Chapter 246 Epstein-Barr Virus
Oncogenesis
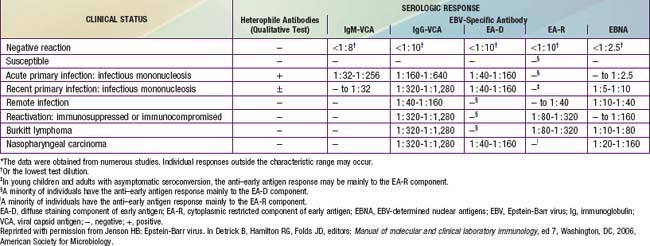
Nasopharyngeal carcinoma occurs worldwide but is 10 times more common in persons in southern China, where it is the most common malignant tumor among adult men. It is also common among whites in North Africa and Inuit in North America. Patients usually present with cervical lymphadenopathy, eustachian tube blockage, and nasal obstruction with epistaxis. All malignant cells of undifferentiated nasopharyngeal carcinoma contain a high copy number of EBV episomes. Persons with undifferentiated and partially differentiated, nonkeratinizing nasopharyngeal carcinomas have elevated EBV antibody titers that are both diagnostic and prognostic. High levels of immunoglobulin A (IgA) antibody to EA and VCA may be detected in asymptomatic individuals and can be used to follow response to tumor therapy (Table 246-1). Cells of well-differentiated, keratinizing nasopharyngeal carcinoma contain a low number of or no EBV genomes; people with this disease have EBV serologic patterns similar to those of the general population.
Endemic (African) Burkitt lymphoma, often found in the jaw, is the most common childhood cancer in equatorial East Africa and New Guinea (Chapter 490.2). The median age at onset is 5 yr. These regions are holoendemic for Plasmodium falciparum malaria and have a high rate of EBV infection early in life. The constant malarial exposure acts as a B-lymphocyte mitogen that contributes to the polyclonal B-lymphocyte proliferation with EBV infection, impairs T-lymphocyte surveillance of EBV-infected B lymphocytes, and increases the risk for development of Burkitt lymphoma. Approximately 98% of cases of endemic Burkitt lymphoma contain the EBV genome, compared with only 20% of cases of nonendemic (sporadic or American) Burkitt lymphoma. Individuals with Burkitt lymphoma have unusually and characteristically high levels of antibody to VCA and EA that correlate with the risk for developing tumor (see Table 246-1).
Failure to control EBV infection may result from host immunologic deficits. The prototype is the X-linked lymphoproliferative syndrome (Duncan syndrome), an X chromosome–linked recessive disorder of the immune system associated with severe, persistent, and sometimes fatal EBV infection (Chapter 118). Approximately two thirds of affected patients, who are male, die of disseminated and fulminating lymphoproliferation involving multiple organs at the time of primary EBV infection. Surviving patients acquire hypogammaglobulinemia, B-cell lymphoma, or both; most of these patients die within 10 yr.
Clinical Manifestations
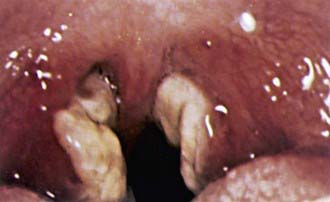
The sore throat is often accompanied by moderate to severe pharyngitis with marked tonsillar enlargement, occasionally with exudates (Fig. 246-1). Petechiae at the junction of the hard and soft palate are frequently seen. The pharyngitis resembles that caused by streptococcal infection. Other clinical findings may include rashes and edema of the eyelids.
Laboratory Tests
Specific Epstein-Barr Virus Antibodies
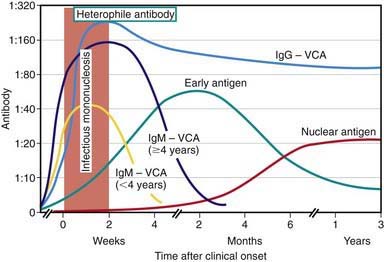
EBV-specific antibody testing is useful to confirm acute EBV infection, especially in heterophile-negative cases, or to confirm past infection and determine susceptibility to future infection. Several distinct EBV antigen systems have been characterized for diagnostic purposes (Fig. 246-2 and Table 246-1). The EBNA, EA, and VCA systems are most useful for diagnostic purposes. The acute phase of infectious mononucleosis is characterized by rapid IgM and IgG antibody responses to VCA in all cases and an IgG response to EA in most cases. The IgM response to VCA is transient but can be detected for at least 4 wk and occasionally for up to 3 mo. The laboratory must take steps to remove rheumatoid factor from the specimen, which may otherwise cause a false-positive IgM VCA result. The IgG response to VCA usually peaks late in the acute phase, declines slightly over the next several weeks to months, and then persists at a relatively stable level for life.
Prognosis
The prognosis for complete recovery is excellent. The major symptoms typically last 2-4 wk followed by gradual recovery. Individuals often harbor multiple strains of EBV, and second infections with a different type of EBV (type 1 or type 2) have been demonstrated in immunocompromised persons, but symptoms or second clinical episodes of infectious mononucleosis have not been documented. Prolonged and debilitating fatigue, malaise, and some disability that may wax and wane for several weeks to 6 mo are common complaints even in otherwise unremarkable cases. Occasional persistence of fatigue for a few years after infectious mononucleosis is well recognized. There is no convincing evidence linking EBV infection or EBV reactivation to chronic fatigue syndrome (Chapter 115).
Alpert G, Fleisher GR. Complications of infection with Epstein-Barr virus during childhood: a study of children admitted to the hospital. Pediatr Infect Dis. 1984;3:304-307.
Balfour HHJr, Holman CJ, Hokanson KM, et al. A prospective clinical study of Epstein-Barr virus and host interactions during acute infectious mononucleosis. J Infect Dis. 2005;192:1505-1512.
Crawford DH, Macsween KF, Higgins CD, et al. A cohort study among university students: identification of risk factors for Epstein-Barr virus seroconversion and infectious mononucleosis. Clin Infect Dis. 2006;43:276-282.
Crawford DH, Swedlow AJ, Higgins C, et al. Sexual history and Epstein-Barr virus infection. J Infect Dis. 2002;186:731-736.
Doja A, Bitnun A, Jones EL, et al. Pediatric Epstein-Barr virus-associated encephalitis: 10-year review. J Child Neurol. 2006;21:384-391.
Fafi-Kremer S, Morand P, Brion JP, et al. Long-term shedding of infectious Epstein-Barr virus after infectious mononucleosis. J Infect Dis. 2005;191:985-989.
Higgins CD, Swerdlow AJ, Macsween KF, et al. A study of risk factors for acquisition of Epstein-Barr virus and its subtypes. J Infect Dis. 2007;195:474-482.
Jenson H. Acute complications of Epstein-Barr virus infectious mononucleosis. Curr Opin Pediatr. 2000;12:263-268.
Jenson H, McIntosh K, Pitt J, et al. Natural history of primary Epstein-Barr virus infection in children of mothers infected with human immunodeficiency virus type 1. J Infect Dis. 1999;179:1395-1404.
Jenson HB, Leach CT, McClain KL, et al. Benign and malignant smooth muscle tumors containing Epstein-Barr virus in children with AIDS. Leuk Lymphoma. 1997;27:303-314.
Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
Njie R, Bell AI, Jia H, et al. The effects of acute malaria on Epstein-Barr virus (EBV) load and EBV-specific T cell immunity in Gambian children. J Infect Dis. 2009;199:31-38.
Sitki-Green D, Covington M, Raab-Traub N. Compartmentalization and transmission of multiple Epstein-Barr virus strains in asymptomatic carriers. J Virol. 2003;77:1840-1847.
Sitki-Green DL, Edwards RH, Covington MM, et al. Biology of Epstein-Barr virus during infectious mononucleosis. J Infect Dis. 2004;189:483-492.
Sokal EM, Hoppenbrouwers K, Vandermeulen C, et al. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J Infect Dis. 2007;196:1749-1753.
Straus SE, Tosato G, Armstrong G, et al. Persisting illness and fatigue in adults with evidence of Epstein-Barr virus infection. Ann Intern Med. 1985;102:7-16.
Sumaya CV, Ench Y. Epstein-Barr virus infectious mononucleosis in children I: clinical and general laboratory findings. Pediatrics. 1985;75:1003-1010.
Sumaya CV, Ench Y. Epstein-Barr virus infectious mononucleosis in children II: heterophile antibody and viral-specific responses. Pediatrics. 1985;75:1011-1019.
Thompson SK, Doerr TD, Hengerer AS. Infectious mononucleosis and corticosteroids. Arch Otolaryngol Head Neck Surg. 2005;131:900-904.
Tierney RJ, Edwards RH, Sitki-Green D, et al. Multiple Epstein-Barr virus strains in patients with infectious mononucleosis: comparison of ex vivo samples with in vitro isolates by use of heteroduplex tracking assays. J Infect Dis. 2006;193:287-297.
Torre D, Tambini R. Acyclovir for treatment of infectious mononucleosis: a meta-analysis. Scand J Infect Dis. 1999;31:543-547.
Tselis AC, Jenson HB, editors. Epstein-Barr virus. New York: Taylor & Francis, 2006.
Williams H, Macsween K, McAulay K, et al. Analysis of immune activation and clinical events in acute infectious mononucleosis. J Infect Dis. 2004;190:63-71.
Yoshida M, Tsuda N, Morihata T, et al. Five patients with localized facial eruptions associated with Gianotti-Crosti syndrome caused by primary Epstein-Barr virus infection. J Pediatr. 2004;145:843-844.