Chapter 29 Epidemiology of Cerebrovascular Disease
Overview
Stroke is the second leading cause of death worldwide, accounting for 4 million deaths in 2004.1 Until recently, stroke ranked as the third leading cause of death in the United States.2 Owing to a reclassification of respiratory diseases using the 10th version of the International Classification of Diseases (ICD-10), preliminary data from the Centers for Disease Control and Prevention (CDC) released on December 9, 2010, ranks cerebrovascular disease as the fourth most common cause of death in the country behind diseases of the heart, cancer, and chronic lower respiratory diseases. On average, every 40 seconds someone in the United States has a stroke, and every 4 minutes someone dies.2 Stroke is also a leading cause of long-term disability in adults. There are a variety of nontreatable and treatable stroke risk factors (Box 29-1).
Stroke Burden
In the United States, an estimated 795,000 persons have a stroke each year, and 7 million individuals 20 years of age or older have had a stroke.2 Ischemic strokes account for 87% of all strokes, with the remaining 13% due to intracranial hemorrhages. About three quarters of these strokes are first cerebrovascular events, and the remainder are recurrent.2 The risk of first ischemic stroke varies by race-ethnicity, increasing from 88 per 100,000 in whites, to 149 per 100,000 in Hispanics/Latinos, to 191 per 100,000 in blacks.2 There were over three quarters of a million hospital discharges for stroke in 2007, and approximately 3.7 million ambulatory care visits attributable to stroke in 2008.3 Up to 70% of cerebrovascular events evaluated in hospitals are ischemic strokes, with 30% due to transient ischemic attacks (TIAs) and hemorrhagic strokes.4 Two thirds of patients hospitalized for stroke are older than age 65; half are older than 70. Importantly, stroke is the second leading cause of death in persons aged 85 and over, who by 2050 will number approximately 20.9 million.5 As measured by disability-adjusted life-years, the burden of stroke relative to other diseases is anticipated to continue to increase worldwide from sixth in 1990 to fourth in 2020.6
A TIA precedes approximately 15% to 23% of ischemic strokes and carries a 90-day stroke risk of 9% to 17%7 and a 25% risk of death over the ensuing year.8 About half of all patients who have a TIA fail to report their symptoms to a healthcare provider.9 With an estimated 240,000 TIAs diagnosed annually in the United States, TIA represents an important target for secondary stroke prevention.
Mortality from stroke accounted for 1 of every 18 deaths in 2007, for a total of 135,952 deaths.2 Mortality in the 5 years after stroke ranges from 27% to 61% across sex-age subgroups.2 Current data on long-term survivorship following ischemic stroke reflects wide ranges of estimated mortality; 13% to 45% at 1 year, 36% to 69% at 5 years, and 31% to 87% at 10 years.10–29 These estimates were primarily obtained from international studies or single regions within the United States and included a relatively small number of cases. Women and minority populations may have higher long-term mortality following stroke, although the evidence is limited, and more research is needed to investigate differences by patient characteristics.13,23,25
In addition to the relatively high mortality from stroke, its aftermath can be devastating. Twenty percent of stroke survivors require institutional care after 3 months, and 15% to 30% are permanently disabled.30 Given the large number of stroke survivors in this country, effective rehabilitation and secondary prevention are important targets for public health intervention.
Cost
Stroke is among the 10 highest contributors to Medicare costs.31 In 2007, the annual cost of stroke in the United States was estimated at $40.9 billion, with $25.2 billion in direct costs.2 Total stroke-related costs were estimated at $68.9 billion in 2009.4 These costs are projected to exceed $2.2 trillion through 2050.32 The mean lifetime cost of ischemic stroke is now estimated at $140,048 in 1999 dollars.33
Regional Patterns of Stroke
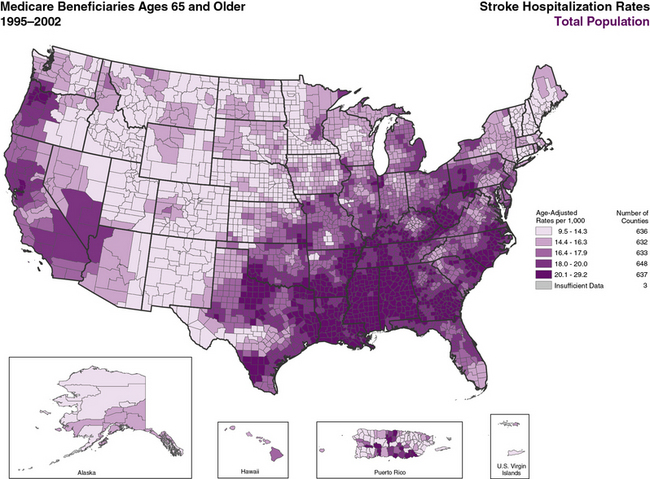
Over the last 50 years, stroke mortality has varied regionally in the United States, with the highest mortality rates in the Southeast, a region of the country termed the “Stroke Belt.”34–38 The Stroke Belt is usually defined as including the eight southern states of North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana, and Arkansas. These geographic differences have been documented since at least 1940,34 and despite some minor shifts35 still persist.36–38 The reason for the existence of the Stroke Belt remains uncertain. Within it, a “buckle” region along the coastal plain of North Carolina, South Carolina, and Georgia has been identified with even higher stroke mortality rates than other portions.39 Overall average stroke mortality is about 20% higher in the Stroke Belt than in the rest of the nation and about 40% higher in the stroke buckle. Individuals living in the southeastern United States have a 50% greater risk of dying from a stroke than residents of other regions.39
Higher stroke mortality rates are now also noted in the Pacific Northwest.40,41 Overall mortality reflects a combination of incidence and case-fatality rates. Stroke incidence is higher in the Southeast,37,42,43 whereas case-fatality rates vary little across the country.37 The CDC published an atlas showing geographic patterns in age-adjusted stroke hospitalization rates by county that are consistent with stroke mortality patterns (Figure 29-1).44 There are also regional differences in recurrent stroke events within the year after stroke hospitalization, even after adjusting for common risk factors.45,46 Causes for these shifts remain unclear, and contemporary national data are needed to monitor incident and prevalent stroke patterns over time, particularly by age, race-ethnicity, and sex subgroups.
Stroke Risk Factors
Demographic Factors
Age
Stroke is rare in young children, occurring at a rate of 4.6 to 6.4 per 100,000 children in the United States.2 The incidence of stroke in adults is strongly age dependent,25 and the rate of adverse outcomes and complications associated with stroke increases with advanced age.47 The mean age of stroke patients in this country is 70 years. The U.S. population is aging, and by the year 2050, the total number of people aged 65 and older will nearly triple, increasing from 34 million in 2000 to 90 million.5,48,49 This means that by 2050, 20% of the population will be 65 years of age or older and at increased risk for stroke. A report released by the CDC in collaboration with the Center for Medicare & Medicaid Services (CMS), the Atlas of Stroke Hospitalizations Among Medicare Beneficiaries, found that 30-day stroke mortality rates varied by age: 9% in patients aged 65 to 74, 13.1% in those aged 74 to 84, and 23% in those aged 85 or older.44 Because stroke is so strongly age dependent, understanding the epidemiology of stroke in the elderly is critical for both clinicians and policy makers.
Sex
Women are older at stroke onset compared with men (75 years vs. 71 years).2 Overall, women have a lower age-adjusted stroke incidence than men, but sex-related differences in stroke risk are modified by age. Data from the Framingham Heart Study demonstrate that compared with white men, white women 45 to 84 years of age have lower stroke risk than men, but this association is reversed in women older than 85, whose risk is higher than men.2 The absolute number of strokes in the population is, however, greater for women because of the increasing risk of stroke with advancing age, combined with women’s longer life expectancy.
A focus on age-adjusted and age-specific stroke mortality rates conceal the greater total number of stroke deaths in women. The excess deaths in women result from the higher mortality in women and their disproportionate representation in the population. About 55% of all strokes occur in women, who have approximately 60% of stroke-related deaths. Not only do more strokes occur in women, the bulk occur in women over the age of 70, who are more likely to be socially isolated, live alone, have fiscal constraints, and higher rates of comorbid disease. The greater burden of stroke deaths in women is predicted to be even greater in the future, based on population projections; an excess of 32,000 stroke deaths in women in 2000 is anticipated to increase to nearly 68,000 excess deaths by 2050.50
The rate of stroke increases during pregnancy, with the majority occurring during the postpartum period. Pregnancy-related intracerebral hemorrhage (ICH) has the highest mortality and morbidity of all stroke types. Risk factors for stroke in pregnancy include advanced maternal age, African American race-ethnicity, migraines, preeclampsia, and gestational hypertension.51 Meta-analyses summarizing over 30 years of studies show that oral contraceptive users have about a twofold increased risk of stroke compared with non-users; hypertension, cigarette smoking, and migraine headaches further increase stroke risk in women taking oral contraceptives.51 Women younger than 50 are generally considered to be at lower risk for stroke than men, although recent studies show that women aged 35 to 64 were almost three times more likely than men to report prior stroke, largely because of higher rates in 45- to 54-year-olds.52 Prevalence of stroke increases as women reach the menopausal transition. Studies suggest that women are protected by endogenous estrogens, but clinical trials have not found a lower risk of either stroke or cardiac events in postmenopausal women treated with exogenous estrogen and progesterone.50
Race-ethnicity
There are prominent stroke race-ethnic disparities. Blacks and Hispanic/Latinos have two to four times the rate of stroke, stroke recurrence, and stroke-related deaths as whites.53–56 Although differences may be more prominent in younger age groups,57 race-ethnic differences remain among older age groups.56,58 Stroke incidence has been decreasing in U.S. whites, but not in U.S. blacks over the past decade, suggesting a worsening of racial disparity in stroke incidence.59
Lifestyle Factors
Diet/nutrition
Numerous studies implicate dietary factors in the risk for stroke. Aspects of diet that have received attention include individual dietary factors such as omega-3 fatty acids, vitamins C and E, potassium, calcium, fatty acids, homocysteine, and sodium, as well as food groups and dietary patterns such as consumption of fruits and vegetables, the Dietary Approaches to Stop Hypertension (DASH) diet, and the Mediterranean diet.60 Intake of antioxidant vitamin supplements such as vitamin C and E do not lower stroke risk. Although some evidence suggests higher total fat intake increases stroke risk,61 higher fish consumption, a marker for omega-3 fatty acid intake, was associated with reduced stroke risk in the Nurses’ Health Study and the Health Professionals’ Follow-up Study.62,63 Higher fruit and vegetable consumption has been associated with lower stroke risk in many studies64–68 and was found to reduce the risk of stroke in a clinical trial.69 A meta-analysis of cohort studies reported a 26% reduction in stroke risk associated with consumption of five or more servings a day of fruits and vegetables compared with consumption of less than three servings a day.70
Reduced sodium and increased potassium intake have also been associated with decreased stroke risk.71–74 The proposed effects of sodium and potassium on stroke risk are likely in part due to their role in lowering blood pressure. The DASH diet, characterized by high intake of fruits and vegetables, consumption of low-fat dairy products, and low intake of saturated and total fat, effectively lowers blood pressure in clinical trials75 and was associated with an 18% lower risk of stroke in the Nurses’ Health Study (highest quintile vs. lowest quintile).76 The Mediterranean style diet has also received considerable attention with regard to its favorable impact on cardiovascular health. Women in the Nurses’ Health Study at the highest vs. lowest quintiles of the Alternate Mediterranean Diet Score had a lower risk of stroke (relative risk [RR] = 0.87; 95% CI, 0.73-1.02) after 20 years of follow-up.77 Analyses in the same group of women evaluating consumption of a so-called prudent diet (high in vegetable, fruit, legume, fish, and whole grain intake) and a so-called Western diet (one high in red and processed meats, refined grains, sweets, and desserts) found a 58% increased risk of stroke in the highest vs. lowest quintile of a Western diet and a 22% lower risk of stroke in the highest vs. lowest quintile of a prudent diet.78
Current dietary guidelines for stroke prevention recommend reduced sodium intake and increased intake of potassium and fruits and vegetables. Unfortunately, according to national surveys conducted in 2005-2006, average adult consumption ranges from 1.1 to 1.8 servings a day of fruit and 1.2 to 2.1 servings a day of vegetables across race-ethnic groups,4 whereas sodium intake exceeds recommendations for 90% of the adult U.S. population.79 Low fruit and vegetable intake is estimated to account for 9% and 11% of stroke deaths in high-income and mid- to low-income countries, respectively.80
Physical inactivity
Lack of regular physical activity is a well-established predictor of early mortality and cardiovascular disease, and regular exercise is associated with a lower risk of stroke in prospective and case-control studies.81–88 The effects of physical activity are thought to be mediated through reductions in blood pressure, control of other cardiovascular risk factors, improvements in glucose tolerance and body weight, reductions in plasma fibrinogen and platelet activity, and elevations in high-density lipoprotein (HDL) cholesterol concentration.88–94 Current guidelines recommend a minimum of 30 minutes per day of moderate-intensity physical activity most days of the week.95,96 In the 2008 National Health and Nutrition Examination Survey (NHANES), only 32.5% of adults aged 18 and older reported they engaged in regular leisure time physical activity.4 Physical inactivity accounts for 8% and 6% of stroke deaths worldwide in high-income and middle- to low-income countries, respectively.80 Participants in the Physicians’ Health Study engaging in vigorous exercise five or more times per week had a 14% lower risk of stroke compared with those engaging in vigorous activity less than 1 time per week.94 The Framingham Heart Study and the Honolulu Heart Program have found similar effects in men.81,82 Studies in women, including the Nurses’ Health Study and the Copenhagen City Heart Study, also report reductions in stroke incidence with increased physical activity.87,88 Among women aged 45 and older in the Women’s Health Study, higher levels of physical activity (measured in kcal/wk) were associated with a 20% to 40% reduction in stroke risk.97 Finally, in the Northern Manhattan Stroke Study, there was an overal 63% reduction in stroke risk associated with physical activity; this protective effect was found in all subgroups of age, sex, and race-ethnicity (white, black, Hispanic). There is some uncertainty as to the dose and intensity of physical activity required for adequate reduction in stroke risk, and comparison studies are limited. An analysis of the Northern Manhattan Study suggested that heavy physical activity was more beneficial than low to moderate activity.86 Other evidence supports the beneficial effect of light exercise. The intensity of physical activity was not related to stroke risk among participants in the Women’s Health Study, although walking time and pace were inversely related to stroke risk.97
Overweight/obesity
Obesity likely increases stroke risk through multiple mechanisms including increased blood pressure, impaired glucose tolerance, and more frequent atherogenic serum lipid levels. Traditionally, weight categorization has been defined according to the body mass index (BMI; weight [kg] divided by the square of height [m]), with 25 and 30 kg/m2 used as cut-points defining overweight and obesity, respectively. In the Framingham cohort, BMI was related to first cerebrovascular events after adjustment for traditional stroke risk factors.98 Relative risks for stroke in an analysis of women aged 30 to 55 in the Nurses’ Health Study ranged from 1.75 for a BMI of 27 to 28.9 kg/m2 to 2.37 for a BMI above 32 kg/m2, and were independent of other risk factors including age, smoking, hormone use, and menopausal status.99
Obesity was also identified as an independent stroke risk factor in the Honolulu Heart Study.100 Abdominal obesity has emerged as an even stronger risk factor for stroke than BMI. In a study of 28,643 male health professionals, the relative risk of stroke was 2.3 times higher in the upper compared with the lower quintiles of waist-to-hip ratio.101 Obesity is a major public health problem in the United States and worldwide. According to a national survey, 67% of U.S. adults are overweight, and 30% are obese.102 As a population attributable risk factor, obesity accounts for an estimated 20% of stroke deaths in high-income countries and 15% in middle- to low-income countries, a risk that is likely to increase in the United States owing to the epidemic of obesity in the young.
Environmental Factors
Air pollution reflects a heterogenous amalgamation of gases, liquids, and particulate matter that has gained attention as a risk factor for stroke. A 2004 American Heart Association (AHA) Scientific Statement on the relationship between particulate matter (PM) air pollution and cardiovascular disease concluded that both short- and long-term exposure to ambient PM increases the risk of acute cardiovascular events.103 With the proliferation of studies on this topic following this Scientific Statement, an update was published in 2010.104 Indeed, most epidemiological studies have corroborated the initial conclusions, generally reporting an elevated risk of cardiovascular events associated with exposure to fine PM less than 2.5 μm in aerodynamic diameter (PM2.5) for susceptible individuals (e.g., the elderly, those with existing cardiovascular conditions). Recent research also suggests a role for ultrafine particulates less than 0.1 μm, co-pollutants such as ozone and nitrogen oxides, and specific sources of pollution such as motor vehicle traffic.
Among the subcategories of cardiovascular disease, the strength of evidence for an association between air pollution and cerebrovascular disease is less than that for heart disease, but the literature is still growing.104 Among more than 60,000 postmenopausal women initially free of cardiovascular disease in the Women’s Health Initiative, each 10 μm/m3 increase in annual PM2.5 exposure was associated with a 28% increased risk of stroke, a 35% increased risk of stroke or fatal cerebrovascular disease, and an 83% increased risk of fatal cerebrovascular disease.105 There was no association between fatal cerebrovascular and long-term PM2.5 exposure in a cohort of over 300,000 adults derived from the American Cancer Society’s Cancer Prevention Study-II.106 Several studies have reported small but statistically significant associations for short-term pollutant exposure. Daily time-series studies from Korea (Seoul),107,108 China (Shanghai),109 and Finland (Helsinki)110 reported associations between elevated levels of air pollution and stroke mortality; however, the associations were found for ischemic but not hemorrhagic stroke mortality in one study,109 and in the warm but not cold seasons in another.110 A U.S. study of Medicare hospitalizations for stroke or cerebrovascular disease in nine cities found that several measures of air pollution within the 0 to 2 days before hospitalization were associated with ischemic but not hemorrhagic stroke. Additional studies using surveillance data from Dijon, France,111 and Corpus Christi, Texas,112 have also linked certain types of air pollutants with the risk of ischemic stroke and TIA.
In addition to acute cardiovascular events, air pollutants potentially contribute to numerous subclinical physiological changes including systemic inflammation and oxidative stress, coagulation/thrombosis, systemic and pulmonary arterial blood pressure, vascular function, atherosclerosis, heart rate variability, cardiac ischemia and repolarization abnormalities, epigenetic changes, and traditional cardiovascular risk factors.104 Such associations provide further support for a relationship between cardiovascular disease and air pollution and provide insight into plausible mechanistic pathways. As a potentially avoidable risk factor, it may be prudent for those at high risk of cardiovascular disease and stroke to limit outdoor air exposure on high pollution days.
Cigarette smoking
Cigarette smoking is consistently identified as a major independent risk factor for ischemic stroke in epidemiological studies including the Framingham Heart Study,113 Cardiovascular Health Study,114 Honolulu Heart Study,115 and INTERSTROKE,116 among many others. In general, cigarette smoking leads to an approximate twofold increase in stroke risk.117 Smoking is also associated with a two- to fourfold increase in risk of subarachnoid hemorrhage (SAH).118–121 Data are inconclusive regarding a relationship between smoking and the risk of parenchymal ICH. Analyses of data from the Hemorrhagic Stroke Project,122 Physicians’ Health Study,120 and the Women’s Health Study119 find an association between smoking and increased ICH risk, whereas analyses from other studies, including a pooled analysis of data from the Atherosclerosis Risk in Communities (ARIC) Study and the Cardiovascular Health Study, find no relationship.123 One meta-analysis even reported a paradoxical protective effect of smoking for ICH risk,124 although a more contemporary review found an approximate 30% increase in ICH risk associated with smoking.125 Using data from the National Health Interview Survey and death certificates for 2000 to 2004, the CDC estimates that nearly 16,000 cerebrovascular deaths annually can be attributed to smoking, equating to approximately 13% of cerebrovascular deaths for men and 8% for women.126
Environmental tobacco smoke (also known as passive or secondhand smoke) is also thought to increase the risk of stroke for lifelong nonsmokers. A meta-analysis of 24 sex-specific estimates from 15 studies found an overall 25% (95% CI, 16%-36%) increased risk of stroke with current spousal exposure (or its nearest equivalent).127 There was no heterogeneity by sex, publication year, or outcome, but risk estimates tended to be lower for prospective studies and those studies with U.S. or European cohorts. There was not an increased risk for SAH, and overall risk estimates were similar when using ever spousal exposure or total exposure rather than current spousal exposure. A 56% (95% CI, 34%-82%) increased risk of stroke was found for the highest level of spousal exposure.
Although data strongly support a relationship between smoking and stroke, the linearity of a dose effect remains uncertain. A number of studies suggest a nonlinear effect, with the stroke risk attributable to smoking increasing sharply at lower cigarette consumption levels and then leveling off as the number of cigarettes per day increases.128 Similarities between stroke risk estimates and changes in biomarkers of cardiovascular risk for active and passive smokers further support a nonlinear effect of cigarette smoking, suggesting a low overall threshold of tobacco smoke exposure.117,129,130
Clinical and experimental studies indicate both acute and chronic effects of cigarette smoking that likely contribute to increased stroke risk.128 Cigarette smoking causes endothelial injury and dysfunction in both the coronary and peripheral arteries. It increases the risk of thrombus generation in atherosclerotic arteries and creates a chronic state of inflammation associated with development of atherosclerosis. Smoking also leads to an atherogenic lipid profile (increased triglycerides and decreased HDL cholesterol), and it is thought to produce insulin resistance that, along with chronic inflammation, can accelerate macrovascular and microvascular complications such as nephropathy. Smoking even just one cigarette increases heart rate, mean blood pressure, and cardiac index and decreases arterial distensibility.131,132 A number of studies also demonstrate a synergistic action of smoking with other cardiovascular risk factors in increasing stroke risk. Such factors include systolic blood pressure,133 vital exhaustion,134 and oral contraceptives135,136 among others.
Smoking cessation is generally accepted as an essential component of primary and secondary stroke prevention.117 Rapid reduction in stroke risk with smoking cessation is often observed, with considerable reductions apparent within 5 years.124,137–141 Although stroke risk is greatly lower for former compared to current smokers, it remains unclear whether risk ever reverts to that of never smokers. The Framingham Study139 and the Nurses’ Health Study137 show a near-complete loss of excess risk, whereas the Honolulu Heart Program,140 British Regional Heart Study,138 and others124,141 report a slight excess risk for former compared to never smokers. Several studies suggest that this excess risk may be retained for up to 2 decades after quitting.138,141 Heavy smokers may have more sustained excess risk compared with light smokers, whose risk falls more rapidly to the level of nonsmokers.138,141 It is possible that past level of smoking rather than years since cessation or duration of smoking might be the determining factor in achieving risk equivalence.
Alcohol Consumption
The effects of alcohol intake on stroke risk appear to be dependent on both level of consumption and stroke type. In the Honolulu Heart Study, there was a very strong dose-response relationship between alcohol intake and intracranial and subarachnoid hemorrhage.142 Alcohol intake also appears to confer risk for ischemic stroke, although effects at low to moderate levels are less clear, with most evidence suggesting a beneficial effect. A study following male health professionals reported increased risk of stroke with an intake of more than two drinks per day, but no clear association with lower levels of consumption.143 Other studies support a J-shaped relationship between alcohol intake and the risk of ischemic stroke, such that low to moderate levels of intake are associated with reduced stroke risk, and heavy consumption is associated with increasingly higher risk.144–150 A meta-analysis of the effects of alcohol (1 drink defined as 12 g of alcohol) and stroke risk reported a 20% to 28% reduced risk of stroke among those consuming fewer than 1 or 1 to 2 drinks a day relative to abstainers.151 In contrast, heavy drinking (> 5 drinks/day) was associated with a 69% increased risk. The population attributable risk associated with alcohol use for stroke mortality in high-income and middle- and low-income countries is estimated at 11% and 5%, respectively.80 Heavy alcohol use may impact risk through increases in blood pressure, higher rates of atrial fibrillation and coagulation disorders, and reductions in cerebral blood flow. At moderate levels of intake, however, alcohol may have beneficial effects through reductions in platelet aggregation and plasma fibrinogen concentration, and improvements in HDL cholesterol levels and endothelial function. Current guidelines recommend moderate drinking of two or less drinks per day for men and one drink or less per day for nonpregnant women who consume alcohol.152
Medically Treatable Risk Factors
Hypertension
Hypertension, or high blood pressure—defined as systolic blood pressure 140 mmHg or greater, diastolic blood pressure 90 mmHg or greater, or requiring antihypertensive medication—occurs in one in three adults in the United States. Hypertension currently affects more than 76 million people in the country. Prevalence is particularly high in individuals older than 65, in whom it exceeds 67%.153,154 Lifetime risk of hypertension in individuals aged 55 years is 90%. African Americans have one of the highest rates of hypertension in the world; prevalence in this group is 32.3%, compared with 23.0% for whites and 21.5% for Hispanics/Latinos. Despite the relative ease of diagnosis and effectiveness of treatment, approximately 20.4% of adults with hypertension are unaware of their condition, 29.1% are not being treated, and 52.2% do not have adequately controlled blood pressure. Control is lower in Mexican Americans (35.2%) compared with non-Hispanic whites (46.1%) and non- Hispanic blacks (46.5%).2
High blood pressure is one of the most important modifiable risk factors for both ischemic and hemorrhagic stroke. Individuals with blood pressures below 120/80 mmHg have approximately half the lifetime risk of stroke of those with hypertension.4 There is a 38% increased relative risk of stroke for every 10 to 20 mmHg increase in systolic blood pressure, and a 34% increased relative risk for every 5 mmHg increase in diastolic blood pressure.155 This increased risk is a graded response with no apparent threshold, even within the normal range. Lowering blood pressure reduces the incidence of stroke.155
Lifestyle changes can effectively lower blood pressure levels. Adherence to the DASH diet (low in sodium, high in potassium and low-fat dairy) lowers blood pressure.69,75 Weight loss is strongly related with improved blood pressure; a meta-analysis found that a 5.1 kg loss of body weight was associated with an average lowering of systolic blood pressure by 4.4 mmHg and diastolic by 3.6 mmHg.156 Treatment with β-blockers lowers the risk for stroke by 29% (RR = 0.71; 95% CI, 0.59-0.86), and diuretics are estimated to reduce the risk by 51% (RR = 0.49; 95% CI, 0.39-0.62).157 Overall reduction in incident stroke with antihypertensive therapy is estimated at 35% to 44%.158 Recent data suggest that reductions in stroke risk may be related to type of antihypertensive used, based on the agent’s relative effects on blood pressure variability.159
Lipids
A clear linear relationship exists between serum cholesterol levels and coronary heart disease; the relationship between serum cholesterol and stroke is less certain. The totality of evidence from large epidemiological studies suggests, at best, a minimal association between increased total cholesterol levels and stroke risk.117,160 A meta-analysis of 45 prospective cohorts involving 450,000 people and 13,000 incident strokes found no relationship between total cholesterol and stroke.161 The cohorts upon which the analyses were based included many middle-aged participants, and stroke subtypes were not specified. Subsequent studies found a small association between higher total or low-density lipoprotein (LDL) cholesterol levels and increasing ischemic stroke risk; paradoxically, lower total or LDL cholesterol has generally been associated with an increased risk of hemorrhagic stroke.117,160 These differing risks may in part explain the lack of a relationship between cholesterol levels and overall stroke rates.
An individual-data meta-analysis of nearly 900,000 patients from 61 studies worldwide found only a weak positive association between total cholesterol and total stroke mortality at ages 40 to 59, with no association at older ages.162 A weak positive association was also found between total cholesterol and ischemic stroke, with a negative association with hemorrhagic stroke. For both total and ischemic stroke mortality, associations were larger at baseline systolic blood pressure below 145 mmHg.
High-density lipoprotein cholesterol protects against atherosclerosis through reverse cholesterol transport, improvement of endothelial function, and antioxidant, antiinflammatory, and antithrombotic effects.163,164 Numerous large cohort studies have evaluated the relationship between serum HDL cholesterol and stroke risk.164 Despite using different HDL cholesterol cut-points and including cohorts of different ages and geography, most of these studies find either a strong and statistically significant inverse relationship165–169 or a trend towards such a relationship.170,171 The risk of atherosclerotic stroke, in particular, may be most strongly related to low HDL cholesterol levels.172–174 A recent large meta-analysis, however, found no evidence for a significant association between HDL cholesterol and stroke mortality, and only a weak positive association between the ratio of total to HDL cholesterol and stroke mortality for those aged 40 to 69.162 There have been no studies showing that pharmacologically raising HDL cholesterol levels decreases stroke risk.
Findings about the relationship between triglycerides and ischemic stroke are conflicting. Fasting levels were not associated with ischemic stroke in the ARIC and other studies,171 but a meta-analysis of prospective studies in the Asia-Pacific region showed a 50% increase in ischemic stroke risk for those with the highest vs. lowest fasting levels.175 In the Copenhagen City Heart Study176 and the Women’s Health Study,177 higher nonfasting triglycerides were associated with increased risk of ischemic stroke. The Women’s Health Study also evaluated fasting triglycerides and found no association with ischemic stroke.177
Although no large consistent association between cholesterol and total stroke has been observed in epidemiological cohorts, data from randomized trials show that statin therapy reduces risk of stroke among patients with established coronary heart disease and in those at increased vascular risk.178,179 One meta-analysis of data from 14 trials including more than 90,000 patients found an approximate 17% to 21% reduction in the relative risk of incident stroke per mmol/L decrease in LDL cholesterol.178 A significant trend with greater proportional reductions in stroke was associated with greater mean absolute LDL cholesterol reductions. Another meta-analysis including nearly 166,000 participants in trials of statins in combination with other preventive efforts also found an approximate 21% decrease in stroke for each mmol/L decrease in LDL cholesterol.179 Incidence of all strokes was reduced by 18%, and a reduction in the risk of recurrent stroke and major cardiovascular events among persons with noncardioembolic stroke was found, with a trend toward a lower incidence of fatal stroke.
Use of statin therapy to reduce cholesterol did not lead to increased risk of hemorrhagic stroke in primary stroke prevention populations. Among those with prior stroke or TIA, statins may be associated with an increased risk of hemorrhagic stroke that partially attenuates their overall benefit. Other lipid-modification therapies including niacin, fibric acid derivatives, bile acid sequestrants, and ezetimibe also have favorable effects on lipid parameters, but evidence in support of such strategies for stroke prevention is not well established.117
Diabetes
Diabetes is a complex metabolic condition with recognized microvascular and macrovascular complications. In 2007, the CDC estimated that 23.5 million Americans had diabetes, 6 million of whom were undiagnosed.180 Prevalence of diabetes among those aged 20 years or older was estimated at 11%, and for those aged 60 years or older, 23%. Diabetes is overrepresented among patients who present with ischemic stroke, with prevalence estimates of 15% to 33%.181 Diabetic patients are more susceptible to development of atherosclerosis and often have other cardiovascular risk factors such as hypertension, dyslipidemia, atrial fibrillation, heart failure, and prior myocardial infarction (MI). Epidemiological studies show the relationship between diabetes and stroke is independent of other such risk factors.
An individual-data meta-analysis based on 97 studies involving nearly 600,000 people worldwide without prior vascular disease found a more than twofold excess risk of incident fatal or first-ever nonfatal ischemic stroke among persons with diabetes, even after adjusting for other vascular risk factors.182 The excess risk with diabetes was similar for unclassified stroke (84% increased risk) but slightly lower for hemorrhagic stroke (56% increased risk). Although diabetes was consistently associated with increased ischemic stroke risk across clinically relevant strata, risks tended to be higher for women, younger adults (age 40-59 years), and those within the highest tertile of BMI. Individual studies report variations in stroke risk by race-ethnicity among younger age groups.117
Diabetes is a strong predictor of stroke outcomes181; it doubles the risk of recurrent stroke, and an estimated 9% of recurrent strokes can be attributed to diabetes. Diabetic patients have higher stroke mortality rates, greater residual disability, and slower recovery after stroke.182,183
Evidence that tight control of glycemic levels lowers stroke (or cardiovascular risk) is lacking.117 Stroke risk, however, can be modified among diabetic patients.117 A comprehensive cardiovascular program that includes hypertension control with an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) reduces stroke risk. Statin treatment, especially among diabetic patients with additional risk factors, decreases the risk of a first or a recurrent stroke. Monotherapy with a fibrate may be helpful in reducing stroke risk, but data are conflicting; addition of a fibrate to statin therapy has not been found to lower stroke risk among diabetic patients.
Atrial Fibrillation
Atrial fibrillation is a major risk factor for ischemic stroke. Prevalence of atrial fibrillation in the United States increases from 0.5% in those aged 50 to 59 years to 1.8% at 60 to 69 years, 4.8% at 70 to 79 years, and 8.8% at 80 to 89 years.117 The population attributable stroke risk for atrial fibrillation increases from 1.5% to 2.8%, 9.9%, and 23.5%, respectively, over these same age groups. About 2.3 million Americans have atrial fibrillation. In a U.S. national bi-racial sample of adult men and women, among individuals with confirmed atrial fibrillation, blacks were approximately one third as likely to be aware as whites that they had the condition.184
In addition to age, a variety of other patient characteristics can affect atrial fibrillation–related stroke risk. A review of seven studies, including six independent cohorts, found the strongest, most consistent risk factors for stroke in persons with atrial fibrillation were a history of prior stroke or TIA (RR = 2.5; 95% CI, 1.8-3.5), increasing age (RR = 1.5 per decade; 95% CI, 1.3-1.7), history of hypertension (RR = 2.0; 95% CI, 1.6-2.5), and diabetes mellitus (RR = 1.7; 95% CI, 1.4-2.0).185 Stroke rates for single independent risk factors were 1.5% to 3% per year for age older than 75, 6% to 9% per year for prior stroke/TIA, 1.5% to 3% per year for history of hypertension, and 2.0% to 3.5% per year for diabetes.
Several published schemes are available to stratify an individual patient’s atrial fibrillation–related stroke risk. A comparison of 12 of these schemes found that they varied considerably.186 Of these, seven were based on extant data and five on expert consensus. Factors most commonly included were previous stroke/TIA (all schemes), patient age (83%), hypertension (83%), and diabetes (83%), with eight additional variables included in one or more schemes. When applied to the same cohort, the fractions of patients categorized by the different schemes varied considerably (proportions of patients categorized) as low risk varied from 9% to 49%, and proportions categorized as high risk varied from 11% to 77%. These differences are not trivial and have important public health and clinical implications.
The so-called CHADS2 scheme is the most commonly used for stroke risk stratification in patients with atrial fibrillation.187 One point is given for congestive heart failure (C), hypertension (H), age older than 75 years (A), and diabetes mellitus (D); and 2 points for a history of prior stroke or TIA. A validation study found that a score of 0 points reflected low risk (0.5%-1.7% per year); 1 point, moderate risk (1.2%-2.2% per year); and 2 points or more, high risk (1.9%-7.6% per year).186 It should be noted, however, that a history of prior stroke or TIA in a patient with atrial fibrillation alone is associated with a high risk of recurrent cerebrovascular events (6%-10% per year).185
Patients with paroxysmal or chronic atrial fibrillation at moderate or high stroke risk are candidates for treatment with an anticoagulant for prevention of stroke. Treatment with adjusted-dose warfarin leads to a 64% (95% CI, 49%-74%) relative reduction in stroke risk vs. placebo, aspirin with 9% (95% CI, 1%-35%) relative reduction vs. placebo, and adjusted-dose warfarin a 39% (95% CI, 19%-53%) relative reduction vs. aspirin.117 Yet many persons with atrial fibrillation who should be anticoagulated go untreated.117 Novel anticoagulants including direct thrombin inhibitors (DTIs) and oral factor Xa inhibitors are now becoming available for treatment of this population of patients.117
Sickle Cell Disease
Prevalence of sickle cell disease (SCD), an autosomal recessive inherited disorder, is 0.25% in blacks and confers a 200- to 400-fold increased relative risk of stroke compared to black children without the condition.117 Prevalence of stroke by age 20 years is approximately 11% in persons homozygous for SCD.188 Transcranial Doppler (TCD) ultrasonography is useful in identifying children with SCD who are at high and low stroke risk. Children with a timed mean TCD velocity in the middle cerebral artery (MCA) greater than 200 cm/sec have a stroke rate in excess of 10% a year, whereas those with velocities below this level have stroke rates of about 1% a year.189 Although a variety of treatments are available, regular red blood cell transfusion is the only preventive intervention proven in randomized trials to prevent stroke in children with SCD.190 Transfusion therapy reduced the risk of stroke from 10% a year to less than 1%.
Sleep-Disordered Breathing
Sleep-disordered breathing (SDB) is highly prevalent in patients with established cardiovascular disease. Obstructive sleep apnea (OSA), one form of SDB, affects an estimated 15 million adult Americans and is present in a large proportion of patients with hypertension and those with other cardiovascular conditions including coronary artery disease (CAD), atrial fibrillation, and stroke.191–197 Obstructive sleep apnea is characterized by repetitive interruption of ventilation during sleep caused by collapse of the pharyngeal airway. A meta-analysis suggests that nearly three fourths of stroke and TIA patients have SDB, with the predominate form being OSA.195 In this review, SDB was more common among men and those with recurrent strokes or strokes of unknown etiology; SDB was less common among patients whose strokes were of cardioembolic etiology. An observational study of consecutive patients who underwent polysomnography, with subsequent verified events (strokes and deaths), found that even after adjusting for age, sex, race-ethnicity, and comorbid conditions, OSA syndrome significantly increased the risk of stroke or death from any cause (adjusted hazard ratio, 1.97; 95% CI, 1.12-3.48).197
In a trend analysis, increased severity of sleep apnea at baseline was associated with an increased risk of development of this composite end point.197 Recent 10-year follow-up data of patients with stroke show an increased risk of death in patients with OSA (adjusted hazard ratio, 1.76; 95% CI, 1.05-2.95) that is independent of age, sex, and other common cardiovascular risk factors.196 Application of noninvasive positive airway pressure ventilation offers patients with sleep apnea the opportunity to increase their rehabilitation potential after stroke, but can be limited by continuous positive airway pressure (CPAP) compliance. Whether patients with stroke and OSA benefit from treatment with CPAP remains to be determined.
Other Risk Factors
Fibrinogen, Clotting Factors, and Inflammation
Evidence supports a role for inflammation in the initiation, progression, and complications of atherosclerosis, as well as being a contributor to destabilization of atherosclerotic lesions.198 A diverse set of proinflammatory factors have been evaluated as a means to add further prognostic information beyond that already provided by traditional risk factors. High-sensitivity C-reactive protein (CRP) is one of the most widely studied of these biomarkers. C-reactive protein is an acute-phase reactant released predominately by hepatocytes in response to inflammatory cytokine stimulation. It is also released in response to systemic inflammation, such as in connective tissue disease and in response to local infections. Despite lack of specificity for the origin of the inflammation, a multitude of epidemiological studies, including the Physicians’ Health Study, the Women’s Health Study, and Framingham, have demonstrated a significant association between elevated CRP and risk of incident and recurrent vascular events, including stroke.117 Risks for those in the highest tertiles/quartiles of CRP concentration ranged between 1.5 and 2 times higher than those in the lowest tertiles/quartiles.
A meta-analysis of individual records from over 50 prospective studies involving over 160,000 participants without preexisting vascular disease found log-transformed CRP concentrations linearly related, with no apparent risk threshold to risk of ischemic stroke.199 One standard deviation increase in log-transformed CRP concentration (threefold increase) was associated with 27% to 44% increased risk of ischemic stroke and 55% to 71% increased risk of vascular mortality, depending upon risk-adjustment factors. Although somewhat controversial, guidelines have tended to suggest that CRP measurement be limited to persons with intermediate cardiovascular risk (10%-20% 10-year risk based upon Framingham Risk Score) as a means to help guide clinical decision making.117,200 The 2011 American Heart Association/American Stroke Association (AHA/ASA) Guidelines for the Primary Prevention of Stroke recommends consideration of a measurement of CRP in patients without cardiovascular disease as a means of identifying patients who may be at increased risk of stroke, although the Guidelines also notes that the usefulness in routine clinical practice is not well established.117 Other markers of inflammation such as lipoprotein-associated protein A2 (LpPLA2) may also be useful.117
In addition to examining the relationship between inflammation and stroke via measurement of proinflammatory factors, studies have also evaluated the occurrence of vascular disease in those with systemic chronic inflammatory conditions, such as rheumatoid arthritis and systemic lupus erythematosus, generally finding an excess risk for cardiovascular events as well as stroke.117 Chronic infection with Helicobacter pylori might promote atherosclerosis, but randomized trials of antibiotics have not shown a benefit for prevention of vascular events. Finally, acute infectious diseases have been studied under the hypothesis that they could trigger a TIA or stroke via possible induction of clotting factors such as fibrinogen or the destabilization of atherosclerotic plaques. Influenza has been associated with increased cardiovascular mortality, and antiviral treatment of influenza within a few days of onset decreases 6-month risk of stroke or TIA. A relationship between influenza vaccination and reduced risk for stroke has also been found.
Fibrinogen, a clotting factor thought to accelerate the thrombotic process, is another potentially useful marker of inflammation for use in vascular disease prediction and prevention.201 An individual-data meta-analysis of 31 prospective studies involving over 150,000 participants without preexisting vascular disease found an approximate log-linear association of usual fibrinogen level and risk of first nonfatal or fatal stroke.201 This association was present within each age group (40-59, 60-69, ≥ 70), with no risk threshold. In analyses adjusted for age, sex, and other established vascular risk factors, risk of stroke was nearly doubled with each 1 g/L increase in usual fibrinogen level. When stroke was categorized into subtypes, the magnitude of association with fibrinogen was present for ischemic stroke and stroke attributed to unspecified causes, but was somewhat lower for hemorrhagic stroke. The role of other abnormal clotting factors (e.g., factor V Leiden, prothrombin 20210A mutations, protein C and protein S deficiencies, lupus anticoagulants, anticardiolipin antibodies) as risk factors for ischemic stroke is unclear.117
Blood Homocysteine Levels
Homocysteine is an intermediary amino acid formed during metabolism of the essential amino acid methionine. Normal plasma levels of homocysteine are 5 to 15 μmol/L. Elevated homocysteine levels, or hyperhomocysteinemia, may result from genetic defects that reduce enzymatic activity in homocysteine metabolism (e.g., homozygosity for the thermolabile variant of methylene tetrahydrofolate reductase [MTHFR; TT genotype]), nutritional deficiencies in vitamin cofactors (e.g., vitamin B6, vitamin B12, folic acid), chronic medical conditions (e.g., chronic renal failure that retards renal clearance of homocysteine), certain medications (e.g., fibrates and nicotinic acid, which are used to treat hypercholesterolemia), or lifestyle behaviors (e.g., smoking).117,202–206
Numerous studies support a modest association between elevated homocysteine levels and atherosclerotic vascular diseases.207–216 One meta-analysis involving 463 nonfatal or fatal stroke or TIA events from 12 prospective studies found that a 25% lower usual homocysteine level (≈︀ 3 μmol/L) was associated with a 19% (95% CI, 5%-31%) lower risk of stroke after adjustment for known cardiovascular risk factors and regression dilution bias.207 Another meta-analysis that involved 676 stroke events from eight prospective studies and adjusted for similar factors found a 59% increased risk of stroke for a 5 μmol/L increase in homocysteine.208 On this basis, a 3 μmol/L decrease in current homocysteine concentrations would be expected to reduce the risk of stroke by 24% (95% CI, 15%-33%). This meta-analysis did not find a statistically significant relationship of the MTHFR TT polymorphism (compared to wild-type CC) with stroke (odds ratio [OR] 1.65; 95% CI, 0.66-4.13), although the seven MTHFR studies included yielded relatively few data. More recent studies continue to report a relationship between hyperhomocysteinemia and stroke, recurrent stroke, and silent brain infarction.210–216 In a larger and more contemporary meta-analysis of data from over 15,000 persons initially free of cardiovascular disease, homozygotes for the T allele of the MTHFR polymorphism had a greater mean homocysteine level and a 26% (95% CI, 1.14-1.40) greater risk of stroke.209 Cohort restrictions by age, race, and geographic location yielded similar results.
Laboratory findings and genetic association studies support the biological plausibility of a causal role for elevated homocysteine in stroke pathogenesis, but results of randomized clinical trials have not established the efficacy of homocysteine-lowering therapies for reduction of stroke risk.117 Clinical trials confirm that vitamins B6, B12, and folic acid lower homocysteine levels, but several large trials of supplementation with these vitamins as a means of lowering homocysteine levels in patients with established cardiovascular disease have generally found no reduction in major vascular events or death. The largest of such trials to date, Vitamins to Prevent Stroke (VITATOPS), was a double-blind placebo-controlled trial including over 8000 patients with recent TIA or stroke from 20 countries who were followed for a median duration of 3.4 years.217 Although VITATOPS found daily administration of B vitamins was safe and lowered homocysteine levels, it was not more effective than placebo in reducing risk of the primary combined end point of stroke, MI, or vascular death (RR = 0.91; 95% CI, 0.82-1.00). Secondary analyses by outcome revealed similar results, except for a reduction in vascular death (RR = 0.86; 95% CI, 0.75-0.99). Results were generally consistent across subgroups, although B vitamins may have a role in reducing risk of vascular events among patients with symptomatic small-vessel intracranial disease. When the VITATOPS findings were included in a meta-analysis with other randomized controlled trials of homocysteine-lowering therapy in patients with and without preexisting cardiovascular disease, trial results were corroborated, with B vitamins not significantly more effective than control for reducing risk of stroke (RR = 0.94; 95% CI, 0.86-1.01) or composite stroke, MI, or vascular death (RR = 0.99; 95% CI, 0.94-1.03).
Migraine
Accumulating evidence suggests a relationship between migraine headache and an increased risk of ischemic stroke. A meta-analysis of 14 studies published through June 2004 reported a pooled RR of 2.16 (95% CI, 1.89-2.48); results were similar for analyses restricted to persons younger than 45 years of age and were consistent among those having migraine with and without aura.218 An updated meta-analysis incorporating nine studies published through January 2009 also reported an increased risk of ischemic stroke among persons with any type of migraine compared to those without migraine (pooled RR = 1.73; 95% CI, 1.31-2.29).219 Stratification by migraine aura status, however, showed that the higher risk of stroke was largely confined to those having migraine with aura (pooled RR = 2.16; 95% CI, 1.53-3.03 for those with aura vs. pooled RR = 1.23; 95% CI, 0.90-1.69 for those without aura).
Additional stratified analyses suggested a greater risk of ischemic stroke for the following groups: women with migraine (pooled RR = 2.08; 95% CI, 1.13-3.84) but not men (pooled RR = 1.37; 95% CI, 0.89-2.11); persons aged younger than 45 years (pooled RR = 2.65; 95% CI, 1.41-4.97), particularly women (pooled RR = 3.65; 95% CI, 2.21-6.04); smokers (pooled RR = 9.03; 95% CI, 4.22-19.34); and women currently using oral contraceptives (pooled RR = 7.02; 95% CI, 1.51-32.68). Three studies each examined the relationship of any migraine to TIA and hemorrhagic stroke; higher risk was found for TIA (pooled RR = 2.34; 95% CI, 1.90-2.88) but not for hemorrhagic stroke (pooled RR = 1.18; 95% CI, 0.87-1.60). Several studies found an association between migraine headache and nonspecific white matter hyperintensities on magnetic resonance imaging (MRI), localized predominately in the posterior circulation white matter or cerebellum.220–223 The clinical significance of these MRI findings is uncertain. There is no evidence that migraine control lowers stroke risk, but as noted earlier, risk of migraine-associated stroke is higher among women who smoke and use oral contraceptives.
Awareness of Stroke Warning Signs and Acute Treatment
Studies indicate that recognition of stroke symptoms is higher in women than men, among whites versus blacks and Hispanics-Latinos, and among people with higher versus lower educational attainment.224 Although awareness of stroke warning signs has improved over time, recognition of multiple warning signs remains low, as does people’s ability to identify tissue-type plasminogen activator (tPA) as an available drug therapy, or the importance of presenting within a window of under 3 hours for treatment.225 Symptoms associated with increased likelihood of calling 9-1-1 include weakness, confusion/decreased level of consciousness, speech/language deficits, and dizziness/coordination/vertigo; however, numbness and headache were not associated with the decision to call.226 Administration of intravenous tissue plasminogen activator (IV-tPA) to selected ischemic stroke patients within 4.5 hours of symptom onset improves the likelihood that they will have an excellent outcome. Despite this benefit, only about 2% of ischemic stroke patients in the United States are treated with IV-tPA.227
Other potential or emerging risk factors not addressed in this chapter are available for review in the AHA Primary Stroke Guidelines.117
1 World Health Organization. World Health Report 2004, Geneva (Switzerland). 2004.
2 Roger V.L., Go A.S., Lloyd-Jones D.M., et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209.
3 Centers for Disease Control and Prevention. National Center for Health Statistics. 2008 National Ambulatory Medical Care Survey and 2008 National Hospital Ambulatory Medical Care Survey. 2008.
4 Lloyd-Jones D., Adams R.J., Brown T.M., et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
5 Department of Health and Human Services. Administration of Aging. Statistics on the Aging Population. Available at: http://www.aoa.gov/prof/Statistics/statistics.aspx Accessed June 10, 2008
6 Murray C.J.L., Lopez A.D., Harvard School of Public HealthWorld Health OrganizationWorld Bank. The global burden of disease : a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Cambridge, MA: Published by the Harvard School of Public Health on behalf of the World Health Organization and the World Bank ; Distributed by Harvard University Press; 1996.
7 Wu C.M., McLaughlin K., Lorenzetti D.L., et al. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167(22):2417–2422.
8 Kleindorfer D., Panagos P., Pancioli A., et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36(4):720–723.
9 Johnston S.C., Fayad P.B., Gorelick P.B., et al. Prevalence and knowledge of transient ischemic attack among US adults. Neurology. 2003;60(9):1429–1434.
10 Bravata D.M., Ho S.Y., Brass L.M., et al. Long-term mortality in cerebrovascular disease. Stroke. 2003;34(3):699–704.
11 Bronnum-Hansen H., Davidsen M., Thorvaldsen P. Long-term survival and causes of death after stroke. Stroke. 2001;32(9):2131–2136.
12 Collins T.C., Petersen N.J., Menke T.J., et al. Short-term, intermediate-term, and long-term mortality in patients hospitalized for stroke. J Clin Epidemiol. 2003;56(1):81–87.
13 Eriksson M., Norrving B., Terent A., et al. Functional outcome 3 months after stroke predicts long-term survival. Cerebrovasc Dis. 2008;25(5):423–429.
14 Hallstrom B., Jonsson A.C., Nerbrand C., et al. Stroke incidence and survival in the beginning of the 21st century in southern Sweden: comparisons with the late 20th century and projections into the future. Stroke. 2008;39(1):10–15.
15 Hankey G.J., Jamrozik K., Broadhurst R.J., et al. Long-term risk of first recurrent stroke in the Perth Community Stroke Study. Stroke. 1998;29(12):2491–2500.
16 Hankey G.J., Jamrozik K., Broadhurst R.J., et al. Five-year survival after first-ever stroke and related prognostic factors in the Perth Community Stroke Study. Stroke. 2000;31(9):2080–2086.
17 Hardie K., Hankey G.J., Jamrozik K., et al. Ten-year survival after first-ever stroke in the Perth community stroke study. Stroke. 2003;34(8):1842–1846.
18 Hartmann A., Rundek T., Mast H., et al. Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study. Neurology. 2001;57(11):2000–2005.
19 Kammersgaard L.P., Olsen T.S. Cardiovascular risk factors and 5-year mortality in the Copenhagen Stroke Study. Cerebrovasc Dis. 2006;21(3):187–193.
20 La Spina P., Savica R., Serra S., et al. Long-term survival and outcome after first stroke in the Sicilian Aeolian Island Archipelago population. Neurol Sci. 2008;29(3):153–156.
21 Lai S.M., Alter M., Friday G., et al. Prognosis for survival after an initial stroke. Stroke. 1995;26(11):2011–2015.
22 Prencipe M., Culasso F., Rasura M., et al. Long-term prognosis after a minor stroke: 10-year mortality and major stroke recurrence rates in a hospital-based cohort. Stroke. 1998;29(1):126–132.
23 Qureshi A.I., Suri M.F., Zhou J., et al. African American women have poor long-term survival following ischemic stroke. Neurology. 2006;67(9):1623–1629.
24 Sacco R.L., Shi T., Zamanillo M.C., et al. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study. Neurology. 1994;44(4):626–634.
25 Sacco R.L., Wolf P.A., Kannel W.B., et al. Survival and recurrence following stroke. The Framingham Study. Stroke. 1982;13(3):290–295.
26 Terent A. Trends in stroke incidence and 10-year survival in Soderhamn, Sweden, 1975-2001. Stroke. 2003;34(6):1353–1358.
27 Tu J.V., Gong Y. Trends in treatment and outcomes for acute stroke patients in Ontario, 1992-1998. Arch Intern Med. 2003;163(3):293–297.
28 von Arbin M., Britton M., de Faire U. Mortality and recurrences during eight years following stroke. J Intern Med. 1992;231(1):43–48.
29 Andersen K.K., Olsen T.S. One-month to 10-year survival in the Copenhagen stroke study: interactions between stroke severity and other prognostic indicators. J Stroke Cerebrovasc Dis. 2011;20(2):117–123.
30 Asplund K., Stegmayr B., Peltonen M. From the twentieth to the twenty-first century: a public health perspective on stroke. In: Ginsberg M.D., Bogousslavsky J., eds. Cerebrovascular disease pathophysiology, diagnosis, and management, vol 2. Malden, MA: Blackwell Science; 1998.
31 Andrews R., Elixhauser A. The national hospital bill: growth trends and 2005 update on the most expensive conditions by payer. Healthcare cost and utilization project–statistical brief #42. Washington, DC: Agency for Healthcare Research and Quality; 2007.
32 Brown D.L., Boden-Albala B., Langa K.M., et al. Projected costs of ischemic stroke in the United States. Neurology. 2006;67(8):1390–1395.
33 Taylor T.N., Davis P.H., Torner J.C., et al. Lifetime cost of stroke in the United States. Stroke. 1996;27(9):1459–1466.
34 Borhani N.O. Changes and geographic distribution of mortality from cerebrovascular disease. Am J Public Health Nations Health. 1965;55:673–681.
35 El-Saed A., Kuller L.H., Newman A.B., et al. Geographic variations in stroke incidence and mortality among older populations in four US communities. Stroke. 2006;37(8):1975–1979.
36 Lanska D.J. Geographic distribution of stroke mortality in the United States: 1939-1941 to 1979-1981. Neurology. 1993;43(9):1839–1851.
37 Lanska D.J., Kryscio R. Geographic distribution of hospitalization rates, case fatality, and mortality from stroke in the United States. Neurology. 1994;44(8):1541–1550.
38 Pickle L.W., Mungiole M., Gillum R.F. Geographic variation in stroke mortality in blacks and whites in the United States. Stroke. 1997;28(8):1639–1647.
39 Howard G. Why do we have a stroke belt in the southeastern United States? A review of unlikely and uninvestigated potential causes. Am J Med Sci. 1999;317(3):160–167.
40 Howard G., Evans G.W., Pearce K., et al. Is the stroke belt disappearing? An analysis of racial, temporal, and age effects. Stroke. 1995;26(7):1153–1158.
41 Howard G., Howard V.J., Katholi C., et al. Decline in US stroke mortality: an analysis of temporal patterns by sex, race, and geographic region. Stroke. 2001;32(10):2213–2220.
42 Gillum R.F., Ingram D.D. Relation between residence in the southeast region of the United States and stroke incidence. The NHANES I Epidemiologic Followup Study. Am J Epidemiol. 1996;144(7):665–673.
43 Howard V.J., Kleindorfer D.O., Judd S.E., et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69(4):619–627.
44 Casper M., Nwaise I., Croft J., et al. Atlas of stroke hospitalizations among medicare beneficiaries. In U.S. Department of Health and Human Services Centers for Disease Control and Prevention. 2008. Atlanta
45 Allen N.B., Holford T.R., Bracken M.B., et al. Geographic variation in one-year recurrent ischemic stroke rates for elderly Medicare beneficiaries in the USA. Neuroepidemiology. 2010;34(2):123–129.
46 Allen N.B., Holford T.R., Bracken M.B., et al. Trends in one-year recurrent ischemic stroke among the elderly in the USA: 1994-2002. Cerebrovasc Dis. 2010;30(5):525–532.
47 Davenport R.J., Dennis M.S., Wellwood I., et al. Complications after acute stroke. Stroke. 1996;27(3):415–420.
48 . 2000-2050 Population projections of the United States by age, sex, race, Hispanic origin, and nativity: 1999-2100. 2000. US Census Bureau. Available at www.census.gov/population/www/projections/natproj.html
49 Day J.C. Population projections of the United States by age, sex, race, and hispanic origin: 1995 to 2050. Washington, DC: U.S. Bureau of the Census, Current, Population Reports, U.S. Government Printing Office; 1996.
50 Reeves M.J., Bushnell C.D., Howard G., et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915–926.
51 Bushnell C.D. Stroke in women: risk and prevention throughout the lifespan. Neurol Clin. 2008;26(4):1161–1176. xi
52 Towfighi A., Markovic D., Ovbiagele B. Persistent sex disparity in midlife stroke prevalence in the United States. Cerebrovasc Dis. 2011;31(4):322–328.
53 Sheinart K.F., Tuhrim S., Horowitz D.R., et al. Stroke recurrence is more frequent in blacks and Hispanics. Neuroepidemiology. 1998;17(4):188–198.
54 Gillum R.F. Epidemiology of stroke in Hispanic Americans. Stroke. 1995;26(9):1707–1712.
55 Sacco R.L., Hauser W.A., Mohr J.P., et al. One-year outcome after cerebral infarction in whites, blacks, and Hispanics. Stroke. 1991;22(3):305–311.
56 Bian J., Oddone E.Z., Samsa G.P., et al. Racial differences in survival post cerebral infarction among the elderly. Neurology. 2003;60(2):285–290.
57 Lackland D.T., Bachman D.L., Carter T.D., et al. The geographic variation in stroke incidence in two areas of the southeastern stroke belt: the Anderson and Pee Dee Stroke Study. Stroke. 1998;29(10):2061–2068.
58 Matchar D.B., Samsa G.P. Secondary and tertiary prevention of stroke. Patient Outcome Research Team (PORT) final report–phase 1, Prepared by Duke University Medical Center under contract no. 290-91-0028. Rockville, MD: Agency for Healthcare Research and Quality. 2000.
59 Kleindorfer D.O., Khoury J., Moomaw C.J., et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41(7):1326–1331.
60 Boden-Albala B., Sacco R.L. Lifestyle factors and stroke risk: exercise, alcohol, diet, obesity, smoking, drug use, and stress. Curr Atheroscler Rep. 2000;2(2):160–166.
61 Boden-Albala B., Elkind M.S., White H., et al. Dietary total fat intake and ischemic stroke risk: the Northern Manhattan Study. Neuroepidemiology. 2009;32(4):296–301.
62 Iso H., Rexrode K.M., Stampfer M.J., et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285(3):304–312.
63 Gillum R.F., Mussolino M.E., Madans J.H. The relationship between fish consumption and stroke incidence. The NHANES I Epidemiologic Follow-up Study (National Health and Nutrition Examination Survey). Arch Intern Med. 1996;156(5):537–542.
64 Bazzano L.A., Serdula M.K., Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr Atheroscler Rep. 2003;5(6):492–499.
65 Johnsen S.P., Overvad K., Stripp C., et al. Intake of fruit and vegetables and the risk of ischemic stroke in a cohort of Danish men and women. Am J Clin Nutr. 2003;78(1):57–64.
66 Sauvaget C., Nagano J., Allen N., et al. Vegetable and fruit intake and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Stroke. 2003;34(10):2355–2360.
67 Steffen L.M., Jacobs D.R.Jr, Stevens J., et al. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78(3):383–390.
68 Joshipura K.J., Ascherio A., Manson J.E., et al. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282(13):1233–1239.
69 John J.H., Ziebland S., Yudkin P., et al. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomised controlled trial. Lancet. 2002;359(9322):1969–1974.
70 He F.J., Nowson C.A., MacGregor G.A. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006;367(9507):320–326.
71 He J., Ogden L.G., Vupputuri S., et al. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA. 1999;282(21):2027–2034.
72 Nagata C., Takatsuka N., Shimizu N., et al. Sodium intake and risk of death from stroke in Japanese men and women. Stroke. 2004;35(7):1543–1547.
73 Khaw K.T., Barrett-Connor E. Dietary potassium and stroke-associated mortality. A 12-year prospective population study. N Engl J Med. 1987;316(5):235–240.
74 Ascherio A., Rimm E.B., Hernan M.A., et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation. 1998;98(12):1198–1204.
75 Appel L.J., Moore T.J., Obarzanek E., et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124.
76 Fung T.T., Chiuve S.E., McCullough M.L., et al. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–720.
77 Fung T.T., Rexrode K.M., Mantzoros C.S., et al. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119(8):1093–1100.
78 Fung T.T., Stampfer M.J., Manson J.E., et al. Prospective study of major dietary patterns and stroke risk in women. Stroke. 2004;35(9):2014–2019.
79 Centers for Disease Control and Prevention. Sodium intake among adults – United States, 2005-2006. MMWR Morb Mortal Wkly Rep. 2010;59(24):746–749.
80 Ezzati M., Hoorn S.V., Lopez A.D., et al. Comparative quantification of mortality and burden of disease attributable to selected risk factors. In: Lopez A.D., Mathers C.D., Ezzati M., et al. Global burden of disease and risk factors. Washington DC: World Bank, 2006.
81 Abbott R.D., Rodriguez B.L., Burchfiel C.M., et al. Physical activity in older middle-aged men and reduced risk of stroke: the Honolulu Heart Program. Am J Epidemiol. 1994;139(9):881–893.
82 Kiely D.K., Wolf P.A., Cupples L.A., et al. Physical activity and stroke risk: the Framingham Study. Am J Epidemiol. 1994;140(7):608–620.
83 Haheim L.L., Holme I., Hjermann I., et al. Risk factors of stroke incidence and mortality. A 12-year follow-up of the Oslo Study. Stroke. 1993;24(10):1484–1489.
84 Gillum R.F., Mussolino M.E., Ingram D.D. Physical activity and stroke incidence in women and men. The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1996;143(9):860–869.
85 Wannamethee G., Shaper A.G. Physical activity and stroke in British middle aged men. BMJ. 1992;304(6827):597–601.
86 Sacco R.L., Gan R., Boden-Albala B., et al. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998;29(2):380–387.
87 Lindenstrom E., Boysen G., Nyboe J. Lifestyle factors and risk of cerebrovascular disease in women. The Copenhagen City Heart Study. Stroke. 1993;24(10):1468–1472.
88 Manson J.E., Colditz G.A., Stampfer M.J., et al. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med. 1991;151(6):1141–1147.
89 Blair S.N., Kampert J.B., Kohl H.W.3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210.
90 Kokkinos P.F., Holland J.C., Pittaras A.E., et al. Cardiorespiratory fitness and coronary heart disease risk factor association in women. J Am Coll Cardiol. 1995;26(2):358–364.
91 Lakka T.A., Salonen J.T. Moderate to high intensity conditioning leisure time physical activity and high cardiorespiratory fitness are associated with reduced plasma fibrinogen in eastern Finnish men. J Clin Epidemiol. 1993;46(10):1119–1127.
92 Williams P.T. High-density lipoprotein cholesterol and other risk factors for coronary heart disease in female runners. N Engl J Med. 1996;334(20):1298–1303.
93 Wang H.Y., Bashore T.R., Friedman E. Exercise reduces age-dependent decrease in platelet protein kinase C activity and translocation. J Gerontol A Biol Sci Med Sci. 1995;1(6):M12–M16.
94 Lee I.M., Hennekens C.H., Berger K., et al. Exercise and risk of stroke in male physicians. Stroke. 1999;30(1):1–6.
95 Pate R.R., Pratt M., Blair S.N., et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–407.
96 Physical activity and cardiovascular health. NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. JAMA. 1996;276(3):241–246.
97 Sattelmair J.R., Kurth T., Buring J.E., et al. Physical activity and risk of stroke in women. Stroke. 2010;41(6):1243–1250.
98 Wilson P.W., Bozeman S.R., Burton T.M., et al. Prediction of first events of coronary heart disease and stroke with consideration of adiposity. Circulation. 2008;118(2):124–130.
99 Rexrode K.M., Hennekens C.H., Willett W.C., et al. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA. 1997;277(19):1539–1545.
100 Burchfiel C.M., Curb J.D., Arakaki R., et al. Cardiovascular risk factors and hyperinsulinemia in elderly men: the Honolulu Heart Program. Ann Epidemiol. 1996;6(6):490–497.
101 Walker S.P., Rimm E.B., Ascherio A., et al. Body size and fat distribution as predictors of stroke among US men. Am J Epidemiol. 1996;144(12):1143–1150.
102 Hedley A.A., Ogden C.L., Johnson C.L., et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847–2850.
103 Brook R.D., Franklin B., Cascio W., et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation. 2004;109(21):2655–2671.
104 Brook R.D., Rajagopalan S., Pope C.A., et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378.
105 Miller K.A., Siscovick D.S., Sheppard L., et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–458.
106 Pope C.A.III, Burnett R.T., Thurston G.D., et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109(1):71–77.
107 Hong Y-C., Lee J-T., Kim H., et al. Effects of air pollutants on acute stroke mortality. Environ Health Perspect. 2002;110(2):187–191.
108 Hong Y-C., Lee J-T., Kim H., et al. Air pollution: a new risk factor in ischemic stroke mortality. Stroke. 2002;33(9):2165–2169.
109 Kan H., Jia J., Chen B. Acute stroke mortality and air pollution: new evidence from shanghai, China. J Occup Health. 2003;45(5):321–323.
110 Kettunen J., Lanki T., Tiittanen P., et al. Associations of fine and ultrafine particulate air pollution with stroke mortality in an area of low air pollution levels. Stroke. 2007;38(3):918–922.
111 Henrotin J.B., Besancenot J.P., Bejot Y., et al. Short-term effects of ozone air pollution on ischaemic stroke occurrence: a case-crossover analysis from a 10-year population-based study in Dijon, France. Occup Environ Med. 2007;64(7):439–445.
112 Lisabeth L.D., Escobar J.D., Dvonch J.T., et al. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann Neurol. 2008;64(1):53–59.
113 Wolf P., D’Agostino R., Belanger A., et al. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318.
114 Manolio T.A., Kronmal R.A., Burke G.L., et al. Short-term predictors of incident stroke in older adults: the Cardiovascular Health Study. Stroke. 1996;27(9):1479–1486.
115 Rodriguez B.L., D’Agostino R., Abbott R.D., et al. Risk of hospitalized stroke in men enrolled in the Honolulu Heart Program and the Framingham Study: a comparison of incidence and risk factor effects. Stroke. 2002;33(1):230–236.
116 O’Donnell M.J., Xavier D., Liu L., et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376(9735):112–123.
117 Goldstein L.B., Bushnell C.D., Adams R.J., et al. Guidelines for the primary prevention of stroke. A guideline for healthcare professionals From the American Heart Association/American Stroke Association. Stroke. 2011;42:517–584.
118 Feigin V.L., Rinkel G.J.E., Lawes C.M.M., et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. 2005;36(12):2773–2780.
119 Kurth T., Kase C.S., Berger K., et al. Smoking and risk of hemorrhagic stroke in women. Stroke. 2003;34(12):2792–2795.
120 Kurth T., Kase C.S., Berger K., et al. Smoking and the risk of hemorrhagic stroke in men. Stroke. 2003;34(5):1151–1155.
121 Feigin V., Parag V., Lawes C.M.M., et al. Smoking and elevated blood pressure are the most important risk factors for subarachnoid hemorrhage in the Asia-Pacific region: an overview of 26 cohorts involving 306, 620 participants. Stroke. 2005;36(7):1360–1365.
122 Feldmann E., Broderick J.P., Kernan W.N., et al. Major risk factors for intracerebral hemorrhage in the young are modifiable. Stroke. 2005;36(9):1881–1885.
123 Sturgeon J.D., Folsom A.R., Longstreth W.T.Jr, et al. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. 2007;38(10):2718–2725.
124 Shinton R., Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ. 1989;298(6676):789–794.
125 Ariesen M.J., Claus S.P., Rinkel G.J.E., et al. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34(8):2060–2065.
126 CDC. Smoking-attributable mortality, years of potential life lost, and productivity losses – United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226–1228.
127 Lee P.N., Forey B.A. Environmental tobacco smoke exposure and risk of stroke in nonsmokers: a review with meta-analysis. J Stroke Cerebrovasc Dis. 2006;15(5):190–201.
128 U.S. Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010.
129 Ambrose J.A., Barua R.S. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–1737.
130 Howard G., Thun M.J. Why is environmental tobacco smoke more strongly associated with coronary heart disease than expected? A review of potential biases and experimental data. Environ Health Perspect. 1999;107:853–858.
131 Kool M., Hoeks A., Struijker Boudier H., et al. Short- and long-term effects of smoking on arterial wall properties in habitual smokers. J Am Coll Cardiol. 1993;22(7):1881–1886.
132 Silvestrini M., Troisi E., Matteis M., et al. Effect of smoking on cerebrovascular reactivity. J Cereb Blood Flow Metab. 1996;16(4):746–749.
133 Nakamura K., Barzi F., Lam T-H., et al. Cigarette smoking, systolic blood pressure, and cardiovascular diseases in the Asia-Pacific region. Stroke. 2008;39(6):1694–1702.
134 Schwartz S.W., Carlucci C., Chambless L.E., et al. Synergism between smoking and vital exhaustion in the risk of ischemic stroke: evidence from the ARIC study. Ann Epidemiol. 2004;14(6):416–424.
135 WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348(9026):498–505.
136 WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Haemorrhagic stroke, overall stroke risk, and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348(9026):505–510.
137 Kawachi I., Colditz G.A., Stampfer M.J., et al. Smoking cessation and decreased risk of stroke in women. JAMA. 1993;269(2):232–236.
138 Wannamethee S.G., Shaper A.G., Whincup P.H., et al. Smoking cessation and the risk of stroke in middle-aged men. JAMA. 1995;274(2):155–160.
139 Wolf P.A., D’Agostino R.B., Kannel W.B., et al. Cigarette smoking as a risk factor for stroke. JAMA. 1988;259(7):1025–1029.
140 Abbott R.D., Yin Y., Reed D.M., et al. Risk of stroke in male cigarette smokers. N Engl J Med. 1986;315(12):717–720.
141 Shinton R. Lifelong exposures and the potential for stroke prevention: the contribution of cigarette smoking, exercise, and body fat. J Epidemiol Community Health. 1997;51(2):138–143.
142 Donahue R.P., Abbott R.D., Reed D.M., et al. Alcohol and hemorrhagic stroke. The Honolulu Heart Program. JAMA. 1986;255(17):2311–2314.
143 Mukamal K.J., Ascherio A., Mittleman M.A., et al. Alcohol and risk for ischemic stroke in men: the role of drinking patterns and usual beverage. Ann Intern Med. 2005;142(1):11–19.
144 Sacco R.L., Elkind M., Boden-Albala B., et al. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281(1):53–60.
145 Hillbom M., Numminen H., Juvela S. Recent heavy drinking of alcohol and embolic stroke. Stroke. 1999;30(11):2307–2312.
146 Gill J.S., Zezulka A.V., Shipley M.J., et al. Stroke and alcohol consumption. N Engl J Med. 1986;315(17):1041–1046.
147 Stampfer M.J., Colditz G.A., Willett W.C., et al. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N Engl J Med. 1988;319(5):267–273.
148 Berger K., Ajani U.A., Kase C.S., et al. Light-to-moderate alcohol consumption and risk of stroke among U.S. male physicians. N Engl J Med. 1999;341(21):1557–1564.
149 Iso H., Baba S., Mannami T., et al. Alcohol consumption and risk of stroke among middle-aged men: the JPHC Study Cohort I. Stroke. 2004;35(5):1124–1129.
150 Malarcher A.M., Giles W.H., Croft J.B., et al. Alcohol intake, type of beverage, and the risk of cerebral infarction in young women. Stroke. 2001;32(1):77–83.
151 Reynolds K., Lewis B., Nolen J.D., et al. Alcohol consumption and risk of stroke: a meta-analysis. JAMA. 2003;289(5):579–588.
152 Anonymous. Report of the Dietary Guidelines Advisory Committee Dietary Guidelines for Americans, 1995. Nutr Rev. 1995;53(12):376–379.
153 Fields L.E., Burt V.L., Cutler J.A., et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44(4):398–404.
154 Chobanian A.V., Bakris G.L., Black H.R., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572.
155 Law M., Wald N., Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess. 2003;7(31):1–94.
156 Neter J.E., Stam B.E., Kok F.J., et al. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42(5):878–884.
157 Psaty B.M., Smith N.L., Siscovick D.S., et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA. 1997;277(9):739–745.
158 Neal B., MacMahon S., Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet. 2000;356(9246):1955–1964.
159 Webb AJ, Fischer U, Mehta Z, et al: Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis, Lancet 13;375(9718):906–915.
160 Amarenco P., Lavallée P., Touboul P-J. Stroke prevention, blood cholesterol, and statins. Lancet Neurol. 2004;3(5):271–278.
161 Prospective Studies Collaboration. Cholesterol, diastolic blood pressure, and stroke: 13,000 strokes in 450,000 people in 45 prospective cohorts. Lancet. 1995;346:1647–1653.
162 Prospective Studies Collaboration. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370(9602):1829–1839.
163 Brewer H.B. Increasing HDL cholesterol levels. N Engl J Med. 2004;350(15):1491–1494.
164 Sanossian N., Saver J.L., Navab M., et al. High-density lipoprotein cholesterol: an emerging target for stroke treatment. Stroke. 2007;38(3):1104–1109.
165 Curb J.D., Abbott R.D., Rodriguez B.L., et al. High density lipoprotein cholesterol and the risk of stroke in elderly men. Am J Epidemiol. 2004;160(2):150–157.
166 Soyama Y., Miura K., Morikawa Y., et al. High-density lipoprotein cholesterol and risk of stroke in Japanese men and women: the Oyabe Study. Stroke. 2003;34(4):863–868.
167 Simons L.A., McCallum J., Friedlander Y., et al. Risk factors for ischemic stroke: Dubbo Study of the Elderly. Stroke. 1998;29(7):1341–1346.
168 Lindenstrom E., Boysen G., Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: the Copenhagen city heart study. BMJ. 1994;309(6946):11–15.
169 Tanne D., Yaari S., Goldbourt U. High-density lipoprotein cholesterol and risk of ischemic stroke mortality: a 21-year follow-up of 8586 men from the Israeli Ischemic Heart Disease Study. Stroke. 1997;28(1):83–87.
170 Wannamethee S.G., Shaper A.G., Ebrahim S. HDL-cholesterol, total cholesterol, and the risk of stroke in middle-aged British men. Stroke. 2000;31(8):1882–1888.
171 Shahar E., Chambless L.E., Rosamond W.D., et al. Plasma lipid profile and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2003;34(3):623–631.
172 Sacco R.L., Benson R.T., Kargman D.E., et al. High-density lipoprotein cholesterol and ischemic stroke in the elderly. JAMA. 2001;285(21):2729–2735.
173 Tirschwell D.L., Smith N.L., Heckbert S.R., et al. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63(10):1868–1875.
174 Johnsen S.H., Mathiesen E.B., Fosse E., et al. Elevated high-density lipoprotein cholesterol levels are protective against plaque progression: a follow-up study of 1952 persons with carotid Atherosclerosis The Tromso Study. Circulation. 2005;112(4):498–504.
175 Asia Pacific Cohort Studies Collaboration. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation. 2004;110(17):2678–2686.
176 Freiberg J.J., Tybjærg-Hansen A., Jensen J.S., et al. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300(18):2142–2152.
177 Bansal S., Buring J.E., Rifai N., et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–316.
178 Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278.
179 Amarenco P., Labreuche J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009;8(5):453–463.
180 CDC: National diabetes fact sheet, 2007. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed December 22, 2010.
181 Furie K.L., Kasner S.E., Adams R.J., et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227–276.
182 The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222.
183 Mankovsky B.N., Ziegler D. Stroke in patients with diabetes mellitus. Diabetes Metab Res Rev. 2004;20(4):268–287.
184 Meschia J.F., Merrill P., Soliman E.Z., et al. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Stroke. 2010;41(4):581–587.
185 Hart R.G., Pearce L.A., Albers G.W., et al. Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. 2007;69:546–554.
186 Hart R.G., Pearce L.A., Halperin J.L., et al. Comparison of 12 risk stratification schemes to predict stroke in patients with nonvalvular atrial fibrillation. Stroke. 2008;39(6):1901–1910.
187 Gage B.F., Waterman A.D., Shannon W., et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870.
188 Ohene-Frempong K., Weiner S.J., Sleeper L.A., et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–294.
189 Adams R.J., McKie V.C., Carl E.M., et al. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann Neurol. 1997;42:699–704.
190 Adams R.J., McKie V.C., Hsu L., et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11.
191 Bagai K. Obstructive sleep apnea, stroke, and cardiovascular diseases. Neurologist. 2010;16(6):329–339.
192 Budhiraja R., Budhiraja P., Quan S.F. Sleep-disordered breathing and cardiovascular disorders. Respir Care. 2010;55(10):1322–1330.
193 Dyken M.E., Im K.B. Obstructive sleep apnea and stroke. Chest. 2009;136(6):1668–1677.
194 Hermann D.M., Bassetti C.L. Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology. 2009;73(16):1313–1322.
195 Johnson K.G., Johnson D.C. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6(2):131–137.
196 Somers V.K., White D.P., Amin R., et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Circulation. 2008;118(10):1080–1111.
197 Yaggi H., Mohsenin V. Sleep apnea and stroke: a risk factor or an association? Sleep Med Clin. 2007;2(4):583–591.
198 Libby P., Ridker P.M. Inflammation and atherothrombosis: from population biology and bench research to clinical practice. J Am Coll Cardiol. 2006;48(9_Suppl_A):A33–A46.
199 The Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–140.
200 Pearson T.A., Mensah G.A., Alexander R.W., et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511.
201 Fibrinogen Studies Collaboration. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality. JAMA. 2005;294(14):1799–1809.
202 Welch G.N., Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–1050.
203 Selhub J., Jacques P.F., Wilson P.W.F., et al. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993;270(22):2693–2698.
204 Dierkes J., Westphal S., Luley C. The effect of fibrates and other lipid-lowering drugs on plasma homocysteine levels. Expert Opin Drug Saf. 2004;3(2):101–111.
205 Rosenson R.S. Antiatherothrombotic effects of nicotinic acid. Atherosclerosis. 2003;171(1):87–96.
206 Bazzano L.A., Jiang H., Munter P., et al. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med. 2003;138(11):891.
207 Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke. JAMA. 2002;288(16):2015–2022.
208 Wald D.S., Law M., Morris J.K. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202.
209 Casas J.P., Bautista L.E., Smeeth L., et al. Homocysteine and stroke: evidence on a causal link from mendelian randomisation. Lancet. 2005;365(9455):224–232.
210 Boysen G., Brander T., Christensen H., et al. Homocysteine and risk of recurrent stroke. Stroke. 2003;34(5):1258–1261.
211 Iso H., Moriyama Y., Sato S., et al. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. 2004;109(22):2766–2772.
212 Kelly P.J., Rosand J., Kistler J.P., et al. Homocysteine, MTHFR 677C→T polymorphism, and risk of ischemic stroke. Neurology. 2002;59(4):529–536.
213 Kim N.K., Choi B.O., Jung W.S., et al. Hyperhomocysteinemia as an independent risk factor for silent brain infarction. Neurology. 2003;61(11):1595–1599.
214 Li Z., Sun L., Zhang H., et al. Elevated plasma homocysteine was associated with hemorrhagic and ischemic stroke, but methylenetetrahydrofolate reductase gene C677T polymorphism was a risk factor for thrombotic stroke: a multicenter case-control study in China. Stroke. 2003;34(9):2085–2090.
215 McIlroy S.P., Dynan K.B., Lawson J.T., et al. Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke. 2002;33(10):2351–2356.
216 Tanne D., Haim M., Goldbourt U., et al. Prospective study of serum homocysteine and risk of ischemic stroke among patients with preexisting coronary heart disease. Stroke. 2003;34(3):632–636.
217 The VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010;9(9):855–865.
218 Etminan M., Takkouche B., Isorna F.C.o., et al. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63.
219 Schürks M., Rist P.M., Bigal M.E., et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009:339.
220 Kruit M.C., Launer L.J., Ferrari M.D., et al. Infarcts in the posterior circulation territory in migraine. The population-based MRI CAMERA study. Brain. 2005;128(9):2068–2077.
221 Kruit M.C., van Buchem M.A., Hofman P.A.M., et al. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291(4):427–434.
222 Scher A.I., Gudmundsson L.S., Sigurdsson S., et al. Migraine headache in middle age and late-life brain infarcts. JAMA. 2009;301(24):2563–2570.
223 Swartz R.H., Kern R.Z. Migraine is associated with magnetic resonance imaging white matter abnormalities: a meta-analysis. Arch Neurol. 2004;61(9):1366–1368.
224 Awareness of stroke warning symptoms–change to N dash. 13 States and the District of Columbia, 2005. MMWR Morb Mortal Wkly Rep. 2008;57(18):481–485.
225 Kleindorfer D., Khoury J., Broderick J.P., et al. Temporal trends in public awareness of stroke: warning signs, risk factors, and treatment. Stroke. 2009;40(7):2502–2506.
226 Kleindorfer D., Lindsell C.J., Moomaw C.J., et al. Which stroke symptoms prompt a 911 call? A population-based study. Am J Emerg Med. 2010;28(5):607–612.
227 Kleindorfer D., Lindsell C.J., Brass L., et al. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke. 2008;39(3):924–928.