CHAPTER 42 Esophageal Neuromuscular Function and Motility Disorders
ESOPHAGEAL MOTOR AND SENSORY FUNCTION
OROPHARYNX AND UPPER ESOPHAGEAL SPHINCTER
The oral cavity and pharynx are critically involved in the task of ingesting food. Within the oral cavity, the lips, teeth, hard palate, soft palate, mandible, floor of the mouth, and tongue serve functions in masticating, containing, and forming food into a bolus suitable for transfer to the pharynx. The pharynx is a hollow cavity separated into three segments (Fig. 42-1): nasopharynx, oropharynx (oral pharynx), and hypopharynx (laryngeal pharynx). The nasopharynx extends from the base of the skull to the distal edge of the soft palate. Although not part of the alimentary tract, muscles in the nasopharynx contribute to elevating the soft palate and sealing the nasopharynx during swallowing, preventing nasopharyngeal regurgitation. The oropharynx extends from the soft palate to the base of the tongue. The inferior margin of the oropharynx is demarcated by the valleculae anteriorly and the mobile tip of the epiglottis posteriorly. The hypopharynx extends from the valleculae to the inferior margin of the cricoid cartilage and includes the upper esophageal sphincter (UES).
The intrinsic muscles of the pharynx are the superior, middle, and inferior pharyngeal constrictors (see Fig. 42-1). The constrictors overlap and insert into a collagenous sheet, the buccopharyngeal aponeurosis. The superior pharyngeal constrictor arises from the pterygoid hamulus, pterygomandibular raphe, mandible, and tongue; passes posteromedially; and inserts to the posterior raphe. The middle constrictor arises from the hyoid bone and stylohyoid ligament, passes posteromedially, and inserts in the posterior median raphe. The inferior constrictor is composed of the thyropharyngeus (superior part) and the cricopharyngeus (inferior part). The thyropharyngeus arises from the thyroid cartilage, passes posteromedially, and inserts in the median raphe. The cricopharyngeus has superior and inferior components, each of which arise bilaterally from the sides of the cricoid lamina; the superior fibers course posteromedially to the median raphe and the inferior fibers loop around the esophageal inlet without a median raphe. Killian’s triangle, a triangular area of thin muscular wall is formed posteriorly between these components of the cricopharyngeus and is the most common site of origin for pharyngeal pulsion diverticuli.
The pharynx also contains five single or paired cartilages: the epiglottic, arytenoid, cuneiform, corniculate, and cricoid (see Fig. 42-1). The spaces formed between the lateral insertion of the inferior constrictor and the lateral walls of the thyroid cartilage are the pyriform sinuses that end inferiorly at the cricopharyngeus muscle, separating the pharynx from the esophagus. The larynx and trachea are suspended in the neck between the hyoid bone superiorly and the sternum inferiorly. A number of muscles, categorized as the laryngeal strap muscles, contribute to this suspension and, together with the intrinsic elasticity of the trachea, permit the larynx to be raised and lowered. The hyoid bone also serves as the base for the tongue that rests on it. Laryngeal movement is crucial to the successful enactment of the swallow response because the laryngeal inlet is closed and physically removed from the bolus path in the course of a swallow. Failure to achieve this synchronized laryngeal movement can result in aspiration.
The pharyngeal muscles are densely innervated with motor fibers coming from nuclei of the trigeminal, facial, glossopharyngeal, and hypoglossal nuclei as well as nucleus ambiguus and spinal segments C1 to C3. The innervation of the major pharyngeal muscles is as follows: mylohyoid, tensor veli palatini, and anterior digastric muscles (trigeminal nerve); stylohyoid and posterior part of the digastric (facial nerve); stylopharyngeus (glossopharyngeal nerve); levator veli palatini, palatopharyngeus, salpingopharyngeus, thryroarytenoid, arytenoid, pharyngeal constrictors, and cricopharyngeus (vagus nerve); thyrohyoid, geniohyoid, and tongue (hypoglossal nerve).1 Nucleus ambiguus is the vagal nucleus responsible for innervation of the striated muscle of the pharynx, larynx, and esophagus.2 All motor neurons within the nucleus ambiguus seem to participate in swallowing with those innervating the esophagus situated rostrally and those innervating the larynx more caudally.3
The muscular components of the UES are the cricopharyngeus, adjacent esophagus, and adjacent inferior constrictor (see Chapter 41, Fig. 41-3). The cricopharyngeus contributes the zone of maximal UES pressure, which is about 1 cm in length.4 The closed sphincter has a slit-like configuration with the cricoid lamina anterior and the cricopharyngeus making up the lateral and posterior walls. Not surprisingly, resting UES pressure is markedly asymmetrical with greatest values anteriorly and posteriorly.5 Neural input via vagal trunks originating in nucleus ambiguus to the UES is required for maintenance of high resting pressure and for the coordination of relaxation with swallowing.6 Cessation of motor neuron firing, or administration of curare, causes relaxation, whereas increased spike activity increases tone. Vagal transection abolishes contractile activity in the cricopharyngeus and inferior pharyngeal constrictor muscles.7
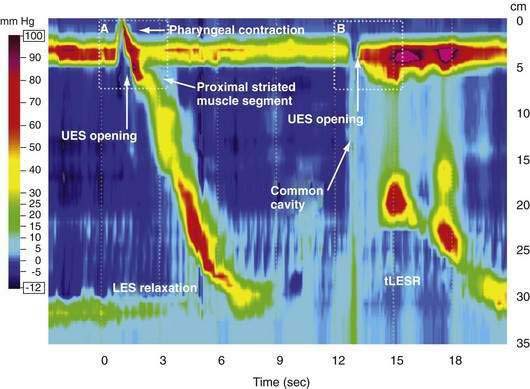
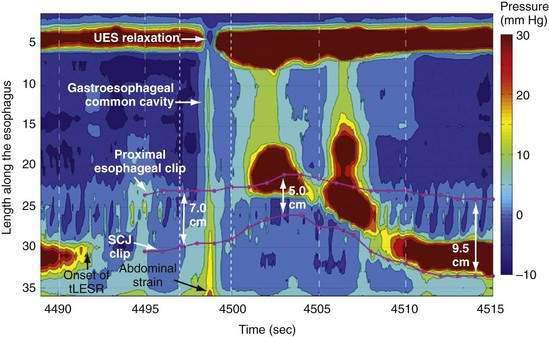
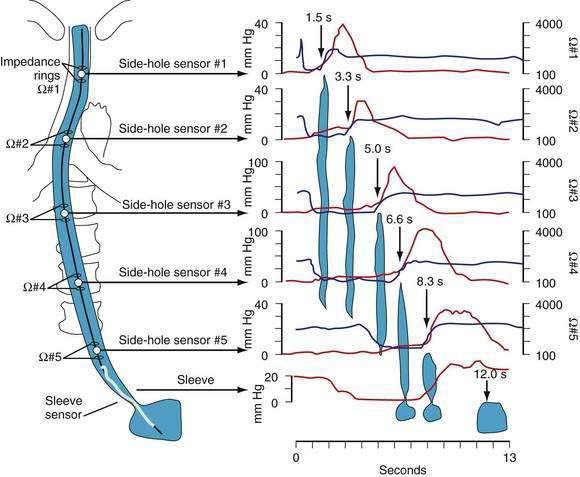
Manometric evaluation of UES function is difficult because it is a short, complex anatomic zone that moves briskly during swallowing. Furthermore, UES pressure measurement is heavily influenced by recording methodology due to its marked asymmetry and to the fact that pharyngeal stimulation by the measurement device stimulates sphincter contraction. Thus, it is not surprising that there is great variability in reported “normal” UES pressure ranges and it is currently impossible to define a meaningful normal range.8 UES relaxation during swallowing also poses substantial recording challenges, making for great variability in technique and interpretation among centers. However, high-resolution manometry using solid state technology has become clinically available, and this technology permits accurate tracking of UES relaxation and intrabolus pressure changes during swallowing (Fig. 42-2).
The main function of the UES is to maintain closure of the proximal end of the esophagus unless opening is required for either swallowing or belching.9 It constitutes an additional barrier to refluxed material entering the pharynx and prevents air from entering the esophagus by contracting in synchrony with inspiration. Inspiratory augmentation is most evident during periods of low UES pressure and can be exaggerated in individuals experiencing globus sensation.10 Balloon distention of the esophagus stimulates UES contraction with the effect being more pronounced with more proximal balloon positions.11 However, when the distension pattern of gas reflux is simulated using a cylindric bag or rapid air injection into the esophagus, UES relaxation rather than contraction occurs.4 Belch-induced UES relaxation is also associated with glottic closure.12 Stress augments UES pressure13 and anesthesia14 or sleep15 virtually eliminates it. Neither experimental acid perfusion of the esophagus16 nor spontaneous gastroesophageal acid reflux alters continuously recorded UES pressure in either normal volunteers15 or in individuals with peptic esophagitis.16
THE PHARYNGEAL SWALLOW
The oral phase of swallowing is largely voluntary and highly variable. Disorders of the oral phase of swallowing occur with many conditions characterized by global neurologic dysfunction such as head trauma, cerebral tumors, or chorea. Detailed discussion of these conditions can be found in texts on swallow evaluation and therapy.17,18 The pharyngeal swallow is the complex coordinated contraction that transfers oral contents into the esophagus. A typical individual swallows about 600 times a day without giving significant thought or effort to the activity.19 Afferent sensory fibers capable of triggering the pharyngeal swallow travel centrally via the internal branch of the superior laryngeal nerve (from the larynx) and via the glossopharyngeal nerve (from the pharynx).20 These sensory fibers converge before terminating in the medullary swallow center.21
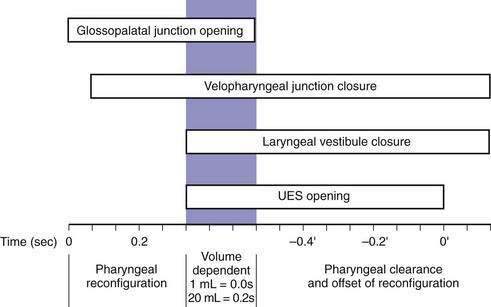
Although understood physiologically as the patterned activation of motor neurons and their corresponding motor units, swallowing is clinically evaluated in mechanical terms and best evaluated by videofluoroscopic or cineradiographic analysis. The pharyngeal swallow rapidly reconfigures pharyngeal structures from a respiratory to an alimentary pathway and then reverses this reconfiguration within one second. The pharyngeal swallow response can be dissected into several closely coordinated actions: (1) nasopharyngeal closure by elevation and retraction of the soft palate, (2) upper esophageal sphincter (UES) opening, (3) laryngeal closure, (4) tongue loading (ramping), (5) tongue pulsion, and (6) pharyngeal clearance. Precise coordination of these actions is an obvious imperative and to some degree the relative timing of these events is affected either by volition or by the volume of the swallowed bolus (Fig. 42-3).22
The most fundamental anatomic reconfiguration required to transform the oropharynx from a respiratory to a swallow pathway is to open the inlet to the esophagus and seal the inlet to the larynx. These events occur in close synchrony. UES opening occurs by laryngeal elevation and anterior traction via the hyoid axis.23,24 The mechanical determinants of laryngeal vestibule closure are laryngeal elevation and anterior tilting of the arytenoid cartilages against the base of the epiglottis.17 Thus, analyzing the efficacy of either of these events inevitably focuses on laryngeal elevation.25 UES relaxation occurs at roughly the same degree of laryngeal elevation regardless of swallow volume, and precedes sphincter opening by about 0.1 second. It is critical to recognize the distinction between UES relaxation and UES opening. UES relaxation occurs due to cessation of excitatory neural input while the larynx is elevating. Once the larynx is elevated, UES opening results from traction on the anterior sphincter wall caused by contraction of the suprahyoid and infrahyoid musculature that also results in a characteristic pattern of hyoid displacement.23,24
The two main determinants of bolus transport out of the oropharynx are the action of the tongue and of the pharyngeal constrictors. Tongue motion varies substantially with swallow conditions and propels most of the bolus into the esophagus prior to the onset of the pharyngeal contraction with larger volume swallows.26 On the other hand, the propagated pharyngeal contraction has similar propagation and vigor regardless of bolus volume.27 However, the propagated pharyngeal contraction is more involved with the process of clearance than of bolus propulsion; it strips the last residue from the pharyngeal walls. Upper esophageal sphincter closure coincides with the arrival of the propagated pharyngeal contraction as evident by the fixed time relationship between these events.24 However, the contractile activity of the sphincter has an added dimension as well, exhibiting increased electromyographic activity during laryngeal descent.28 The magnitude of this postdeglutitive contraction is further augmented by either sphincteric or proximal esophageal distention resulting in a grabbing effect such that the sphincter and laryngeal descent complement each other to clear residue from the hypopharynx.29 This clearing function probably acts to minimize the risk of post-swallow aspiration by preventing residual material from adhering to the laryngeal inlet when respiration resumes.
ESOPHAGUS
The esophagus is a 20- to 22-cm muscular tube with a wall composed of skeletal and smooth muscle (see Chapter 41, Fig. 41-1). The proportion of each muscle type is species dependent, but in man, the proximal 5% is striated, the middle 35% to 40% is mixed with an increasing proportion of smooth muscle distally, and the distal 50% to 60% is entirely smooth muscle.30 The bundles of the outer (longitudinal) muscle arise from the cricoid cartilage receiving slips from the cricopharyngeus and pass dorsolaterally to fuse posteriorly about 3 cm distal to the cricoid cartilage. This arrangement results in a posterior triangular area devoid of longitudinal muscle, Laimer’s triangle. Distal to Laimer’s triangle the longitudinal muscles form a continuous sheath of uniform thickness around the esophagus.31 The adjacent, inner muscle layer is formed of circular or, more precisely, helical muscle also forming a sheath of uniform thickness throughout the length of the esophageal body. The overlapping helices exhibit decreasing degree of helicity moving distally ranging from 60 degrees in the proximal esophagus to nearly 0 degrees in the most distal esophagus.32
The extrinsic innervation of the esophagus is via the vagus nerve (see Chapter 41). Fibers innervating the striated muscle are axons of lower motor neurons with cell bodies situated in nucleus ambiguus, whereas the smooth muscle esophagus is innervated by the dorsal motor nucleus of the vagus.33,34 Efferent nerve fibers reach the cervical esophagus by the pharyngoesophageal nerve35 and histologic studies show that vagal efferents synapse directly on striated muscle neuromuscular junctions.36,37 The vagus nerves also provide sensory innervation. In the cervical esophagus this is via the superior laryngeal nerve with cell bodies in the nodose ganglion, whereas in the remainder of the esophagus sensory fibers travel via the recurrent laryngeal nerve or, in the most distal esophagus, via the esophageal branches of the vagus. Histologic studies demonstrate many free nerve endings in the mucosa, submucosa, and muscular layers.2,38 Additionally, a few encapsulated structures resembling spindles have been described in humans. These vagal afferents are strongly stimulated by esophageal distention.
The esophagus also contains an autonomic nerve network, the myenteric plexus, located between the longitudinal and circular muscle layers.39 Myenteric plexus neurons are sparse in the proximal esophagus and their function in that region is unclear because the striated muscle is directly controlled by somatic motor fibers. On the other hand, the thoracic esophagus receives innervation from preganglionic neurons in the dorsal motor nucleus of the vagus that then synapse in myenteric plexus ganglia, relay neurons between the vagus and the smooth muscle. The ganglia of the myenteric plexus are more numerous in the distal esophagus than in the striated muscle region, but throughout, they are still far less dense and smaller than in other regions of the gut.40,41 A second nerve network, the submucosal or Meissner’s plexus, is situated between the muscularis mucosa and the circular muscle layer, but this is exceedingly sparse with few ganglia in the human esophagus.41
Properties of Esophageal Peristalsis
The esophagus does not normally exhibit spontaneous contractions and its intraluminal pressure closely reflects pleural pressure, becoming negative during inspiration. However, swallowing or focal distention initiates peristalsis. Primary peristalsis is that which is initiated by a swallow and traverses the entire length of the esophagus; secondary peristalsis can be elicited in response to focal esophageal distention with air, fluid, or a balloon, beginning at the point of distention.42 The mechanical correlate of peristalsis is a stripping wave that milks the esophagus clean from its proximal to distal end. The propagation of the stripping wave corresponds closely with that of the manometrically recorded contraction such that the point of the inverted “V” seen fluoroscopically at each esophageal locus occurs concomitantly with the upstroke of the pressure wave. The likelihood of achieving complete esophageal emptying from the distal esophagus is inversely related to peristaltic amplitude such that emptying becomes progressively impaired with peristaltic amplitudes of 30 mm Hg or less.43
Another essential feature of peristalsis is deglutitive inhibition. A second swallow, initiated while an earlier peristaltic contraction is still progressing in the proximal esophagus, causes rapid and complete inhibition of the contraction induced by the first swallow.44 If the first peristaltic contraction has reached the distal esophagus, it may proceed distally for a few seconds after the second swallow, but its amplitude then diminishes until it disappears.45 Deglutitive inhibition in the distal esophagus is attributable to hyperpolarization of the circular smooth muscle and is mediated via inhibitory ganglionic neurons in the myenteric plexus. Deglutitive inhibition can be demonstrated experimentally in the esophagus by creation of an artificial high-pressure zone with an intraluminal balloon.46 The artificial high-pressure zone is created by distending the esophageal lumen with a balloon and recording intraluminal pressure between the balloon and the esophageal wall. Once the high pressure zone is established in the normally flaccid tubular esophagus, deglutitive inhibition is evident by relaxation of the artificial high pressure zone commencing concurrently with the swallow.
The physiologic control mechanisms governing the striated and smooth esophageal musculature are distinct. The striated muscle of the esophagus receives exclusively excitatory vagal innervation and its peristaltic contraction results from sequential activation of motor units in a craniocaudal sequence. These fibers release acetylcholine and stimulate nicotinic cholinergic receptors on the motor endplates of the striated muscle cells. Physiologic evidence of this arrangement was provided by an ingenious series of experiments using the nerve suture technique.2 In these experiments, the vagal branch innervating the esophagus was severed and the central end anastomosed to the peripheral end of the also severed spinal accessory nerve. Thus, after a period of nerve regeneration, the vagal branch effectively innervates the sternocleidomastoid and trapezius muscles. Occurrence of excitatory vagal discharges can then be surmised from the contractile activity of these readily accessible muscles. Nerve suture experiments demonstrated several properties of vagal control of esophageal striated muscle: (1) vagal efferent fibers exhibit no spontaneous discharge but fire in spike bursts during primary or secondary peristalsis; (2) once activated, vagal fibers innervating different levels of the esophagus fire sequentially, demonstrating peristaltic programming by the medullary swallow center; (3) peristaltic vagal motor discharges are potentiated by stimulation of afferent fibers from the esophagus (designed to mimic the effect of a bolus being pushed ahead of the contraction); (4) peristaltic vagal motor discharges are stronger during primary than secondary peristalsis; and (5) vagal motor fibers are inhibited during the pharyngeal stage of deglutition or after distention of a proximal esophageal segment supporting the notion that deglutitive inhibition has a central origin. Thus, there is substantial evidence that peristalsis in the striated muscle esophagus is controlled by the swallowing center of the medulla in much the same way as is the oropharyngeal musculature.
The vagus nerves also exhibit control of primary peristalsis in the smooth muscle esophagus. Deviation of a swallowed bolus at the level of the cervical esophagus (thereby eliminating the potential for bolus-initiated afferent feedback) does not eliminate the primary peristaltic contraction in the distal esophagus.47 Furthermore, primary peristalsis of the smooth muscle persists even after curarization. Because curarization paralyzes the oropharyngeal and cervical esophagus, the persistence of distal peristalsis in these experiments strongly suggests that it is triggered by the medullary swallowing center that can elicit the entire motor sequence of primary peristalsis without receiving afferent feedback.
The mechanism of vagal control of the smooth muscle esophagus is more complex than that of the striated muscle because vagal fibers synapse on myenteric plexus neurons rather than directly on muscle cells. Experimentally, vagal stimulation either excites or inhibits esophageal musculature depending on the stimulation parameters used.48,49 In the esophagus of the opossum, vagal or swallow-induced stimulation causes depolarization with superimposed spikes on longitudinal muscle but an initial hyperpolarization followed by depolarization and spike burst on circular muscle.50 With swallowing initiated by superior laryngeal nerve stimulation, the response characteristics of single nerve fibers participating in smooth muscle peristalsis could be divided into two groups.51 Activity of short latency vagal fibers correlated temporally with the onset of the deglutitive inhibition while the activity of long latencies fibers was temporally correlated with the onset of contraction at each esophageal locus. Thus, activity of neurons in the dorsal motor nucleus of the vagus reflects several properties of primary peristalsis in the smooth muscle esophagus including deglutitive inhibition and both the speed and vigor of peristaltic contraction.
Control of peristalsis may also arise in the myenteric plexus. Stimulation of decentralized vagal efferents evokes peristalsis similar to that seen with swallowing and this is obliterated with transection across the smooth muscle esophagus, suggesting that an intact intramural neural myenteric plexus is necessary for peristaltic propagation. In contrast, transection across the striated muscle in the proximal esophagus does not inhibit peristaltic progression across the transection site or distally.47 Further evidence supporting the potential autonomy of peripheral mechanisms is that distention anywhere within the smooth muscle esophagus will elicit secondary peristalsis despite extrinsic denervation.52
Regardless of central or ganglionic control, esophageal smooth muscle contraction is ultimately elicited by ganglionic cholinergic neurons. Swallow-induced peristalsis is highly atropine sensitive, can be augmented by cholinergic agonists, and is inhibited by acetylcholinesterase.53,54 Less clear, however, are the control mechanisms for the direction and velocity of the peristaltic wavefront. Nerve conduction studies indicate that neural stimuli initiated by swallowing propagate with a speed of 5 to 6 m per second and therefore reach the ganglionic neurons along the length of the esophagus essentially simultaneously.51 However, the latency between the arrival of the vagal stimulus and muscle contraction progressively increases moving aborally. In humans, the latent period is two seconds in the proximal smooth muscle esophagus and five to seven seconds just proximal to the lower esophageal sphincter (LES). The in vitro correlate of this is that when electrically stimulated, distal esophageal muscle strips exhibit longer latencies to contraction than do strips from the proximal esophagus.55,56 The latency gradient can be changed by varying vagal stimulation parameters or by pharmacologic manipulation suggesting it to be the result of an interaction between the initial inhibition and subsequent excitation of esophageal smooth muscle.57 The current hypothesis is that peristaltic direction and velocity result from a neural gradient along the esophagus, wherein excitatory ganglionic neurons dominate proximally and inhibitory ganglionic neurons dominate distally. This organization is supported by the demonstration with pressure topography plotting of two subsegments within the smooth muscle segment, the first of which is strongly reactive to stimulation with cholinergic drugs.58,59 The primary inhibitory neurotransmitter (formerly referred to as the nonadrenergic, noncholinergic transmitter) is nitric oxide (NO) produced from l-arginine by the enzyme NO synthase in myenteric neurons.60,61 NO synthase inhibitors reduce the latency to contraction in vivo in response to swallowing.62,63 In addition to NO neurons, there is also evidence for a role of vasoactive intestinal polypeptide (VIP)–containing neurons in the initial inhibition.64,65
Sympathectomy of the esophagus has no apparent effect on peristalsis.66,67 On the other hand, bilateral vagotomy results in paralysis of the striated muscle segment. Severing only the afferent nerve supply to the striated muscle abolishes secondary peristalsis while leaving primary peristalsis intact, highlighting the role of central programming in the latter and the necessity of afferent sensory signals in the former.2 Recordings from the cervical esophageal vagal afferents show these to be highly sensitive to intraluminal distention, implicating them as the sensory basis for secondary peristalsis. In the smooth muscle segment of the esophagus, vagal cooling or vagotomy reduces the amplitude of primary peristalsis68,69 but does not affect secondary peristalsis.70
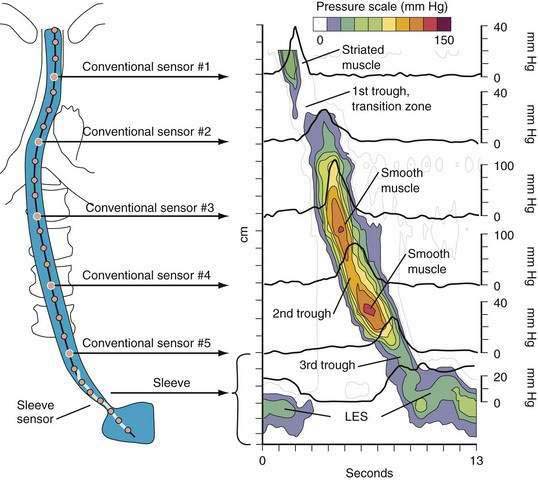
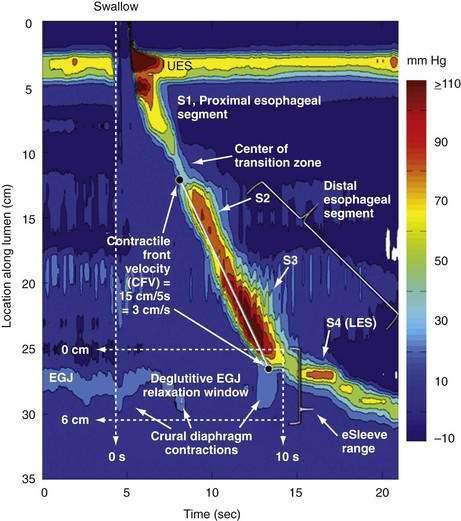
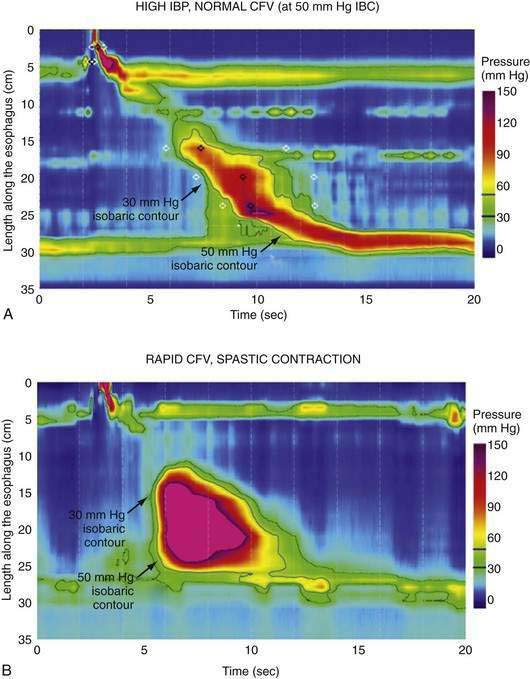
A tool introduced in the study of esophageal physiology is high-resolution manometry or, more precisely, high-resolution esophageal pressure topography. High-resolution esophageal pressure topography allows the imaging of esophageal contractile activity as a continuum not only in time but also along the length of the esophagus. Figure 42-4 illustrates the conversion from conventional manometric study to high-resolution esophageal pressure topography. Note how high-resolution esophageal pressure topography reveals that the vigor and propagation of peristalsis along the length of the esophagus is not seamless. Rather, there is a distinct transition zone between the striated and smooth muscle segments characterized by the minimal peristaltic amplitude, a slight delay in progression, and an increased likelihood of failed transmission.71 Detailed modeling studies suggest this to be the transition point between two distinct contraction waves governing the proximal and distal esophagus respectively.72 The topographic analysis also reveals a segmental characteristic of peristaltic progression through the smooth muscle esophagus with two distinct contractile segments separated by a pressure trough followed by the LES, which contracts with vigor and persistence quite dissimilar to the adjacent smooth muscle esophagus.73
The longitudinal muscle of the esophagus also contracts during peristalsis with the net effect of transiently shortening the esophagus by 2 to 2.5 cm.74 Similar to the pattern of circular muscle contraction, longitudinal muscle contraction is propagated distally as an active segment at a rate of 2 to 4 cm per second.75 The segment of contracting longitudinal muscle precedes, but overlaps with the contracting segment of circular muscle. Thus, within a given esophageal segment, the contraction of the longitudinal and circular muscle are slightly out of phase with each other. Propulsive force occurs in the zone of overlap as the delayed circular muscle contraction “catches up” with the distal longitudinal muscle contraction.75
Central mechanisms also control the contractions of esophageal longitudinal muscle. Swallowing induces peristaltic sequences with gradual activation of longitudinal muscle progressing from orad to caudad. This progression is associated with a progressive increase in latency similar to that seen with the circular smooth muscle esophagus.76 However, unlike the responses observed in the circular muscle, stimulation of decentralized vagal efferent fibers causes simultaneous contractions in the longitudinal muscle layer, suggesting this muscle layer is free of inhibitory neuron control.57
ESOPHAGOGASTRIC JUNCTION
The anatomy of the esophagogastric junction (EGJ) is complex (see Chapter 41). The distal end of the esophagus is anchored to the diaphragm by the phrenoesophageal membrane that inserts circumferentially into the esophageal musculature close to the squamocolumnar junction (SCJ). The esophagus then traverses the diaphragmatic hiatus and joins the stomach in almost a tangential fashion. Thus, there are three significant contributors to the EGJ high-pressure zone: the LES, the crural diaphragm, and the muscular architecture of the gastric cardia that constitutes the distal aspect of the EGJ high-pressure zone.
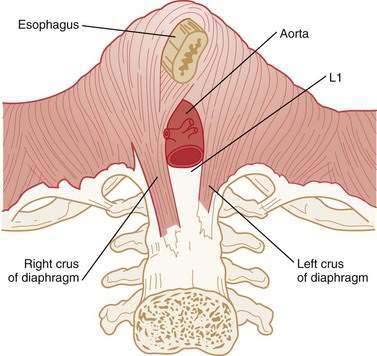
The LES is a 3- to 4-cm segment of tonically contracted smooth muscle at the distal extreme of the tubular esophagus. Surrounding the LES at the level of the SCJ is the crural diaphragm, most commonly bundles of the right diaphragmatic crus forming a teardrop-shaped canal about 2 cm long on its major axis (Fig. 42-5).77,78 Elegant anatomic studies suggest that the component of the EGJ high-pressure zone distal to the SCJ is largely attributable to the opposing sling and clasp fibers of the middle layer of gastric cardia musculature.77,79,80 In this region, the lateral wall of the esophagus meets the medial aspect of the dome of the stomach at an acute angle, defined as the angle of His. Viewed intraluminally, this region extends within the gastric lumen, appearing as a fold that has been conceptually referred to as a flap valve because increased intragastric pressure forces it closed, sealing off the entry to the esophagus.81,82
Physiologically, the EGJ high-pressure zone is attributable to a composite of both the LES and the surrounding crural diaphragm. Concurrent fluoroscopy and manometry, aided by the placement of an endoclip at the SCJ, localized the EGJ high-pressure zone as extending 1 to 1.5 cm proximal to the SCJ and about 2 cm distal to it.83 Manometric and intraluminal ultrasound studies suggest that axial asymmetry of the pressure profile is attributable to the varying thickness of the muscularis propria, whereas the radial pressure asymmetry results from asymmetrical compression by the surrounding crural diaphragm.83,84
Resting LES tone ranges from 10 to 30 mm Hg relative to intragastric pressure with considerable temporal fluctuation. The mechanism of LES tonic contraction is likely a property of the muscle itself and of nerves affecting the sphincter. This conclusion is supported by the observation that pressure within the sphincter is minimally affected by the elimination of neural activity by close intra-arterial injection of tetrodotoxin.85 Myogenic LES tone varies directly with membrane potential86 and superimposed electrical spike activity that leads to an influx of Ca++. Resting membrane potential of the LES is slightly greater (i.e., less negative) than that of the adjacent esophagus.87 Modulation of spike activity and membrane potential are not completely understood; however, it is believed that spike activity may be regulated by K+– and Ca++-activated Cl− channels.88 Sphincter tone may be maintained by inositol phosphate-mediated continuous release of intracellular calcium.89,90 Inositol phosphate concentrations are higher in the LES than in adjacent esophagus.
Apart from myogenic factors, LES pressure is also modulated by intra-abdominal pressure, gastric distention, peptides, hormones, foods, and many medications. Large fluctuations of LES pressure occur with the migrating motor complex (MMC); during phase III, LES pressure may exceed 80 mm Hg. Lesser fluctuations occur throughout the day with pressure decreasing in the post-prandial state and increasing during sleep.91 As mentioned, superimposed on the myogenic LES contraction, input from vagal, adrenergic, hormonal, and mechanical influences will alter LES pressure. Vagal influence is similar to that of the esophageal body with vagal stimulation activating excitatory and inhibitory myenteric neurons.49 Dogs provide an interesting model for studying this because they have an entirely striated muscle esophagus except for a smooth muscle band at the LES. Vagal fibers innervating the dog LES are of two types: (1) spontaneously active fibers that exhibit a sudden increase with swallowing, abruptly cease firing when the peristaltic contraction arrives, and then resume a spontaneous rate; and (2) spontaneously active fibers that cease activity with swallowing and resume normal activity when the bolus reaches the stomach.92 Thus, the LES pressure at any instant reflects the balance between excitatory and inhibitory neural input and altering the pattern of vagal discharge can result in a swallow-mediated LES relaxation. Data on humans suggest that, similar to the dog, basal LES tone is partially generated by cholinergic input.93 The excitatory and inhibitory intramural neurons are acetylcholine sensitive with nicotinic and muscarinic receptors.94 At the LES, the excitatory neurons release acetylcholine, whereas the inhibitory neurons mainly use NO as a neurotransmitter.
Adrenergic influences on LES pressure are complex and mostly mediated through actions on the myenteric neurons.34,95 Sympathetic fibers from the stellate and proximal thoracic ganglia follow the splanchnic nerve, form a recurrent loop through the celiac ganglion, and then synapse on both the excitatory and inhibitory myenteric neurons expressing α-adrenergic receptors. Adrenergic stimulation increases LES pressure by activating excitatory neurons and reducing inhibitory neuron activity. Adrenergic action on the esophageal body is the opposite of that on the LES, with direct inhibition of the muscle and inhibition of the excitatory myenteric neurons.
The crural diaphragm is also a major contributor to EGJ pressure (see Fig. 42-5). Even after esophagogastrectomy, with removal of the smooth muscle LES, a persistent EGJ pressure of about 6 mm Hg can be demonstrated during expiration.96 During inspiration there is substantial augmentation of EGJ pressure attributable to crural diaphragm contraction. Experimentally, the inspiratory augmentation of EGJ pressure can be increased even more with enhanced respiratory effort or, conversely, can be eliminated by manual ventilation. The augmentation of LES pressure observed during sustained inspiration corresponds both temporally and quantitatively with the augmentation of crural electromyographic activity, and this augmented EGJ pressure can obscure intrinsic LES relaxation induced by esophageal distention.97 Crural diaphragm contraction is also augmented during abdominal compression, straining, or coughing.98 On the other hand, during esophageal distention, vomiting, and belching, electrical activity in the crural diaphragm is selectively inhibited despite continued respiration demonstrating a control mechanism independent of the costal diaphragm.99,100 This reflex inhibition of crural activity is eliminated with vagotomy.
Lower Esophageal Sphincter Relaxation
The neural mediation of LES relaxation has been studied extensively.94,101,102 LES relaxation can be triggered by distention from either side of the esophagogastric junction or by swallowing.103 Relaxation induced by esophageal distention is modulated by bolus volume and is unaffected by vagotomy, demonstrating it to be an intramural process. Relaxation is, however, antagonized by tetrodotoxin proving that it is mediated by postganglionic nerves.104 Deglutitive LES relaxation is mediated by the vagus nerve, which synapses with myenteric plexus inhibitory neurons. Ganglionic transmission is through nicotinic and muscarinic acetylcholine receptors and can be blocked by a combination of hexamethonium (i.e., nicotinic blocker) and atropine (i.e., muscarinic blocker).
Current evidence implicates NO as the main neurotransmitter in the postganglionic neurons responsible for LES relaxation. NO is produced by NO synthase from the precursor amino acid l-arginine. Neuronal NO synthase is a soluble cytosolic enzyme and has been identified in neurons of the myenteric plexus, co-localizing with VIP, which may be a second inhibitory neurotransmitter in the LES as well as in the esophageal body.105,106 NO is released with neural stimulation in the esophagus, LES, and stomach.60,107–109 In the LES, NO has a marked inhibitory effect, and multiple in vitro and in vivo studies have shown that NO synthase inhibitors block neurally mediated LES relaxation.
Although the evidence implicating NO as the main inhibitory transmitter facilitating LES relaxation is very convincing, NO may not work alone. VIP-containing neurons have been demonstrated in the submucosal plexus and VIP relaxes the LES by direct muscle action.110–114 Electrical stimulation of LES muscle strips also causes LES relaxation and release of VIP into the muscle bath. Furthermore, VIP antiserum partially reduces LES relaxation evoked by vagal or field stimulation.65,114 It is thought that VIP acts on NO synthase containing neural terminals as a prejunctional neurotransmitter, facilitating the release of NO and on gastric muscle cells to stimulate production of NO by the muscle.115–119 In addition to VIP, there is some evidence that peptide histidine isoleucine (PHI) in the cat and, to a lesser extent, calcitonin gene-related peptide (CGRP) in the opossum may participate as inhibitory neurotransmitters.114,120 Like VIP, PHI and CGRP relax the LES by a direct action on the muscle.114,120,121 PHI is of interest because it is derived from the same precursor as VIP and coexists with VIP in the same neurons.122
The notion that multiple neurotransmitters may interact to produce LES relaxation may resolve inconsistencies and discrepancies that follow from the assumption that any one neurotransmitter is uniquely responsible for LES relaxation. Reports of the co-localization of NO synthase, VIP, pituitary adenylate cyclase activating peptide (PACAP), CGRP, and galanin in myenteric neurons of the distal esophagus support this concept.123–125
Transient Lower Esophageal Sphincter Relaxation
During rest the EGJ must contain gastric juice, but also be able to transiently relax and permit gas venting of the stomach without allowing reflux of gastric juice and food. These functions are accomplished by prolonged LES relaxations that occur transiently without swallowing or peristalsis. These transient LES relaxations (tLESRs) are thought to be an important mechanism in the pathogenesis of GERD (see Chapter 43). tLESRs are a complex reflex distinguishable from swallow-induced relaxation in several ways: (1) a prolonged (more than 10 seconds) LES relaxation independent of a pharyngeal swallowing, (2) contraction of the distal esophageal longitudinal muscle causing esophageal shortening, (3) absence of synchronized peristalsis, and (4) crural diaphragm inhibition, which is not the case with swallow-induced relaxation (Fig. 42-6).126,127 tLESRs occur most frequently in the postprandial state during gastric accommodation attributable to vagally mediated receptive relaxation of the fundus (see Chapter 48). In the setting of the completely relaxed EGJ during tLESRs, even the minimal gastroesophageal pressure gradients observed with gastric distention (3 to 4 mm Hg) are sufficient to facilitate gas venting of the stomach (belching). Thus, tLESRs are the physiologic mechanism of belching.128,129
Proximal gastric distention is the major stimulus for tLESR. Distention stimulates mechanoreceptors (intraganglionic lamellar endings) in the proximal stomach activating vagal afferent fibers projecting to the nucleus of the solitary tract.127,130,131 The afferent and efferent neural pathways responsible for swallow and non-swallow LES relaxations have been compared in the mouse. The afferent arm of swallow-induced relaxation lies in the pharyngeal and superior laryngeal nerves, with the central neural circuit in the medullary subnuclei.132–135 Non–swallow-induced relaxations, in contrast, are initiated through gastric afferents in the subdiaphragmatic vagus and activate neurons in the caudal part of the dorsal motor nucleus.136 The efferent limb of both swallow and non-swallow LES relaxations lies in the preganglionic vagal inhibitory pathway to the LES. Both types of relaxation can be blocked by bilateral cervical vagotomy, cervical vagal cooling, or NO synthase inhibitors.127,137 Vagal outflow from the dorsal motor nucleus completely inhibits both the LES and the crural diaphragm, an important distinction from swallow-induced LES relaxation, which is not associated with concomitant inhibition of the crural diaphragm.
tLESRs triggered by gastric distention likely use NO and cholecystokinin (CCK) as neurotransmitters, as evidenced by increased tLESR frequency after intravenous CCK and blockade by either NO synthase inhibitors or CCK-A receptor antagonists.138–141 The CCK (and fatty meal) augmentation of tLESR frequency is mediated through CCK-A receptors.142,143 Muscarinic receptor involvement in the tLESR pathway is suggested by inhibition of tLESRs by atropine.144–147 Finally γ-aminobutyric acid (GABA)B agonists, such as baclofen, inhibit tLESRs (see Chapter 43), possibly by acting on peripheral receptors and receptors located in the dorsal motor nucleus of the vagus.148–153
ESOPHAGEAL SENSATION
The human esophagus can sense mechanical, electrical, chemical, and thermal stimuli. These stimuli are perceived as chest pressure, warmth, or pain with substantial overlap in perception among stimuli.154,155 Esophageal sensation is carried via both the vagal and spinal afferent nerves. The associated vagal neurons are located in the nodose and jugular ganglia while the corresponding spinal neurons are located in thoracic and cervical dorsal root ganglia. Vagal afferents to the upper one third of the esophagus are carried in the superior laryngeal nerve, whereas those to the remainder of the esophagus and LES are carried in vagal branches.156 The spinal afferents are contained in the thoracic splanchnic nerves projecting onto the lower cervical to upper lumbar spinal segments.156,157 Compared with vagal afferent fibers, relatively little is known about esophageal spinal afferents, but spinal pathways are thought to be primarily nociceptive. Supportive of that concept, prolonged acid perfusion produces esophageal hypersensitivity to distention via spinal sensitization.158,159 Esophageal sensations are usually perceived substernally; in the instance of pain, radiation to the midline of the back, shoulders, and jaw is very analogous to cardiac pain. These similarities are likely due to convergence of sensory afferent fibers from the heart and esophagus in the same spinal pathways, even to the same dorsal horn neurons in some cases.160
Vagal sensory endings in the esophagus consist of free nerve endings, intraganglionic laminar endings (IGLEs) within the myenteric ganglia, and intramuscular arrays (IMAs) within the muscularis propria. Labeling studies demonstrated the densest innervation of free endings between the muscularis mucosa and muscularis propria along the entire length of the esophagus.161 Electron and confocal microscopy reveal that most vagal afferents terminating in the myenteric ganglia do so in specialized laminar structure that encapsulates myenteric ganglia (IGLEs).162 One vagal afferent axon may end in several IGLEs. Combined electrophysiologic and tracer studies have demonstrated that the majority of the tension-sensitive esophageal afferents emanate from IGLEs.163 These endings detect passive and active tension of hollow viscera. In addition to IGLEs, another specialized vagal axonal ending primarily found in the longitudinal and circular smooth muscle forms a branching array parallel to the muscle fibers (IMAs).164 Although more ubiquitous in other parts of the gut, these are essentially restricted to the LES in the esophagus.165 IMAs maintain a close network with interstitial cells of Cajal and it appears that ICCs serve a trophic function.166 Functionally, IMAs appear to be stretch-sensitive endings, sensitive to changes in the muscle length.167
With IGLE and IMA sensory endings concentrated deeply within the muscularis propria beneath a relatively impermeable mucosa, it seems unlikely that intraluminal acid can directly stimulate them. However, these afferents easily respond to chemical mediators such as 5-hydroxytryptamine or alpha, beta-methylene adenosine triphosphate (ATP) as well as to mucosally applied bile or capsaicin,168,169 suggesting that these chemicals induce the release of some endogenous substance that in turn excites the muscle afferents. Supportive of that concept, muscle afferents have been shown to be sensitive to the selective purinergic P2X3 agonist alpha, beta-methylene ATP163,170 and immunohistochemical studies have documented the presence of P2X3 receptors in IGLEs implicating direct activation of purinergic P2X2 and P2X3 receptors as an initiating sensory event.171,172
With respect to free nerve endings, acid can excite esophageal vagal and spinal afferents by activating two proton-gated channels: transient receptor potential vanilloid-1 (TRPV1) and acid-sensing ion channels (ASICs).173–176 Capsaicin, a derivative of chili pepper, excites afferent fibers by activating the TRPV1 channels, which can also initiate a positive feedback loop of increased (neurogenic) inflammation via the release of neuropeptides and inflammatory substances.177,178 ASICs are the other major receptor class that are sensitive to acid, although it is doubtful that acid is their natural ligand; ASICs are probably more involved in mechanotransduction.176
Owing to its significance in the pathogenesis of reflux disease, there has been substantial interest in modulating the tLESR reflex (see Chapter 43). The current concept is that vagal afferent endings terminating in IGLEs located in the proximal stomach are primarily responsible for initiating the reflex, which is then mediated through the medulla and back to the esophagus and diaphragm via vagal efferent and the phrenic nerves.179 Pharmacologic and physiologic studies have demonstrated that the mechanotransduction properties of tension-sensitive vagal afferent fibers can be attenuated by the GABAB receptor agonist baclofen, thereby reducing the frequency of tLESR.180 Glutamate receptors are also present in vagal and spinal sensory afferent fibers, and metabotropic glutamate receptor antagonists (especially mGluR5 antagonists) have been shown to inhibit tLESR.153
ESOPHAGEAL MOTILITY DISORDERS
A working, albeit restrictive, definition of an esophageal motility disorder is an esophageal disease attributable to neuromuscular dysfunction that causes symptoms referable to the esophagus, most commonly dysphagia, chest pain, or heartburn. Employing this definition, there are relatively few firmly established primary esophageal motility disorders: achalasia, distal esophageal spasm (DES), and gastroesophageal reflux disease (GERD). GERD is clearly the most prevalent among the group and, fittingly, it is addressed in detail elsewhere in this text (see Chapter 43). Esophageal motility disorders also can be secondary phenomena in which case esophageal dysfunction is part of a more global disease: pseudoachalasia, Chagas disease, and scleroderma. Dysphagia attributable to pharyngeal or UES dysfunction can be included in a discussion of esophageal motor disorders, but this is usually as a manifestation of a more global neuromuscular disease process. The major focus of this chapter is on the primary esophageal motility disorders, particularly achalasia. However, mention is made of the secondary motility disorders and proximal pharyngoesophageal dysfunction when important unique features exist.
EPIDEMIOLOGY
Estimates of the prevalence of dysphagia among individuals older than 50 years of age range from 16% to 22%181,182 with most of this related to oropharyngeal dysfunction. Within health care institutions, it is estimated that up to 13% of hospitalized patients and 60% of nursing home residents183 have feeding problems, most of which are attributed to oropharyngeal dysfunction as opposed to esophageal dysfunction. Most oropharyngeal dysphagia is related to impaired neuromuscular function; the prevalence of the most common anatomic etiology, Zenker’s diverticulum (discussed in Chapter 23), is estimated to range from a meager 0.01% to 0.11% of the U.S. population, with a peak incidence in men between the seventh and ninth decades.184 The consequences of oropharyngeal dysphagia are severe: volume depletion, malnutrition, aspiration, choking, pneumonia, and death. In fact, mortality of nursing residents with dysphagia and aspiration can be as high as 45% over one year.185 As the U.S. population continues to age, oropharyngeal dysphagia will become an increasing problem associated with complex medical and ethical issues.
Achalasia is the most easily recognized and best-defined motor disorder of the esophagus. The annual incidence of achalasia is about 1/100,000 population in the United States and Europe,186,187 affecting both genders equally and usually presenting between ages 25 and 60.188 Because achalasia is a chronic condition, its prevalence greatly exceeds its incidence, with prevalence estimates in Europe ranging from 7.1/100,000 in Wales to 13.4/100,000 in Ireland.189 Reports of familial clustering of achalasia raise the possibility of genetic predisposition. Achalasia has been reported in one pair of monozygotic twins,190 in siblings,191 and in children of affected parents.192 However, a genetic determinant for achalasia is not strong.193 Emphasizing this point, a survey of 1012 first-degree relatives of 159 achalasic patients identified no affected relatives.194 Familial adrenal insufficiency with alacrima is a rare genetic achalasia syndrome. This condition is inherited as an autosomal recessive disease that manifests itself with the childhood onset of autonomic nervous system dysfunction including achalasia, alacrima, sinoatrial dysfunction, abnormal pupillary responses to light, and delayed gastric emptying.195 It is caused by mutations in AAAS, a gene which encodes a protein known as ALADIN.
No population-based studies exist on the incidence or prevalence of esophageal motility disorders other than achalasia. Thus, the only way to estimate the incidence or prevalence of spastic disorders is to examine data on populations at risk and reference the observed frequency of spastic disorders to the incidence of achalasia which, as detailed earlier, is about 1 per 100,000 population. Doing so, the prevalence of DES is similar to that of achalasia (or much lower if more restrictive diagnostic criteria are used). Populations at risk for motility disorders are patients with chest pain or dysphagia, so it is among these patients that extensive manometric data have been collected. Manometric abnormalities are prevalent among these groups,196–205 but in most cases the manometric findings are of unclear significance.197
PATHOGENESIS
Oropharyngeal Dysphagia
If the etiology of oropharyngeal dysphagia is not readily apparent after initial evaluation for anatomic disorders, evidence of functional abnormalities should be sought. Primary neurologic or muscular diseases involving the oropharynx are often associated with dysphagia. Thus, whereas esophageal dysphagia usually results from esophageal diseases, oropharyngeal dysphagia frequently results from neurologic or muscular diseases, with oropharyngeal dysfunction being just one pathologic manifestation. Although the specifics vary from disease to disease, the net effect on swallowing can be analyzed according to the mechanical description of the swallow outlined earlier. Table 42-1 summarizes the mechanical elements of the swallow along with the manifestation and consequence of dysfunction and provides representative pathologic conditions in which they are likely encountered. Neurologic examination may indicate cranial nerve dysfunction, neuromuscular disease, cerebellar dysfunction, or an underlying movement disorder. Functional abnormalities can be attributable to dysfunction of intrinsic musculature, peripheral nerves, or central nervous system control mechanisms. Of note, contrary to popular belief, the gag reflex is not predictive of pharyngeal swallowing efficiency or aspiration risk. The gag reflex is absent in 20% to 40% of normal adults.206
Table 42-1 Affected Phases, Manifestations, and Typical Disease Conditions Causing Oropharyngeal Dysphagia
| AFFECTED PHASE OF THE OROPHARYNGEAL SWALLOW | MANIFESTATION OF DYSFUNCTION | ASSOCIATED DISEASE(S) |
|---|---|---|
| Nasopharyngeal closure |
UES, upper esophageal sphincter.
Evident in Table 42-1, oropharyngeal dysphagia is frequently the result of neurologic or muscular diseases. Neurologic diseases can damage the neural structures requisite for either the afferent or efferent limbs of the oropharyngeal swallow. Virtually any neuromuscular disease can cause dysphagia. Because there is nothing unique to neurons controlling swallowing, their involvement in disease processes is usually random. Furthermore, in most instances, functions mediated by adjacent neuronal structures are concurrently involved. The following discussion focuses on neuromuscular pathologic processes most commonly encountered. These entities are also discussed in Chapter 35.
Stroke
Aspiration pneumonia has been estimated to inflict a 20% death rate in the first year after a stroke, and 10% to 15% each year thereafter.207 It is usually not the first episode of aspiration pneumonia, but the subsequent recurrences over the years that eventually causes death.208 The ultimate cause of aspiration pneumonia is dysphagia leading to aspiration that can occur by at least three mechanisms: absence or severe delay in triggering the swallow, reduced lingual control, or weakened laryngopharyngeal musculature.17 Conceptually, these mechanisms can involve motor or sensory impairments. Cortical strokes are less likely to result in severe dysphagia than brainstem strokes.209 Cortical strokes are also more likely to demonstrate neurologic recovery. Of 86 consecutive patients who sustained an acute cerebral infarct, 37 (43%) experienced dysphagia when evaluated within four days of the event. However, 86% of these patients were able to swallow normally two weeks later,209 with recovery resulting from contralateral areas taking over the lost function.210 Failure to recover swallowing function was more likely among patients incurring larger strokes or patients who have had prior infarcts.
Poliomyelitis
Most cases of poliomyelitis involve only the spinal cord. However, the fatality rate from bulbar polio far exceeds that of spinal disease, primarily a consequence of respiratory depression. Bulbar poliomyelitis is also associated with dysphagia. In one analysis of the persistent sequelae of bulbar poliomyelitis, 28 of 47 patients (60%) had recurrent or continued involvement of the pharynx 17 or more months after their acute illness.211 Speech and swallowing dysfunction result from weakness of the pharyngeal musculature.212 Neurologists have also observed an increasing number of patients with new paretic symptoms traceable to their remote polio infection 30 to 40 years earlier. The new, slowly progressive post-polio muscular atrophy may occur in muscles that were clinically unaffected by the acute illness.211 One investigation studied 13 patients with post-polio dysphagia and demonstrated palatal, pharyngeal, and laryngeal weakness.213 More than half of the patients evaluated demonstrated silent aspiration.
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a progressive neurologic disease characterized by degeneration of motor neurons in the brain, brainstem, and spinal cord. Specific symptoms are dependent on the locations of affected motor neurons and the relative severity of involvement. When the degenerative process involves the cranial nerve nuclei, swallowing difficulties ensue. Oropharyngeal dysfunction characteristically begins with the tongue and progresses to involve the pharyngeal and laryngeal musculature. Patients experience choking attacks, become volume depleted and/or malnourished, and incur aspiration pneumonia. The decline in swallowing function is progressive and predictable, invariably leading to gastrostomy feeding. Patients often die as a consequence of their swallowing dysfunction in conjunction with respiratory depression.214
Parkinson’s Disease
Although only 15% to 20% of patients with Parkinson’s disease complain of swallowing problems, more than 95% have demonstrable defects when studied videofluoroscopically.215 This disparity suggests that patients compensate in the early stages of the disease and complain of dysphagia only when it becomes severe. Abnormalities include repetitive lingual pumping prior to initiation of a pharyngeal swallow, piecemeal swallowing, and oral residue after the swallow. Patients may also exhibit a delayed swallow response and a weak pharyngeal contraction, resulting in vallecular and pyriform sinus residue. Recent data suggest this to be related to the combination of incomplete UES relaxation and a weakened pharyngeal contraction.215
Tumors
Medullary or vagal tumors are potentially debilitating with respect to swallowing. Astrocytomas are the most common tumor subtype affecting adults whereas medulloblastomas are the most common type encountered in children.216 The morbidity of these tumors is often substantially increased as a result of the relative inaccessibility of the medulla to surgery. Unilateral lesions of the vagus can result in hemiparesis of the soft palate and pharyngeal constrictors, as well as of the laryngeal musculature. Surgical manipulation of this region can even result in complete loss of the pharyngeal swallow response.217 The recurrent laryngeal nerves can be injured as a result of thyroid surgery, aortic aneurysms, pneumonectomy, primary mediastinal malignancies, or metastatic lesions to the mediastinum. Owing to its more extensive loop in the chest, the left recurrent laryngeal nerve is more vulnerable than the right to involvement with mediastinal node malignancy. Unilateral recurrent laryngeal nerve injury results in unilateral adductor paralysis of the vocal cords. This defect can result in aspiration during swallowing because of impaired laryngeal closure. It is rare, however, to have any primary pharyngeal dysfunction resultant from recurrent laryngeal nerve injury.218
Oculopharyngeal Dystrophy
Oculopharyngeal muscular dystrophy is a syndrome characterized by progressive dysphagia and ptosis. Historically, afflicted patients reaching age 50 typically died of starvation resulting from pharyngeal paralysis.219 The disease is now known to be a form of muscular dystrophy and is inherited as an autosomal dominant disorder with occurrences clustered in families of French-Canadian descent. Genetic studies of an afflicted family indicate linkage to chromosome 14, perhaps involving the region coding for cardiac alpha or beta myosin heavy chains.220 Oculopharyngeal dystrophy affects the striated pharyngeal muscles and the levator palpebrae. Although other forms of muscular dystrophy occasionally affect the pharyngeal constrictors, this is rarely a dominant manifestation. The first symptom of oculopharyngeal dystrophy is usually ptosis that slowly progresses and eventually dominates the patient’s appearance. Dysphagia may begin before, be concomitant with, or occur after ptosis. The dominant functional abnormalities are of a weak or absent pharyngeal contraction with hypopharyngeal stasis.219 Dysphagia is slowly progressive, but may ultimately lead to starvation, aspiration pneumonia, or asphyxia.
Myotonic Dystrophy
Myotonic dystrophy is a rare disorder characterized by prolonged contraction and difficulty in relaxation of affected skeletal musculature. Recent investigations suggest that even though only half of the patients complain of dysphagia, pharyngeal and esophageal motor abnormalities can be universally demonstrated. The pattern of abnormality is of a weakened pharyngeal contraction, absent peristalsis in the striated muscle esophagus, and diminished or absent peristalsis in the smooth muscle segment of the esophagus. Myotonia has not been demonstrated in any part of the esophagus.29 Thus, the risk of aspiration in this disease is similar to other forms of muscular dystrophy. Aspiration can occur during the swallow due to poor pharyngeal clearance combined with concurrent weakness of the laryngeal elevators or after the swallow when the substantial pharyngeal residue might fall into the reopened airway.
Myasthenia Gravis
Myasthenia gravis is a progressive autoimmune disease characterized by high circulating levels of acetylcholine receptor antibody and destruction of acetylcholine receptors at neuromuscular junctions. Musculature controlled by the cranial nerves is almost always involved, particularly the ocular muscles. Dysphagia is prominent in more than a third of cases and, in unusual instances, can be the initial and dominant manifestation of the disease.17 In mild cases, dysphagia may not be evident until after 15 to 20 minutes of eating. Classically, manometric studies reveal a progressive deterioration in the amplitude of pharyngeal contractions with repeated swallows. Peristaltic amplitude recovers with rest or following the administration of 10 mg edrophonium chloride, an acetylcholinesterase inhibitor. In more advanced cases, the dysphagia can be profound and associated with nasopharyngeal regurgitation and nasality of the voice, even to the extent of being confused with bulbar ALS or a brainstem stroke.221
Hypopharyngeal (Zenker’s) Diverticulum and Cricopharyngeal Bar
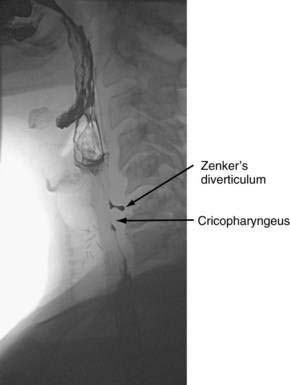
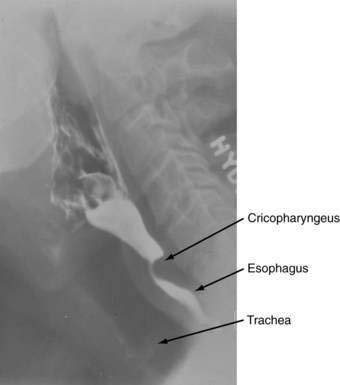
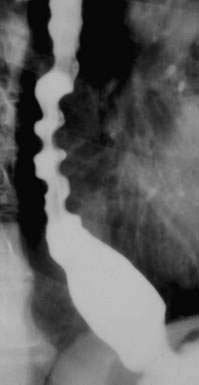
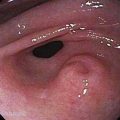
Hypopharyngeal diverticulum and cricopharyngeal bars are closely related disease entities in that it is a cricopharyngeal bar that can result in diverticulum formation. Zenker’s diverticulum (Fig. 42-7), is discussed in Chapter 23. Zenker’s diverticulum originates in the midline posteriorly at Killian’s dehiscence, a point of pharyngeal wall weakness between the oblique fibers of the inferior pharyngeal constrictor and the transverse cricopharyngeus muscle (see Fig. 42-6).222 Other locations of acquired pharyngeal diverticula include (1) the lateral slit separating the cricopharyngeus muscle from the fibers of the proximal end of the esophagus through which the recurrent laryngeal nerve and its accompanying vessels run to supply the larynx; (2) at the penetration of the inferior thyroid artery into the hypopharynx; and (3) at the junction of the middle and inferior constrictor muscles. The unifying theme of these locations is that they are sites of potential weakness of the muscular lining of the hypopharynx through which the mucosa herniates, leading to a “false” diverticulum. The best-substantiated explanation for the development of diverticula is that they form as a result of a restrictive myopathy associated with diminished compliance of the cricopharyngeus muscle. Surgical specimens of cricopharyngeus muscle strips from 14 patients with hypopharyngeal (Zenker’s) diverticula demonstrated structural changes that would decrease UES compliance and opening.223 The cricopharyngeus samples from these patients had “fibro-adipose tissue replacement and (muscle) fiber degeneration.” Thus, although the muscle relaxes normally during a swallow, it cannot distend normally, resulting in the appearance of a cricopharyngeal indentation, or bar, during a barium swallow (Fig. 42-8). Diminished sphincter compliance necessitates increased hypopharyngeal intrabolus pressure to maintain trans-sphincteric flow through the smaller UES opening. The increased stress on the hypopharynx from the increased intrabolus pressure may ultimately result in diverticulum formation.
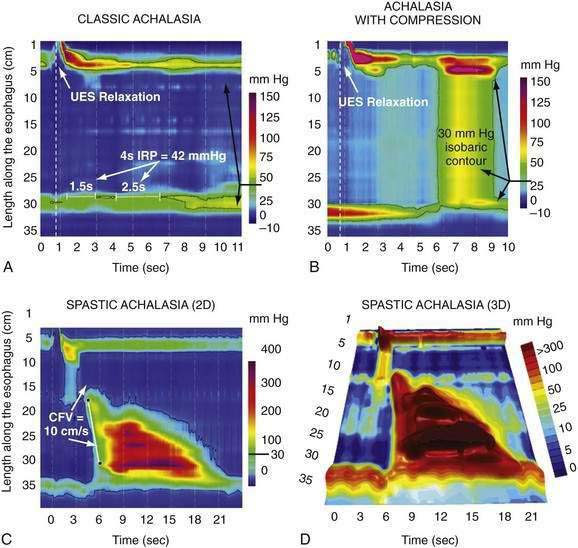
Achalasia
Achalasia is characterized by impaired LES relaxation with swallowing, and aperistalsis in the smooth muscle esophagus. The resting LES pressure is elevated in about 60% of cases. If there are nonperistaltic, spastic contractions in the esophageal body, the disease is referred to as vigorous achalasia or, more recently, spastic achalasia.224 These physiologic alterations result from damage to the innervation of the smooth muscle segment of the esophagus (including the LES). Proposed neuroanatomic changes responsible for achalasia include loss of ganglion cells within the myenteric (Auerbach’s) plexus, degeneration of the vagus nerve, and degeneration of the dorsal motor nucleus of the vagus. Of these three possibilities, only the loss of ganglion cells is well substantiated. Several observers report fewer ganglion cells and ganglion cells surrounded by mononuclear inflammatory cells in the smooth muscle esophagus of achalasics.225 One report additionally noted ganglion cell degeneration extending into the proximal stomach in half of 34 specimens analyzed.226 The degree of ganglion cell loss parallels the duration of disease such that ganglion cells are almost absent in patients afflicted for 10 or more years.227 A morphologic study of 42 esophagi resected from patients with advanced achalasia reported reduced numbers of ganglion cells and inflammation within the myenteric plexus in all cases.228
The ultimate cause of ganglion cell degeneration in achalasia is gradually being unraveled, with increasing evidence pointing toward an autoimmune process attributable to a latent infection with human herpes simplex virus 1 (HSV-1) in genetically susceptible individuals.229,230 Immunohistochemical analysis of the myenteric plexus infiltrate in achalasia patients revealed that the majority of inflammatory cells are either resting or activated cytotoxic T cells.231 In addition, immunoglobulin M (IgM) antibodies and evidence of complement activation have been demonstrated within myenteric ganglia.232 Antibodies against myenteric neurons have been repeatedly shown in serum of achalasia patients,233,234 especially in patients with HLA DQA1* 0103 and DQB1* 0603 alleles.235 The trigger for initiating the autoimmune response leading to the development of achalasia is suspected to be a viral infection, but studies implicating varicella zoster or measles virus have been contradictory.232,236,237 However, an elegant recent study provided strong evidence implicating HSV-1 as the culprit.230 T cells of achalasia patients exhibited clonal expansion within the myenteric plexus of the LES, and were activated by HSV-1 antigens, but not by cytomegaloviral, adenoviral, or enteroviral antigens. Furthermore, HSV-1 antibodies and HSV-1 deoxyribonucleic acid (DNA) were isolated in 84% and 63% of achalasic patients, respectively, potentially implicating HSV-1 in the majority of achalasia cases. Interestingly, HSV-1 was also detected in LES tissue from non-achalasic organ donors, suggesting that the development of achalasia is dependent on both the virus and a genetic predisposition as indicated by the specific HLA associations. Achalasia may also be associated with degenerative neurologic disorders such as Parkinson’s disease. Patients with both achalasia and Parkinson’s disease were noted to have Lewy bodies (intracytoplasmic hyaline or spherical eosinophilic inclusions) in the degenerating ganglion cells of the myenteric plexus.238
Physiologic studies in individuals with achalasia also suggest dysfunction consistent with postganglionic denervation of esophageal smooth muscle. Such damage can affect excitatory ganglion neurons (cholinergic), inhibitory ganglion neurons (NO ± VIP), or both. Consider first the excitatory ganglion neurons. Muscle strips from the circular layer of the esophageal body of achalasic patients contract when directly stimulated by acetylcholine but fail to respond to ganglionic stimulation by nicotine, indicating a postganglionic excitatory defect. However, it is likely that loss of excitatory innervation is variable among achalasic patients. Partial preservation of the postganglionic cholinergic pathway is suggested by the observations that an achalasic patient’s LES pressure increases after administration of the acetycholinesterase inhibitor, edrophonium, and decreases after administration of the muscarinic antagonist, atropine.239 These observations are crucial to understanding why botulinum toxin may have therapeutic benefit in achalasia (see section on treatment).
Regardless of excitatory ganglion neuron impairment, it is clear that inhibitory ganglion neuron dysfunction is an early manifestation of achalasia. These neurons mediate deglutitive inhibition (including LES relaxation) and the sequenced propagation of esophageal peristalsis; their absence offers a unifying hypothesis for the key physiologic abnormalities of achalasia, namely, impaired LES relaxation and aperistalsis. Inhibitory ganglion neurons use NO as a neurotransmitter, and patients with achalasia have been shown to lack NO synthase in the gastroesophageal junction.240 VIP may be a co-transmitter in these neurons and immunohistochemical studies have demonstrated a marked reduction of VIP-staining neurons in achalasic individuals.112
A multitude of evidence supports impaired physiologic function of post-ganglionic inhibitory innervation in the smooth muscle esophagus of achalasic patients. Muscle strips from their LES do not relax in response to ganglionic stimulation as they do in normal controls241 and CCK, which normally stimulates the inhibitory ganglion neurons, thereby reducing LES pressure, paradoxically increases the LES pressure in achalasics.242 Impaired inhibitory innervation of the smooth muscle esophagus above the LES is more difficult to demonstrate because of the absence of resting tone in this region. However, in a clever experiment, Sifrim and colleagues used an intraesophageal balloon to create a high-pressure zone in the tubular esophagus that then relaxed with the onset of deglutitive inhibition. This deglutitive relaxation in the esophageal body was absent in early, nondilated cases of achalasia.243
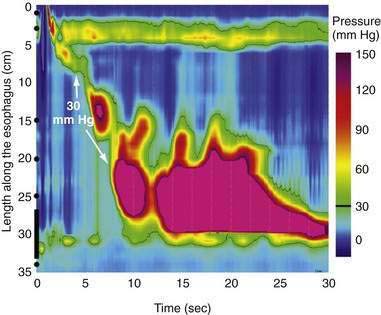
Distal Esophageal Spasm
The term “diffuse esophageal spasm” and our present concept of this entity dates to Fleshler’s 1967 description of a “clinical syndrome characterized by symptoms of substernal distress or dysphagia or both, the roentgenographic appearance of localized, nonprogressive waves (tertiary contractions), and an increased incidence of nonperistaltic contractions recorded by intraluminal manometry.”244 Because only the smooth muscle esophagus is affected, the entity was subsequently more precisely labeled “distal esophageal spasm.”245,246 Clearly, distal esophageal spasm is a disorder of peristalsis. However, in most afflicted patients, the esophagus retains the ability to propagate normal peristaltic contractions the majority of the time suggesting that the neuromuscular pathology is more subtle than with achalasia. Partly because of this fact, the criteria for diagnosing DES remain variable and confusing.245
The neuromuscular pathology responsible for DES is unknown and there are no known risk factors or other conditions associated with DES. Furthermore, because neither the esophageal muscularis propria or myenteric plexus is readily accessible for biopsy and patients with spastic disorders of the esophagus rarely undergo esophageal surgery, only a paucity of pathologic material has been available for analysis. The most striking reported pathologic change is diffuse muscular hypertrophy or hyperplasia in the distal two thirds of the esophagus. Muscular thickening of up to 2 cm has been reported in patients with clinical and manometric evidence of DES.247 However, there are other well-documented cases of spasm in which esophageal muscular thickening was not found at thoracotomy248 and still other instances of patients with muscular thickening not associated with DES symptoms.249 Similarly, little evidence of neuropathology has been reported; diffuse fragmentation of vagal filaments, increased endoneural collagen, and mitochondrial fragmentation have been described, but the significance of these findings is unclear.250
Despite the absence of defined histopathology, physiologic evidence implicates myenteric plexus neuronal dysfunction in spastic disorders of the esophagus. During peristalsis, vagal impulses reach the entire smooth muscle segment of the esophagus simultaneously and activate myenteric plexus neurons between the longitudinal and circular muscle layers.51 Ganglionic neurons then intervene between the efferent vagal fibers and the smooth muscle, belonging to either an inhibitory population that hyperpolarizes the muscle cell membrane and inhibits contraction or to an excitatory population that depolarizes the membrane, thereby prompting contraction. Thus, the instantaneous activity of the musculature at each esophageal locus is determined by the balance between these controlling influences from the myenteric plexus. Experimental evidence suggests heterogeneity among patients with spastic disorders, such that some primarily exhibit a defect of inhibitory interneuron function, whereas in others the defect is of excess excitation.
Two in vivo experiments implicate a defect of myenteric plexus inhibitory interneuron function in the genesis of simultaneous contractions in the distal esophagus. In one, the propagation of a swallow-induced contraction was timed in normal subjects and in a group of patients with a simultaneous contraction in the distal esophagus.251 Within the proximal esophagus the two groups exhibited similar contraction propagation, consistent with this timing being the result of the sequenced activation of motor units by vagal efferent nerves programmed within the medullary swallow center. However, once entering the smooth muscle segment, the patients’ contractions diverged from those of the normal subjects, resulting in a simultaneous contraction in the distal esophagus. The distal esophageal contractions were otherwise normal, but the progressive delay of initiation of the contraction at more distal loci, a function attributable to increasing dominance of inhibitory interneurons in the distal esophagus, was absent. Furthermore, if these patients swallowed twice within a five-second interval, there was no deglutitive inhibition of the first peristaltic contraction within the smooth muscle esophagus, as is observed in normal subjects. A second experiment demonstrating impaired deglutitive inhibition in DES comes from work using an artificial high-pressure zone within the distal esophagus. Patients with motor disorders characterized by rapidly propagating or simultaneous contractions exhibited only partial relaxation of the artificial high-pressure zone, proportional to the impairment of propagation velocity.243 Taken together these findings strongly suggest that one potential neuropathologic process in DES is a selective, intermittent dysfunction of myenteric plexus inhibitory interneurons.
A second group of patients in the analysis of Behar and Biancani had normal propagation latency but exhibited frequent spontaneous distal esophageal contractions. These patients had significantly longer and higher-amplitude contractions at each locus within the distal esophagus.251 Patients with peristaltic disorders characterized by excess excitation demonstrate heightened sensitivity to stimulation with cholinergic agents,112,252 the cholinesterase inhibitor edrophonium,253 pentagastrin,254 and ergonovine.255 An electromyographic correlate of this excitability is found from bipolar ring electrode recordings from the distal esophagus.256 Whereas normal individuals uniformly exhibited spiking activity prior to each esophageal contraction, DES patients exhibited spike-independent spontaneous esophageal contractions.
The preceding discussion suggests that the physiologic abnormalities of patients with spastic disorders are heterogeneous, but all are characterized by an imbalance between the excitatory and inhibitory influences on the esophageal smooth muscle. The suggestion of an impairment of the pathway of deglutitive inhibition is particularly interesting in that it places DES in a pathophysiologic continuum with achalasia, consistent with documented case reports of patients undergoing this evolution.257 Furthermore, there are marked similarities between spastic achalasia and DES, both characterized by rapidly propagated contractions in the distal esophagus, with the only differences being involvement of the LES and constancy of the disorder in vigorous achalasia. Similar to achalasia, the simultaneous contractions typifying DES impair bolus transit through the esophagus, potentially explaining the associated dysphagia.258
CLINICAL FEATURES
Dysphagia is a fundamental symptom of esophageal motility disorders. Esophageal, as opposed to oropharyngeal, dysphagia is suggested by the absence of associated aspiration, cough, nasopharyngeal regurgitation, dry mouth, drooling, pharyngeal residue following swallow, or co-occurring neuromuscular dysfunction (e.g., weakness, paresthesia, slurred speech). The associated conditions of heartburn, esophagopharyngeal regurgitation, chest pain, odynophagia, or intermittent esophageal obstruction suggest esophageal dysphagia. However, an important limitation of the patient history with esophageal dysphagia is that a patient’s identification of the location of obstruction is of limited accuracy. Specifically, a distal esophageal obstruction caused by an esophageal ring or achalasia often is perceived as cervical dysphagia, such that patients correctly localize distal dysfunction only 60% of the time.259 Because of this subjective difficulty in distinguishing proximal from distal lesions within the esophagus, an evaluation for cervical dysphagia should encompass the entire length of the esophagus.
Achalasia
Clinical manifestations of achalasia may include dysphagia, regurgitation, chest pain, hiccups, halitosis, weight loss, aspiration pneumonia, and heartburn. All patients have solid food dysphagia; the majority of patients also have variable degrees of liquid dysphagia. The onset of dysphagia is usually gradual, with the duration of symptoms averaging two years at presentation.188 The severity of dysphagia fluctuates, but eventually plateaus. With long-standing disease there is progressive esophageal dilatation, and regurgitation becomes frequent when large amounts of food and fluid are retained in the dilated esophagus. The regurgitant is often recognized as food that has been eaten hours, or even days, previously. It tends to be nonbilious, non-acid, and mixed with copious amounts of saliva. Patients often fail to recognize the slimy mucoid regurgitant as saliva, being unfamiliar with its normal consistency. Chest pain is a frequent complaint early in the course of achalasia, occurring in approximately two thirds of patients.260 Its etiology is unknown, but is speculated to be related to the occurrence of esophageal spasm (more recently, spasm of longitudinal muscle) or to the process of esophageal dilatation associated with disease progression. Treatment of achalasia (discussed later) is less effective in relieving chest pain than it is in relieving dysphagia or regurgitation. However, unlike dysphagia or regurgitation, chest pain may spontaneously improve or disappear over time.260
An estimated 10% of people with achalasia have bronchopulmonary complications as a result of regurgitation and aspiration; in some instances, it is these complications rather than dysphagia that prompts them to seek medical care.261 Another interesting, but fortunately rare, symptom of achalasia is airway compromise and stridor as a result of the dilated esophagus compressing the membranous trachea in the neck.262 This is hypothesized to result from dysfunction of the belch reflex.263
It is paradoxical that many achalasic patients complain of heartburn, even after the onset of dysphagia.264 Although gastroesophageal reflux may be a common sequela of the treatments for achalasia, it seems physiologically inconsistent to simultaneously have dysphagia from impaired LES relaxation and reflux from excessive LES relaxation. In support of this skepticism, ambulatory 24-hour esophageal pH studies of achalasic patients have only shown periods of esophageal acidification caused by the bacterial fermentation of retained food in the esophagus rather than discrete gastroesophageal reflux events.265 Furthermore, prolonged LES recordings have shown nearly a complete absence of transient LES relaxations in achalasics.266 However, there are occasional exceptions to this, evident from a well-documented case of an achalasic patient with intact transient LES relaxation despite the absence of deglutitive LES relaxation.267 The cause of the heartburn often reported by patients with achalasia is unknown.
Distal Esophageal Spasm
Esophageal chest pain is very similar in character to angina and is often described as crushing or squeezing in character, radiating to the neck, jaw, arms, or midline of the back. Pain episodes may last from minutes to hours, but continued swallowing is not always impaired. The mechanism producing esophageal pain is poorly understood. Recent data suggest that it may be related to sustained contraction of esophageal longitudinal muscle.268
Chest pain is also prevalent in patients subsequently found to have manometric abnormalities that are insufficient to establish a diagnosis of achalasia or DES. Among such individuals, there is a high prevalence of reflux and of psychiatric diagnoses, particularly anxiety and depression.197 Evidence also suggests a lower visceral pain threshold in this group, and symptoms of irritable bowel syndrome (Chapter 118) may be seen in more than 50% of these patients.269
DIFFERENTIAL DIAGNOSIS
Achalasia
The differential diagnosis of achalasia includes other esophageal motility disorders, with functional attributes overlapping those of achalasia and diseases of distinct pathophysiology that duplicate the functional consequences of achalasia. With respect to other motility disorders, there are many similarities between DES and achalasia, especially the subtype of spastic achalasia. In fact, the only distinction between these entities is the demonstration of incomplete LES relaxation in vigorous achalasia. Thus, some have speculated that DES and vigorous (spastic) achalasia may represent early disease and subsequently evolve into full-fledged achalasia.257 Testing this hypothesis, a report on a prospective cohort of patients diagnosed with esophageal spasm between 1992 and 2003 revealed that achalasia was subsequently diagnosed in only one.270 Given that rarity and the possibility of the case initially being misdiagnosed, it seems reasonable to conclude that at most only a small minority of DES cases are part of the continuum with achalasia.
Chagas Disease
Esophageal involvement in Chagas disease (see Chapter 109), which is endemic in areas of central Brazil, Venezuela, and northern Argentina, can be indistinguishable from idiopathic achalasia. An estimated 20 million South Americans are infected. Due to immigration, about 500,000 people in the United States are believed infected. Chagas disease is spread by the bite of reduvid (kissing) bug that transmits the parasitic protozoan, Trypanosoma cruzi. An acute septicemic phase of the illness follows that varies in severity from going unnoticed to being fatal.271 The chronic phase of the disease develops up to 20 years after infection and results from destruction of autonomic ganglion cells throughout the body, including the heart, gut, urinary tract, and respiratory tract. Chronic cardiomyopathy with conduction system disturbances and arrhythmias is the most common cause of death. Within the digestive tract, the organs most commonly affected are the esophagus, duodenum, and colon. The severity of esophageal dysfunction is directly proportional to the degree of intramural ganglion cell loss. Abnormal peristalsis is first detectable after 50% of ganglion cells are destroyed, whereas esophageal dilatation occurs only after 90% are destroyed. Paralleling this, the initial dysfunction is confined to the esophageal body, with LES dysfunction occurring late in the course of the disease.271 The most obvious clinical distinction between idiopathic achalasia and esophageal involvement in Chagas disease is evidence of additional organ involvement (megaureter, cardiomyopathy, megaduodenum, megacolon, megarectum) in Chagas disease. With respect to esophageal pathology, the two are otherwise indistinguishable. The diagnosis of Chagas disease is made in the acute phase by visualizing the parasite in a blood smear. In the chronic phase, the diagnosis is confirmed by serologic tests using complement fixation or polymerase chain reaction. The treatment of the achalasia syndrome in Chagas disease is similar to that for idiopathic achalasia (discussed later). Treatment of the infection itself is of limited efficacy in the acute phase and of no proven efficacy with chronic disease.
Pseudoachalasia
Neither the radiographic nor the manometric features of achalasia are specific for idiopathic achalasia or achalasia associated with Chagas disease. Tumor-related pseudoachalasia accounts for up to 5% of cases with manometrically defined achalasia. Pseudoachalasia is more likely in older age groups (sixth decade and beyond), in patients with recent onset of symptoms (within the past year), and in those with early weight loss in excess of 7 kg.188 However, even though these criteria make pseudoachalasia more likely, they still have a poor predictive value.272 Tumor infiltration (especially carcinoma in the gastric fundus) can completely mimic the functional impairment seen with idiopathic achalasia.273 It is because of this potential pitfall that a thorough anatomic examination including endoscopy should be done as part of the diagnostic evaluation of every new case of achalasia. A clue to the presence of pseudoachalasia on endoscopic examination is of more than the slightest resistance of passage of the endoscope across the gastroesophageal junction. In idiopathic achalasia, the endoscope should pop through with only gentle pressure required. If suspicion of pseudoachalasia is high, endoscopic biopsy, computerized tomography, magnetic resonance imaging, or endoscopic ultrasound should be considered for further evaluation, depending on the individual circumstances.
Adenocarcinoma of the gastroesophageal junction accounts for more than one half of pseudoachalasia cases, with myriad other tumors and miscellaneous conditions accounting for the remainder. Within the spectrum of malignancies, pancreatic, hepatoma, lung (small cell or non–small cell), esophageal squamous cell, prostate, and lymphoma cases have been reported.188 These tumors produce an achalasia syndrome by infiltrating the wall of the esophagus at the gastroesophageal junction, causing a malignant obstruction at the LES with proximal esophageal dilatation.273 Similarly, pseudoachalasia has also been reported to result from esophageal infiltration by amyloid,274 eosinophilic gastroenteritis,275 and sarcoidosis.276 Although often speculated in the literature, it is less certain, and certainly much less common, that an achalasic syndrome occurs as a paraneoplastic syndrome without direct tumor stenosis of the gastroesophageal junction.188
Postsurgical
Dysphagia is common following fundoplication in the early postoperative period and patients are often advised to consume soft diets for the first two to four weeks. Dysphagia that persists beyond two to four weeks should be evaluated with an upper endoscopy or barium esophagogram to assess the integrity of the wrap and evaluate for possible paraesophageal hernia. Subjects without an overt mechanical disruption should be evaluated with manometry to assess peristaltic function, LES pressure, and LES relaxation to determine whether the wrap is too tight or an underlying motility disorder, such as achalasia, exists. Diagnosing achalasia in the context of fundoplication can be difficult, because aperistalsis and impaired LES relaxation can be seen in both entities. In order to distinguish mechanical obstruction due to an obstructive fundoplication (or crural repair) from achalasia, one can administer amyl nitrite during manometry and observe the effect on the EGJ high-pressure zone. The mechanical effect of a fundoplication is less affected by the smooth-muscle relaxing effects of the amyl nitrite than the hypertensive sphincter of a person with achalasia.277
Bariatric surgery, especially laparoscopic adjustable gastric banding, can be complicated by development of a pseudoachalasia syndrome. A recent report examined 121 patients a year after this procedure and found that 14% of them had esophageal dilatation in excess of 3.5 cm. Affected patients developed an achalasia-type syndrome with dysphagia and vomiting.278 This form of pseudoachalasia is usually, but not always, reversible with removal of the gastric band.279
DIAGNOSTIC METHODS
Endoscopy
Upper endoscopy should be the first test for evaluating new onset dysphagia because it combines the ability to detect most structural causes of dysphagia with the ability to obtain biopsies. The increasing recognition of eosinophilic esophagitis (Chapter 27) as a confounding clinical entity has increased the potential value of biopsies when performing upper endoscopy in the evaluation of dysphagia.280 The endoscopist should have a very low threshold for obtaining multiple (preferably five) esophageal mucosal biopsy specimens to evaluate for eosinophilic esophagitis even with a normal appearing esophageal mucosa.281 Additionally, should a stricture or mucosal ring be detected, dilation can be accomplished in the same session. However, even though upper endoscopy is an excellent tool for evaluating dysphagia, it has substantial limitations in assessing extraluminal structures and abnormal esophageal motility. It also has the potential to miss subtle obstructing lesions, such as webs and rings.
Contrast Imaging
Contrast studies of the oropharynx and esophagus are useful in assessing dysphagia after endoscopy if the latter was inconclusive or instead of it, if endoscopy is not readily available. Videofluoroscopy is particularly useful for a functional evaluation of the oropharyngeal phase of swallowing following an examination for anatomic explanations. Frequently referred to as a modified barium swallow, Logemann has described a protocol composed of a series of swallow tasks.17 Images are obtained in a lateral projection, framed to include the oropharynx, palate, proximal esophagus, and proximal airway. These images are then evaluated with respect to four major categories of oropharyngeal dysfunction: (1) inability or excessive delay in initiation of pharyngeal swallowing; (2) aspiration; (3) nasopharyngeal regurgitation; and (4) residue of the ingestate within the pharyngeal cavity after swallowing. Furthermore, the procedure allows for evaluation of the efficacy of various compensatory dietary modifications, postures, and swallowing maneuvers in compensating for observed swallowing dysfunction.
A barium esophagogram can also provide useful information regarding UES function, peristalsis, and bolus clearance through the EGJ. With advanced cases of achalasia (Fig. 42-9) the findings are somewhat obvious and it is only necessary clinically to differentiate between primary and secondary etiologies. However, with good technique, normal peristalsis can also be verified with 91% to 95% specificity.282,283 Peristalsis is best evaluated in the prone position so that clearance does not occur by gravity. In the prone position, the primary peristaltic wave manifests as an inverted “V” (∧), the peak of which represents the tail of the bolus. Luminal closure at the tail of the bolus corresponds closely to the leading edge, or upstroke, of the peristaltic wavefront as recorded manometrically (Fig. 42-10). Peristaltic abnormalities are inferred by retrograde escape of the bolus through the peristaltic wavefront resulting in incomplete esophageal emptying. Normally the EGJ will become widely patent when the bolus reaches this area and impaired relaxation can be inferred when either a smooth tapering is noted at the EGJ or bolus transit across the EGJ is impeded. Alternatively, fluoroscopy will occasionally demonstrate spastic contractions, evident by a corkscrew appearance (Fig. 42-11).
Esophageal Manometry (High-Resolution Esophageal Pressure Topography)
Esophageal manometry is a test in which intraluminal pressure sensors, either water perfused or solid state, are positioned within the esophagus to quantify the contractile characteristics of the esophagus and segregate it into functional regions. The concept of high-resolution esophageal manometry is to use a sufficient number of pressure sensors within the esophagus such that intraluminal pressure can be monitored as a continuum along the length of the esophagus, much as time is viewed as a continuum in line tracings of conventional manometry such as those in Figure 42-10.
When high-resolution manometry is coupled with sophisticated algorithms to display the manometric data as pressure topography plots, esophageal contractility is visualized with isobaric conditions among sensors indicated by isocoloric regions on the pressure topography plots. Figure 42-12 depicts a normal swallow in a high-resolution esophageal pressure topography plot encompassing both sphincters (UES and LES) and the intervening esophagus. The relative timing of sphincter relaxation and segmental contraction as well as the position of the transition zone are all readily demonstrated.
The manometric evaluation of deglutitive EGJ relaxation is probably the most important measurement made during clinical esophageal manometry. Incomplete EGJ relaxation is an essential feature in the diagnosis of achalasia and achalasia is not only the best-defined esophageal motor disorder, but also the one with the most specific treatments. Despite this cardinal significance, there was no convention for defining incomplete deglutitive EGJ relaxation with conventional manometry. Furthermore, numerous potential confounding factors exist including crural diaphragm contraction during relaxation, deglutitive esophageal shortening, hiatal hernia, sphincter radial asymmetry, and movement of the recording sensor relative to the EGJ.8 With high-resolution esophageal pressure topography, this situation is greatly improved. A study comparing criteria for detecting impaired deglutitive EGJ relaxation within the deglutitive relaxation window (see Fig. 42-12) in a large group of patients and control subjects concluded that the optimal measure for quantifying deglutitive relaxation was the integrated relaxation pressure (IRP), with normal being defined as 15 mm Hg or less.284 Conceptually, the IRP is the average EGJ pressure for the four seconds of greatest relaxation within the relaxation window. This single measure of deglutitive EGJ relaxation exhibited 98% sensitivity and 96% specificity for distinguishing well-defined achalasia patients from control subjects and patients with other diagnoses.284
Apart from improving the sensitivity of manometry in the detection of achalasia, high-resolution esophageal pressure topography has also defined a clinically relevant subclassification of achalasia.224 A diagnosis of achalasia requires both aperistalsis and impaired deglutitive EGJ relaxation. In its most obvious form this occurs in the setting of esophageal dilatation with negligible pressurization within the esophagus (Fig. 42-13A). However, despite there being no peristalsis, there can still be substantial pressurization within the esophagus. In fact, a very common pattern encountered is achalasia with esophageal compression and panesophageal pressurization (see Fig. 42-13B). The other, less common pattern is of spastic achalasia in which there is a spastic contraction within the distal esophageal segment (see Fig. 42-13C and D). In a series of 99 consecutive patients with newly diagnosed achalasia, 21 had the classical pattern in Figure 42-13A, 49 had the panesophageal pressurization pattern of Figure 42-13B, and 29 had the spastic achalasia pattern of Figure 42-13C.224 Logistic regression analysis found panesophageal pressurization (see Fig. 42-13B) to be a predictor of response to treatment, whereas spastic achalasia (see Fig. 42-13C) and pretreatment esophageal dilatation were predictive of a poorer response to treatment.
Following the analysis of the EGJ, a swallow is further categorized by the characteristics of the distal esophageal contraction. This analysis is largely facilitated by the generation of a pressure topography plot highlighting the 30 mm Hg isobaric contour. Under circumstances of normal deglutitive EGJ relaxation, the 30 mm Hg pressure threshold reliably delineates the wavefront of the peristaltic contraction.285 Contractile front velocity (CFV) is calculated from the 30 mm Hg isobaric contour plots by calculating the slope of the line connecting the 30 mm Hg isobaric contour at the proximal margin of the first subsegment and the distal margin of the second subsegment (see Fig. 42-12). A CFV of more than 8 cm/second is indicative of a spastic contraction.285,286
Although the CFV is easily definable in the circumstance of normal EGJ relaxation, it is more complex when EGJ relaxation is impaired (Fig. 42-14). With impaired EGJ relaxation, there is compartmentalized pressurization between the contractile front of the distal esophageal contraction and the EGJ with a high intrabolus pressure residing between the two. In such instances, the slope of the 30 mm Hg isobaric contour is no longer indicative of the CFV but now indicates increased intrabolus pressure as a result of functional obstruction at the EGJ. In such circumstances, the algorithm for computing CFV defaults to computing the slope of an isobaric contour line of magnitude greater than the EGJ relaxation pressure (e.g., 50 mm Hg in a given patient) so as to consistently represent the timing of luminal closure (see Fig. 42-14).
Apart from a rapid CFV, other common abnormalities of the distal esophageal contraction are weak or absent peristalsis. In such instances, the 30 mm Hg isobaric contour is either discontinuous or absent, reflective of either focal or diffuse hypotensive contraction within the distal segment. Each swallow is thus characterized as normal (intact 30 mm Hg isobaric contour and a CFV < 8 cm/second), hypotensive (3 cm or greater defect in the 30 mm Hg isobaric contour), or absent (complete failure of contraction with no pressure domain > 30 mm Hg). Weak or absent peristalsis is a risk factor for impaired bolus clearance, but, whether impaired bolus clearance occurs depends on the balance between the severity of weakness and the magnitude of outflow resistance at the EGJ.287
Once swallows are characterized by the integrity of deglutitive EGJ relaxation and normality of the CFV, the distal esophageal contraction is further analyzed for the vigor of contraction using a newly developed measure for high-resolution esophageal pressure topography, the distal contractile integral (DCI). The DCI integrates the length, vigor, and persistence of the two subsegments of the distal esophageal segment contraction, expressed as mm Hg·s·cm. A DCI value greater than 5000 mm Hg·s·cm is considered elevated.288 Adopting the nomenclature “nutcracker esophagus” from conventional manometry, this is the high-resolution manometry criterion defining hypertensive peristalsis and was seen in 9% of a 400-patient series.285 However, there was substantial heterogeneity as to the locus of the hypertensive contraction within this group, potentially involving either or both of the subsegments within the distal esophageal contraction. Similarly, the LES can exhibit a hypertensive postdeglutitive contraction, defined as exceeding 180 mm Hg. Furthermore, one particularly interesting subgroup, defined by having a higher threshold DCI (>8000 mm Hg·s·cm), exhibited repetitive high-amplitude contractions and was clinically distinguishable by the uniform association with dysphagia or chest pain. Similar to DES, this “spastic nutcracker” pattern is very rare, found in only 12 (3%) of this 400-patient series (Fig. 42-15).
Following analysis of individual swallows by the criteria outlined, the component results are synthesized into a global manometric diagnosis by the criteria detailed in Table 42-2. Patients with normal EGJ relaxation, normal CFV, and a DCI less than 5000 mm Hg·s·cm are normal. The abnormalities encountered are described in specific functional terms with the intent that these then be interpreted within the clinical context of the patient. The classification detailed in Table 42-2 represents an incremental update on the Chicago classification,289 the task of a newly convened international working group focused on the standardization of the performance and interpretation of high-resolution esophageal pressure topography studies.
Table 42-2 The Chicago Classification of Distal Esophageal Motility Disorders
| With Normal EGJ Relaxation (Mean Integrated Relaxation Pressure <15 mm Hg) | |
|---|---|
| DISORDER | CRITERIA |
| Aperistalsis | 100% of swallows with absent peristalsis |
| Hypotensive peristalsis | |
| Intermittent | More than 30% of swallows with peristaltic defects ≥3 cm in 30 mm Hg pressure isocontour |
| Frequent | 70% or more of swallows with peristaltic defects ≥3 cm in 30 mm Hg pressure isocontour |
| Hypertensive peristalsis | Normal CFV, mean DCI >5000 and <8000 mm Hg·s·cm or LES after-contraction >180 mm Hg |
| Spastic nutcracker | Normal CFV, mean DCI >8000 mm Hg·s·cm (Fig. 42-15) |
| Distal esophageal spasm (DES) | Normal EGJ relaxation and spasm (CFV >8 cm/s) with ≥20% of swallows (see Fig. 42-14B) |
| With Impaired EGJ Relaxation (Mean Integrated Relaxation Pressure ≥15 mm Hg) | |
|---|---|
| DISORDER | CRITERIA |
| Achalasia | |
| Classic achalasia | Impaired EGJ relaxation and aperistalsis (see Fig 42-13A) |
| Achalasia with esophageal compression | Impaired EGJ relaxation, aperistalsis, and panesophageal pressurization with ≥20% of swallows (see Fig. 42-13B) |
| Spastic achalasia | Impaired EGJ relaxation, aperistalsis, and spasm (CFV >8 cm/s) with ≥20% of swallows (see Fig. 42-13C and D) |
| Functional EGJ obstruction* | IBP >30 mm Hg compartmentalized between the peristaltic wavefront (normal or nutcracker) and EGJ (see Fig. 42-14A) |
CFV, contractile front velocity; DCI, distal contractile integral; EGJ, esophagogastric junction; IBP, intrabolus pressure; LES, lower esophageal sphincter.
Intraluminal Impedance Measurement
Intraluminal impedance monitoring was described more than a decade ago as a method to assess intraluminal bolus transit without using fluoroscopy. The technique uses an intraluminal catheter with multiple, closely spaced pairs of metal rings (see Fig. 42-10). An alternating current is applied across each pair of adjacent rings and the resultant current flow between the rings is dependent on the impedance of the tissue and luminal content between the rings. Impedance decreases when the electrodes are bridged by liquid and increases when they are surrounded by air thereby providing data on the direction, content, and completeness of bolus transit. Validation data suggest that liquid bolus entry at the level of an electrode pair is indicated by a 50% drop in impedance and return of the impedance tracing to 50% of baseline correlates with the passage of the tail of the bolus on fluoroscopy, also indicated by the contractile upstroke noted on manometry (see Fig. 42-10). Validation studies of intraluminal impedance measurement against videofluoroscopy have shown excellent concordance in ascertaining bolus transit, reporting agreement in 97% (83/86) of swallows analyzed.290
Intraluminal impedance measurement has also recently been combined with manometry to assess the efficacy of esophageal emptying as a function of distal peristaltic amplitude. In a receiver operating characteristic (ROC) analysis of a large number of swallows, a 30 mm Hg cutoff had 85% sensitivity and 66% specificity for identifying incomplete bolus transit.291 With diminishing peristaltic amplitudes, the sensitivity progressively decreased and the specificity progressively increased. This study illustrates the complementary nature of manometry and impedance testing in assessing esophageal function and may develop into a valuable clinical tool for the assessment of dysphagia.
Sensory Testing
Esophageal sensory nerves play a key role in determining symptoms of esophageal neuromuscular diseases because the esophagus is sensitive to a variety of stimuli including mechanical (elicited by luminal distention or high-amplitude contractions), chemical (acid or other constituents of reflux), and temperature.292 Typically the visceral input is not perceived consciously. However, some patients may experience symptoms attributed to hyperalgesia (exaggerated pain perception) or allodynia (perception of pain to a stimulus that is usually not painful).155,293 Esophageal symptoms may be described as burning, pressing, pricking, or heat sensations. Nevertheless, these symptoms are not specific to a given stimulus, and substantial overlap in perception among stimuli is common.
Although the precise mechanism by which an esophageal stimulus causes pain or the perception of dysphagia is unclear, methodologies devised to evoke or stimulate pain by simulating physiologic events are available to assess the possible relationship between ongoing symptoms and suspected causes. These tests typically use forms of distention studies (balloon, barostat, impedance planimetry, or volume challenges) or direct mucosal stimulation (chemical, electrical, or thermal). Balloon distention studies have shown that esophageal distention can provoke chest pain and that patients with esophageal chest pain tend to have lower threshold volumes for both first perception and first pain perception compared with controls.294,295 Combining impedance planimetry with balloon distention allowed other investigators to correlate biomechanical properties of the esophagus with the generation of chest pain.296,297 Results of those studies suggested that the tension-strain curve in chest pain patients was shifted to the left when compared with controls, a finding consistent with reduced compliance of the esophageal wall.269
TREATMENT
Oropharyngeal Dysphagia
Disorders Amenable to Surgery
The most common surgical treatment for oropharyngeal dysphagia is cricopharyngeal myotomy but the efficacy of myotomy in neurogenic or myogenic dysphagia is variable. Most series evaluating the efficacy of myotomy in these circumstances are uncontrolled and lack validated or even specific outcome measures. Thus, although an overall favorable response rate in excess of 60% is reported in this literature, there are no validated criteria for patient selection. Theoretically, the functional limitation faced by patients with neurogenic or myogenic dysphagia is of weak pharyngeal propulsion and the potential benefit of myotomy in that circumstance is less obvious than in the case of obstruction at the level of the cricopharyngeus.298
Patterns of Oropharyngeal Dysphagia Amenable to Swallow Therapy
Identifying potential treatments for oropharyngeal dysphagia begins with definition of the aberrant physiology as categorized in Table 42-1. This is best accomplished with a videofluoroscopic swallowing study that first characterizes a patient’s swallow dysfunction and then proceeds to test the effectiveness of selected compensatory or therapeutic treatment strategies. Compensatory treatments include postural changes, modifying food delivery or consistency, or the use of prosthetics. For instance, head turning can eliminate aspiration or pharyngeal residue by favoring the more functional side in patients with hemiparesis.17 Similarly, diet modifications can reduce the “difficulty” of the swallow. Therapeutic strategies are designed to alter the physiology of the swallow, usually by improving the range of motion of oral or pharyngeal structures using voluntary control of oropharyngeal movement during a swallow. Depending on the severity of the impairment, level of motivation, and global neurologic integrity, defective elements of the swallow can be selectively rehabilitated. For a detailed description of the techniques and limitations of swallow therapy, the reader is referred to treatises on the topic.17,299
Evaluating Aspiration Risk
Oropharyngeal dysphagia is responsible for an estimated 40,000 deaths a year due to aspiration pneumonia.300 Videoflouroscopy is considered the most sensitive test for detecting aspiration, reportedly detecting instances not evident by bedside evaluation in 42% to 60% of patients. However, despite the logical association between deglutitive aspiration and the subsequent development of pneumonia, this sequence is not inevitable. In fact, available data suggest that radiographic aspiration has a positive predictive value of only 19% to 68% and a negative predictive value of 55% to 97% for pneumonia.300 Nonetheless, the balance of evidence suggests that detection of aspiration is a predictor of pneumonia risk, and that its detection dictates that compensatory swallowing strategies, non-oral feeding or corrective surgery be instituted. Whether non-oral feeding eliminates the risk of aspiration is controversial. In one study of 22 patients with radiographic aspiration, pneumonia and death were more frequent among patients who received feeding tubes.185 This suggests that aspiration of oral secretions may be the essential element in pneumonia risk and has led some to consider procedures such as tracheostomy to protect the airway.
Hypopharyngeal (Zenker’s) Diverticulum and Cricopharyngeal Bar
The treatment of hypopharyngeal diverticulum is cricopharyngeal myotomy with or without a diverticulectomy (see Chapter 23). Cricopharyngeal myotomy reduces both the resting sphincter tone and resistance to flow across the UES. A study found that the compliance of the sphincter following diverticulectomy with myotomy was restored to normal following surgery, as indicated by normal hypopharyngeal intrabolus pressure during swallowing.301 Good or excellent results are reported in 80% to 100% of Zenker’s patients treated by transcervical myotomy combined with diverticulectomy or diverticulopexy.299 There are instances in which a limited procedure would be adequate, but a definitive approach to the problem of pulsion diverticula should generally involve myotomy and diverticulectomy. Diverticulectomy alone risks recurrence because the underlying stenosis at the level of the cricopharyngeus is not remedied. Similarly, myotomy alone may not solve the problem of food accumulation within the diverticulum, with attendant regurgitation and aspiration. Small diverticula may, however, disappear spontaneously following myotomy.
A more recent trend is to treat Zenker’s diverticula via either rigid or flexible endoscopy. With both techniques, the principle is to divide the septum between the lumen of the diverticulum and the lumen of the esophagus. The division allows food and liquid to flow out of the diverticulum distal to the cricopharyngeus (which was within the septum) rather than to accumulate within the diverticulum. In the case of rigid endoscopy the procedure is performed under general anesthesia with a stapling device. In the case of flexible endoscopy the procedure is performed under light sedation with a needle knife, argon plasma coagulation, or hot biopsy forceps. Controlled trials have not been done comparing the two procedures, but a recent summary of 376 reported cases treated with flexible endoscopic methods found treatment to result in clinical resolution in 43% to 100% of cases among series.184
Whether a cricopharyngeal bar in the absence of a diverticulum requires treatment is less clear. Certainly, if dysphagia is present and combined fluoroscopic/manometric analysis demonstrates reduced sphincter opening in conjunction with elevated upstream intrabolus pressure, there is good rationale for treatment. One recent uncontrolled series suggests that in this scenario dilation with a large-caliber bougie may be efficacious in relieving dysphagia and this approach is certainly a reasonable treatment option prior to myotomy.302
Achalasia
Pharmacologic treatments, on the whole, are not very effective, making them more appropriate as temporizing maneuvers than definitive therapies. The definitive treatments of achalasia are disruption of the LES either surgically (Heller myotomy) or with a pneumatic dilator. Which of these is the optimal approach remains an issue of debate given the paucity of randomized controlled trials with accepted criteria for assessing efficacy. A further limitation of the previous studies is failing to stratify patients by disease severity or, as more recently defined, by disease subtype.224 High-resolution esophageal pressure topography allows the subtyping of achalasia into three distinct patterns: I, classic achalasia; II, achalasia with compression; and III, spastic achalasia (see Fig. 42-13). From a conceptual vantage point types I and II represent a continuum, with type II representing early disease before the progression of esophageal dilatation characteristic of type I. Type III, on the other hand, is a subtype characterized by spasm of the distal esophagus. The significance of these disease subtypes is in how differently they responded to therapy, be it botulinum toxin injection, pneumatic dilation, or Heller myotomy. In a series of 99 new cases of achalasia, the overall treatment response was 56% with type I, 96% with type II, and only 29% with type III.
Pharmacologic Therapy
Smooth muscle relaxants such as nitrates or calcium channel blockers, administered sublingually immediately prior to eating can relieve dysphagia in achalasia by reducing the LES pressure. Amyl nitrite,303 sublingual nitroglycerin, theophylline, and β2-adrenergic agonists304 have also been tried. The largest reported experience has been with isosorbide dinitrate (Isordil) and nifedipine.305 Isosorbide dinitrate, 5 to 10 mg sublingually before meals, reduces LES pressure by 66% for about 90 minutes, with the degree of dysphagia relief paralleling the magnitude of the LES response over the 19-month trial.306 Side effects, particularly headache, are common. Placebo-controlled trials have not been reported.
Calcium channel blockers (diltiazem, nifedipine, verapamil) reduce LES pressure by 30% to 40% for more than an hour.306,307 The largest clinical experience in achalasia has been with nifedipine (Procardia). Nifedipine, 10 mg sublingually (capsules are crushed in the mouth) administered before meals (30 to 40 mg per day) was studied in 29 patients with early achalasia (prior to esophageal dilatation) in a placebo-controlled trial. Nifedipine was significantly better that placebo (which had no benefit), with good results in 70% of achalasic patients followed for 6 to 18 months.305 However, subsequent placebo-controlled crossover trials have found only minimal benefit with nifedipine.308 Side effects of nifedipine include flushing, dizziness, headache, peripheral edema, and orthostasis.
Sildenafil (Viagra) is another smooth muscle relaxant that can decrease LES pressure in patients with achalasia by blocking phosphodiesterase type 5, the enzyme that destroys cyclic guanosine monophosphate induced by NO. A double-blind placebo controlled trial found that 50 mg of sildenafil significantly reduced LES pressure and relaxation pressure when compared with placebo.309 The effect peaked at 15 to 20 minutes after administration and persisted for less than one hour. Although conceptually appealing, the practicality of using sildenafil clinically is limited by its cost that is rarely, if ever, covered by health care insurance.
Botulinum Toxin Injection
The initial landmark study of botulinum toxin in achalasia reported that intrasphincteric injection of 80 units of botulinum toxin decreased LES pressure by 33% and improved dysphagia in 66% of patients for a six-month period.310 Botulinum toxin irreversibly inhibits the release of acetylcholine from presynaptic cholinergic terminals, effectively eliminating the neurogenic component of LES pressure. However, because this inhibitory effect is eventually reversed by the growth of new axons, botulinum toxin is not a long-lasting therapy. The technique involves injecting divided doses of botulinum toxin into four quadrants of the LES with a sclerotherapy catheter. Side effects are rare, but include chest discomfort for several days and rash. Although many patients initially experience a good response, there is minimal continued efficacy at one year.311–313 Repeat injection can be effective for a reasonable subset of patients, but the injection leads to a local inflammatory reaction and fibrosis, ultimately limiting this strategy. Doses greater than 100 units do not have increased efficacy.314 Studies comparing botulinum toxin injection to pneumatic dilation suggest that the expense of repeated injection outweighs the potential economic benefits of added safety, unless the patient’s life expectancy is minimal.315 Thus, this option is mainly reserved for older adults or frail individuals who are poor risks for definitive treatments.
Pneumatic Dilation
Therapeutic dilation for achalasia requires distention of the LES to a diameter of at least 3 cm to produce a lasting reduction of LES pressure, presumably by partially disrupting the circular muscle of the sphincter. Dilation with an endoscope, standard bougies (up to 60 French), or with through-the-scope balloon dilators (up to 2 cm) provides very temporary benefit at best. Only dilators specifically designed to treat achalasia achieve adequate diameter for lasting effectiveness. The basic element of an achalasia dilator is a long, noncompliant, cylindrical balloon that can be positioned across the LES fluoroscopically (Rigiflex dilator) or endoscopically (Witzel dilator) and then inflated to a characteristic diameter in a controlled fashion using a handheld manometer. There is general agreement that pneumatic dilation can be done on an outpatient basis with the patient under conscious sedation. The technique of pneumatic dilation is variable among practitioners in terms of patient preparation, parameters of balloon inflation, and postdilation monitoring. In patients with substantial esophageal retention, it is useful to impose a liquid diet for one or more days prior to the procedure. Reported balloon inflation periods range from several seconds to five minutes.316 Although there is minimal methodologic consistency among authors, a cautious approach of beginning with a small-diameter dilator (3 cm) and progressing to larger diameters (3.5 and 4 cm) only when the smaller dilator proved ineffective is fairly universal. As for inflation pressures, these are of minimal relevance with modern noncompliant balloon dilators because they do not distend beyond their specified diameter regardless of inflation pressure. Hence, it is simply necessary to observe under fluoroscopy that the balloon is properly positioned to capture the LES, observed as the “waist” of the hourglass-shaped balloon silhouette and that the waist fully effaces as the inflation proceeds. As for technical details of the procedure other than balloon diameter, there is minimal evidence that they influence outcome.
The major complication of pneumatic dilation is esophageal perforation (see Chapter 40); mortality is fortunately rare.317 The reported incidence of esophageal perforation consequent from pneumatic dilation ranges between 1% and 5%261,316 with a global average of 3%. Because most perforations are readily evident or at least suspected within an hour of the procedure, patients should be observed closely for signs of an esophageal leak for at least two hours after pneumatic dilation. Alternatively, some practitioners routinely obtain a fluoroscopic examination of the esophagus following pneumatic dilation to ensure that perforation has not occurred. Usually, water-soluble contrast is given first, followed by barium. If a perforation appears small and contained or intramural, conservative management in the hospital consisting of close observation while maintaining the patient on nothing per mouth status and administering intravenous antibiotics is appropriate.261 If a perforation is substantial, or if worsening pain and fever occur during observation of what was thought to be a small perforation, surgical repair should be pursued expediently. Patients with a perforation from pneumatic dilation that is recognized and promptly treated surgically (within six to eight hours) have outcomes comparable with those of patients undergoing elective Heller myotomy.318
The best predictor of efficacy following a pneumatic dilation is the postdilation LES pressure; neither sphincter relaxation nor peristaltic function is significantly changed. A postdilation LES pressure less than 10 mm Hg is associated with prolonged remission, whereas a postdilation LES pressure greater than 20 mm Hg predicts that little benefit will occur from the procedure.319 In instances of an unsatisfactory result, it is reasonable to perform a subsequent dilation within a matter of weeks using an incrementally larger dilator. If the benefit of dilation persisted for a year or more, it is neither unusual nor dangerous to repeat pneumatic dilation as necessary. The clinical efficacy of dilation has been reported to range from 32% to 98%.311 Patients having a poor initial result or rapid recurrence of symptoms have diminished likelihood of responding to additional dilations.311 Subsequent response to surgical myotomy is not influenced by the history of previous dilations.261
Heller Myotomy
Current surgical procedures for treating achalasia are variations on the esophagomyotomy described by Heller in 1913 consisting of an anterior and posterior myotomy performed through either a laparotomy or a thoracotomy.311 Subsequently, this was modified to an anterior myotomy via thoracotomy. The appeal of myotomy is that it offers a more predictable method of reducing LES pressure than does pneumatic dilation.320 Although clearly efficacious, open Heller myotomy is associated with considerable morbidity related to thoracotomy, which led most patients to pursue pneumatic dilation as the initial intervention. However, adoption of the laparoscopic approach for achalasia surgery has led many practitioners to reconsider this.
Published series of the efficacy of Heller myotomy in treating achalasia report good to excellent results in 62% to 100% of patients, with persistent dysphagia troubling less than 10% of patients.311 Recent studies suggest that a laparoscopic approach is associated with similar efficacy, reduced morbidity, and shorter hospital stay when compared with myotomy via thoracotomy, laparotomy, or thoracoscopy.311,321–325 The overall mortality from Heller myotomy is less than 2%. Historically, postmyotomy reflux in achalasic patients could be particularly severe, making this a hotly disputed detail of the surgical technique.326 However, with the broad use of proton pump inhibitors, reflux is usually easily controlled, making these complications very unlikely. Thus, laparoscopic Heller myotomy combined with a partial fundoplication (Toupet or Dor) has become the preferred surgical procedure for achalasia. An unsatisfactory result following Heller myotomy can result from incomplete myotomy, scarring of the myotomy, functional esophageal obstruction from the antireflux component of the operation, paraesophageal hernia, or severe esophageal dilatation.
Heller Myotomy versus Medical Treatment
Although pharmacologic therapy is simple and safe, it is increasingly clear that this should be reserved for use as a temporizing measure while more definitive therapy is being considered. Thus, practically speaking, the therapeutic choice is between pneumatic dilation and laparoscopic Heller myotomy as the primary therapy for achalasia. However, there are as yet no prospective controlled trials comparing these treatments. One controlled trial compares pneumatic dilation to myotomy via thoracotomy. That study reported 95% symptom resolution with myotomy and 51% symptom resolution in the dilation group, but the study was criticized for the methodology of pneumatic dilation used.327 Most case series report symptom resolution in approximately 70% of patients with pneumatic dilation, substantially higher than the 51% reported in the controlled trial, but still substantially lower than that reported in uncontrolled series of laparoscopic Heller myotomy (85% to 91%). Furthermore, although laparoscopic Heller myotomy is invasive, its morbidity and mortality are low. On the other hand, pneumatic dilation has a reported perforation rate that averages 3%, an incidence that probably exceeds that sustained by clinicians with substantial experience. Even though these patients do well if the perforation is recognized and addressed promptly, they may require a thoracotomy. Thus, it appears that cogent arguments can be made for each of these therapies and likely one should assess the local skills available, as well a patient preference, in selecting the most appropriate initial therapy.
Treatment Failures
Persistent dysphagia after treatment suggests treatment failure and should be evaluated with some combination of endoscopy, esophageal manometry, and fluoroscopic imaging. Endoscopy may detect esophagitis, stricture, paraesophageal hernia, or anatomic deformity. Manometry may be useful to quantify residual LES pressure, with values exceeding 10 mm Hg arguing for further therapy targeting the LES. Fluoroscopy is useful to identify anatomic problems as well as to evaluate esophageal emptying by using a timed barium swallow, a standardized method of measuring the height of the esophageal barium column one and five minutes after ingestion.328 In some instances these evaluations will lead to further intervention. In the case of a patient not previously operated on this could potentially be either repeat dilation or Heller myotomy. In patients who have already undergone myotomy, detection of an excessively short myotomy or functional esophageal obstruction from the antireflux component of the surgery usually requires reoperation, but pneumatic dilation can be pursued as an alternative. Reoperation, in general, is less effective than an initial operation for any indication in achalasia.329
Occasionally patients fail to respond to optimally performed dilation or myotomy and require alternative approaches. In extremely advanced or refractory cases of achalasia, esophageal resection with gastric pull-up or interposition of a segment of transverse colon or small bowel may be the only surgical option.330 Indications for this intervention include unresolvable obstructive symptoms, starvation, chronic aspiration, cancer, and perforation during dilation. Although excellent long-term functional results can be achieved, the reported mortality rate of this surgery is about 4%, consistent with the mortality rate of esophagectomy done for other indications.
Risk of Squamous Cell Cancer
Numerous series report cases of squamous cell carcinoma developing in the achalasic esophagus (see Chapter 46).331 The relative risk of developing squamous cell cancer has been estimated to be 33-fold relative to the non-achalasic population.332 The pathogenesis of the carcinoma is obscure, but stasis esophagitis is the likely precipitating factor. The tumors develop many years after the diagnosis of achalasia and usually arise in a greatly dilated esophagus, often in the middle third of the esophagus. Symptoms attributable to the cancer can be delayed, and the neoplasms are often large and advanced at the time of detection. These considerations raise the issue of surveillance endoscopy in achalasic individuals to detect early squamous cell cancer. However, an elegant analysis of a database encompassing the entire Swedish population of 1062 achalasic patients suggests that after discounting incident carcinomas, the overall odds ratio of squamous cell cancer for these people compared with age-matched controls was 17, corresponding to a 0.15% incidence of squamous cell cancer among the achalasic subjects.333 The authors calculated that if surveillance endoscopy was done annually, 406 examinations would need to be done in men and 2220 in women before one potentially treatable tumor was found. However, even that calculation is optimistic given that detection of a small cancer in a massively dilated esophagus with retained food and stasis esophagitis is far from ensured. Given these considerations, a surveillance program is currently not the standard of practice.
Distal Esophageal Spasm
Despite the dogma of treatment with smooth muscle relaxants, minimal controlled data exist regarding pharmacologic therapy of DES. Long-term studies are not available, and the entire basis for this therapy is anecdotal. Furthermore, most instances of esophageal chest pain are attributable to reflux rather than DES, and reflux symptoms will likely be made worse by treating with smooth muscle relaxants. Uncontrolled trials of small numbers of DES patients report clinical response to nitrates,334 calcium channel blockers,335 hydralazine,336 botulinum toxin,337 and anxiolytics. The only controlled trial showing efficacy was with the anxiolytic trazodone, suggesting that reassurance and control of anxiety are important therapeutic goals.338 Also consistent with that conclusion, success has been reported using behavioral modification and biofeedback.339
Although the rationale for dilation is unclear, use of bougie dilators has been suggested as a therapy for dysphagia or chest pain in patients with spastic disorders. However, in the only controlled trial of this therapy, dilation with an 8-mm “placebo” dilator was as effective as an 18-mm “therapeutic” dilator in producing transient symptom relief.340 Alternatively, pneumatic dilation has been used in DES patients with severe dysphagia. In one practitioner’s experience, 45% of DES patients noted relief from pneumatic dilation, compared with 80% of achalasic patients.261 In another series of nine patients with DES and LES dysfunction treated with pneumatic dilation, dysphagia but not chest pain was improved during 37 months of observation.341 However, it is not clear that the patients who benefited by pneumatic dilation in these series would not be more properly categorized as spastic achalasia, emphasizing the need for accurate manometric classification.224
If dysphagia becomes so severe in DES that weight loss is observed or if pain becomes unbearable, surgical therapy consisting of a Heller myotomy across the LES with proximal extension of the incision up the distal esophagus to include the involved area of spasm or even esophagectomy should be considered.186,342 However, there are no controlled studies of these procedures in well-defined DES patients and the indication is, fortunately, extremely rare.
Esophageal Hypersensitivity
Pharmacologic Treatments
Numerous neuropeptides and pharmacologic agents can reduce chemical and mechanical visceral sensitivity suggesting a possible role in the treatment of esophageal pain syndromes. Although data on these agents specific to esophageal motor disorders are sparse, there is substantial literature focused on the treatment of noncardiac chest pain with or without motor abnormalities, and it is reasonable to generalize these findings to the treatment of esophageal hypersensitivity (see Chapter 12).
Antidepressants are the most common medications prescribed for visceral pain modulation or chest pain of esophageal origin. Among antidepressants, the tricyclic antidepressants (TCAs) are the best studied. The mechanism of action for this therapeutic benefit is unknown because these agents act centrally as well as peripherally and have multiple receptor targets (acetylcholine, histamine, α-adrenergic). In a randomized placebo-controlled study, imipramine at a dose of 50 mg nightly was shown to be effective in reducing chest pain in patients with normal coronary angiograms.343 Similar results have been reported with other TCAs, and treatment with these agents at doses lower than those used for mood altering effects is common. Typical starting doses for TCAs (amitriptyline, nortriptyline) are 10 to 25 mg at bedtime with escalation of 10- to 25-mg increments to a target of 50 to 75 mg.344 Low-dose trazodone also has been used to treat noncardiac chest pain associated with esophageal dysmotility.338 In a double-blind placebo-controlled study in patients with noncardiac chest pain, the group taking 100 to 150 mg of trazodone had significant symptomatic improvement and less residual distress related to their esophageal symptoms. Esophageal motor function was not altered.
Recent data also support the effectiveness of selective serotonin reuptake inhibitors (SSRIs) in the treatment of esophageal hypersensitivity. Intravenous citalopram at a dose of 20 mg was studied in a randomized, double-blinded, crossover study and found to significantly reduce both chemical (acid perfusion) and mechanical (balloon distention) esophageal sensitivity.345 Although clinical trials are not yet available, mechanistic studies assessing other SSRIs have also yielded encouraging results. Along similar lines, there has been a substantial interest in developing serotonin (5-HT) medications.293,346 5-HT3 antagonists and 5-HT4 agonists have been the most extensively studied given their effects on gut motility and as treatments for nausea. Unfortunately, several of these medications have proven to have unacceptable risks related to cardiac dysrhythmias or gut ischemia that led to their withdrawal.
Theophylline has shown promising effects in the treatment of noncardiac chest pain, presumably by adenosine receptor blockade. In a recent placebo-controlled double-blind study, sensory and biomechanical properties of the esophagus were assessed using impedance planimetry in 16 patients with esophageal hypersensitivity.347 Chest pain thresholds increased after intravenous theophylline and the esophageal wall was shown to relax and become more distensible. In a parallel study using oral theophylline and placebo in 24 chest pain patients there was a significant reduction in chest pain episodes, chest pain duration, and chest pain severity in the theophylline group.347 Although limited, these are very promising results for patients with symptoms thought to be attributable to mechanical hypersensitivity.
Behar J, Biancani P. Pathogenesis of simultaneous esophageal contractions in patients with motility disorders. Gastroenterology. 1993;105:111-18. (Ref 251.)
Cook IJ, Kahrilas PJ. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology. 1999;116:455-78. (Ref 299.)
Kahrilas PJ, Clouse RE, Hogan WJ. American Gastroenterological Association technical review on the clinical use of esophageal manometry [comment]. Gastroenterology.. 1994;107:1865-84. (Ref 197.)
Logemann J. Evaluation and treatment of swallowing disorders. Austin, Tex: Pro-Ed Inc; 1998. (Ref 17.)
Massey BT, Dodds WJ, Hogan WJ, et al. Abnormal esophageal motility. An analysis of concurrent radiographic and manometric findings. Gastroenterology. 1991;101:344-54. (Ref 258.)
Ott DJ, Richter JE, Chen YM, et al. Esophageal radiography and manometry: Correlation in 172 patients with dysphagia. AJR Am J Roentgenol. 1987;149:307-11. (Ref 282.)
Pandolfino JE, Ghosh SK, Rice J, et al. Classifying esophageal motility by pressure topography characteristics: A study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103:27-37. (Ref 285.)
Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: A new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526-33. (Ref 224.)
Sifrim D, Janssens J, Vantrappen G. A wave of inhibition precedes primary peristaltic contractions in the human esophagus. Gastroenterology. 1992;103:876-82. (Ref 46.)
Vaezi MF, Richter JE, Wilcox CM, et al. Botulinum toxin versus pneumatic dilatation in the treatment of achalasia: A randomised trial. Gut. 1999;44:231-9. (Ref 312.)
Vela MF, Richter JE, Wachsberger D, et al. Complexities of managing achalasia at a tertiary referral center: Use of pneumatic dilatation, Heller myotomy, and botulinum toxin injection. Am J Gastroenterol. 2004;99:1029-36. (Ref 329.)
1. Weisbrodt NW. Neuromuscular organization of esophageal and pharyngeal motility. Arch Intern Med. 1976;136:524-31.
2. Roman C, Gonnella J. Extrinsic control of digestive tract motility. In: Johnson L, editor. Physiology of the gastrointestinal tract. 2nd ed. New York: Raven Press; 1987:507-553.
3. Doty RW, Richmond WH, Storey AT. Neural organization of deglutition. In: Code CF, editor. Handbook of Physiology, Section 6: Alimentary Canal, Vol IV Motility. sec ed. Washington DC: American Physiological Society; 1968:1861-1902.
4. Kahrilas PJ, Dodds WJ, Dent J, et al. Upper esophageal sphincter function during belching. Gastroenterology. 1986;91:133-40.
5. Welch RW, Gates GA, Luckmann KF, et al. Change in the force-summed pressure measurements of the upper esophageal sphincter prelaryngectomy and postlaryngectomy. Ann Otol Rhinol Laryngol. 1979;88:804-8.
6. Yoshida Y, Miyazaki T, Hirano M, et al. Localization of efferent neurons innervating the pharyngeal constrictor muscles and the cervical esophagus muscle in the cat by means of the horseradish peroxidase method. Neurosci Lett. 1981;22:91-5.
7. Asoh R, Goyal RK. Manometry and electromyography of the upper esophageal sphincter in the opossum. Gastroenterology. 1978;74:514-20.
8. Pandolfino JE, Kahrilas PJ. AGA technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128:209-24.
9. Winans CS. The pharyngoesophageal closure mechanism: A manometric study. Gastroenterology. 1972;63:768-77.
10. Kwiatek MA, Mirza F, Kahrilas PJ, Pandolfino JE. Hyperdynamic upper esophageal sphincter pressure: A manometric observation in patients reporting globus sensation. Am J Gastroenterol. 2009;104:289-98.
11. Kahrilas PJ, Dodds WJ, Hogan WJ. Dysfunction of the belch reflex. A cause of incapacitating chest pain. Gastroenterology. 1987;93:818-22.
12. Shaker R, Dodds WJ, Ren J, et al. Esophagoglottal closure reflex: A mechanism of airway protection. Gastroenterology. 1992;102:857-61.
13. Cook IJ, Dent J, Shannon S, Collins SM. Measurement of upper esophageal sphincter pressure. Effect of acute emotional stress. Gastroenterology. 1987;93:526-32.
14. Vanner RG, Pryle BJ, O’Dwyer JP, Reynolds F. Upper oesophageal sphincter pressure during inhalational anaesthesia. Anaesthesia. 1992;47:950-4.
15. Kahrilas PJ, Dodds WJ, Dent J, et al. Effect of sleep, spontaneous gastroesophageal reflux, and a meal on upper esophageal sphincter pressure in normal human volunteers. Gastroenterology. 1987;92:466-71.
16. Vakil NB, Kahrilas PJ, Dodds WJ, Vanagunas A. Absence of an upper esophageal sphincter response to acid reflux. Am J Gastroenterol. 1989;84:606-10.
17. Logemann J. Evaluation and treatment of swallowing disorders. Austin, Tex: Pro-Ed Inc; 1998.
18. Perlman AL, Booth BM, Grayhack JP. Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia. 1994;9:90-5.
19. Lear CS, Flanagan JBJr., Moorrees CF. The frequency of deglutition in man. Arch Oral Biol. 1965;10:83-100.
20. Sinclair WJ. Role of the pharyngeal plexus in initiation of swallowing. Am J Physiol. 1971;221:1260-3.
21. Miller AJ. The search for the central swallowing pathway: the quest for clarity. Dysphagia. 1993;8:185-94.
22. Lin S, Chen J, Hertz P, Kahrilas PJ. Dynamic reconstruction of the oropharyngeal swallow using computer based animation. Comput Med Imaging Graph. 1996;20:69-75.
23. Cook IJ, Dodds WJ, Dantas RO, et al. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol. 1989;257:G748-59.
24. Jacob P, Kahrilas PJ, Logemann JA, et al. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97:1469-78.
25. Kahrilas PJ, Dodds WJ, Dent J, et al. Upper esophageal sphincter function during deglutition. Gastroenterology. 1988;95:52-62.
26. Wein B, Bockler R, Klajman S. Temporal reconstruction of sonographic imaging of disturbed tongue movements. Dysphagia. 1991;6:135-9.
27. Ekberg O, Olsson R, Sundgren-Borgstrom P. Relation of bolus size and pharyngeal swallow. Dysphagia. 1988;3:69-72.
28. Code CF, Schlegel JF. Motor action of the esophagus and its sphincters. In: Code CF, editor. Handbook of Physiology, Section 6: Alimentary Caual, Vol IV: Motility. sec ed. Washington DC: American Physiological Society; 1968:1811-1820.
29. Swick HM, Werlin SL, Dodds WJ, Hogan WJ. Pharyngoesophageal motor function in patients with myotonic dystrophy. Ann Neurol. 1981;10:454-7.
30. Meyer GW, Austin RM, Brady CE3rd, Castell DO. Muscle anatomy of the human esophagus. J Clin Gastroenterol. 1986;8:131-4.
31. Christensen J. Motor functions of the pharynx and esophagus. In: Johnson LR, editor. Physiology of the gastrointestinal tract. New York: Raven Press; 1987:595-612.
32. Gilbert RJ, Gaige TA, Wang R, et al. Resolving the three-dimensional myoarchitecture of bovine esophageal wall with diffusion spectrum imaging and tractography. Cell Tissue Res. 2008;332:461-8.
33. Higgs B, Kerr FW, Ellis FHJr. The experimental production of esophageal achalasia by electrolytic lesions in the medulla. J Thorac Cardiovasc Surg. 1965;50:613-25.
34. Gonella J, Niel JP, Roman C. Mechanism of the noradrenergic motor control on the lower oesophageal sphincter in the cat. J Physiol. 1980;306:251-60.
35. Hwang K, Grossman MI, Ivy AC. Nervous control of the cervical esophagus. Am J Physiol. 1948;154:343-57.
36. Floyd K. Cholinesterase activity in sheep esophageal muscle. J Anat (Lond). 1973;116:357-73.
37. Samarasinghe DD. Some observations on the innervation of the striated muscle in the mouse esophagus. J Anat (Lond). 1972;112:173-84.
38. Abe S. On the histology and the innervation of the esophagus and the first forestomach of the goat. Arch Histol Jpn. 1959;16:109-29.
39. Kuntz A. The Autonomic Nervous System, 3rd ed. Philadelphia, Pa: Lea & Febiger; 1945.
40. Christensen J, Robison BA. Anatomy of the myenteric plexus of the opossum esophagus. Gastroenterology. 1982;83:1033-42.
41. Christensen J, Rick GA, Robison BA, et al. Arrangement of the myenteric plexus throughout the gastrointestinal tract of the opossum. Gastroenterology. 1983;85:890-9.
42. Schoeman MN, Holloway RH. Secondary oesophageal peristalsis in patients with non-obstructive dysphagia. Gut. 1994;35:1523-8.
43. Kahrilas PJ, Dodds WJ, Hogan WJ. Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology. 1988;94:73-80.
44. Hellemans J, Vantrappen G, Valembois P, et al. Electrical activity of striated and smooth muscle of the esophagus. Am J Dig Dis. 1968;13:320-34.
45. Meyer GW, Castell DO. Current concepts of esophageal function. Am J Otolaryngol. 1980;1:440-6.
46. Sifrim D, Janssens J, Vantrappen G. A wave of inhibition precedes primary peristaltic contractions in the human esophagus. Gastroenterology. 1992;103:876-82.
47. Janssens J, De Wever I, Vantrappen G, Hellemans J. Peristalsis in smooth muscle esophagus after transection and bolus deviation. Gastroenterology. 1976;71:1004-9.
48. Diamant NE, El-Sharkawy TY. Neural control of esophageal peristalsis. A conceptual analysis. Gastroenterology. 1977;72:546-56.
49. Gonella J, Niel JP, Roman C. Vagal control of lower oesophageal sphincter motility in the cat. J Physiol. 1977;273:647-64.
50. Sugarbaker DJ, Rattan S, Goyal RK. Mechanical and electrical activity of esophageal smooth muscle during peristalsis. Am J Physiol. 1984;246:G145-50.
51. Gidda JS, Goyal RK. Swallow-evoked action potentials in vagal preganglionic efferents. J Neurophysiol. 1984;52:1169-80.
52. Christensen J, Lund GF. Esophageal responses to distension and electrical stimulation. J Clin Invest. 1969;48:408-19.
53. Dodds WJ, Dent J, Hogan WJ, Arndorfer RC. Effect of atropine on esophageal motor function in humans. Am J Physiol. 1981;240:G290-6.
54. Paterson WG, Hynna-Liepert TT, Selucky M. Comparison of primary and secondary esophageal peristalsis in humans: effect of atropine. Am J Physiol. 1991;260:G52-7.
55. Weisbrodt NW, Christensen J. Gradients of contractions in the opossum esophagus. Gastroenterology. 1972;62:1159-66.
56. Crist J, Surprenant A, Goyal RK. Intracellular studies of electrical membrane properties of opossum esophageal circular smooth muscle. Gastroenterology. 1987;92:987-92.
57. Gidda JS, Cobb BW, Goyal RK. Modulation of esophageal peristalsis by vagal efferent stimulation in opossum. J Clin Invest. 1981;68:1411-19.
58. Staiano A, Clouse RE. The effects of cisapride on the topography of oesophageal peristalsis. Aliment Pharmacol Ther. 1996;10:875-82.
59. Fox M, Menne D, Stutz B, et al. The effects of tegaserod on oesophageal function and bolus transport in healthy volunteers: Studies using concurrent high-resolution manometry and videofluoroscopy. Aliment Pharmacol Ther. 2006;24:1017-27.
60. Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768-70.
61. Fang S, Christensen J. Distribution of NADPH diaphorase in intramural plexuses of cat and opossum esophagus. J Auton Nerv Syst. 1994;46:123-33.
62. Murray J, Du C, Ledlow A, et al. Nitric oxide: mediator of nonadrenergic noncholinergic responses of opossum esophageal muscle. Am J Physiol. 1991;261:G401-6.
63. Yamashita Y, Kako N, Homma K, et al. Neuropeptide release from the isolated, perfused, lower esophageal sphincter region of the rabbit and the effect of vasoactive intestinal peptide on the sphincter. Surgery. 1992;112:227-33.
64. Uddman R, Alumets J, Edvinsson L, et al. Peptidergic (VIP) innervation of the esophagus. Gastroenterology. 1978;75:5-8.
65. Goyal RK, Rattan S, Said SI. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurones. Nature. 1980;288:378-80.
66. Burgess JN, Schlegel JF, Ellis FHJr. The effect of denervation of feline esophageal function and morphology. J Surg Res. 1972;12:24-33.
67. Greenwood RK, Schlegel JF, Code CF, Ellis FHJr. The effect of sympathectomy, vagotomy and esophageal interruption on the canine gastroesophageal sphincter. Thorax. 1962;17:310-19.
68. Ryan JP, Snape WJJr, Cohen S. Influence of vagal cooling on esophageal function. Am J Physiol. 1977;232:E159-64.
69. Reynolds JC, Ouyang A, Cohen S. A lower esophageal sphincter reflex involving substance P. Am J Physiol. 1984;246:G346-54.
70. Kravitz JJ, Snape WJJr, Cohen S. Effect of thoracic vagotomy and vagal stimulation on esophageal function. Am J Physiol. 1966;238:233-8.
71. Brasseur JG. Mechanical studies of the esophageal function. Dysphagia. 1993;8:384-6.
72. Ghosh SK, Janiak P, Schwizer W, et al. Physiology of the esophageal pressure transition zone: Separate contraction waves above and below. Am J Physiol Gastrointest Liver Physiol. 2006;290:G568-76.
73. Clouse RE, Staiano A. Topography of normal and high-amplitude esophageal peristalsis. Am J Physiol. 1993;265:G1098-107.
74. Edmundowicz SA, Clouse RE. Shortening of the esophagus in response to swallowing. Am J Physiol. 1991;260:G512-16.
75. Pouderoux P, Lin S, Kahrilas PJ. Timing, propagation, coordination, and effect of esophageal shortening during peristalsis. Gastroenterology. 1997;112:1147-54.
76. Sugarbaker DJ, Rattan S, Goyal RK. Swallowing induces sequential activation of esophageal longitudinal smooth muscle. Am J Physiol. 1984;247:G515-19.
77. Kahrilas PJ. Anatomy and physiology of the gastroesophageal junction. Gastroenterol Clin North Am. 1997;26:467-86.
78. Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med. 1997;336:924-32.
79. Liebermann-Meffert D, Allgower M, Schmid P, Blum AL. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979;76:31-8.
80. Brasseur JG, Ulerich R, Dai Q, et al. Pharmacological dissection of the human gastro-oesophageal segment into three sphincteric components. J Physiol. 2007;580:961-75.
81. Thor KB, Hill LD, Mercer DD, Kozarek RD. Reappraisal of the flap valve mechanism in the gastroesophageal junction. A study of a new valvuloplasty procedure in cadavers. Acta Chir Scand. 1987;153:25-8.
82. Hill LD, Kozarek RA, Kraemer SJ, et al. The gastroesophageal flap valve: In vitro and in vivo observations. Gastrointest Endosc. 1996;44:541-7.
83. Kahrilas PJ, Lin S, Chen J, Manka M. The effect of hiatus hernia on gastro-oesophageal junction pressure. Gut. 1999;44:476-82.
84. Liu J, Parashar VK, Mittal RK. Asymmetry of lower esophageal sphincter pressure: Is it related to the muscle thickness or its shape? Am J Physiol. 1997;272:G1509-17.
85. Goyal RK, Rattan S. Genesis of basal sphincter pressure: Effect of tetrodotoxin on lower esophageal sphincter pressure in opossum in vivo. Gastroenterology. 1976;71:62-7.
86. Papasova M, Milousheva E, Bonev A, et al. On the changes in the membrane potential and the contractile activity of the smooth muscle of the lower esophageal and ileo-caecal sphincters upon increased K in the nutrient solution. Acta Physiol Pharmacol Bulg. 1980;6:41-9.
87. Zelcer E, Weisbrodt NW. Electrical and mechanical activity in the lower esophageal sphincter of the cat. Am J Physiol. 1984;246:G243-7.
88. Zhang Y, Miller DV, Paterson WG. Opposing roles of K(+) and Cl(−) channels in maintenance of opossum lower esophageal sphincter tone. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1226-34.
89. Biancani P, Harnett KM, Sohn UD, et al. Differential signal transduction pathways in cat lower esophageal sphincter tone and response to ACh. Am J Physiol. 1994;266:G767-74.
90. Hillemeier C, Bitar KN, Sohn U, Biancani P. Protein kinase C mediates spontaneous tone in the cat lower esophageal sphincter. J Pharmacol Exp Ther. 1996;277:144-9.
91. Dent J, Dodds WJ, Friedman RH, et al. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65:256-67.
92. Miolan JP, Roman C. Discharge of efferent vagal fibers supplying gastric antrum: Indirect study by nerve suture technique. Am J Physiol. 1978;235:E366-73.
93. Dodds WJ, Christensen J, Dent J, et al. Esophageal contractions induced by vagal stimulation in the opossum. Am J Physiol. 1978;235:E392-401.
94. Goyal RK, Rattan S. Nature of the vagal inhibitory innervation to the lower esophageal sphincter. J Clin Invest. 1975;55:1119-26.
95. Gonella J, Niel JP, Roman C. Sympathetic control of lower oesophageal sphincter motility in the cat. J Physiol. 1979;287:177-90.
96. Klein WA, Parkman HP, Dempsey DT, Fisher RS. Sphincterlike thoracoabdominal high pressure zone after esophagogastrectomy. Gastroenterology. 1993;105:1362-9.
97. Mittal RK, Rochester DF, McCallum RW. Sphincteric action of the diaphragm during a relaxed lower esophageal sphincter in humans. Am J Physiol. 1989;256:G139-44.
98. Mittal RK, Fisher M, McCallum RW, et al. Human lower esophageal sphincter pressure response to increased intra-abdominal pressure. Am J Physiol. 1990;258:G624-30.
99. De Troyer A, Sampson M, Sigrist S, Macklem PT. Action of costal and crural parts of the diaphragm on the rib cage in dog. J Appl Physiol. 1982;53:30-9.
100. Altschuler SM, Boyle JT, Nixon TE, et al. Simultaneous reflex inhibition of lower esophageal sphincter and crural diaphragm in cats. Am J Physiol. 1985;249:G586-91.
101. Goyal RK, Rattan S. Mechanism of the lower esophageal sphincter relaxation. Action of prostaglandin E1 and theophylline. J Clin Invest. 1973;52:337-41.
102. Christensen J. Pharmacology of the esophageal motor function. Annu Rev Pharmacol. 1975;15:243-58.
103. Schulze-Delrieu K, Percy WH, Ren J, et al. Evidence for inhibition of opossum LES through intrinsic gastric nerves. Am J Physiol. 1989;256:G198-205.
104. Paterson WG, Rattan S, Goyal RK. Esophageal responses to transient and sustained esophageal distension. Am J Physiol. 1988;255:G587-95.
105. Murthy KS, Zhang KM, Jin JG, et al. VIP-mediated G protein-coupled Ca2+ influx activates a constitutive NOS in dispersed gastric muscle cells. Am J Physiol. 1993;265:G660-71.
106. Nathan C, Xie QW. Nitric oxide synthases: Roles, tolls, and controls. Cell. 1994;78:915-18.
107. Furness JB, Bornstein JC, Murphy R, Pompolo S. Roles of peptides in transmission in the enteric nervous system. Trends Neurosci. 1992;15:66-71.
108. Murray J, Bates JN, Conklin JL. Nerve-mediated nitric oxide production by opossum lower esophageal sphincter. Dig Dis Sci. 1994;39:1872-6.
109. Murray JA, Clark ED. Characterization of nitric oxide synthase in the opossum esophagus. Gastroenterology. 1994;106:1444-50.
110. Berezin I, Allescher HD, Daniel EE. Ultrastructural localization of VIP-immunoreactivity in canine distal oesophagus. J Neurocytol. 1987;16:749-57.
111. Wattchow DA, Furness JB, Costa M, et al. Distributions of neuropeptides in the human esophagus. Gastroenterology. 1987;93:1363-71.
112. Aggestrup S, Uddman R, Sundler F, et al. Lack of vasoactive intestinal polypeptide nerves in esophageal achalasia. Gastroenterology. 1983;84:924-7.
113. Rattan S, Said SI, Goyal RK. Effect of vasoactive intestinal polypeptide. Proc Soc Exp Biol Med. 1977;155:40-3.
114. Biancani P, Beinfeld MC, Hillemeier C, Behar J. Role of peptide histidine isoleucine in relaxation of cat lower esophageal sphincter. Gastroenterology. 1989;97:1083-9.
115. Teng B, Murthy KS, Kuemmerle JF, et al. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol. 1998;275:G342-51.
116. Murthy KS, Grider JR, Jin JG, Makhlouf GM. Interplay of VIP and nitric oxide in the regulation of neuromuscular activity in the gut. Arch Int Pharmacodyn Ther. 1995;329:27-38.
117. Jin JG, Murthy KS, Grider JR, Makhlouf GM. Stoichiometry of neurally induced VIP release, NO formation, and relaxation in rabbit and rat gastric muscle. Am J Physiol. 1996;271:G357-69.
118. Murthy KS, Grider JR, Jin JG, Makhlouf GM. Interplay of VIP and nitric oxide in the regulation of neuromuscular function in the gut. Ann N Y Acad Sci. 1996;805:355-62.
119. Mashimo H, He XD, Huang PL, Fishman MC, Goyal RK. Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. J Clin Invest. 1996;98:8-13.
120. Rattan S, Gonnella P, Goyal RK. Inhibitory effect of calcitonin gene-related peptide and calcitonin on opossum esophageal smooth muscle. Gastroenterology. 1988;94:284-93.
121. Uc A, Murray JA, Conklin JL. Effects of calcitonin gene-related peptide on opossum esophageal smooth muscle. Gastroenterology. 1997;113:514-20.
122. Lundberg JM, Fahrenkrug J, Larsson O, Anggard A. Corelease of vasoactive intestinal polypeptide and peptide histidine isoleucine in relation to atropine-resistant vasodilation in cat submandibular salivary gland. Neurosci Lett. 1984;52:37-42.
123. Ny L, Alm P, Larsson B, et al. Nitric oxide pathway in cat esophagus: Localization of nitric oxide synthase and functional effects. Am J Physiol. 1995;268:G59-70.
124. Ny L, Alm P, Ekstrom P, et al. Nitric oxide synthase-containing, peptide-containing, and acetylcholinesterase-positive nerves in the cat lower oesophagus. Histochem J. 1994;26:721-33.
125. Singaram C, Sengupta A, Sweet MA, et al. Nitrinergic and peptidergic innervation of the human oesophagus. Gut. 1994;35:1690-6.
126. Holloway RH, Penagini R, Ireland AC. Criteria for objective definition of transient lower esophageal sphincter relaxation. Am J Physiol. 1995;268:G128-33.
127. Mittal RK, Holloway RH, Penagini R, et al. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601-10.
128. Pandolfino JE, Zhang QG, Ghosh SK, et al. Transient lower esophageal sphincter relaxations and reflux: mechanistic analysis using concurrent fluoroscopy and high-resolution manometry. Gastroenterology. 2006;131:1725-33.
129. McNally EF, Kelly JEJr, Ingelfinger FJ. Mechanism of belching: Effects of gastric distension with air. Gastroenterology. 1964;46:254-9.
130. Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985;238:473-88.
131. Altschuler SM, Bao XM, Bieger D, et al. Viscerotopic representation of the upper alimentary tract in the rat: Sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248-68.
132. Jean A. Brainstem organization of the swallowing network. Brain Behav Evol. 1984;25:109-16.
133. Kessler JP, Jean A. Identification of the medullary swallowing regions in the rat. Exp Brain Res. 1985;57:256-63.
134. Jean A. Control of the central swallowing program by inputs from the peripheral receptors. A review. J Auton Nerv Syst. 1984;10:225-33.
135. Bieger D. The brainstem esophagomotor network pattern generator: A rodent model. Dysphagia. 1993;8:203-8.
136. Sang Q, Goyal RK. Lower esophageal sphincter relaxation and activation of medullary neurons by subdiaphragmatic vagal stimulation in the mouse. Gastroenterology. 2000;119:1600-9.
137. Martin CJ, Patrikios J, Dent J. Abolition of gas reflux and transient lower esophageal sphincter relaxation by vagal blockade in the dog. Gastroenterology. 1986;91:890-6.
138. Hirsch DP, Holloway RH, Tytgat GN, Boeckxstaens GE. Involvement of nitric oxide in human transient lower esophageal sphincter relaxations and esophageal primary peristalsis. Gastroenterology. 1998;115:1374-80.
139. Boulant J, Mathieu S, D’Amato M, et al. Cholecystokinin in transient lower oesophageal sphincter relaxation due to gastric distension in humans. Gut. 1997;40:575-81.
140. Zerbib F, Bruley Des Varannes S, Scarpignato C, et al. Endogenous cholecystokinin in postprandial lower esophageal sphincter function and fundic tone in humans. Am J Physiol. 1998;275:G1266-73.
141. Boeckxstaens GE, Hirsch DP, Fakhry N, et al. Involvement of cholecystokinin A receptors in transient lower esophageal sphincter relaxations triggered by gastric distension. Am J Gastroenterol. 1998;93:1823-8.
142. Ledeboer M, Masclee AA, Biemond I, Lamers CB. Effect of medium- and long-chain triglycerides on lower esophageal sphincter pressure: role of CCK. Am J Physiol. 1998;274:G1160-5.
143. Holloway RH, Lyrenas E, Ireland A, Dent J. Effect of intraduodenal fat on lower oesophageal sphincter function and gastro-oesophageal reflux. Gut. 1997;40:449-53.
144. Mittal RK, Holloway R, Dent J. Effect of atropine on the frequency of reflux and transient lower esophageal sphincter relaxation in normal subjects. Gastroenterology. 1995;109:1547-54.
145. Mittal RK, Chiareli C, Liu J, et al. Atropine inhibits gastric distension and pharyngeal receptor mediated lower oesophageal sphincter relaxation. Gut. 1997;41:285-90.
146. Lidums I, Checklin H, Mittal RK, Holloway RH. Effect of atropine on gastro-oesophageal reflux and transient lower oesophageal sphincter relaxations in patients with gastro-oesophageal reflux disease. Gut. 1998;43:12-16.
147. Lidums I, Hebbard GS, Holloway RH. Effect of atropine on proximal gastric motor and sensory function in normal subjects. Gut. 2000;47:30-6.
148. Blackshaw LA, Staunton E, Lehmann A, Dent J. Inhibition of transient LES relaxations and reflux in ferrets by GABA receptor agonists. Am J Physiol. 1999;277:G867-74.
149. Lehmann A, Antonsson M, Bremner-Danielsen M, et al. Activation of the GABA(B) receptor inhibits transient lower esophageal sphincter relaxations in dogs. Gastroenterology. 1999;117:1147-54.
150. Lidums I, Lehmann A, Checklin H, et al. Control of transient lower esophageal sphincter relaxations and reflux by the GABA(B) agonist baclofen in normal subjects. Gastroenterology. 2000;118:7-13.
151. Hornby PJ, Abrahams TP. Central control of lower esophageal sphincter relaxation. Am J Med. 2000;108(Suppl 4a):90S-8.
152. Poorkhalkali N, Rich HG, Jacobson I, et al. Chronic oesophagitis in the cat. Scand J Gastroenterol. 2001;36:904-9.
153. Frisby CL, Mattsson JP, Jensen JM, et al. Inhibition of transient lower esophageal sphincter relaxation and gastroesophageal reflux by metabotropic glutamate receptor ligands. Gastroenterology. 2005;129:995-1004.
154. Drewes AM, Schipper KP, Dimcevski G, et al. Multimodal assessment of pain in the esophagus: A new experimental model. Am J Physiol Gastrointest Liver Physiol. 2002;283:G95-103.
155. Drewes AM, Schipper KP, Dimcevski G, et al. Multi-modal induction and assessment of allodynia and hyperalgesia in the human oesophagus. Eur J Pain. 2003;7:539-49.
156. Khurana RK, Petras JM. Sensory innervation of the canine esophagus, stomach, and duodenum. Am J Anat. 1991;192:293-306.
157. Collman PI, Tremblay L, Diamant NE. The distribution of spinal and vagal sensory neurons that innervate the esophagus of the cat. Gastroenterology. 1992;103:817-22.
158. Sarkar S, Aziz Q, Woolf CJ, et al. Contribution of central sensitisation to the development of non-cardiac chest pain. Lancet. 2000;356:1154-9.
159. Sarkar S, Hobson AR, Furlong PL, et al. Central neural mechanisms mediating human visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1196-202.
160. Qin C, Chandler MJ, Foreman RD. Esophagocardiac convergence onto thoracic spinal neurons: Comparison of cervical and thoracic esophagus. Brain Res. 2004;1008:193-7.
161. Neuhuber WL. Sensory vagal innervation of the rat esophagus and cardia: A light and electron microscopic anterograde tracing study. J Auton Nerv Syst. 1987;20:243-55.
162. Rodrigo J, Hernandez CJ, Pedrosa JA, et al. Ganglionic cytoarchitecture of the esophagus in the cat and the rhesus monkey. Bull Assoc Anat (Nancy). 1975;59:985-94.
163. Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol. 2003;553:575-87.
164. Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: Morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261-76.
165. Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol. 2000;421:302-24.
166. Fox EA, Phillips RJ, Martinson FA, et al. C-Kit mutant mice have a selective loss of vagal intramuscular mechanoreceptors in the forestomach. Anat Embryol (Berl). 2001;204:11-26.
167. Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1-26.
168. Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol. 1998;512:907-16.
169. Blackshaw LA, Page AJ, Partosoedarso ER. Acute effects of capsaicin on gastrointestinal vagal afferents. Neuroscience. 2000;96:407-16.
170. Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol. 2005;563:831-42.
171. Castelucci P, Robbins HL, Furness JB. P2X(2) purine receptor immunoreactivity of intraganglionic laminar endings in the mouse gastrointestinal tract. Cell Tissue Res. 2003;312:167-74.
172. Wang ZJ, Neuhuber WL. Intraganglionic laminar endings in the rat esophagus contain purinergic P2X2 and P2X3 receptor immunoreactivity. Anat Embryol (Berl). 2003;207:363-71.
173. Patterson LM, Zheng H, Ward SM, Berthoud HR. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 2003;311:277-87.
174. Ward SM, Bayguinov J, Won KJ, et al. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465:121-35.
175. Banerjee B, Medda BK, Lazarova Z, et al. Effect of reflux-induced inflammation on transient receptor potential vanilloid one (TRPV1) expression in primary sensory neurons innervating the oesophagus of rats. Neurogastroenterol Motil. 2007;19:681-91.
176. Page AJ, Brierley SM, Martin CM, et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408-15.
177. De Petrocellis L, Chu CJ, Moriello AS, et al. Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels. Br J Pharmacol. 2004;143:251-6.
178. De Petrocellis L, Di Marzo V. Lipids as regulators of the activity of transient receptor potential type V1 (TRPV1) channels. Life Sci. 2005;77:1651-66.
179. Lehmann A. Novel treatments of GERD: Focus on the lower esophageal sphincter. Eur Rev Med Pharmacol Sci. 2008;12:103-10.
180. Page AJ, Blackshaw LA. GABA(B) receptors inhibit mechanosensitivity of primary afferent endings. J Neurosci. 1999;19:8597-602.
181. Bloem BR, Lagaay AM, van Beek W, et al. Prevalence of subjective dysphagia in community residents aged over 87. BMJ. 1990;300:721-2.
182. Lindgren S, Janzon L. Prevalence of swallowing complaints and clinical findings among 50- to 79-year-old men and women in an urban population. Dysphagia. 1991;6:187-92.
183. Siebens H, Trupe E, Siebens A, et al. Correlates and consequences of eating dependency in institutionalized elderly. J Am Geriatr Soc. 1986;34:192-8.
184. Ferreira LE, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus. 2008;21:1-8.
185. Croghan JE, Burke EM, Caplan S, Denman S. Pilot study of 12-month outcomes of nursing home patients with aspiration on videoflouroscopy. Dysphagia. 1994;9:141-6.
186. Ellis FHJr. Esophagomyotomy for noncardiac chest pain resulting from diffuse esophageal spasm and related disorders. Am J Med. 1992;92:129S-31.
187. Howard PJ, Maher L, Pryde A, et al. Five year prospective study of the incidence, clinical features, and diagnosis of achalasia in Edinburgh. Gut. 1992;33:1011-15.
188. Kahrilas PJ, Kishk SM, Helm JF, et al. Comparison of pseudoachalasia and achalasia. Am J Med. 1987;82:439-46.
189. Mayberry JF. Epidemiology and demographics of achalasia. Gastrointest Endosc Clin North Am. 2001;11:235-48. v
190. Stein DT, Knauer CM. Achalasia in monozygotic twins. Dig Dis Sci. 1982;27:636-40.
191. Bosher LP, Shaw A. Achalasia in siblings. Clinical and genetic aspects. Am J Dis Child. 1981;135:709-10.
192. Frieling T, Berges W, Borchard F, et al. Family occurrence of achalasia and diffuse spasm of the oesophagus. Gut. 1988;29:1595-602.
193. Eckrich JD, Winans CS. Discordance for achalasia in identical twins. Dig Dis Sci. 1979;24:221-4.
194. Mayberry JF, Atkinson M. A study of swallowing difficulties in first degree relatives of patients with achalasia. Thorax. 1985;40:391-3.
195. Stuckey BG, Mastaglia FL, Reed WD, Pullan PT. Glucocorticoid insufficiency, achalasia, alacrima with autonomic motor neuropathy. Ann Intern Med. 1987;106:61-3.
196. Traube M, Albibi R, McCallum RW. High-amplitude peristaltic esophageal contractions associated with chest pain. Jama. 1983;250:2655-9.
197. Kahrilas PJ, Clouse RE, Hogan WJ. American Gastroenterological Association technical review on the clinical use of esophageal manometry. Gastroenterology. 1994;107:1865-84.
198. Ferguson SC, Hodges K, Hersh T, Jinich H. Esophageal manometry in patients with chest pain and normal coronary arteriogram. Am J Gastroenterol. 1981;75:124-7.
199. Benjamin SB, Richter JE, Cordova CM, et al. Prospective manometric evaluation with pharmacologic provocation of patients with suspected esophageal motility dysfunction. Gastroenterology. 1983;84:893-901.
200. Katz PO, Dalton CB, Richter JE, et al. Esophageal testing of patients with noncardiac chest pain or dysphagia. Results of three years’ experience with 1161 patients. Ann Intern Med. 1987;106:593-7.
201. Bancewicz J, Osugi H, Marples M. Clinical implications of abnormal oesophageal motility. Br J Surg. 1987;74:416-19.
202. Breumelhof R, Nadorp JH, Akkermans LM, Smout AJ. Analysis of 24-hour esophageal pressure and pH data in unselected patients with noncardiac chest pain. Gastroenterology. 1990;99:1257-64.
203. Hewson EG, Sinclair JW, Dalton CB, Richter JE. Twenty-four-hour esophageal pH monitoring: the most useful test for evaluating noncardiac chest pain. Am J Med. 1991;90:576-83.
204. Clouse RE, Staiano A. Manometric patterns using esophageal body and lower sphincter characteristics. Findings in 1013 patients. Dig Dis Sci. 1992;37:289-96.
205. Bassotti G, Pelli MA, Morelli A. Clinical and manometric aspects of diffuse esophageal spasm in a cohort of subjects evaluated for dysphagia and/or chest pain. Am J Med Sci. 1990;300:148-51.
206. Davies AE, Kidd D, Stone SP, MacMahon J. Pharyngeal sensation and gag reflex in healthy subjects. Lancet. 1995;345:487-8.
207. Scmidt EV, Smirnov VE, Ryabova VS. Results of the seven-year prospective study of stroke patients. Stroke. 1988;19:942-9.
208. Gresham SL. Clinical assessment and management of swallowing difficulties after stroke. Med J Aust. 1990;153:397-9.
209. Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. BMJ (Clin Res Ed). 1987;295:411-14.
210. Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology. 1998;114:559-78.
211. Dalakas MC, Elder G, Hallett M, et al. A long-term follow-up study of patients with post-poliomyelitis neuromuscular symptoms. N Engl J Med. 1986;314:959-63.
212. Bosma JF. Studies of disability of the pharynx resultant from poliomyelitis. Ann Otol Rhinol Laryngol. 1953;62:529-47.
213. Buchholz D. Dysphagia in post-polio patients. Birth Defects Orig Artic Ser. 1987;23:55-62.
214. Mackay R. Course and prognosis of ALS. Arch Neurol. 1963;81:117.
215. Ali GN, Wallace KL, Schwartz R, et al. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology. 1996;110:383-92.
216. Newton HB, Newton C, Pearl D, Davidson T. Swallowing assessment in primary brain tumor patients with dysphagia. Neurology. 1994;44:1927-32.
217. Kahrilas PJ, Logemann JA, Gibbons P. Food intake by maneuver; an extreme compensation for impaired swallowing. Dysphagia. 1992;7:155-9.
218. Henderson RD, Boszko A, VanNostrand AW, Pearson FG. Pharyngoesophageal dysphagia and recurrent laryngeal nerve palsy. J Thorac Cardiovasc Surg. 1974;68:507-12.
219. Duranceau A. Oropharyngeal dysphagia and disorders of the upper esopharyngeal sphincter. Ann Chir Gynaecol. 1995;84:225-33.
220. Brais B, Xie YG, Sanson M, et al. The oculopharyngeal muscular dystrophy locus maps to the region of the cardiac alpha and beta myosin heavy chain genes on chromosome 14q11.2-q13. Hum Mol Genet. 1995;4:429-34.
221. Khan OA, Campbell WW. Myasthenia gravis presenting as dysphagia: Clinical considerations. Am J Gastroenterol. 1994;89:1083-5.
222. Wilson CP. Diverticula of the pharynx. J R Coll Surg Edinb. 1959;4:236-52.
223. Cook IJ, Blumbergs P, Cash K, et al. Structural abnormalities of the cricopharyngeus muscle in patients with pharyngeal (Zenker’s) diverticulum. J Gastroenterol Hepatol. 1992;7:556-62.
224. Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: A new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526-33.
225. Csendes A, Smok G, Braghetto I, et al. Gastroesophageal sphincter pressure and histological changes in distal esophagus in patients with achalasia of the esophagus. Dig Dis Sci. 1985;30:941-5.
226. Csendes A, Smok G, Braghetto I, et al. Histological studies of Auerbach’s plexuses of the oesophagus, stomach, jejunum, and colon in patients with achalasia of the oesophagus: Correlation with gastric acid secretion, presence of parietal cells and gastric emptying of solids. Gut. 1992;33:150-4.
227. Cash BD, Wong RK. Historical perspective of achalasia. Gastrointest Endosc Clin North Am. 2001;11:221-34. v
228. Goldblum JR, Whyte RI, Orringer MB, Appelman HD. Achalasia. A morphologic study of 42 resected specimens. Am J Surg Pathol. 1994;18:327-37.
229. Boeckxstaens GE. Achalasia: Virus-induced euthanasia of neurons? Am J Gastroenterol. 2008;103:1610-12.
230. Facco M, Brun P, Baesso I, et al. T cells in the myenteric plexus of achalasia patients show a skewed TCR repertoire and react to HSV-1 antigens. Am J Gastroenterol. 2008;103:1598-609.
231. Clark SB, Rice TW, Tubbs RR, et al. The nature of the myenteric infiltrate in achalasia: An immunohistochemical analysis. Am J Surg Pathol. 2000;24:1153-8.
232. Storch WB, Eckardt VF, Junginger T. Complement components and terminal complement complex in oesophageal smooth muscle of patients with achalasia. Cell Mol Biol (Noisy-le-grand). 2002;48:247-52.
233. Storch WB, Eckardt VF, Wienbeck M, et al. Autoantibodies to Auerbach’s plexus in achalasia. Cell Mol Biol (Noisy-le-grand). 1995;41:1033-8.
234. Moses PL, Ellis LM, Anees MR, et al. Antineuronal antibodies in idiopathic achalasia and gastro-oesophageal reflux disease. Gut. 2003;52:629-36.
235. Ruiz-de-Leon A, Mendoza J, Sevilla-Mantilla C, et al. Myenteric antiplexus antibodies and class II HLA in achalasia. Dig Dis Sci. 2002;47:15-19.
236. Robertson CS, Martin BA, Atkinson M. Varicella-zoster virus DNA in the oesophageal myenteric plexus in achalasia. Gut. 1993;34:299-302.
237. Castagliuolo I, Brun P, Costantini M, et al. Esophageal achalasia: is the herpes simplex virus really innocent? J Gastrointest Surg. 2004;8:24-30.
238. Qualman SJ, Haupt HM, Yang P, Hamilton SR. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology. 1984;87:848-56.
239. Holloway RH, Dodds WJ, Helm JF, et al. Integrity of cholinergic innervation to the lower esophageal sphincter in achalasia. Gastroenterology. 1986;90:924-9.
240. Mearin F, Mourelle M, Guarner F, et al. Patients with achalasia lack nitric oxide synthase in the gastro-oesophageal junction. Eur J Clin Invest. 1993;23:724-8.
241. Misiewicz JJ. Pathophysiology of achalasia of the cardia. Postgrad Med J. 1974;50:207-8.
242. Dodds WJ, Dent J, Hogan WJ, et al. Paradoxical lower esophageal sphincter contraction induced by cholecystokinin-octapeptide in patients with achalasia. Gastroenterology. 1981;80:327-33.
243. Sifrim D, Janssens J, Vantrappen G. Failing deglutitive inhibition in primary esophageal motility disorders. Gastroenterology. 1994;106:875-82.
244. Fleshler B. Diffuse esophageal spasm. Gastroenterology. 1967;52:559-64.
245. Richter JE, Castell DO. Diffuse esophageal spasm: a reappraisal. Ann Intern Med. 1984;100:242-5.
246. Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145-51.
247. Gillies M, Skyring A. The clinical application of oesophageal motility studies. Med J Aust. 1968;1:1075-9.
248. Ellis FHJr, Schlegel JF, Code CF, Olsen AM. Surgical treatment of esophageal hypermotility disturbances. JAMA. 1964;188:862-6.
249. Demian SD, Vargas-Cortes F. Idiopathic muscular hypertrophy of the esophagus. Postmortem incidental finding in six cases and review of the literature. Chest. 1978;73:28-32.
250. Gillies M, Nicks R, Skyring A. Clinical, manometric, and pathlogical studies in diffuse oesophageal spasm. BMJ. 1967;2:527-30.
251. Behar J, Biancani P. Pathogenesis of simultaneous esophageal contractions in patients with motility disorders. Gastroenterology. 1993;105:111-18.
252. Mellow M. Symptomatic diffuse esophageal spasm. Manometric follow-up and response to cholinergic stimulation and cholinesterase inhibition. Gastroenterology. 1977;73:237-40.
253. Richter JE, Hackshaw BT, Wu WC, Castell DO. Edrophonium: a useful provocative test for esophageal chest pain. Ann Intern Med. 1985;103:14-21.
254. Orlando RC, Bozymski EM. The effects of pentagastrin in achalasia and diffuse esophageal spasm. Gastroenterology. 1979;77:472-7.
255. Eastwood GL, Weiner BH, Dickerson WJ2nd, et al. Use of ergonovine to identify esophageal spasm in patients with chest pain. Ann Intern Med. 1981;94:768-71.
256. Ouyang A, Reynolds JC, Cohen S. Spike-associated and spike-independent esophageal contractions in patients with symptomatic diffuse esophageal spasm. Gastroenterology. 1983;84:907-13.
257. Vantrappen G, Janssens J, Hellemans J, Coremans G. Achalasia, diffuse esophageal spasm, and related motility disorders. Gastroenterology. 1979;76:450-7.
258. Massey BT, Dodds WJ, Hogan WJ, et al. Abnormal esophageal motility. An analysis of concurrent radiographic and manometric findings. Gastroenterology. 1991;101:344-54.
259. Edwards DA. History and symptoms of esophageal disease. In: Vantrappen G, editor. Diseases of the Esophagus. Berlin: Springer-Velag; 1974:103.
260. Eckardt VF, Stauf B, Bernhard G. Chest pain in achalasia: Patient characteristics and clinical course. Gastroenterology. 1999;116:1300-4.
261. Vantrappen G, Hellemans J. Treatment of achalasia and related motor disorders. Gastroenterology. 1980;79:144-54.
262. Panzini L, Traube M. Stridor from tracheal obstruction in a patient with achalasia. Am J Gastroenterol. 1993;88:1097-100.
263. Massey BT, Hogan WJ, Dodds WJ, Dantas RO. Alteration of the upper esophageal sphincter belch reflex in patients with achalasia. Gastroenterology. 1992;103:1574-9.
264. Spechler SJ, Souza RF, Rosenberg SJ, et al. Heartburn in patients with achalasia. Gut. 1995;37:305-8.
265. Smart HL, Foster PN, Evans DF, et al. Twenty four hour oesophageal acidity in achalasia before and after pneumatic dilatation. Gut. 1987;28:883-7.
266. Holloway RH, Wyman JB, Dent J. Failure of transient lower oesophageal sphincter relaxation in response to gastric distension in patients with achalasia: evidence for neural mediation of transient lower oesophageal sphincter relaxations. Gut. 1989;30:762-7.
267. Hirano I, Tatum RP, Shi G, et al. Manometric heterogeneity in patients with idiopathic achalasia. Gastroenterology. 2001;120:789-98.
268. Pehlivanov N, Liu J, Mittal RK. Sustained esophageal contraction: A motor correlate of heartburn symptom. Am J Physiol Gastrointest Liver Physiol. 2001;281:G743-51.
269. Rao SS, Gregersen H, Hayek B, et al. Unexplained chest pain: the hypersensitive, hyperreactive, and poorly compliant esophagus. Ann Intern Med. 1996;124:950-8.
270. Khatami SS, Khandwala F, Shay SS, Vaezi MF. Does diffuse esophageal spasm progress to achalasia? A prospective cohort study. Dig Dis Sci. 2005;50:1605-10.
271. Bettarello A, Pinotti HW. Oesophageal involvement in Chagas disease. Clin Gastroenterol Hepatol. 1976;5:103.
272. Sandler RS, Bozymski EM, Orlando RC. Failure of clinical criteria to distinguish between primary achalasia and achalasia secondary to tumor. Dig Dis Sci. 1982;27:209-13.
273. Song CW, Chun HJ, Kim CD, et al. Association of pseudoachalasia with advancing cancer of the gastric cardia. Gastrointest Endosc. 1999;50:486-91.
274. Costigan DJ, Clouse RE. Achalasia-like esophagus from amyloidosis. Successful treatment with pneumatic bag dilatation. Dig Dis Sci. 1983;28:763-5.
275. Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology. 1978;74:1298-301.
276. Dufresne CR, Jeyasingham K, Baker RR. Achalasia of the cardia associated with pulmonary sarcoidosis. Surgery. 1983;94:32-5.
277. Dodds WJ, Stewart ET, Kishk SM, et al. Radiologic amyl nitrite test for distinguishing pseudoachalasia from idiopathic achalasia. AJR Am J Roentgenol. 1986;146:21-3.
278. Milone L, Daud A, Durak E, et al. Esophageal dilation after laparoscopic adjustable gastric banding. Surg Endosc. 2008;22:1482-6.
279. Facchiano E, Scaringi S, Sabate JM, et al. Is esophageal dysmotility after laparoscopic adjustable gastric banding reversible? Obes Surg. 2007;17:832-5.
280. Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342-63.
281. Garrean CP, Gonsalves N, Hirano I. Bravo pH testing on and off treatment with immediate-release omeprazole. Gastroenterol Hepatol. 2007;3:4-7.
282. Ott DJ, Richter JE, Chen YM, et al. Esophageal radiography and manometry: Correlation in 172 patients with dysphagia. AJR Am J Roentgenol. 1987;149:307-11.
283. Ott DJ, Richter JE, Chen YM, et al. Radiographic and manometric correlation in achalasia with apparent relaxation of the lower esophageal sphincter. Gastrointest Radiol. 1989;14:1-5.
284. Ghosh SK, Pandolfino JE, Rice J, et al. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: A quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293:G878-85.
285. Pandolfino JE, Ghosh SK, Rice J, et al. Classifying esophageal motility by pressure topography characteristics: A study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103:27-37.
286. Hewson EG, Dalton CB, Richter JE. Comparison of esophageal manometry, provocative testing, and ambulatory monitoring in patients with unexplained chest pain. Dig Dis Sci. 1990;35:302-9.
287. Ghosh SK, Kahrilas PJ, Lodhia N, Pandolfino JE. Utilizing intraluminal pressure differences to predict esophageal bolus flow dynamics. Am J Physiol Gastrointest Liver Physiol. 2007.
288. Ghosh SK, Pandolfino JE, Zhang Q, et al. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G988-97.
289. Kahrilas PJ, Ghosh SK, Pandolfino JE. Esophageal motility disorders in terms of pressure topography: The Chicago Classification. J Clin Gastroenterol. 2008;42:627-35.
290. Imam H, Sanmiguel C, Larive B, et al. Study of intestinal flow by combined videofluoroscopy, manometry, and multiple intraluminal impedance. Am J Physiol Gastrointest Liver Physiol. 2004;286:G263-70.
291. Tutuian R, Castell DO. Clarification of the esophageal function defect in patients with manometric ineffective esophageal motility: Studies using combined impedance-manometry. Clin Gastroenterol Hepatol. 2004;2:230-6.
292. Drewes AM, Gregersen H. Multimodal pain stimulation of the gastrointestinal tract. World J Gastroenterol. 2006;12:2477-86.
293. Mulak A. Testing of visceral sensitivity. J Physiol Pharmacol. 2003;54:55-72.
294. Richter JE, Barish CF, Castell DO. Abnormal sensory perception in patients with esophageal chest pain. Gastroenterology. 1986;91:845-52.
295. Clouse RE, McCord GS, Lustman PJ, Edmundowicz SA. Clinical correlates of abnormal sensitivity to intraesophageal balloon distension. Dig Dis Sci. 1991;36:1040-5.
296. Rao SS, Hayek B, Summers RW. Impedance planimetry: An integrated approach for assessing sensory, active, and passive biomechanical properties of the human esophagus. Am J Gastroenterol. 1995;90:431-8.
297. Patel RS, Rao SS. Biomechanical and sensory parameters of the human esophagus at four levels. Am J Physiol. 1998;275:G187-91.
298. St Guily JL, Perie S, Willig TN, et al. Swallowing disorders in muscular diseases: functional assessment and indications of cricopharyngeal myotomy. Ear Nose Throat J. 1994;73:34-40.
299. Cook IJ, Kahrilas PJ. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology. 1999;116:455-78.
300. Aviv JE, Martin JH, Sacco RL, et al. Supraglottic and pharyngeal sensory abnormalities in stroke patients with dysphagia. Ann Otol Rhinol Laryngol. 1996;105:92-7.
301. Cook IJ, Gabb M, Panagopoulos V, et al. Pharyngeal (Zenker’s) diverticulum is a disorder of upper esophageal sphincter opening. Gastroenterology. 1992;103:1229-35.
302. Wang AY, Kadkade R, Kahrilas PJ, Hirano I. Effectiveness of esophageal dilation for symptomatic cricopharyngeal bar. Gastrointest Endosc. 2005;61:148-52.
303. Cohen S, Lipshutz W. Lower esophageal sphincter dysfunction in achalasia. Gastroenterology. 1971;61:814-20.
304. Wong RK, Maydonovitch C, Garcia JE, et al. The effect of terbutaline sulfate, nitroglycerin, and aminophylline on lower esophageal sphincter pressure and radionuclide esophageal emptying in patients with achalasia. J Clin Gastroenterol. 1987;9:386-9.
305. Bortolotti M, Labo G. Clinical and manometric effects of nifedipine in patients with esophageal achalasia. Gastroenterology. 1981;80:39-44.
306. Gelfond M, Rozen P, Gilat T. Isosorbide dinitrate and nifedipine treatment of achalasia: A clinical, manometric and radionuclide evaluation. Gastroenterology. 1982;83:963-9.
307. Traube M, Hongo M, Magyar L, McCallum RW. Effects of nifedipine in achalasia and in patients with high-amplitude peristaltic esophageal contractions. JAMA. 1984;252:1733-6.
308. Traube M, Dubovik S, Lange RC, McCallum RW. The role of nifedipine therapy in achalasia: Results of a randomized, double-blind, placebo-controlled study. Am J Gastroenterol. 1989;84:1259-62.
309. Bortolotti M, Mari C, Lopilato C, et al. Effects of sildenafil on esophageal motility of patients with idiopathic achalasia. Gastroenterology. 2000;118:253-7.
310. Pasricha PJ, Ravich WJ, Hendrix TR, et al. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995;332:774-8.
311. Spiess AE, Kahrilas PJ. Treating achalasia: From whalebone to laparoscope. JAMA. 1998;280:638-42.
312. Vaezi MF, Richter JE, Wilcox CM, et al. Botulinum toxin versus pneumatic dilatation in the treatment of achalasia: a randomised trial. Gut. 1999;44:231-9.
313. Kolbasnik J, Waterfall WE, Fachnie B, et al. Long-term efficacy of Botulinum toxin in classical achalasia: A prospective study. Am J Gastroenterol. 1999;94:3434-9.
314. Annese V, Bassotti G, Coccia G, et al. A multicentre randomised study of intrasphincteric botulinum toxin in patients with oesophageal achalasia. GISMAD Achalasia Study Group. Gut. 2000;46:597-600.
315. Panaccione R, Gregor JC, Reynolds RP, Preiksaitis HG. Intrasphincteric botulinum toxin versus pneumatic dilatation for achalasia: A cost minimization analysis. Gastrointest Endosc. 1999;50:492-8.
316. Kurlander DJ, Raskin HF, Kirsner JB, Palmer WL. Therapeutic value of the pneumatic dilator in achalasia of the esophagus. Long term results in sixty-two living patients. Gastroenterology. 1963;45:604-13.
317. Vantrappen G, Hellemans J. Oesophageal spasm and other muscular dysfunction. Clin Gastroenterol. 1982;11:453-77.
318. Schwartz HM, Cahow CE, Traube M. Outcome after perforation sustained during pneumatic dilatation for achalasia. Dig Dis Sci. 1993;38:1409-13.
319. Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
320. Csendes A, Braghetto I, Henriquez A, Cortes C. Late results of a prospective randomised study comparing forceful dilatation and oesophagomyotomy in patients with achalasia. Gut. 1989;30:299-304.
321. Hunter JG, Trus TL, Branum GD, Waring JP. Laparoscopic Heller myotomy and fundoplication for achalasia. Ann Surg. 1997;225:655-64.
322. Rosati R, Fumagalli U, Bona S, et al. Evaluating results of laparoscopic surgery for esophageal achalasia. Surg Endosc. 1998;12:270-3.
323. Patti MG, Pellegrini CA, Horgan S, et al. Minimally invasive surgery for achalasia: an 8-year experience with 168 patients. Ann Surg. 1999;230:587-93.
324. Yamamura MS, Gilster JC, Myers BS, et al. Laparoscopic Heller myotomy and anterior fundoplication for achalasia results in a high degree of patient satisfaction. Arch Surg. 2000;135:902-6.
325. Stewart KC, Finley RJ, Clifton JC, et al. Thoracoscopic versus laparoscopic modified Heller Myotomy for achalasia: Efficacy and safety in 87 patients. J Am Coll Surg. 1999;189:164-9.
326. Agha FP, Keren DF. Barrett’s esophagus complicating achalasia after esophagomyotomy. A clinical, radiologic, and pathologic study of 70 patients with achalasia and related motor disorders. J Clin Gastroenterol. 1987;9:232-7.
327. Richter JE. Surgery or pneumatic dilatation for achalasia: A head-to-head comparison. Now are all the questions answered? Gastroenterology. 1989;97:1340-1.
328. Vaezi MF, Baker ME, Richter JE. Assessment of esophageal emptying post-pneumatic dilation: Use of the timed barium esophagram. Am J Gastroenterol. 1999;94:1802-7.
329. Vela MF, Richter JE, Wachsberger D, et al. Complexities of managing achalasia at a tertiary referral center: Use of pneumatic dilatation, Heller myotomy, and botulinum toxin injection. Am J Gastroenterol. 2004;99:1029-36.
330. Peters JH, Kauer WK, Crookes PF, et al. Esophageal resection with colon interposition for end-stage achalasia. Arch Surg. 1995;130:632-6.
331. Chuong JJ, DuBovik S, McCallum RW. Achalasia as a risk factor for esophageal carcinoma. A reappraisal. Dig Dis Sci. 1984;29:1105-8.
332. Meijssen MA, Tilanus HW, van Blankenstein M, et al. Achalasia complicated by oesophageal squamous cell carcinoma: A prospective study in 195 patients. Gut. 1992;33:155-8.
333. Sandler RS, Nyren O, Ekbom A, et al. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA. 1995;274:1359-62.
334. Orlando RC, Bozymski EM. Clinical and manometric effects of nitroglycerin in diffuse esophageal spasm. N Engl J Med. 1973;289:23-5.
335. Thomas E, Witt P, Willis M, Morse J. Nifedipine therapy for diffuse esophageal spasm. South Med J. 1986;79:847-9.
336. Mellow MH. Effect of isosorbide and hydralazine in painful primary esophageal motility disorders. Gastroenterology. 1982;83:364-70.
337. Miller LS, Parkman HP, Schiano TD, et al. Treatment of symptomatic nonachalasia esophageal motor disorders with botulinum toxin injection at the lower esophageal sphincter. Dig Dis Sci. 1996;41:2025-31.
338. Clouse RE, Lustman PJ, Eckert TC, et al. Low-dose trazodone for symptomatic patients with esophageal contraction abnormalities. A double-blind, placebo-controlled trial. Gastroenterology. 1987;92:1027-36.
339. Latimer PR. Biofeedback and self-regulation in the treatment of diffuse esophageal spasm: a single-case study. Biofeedback Self Regul. 1981;6:181-9.
340. Winters C, Artnak EJ, Benjamin SB, Castell DO. Esophageal bougienage in symptomatic patients with the nutcracker esophagus. A primary esophageal motility disorder. JAMA. 1984;252:363-6.
341. Ebert EC, Ouyang A, Wright SH, et al. Pneumatic dilatation in patients with symptomatic diffuse esophageal spasm and lower esophageal sphincter dysfunction. Dig Dis Sci. 1983;28:481-5.
342. Filipi CJ, Hinder RA. Thoracoscopic esophageal myotomy—A surgical technique for achalasia diffuse esophageal spasm and “nutcracker esophagus.”. Surg Endosc. 1994;8:921-5.
343. Cannon RO3rd, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330:1411-17.
344. Shapiro M, Green C, Faybush EM, et al. The extent of oesophageal acid exposure overlap among the different gastro-oesophageal reflux disease groups. Aliment Pharmacol Ther. 2006;23:321-9.
345. Broekaert D, Fischler B, Sifrim D, et al. Influence of citalopram, a selective serotonin reuptake inhibitor, on oesophageal hypersensitivity: A double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2006;23:365-70.
346. Miner PBJr, Rodriguez-Stanley S, Proskin HM, et al. Tegaserod in patients with mechanical sensitivity and overlapping symptoms of functional heartburn and functional dyspepsia. Curr Med Res Opin. 2008;24:2159-72.
347. Rao SS, Mudipalli RS, Mujica V, et al. An open-label trial of theophylline for functional chest pain. Dig Dis Sci. 2002;47:2763-8.