Chapter 192 Escherichia coli
Escherichia coli are important causes of enteric infections as well as urinary tract infections (Chapter 532), sepsis and meningitis in the newborn (Chapter 103), and bacteremia and sepsis in immunocompromised patients (Chapter 171) and in patients with intravascular devices (Chapter 172).
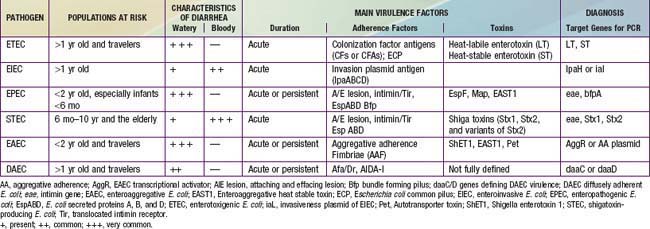
Because E. coli are normal fecal flora, pathogenicity is defined by demonstration of virulence characteristics and association of those traits with illness (Table 192-1). The mechanism by which E. coli produces diarrhea typically involves adherence of organisms to a glycoprotein or glycolipid receptor, followed by production of some noxious substance that injures or disturbs the function of intestinal cells. The genes for virulence properties and for antibiotic resistance are often carried on transferable plasmids, pathogenicity islands, or bacteriophages. In the developing world, the various diarrheagenic E. coli cause frequent infections in the first few years of life. They occur with increased frequency during the warm months in temperate climates and during rainy season months in tropical climates. Most diarrheagenic E. coli strains (except STEC) require a large inoculum of organisms to induce disease. Infection is most likely when food-handling or sewage-disposal practices are suboptimal. The diarrheagenic E. coli are also important in North America and Europe, although their epidemiology is less well defined in these areas than in the developing world. Recent data in North America suggest that the various diarrheagenic E. coli could be the etiology of as much as 30% of infectious diarrhea in children <5 yr of age.
Shiga Toxin–Producing Escherichia Coli
STEC have been shown to cause a wide spectrum of diseases. STEC infections may be asymptomatic. Patients who develop intestinal symptoms can have mild diarrhea or severe hemorrhagic colitis. The gastrointestinal illness is characterized by abdominal pain with diarrhea that is initially watery but within a few days can become blood-streaked or grossly bloody. Although this pattern resembles that of shigellosis or EIEC disease, it differs in that fever is an uncommon manifestation. Most persons infected with STEC recover from the infection without further complication. However, 5-10% of children with STEC hemorrhagic colitis go on within a few days to develop systemic complications such as hemolytic-uremic syndrome (HUS), characterized by acute kidney failure, thrombocytopenia, and microangiopathic hemolytic anemia (Chapter 512). Severe illness occurs most often among children from 6 months to 10 years of age. The elderly can also develop HUS or thrombotic thrombocytopenic purpura.
Treatment
The STEC represent a particularly difficult therapeutic dilemma; many antibiotics can induce toxin production and phage-mediated bacterial lysis with toxin release. Antibiotics should not be given for STEC infection because they can increase the risk of hemolytic-uremic syndrome (Chapter 512).
Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metalol-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:750-751.
Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenem-producing Enterobacteriaceae in acute facilities. MMWR Morb Mortal Wkly Rep. 2009;58:256-260.
Chen HD, Frankel G. Enteropathogenic Escherichia coli: unraveling pathogenesis. FEMS Micro Rev. 2005;29:83-98.
Cohen MB, Nataro JP, Bernstein DI, et al. Prevalence of diarrheagenic E. coli in acute childhood enteritis; a prospective controlled study. J Pediat. 2005;146:54-61.
Donnenberg MS, Whittam TS. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. J Clin Invest. 2001;107:539-548.
Garmendia J, Frankel G, Crepin VF. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect Immun. 2005;73:2573-2585.
Guion CE, Ochoa TJ, Walker CM, et al. Detection of diarrheagenic Escherichia coli by use of melting-curve analysis and real-time multiplex PCR. J Clin Microbiol. 2008;46:1752-1757.
Harrington SM, Dudley EG, Nataro JP. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol Lett. 2006;254:12-18.
Le Bouguenec C, Servin AL. Diffusely adherent E. coli strains expressing AFA/Dr adhesins (AFA/Dr DAEC): hitherto unrecognized pathogens. FEMS Microbiol Lett. 2006;256:185-194.
Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Micro Rev. 1998;11:142-201.
Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18:465-483.
Stenutz R, Weintraub A, Widmalm G. The structures of E. coli O-polysaccharide antigens. FEMS Microbiol Rev. 2006;30:382-403.