Epithelial Neoplasms of the Small Intestine
Amy E. Noffsinger
Introduction
Epithelial neoplasms develop far less frequently in the small intestine than in the colon, despite the fact that the small intestine has a larger epithelial surface area and a higher rate of cellular turnover. Overall, only 2% of all malignant neoplasms of the gastrointestinal (GI) tract occur in the small intestine.1 In contrast, 57% of neoplasms arise in the colon. A number of hypotheses have been proposed to explain the relative rarity of small bowel adenomas and carcinomas.2 First, the transit time of substances through the small intestine is relatively short compared with the colon, resulting in brief contact time between the mucosa and the luminal contents. Second, unlike the colon, the small intestine does not contain a large quantity of bacteria. Bacteria are known to convert bile salts into potential carcinogens. Third, the luminal contents are more liquid in the small intestine than in the colon. As a result, potentially carcinogenic luminal substances are diluted and the risk of mechanical trauma is reduced. Fourth, the small intestine is rich in lymphoid tissue, which provides a potentially high level of immunosurveillance against neoplastic cells. Finally, mucosal enzymes may help detoxify potentially carcinogenic substances in the luminal contents.2
Epithelial tumors in the small intestine are most commonly located in the duodenum, usually in the vicinity of the ampulla of Vater.3–5 This finding suggests that biliary or pancreatic secretions may play a role in their development, possibly as a result of the carcinogenic effect of bile. Alternatively, constant influx of alkaline bile or acidic pancreatic juice may cause cell damage. Epithelial neoplasms also occur in the jejunum and in the ileum, but much less commonly.6 A number of diseases predispose individual patients to the development of small-intestinal adenomas and carcinomas, including familial adenomatous polyposis (FAP), Crohn’s disease (CD),7 and celiac disease.8,9 The risk for small-intestinal carcinomas may also be increased in individuals with Peutz-Jeghers syndrome10,11 or juvenile polyposis syndrome,12,13 and in patients with long-standing ileostomies.14,15
Small-intestinal epithelial tumors are most commonly glandular, although other forms of neoplasia are also seen. Tumors arising in the ampulla of Vater are separately classified from those arising elsewhere in the small intestine because significant treatment and prognostic differences exist for this group of tumors. Boxes 26.1 and 26.2 summarize the World Health Organization’s (WHO) histologic classification of epithelial tumors of the small intestine and the ampullary region, respectively.16
Small-Intestinal Neoplasia in Polyposis and Hereditary Cancer Syndromes
Familial Adenomatous Polyposis
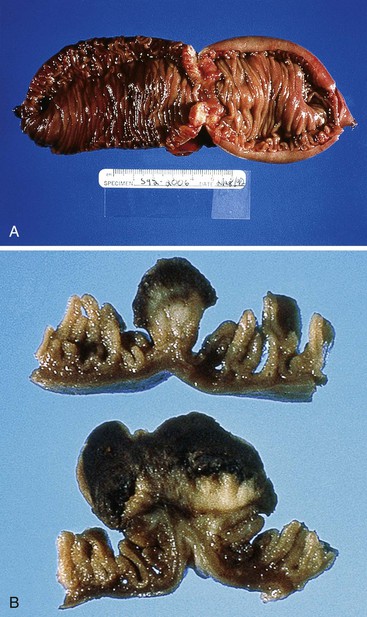
FAP is associated with adenomatous polyps of the intestinal tract, as well as fundic gland polyps of the stomach. In the small intestine, most FAP-associated lesions arise in the duodenum and tend to cluster around the ampulla of Vater.17–19 They are usually multiple and may be numerous (>20 to 50) in some patients.20 Adenomas are often small, sessile, and tubular, usually measuring less than 1 cm in diameter.19 A “serrated” ampullary adenoma in a patient with FAP has been reported,21 but this morphology is not typical of small-intestinal adenomas. Ileal adenomas also occur in patients with FAP.22
Patients with FAP who have multiple duodenal adenomas have a 100- to 300-fold increased lifetime risk for duodenal or periampullary cancer compared with the general population.23,24 In fact, periampullary adenocarcinoma is the most common extracolonic malignant neoplasm in FAP. As a result, patients with known FAP should undergo endoscopic surveillance with biopsy examination of grossly normal duodenal and ampullary mucosa to identify potential early precancerous lesions.25 Patients who have only a small number of adenomas should be screened at least every 3 years. In patients whose lesions are numerous or large, the screening interval should be shortened to 1 year.
Peutz-Jeghers Syndrome
Peutz-Jeghers syndrome is an autosomal dominant inherited syndrome characterized by mucocutaneous pigmentation and GI polyposis. The disorder has been linked to germline mutations in the serine/threonine kinase gene STK11 (also called LKB1) located on chromosome 19p13.3.26,27 The most common cancers in this group of patients are gastrointestinal in origin, arising from gastroesophageal, small-intestinal, colonic, and pancreatic sites.28 The risk for small-intestinal adenocarcinoma among patients with Peutz-Jeghers syndrome is estimated to be as much as 400 times that of the general population.29
Lynch Syndrome
Lynch syndrome (also called hereditary nonpolyposis colorectal cancer [HNPCC]) is an autosomal dominant genetic disorder that confers a high risk for both colorectal and endometrial tumors. It occurs as a result of germline mutation in one of the four genes in DNA mismatch repair pathways. Patients with Lynch syndrome also exhibit a risk for tumors in other sites, albeit at a lower rate than for colorectal and endometrial cancers. These include urothelial cancers, biliary tumors, and gastric and small-intestinal adenocarcinomas.30–32 The relative risk of small bowel adenocarcinoma in patients with Lynch syndrome is estimated to be 100 times greater than that in the general population, and in one quarter of cases the small bowel tumor is the presenting neoplasm of the syndrome.30,33,34 Unlike patients with FAP, adenomas or adenocarcinomas at any location throughout the small bowel may develop in patients with Lynch syndrome, although a duodenojejunal predominance has been reported.34
Adenomatous Polyps
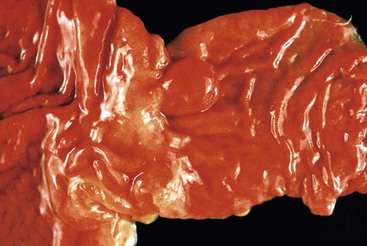
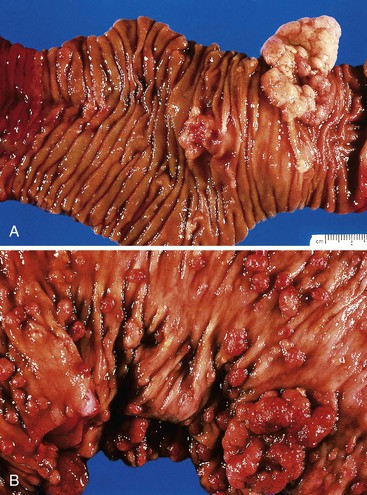
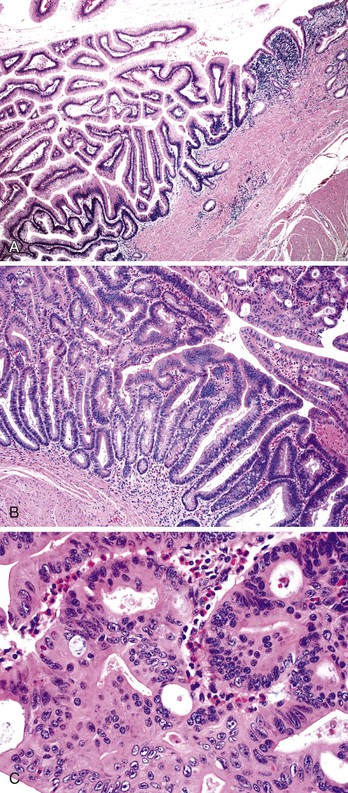
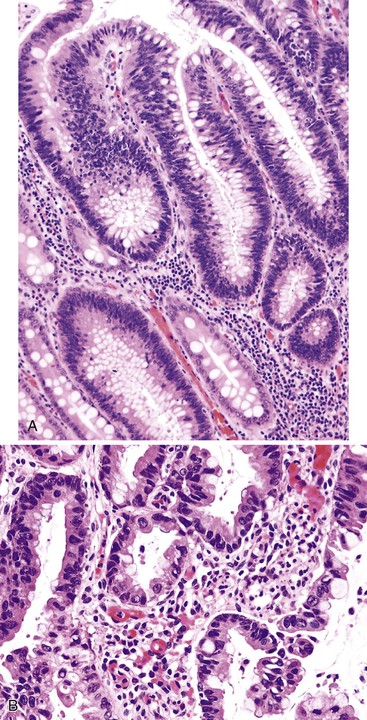
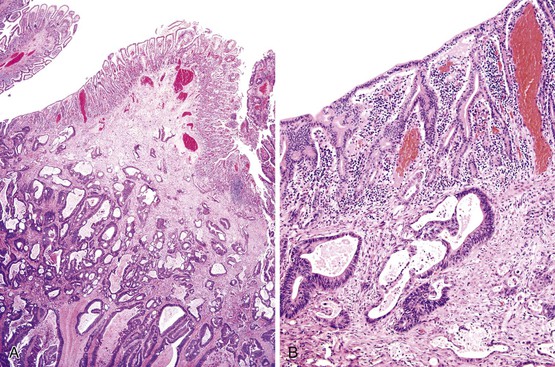
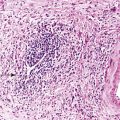
Small-intestinal adenomas are rare,35 accounting for fewer than 0.05% of all intestinal adenomas. Adenomas peak in incidence in the seventh decade of life but may occur at any age. Most adenomas are asymptomatic. They are usually discovered incidentally in individuals who have undergone endoscopic examination for other reasons. Adenomas that are symptomatic typically involve the region of the ampulla of Vater and manifest with biliary colic and obstruction, acute cholangitis, or pancreatitis.35 Intestinal obstruction, bleeding, nausea, vomiting, anorexia, weight loss, pain, or intussusception may also develop, depending on the size and location of the lesion.36 Small-intestinal adenomas resemble those that arise in the colon in gross and microscopic characteristics (Fig. 26.1). They are usually lobulated and soft, and they may be sessile, pedunculated, villous, or tubular. A higher proportion of small-intestinal lesions tend to be villous compared with adenomas of the colon; this is most likely a reflection of the underlying villous architecture of the small bowel. Tubular adenomas tend to be small, varying from 0.5 to 3 cm in maximum diameter. Villous adenomas are often larger, sometimes reaching 8 cm or larger. Small-intestinal adenomas are usually single36 but can be multiple. The finding of multiple adenomas in the small intestine is rare in patients without a hereditary polyposis syndrome. As a result, identification of multiple lesions should raise a suspicion of FAP (Fig. 26.2).
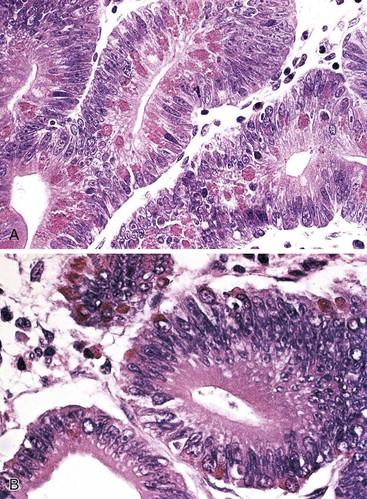
Histologically, small-intestinal adenomas may demonstrate tubular, tubulovillous, or villous growth patterns. They are composed of tall columnar epithelial cells with elongated, crowded, hyperchromatic nuclei arranged in a “picket fence” pattern. Immature goblet cells may be present. In addition, endocrine cells, squamous cells, and particularly Paneth cells may be numerous (Fig. 26.3). Mitoses, normally seen only in the base of the crypts, may occur at all levels of the adenomatous crypts and villi. Normal-appearing lamina propria is usually present between the neoplastic crypts.
Small-intestinal adenomas can display varying degrees of dysplasia, ranging from low to high grade, and may show intramucosal or invasive carcinoma. While the degree of dysplasia increases, one also tends to see an increased ratio of nucleus to cytoplasm in the cells, loss of cell polarity, and an increased mitotic rate. Prominent crypt budding, nuclear stratification, and loss of mucinous differentiation may herald progression to malignancy.
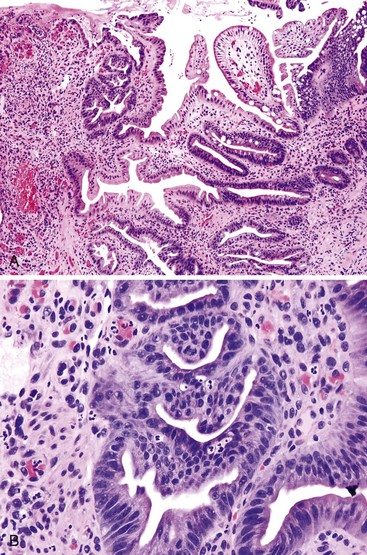
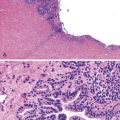
It is important to distinguish regenerative atypia associated with surface erosion from an adenoma (Fig. 26.4). Regenerating cells tend to mature toward the surface, whereas adenomas do not. The presence of Paneth or endocrine cells in the superficial portions of the lesion is almost always associated with a neoplastic alteration. Prominent acute inflammation with congested capillaries and fibrin deposition, especially when superficial, should alert the examiner to the possibility of regenerative atypia.
Preinvasive Ampullary Neoplasia
Most preinvasive neoplasms in the region of the ampulla of Vater represent intestinal-type adenomas, and approximately 80% of all small-intestinal adenomas arise at this site.35 Ampullary adenomas resemble their nonampullary counterparts, as described earlier.
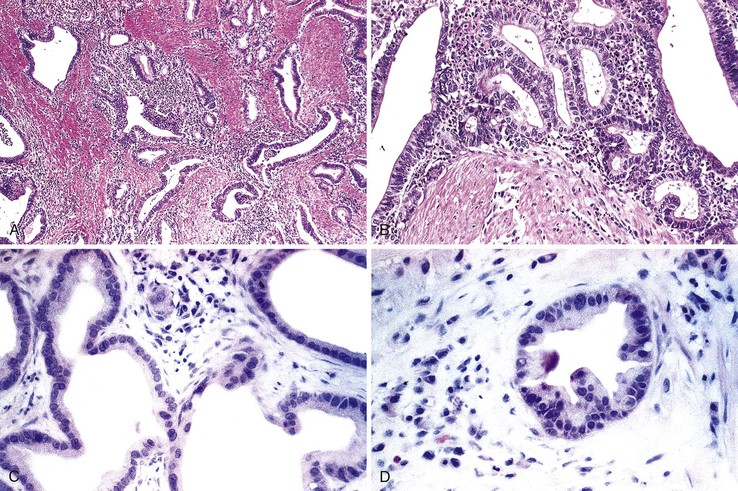
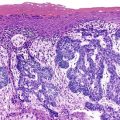
Pancreaticobiliary-type ampullary adenocarcinomas are thought to arise from noninvasive papillary and flat intraductal neoplasia reminiscent of that seen in the bile duct and pancreas (Fig. 26.5). These preinvasive lesions are encountered infrequently and are usually associated with a coexisting invasive carcinoma. The clinical features of these intraductal papillary lesions are not well characterized, primarily because of their rarity. Histologically, these neoplasms consist of complex, arborizing papillary structures lined by variably atypical epithelial cells. Almost all pancreaticobiliary noninvasive papillary neoplasms have focal high-grade dysplasia, and many have an associated invasive carcinoma.37–39 The invasive component most commonly has a tubular growth pattern and is of pancreaticobiliary type, although intestinal type adenocarcinomas may occasionally arise from these papillary precursors.
Although most ampullary adenocarcinomas probably arise from preexisting adenomas or pancreaticobiliary noninvasive papillary neoplasms, a small subset appear to arise from areas of flat high-grade intraductal neoplasia (dysplasia). Microscopically, the dysplastic epithelium may have a truly flat architecture, or it may have a micropapillary growth pattern. Flat dysplasia is almost always found adjacent to invasive adenocarcinoma and is rarely seen in isolation.40,41
Small-Intestinal Adenocarcinoma
Clinical Features and Associations
More than half of all small-intestinal carcinomas arise in the duodenum, even though this organ constitutes only 4% of the entire length of the small intestine.5,42,43 The incidence of duodenal adenocarcinoma has risen in recent years, most likely because of the increased use of upper endoscopy44 and the development of newer techniques such as video capsule endoscopy, double-balloon enteroscopy, and computed tomographic (CT) enterography.45–47 Most small-intestinal carcinomas arise in the region of the ampulla of Vater. A smaller percentage of tumors arise in the jejunum, particularly in the first 30 cm distal to the ligament of Treitz. Ileal carcinomas are the least common, except in patients with CD. Small-intestinal carcinomas occur more frequently in men than in women and affect blacks more often than whites.3,44
Some diseases (e.g., FAP) are associated with an increased incidence of small-intestinal carcinomas (Box 26.3). Cancers that arise in the upper GI tract, and especially in the periampullary region, represent a major cause of death in these patients. As discussed previously, patients with hereditary polyposis and familial cancer syndromes including FAP, Lynch syndrome, and Peutz-Jeghers syndrome are at increased risk for the development of small-intestinal tumors. In addition, patients with celiac disease have an 80-fold increased incidence of small-intestinal adenocarcinomas compared with the general population.8 In one study of 175 patients with adenocarcinoma of the small bowel, 13% had celiac disease.9 The diagnosis of celiac disease preceded that of adenocarcinoma in 63% of these patients. Tumors in these patients often arise in the jejunum.47
Ileal adenocarcinomas develop with increased frequency in individuals with long-standing CD (see later discussion). However, in these patients, adenocarcinomas typically arise in the setting of dysplasia (flat or polypoid) rather than in preexisting adenomas.48
Most small-intestinal carcinomas manifest in patients between 60 and 70 years of age.49,50 However, most tumors that arise in the setting of a hereditary cancer syndrome are seen in younger individuals. Patients may have presenting symptoms of intestinal obstruction, bleeding, intussusception, or perforation. Ampullary carcinomas often manifest with bile duct obstruction, pancreatitis, and jaundice. Pancreatitis may also develop secondary to pancreatic outflow obstruction.
Pathologic Features
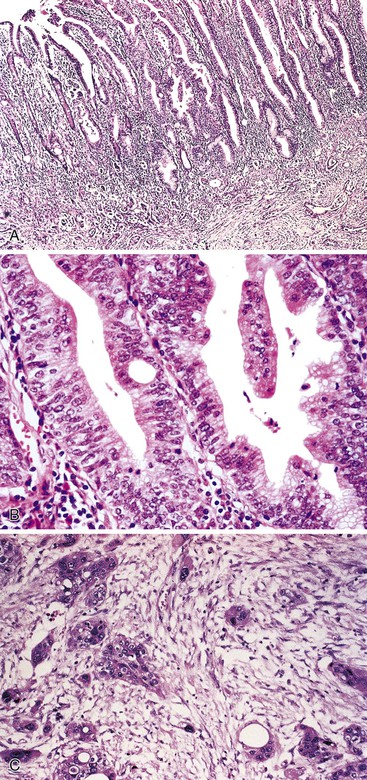
Small-intestinal carcinomas may have a flat, stenotic, ulcerative, infiltrative, or polypoid gross appearance (Fig. 26.6). Tumors typically range from 1 to 15 cm in diameter. Larger lesions tend to be found in the more distal portions of the small bowel, because lesions in that area often fail to produce symptoms until they are advanced.
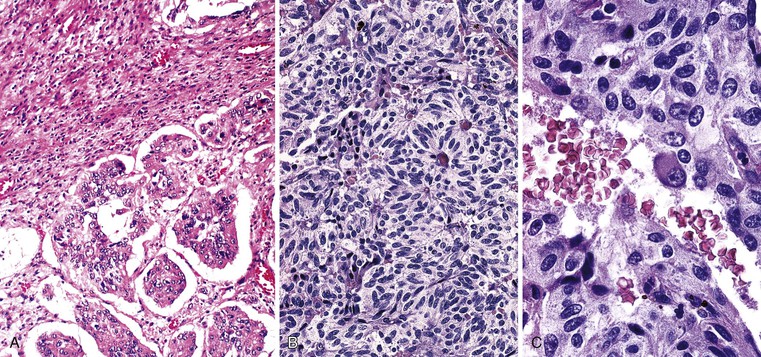
Small-intestinal adenocarcinomas are similar, histologically, to those that develop elsewhere in the GI tract. However, because small-intestinal cancers usually arise from preexisting adenomas, one may see residual adenomatous changes in the adjacent or overlying epithelium, particularly in smaller lesions. More often, the cancer has overgrown the adenomatous component at the time of diagnosis, especially in tumors that arise in sites other than the ampulla of Vater. Identification of an associated preinvasive lesion allows one to be relatively certain that the tumor is primary to that location. However, some metastatic carcinomas induce significant cytologic atypia in adjacent non-neoplastic small-intestinal epithelium that can resemble adenomatous change (Fig. 26.7).
Adenocarcinomas are characterized by cellular and nuclear pleomorphism, loss of epithelial polarity, gland-in-gland architecture, and invasion into adjacent tissues. Most small-intestinal adenocarcinomas are moderately differentiated and demonstrate variable degrees of mucin production. Approximately 20% of tumors are poorly differentiated and contain signet ring cells. Other tumors display a prominent extracellular mucinous component. Neoplasms in which more than 50% of the tumor is mucinous should be designated as mucinous adenocarcinomas, because these tumors tend to have a poorer prognosis than typical gland-forming lesions. Neoplastic endocrine cells and Paneth cells are often present. Squamous cells may also be identified but are less common. The presence of endocrine, Paneth, or squamous cells in a carcinoma has no prognostic significance.
Immunohistochemical Features
Small-intestinal adenocarcinomas show more variable expression of cytokeratin 7 (CK7) than do colorectal carcinomas. In one study, diffuse positive CK7 immunoreactivity was identified in 54% of nonampullary small-intestinal adenocarcinomas, and focal positivity was present in the remaining 46% of cases.51 In the same study, 67% of cases expressed CK20. Expression of MUC1, MUC2, and MUC5AC occurs in 53%, 57% and 40% of small bowel adenocarcinomas, respectively.52 Expression of villin is observed in 67% of cases, but the staining is often focal. CDX2 staining is identified in 60% of small-intestinal adenocarcinomas, and the pattern of staining is usually diffuse, similar to colorectal carcinomas.52 Ninety-six percent of small-intestinal adenocarcinomas are negative for alpha methylacyl–coenzyme A racemase (AMACR).53
Ampullary Adenocarcinomas
The ampulla of Vater represents the site in the small intestine where most carcinomas arise. The incidence of cancers at this site has increased in the past 4 decades. Ampullary adenocarcinomas are more common in men than in women.54 Patients most often have painless jaundice at presentation. Because even small tumors in this location can result in biliary obstruction, many tumors arising in this site are diagnosed at a relatively early stage.
Histologic Features
The ampulla is an area in which two types of epithelium converge, that of the duodenum and that of the common bile duct. As a result, carcinomas that arise in this region may be of either intestinal or pancreaticobiliary type. Intestinal-type cancers represent the most common histologic type of ampullary carcinoma, accounting for 85% of cases.55 The pancreaticobiliary type is the most common of the remaining tumors, although other unusual histologic tumor types may occur, including mucinous, signet ring cell, adenosquamous, clear cell, and neuroendocrine carcinomas. Distinction between the two major histologic types of ampullary adenocarcinoma is important, because many studies have shown a significantly poorer prognosis in tumors of the pancreaticobiliary type.54,56–58
Intestinal-type ampullary adenocarcinomas are histologically indistinguishable from adenocarcinomas that occur elsewhere in the small intestine or colon (Fig. 26.8). The histologic pattern varies, from well-formed glandular or tubular structures to cribriform areas or solid nests of tumor cells. As in their colonic counterparts, the glands often contain necrotic or apoptotic debris, so-called dirty necrosis.
Pancreaticobiliary ampullary carcinomas closely resemble pancreatic ductal adenocarcinomas or primary adenocarcinomas of the extrahepatic bile ducts. These tumors consist of small, simple or branched glands surrounded by abundant desmoplastic stroma (Fig. 26.9). The cells lining the neoplastic glands are usually cuboidal to low columnar in shape, and they are typically arranged in a single layer. Intraluminal necrotic debris is infrequently present. Most tumors demonstrate well-formed glandular structures, although less differentiated tumors may contain small clusters and solid nests of tumor cells.
Immunohistochemical Features
The immunoprofile of ampullary adenocarcinoma varies depending on whether the tumor is of the intestinal or the pancreaticobiliary histologic type. Intestinal-type ampullary adenocarcinomas are usually CK20 positive (80% to 91%) and CK7 negative (73% to 82%).59–61 In addition, 100% of these tumors are positive for CDX2.62 They variably express MUC1 (18% to 60%), MUC2 (47% to 82%),61,62 and carcinoembryonic antigen (CEA; 53%).61 Forty percent express CA19-9.
In contrast, the pancreaticobiliary-type ampullary tumors are typically CK20 and CDX2 negative (92% and 83%, respectively) and CK7 positive (96%), and they more commonly express MUC1 (83%) and MUC2 (100%) proteins and CA 19-9 (79%).59–62 Twenty-nine percent express CEA.61 Most ampullary adenocarcinomas are negative for AMACR regardless of whether they are of the intestinal or the pancreaticobiliary type.53
Staging
The American Joint Committee on Cancer (AJCC) staging system for ampullary adenocarcinomas differs from that of other epithelial tumors of the small intestine. Criteria for determining the pathologic stage are outlined in Table 26.1.
Table 26.1
American Joint Committee on Cancer Staging System for Carcinomas of the Ampulla of Vater
| Primary Tumor (T) | |||
| TX | Primary tumor cannot be assessed | ||
| T0 | No evidence of primary tumor | ||
| Tis | Carcinoma in situ | ||
| T1 | Tumor limited to ampulla of Vater or sphincter of Oddi | ||
| T2 | Tumor invades duodenal wall | ||
| T3 | Tumor invades pancreas | ||
| T4 | Tumor invades peripancreatic soft tissues or other adjacent organs or structures other than pancreas | ||
| Regional Lymph Nodes (N) | |||
| NX | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastasis | ||
| N1 | Regional lymph node metastasis | ||
| Distant Metastasis (M) | |||
| MX | Distant metastasis cannot be assessed | ||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis | ||
| Stage Grouping | |||
| Stage 0 | Tis | N0 | M0 |
| Stage IA | T1 | N0 | M0 |
| Stage IB | T2 | N0 | M0 |
| Stage IIA | T3 | N0 | M0 |
| Stage IIB | T1 | N1 | M0 |
| T2 | N1 | M0 | |
| T3 | N1 | M0 | |
| Stage III | T4 | Any N | M0 |
| Stage IV | Any T | Any N | M1 |
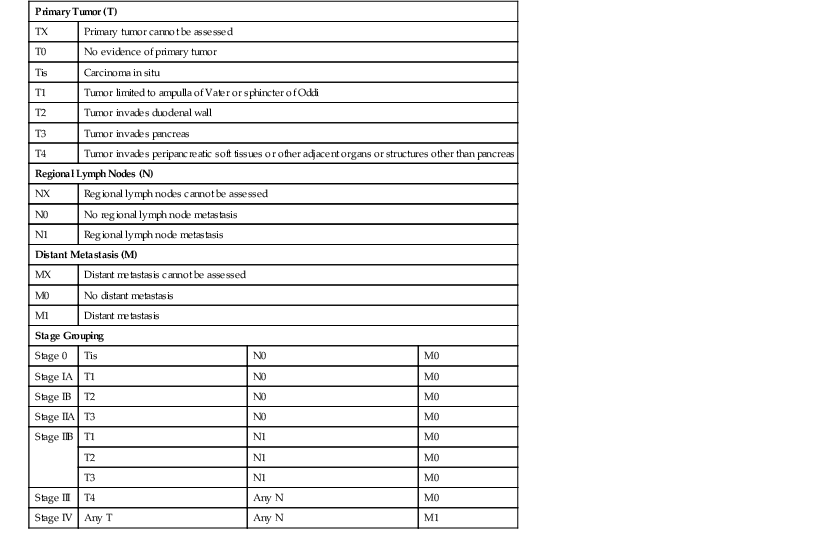
From Edge SB, Byrd DR, Compton CC, et al., eds. AJCC Cancer Staging Manual. 7th ed. New York: Springer-Verlag; 2010. Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. Published by Springer Science and Business Media LLC, www.springerlink.com.
Molecular Features
Most small-intestinal and ampullary adenocarcinomas, similar to colonic adenocarcinomas, are believed to arise from an adenoma–carcinoma sequence in which genetic alterations progressively accumulate, leading to cancer development. As in the colon, an exception is neoplasms that arise in association with inflammatory bowel disease; such tumors arise through a dysplasia–carcinoma sequence. Small-intestinal adenocarcinomas share many common molecular alterations with colorectal adenocarcinomas, but significant differences exist.
KRAS Mutations
The KRAS oncogene encodes a binding protein that plays a key role in transmitting signals from extracellular growth factors to the cell nucleus. KRAS mutations occur commonly in colorectal carcinomas and are also frequently identified in colorectal adenomas. Therefore, mutations in this gene are thought to represent an early change in the adenoma–carcinoma sequence in the colon. KRAS mutations are also found in small-intestinal adenocarcinomas, occurring in 14% to 83% of cases.63–67 The reported wide variation in mutation frequency in different studies may be related to the fact that combined tumors from duodenal and other small-intestinal locations are often included in the analyses. In general, KRAS mutations are more frequent in duodenal neoplasms than in those that arise in other small bowel sites. KRAS mutations have also been detected in small-intestinal adenomas,63 suggesting that KRAS may play a similar role in both the small-intestinal and the colorectal adenoma–carcinoma sequence.
TP53 Alterations
TP53 (formerly p53) is the gene most commonly mutated in human cancers. Its function is to facilitate DNA repair before cell replication or, if DNA damage is severe, to initiate apoptosis in the affected cell. As in the colon, TP53 mutation is a late event in the neoplastic progression of small-intestinal adenocarcinomas.63,68 Overexpression or mutation of TP53 is identified in 20% to 53% of cases.52,63,67,69,70
APC and β-Catenin Mutations
Both the APC gene product and β-catenin (encoded by CTNNB1) represent important proteins in the WNT signaling pathway. APC binds β-catenin, which leads to its degradation and prevents it from interacting with nuclear transcription factors that initiate cell proliferation. Although APC mutations are frequent in both colorectal adenomas and adenocarcinomas, they are rare in small-intestinal adenocarcinomas.62,63,69 Abnormal expression of β-catenin, however, occurs in as many as 81% of these tumors,69 suggesting that mutations in β-catenin play a role in small-intestinal adenocarcinoma.71 One study suggests that loss of β-catenin expression in ampullary carcinomas is associated with poor prognosis and is a predictor of disease recurrence.72
Mismatch Repair Genes
Defects in DNA mismatch repair result in high-frequency microsatellite instability (MSI) in colorectal and other cancers and are a characteristic alteration identified in tumors from patients with HNPCC. MLH1 and MSH2 are the genes most commonly mutated in patients with HNPCC. These genes are also inactivated in a proportion of sporadic colorectal carcinomas, usually through epigenetic events such as promoter hypermethylation. The overall result is an increased mutation frequency in affected cells. Small bowel cancers occur with increased frequency among patients with HNPCC,73–76 suggesting that mismatch repair gene defects likely play a role in sporadic small-intestinal adenocarcinomas as well. MSI has been reported in 18% to 35% of small bowel carcinomas.77,78 One study suggests that the frequency of MSI and methylation abnormalities is even higher in small-intestinal adenocarcinomas that arise in the setting of celiac disease.79 The rate of MSI in ampullary adenocarcinomas may be lower, with high-frequency MSI occurring in as many as 10% of tumors.80–82
Epigenetic Alterations
As with adenocarcinomas of the colon, a subset of small-intestinal adenocarcinomas show methylation abnormalities. In a study of 37 small bowel adenocarcinomas, 24 tumors were found to show abnormal methylation patterns in at least one of the loci studied.77 Eleven tumors were classified as CpG island methylator phenotype-high (CIMP-H), and 13 were CIMP-low.77 As in the colon, CIMP-H status was strongly associated with the high-frequency MSI phenotype.
SMAD4 Mutations
The SMAD4 (DPC4) gene product was first identified as an important tumor suppressor protein in pancreatic adenocarcinomas. It is part of the transforming growth factor-β signaling pathway, where it plays a role in growth suppression. Deletion or loss of SMAD4 expression occurs in approximately 50% of pancreatic cancers and in 3% to 50% of colorectal cancers.83–85 SMAD4 alterations occur in 24% of nonampullary small-intestinal adenocarcinomas and 34% of ampullary carcinomas.86,87
Prognosis
The prognosis of small-intestinal carcinomas is poor. In one study,42 the median overall survival time was 20.1 months, with a 5-year overall survival rate of 26%. The primary reason is that patients are often asymptomatic until late in the course of the disease, and metastases are often present at the time of diagnosis. In general, ampulla of Vater tumors have a better prognosis than distal tumors,88,89 presumably because they become symptomatic early and therefore tend to be removed at a less advanced stage of growth. Other prognostic factors include tumor size, surgical resectability of the tumor,90,91 presence of lymphatic or vascular invasion, nodal involvement, depth of invasion into the bowel wall, and presence or absence of invasion into adjacent structures. The tumor-node-metastasis (TNM) staging system for small-intestinal carcinoma is outlined in Table 26.2.
Table 26.2
American Joint Committee on Cancer Tumor-Node-Metastasis Classification of Small-Intestinal Carcinomas
| Primary Tumor (T) | |||
| TX | Primary tumor cannot be assessed | ||
| T0 | No evidence of primary tumor | ||
| Tis | Carcinoma in situ | ||
| T1a | Tumor invades lamina propria | ||
| T1b | Tumor invades submucosa* | ||
| T2 | Tumor invades muscularis propria | ||
| T3 | Tumor invades through the muscularis propria into the subserosa or nonperitonealized perimuscular tissue (mesentery or retroperitoneum) with extension ≤2 cm* | ||
| T4 | Tumor perforates the visceral peritoneum or directly invades other organs or structures (includes other loops of small intestine, mesentery, or retroperitoneum >2 cm, and abdominal wall by way of serosa; for duodenum only, invasion of pancreas or bile duct) | ||
| Regional Lymph Nodes (N) | |||
| NX | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastasis | ||
| N1 | Metastasis in 1-3 regional lymph nodes | ||
| N2 | Metastasis in ≥4 regional lymph nodes | ||
| Distant Metastasis (M) | |||
| MX | Distant metastasis cannot be assessed | ||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis | ||
| Stage Grouping | |||
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| T2 | N0 | M0 | |
| Stage IIA | T3 | N0 | M0 |
| Stage IIB | T4 | N0 | M0 |
| Stage IIIA | Any T | N1 | M0 |
| Stage IIIB | Any T | N2 | M0 |
| Stage IV | Any T | Any N | M1 |
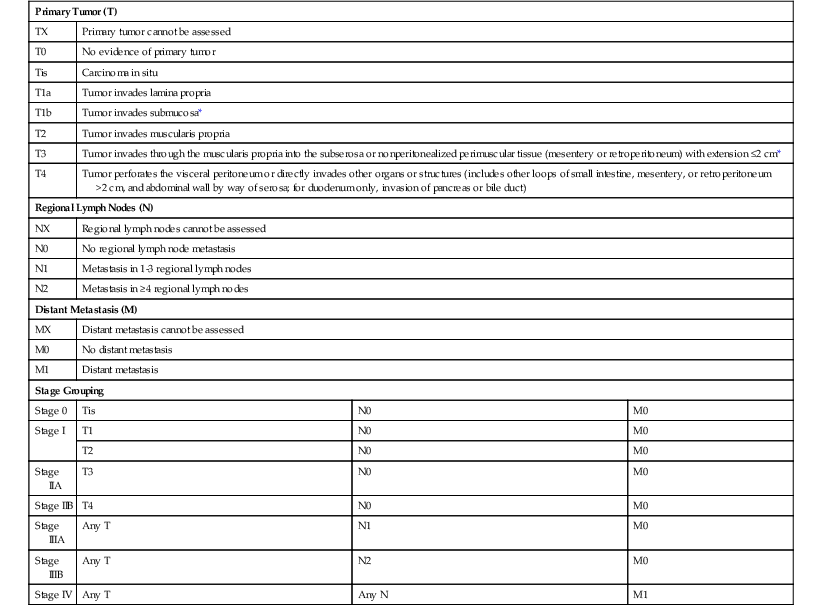
* For jejunum and ileum, the nonperitonealized perimuscular tissue is part of the mesentery; for duodenum in areas where serosa is lacking, it is part of the interface with the pancreas.
From Edge SB, Byrd DR, Compton CC, et al., eds. AJCC Cancer Staging Manual. 7th ed. New York: Springer-Verlag; 2010. Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. Published by Springer Science and Business Media LLC, www.springerlink.com.
Crohn’s Disease–Associated Adenocarcinoma
Clinical Features
Patients with CD are at increased risk for carcinoma of the colon and small intestine.92–94 The incidence of small-intestinal carcinoma in CD is 40- to 86-fold greater than that observed in the general population.95,96 The cancer risk correlates positively with disease duration and the anatomic extent of the inflammatory process. Risk factors for development of small-intestinal carcinoma in individuals with CD include surgically excluded loops of small bowel, chronic fistulous disease, and male sex. In one study, adenocarcinoma risk was found to be lower in CD patients who had undergone small bowel resection or who had used salicylates for longer than 2 years.97 The mortality rate in CD-associated carcinoma is approximately 80%.
Unlike sporadic small-intestinal carcinomas that commonly involve the duodenum, small-intestinal carcinomas that develop in CD arise in areas involved by inflammatory disease. Thirty percent of these tumors arise in the jejunum, and 70% arise in the ileum.
Pathologic Features of Dysplasia
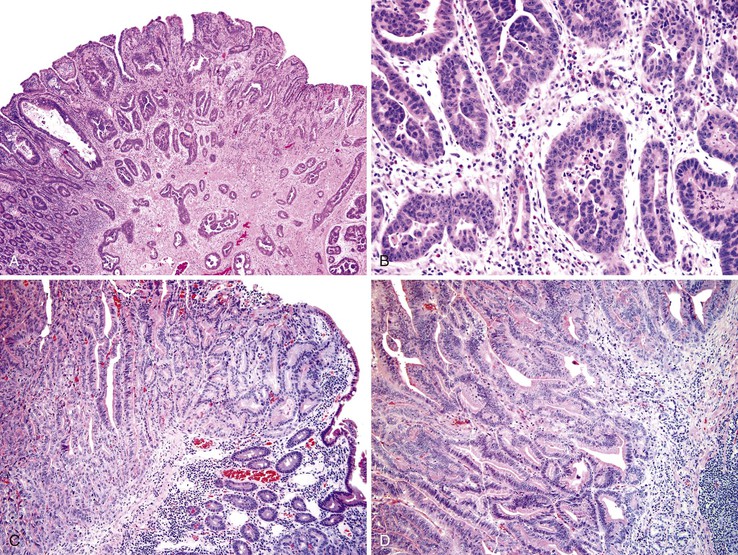
It is now believed that dysplasia precedes cancer development in CD, as in ulcerative colitis.98–100 However, the diagnosis of dysplasia is sometimes difficult because of recurrent and persistent inflammatory changes associated with the underlying inflammatory bowel disease. Grossly, dysplasia may appear flat or elevated (polypoid). Loss of the normal pattern of mucosal folds may be the only gross manifestation of dysplasia, although a granular or pebbly appearance is not uncommon. On occasion, plaques, nodules, and other irregular polypoid lesions may be identified. Histologically, the diagnosis of dysplasia is based on identification of a combination of architectural and cytologic features.101 Architectural alterations may result in a configuration that resembles adenoma. Cytologic abnormalities consist primarily of cellular and nuclear pleomorphism, nuclear hyperchromasia, loss of polarity, and nuclear stratification.
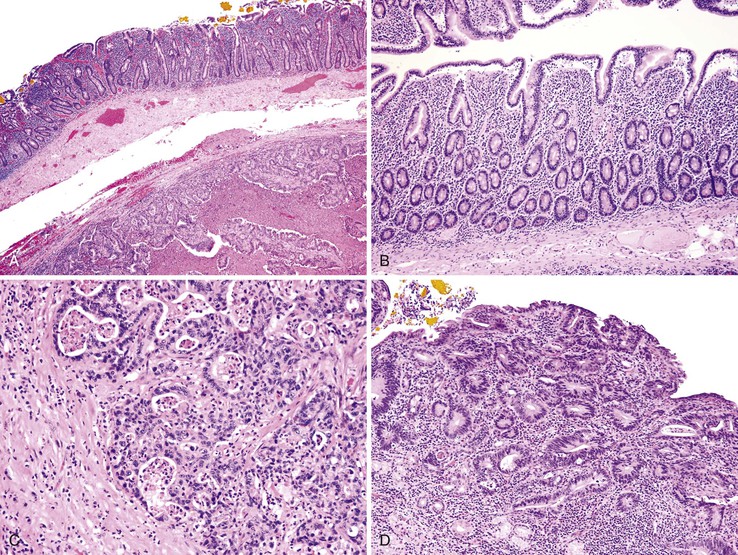
Dysplasia is generally classified as low grade or high grade (including carcinoma in situ). Although invasive carcinoma is more commonly observed in individuals with high-grade dysplasia, it may also be associated with lesser degrees of dysplasia. Low-grade dysplasia is characterized by the presence of tall epithelial cells with elongated, hyperchromatic, pseudostratified nuclei that fail to differentiate into normal goblet or absorptive cells at the mucosal surface (Fig. 26.10). Dystrophic goblet cells may also be present. In low-grade dysplasia, normal basal polarity of the nuclei is maintained. In contrast, high-grade dysplasia demonstrates true nuclear stratification and a greater degree of cytologic atypia (see Fig. 26.10, B). In high-grade dysplasia, the nuclei lose their polarity; instead of being elongated with the long axis of the nucleus oriented perpendicular to the basement membrane, they are often round and contain prominent nucleoli.
Pathologic Features of Adenocarcinoma
Most carcinomas develop in areas of macroscopically identifiable inflammatory bowel disease. Grossly, they resemble ordinary, sporadic intestinal carcinomas. Histologically, adenocarcinomas that arise in CD also resemble sporadic tumors. They may show any degree of differentiation, but in patients with CD, there is a higher proportion of poorly differentiated and mucinous tumors compared with the sporadic type.
Differential Diagnosis
In some cases, CD-associated adenocarcinoma is difficult to distinguish from pseudoinvasion, which entails misplacement of epithelium in the submucosa or muscularis that develops as a result of recurrent injury, ulceration, and repair.102 This represents a form of ileitis cystica profunda. Histologically, epithelial misplacement (pseudoinvasion) is characterized by mucus-filled cysts in the submucosa, muscularis propria, or serosa (Fig. 26.11). The cysts are lined by cuboidal to columnar epithelium–containing goblet cells, enterocytes, and Paneth cells and are normally associated with a rim of lamina propria. On occasion, the cyst lining may regress from pressure atrophy. Features that help rule out malignancy include the absence of desmoplasia and the presence of a rim of lamina propria surrounding misplaced epithelium. Marked cytologic atypia, desmoplasia, and angular, irregularly shaped glands are characteristics of invasive adenocarcinoma.
In diagnostically difficult cases, careful sampling and evaluation of the surface epithelium may help resolve the diagnostic dilemma, particularly if dysplastic epithelium is present.
Celiac Disease–Associated Adenocarcinoma
The rate of development of small bowel malignant neoplasms among patients with celiac disease is increased as much as 80-fold compared with the general population.8,65 Adenocarcinoma of the duodenum and proximal jejunum is the most common nonlymphomatous type of malignancy associated with celiac disease, accounting for more than 20% of all small bowel malignant neoplasms in patients with this disorder103 (Fig. 26.12). The risk of cancer is highest after 2 years of disease; the tumors may be multifocal.103–105 Dysplasia similar to that seen in patients with CD106 may be observed in celiac disease–associated adenocarcinomas as well (see Fig. 26.12, D).
Other Types of Carcinoma
Hepatoid Carcinoma
Rarely, ampullary cancers may display unusual histologic patterns or produce unusual proteins, such as α-fetoprotein (AFP). These tumors are usually moderately to poorly differentiated and resemble gastric AFP-producing hepatoid tumors.107,108 Histologically, they demonstrate solid, papillary, and tubular growth patterns. Clear cell areas may also be present. α1-Antitrypsin-immunoreactive hyaline droplets and bile are found in some cases.107 In addition to hepatoid areas, one usually sees other areas of mucin production and other features of “adenocarcinoma,” such as CEA positivity. Immunohistochemical analysis for antibodies against α-chymotrypsin, prealbumin, transferrin, and AFP is positive, at least focally, in most cases.108 Elevated AFP may also be detectable in the serum of affected patients.
Choriocarcinoma
Primary choriocarcinomas have been reported in the small intestine but are extremely rare.109,110 Grossly, these tumors often appear hemorrhagic and partially necrotic. Histologically, they are composed of aggregates of relatively uniform eosinophilic cells with basophilic vesicular nuclei. Multinucleated syncytial cells with irregular cytoplasmic margins and bizarre, anaplastic nuclei are scattered among more uniform smaller cells. Cytotrophoblastic cells are also seen among the syncytial cells. Vascular invasion is often present. Small-intestinal choriocarcinomas produce human chorionic gonadotropin and human placental lactogen, both of which can be documented by immunohistochemistry. Most of the reported cases of choriocarcinoma have been associated with an adenocarcinoma or anaplastic large cell carcinoma, suggesting that these tumors may arise from multipotential stem cells. Before a diagnosis of primary intestinal choriocarcinoma is established, ectopic pregnancy, teratoma, and metastatic disease from an unrecognized primary tumor must be excluded.
Small Cell Carcinoma
Rarely, small cell neuroendocrine carcinomas, similar to those in the lung or large intestine, can arise in the small intestine.111–113 Patients with small cell carcinoma are often older men in their fifth to eighth decades of life. These tumors are highly aggressive. Most patients die within 1 year after diagnosis.112 Histologically, these tumors are composed of small anaplastic cells with hyperchromatic nuclei and scant cytoplasm. The cells form broad sheets, solid nests, and ribbon-like strands. They may resemble lymphoma, a tumor that statistically is much more common in the small intestine than small cell carcinoma. Immunohistochemistry and special stains can usually help resolve this differential diagnosis. Small cell carcinomas display immunoreactivity for neuron-specific enolase (NSE), Leu-7, chromogranin A, neurofilament protein, synaptophysin, and low-molecular-weight cytokeratin CAM 5.2.
Adenosquamous Carcinoma
Primary adenosquamous carcinomas of the small intestine are extremely rare malignant neoplasms composed of a combination of malignant glandular and squamous elements.114 Both the glandular and the squamous component are thought to arise from a single multipotential stem cell, presumably located at the base of the crypts.115,116 Some studies suggest that adenosquamous carcinomas in the colon are more aggressive than pure adenocarcinomas.115,117 Whether this fact is also true of small-intestinal adenosquamous carcinomas is unknown.
Squamous Cell Carcinoma
Primary squamous cell carcinoma involving the small intestine is extremely rare118 and usually develops in congenital anomalies such as intestinal duplications or Meckel diverticula.119 Squamous cell carcinomas more commonly represent metastases from other sites, such as the cervix or lung.
Sarcomatoid Carcinoma
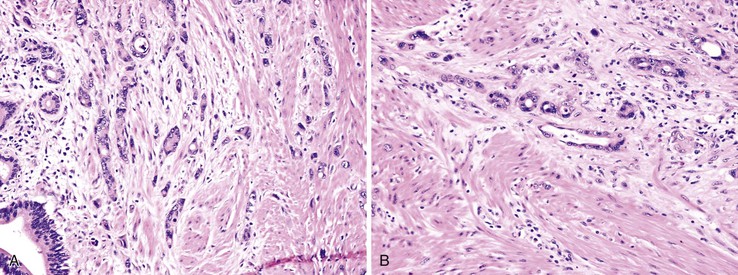
Sarcomatoid carcinoma of the small bowel is rare, with fewer than 25 cases reported to date. These neoplasms primarily affect patients in their sixth decade of life, although younger patients may be affected. Small-intestinal sarcomatoid carcinomas have primarily been reported in the distal small intestine.120–126 They may appear grossly polypoid or endophytic with central ulceration, and they are commonly large (average, 7 cm) at the time of diagnosis. Histologically, these neoplasms may appear biphasic, with admixed epithelioid and mesenchymal elements, or monophasic, composed solely of mesenchymal-type spindle cells. Some tumors demonstrate areas containing anaplastic, bizarre tumor giant cells. Immunohistochemically, both epithelioid and spindle cell components of these tumors show positivity for cytokeratin. Usually, both components also show positivity for vimentin. Focal immunoreactivity for neuroendocrine markers may also be observed.123 These tumors are negative for CD117 and DOG-1.
Carcinoma in Ileostomies
Adenocarcinomas may arise in ileostomy sites in patients who have undergone bowel resection for FAP or inflammatory bowel disease.14,127,128 In patients with polyposis, carcinomas develop in adenomatous polyps.129 Tumors that arise in ileostomies of patients with inflammatory bowel disease occur in those who have had either antecedent backwash ileitis or dysplasia.14,130 In most cases, the cancers develop many years after creation of the ileostomy.131
Carcinoma in Heterotopic Pancreas
Pancreatic heterotopia is the most common congenital abnormality to involve the small intestine. Heterotopic pancreas usually remains asymptomatic, although secondary changes can lead to symptom production. A rare complication is the development of adenocarcinoma, which usually arises from the ductular component of the lesion. Histologically, carcinomas that arise in heterotopic pancreas show the full range of histologic changes that occur in ordinary pancreatic carcinomas.135,136
Metastatic Carcinoma
Metastatic tumors are significantly more common than primary neoplasms in the small intestine.137,138 Metastatic carcinomas from many sites may affect the small intestine, although melanoma and lung, breast, colon, and renal cell carcinomas are the most common. Tumors from the mesentery, pancreas, stomach, or colon may spread to the small intestine directly. Metastases from carcinomas of the testes, adrenal glands, ovary, stomach, uterus, cervix, and liver have also been reported. Of these, ovarian tumors are most likely to cause widespread serosal implants.
Secondary adenocarcinomas may closely mimic primary small-intestinal carcinomas both grossly and microscopically. Grossly, secondary tumors often manifest as intramural masses; they may form submucosal nodules or plaques or even produce a polypoid structure or sessile mucosal lesion. Presenting symptoms may include obstruction, intussusception, or perforation. Napkin ring–like circumferential stenotic lesions may also develop and can lead to localized serosal retraction and intestinal kinks. Metastatic lesions may have an infiltrative growth pattern, in which case they may simulate CD or an ischemic stricture.
Differentiation of metastatic from primary carcinomas of the small intestine is sometimes difficult. If the majority of the neoplastic cells are deep within the wall of the small bowel and there is little involvement of the mucosa, the lesion is most likely metastatic (Fig. 26.13). Adenomatous or dysplastic change in the epithelium overlying or adjacent to the invasive tumor strongly favors a small-intestinal primary. However, secondary adenocarcinomas, especially pancreatic carcinoma, can rarely induce marked epithelial atypia simulating adenomatous change in epithelium adjacent to the carcinoma (see Fig. 26.7). The cells demonstrate cytologic features of malignancy and often appear more disorderly than adenomatous epithelium. However, the pseudostratification of nuclei, typical of adenomatous epithelium, is usually absent. It is unclear whether this epithelial atypia represents neoplastic transformation of the native intestinal epithelium or pagetoid spread of neoplastic cells.
Gangliocytic Paraganglioma
In 1962, Taylor and Helwig139 described a cohort of polypoid duodenal tumors that they initially regarded as benign nonchromaffin paragangliomas, even though the tumors also contained ganglion cells. Later, Kepes and Zacharias140 introduced the term gangliocytic paraganglioma for this tumor (see also Chapters 21 and 30). Some debate exists as to whether the lesion represents a hamartomatous or neoplastic proliferation of endodermally derived epithelial cells originating from the ventral primordium of the pancreas, combined with neuroectoderm-derived ganglion cells, spindle cells, and Schwann cells. Origin from stray embryonic cells that have migrated into the duodenal wall, recruiting nerves, ganglion cells, and smooth muscle cells, has also been suggested on the basis of the resemblance of these tumors to endodermal-neuroectodermal complexes. Another possibility is that the lesion represents a pancreatic tumor that develops from ganglion–islet cell complexes with secondary involvement of the duodenum.
Most gangliocytic paragangliomas arise in the second portion of the duodenum, especially at the level of the ampulla of Vater. However, some develop in the jejunum.139–144 These tumors usually manifest in the patient’s fifth to sixth decades of life, although patients range in age from 15 to 84 years.145 The male-to-female ratio is 1.8 : 1. Most patients have symptoms similar to those of peptic ulcer disease, including abdominal pain, nausea, vomiting, and, most commonly, upper GI tract bleeding.145 Obstructive jaundice may occur in association with periampullary lesions. These neoplasms are considered to be of uncertain malignant potential. Lymph node metastasis is observed in 6.9% of patients.145 Liver metastasis is rare but has been reported.143,146 However, metastatic disease may not be an indicator of aggressive disease, because no deaths have occurred in association with metastatic spread.
Gangliocytic paragangliomas usually appear grossly as small, polypoid, submucosal lesions. The overlying mucosal surface is frequently ulcerated. One sees a mixture of histologic patterns that includes paraganglioma, neurofibroma with proliferating neural processes, and Schwann cells, ganglion cells, and epithelioid cells arranged in clusters, nests, or trabeculae resembling a carcinoid tumor (Fig. 26.14). The neoplasm often extends into the duodenal mucosa.
Most gangliocytic paragangliomas are hormonally inactive. However, immunohistochemical studies have revealed a wide range of polypeptide hormones in the epithelioid cells, such as insulin, glucagon, leu-enkephalin, pancreatic polypeptide, somatostatin, vasoactive intestinal peptide, and serotonin,141,147 as well as NSE, chromogranin, and synaptophysin. The ganglion cells stain with antibodies against NSE, neurofilament, synaptophysin, and CD56.148 S100 immunoreactivity is often detected in the sustentacular cells that typically surround the epithelioid cells of the tumor.141,142,147