CHAPTER 45 Epistaxis
Epistaxis is the commonest otolaryngologic emergency, affecting up to 60% of the population in their lifetimes, with 6% of cases requiring medical attention.1 It has been estimated that nosebleeds affect 108 per 100,000 population per year.2 In England and Wales an average of 10.2 per 100,000 patients with nosebleed are admitted, for an average stay of 2.9 days, in a 3-month period3; and in the United States 17 per 100,000 or 6% of patients with nosebleed are admitted.4 There are peaks in incidence for patients both younger than 10 years old and older than 40 years.4–6 The etiology of epistaxis in the majority of patients is idiopathic,7 followed in frequency by primary neoplasms and traumatic or iatrogenic causes.
Decisions about the optimum therapeutic intervention and timing are often made on an ad hoc basis, and most units do not have a protocol (systematic algorithm) for the management of epistaxis,8 despite a 2005 published protocol aimed at junior doctors.9 However, this protocol does not contain the timing of surgical intervention. The management of a patient with epistaxis ranges from resuscitation, through direct visualization and cautery, nasal packing, and surgery (both endoscopic and external) to embolization.
Vascular Anatomy
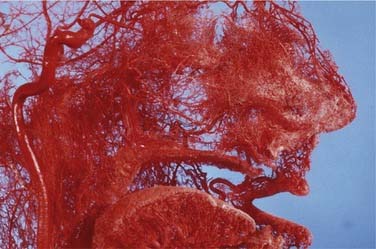
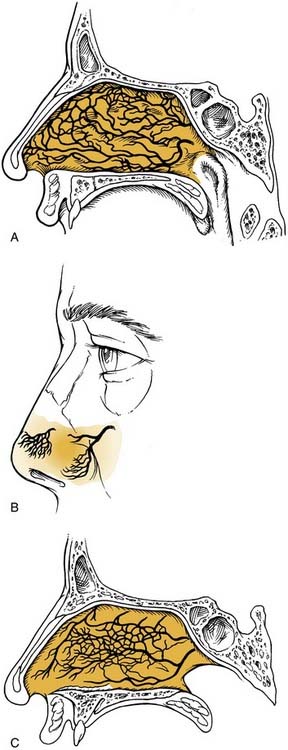
The nasal cavity is extremely vascular. Terminal branches of the external and internal carotid arteries supply the mucosa of the nasal cavity with frequent anastomoses between these systems (Fig. 45-1). The anterior nasal septum is the site of a plexus of vessels called Little’s or Kiesselbach’s area, which is supplied by both systems.
The terminal branches of the external carotid artery that supply the nasal cavity are the facial artery and the internal maxillary artery. The facial artery supplies the superior labial artery, which enters the nose and supplies the anterior nasal septum. The internal maxillary artery courses within the pterygopalatine fossa and terminates in the sphenopalatine, descending palatine, pharyngeal, infraorbital, and posterior superior alveolar arteries. The branching of the sphenopalatine artery when it enters the nasal cavity is a key point in the understanding of the management of posterior nosebleeds. The sphenopalatine artery enters the nasal cavity through the sphenopalatine foramen and then divides into conchal (posterior-lateral) and septal (posterior-medial) branches.10,11 The descending palatine artery courses through the greater palatine canal and becomes the greater palatine artery, entering the nose through the incisive foramen, where it supplies the anterior inferior septum and anastomoses with medial branches of the sphenopalatine artery. Importantly, the vidian artery has a significant anastomosis between the internal carotid artery and a branch of the sphenopalatine artery and therefore with the external carotid system (Fig. 45-2).
The internal carotid artery supplies the nasal mucosa via the ethmoidal branches of the ophthalmic artery. The ophthalmic artery is the first branch of the internal carotid artery. The posterior ethmoidal artery passes through the posterior ethmoidal canal into the anterior cranial fossa and divides into lateral and medial branches, supplying the superior part of the posterior septum and lateral nasal wall. The anterior ethmoidal artery enters the nasal cavity through the anterior ethmoidal canal and passes anteromedially to the area of the anterior skull base (Fig. 45-3). Where it crosses the anterior ethmoid roof to reach the fovea ethmoidalis and cribriform plate, a nasal branch supplies the anterior superior part of the septum (Fig. 45-4) and its other branch—the anterior meningeal artery—enters intracranially.
A fundamental aspect of the understanding of the vascular anatomy and its importance to epistaxis is the fact that various anastomoses on the ipsilateral side between the internal and external carotid systems exist as well as crossover to the contralateral side. The rich anastomoses underlie the importance of a strategy to address the most distal site of any bleeding (Figure 45-5).
Management
Initial Assessment
Obtaining intravenous access, checking for and correcting any clotting abnormalities, and taking blood for “group and save” and/or crossmatching may be required. In our unit patients admitted via the emergency department can be “fast-tracked” to the otorhinolaryngologic emergency unit if stable (Box 45-1). This practice helps avoid unnecessary and counterproductive nasal packing in the emergency department as well as transfer of patients before they are fit enough to travel.
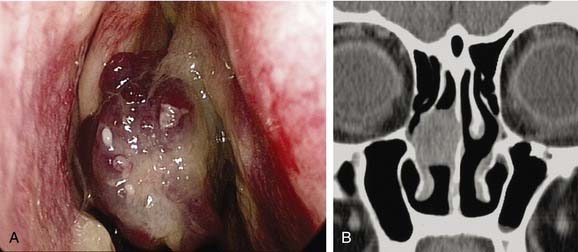
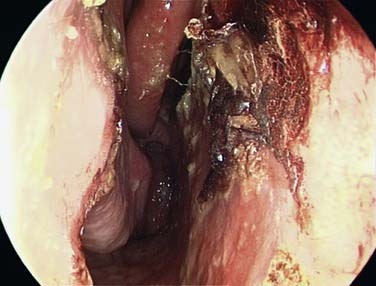
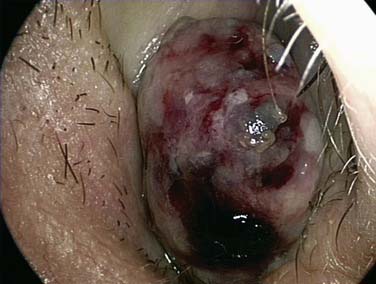
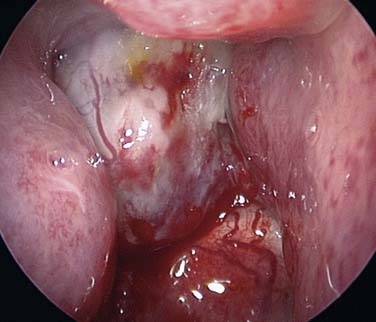
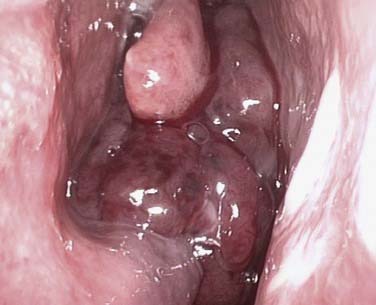
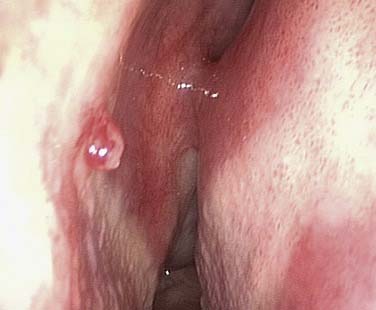
It is important to establish both the site and the cause (Box 45-2; Figs. 45-6 to 45-12) of bleeding. The philosophy of this approach can be summarized as follows:
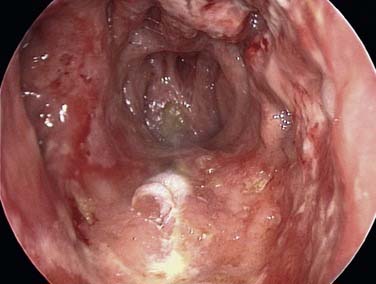
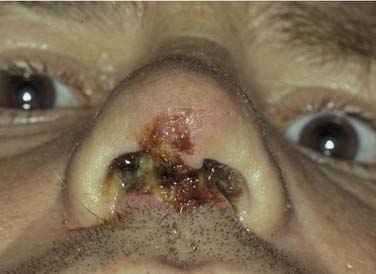
Figure 45-12. Total septal perforation causing epistaxis and crusting in a patient with cocaine abuse.
The clinician must remember that epistaxis is frequently idiopathic but can be a manifestation of a possible underlying pathology (see Fig. 45-12). Your patient should undergo further investigation according to the history.
Epistaxis in Children
Young children usually bleed from a vessel just inside the nose at the mucocutaneous junction on the septum, and the bleeding invariably stops spontaneously. In children with epistaxis in whom no prominent vessel can be seen, the regular local application of a cream can help,12 but petroleum jelly (Vaseline) alone does not.13 A Cochrane Collaboration systematic analysis of the efficacy of topical treatments for idiopathic epistaxis in children concluded that the best treatment still must be defined.14 As many as 5% to 10% of children with recurrent nosebleeds may have undiagnosed von Willebrand’s disease.15,16 Children who have leukemia or are undergoing chemotherapy often have epistaxis associated with thrombocytopenia. Older children, adolescents, and adults often bleed from Little’s area or a maxillary spur.
Epistaxis in Adults
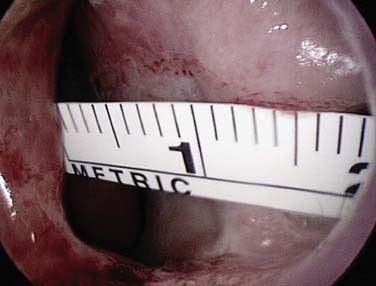
The caudal end of the septum, where several branches of the external and internal carotid anastomose in Little’s area or Kiesselbach’s plexus, is the most common site of bleeding in adults.17 Less commonly bleeding, comes from further back on the septum, and a septal deviation may make it difficult to visualize (Fig. 45-13). Some patients with seasonal allergic rhinitis complain of more nosebleeds in the hay fever season, and topical nasal steroids aggravate the bleeding in approximately 4% of users. Many people believe that a nosebleed signifies a release of pressure and may herald a stroke, and it is important for the clinician to address these anxieties for the patient. Although many patients are found to be hypertensive during nosebleeds, few remain so on follow-up. The association between hypertension and epistaxis is disputed.18 Many clinicians report that hypertension is not related to nosebleed.19–21 However, nosebleeds in patients with hypertension are more likely to lead to admission and to be associated with comorbidity.22
Bleeding disorders can manifest de novo as nosebleeds, although this situation is rare. A range of drugs has been linked with epistaxis, warfarin being one of the most common.3,23 Nearly a third of patients admitted with epistaxis who were taking warfarin had an international normalized ratio (INR) value above the upper limit of the therapeutic range.24 The need to reverse anticoagulation is uncertain as long as the INR value is within the therapeutic range.25 In over-anticoagulated patients, fresh frozen plasma, clotting factor extracts, and vitamin K help. Vitamin K takes more than 6 hours to work, however, and it can delay anticoagulation for 7 days after warfarin is started. If the INR value is greater than 4, the warfarin should be stopped, and fresh frozen plasma given.23 Clotting factor extracts should be given with extreme caution because of the risk of thromboembolic complications. Tranexamic acid, an antifibrinolytic agent, has not been shown to help.26 Other drugs associated with bleeding are aspirin, which interferes with platelet function for up to 7 days, clopidogrel, and nonsteroidal anti-inflammatory drugs.27,28 In patients who do not have a history of a bleeding disorder or undergoing anticoagulant therapy, routine clotting studies do not add to the management.22,24 There is a higher incidence of epistaxis in patients with a high alcohol intake, even when there is no laboratory evidence of a coagulation abnormality.29,30
Topical Treatment
A randomized controlled trial of silver nitrate cautery with topical antiseptic nasal carrier cream versus topical alone showed both to be effective.31 A study of patients applying weekly triamcinolone 0.025% and daily petroleum jelly reported that 89% of patients had no further bleeding.32 Various hemostatic compounds have been used but without consistent evidence of their efficacy. Collagen-derived particles with bovine-derived thrombin have been found to be better than nasal packs.33
Cautery
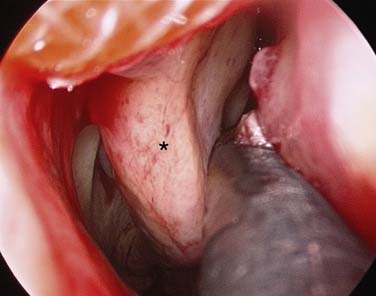
Most anterior epistaxis can be controlled with identification of the bleeding point and cautery using a headlamp. The vast majority of posterior bleeding sites can be identified by endoscopy without the use of general anesthesia.34 The majority of posterior idiopathic bleeds are from the septum, usually from the septal branch of the sphenopalatine artery as it runs submucosally (Fig. 45-14), although some workers report bleeding from the lateral aspect of the middle or inferior meatus or the posterior end of the turbinates (Fig. 45-15).35 When the site of bleeding cannot clearly be identified with a headlamp, the use of a rigid nasal endoscope by an experienced endoscopist is best. The key is to identify the site of the bleeding and gain control using silver nitrate cautery or bipolar suction diathermy. Studies have shown that the use of endoscopic bipolar diathermy treats most cases of epistaxis.36,37 Preparing the nose with lignocaine and epinephrine or cocaine for its decongestant and anesthetic effects often helps. It is worth noting that cophenylcaine has a significant decongestant effect at 6 minutes and a maximum anesthetic effect after 9 minutes,38 so time must be allowed for it to take effect. An injection of local anesthetic and epinephrine gives better analgesia if there is a sizable vessel that needs bipolar diathermy; otherwise the patient may feel a “smarting” sensation.
Rarely, nasal tumors can manifest as epistaxis, so it is important to check for a nasal mass, especially beyond a septal deviation. If there is crusting on the septum, it is worth asking whether it has been a problem. Crusting can follow an abrasion, picking, or a vasculitis such as Wegener’s granulomatosis.39 If it looks likely that the problem is being perpetuated by the patient’s picking or blowing any crust off, it is usually not helpful to confront the patient about this possibility. Instead, the clinician should casually mention that nose-picking is very common (“You see people doing it in cars or waiting for a train, and many people do it when nobody is watching”), and say that dried crusts are irritating, and then ask whether the patient has to remove the crusts; the patient may confess to doing so, and some progress can be made.
The majority of posterior idiopathic bleeds are from the septum, usually from the septal branch of the sphenopalatine artery as it runs submucosally. The key is to identify the site of the bleeding and gain control using bipolar suction diathermy. A severe septal deviation can make it difficult to define the bleeding point.40 Controlling the bleeding avoids the discomfort associated with nasal packing and also avoids hospital admission.40 A U.K. cost-benefit analysis of 38 adult patients with epistaxis concluded that £6804 (≈$13,600) could be saved by avoiding admission in 28 patients.36 Endoscopic cauterization achieves hemostasis in more than 80% of patients with posterior epistaxis at the first attempt and more than 90% after a second attempt.29 The bleeding point can be cauterized with the help of nasal endoscopy; this procedure has a reported failure rate of 17% to 33%.41,42 Complications associated with this procedure are uncommon, but there are isolated reports of palatal numbness from thermal damage to the greater palatine nerve, damage to the lacrimal duct, and possible damage to the optic nerve if cautery is used in a patient who already has undergone an ethmoidectomy.43
Nasal Packing
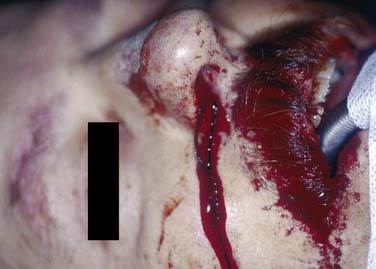
If an anterior pack fails and there is no access to an experienced surgeon who can examine, localize, and cauterize the bleeding point, more packing to tamponade the bleeding point may be required. This procedure requires good local anesthesia and analgesia to enable adequate pressure to be applied with the packs in position. Various proprietary packs are available. The insertion of a nasal pack has conventionally meant that the patient has to be admitted, although one study discharged 46 of 62 patients whose nasal airways had been packed, with outpatient follow-up arranged for 48 hours later. However, 28% of those discharged had unplanned returns to the emergency department.44 If anterior packing fails, a posterior balloon may have to be placed and inflated in the postnasal space. An anterior pack is then placed, and gentle traction used to pull the balloon forward against the anterior pack; this arrangement is held by placement of a clip over the catheter anteriorly as it emerges through the anterior pack.45 It is vital that any clip used to secure the catheter does not rest on the skin of the nostril; a clip in this position could produce necrosis of the area in as little as 4 hours (Fig. 45-16). The morbidity and physical discomfort associated with nasal packing includes pain, hypoxia, alar necrosis, and toxemia, and is well described in the literature.46–49 Unfortunately, nasal packing is used routinely in some units where expertise in nasal endoscopy is not available; for example, in only 7% of ear, nose, and throat emergency departments in the United Kingdom is suction diathermy available.8 Packing not only traumatizes the nasal lining but also can cause cardiorespiratory complications and local infection.46
The role of prophylactic systemic antibiotics in patients who have nasal packs is not well established. There appears to be wide variation in current practice in England.50 The main concern is to avoid toxic shock syndrome. We would prescribe antibiotics if the patient has any cardiac anomalies that would require antibiotic prophylaxis for surgery.
Posterior epistaxis controlled by posterior nasal packing has a failure rate between 26% and 52% and a complication rate between 2% and 68.8%.43,51 The complications noted in two studies were synechia, angina, periorbital cellulitis, sinusitis, toxic shock syndrome, hypoxia, and otitis media.43,51 “Gone are the days when a patient had an uncomfortable posterior nasal pack inserted then spent several days on the ward only to bleed again on its removal.”52 Endoscopic sphenopalatine artery ligation (ESPAL; see later) has replaced the need for posterior nasal packs, other than in an emergency situation, to control profuse bleeding. The goal is to achieve a high success rate and a low morbidity. Especially elderly patients with multiple medical problems (arteriosclerosis, hypertension, diabetes, hepatic and renal disease) tolerate packing poorly, and complications occur frequently; therefore, in such patients, the clinician should consider an early surgical intervention rather than packing of the nose.49
Maxillary Artery Ligation
Another technique to control posterior epistaxis is ligation of the internal maxillary artery in the pterygopalatine fossa.53 It has a reported success rate of approximately 90%.54 Treatment failures are due to the difficulty in finding the artery and its branches. The technique is also associated with a complication rate of 28%.54 Because this procedure is done through a Caldwell-Luc approach, complications include sinusitis, facial pain, oroantral fistula, and facial and dental paresthesia; also, dissection in pterygopalatine fossa can result in blindness, ophthalmoplegia, and decreased lacrimation.41
Ligation of the External Carotid Artery
Ligation of the external carotid artery has been advocated, but the rich anastomoses of vessels in the nose means that this procedure is not very effective.55 Ligation of the external carotid artery prevents embolization that may be desirable because of bleeding that is in the distribution of that vessel but not in the vessels supplied by it, which are also supplied by cross-anastomoses from contralateral vessels. There are reports of cerebrovascular ischemia and infarction following external carotid ligation in elderly atherosclerotic patients, whose cerebral circulation has partially relied on anastomotic connections from the external to the internal carotid system.
The Role of Endoscopic Sphenopalatine Artery Ligation
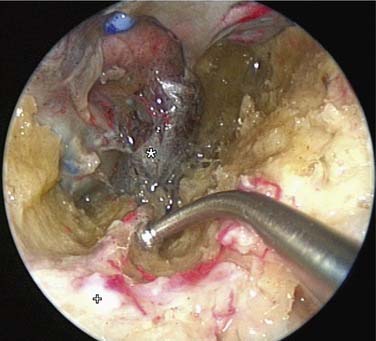
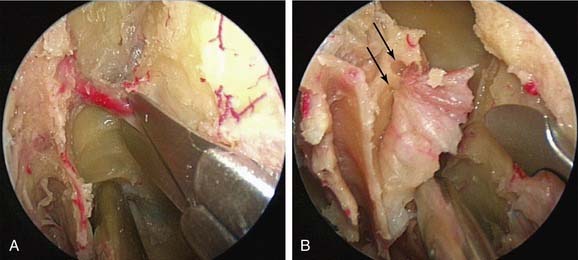
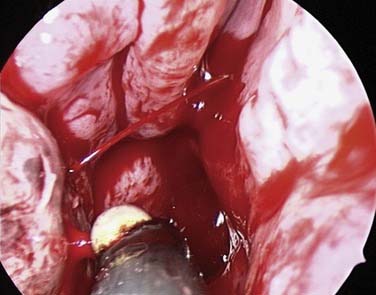
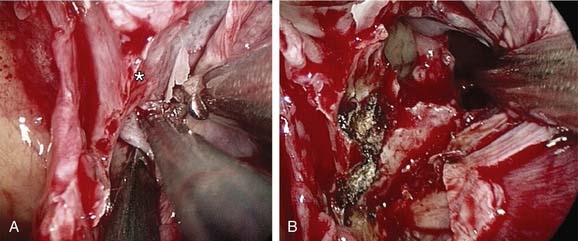
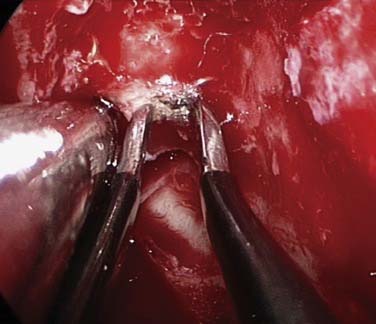
If bleeding cannot be controlled after endoscopic examination and cautery and/or nasal packing, then examination with use of either a general anesthetic or a local anesthetic with sedation is indicated. Bipolar diathermy of any bleeding points or ESPAL is then the treatment of choice.9 Clipping or diathermy of the sphenopalatine artery is now the accepted treatment for management of persistent posterior epistaxis.40,43,56–60 The morbidity associated with ESPAL is low compared with that for ligation of the maxillary or external carotid artery and for embolization. Pooled case-series data have shown that epistaxis was controlled by this technique in 98% of patients.61 The endoscopic sinus surgeon should locate the sphenopalatine artery at the level of the crista ethmoidalis (Fig. 45-17).62 An incision is made over the posterior fontanelle, a submucosal flap is lifted, and the anterior branch is identified with its origin just posterior to the crista ethmoidalis. The anterior branch is then identified and then undergoes clipping or diathermy (Fig. 45-18). In the majority of cases the sphenopalatine foramen opens into the middle and superior meatus.62
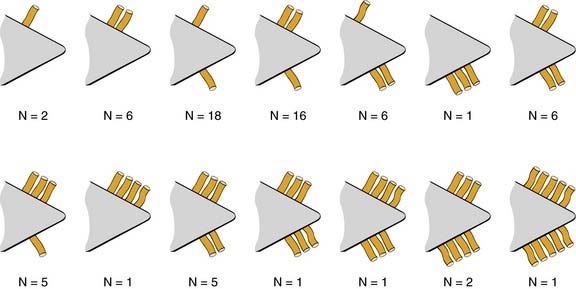
The sphenopalatine artery normally starts to branch lateral to the crista ethmoidalis, and these branches vary widely. It is important that the surgeon who undertakes ligation or cautery of the artery is aware that more than 97% of individuals have two or more branches medial to the crista ethmoidalis, 67% have three or more branches, and 35% have four or more branches.63 The endoscopic sinus surgeon views the sphenopalatine artery at the level of the sphenopalatine foramen or a few millimeters medial to it. With a mucosal incision made in the posterior fontanelle area, anterior to the horizontal part of the base middle turbinate as it joins the lateral nasal wall, a submucosal flap is lifted, and the artery is identified posterior to the crista ethmoidalis and clipped. Some workers describe identifying the artery and clipping it without further dissection to verify whether there were any other branches.56,59,60 The mistaken belief that the artery enters as a single trunk into the nose may have led to the failure of these procedures. One study specifically looked at the branching pattern of this artery from the endoscopic surgeon’s point of view.41 The researchers found two branches of the sphenopalatine artery near the sphenopalatine foramen. In their study, 16% of arteries branched within the foramen, allowing the two arteries to exit together; in 42%, the branches exited much more posteriorly; and in the remaining 42%, the septal branch exited through a separate foramen posterior to the sphenopalatine foramen.41 Simmen and colleagues63 reported that in more than 97% of cadaver specimens examined there were 2 or more branches medial to the crista ethmoidalis, in 30% there were 3 branches, and in one specimen there were 10 branches. The variations in 77 specimens are shown in Figure 45-19.
Various reports have claimed success rates for ESPAL between 92% and 100% in controlling epistaxis.57,59,61 Failure to clip all the branches of the sphenopalatine artery may be the reason for continued epistaxis after the procedure. All divisions of the sphenopalatine artery take place in the pterygomaxillary fissure, and the branches enter the nasal fossa as separate blood vessels. The arrangement of these branches in relation to the sphenopalatine foramen varies widely. The largest and most anteriorly based branch is the posterior lateral nasal branch. This must be clipped and divided so that the flap can be lifted superiorly to locate the nasopalatine branch, which must be clipped and divided as well. Because there are such wide variations in the branching pattern of the sphenopalatine artery, careful dissection is required even after these two branches are clipped. A thorough search by careful dissection should be made all around the sphenopalatine foramen to find any other branches that can be clipped. Clipping or diathermy of the sphenopalatine artery has a failure rate between 0% and 8%61 and is not associated with any serious complications. The main complication, as previously mentioned, is failure to control epistaxis, which is usually due to the surgeon’s failure to clip all the branches of the sphenopalatine artery. The arrangements of these branches are unpredictable, as seen in our study, and can vary on the two sides in the same individual. In addition the branches of this artery can come out through separate foramina, and a surgeon who is not aware of this possibility can miss a few branches. Other complications are uncommon; they include nasal crusting, palatal numbness, acute rhinosinusitis, decreased lacrimation, and septal perforation.59 A long-term study of ESPAL showed a 93% success rate.64
We do not advocate packing and repacking for refractory epistaxis.
Embolization
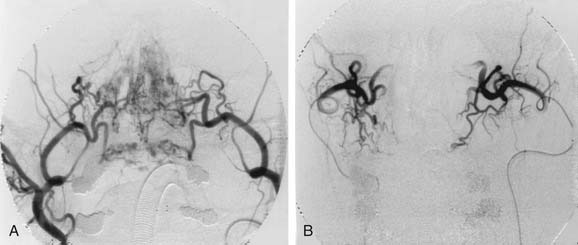
Arterial embolization has been shown to be effective in the treatment of intractable epistaxis.65,66 However, the procedure carries a risk of complications, which include cerebrovascular accident, hemiplegia, ophthalmoplegia, facial nerve palsy, seizures, and soft tissue necrosis.67–70 The analysis of 31 cases of embolization of internal maxillary artery by Siniluoto and colleagues71 did not find any major and persistent complications. Because interventional neuroradiology is increasingly available, embolization has become an option when initial treatment fails. It is also important to state at this point that the arterial embolization technique is effective mainly for the external carotid artery supply and very dangerous for the internal carotid supply. Embolization for bleeding from the terminal branches of the ophthalmic artery is therefore not advocated because of the high risk of blindness due to reflux of the embolic materials.72
We would advocate the use of this technique for patients with refractory epistaxis who are unfit for surgery or in whom surgery has failed to control the bleeding (Fig. 45-20). Persistent posterior epistaxis can be controlled by percutaneous embolization of bleeding arteries. The success rate for this procedure is between 71% and 95%, and the complication rate is 27%.41,54,71
Bleeding from the Anterior Ethmoidal Artery
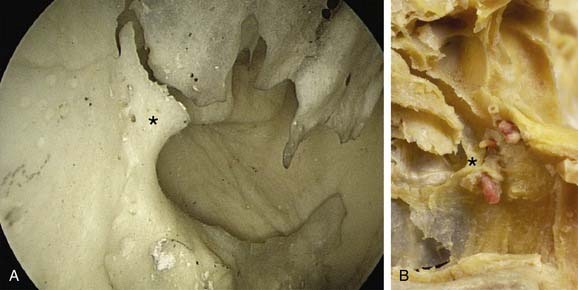
An uncommon cause of severe epistaxis is associated with a nasoethmoid fracture when the bleeding originates from the anterior ethmoidal artery. Patients with anterior ethmoidal artery epistaxis form a distinct subgroup whose management differs in that they require surgery to the anterior ethmoidal artery, via either a traditional external approach or an endoscopic approach, because the bleeding rarely subsides with conservative measures (Fig. 45-21).73
The anterior ethmoidal artery can also be damaged during sinus surgery. It is at risk as it passes through the roof of the ethmoid sinuses on its way from the orbit to the anterior cranial fossa.74 Damage to the artery can lead to profuse epistaxis and intraorbital bleeding, and, at times, intracranial bleeding.75 The development of a retro-orbital hemorrhage is an emergency because it can even lead to blindness. The bony canal in which the anterior ethmoidal artery crosses the skull base can be dehiscent, making it vulnerable to damage.76 The rate of dehiscence of the anterior ethmoidal artery has been reported as 11% to 40%.77
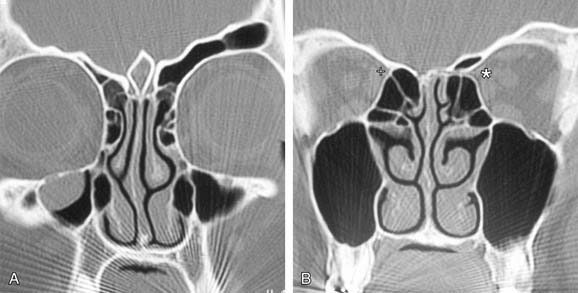
There are bony protrusions from the lateral end of the bony canal of the artery where it emerges from the orbit and medially where it goes through the lateral lamella. Some authorities have stated that the artery lies behind the frontal recess and can act as a landmark in the surgical approach to this area.74, 78–80 Stammberger76 located the anterior ethmoid artery 1 to 2 mm behind the junction of the posterior wall of the frontal recess and the anterior ethmoid air cells.76 Simmen and associates81 showed a mean distance from the posterior wall of the frontal recess to the artery of 11 mm (range 6-15 mm). In most cases the position of the artery is mirrored on the contralateral side. However, the position is very variable, so it is not safe to use the artery as a landmark for any endoscopic intervention, especially in locating the frontal recess. The anterior ethmoidal artery is always seen between the second and third lamellae, and the most common site to find it is in the suprabullar recess (85%). If pneumatization of the ethmoid sinus is marked, the artery is likely to be lying below the skull base and is more prone to surgical damage. Preoperative knowledge of the suprabullar recess and supraorbital cells through careful interpretation of computed tomography scans is helpful in avoiding damage to the anterior ethmoidal artery (Fig. 45-22).
Hot Water Irrigation for Epistaxis
Hot water irrigation as a noninvasive treatment for posterior epistaxis has received some renewed attention.82 Obstetricians used hot water to irrigate a postpartum bleeding wound in the 19th century, and Guice83 introduced this procedure for staunching blood in cases of epistaxis in 1879. Studies by Stangerup and colleagues84 involving experiments with rabbits showed that irrigating the nose at 40° to 46°C does not lead to any histologic changes of the mucosa. Temperatures higher than 46°C result in vasodilatation and, in particular, edema of the mucosa. Only temperatures above 52°C trigger necrosis. It can be assumed that the temperature-induced mucosal edema leads to local compression of the bleeding vessel and at the same time may propagate the cascade for hemostasis.84
After an examination with nasal endoscopy, topical anesthesia of the relevant nasal cavity is administered using 4% tetracaine. Then Stangerup’s modified bladder catheter is inserted in the affected nasal cavity. The balloon is inflated in the epipharynx and the catheter gently pulled back until the choana is blocked off. The affected nasal cavity is continuously irrigated with tap water at a temperature of 50°C. The treatment lasts approximately 3 minutes, with the patient sitting upright so that the irrigation water can flow forward from the nose into a basin. After the irrigation with 500 mL of hot water, the catheter can be removed. In a prospective study involving 84 patients, Schlegel and colleagues82 showed a success rate of 82% for hot water irrigation without any complications. This technique avoids painful packing, hospitalization, and immediate surgery but also allows the patient to breath normally through the open nasal cavity after the treatment.82–84
Management of Epistaxis in Patients with Hereditary Hemorrhagic Telangiectasia
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant multisystemic disorder characterized by telangiectasia that affects cutaneous and mucosal surfaces as well as arteriovenous malformations in the pulmonary, cerebral, and hepatic circulations (Fig. 45-23). The most common symptom in patients with HHT is epistaxis, which affects more than 90% of individuals. The severity of attacks is variable; these episodes of epistaxis can be functionally and socially debilitating for the patient and may require frequent hospital admission; and for some patients, the bleeding is intractable.85 Many methods of treatment for epistaxis due to HHT have been described in the literature, including medical strategies, such as the use of hormonal manipulation and the use of antifibrinolytic agents, as well as surgical options, including laser coagulation (see Fig. 45-23),86 septodermoplasty,87 and nasal closure.88 No one method has proved itself entirely successful or without significant side effects, however.
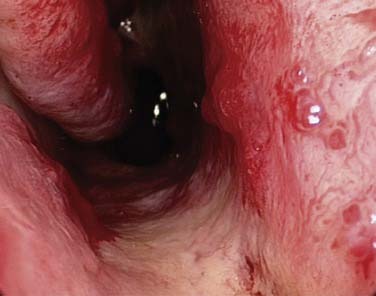
Figure 45-23. Hereditary hemorrhagic telangiectasia with arteriovenous malformations, on an endoscopic view of the right nasal cavity.
A prospective study by Hitchings and coworkers85 investigated the effect of various surgical options on quality of life scores in patients with HHT and epistaxis. The researchers concluded that nasal closure should be offered to patients with moderate to severe epistaxis that has proved unresponsive to other treatment options, either bipolar (see Fig. 45-23) or laser coagulation treatment (see Fig. 45-24), or septodermoplasty; 88% (7/8) of the patients in their series who were treated with nasal closure reported a complete cessation of nosebleeds. Closure of the nasal cavity—Young’s procedure—is based on the principle that the absence of desiccating airflow through the nasal cavity prevents the breakdown of mucosa overlying the fragile telangiectasias. An alternative to nasal closure is the use of nasal obturators.89
Summary
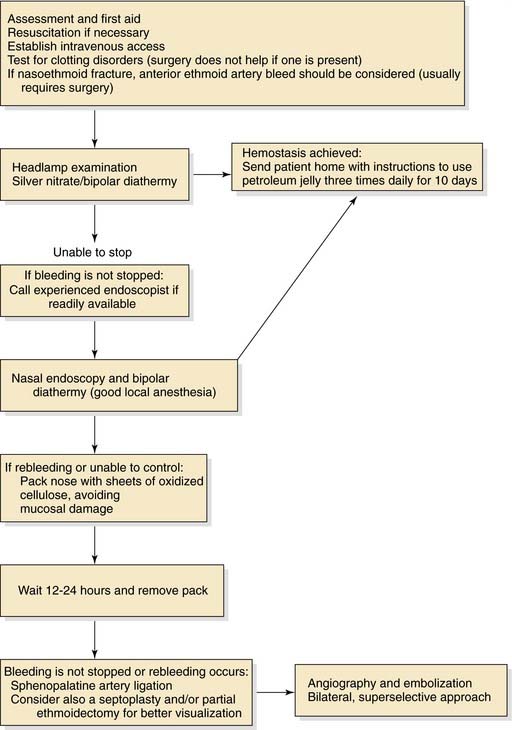
The role of rigid nasal endoscopy as part of the initial assessment of epistaxis, with direct visualization and control of the bleeding point, is effective in the majority of patients and reduces the need for nasal packing. Endoscopic sphenopalatine artery ligation is well established as the treatment of choice when cautery and nasal packing fail. Figure 45-25 presents a protocol for management of epistaxis.
Hitchings AE, Lennox PA, Lund VJ, et al. The effect of treatment for epistaxis secondary to hereditary hemorrhagic telangiectasia. Am J Rhinol. 2005;19:75-78.
Leong SCL, Roe RJ, Karkanevatos A. No frills management of epistaxis. Emerg Med J. 2005;22:470-472.
Lund VJ. Treatment algorithm for the management of epistaxis in hereditary hemorrhagic telangiectasia. Am J Rhinol. 1999;13:319-322.
Pope LE, Hobbs CG. Epistaxis: an update on current management. Postgrad Med J. 2005;81:309-314.
Schlegel C, Siekmann U, Linder H. Non-invasive treatment of intractable posterior epistaxis with hot-water irrigation. Rhinology. 2006;44:90-93.
Simmen D, Raghavan U, Manestar M, et al. The anatomy of the sphenopalatine artery for the endoscopic sinus surgeon. Am J Rhinol. 2006;20:502-505.
Simmen D, Raghavan U, Briner HR, et al. The surgeon’s view of the anterior ethmoid artery. Clin Otolaryngol. 2006;31:187-191.
Woolford TJ, Jones NS. Endoscopic ligation of anterior ethmoidal artery in treatment of epistaxis. J Laryngol Otology. 2000;114:858-860.
1. Small M, Murray JAM, Maran AGD. A study of patients with epistaxis requiring admission to hospital. Health Bull (Edinb). 1982;40:20-29.
2. O’Donnell M, Robertson G, McGary GW. A new bipolar diathermy probe for the outpatient management of adult acute epistaxis. Clin Otolaryngol. 1999;24:537-541.
3. Kotecha B, Fowler S, Harkness P, et al. Management of epistaxis: A national survey. Ann R Coll Surg Engl. 1996;78:444-446.
4. Pallin DJ, Chng YM, McKay MP, et al. Epidemiology of epistaxis in U.S. emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46(1):77-81.
5. Petruson B. Epistaxis in childhood. Rhinology. 1979;17:83-90.
6. Kucik CJ, Clenney T. Management of epistaxis. Am Fam Physician. 2005;71:305-311.
7. McGarry GW. Clinical aspects of adult idiopathic epistaxis. MD thesis held in the special collection at the University of Glasgow, 1996.
8. Duvvi S, Khattab A, Khalil HS, et al. Short falls in epistaxis management: a nationwide survey in UK. Clin Otolaryngol. 2006;31:560-561.
9. Pope LE, Hobbs CG. Epistaxis: an update on current management. Postgrad Med J. 2005;81:309-314.
10. Pearson B, MaxKenzie R, Goodman W. The anatomical basis of transantral ligation of the maxillary artery in severe epistaxis. Laryngoscope. 1969;79:969-984.
11. Lee HY, Kim HU, Kim SS, et al. Surgical anatomy of the sphenopalatine artery in lateral nasal wall. Laryngoscope. 2002;112:1813-1818.
12. Kubba H, MacAandie C, Botma M. A prospective single-blind randomized controlled trial of antiseptic cream for recurrent epistaxis in childhood. Clin Otolaryngol Allied Sci. 2001;26(6):465-468.
13. Loughran S, Spinou E, Clement W, et al. A prospective single-blind, randomized controlled trial of petroleum jelly/Vaseline for recurrent paediatric epistaxis. Clin Otolaryngol. 2004;29:266-269.
14. Burton MJ, Doree CJ. Interventions for recurrent idiopathic epistaxis (nosebleeds) in children. The Cochrane Collaboration. Cochrane Ear, Nose and Throat Disorders Group website. 1 October 2006. Available at http://www.cochrane.org/reviews/en/ab04461.html
15. Kiley V, Stuart JJ, Johnson CA. Coagulation studies in children with isolated recurrent epistaxis. J Pediatrics. 1982;100:579-581.
16. Katsanis E, Koon-Hung L, Hsu M. Prevalence and significance of mild bleeding disorders in children with recurrent epistaxis. J Pediatr. 1988;113:73-76.
17. Chiu T, Dunn JS. An anatomical study of the arteries of the anterior nasal septum. Otolaryngol Head Neck Surg. 2006;134:33-36.
18. Temmel AF, Quint C, Toth J. Debate about blood pressure and epistaxis will continue. BMJ. 2001;322(7295):1181.
19. Poulsen P. Epistaxis—examination of hospitalised patients. J Laryngol Otol. 1984;98:277-279.
20. Weiss NS. The relation of high blood pressure to headache and epistaxis and selected other symptoms. N Engl J Med. 1972;287:631.
21. Fuchs FD, Moreira LB, Pires CP, et al. Absence of association between hypertension and epistaxis: a population based study. Blood Press. 2003;12:145-148.
22. Jackson KR, Jackson RT. Factors associated with active, refractory epistaxis. Arch Otolaryngol Head Neck Surg. 1988;114:862-865.
23. Choudhury N, Sharp HR, Mir N, et al. Epistaxis and oral anticoagulant therapy. Rhinology. 2004;42:92-97.
24. Thaha MA, Nilssen ELK, Holland S, et al. Routine coagulation screening in the management of emergency admission for epistaxis—is it necessary? J Laryngol Otol. 2000;114:38-40.
25. Srinvasan V, Patel H, John DG, et al. Warfarin and epistaxis: should warfarin always be discontinued? Clin Otolaryngol. 1997;22:542-544.
26. Tibbelin A, Aust R, Bende M. Effects of local tranexamic acid gel in the treatment of epistaxis. ORL. J Otorhinolaryngol Relat Spec. 1995;57:207-209.
27. Livesey JR, Watson MG, Kelly M, et al. Do patients with epistaxis have drug-induced platelet dysfunction? Clin Otolaryngol. 1995;20:407-410.
28. Watson MG, Shenoi PM. Drug induced epistaxis? J R Soc Med. 1990;83:162-164.
29. Frikart L, Agrifoglio A. Endoscopic treatment of posterior epistaxis. Rhinology. 1998;36:59-61.
30. McGarry GW, Gatehouse S, Vernham G. Idiopathic epistaxis, haemostasis and alcohol. Clin Otolaryngol. 1995;20:174-177.
31. Murthy P, Nilssen ELK, Roa S, et al. A randomised clinical trial of antiseptic nasal carrier cream and silver nitrate cautery in the treatment of recurrent anterior epistaxis. Clin Otolaryngol. 1999;24:228-231.
32. London SD, Lindsey WH. A reliable medical treatment for recurrent mild anterior epistaxis. Laryngoscope. 1999;109:1535-1537.
33. Mathiasen RA, Cruz RM. Prospective, randomized, controlled clinical trial of a novel matrix haemostatic sealant in patients with acute anterior epistaxis. Laryngoscope. 2005;115:899-902.
34. Chiu TW, McGarry GW. Prospective clinical study of bleeding sites in idiopathic adult posterior epistaxis. Otolaryngol Head Neck Surg. 2007;137:390-393.
35. Thornton MA, Mahesh BN, Lang J. Posterior epistaxis: identification of common bleeding sites. Laryngoscope. 2005;115:588-590.
36. Ahmed A, Woolford TJ. Endoscopic bipolar diathermy in the management of epistaxis: an effective and cost-effective treatment. Clin Otolaryngol. 2003;28:273-275.
37. O’Donnell M, Robertson G, McGarry GW. A new bipolar diathermy probe for the outpatient management of adult acute epistaxis. Clin Otolaryngol. 1999;24:537-541.
38. Vaghela HM. Foley catheter posterior nasal packing. Clin Otolaryngol. 2005;30:209.
39. Sproson E, Lanyon P, Al-Deiri M. Lessons learnt in the management of Wegener’s granulomatosis: long-term follow-up of 60 patients. Rhinology. 2007;45:63-67.
40. McGarry GW. Nasal endoscope in posterior epistaxis: a preliminary evaluation. J Laryngol Otol. 1991;105:428-431.
41. Schwartzbauer HR, Shete M, Tami TA. Endoscopic anatomy of the sphenopalatine and posterior nasal arteries: implications for the endoscopic management of epistaxis. Am J Rhinol. 2003;17(1):63-66.
42. Barlow DW, Deleyiannis FWB, Pinczower EF. Effectiveness of surgical management of epistaxis at a tertiary care centre. Laryngoscope. 1997;107:210-240.
43. O’Leary-Stickney K, Makielski K, Weymuller EA. Rigid endoscopy for the control of epistaxis. Arch Otolaryngol Head Neck Surg. 1992;118:966-967.
44. Van Wyk FC, Massey S, Worley G, et al. Do all epistaxis patients with a nasal pack need admission? A retrospective study of 116 patients managed in accident and emergency according to a peer reviewed protocol. J Laryngol Otol. 2007;121:222-227.
45. Wurman LH, Sack JF, Flannery JV, et al. The management of epistaxis. Am J Otolaryngol. 1992;13-14:193-209.
46. Fairbanks D. Complications of nasal packing. Otolaryngol Head Neck Surg. 1986;94:412-415.
47. Cassini NJ, Biller HF, Ogura JH. Changes in arterial oxygen tension and pulmonary mechanics with use of posterior packing in epistaxis. Laryngoscope. 1971;81:1261-1266.
48. Herzon FS. Bacteraemia and local infections with nasal packing. Arch Otolaryngol Head Neck Surgery. 1971;114:862-865.
49. Simmen D, Heinz B. Epistaxis strategy—experiences with the last 360 hospitalizations [in German]. Laryngorhinootologie. 1998;77:100-106.
50. Biswas D, Wilson H, Mal R. Use of systemic prophylactic antibiotics with anterior nasal packing in England, UK. Clin Otolaryngol. 2006;31:566-567.
51. Schaitkin B, Strauss M, Houck JR. Epistaxis: medical versus surgical therapy: a comparison of efficacy, complications and economic considerations. Laryngoscope. 1987;97:1392-1396.
52. Gouglas R, Wormald P-J. Update on epistaxis. Curr Opin Otolaryngol Head Neck Surg. 2007;15:180-183.
53. Busch RF. A new vascular clip applier for internal maxillary and ethmoid artery ligations. Otolaryngol Head Neck Surg. 1992;107:129-130.
54. Strong EB, Bell DA, Johnson LP, et al. Intractable epistaxis: transantral ligation vs embolisation: efficacy review and cost analysis. Otolaryngol Head Neck Surg. 1995;113:674-678.
55. Waldron J, Stafford N. Ligation of the external carotid artery for severe epistaxis. J Otolaryngol. 1992;21:249-251.
56. Ram B, White PS, Saleh HA, et al. Endoscopic endonasal ligation of the sphenopalatine artery. Rhinology. 2000;38:147-149.
57. Wormald PJ, Wee DTH, van Hasselt CA. Endoscopic ligation of the sphenopalatine artery for refractory posterior epistaxis. Am J Rhinol. 2000;14(4):261-264.
58. O’Flynn PE, Shadaba A. Management of posterior epistaxis by endoscopic clipping of the sphenopalatine artery. Clin Otolaryngol. 2000;25:374-377.
59. Snyderman CH, Goldman SA, Carrau RL, et al. Endoscopic sphenopalatine artery ligation is an effective method of treatment for posterior epistaxis. Am J Rhinol. 1999;13(2):138-140.
60. Bolger WE, Borgie RC, Melder P. The role of the crista ethmoidalis in endoscopic sphenopalatine artery ligation. Am J Rhinol. 1999;13(2):82-86.
61. Kumar S, Shetty A, Rockey J, et al. Contemporary surgical treatment of epistaxis: what is the evidence for sphenopalatine artery ligation? Clin Otolaryngol Allied Sci. 2003;28:360-363.
62. Wareing MJ, Padgham ND. Osteologic classification of the sphenopalatine foramen. Laryngoscope. 1998;108:125-127.
63. Simmen D, Raghavan U, Manestar M, et al. The anatomy of the sphenopalatine artery for the endoscopic sinus surgeon. Am J Rhinol. 2006;20:502-505.
64. Abdelkader M, Leong SC, White PS. Endoscopic control of the sphenopalatine artery for epistaxis: long-term results. J Laryngol Otol. 2007;121:759-762.
65. Scaramuzzi N, Walsh RM, Brennan P, et al. Treatment of intractable epistaxis using arterial embolization. Clin Otolaryngol Allied Sci. 2001;26:307-309.
66. Elahi MM, Parnes LS, Fox AJ, et al. Therapeutic embolisation in the treatment of intractable epistaxis. Arch Otolaryngol Head Neck Surg. 1995;121:65-69.
67. Tseng EY, Narducci CA, Willing SJ, et al. Angiographic embolization for epistaxis: a review of 114 cases. Laryngoscope. 1998;108:615-619.
68. Lin S. Arterial embolization leading to fatal cerebral infarction (a case report) [in Chinese]. Chung Hua Erh Pi Yen Hou Ko Tsa Chih. 1994;29(4):209-210.
69. De Vries N, Versluis RJJ, Valk J, et al. Facial nerve paralysis following embolisation for severe epistaxis (case report and review of the literature). J Laryngol Otol. 1986;100:207-210.
70. Christensen NP, Smith DS, Barnwell SL, et al. Arterial embolization in the management of posterior epistaxis. Otol Head Neck Surg. 2005;133:748-753.
71. Siniluoto TMJ, Leinonen ASS, Kartunen AI, et al. Embolization for the treatment of posterior epistaxis. Arch Otolaryngol Head Neck Surg. 1993;119:837-841.
72. Koh E, Frazzini VI, Kagetsu NJ. Epistaxis—vascular anatomy, origins and endovascular treatment. Am J Roentgenol. 2000;174:845-851.
73. Woolford TJ, Jones NS. Endoscopic ligation of the anterior ethmoid artery in treatment of epistaxis. J Laryngol Otol. 2000;114:858-860.
74. Polavaran R, Devaiah AK, Sakai O, et al. Anatomical variation and pearls—functional endoscopic sinus surgery. Otolaryngol Clin North Am. 2004;37:221-242.
75. Lee WC, Ku PK, van Hasselt CA. New guidelines for endoscopic localisation of the anterior ethmoidal artery: a cadaveric study. Laryngoscope. 2000;110:1173-1178.
76. Stammberger H. Functional Endoscopic Sinus Surgery: The Messerklinger Technique. St Louis: Mosby–Year Book; 1991.
77. Moon HJ, Kim HU, Lee JG, et al. Surgical anatomy of the anterior ethmoid roof. Laryngoscope. 2001;111:900-904.
78. Ohnishi T, Tachibana T, Kaeko Y, et al. High-risk areas in endoscopic sinus surgery and prevention of complications. Laryngoscope. 1993;103:1181-1185.
79. Moon HJ, Kim HU, Lee JG, et al. Surgical anatomy of the anterior ethmoid roof. Laryngoscope. 2001;111:900-904.
80. Kirchner JA, Yanagisawa E, Crelin ES. Surgical anatomy of the ethmoidal arteries. Arch Otolaryngol. 1961;74:382-386.
81. Simmen D, Raghavan U, Briner HR, et al. The surgeon’s view of the anterior ethmoid artery. Clin Otolaryngol. 2006;31:187-191.
82. Schlegel C, Siekmann U, Linder T. Non-invasive treatment of intractable posterior epistaxis with hot-water irrigation. Rhinology. 2006;44:90-93.
83. Guice NL. Hot water in epistaxis. Mississippi Valley Medical Monthly. 1884;2:3-4.
84. Stangerup SE, Dommerby H, Siim C, et al. New modification of hot water irrigation in the treatment of posterior epistaxis. Arch Otolaryngol Head Neck Surg. 1999;125:686-690.
85. Hitchings AE, Lennox PA, Lund VJ, et al. The effect of treatment for epistaxis secondary to hereditary hemorrhagic telangiectasia. Am J Rhinol. 2005;19:75-78.
86. Lennox PA, Harries M, Lund VJ, et al. Retrospective study of the role of the argon laser in the management of epistaxis secondary to hereditary haemorrhagic telangiectasia. J Laryngol Otol. 1997;111:34-37.
87. Saunders WH. Septal dermoplasty for the control of nose bleeds in hereditary hemorrhagic telangiectasia. Trans Am Ophthalmol Otol. 1960;64:500-506.
88. Gluckmann JL, Portugal LG. Modified Young’s procedure for refractory epistaxis due to hereditary hemorrhagic telangiectasia. Laryngoscope. 1994;104:1174-1177.
89. Woolford T, Loke D, Bateman ND. The use of a nasal obturator in hereditary haemorrhagic telangiectasia: an alternative to Young’s procedure. J Laryngol Otol. 2002;116(6):445-446.