Chapter 50 Erythromelalgia
Definition and Historical Perspective
Erythromelalgia is a rare condition of the extremities characterized by the triad of redness, warmth, and pain. The symptom complex of intermittent acral warmth, pain, and erythema that defines erythromelalgia has been well documented in the medical literature for more than 150 years. Graves1 described cases of “hot and painful legs” in 1834. The term erythromelalgia was coined in 1878 by Mitchell2 from erythros (red), melos (extremity), and algos (pain); some have since referred to it as Mitchell’s disease. As we discover more about the link between a vasculopathy and neuropathy in this syndrome, it seems that Mitchell was prophetically accurate when he entitled the original manuscript “On a Rare Vasomotor Neurosis of the Extremities.” Smith and Allen3 emphasized another essential component of this syndrome when they renamed it erythermalgia in 1938 to denote the heat (thermé) in the affected extremity during periods of redness. Although many authors agree that erythermalgia is perhaps more accurate, erythromelalgia is the term most commonly used, and it is the term used in this chapter.
Although poorly characterized inititally,4–6 there have been considerable advances in the characterization of this clinical syndrome, with large case series7–10 published. Although the condition is mysterious, it is not as mysterious as was once believed.9,10 It has been argued that William Harvey could have had erythromelalgia, not gout.11 Much of our current understanding of erythromelalgia derives from the larger case series reported.7,8,10,12,13
Nomenclature
Considerable confusion exists regarding the nomenclature of erythromelalgia.14 Many terms have been used, and some authors have proposed that these terms should refer to different forms of erythromelalgia, as detailed later. However, these synonyms are not widely used, and most authors now use the term erythromelalgia as originally used by Silas Weir Mitchell (1829-1914). Related names used by some include Weir-Mitchell’s disease, Mitchell’s disease, and acromelalgia. Michiels et al.15 proposed that the term erythromelalgia be restricted to cases due to myeloproliferative disorders responsive to aspirin therapy. They used the term erythermalgia to describe idiopathic conditions or conditions due to other diseases that are unresponsive to aspirin therapy. An unwieldy term, erythermomelalgia, accounts for the four cardinal symptoms and signs of the condition, but it is not in general use.16 Erythralgia has been used.5,17 Erythroprosopalgia, derived from prosopon (face), is used in the German literature to describe facial erythromelalgia.5,14,17
Criteria for Diagnosis
No objective criteria exist for the diagnosis of erythromelalgia, making it difficult to interpret some of the cases reported in the literature.14 The diagnosis is most often clinically based, dependent on the medical history and physical findings, because no objective diagnostic or laboratory tests are available,14 and because the physical findings of erythromelalgia may be absent owing to the frequently intermittent nature of the condition.8
Different diagnostic criteria have been suggested by different authors. Weir Mitchell2,8 applied the three inclusion criteria used in the original description of the syndrome: red, hot, and painful extremities. Brown18 added three additional criteria in 1932: induction and exacerbation of symptoms by warming, relief by cooling, and unresponsiveness to therapy. The criteria were described as follows: (1) during attacks (bilateral or symmetrical burning pain in hands and feet), affected parts are flushed, congested, and warm; (2) attacks are initiated or aggravated by standing, exercising, or exposing the extremity to temperatures warmer than 34 °C; (3) symptoms are relieved by elevation of the extremity or exposure of the extremity to cold; and (4) the condition is refractory to treatment. Thompson et al.19 suggested the following five criteria: (1) burning extremity pain, (2) pain aggravated by warming, (3) pain relieved by cooling, (4) erythema of the affected skin, and (5) increased temperature of the affected skin. These five criteria have been used in several publications.10,14,20–23
Lazareth et al.24 used three major and two of four minor criteria to satisfy the diagnosis. Major criteria were paroxysmal pain, burning pain, and redness of affected skin. Minor criteria were typical precipitating factors (heat exposure, effort), typical relieving factors (cold, rest), elevated skin temperature in affected skin, and response of symptoms to acetylsalicylic acid. Drenth et al.25–28 distinguished three types of red, congested, and painful conditions of the extremities that must be distinguished for effective treatment according to their cause: (1) erythromelalgia in thrombocythemia, (2) primary erythermalgia, and (3) secondary erythermalgia. Kurzrock and Cohen29 used a classification of early-onset erythromelalgia and late-onset erythromelalgia, irrespective of the cause.
Littleford et al.30 used a classification of type 1 erythromelalgia (the typical form) and type 2 erythromelalgia (the abortive form), in which the burning nature of the pain is absent and symptomatic relief is not always provided by cooling or elevation of the limb. Mørk and Kvernebo14 made the following distinctions: (1) Syndrome is used when initial and gradual symptoms localized to the feet and legs appear in childhood or adolescence, and when there is a family history of erythromelalgia; phenomenon is used for all other cases. (2) Erythromelalgia is primary when it is idiopathic. It is secondary when symptoms are caused by a primary disease such as a hemorrheological, metabolic, connective tissue, musculoskeletal, or infective disease; are induced by drugs; or are part of a paraneoplastic phenomenon. (3) Acute is used when symptoms reach maximal strength within 1 month after onset of symptoms. (4) Borderline erythromelalgia, erythromelalgia, and severe erythromelalgia may be useful.14
Clinical Presentation
The essential elements of this clinical syndrome, as described by its name, are intermittent (occasionally continuous) redness of an acral area (i.e., extremities, head and neck area) associated with heat and pain. Common terms used to describe the pain include “piercing,” “burning,” and “discomfort.”8 The pain and burning sensation can be extremely severe. Patients report that they make major adjustments to their lifestyles to avoid precipitating an event. During an episode, they try to cool their feet in many ways, sometimes resorting to extraordinary measures to alleviate the pain, such as putting their feet in ice or walking barefoot in snow.
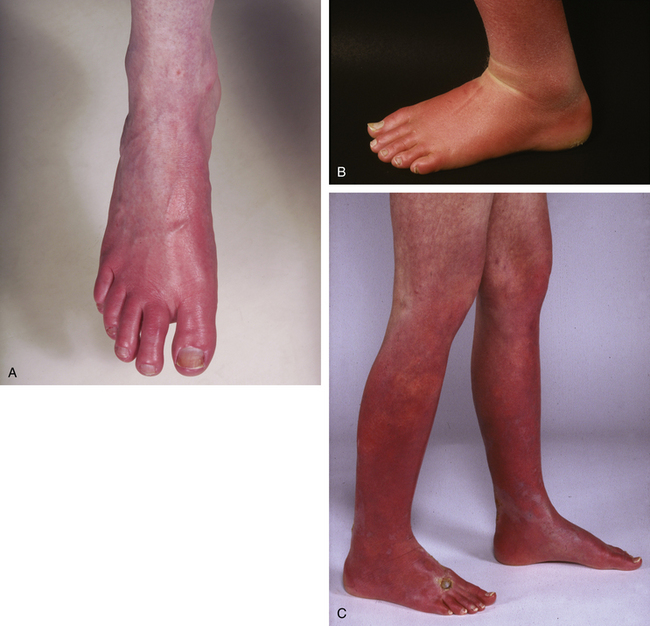
Erythromelalgia involves the feet in most circumstances (Fig. 50-1A-B); a minority of these patients have similar symptoms involving the hands.8 Occasionally, only the hands are involved. Erythromelalgia may extend proximally to the knees in the lower extremities (Fig. 50-1C) and to the elbows in the upper extremities. Involvement of the extremities is generally symmetrical. Rarely, erythromelalgia involves the ears and face. In the largest reported series (168 patients), symptoms predominantly involved feet (148 patients, 88.1%) and hands (43 patients, 25.6%).8
In the majority of patients, symptoms are intermittent; episodes, precipitated by specific triggers, can last from minutes to hours. In a minority of patients, erythromelalgia symptoms are continuous, although they may wax and wane. Patients with continuous symptoms usually report that their symptoms started intermittently and then became more frequent and prolonged until they were continuous. In the series of 168 patients,8 symptoms were intermittent in 163 patients (97%) and continuous in 5 (3%).
Aspirin may dramatically relieve symptoms in a subset of patients with underlying myeloproliferative disease, but otherwise aspirin is rarely effective. Other agents that have been reported to relieve symptoms are presented later in the section on treatment. Many patients report that plunging their feet into ice water during an episode relieves their symptoms. Patients frequently report that the affected extremities must be exposed to cold surfaces or air-conditioned rooms or be immersed in buckets of cool or ice water to relieve their symptoms. A decrease in local temperature may decrease the severity of erythromelalgia or even abort an episode. Some patients sleep with their extremities outside the bedcovers, and some engage in unusual behaviors such as sleeping with their feet out a window, putting their feet in a refrigerator, walking barefoot in the snow, or storing shoes in a freezer. Kvernebo10 described a patient who, for almost 25 years day and night, lived with a bucket of ice water at her side, immersing her feet intermittently for 15 to 30 minutes an hour. Thus, in what superficially appears to be the antithesis of Raynaud’s phenomenon, patients seek relief by cooling the affected extremity.
Symptoms of erythromelalgia are intermittent, and the clinical examination is often normal. If the patient is examined during an episode of erythromelalgia, the affected extremity is tender, erythematous, and objectively hot. In up to two thirds of patients, affected extremities are discolored (blue/cyanotic, red, or mottled) and cool or cold to the touch, with varying degrees of discomfort between episodes. Raynaud’s phenomenon is not uncommon between episodes, occurring in 15% of one series.31
Erythromelalgia predominantly affects individuals who are white and of any age. In the largest published series,8 all 168 patients were white, the female-to-male ratio was approximately 3:1, and the mean age was 55.8 years (range, 5-91 years). Symptoms had been present since childhood in seven patients (4.2%), and six patients (3.6%) had a first-degree relative with erythromelalgia.
Erythromelalgia can also occur in the pediatric age group. In the largest pediatric series reported—32 patients (girls, 22 [69%]) seen at the Mayo Clinic32—mean age was 14.1 years (range, 5-18 years), and the diagnosis was delayed; mean time to diagnosis was 5.2 years. Seven patients (22%) had a first-degree relative with erythromelalgia; four were from the same family. Physical activity was limited in 21 patients (66%), and school attendance was affected in 11 patients (34%). Hypertension was not a feature of these patients. In contrast, Drenth et al.33 described nine children in whom erythromelalgia was transient (seven girls and two boys, mean age 11.6 years); in seven, hypertension was directly related to the symptoms, and treatment of the hypertension with intravenous (IV) sodium nitroprusside relieved symptoms.
Diagnosis
1. Examine and assess the patient both during an episode and between episodes. Ask the patient to engage in an activity, such as climbing stairs, that will precipitate an episode.
2. If it is not possible to examine a patient during an episode, ask the patient to obtain a photograph of the affected areas during an episode.
Classification
Most authors agree on the fundamentals of the diagnosis of erythromelalgia, but there are many described criteria for diagnosis and many subclassifications of erythromelalgia. Use of these subclassifications may depend on whether one is a “lumper” or “splitter.”28,29,34 The most popular classification of erythromelalgia is into primary and secondary forms.
Secondary Erythromelalgia
Potential causes of secondary erythromelalgia are presented in Box 50-1. Erythromelalgia has been reported in association with myeloproliferative diseases, blood disorders, drugs, infectious diseases, food ingestion (mushrooms), neoplasms, connective tissue disease, physiological conditions (pregnancy), and neuropathies. An epidemic in China has been described.66 The relationship of many underlying disorders to erythromelalgia is sometimes unclear, and the disorder may be a coincidental comorbidity rather than an underlying disease.
Among the reported series, the association with myeloproliferative disease seems most constant.8,35–37 Evidence of underlying myeloproliferative disease should be sought at diagnosis and subsequently. Erythromelalgia can herald the onset of underlying myeloproliferative disease. In one series, erythromelalgia was the presenting symptom of essential thrombocythemia in 26 of 40 patients (65%)36; in another series, erythromelalgia was the presenting symptom in 11 of 268 patients with thrombocythemia (4%).37
Incidence
In a population-based study from Olmsted County, Minnesota, the overall age- and sex-adjusted incidence rate (95% confidence interval [95% CI]) was calculated to be 1.3 (0.8-1.7) per 100,000 persons per year. The incidence of primary and secondary erythromelalgia was 1.1 (0.7-1.5) and 0.2 (0.02-0.4) per 100,000 persons per year, respectively.89 The incidence was noted to have increased over the past 3 decades. This incidence was approximately five times higher than that reported from Norway, where the incidence was calculated to be 2.5 to 3.3 per 1 million inhabitants per year in the Norwegian population, with a corresponding annual prevalence of 18 to 20 per 1 million.10 Cases of borderline erythromelalgia were not included in these figures.9,10
Pathophysiology
The pathophysiology of erythromelalgia is not clearly understood. Part of the difficulty in understanding this disorder has been the heterogeneity of the affected population.90 The underlying pathological mechanisms most likely involve a complex dysregulation of cutaneous blood flow that ultimately results in microvascular ischemia. Determining the nature of this dysfunction has also been challenging because control of cutaneous blood flow depends on an intricate interplay of systemic and local signals and is not completely understood.90 A small-fiber neuropathy likely contributes to this dysregulation.91,92
Erythromelalgia: a Vasculopathy?
Thermoregulatory control of human skin blood flow is vital to maintenance of normal body temperatures during challenges to thermal homeostasis. Sympathetic neural control of skin blood flow includes the noradrenergic vasoconstrictor system and a sympathetic active vasodilator system, the latter being responsible for 80% to 90% of the substantial cutaneous vasodilation that occurs with whole-body heat stress. With body heating, the magnitude of skin vasodilation is striking; skin blood flow can reach 6 to 8 L/min during hyperthermia.93
Erythromelalgia is a cutaneous microvascular disorder. Pathophysiology appears to relate to disorders of local or reflex thermoregulatory control of skin circulation.93 Two paradoxical observations concerning blood flow during an episode of erythromelalgia have been made. During symptoms, there is increased blood flow. Sandroni et al.,92 Mørk et al.,9 and Kvernebo10 confirmed that the observed erythema and warmth are associated with increased blood flow. Using laser Doppler, Sandroni et al. measured blood flow during symptoms and demonstrated increased perfusion during attacks. Paradoxically, however, this increased blood flow is accompanied by local hypoxia. Although there is increased perfusion during attacks, the values for transcutaneous oxygen tension are critically low, low, or unchanged—in other words, during symptoms, transcutaneous oximetry values decrease or do not change.9,10,91,92 To explain this paradox, Mørk et al.23 theorized and demonstrated that the increased blood flow is probably due to shunting through arteriovenous anastomoses, which results in hypoperfusion of the more superficial nutritive capillaries. If available blood is shunted away from normal skin capillaries, the skin will be hypoxic. Mørk et al. demonstrated that despite increased overall blood flow to the skin, the induction of erythromelalgia symptoms is accompanied by decreased perfusion of the superficial vascular plexus, as evidenced by a decreased density of skin capillaries. Thus their hypothesis is that dilation of arteriovenous anastomoses is directly responsible for shunting nutritive blood flow away from the superficial vascular plexus. Furthermore, Mørk et al.21 postulated that erythromelalgia is not a disease, but rather a physiological response to stimuli such as infection, trauma, or tumor, and symptoms are caused by tissue hypoxia induced by maldistribution of microvascular blood flow in the skin, with increased thermoregulatory flow and inadequate perfusion.
Sandroni et al.92 theorized that the effects of diminished perfusion could be exacerbated by increased metabolic demands in response to hyperthermia, ultimately resulting in hypoxic tissue damage and pain. Pain relief by cooling could be explained by a resultant decrease in the metabolic rate and a corresponding decrease in the need for oxygen.
Littleford et al.30 described an underlying vasoconstrictor tendency in patients with erythromelalgia that may be related to functional or structural changes in skin microvessels, and noted that basal skin erythrocyte flux and skin temperature were lower in patients with a history of erythromelalgia than in controls. As noted earlier, Raynaud’s phenomenon has been described in patients with erythromelalgia.94,95 Acrocyanosis has also been described.94 Davis et al.91 have also noted that at baseline, the skin is cool and occasionally cyanotic between episodes in two thirds of patients.31
Erythromelalgia: a Neuropathy?
Several lines of evidence suggest that a neuropathy is associated with erythromelalgia, since the disorder has been described in association with many types of neuropathy. Both large- and small-fiber neuropathies are observed in a large proportion of patients with erythromelalgia (see Box 50-1).91,92,96
Among 57 patients with erythromelalgia who were evaluated with use of an autonomic reflex screen, results for 49 (86%) were abnormal, indicating a small-fiber neuropathy. The most common abnormalities were sudomotor abnormalities (i.e., absent or reduced sweat production).91 In an earlier series, findings were similar for 17 of 27 patients (63%); whether the observed neuropathy led to erythromelalgia, or vice versa, is unclear.92,98 In support of this, thermoregulatory sweat testing results were abnormal in 28 (88%) of 32 patients, and quantitative sudomotor axon reflex test results were abnormal in 22 patients (69%); abnormalities noted on thermoregulatory sweat testing varied from local hypohidrosis or anhidrosis to global anhidrosis.96
Conversely, in a series of 321 cases of disorders of autonomic neuropathy, the majority had erythromelalgia.99 Orstavik et al.100 used erythromelalgia as a model to study chronic pain and found changes in the conductive properties of C fibers in patients with erythromelalgia that were indicative of a small-fiber neuropathy. Additionally, an active contribution of mechanoinsensitive fibers to chronic pain was postulated. Uno and Parker101 reported that the density of both acetylcholinesterase-positive and catecholamine-containing nerve terminals in the periarterial and sweat gland plexuses was much less in the skin of the erythermalgic foot than in the unaffected skin of the same patient, and much less than in the foot skin of a healthy person.
Layzer102 wrote that it seems plausible to regard erythromelalgia as a problem of polymodal C-fiber receptors in sensitized skin. The threshold of C fibers to activation by heat would decrease to between 32 °C and 36 °C; activated C fibers would cause vasodilation by axon reflexes, resulting in redness, heat, and swelling. With cooling, the threshold for the nociceptors would increase.
Kazemi et al.97 reported that 72.7% of the patients studied had abnormal sympathetic reflexes, which may result from an abnormality of the sympathetic nerves. Normal sympathetic nerve activity in skin without an associated vasoconstriction response has been found in a patient.103 Littleford et al.104 also noted findings suggesting that patients with erythromelalgia have diminished sympathetic vasoconstrictor responses to both cold challenge of the contralateral arm and inspiratory gasp; an interplay between neural and vasoactive agents was postulated.
Inherited Erythromelalgia
There is a subset of erythromelalgia that is inherited. In the largest reported series, the proportion of cases that are inherited was approximately 5%.8 Inheritance in familial autosomal dominant (29 persons were affected in five generations) and X-linked dominant fashions have been reported. Clinical onset in familial cases usually occurs in childhood, most frequently prior to the age of 5 or 6, but occasionally is seen up to 10 or 12 years of age and, in rare families, at even older ages.
Gain-of-function mutations in sensory nerves and consequent nerve hyperexcitability
In the inherited forms of erythromelalgia, there have been new developments in understanding the disease. It now appears that mutations in particular sodium channels in the nociceptors of sensory nerves lead to firing of nerves with little provocation; in other words, sensory nerves are hyperexcitable. In 2001, Drenth et al. investigated DNA from five families with hereditary erythromelalgia using linkage analysis and located the gene responsible to chromosome 2q31-32.105 Based on this work, Yang et al. subsequently reported that mutations in the gene SCN9A on this chromosome caused primary erythromelalgia.106 Cummins et al. showed that functionally this gene encoded the Nav 1.7 sodium channel; mutations in this gene leads to altered channel function in nerves.107 These mutations are preferentially expressed by the dorsal root ganglion and sympathetic ganglion neuron. In 2005, Michiels et al. reported that a mutation in this gene occurred in all five affected members of a Flemish family and in none of five unaffected members.108 Han et al. described a patient with onset of erythromelalgia in the second decade and suggested that mutations that lead to lesser changes in sodium channel activation are associated with lesser neuron excitability and later onset of clinical signs.109
The implications of these findings are significant, since these mutated sodium channels could be a therapeutic target, and more widely, other pain syndromes also involve altered sodium channel function.110 In an editorial in 2005, Waxman and Dib-Hajj stated, “Erythromelalgia is the first human disorder in which it has been possible to associate an ion channel mutation with chronic neuropathic pain … erythromelalgia may emerge as a model disease that holds more general lessons about the molecular neurobiology of chronic pain.”111
It is important to bear in mind that these findings have been described in the inherited form of erythromelalgia thus far and not the more common noninherited forms. Additionally, SCN9A mutation is not always present in inherited erythromelalgia. Drenth et al. identified this mutation in only 1 of 15 patients with a family history of the disease.112
Erythromelalgia associated with a myeloproliferative disease
Erythromelalgia in the setting of thrombocythemia or myeloproliferative diseases seems to be a separate entity, although it presents similarly to other forms of erythromelalgia. Recognition of the associated myeloproliferative disease is vital because in these specific types of erythromelalgia, aspirin provides immediate and long-lived relief from symptoms. Thrombin, platelet function, and genetics have been considered in studies of erythromelalgia. Van Genderen et al.113 noted that thrombocythemia-associated erythromelalgia may develop despite treatment with oral anticoagulants or heparin, suggesting that generation of thrombin is not a prerequisite for development of erythromelalgia. Disordered platelet function affecting the microvasculature has been implicated in thrombocythemia-related erythromelalgia.114
Pathophysiological Controversies
Several questions about erythromelalgia remain. Does a neuropathy cause the vasculopathy, or does the vasculopathy cause a neuropathy?91 What causes the neuropathy if it is not caused by the vasculopathy? In the inherited form, mutations in the sodium channel lead to hyperexcitability of sensory nerves. Similar mutations have not been described in the sporadic form, which accounts for 95% of cases. What is the pathophysiology in these forms? How does dysfunction of the precapillary sphincter affect this disease? Schechner90 pointed out that it is unknown whether shunting of blood through arteriovenous anastomoses alone can induce hypoxia severe enough to induce pain, particularly in areas that contain few arteriovenous anastomoses. Potentially inadequate compensatory dilation, or even inappropriate constriction of the precapillary sphincter, may compound the effects of the relative hypoperfusion. What factors are responsible for vascular dysfunction? Both autonomic neuropathy21,92 and endothelial injury115 have been observed in patients with erythromelalgia, but it is not known whether this damage to critical vasoregulatory components is primary or secondary to chronic hypoxia.
Differential Diagnosis
Any condition causing extremity pain could be mistaken for erythromelalgia. In particular, unwarranted diagnosis of erythromelalgia can result from any clinical situation that includes burning sensations in the limbs.116 However, the syndrome of erythromelalgia is specific for red, hot extremities. The following conditions are included in the differential diagnosis:
• Neuropathies: peripheral neuropathy, small-fiber neuropathy, reflex sympathetic dystrophy.
• Vascular: large vessel disease, small-vessel disease, Raynaud’s phenomenon, Raynaud’s disease, arterial insufficiency, venous insufficiency (which can produce sensations of warm feet, often at bedtime, with edema and an increase in local heat).
• Metabolic: painful crises associated with Fabry’s disease (a hereditary sphingolipidosis transmitted on chromosome X that occurs predominantly in men, often starting early in childhood with a burning sensation in the limbs).
• Skin: dermatitis, immersion foot.
• Exogenous: acrodynia (a rare disease caused by excessive mercury intake and confirmed by high mercury levels in the urine, in which the main sign is vasomotor impairment in the limbs, and the red hands and feet have an intense, paroxysmal, burn-type pain).
Investigations
To investigate the possibility of erythromelalgia, the clinician should take the following steps:
1. Get a detailed history, and perform a physical examination with respect to each element of the history outlined earlier.
2. If signs of erythromelalgia are not present during the examination, ask the patient to photograph the affected area when symptoms are apparent.
3. Evaluate the results of a complete blood cell (CBC) count, including total and differential leukocyte counts.
4. Investigate the possibility of underlying disease, as indicated by the patient’s age, history, and physical examination.
5. Consider further investigations as outlined in Box 50-2, especially for small-fiber neuropathy and large-fiber neuropathy, and for noninvasive vascular studies during symptoms and between symptoms (as detailed by Davis et al.91) to better define the pathophysiology. Results of these tests are useful to confirm the diagnosis and help guide therapy.
6. In the inherited form, molecular genetic testing for mutations in SCN9A is available on a clinical basis.
![]() Box 50-2 Investigation Protocol for Patients Presenting with Erythromelalgia
Box 50-2 Investigation Protocol for Patients Presenting with Erythromelalgia
Neurological Evaluation
Modified from Davis MDP, Sandroni P, Rooke TW, et al: Erythromelalgia: vasculopathy, neuropathy, or both? A prospective study of vascular and neurophysiologic studies in erythromelalgia. Arch Dermatol 139:1337, 2003.91
Biopsy Findings
Reports of skin biopsies for erythromelalgia are scant. In the series reported by Davis et al.,8 only 12 of the 168 patients with primary erythromelalgia had a biopsy, and the biopsy specimens showed no specific abnormalities. Three cases of primary erythromelalgia were reported by Drenth et al.,115 and similarly, the biopsy specimens showed nonspecific changes.
The histopathological changes in cases of erythromelalgia related to thrombocythemia have been characterized by Michiels and associates.36 Arteriolar inflammation, fibromuscular intimal proliferation, and thrombotic occlusions were noted.117 Croue et al.118 noted similar changes in a case of erythromelalgia related to essential thrombocythemia. Biopsies from a few patients with drug-induced erythromelalgia have been described. Biopsies from a patient with verapamil-induced erythromelalgia showed mild perivascular mononuclear infiltrate and moderate perivascular edema.50 Among three patients with erythromelalgia due to bromocriptine, biopsies showed a prominent perivascular lymphocytic infiltration and perivascular edema of the dermis without frank vasculitis.58
Natural History and Prognosis
Information concerning the prognosis of this condition is limited.8,12 In a study of the natural history of erythromelalgia in which 168 patients were studied, with a mean follow-up of 8.7 years (range, 1.3-20 years), Kaplan-Meier survival curves showed a significant decrease in survival compared with expected survival among people matched for age and sex from the U.S. general population (P<.001).8 Of 94 patients questioned about their symptoms, 30 (31.9%) reported that their symptoms had worsened, 25 (26.6%) had not changed, 29 (30.9%) had improved, and 10 (10.6%) had completely resolved.
At the time of the most recent follow-up, 45 of the 168 patients (26.8%) had died. Causes of death included myeloproliferative disease, cardiovascular disease, and cancer. Importantly, three patients with severe symptoms had committed suicide. In a series of patients with pediatric erythromelalgia, one patient had committed suicide.32
Kalgaard et al.12 reported that about two thirds of 87 patients studied had primary cases, and about three quarters had some form of chronic condition. Over time in the patients with erythromelalgia, the condition gradually became worse. In patients with primary or secondary acute erythromelalgia, the condition improved, and in patients with primary or secondary chronic erythromelalgia, the condition remained stable.
Thus overall, it can be concluded from these studies that some patients become worse, some have a stable course, and some get better or even have full resolution of erythromelalgia with time. It is notable that some patients experienced cure.8
Quality of Life
Erythromelalgia has a markedly negative effect on quality of life. One study has directly measured quality-of-life parameters.8 The Medical Outcomes Study 36-Item Short Form Health Survey was used, and the results of this questionnaire were compared with scores obtained from a cohort from the U.S. general population. The questionnaire is a standard survey that measures health-related quality-of-life outcomes and measures each of eight health concepts (or domains) on a five-point Likert scale: physical functioning, role limitations due to physical disease, bodily pain, general health, vitality (energy and fatigue), social functioning, role limitations due to emotional problems, and mental health (psychological stress and psychological well-being). Scores for all but one of the health domains were significantly less in the study population than in the U.S. general population. The lowest scores were in the physical functioning domain.
Management
Management of erythromelalgia is difficult. There are no randomized controlled studies of treatments for erythromelalgia, and no single treatment is effective in all cases. The literature is replete with case reports and small case series describing a response to one treatment or another. When a larger group of erythromelalgia patients was surveyed, the majority reported that no treatment was very effective. Various treatments used in the management of erythromelalgia have been reviewed.119
One algorithmic approach to the management of erythromelalgia is as follows:
Aspirin3,36,42 has been reported to abolish erythromelalgia, especially in initial reports of the syndrome. It has become increasingly evident that aspirin may be effective in erythromelalgia due to myeloproliferative disease, but it is rarely effective in other forms of erythromelalgia.8,124
Increasingly, drugs acting on the nervous system are reported to be useful in inducing remission of disease or in controlling symptoms. These include selective serotonin reuptake inhibitors (SSRIs) such as venlafaxine and sertraline,125,126 tricyclic antidepressants such as amitriptyline,77 and anticonvulsants such as gabapentin.127 Intravenous lidocaine followed by oral mexiletine128 induced remission in a patient with long-standing erythromelalgia. Topical capsaicin was helpful in one report129 but not in others.8 Benzodiazepines such as clonazepam are effective occasionally.72 Sympathectomy and sympathetic nerve blocks have been reported to both relieve and exacerbate erythromelalgia.16,130–133 Stereotactic thalamotomy,134 ankle nerve crushing and neurectomy,135 and spinal cord stimulation136 have been reported to be helpful. Drugs acting on the vascular system (vasoactive drugs) may be effective. β-Blockers such as propranolol137 and labetalol33 have been reported to be successful in single cases. Calcium antagonists have been reported to both relieve and exacerbate erythromelalgia.8,124 Various doses of magnesium have been reported to relieve symptoms.138 Sodium nitroprusside infusions may be helpful in children with erythromelalgia,139–142 but one adult experienced worsening of the disease with this medication.124 Prostaglandin E1(PGE1), a potent vasodilator and platelet inhibitor administered intravenously, has successfully induced remission.10,30,139 Iloprost, a synthetic prostacyclin analog, improved patients’ symptoms more than placebo did.143 Use of ergot alkaloids, such as methysergide maleate, has been described in isolated case reports.82,144 Low-molecular-weight heparin (LMWH) has been reported to exacerbate erythromelalgia.140
Control of pain is an extremely important factor in erythromelalgia. Anesthetics have been used, including topical lidocaine (lidocaine patch),145 a combination of topical amitriptyline and ketamine,122 and epidural infusion of narcotic analgesic medications such as bupivacaine, sometimes in combination with other narcotic drugs.146–149 Narcotic analgesic drugs may be administered by various routes—oral, intravenous, intramuscular (IM),150 epidural,149 or intrathecal,151 alone or in combination with other drugs. Nonsteroidal antiinflammatory drugs (NSAIDs) such as piroxicam152 may be helpful occasionally.
Pizotyline, a benzocycloheptathiophene derivative used primarily for migraine prophylaxis, has been used for erythromelalgia.33,153,154 Systemic corticosteroids, including prednisone155 and prednisolone,156 and growth hormone157 have been useful in single cases. Antihistamines such as cyproheptadine158 have been reported to help, but many patients find them unhelpful.
A combination of pharmacological interventions may be of benefit.63,159 Nonmedicinal therapies such as acupuncture, biofeedback, hypnosis, and magnets have been variably effective.124,160
Pain rehabilitation is a useful method for managing pain-related impairment in physical and emotional functioning in patients with severe forms of erythromelalgia.123
1 Graves R.J. Clinical lectures on the practice of medicine. Dublin: Fannin; 1834.
2 Mitchell S.W. On a rare vaso-motor neurosis of the extremities and on the maladies with which it may be confounded. Am J Med Sci. 1878;76:17.
3 Smith L.A., Allen E.V. Erythermalgia (erythromelalgia) of the extremities: a syndrome characterized by redness, heat, and pain. Am Heart J. 1938;16:175.
4 Housley E. What is erythromelalgia and how should it be treated? BMJ. 1986;293:117.
5 Lewis T. Clinical observations and experiments relating to burning pain in extremities and to so-called “erythromelalgia” in particular. Clin Sci. 1933;1:175.
6 Snapper I., Kahn A.I. Bedside medicine, ed 2. London: William Heinemann Medical Books; 1967. p 106
7 Babb R.R., Alarcon-Segovia D., Fairbairn J.F.II. Erythermalgia: review of 51 cases. Circulation. 1964;29:136.
8 Davis M.D., O’Fallon W.M., Rogers R.S.III, et al. Natural history of erythromelalgia: presentation and outcome in 168 patients. Arch Dermatol. 2000;136:330.
9 Mørk C., Kalgaard O.M., Kvernebo K. Erythromelalgia: a clinical study of 102 cases (abstract). Australas J Dermatol. 1997;38(Suppl 2):50.
10 Kvernebo K. Erythromelalgia: a condition caused by microvascular arteriovenous shunting. VASA J Vasc Dis Suppl. 1998;51:1.
11 Hart F.D. William Harvey and his gout. Ann Rheum Dis. 1984;43:125.
12 Kalgaard O.M., Seem E., Kvernebo K. Erythromelalgia: a clinical study of 87 cases. J Intern Med. 1997;242:191.
13 Levesque H. Classification of erythermalgia [French]. J Mal Vasc. 1996;21:80.
14 Mørk C., Kvernebo K. Erythromelalgia: a mysterious condition? Arch Dermatol. 2000;136:406.
15 Michiels J.J., Drenth J.P., Van Genderen P.J. Classification and diagnosis of erythromelalgia and erythermalgia. Int J Dermatol. 1995;34:97.
16 Zoppi M., Zamponi A., Pagni E., et al. A way to understand erythromelalgia. J Auton Nerv Syst. 1985;13:85.
17 Regli F. Facial neuralgia and vascular facial pain [German]. Praxis. 1969;58:210.
18 Brown G.E. Erythromelalgia and other disturbances of the extremities accompanied by vasodilatation and burning. Am J Med Sci. 1932;183:468.
19 Thompson G.H., Hahn G., Rang M. Erythromelalgia. Clin Orthop. 1979;144:249.
20 Mørk C., Asker C.L., Salerud E.G., et al. Microvascular arteriovenous shunting is a probable pathogenetic mechanism in erythromelalgia. J Invest Dermatol. 2000;114:643.
21 Mørk C., Kalgaard O.M., Kvernebo K. Impaired neurogenic control of skin perfusion in erythromelalgia. J Invest Dermatol. 2002;118:699.
22 Mørk C., Kalgaard O.M., Myrvang B., et al. Erythromelalgia in a patient with AIDS. J Eur Acad Dermatol Venereol. 2000;14:498.
23 Mørk C., Kvernebo K., Asker C.L., et al. Reduced skin capillary density during attacks of erythromelalgia implies arteriovenous shunting as pathogenetic mechanism. J Invest Dermatol. 2002;119:949.
24 Lazareth I., Fiessinger J.N., Priollet P. Erythermalgia, rare acrosyndrome: 13 cases [French]. Presse Med. 1988;17:2235.
25 Drenth J.P., Michiels J.J., van Joost T. Primary and secondary erythermalgia [Dutch]. Ned Tijdschr Geneeskd. 1994;138:2231.
26 Drenth J.P., Michiels J.J. Erythromelalgia and erythermalgia: diagnostic differentiation. Int J Dermatol. 1994;33:393.
27 Drenth J.P., Michiels J.J. Erythromelalgia versus primary and secondary erythermalgia. Angiology. 1994;45:329.
28 Drenth J.P., van Genderen P.J., Michiels J.J. Thrombocythemic erythromelalgia, primary erythermalgia, and secondary erythermalgia: three distinct clinicopathologic entities. Angiology. 1994;45:451.
29 Kurzrock R., Cohen P.R. Classification and diagnosis of erythromelalgia. Int J Dermatol. 1995;34:146.
30 Littleford R.C., Khan F., Belch J.J. Skin perfusion in patients with erythromelalgia. Eur J Clin Invest. 1999;29:588.
31 Davis M.D., Wilkins F., Rooke T.W. Between episodes of erythromelalgia: a spectrum of colors. Arch Dermatol. 2006;142:1085.
32 Cook-Norris R.H., Tollefson M.M., Cruz-Inigo A.E., et al. Pediatric erythromelalgia: a retrospective review of 32 cases evaluated at Mayo Clinic over a 37-year period. J Am Acad Dermatol. 66(416), 2012.
33 Drenth J.P.H., Michiels J.J., Özsoylu S. Erythermlgia Multidisciplinary Study Group: acute secondary erythermalgia and hypertension in children. Eur J Pediatr. 1995;154:882.
34 Michiels J.J., Drenth J.P. Erythromelalgia and erythermalgia: lumpers and splitters. Int J Dermatol. 1994;33:412.
35 Kurzrock R., Cohen P.R. Erythromelalgia and myeloproliferative disorders. Arch Intern Med. 1989;149:105.
36 Michiels J.J., Abels J., Steketee J., et al. Erythromelalgia caused by platelet-mediated arteriolar inflammation and thrombosis in thrombocythemia. Ann Intern Med. 1985;102:466.
37 Itin P.H., Winkelmann R.K. Cutaneous manifestations in patients with essential thrombocythemia. J Am Acad Dermatol. 1991;24:59.
38 Michiels J.J., ten Kate F.J. Erythromelalgia in thrombocythemia of various myeloproliferative disorders. Am J Hematol. 1992;39:131.
39 McCarthy L., Eichelberger L., Skipworth E., et al. Erythromelalgia due to essential thrombocythemia. Transfusion. 2002;42:1245.
40 Michiels J.J., van Genderen P.J., Lindemans J., et al. Erythromelalgic, thrombotic and hemorrhagic manifestations in 50 cases of thrombocythemia. Leuk Lymphoma. 1996;22(Suppl 1):47.
41 Naldi L., Brevi A., Cavalieri d’Oro L., et al. Painful distal erythema and thrombocytosis: erythromelalgia secondary to thrombocytosis. Arch Dermatol. 1993;129:105.
42 Ongenae K., Janssens A., Noens L., et al. Erythromelalgia: a clue to the diagnosis of polycythemia vera. Dermatology. 1996;192:408.
43 Kudo I., Soejima K., Morimoto S., et al. Polycythemia vera associated with erythromelalgia [Japanese]. Rinsho Ketsueki. 1988;29:1055.
44 Coppa L.M., Nehal K.S., Young J.W., et al. Erythromelalgia precipitated by acral erythema in the setting of thrombocytopenia. J Am Acad Dermatol. 2003;48:973.
45 Mehle A.L., Nedorost S., Camisa C. Erythromelalgia. Int J Dermatol. 1990;29:567.
46 Yosipovitch G., Krause I., Blickstein D. Erythromelalgia in a patient with thrombotic thrombocytopenic purpura. J Am Acad Dermatol. 1992;26:825.
47 Rey J., Cretel E., Jean R., et al. Erythromelalgia in a patient with idiopathic thrombocytopenic purpura (letter). Br J Dermatol. 2003;148:177.
48 Thami G.P., Bhalla M. Erythromelalgia induced by possible calcium channel blockade by ciclosporin. BMJ. 2003;326:910.
49 Wagner D.R., Spengel F., Middeke M. Erythromelalgia unmasked during norephedrine therapy: a case report. Angiology. 1993;44:244.
50 Drenth J.P., Michiels J.J., Van Joost T., et al. Verapamil-induced secondary erythermalgia. Br J Dermatol. 1992;127:292.
51 Levesque H., Moore N., Wolfe L.M., et al. Erythromelalgia induced by nicardipine (inverse Raynaud’s phenomenon?). BMJ. 1989;298:1252.
52 Drenth J.P. Erythromelalgia induced by nicardipine (letter). BMJ. 1989;298:1582.
53 Albers G.W., Simon L.T., Hamik A., et al. Nifedipine versus propranolol for the initial prophylaxis of migraine. Headache. 1989;29:215.
54 Brodmerkel G.J.Jr. Nifedipine and erythromelalgia (letter). Ann Intern Med. 1983;99:415.
55 Fisher J.R., Padnick M.B., Olstein S. Nifedipine and erythromelalgia. Ann Intern Med. 1983;98:671.
56 Monk B.E., Parkes J.D., Du Vivier A. Erythromelalgia following pergolide administration. Br J Dermatol. 1984;111:97.
57 Dupont E., Illum F., Olivarius B.F. Bromocriptine and erythromelalgia-like eruptions (letter). Neurology. 1983;33:670.
58 Eisler T., Hall R.P., Kalavar K.A., et al. Erythromelalgia-like eruption in parkinsonian patients treated with bromocriptine. Neurology. 1981;31:1368.
59 Calne D.B., Plotkin C., Williams A.C., et al. Long-term treatment of parkinsonism with bromocriptine. Lancet. 1978;1:735.
60 Dolan C.K., Hall M.A., Turlansky G.W. Secondary erythermalgia in an HIV-1-positive patient. AIDS Read. 2003;113:91.
61 Itin P.H., Courvoisier S., Stoll A., et al. Secondary erythermalgia in an HIV-infected patient: is there a pathogenic relationship? Acta Derm Venereol. 1996;76:332.
62 Rabaud C., Barbaud A., Trechot P. First case of erythermalgia related to hepatitis B vaccination. J Rheumatol. 1999;26:233.
63 Confino I., Passwell J.H., Padeh S. Erythromelalgia following influenza vaccine in a child. Clin Exp Rheumatol. 1997;15:111.
64 Clayton C., Faden H. Erythromelalgia in a twenty-year-old with infectious mononucleosis. Pediatr Infect Dis J. 1993;12:101.
65 Zheng Z.M., Specter S., Zhang J.H., et al. Further characterization of the biological and pathogenic properties of erythromelalgia-related poxviruses. J Gen Virol. 1992;73:2011.
66 Mo Y.M. An epidemiological study on erythromelalgia [Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 1989;10:291.
67 Mørk C., Kalgaard O.M., Kvernebo K. Erythromelalgia as a paraneoplastic syndrome in a patient with abdominal cancer (letter). Acta Derm Venereol. 1999;79:394.
68 Kurzrock R., Cohen P.R. Paraneoplastic erythromelalgia. Clin Dermatol. 1993;11:73.
69 Levine A.M., Gustafson P.R. Erythromelalgia: case report and literature review. Arch Phys Med Rehabil. 1987;68:119.
70 Lantrade P., Didier A., Ille H., et al. Thymome malin et acrosyndromes vasculaires paroxystiques: une observation. Ann Med Interne (Paris). 1980;131:228.
71 Cailleux N., Levesque H., Courtois H. Erythermalgia and systemic lupus erythematosus [French]. J Mal Vasc. 1996;21:88.
72 Kraus A. Erythromelalgia in a patient with systemic lupus erythematosus treated with clonazepam. J Rheumatol. 1990;17:120.
73 Michiels J.J. Erythermalgia in SLE. J Rheumatol. 1991;18:481.
74 Alarcon-Segovia D., Diaz-Jouanen E. Case report: erythermalgia in systemic lupus erythematosus. Am J Med Sci. 1973;266:149.
75 Drenth J.P., Michiels J.J., Van Joost T., et al. Erythermalgia secondary to vasculitis. Am J Med. 1993;94:549.
76 Garrett S.J., Robinson J.K. Erythromelalgia and pregnancy. Arch Dermatol. 1990;126:157.
77 Herskovitz S., Loh F., Berger A.R., et al. Erythromelalgia: association with hereditary sensory neuropathy and response to amitriptyline. Neurology. 1993;43:621.
78 Staub D.B., Munger B.L., Uno H., et al. Erythromelalgia as a form of neuropathy. Arch Dermatol. 1992;128:1654.
79 Nagamatsu M., Ueno S., Teramoto J., et al. A case of erythromelalgia with polyneuropathy [Japanese]. Nippon Naika Gakkai Zasshi. 1989;78:418.
80 Tridon P., Vidailhet C., Schweitzer F. The acrodynic form of the Riley-Day syndrome [French]. Pediatrie. 1989;44:455.
81 Cendrowski W. Secondary erythromelalgia in multiple sclerosis [Polish]. Wiad Lek. 1988;41:1477.
82 Vendrell J., Nubiola A., Goday A., et al. Erythromelalgia associated with acute diabetic neuropathy: an unusual condition. Diabetes Res. 1988;7:149.
83 Kikuchi I., Inoue S., Tada S. A unique erythermalgia in a patient with von Recklinghausen neurofibromatosis. J Dermatol. 1985;12:436.
84 van Genderen P.J., Michiels J.J., Drenth J.P. Hereditary erythermalgia and acquired erythromelalgia. Am J Med Genet. 1993;45:530.
85 Finley W.H., Lindsey J.R.Jr, Fine J.D., et al. Autosomal dominant erythromelalgia. Am J Med Genet. 1992;42:310.
86 Michiels J.J., van Joost T., Vuzevski V.D. Idiopathic erythermalgia: a congenital disorder. J Am Acad Dermatol. 1989;21:1128.
87 Cohen I.J., Samorodin C.S. Familial erythromelalgia. Arch Dermatol. 1982;118:953.
88 Krebs A., Andres H.U. On the clinical features of erythromelalgia: familial incidence of an idiopathic form in mother and daughter [German]. Schweiz Med Wochenschr. 1969;99:344.
89 Reed K.B., Davis M.D. Incidence of erythromelalgia: a population-based study in Olmsted County, Minnesota. J Eur Acad Dermatol Venereol. 2009;23:13.
90 Schechner J. Red skin re-read. J Invest Dermatol. 2002;119:781.
91 Davis M.D.P., Sandroni P., Rooke T.W., et al. Erythromelalgia: vasculopathy, neuropathy, or both? A prospective study of vascular and neurophysiologic studies in erythromelalgia. Arch Dermatol. 2003;139:1337.
92 Sandroni P., Davis M.D.P., Harper C.M.Jr, et al. Neurophysiologic and vascular studies in erythromelalgia: a retrospective analysis. J Clin Neuromuscul Dis. 1999;1:57.
93 Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78:603.
94 Lazareth I., Priollet P. Coexistence of Raynaud’s syndrome and erythromelalgia (letter). Lancet. 1990;335:1286.
95 Slutsker. G.E. Coexistence of Raynaud’s syndrome and erythromelalgia (letter). Lancet. 1990;335:853.
96 Davis M.D., Genebriera J., Sandroni P., et al. Thermoregulatory sweat testing in patients with erythromelalgia. Arch Dermatol. 2006;142:1583–1588.
97 Kazemi B., Shooshtari S.M., Nasab M.R., et al. Sympathetic skin response (SSR) in erythromelalgia. Electromyogr Clin Neurophysiol. 2003;43:165.
98 Davis M.D., Rooke T.W., Sandroni P. Mechanisms other than shunting are likely contributing to the pathophysiology of erythromelalgia. J Invest Dermatol. 2000;115:1166.
99 Liu Y. A study of erythromelalgia in relation to the autonomic nervous system: report of 321 cases of functional disorders of the autonomic nervous system [Chinese]. Zhonghua Shen Jing Jing Shen Ke Za Zhi. 1990;23:47.
100 Orstavik K., Weidner C., Schmidt R., et al. Pathological C-fibres in patients with a chronic painful condition. Brain. 2003;126:567.
101 Uno H., Parker F. Autonomic innervation of the skin in primary erythermalgia. Arch Dermatol. 1983;119:65.
102 Layzer R.B. Hot feet: erythromelalgia and related disorders. J Child Neurol. 2001;16:199.
103 Sugiyama Y., Hakusui S., Takahashi A., et al. Primary erythromelalgia: the role of skin sympathetic nerve activity. Jpn J Med. 1991;30:564.
104 Littleford R.C., Khan F., Belch J.J. Impaired skin vasomotor reflexes in patients with erythromelalgia. Clin Sci (Lond). 1999;96:507.
105 Drenth J.P., Finley W.H., Breedveld G.J., et al. The primary erythermalgia-susceptibility gene is located on chromosome 32. Am J Hum Genet. 2001;68:1277.
106 Yang Y., Wang Y., Li S., et al. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet. 2004;41:171.
107 Cummins T.R., Dib-Hajj S.D., Waxman S.G. Electrophysiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy. J Neurosci. 2004;24:8232.
108 Michiels J.J., te Morsche R.H., Jansen J.B., et al. Autosomal dominant erythermalgia associated with a novel mutation in the voltage-gated sodium channel alpha subunit Nav1.7. Arch Neurol. 2005;62:1587.
109 Han C., Dib-Hajj S.D., Lin Z., et al. Early- and late-onset inherited erythromelalgia: genotype-phenotype correlation. Brain. 2009;132:1711.
110 Estacion M., Waxman S.G., Dib-Hajj S.D. Effects of ranolazine on wild-type and mutant hNav1.7 channels and on DRG neuron excitability. Mol Pain. 2010;6:35.
111 Waxman S.G., Dib-Hajj S. Erythermalgia: molecular basis for an inherited pain syndrome. Trends Mol Med. 2005;11:555.
112 Drenth J.P., Te Morsche R.H., Mansour S., et al. Primary erythermalgia as a sodium channelopathy: screening for SCN9A mutations: exclusion of a causal role of SCN10A and SCN11A. Arch Dermatol. 2008;144:320.
113 van Genderen P.J., Lucas I.S., van Strik R., et al. Erythromelalgia in essential thrombocythemia is characterized by platelet activation and endothelial cell damage but not by thrombin generation. Thromb Haemost. 1996;76:333.
114 Kurzrock R., Cohen P.R. Erythromelalgia: review of clinical characteristics and pathophysiology. Am J Med. 1991;91:416.
115 Drenth J.P., Vuzevski V., Van Joost T., et al. Cutaneous pathology in primary erythermalgia. Am J Dermatopathol. 1996;18:30.
116 Lazareth I. False erythermalgia [French]. J Mal Vasc. 1996;21:84.
117 Michiels J.J., ten Kate F.W., Vuzevski V.D., et al. Histopathology of erythromelalgia in thrombocythaemia. Histopathology. 1984;8:669.
118 Croue A., Gardembas-Pain M., Verret J.L., et al. Histopathologic lesions in erythromelalgia during essential thrombocythemia [French]. Ann Pathol. 1993;13:128.
119 Davis M.D., Rooke T. Erythromelalgia. Curr Treat Options Cardiovasc Med. 2002;4:207.
120 Davis M.D., Sandroni P. Lidocaine patch for pain of erythromelalgia: follow-up of 34 patients. Arch Dermatol. 2005;141:1320.
121 Davis M.D., Sandroni P. Lidocaine patch for pain of erythromelalgia. Arch Dermatol. 2002;138:17–19.
122 Sandroni P., Davis M.D. Combination gel of 1% amitriptyline and 0.5% ketamine to treat refractory erythromelalgia pain: a new treatment option? Arch Dermatol. 2006;142:283.
123 Durosaro O., Davis M.D., Hooten W.M., et al. Intervention for erythromelalgia, a chronic pain syndrome: comprehensive pain rehabilitation center, Mayo Clinic. Arch Dermatol. 2008;144:1578.
124 Cohen J.S. Erythromelalgia: new theories and new therapies. J Am Acad Dermatol. 2000;43:841.
125 Rudikoff D., Jaffe I.A. Erythromelalgia: response to serotonin reuptake inhibitors. J Am Acad Dermatol. 1997;37:281.
126 Moiin A., Yashar S.S., Sanchez J.E., et al. Treatment of erythromelalgia with a serotonin/noradrenaline reuptake inhibitor. Br J Dermatol. 2002;146:336.
127 McGraw T., Kosek P. Erythromelalgia pain managed with gabapentin. Anesthesiology. 1997;86:988.
128 Kuhnert S.M., Phillips W.J., Davis M.D. Lidocaine and mexiletine therapy for erythromelalgia. Arch Dermatol. 1999;135:1447.
129 Muhiddin K.A., Gallen I.W., Harries S., et al. The use of capsaicin cream in a case of erythromelalgia. Postgrad Med J. 1994;70:841.
130 Postlethwaite J.C. Lumbar sympathectomy: a retrospective study of 142 operations on 100 patients. Br J Surg. 1973;60:878.
131 Shiga T., Sakamoto A., Koizumi K., et al. Endoscopic thoracic sympathectomy for primary erythromelalgia in the upper extremities. Anesth Analg. 1999;88:865.
132 Takeda S., Tomaru T., Higuchi M. A case of primary erythromelalgia (erythermalgia) treated with neural blockade [Japanese]. Masui. 1989;38:388.
133 Seishima M., Kanoh H., Izumi T., et al. A refractory case of secondary erythermalgia successfully treated with lumbar sympathetic ganglion block. Br J Dermatol. 2000;143:868.
134 Kandel E.I. Stereotactic surgery of erythromelalgia. Stereotact Funct Neurosurg. 1990;54–55:96.
135 Sadighi P.J., Arbid E.J. Neurectomy for palliation of primary erythermalgia. Ann Vasc Surg. 1995;9:197.
136 Graziotti P.J., Goucke C.R. Control of intractable pain in erythromelalgia by using spinal cord stimulation. J Pain Symptom Manage. 1993;8:502.
137 Bada J.L. Treatment of erythromelalgia with propranolol. Lancet. 1977;2:412.
138 Cohen J.S. High-dose oral magnesium treatment of chronic, intractable erythromelalgia. Ann Pharmacother. 2002;36:255.
139 Kvernebo K., Seem E. Erythromelalgia—pathophysiological and therapeutic aspects: a preliminary report. J Oslo City Hosp. 1987;37:9.
140 Conri C.L., Azoulai P., Constans J., et al. Erythromelalgia and low molecular weight heparin [French]. Therapie. 1994;49:518.
141 Ozsoylu S., Coskun T. Sodium nitroprusside treatment in erythromelalgia. Eur J Pediatr. 1984;141:185.
142 Stone J.D., Rivey M.P., Allington D.R. Nitroprusside treatment of erythromelalgia in an adolescent female. Ann Pharmacother. 1997;31:590.
143 Kalgaard O.M., Mørk C., Kvernebo K. Prostacyclin reduces symptoms and sympathetic dysfunction in erythromelalgia in a double-blind randomized pilot study. Acta Derm Venereol. 2003;83:442.
144 Pepper H. Primary erythermalgia: report of a patient treated with methysergide maleate. JAMA. 1968;203:1066.
145 Davis M.D., Sandroni P. Lidocaine patch for pain of erythromelalgia. Arch Dermatol. 2002;138:17.
146 Stricker L.J., Green C.R. Resolution of refractory symptoms of secondary erythermalgia with intermittent epidural bupivacaine. Reg Anesth Pain Med. 2001;26:488.
147 D’Angelo R., Cohen I.T., Brandom B.W. Continuous epidural infusion of bupivacaine and fentanyl for erythromelalgia in an adolescent. Anesth Analg. 1992;74:142.
148 Rauck R.L., Naveira F., Speight K.L., et al. Refractory idiopathic erythromelalgia. Anesth Analg. 1996;82:1097.
149 Mohr M., Schneider K., Grosche M., et al. Cervical epidural infusion of morphine and bupivacaine in severe erythromelalgia [German]. Anasthesiol Intensivmed Notfallmed Schmerzther. 1994;29:371.
150 Trapiella Martinez L., Quiros J.F., Caminal Montero L., et al. Treatment of erythromelalgia with buprenorphine (letter) [Spanish]. Rev Clin Esp. 1997;197:792.
151 Macres S., Richeimer S. Successful treatment of erythromelalgia with intrathecal hydromorphone and clonidine. Clin J Pain. 2000;16:310.
152 Calderone D.C., Finzi E. Treatment of primary erythromelalgia with piroxicam. J Am Acad Dermatol. 1991;24:145.
153 H’Mila R., Samoud A., Souid M., et al. Erythermalgia: a rare vascular acrosyndrome [French]. Arch Fr Pediatr. 1991;48:555.
154 Guillet M.H., Le Noach E., Milochau P., et al. Familial erythermalgia treated with pizotifen [French]. Ann Dermatol Venereol. 1995;122:777.
155 Drenth J.P., Michiels J.J. Treatment options in primary erythermalgia? (letter). Am J Hematol. 1993;43:154.
156 Kasapcopur O., Akkus S., Erdem A., et al. Erythromelalgia associated with hypertension and leukocytoclastic vasculitis in a child. Clin Exp Rheumatol. 1998;16:184.
157 Cimaz R., Rusconi R., Fossali E., et al. Unexpected healing of cutaneous ulcers in a short child. Lancet. 2001;358:211.
158 Sakakibara R., Fukutake T., Kita K., et al. Treatment of primary erythromelalgia with cyproheptadine. J Auton Nerv Syst. 1996;58:121.
159 Suh D.H., Kim S.D., Ahn J.S., et al. A case of erythromelalgia successfully controlled by systemic steroids and pentazocine: is it related to a unique subtype of neutrophilic dermatosis? J Dermatol. 2000;27:204.
160 Chakravarty K., Pharoah P.D., Scott D.G., et al. Erythromelalgia: the role of hypnotherapy. Postgrad Med J. 1992;68:44.