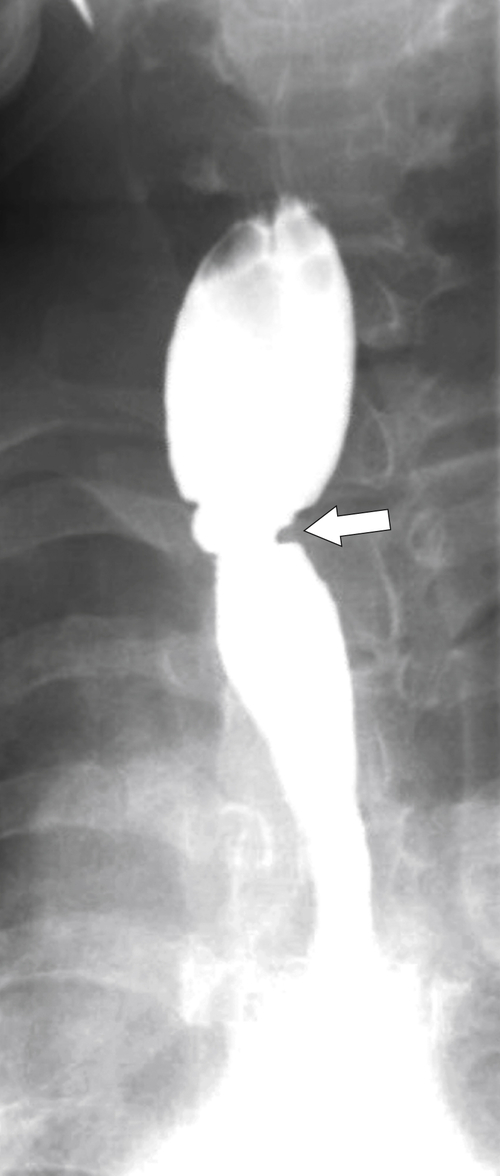
Hiatal Hernias
Hiatal hernias are actually an extension of the stomach into the chest as opposed to a primary esophageal abnormality. They are differentiated into sliding (axial) or rolling (paraesophageal) types. In the former, which are far more common (up to 95% of all hiatal hernias), the upper gastric cardia and B-ring (lower esophageal mucosal ring) “slide” up through the diaphragmatic hiatus, typically more than 2 cm. The GE junction therefore lies above the diaphragm in the chest. Most sliding hernias are small and may not be observed at UGI contrast studies unless the patient is examined
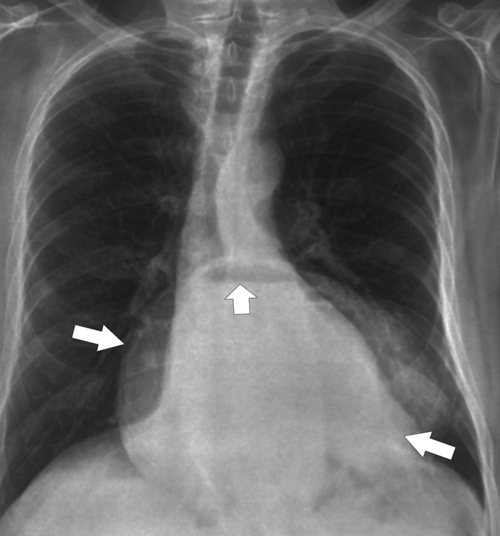
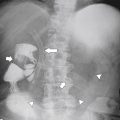
carefully (usually in the prone oblique position), and many are self-reducible in the erect position. Their significance, even when small, is that patients can have GERD with the resulting symptoms and potential complications. The typical sliding hernia at UGI study demonstrates several cardiac folds passing up into the chest, which may reduce back into the stomach when the patient is upright (Fig. 1-17). There may be a kink in the hiatal hernia because of compression by the adjacent diaphragm. Sliding hiatal hernias can be large with almost the whole stomach being in the chest, but the antrum pylorus remains within the abdomen (Fig. 1-19).
Figure 1-19 Chest radiograph in a 56-year-old man with a large hiatal hernia (large arrows) and gastric fluid level (small arrow). Most of the stomach resides in the chest.
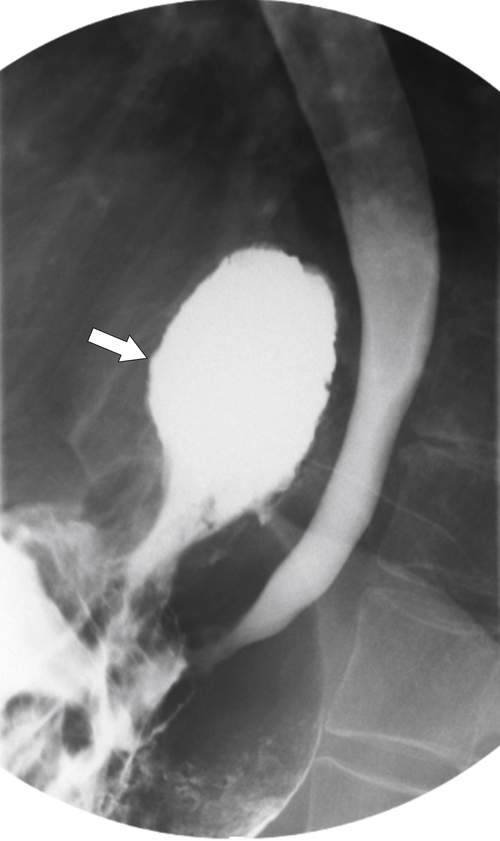
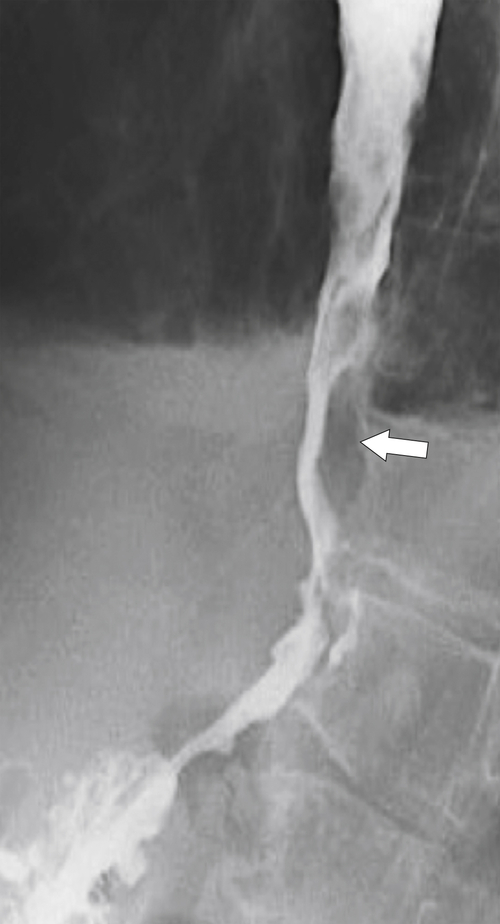
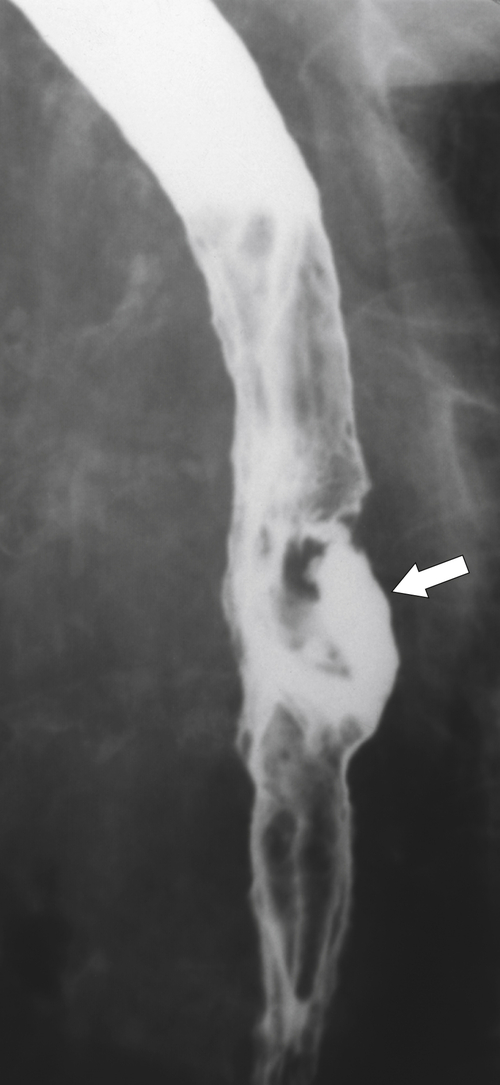
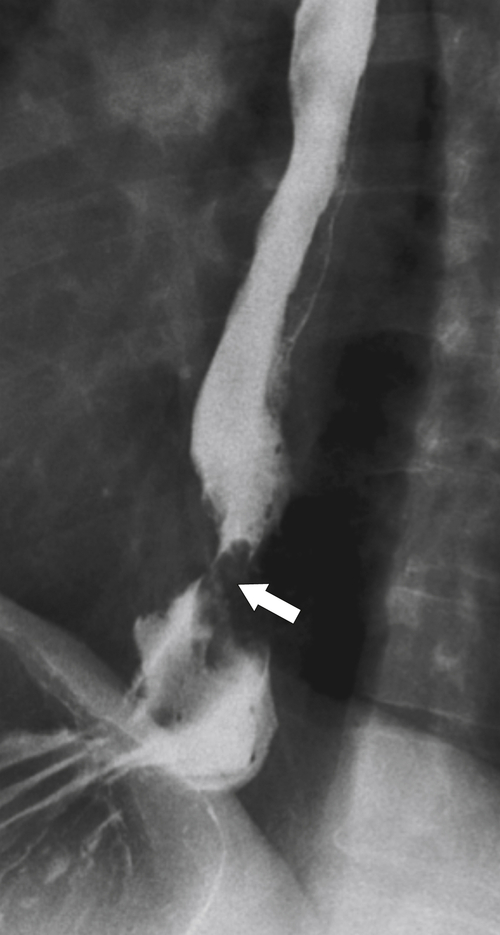
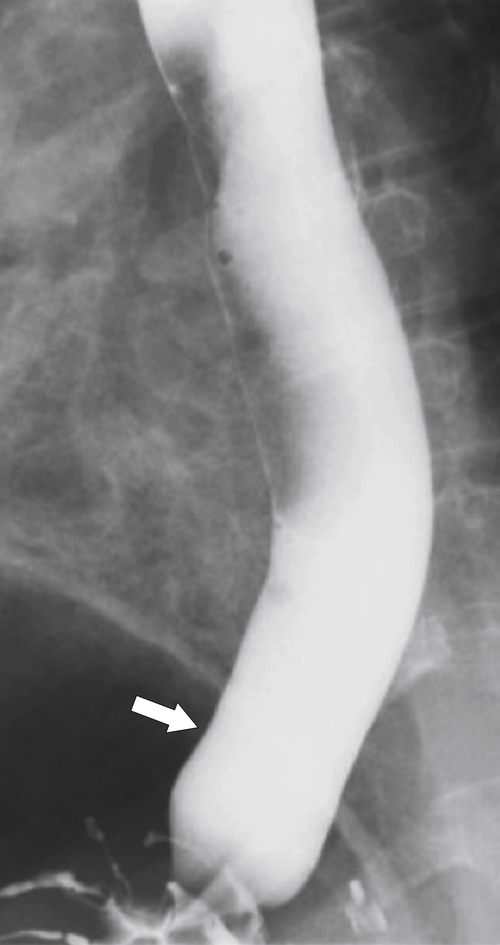
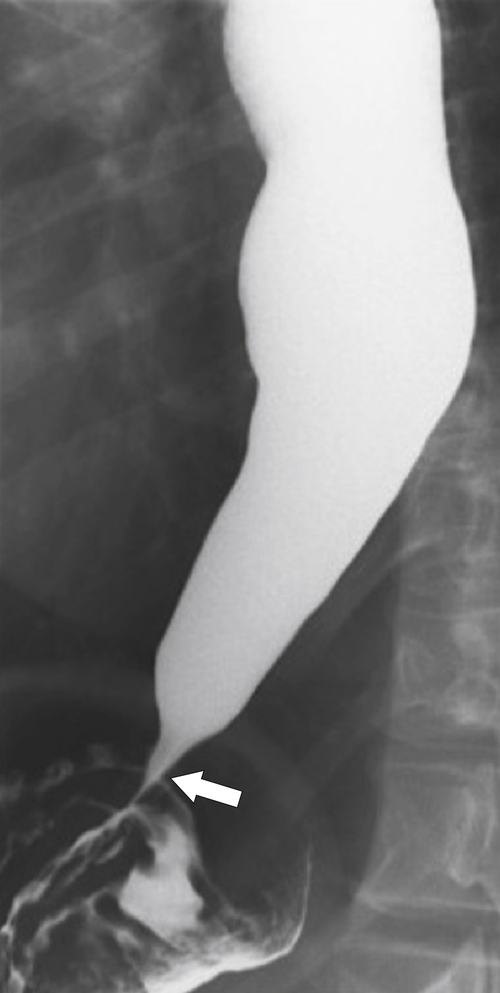
Far less common are paraesophageal hernias (rolling hernias) in which the GE junction remains within the abdomen, so reflux is much less likely to occur compared with sliding hiatal hernias. Rather, the gastric fundus passes up into the chest and lies to the left of the lower esophagus (
Fig. 1-20). These are generally irreducible but are more likely to be asymptomatic compared with sliding hiatal hernia, due to GERD in the more common sliding hernias. On the other hand, a variant of the paraesophageal hernia occurs when the whole stomach lies “upside down” in the chest because of volvulus, which may be obstructing or nonobstructing (see “Gastric Volvulus” in Chapter 2) and which is at greater risk of strangulation and perforation (Fig. 1-21). This is also the case with the even rarer combination of a sliding hernia and paraesophageal hernia, whereby the GE junction lies in the chest along with the gastric cardia (Fig. 1-22). Therefore, the GE junction lies in the chest with the herniated gastric fundus lying alongside and to the left of the lower esophagus.
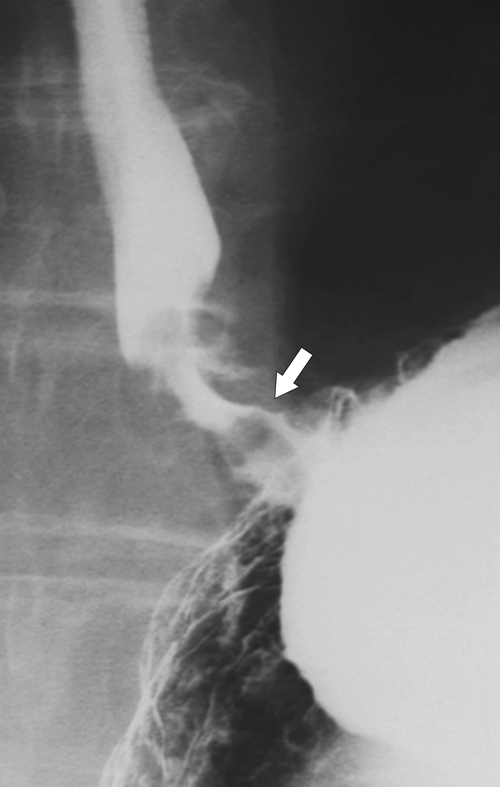
Figure 1-20 Barium swallow in a 48-year-old woman with a paraesophageal hernia (arrow).
Figure 1-21 UGI series (A) and coronal noncontrast CT (B) in a 52-year-old woman with a nonobstructing organoaxial volvulus resulting in an “upside down” stomach.
Figure 1-22 UGI series in an 86-year-old woman with a sliding hernia with the GE junction in the chest (large arrow) and paraesophageal hiatal hernia (small arrow).
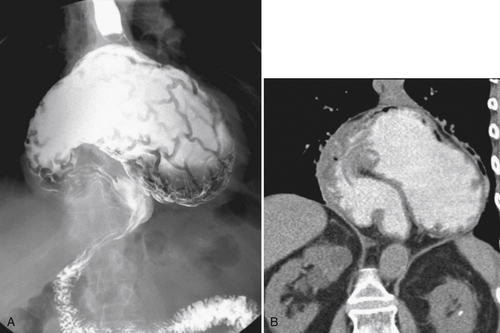
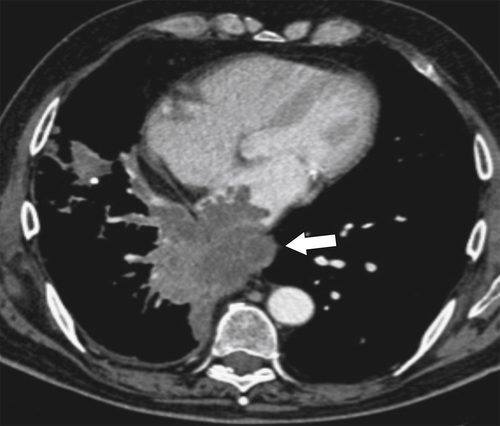
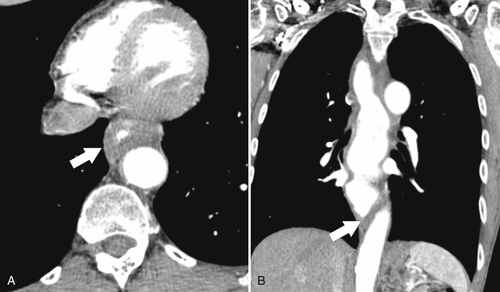
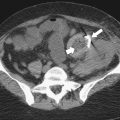
Although hernias are best appreciated by UGI series, they are frequently visualized incidentally by CT, which will demonstrate a dilated lower esophagus (actually represents the gastric cardia in the chest) in the lower chest with a variable proportion of mesenteric fat surrounding the herniated stomach (
Fig. 1-23). Consequently, there is a slight widening of the diaphragmatic hiatus (>15 mm). A paraesophageal hernia can also be appreciated with the herniated cardia lying alongside the lower esophagus (Fig. 1-24).
Figure 1-23 Axial (A) and coronal (B) contrast-enhanced CT in a 77-year-old woman with a sliding hiatal hernia (arrows).
Figure 1-24 UGI swallow (A) and axial (B) and coronal (C) noncontrast CT in a 60-year-old woman with a combined sliding and paraesophageal hernia (arrows). At CT the GE junction is in the chest.
Extramural Esophageal Impressions
Extramural esophageal impressions cause smooth and obtuse impressions on the esophagus similar to the extramural masses throughout the GI tract. Most causes are benign (
Table 1-1). External malignant masses may ultimately distort the mucosal contour but only after transmural metastatic invasion.
Table 1-1
Extrinsic Esophageal Masses
| Benign |
Malignant |
Postcricoid impression
Cricopharyngeal spasm
Cervical osteophytic disease
Retropharyngeal masses (e.g., goiter)
Cardiomegaly
Vascular anomalies (aberrant vessels, aneurysms, vascular rings)
Mediastinal adenopathy (e.g., tuberculosis) |
Mediastinal adenopathy (metastases)
Lung cancer
Lung metastases |
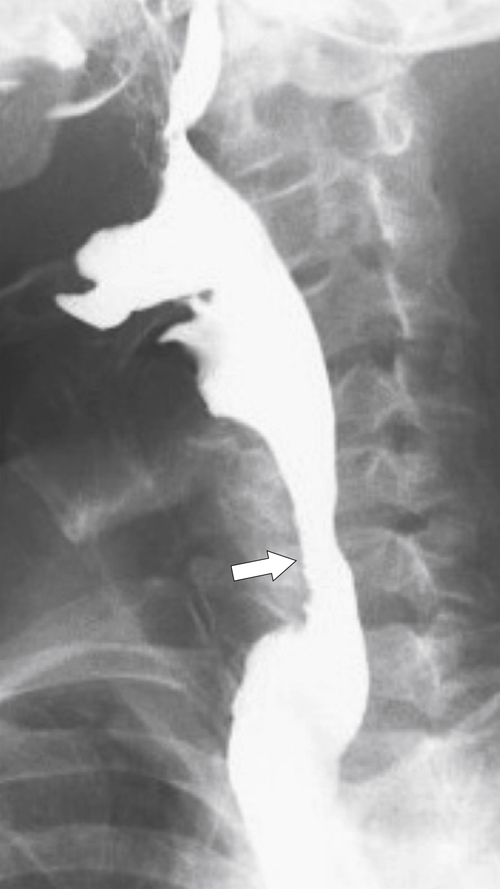
Cricopharyngeal Spasm
This posterior esophageal impression is secondary to hypertrophy and spasm of the cricopharyngeus muscle, the lower portion of the inferior pharyngeal constrictor muscle located posteriorly at the level of C5-6 vertebral body (
Figs. 1-16 and
1-25). Normally, after the primary peristaltic wave is initiated, the cricopharyngeus muscle relaxes, allowing the food bolus to pass. Failure to relax due to hypertrophy or spasm is a relatively common cause of dysphagia because the protruding muscle causes localized esophageal dysmotility and relative stricture formation. Usually, symptoms are relatively mild (many patients do not present to their physician) but can result in more marked symptoms depending on the severity of the muscular protrusion. A Zenker diverticulum may ultimately result from the localized increased intraesophageal pressure.
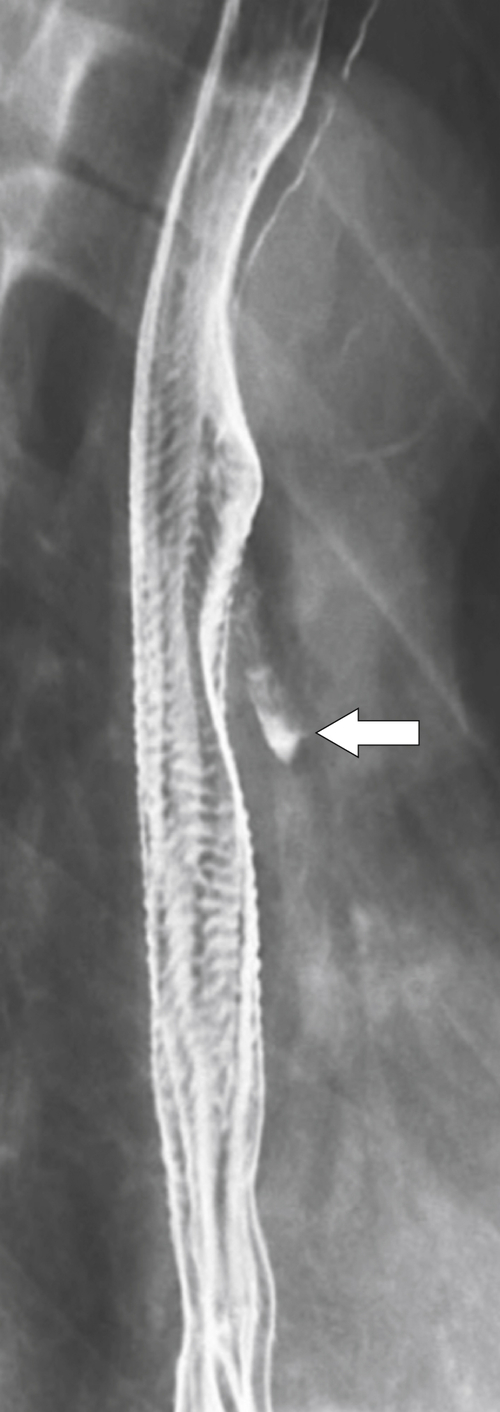
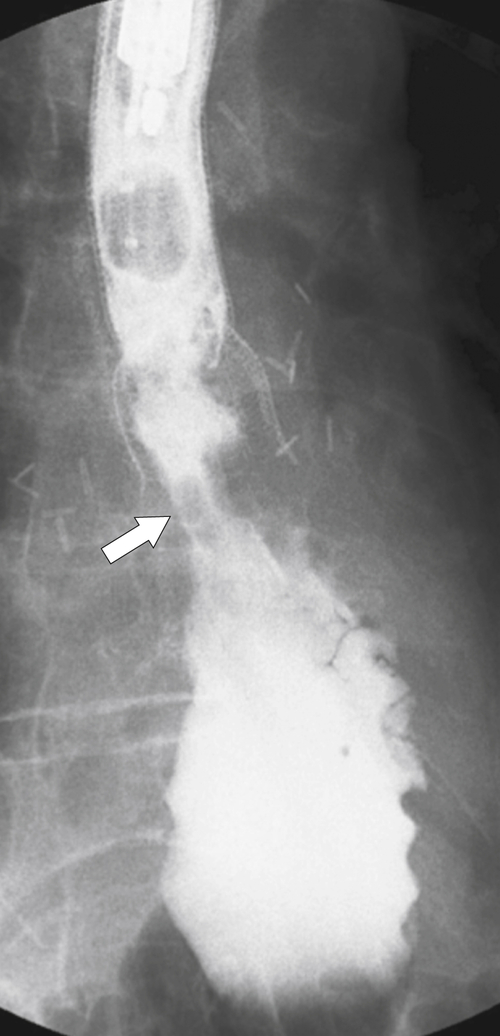
Figure 1-25 UGI swallow in a 56-year-old man with cricopharyngeus spasm (arrow).
Vertebral Osteophytic Disease
Anterior vertebral osteophytes or, rarely, anterior cervical disc herniation can cause discrete posterior esophageal indentations, which are usually smooth and sometimes marked (
Fig. 1-26).
Figure 1-26 UGI swallow in a 75-year-old woman with pharyngeal anterior displacement due to vertebral osteophyte disease (arrow).
Retropharyngeal Masses
The cervical esophagus abuts the vertebral bodies, so pharyngeal disease that extends inferiorly and posterior to the esophagus will produce esophageal deviation. Such retropharyngeal masses include goiter, abscess, hematoma, lymphadenopathy, and parathyroid enlargement (
Figs. 1-27 and
1-28).
Figure 1-27 UGI swallow in a 46-year-old woman with extramural compression of the esophagus (arrow) due to a goiter.
Figure 1-28 UGI swallow (A) and sagittal noncontrast CT (B) in a 54-year-old woman with extramural compression and deviation of the esophagus due to a retropharyngeal abscess that contains gas (arrows).
Vascular Impressions
Normally the aortic arch and left main bronchus cause smooth external compressions of the esophagus. However, ectatic or aneurysmal dilatation can cause deviation of the esophagus. There are also several congenital vascular anomalies that produce esophageal compressions, which are generally smooth and obliquely angled. Their diagnoses should be suspected by their location either by UGI examination or CT, although contrast-enhanced CT (CECT) (or MRI) will delineate the exact origin and course of the aberrant vessels.
A double aortic arch, the most common form of vascular ring, passes on both sides of the trachea and joins posterior to the esophagus. Barium studies of the esophagus demonstrate bilateral impressions anteriorly and a smooth posterior impression. A right-sided aortic arch, of which there are several types, is recognized by smooth leftward displacement of the barium-filled esophagus in the midthoracic region.
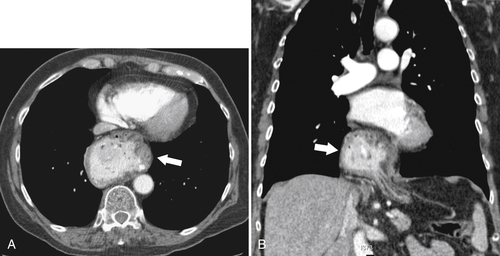
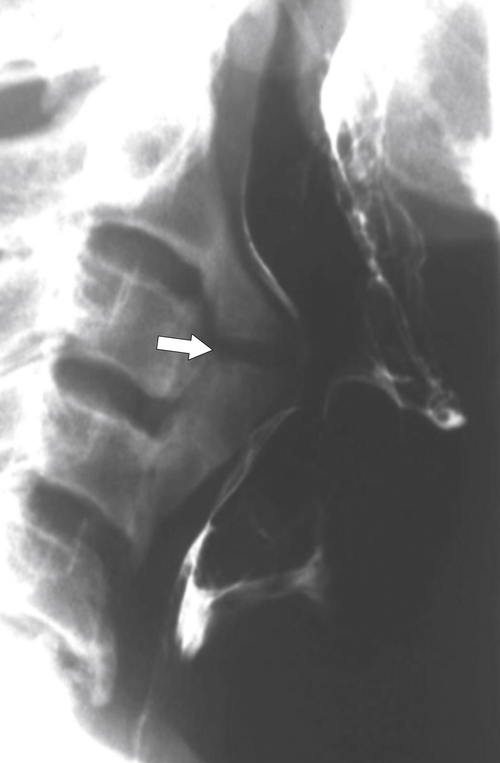
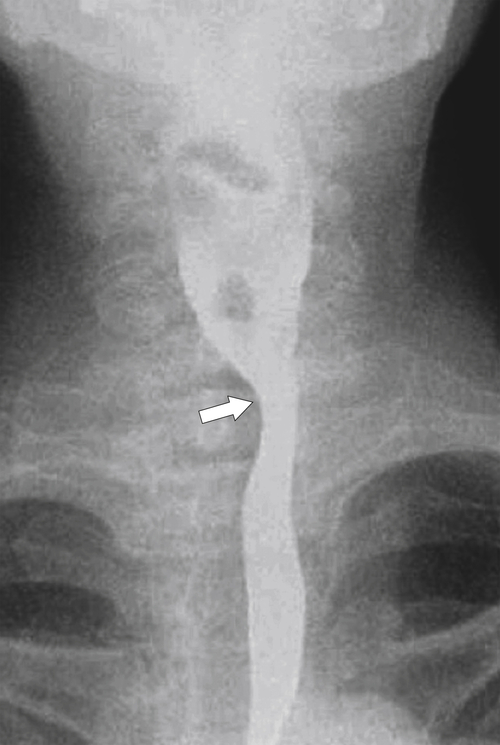
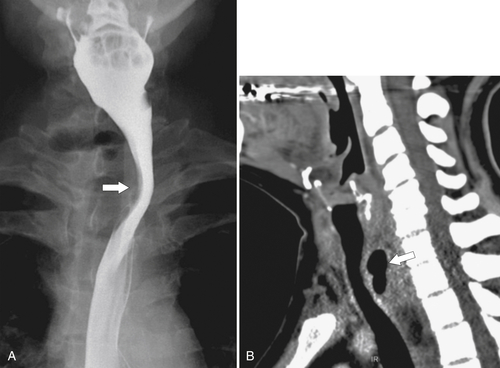
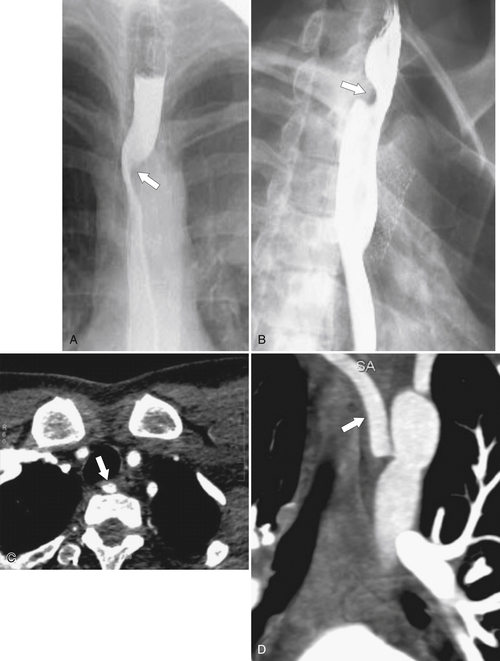
An aberrant right subclavian artery occurs in approximately 1 in 200 individuals and is due to the aberrant origin of the right subclavian artery, usually from a left-sided aortic arch, such that the vessel now has to reach the right axilla by crossing behind the
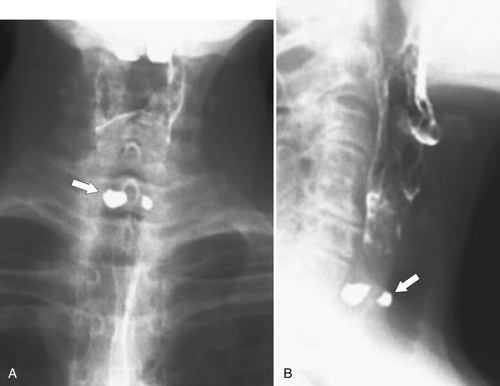
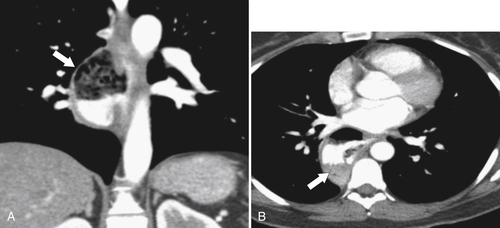
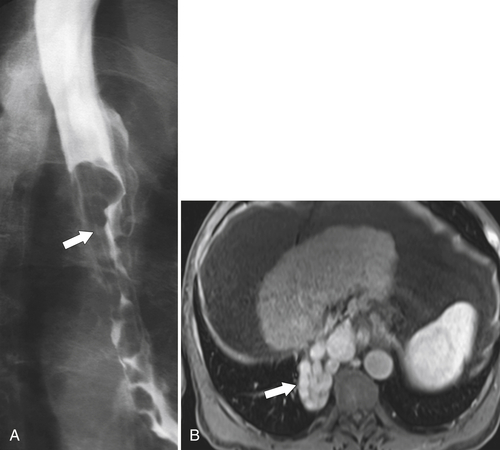
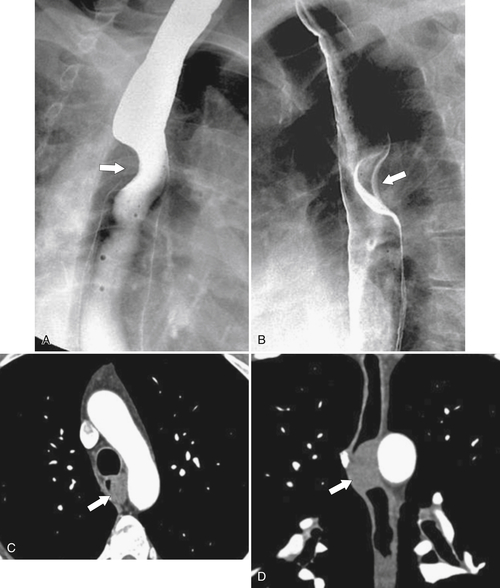
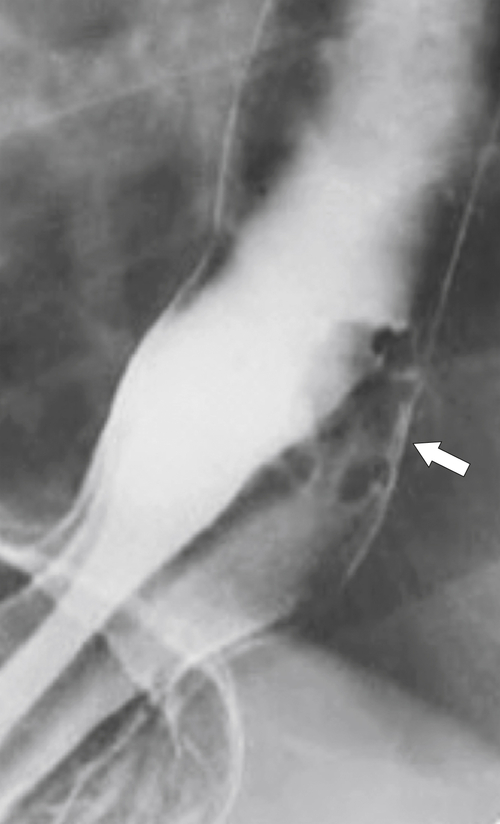
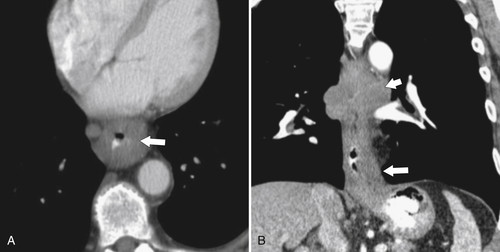
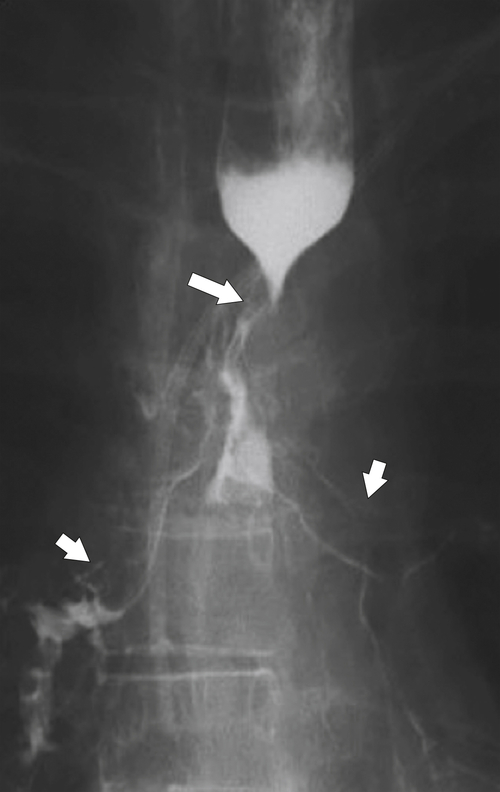
esophagus at an oblique angle. Less commonly, it arises from a right aortic arch. It may be asymptomatic but can cause dysphagia (also known as dysphagia lusoria∗). The diagnosis is best made by arterial phase CT where the aberrant vessel is readily observed crossing behind the esophagus (Fig. 1-29). At UGI examination, there is a classic smooth posterior impression angled upward from the left to the right (Fig. 1-29). An aberrant left subclavian artery is part of a right-sided arch and has similar, but reverse, findings to an aberrant right subclavian artery because it passes from the right and behind the esophagus as it traverses toward the left axilla (Fig. 1-30).
Figure 1-29 AP (A) and lateral oblique (B) UGI swallow and axial (C) and coronal (D) contrast-enhanced CT in a 35-year-old man. UGI demonstrates a slanting esophageal impression due to an aberrant right subclavian artery (arrows, A and B). CT demonstrates the aberrant vessel passing posterior to the esophagus on the axial view and cephalad to the right on coronal MIP (maximum intensity projection) imaging (arrows, C and D).
Figure 1-30 AP (A) and lateral (B) barium swallow in a 17-year-old male adolescent with aberrant left subclavian artery. The vessel passes posteriorly and obliquely up and to the left of the esophagus (arrows).
An aberrant left pulmonary artery is an anomaly resulting from the left lung being supplied from the right pulmonary artery, rather than the left pulmonary artery. Because of the location of its origin, this aberrant left pulmonary artery has to cross to the left side of the mediastinum and in doing so causes an anterior extrinsic compression of the thoracic esophagus at the level of the carina, giving the name to the so-called pulmonary sling.
Extramural Mediastinal Masses
Mediastinal lymphadenopathy (benign or malignant) can cause anterior extrinsic impressions on the esophagus, which it may deviate if it is large enough. Other mediastinal masses that cause anterior esophageal impressions include bronchogenic and duplication cysts. Cardiomegaly, particularly left atrial enlargement, causes esophageal deviation if it is significantly dilated. Traction anomalies, including pulmonary fibrotic disease, also cause esophageal deviation (
Fig. 1-31).
Figure 1-31 UGI swallow in a 56-year-old man with right-sided esophageal deviation (arrow) due to traction from diffuse right-sided fibrotic pulmonary tuberculosis.
Submucosal Esophageal Masses
Esophageal submucosal masses cause smooth esophageal impressions but are less obtuse than extraluminal masses. There are several well-recognized causes, but appearances at UGI series usually cannot be distinguished from one another (
Table 1-2). CT may help, but small, benign masses generally cannot be differentiated, either. However, when masses are malignant or large, some CT features can aid the diagnosis.
Table 1-2
Esophageal Submucosal Masses
| Benign |
Malignant |
| Varices |
Metastases |
| Cyst |
Lymphoma |
| GIST |
Kaposi sarcoma |
| Hemangioma |
Malignant GIST |
| Lipoma |
|
| Neurofibroma |
|
| Fibrovascular polyp |
|
| Granular cell tumor |
|
GIST, Gastrointestinal stromal tumor.
Benign Submucosal Masses
Esophageal Varices
Esophageal varices are most commonly secondary to increased portal venous pressure (usually hepatic cirrhosis) with varices in the lower esophagus; these are sometimes termed
uphill varices because of their caudal to cephalad direction of blood flow. Increased venous pressure is relieved by diverting blood away from the obstruction to the lower pressure of the esophageal venous system, usually the middle to lower third of the esophagus (
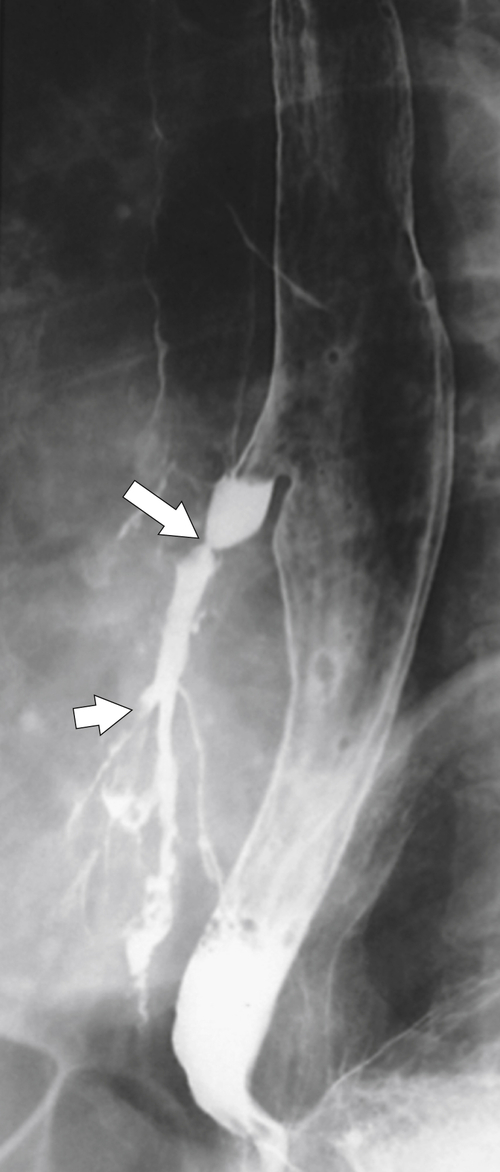
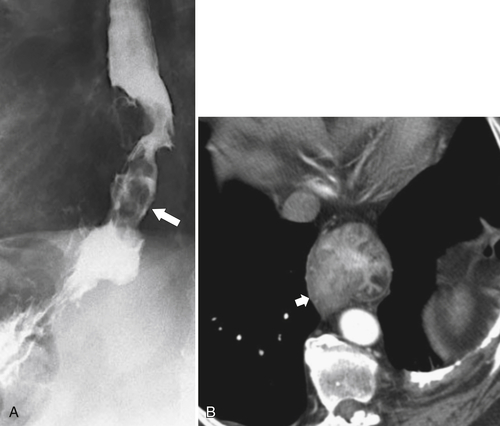
Fig. 1-32). Venous return from the portal vein is via the coronary vein (left gastric) into the lower esophageal plexus with blood flowing with a cephalad direction and then from esophageal veins into the azygous vein and superior vena cava (SVC). As part of the same venous plexus, it is often associated with gastric fundal varices.
Figure 1-32 UGI examination (A) and axial contrast-enhanced T1-weighted MRI (B) in a 53-year-old man with a serpiginous esophageal filling defect caused by large esophageal varices (arrows), a complication of cirrhosis. Note the liver atrophy and diffuse ascites.
The far less common “downhill” varices form from the upper to middle third of the esophagus and are secondary to superior vena caval obstruction. Blood in “downstream” varices enters the upper esophageal plexus in a caudal direction of flow and reenters the SVC through the azygos in the thoracic esophagus. If the azygos is also obstructed (or the lower SVC), then blood continues in a caudal direction to connect with the coronary vein, then the portal system, and finally the inferior vena cava (IVC). Varices in these circumstances occur along the length of the entire esophagus.
Despite the increased venous pressures that precipitate esophageal variceal formation, esophageal varices are best visualized with the patient horizontal because the venous pressure in the upright position is not sufficient to distend the varicosities. Aside from EGD, they are best visualized when the patient is prone while drinking barium as serpiginous submucosal impressions either in the lower (uphill) or mid to upper (downhill) esophagus. They are also usually well visualized by contrast-enhanced CT or MRI, particularly in the portal venous phase after contrast enhancement as prominent submucosal contrast-filled venous plexuses (
Fig. 1-32). Without the use of intravenous (IV) contrast, the esophageal wall will simply appear thickened and therefore difficult to differentiate from other esophageal abnormalities. The findings need to be differentiated from varicoid carcinoma that can also present with serpiginous submucosal fold findings (
Fig. 1-33).
Figure 1-33 UGI swallow of the lower esophagus in an 84-year-old man with an infiltrative esophageal adenocarcinoma extending for several centimeters with a varicoid pattern (arrow).
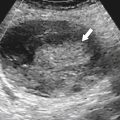
Esophageal Cysts
Esophageal cysts represent foregut duplication cysts (congenital) or retention cysts (acquired). The former are usually discovered incidentally, whereas the much rarer retention cysts may be more symptomatic, arising from dilated mucous glands, usually in the distal esophagus.
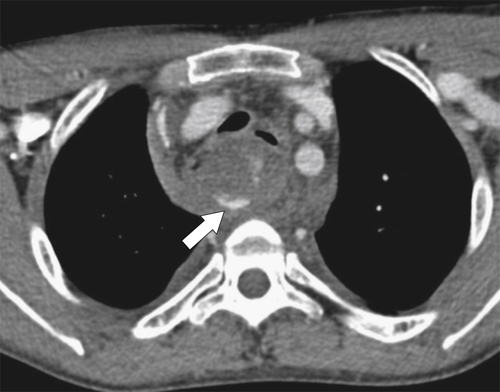
Figure 1-34 Axial contrast-enhanced CT in a 10-year-old girl with a hypodense 1.8-cm mass (arrow) contiguous with the esophagus due to an esophageal duplication cyst.
Esophageal Submucosal Benign Tumors
Table 1-3
Submucosal Benign Esophageal Tumors
| Tumor type |
Features |
| GIST |
Most common; smaller; may contain amorphous calcification at CT; can be large |
| Hemangioma |
Reddish blue multinodular mass |
| Lipoma |
Fat density on CT |
| Neurofibroma |
May be associated with disease in multiple anatomical sites |
| Granulosa cell tumor |
Broader-based mass |
| Hamartoma |
Contains multiple tissue types (bone, cartilage, fat, muscle) |
CT, Computed tomography; GIST, gastrointestinal stromal tumor.
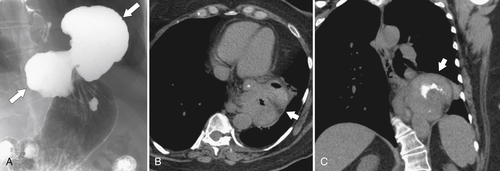
Figure 1-35 Single- (A) and double-contrast (B) UGI series and axial (C) and coronal (D) contrast-enhanced CT views in a 50-year-old man with a smooth submucosal esophageal impression (arrows) due to a GIST.
Figure 1-36 AP (A) and lateral (B) UGI swallow in a 31-year-old woman with a large submucosal esophageal impression (arrows) due to a GIST.
Fibrovascular Polyp
Fibrovascular polyp is an unusual, benign, submucosal neoplasm of the mid and upper esophagus and consists of fibrovascular and adipose tissue (
Fig. 1-37). It may have a long stalk that then elongates in tubular fashion down the length of the esophagus. It sometimes bleeds and can be so long that it can be regurgitated into the pharynx, rarely obstructing the larynx.
Figure 1-37 AP (A) and lateral (B) UGI swallow in a 50-year-old man with an irregular filling esophageal defect due to a fibrovascular polyp (arrows).
Submucosal Hemorrhage
Submucosal hemorrhage is usually iatrogenic from endoscopic procedures or nasogastric tube placement but can be spontaneous in patients with bleeding disorders. The appearances are rarely observed at barium studies but are identified at CT, usually as a circumferential esophageal soft tissue mass, which may be hyperdense if imaged shortly after the acute hemorrhagic event (
Fig. 1-38).
Figure 1-38 Axial contrast-enhanced CT in a 66-year-old woman with esophageal hematoma and acute hemorrhage (arrow).
Malignant Submucosal Masses
These masses are rare and include metastases (most commonly melanoma, lymphoma, breast) or primary tumors, including sarcomas, mainly Kaposi sarcoma, or malignant GISTs. They often cause mucosal irregularity and ulceration if they invade through the muscularis mucosae and into the mucosa.
Mucosal Abnormalities
Both benign and malignant mucosal abnormalities are well delineated by good single- and double-contrast technique (
Table 1-4). Normal esophageal mucosal folds are mostly longitudinal and extend the length of the esophagus and are best seen on collapsed rather than distended double-contrast views at UGI examination (Fig. 1-39). Conversely, transverse folds, which are also normal, are more difficult to identify and are better seen on double-contrast views. Transverse folds in the esophagus, when observed, are sometimes referred to as a feline esophagus, mimicking the normal esophageal folds observed in cats (Fig. 1-40). Thickened edematous benign folds are often seen with esophagitis from any cause, the most common being GERD (Fig. 1-41).
Table 1-4
Esophageal Mucosal Abnormalities
| Benign |
Malignant |
Esophagitis (peptic, infectious, caustic, inflammatory, drugs, iatrogenic, radiation, Crohn, Behçet)
Ectopic gastric mucosa
Neoplastic (leukoplakia, squamous papilloma)
Glycogenic acanthosis
Tylosis
Epidermolysis bullosa/pemphigoid |
Esophageal carcinoma
Kaposi sarcoma
Malignant GIST
Metastases |
GIST, Gastrointestinal stromal tumor.
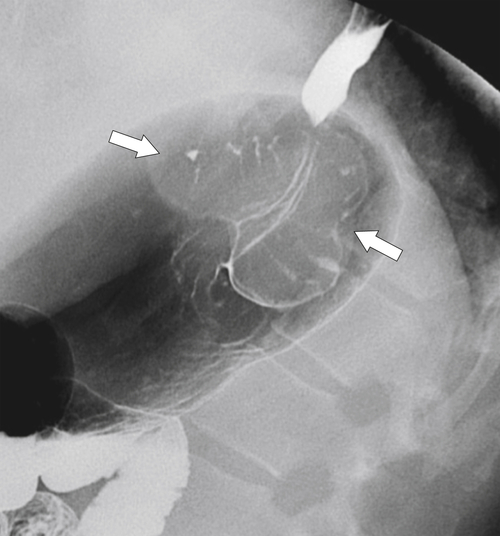
Figure 1-39 Barium swallow demonstrating multiple longitudinal esophageal folds.
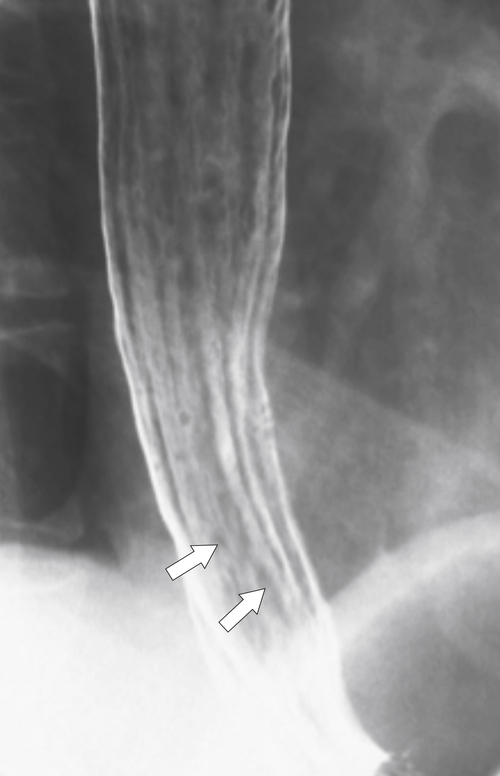
Figure 1-40 UGI swallow in a 77-year-old man with multiple fine transverse folds due to a feline esophagus. There is slight aspiration in the trachea and left main bronchus (arrow).
Figure 1-41 UGI swallow in a 56-year-old man demonstrating thickening longitudinal esophageal folds and subtle nodular change (arrows) due to reflux esophagitis.
Benign Mucosal Disease
Esophagitis
Esophagitis represents the most common mucosal abnormality in clinical practice predominantly because it is due to a wide variety of causes (
Table 1-5). Barium studies can be diagnostic and should be performed if endoscopy is contraindicated. Some findings are subtle, and close observation of the whole esophagus is mandatory. Once an abnormality is identified, additional spot views in multiple tangential planes should be taken to maximize the ability of the examination to define the precise cause.
Table 1-5
Causes of Esophagitis
| Infectious |
Herpes
Candida infection
CMV
HIV
Fungal infection
Tuberculosis |
| Iatrogenic |
Radiation therapy
Nasogastric tube |
| Drugs |
Tetracycline
Nonsteroidal antiinflammatory drug
Potassium
Iron |
| Chemical |
Reflux esophagitis
Corrosives |
| Inflammatory |
Crohn disease
Scleroderma
Pemphigoid
Epidermolysis bullosa |
CMV, Cytomegalovirus; HIV, human immunodeficiency virus.
Infectious Esophagitis
Infectious esophagitis is most commonly identified in the immunocompromised host (from any cause) and includes herpes simplex, candidiasis, and cytomegalovirus (CMV).
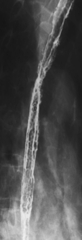
Candida albicans causes the most common infectious esophagitis, which presents with diffuse and painful dysphagia. Most patients are immunosuppressed from either chemotherapy or immune deficiencies, particularly human immunodeficiency virus (HIV), and it is a relatively common manifestation in patients with acquired immune deficiency syndrome (AIDS). The disease may coexist with herpes or CMV esophagitis. Patients with disorders of esophageal motility and the resulting stasis (scleroderma, achalasia, and strictures) are also at risk for Candida infection. As its name suggests, the esophageal mucosa is coated with diffuse white (albicans) plaques, which can be present from the tongue (oral thrush) to the lower esophagus. Gastric hyperacidity generally prevents it from spreading below the GE junction.
The imaging findings may lag clinical findings, either with the onset of disease or after treatment (oral antifungal agents are highly effective at treating the disease). When imaging findings are present, they are usually seen from the mid to upper esophagus with multiple and diffuse raised mucosal plaques, sometimes with pseudomembranes and ulcer formation with severe disease (
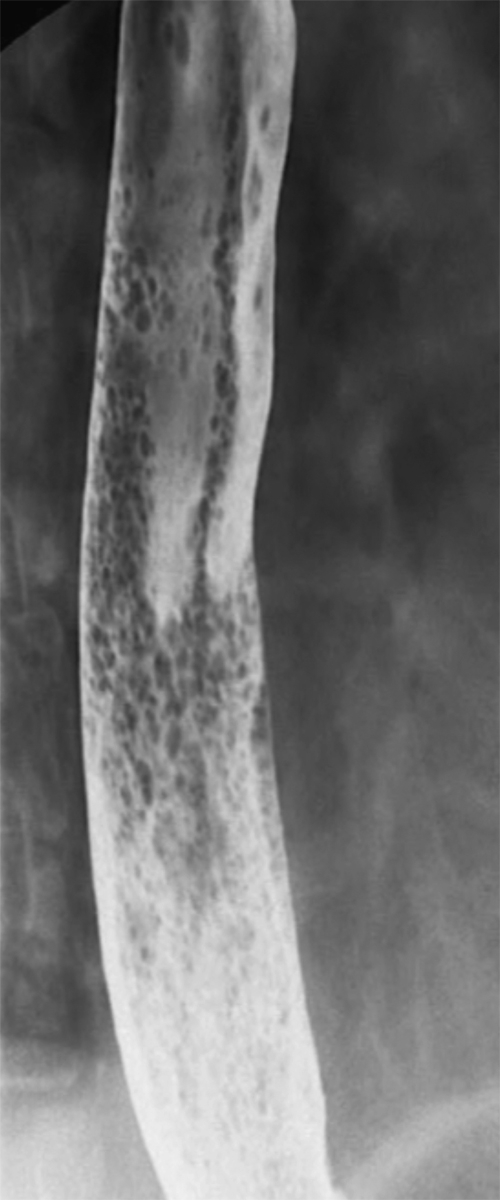
Fig. 1-42). The appearances can sometimes be confused with glycogenic acanthoses, which have randomly deposited mucosal abnormalities, whereas a Candida infection tends to have linear mucosal plaques (Figs. 1-42 and 1-43).
Figure 1-42 UGI swallow in 43-year-old woman undergoing chemotherapy for breast cancer and Candida esophagitis. There is a “shaggy,” plaque-like appearance to the esophageal mucosa.
Figure 1-43 UGI swallow in a 74-year-old woman with multiple, predominantly rounded or ovoid, randomly distributed mucosal defects due to glycogenic acanthosis.
Viral Esophagitis
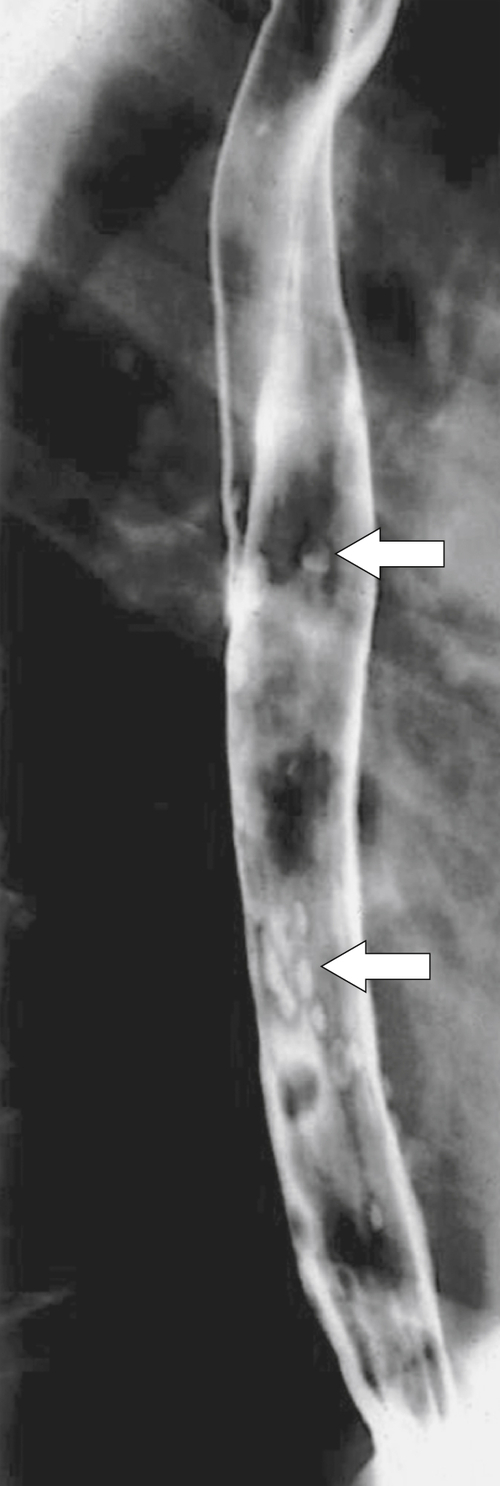
Herpes, CMV, and HIV esophagitis also typically follow immunocompromised states but can also develop when the esophageal mucosa is damaged from radiation therapy used to treat mediastinal malignancies. With herpes infection, UGI examination will demonstrate multiple small and discrete ulcers separated by normal mucosa, unlike
Candida infection, in which the mucosa is diffusely involved (
Fig. 1-44). CMV and HIV tend to have fewer ulcers, and sometimes there is only a single larger ulcer. Rarely, human papillomavirus and Epstein-Barr∗ virus can also infect the esophagus.
Figure 1-44 Barium swallow in a 33-year-old man with immunosuppression showing multiple punctate esophageal ulcers (arrows) due to herpes esophagitis.
Fungus
Fungal ulcers result from extension from the mouth and pharynx and demonstrate mucosal, plaque-like disease. The diffusion of barium between these plaques may give the appearance of ulceration; however, this is pseudoulceration.
Tuberculosis
Tuberculosis may result from direct mediastinal extension with ulceration secondary to the extrinsic invasion of the disease, but it can occasionally occur within the esophagus as a result of swallowing infected sputum.
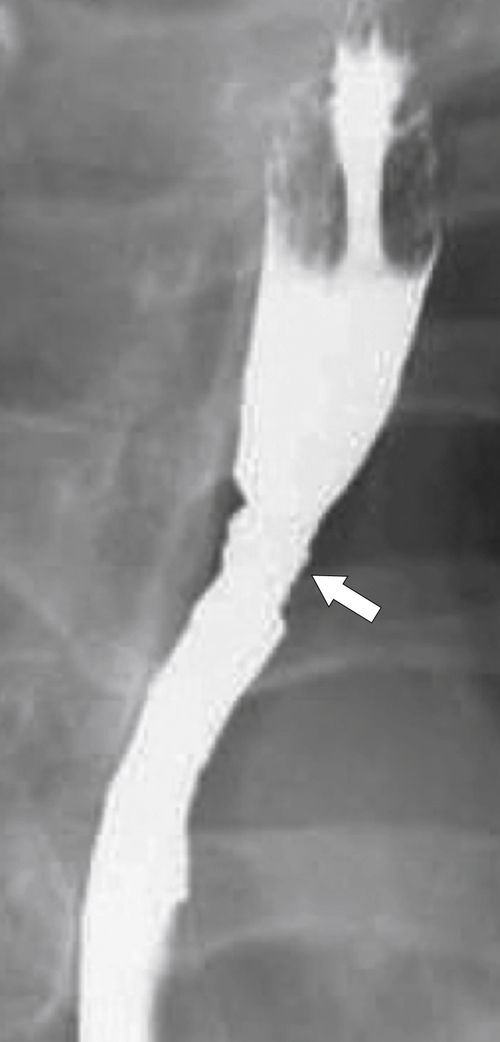
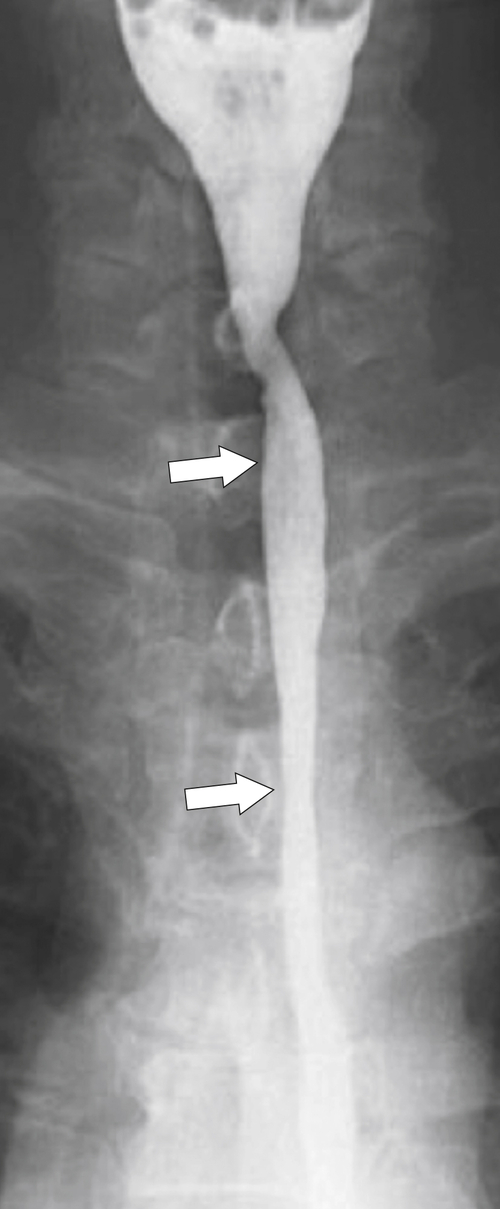
Reflux Esophagitis
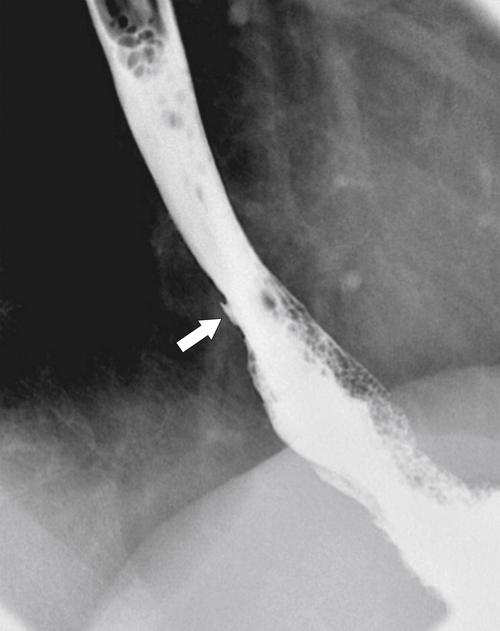
Reflux esophagitis is a common disease due to the widespread incidence of GERD; up to 20% of Westerners will have GERD at some time. Any condition that enhances either the production of acid or facilitation of reflux through the GE junction predisposes the patient to lower esophageal ulceration. Therefore, hyperacidity due to Zollinger-Ellison syndrome compounded by GERD can lead to severe esophagitis and ulcer formation. Patients may be asymptomatic, but most will complain of some pain (heartburn) at some time. The disease can be acute and self-limiting or often chronic, requiring adjustment of food intake, antacid medication, and possible histamine (H2) blocker or proton pump inhibitor treatment. The lower esophageal sphincter tone is generally weakened, permitting free retrograde flow of gastric juice. The irritation caused by refluxing gastric acidic juice readily inflames the mucosa and is usually confined to the lower esophagus.
Imaging findings in acute disease include decreased primary peristaltic waves and, sometimes, increased tertiary contractions. There may be an associated esophageal spasm, which tends to give the esophagus a shortened appearance. There is mucosal edema, which can lead to thickened folds, either vertical or transverse, and erosive disease, which is manifested by subtle nodular or granular changes (
Fig. 1-41). Further erosive disease can lead to ulceration, seen as subtle, tiny collections of barium with surrounding edematous edges and thickened folds, which may radiate away from the ulcer (
Fig. 1-45).
Figure 1-45 Barium swallow in a 63-year-old man with subtle esophageal ulcers (arrow) caused by reflux esophagitis. There is also a granular appearance to the lower esophagus.
With more chronic disease, a hiatal hernia is almost always present. The mucosa can appear puckered or serrated because of edema and spasm. Pseudodiverticula are classic signs indicative of chronic inflammation (
Figs. 1-11 and
1-46). Continued inflammation will ultimately lead to stricture formation, usually of the lower esophagus, represented by a short smooth narrowing typically 1 to 3 cm in length (Fig. 1-47). More severe strictures can develop, with or without ulceration (Fig. 1-48). A benign inflammatory pseudopolyp can also develop, representing a profusely enlarged esophageal fold at the GE junction (see Fig. 1-18).
Figure 1-46 Barium swallow in a 43-year-old man with multiple pseudodiverticula (long arrow). The patient also has underlying candidiasis (short arrow).
Figure 1-47 UGI swallow in a 57-year-old man with chronic lower esophagitis with a short smooth stricture (short arrow), fold thickening (arrowhead), and small ulcerations (long arrow).
Figure 1-48 Barium swallow in a 59-year-old woman with thickened longitudinal folds due to reflux esophagitis and a lower esophageal irregularity and narrowing (arrow) due to a peptic stricture.
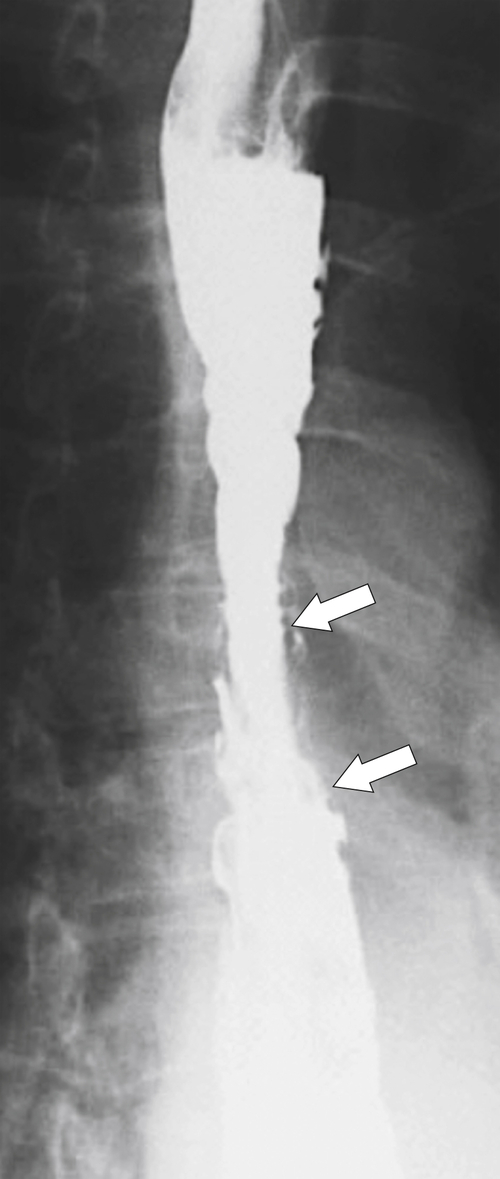
The greatest risk from GERD is the development of a Barrett
∗ esophagus. This occurs in about 10% of patients with GERD (most also have a hiatal hernia) and chronic esophagitis; therefore it is relatively common in the population. Some patients are
asymptomatic, so the incidence may well be higher. It is more common in males, possibly because it is more commonly associated with centripetal obesity. It requires a histological diagnosis because it represents squamous metaplasia to gastric columnar epithelium, a premalignant condition that can progress to adenocarcinoma in about 0.5% of patients per year. Therefore its detection and follow-up are important. Any patient with symptomatic prolonged GERD should be periodically screened (either with EGD or UGI examination) to determine any presence of the abnormality. Once detected, annual EGD procedures with biopsies of the affected area should be performed to rule out the presence of dysplastic squamous cells.At UGI examination, Barrett esophagus is recognized by a focal mucosal nodularity, a reticular mucosal pattern, or ulceration and stricture formation in the mid to lower esophagus (
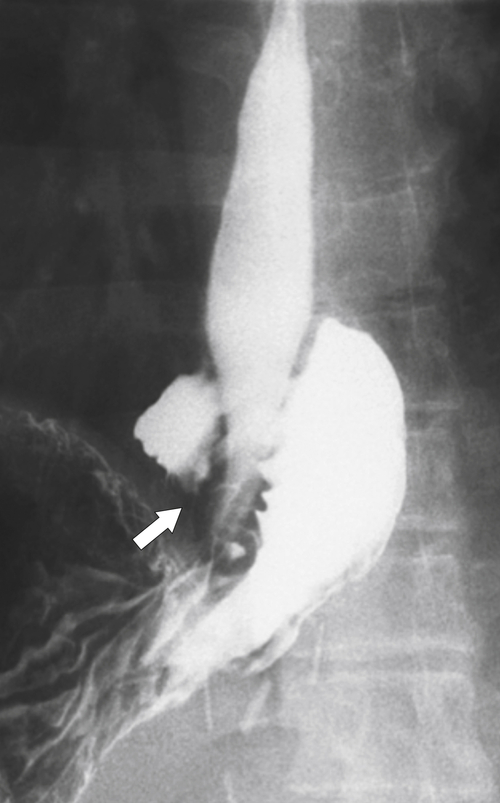
Fig. 1-49). Many appear as simple, smooth strictures, but others demonstrate short, narrower strictures, with or without ulceration (Fig. 1-50). Because there is frequent monitoring (usually endoscopy), early esophageal carcinoma can be detected (Fig. 1-51).
Figure 1-49 Barium swallow in a 61-year-old woman with a midesophageal smooth stricture (arrow) due to Barrett esophagus.
Figure 1-50 UGI swallow in a 61-year-old man with a tight, short midesophageal stricture (long arrow) and ulcer (short arrow) due to Barrett esophagus.
Figure 1-51 UGI swallow in a 60-year-old woman with midesophageal stricture due to Barrett esophagus and a plaque-like mucosal defect due to early adenocarcinoma (arrow).
Less commonly, biliary reflux can also cause erosions and ulcers of the lower esophagus and is a result of disordered bowel mechanics from partial or complete gastric resections with cholodocho-enteric anastomoses. The alkaline bile refluxes into the lower esophagus, which is as toxic as gastric acid and readily causes edema, erosions, ulceration, and ultimately stricture formation. It is usually best treated by diverting bile away from the stomach remnant and esophagus with a Roux
∗-en-Y loop of gastroduodenostomy.
Prolonged nasogastric tube insertion interferes with esophageal sphincter closure, and continuous reflux can occur in addition to failure to clear the lower esophagus from refluxed acid due to dysmotility and abnormal peristalsis. Changes of esophagitis mimic those of the more common GERD due to hiatal hernia.
Drug-Induced Esophagitis
Some drugs are caustic to the esophagus if they have prolonged contact with the mucosa because of dysmotility or some other local stricture. Alternatively, if these medications are taken with patients in the supine or prone position before the patients sleep, they can be held up at the level of the aortic arch, left main bronchus, or distal esophagus. These medications include tetracyclines, potassium chloride, quinidine, and nonsteroidal antiinflammatory drugs (NSAIDs). Imaging findings consist of multiple small ulcers that are variable in shape and shallow in depth (
Fig. 1-52). Some ulcers are larger and appear more sinister but will ultimately heal by fibrosis and stricture formation (Figs. 1-53 and 1-54). Acute alcoholic binges may result in esophageal ulceration, typically of the mid to lower esophagus, although these do not usually lead to stricture formation.
Figure 1-52 UGI swallow in a 47-year-old man with midesophageal ulceration (arrow) resulting from tetracycline administration.
Figure 1-53 Barium swallow in a 47-year-old woman with a large midesophageal ulcer (arrow) due to ulceration from indomethacin ingestion.
Figure 1-54 UGI swallow in a 38-year-old woman with a short narrowing (arrow) in the upper esophagus secondary to fibrotic stricture caused by nonsteroidal antiinflammatory drugs.
Caustic or Corrosive Esophagitis
Strong liquid alkali or acid ingestion (e.g., lye, a concentrated sodium hydroxide, ammonium chloride, silver nitrate, and other acids) causes a severe erosive and diffuse ulceration of the esophagus, which can be fatal in the acute stage. The stomach and duodenum can be affected if sufficient quantities are swallowed and reach these anatomical regions, but the acute manifestations are most common in the esophagus.
In the acute phase, the esophagus is atonic with extensive ulceration, which may perforate, leading to gas in the mediastinum (
Fig. 1-55). It tends to heal by fibrosis and stricture formation (often very narrowed) primarily of the midesophagus, either focally or as a diffusely long stricture (Fig. 1-56). This may retract the stomach partially into the chest because of associated esophageal shortening from the stricture. The ulceration and stricture formation are premalignant for esophageal squamous carcinoma, which may develop 20 years after the ingestion. This would require lifelong repeat endoscopies to exclude malignant transformation in these patients.
Figure 1-55 UGI barium swallow in a 21-year-old woman with recent ingestion of lye demonstrating esophageal narrowing due to edema and ulcers (arrows).
Figure 1-56 UGI swallow in a 55-year-old man with a long esophageal stricture (arrows) due to previous caustic ingestion.
Radiation Esophagitis
Radiation esophagitis is less common than in the past because of more recently refined radiation treatment protocols. In the acute phase, there are esophageal edema and a friable esophagus that may cause not only an ulcer, but also a stricture. Fistula formation may then occur between the esophagus and the underlying mediastinal tumor that is being treated or the adjacent normal mediastinum or lung. Healing is by stricture formation, which can be difficult to differentiate from other chronic strictures, although a history of radiation is suggestive (
Fig. 1-57).
Figure 1-57 Barium swallow in a 65-year-old man with prior radiation for thyroid cancer, demonstrating a radiation stricture (arrow) in the upper esophagus.
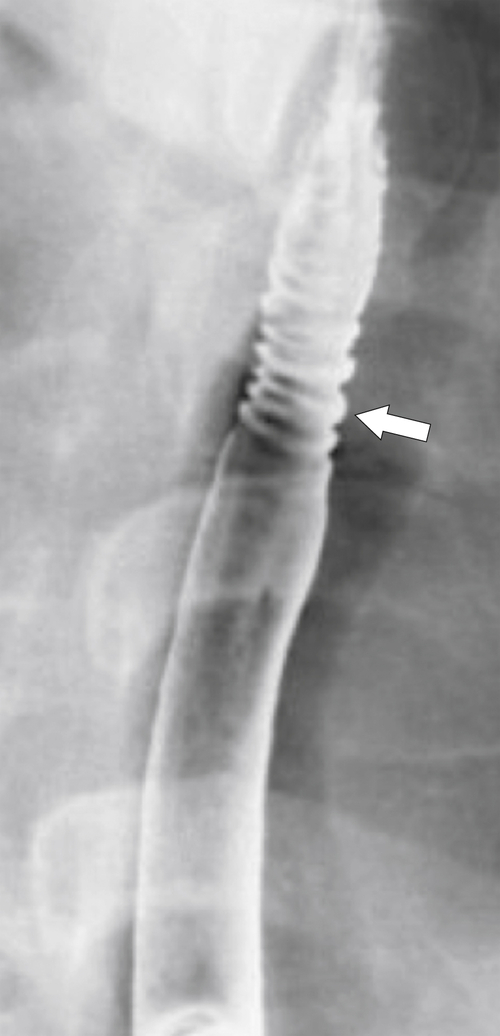
Eosinophilic Esophagitis
Eosinophilic esophagitis is a poorly understood inflammatory disease that occurs in patients with hyperallergenic conditions with associated hypereosinophilia. Histologically, there is eosinophilic infiltration of all layers of the esophageal wall, and a diagnosis is made at esophageal biopsy. It is associated with other GI eosinophilic conditions (e.g., eosinophilic gastroenteritis). At imaging, there may be diffuse esophageal nodularity, which histologically is filled with eosinophils (
Fig. 1-58). More often, however, ulceration and stricture formation are observed (Fig. 1-59). The most specific imaging finding is a “ringed” esophagus due to narrow, fine circumferential focal stricture formation (Fig. 1-60).
Figure 1-58 Barium swallow in a 33-year-old woman with diffuse mild narrowing and nodularity (arrow) due to eosinophilic esophagitis.
Figure 1-59 Barium swallow in a 23-year-old man with an upper esophageal stricture (arrow) due to eosinophilic esophagitis.
Figure 1-60 UGI swallow in a 43-year-old woman with a ring-appearing esophagus (arrow) due to eosinophilic esophagitis.
Behçet Disease
Behçet
∗ disease is a systemic disease affecting mucous membranes (aphthous ulcers), the skin (erythema nodosum and pyoderma gangrenosum), the eyes (uveitis and optic atrophy), the penis (genital ulcerations), the lungs (pleuritis), the joints (arthritis), the brain (dural sinus thrombosis), and the gut. Its cause is poorly understood, but it is thought to be an immune response to external agents rather than autoimmune, although this is unsubstantiated. It commonly affects the esophagus with findings ranging from esophagitis with or without ulceration and stricture formation.
Pemphigoid
An extension of the epidermal manifestation of this disease can extend to the esophagus with mucosal webs (early in the disease) or bullae (blebs), which are recognized as small nodular mucosal lesions at EGD and UGI examination. These readily ulcerate, and chronic disease can result in esophageal stricture, mostly in the upper esophagus (
Fig. 1-61). Some strictures can be very narrow, and a “jet” of barium may be seen below the stricture (Fig. 1-62). There is a risk of malignant degeneration to squamous cell carcinoma.
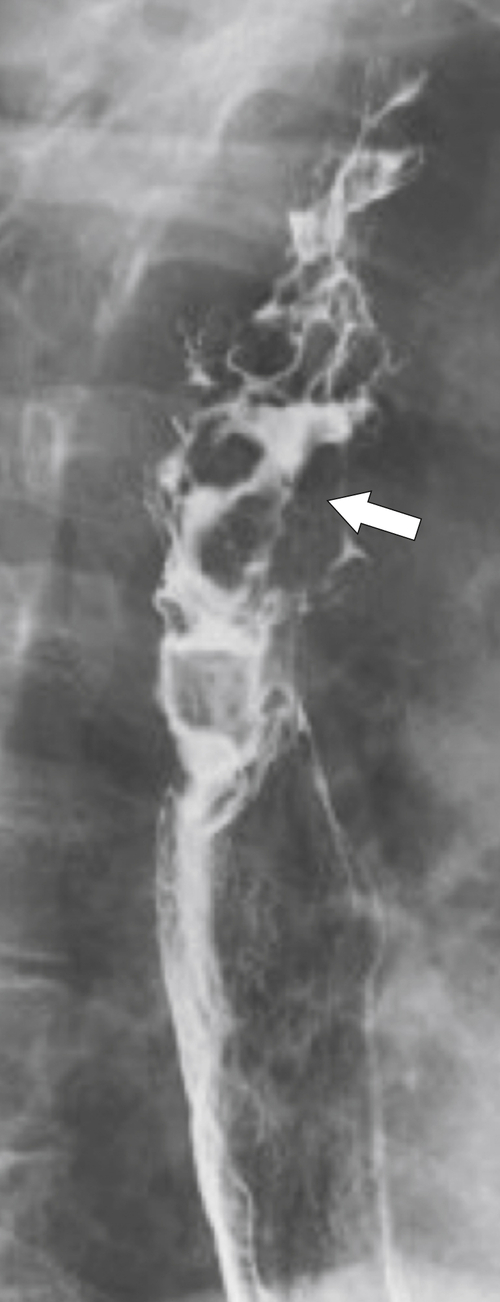
Figure 1-61 Barium swallow of the upper third of the esophagus in a 55-year-old woman demonstrating diffuse cervical and upper thoracic strictures (arrows) (tight and circumferential) due to pemphigoid.
Figure 1-62 UGI series in a 49-year-old woman with pemphigoid and a tight stricture (arrow) with a barium “jet” caudally.
Epidermolysis Bullosa
Epidermolysis bullosa is similar to pemphigoid with multiple small esophageal bullae that form in the upper esophagus in conjunction with epidermal disease. These ulcerate and result in strictures on healing, with either tight focal strictures or longer tapered strictures (
Figs. 1-63 and
1-64). There is a small risk of malignant degeneration.
Figure 1-63 Oblique UGI swallow of the upper esophagus in a 59-year-old woman with a tight stricture (arrow) due to epidermolysis bullosa. The appearance is not dissimilar to pemphigoid.
Figure 1-64 UGI in a 49-year-old woman with an upper esophageal stricture (arrow) due to epidermolysis bullosa.
Tylosis
Tylosis is a rare autosomal dominant disease characterized by hyperkeratinization of the palms of the hands (and sometimes the soles of the feet) with a high risk for squamous esophageal carcinoma (approximately 95% of patients aged 70 years) secondary to esophageal leukoplakia and dysplasia. In the esophagus, focal, small, nonspecific ulcerations can occur along any length of the esophagus, stomach, and small bowel. In the colon, it can produce a colitis similar to ulcerative colitis and is one of the potential causes of a toxic megacolon (see
“Thumb-Printing and Toxic Megacolon” in
Chapter 5).
Glycogenic Acanthosis
Glycogenic acanthosis is a condition typically seen in the elderly, characterized by small (2 to 10 mm), multiple, disparate, plaque-like mucosal lesions throughout the esophagus, which can be numerous or scattered (
Figs. 1-43 and
1-65). These are filled with glycogen deposits, hence their name. Diagnosis is benign, and it is usually detected incidentally and is mostly asymptomatic.
Figure 1-65 UGI swallow in an 85-year-old man with multiple small scattered esophageal plaques (arrow) due to glycogenic acanthosis.
Ectopic Gastric Mucosa
Gastric heterotopia (or ectopic mucosa) in the upper esophagus is present in about 4% of patients and is usually asymptomatic, unlike Barrett esophagus. Ectopic gastric mucosa can produce small, ring-like indentations of the esophagus, usually at the level of the thoracic inlet.
Neoplastic Esophageal Mucosal Abnormalities
Benign
Squamous Papillomas and Papillomatosis
Squamous papilloma is a rare condition, thought to be mostly benign, but it can be premalignant with the development of squamous carcinoma. A papilloma may be single, but more than one is known as papillomatosis. Squamous papillomas are associated with acanthosis nigricans, and at imaging, they appear as single or multiple small polyps.
Villous Adenoma
These adenomas are rarely identified. Like villous adenomatous polyps elsewhere in the GI tract, they appear as frond-like mucosal masses and have premalignant potential (
Fig. 1-66).
Figure 1-66 Barium swallow of the lower esophagus in a 63-year-old woman with an irregular mucosal mass (arrow) due to a villous adenoma.
Hamartoma
Cowden’s
∗ disease or multiple hamartoma syndrome, is a rare autosomal dominant-inherited disease that produces widespread hamartomatous disease, particularly of the skin. When it affects the esophagus, multiple small mucosal nodularities can be identified. Similar manifestations can be observed in the colon. These hamartomas are premalignant throughout the body, and frequent patient screening is required.
Leukoplakia
Leukoplakia is due to hyperplastic squamous epithelium that occurs most commonly in the oropharynx because of excessive tobacco use. In the oropharynx it is premalignant, but in the esophagus, where it is less commonly observed, it has debatable malignant potential. When observed, it produces small, whitish, plaque-like mucosal esophageal abnormalities.
Malignant
Carcinoma
Carcinoma of the esophagus continues to have high mortality because the development of these tumors is indolent and therefore the tumors present late. Early detection, however, has a 90% 5-year survival. There are multiple predisposing factors (
Box 1-1). Males are four times more likely than women to be affected, mostly because of smoking and alcohol habits. Patients present with dysphagia (difficulty swallowing), particularly of solids, and odynophagia (painful swallowing), pain, weight loss, and anorexia.
Box 1-1 Risk Factors for the Development of Esophageal Cancer
Alcohol
Tobacco
Chronic reflux esophagitis/Barrett complex
HPV infection
Achalasia
Caustic ingestion (lye)
Radiation
Head and neck cancer
Plummer-Vinson syndrome
Celiac disease
Tylosis (hyperkeratosis)
Pemphigoid
Epidermolysis bullosa
HPV, Human papillomavirus.
Table 1-6
Esophageal Carcinoma
| Type |
Frequency |
| Squamous |
Worldwide, 90%; Western Europe/United States, 50% |
| Adenocarcinoma |
Worldwide, 5%-10%; Western Europe/United States, 35%-50% |
| Spindle cell carcinoma |
<1% |
| Carcinosarcoma |
<1% |
| Primary malignant melanoma |
<1% |
| Oat cell carcinoma |
<1% |
Table 1-7
Staging of Esophageal Carcinoma
| Stage |
Disease Extension |
| 0 |
Carcinoma in situ |
| I |
Lamina propria/submucosa |
| IIA |
Transmural but not further extension |
| IIB |
Transmural and regional lymph nodes |
| III |
Regional lymph nodes (or other nodes), invasion of adjacent structures |
| IVB |
Regional or other nodes |
| IVB |
Regional or other nodes and distant metastases |
The findings at UGI examination depend on the timing of the diagnosis. Early findings may demonstrate small sessile polyps or plaque-like lesions, although with adenocarcinoma, there is usually an associated Barrett esophagus with small plaque-like lesions or more marked irregularity along the Barrett stricture (see
Fig. 1-51). Later presentations produce larger lesions, which infiltrate along the length of affected esophagus, with a shelf-like abrupt transition from normal to abnormal esophageal mucosa (not dissimilar to its colonic adenocarcinoma “apple core” counterpart), with resultant luminal stricture formation (
Fig. 1-67). The mucosa is distorted, blunted, or destroyed, and nodular masses can arise, which can ulcerate (Fig. 1-68). Other lesions can present as flat ulcers surrounded by an edematous rim. A rarer type is varicoid carcinoma, whereby the neoplasm extends submucosally along a length of esophagus, giving the appearance of varicosities or thickened folds (see Fig. 1-33). The mucosal folds may not be as smooth as those in esophageal varices, and this result gives a clue to the diagnosis. The patient may not have a history of cirrhosis. Some gastric adenocarcinomas invade the lower esophagus and extend a few centimeters above the GE junction (Fig. 1-69). At UGI examination, it can be difficult to know for certain whether these tumors are primarily esophageal extending to the GE junction or vice versa, and the diagnosis may only be made at endoscopy and biopsy.
Figure 1-67 UGI swallow (A) and axial contrast-enhanced CT (B) of the lower esophagus in a 68-year-old man with marked mucosal irregularity (A; arrow) typical of esophageal carcinoma. The lesion is less evident on CT, with eccentric wall thickening (B; arrow).
Figure 1-68 UGI swallow of the midesophagus in a 54-year-old man with nodular distortion of the mucosal folds (arrow) due to esophageal carcinoma.
Figure 1-69 UGI swallow in a 59-year-old woman with irregular and distorted lower esophageal mucosa due to adenocarcinoma of the gastric cardia infiltrating up into the lower esophagus (arrow).
At CT, the primary tumor can be harder to identify unless it is large, particularly if the esophagus is undistended and empty of oral contrast medium. However, CT is far superior to UGI examination for the identification of any local extension, including regional lymphadenopathy (
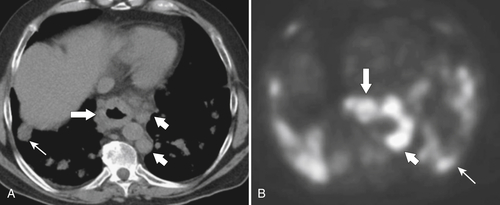
Fig. 1-70). In stages I and II and early stage III, tumors will generally not be identified at CT, although simple esophageal thickening may be present (Table 1-7). Once a tumor develops later in stage III, the mass should be detected by CT as the tumor invades the surrounding mediastinal structures and enlarges regional lymph nodes (Fig. 1-71). Stage IV disease should demonstrate distant dissemination of the disease, particularly to the lungs, liver, and lymph nodes. The detection of these lymph nodes is more readily identifiable by PET/CT, however, which may also aid in detecting more distant disease (particularly lymph nodes) (Fig. 1-71).
Figure 1-70 Axial (A) and coronal (B) contrast-enhanced CT in a 71-year-old woman with a lower esophageal carcinoma over several centimeters (large arrow) with mediastinal adenopathy (small arrow).
Figure 1-71 Axial noncontrast CT (A) and PET (B) in a 70-year-old woman with stage IV esophageal carcinoma (large arrows) with multiple paraesophageal lymph nodes (small arrows) and lung metastases (thin arrow).
Adenocarcinomas look similar to squamous cell carcinomas, even when arising in a Barrett esophagus. By the time the patient is seen, the Barrett stricture has generally been obliterated by the malignant process (
Fig. 1-72).
Figure 1-72 UGI swallow in a 67-year-old man with a lower esophageal adenocarcinoma (arrow) arising in a Barrett esophagus.
Other carcinomas are rare, and diagnosis will only be made at histological examination. Spindle cell carcinoma is a rare variant of squamous cell carcinoma due to mesenchymal metaplastic transformation. These cells are typically large and polypoid. Oat cell carcinoma, primary esophageal melanoma, and carcinosarcoma are even rarer and present similarly to spindle
cell carcinoma. Superficial spreading carcinoma is a variant of squamous cell carcinoma that is characterized by small mucosal nodules rather than plaque-like or polypoid masses. It has a better prognosis because it is usually confined to the mucosa and submucosa.
Kaposi Sarcoma
Kaposi
∗ sarcoma is caused by a herpes virus and is a systemic disease that typically manifests with purplish skin papular lesions. There are four subtypes, which were originally described in
middle-aged men of Mediterranean and Jewish descent who had vascular cutaneous lesions. This same disease is now observed more commonly in immunocompromised patients, particularly those with AIDS. The former type has a more benign and indolent course, but the type that results from immunosuppression has a more aggressive course. Both the respiratory and GI tracts can be involved, particularly with the immune-suppressed form, with single or multiple submucosal lesions, which often have central ulceration (see Chapter 2).
Metastatic Disease
Metastatic disease results either from local extension (including the larynx, thyroid, and lung), mediastinal nodes (e.g., lymphoma), and gastric extension into the esophagus or rarely from hematogenous spread (breast, melanoma) (
Fig. 1-73). In malignancies that directly invade the esophagus from local structures, there may be a smooth extrinsic compression before mucosal nodularity and ulceration are seen, confirming that the malignancy has extended transmurally. As ulceration and necrosis develop, fistula formation can occur, which rarely results from hematogenous metastatic spread (Fig. 1-74). Hematogenous spread from breast cancer typically produces short eccentric strictures, whereas those from melanoma produce submucosal “bull’s-eye” ulcerating lesions, as elsewhere in the GI tract. Lymphomatous extension from mediastinal nodes often causes less ulceration; rather, it may produce a more smooth-tapered, achalasia-like appearance.
Figure 1-73 Axial contrast-enhanced CT in a 66-year-old woman with esophageal invasion (arrow) by a large lung cancer.
Figure 1-74 Nonionic contrast media UGI swallow in a 76-year-old man with metastatic lymphadenopathy to the esophagus, stricture formation (large arrow) from lung cancer, and a bronchoesophageal fistula (small arrows).
Esophageal Motility Disorders
Normal Peristalsis
Peristalsis is a complex neuromuscular and involuntary event involving a primary peristaltic wave that is initiated at the moment of swallowing that propels the food bolus caudally with progressive stripping (or propulsive) waves. This wave is soon followed by the secondary peristaltic wave that results from esophageal distention by the food bolus and is a locally initiated propulsive wave in the area immediately adjacent to the food bolus. Under normal conditions, the result is a smooth, wave-like contraction from upper to lower esophagus. Tertiary contractions are nonpropulsive, random, and disorganized contractions observed more often in the elderly. Dysmotility disorders are caused by disparate causes but are probably most common after stroke, given its frequency in the population (
Table 1-8).
Table 1-8
Causes of Esophageal Dysmotility
| Neurological disease |
Brainstem infarction
Bulbar palsy
Multiple sclerosis
Peripheral neuropathies
Myasthenia gravis |
| Local muscular spasm |
Cricopharyngeus spasm∗ |
| Muscular dysfunction |
Dermatomyositis
Muscular dystrophy
Scleroderma∗
Achalasia (primary and secondary)∗
Diffuse esophageal spasm |
| Inflammatory conditions |
Severe esophagitis (e.g., radiation, caustic, GERD) |
| Systemic disease |
Hyperthyroidism, hypothyroidism, amyloidosis, diabetes |
| Drugs |
Anticholinergics (e.g., atropine) |
GERD, Gastrointestinal reflux disease.
∗See discussion in this chapter.
Neurogenic Dysmotility
Central or peripheral neuropathies can affect esophageal peristalsis and lead to major dysmotility. It is not uncommon after stroke for the patient to have swallowing difficulties, often with aspiration and its consequent complications. Other causes are listed in
Table 1-8. Many of these causes can be sufficiently debilitating that the patient will ultimately require enteral or parenteral feeding; many patients require the placement of gastric or jejunal feeding tubes.
Scleroderma
This autoimmune and chronic systemic disease of uncertain etiology affects the skin, lungs, heart, kidneys, and central nervous
system (CNS), and commonly affects the GI tract (second most common manifestation after the skin). It is part of the CREST (calcinosis, Raynaud∗ phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) syndrome. Within the GI tract, the esophagus is most commonly affected (approximately 90% of cases), although small bowel involvement is also common (50%). There is a progressive smooth muscular atrophy secondary to the chronic fibrosing process that affects the lower two thirds of the esophagus (the upper third is striated muscle). The bowel progressively loses function and becomes dilated with the loss of normal peristaltic activity (Fig. 1-75). The resulting fibrosis leads to esophageal widening and distention rather than stricture formation, although GERD is common because the GE junction is typically widely patent. The resulting ulceration can cause stricture formation and even a Barrett esophagus. Dermatomyositis can give similar dysmotility appearances to scleroderma.
Figure 1-75 Barium swallow in a 49-year-old woman with smooth diffuse esophageal dilatation due to scleroderma (arrow).
Achalasia
Achalasia is a smooth muscle motility disorder of the lower esophagus that can be primary or secondary. Primary achalasia is a disease, usually starting in young adulthood, that affects the lower esophagus because of a reduction in myenteric ganglia in Auerbach
† (or myenteric) plexus that provide motor function to the lower esophageal sphincter. The lower esophageal sphincter then fails to relax, with the proximal esophagus steadily dilating over months and years as it attempts to propel food through the strictured lower esophagus. Clinically, the resulting stasis can lead to halitosis, aspiration, and secondary infection with candidiasis. It is also a premalignant condition with the risk of secondary squamous carcinoma development, although the diagnosis can be difficult because the patient has a long history of dysphagia and a small tumor can be easily missed for surrounding food debris.
Severe forms can even be recognized on a plain chest radiograph with a posterior mediastinal fluid-filled dilated esophagus, which should be confirmed with UGI examination or ultimately EGD because biopsy of the affected esophageal segment confirms the disease. Esophageal dysmotility at UGI examination varies from mild to almost the complete absence of peristalsis in severe forms. A diffusely dilated esophagus is typically identified as a tight stricture at the GE junction with a “rat-tailed” or “bird-beak”
appearance (Fig. 1-76). Observation under fluoroscopy may not demonstrate the passage of barium into the stomach unless the patient is upright and gravity increases the lower esophageal fluid/barium pressure to overcome the resistance created by the GE junction stricture. However, the passage of barium through the GE sphincter is often slow, if it happens at all. Patients are usually treated with regular endoscopic dilatation where any underlying mucosal malignant change can be observed.
Figure 1-76 Barium swallow in a 53-year-old man with achalasia with a diffusely widened esophagus and “rat-tail” stricture at the GE junction (arrow).
Secondary Achalasia
Secondary achalasia, also known as
pseudoachalasia, essentially produces the same symptoms and radiological findings as primary achalasia but is caused by invasion of the myenteric plexuses from lower esophageal or GE junction malignancies, particularly carcinoma; lymphoma or even metastatic disease is also recognized (
Fig. 1-77). It is therefore seen in older patients and anyone presenting for the first time with symptoms. Radiological findings of achalasia in adults of middle age or in the elderly should raise suspicion of an underlying malignancy. The diagnosis is usually made with EGD and biopsy of the affected segment.
Figure 1-77 Axial (A) and coronal (B) contrast-enhanced CT in a 67-year-old woman with a dilated esophagus tapering to a narrow, smooth stricture at the GE junction (arrows) from submucosal esophageal invasion by gastric cancer.
Other causes include scleroderma, severe peptic stricture, surgical postvagotomy, and Chagas
∗ disease. The latter is produced by the trypanosome,
Trypanosoma cruzi, which is a protozoal infection typically found in poorer living conditions in South America. Infection results from fecal excretion of the protozoa by the reduviid bug subdermally. The resulting complications of infection can take up to 10 years to manifest and affect the esophagus, duodenum, colon, and heart. There is diffuse dilatation of the infected organs secondary to neurotoxic damage of intramural autonomic sympathetic ganglion cells. At imaging, the esophageal abnormalities can appear identical to those of achalasia, although unlike that disease, esophageal contractions can be observed in Chagas disease.
Diffuse Esophageal Spasm
Diffuse esophageal spasm is characterized by repetitive involuntary unexpected esophageal contractions (although hot or cold fluids can trigger the symptoms) that result in intense dysphagia. This spasm can be diagnosed through the observation of these contractions at UGI examination and fluoroscopy with the appearance of a “cork-screw” spasmodic esophagus (
Fig. 1-78). The lower esophageal sphincter functions normally.
Figure 1-78 Barium swallow in a 77-year-old man with a “corkscrew” esophagus due to esophageal spasm.
Presbyesophagus
Presbyesophagus is commonly seen in the elderly in whom there is reduced normal peristaltic activity but increased, haphazard, aperistaltic contractions. The lower esophageal sphincter is usually functioning normally. It is a diagnosis primarily made by fluoroscopic barium examination.
Trauma
Traumatic disruption to the esophageal mucosa is usually iatrogenic (nasogastric tube insertion, endoscopic perforation, or sclerotherapy for esophageal varices) but can be secondary to ingested fish bones or other sharp foods (e.g., corn chips) (
Fig. 1-79). Profuse vomiting with a tear (Mallory-Weiss tear) or frank perforation (Boerhaave syndrome) leads to ulceration and delayed stricture formation in some patients.
Figure 1-79 Barium swallow in an 88-year-old woman with a linear ulcer (arrow) in the midesophagus due to trauma from a swallowed toothpick.
Esophageal Foreign Body
There is usually a temporal history of dysphagia related to food ingestion. Typical objects include fish or chicken bones or chunks of meat in adults, but in children, almost anything that can be swallowed has been described (
Fig. 1-80). Fish or chicken bones typically become lodged in the upper esophagus just below the cricopharyngeus muscle, whereas meat chunks become stuck above previous strictures (Schatzki ring, peptic or neoplastic lesion). Lateral plain film might identify calcified elements, and UGI examination identifies linear or curved foreign bodies. UGI studies will readily identify larger food boluses, but smaller material can be hard to identify (Fig. 1-81). CT is more likely to identify calcified structures and any related edema, hemorrhage, or abscess (if the foreign body has penetrated the esophageal wall).
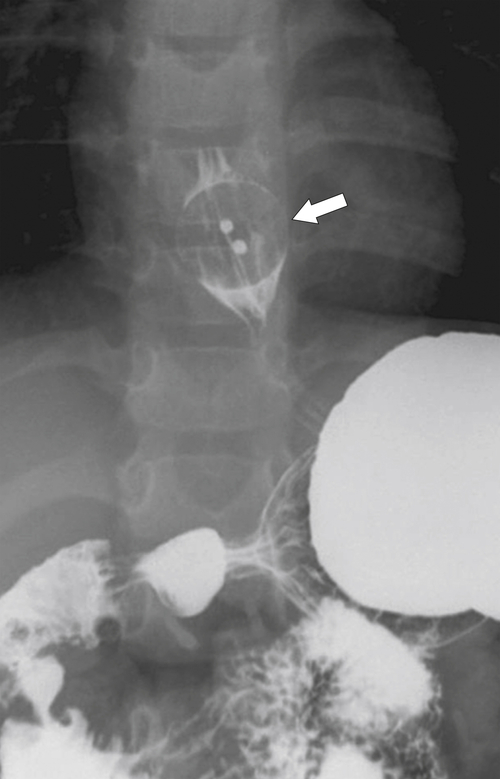
Figure 1-80 Barium swallow in a 5-year-old boy with an ingested coat button (arrow).
Figure 1-81 UGI swallow in a 70-year-old man with a foreign body (steak matter) (arrow) completely occluding the esophagus.
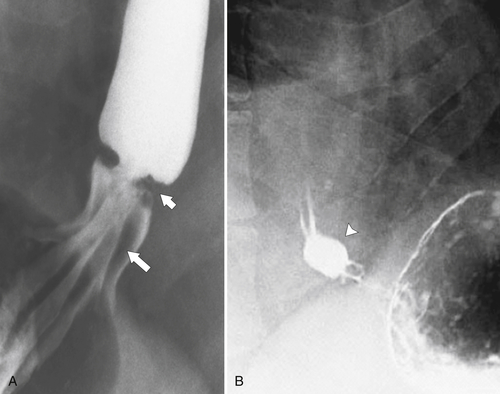
Mallory-Weiss Tear
Mallory-Weiss
∗ tear is a rent in the lower esophageal mucosa after prolonged vomiting and retching. Patients usually have hematemesis, melena, or both. This rent is only a mucosal tear
(rather than full thickness from Boerhaave∗ syndrome) and is usually self-limiting. The diagnosis can be made by EGD, but a UGI study with water-soluble contrast media may demonstrate a thin, linear collection adjacent to the esophagus. Because the tear is mucosal, though, it is commonly not visualized. If no tear is identified or better visualization is required, then barium can subsequently be used because there is essentially little risk of complete perforation. However, some tears can be more extensive and lead to submucosal hemorrhage, which is recognized as a smooth submucosal mass. At CT, there may be a small linear track of water-soluble contrast medium adjacent to the lower esophagus with or without a small area of gas (Fig. 1-82). This could also identify any submucosal hemorrhage and rule out a complete esophageal wall tear (Boerhaave syndrome).
Figure 1-82 Axial contrast-enhanced CT in a 48-year-old man with a small ring of submucosal gas (arrows) due to a Mallory-Weiss tear.
Boerhaave Syndrome
This is a more severe version of the Mallory-Weiss tear due to a full transmural esophageal perforation also precipitated by a marked sudden increase in esophageal pressure from retching, vomiting, defecation, weight lifting, and seizures. Similar findings can be seen after instrumentation (e.g., perforating EGD, stent placements, or dilatation procedures). There may be mediastinal soft tissue widening and pneumomediastinum by plain radiography due to hemorrhage, fluid, or gas (
Fig. 1-83). As the perforation is usually at the left lower esophagus, there may be a left pleural effusion or a left hydropneumothorax (Fig. 1-84). A diagnosis can be made at UGI examination, but barium is initially contraindicated if this condition is suspected because it is highly toxic in the mediastinum and can cause a severe mediastinitis. Rather, water-soluble nonionic contrast should be administered, which may demonstrate a local leak and extravasation of contrast material. Only after no leak is identified should barium be administered to fully exclude a small, localized leak (if no leak is identified with water-soluble contrast medium, then a large leak is excluded). These findings are better demonstrated by CT, however, with the use of oral water-soluble contrast material, which may demonstrate both the site of the leak and mediastinal gas (emphysema) resulting from the perforation (Fig. 1-84). This may also identify mediastinal fluid, including a pericardial effusion, periesophageal fluid, and pleural fluid. Urgent surgical repair is required to prevent severe mediastinitis and possible death; there is a 70% mortality if this rupture is not treated within 24 hours.
Figure 1-83 Plain radiograph (A) and nonionic contrast UGI swallow (B) of the lower chest in a 36-year-old man after severe vomiting demonstrating mediastinal gas (large arrow). At UGI swallow an esophageal perforation is confirmed with leak of contrast into the mediastinum (small arrow) due to a complete (Boerhaave) tear.
Figure 1-84 Axial (A) and coronal (B) contrast-enhanced CT in a 44-year-old man with Boerhaave syndrome with mediastinal gas and extraluminal fluid (arrows) due to esophageal perforation.
Aortoenteric Fistula
Aortic fistula is secondary to midthoracic invasion of the esophagus from aortic aneurysms, either from atherosclerotic disease or from mycotic or postsurgical complications (e.g, infected aortic grafts). As expected, patients have profuse, bright red hematemesis, and it is often fatal. Contrast-enhanced CT without oral contrast is the investigation of choice.
Postoperative and Interventional Procedures
There are several therapeutic endoscopic esophageal procedures that are performed for either the treatment or relief of benign or malignant esophageal obstruction. These include balloon dilatation for the relief of benign strictures (e.g., achalasia, postcaustic ingestion, drug-induced lesion) and sclerotherapy for the treatment of esophageal varices. Laser therapy is used for debulking malignant disease, and a metallic or plastic stent placement is used to bypass malignant strictures in patients with inoperable esophageal cancer (
Fig. 1-85).
Figure 1-85 Barium swallow in a 74-year-old man with recurrent esophageal cancer and stent placement to relieve dysphagia. There is irregularity of the esophageal lumen (arrow), but contrast passes through to the stomach.
Hairy Esophagus
As its name suggests, hairy esophagus is a rare manifestation of skin grafting for pharyngoesophageal reconstructive surgery that presents with hair follicles inside the esophagus.
Fundoplication
Fundoplications constitute a variety of surgical procedures that are aimed at preventing intractable GERD. The fundamental principle is to tighten or compress the lower esophageal sphincter by “wrapping” a portion of the proximal stomach around the lower esophagus, thereby preventing gastric contents from
refluxing up into the esophagus. The most common procedure is the Nissen∗ fundoplication, which is a complete 360-degree wrap (others are partial wraps of approximately 240 to 270 degrees). Complications usually arise because the wrap is too tight or too loose. In the former, there is delayed esophageal emptying, which can be observed at UGI examination, or there can be free reflux and a widened GE junction if the wrap is too loose. Wraps become too loose when the sutures become disrupted and the wrap begins to unfold itself. The wrap can also slide caudally to constrict the middle aspect of the stomach (similar to an hourglass appearance). Conversely, the wrap may herniate into the chest.The normal appearances with UGI examination demonstrate a smooth tapering of the lower esophagus as it passes through the wrap, which itself may be outlined by barium (
Fig. 1-86). Between the esophagus and the wrap is a radiolucent circular band of the constricting gastric wall around the esophagus (Fig. 1-87). At CT, there is a soft tissue mass-like density around the lower esophagus for a few centimeters. Complications, however, are best evaluated by UGI series because CT will generally be unable to determine whether the wrap is too loose or too tight. If the wrap is too tight, the esophagus may be distended, and if it is too loose, the wrap can be seen at barium swallow unfolding and GERD will return (Figs. 1-88 and 1-89). Nonwrap complications are common to most other surgical procedures and include hemorrhage, fluid collections, and abscess in the chest or abdomen due to leaks.
Figure 1-86 UGI swallow in a 44-year-old woman with a normal Nissen fundoplication. There is a smooth tapering to the lower esophagus. The “wrap” surrounds the lower esophagus (arrow).
Figure 1-87 UGI swallow in a 51-year-old woman with a fundal filling defect (arrows) due a normal Nissen fundoplication.
Figure 1-88 UGI swallow in a 41-year-old man with a Nissen fundoplication. There is smooth narrowing of the lower esophagus due to the “wrap” being too tight (arrow). The esophagus is also dilated.
Figure 1-89 Barium swallow in a 38-year-old woman with partial unwrapping of the fundoplication (arrow).
Suggested Readings
Asrani A. et al. Urgent findings on portable chest radiography: what the radiologist should know. AJR Am J Roentgenol 2011;196:S45–S61.
Bird-Lieberman E.L. et al. Early diagnosis of oesophageal cancer. Br J Cancer 2009;101(1):1–6.
Bizekis C. et al. Initial experience with minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2006;82(2):402–406: discussion 406-407.
Bleshman M.H. et al. The inflammatory esophagogastric polyp and fold. Radiology 1978;128:589–593.
Buecker A. et al. Esophageal perforation: comparison of use of aqueous and barium-containing contrast media. Radiology 1997;202(3):683–686.
Cheng H.T. et al. Caustic ingestion in adults: the role of endoscopic classification in predicting outcome. BMC Gastroenterol 2008;8:31.
De Schipper J.P. et al. Spontaneous rupture of the oesophagus: Boerhaave’s syndrome in 2008. Literature review and treatment algorithm. Dig Surg 2009;26(1):1–6.
Dibble C. et al. Detection of reflux esophagitis on double-contract esophagrams and endoscopy using the histologic findings as the gold standard. Abdom Imaging 2004;29(4):421–425.
Doo E.Y. et al. Oesophageal strictures caused by the ingestion of corrosive agents: effectiveness of balloon dilatation in children. Clin Radiol 2009;64(3):265–271.
Ekberg O. et al. Dysfunction of the cricopharyngeal muscle: a cineradiographic study of patients with dysphagia. Radiology 1982;143:481–486.
Freeman R.K. et al. Esophageal stent placement for the treatment of spontaneous esophageal perforations. Ann Thorac Surg 2009;88(1):194–198.
Ghahremani G.G. et al. Glycogenic acanthosis of the esophagus: radiographic and pathologic features. Gastrointest Radiol 1984;9(2):93–98.
Grant P.D. et al. Pharyngeal dysphagia: what the radiologist needs to know. Curr Probl Diagn Radiol 2009;38(1):17–32.
Grishaw E.K. et al. Functional abnormalities of the esophagus: a prospective analysis of the radiographic findings relative to age and symptoms. AJR Am J Roentgenol 1996;167(3):719–723.
Gupta S. et al. Usefulness of barium strictures of the esophagus. AJR Am J Roentgenol 2003;180(3):737–744.
Hamilton J.M. et al. Severe injuries from coin cell battery ingestions: 2 case reports. J Pediatr Surg 2009;44(3):644–647.
Huang S.Y. et al. Large hiatal hernia with floppy fundus: clinical and radiographic findings. AJR Am J Roentgenol 2007;188(4):960–964.
Insko E.K. et al. Benign and malignant lesions of the stomach: evaluation of CT criteria for differentiation. Radiology 2003;228(1):166–171.
Iyer R.B. et al. Diagnosis, staging, and follow-up of esophageal cancer. AJR Am J Roentgenol 2003;181(3):785–793.
Jiang G. et al. Thoracoscopic enucleation of esophageal leiomyoma: a retrospective study in 40 cases. Dis Esophagus 2009;22(3):279–283.
Kang H.K. et al. Three-dimensional multi-detector row CT portal venography in the evaluation of portosystemic collateral vessels in liver cirrhosis. Radiographics 2002;22(5):1053–1061.
Kent M.S. et al. Revisional surgery after esophagectomy: an analysis of 43 patients. Ann Thorac Surg 2008;86(3):975–983: discussion 967-974.
Kim T.J. et al. Multimodality assessment of esophageal cancer: preoperative staging and monitoring of response to therapy. Radiographics 2009;29(2):403–421.
Kim T.J. et al. Postoperative imaging of esophageal cancer: what chest radiologists need to know. Radiographics 2007;27(2):409–429.
Kim Y.J. et al. Esophageal varices in cirrhotic patients: evaluation with liver CT. AJR Am J Roentgenol 2007;188(1):139–144.
Kwee R.M. Prediction of tumor response to neoadjuvant therapy in patients with esophageal cancer with use of 18F FDG PET: a systematic review. Radiology 2010;254(3):707–717.
Lahcene M. et al. Esophageal dysmotility in scleroderma: a prospective study of 183 cases. Gastroenterol Clin Biol 2009;33(6-7):466–469.
Lee S.S. et al. Superficial esophageal cancer: esophagographic findings correlated with histopathologic findings. Radiology 2005;236(2):535–544.
Levine M.S. et al. Diseases of the esophagus: diagnosis with esophagography. Radiology 2005;237(2):414–427.
Levine M.S. et al. Esophageal intramural pseudodiverticulosis: a reevaluation. AJR Am J Roentgenol 1986;147:1165–1170.
Levine M.S. et al. Double-contrast upper gastrointestinal examination: technique and interpretation. Radiology 1988;168(3):593–602.
Levine M.S. et al. Barium studies in modern radiology: do they have a role? Radiology 2009;250(1):18–22.
Levine M.S. Reflux esophagitis and Barrett’s esophagus. Semin Roentgenol 1994;29(4):332–340.
Mahgerefteh S.Y. et al. Radiologic imaging and intervention for gastrointestinal and hepatic complications of hematopoietic stem cell transplantation. Radiology 2011;258(3):660–671.
Mann N.S. et al. Barrett’s esophagitis in patients with symptomatic reflux esophagitis. Am J Gastroenterol 1989;84(12):1494–1496.
Mauro M.A. et al. Epidermolysis bullosa: radiographic findings in 16 cases. AJR Am J Roentgenol 1987;149:925–927.
Newcomer M.K. et al. Complications of upper gastrointestinal endoscopy and their management. Gastrointest Endosc Clin N Am 1994;4:551.
Ntoumazios S.K. et al. Esophageal involvement in scleroderma: gastroesophageal reflux, the common problem. Semin Arthritis Rheum 2006;36(3):173–181.
Ott D.J. et al. Esophagogastric region and its rings. AJR Am J Roentgenol 1984;142(2):281–287.
Rantanen T.K. et al. Gastroesophageal reflux disease as a cause of death is increasing: analysis of fatal cases after medical and surgical treatment. Am J Gastroenterol 2007;102(2):246–253.
Rubesin S. et al. Granular cell tumors of the esophagus. Gastrointest Radiol 1985;10(1):11–15.
Rubesin S.E. et al. Killian-Jamieson diverticula: radiographic findings in 16 patients. AJR Am J Roentgenol 2001;177(1):85–89.
Rubesin S.E. et al. The tailored double-contrast pharyngogram. Crit Rev Diagn Imaging 1988;28(2):133–179.
Samadi F. et al. Feline esophagus and gastroesophageal reflux. AJR Am J Roentgenol 2010;194(4):972–976.
Sass D.A. et al. Portal hypertension and variceal hemorrhage. Med Clin North Am 93(4):837-853, vii-viii, 2009. Savarino E et al: Gastroesophageal reflux and pulmonary fibrosis in scleroderma: a study using pH-impedance monitoring. Am J Respir Crit Care Med 2009;179(5):408–413.
Schneider J.H. et al. Transient lower esophageal sphincter relaxation and esophageal motor response. J Surg Res 2010;159(2):714–719.
Sydow B.D. et al. Radiographic findings and complications after surgical or endoscopic repair of Zenker’s diverticulum in 16 patients. : . AJR Am J Roentgenol 2001 Nov;177(5):1067–1071.
Umeoka S. et al. Esophageal cancer: evaluation with triple-phase dynamic CT—initial experience. Radiology 2006;239(3):777–783.
Warshauer D.M. et al. Imaging manifestations of abdominal sarcoidosis. AJR Am J Roentgenol 2004;182(1):15–28.
Westerterp M. et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy—systematic review. Radiology 2005;236(3):841–851.
White S.B. et al. The small-caliber esophagus: radiographic sign of idiopathic eosinophilic esophagitis. Radiology 2010;256(1):127–134.
Williams V.A. et al. Achalasia of the esophagus: a surgical disease. J Am Coll Surg 2009;208(1):151–162.
Yamabe Y. et al. Tumor staging of advanced esophageal cancer: combination of double-contrast esophagography and contrast-enhanced CT. AJR Am J Roentgenol 2008;191(3):753–757.
Yu N.C. et al. Detection and grading of esophageal varices on liver CT: comparison of standard and thin-section multiplanar reconstructions in diagnostic accuracy. AJR Am J Roentgenol 2011;197(3):643–649.
∗ Richard Schatzki (1901-1992), American radiologist.
∗ Antonio Maria Valsalva (1666-1723), Italian anatomist.
∗ Friedrich Albert von Zenker (1825-1898), German pathologist.
∗ Gustav Killian (1860-1921), German surgeon; Edward Bald Jamieson (1876-1956), Scottish anatomist.
∗ Henry S. Plummer (1874-1936), American physician; Porter P. Vinson (1890-1959), American physician.
∗ lusoria: Latin for “freak,” as in “freak of nature.”
∗ Michael A. Epstein (1921-2006), British pathologist and virologist; Yvonne M. Barr (1932- ), British virologist.
∗ Norman Barrett (1903-1979), British surgeon.
∗ César Roux (1857-1934), Swiss surgeon.
∗ Hulusi Behçet (1889-1948), Turkish dermatologist.
∗ Cowden’s syndrome was first mentioned in 1963; Cowden was the first patient seen with the condition.
∗ Moritz Kaposi (1837-1902), Hungarian dermatologist.
∗ Maurice Raynaud (1834-1881), French internal medicine physician.
† Leopold Auerbach (1828-1897), German anatomist.
∗ Carlos Chagas (1879-1934), Brazilian physician.
∗ G. Kenneth Mallory (1900-1986), American pathologist; Soma Weiss (1898-1942), American physician.
∗ Herman Boerhaave (1668-1738), Dutch physician.
∗ Rudolf Nissen (1896-1981), German surgeon.