Chapter 82
Erectile Dysfunction
Boback M. Berookhim, John P. Mulhall
Based on a chapter in the seventh edition by Ralph G. DePalma
Erectile dysfunction (ED) is the recurrent or consistent inability to obtain or to maintain a penile erection sufficient for satisfactory sexual performance.1 A common problem with significant negative effects on the quality of life of both the patient and his partner, it is associated with poor relationship satisfaction, negative general health perceptions, and role limitations due to physical and emotional problems.2
Epidemiology
Estimates on the prevalence of ED vary; however, it is estimated that at least 20 million men in the United States experience the condition.3 The Massachusetts Male Aging Study has reported a combined prevalence of ED in 52% of men older than 40 years, with an increase in the rate of moderate dysfunction from 17% to 34% and of complete ED from 5% to 15% between the ages of 40 and 70 years, respectively.4 Another study, looking at data from the National Health and Nutrition Examination Survey, reported a combined prevalence of ED in 18% of men older than 20 years, with an increase from 7% of the population between the ages of 20 and 29 years to 78% of those older than 75 years.5 The prevalence of ED appears to be similar among different ethnic groups; a different population-based study demonstrated a 22% rate of ED in white men older than 40 years, 24% rate among black men, and 20% rate among Hispanic men.6 Differences noted in the prevalence rates across these studies are partially due to nonstandardized definitions of the severity of ED, especially given the frequently subjective nature of this reported complaint.
Physiology of Penile Erection
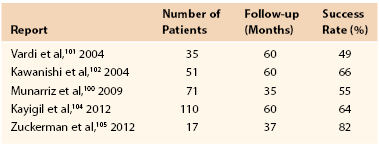
The penis is a composite structure: the paired corpora cavernosa dorsally and the ventral corpus spongiosum, which contains the urethra. The corpora cavernosa are surrounded by the tunica albuginea, a bilayered structure that supports the cavernous tissue and provides the flexibility, rigidity, and strength of the penis. The spongiosum lacks the outer layer of the tunica, allowing a low-pressure urethra during erection. Blood flow to the penis typically originates from the internal pudendal artery, a branch of the internal iliac artery. Distally, it then becomes the common penile artery, where it subdivides into the dorsal, cavernosal, and bulbourethral arteries. Accessory pudendal arteries, arising from the external iliac, obturator, vesical, and femoral arteries, frequently contribute significantly to the arterial supply to the penis.7 The cavernosal arteries affect tumescence of the penis and end at the helicine arteries, which open into the endothelium-lined lacunar spaces. Venous drainage begins within the lacunar spaces, which drain into subtunical venules and emerge as emissary veins. The deep venous system continues with these emissary veins draining into the cavernosal, deep dorsal, or spongiosal veins until they join either the prostatic venous plexus or the internal pudendal veins. Superficial veins coalesce to form the superficial dorsal vein, which drains into the great saphenous veins (Fig. 82-1).
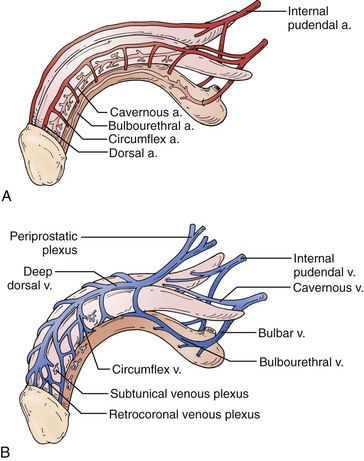
Figure 82-1 Penile vascular anatomy. A, Penile arterial supply. B, Penile venous drainage. (From Wein AJ: Campbell-Walsh urology, ed 10, Philadelphia, 2011, Saunders Elsevier, pp 691-692.)
Erection is the result of an integrative physiologic complex of psychologic, neuronal, hormonal, vascular, and cavernous smooth muscle systems that begins in the brain. Relevant innervation to the penis includes the somatic nerves, responsible for sensation and contraction of the bulbocavernosus and ischiocavernosus muscles, and the parasympathetic and sympathetic autonomic nerves located at levels S2-4 and T12-L2, respectively, which are instrumental in tumescence. These segments form the hypogastric and pelvic plexuses. Those fibers that innervate the penis compose the cavernous nerve, which travels along the posterolateral aspect of the prostate and then accompanies the membranous urethra through the urogenital diaphragm.8 The cavernous nerves then innervate the helicine arteries and trabecular smooth muscle.9
The cavernous smooth muscle plays a key role in erectile function and remains contracted in the flaccid state of the penis under α-adrenergic control. The erectile process begins with sexual stimulation, which increases parasympathetic activity and stimulates the release of neurotransmitters from cavernous nerve terminals or from the endothelium of the cavernosal arteries, resulting in relaxation of the penile smooth muscle.10,11 This is mediated by the nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) pathway, ultimately resulting in increased blood flow through the penile arteries (Fig. 82-2).12,13 This blood flow then expands the sinusoids, compressing subtunical venules and peripheral sinusoids and ultimately reducing venous outflow (veno-occlusive mechanism). This leads to an increase in intracavernous pressure, resulting in an erection.
Pathophysiology of Erectile Dysfunction
Given the interaction of multiple different physiologic systems required for adequate erectile function, it is easy to appreciate that derangements in any one system can serve as a cause of ED. ED is classified according to its likely origin: psychogenic, neurogenic, endocrinologic, vasculogenic, and drug induced. It is often multifactorial in nature.
Psychogenic Erectile Dysfunction
Psychogenic (nonorganic) ED is generally assumed to be ED predominantly or exclusively related to psychologic or interpersonal factors.14 It is estimated to affect 10% to 15% of all men with ED. It has been further classified as situational psychogenic ED, which is noted to be partner related, performance related, or associated with psychologic distress, or generalized psychogenic ED, secondary to a lack of sexual arousability or a disorder of sexual intimacy.15 Psychogenic ED is an epinephrine-mediated phenomenon and is a diagnosis of exclusion, once physical (organic) factors have been ruled out. The clinical features include sudden-onset ED, with intermittency of function or a situational nature to the erectile problems, and good nocturnal erections.16
Neurogenic Erectile Dysfunction
Neurogenic ED, an uncommon entity, is secondary to events disrupting central neural networks or the peripheral nerves involved in sexual function. It is further subclassified into supraspinal, spinal, and peripheral neurogenic ED.17 Supraspinal ED is generally caused by tumor, stroke, Parkinson’s disease, dementia, and temporal lobe epilepsy.17 Spinal causes include multiple sclerosis, spinal cord injury, transverse myelitis, myelodysplasia, and lumbar disk disease or surgery.17–19 Peripheral causes include diseases associated with lower motor neuron lesions, trauma, pelvic disease, and radical pelvic surgery.17 Pelvic surgery, particularly radical prostatectomy, has a high risk of cavernous nerve injury and has been demonstrated to have a significant effect on erectile function.20
Endocrinologic Erectile Dysfunction
Androgens, specifically testosterone, have been linked to sexual desire, orgasmic function, and erectile function. However, low serum testosterone concentration has not been clearly linked to the presence or severity of ED.21,22 Animal models have shown that testosterone is important in the regulation of the expression of NO synthase and phosphodiesterase type 5 (PDE5) inside the penis.23 Hyperprolactinemia, leading to the inhibition of luteinizing hormone, is associated with low libido and possibly ED. Profound hypothyroidism (low luteinizing hormone levels) and profound hyperthyroidism (high estradiol levels) may be associated with sexual dysfunction.
Vasculogenic Erectile Dysfunction
ED and cardiovascular disease share common risk factors, including hypertension, diabetes mellitus, hypercholesterolemia, obesity, and smoking, leading to the concept that ED is another manifestation of vascular disease.24 A large patient survey of more than 7500 patients with hypertension and diabetes demonstrated ED in 67% of patients with hypertension alone, 71% of patients with diabetes alone, and 78% of patients with both conditions.25 A Spanish study of more than 2400 patients has demonstrated each of these to be independently associated with ED, with age-adjusted odds ratios of having ED of 4.0 in diabetics, 1.58 in patients with hypertension, 1.63 in patients with high cholesterol, 2.63 in patients with peripheral vascular disease, and 2.5 among smokers.26 ED patients have also been noted to have significantly higher plasma levels of low-density lipoprotein compared with normal counterparts.27
The Princeton III consensus guidelines recognize ED as a strong predictor of cardiovascular disease and specifically of coronary artery disease.28 Data from a meta-analysis of prospective cohort studies evaluating ED and the risk of cardiovascular disease demonstrated a combined relative risk for men with ED of 1.48 for overall cardiovascular disease, 1.46 for coronary artery disease, 1.35 for stroke, and 1.19 for all-cause mortality.29 In addition, ED has been described as an independent marker of cardiovascular events and all-cause mortality after adjustment for age, weight, hypertension, diabetes, hyperlipidemia, and cigarette smoking.30 ED is also an independent predictor for peripheral arterial disease (odds ratio, 1.97), with a significant stepwise increase in prevalence of peripheral arterial disease with increasing severity of ED (28% of men with mild ED, 33% with moderate ED, and 40% with severe ED).31
Looking specifically at patients with comorbid ED and cardiovascular disease, a number of theories have been proposed to explain the pathophysiologic association between these two disease states. Given that atherosclerosis is a systemic disease, with all vascular beds affected to the same extent, the artery size hypothesis proposes that symptoms are manifested at different time points according to the diameter of the arterial blood supply.32 Accordingly, a 50% stenosis of the coronary arteries would amount to a nearly complete occlusion of the penile arteries despite an equal amount of total atherosclerotic plaque in both vessels. In addition to the flow-limiting stenosis, ED due to arterial insufficiency is related to lower oxygen tension in intracavernous blood.33 This has been associated with possibly decreased prostaglandin E1 and E2 formation, leading to transforming growth factor-β1–induced collagenization of cavernous smooth muscle.17,34 Increases in the connective tissue have been correlated with failure of the veno-occlusive mechanism required for good erectile function.35
Endothelial dysfunction serves as an additional link between ED and cardiovascular disease. NO produced by the endothelium is necessary for the increased flow and vasodilatation of the penile arteries, which is necessary for erection. In both ED and cardiovascular disease, a deficiency of NO is brought about by impaired production or increased degradation, thereby decreasing vasodilatation and modulation of smooth muscle cells and inhibiting cellular adhesion.36,37 Among ED patients without evidence of cadiovascular disease, endothelial dysfunction has been noted within the penile vasculature but not in the small arteries of the forearm, indicating that penile endothelial dysfunction may occur earlier than in other vascular beds.38
ED has also been demonstrated to be a strong predictor of subsequent cardiovascular events. Data from the Prostate Cancer Prevention Trial demonstrated that incident ED was associated with a 25% increased likelihood of subsequent cardiovascular events during the 5-year study follow-up and that men with incident ED during the study period were at a 45% increased risk of cardiovascular events.39 This association was similar to the risk associated with current smoking or a family history of myocardial infarction. Another study addressing men referred with ED and vasculogenic ED documented on penile ultrasonography demonstrated a 20% rate of abnormalities on stress echocardiography in cardiac evaluation.40
In addition to arterial causes, as indicated before, vasculogenic ED is also often the result of inadequate venous occlusion.41 Corporal veno-occlusive dysfunction may be caused by the development of large venous channels draining the cavernous tissue or be a result of degenerative or functional changes in the tunica albuginea, as is seen in Peyronie’s disease.42,43
Drug-Induced Erectile Dysfunction
Antihypertensives, psychotropics, and antiandrogens are the primary drug classes associated with ED. Among the antihypertensives, multiple studies have demonstrated significant increases in ED among patients receiving thiazides compared with placebo.44,45 Studies of β-adrenergic antagonists demonstrate mixed results with respect to their potentially causing ED; nonselective drugs, such as propranolol, show clear associations with ED, and agents with higher selectivity for the β1-adrenoreceptor, such as acebutolol, show a reduction in ED compared with placebo.46 Angiotensin receptor blockers have been consistently shown to have pro-erectile effects.47 Antidepressants, specifically selective serotonin reuptake inhibitors, can negatively affect all steps of the sexual response cycle, most notably ejaculatory latency. Differences are noted in the incidence of ED among different medications, with higher rates noted in patients taking paroxetine.48 Antiandrogens cause partial or complete blockade of circulating androgens either through inhibition of production or by antagonism at the level of the androgen receptor and are frequently used in the treatment of prostate cancer. Use of these medications is generally associated with decreased sexual desire, although medical castration with luteinizing hormone–releasing hormone agonists or antagonists and nonsteroidal antiandrogens such as flutamide and bicalutamide can also contribute to veno-occlusive dysfunction.49 Other classes of drugs associated with ED include 5α-reductase inhibitors (finasteride, dutasteride) and digoxin.50,51
Assessment of Erectile Dysfunction
History and Physical Examination
ED is often multifactorial in origin, and the initial evaluation of ED must include a complete medical, psychosocial, and sexual history.52 Care should be taken to assess for signs and symptoms of possible underlying conditions, with a detailed review of current medications, to identify possibly reversible and underlying treatable disorders causing ED. One study of more than 270,000 ED patients demonstrated a 68% prevalence of a significant underlying condition at the time of ED presentation.53
Given the often sensitive subject matter, clinicians should give consideration to use of validated questionnaires to ease into the conversation, including the International Index of Erectile Function (IIEF) and the abridged, five-item version of the IIEF, the Sexual Health Inventory for Men. These have been demonstrated to accurately diagnose and quantify the severity of the ED complaint.54,55 Descriptive measures should be used for rigidity and sustainability of erections during sexual arousal, intermittency or any situational aspect of erections, and presence of nocturnal erections to further define underlying causes of ED. Patients complaining of sudden-onset ED with loss of sustaining capability and strong nocturnal erections are more likely to have nonorganic ED secondary to psychogenic causes. Frequently, physical examination will not reveal a specific cause of ED. A general examination, with evaluation of blood pressure, heart rate, abdominal pulsations, peripheral pulses, and male secondary sex characteristics, including the presence of gynecomastia, and a focused genital examination should be performed.52,56 Attention should be paid to the absence of femoral pulses, possibly indicative of aortoiliac arterial occlusive disease. This can be a marker of decreased penile arterial flow through the hypogastric (internal iliac) arteries. Genital examination should include examination of the penis, specifically the size and position of the urethral meatus; determination of the presence of tunical plaques; evaluation of testicular size and consistency; and a digital rectal examination in the appropriately aged man.56
Laboratory Evaluation and Adjunctive Testing
According to the International Consultation on Sexual Medicine committee, recommended laboratory tests to confirm the presence of underlying diseases include fasting blood glucose concentration, lipid profile, and serum testosterone level, with optional examinations such as thyroid function testing based on the clinical scenario.52,56 The Princeton III consensus statement recommends that all men with organic ED older than 30 years be considered at increased cardiovascular disease risk until recommended checks suggest otherwise and further recommends resting electrocardiography and serum creatinine level in addition to the previously described testing in men without known cardiovascular disease.28 It also recommends, in consultation with a cardiologist or primary care physician, performance of a thorough noninvasive and, when indicated, invasive evaluation of cardiovascular disease status, including measurement of biomarkers, physiologic stress testing for ischemia, and anatomic clarification by coronary computed tomographic angiography.28
Nocturnal penile tumescence monitoring, to study nocturnal erectile quality, may be used both in a sleep laboratory setting and with a portable, home-use device such as the RigiScan (Timm Medical Technologies, Minneapolis, Minn).57 It allows the monitoring of rigidity, tumescence, and number and duration of erectile events.58 Testing, however, does not indicate the cause and severity of ED and does not evaluate wakeful, sexually relevant erections. In contemporary practice, its routine use for diagnostic purposes is significantly limited.
Vascular Evaluation
Vascular studies in the patient with ED are designed to define the presence of arteriogenic ED or veno-occlusive dysfunction.
Penile-Brachial Pressure Index
The penile-brachial pressure index (PBI) refers to the ratio of penile systolic pressure to brachial systolic pressure and has been used to evaluate for the presence of significant hemodynamic occlusions proximal to the penile arteries contributing to ED (e.g., in the aorta or iliac arteries). Penile systolic blood pressure is measured by applying a small pediatric blood pressure cuff to the base of the flaccid penis and measuring the systolic blood pressure with a Doppler probe. The validity of this technique in the evaluation of ED has been called into question, given significant interobserver variability, failure to measure pressure in the erect state, and concerns with false-positive diagnosis of arterial insufficiency. In a study of 88 patients referred for evaluation of claudication, the sensitivity of PBI to determine clinically meaningful arterial stenosis or occlusion in the hypogastric circulation was determined with use of angiography as the “gold standard.”59 By use of a receiver operating characteristic curve, the only area significantly different from random choice was the detection of bilateral occlusion with PBI. On the basis of analysis of the positive and negative predictive values, the authors reported an inability to discriminate normal from abnormal angiographic results using a PBI threshold of 0.6. Thus, its use in the evaluation of ED is primarily of historical significance.59,60
Office Injection Testing
Intracavernosal injection testing is performed by injection of a vasodilatory drug (or cocktail of drugs), combined with genital or audiovisual stimulation, in a clinical setting. The erectile response is then evaluated by a clinician to rate both rigidity and duration of response. Patients are monitored for detumescence while in the office after the test is performed, and those who do not spontaneously detumesce within 1 hour of injection are given intracavernous injections of a diluted phenylephrine solution. Vasodilatory medications used include prostaglandin E1 (PGE1) alone and a combination of medications including PGE1, papaverine, and phentolamine. Whereas intracavernosal injection testing has been used in an attempt to differentiate organic from psychogenic ED, it is now accepted that its only value is to define a functional veno-occlusive mechanism in men who develop a rigid and sustained erection.61
Failure to obtain a rigid erection may indicate vascular disease but may also be the result of an excessive sympathetic response associated with anxiety during intracavernosal injection testing.62 Currently, the intracavernosal injection test is seldom performed and generally considered obsolete.
Duplex Ultrasonography of the Penis
Doppler duplex ultrasound (DUS) of the penis is a reliable and noninvasive diagnostic method for evaluating ED that allows objective quantification of the blood flow to the penis. It provides a physiologic diagnosis to guide therapy in patients with a poor response to oral ED therapy, differentiates psychogenic from organic ED, aids in the evaluation of the young man with primary ED or a history of pelvic trauma, and suggests the need for cardiovascular evaluation in the man with vasculogenic ED without overt cardiovascular disease risk factors.63
Given the significant influence of psychologic and environmental factors on erectile function, Doppler DUS should be performed in a quiet and comfortable room, isolated from intrusion and other distractions. An erection is then pharmacologically induced by intracavernosal injection with vasoactive medications. If a good erection is not achieved, the dose should be repeated to ensure maximal smooth muscle relaxation for accurate interpretation of the results. Rigidity and sustainability of the erection are noted by the examiner. Doppler DUS is performed with a high-resolution linear array ultrasound transducer, with a frequency between 7.5 and 12 MHz. With the patient in the supine position, the penis is scanned and the location of the left and right cavernosal arteries is identified. Peak systolic velocity (PSV), end-diastolic velocity (EDV), and resistive index (RI) are measured. The penis is then evaluated with use of B-mode images to observe for the presence of tunical plaque (Peyronie’s disease), fibrosis, and calcification either in the vasculature or in the plaque itself.
Various criteria have been suggested for normal PSV values, but it is generally accepted that the patients with PSV below 25 cm/s have evidence of arteriogenic ED.64 This level has been noted to have a sensitivity of 100% and a specificity of 86% among patients with abnormalities on pudendal angiography.65 Use of values of 35 cm/s decreases the sensitivity to 76% but increases specificity to 92%.66 Significant asymmetry of PSV, with a difference of more than 10 cm/s between the right and left sides, suggests a significant atherosclerotic lesion or iatrogenic or surgical cause of a decrease in arterial flows.66 Veno-occlusive dysfunction is evaluated by EDV, in the presence of normal arterial inflow. In general, an EDV of greater than 5 cm/s is accepted as the measurement at which corporal veno-occlusive dysfunction is present (Fig. 82-3).63,66 Given concerns for the specificity of EDV alone for the diagnosis of corporal veno-occlusive dysfunction in patients with arterial insufficiency, RI has been used with a cut point of less than 0.75 as abnormal.63,67 However, RI, calculated as the difference between PSV and EDV divided by the PSV, is ultimately dependent on PSV. Patients with good arterial inflow and significantly elevated EDV may still have RI values within the normal range, and as such, RI alone should not be used for diagnostic purposes.
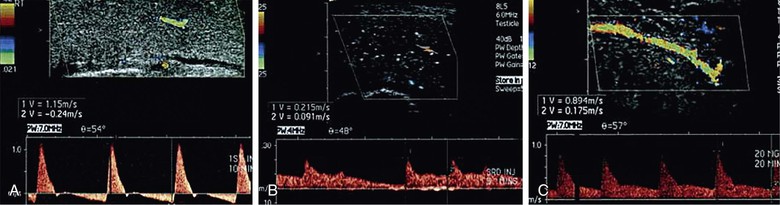
Figure 82-3 Color Doppler duplex ultrasound of the penis. A, Normal erectile hemodynamics: Doppler waveform analysis demonstrating cavernosal artery peak systolic velocity of 115 cm/s and end-diastolic velocity of −24 cm/s, representing normal erectile hemodynamics. B, Mixed vasculogenic erectile dysfunction: Doppler waveform analysis demonstrating cavernosal artery peak systolic velocity of 21.5 cm/s and end-diastolic velocity of 9.1 cm/s, indicating arterial insufficiency and corporal veno-occlusive dysfunction. C, Corporal veno-occlusive dysfunction: Doppler waveform analysis demonstrating cavernosal artery peak systolic velocity of 89.4 cm/s and end-diastolic velocity of 17.5 cm/s. End-diastolic velocity greater than 6 cm/s is an indication of corporal veno-occlusive dysfunction. (From Wilkins CJ, et al: Colour Doppler ultrasound of the penis. Clin Radiol 58:514-523, 2003.)
As described before, accurate assessment of penile hemodynamics requires maximal smooth muscle relaxation, ensuring that the sympathetic response (anxiety, stress) experienced with intracavernosal injection is minimized. As such, the patient’s erectile quality should be assessed throughout the examination and compared with the “bedroom quality erection,” which corresponds to the best erection hardness obtained at home without the use of a medication.68 Assessment of PSV and EDV in this situation is most likely to provide accurate results. Also, as noted, asymmetry between right and left PSV and EDV values must take erectile rigidity into account, as it is not uncommon to have a patient experience detumescence in the process of completing the study.
Dynamic Infusion Cavernosometry and Cavernosography
Dynamic infusion cavernosometry and cavernosography is the most accurate assessment of erectile hemodynamics. Given the widespread availability of Doppler DUS and the specialized equipment and training needed to perform dynamic infusion cavernosometry and cavernosography, its clinical use is limited. It is now generally used in young, healthy men with a history of perineal or pelvic trauma being considered candidates for penile revascularization; in young men with ED failing to respond to erectogenic pharmacotherapy, presumed to be secondary to corporal veno-occlusive dysfunction, and faced with penile implant surgery as their only option; in young men with primary ED (never had a rigid erection) to rule out corporal veno-occlusive dysfunction; in medicolegal cases in which a definitive diagnosis is needed; and in patients with Peyronie’s disease and comorbid ED, in whom the presence of corporal veno-occlusive dysfunction will ultimately change surgical management.69
Dynamic infusion cavernosometry involves placement of a butterfly needle in each corporal body, one connected to a pressure transducer and the other to a server-controlled pump for heparinized saline infusion.69,70 After the injection of vasoactive drugs (in doses significantly higher than those used for Doppler DUS), erection ensues, and a number of parameters are recorded: equilibrium pressure within the corpus cavernosum, which is an assessment of intracavernosal pressure development within the corpus cavernosum 10 to 15 minutes after vasoactive agent injection; cavernosal artery inflow gradient, which is the difference between the brachial artery systolic pressure and the cavernosal artery occlusion pressure, measured on both sides; flow to maintain, defined as the flow of saline required to maintain a given intracavernosal pressure; and pressure decay, the pressure drop occurring during a 30-second period after the intracavernosal pressure is raised to 150 mm Hg. The dose of the vasoactive agent is repeated as needed if flow to maintain or pressure decay values are abnormal.71 If the cavernosometry demonstrates corporal veno-occlusive dysfunction, cavernosography may be performed, in which nonionic radiopaque dye is injected intracavernosally and a radiograph is obtained to demonstrate the site of venous drainage.
Standardized data for results of dynamic infusion cavernosometry and cavernosography are not available. However, normal values are as follows: cavernosal artery occlusion pressure of less than 30 mm Hg, flow to maintain of less than 5 mL/min, and pressure decay of less than 45 mm Hg during 30 seconds.70 Whereas abnormal values aid in the diagnosis of corporal veno-occlusive dysfunction after dynamic infusion cavernosometry and cavernosography, additional data are available through the relationships between these parameters. Plotting the flow to maintain against the intracavernosal pressure graphically generates one of two patterns, a curvilinear pattern and a parabolic pattern. Patients with a linear pattern are generally accepted to have genuine evidence of corporal veno-occlusive dysfunction, whereas those with a parabolic pattern generally have abnormal veno-occlusive parameters secondary to excess sympathetic tone.69
Selective Internal Pudendal Angiography and Penile Angiography
Penile arteriography is an anatomic study that is an essential component of the evaluation for any patient under consideration for penile revascularization surgery. Such patients have pure arterial insufficiency without corporal veno-occlusive dysfunction, focal occlusion of one or both common penile or cavernosal arteries, perforating branches traveling from the dorsal to the cavernosal artery, at least one patent inferior epigastric artery of sufficient length to serve as a donor artery, and at least one patent dorsal artery to act as a recipient artery. Another indication is in patients with high-flow priapism, in which angioembolization of the cavernosal artery–corpus cavernosal fistula is potentially curative.
A technically challenging and invasive procedure, pudendal and penile angiography requires an endovascular specialist with skill at cannulating both the internal pudendal arteries and the inferior epigastric arteries, which are used for revascularization.63 Arterial inflow to the penis should be maximized with the use of an intracavernosal injection-delivered vasoactive agent, usually administered before the injection of contrast material. The injection should be timed to allow maximal arterial inflow to the penis but not be administered at such a time point as to cause reduced arterial flow at full rigidity. Selective catheterization of the internal pudendal artery is performed to increase detail (Fig. 82-4).72
Figure 82-4 Selective internal pudendal angiography. Left, Internal pudendal angiogram demonstrating patent dorsal and cavernosal arteries of normal caliber. Right, Internal pudendal angiogram demonstrating patent dorsal artery with occlusion of the cavernosal artery. (Courtesy Irwin Goldstein, MD, San Diego Sexual Medicine, Alvarado Hospital, San Diego, Calif)
Despite the anatomic detail provided by arteriography, it represents only a roadmap and is not a functional test. Thus, the results must be interpreted in light of the functional results obtained by Doppler DUS or dynamic infusion cavernosometry and cavernosography.
Treatment of Erectile Dysfunction
Given the safe and effective oral therapies widely available for treatment of ED, a process of care model has been established to guide the practitioner through a structured treatment algorithm (Fig. 82-5).73 After adverse medication effects have been ruled out, patients with presumed organic ED are first referred for lifestyle modification, targeting those factors that are commonly associated with ED and frequently cardiovascular disease, including smoking cessation, weight loss, and increased aerobic exercise. Numerous trials have demonstrated improvements in erectile function with smoking cessation, giving the clinician additional evidence to motivate a patient to quit smoking.74 In a randomized, controlled trial of obese Italian men, participants were randomized to receive intervention based on detailed advice for a 10% weight loss (including diet and exercise) or control, in which no advice was given.75 At 2-year follow-up, those patients in the intervention group had significant weight loss and reported significant improvements in erectile function defined by the IIEF.
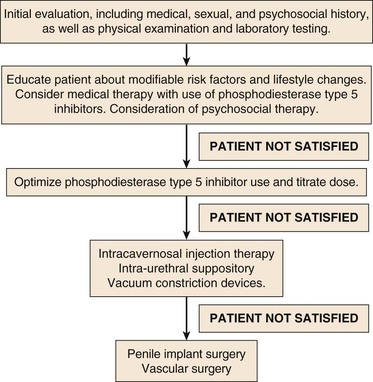
Figure 82-5 Process of care model for treatment of ED. A goal-oriented treatment approach to ED, with a stepwise escalation of therapeutic modalities in patients unsatisfied with treatment results.
Among patients with psychogenic ED, psychosocial interventions have demonstrated some improvement in erectile function when they are used in combination with sildenafil therapy compared with sildenafil therapy alone.76
PDE5 inhibitors, described next, complete the available first-line therapeutic modalities for ED. Vacuum constriction devices, although once considered first line, are now generally considered a second-line treatment of ED, in addition to intracavernosal injection and intraurethral alprostadil. Surgical therapy in the present day is considered a third-line therapeutic modality and includes penile implant surgery and vascular reconstructive surgery.
Phosphodiesterase Type 5 Inhibitors
The PDE5 inhibitor sildenafil citrate (Viagra) was the first oral drug for ED treatment approved by the U.S. Food and Drug Administration (FDA) in 1998. It dramatically changed the treatment of ED compared with historical therapeutic modalities. With similar mechanisms of action, four PDE5 inhibitors have been approved to date by the FDA: sildenafil (Viagra), vardenafil hydrochloride (Levitra or Staxyn), tadalafil (Cialis), and avanafil (Stendra).
Mechanism of Action and Means of Use
As suggested by their name, PDE5 inhibitors work by inhibiting the PDE5 enzyme, which degrades cGMP, the downstream effector of NO. Prolonged activity of cGMP decreases intracellular calcium concentration and maintains smooth muscle relaxation, thus leading to an erection. Means of use for the tablets, including the time to maximum concentration, and the half-life of each medication vary (Table 82-1). The choice of optimal PDE5 inhibitor for the individual patient depends on the sexual dynamics of the patient, particularly the frequency and predictability of sexual activity, as well as the presence of comorbid conditions that may potentially be treated with a daily dose of PDE5 inhibitor, such as tadalafil for concomitant benign prostatic hyperplasia.77
Outcomes
Significant research has addressed the efficacy of PDE5 inhibitors. The majority of the drug trials in ED are short term, lasting less than 12 weeks. Across these studies, sildenafil was more effective than placebo with per-patient sexual intercourse success rates of 69% (range, 52%-85%) versus 36% (range, 19%-68%), respectively.78 Sexual intercourse success rates with vardenafil and tadalafil have been shown to be similar (68% and 69%, respectively), although not a single published trial comparing all agents exists.78 In pooled estimates, use of sildenafil, vardenafil, and tadalafil had an approximate 2.5 times increased likelihood of improvement in erectile quality compared with placebo in general ED patients.78 The ability of these agents to permit sexual intercourse depends on the underlying cause of ED; men with psychogenic ED respond better than do men with veno-occlusive dysfunction.79 The poorest responders to PDE5 inhibitors, in general, are men with cavernous nerve dysfunction, such as diabetics and patients after radical prostatectomy, largely as a result of their inability to mount a nitrergic response on sexual arousal.
Adverse Events and Contraindications
Side effect profiles tend to be similar across the PDE5 inhibitors. The most commonly reported effects are headache, flushing, nasal congestion, heartburn, and temporary alteration in color vision, all of which can frequently be easily managed with other over-the-counter medications.78 Other side effects include myalgia, nausea, diarrhea, vomiting, and dizziness with risk of serious cardiovascular events in 0.2% to 0.5% of patients receiving sildenafil, vardenafil, or tadalafil versus 0.1% to 0.2% with placebo.78 Among these medications, the incidence of adverse effects is similar (range, 24%-34%), with discontinuation secondary to adverse effects ranging from 0.53% to 8%.78 Ischemic nonarteritic optic neuropathy and priapism have been reported rarely among PDE5 inhibitor users, with no clear causal link with PDE5 inhibitor use established.78
PDE5 inhibitors are contraindicated in patients who use nitrate medications because of a risk of severe hypotension.80 Although it is not specifically contraindicated, patients with serious cardiovascular disease should use PDE5 inhibitors with caution, primarily owing to risks associated specifically with physical exertion (sexual activity) as opposed to the PDE5 inhibitors themselves. Vardenafil use is contraindicated among patients taking class Ia or class III antiarrhythmics or with prolonged QT syndrome.81
Intracavernosal Injection Therapy
Intracavernosal pharmacotherapy, accidentally discovered and first described for treatment of ED in 1982, is recognized as a safe and effective treatment strategy.82 It is generally relegated to second-line therapy for ED and can prove effective in up to 90% of ED patients and in more than 50% of those patients who fail to respond to PDE5 inhibitors.
Mechanism of Action and Means of Use
There are three main drugs (although many others exist) used in intracavernosal pharmacotherapy: PGE1, phentolamine, and papaverine. These medications are frequently used in combination (trimix: PGE1, phentolamine, papaverine; bimix: phentolamine, papaverine) for increasing efficacy and potency. PGE1 (the FDA-approved version is known as alprostadil and marketed as Caverject or Edex) activates prostaglandin receptors, resulting in an increase in the intracellular concentration of cyclic adenosine monophosphate (cAMP) in the cavernosal smooth muscle.83 Phentolamine is a nonselective α-adrenoceptor antagonist that induces relaxation of cavernosal smooth muscle.84 Papaverine acts as a nonspecific phosphodiesterase inhibitor and initiates an increase in both intracellular cAMP and cGMP, resulting in penile erection.82 Use of the therapy involves direct injection of these vasoactive medications into the corpora cavernosa by the patient. The patient is trained to self-administer the medication at home. Responses are generally seen within 5 to 10 minutes, with dose-dependent changes in duration and rigidity of the erection.
Outcomes
Used in patients who generally have ED refractory to PDE5 inhibitor therapy, intracavernosal therapy is highly efficacious, with an 89% response rate (patients are able to achieve a penetration-hardness erection capable of sexual intercourse) and at least a 24% discontinuation rate across ED patients at 36-month follow-up.85 Discontinuation rates and reasons vary according to the patient population and can be secondary to recovery of erections after radical prostatectomy, progression of ED such that the patient requires escalating medication doses, and declining interest in sexual activity. Patients with a history of radical prostatectomy, combination radical prostatectomy and pelvic radiation therapy, diabetes, and a long preexisting history (>5 years) of untreated ED are more likely to fail to respond to the therapy.85 True failure generally is a clinical indicator of the presence of cavernosal smooth muscle damage (veno-occlusive dysfunction).
Adverse Events and Contraindications
The predominant concern for the therapy is priapism. Other minor adverse effects include pain, ecchymosis, and hematoma formation. Priapism rates vary in the literature from 0.25% to 7.3%, with figures in the lower part of the range in centers with careful patient selection and superior patient education and counseling.85 Aching or burning penile pain tends to be reported more often with the use of PGE1. Reversal of a prolonged erection is accomplished with the intracavernosal administration of an α-adrenergic agonist agent (phenylephrine).
Intraurethral PGE1 Suppository
Intraurethral PGE1 in the form of alprostadil, known as MUSETM, is an alternative method for delivery of medication directly to the corporal bodies. The drug is absorbed through the urethral mucosa and into the corpora cavernosa, where it has a mechanism of action similar to that of intracavernosal alprostadil. A small plastic device is inserted into the urethral meatus by the patient, and the pellet is deposited into the urethra. Efficacy is variable, with small trials suggesting superior efficacy for intracavernosal therapy versus intraurethral administration.86,87 Penile pain is reported in 33% of men; approximately 5% of patients report urethral bleeding.88
Vacuum Constriction Devices
Vacuum constriction devices, also known as vacuum erection devices, have been approved by the FDA since 1982.89 Vacuum erection devices consist of a suction cylinder and pump, which induces a vacuum around the corporal bodies, with a resultant increase in corporal blood inflow. This requires the application of a compression band around the base of the penis to restrict venous drainage and to maintain rigidity. Compression rings cannot be left in place for longer than 30 minutes to avoid ischemic injury to cavernosal tissue. Patient satisfaction is generally low; up to 70% of men discontinue treatment at a median of 1 month of use owing to the cumbersome application of the device and the cooler and cyanotic nature of the vacuum erection device–erected penis.90
Penile Implant Surgery
Penile implant, or penile prosthesis surgery, represents the first available treatment option for patients presenting with organic ED, with the first inflatable device described in 1973.91 It remains an excellent option for patients who have attempted and failed to achieve satisfactory results with other modalities, have grown weary of second-line therapy use (intracavernosal injection, intraurethral alprostadil, vacuum erection device), or are not deemed candidates for vascular surgical options (see later). Implants act solely to allow a patient to achieve a rigid erection on demand and have no effect on a patient’s sensation, ability to ejaculate, or achievement of orgasm.
Technical Considerations
There are two main classes of implants: malleable (or semirigid) and hydraulic (or inflatable). Semirigid prostheses consist of two malleable rods that are placed into the corpora cavernosa, which are manually shaped by the patient at the onset of sexual activity and are re-shaped after sexual relations are completed. Inflatable implants are further subdivided and classified according to the configuration of the prosthesis: a two-piece inflatable, which consists of two cylinders attached to a scrotal pump; and a three-piece inflatable, which consists of two cylinders attached to a scrotal pump and a separate reservoir that is generally placed extraperitoneally in the space of Retzius. Choice of implant is both surgeon and patient dependent. Malleable implants are less commonly employed in the modern implant era and tend to be implanted by the occasional implanter, with some uncommon exceptions. Our practice is to preferentially place three-piece implants, given their superior rigidity and girth, better concealability, and zero rigidity nature in the deflated state. Two-piece implants are generally placed in patients who have an obliterated extraperitoneal space, have small bowel loops abutting their deep inguinal ring (patients after radical cystoprostatectomy), have a renal transplant, or have poor grip strength and may have difficulty deflating a three-piece implant.
Outcomes and Adverse Events
The satisfaction of the patient and his partner is generally high after penile implant surgery; satisfaction rates vary between 75% and 97%, depending on the patient population and implant used.91 This is mostly related to the excellent rigidity and spontaneity of these devices. The most significant complication of penile implant surgery is infection, with data from the most recent generation of penile prostheses varying from 0.7% to 3%.92,93 Treatment of infection requires complete device removal, which can lead to difficulty with subsequent re-implantation due to scarring and decreased penile length and girth. Protocols for salvage implantation (infected device removal with simultaneous re-implantation) demonstrate an 84% success rate.94 Additional complications include component erosion (most often occurring in the infected implant), reservoir herniation, visceral erosion, device autoinflation, cylinder migration, and device malfunction. Five-year mechanical failure rates range from 0% to 9% for currently available prostheses.91
Penile Revascularization Surgery
Given the myriad causes of ED, penile revascularization (penile artery bypass surgery) currently serves as one of only two modalities with the potential to cure the ED patient without the future necessity of vasoactive medications or implant surgery (the other is crural ligation surgery). Penile revascularization, unlike revascularization in patients with coronary disease or peripheral artery insufficiency, however, is not successful in patients with cadiovascular disease; these patients have a significant underlying element of endothelial dysfunction and corporal smooth muscle dysfunction as the main factors leading to ED.91 In addition, patients with ED secondary to diffuse atherosclerosis will have significant disease present in the small penile arteries, thereby ruling out an adequate recipient vessel.95 In light of this fact, patient selection for revascularization surgery is of paramount importance as it is most likely to be successful in a highly selected group of young men with an isolated arterial stenosis after pelvic trauma. Inclusion criteria for consideration for revascularization generally include young men (age is poorly defined, although most authorities would not perform revascularization on men older than 40 years), absence of vascular risk factors, absence of evidence for other causes of ED, absence of evidence of corporal veno-occlusive dysfunction with evidence of pure arterial insufficiency (as described before), and presence of focal occlusive disease in the common penile artery or cavernosal artery that is amenable to distal bypass by the previously outlined arteriographic criteria.91,96
Technical Considerations
The inferior epigastric artery has been identified as the ideal donor vessel for bypass. Many bypass procedures have been identified (historically more than 20 procedures in total), but the most common approach is to perform inferior epigastric artery to dorsal artery bypass.95 Given the paucity of data pertaining to the use of venous target vessels, the arterial revascularization, modified from Goldstein and colleagues, is reported here (Fig. 82-6).96,97
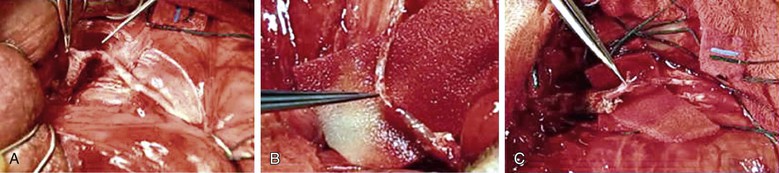
Figure 82-6 Penile revascularization. A, Isolated and mobilized dorsal artery of the penis just distal to the fundiform ligament. B, Transected inferior epigastric artery transferred to the dorsum of the penis. C, Microsurgical anastomosis between the inferior epigastric artery and the dorsal penile artery. (Courtesy Irwin Goldstein, MD, San Diego Sexual Medicine, Alvarado Hospital, San Diego, Calif)
A variety of scrotal incisions may be made to expose the dorsal artery at the base of the penile shaft. An abdominal incision is made to expose the rectus muscle, which is reflected medially, and the inferior epigastric artery is identified. It is then mobilized from its origin near the external iliac artery to a point near the umbilicus, where it is transected. It is then transferred through the posterior wall of the inguinal canal onto the dorsum of the penis. The abdominal incision is then closed. The adventitial layer of the distal aspect of the inferior epigastric artery and proximal aspect of the dorsal artery are stripped with microscissors, and a microsurgical simple interrupted anastomosis is performed with 10-0 nylon sutures. After completion of the anastomosis and after complete hemostasis is achieved, the penis is replaced into its anatomic position and the wound is closed.
Endovascular management of focal arterial occlusion has also been previously studied. The majority of the reports published in the 1980s used balloon angioplasty without stenting.98 Results are variable and poorly defined.
Outcomes and Complications
Data on outcomes after penile revascularization are sparse; most studies are limited by varying inclusion and exclusion criteria, lack of validated markers to evaluate success, and generally short follow-up.95 In addition, the published studies have discrepant patient populations, use different anastomotic and bypass techniques, and frequently do not assess for long-term patency. As a result, practice guidelines from the American Urological Association consider penile revascularization unproven and controversial.99 Among these studies, subjective success rates vary by the type of revascularization procedure, definition of successful outcome, and patient population but range from 50% to 82%100–105 (Table 82-2). Looking specifically at arterial revascularization surgery as described before, a review of 71 patients with 34-month mean follow-up demonstrated that 55% of men had IIEF scores qualifying them as having normal erectile function without the use of PDE5 inhibitors.100
The most frequently reported complications include failure to improve erectile function, inguinal hernias, penile shortening, loss of penile sensation, and in cases in which arterialization of the dorsal vein is used, glans hyperemia.91,100
Surgery for Veno-Occlusive Dysfunction
Current guidelines by the American Urological Association do not recommend surgery to limit the venous outflow of the penis, given an absence of clear data demonstrating benefit to patients.99 Corporal veno-occlusive dysfunction is now considered the result of damage to the cavernosal smooth muscle, and thus the venous leak is a symptom of an underlying pathologic process that is not generally correctable through venous ligation.91,95 Despite this, venous ligation surgery has been attempted for decades with poor long-term results. However, crural ligation surgery among highly selected young patients with isolated crural venous leak (generally secondary to blunt perineal trauma) has demonstrated some medium-term efficacy.106 In one study of 15 patients with isolated crural venous leak demonstrated on dynamic infusion cavernosometry and cavernosography, with a mean age of 29 years, crural ligation surgery resulted in unassisted sexual intercourse in 71% of men, and 93% of men reported improved erectile function as quantified by the IIEF.106 This confirmed results from a similar population of 11 patients, with a mean age of 28 years, with 82% improvement in erectile function.107
Future Therapeutic Strategies
Given the significant amount of research that has been performed on ED during the past decade, increased understanding of the physiology of erection and pathophysiology of ED has identified myriad potential targets for ED. Studies focusing on medications targeting the central nervous system as well as innovative methods for targeting the NO/cGMP pathway, as PDE5 inhibitors do, are currently ongoing.108 Gene therapy, focusing on signaling pathways for penile erection, has been effective in improving ED in a variety of animal models.109 Two of the more exciting and promising therapeutic modalities currently evaluated in men are described here; an exhaustive discussion of all future therapies is beyond the scope of this chapter.
Endovascular Stenting: ZEN Trial
Initial results from the ZEN (zotarolimus-eluting peripheral stent system for the treatment of erectile dysfunction in men with suboptimal response to PDE5 inhibitors) trial were published in late 2012, addressing the safety and feasibility of a balloon-expandable drug-eluting stent for patients with atherosclerotic ED and focal internal pudendal artery stenoses.110 Patients with atherosclerotic ED and a suboptimal response to PDE5 inhibitors as well as specific clinical, Doppler DUS, and angiographic factors were prospectively enrolled in this trial, with a safety endpoint of a major adverse event 30 days after the procedure and a feasibility endpoint of four-point improvement in IIEF erectile function domain scores in more than 50% of patients at 3 months. Thirty subjects with a mean age of 60 years were studied, with a total of 45 stents placed. The majority of patients had known cardiovascular risk factors. Safety endpoints were met in all subjects, with 59% of patients in an intention-to-treat analysis meeting the feasibility endpoint at 3 months (unchanged at 6 months). Restenosis greater than 50% of the post-treatment luminal diameter was noted in 34% of patients at 6-month follow-up (32 lesions evaluated). Longer term results are pending.
Stem Cell Therapy
The use of pluripotent stem cells as a curative therapy for ED has been evaluated in a number of animal studies and is currently being investigated in at least one phase I human clinical trial.111 Stem cells are believed to be able to differentiate into the various cell types instrumental in erectile function, and as such it was hypothesized that systemic injection or intracavernosal injection of stem cells may replenish lost or deteriorating tissues contributing to ED, although this mechanism has not been proved in animals. A number of stem cell types, derived from bone marrow, adipose tissue, skeletal muscle, embryonic cells, endothelial progenitor cells, and umbilical cord blood, have been used in rats, with promising results.111 One report of seven human patients undergoing treatment and three control patients with a long history of diabetes (mean, 29 years) and ED refractory to medical therapy investigated the benefit of intracavernosal injection of umbilical cord blood stem cells. In the experimental group, six patients reported some increase in rigidity, with two patients able to achieve penetration and maintenance with use of 100 mg of sildenafil at 6-month follow-up compared with no improvement in the control group.112 Further research is obviously needed to prove feasibility of this treatment modality in men.
Erectile Dysfunction in Men Undergoing Vascular Surgery
Prevalence of Erectile Dysfunction in the Vascular Surgery Population
Given the significant cardiovascular risk factors and comorbidities present among patients requiring vascular surgical interventions, it comes as no surprise that ED is a frequent and common complaint in patients both before and after abdominal aortic aneurysm (AAA) repair and treatment of aortoiliac disease. One study, looking at the prevalence of ED among a population of 137 vascular surgery patients, identified a 90% rate of ED (defined as IIEF erectile function domain score of less than 25), with moderate or severe ED in 70% of cases.113 Among patients with an untreated AAA, the rate of moderate or severe ED was 82%. Another study looking at self-report of preoperative sexual dysfunction in 56 AAA patients using the Sexual Health Inventory for Men scores described a 27% rate of ED in patients undergoing elective open AAA repair, 63% for those undergoing endovascular repair of AAA (EVAR), and 45% for those undergoing open repair for AAA rupture.114 These figures are considerably higher than historically reported preoperative ED rates in AAA and aortoiliac occlusive disease patients, ranging from 22% to 39%, as previous studies had variable definitions of ED without use of validated questionnaires or defined ED as only the most severe ED cases (IIEF erectile function domain score of less than 11).115,116
Erectile Function Outcomes after Definitive Therapy
Outcomes after AAA repair have recently been more extensively studied following a pair of randomized trials. The landmark Aneurysm Detection and Management study randomized 1136 patients with 4.0- to 5.4-cm AAA to surveillance versus immediate open repair.117 Patients were evaluated by a nurse to rate their sexual function as normal versus impotent, without use of questionnaires. “Impotence” rates were significantly higher across patients randomized to immediate repair from 18 months to 4-year follow-up. Across groups, a steady and significant increase in ED was also noted, pointing to disease progression over time. Another rigorously performed study randomized 881 AAA patients to EVAR and open AAA repair and evaluated sexual function outcomes by use of the IIEF erectile function domain score.118 Of note, patients had significant ED at baseline, with a mean IIEF score of 11.4 for the EVAR group and 10.3 for the open group preoperatively. At 1 year, there was a mean decline in IIEF of 2.5 and 2.3 for the EVAR group and the open group, respectively, and a decline of 3 and 2.9 points, respectively, at 2 years, demonstrating no significant difference between the EVAR and open AAA repair groups. In a vasculopathic population, such a decrease is likely to be age related and not related to the surgical intervention.
ED rates after open and endovascular aortoiliac procedures have also been evaluated in a single retrospective study of 116 patients who underwent open aortoiliac reconstruction and percutaneous transluminal balloon angioplasty or stenting of the common or external iliac artery.119 Patients were evaluated with the IIEF postoperatively and were asked to recall their sexual function before surgery. Significant decline in erectile function was recorded in both groups.
ED after vascular repair has been associated with bilateral internal iliac interruption during EVAR, as would be expected.120 Internal iliac embolization before EVAR has also demonstrated significant increases in reported ED rates across a number of studies.121 As such, preservation of the internal iliac artery has been recommended when possible. In addition, adjunct internal iliac revascularization before EVAR may improve erectile outcomes, with reports of no change in sexual function and even improvement in erectile ability in a single patient after this procedure.122
Given these findings, it would be prudent for the vascular surgeon to consider counseling of patients for erectile function changes after vascular surgical procedures. A previous report has shown that 91% of patients undergoing open AAA repair or EVAR do not recall receiving preoperative information about the risk of a possible negative impact on sexual function.123
Conclusions
ED, a highly prevalent condition and a potential marker for significant vascular disease, is frequently unaddressed by vascular surgery practitioners despite the significant overlap of risk factors between ED and cardiovascular and peripheral vascular disease. Although treatment is often easily achieved with use of PDE5 inhibitors, diagnostic evaluation in a subset of patients may help to better direct patient care and can provide prognostic information about potential response to therapy. Further study with validated instruments to quantify erectile function is needed to fully appreciate the effect of vascular reconstructive surgery on ED rates.
Acknowledgments
We would like to gratefully acknowledge the assistance of Irwin Goldstein, MD, Director of Sexual Medicine at San Diego Sexual Medicine, Alvarado Hospital, San Diego, Calif, in providing us with images from selective internal pudendal arteriography and intraoperative photographs from his extensive experience with penile revascularization.
Selected Key References
Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401.
This seminal article was the first to describe the role of nitric oxide in achieving a penile erection and forms the basis for the targeting of current medical therapy for ED.
Coombs PG, Heck M, Guhring P, Narus J, Mulhall JP. A review of outcomes of an intracavernosal injection therapy programme. BJU Int. 2012;110:1787–1791.
The authors report on their significant experience with intracavernosal injection therapy and describe predictors of intracavernosal therapy failures or long-term discontinuation.
Goldstein I, Bastuba M, Lurie A, Lubisich J. Penile revascularization. J Sex Med. 2008;5:2018.
The authors provide a beautifully illustrated report outlining their technique for penile arterial revascularization.
Lin CS, Xin ZC, Wang Z, Deng C, Huang YC, Lin G, Lue TF. Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev. 2012;21:343.
This report provides an exhaustive review of all of the stem cell therapy trials conducted to date in ED and postulates the mechanism of action of this exciting potential therapeutic modality.
Nehra A, Jackson G, Miner M, Billups KL, Burnett AL, Buvat J, Carson CC, Cunningham GR, Ganz P, Goldstein I, Guay AT, Hackett G, Kloner RA, Kostis J, Montorsi P, Ramsey M, Rosen R, Sadovsky R, Seftel AD, Shabsigh R, Vlachopoulos C, Wu FC. The Princeton III Consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc. 2012;87:766.
Tsertsvadze A, Fink HA, Yazdi F, MacDonald R, Bella AJ, Ansari MT, Garritty C, Soares-Weiser K, Daniel R, Sampson M, Fox S, Moher D, Wilt TJ. Oral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: a systematic review and meta-analysis. Ann Intern Med. 2009;151:650.
This article provides a thorough and in-depth review of the multitude of trials conducted with phosphodiesterase-5 inhibitors for the treatment of ED.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83.
2. Litwin MS, et al. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998;13:159.
3. Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802.
4. Feldman HA, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54.
5. Saigal CS, et al. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. 2006;166:207.
6. Laumann EO, et al. Prevalence and correlates of erectile dysfunction by race and ethnicity among men aged 40 or older in the United States: from the Male Attitudes Regarding Sexual Health survey. J Sex Med. 2007;4:57.
7. Breza J, et al. Detailed anatomy of penile neurovascular structures: surgical significance. J Urol. 1989;141:437.
8. Lepor H, et al. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosa: a detailed anatomical study of the adult male pelvis. J Urol. 1985;133:207.
9. Paick JS, et al. Anatomy of cavernous nerves distal to prostate: microdissection study in adult male cadavers. Urology. 1993;42:145.
10. Ignarro LJ, et al. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990;170:843.
11. Levin RM, et al. Comparative studies on rabbit corpus cavernosal contraction and relaxation. An in vitro study. J Androl. 1994;15:36.
12. Burnett AL, et al. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401.
13. Gupta S, et al. Possible role of Na+-K+-ATPase in the regulation of human corpus cavernosum smooth muscle contractility by nitric oxide. Br J Pharmacol. 1995;116:2201.
14. Rosen RC. Psychogenic erectile dysfunction. Classification and management. Urol Clin North Am. 2001;28:269.
15. Lizza EF, et al. Definition and classification of erectile dysfunction: report of the Nomenclature Committee of the International Society of Impotence Research. Int J Impot Res. 1999;11:141.
16. Deveci S, et al. Can the International Index of Erectile Function distinguish between organic and psychogenic erectile function? BJU Int. 2008;102:354.
17. Saenz de Tejada I, et al. Pathophysiology of erectile dysfunction. J Sex Med. 2005;2:26.
18. Beck RO, et al. Genitourinary dysfunction in multiple system atrophy: clinical features and treatment in 62 cases. J Urol. 1994;151:1336.
19. Courtois FJ, et al. Erectile mechanism in paraplegia. Physiol Behav. 1993;53:721.
20. Mulhall JP. Penile rehabilitation following radical prostatectomy. Curr Opin Urol. 2008;18:613.
21. Rhoden EL, et al. The relationship of serum testosterone to erectile function in normal aging men. J Urol. 2002;167:1745.
22. Corona G, et al. Aging and pathogenesis of erectile dysfunction. Int J Impot Res. 2004;16:395.
23. Traish AM, et al. Effects of medical or surgical castration on erectile function in an animal model. J Androl. 2003;24:381.
24. Sullivan ME, et al. Nitric oxide and penile erection: is erectile dysfunction another manifestation of vascular disease? Cardiovasc Res. 1999;43:658.
25. Giuliano FA, et al. Prevalence of erectile dysfunction among 7689 patients with diabetes or hypertension, or both. Urology. 2004;64:1196.
26. Martin-Morales A, et al. Prevalence and independent risk factors for erectile dysfunction in Spain: results of the Epidemiologia de la Disfuncion Erectil Masculina Study. J Urol. 2001;166:569.
27. Sullivan ME, et al. Fibrinogen, lipoprotein (a) and lipids in patients with erectile dysfunction. A preliminary study. Int Angiol. 2001;20:195.
28. Nehra A, et al. The Princeton III Consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc. 2012;87:766.
29. Dong JY, et al. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58:1378.
30. Gupta BP, et al. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med. 2011;171:1797.
31. Polonsky TS, et al. The association between erectile dysfunction and peripheral arterial disease as determined by screening ankle-brachial index testing. Atherosclerosis. 2009;207:440.
32. Montorsi P, et al. The artery size hypothesis: a macrovascular link between erectile dysfunction and coronary artery disease. Am J Cardiol. 2005;96:19M.
33. Tarhan F, et al. Cavernous oxygen tension in the patients with erectile dysfunction. Int J Impot Res. 1997;9:149.
34. Moreland RB, et al. PGE1 suppresses the induction of collagen synthesis by transforming growth factor-beta 1 in human corpus cavernosum smooth muscle. J Urol. 1995;153:826.
35. Nehra A, et al. Cavernosal expandability is an erectile tissue mechanical property which predicts trabecular histology in an animal model of vasculogenic erectile dysfunction. J Urol. 1998;159:2229.
36. Tamler R, et al. Assessment of endothelial function in the patient with erectile dysfunction: an opportunity for the urologist. Int J Impot Res. 2008;20:370.
37. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115.
38. Kaiser DR, et al. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J Am Coll Cardiol. 2004;43:179.
39. Thompson IM, et al. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996.
40. Mulhall J, et al. Vasculogenic erectile dysfunction is a predictor of abnormal stress echocardiography. J Sex Med. 2009;6:820.
41. Wespes E, et al. Venous impotence: pathophysiology, diagnosis and treatment. J Urol. 1993;149:1238.
42. Christ GJ, et al. Pharmacological studies of human erectile tissue: characteristics of spontaneous contractions and alterations in alpha-adrenoceptor responsiveness with age and disease in isolated tissues. Br J Pharmacol. 1990;101:375.
43. Chung E, et al. Penile duplex ultrasonography in men with Peyronie’s disease: is it veno-occlusive dysfunction or poor cavernosal arterial inflow that contributes to erectile dysfunction? J Sex Med. 2011;8:3446.
44. Chang SW, et al. The impact of diuretic therapy on reported sexual function. Arch Intern Med. 1991;151:2402.
45. Grimm RH Jr, et al. Long-term effects on sexual function of five antihypertensive drugs and nutritional hygienic treatment in hypertensive men and women. Treatment of Mild Hypertension Study (TOMHS). Hypertension. 1997;29:8.
46. Croog SH, et al. The effects of antihypertensive therapy on the quality of life. N Engl J Med. 1986;314:1657.
47. Segal RL, et al. Irbesartan promotes erection recovery after nerve-sparing radical retropubic prostatectomy: a retrospective long-term analysis. BJU Int. 2012;110:1782.
48. Kennedy SH, et al. Antidepressant-induced sexual dysfunction during treatment with moclobemide, paroxetine, sertraline, and venlafaxine. J Clin Psychiatry. 2000;61:276.
49. Mazzola CR, et al. Androgen deprivation therapy before radical prostatectomy is associated with poorer postoperative erectile function outcomes. BJU Int. 2012;110:112.
50. Gupta S, et al. A possible mechanism for alteration of human erectile function by digoxin: inhibition of corpus cavernosum sodium/potassium adenosine triphosphatase activity. J Urol. 1998;159:1529.
51. Moinpour CM, et al. Longitudinal analysis of sexual function reported by men in the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2007;99:1025.
52. Hatzichristou D, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7:337.
54. Rosen RC, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822.
55. Rosen RC, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319.
56. Ghanem HM, et al. SOP: physical examination and laboratory testing for men with erectile dysfunction. J Sex Med. 2013;10:108.
57. Wasserman MD, et al. The differential diagnosis of impotence. The measurement of nocturnal penile tumescence. JAMA. 1980;243:2038.
58. Bradley WE, et al. New method for continuous measurement of nocturnal penile tumescence and rigidity. Urology. 1985;26:4.
59. Mahe G, et al. A normal penile pressure cannot rule out the presence of lesions on the arteries supplying the hypogastric circulation in patients with arterial claudication. Vasc Med. 2009;14:331.
60. Aitchison M, et al. Is the penile brachial index a reproducible and useful measurement? Br J Urol. 1990;66:202.
61. Virag R, et al. Intracavernous injection of papaverine as a diagnostic and therapeutic method in erectile failure. Angiology. 1984;35:79.
62. Aversa A, et al. Anxiety-induced failure in erectile response to intracorporeal prostaglandin-E1 in non-organic male impotence: a new diagnostic approach. Int J Androl. 1996;19:307.
63. Sikka SC, et al. Standardization of vascular assessment of erectile dysfunction: standard operating procedures for duplex ultrasound. J Sex Med. 2013;10:120.
64. Lue TF, et al. Vasculogenic impotence evaluated by high-resolution ultrasonography and pulsed Doppler spectrum analysis. Radiology. 1985;155:777.
65. Quam JP, et al. Duplex and color Doppler sonographic evaluation of vasculogenic impotence. AJR Am J Roentgenol. 1989;153:1141.
66. Benson CB, et al. Correlation of duplex sonography with arteriography in patients with erectile dysfunction. AJR Am J Roentgenol. 1993;160:71.
67. Wilkins CJ, et al. Colour Doppler ultrasound of the penis. Clin Radiol. 2003;58:514.
68. Teloken PE, et al. The false diagnosis of venous leak: prevalence and predictors. J Sex Med. 2011;8:2344.
69. Vardi Y, et al. Cavernosometry: is it a dinosaur? J Sex Med. 2008;5:760.
70. Glina S, et al. SOP: corpus cavernosum assessment (cavernosography/cavernosometry). J Sex Med. 2013;10:111.
71. Mulhall JP, et al. Improving the accuracy of vascular testing in impotent men: correcting hemodynamic alterations using a vasoactive medication re-dosing schedule. J Urol. 2001;166:923.
72. Gray RR, et al. Investigation of impotence by internal pudendal angiography: experience with 73 cases. Radiology. 1982;144:773.
73. The process of care model for evaluation and treatment of erectile dysfunction. The Process of Care Consensus Panel. Int J Impot Res. 1999;11:59.
74. Guay AT, et al. Cessation of smoking rapidly decreases erectile dysfunction. Endocr Pract. 1998;4:23.
75. Esposito K, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291:2978.
76. Melnik T, et al. Psychosocial interventions for erectile dysfunction. Cochrane Database Syst Rev. 2007;(3).
77. Corona G, et al. Phosphodiesterase type 5 (PDE5) inhibitors in erectile dysfunction: the proper drug for the proper patient. J Sex Med. 2011;8:3418.
78. Tsertsvadze A, et al. Oral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: a systematic review and meta-analysis. Ann Intern Med. 2009;151:650.
79. Mulhall J, et al. Sildenafil citrate response correlates with the nature and the severity of penile vascular insufficiency. J Sex Med. 2005;2:104.
80. Eardley I, et al. Pharmacotherapy for erectile dysfunction. J Sex Med. 2010;7:524.
81. Morganroth J, et al. Evaluation of vardenafil and sildenafil on cardiac repolarization. Am J Cardiol. 2004;93:1378.
82. Virag R. Intracavernous injection of papaverine for erectile failure. Lancet. 1982;2:938.
83. Angulo J, et al. Rationale for the combination of PGE1 and S-nitroso-glutathione to induce relaxation of human penile smooth muscle. J Pharmacol Exp Ther. 2000;295:586.
84. Traish A, et al. Phentolamine mesylate relaxes penile corpus cavernosum tissue by adrenergic and non-adrenergic mechanisms. Int J Impot Res. 1998;10:215.
85. Coombs PG, et al. A review of outcomes of an intracavernosal injection therapy programme. BJU Int. 2012;110:1787.
86. Urciuoli R, et al. Prostaglandin E1 for treatment of erectile dysfunction. Cochrane Database Syst Rev. 2004;(2).
87. Porst H. Transurethral alprostadil with MUSE (medicated urethral system for erection) vs intracavernous alprostadil—a comparative study in 103 patients with erectile dysfunction. Int J Impot Res. 1997;9:187.
88. Padma-Nathan H, et al. Treatment of men with erectile dysfunction with transurethral alprostadil. Medicated Urethral System for Erection (MUSE) Study Group. N Engl J Med. 1997;336:1.
89. Blackard CE, et al. Use of vacuum tumescence device for impotence secondary to venous leakage. Urology. 1993;41:225.
90. Dutta TC, et al. Vacuum constriction devices for erectile dysfunction: a long-term, prospective study of patients with mild, moderate, and severe dysfunction. Urology. 1999;54:891.
91. Hellstrom WJ, et al. Implants, mechanical devices, and vascular surgery for erectile dysfunction. J Sex Med. 2010;7:501.
92. Carson CC 3rd. Efficacy of antibiotic impregnation of inflatable penile prostheses in decreasing infection in original implants. J Urol. 2004;171:1611.
93. Wolter CE, et al. The hydrophilic-coated inflatable penile prosthesis: 1-year experience. J Sex Med. 2004;1:221.
94. Mulcahy JJ. Long-term experience with salvage of infected penile implants. J Urol. 2000;163:481.
95. Sohn M, et al. Standard operating procedures for vascular surgery in erectile dysfunction: revascularization and venous procedures. J Sex Med. 2013;10:172.
96. Munarriz R, et al. Penile arterial reconstruction. Graham SD, Glenn JF. Glenn’s urologic surgery. ed 6. Lipincott Williams & Wilkins: Philadelphia; 2004:573–581.
97. Goldstein I, et al. Penile revascularization. J Sex Med. 2008;5:2018.
98. Rogers JH, et al. Endovascular therapy for vasculogenic erectile dysfunction. Curr Treat Options Cardiovasc Med. 2012;14:193.
99. Montague DK, et al. Chapter 1: The management of erectile dysfunction: an AUA update. J Urol. 2005;174:230.
100. Munarriz R, et al. Microvascular arterial bypass surgery: long-term outcomes using validated instruments. J Urol. 2009;182:643.
101. Vardi Y, et al. Evaluation of penile revascularization for erectile dysfunction: a 10-year follow-up. Int J Impot Res. 2004;16:181.
102. Kawanishi Y, et al. Penile revascularization surgery for arteriogenic erectile dysfunction: the long-term efficacy rate calculated by survival analysis. BJU Int. 2004;94:361.
103. Kayigil O, et al. Is deep dorsal vein arterialization effective in elderly patients? Int Urol Nephrol. 2008;40:125.
104. Kayigil O, et al. Penile revascularization in vasculogenic erectile dysfunction (ED): long-term follow-up. BJU Int. 2012;109:109.
105. Zuckerman JM, et al. Outcome of penile revascularization for arteriogenic erectile dysfunction after pelvic fracture urethral injuries. Urology. 2012;80:1369.
106. Flores S, et al. Outcomes of crural ligation surgery for isolated crural venous leak. J Sex Med. 2011;8:3495.
107. Rahman NU, et al. Crural ligation for primary erectile dysfunction: a case series. J Urol. 2005;173:2064.
108. Decaluwe K, et al. New therapeutic targets for the treatment of erectile dysfunction. J Sex Med. 2011;8:3271.
109. Harraz A, et al. Emerging gene and stem cell therapies for the treatment of erectile dysfunction. Nat Rev Urol. 2010;7:143.
111. Lin CS, et al. Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev. 2012;21:343.
112. Bahk JY, et al. Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: preliminary report of 7 cases. Exp Clin Transplant. 2010;8:150.
113. Falkensammer J, et al. Prevalence of erectile dysfunction in vascular surgery patients. Vasc Med. 2007;12:17.
114. Koo V, et al. Pilot study of sexual dysfunction following abdominal aortic aneurysm surgery. J Sex Med. 2007;4:1147.
115. Jimenez JC, et al. Sexual dysfunction in men after open or endovascular repair of abdominal aortic aneurysms. Vascular. 2004;12:186.
116. Lee ES, et al. Incidence of erectile dysfunction after open abdominal aortic aneurysm repair. Ann Vasc Surg. 2000;14:13.
117. Lederle FA, et al. Quality of life, impotence, and activity level in a randomized trial of immediate repair versus surveillance of small abdominal aortic aneurysm. J Vasc Surg. 2003;38:745.
118. Lederle FA, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302:1535.
119. Karkos CD, et al. Erectile dysfunction after open versus angioplasty aortoiliac procedures: a questionnaire survey. Vasc Endovascular Surg. 2004;38:157.
120. Mehta M, et al. Unilateral and bilateral hypogastric artery interruption during aortoiliac aneurysm repair in 154 patients: a relatively innocuous procedure. J Vasc Surg. 2001;33:S27.
121. Lin PH, et al. Hypogastric artery preservation during endovascular aortic aneurysm repair: is it important? Semin Vasc Surg. 2009;22:193.
122. Faries PL, et al. Internal iliac artery revascularization as an adjunct to endovascular repair of aortoiliac aneurysms. J Vasc Surg. 2001;34:892.
123. Pettersson M, et al. Prospective follow-up of sexual function after elective repair of abdominal aortic aneurysms using open and endovascular techniques. J Vasc Surg. 2009;50:492.

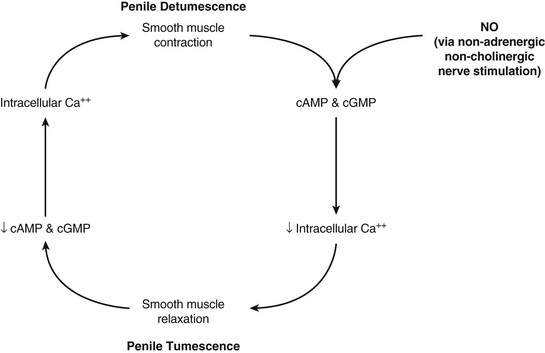
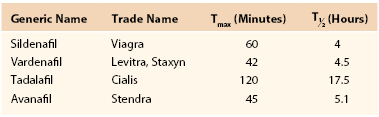
 , half-life, time required for elimination of half of the medication from plasma.
, half-life, time required for elimination of half of the medication from plasma.