Chapter 485 Epidemiology of Childhood and Adolescent Cancer
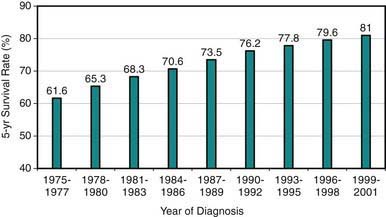
Cancer in patients <19 yr of age is uncommon, with an age-adjusted annual incidence rate of 16.6/100,000, representing only about 1% of all new cancer cases in a year in the USA or 18,000 new cases/yr in 2006. Although relative 5-yr survival rates have improved from 61% in 1977 to 81.6% in 2005 in all age groups (Fig 485-1), malignant neoplasms remain the leading cause of disease-related (noninjury) mortality (12.8%) among persons 1-14 yr of age. There are 1500-1600 cancer-related deaths annually in the USA among children <15 yr of age. The relative contribution of cancer to the overall mortality in 15-19 yr olds is lower. Multi-institutional cooperative clinical trials investigating novel therapies and investigating ways to improve survival rates even further and to decrease treatment-related long-term complications are ongoing. Because increasingly more patients survive their disease, clinical investigation also is focusing on the quality of life among survivors and the late outcomes of therapy experienced by pediatric and adult survivors of childhood cancer. The National Cancer Institute estimates that in 1977 there were 269,700 persons alive (in all age groups) who had survived childhood cancer, corresponding to 1/810 of persons <20 yr of age and 1/1,000 of persons 20-39 yr of age in the US population.
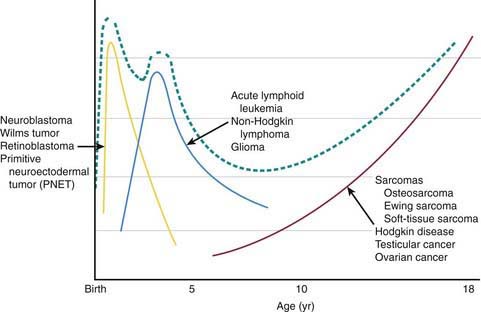
Pediatric cancers differ markedly from adult malignancies in both prognosis and distribution by histology and tumor site. Lymphohematopoietic cancers (i.e., acute lymphoblastic leukemia, lymphomas) account for ~40%, nervous system cancers for ~30%, and embryonal tumors and sarcomas for ~10% each among the broad categories of childhood cancers (Table 485-1). In contrast, epithelial tumors of organs such as lung, colon, breast, and prostate, which are commonly seen among adults, are rare malignancies in children. Unlike incidence patterns in adults, where cancer rates tend to increase rapidly with increasing age, a relatively wide age range exists in the pediatric age group, with 2 peaks: the 1st in early childhood and the 2nd in adolescence (Fig 485-2). During the 1st year of life, embryonal tumors such as neuroblastoma, nephroblastoma, retinoblastoma, rhabdomyosarcoma, hepatoblastoma, and medulloblastoma are most common (Figs 485-3 and 485-4). These tumors are much less common in older children and adults after cell differentiation processes have slowed considerably. Embryonal tumors, acute leukemias, non-Hodgkin lymphomas, and gliomas peak in incidence from 2-5 yr of age. As children age, bone malignancies, Hodgkin disease, gonadal germ cell malignancies (testicular and ovarian carcinomas), and other carcinomas increase in incidence. Adolescence is a transitional period between the common early childhood malignancies and characteristic carcinomas of adulthood (see Fig 485-4).
Table 485-1 AGE-ADJUSTED INCIDENCE AND SURVIVAL RATES OF MALIGNANT NEOPLASMS BY TUMOR TYPE AMONG US CHILDREN
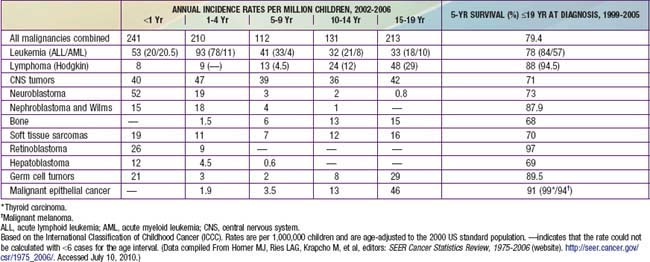
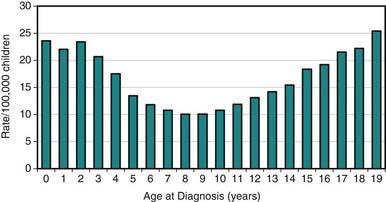
Figure 485-2 Age-specific cancer incidence rates per 100,000 children between 2002-2006 within the USA.
(From Horner MJ, Ries LAG, Krapcho M, et al, editors: SEER cancer statistics review, 1975-2006, Bethesda, MD, 2008, National Cancer Institute.)
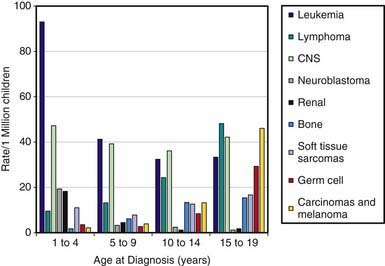
(From Horner MJ, Ries LAG, Krapcho M, et al, editors: SEER Cancer Statistics Review, 1975-2006 (website). http://seer.cancer.gov/csr/1975_2006/. Accessed July 10, 2010.)
Childhood neoplasms include a diverse array of malignant tumors, termed “cancers,” and nonmalignant tumors arising from disorders of genetic processes involved in control of cellular growth and development. Although many genetic conditions are associated with increased risks for childhood cancer, such conditions are believed to account for <5% of all occurrences (Chapter 486). The most notable genetic conditions that impart susceptibility to childhood cancer are neurofibromatosis types 1 and 2, Down syndrome, Beckwith-Wiedemann syndrome, tuberous sclerosis, von Hippel-Lindau disease, xeroderma pigmentosum, ataxia-telangiectasia, nevus basal cell carcinoma syndrome, and Li-Fraumeni (p53) syndrome. The varying incidence patterns of individual childhood cancers around the world imply additional genetic and epidemiologic risk factors that remain uncharacterized.
Compared with adult epithelial tumors, an extremely small fraction of pediatric cancers appear to be explained by known environmental exposures (Table 485-2). Ionizing radiation exposure and several chemotherapeutic agents explain only a small number of pediatric cases (Chapter 699). The association between fetal exposures and pediatric cancer is largely not established, with the exception of maternal diethylstilbestrol intake during pregnancy and subsequent vaginal adenocarcinoma in adolescent daughters. Environmental exposures that have been studied without convincing evidence for a causal role include non-ionizing power frequency electromagnetic fields, pesticides, parental occupational chemical exposures, dietary factors, and environmental cigarette smoke. Viruses have been associated with certain pediatric cancers, such as polyomaviruses (BK, JC, SV40) associated with brain cancer and Epstein-Barr virus with non-Hodgkin lymphoma, but the etiologic importance remains unclear. Because the etiology of cancer in children still is poorly understood, for the most part, epidemiology studies have recognized that the likely mechanism is multifactorial, possibly resulting from potential interactions between genetic susceptibility traits and environmental exposures. Ongoing studies are investigating the role of polymorphisms of genes encoding enzymes, which function in the activation or metabolism of xenobiotics, protection of cells against oxidative stress, DNA repair, and/or immune modulation.
Table 485-2 KNOWN RISK FACTORS FOR SELECTED CHILDHOOD CANCERS
| CANCER TYPE | RISK FACTOR | COMMENTS |
|---|---|---|
| Acute lymphoid leukemia | Ionizing radiation | Although primarily of historical significance, prenatal diagnostic x-ray exposure increases risk. Therapeutic irradiation for cancer treatment also increases risk. |
| Race | White children have a 2-fold higher rate than black children in the USA. | |
| Genetic factors* | Down syndrome is associated with an estimated 10- to 20-fold increased risk. NF1, Bloom syndrome, ataxia-telangiectasia, and Langerhans cell histiocytosis, among others, are associated with an elevated risk. |
|
| Acute myeloid leukemias | Chemotherapeutic agents | Alkylating agents and epipodophyllotoxins increase risk. |
| Genetic factors* | Down syndrome and NF1 are strongly associated. Familial monosomy 7 and several other genetic syndromes are also associated with increased risk. |
|
| Brain cancers | Therapeutic ionizing radiation to the head | With the exception of cancer radiation therapy, higher risk from radiation treatment is essentially of historical importance. |
| Genetic factors* | NF1 is strongly associated with optic gliomas, and, to a lesser extent, with other central nervous system tumors. Tuberous sclerosis and several other genetic syndromes are associated with increased risk. |
|
| Hodgkin disease | Family history | Monozygotic twins and siblings are at increased risk. |
| Infections | EBV is associated with increased risk. | |
| Non-Hodgkin lymphoma | Immunodeficiency | Acquired and congenital immunodeficiency disorders and immunosuppressive therapy increase risk. |
| Infections | EBV is associated with Burkitt lymphoma in Africa. | |
| Osteosarcoma | Ionizing radiation | Cancer radiation therapy and high radium exposure increase risk. |
| Chemotherapy | Alkylating agents increase risk. | |
| Genetic factors* | Increased risk is apparent with Li-Fraumeni syndrome and hereditary retinoblastoma. | |
| Ewing sarcoma | Race | White children have about a 9-fold higher incidence rate than black children in the USA. |
| Neuroblastoma | No established risk factors. | |
| Retinoblastoma | Genetic factors* | No established other risk factors. |
| Wilms tumor | Congenital anomalies | Aniridia, Beckwith-Wiedemann syndrome, and other congenital and genetic conditions are associated with increased risk. |
| Race | Asian children reportedly have about half the rates of white and black children. | |
| Renal medullary carcinoma | Sickle cell trait | Etiology unknown. |
| Rhabdomyosarcoma | Congenital anomalies and genetic conditions | Li-Fraumeni syndrome and NF1 are believed to be associated with increased risk. There is some concordance with major birth defects. |
| Hepatoblastoma | Genetic factors* | Beckwith-Wiedemann syndrome, hemihypertrophy, Gardner syndrome, and family history of adenomatous polyposis are associated with increased risk. |
| Leiomyosarcoma | Immunosuppression and EBV infection | EBV is associated with leiomyosarcoma for all forms of congenital and acquired immunosuppression but not leiomyosarcoma among immunocompetent persons. |
| Malignant germ cell tumors | Cryptorchidism | Cryptorchidism is a risk factor for testicular germ cell tumors. |
EBV, Epstein-Barr virus; NF1, neurofibromatosis type 1.
* See Chapter 486, Table 486-2.
From Gurney JG, Bondy ML: Epidemiology of childhood cancer. In Pizzo PA, Poplack DG, editors: Principles and practice of pediatric oncology, ed 5, Philadelphia, 2006, Lippincott Williams & Wilkins, p 11.
Curative therapy with chemotherapy, radiation, and/or surgery can adversely affect a child’s development and result in serious long-term medical and psychosocial effects in childhood and adulthood. Potential adverse late effects include subsequent second malignancy, early mortality, infertility, reduced stature, cardiomyopathy, pulmonary fibrosis, osteoporosis, neurocognitive impairment, affective mood disorders, and altered social functioning. Much has been learned about the incidence of late effects from large multisite cohort studies such as the Childhood Cancer Survivor Study, an ongoing study of medical and psychosocial outcomes in survivors (www.cancer.umn.edu/ltfu).
Given the relative rarity of specific types of childhood cancer and the sophisticated technology and expertise required for diagnosis, treatment, and monitoring of late effects, all children with cancer should be treated on standardized clinical protocols in pediatric clinical research settings whenever possible. Promoting such treatment, the Children’s Oncology Group is a multi-institutional research consortium that facilitates cooperative clinical, biologic, and epidemiologic research in more than 200 affiliated institutions in the United States, Canada, and other countries (www.curesearch.org/). Coordinated participation in such research trials has been a major factor in the increased survival for many children with cancer. Such ongoing efforts are critical to better understand the etiology of childhood cancers, improve survival for malignancies with a poor prognosis, and maximize the quality of life for survivors.
Acknowledgment
The author would like to acknowledge the work of Nina S. Kadin-Lottick on Chapter 491, “Epidemiology of Childhood and Adolescent Cancer,” in the previous editions of this work.
Bliek J, Maas S, Alders M, et al. Epigenotype, phenotype, and tumors in patients with isolated hemihyperplasia. J Pediatr. 2008;153:95-100.
Buffler PA, Kwan ML, Reynolds P, et al. Environmental and genetic risk factors for childhood leukemia: appraising the evidence. Cancer Invest. 2005;1:60-75.
Centers for Disease Control and Prevention. Trends in childhood cancer mortality—United States, 1990–2004. JAMA. 2008;299:626-627.
Christiani DC. Combating environmental causes of cancer. N Engl J Med. 2011;364(9):791-793.
Clarke AJ, Gaff C. Challenges in the genetic testing of children for familial cancers. Arch Dis Child. 2008;93:911-914.
Croce CM. Oncogenes and cancer. N Engl J Med. 2008;358:502-511.
Engel ME, Hiebert SW. The enemy within: dormant retroviruses awaken. Nature Med. 2010;16:517-518.
Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705-2715.
Gurney JG, Bondy ML. Epidemiology of childhood cancer. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2006:1-13.
Haigis KM, Sweet-Cordero A. New insights into oncogenic stress. Nat Genet. 2011;43(3):177-178.
Heron MP, Hoyert DL, Murphy SL, et al. Deaths: final data for 2006. National vital statistics reports, vol 57 no 14. National Center for Health Statistics, Hyattsville, MD, 2009.
Hewitt M, Ganz PA. Implementing cancer survivorship care planning: an Institute of Medicine National Cancer Policy Forum workshop summary. Washington, DC: The National Academies Press; 2007.
Hirschel B, Perneger T. Immunity, infection, and cancer. Lancet. 2007;370:6-7.
Hitchins MP, Wong JJL, Suthers G, et al. Inheritance of a cancer-associated MLH1 germ-line epimutation. N Engl J Med. 2007;356:697-705.
Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2006 (website). editors http://seer.cancer.gov/csr/1975_2006/ Accessed July 10, 2010
Isoda T, Ford AM, Tomizawa D, et al. Immunologically silent cancer clone transmission from mother to offspring. Proc Natl Acad Sci U S A. 2009;106:17882-17885.
Lancashire ER, Frobisher C, Reulen RC, et al. Educational attainment among adult survivors of childhood cancer in Great Britain: a population-based cohort study. J Natl Cancer Inst. 2010;102:254-270.
The Lancet. Preventable cancer in the USA. Lancet. 2010;375:1665.
Li J, Thompson TD, Miller JW, et al. Cancer incidence among children and adolescents in the United States, 2001–2003. Pediatrics. 2008;121:e1470-e1477.
Merks JH, Caron HN, Hennekam RC. High incidence of malformation syndromes in a series of 1,073 children with cancer. Am J Med Genetics. 2005;134A:132-143.
Merks JH, Ozgen HM, Koster J, et al. Prevalence and patterns of morphological abnormalities in patients with childhood cancer. JAMA. 2008;299:61-69.
Mutafoğlu-Uysal K, Günes D, Tüfekçi O, et al. The incidence of congenital malformations in children with cancer. Turk J Pediatr. 2009;51:444-452.
Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4401-4409.
National Center for Health Statistics. Health, United States, 2008 With Special Feature on the Health of Young Adults (website). www.cdc.gov/nchs/data/hus/hus08.pdf. Accessed July 10, 2010
Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572-1582.
Rajaraman P, Simpson J, Neta G, et al. Early life exposure to diagnostic radiation and ultrasound scans and risk of childhood cancer: case control study. BMJ. 2011;342:424.
Reid S, Schindler D, Hanenberg H, et al. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nature Genetics. 2007;39:162-164.
Ries LAG, Smith MA, Gurney JG, et al. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995 (website). editors http://seer.cancer.gov/publications/childhood/index.html Accessed July 10, 2010
Wasilewski-Masker K, Mertens AC, Patterson B, et al. Severity of health conditions identified in a pediatric cancer survivor program. Pediatr Blood Cancer. 2010;54:976-982.