CHAPTER 106 Epidemiology of Brain Tumors*
Descriptive Epidemiology
Brain tumor incidence rates have increased over time, with the rate of increase depending on histologic subtype, sex, age, race, and geographic area. For all central nervous system (CNS) tumors, of which brain tumors represent approximately 88%, the age-adjusted average annual (2000 to 2004) incidence rate for females (17.2 per 100,000) is greater than that for males (15.8 per 100,000).1 Table 106-1 shows the age-adjusted average annual (2000 to 2004) incidence rates and median ages at diagnosis for the major histologic groupings and their selected common histologic subtypes of brain tumors.
TABLE 106-1 Number of Cases, Median Ages at Diagnosis, and Age-Adjusted, Average Annual (2000-2004) Incidence Rates* of Primary Brain Tumors (Major Histologic Groupings and Selected Histologic Subtypes) According to Sex
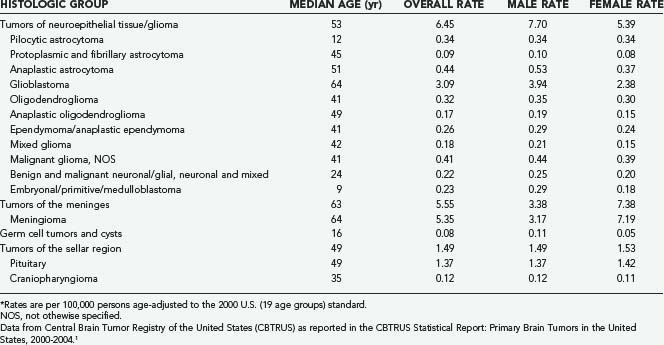
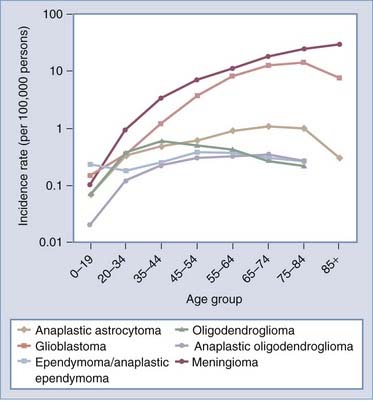
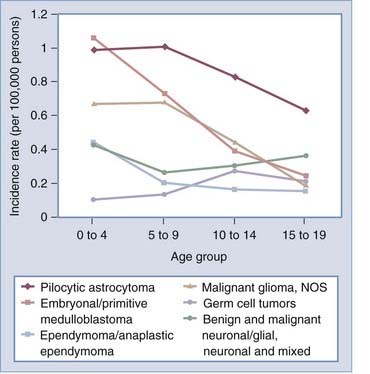
Glioma, which consists of a number of histologic subtypes, and meningioma have age-adjusted average annual incidence rates of 6.45 and 5.55 per 100,000 population, respectively.1,2 As shown in Table 106-1, men have higher incidences rates of glioma, germ cell tumors, and cysts, whereas women have higher meningioma incidence rates. In the United States, the median age at diagnosis of a primary brain or CNS tumor between 2000 and 2004 was 57 years.1 For all major histologic groups except germ cell tumors and cysts, incidence rates increase with age. Average annual incidence rates by age at diagnosis for selected histologic types common in adults and children/adolescents (aged 0 to 19 years) are shown in Figures 106-1 and 106-2, respectively. In adults (Fig. 106-1), incidence rates of meningioma and glioblastoma increase with advancing age, except for a decline in the incidence rate of glioblastoma in those 85 years and older. In children/adolescents (Fig. 106-2), incidence rates of all non–germ cell histologic types decrease throughout childhood and adolescence, whereas the incidence of germ cell tumors reaches a peak during the adolescent years.
Survival Time from Diagnosis of Glioma and Meningioma
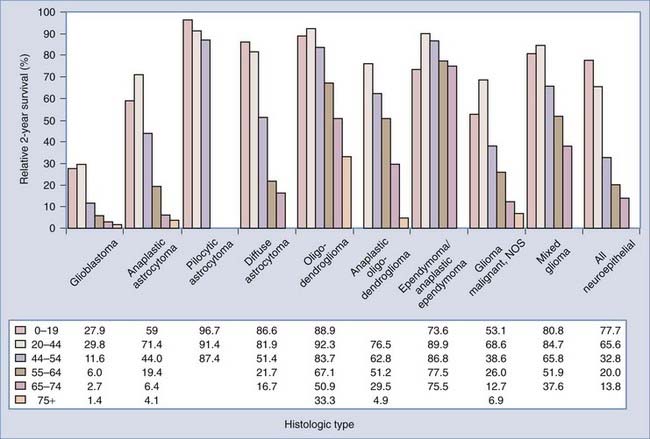
Survival time varies greatly by histologic type and age at diagnosis, as shown in Figure 106-3. For each age group, relative 2-year survival is lowest for patients with glioblastoma. In general, within histologic types survival time decreases with age. The mechanisms for the strong, consistent inverse association between age and survival are poorly understood and deserve further exploration. In patients in whom primary malignant brain tumors were diagnosed between 2000 and 2005, only 39.1% and 31.0% will be alive 2 and 5 years, respectively, from the time of diagnosis.3 Although the prognosis is poor for most patients with malignant brain tumors, 2-year survival rates increased from 28.6% in 1975 to 38.2% in 2004.3 The largest improvements in survival occurred in patients younger than 65 years in whom tumors other than anaplastic astrocytoma and glioblastoma were diagnosed. This may be attributed not necessarily to improvements in treatment but to improvements in imaging technology that allow earlier identification of tumors. There has been little change in the poor survival of patients with glioblastoma. Because glioblastoma appears to have only a brief preclinical period, improvements in survival will probably be attained only with technologies outside the current treatment paradigm, which includes surgery, radiation therapy, and alkylating chemotherapy. The concomitant addition of and maintenance with the chemotherapeutic agent temozolomide (Temodar) has improved the median survival time for glioblastoma patients by 2.5 months.4
Population-based data from Norway and Finland suggest that survival of patients with meningioma also improved between the 1950s and 1990s,5,6 and it is possible that this improvement is likewise associated with advances in imaging technology. McCarthy and colleagues estimated a 69% 5-year survival rate for meningioma, with those younger at diagnosis having a more favorable prognosis (5-year survival probabilities were 81% versus 56% for patients in whom meningioma was diagnosed before versus after 65 years of age).7 Survival from meningioma is generally poorer for patients with malignant versus benign histology, with a 5-year survival probability of 55% versus 70%7; however, the vast majority (96%) of meningiomas are not malignant.
Environmental Risk Factors
Ionizing Radiation
Sources of exposure to ionizing radiation include therapeutic and diagnostic medical procedures, occupation, atmospheric testing of nuclear weapons, and proximity to atomic bomb explosions in Japan. Survivors of the bombing of Hiroshima have elevated incidence rates of meningioma that increase with the estimated dose of radiation to the brain.8 These survivors also have higher incidence rates of glioma, schwannoma, and pituitary tumors, although there is no increased risk for brain tumors in survivors who were exposed in utero.9 The use of ionizing radiation to treat tinea capitis and skin hemangioma in infants and children has been associated with significantly elevated relative risks for nerve sheath tumors, pituitary adenoma, meningioma, and glioma.9,10 However, there are mixed results from studies involving other sources of exposure to diagnostic and therapeutic irradiation of the head and neck.11,12 One study found that radiographs performed 15 to 40 years preceding diagnosis appeared to increase the risk for meningioma,13 and another study found meningioma risk to be associated with radiographs before the age of 20 years or taken before the year 1945.14 Further evidence possibly supporting an effect of ionizing radiation on brain tumor risk includes the observation that second primary brain tumors occur more frequently than expected in patients previously treated for brain tumors with radiation therapy; the standardized incidence ratio for second CNS tumors in brain tumor patients treated by surgery alone is 2.0 (95% confidence interval [CI], 1.2 to 3.2) versus 5.1 (95% CI, 2.5 to 9.4) for patients treated by radiotherapy with or without surgery or chemotherapy, or both.15 It is also possible that these results can be attributed to the fact that people with higher grade tumors are more likely to both receive radiation therapy and have a recurrence. Results from case-control studies of exposure to ionizing radiation and glioma risk are varied, perhaps because of underreporting of exposure, imprecise estimates of age at first exposure, or a low prevalence of exposure to high doses of ionizing radiation.16 The consistent and strong results from prospective studies of people exposed to ionizing radiation provide unquestionable evidence of a linear dose-response association between ionizing radiation exposure and glioma risk.16 Future studies should consider the potential for interaction between ionizing radiation and both age at exposure and genetic variation that may mediate the exposure. Regardless of the strong evidence for an association between ionizing radiation and brain tumors, therapeutic doses of ionizing radiation probably contribute to the development of only a small proportion of brain tumors because exposure to therapeutic levels of ionizing radiation is rare and the vast majority of glioma and meningioma patients report no such exposure; in one study, between 1% and 3% of glioma and meningioma patients, as well as controls, reported a history of at least one therapeutic dose of ionizing radiation before diagnosis of their brain tumor.17 Elucidating a possible role of more common radiation exposure, such as that resulting from dental radiographs, will require reliable assessment of exposure.
Cellular Telephone Use
Public concern over the potential health effects of cell phones has prompted studies focused on exposure to radiofrequency fields and brain tumor risk. In general, risk for meningioma does not appear to be increased as a result of cell phone use18–20; however, there is some limited evidence that use of an analog cell phone,21 especially of longer duration (>10 years),22 may increase meningioma risk (although exposure to analog cell phones has greatly diminished because they have largely been replaced by digital cell phones). Mixed results have been reported for association between cell phone use and acoustic neuroma risk,21–26 yet relative risks from studies of more than one histologic type of brain tumor have been greater for acoustic neuroma than for meningioma and glioma, especially when cell phone use is on the same side of the head as the tumor (ipsilateral).25
A relatively large number of epidemiologic studies of cell phone use and glioma risk have been conducted. Results from these studies have suggested that short-term cell phone use is probably not associated with risk for glioma.18–20,22,27–35 There are limited data and inconsistent results pertaining to long-term use and glioma risk,18–20,22,28–31,34,36 with the most compelling results suggesting evidence of increased glioma risk as a result of ipsilateral cell phone use.19,22,29,31,34 However, these results have probably been affected by small sample size and selection and recall bias.20,37 Some of the increased risk resulting from ipsilateral cell phone use may be attributable to recall bias because contralateral cell phone use appears to reduce risk; in the absence of recall bias, one would not expect cell phone use to decrease risk. The largest population-based case-control study reported to date (1522 glioma cases and 3301 controls), conducted in five Nordic countries and the United Kingdom, found no consistent evidence overall of increased risk for glioma related to the use of cell phones, nor was increased glioma risk found in the most highly exposed group.24 Thus far, no study has demonstrated irrefutable evidence of an association between long-term cell phone use and increased glioma risk. However, if the latency period is at least 5 years long, earlier studies lacked sufficient numbers of long-term cell phone users to adequately evaluate the relationship. The association of glioma risk with long-term cell phone use has not yet been convincingly demonstrated but will continue to be examined in the context of more refined studies with greater statistical power because of the increasing number of people who are long-term cell phone users and the potential release of individual records by cell phone companies for better assessment of exposure.
Risk and Preventive Factors for Which Evidence Is Inconclusive
Numerous dietary, experiential, and environmental factors studied in relation to brain tumor risk have shown inconsistent associations, that is, one or more positive studies but some with no association found. Such factors include head injury and trauma,38–43 dietary calcium intake (for glioma),44,45 dietary intake of N-nitroso compounds (for glioma and meningioma),46–49 dietary antioxidant intake (for glioma),44,46,47,49 dietary maternal intake of N-nitroso compounds (for childhood brain tumors),38,43 dietary maternal and early life intake of antioxidants (for childhood brain tumors), maternal folate supplementation (for primitive neuroectodermal tumors),38,50 tobacco smoking (for glioma and meningioma),38,47,51 alcohol consumption (for glioma, meningioma, and childhood brain tumors),9,52 and exposure to electromagnetic fields (for childhood and adult brain tumors).38 In addition to small study sample sizes, possible explanations for the inconsistent findings include invalid or imprecise measurements of exposure (resulting from the use of self-reported or proxy-reported exposure and from lack of validation of the exposure), unfocused hypotheses (resulting from studies of large numbers of exposures without a specific rationale for examining many exposures or from studies in which relationships between exposure and brain tumor risk are examined without respect to known confounding or modifying factors), inherited or developmental variations in metabolic or DNA repair pathways that modify the effect of environmental factors on brain tumor risk, and unaccounted-for protective environmental exposures or conditions (e.g., allergy). The levels generally encountered for some examined exposures (e.g., chemical compounds) may also often be too low to have a measurable impact on brain tumor risk. Continued progress in understanding risk factors is dependent on the construction of large studies with better assessment of exposure, along with analysis of genetic factors that influence the effects of such exposure.
Reproductive and Menstrual Factors
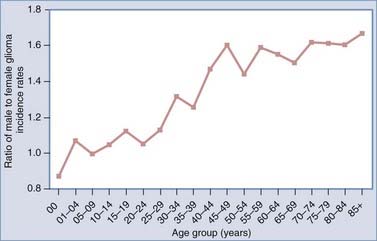
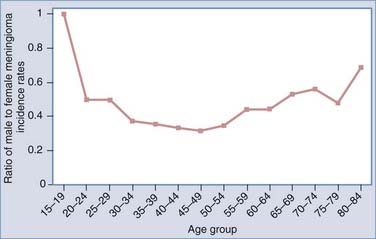
In part because females have a lower risk for glioma and a greater risk for meningioma, investigators have examined factors associated with endogenous and exogenous female hormonal status as potentially being related to brain tumor risk, including menopausal status, ages at menarche and menopause, parity, and use of oral contraceptives and hormone replacement therapy (HRT). Estrogen and other female hormone levels are greater in general and without respect to exogenous estrogen sources in women between the ages of menarche and menopause; therefore, investigators have examined age- and sex-specific glioma and meningioma incidence rates to look for patterns in rates potentially related to these hormones. One study suggested that the gender differential in glioma rates occurred primarily from menarche to menopause and decreased in postmenopausal age groups,53 whereas another showed that postmenopausal women had an increased risk for glioma and acoustic neuroma.54 However, the latter notion is not fully supported by Figure 106-4, which shows that glioma incidence rates derived from the Surveillance, Epidemiology, and End Results (SEER) program are greater in males within each age group and that the ratio of male-to-female incidence rates increases with advancing age and reaches a plateau after the age of 40, with males having at least a 40% greater risk than females for all age groups 40 years and older. For meningioma, in general, there are consistent results suggesting greater risk in women during their reproductive years,5,54,55 and these results are supported by Figure 106-5, which shows that SEER meningioma incidence rates are greater in females (indicated by lower male-to-female incidence rate ratios) and the greatest differential occurs during the approximate ages of 30 to 54 years.
There is no consistent or convincing evidence that parity is associated with risk for either glioma54,56–59 or meningioma.54,60 Two recent studies have suggested a possible increase in glioma risk as a result of later (14 years or older versus younger than 12 years) age at menarche,57,59 but more studies are needed to confirm this relationship. Furthermore, inconsistent results pertaining to oral contraceptive use and HRT have been reported for both glioma55,57,59 and meningioma55,57 risk. Results from a population-based case-control study conducted by Wigertz and coworkers revealed elevated meningioma risk in women who had used long-acting hormonal contraceptives (odds ratio [OR] for at least 10 years of use, 2.7; 95% CI, 0.9 to 7.5) and postmenopausal women who had ever received HRT (OR, 1.7; 95% CI, 1.0 to 2.8).55 These authors also reported that the use of oral contraceptives and HRT had no impact on glioma risk.55 Female hormones probably play a role in the development of some meningiomas, which may partially explain why women have lower glioma incidence rates, but our understanding of the mechanisms governing their role is limited, perhaps in part because the menstrual and reproductive factors that have been examined are insufficient to accurately characterize lifetime estrogen or other hormonal exposure.
Glioma, Allergic Conditions, Infections, and Associated Immunologic Factors
Since 1990, the results of 10 case-control and 1 of 2 cohort studies have shown that self-reported allergies are inversely related to glioma risk. Linos and associates conducted a formal meta-analysis of a subset of these studies and concluded that the strong inverse association between self-reported allergies and glioma (OR, 0.61; 95% CI, 0.55 to 0.67) is probably not attributable to methodologic bias alone.61 Further evidence of this inverse association was contributed by Wiemels and coworkers, who found that total IgE levels were lower in glioma patients than in controls.62
Although mechanisms governing potential protection have not been identified, they may arise from the anti-inflammatory effects of interleukin-4 (IL- 4) and IL-13 cytokines involved in allergic and autoimmune disease63 or from increased tumor immunosurveillance in those with allergies and autoimmune disease.64 It is also possible that the inverse association results from immune suppression by the preclinical tumor.
In addition to their role in allergic conditions, IL-4 and IL-13 cytokines also inhibit the growth of glioma cell lines. Although IL-4 is not expressed in the normal adult brain, it is strongly expressed during brain injury,65 where invading T cells may be a source of this cytokine.66 Barna and associates found that three normal astrocytic, two low-grade astrocytoma, and three of four glioblastoma cell lines that they evaluated expressed IL-4Rα receptors.66 However, IL-4 suppresses DNA synthesis and cell proliferation only in the normal astrocytic and low-grade astrocytoma cell lines but not in the glioblastoma cell lines. Their results suggest that IL-4 could play a role in the inhibition of glioblastomas that arise from astrocytomas but may not be involved in glioblastomas that arise from other pathways.67 In support of a role for IL-4 in glioma pathology, Faber and colleagues reported that IL-4 increases the number of T-cell precursors in glioblastoma patients.68 Saleh and associates attributed the growth-inhibiting properties of mouse IL-4 on implanted C6 glioma cell lines to its ability to promote eosinophil infiltration and inhibit angiogenesis.69 Furthermore, they observed that C6 cell gliomas implanted in rats that produce retroviral IL-4 are rapidly eradicated.70
In view of a possible role of IL-4 and IL-13 in both allergic conditions and glioma, Schwartzbaum and coworkers identified polymorphisms of the IL-4Rα and IL-13 genes that increase risk for allergic conditions.71 Although these germline genetic variants are not sensitive indicators of the presence of allergic conditions, they do provide a measure of risk for these conditions free of recall bias. The working hypothesis was that in individuals with IL-4Rα or IL-13 polymorphisms that increase risk for allergic conditions, glioblastoma risk would be decreased. Using data from a small case-control study (111 glioblastoma cases, 422 controls), the authors found results consistent with their hypothesis. Each of the two IL-4Rα and IL-13 single nucleotide polymorphisms (SNPs) associated with increased risk for allergic conditions were also related to decreased glioblastoma risk. Wiemels and coauthors confirmed their finding for one of the IL-13 SNPs in a larger case-control study of glioma (456 glioma cases, 541 controls).72 Furthermore, they reported that this IL-13 SNP was inversely associated with IgE levels in controls (P = .04). However, they did not find associations between IL-4Rα SNPs and glioma as had Schwartzbaum and associates, but they did see a borderline association between an IL-4Rα haplotype (OR, 1.5; 95% CI, 1.0 to 2.3) and glioma. They also found that a rare IL-4 haplotype was associated with decreased glioma risk (OR, 0.23; 95% CI, 0.07 to 0.83).
A larger study of the original four IL-4Rα and IL-13 genetic variants by Schwartzbaum and colleagues did not provide strong support for their original observations. Nonetheless, they found an IL-4Rα haplotype associated with glioblastoma (OR, 2.26; 95% CI, 1.13 to 4.52) and inversely related to self-report of hayfever or asthma in controls (OR, 0.39; 95% CI, 0.16 to 0.98).73 Although Wiemels and associates also found suggestive evidence of an association between an IL-4Rα haplotype and glioma, when they restricted their haplotype to the same IL-4Rα SNPs that Schwartzbaum and coworkers examined, they observed no evidence of an association with glioma (OR, 1.13; 95% CI, 0.83 to 1.53).
The tumor itself may also have mechanisms that inhibit the ability of the immune system to eradicate it. In recent in vitro studies of glioma, human glioma cell lines were found to secrete immunosuppressive cytokines that can selectively recruit regulatory T cells into the tumor microenvironment.74 In addition, Chahlavi and associates demonstrated that glioma cell lines mediate immunosuppression by promoting T-cell death through tumor-associated antigens and gangliosides.75 Two of the major immunosuppressive cytokines that are present in both the glioma microenvironment and peripheral blood of glioma patients, IL-10 and transforming growth factor-β, induce immune tolerance and thereby inhibit allergy and asthma.76 Elevated IgE concentrations may therefore indicate low levels of immunosuppression and the resulting ability to conduct antitumor immunosurveillance against incipient glioma. Alternatively, the relative absence of allergies in glioma patients may show merely that these tumor-induced cytokines have suppressed the immune system.
Reduced glioma risk has also been attributed to a reported history of varicella-zoster virus (VZV) infections and positive IgG to VZV.77–79 Papovaviruses including simian virus 40 [SV40], JC and BK viruses, adenoviruses, retroviruses, herpes and influenza viruses, and parasitic infections (Toxoplasma gondii) have also been investigated in relation to the genesis of glioma in experimental animals and in limited epidemiologic studies; however, the potential risk from these agents has generally been inadequately addressed in epidemiologic studies.80,81 With relative consistency, results from two case-control series suggest that previous clinical disease associated with VZV infection and anti-VZV IgG levels may be inversely associated with adult glioma risk.77–79 It might be the specific nature of the immune system’s response to antigens and not exposure to the antigen per se that is responsible for this inverse association with glioma.62
At present, there is no strong epidemiologic evidence suggesting that human cytomegalovirus (HCMV) plays a role in the development of glioma. However, HCMV nucleic acids and proteins have been found in the tumors of glioblastoma patients, HCMV DNA has also been found in the peripheral blood of glioblastoma patients,82 and Scheurer and coauthors reported detection of HCMV infection in 21 of 21 patients with glioblastoma.83 However, Wrensch and colleagues79 and Poltermann and associates84 reported that antibody positivity to HCMV in glioma patients was not different from that in controls and the general population, respectively. The presence of HCMV gene products in blood or tumor tissue may result from reactivation of infection or from infected tumor cells shedding viral DNA.82 Renewed interest in HCMV may spark new epidemiologic studies, and these studies should consider the potential importance of low-level infection, which requires stringent technical conditions to adequately detect such infection.83
With respect to SV40, between 1955 and 1963 an unknown proportion of all inactivated and live polio vaccines distributed were contaminated with SV40.85 In Germany, where children were monitored over a 20-year period, those inoculated with the polio vaccine contaminated with SV40 had a higher incidence of glioblastoma, medulloblastoma, and some less common brain tumor types than did children not given the contaminated vaccine.86 In the United States, no difference in brain tumor risk was found for glioma or meningioma between the two groups of children,87 but one study reported that the incidence of ependymoma was 37% greater in children receiving the contaminated vaccine.85 Results pertaining to infections should be validated by studies in which serologic measurement of viral or bacterial exposure is ascertained before the development of brain tumors and in which there is serologic or symptom-based confirmation of infection.
Results pertaining to human leukocyte antigens (HLAs)—cell surface molecules that modulate immune responses, in part by presenting antigenic peptides to T lymphocytes—also suggest the importance of immunologic responses in glioma development. Tang and colleagues showed that glioblastoma is positively associated with the HLA genotype B*13 and the HLA haplotype B*07-Cw*07 (P = .01 and P < .001, respectively) and is inversely associated with the genotype Cw*01.88 Interestingly, if confirmed, these results could partially explain the increased glioblastoma incidence in whites because B*07 and B*07-Cw*07 are more common in whites than nonwhites. Guerini and associates compared a small group of glioma patients in northern Italy with control organ donors from the same region and demonstrated a positive association between HLA-DRB1*14 and the presence of symptomatic cerebral glioma.89 Facoetti and coworkers found that HLA class I antigens were lost in approximately half of glioblastoma tumors but in only 20% of grade 2 astrocytoma tumors, selective HLA-A2 antigen loss was observed in approximately 80% of glioblastoma lesions and half of the grade 2 astrocytoma tumors, and HLA class I antigen loss was significantly (P < .025) correlated with tumor grade.90 Studies of HLA may contribute to our understanding of the immune escape mechanisms used by glioma because HLA antigens mediate interactions of tumor cells with the host immune response; furthermore, HLA antigen defects in astrocytoma brain tumors may explain the relatively poor clinical response rates observed in the majority of the T-cell–based immunotherapy clinical trials.90
Genetic Factors
Glioma and meningioma are thought to develop through the progressive accumulation of genetic and epigenetic alterations that permit cells to evade normal regulatory mechanisms and escape destruction by the immune system.2,80,81 Diseases or syndromes associated with rare mutations in highly penetrant genes (including neurofibromatosis types 1 and 2 [NF1, NF2], tuberous sclerosis, retinoblastoma, Li-Fraumeni cancer family syndrome, and Turcot’s syndrome) are known to increase risk for glioma.43,91 However, in a study of 500 glioma patients, less than 1% had a known hereditary syndrome.92 Although familial aggregation of brain tumors, especially glioma, has been demonstrated, it can be difficult to distinguish shared environmental exposure from inherited characteristics. Grossman and coworkers showed that brain tumors occur frequently in families with no known predisposing hereditary disease and that the pattern of occurrence in many families suggests environmental causes.93 Results presented by Malmer and associates suggest that first-degree relatives, not spouses, have a significantly increased risk for brain tumors.94
Genetic Factors and Glioma Risk
Studies of polymorphic variation in detoxification, DNA stability and repair, and cell cycle regulation have produced some persuasive results with respect to risk for cancer at some sites that have framed new hypotheses and propelled new lines of inquiry concerning the causes of brain tumors. Cytochrome P-450 (CYP) and glutathione-S-transferase (GST) are involved in the metabolism of many electrophilic compounds, including carcinogens, mutagens, cytotoxic drugs, metabolites, and products of reactive oxidation. For brain tumors, studies of CYP and GST have produced mixed results. Results from a recent meta-analysis of eight studies that included 1630 glioma patients, 245 meningioma patients, and 7151 controls suggest that although the T1 null genotype is significantly associated with nearly double the risk for meningioma, there are no associations between any of the GSTP1 105 and GSTP1 114 SNPs and glioma risk; however, none of the authors whose work was summarized had conducted haplotype analysis. Wrensch and colleagues found little evidence of a general association of GST polymorphisms with glioma but did show an association of GSTT1 deletion and glioma with p53 mutations.95 In a large Nordic and British population-based case-control study, Schwartzbaum and coauthors reported no associations between the GSTM3, GSTP1 NQ01, CYP1A1, GSTM1 or GSTT1 polymorphisms and adult brain tumor risk; however they found a weak association between the G-C (Val-Ala) GSTP1 105/114 haplotype and glioma.96
Because DNA repair is important in maintaining DNA integrity, inherited variation in components of DNA repair pathways has been extensively studied with respect to cancer. Associations with glioma have been reported for variants in ERCC1,97,98 ERCC2,98–100 the nearby gene glioma tumor suppressor candidate of unknown function (GLTSCR1),100 PRKDC (also known as XRCC7—a gene involved in nonhomologous end joining double-strand break repair),101 and MGMT (O6-methylguanine-DNA methyltransferase, a DNA repair enzyme),102,103 but there are too few studies to assess consistency. The AA or AC versus CC genotype in nucleotide 8092 of ERCC1 has been shown to increase risk for oligoastrocytoma,97 whereas the AA genotype (C-to-A polymorphism [R156R]) of ERCC2 was more prevalent than the CC or CA genotypes in patients with glioblastoma, astrocytoma, or oligoastrocytoma than in controls.99 The ERCC2–exon 22 T-allele prevalence was 35% in a group of oligodendroglioma patients and 18% in controls, and alterations in GLTSCR1 (or a closely linked gene) have been associated with risk for oligodendroglioma.100 Wang and associates found that the TT genotype of XRCC7 was more common in glioma patients than in controls.101 Regarding the MGMT gene, results presented by Wiencke and coworkers suggest that an inherited factor involving the repair of methylation and other alkylation damage may be associated with the development of gliomas that have neither TP53 mutations nor TP53 protein overexpression.103 A combined heterozygous state consisting of V1 and a wild allele of the MGMT gene may contribute to the de novo occurrence of glioblastoma.102 DNA repair is complex and involves more than 130 known genes; therefore, studies focusing on constellations of variants involved in DNA repair pathways, as well as their interactions, might help elucidate the role of variants in the genesis and progression of glioma.
Dysregulation of the cell cycle (control of proliferation and apoptosis) is a hallmark feature of most gliomas,104 and MDM2 is a key molecule in maintaining the fidelity of this process. In one study, the G variant of SNP309 in the MDM2 promoter led to higher expression of MDM2 with concomitant reduced expression of TP53 and was significantly associated with earlier age at tumor development and multiple tumor sites in participants with the Li-Fraumeni syndrome,105 of which brain tumors are one component. MDM2 appears to regulate TP53 expression negatively,106 and an inverse association between TP53 and MDM2 expression has been reported by Wiencke and associates among others.107 Results from a recent study suggest that SNP309, in the promoter region of MDM2 and related to MDM2 protein expression levels, is not associated with histologic grade of glioma, age at onset, p53 mutation rate, or gliomagenesis.108 Associations between MDM2 and TP53 remain poorly understood and should be examined in studies with large sample size because of the potential need for smaller subgroup analyses.
Genetic Factors and Risk for Meningioma
Mutations in the NF2 gene and loss of chromosome 22q are the most common genetic alterations associated with the initiation of meningiomas.109,110 Mutations in the NF2 gene probably account for the formation of more than half of all meningiomas.110 Meningiomas that are not associated with NF2 are poorly understood. Although the underlying genetic causes are unknown, meningioma growth is sustained by dysregulated expression of steroid hormones, growth factors, and their receptors and activation of signal transduction cascades. Results from recent studies that may be important in framing future work include those suggesting the following: a candidate common loss-of-heterozygosity (LOH) region on 1p36.11 might harbor tumor suppressor genes related to malignant progression of meningioma111; changes in the APC gene may play a role in meningioma formation112; the genotype combination of CC-CG-CC formed by three polymorphisms in p53 might increase meningioma risk, but only in those with a family history of cancer113; and SNPs in the Ki-ras and ERCC2 genes may be involved in meningioma formation, and SNPs in cyclin D1 and p16 may mark genes that have an inverse effect on the risk for development of both radiation-associated and sporadic meningioma.
One of the most intriguing findings concerning genetic factors related to meningioma resulted from a recent analysis of 136 DNA repair genes that were examined because of the well-known association of meningioma with exposure to ionizing radiation.114 Bethke and colleagues found that the SNP rs4968451, which maps to the gene encoding breast cancer susceptibility gene 1–interacting protein 1 (BRIP1), was consistently associated, across five studies, with an increased risk for the development of meningioma.114 This relationship is compelling because (1) greater than a fourth of the European population are carriers of at-risk genotypes for rs4968451 and thus the variant is likely to contribute to 16% of meningiomas, and (2) BRIP1 encodes a helicase that interacts with BRCA1 and has BRCA1-dependent DNA repair and checkpoint functions, and this provides a possible explanation for the fact that both breast cancer and meningioma most commonly occur in women aged 50 to 70 years and that women with breast cancer have an approximately 50% greater risk for meningioma and vice versa.114 The results of Bethke and coworkers show a strong association across studies, and they demonstrate the functional importance of an SNP.114
Table 106-2 summarizes adult glioma and meningioma nonoccupational risk factors for which there is sufficient evidence to estimate likelihoods of association, strengths, and directions of associations. These summary likelihoods result only from surveillance and epidemiologic studies. Risk factors for which, at present, there is insufficient evidence have been described earlier. Note that there is insufficient evidence for all constitutive polymorphisms described previously and for some molecular factors related to immune function. During the next decade, burgeoning research in these areas will probably result in adequate evidence to estimate summary likelihoods of association.
TABLE 106-2 Adult Glioma and Meningioma Nonoccupational Risk Factors for Which There Is Sufficient Evidence to Estimate Likelihoods of Association, Strengths, and Directions of Associations Resulting from Surveillance and Epidemiologic Studies
| FACTOR | LIKELIHOOD/STRENGTH/DIRECTION | |
|---|---|---|
| Glioma | Meningioma | |
| Increasing age1,3 | High/high/positive | High/high/positive |
| Male sex1,3 | High/low/positive | High/low/negative |
| White race1,3 | High/moderate/positive | Low/none/none |
| Syndromes associated with rare mutations43,91–94 | High/high/positive | High/high/positive |
| Common inherited variation in chromosome 5p15.33 (TERT), 9p21.3 (CDKN2B), 8q24, 11q23 (PHLDB1), and 20q13.3 (RTEL1)173,174 | High/low-moderate/varies | Not established |
| High-dose ionizing radiation8–17 | High/high/positive | High/low/positive |
| Epilepsy/seizures (probably not causal, but an early symptom)115–117 | High/low/positive | Moderate/low/positive |
| Allergies/asthma/elevated IgE62,71–73,118–121 | Moderate/low/negative | Not established |
| Familial aggregation/family history2,80,81,92–94,122–124 | Moderate/low/positive | Moderate/low/positive |
| Estrogen/reproductive/menstrual factors (including exogenous hormone use)54,56–59,125,126 | Not established | High/low/positive |
| Mutagen sensitivity127–131 | Moderate/low/positive | Not established |
| History of varicella-zoster virus infection/anti-VZV IgG77–79 | Moderate/low/negative | Not established |
| Head injury/trauma39–43 | Low/none/none | Low/none/none |
| Cellular telephone use11,17–20,23–26,30,31,33–36,132–137 | Not established | Low/none/none |
| Dental radiographs12–14,126,138 | Low/none/none | Low/none/none |
| Filtered cigarette smoking47,51,139,140 | Low/none/none | Low/none/none |
| Alcohol consumption40,47,51,140–143 | Low/none/none | Low/none/none |
| Previous cancer diagnosis15,92 | Low/none/none | Not established |
| Electromagnetic fields from residential power144 | Low/none/none | Not established |
Prognostic Factors for Glioma, Including Glioblastoma
Because many brain tumors, especially glioblastoma, have a poor prognosis, investigators have sought to identify factors associated with probability of survival and survival time in order to inhibit mechanisms associated with tumor progression. The following are associated with glioblastoma prognosis: age, Karnofsky performance scale score, extent of resection, capacity for complete resection, degree of necrosis, enhancement on preoperative magnetic resonance imaging studies, volume of residual disease, therapeutic approach, preoperative and postoperative tumor size, noncentral tumor location (defined as infiltration of the splenium, basal ganglia, thalamus, or midbrain), patient deterioration, patient condition before radiation therapy, and presurgical serum albumin level.145–148 Furthermore, the results of a large study using SEER data highlight the potential importance of familial or spousal support in the prognosis; survival probability was lower in unmarried glioblastoma patients (6 months) than in married patients (7 months), even after statistical adjustment for other prognostic factors, including treatment (although unmarried patients were also less likely to undergo both surgery and radiation therapy).149 In addition, results from a recent study suggest that persistent hyperglycemia 1 to 3 months after surgical resection as part of the treatment of anaplastic astrocytoma and glioblastoma multiforme decreases survival probability (relative risk, 1.79; 95% CI, 1.05 to 3.05); the median survival of those with hyperglycemia was 5 months as compared with 11 months for normoglycemic patients.150 These results are important because clinical glucose management after tumor resection may prolong survival time.
Recent efforts to identify prognostic factors for glioma have focused on tumor molecular markers, serologic factors, and inherited genetic variation. For oligodendroglioma, it is now well established that the combined tumor loss of 1p and 19q confers a more favorable prognosis.151 Results submitted by Yang and coauthors showed that two genotypes associated with the 19q deletion region, GLTSCR1–exon 1 and ARCC2–exon 22, are independent predictors of glioma survival.100 There is some evidence that the following are prognostic indicators for glioblastoma and other glioma subtypes: p53 mutation and expression,152–161 overexpression or amplification of the epidermal growth factor receptor (EGFR),152–156161 CDKN2A alterations and deletions,152,153,155 and MDM2 amplifications.152,155,160–162 Simmons and associates demonstrated the complex relationship between survival and age at diagnosis, p53, and EGFR in glioblastoma patients.163 In younger patients, survival was shorter in those whose tumors overexpressed EFGR but had normal p53 immunohistochemistry,163 although when interpreting their findings it should be remembered that post hoc subgroup analysis increases the risk for false-positive findings.164 Age-dependent associations between glioblastoma survival and 1p and CDKN2A have also been demonstrated.153 Expression of p53 protein probably decreases with advancing age,153,163 and the association between p53 expression and survival from glioblastoma may be hidden when confounding by age is adjusted statistically. In addition, loss of heterozygosity on chromosome 10q has been associated with shorter duration of survival from glioblastoma,158,165 and the combined LOH on 1p and 19q may afford glioblastoma patients a more favorable prognosis.158 There may be a strong association between different genotypes of human telomerase MNS16A and glioblastoma survival time (24.7 months’ median survival time for the SS genotype versus 14.0 months and 13.1 months for the SL and LL genotypes, respectively).166 These results are promising because human telomerase MNS16A may be exploitable as a biomarker of treatment success.
Recently, Wrensch and coauthors reported that the GST (θ)1 deletion bestowed a less favorable glioma prognosis whereas higher glioma survival was afforded to glioma patients with the ERCC1 (a DNA excision repair gene) C8092A polymorphism.161 EGFR expression in patients with anaplastic astrocytoma was associated with a nearly threefold poorer survival.161 Importantly, glioblastoma patients with elevated IgE lived 9 months longer than did those with lower or normal IgE levels.161 This finding is especially important because it may implicate immunologic factors in the prognosis of patients with glioblastoma.161 Glioblastoma patients with higher IgE levels may have better antitumor defenses or less aggressive tumors with weaker anti-immunologic effects, or IgE itself may have antitumor activity through direct activity on glioma or other nearby cells.161 Associations between glioma prognosis and atopic allergy, in which IgE is increased, should be studied. As discussed earlier, there is consistent and compelling evidence of protection against glioma as a result of allergies and immune-related conditions. Further suggesting the importance of immunologic factors in the prognosis of patients with glioblastoma, a recent report indicates that amplification of IL-6, a cytokine that may promote glioblastoma, is significantly associated with decreased glioblastoma survival.167 Analyses of atopy, IgE, and cytokines in relation to glioma prognosis may help us better understand the complex nature of the immunologic response to the genesis of glioma, including secreted tumor-specific factors and host immune responses, and such investigations may also have important implications for immunologic therapy for glioma. In addition, brain tumors, like all cancers, must evade immune rejection with presumably similar mechanisms as any foreign tissue growth. Future studies should also include the examination of T-cell activities such as that of regulatory T cells, which have been associated with tissue graft acceptance, as well as brain tumor prognosis.168,169
In a pilot study, Wrensch and colleagues genotyped 112 incident and population-based glioblastoma patients with the ParAllele assay panel consisting of approximately 10,000 nonsynonymous coding SNPs, identified 17 SNPs potentially related to either age at diagnosis of glioblastoma or survival, and genotyped 16 of these SNPs by conventional polymerase chain reaction methods in an independent group of 195 glioblastoma patients.170 Among the 195 glioblastoma patients, they found that that only 1 SNP, rs8057643 (located on 16p13.2), was significantly associated with age at diagnosis of glioblastoma and that time to death was related to number of T alleles after statistical adjustment for gender.
Prognostic Factors for Meningioma
Prognostic factors for patients with meningioma have not been thoroughly studied, perhaps because these patients typically have a more favorable prognosis than those in whom glioma is diagnosed. Generally, like glioma, age at diagnosis and histologic characteristics contribute to the prognosis. The results from a large study of 9000 patients revealed the following prognostic factors for benign meningioma: age, tumor size, and surgical and radiation treatments; in contrast, for malignant meningioma, the prognostic factors included only age and surgical and radiation treatments.95 Abnormalities in chromosome 14 may also affect the prognosis of patients with meningioma.171 Loss of the tumor suppressor in lung cancer-1 (TSLC1) protein has been associated with decreased meningioma survival.172 We know very little about factors that determine prognosis for meningioma patients, but studies of SNPs and other genetic characteristics are under way. The recent requirement of central cancer registries to report benign brain tumors may increase our knowledge of factors related to prognosis for patients with meningioma.
Important Update
Very recently, two published genome-wide association studies for glioma, based on five sets of genome-wide genotyping in glioma cases and several control groups, discovered and confirmed the following important five regions for glioma risk: chromosome 5p15.33 (TERT), 9p21.3 (CDKN2B), 8q24, 11q23 (PHLDB1), and 20q13.3 (RTEL1).173,174 These new discoveries reflect tremendous opportunities for discovering mechanisms of glioma development.
Aldape K, Burger PC, Perry A. Clinicopathologic aspects of 1p/19q loss and the diagnosis of oligodendroglioma. Arch Pathol Lab Med. 2007;131:242.
Bethke L, Murray A, Webb E, et al. Comprehensive analysis of DNA repair gene variants and risk of meningioma. J Natl Cancer Inst. 2008;100:270.
Blettner M, Schlehofer B, Samkange-Zeeb F, et al. Medical exposure to ionising radiation and the risk of brain tumours: Interphone Study Group, Germany. Eur J Cancer. 2007;43:1990.
Lahkola A, Auvinen A, Raitanen J, et al. Mobile phone use and risk of glioma in 5 North European countries. Int J Cancer. 2007;120:1769.
Linos E, Raine T, Alonso A, et al. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99:1544.
Malmer B, Feychting M, Lonn S, et al. p53 Genotypes and risk of glioma and meningioma. Cancer Epidemiol Biomarkers Prev. 2005;14:2220.
Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892.
Pecina-Slaus N, Nikuseva Martic T, Tomas D, et al. Meningiomas exhibit loss of heterozygosity of the APC gene. J Neurooncol. 2008;87:63.
Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5:1045.
Schwartzbaum J, Ahlbom A, Malmer B, et al. Polymorphisms associated with asthma are inversely related to glioblastoma multiforme. Cancer Res. 2005;65:6459.
Schwartzbaum JA, Ahlbom A, Lonn S, et al. An international case-control study of interleukin-4Ralpha, interleukin-13, and cyclooxygenase-2 polymorphisms and glioblastoma risk. Cancer Epidemiol Biomarkers Prev. 2007;16:2448.
Schwartzbaum JA, Ahlbom A, Lonn S, et al. An international case-control study of glutathione transferase and functionally related polymorphisms and risk of primary adult brain tumors. Cancer Epidemiol Biomarkers Prev. 2007;16:559.
Schwartzbaum JA, Fisher JL, Aldape KD, et al. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494.
Silvera SAN, Miller AB, Rohan TE. Hormonal and reproductive factors and risk of glioma: a prospective cohort study. Int J Cancer. 2006;118:1321.
Simon M, Bostrom JP, Hartmann C. Molecular genetics of meningiomas: from basic research to potential clinical applications. Neurosurgery. 2007;60:787.
Tchirkov A, Khalil T, Chautard E, et al. Interleukin-6 gene amplification and shortened survival in glioblastoma patients. Br J Cancer. 2007;96:474.
Wiemels JL, Wiencke JK, Kelsey KT, et al. Allergy-related polymorphisms influence glioma status and serum IgE levels. Cancer Epidemiol Biomarkers Prev. 2007;16:1229.
Wiemels JL, Wiencke JK, Patoka J, et al. Reduced immunoglobulin E and allergy among adults with glioma compared with controls. Cancer Res. 2004;64:8468.
Wrensch M, Fisher JL, Schwartzbaum JA, et al. The molecular epidemiology of gliomas in adults. Neurosurg Focus. 2005;19(5):E5.
Wrensch M, Kelsey KT, Liu M, et al. ERCC1 and ERCC2 polymorphisms and adult glioma. Neuro Oncol. 2005;7:495.
Wrensch M, Kelsey KT, Liu M, et al. Glutathione-S-transferase and adult glioma. Cancer Epidemiol Biomarkers Prev. 2004;13:461.
Wrensch M, McMillan A, Wiencke J, et al. Nonsynonymous coding single-nucleotide polymorphisms spanning the genome in relation to glioblastoma survival and age at diagnosis. Clin Cancer Res. 2007;13:197.
Wrensch M, Wiencke JK, Wiemels J, et al. Serum IgE, tumor epidermal growth factor receptor expression, and inherited polymorphisms associated with glioma survival. Cancer Res. 2006;66:4531.
Yang P, Kollmeyer TM, Buckner K, et al. Polymorphisms in GLTSCR1 and ERCC2 are associated with the development of oligodendrogliomas. Cancer. 2005;103:2363.
Yong Z, Chang L, Mei YX, et al. Role and mechanisms of CD4+CD25+ regulatory T cells in the induction and maintenance of transplantation tolerance. Transpl Immunol. 2007;17:120.
1 Central Brain Tumor Registry of the United States. Statistical Report: Primary Brain Tumors in the United States, 2000-2004. 2008.
2 Fisher JL, Schwartzbaum J, Wrensch M, et al. Epidemiology of brain tumors. Neurol Clin. 2007;25:867.
3 SEER: Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases, Nov 2007 Sub (1973-2005 varying)—Linked To County Attributes—Total U.S., 1969-2005 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on the November 2007 submission, 2008.
4 Cohen MH, Johnson JR, Pazdur R. Food and Drug Administration Drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res. 2005;11:6767.
5 Helseth A. Incidence and survival of intracranial meningioma patients in Norway. Neuroepidemiology. 1997;16:1963-1992.
6 Sankila R, Kallio M, Jaaskelainen J, et al. Long-term survival of 1986 patients with intracranial meningioma diagnosed from 1953 to 1984 in Finland. Comparison of the observed and expected survival rates in a population-based series. Cancer. 1992;70:1568.
7 McCarthy BJ, Davis FG, Freels S, et al. Factors associated with survival in patients with meningioma. J Neurosurg. 1998;88:831.
8 Shintani T, Hayakawa N, Hoshi M, et al. High incidence of meningioma among Hiroshima atomic bomb survivors. J Radiat Res. 1999;40:49.
9 Preston-Martin S. Epidemiology of primary CNS neoplasms. Neurol Clin. 1996;14:273.
10 Juven Y, Sadetzki S. A possible association between ionizing radiation and pituitary adenoma: a descriptive study. Cancer. 2002;95:397.
11 Hardell L, Mild KH, Pahlson A, et al. Ionizing radiation, cellular telephones and the risk for brain tumours. Eur J Cancer Prev. 2001;10:523.
12 Wrensch M, Miike R, Lee M, et al. Are prior head injuries or diagnostic X-rays associated with glioma in adults? The effects of control selection bias. Neuroepidemiology. 2000;19:234.
13 Longstreth WTJ, Phillips LE, Drangsholt M, et al. Dental X-rays and the risk of intracranial meningioma: a population-based case-control study. Cancer. 2004;100:1026.
14 Preston-Martin S, Yu MC, Henderson BE, et al. Risk factors for meningiomas in men in Los Angeles County. J Natl Cancer Inst. 1983;70:863.
15 Salminen E, Pukkala E, Teppo L. Second cancers in patients with brain tumours—impact of treatment. Eur J Cancer. 1999;35:102.
16 Sadetzki S. Exposure to ionizing radiation and glioma risk. In: Percy RC, ed. Principles and Practices of Neuro-Oncology: A Multidisciplinary Approach. New York: Demos Medical Publishing (in press).
17 Blettner M, Schlehofer B, Samkange-Zeeb F, et al. Medical exposure to ionising radiation and the risk of brain tumours: Interphone Study Group, Germany. Eur J Cancer. 2007;43:1990.
18 Christensen HC, Schuz J, Kosteljanetz M, et al. Cellular telephones and risk for brain tumors: a population-based, incident case-control study. Neurology. 2005;64:1189.
19 Lonn S, Ahlbom A, Hall P, et al. Long-term mobile phone use and brain tumor risk. Am J Epidemiol. 2005;161:526.
20 Schuz J, Bohler E, Berg G, et al. Cellular phones, cordless phones, and the risks of glioma and meningioma (Interphone Study Group, Germany). Am J Epidemiol. 2006;163:512.
21 Hardell L, Carlberg M, Hansson Mild K. Pooled analysis of two case-control studies on the use of cellular and cordless telephones and the risk of benign brain tumours diagnosed during 1997-2003. Int J Oncol. 2006;28:509.
22 Hardell L, Carlberg M, Hansson Mild K. Case-control study on cellular and cordless telephones and the risk for acoustic neuroma or meningioma in patients diagnosed 2000-2003. Neuroepidemiology. 2005;25:120.
23 Christensen HC, Schuz J, Kosteljanetz M, et al. Cellular telephone use and risk of acoustic neuroma. Am J Epidemiol. 2004;159:277.
24 Kundi M, Mild K, Hardell L, et al. Mobile telephones and cancer—a review of epidemiological evidence. J Toxicol Environ Health B Crit Rev. 2004;7:351.
25 Schoemaker MJ, Swerdlow AJ, Ahlbom A, et al. Mobile phone use and risk of acoustic neuroma: results of the Interphone case-control study in five North European countries. Br J Cancer. 2005;93:842.
26 Takebayashi T, Akiba S, Kikuchi Y, et al. Mobile phone use and acoustic neuroma risk in Japan. Occup Environ Med. 2006;63:802.
27 Auvinen A, Hietanen M, Luukkonen R, et al. Brain tumors and salivary gland cancers among cellular telephone users. Epidemiology. 2002;13:356.
28 Feychting M, Anders A. Radiofrequency fields and glioma. In: Percy RC, ed. Principles and Practices of Neuro-Oncology: A Multidisciplinary Approach. New York: Demos Medical Publishing (in press).
29 Hardell L, Mild KH, Carlberg M. Case-control study on the use of cellular and cordless phones and the risk for malignant brain tumours. Int J Radiat Biol. 2002;78:931.
30 Hardell L, Nasman A, Pahlson A, et al. Use of cellular telephones and the risk for brain tumours: A case-control study. Int J Oncol. 1999;15:113.
31 Hepworth SJ, Schoemaker MJ, Muir KR, et al. Mobile phone use and risk of glioma in adults: case-control study. BMJ. 2006;332:883.
32 Inskip PD, Tarone RE, Hatch EE, et al. Cellular-telephone use and brain tumors. N Engl J Med. 2001;344:79.
33 Johansen C, Boice JJ, McLaughlin J, et al. Cellular telephones and cancer—a nationwide cohort study in Denmark. J Natl Cancer Inst. 2001;93:203.
34 Lahkola A, Auvinen A, Raitanen J, et al. Mobile phone use and risk of glioma in 5 north European countries. Int J Cancer. 2007;120:1769.
35 Muscat JE, Malkin MG, Thompson S, et al. Handheld cellular telephone use and risk of brain cancer. JAMA. 2000;284:3001.
36 Ahlbom A, Green A, Kheifets L, et al. Epidemiology of health effects of radiofrequency exposure. Environ Health Perspect. 2004;112:1741.
37 Hardell L, Carlberg M, Hansson Mild K. Pooled analysis of two case-control studies on use of cellular and cordless telephones and the risk for malignant brain tumours diagnosed in 1997-2003. Int Arch Occup Environ Health. 2006;79:630.
38 Baldwin RT, Preston-Martin S. Epidemiology of brain tumors in childhood—a review. Toxicol Appl Pharmacol. 2004;199:118.
39 Hochberg F, Toniolo P, Cole P. Head trauma and seizures as risk factors of glioblastoma. Neurology. 1984;34:1511.
40 Hu J, Johnson KC, Mao Y, et al. Risk factors for glioma in adults: a case-control study in northeast China. Cancer Detect Prev. 1998;22:100.
41 Inskip PD, Mellemkjaer L, Gridley G, et al. Incidence of intracranial tumors following hospitalization for head injuries (Denmark). Cancer Causes Control. 1998;9:109.
42 Preston-Martin S, Pogoda JM, Schlehofer B, et al. An international case-control study of adult glioma and meningioma: the role of head trauma. Int J Epidemiol. 1998;27:579.
43 Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278.
44 Hu J, La Vecchia C, Negri E, et al. Diet and brain cancer in adults: a case-control study in northeast China. Int J Cancer. 1999;81:20.
45 Tedeschi-Blok N, Schwartzbaum J, Lee M, et al. Dietary calcium consumption and astrocytic glioma: the San Francisco Bay Area Adult Glioma Study, 1991-1995. Nutr Cancer. 2001;39:196.
46 Chen H, Ward MH, Tucker KL, et al. Diet and risk of adult glioma in eastern Nebraska, United States. Cancer Causes Control. 2002;13:647.
47 Lee M, Wrensch M, Miike R. Dietary and tobacco risk factors for adult onset glioma in the San Francisco Bay Area (California, USA). Cancer Causes Control. 1997;8:13.
48 Preston-Martin S, Henderson BE. N-nitroso compounds and human intracranial tumours. IARC Sci Publ. 1984;57:887.
49 Schwartzbaum JA, Fisher JL, Goodman J, et al. Hypotheses concerning roles of dietary energy, cured meat, and serum tocopherols in adult glioma development. Neuroepidemiology. 1999;18:156.
50 Bunin GR, Kuijten RR, Buckley JD, et al. Relation between maternal diet and subsequent primitive neuroectodermal brain tumors in young children. N Engl J Med. 1993;329:536.
51 Hu J, Little J, Xu T, et al. Risk factors for meningioma in adults: a case-control study in northeast China. Int J Cancer. 1999;83:299.
52 Wrensch M, Bondy ML, Wiencke J, et al. Environmental risk factors for primary malignant brain tumors: a review. J Neurooncol. 1993;17:47.
53 McKinley BP, Michalek AM, Fenstermaker RA, et al. The impact of age and sex on the incidence of glial tumors in New York state from 1976 to 1995. J Neurosurg. 2000;93:932.
54 Schlehofer B, Blettner M, Wahrendorf J. Association between brain tumors and menopausal status. J Natl Cancer Inst. 1992;84:1346.
55 Wigertz A, Lonn S, Mathiesen T, et al. Risk of brain tumors associated with exposure to exogenous female sex hormones. Am J Epidemiol. 2006;164:629.
56 Cantor KP, Lynch CF, Johnson D. Reproductive factors and risk of brain, colon, and other malignancies in Iowa (United States). Cancer Causes Control. 1993;4:505.
57 Hatch EE, Linet MS, Zhang J, et al. Reproductive and hormonal factors and risk of brain tumors in adult females. Int J Cancer. 2005;114:797.
58 Lambe M, Coogan P, Baron J. Reproductive factors and the risk of brain tumors: a population-based study in Sweden. Int J Cancer. 1997;72:389.
59 Silvera SAN, Miller AB, Rohan TE. Hormonal and reproductive factors and risk of glioma: a prospective cohort study. Int J Cancer. 2006;118:1321.
60 Jhawar BS, Fuchs CS, Colditz GA, et al. Sex steroid hormone exposures and risk for meningioma. J Neurosurg. 2003;99:848.
61 Linos E, Raine T, Alonso A, et al. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99:1544.
62 Wiemels JL, Wiencke JK, Patoka J, et al. Reduced immunoglobulin E and allergy among adults with glioma compared with controls. Cancer Res. 2004;64:8468.
63 Dinarello CA. Setting the cytokine trap for autoimmunity. Nat Med. 2003;9:20.
64 Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991.
65 Liu H, Prayson RA, Estes ML, et al. In vivo expression of the interleukin 4 receptor alpha by astrocytes in epilepsy cerebral cortex. Cytokine. 2000;12:1656.
66 Barna BP, Estes ML, Pettay J, et al. Human astrocyte growth regulation: interleukin-4 sensitivity and receptor expression. J Neuroimmunol. 1995;60:75.
67 Louis DN. A molecular genetic model of astrocytoma histopathology. Brain Pathol. 1997;7:755.
68 Faber C, Terao E, Morga E, et al. Interleukin-4 enhances the in vitro precursor cell recruitment for tumor-specific T lymphocytes in patients with glioblastoma. J Immunother. 2000;23:11.
69 Saleh M, Davis ID, Wilks AF. The paracrine role of tumor-derived mIL-4 on tumour-associated endothelium. Int J Cancer. 1997;72:664.
70 Saleh M, Wiegmans A, Malone Q, et al. Effect of in situ retroviral interleukin-4 transfer on established intracranial tumors. J Natl Cancer Inst. 1999;91:438.
71 Schwartzbaum J, Ahlbom A, Malmer B, et al. Polymorphisms associated with asthma are inversely related to glioblastoma multiforme. Cancer Res. 2005;65:6459.
72 Wiemels JL, Wiencke JK, Kelsey KT, et al. Allergy-related polymorphisms influence glioma status and serum IgE levels. Cancer Epidemiol Biomarkers Prev. 2007;16:1229.
73 Schwartzbaum JA, Ahlbom A, Lonn S, et al. An international case-control study of interleukin-4Ralpha, interleukin-13, and cyclooxygenase-2 polymorphisms and glioblastoma risk. Cancer Epidemiol Biomarkers Prev. 2007;16:2448.
74 Jordan JT, Sun W, Hussain SF, et al. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57:123.
75 Chahlavi A, Rayman P, Richmond AL, et al. Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res. 2005;65:5428.
76 Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006;212:238.
77 Wrensch M, Weinberg A, Wiencke J, et al. Does prior infection with varicella-zoster virus influence risk of adult glioma? Am J Epidemiol. 1997;145:594.
78 Wrensch M, Weinberg A, Wiencke J, et al. Prevalence of antibodies to four herpesviruses among adults with glioma and controls. Am J Epidemiol. 2001;154:161.
79 Wrensch M, Weinberg A, Wiencke J, et al. History of chickenpox and shingles and prevalence of antibodies to varicella-zoster virus and three other herpesviruses among adults with glioma and controls. Am J Epidemiol. 2005;161:929.
80 Schwartzbaum JA, Fisher JL, Aldape KD, et al. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494.
81 Wrensch M, Fisher JL, Schwartzbaum JA, et al. The molecular epidemiology of gliomas in adults. Neurosurg Focus. 2005;19(5):E5.
82 Mitchell DA, Xie W, Schmittling R, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10:10.
83 Scheurer ME, Bondy M, Aldape K, et al. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol. 2008;116:79.
84 Poltermann S, Schlehofer B, Steindorf K, et al. Lack of association of herpesviruses with brain tumors. J Neurovirol. 2006;12:90.
85 Fisher SG, Weber L, Carbone M. Cancer risk associated with simian virus 40 contaminated polio vaccine. Anticancer Res. 1999;19:2173.
86 Geissler E, Staneczek W. SV40 and human brain tumors. Arch Geschwulstforsch. 1988;58:129.
87 Strickler HD, Rosenberg PS, Devesa SS, et al. Contamination of poliovirus vaccines with simian virus 40 (1955-1963) and subsequent cancer rates. JAMA. 1998;279:292.
88 Tang J, Shao W, Dorak MT, et al. Positive and negative associations of human leukocyte antigen variants with the onset and prognosis of adult glioblastoma multiforme. Cancer Epidemiol Biomarkers Prev. 2005;14:2040.
89 Guerini FR, Agliardi C, Zanzottera M, et al. Human leukocyte antigen distribution analysis in north Italian brain glioma patients: an association with HLA-DRB1*14. J Neurooncol. 2006;77:213.
90 Facoetti A, Nano R, Zelini P, et al. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res. 2005;11:8304.
91 Bondy M, Wiencke J, Wrensch M, et al. Genetics of primary brain tumors: a review. J Neurooncol. 1994;18:69.
92 Wrensch M, Lee M, Miike R, et al. Familial and personal medical history of cancer and nervous system conditions among adults with glioma and controls. Am J Epidemiol. 1997;145:581.
93 Grossman SA, Osman M, Hruban R, et al. Central nervous system cancers in first-degree relatives and spouses. Cancer Invest. 1999;17:299.
94 Malmer B, Henriksson R, Gronberg H. Familial brain tumours—genetics or environment? A nationwide cohort study of cancer risk in spouses and first-degree relatives of brain tumour patients. Int J Cancer. 2003;106:260.
95 Wrensch M, Kelsey KT, Liu M, et al. Glutathione-S-transferase and adult glioma. Cancer Epidemiol Biomarkers Prev. 2004;13:461.
96 Schwartzbaum JA, Ahlbom A, Lonn S, et al. An international case-control study of glutathione transferase and functionally related polymorphisms and risk of primary adult brain tumors. Cancer Epidemiol Biomarkers Prev. 2007;16:559.
97 Chen P, Wiencke J, Aldape K, et al. Association of an ERCC1 polymorphism with adult-onset glioma. Cancer Epidemiol Biomarkers Prev. 2000;9:843.
98 Wrensch M, Kelsey KT, Liu M, et al. ERCC1 and ERCC2 polymorphisms and adult glioma. Neuro Oncol. 2005;7:495.
99 Caggana M, Kilgallen J, Conroy JM, et al. Associations between ERCC2 polymorphisms and gliomas. Cancer Epidemiol Biomarkers Prev. 2001;10:355.
100 Yang P, Kollmeyer TM, Buckner K, et al. Polymorphisms in GLTSCR1 and ERCC2 are associated with the development of oligodendrogliomas. Cancer. 2005;103:2363.
101 Wang L-E, Bondy ML, Shen H, et al. Polymorphisms of DNA repair genes and risk of glioma. Cancer Res. 2004;64:5560.
102 Inoue R, Isono M, Abe M, et al. A genotype of the polymorphic DNA repair gene MGMT is associated with de novo glioblastoma. Neurol Res. 2003;25:875.
103 Wiencke JK, Aldape K, McMillan A, et al. Molecular features of adult glioma associated with patient race/ethnicity, age, and a polymorphism in O6-methylguanine-DNA-methyltransferase. Cancer Epidemiol Biomarkers Prev. 2005;14:1774.
104 Ichimura K, Ohgaki H, Kleihues P, et al. Molecular pathogenesis of astrocytic tumours. J Neurooncol. 2004;70:137.
105 Bond GL, Hu W, Bond EE, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591.
106 Bond GL, Hu W, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Targets. 2005;5:3.
107 Halatsch ME, Schmidt U, Unterberg A, et al. Uniform MDM2 overexpression in a panel of glioblastoma multiforme cell lines with divergent EGFR and p53 expression status. Anticancer Res. 2006;26:4191.
108 Tsuiki H, Nishi T, Takeshima H, et al. Single nucleotide polymorphism 309 affects murin-double-minute 2 protein expression but not glioma tumorigenesis. Neurol Med Chirurg. 2007:47-203.
109 Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5:1045.
110 Simon M, Bostrom JP, Hartmann C. Molecular genetics of meningiomas: from basic research to potential clinical applications. Neurosurgery. 2007;60:787.
111 Guan Y, Hata N, Kuga D, et al. Narrowing of the regions of allelic losses of chromosome 1p36 in meningioma tissues by an improved SSCP analysis. Int J Cancer. 2008;122:1820.
112 Pecina-Slaus N, Nikuseva Martic T, Tomas D, et al. Meningiomas exhibit loss of heterozygosity of the APC gene. J Neurooncol. 2008;87:63.
113 Malmer B, Feychting M, Lonn S, et al. p53 Genotypes and risk of glioma and meningioma. Cancer Epidemiol Biomarkers Prev. 2005;14:2220.
114 Bethke L, Murray A, Webb E, et al. Comprehensive analysis of DNA repair gene variants and risk of meningioma. J Natl Cancer Inst. 2008;100:270.
115 Adelow C, Ahlbom A, Feychting M, et al. Epilepsy as a risk factor for cancer. J Neurol Neurosurg Psychiatry. 2006;77:784.
116 Schlehofer B, Blettner M, Preston-Martin S, et al. Role of medical history in brain tumour development. Results from the International Adult Brain Tumour Study. Int J Cancer. 1999;82:155.
117 Schwartzbaum J, Jonsson F, Ahlbom A, et al. Prior hospitalization for epilepsy, diabetes, and stroke and subsequent glioma and meningioma risk. Cancer Epidemiol Biomarkers Prev. 2005;14:643.
118 Brenner AV, Linet MS, Fine HA, et al. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int J Cancer. 2002;99:252.
119 Schoemaker MJ, Swerdlow AJ, Hepworth SJ, et al. History of allergies and risk of glioma in adults. Int J Cancer. 2006;119:2165.
120 Turner MC, Chen Y, Krewski D, et al. An overview of the association between allergy and cancer. Int J Cancer. 2006;118:3124.
121 Wiemels JL, Wiencke JK, Sison JD, et al. History of allergies among adults with glioma and controls. Int J Cancer. 2002;98:609.
122 Malmer B, Gronberg H, Bergenheim AT, et al. Familial aggregation of astrocytoma in northern Sweden: an epidemiological cohort study. Int J Cancer. 1999;81:366.
123 Malmer B, Henriksson R, Gronberg H. Different aetiology of familial low-grade and high-grade glioma? A nationwide cohort study of familial glioma. Neuroepidemiology. 2002;21:279.
124 Wrensch MR, Barger GR. Familial factors associated with malignant gliomas. Genet Epidemiol. 1990;7:291.
125 Cicuttini FM, Hurley SF, Forbes A, et al. Association of adult glioma with medical conditions, family and reproductive history. Int J Cancer. 1997;71:203.
126 Longstreth WTJ, Dennis LK, McGuire VM, et al. Epidemiology of intracranial meningioma. Cancer. 1993;72:639.
127 Berwick M, Vineis P. Markers of DNA repair and susceptibility to cancer in humans: an epidemiologic review. J Natl Cancer Inst. 2000;92:874.
128 Bondy ML, Kyritsis AP, Gu J, et al. Mutagen sensitivity and risk of gliomas: a case-control analysis. Cancer Res. 1996;56:1484.
129 Bondy ML, Wang LE, El-Zein R, et al. Gamma-radiation sensitivity and risk of glioma. J Natl Cancer Inst. 2001;93:1553.
130 El-Zein R, Bondy ML, Wang LE, et al. Risk assessment for developing gliomas: a comparison of two cytogenetic approaches. Mutat Res. 2001;490:35.
131 Sigurdson AJ, Bondy ML, Hess KR, et al. Gamma-ray mutagen sensitivity and survival in patients with glioma. Clin Cancer Res. 1998;4:3031.
132 Hardell L, Carlberg M, Hansson Mild K. Use of cellular telephones and brain tumour risk in urban and rural areas. Occup Environ Med. 2005;62:390.
133 Hardell L, Carlberg M, Mild KH. Case-control study of the association between the use of cellular and cordless telephones and malignant brain tumors diagnosed during 2000-2003. Environ Res. 2006:100-232.
134 Kane RC. Cellular telephones and brain tumors. N Engl J Med. 2001;344:1332.
135 Lonn S, Ahlbom A, Hall P, et al. Mobile phone use and the risk of acoustic neuroma. Epidemiology. 2004;15:653.
136 McWhirter WR, Dobson C. Brain tumours and mobile phones? Med J Aust. 1998;168:309.
137 Rothman KJ, Loughlin JE, Funch DP, et al. Overall mortality of cellular telephone customers. Epidemiology. 1996;7:303.
138 Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197.
139 Blowers L, Preston-Martin S, Mack WJ. Dietary and other lifestyle factors of women with brain gliomas in Los Angeles County (California, USA). Cancer Causes Control. 1997;8:5.
140 Hurley SF, McNeil JJ, Donnan GA, et al. Tobacco smoking and alcohol consumption as risk factors for glioma: a case-control study in Melbourne, Australia. J Epidemiol Community Health. 1996;50:442.
141 Boeing H, Schlehofer B, Blettner M, et al. Dietary carcinogens and the risk for glioma and meningioma in Germany. Int J Cancer. 1993;53:561.
142 Efird JT, Friedman GD, Sidney S, et al. The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: cigarette smoking and other lifestyle behaviors. J Neurooncol. 2004;68:57.
143 Preston-Martin S, Mack W, Henderson BE. Risk factors for gliomas and meningiomas in males in Los Angeles County. Cancer Res. 1989;49:6137.
144 Wrensch M, Yost M, Miike R, et al. Adult glioma in relation to residential power frequency electromagnetic field exposures in the San Francisco Bay area. Epidemiology. 1999;10:523.
145 Jeremic B, Milicic B, Grujicic D, et al. Multivariate analysis of clinical prognostic factors in patients with glioblastoma multiforme treated with a combined modality approach. J Cancer Res Clin Oncol. 2003;129:477.
146 Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001:95-190.
147 Lutterbach J, Sauerbrei W, Guttenberger R. Multivariate analysis of prognostic factors in patients with glioblastoma. Strahlenther Onkol. 2003;179:8.
148 Schwartzbaum J, Lal P, Evanoff W, et al. Presurgical serum albumin levels predict survival time from glioblastoma multiforme. J Neurooncol. 1999;43:35.
149 Chang S, Barker FG2nd. Marital status, treatment, and survival in patients with glioblastoma multiforme. Cancer. 2008;104:1975.
150 McGirt MJ, Chaichana KL, Gathinji M, et al. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008;63:286.
151 Aldape K, Burger PC, Perry A. Clinicopathologic aspects of 1p/19q loss and the diagnosis of oligodendroglioma. Arch Pathol Lab Med. 2007;131:242.
152 Backlund LM, Nilsson BR, Liu L, et al. Mutations in Rb1 pathway–related genes are associated with poor prognosis in anaplastic astrocytomas. Br J Cancer. 2005;93:124.
153 Batchelor TT, Betensky RA, Esposito JM, et al. Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin Cancer Res. 2004;10:228.
154 Deb P, Sharma MC, Mahapatra AK, et al. Glioblastoma multiforme with long term survival. Neurol India. 2005;53:329.
155 Houillier C, Lejeune J, Benouaich-Amiel A, et al. Prognostic impact of molecular markers in a series of 220 primary glioblastomas. Cancer. 2006;106:2218.
156 Layfield LJ, Willmore C, Tripp S, et al. Epidermal growth factor receptor gene amplification and protein expression in glioblastoma multiforme: prognostic significance and relationship to other prognostic factors. Appl Immunohistochem Mol Morphol. 2006;14:91.
157 McLendon RE, Herndon JE2nd, West B, et al. Survival analysis of presumptive prognostic markers among oligodendrogliomas. Cancer. 2005;104:1693.
158 Schmidt MC, Antweiler S, Urban N, et al. Impact of genotype and morphology on the prognosis of glioblastoma. J Neuropathol Exp Neurol. 2002;61:321.
159 Stander M, Peraud A, Leroch B, et al. Prognostic impact of TP53 mutation status for adult patients with supratentorial World Health Organization Grade II astrocytoma or oligoastrocytoma: a long-term analysis. Cancer. 2004;101:1028.
160 Ushio Y, Tada K, Shiraishi S, et al. Correlation of molecular genetic analysis of p53, MDM2, p16, PTEN, and EGFR and survival of patients with anaplastic astrocytoma and glioblastoma. Front Biosci. 2003;8:e281.
161 Wrensch M, Wiencke JK, Wiemels J, et al. Serum IgE, tumor epidermal growth factor receptor expression, and inherited polymorphisms associated with glioma survival. Cancer Res. 2006;66:4531.
162 Ranuncolo SM, Varela M, Morandi A, et al. Prognostic value of Mdm2, p53 and p16 in patients with astrocytomas. J Neurooncol. 2004;68:113.
163 Simmons ML, Lamborn KR, Takahashi M, et al. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61:1122.
164 Wacholder S, Chanock S, Garcia-Closas M, et al. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96:434.
165 Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892.
166 Wang L, Wang LE, El-Zein R, et al. Human telomerase genetic variation predicts survival of patients with glioblastoma multiforme [abstract 2823]. Proc Am Assoc Cancer Res. 2005:46.
167 Tchirkov A, Khalil T, Chautard E, et al. Interleukin-6 gene amplification and shortened survival in glioblastoma patients. Br J Cancer. 2007;96:474.
168 Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294.
169 Yong Z, Chang L, Mei YX, et al. Role and mechanisms of CD4+CD25+ regulatory T cells in the induction and maintenance of transplantation tolerance. Transpl Immunol. 2007;17:120.
170 Wrensch M, McMillan A, Wiencke J, et al. Nonsynonymous coding single-nucleotide polymorphisms spanning the genome in relation to glioblastoma survival and age at diagnosis. Clin Cancer Res. 2007;13:197.
171 Maillo A, Orfao A, Sayagues JM, et al. New classification scheme for the prognostic stratification of meningioma on the basis of chromosome 14 abnormalities, patient age, and tumor histopathology. J Clin Oncol. 2003;21:3285.
172 Surace EI, Lusis E, Murakami Y, et al. Loss of tumor suppressor in lung cancer-1 (TSLC1) expression in meningioma correlates with increased malignancy grade and reduced patient survival. J Neuropathol Exp Neurol. 2004;63:1015.
173 Sanjay S, Hosking FJ, Robertson LB, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nature Genetics. 2009;41(8):899-904.
174 Wrensch M, Jenkins RB, Chang JS, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nature Genetics. 2009;41(8):905-908. published online ahead of print July 5