chapter 7
Episodic Disturbances of Neurological Function
The assessment of patients with intermittent disturbances of neurological function is one of the most interesting and challenging aspects of clinical neurology. One needs to be an amateur detective like Sherlock Holmes, whom Arthur Conan Doyle modelled on Dr Joseph Bell, one of his teachers at the medical school of Edinburgh University. Dr Bell was a master at observation, logic, deduction and diagnosis [1]. This chapter discusses the various causes of episodic disturbance of neurological function. There is only a brief discussion of epilepsy and cerebrovascular disease as they are covered in more detail in Chapter 8, ‘Seizures and epilepsy’, and Chapter 10, ‘Cerebrovascular disease’, respectively. Vertigo is discussed in this chapter as most often it is an episodic disturbance, but mainly because it seemed to fit better in this chapter than in any other.
THE HISTORY AND INTERMITTENT DISTURBANCES
Most inexperienced clinicians simply ascertain the nature of the symptoms. They do not determine their exact distribution, the mode of onset and progression of each and every symptom, particularly in relation to the other symptoms, nor the circumstances under which symptoms occur, which often provides the vital clue to the diagnosis.
The recommended method of taking a history is different from that described in Chapter 2, ‘The neurological history’. It is far more useful to ask the patient to provide a detailed account of several individual episodes.
As you take the history question the patient about the symptoms:
A suggested method of history taking
Begin by asking the patient the following questions:
1. Tell me about the last episode you had: what were you doing at the time it commenced?
2. What was the very first symptom that you noticed?
3. From the moment you first noticed that symptom, was it at its most severe or did it become more intense or spread to involve other parts of the body?
4. If it did, how long did it take to spread or reach maximum intensity?
6. What was the next symptom that you noticed?
7. How long after the first symptom did it commence?
8. Was the initial symptom showing signs of improving or worsening before this symptom began?
9. How long did this symptom take to develop in terms of maximum intensity or extent of involvement of the body?
Cases involving single episodes
On the surface this does appear to be an epileptic seizure preceded by an olfactory aura (see Chapter 8, ‘Seizures and epilepsy’, for further discussion of the term ‘aura’, which is used to describe the initial symptom(s) of a seizure, often referred to as the warning symptoms). However, this doctor was too quick in jumping to the diagnosis of epilepsy and used only the nature of the symptoms to make a diagnosis, recalling that some seizures are associated with an altered smell. The correct diagnosis was apparent when a detailed history was obtained.
Cases involving recurrent episodes or transient symptoms
In patients with recurrent episodes or transient neurological symptoms, establish whether all episodes were identical and whether the symptoms varied from one episode to another by asking: ‘Are all your turns the same or are some different to the others?’ If the episodes varied, it is more rewarding to ask about several individual episodes. This is most relevant in some patients with epilepsy who may have multiple types of seizures (see Chapter 8, ‘Seizures and epilepsy’). If the events were all identical, it is possible to use the approach of asking the patient to imagine having an episode right now in front of you and using the questioning technique described above to obtain a detailed description of each and every symptom. This approach could miss the diagnosis when the circumstances under which these episodes occurred provide the vital clue to the diagnosis. This is highlighted by the next two cases.
Note the same error: only the nature of the symptoms was obtained. The weakness in all four limbs combined with true vertigo (the room spinning), diplopia (double vision) and dysarthria (slurred speech) clearly points to involvement of the brain stem. The intermittent nature of the symptoms combined with the age of the patient suggests the diagnosis of vertebrobasilar insufficiency (VBI, transient cerebral ischaemia in the posterior circulation). To the experienced clinician the transient loss of consciousness (LOC) in two of the episodes is atypical and would raise doubts about this being primarily related to cerebral vascular disease. (LOC is extremely rare in patients with VBI. See Chapter 10, ‘Cerebrovascular disease’.)
A more detailed history obtained the vital clue.
The detailed description of the three individual events revealed that they all occurred with exercise, and cerebral ischaemia related to vascular disease does not occur in such a predictable manner.1 Examination of the patient demonstrated severe aortic stenosis, and the explanation for her symptoms was exercise-induced hypotension due to poor cardiac output with initial selective involvement of the posterior circulation causing the focal symptoms and the subsequent global cerebral ischaemia resulting in LOC.
The patient described in Case 7.4 alerted this author to the importance of obtaining a blow-by-blow description of each of the episodes. She had recently been discharged from hospital without a clear diagnosis and after having undergone extensive investigation over a 2-week period. The patient was an 85-year-old woman who would be called in the trade ‘a poor historian’.
After the patient described two further episodes it became clear that every one occurred about midday and only when she stood up. Six months beforehand she had been placed on prazosin, a drug for hypertension and one that is often associated with postural hypotension. Her blood pressure fell from 170/100 lying to 110/65 on standing and her symptoms resolved upon cessation of this drug.
GENERAL PRINCIPLES OF CLASSIFICATION OF INTERMITTENT DISTURBANCES
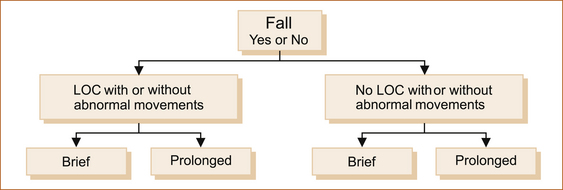
There are many ways to classify intermittent disturbances of neurological function. The traditional approach is to classify them according to the aetiology or underlying pathological basis. On the other hand, the simplest classification from the clinical point of view depends on what can readily be observed during episodes and is shown in Figure 7.1. Patients:
• fall (or slump if seated) or do not fall
• have a ‘blackout’ or they do not, whether they fall or not
• may or may not experience abnormal movements under any of these circumstances
• may experience episodes that vary in duration from seconds to minutes or even hours.
The various causes of intermittent disturbances can be differentiated along these lines.
Most episodes in patients who fall with or without LOC are brief. The exceptions are:
• the very rare prolonged tonic–clonic seizure lasting many minutes
• syncope with a prolonged aura
• a secondary head injury causing LOC complicating the fall, whatever the cause.
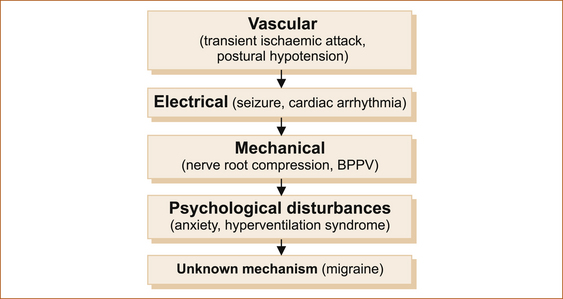
Most intermittent disturbances result from one of the mechanisms illustrated in Figure 7.2.
EPISODIC DISTURBANCES WITH FALLING
Falling with loss of consciousness
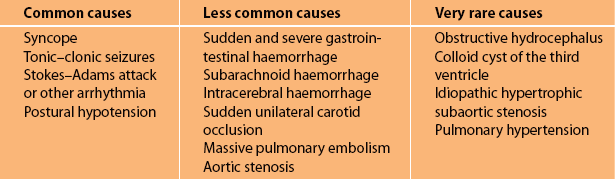
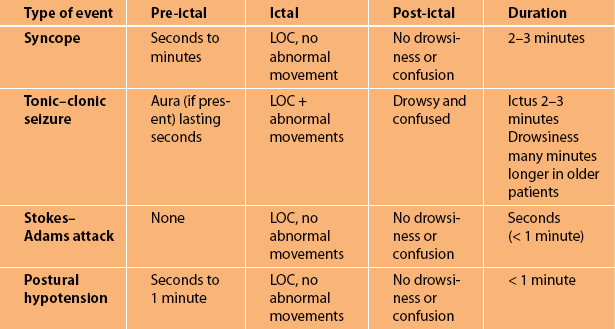
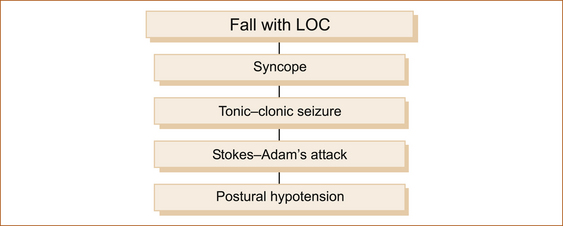
The four most common causes of transient LOC with falling are given in Figure 7.3. A complete summary of the numerous causes of transient LOC associated with a fall are listed in Table 7.1. Table 7.2 summarises the main clinical features of the common causes. Note that all are brief in duration.
Syncope (fainting, vasovagal or neurocardiogenic syncope)
Although syncope can afflict anyone of any age it tends to occur more commonly in young adults [2]. The patient is almost invariably standing, occasionally sitting and very, very rarely in a recumbent position. There is often, but not always, a trigger such as pain, alcohol, stressful situations, the sight of blood or being in a hot crowded environment.
• Immediately before ictus: There are several warning (pre-ictal) symptoms that increase in intensity over a period lasting between 30 seconds and 2 minutes after they first appear. These warning symptoms are referred to as pre-syncope and include light-headedness, nausea and feeling hot and clammy. If the symptoms worsen the patient becomes sweaty, their vision darkens and their hearing dims.
• During ictus: The patient subsequently loses consciousness (ictus), the eyes are closed, they are very pale and there are no abnormal movements unless the patient suffers a secondary seizure that usually consists of a very brief tonic seizure lasting less than 20 seconds. In some patients syncope can occur with little or no warning, mimicking a Stokes–Adams attack (see below). Patients with shorter duration of warning symptoms may suffer traumatic injuries [3].
• After ictus: The patient rapidly regains consciousness (within 10–30 seconds) and, although they wonder what has happened, they are neither confused nor drowsy and can carry on a sensible conversation almost immediately after the episode, even when there has been a brief secondary seizure.
Unlike epilepsy, many patients who suffer from syncope can prevent LOC by lying or sitting down quickly when they experience the warning symptoms. This is an important diagnostic clue. Where there is uncertainty advise the patient to lie down immediately when the episode next occurs to see if LOC can be prevented by elevating the legs so that they are above the level of the head. There is a very rare condition in elderly patients where syncope can be related to carotid sinus sensitivity [4].
SOME NOTES OF CAUTION
1. Patients and eyewitnesses often have difficulty estimating time, and ‘funny turns’ always seem to last longer than they actually do.
2. Pallor by itself is not overly useful, as patients are invariably described as being pale or a dreadful colour with all types of funny turns of differing causes. Having said this, extreme pallor associated with sweating is very suggestive of a cardiovascular cause.
3. Feeling the pulse quickly is very difficult, even for people who are trained such as medical practitioners and nurses; the apparent absence of a pulse does not necessarily imply an arrhythmia.
4. Eyewitnesses and patients often interpret having no recollection of the event as post-ictal confusion.
5. Regarding confusion, it is very important to clarify what observers and patients mean when they say the patient was confused after the episode.
Tonic–clonic seizures
Only a few brief principles are discussed here, as Chapter 8, ‘Seizures and epilepsy’, deals with the subject of epilepsy in detail.
• Immediately before ictus: The pre-ictal phase or aura if present is very brief, lasting only a few seconds. In many patients with tonic–clonic seizures there is no warning before they lose consciousness.
• During ictus: The patient will fall to the ground and there is a brief stiffening of the limbs (tonic phase) lasting 10–20 seconds followed by jerking (clonic phase) of the limbs lasting on average 5–30 seconds. The duration of impaired consciousness is brief. Most tonic–clonic seizures last approximately 1 minute, although they can last as long as 10 minutes [5]. The eyes are usually open during the seizure and observers often say the eyes rolled up into the top of the head. The patient may bite their lip, cheek or tongue and they may suffer incontinence of urine and/or faeces during the seizure.
• After ictus: The post-ictal period is associated with drowsiness and confusion lasting from 30 seconds to several minutes [5]. The period of post-ictal drowsiness and confusion may be as long as 24 hours or even up to 1 week following prolonged seizures and in older patients [6].
Stokes–Adam’s attack
This predominantly occurs in the elderly, although very rarely Stokes–Adam’s attacks can occur in younger patients. These episodes are usually related to a bradyarrhythmia or complete heart block, although similar symptoms can occur with a tachyarrhythmia if it results in sudden hypotension [7].
• Immediately before ictus: There is no warning.
• During ictus: The patient suddenly finds themselves on the ground, wondering what has happened. They do NOT recall falling. The period of impaired consciousness (ictus) is very brief, usually only a matter of seconds, certainly less than 1 minute [8].
Postural hypotension associated with loss of consciousness
• Immediately before ictus: The ‘pre-ictal’ symptoms are actually the initial symptoms of the event and are similar to those seen with syncope.
• During ictus: The LOC, if it occurs, is momentary. No abnormal movements.
The other causes of transient LOC listed in Table 7.1 are very rare and usually obvious because of the associated symptoms or circumstances when a very detailed history is obtained. Syncope due to aortic stenosis, idiopathic hypertrophic subaortic stenosis and pulmonary hypertension is precipitated by exertion. With these conditions, LOC can be avoided if the patient stops exercising with the very first symptom. There may also be associated dyspnoea with or without chest pain. Subarachnoid and intracerebral haemorrhage or a colloid cyst of the third ventricle will have associated severe explosive headache and vomiting. Pulmonary embolism causing a fall with LOC will be associated with severe chest pain, dyspnoea and hypotension. Gastrointestinal haemorrhage will be associated with haematemesis and/or melaena. The melaena may not be apparent when the patient is initially assessed.
FALLING WITHOUT LOSS OF CONSCIOUSNESS
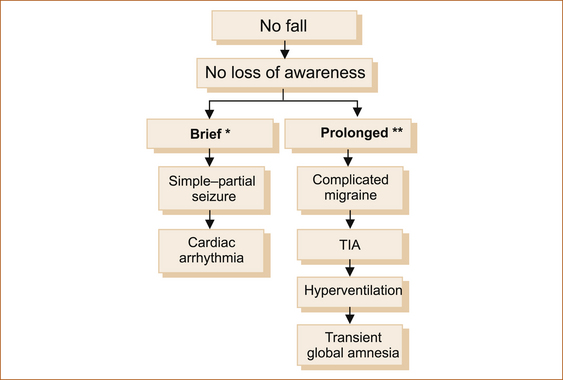
Some patients will experience a fall but do not lose consciousness. There are three common causes as shown in Figure 7.4.
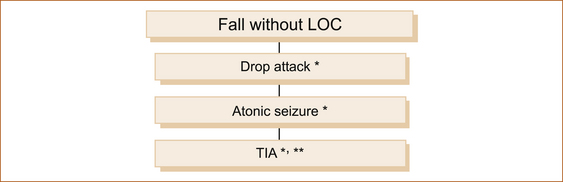
FIGURE 7.4 Causes of a fall without loss of consciousness
TIA = transient ischaemic attack
∗Brief = seconds to less than 2 minutes
∗∗Prolonged = many minutes to hours
Note: Patients with a TIA will fall only if the episode results in weakness of the leg(s) or the neurological symptoms are associated with severe vertigo. More commonly, patients with a TIA do not experience LOC nor do they fall. (See later in this chapter for more on TIA.)
Drop attacks
• Immediately before ictus: There is no warning.
• During ictus: The patient suddenly feels the legs go out from underneath them. The patient is able to say that they have fallen. The fall or ictus is very brief, lasting only a matter of seconds. The patient does not lose consciousness and may or may not feel themselves falling.
• After ictus: There are no post-ictal symptoms unless the patient has been injured in the fall, or is elderly with some physical disability. The patient is able to arise immediately and resume normal activities. In these episodes a patient may suffer serious injuries.
Drop attacks occur predominantly in middle-aged to elderly females. These falls may well relate to the same mechanisms that cause syncope in the elderly [9]. Drop attacks also occur in patients with advanced Ménière’s disease [10], although if less severe the patient may simply experience an acute loss of balance without falling. Drop attacks are clinically identical to atonic seizures except that the latter are extremely rare in adults who have not had epilepsy (Lennox–Gastaut syndrome) in childhood.
Atonic seizures
• Immediately before ictus: There is no warning.
• During ictus: Very rarely there may be a momentary myoclonic jerk of the limbs preceding the sudden fall. The ictus is brief, usually lasting only seconds, or occasionally up to 1 minute. If an attack lasts for 1 minute there may be an associated LOC. No abnormal movements. Atonic seizures rarely, if ever, occur as an isolated phenomenon and are almost invariably associated with other types of seizures (see Chapter 8, ‘Seizures and epilepsy’).
EPISODIC DISTURBANCES WITHOUT FALLING
Loss of awareness (consciousness) without falling
Most people interpret LOC as a dramatic event with profound impairment of consciousness and a collapse. In patients with impaired consciousness without falling perhaps a better term would be loss of awareness, where ‘the lights are on but no one is at home’ or, as a farmer once commented about his son, ‘there are no sheep in the top paddock for 30 seconds’.2 The patient remains sitting, standing or lying. They simply go off the air for a short period, unresponsive to external stimuli.
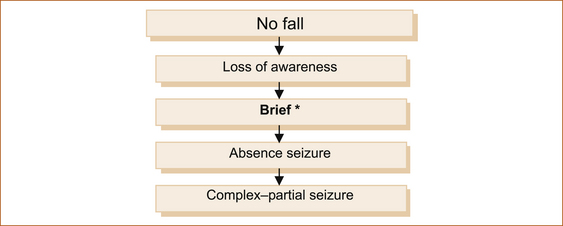
Figure 7.5 shows the more common intermittent disturbances of neurological function associated with a loss of awareness but no fall. The duration of the episodes is usually brief, seconds to less than 2–3 minutes.
BRIEF EPISODES
Absence seizure: Absence epilepsy is almost exclusively a problem in childhood. Very rarely it may present in adulthood, but in the form of recurrent absence seizures termed absence status (see Chapter 8, ‘Seizures and epilepsy’).
• Immediately before ictus: There is no warning.
• During ictus: The ictus is very brief, usually 4–9 (range 1–44) seconds [11]. During the seizure, the eyes are open and the patient stares into space without any abnormal movements apart from frequent blinking.
• After ictus: No symptoms, the patient behaves as if nothing had happened (unless driving, cycling or operating machinery where the seizure may result in an accident).
• Immediately before ictus: Most but not all patients with complex–partial seizures will experience brief pre-ictal symptoms (aura) lasting seconds. The nature of the symptoms during the aura reflects the site of origin of the seizure within the brain and is discussed in Chapter 8, ‘Seizures and epilepsy’.
• During ictus: During the ictus the patient remains in the same posture, stares into space and is unresponsive for approximately 1–3 minutes. Very rarely, complex–partial seizures can last up to 16 minutes [5]. Minor abnormal movements, especially of the mouth (lip-smacking), termed automatisms are not uncommon (see Chapter 8, ‘Seizures and epilepsy’).
• After ictus: There is a period of post-ictal confusion lasting several minutes, occasionally much longer.
PROLONGED EPISODES
Non-convulsive status epilepsy (NCSE) causes prolonged episodes lasting hours to days [12]. It presents more with confusion rather than unresponsiveness. Patients in NCSE may exhibit a wide range of clinical presentations including subtle memory deficits, bizarre behaviour, psychosis or coma. Absence status and complex partial status are the two primary types of NCSE. This is discussed in more detail in Chapter 8, ‘Seizures and epilepsy’.
Absence status epilepsy: There are no pre-ictal symptoms of absence status epilepsy. This manifests as a prolonged period of depression in mental state that may not be noticed by eyewitnesses because it is mild. Often there is associated repetitive blinking.
Complex–partial status epilepsy: Complex–partial status epilepsy manifests as a fluctuating mental state with confusion related to repeated typical and at times atypical complex–partial seizures, with or without clearing of consciousness between the episodes [13]. Prolonged confusion and episodic stereotyped repetitive automatisms with fluctuating impairment of consciousness lasting days has also been described [12].
No fall and no loss of consciousness
Some intermittent disturbances are not associated with either LOC or falling. The diagnosis is based on the duration of the episode as well as the nature of the symptoms. In this setting there are some problems that produce symptoms lasting hours and occasionally days. Figure 7.6 shows the more common intermittent disturbances of neurological function that are not associated with a fall or loss of awareness (consciousness), distinguishing brief from prolonged episodes.
BRIEF EPISODES
• Immediately before ictus: There are no pre-ictal symptoms in a simple-partial seizure.
• During ictus: The ictus consists of brief stereotyped episodes lasting from 30 seconds to 3 minutes, and rarely up to 8 minutes [5]. There is no loss of awareness and in most instances the patient can continue normal activities during the episode but often chooses to halt them momentarily until the symptoms pass. The patient can describe all the symptoms; the nature of the symptoms reflects the site of origin within the cerebral hemisphere (see Chapter 8, ‘Seizures and epilepsy’).
• After ictus: There is no post-ictal confusion or drowsiness.
• Immediately before ictus: There are no symptoms.
• During ictus: Although a patient presenting with palpitations with or without dyspnoea or chest pain presents little difficulty, many patients experience the symptoms related to hypotension secondary to the arrhythmia without being aware of the altered cardiac rhythm and in the absence of dyspnoea or chest pain. Here the diagnosis can present some difficulty, as the symptoms are non-specific and include light-headedness, nausea, sweating, blurred vision and a sensation of feeling unwell. The one potential clue is that these symptoms, which seem like hypotension, unlike postural hypotension or syncope may occur in the recumbent or sitting posture.
PROLONGED EPISODES
Complicated migraine: This is discussed in more detail in Chapter 9, ‘Headache and facial pain’. In essence, the symptoms come on gradually over minutes to less than 2 hours in 97% of patients [14] and persist on average for 24 hours but may persist for days [15]. The most important diagnostic feature, and the one that differentiates migraine from cerebral ischaemia, is the partial or complete resolution of the initial or early symptoms before the later symptoms have either appeared or fully evolved. In contrast, in cerebral ischaemia the symptoms are either of maximum intensity and distribution at onset or a cumulative neurological deficit develops with a stroke in evolution (see Chapter 10, ‘Cerebrovascular disease’). The second clue is that the symptoms spread from their original site to other parts of the body, reflecting the spreading cortical depression of Leão. This is typically seen with the visual aura of migraine where the scotoma enlarges and moves across the visual field. The third clue is that the aura of migraine typically develops over 5 or more minutes and, when there is more than one symptom, they occur in succession [16].
Transient cerebral ischaemia attack including vertebrobasilar insufficiency: The great majority of patients with cerebral ischaemia, even those with widespread symptoms of VBI such as diplopia, dysarthria, visual loss and motor and sensory symptoms, can describe their symptoms and clearly have not lost consciousness or awareness. The exception is the patient with VBI where there is medial temporal lobe or thalamic involvement with amnesia for the event [17]. If the degree of weakness is severe patients with a TIA may fall if they are standing at the time of onset.
• Immediately before ictus: There are no clear-cut pre-ictal symptoms, although some patients describe being off-colour in the preceding few days.
• During ictus: The ‘ictal’ symptoms last from minutes to hours (by definition up to 24 hours, but usually 3–4 hours [18]). The nature of the symptoms depends on the vascular territory affected (carotid versus vertebrobasilar, large vessel versus small vessel). This is discussed in more detail in Chapter 10, ‘Cerebrovascular disease’.
Hyperventilation syndrome: Hyperventilation syndrome is a very common clinical problem that is often under-recognised.3 Hyperventilation syndrome is characterised by a variety of somatic symptoms induced by physiologically inappropriate hyperventilation and usually reproduced in whole or in part by voluntary hyperventilation [19]. There is no pre-ictal or post-ictal phase.
• Immediately before ictus: There are no symptoms.
• During ictus: The symptoms gradually increase in intensity and then fluctuate in severity as the episode continues. The ictus consists of light-headedness that increases in severity over minutes and persists for hours, fluctuating in intensity during that period of time. There are often, but not invariably, associated symptoms with a sense of heaviness in the chest. Chest pain is rare; patients describe tightness in the chest. Occasionally, patients complain of shortness of breath; more often they complain of an inability to get enough air into the lungs, which is often associated with dryness of the mouth.
When the symptoms become more severe, tingling that can at times be unilateral develops almost simultaneously around the mouth (peri-oral) and in the hands and/or feet. The tingling develops and increases in severity after the light-headedness has commenced and while it is still present. The tingling remains confined to the hands, feet and around the mouth. Unlike migraine, the paraesthesia does not migrate from one part of the body to another, although occasionally the tingling may commence in the foot or hands and spread to other parts of the body. If the patient has very severe hyperventilation the hands and wrists can develop an involuntary flexion termed ‘carpopedal spasm’. Very rarely, subsequent LOC can occur. The patient may state that the limbs and body are shaking, suggesting epilepsy, but clarification of this symptom reveals that it is trembling rather than the involuntary jerking of epilepsy. The patient is fully alert during the time the limbs are shaking, which is not a feature of tonic–clonic epilepsy. In one study similar previous episodes were reported in 74% [20].
Patients may have a background history of tension headache and neck discomfort, but in many cases hyperventilation appears as a recurrent symptom and is not necessarily associated with recent provocative stress [21]. A number of patients develop this problem after attending relaxation classes where they are instructed to take deep breaths in order to relax!4 The symptoms can be reproduced by asking the patient to take deep breaths (not panting) for 2–5 minutes. Alternatively, blood gases measured during an episode should reveal a low carbon dioxide (CO2) level.
Transient global amnesia: Transient global amnesia (TGA) was first described in 1956 [22] and is a curious clinical syndrome characterised by the abrupt onset of severe anterograde amnesia [23]. It lasts several hours and is seen most often in the middle-aged or elderly. These patients are often not aware of any problems and are brought to medical attention by a concerned relative or an observer. During the attack the patient remains alert and communicative but keeps asking the same questions over and over again. Their personal identity is preserved and there are no focal neurological or epileptic features. Apart from short-term memory loss, the patient behaves as if nothing else is wrong; they can talk, walk etc. The ability to lay down new memories gradually recovers as the period of amnesia shrinks. There is a residual amnesia for events near the onset of the episode.
There are strict criteria for the diagnosis of TGA [24]:
1. The attack must be witnessed if it is to be diagnosed with a degree of certainty. Clear-cut anterograde amnesia must be present during the attack.
2. Clouding of consciousness and loss of personal identity must be absent.
3. The cognitive impairment must be limited to amnesia.
4. There should be no accompanying focal neurological symptoms.
5. Epileptic seizures must be absent.
6. Attacks must resolve within 24 hours and patients with recent head injury or known active epilepsy are excluded.
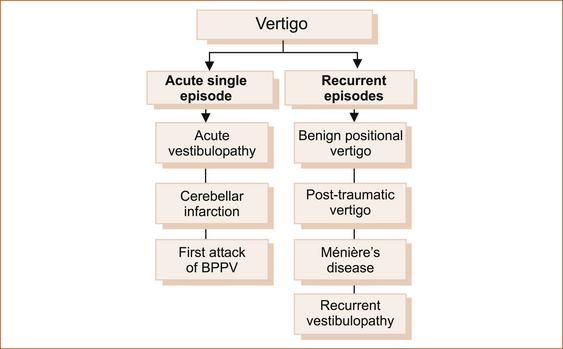
Vertigo: Dizziness and giddiness are very non-specific terms that are commonly used by patients to describe their symptoms. Four types of dizziness have been defined: vertigo, pre-syncope, disequilibrium, and other [25]. Vertigo is a false sensation that the body or the environment is moving (head or room spinning). This is true vertigo and indicates a problem within the peripheral vestibular system (labyrinth or vestibular nerve) or the central vestibular connections in the brainstem or cerebellum. Table 7.3 lists the distinguishing features of central vertigo and peripheral vertigo as described by Swartz and Longwell [26]. Cerebellar infarction is discussed in greater detail in Chapter 10, ‘Cerebrovascular disease’.
TABLE 7.3
Central versus peripheral vertigo
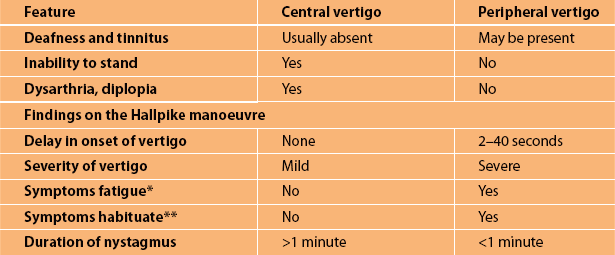
∗Fatigue refers to the abatement of the vertigo and nystagmus after provocation while the head is still in the position that precipitated the symptoms.
∗∗Habituation refers to a lessening of the severity of the symptoms with repeated Hallpike’s testing. Nystagmus is a rapid, involuntary, oscillatory motion of the eyeball.
Vertigo essentially presents either as an acute severe episode or as recurrent attacks over months to years. Whatever the cause, vertigo is almost invariably associated with variable degrees of nausea and vomiting. Figure 7.7 shows the more common causes of vertigo. The two main causes of acute severe vertigo are ‘acute vestibulopathy’ (vestibular neuronitis, labyrinthitis) and cerebellar infarction [27].
Apart from cerebellar infarction, most conditions that cause vertigo do not have ‘gold standard’ tests to confirm the clinical diagnosis. Ménière’s disease also lacks a diagnostic test but the syndrome is well defined in patients subsequently found to have the typical pathology in the ears, so the term has been retained.
As an isolated symptom, vertigo is most often peripheral in origin (inner ear or vestibular nerve including the root’s entry zone in the brain stem) with benign positional vertigo, acute vestibular neuronitis and Ménière’s disease accounting for 93% of patients with vertigo presenting to primary care physicians [28]. Very occasionally vertigo is central in origin, affecting the vestibular connections within the brainstem, but there are almost always other neurological symptoms and/or signs referable to the brainstem.
Acute single episode: Although the two conditions discussed in this section are monophasic illnesses, they are important causes of vertigo and it seems appropriate to include them in this chapter with the other causes of vertigo that result in episodic symptoms. In theory, patients with an initial attack of benign paroxysmal positional vertigo could present as an acute single episode; in reality these patients rarely if ever present after the first episode.
ACUTE VESTIBULOPATHY: The use of the term ‘vestibulopathy’ reflects the unknown aetiology of this clinical syndrome and, as already explained, is preferred to the terms ‘labyrinthitis’ or ‘vestibular neuronitis’ which imply a site of pathology and an infective or inflammatory process that is not proven [29]. By definition vestibular neuritis (or neuronitis) is confined to the vestibular system and hearing is unaffected, while labyrinthitis is a process that is thought to affect the inner ear as a whole or the 8th nerve as a whole and where hearing may be reduced or distorted in tandem with vertigo [30].
• Immediately before ictus: There may be an antecedent upper respiratory infection. In some patients a vague sense of imbalance may precede by some hours to days the more severe vertigo.
• During ictus: Vertigo typically develops slowly over a period of hours, is severe for a few days and then subsides over the course of a few weeks. Nausea and vomiting are marked but there is no tinnitus or deafness. The patients prefer to lie completely immobile on the side opposite to the affected ear as the slightest movement exacerbates the vertigo. There is unidirectional nystagmus with the fast phase to the unaffected ear. It can be suppressed by visual fixation (asking the patient to stare at an object). Examination of one retina with the ophthalmoscope while the other eye is covered can elicit the nystagmus as this removes visual fixation. Other than nystagmus there are no focal neurological symptoms or signs.
Patients with an acute peripheral vestibular lesion as opposed to cerebellar infarction typically can stand, although they will veer toward the side of the lesion, especially if asked to walk on the spot with their eyes closed [31]. They may notice that their vision is disturbed or jumpy on looking to a particular side. This is termed oscillopsia, the symptom of nystagmus. Very rarely, anticlockwise rotary nystagmus with vertical (not horizontal) diplopia may occur (a skew deviation), related to dysconjugate larger deviation of the ipsilateral eye [32, 33]. This may give the impression that the vertigo is of central origin. Skew deviation can also occur with brainstem lesions and in these circumstances it can be difficult to differentiate between a central and a peripheral cause for the vertigo.
• After ictus: Some patients can have residual non-specific post-ictal giddiness and imbalance that lasts for months. Benign paroxysmal positional vertigo may develop as a sequela.
CEREBELLAR INFARCTION/HAEMORRHAGE:
• Immediately before ictus: There are no ‘pre-ictal’ symptoms.
• During ictus: Onset is sudden if ischaemic or over many minutes if related to haemorrhage. ‘Ictal’ symptoms last for days. Although the patient may present with severe vertigo, cerebellar infarction or haemorrhage should be suspected in patients who present with:
Unlike acute vestibulopathy, the patient is unable to stand. When there is associated dysarthria, diplopia and limb ataxia, the diagnosis of a central cause for the vertigo is apparent.
• After ictus: Residual symptoms will occur if recovery is incomplete.
In both cerebellar infarction and acute vestibulopathy the vomiting may be so severe and repeated that a Mallory–Weiss tear in the lower end of the oesophagus may occur and haematemesis may be the presenting symptom. It is not uncommon for these patients to be misdiagnosed with an acute gastrointestinal problem and admitted under the gastroenterology unit. The presence of severe vertigo or an inability to stand in these patients should alert the clinician to the correct diagnosis.
The head impulse test [34] detects severe unilateral loss of semicircular canal function clinically. It can distinguish between vestibular neuritis and cerebellar infarction as it is normal in a patient with cerebellar infarction but abnormal in a patient with vestibular neuritis or acute vestibulopathy. The head thrust test consists of holding the patient’s face with both hands with the patient’s head turned to one side slightly past the midline and then rapidly thrusting it to just past the midline on the opposite side. The patient is asked to fixate on a distant object. When the subject’s head is turned to the side of the lesion, the vestibular ocular reflex is deficient and the eyes will move with the head so that they no longer fix on the point in the distance. A CT scan will detect haemorrhage but may be normal in the early hours after an infarct. An MRI scan will detect the infarction earlier.
POSITIONAL VERTIGO: Benign paroxysmal positional vertigo (BPPV) was first described in detail by Barany in 1921 [35]. This usually, but not invariably, occurs in the middle-aged to elderly where it is related to small deposits of calcium on the hair cells in the vestibule. Positional vertigo can also occur after a head injury or an acute vestibulopathy.
• Pre-ictal: There are no warning (pre-ictal) symptoms.
• During ictus: Patients describe paroxysms of true vertigo precipitated by head movement such as:
The symptoms and the associated delayed onset of nystagmus that abates with maintenance of the fixed posture can be precipitated by the positioning test or Hallpike manoeuvre [36]:
1. The examiner should describe to the patient what the test involves and reassure them that it is safe and painless, but that it may reproduce their symptoms and make them very giddy. The patient should be reassured that they will not be allowed to fall off the examination couch.
2. Ask the patient to sit on an examination couch so that when they lie down the head is over and below the end of the couch. The movement has to be quick and the examiner needs to ensure that the patient does not suffer from back or neck problems before doing this procedure. Ensure that the back and neck are supported during the procedure.
3. When this test is performed the patient will want to close the eyes but must be encouraged to keep them open so that the nystagmus can be seen. Commence with the head looking to one side and then rapidly lie the patient down from the sitting position. If the problem is benign positional vertigo affecting the right ear, there is the delayed onset of a fast-phase clockwise (as the patient sees it, anti-clockwise from the examiner’s perspective) torsional nystagmus with the affected right ear dependent or lower.
4. To complete the procedure the patient is returned to the seated position and the eyes are observed for reversal in the direction of the nystagmus, in this case a fast-phase anticlockwise nystagmus. The nystagmus settles within 30 seconds if the patient stays still in that position [36].
The rationale behind this is the observations of Schuknecht and Ruby who described small deposits of calcium (otoconia) on the hair cells, most often within the posterior semi-circular canals, as the cause of this problem [37]. These deposits are flushed out of the semi-circular canals using the Epley manoeuvre or a similar particle repositioning manoeuvre. The condition can be cured in 80% of patients, using the Canalith Repositioning Procedure or Epley manoeuvre [38]. This requires identification of whether it is the right or left ear in which the problem occurs and this is not always possible, particularly if the patient is having a good day and the Hallpike manoeuvre is negative. If one cannot provoke vertigo with the Hallpike manoeuvre, one cannot cure it with the Epley manoeuvre. In these circumstances the options are to bring the patient back on a bad day or alternatively recommend that they deliberately precipitate the symptoms many times in the morning and the evening until the problem resolves, using the Brandt–Daroff exercises [39]. This problem can recur on more than one occasion months or even years later.
MÉNIÈRE’S DISEASE: The term Ménière’s disease is used to define the classic triad of:
Ménière’s disease is manifested by episodic true vertigo associated with nausea and vomiting lasting longer than 1 hour and usually a few hours, together with a sense of fullness in the ear. Tinnitus may occur, and transient deafness during the attack that improves following the episode is a pathognomonic (this is a term that indicates that only one condition can cause the problem) symptom of Ménière’s that can occur in two-thirds of patients [40]. The tinnitus and deafness may persist for days. The symptoms increase in intensity over several minutes and may continue to increase for up to half an hour. There may be a further period of up to half an hour of a sense of instability before the onset of the severe true vertigo with a sense of rotation. The patient prefers to lie still with the affected ear uppermost, but the vertigo persists even if the patient remains motionless. This condition recurs at variable intervals, as frequently as several attacks within a week or none for some years. Two episodes of vertigo in 1 day are incompatible with the diagnosis of Ménière’s. Repeated attacks usually lead to progressive hearing loss over many years. In the early stages tinnitus, hearing impairment and/or fullness in the ear may appear prior to the onset of the first vertigo attack and vertigo can occur without tinnitus and deafness [41].
Three stages are identified in Ménière’s disease:
• Stage I. In the early phase the predominant symptom is vertigo, characteristically rotatory or rocking, and it is associated with nausea or vomiting. The episode is often preceded by an aura of fullness or pressure in the ear or side of the head and usually lasts from 20 minutes to several hours. Between the attacks hearing reverts to normal and examination of the patient during this period of remission invariably shows normal results.
• Stage II. As the disease advances the hearing loss becomes established but continues to fluctuate. The deafness is sensorineural and initially affects the lower pitches. The paroxysms of vertigo reach their maximum severity and then tend to become less severe. The period of remission is highly variable, often lasting for several months.
• Stage III. In the last stage of the disorder the hearing loss stops fluctuating and progressively worsens; both ears tend to be affected so that the prime disability is deafness. The episodes of vertigo diminish and then disappear, although the patient may be unsteady, especially in the dark. [42].
RECURRENT VESTIBULOPATHY: Essentially, patients have recurrent isolated vertigo of unknown cause and without headache, neurological or auditory symptoms. Patients experience recurrent episodes of vertigo, with nausea and vomiting lasting hours or sometimes days [43]. These episodes occur at variable intervals and do not display the features of Ménière’s syndrome, such as transient deafness and tinnitus during the attacks, and patients do not subsequently develop deafness. The precise aetiology of these episodes has not been established. At the time of writing there is a strong body of opinion that considers these episodes to be migrainous [44–50]. The increased incidence of migraine in patients with vertigo and vice versa and the response to ‘migraine therapy’ is cited as evidence for the link between migraine and vertigo. Diagnostic criteria have been proposed [47]. The evidence is circumstantial and, as there is no gold standard diagnostic test for migraine or migrainous vertigo, the episodes have been referred to as recurrent vestibulopathy.
Fleeting symptoms: Patients are occasionally encountered who describe fleeting symptoms lasting only 1 or 2 seconds. There may be a momentary sensation of impending LOC, particularly when a person is relaxed, referred to as the blip syndrome [51]. There may be transient symptoms of pain such as ice-pick headache (see Chapter 9, ‘Headache and facial pain’) or there may be transient sensory symptoms. These often defy explanation and are benign, and all investigations are normal5.
REFERENCES
1. Official website of the Sir Arthur Conan Doyle Literary Estate, 2006. Available: http://www.sherlockholmesonline.org/Biography/index.htm (14 Dec 2009).
2. Sheldon, R.S., Sheldon, A.G., Connolly, S.J., et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49–54.
3. Ammirati, F., Colivicchi, F., Velardi, A., et al. Prevalence and correlates of syncope-related traumatic injuries in tilt-induced vasovagal syncope. Ital Heart J. 2001;2:38–41.
4. Brignole, M., Alboni, P., Benditt, D., et al. Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J. 2001;22:1256–1306.
5. Jenssen, S., Gracely, E.J., Sperling, M.R. How long do most seizures last? A systematic comparison of seizures recorded in the epilepsy monitoring unit. Epilepsia. 2006;47:1499–1503.
6. Godfrey, J.W., Roberts, M.A., Caird, F.I. Epileptic seizures in the elderly. II: Diagnostic problems. Age Ageing. 1982;11:29–34.
7. Johansson, B.W. Long-term ECG in ambulatory clinical practice. Analysis and 2-year follow-up of 100 patients studied with a portable ECG tape recorder. Eur J Cardiol. 1977;5:39–48.
8. Harbison, J., Newton, J.L., Seifer, C., et al. Stokes Adams attacks and cardiovascular syncope. Lancet. 2002;359:158–160.
9. Kenny, R.A., Traynor, G. Carotid sinus syndrome – clinical characteristics in elderly patients. Age Ageing. 1991;20:449–454.
10. Kentala, E., Havia, M., Pyykko, I. Short-lasting drop attacks in Ménière’s disease. Otolaryngol Head Neck Surg. 2001;124:526–530.
11. Sadleir, L.G., Farrell, K., Smith, S., et al. Electroclinical features of absence seizures in childhood absence epilepsy. Neurology. 2006;67:413–418.
12. Escueta, A.V., Boxley, J., Stubbs, N., et al. Prolonged twilight state and automatisms: A case report. Neurology. 1974;24:331–339.
13. Ellis, J.M., Lee, S.I. Acute prolonged confusion in later life as an ictal state. Epilepsia. 1978;19:119–128.
14. Pryse-Phillips, W., Aube, M., Bailey, P., et al. A clinical study of migraine evolution. Headache. 2006;46:1480–1486.
15. Kelman, L. Pain characteristics of the acute migraine attack. Headache. 2006;46:942–953.
16. Kirchmann, M. Migraine with aura: New understanding from clinical epidemiologic studies. Curr Opin Neurol. 2006;19:286–293.
17. Akiguchi, I., Ino, T., Nabatame, H., et al. Acute-onset amnestic syndrome with localized infarct on the dominant side – comparison between anteromedial thalamic lesion and posterior cerebral artery territory lesion. Jpn J Med. 1987;26:15–20.
18. Crisostomo, R.A., Garcia, M.M., Tong, D.C. Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack: Correlation with clinical characteristics. Stroke. 2003;34:932–937.
19. Lewis, R.A., Howell, J.B. Definition of the hyperventilation syndrome. Bull Eur Physiopathol Respir. 1986;22:201–205.
20. Saisch, S.G., Wessely, S., Gardner, W.N. Patients with acute hyperventilation presenting to an inner-city emergency department. Chest. 1996;110:952–957.
21. Fejerman, N. Nonepileptic disorders imitating generalized idiopathic epilepsies. Epilepsia. 2005;46(Suppl 9):S80–S83.
22. Courjon, J., Guyotat, J. [Amnesic strokes.]. J Med Lyon. 1956;37:697–701.
23. Fisher, C.M., Adams, R.D. Transient global amnesia. Acta Neurol Scand. 1964;40(Suppl 9):S1–S83.
24. Hodges, J.R., Warlow, C.P. Syndromes of transient amnesia: Towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53:834–843.
25. Drachman, D.A., Hart, C.W. An approach to the dizzy patient. Neurology. 22, 1972. [323–234].
26. Swartz, R., Longwell, P. Treatment of vertigo. Am Fam Physician. 2005;71:1115–1122.
27. Halmagyi, G.M. Diagnosis and management of vertigo. Clin Med. 2005;5:159–165.
28. Hanley, K., O’Dowd, T. Symptoms of vertigo in general practice: A prospective study of diagnosis. Br J Gen Pract. 2002;52:809–812.
29. Ryu, J.H. Vestibular neuritis: An overview using a classical case. Acta Otolaryngol Suppl. 1993;503:25–30.
30. Silvoniemi, P. Vestibular neuronitis. An otoneurological evaluation. Acta Otolaryngol Suppl. 1988;453:1–72.
31. Fukuda, T. The stepping test: Two phases of the labyrinthine reflex. Acta Otolaryngol. 1959;50:95–108.
32. Brandt, T.H., Dieterich, M. Different types of skew deviation. J Neurol Neurosurg Psychiatry. 1991;54:549–550.
33. Safran, A.B., Vibert, D., Issoua, D., et al. Skew deviation after vestibular neuritis. Am J Ophthalmol. 1994;118:238–245.
34. Halmagyi, G.M., Curthoys, I.S. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739.
35. Barany, R. Diagnose von Krankheitserscheinungen im Bereiche des Otolithenapparates. Acta Otolaryng. 1921;2:434–437.
36. Dix, M.R., Hallpike, C.S. The pathology, symptomatology and diagnosis of certain common disorders of the vestibular system. Proc R Soc Med. 1952;45:341–354.
37. Schuknecht, H.F., Ruby, R.R. Cupulolithiasis. Adv Otorhinolaryngol. 1973;20:434–443.
38. Epley, J.M. The canalith repositioning procedure: For treatment of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 1992;107:399–404.
39. Brandt, T., Daroff, R.B. Physical therapy for benign paroxysmal positional vertigo. Arch Otolaryngol. 1980;106:484–485.
40. Havia, M., Kentala, E., Pyykko, I. Hearing loss and tinnitus in Ménière’s disease. Auris Nasus Larynx. 2002;29:115–119.
41. Tokumasu, K., Fujino, A., Naganuma, H., et al. Initial symptoms and retrospective evaluation of prognosis in Ménière’s disease. Acta Otolaryngol Suppl. 1996;524:43–49.
42. Saeed, S.R. Fortnightly review: Diagnosis and treatment of Ménière’s disease. BMJ. 1998;316:368–372.
43. Lee, H., Sohn, S.I., Jung, D.K., et al. Migraine and isolated recurrent vertigo of unknown cause. Neurol Res. 2002;24:663–665.
44. Maione, A. Migraine-related vertigo: Diagnostic criteria and prophylactic treatment. Laryngoscope. 2006;116:1782–1786.
45. Eggers, S.D. Migraine-related vertigo: Diagnosis and treatment. Curr Neurol Neurosci Rep. 2006;6:106–115.
46. Neuhauser, H.K., Lempert, T. Diagnostic criteria for migrainous vertigo. Acta Otolaryngol. 2005;125:1247–1248.
47. Neuhauser, H., Leopold, M., von Brevern, M., et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001;56:436–441.
48. Gupta, V.K. Migraine-related vertigo: The challenge of the basic sciences. Clin Neurol Neurosurg. 2005;108:109–110. [reply 111–112].
49. Lempert, T., Neuhauser, H. Migrainous vertigo. Neurol Clin. 2005;23:715–730. [vi].
50. Brantberg, K., Trees, N., Baloh, R.W. Migraine-associated vertigo. Acta Otolaryngol. 2005;125:276–279.
51. Lance, J.W. Transient sensations of impending loss of consciousness: The “blip” syndrome. J Neurol Neurosurg Psychiatry. 1996;60:437–438.
1Except in the very rare instance of subclavian steal syndrome where stenosis of the left subclavian artery proximal to the vertebral artery means that the left arm ‘steals’ blood from the brainstem when it is exercising.
2Two descriptions by relatives of patients with absence and complex–partial seizures.