CHAPTER 4 Epidemiology and Natural History of Meningiomas
INTRODUCTION
In this chapter, we (1) provide descriptive data on the impact of meningiomas; (2) describe the natural history of these tumors; and (3) review risk and protective factors. Throughout, we critically evaluate the literature and identify gaps in knowledge. We identified studies on natural history and risk factors through MEDLINE using the PubMed system to retrieve articles published through February, 2008. We conducted searches, restricted to articles written in English, using the key words “meningioma” or “meningiomas” in conjunction with “biology,” “natural history,” “long-term,” “outcome,” surgery/microsurgery, “radiotherapy,” “radiation (ionizing),” “radiation effects,” cellular/mobile telephone/s, “occupation,” “head trauma,” “head injury,” “allergy,” breast cancer/carcinoma, “oral contraceptives,” hormone/estrogen replacement therapy, “hormone receptors,” genetic/s, and “epidemiology.” We obtained additional references from those articles and from several recent epidemiologic reviews of brain tumors.1–4 We obtained descriptive statistics from articles identified through PubMed key word searches combining meningioma/s with “incidence,” “prevalence,” “survival,” “recurrence,” and “descriptive epidemiology,” and from the most recent report of the Central Brain Tumor Registry of the United States (CBTRUS) that is based on voluntary reporting by 18 registries from 1998 to 2002.5
DESCRIPTIVE STATISTICS
By histology, meningiomas were the most frequent primary brain and central nervous system (CNS) tumors reported to CBTRUS between 1998 and 2002, accounting for 19,190 (30.1%) of all 63,698 tumors reported (Fig. 4-1). Ninety-three percent of the meningiomas were nonmalignant.5
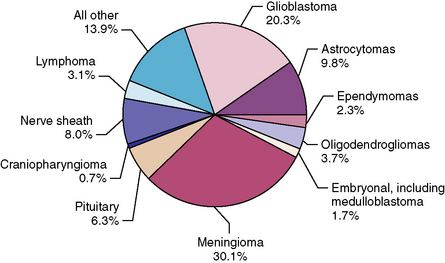
FIGURE 4-1 Histologic distribution of primary brain tumors and CNS tumors, CBTRUS 1998–2002, n = 63,698.
Incidence
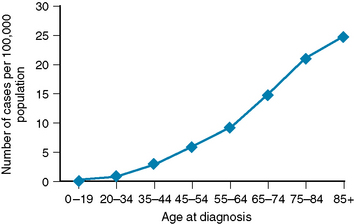
CBTRUS rates per 100,000 person years, age-adjusted to the 2000 U.S. standard population, demonstrated an overall meningioma incidence rate of 4.52. Rates differed little by race/ethnicity (4.46 in non-Hispanic whites; 4.58 in non-Hispanic blacks, and 4.61 in Hispanics of any race), but more than twice as many new cases were diagnosed among women than men (6.01 vs. 2.75).5 Meningiomas are uncommon in children, accounting for approximately 3% of all childhood tumors; their incidence increases linearly with age (Fig. 4-2). Mean age at diagnosis was 64 years.5
In the United States, data collected by CBTRUS between 1985 and 1994 from six population-based registries did not show an increase in incidence of meningioma,6 nor did a study of the population of Rochester, Minnesota, 1950–1990.7 However, data from the Danish Cancer Registry (1943–1997) demonstrated an increase in new cases of meningioma from 0.61 to 2.42 per 100,000 population, with an accelerating increase over time.8 A similar trend was observed across Denmark, Sweden, Norway, and Finland between 1968 and 1997,9 whereas in Japan, based on 1973–1993 data, an increase in incidence was seen before 1980, followed by stable subsequent rates.10 Where an increasing trend has been observed, it has been attributed to increased use of advanced imaging techniques, increased exposure to potential risk factors,9,10 and differential histologic classification of meningioma over time.8
Survival, Prevalence, and Recurrence
Data from the Hospital-based National Cancer Data Base collected from 1985 to 1988 and 1990 to 1992 estimated 5-year survival rates for benign, atypical, and malignant meningiomas in the United States at 70.1%, 74.5%, and 54.6%.11,12 Population-based data from Finland, Australia, and Sweden have found that 5-year survival rates for all meningioma histologic subtypes combined ranged from 73% to 94%.13–15 This relatively high 5-year survival is reflected in the number of prevalent cases. Registry data from Connecticut and Utah estimated that 138,000 individuals were living with this tumor in the United States in the year 2000, a prevalence rate of 50.4 per 100,000 population.16 In addition, meningiomas may recur. At 5 years, 19.2% of persons with benign tumors and 32.4% of persons with malignant meningioma had suffered a recurrence of symptoms.11
These data likely represent a lower limit of the number of persons with meningioma, as many patients presumed to have such a lesion are managed conservatively (i.e., without surgical intervention and pathologic confirmation), and hence may not be included in national databases that produce estimates of tumor incidence and prevalence. The different incident trends across nations reported here may reflect real differences, but are difficult to compare owing to differences in the time periods assessed and in the quality and methods of reporting. The Danish Cancer Registry is considered valid and 95% to 99% complete.8 In contrast, case reporting in the United States may be hampered by information and selection biases. Although CBTRUS has worked collaboratively with state cancer registries since the 1980s to collect information on all primary brain tumors, including tumors of benign and uncertain behavior, such reporting was voluntary and necessarily incomplete until recently, primarily reflecting patterns for the white population of the northeastern United States.6 In 2004, the United States Congress passed the Benign Brain Tumor Registry Amendment Act (Public Law 107-206) that mandated all United States cancer registries within the National Program of Cancer Registries (NPCR) to collect data on nonmalignant brain tumors. The accuracy of future population estimates in the United States will improve once these data become available.
NATURAL HISTORY AND LONG-TERM FOLLOW-UP
Some meningiomas may be asymptomatic and found incidentally. Other meningiomas may cause devastating symptoms with relatively abrupt onset. Or, because of the slow growth, some tumors may cause more subtle neurologic symptoms including difficulty concentrating or finding words and weakness or numbness in arms or legs with resultant problems with gait and walking.1 In addition, whereas more than 90% of meningiomas are benign (WHO Grade I), approximately 5% are atypical/borderline, and 3% to 5% are malignant.1 In addition, these tumors differ in size, site, and relationship to important vascular and neural structures.17 These varied presentations require different treatment strategies, each with associated risks and benefits. Case series that examine long-term outcomes of patients who were conservatively managed, and those who received surgery or radiation therapy, or both, may aid decision making regarding treatment options. We reviewed long-term follow-up studies of tumor progression and recurrence, survival, symptoms, and quality of life among patients with meningioma by treatment modality and histologic grade.
Incidental Findings and Conservative Management
With increasing use of magnetic resonance imaging (MRI) and computed tomography (CT) in clinical settings, asymptomatic meningiomas are more often coming to medical attention,18 with attendant questions about their clinical management. Several studies with fairly small samples (n = 17–67) have reported on patients with conservatively treated, incidental and asymptomatic tumors across meningioma sites. During mean follow-up times that ranged from 2.7 to 6.2 years, the proportion of patients who became symptomatic was small, ranging from 0% to 16%.19–22 In addition, during mean follow-up of 1.3 to more than 5 years, a majority of patients (between 63% and 100%) demonstrated no or limited (<1 cm3 per year) tumor growth.18,20–24 However, there was substantial variability. For example, in a study of 41 patients over a 3.6-year follow-up, the range in growth rate was 0.48% to 72% and calculated tumor doubling time varied from 1.27 to 143.5 years.18
Whereas the aforementioned studies examined the natural history of meningiomas across tumor locations in asymptomatic individuals, the natural history of skull base tumors, specifically, was examined in cohorts of conservatively managed patients, many of whom were symptomatic but did not undergo more aggressive treatments owing to advanced age, patient preference, medical contraindications, or tumors considered inoperable. These patients presented with symptoms that included headaches, dizziness and vertigo, seizures, hearing and vision loss, facial palsy, trigeminal neuropathy, swallowing problems, and gait disturbance.25,26 Of 21 consecutive patients with petroclival tumors followed on average 6.8 years, tumor growth was observed in 76% of cases, 58% experienced functional deterioration, and two succumbed to tumor-related deaths.26 Among 40 patients with petroclival, cavernous sinus, and anterior clinoid tumors at 10-year radiographic follow-up, 58% of tumors evidenced some growth. After a mean 6.9 years of clinical follow-up, 11 patients (28%) experienced new or worsening neuropathy; 23 (58%) developed paralysis or long tract signs; 2 (5%) had lost sight in one eye; and two became disabled.25
Surgery and Radiation Treatments
Benign tumors
Among selected case series that included exclusively or predominantly benign meningiomas, a study of 315 patients treated at Karolinski Hospital in Sweden for meningiomas of the cranial base is notable for its long follow-up (mean = 18 years) and historic cohort (1947–1982), against which more recent series can be compared. In that study, at 5 years, 4% of patients who had undergone Simpson Grade I and II surgeries and 25% to 45% of those with Grade III to Grade V surgeries had experienced symptomatic recurrence. By 20-year follow-up, 100% of tumors among those with Grade IV and V surgeries had symptomatic progression.27 More recently, two relatively large studies, one from the Mayo Clinic (n = 581; 1978–1988),28 and one from the University of Florida (n = 262; 1964–1992)29 examined outcomes across intracranial tumor sites. In the Mayo Clinic series, gross tumor resection (GTR) was possible in 80% of cases and resulted in estimates of progression-free survival (PFS) of 88% at 5 years and 75% at 10 years. Where only subtotal resection (STR) was possible, 5- and 10-year PFS were far lower: 61% and 38%, respectively.28 Similarly, among those treated solely with surgery, local control and cause-specific survival in the University of Florida series were higher after GTR than STR. However, STR cases who received adjuvant RT had outcomes as favorable as those with GTR: 87% local control and 86% cause-specific survival at 15 years.29
Recurrence and progression in GTR and STR were assessed among a number of case series that focused specifically on tumors of the skull base, where surgery can be technically challenging.30 Twelve studies of petroclival tumors reviewed by Little and colleagues, with mean follow-up of 14 to 67 months, demonstrated recurrence/progression ranging from 0% to 42%, again related to the extent of resection.31 As reviewed by Sindou and colleagues, studies of recurrence/progression of tumors of the cavernous sinus treated solely with surgery ranged from 10% to 14% with mean follow-up of 24 to 96 months; recurrence/progression among cases treated with surgery and RT ranged from 6.5% to 19% at 40- to 73-month follow-up.32 The effectiveness of stereotactic radiosurgery as primary or adjuvant treatment for tumors mainly located in the cranial base was reviewed by Goldsmith; 5-year PFS ranged from 86% to 98%. However, as primary treatment, the latter modality is restricted to smaller tumors.17
In addition to reports of tumor growth and survival, some investigators have undertaken studies of quality of life among meningioma patients overtime. The Karnofsky Performance Scale (KPS),33 which ranges from 0 (lowest) to 100 (highest), measures physical functioning and was used in several studies. Among surgically treated patients, preoperative mean KPS scores ranged from 70-90.34,35 Postoperatively, KPS scores tended to decline or remain at preoperative levels.34–36 However, functioning may improve gradually. In one study, KPS scores 1 year after treatment were higher than preoperative scores, but even in that study, all patients had at least one impairment including diplopia (72%), hearing loss (48%), facial numbness (45%), or balance problems (38%).36 Also at 1-year follow-up, two small studies that utilized the well-validated SF-36 that encompasses domains of physical functioning, role limitation, bodily pain, vitality, social functioning, and mental health37–39 found that 39% to 75% of meningioma patients were functioning below accepted norms.40,41 At mean follow-up of 33 months among 164 patients surgically treated at Brigham and Women’s Hospital, 47% expressed frustration over not being able to do things they used to do, although 87% described themselves as “quite a bit” or “very much” independent; and 77% said they were “quite a bit” or “very much” content with their quality of life.42 Of 82 individuals who were treated for meningioma in Austria between 1977 and 1993, 60% reported mild to moderate impairment in quality of life, and 20% suffered from moderate to severe physical handicaps or impairment in energy level.43
Atypical and malignant tumors
We located seven cases series reported since 1995 that assessed long-term outcomes among patients with atypical and/or malignant meningiomas. These were relatively small studies that included from 22 to 119 cases, reflecting the rarity of the more aggressive tumors. Median follow-up time ranged from 3.5 to 8 years. As one might expect, PFS was higher among those with benign than atypical and malignant meningiomas. In addition, as shown in Table 4-1, PFS among those with atypical meningiomas was higher than PFS among those with malignant tumors at 5 and 10 years.44–47 In addition, across studies, overall 5- and 10-year survival was also higher for those with atypical than malignant meningiomas.44–49
TABLE 4-1 Recurrence/progression-free and overall survival among patients with atypical and malignant meningiomas: Selected case series since 1995.44–49
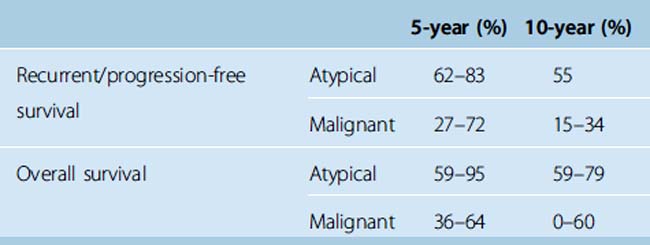
As with benign tumors, those with more completely resected atypical and malignant tumors had better outcomes.44,49 Post-treatment symptoms and quality of life were described in a small series from Thomas Jefferson Hospital where, based on Eastern Cooperative Oncology Group (ECOG) performance status, 18% of patients improved, 77% demonstrated no change, and 6% declined in functioning after adjuvant radiotherapy. In the post-treatment period, 54% of patients complained of limb weakness, 18% each became blind or aphasic, and 24% experienced memory loss.48
RISK AND PROTECTIVE FACTORS
Epidemiologic investigations, primarily case control and cohort studies, have examined the potential impact of a range of exposures potentially associated with meningioma. We focus on the factors that have received considerable attention and on developing areas of study that show promise. The current state of knowledge of the impact of these exposures is summarized in Table 4-2.
| Exposure | Impact |
|---|---|
| Ionizing Radiation |
Ionizing Radiation
Ionizing radiation is one of few established risk factors for brain tumors.1,3,4,50 Evidence supporting a link between this exposure and meningioma has been mounting for more than years,51 based largely on studies of (1) atomic bomb survivors; (2) the effects of radiation therapy; and (3) the effects of diagnostic radiographs. Radiation may be implicated in neoplastic transformation and tumor development via production of base-pair alterations and disruption of DNA that is not repaired before DNA replication.52 Radiation in medical and dental settings is typically measured in grays (Gy). Low (<10 Gy); moderate (10–20 Gy); and high (>20 Gy) treatment doses have been studied in relation to meningioma risk.52 Sieverts (Svs), which are dose-equivalent, are typically used to assess exposure among atomic bomb survivors. In an acute exposure, greater than 4 Svs is considered a lethal dose.53
Studies that examined meningioma risk among atomic bomb survivors in Nagasaki (1973–1992)54 and in Hiroshima (1975–1992)55 found increasing incidence of meningioma with decreasing distance from the hypocenter of the explosion. The Hiroshima study also found increased incidence of meningioma with exposure to higher doses of radiation at the time of the blast.55 A more recent study by Preston and colleagues, based on data collected between 1958 and 1995 from the Life Span Study (LSS) of 80,160 individuals in Hiroshima and Nagasaki at the time of the explosion, found a significant relationship between radiation dose and risk of all nervous system tumors combined. However, the risk of meningioma examined separately, albeit elevated, was not significant. These investigators estimated that the vast majority of survivors included in the LSS were exposed to radiation doses of less than 1 Sv and that a minority of identified nervous system tumors in that cohort (14%) were related to radiation exposure.56 In keeping with that conclusion, Yonehara and colleagues found that the clinical characteristics of the central nervous system tumors in the LSS population were more consistent with the characteristics of “spontaneous” than radiation-induced tumors.53
Harrison and colleagues provided criteria to distinguish spontaneous meningiomas from tumors that were radiation-induced in medical settings (Table 4-3),52 modified from criteria originally developed by Cahan to identify radiation-induced sarcoma.57
TABLE 4-3 Diagnostic criteria for radiation-induced meningioma.
| The meningioma must: |
Harrison MJ, Wolfe DE, Lau TS, Mitnick RJ, Sachdev VP. Radiation-induced meningiomas:experience at the Mount Sinai Hospital and review of the Literature; J Neurosurgery 1991;75(4):564–74.
Common features that distinguish spontaneous from radiation-induced meningiomas may include younger age at diagnosis,52,58–60 shorter latency period,52 multiple lesions,52,58–60 relatively high recurrence,52,60 and greater likelihood of atypical and malignant meningiomas.52,58,59,61,62 One might expect that equal proportions of males and females would be diagnosed with these tumors if they were indeed caused by radiation and members of both sexes were equally susceptible.58 However, the data are inconsistent, with some studies demonstrating a female preponderance, some a male preponderance, and some a difference in male/female ratio based on radiation dose.52,58,63
The data supporting the existence of radiation-induced meningioma among patients who received cranial radiotherapy is compelling. Studies of survivors of childhood cancers, in particular, have demonstrated strong associations between high-dose radiation treatments and development of secondary neoplasms, including meningiomas.64–69 These include a large retrospective study of 2169 children and adolescents treated for acute lymphoblastic leukemia at St. Jude’s Research Hospital between 1962 and 199866 and the Childhood Cancer Survivor Study, a cohort of 14,361 individuals with a history of cancer before the age of 22 years, treated at one of 26 collaborating hospitals between 1970 and 1986.68 In the latter study, the risk of developing meningioma with any dose of radiation therapy was significantly elevated (odds ratio [OR]: 9.94; 95% confidence interval [95% CI]: 1.54–29.7). In addition, the risk increased with radiation dose; at treatment doses of 30 to 49.9 Gy, the OR was 96.3 (CI: 10.32–899.3).68 Investigators have noted a long latency period for development of meningiomas after the original diagnosis.65,66,68 In the Childhood Cancer Survivor Study, median time to occurrence from original diagnosis was 17 years for meningioma, nearly twice the time to occurrence of glioma,68 underscoring the need for long-term follow-up to adequately assess risk. Although younger age has been associated with shorter latency67 and with greater risk,64 adults who underwent cranial radiotherapy were also at risk.52,70,71 In a study that included 200 cases and 400 controls, those with meningioma were 3.7 times more likely to have had radiation treatments for any condition (CI: 1.5–9.5) and 11.8 times more likely to have radiation treatment for a neoplastic condition CI: 1.5–∞).70
In the aforementioned studies, many participants were exposed to high doses of radiation, but there is evidence that relatively low doses may also be implicated, particularly among children. Studies of the Tinea Capitis Cohort are among the most well known in the field. This cohort included 10,834 children who received relatively low-dose radiation (mean 1.5 Gy) for treatment of ringworm in Israel over a 5-day period between 1948 and 1960. The comparison groups included matched population and sibling controls.63 Follow-up studies published in 198872 and 200563 found an excess relative risk of meningioma of 5.01 (95% CI: 2.66–9.80)63 and a relative risk of 9.5 (95% CI: 3.5–25.7)72 in the irradiated group. Moreover, risk increased with increasing dose.63,72
In addition to risks posed by radiotherapy, there are potential risks of diagnostic medical and dental radiographs. Several studies conducted by Preston-Martin and colleagues and reported in the 1980s, found increased risk of meningioma with receipt of full-mouth dental radiograph series before 1960 and with frequency of full-mouth dental radiographs.73–76 Subsequent studies conducted in Australia, Germany, and Sweden, however, demonstrated no or equivocal evidence of an association between dental radiographs and meningioma.77–79 The most recent case control studies demonstrated conflicting findings. Among 200 cases diagnosed between 1995 and 1998 in Washington state, having six or more full-mouth radiograph series performed 15 to 40 years before diagnosis was linked with meningioma risk (OR: 2.06, 95% CI: 1.03–4.17), although a dose–response relationship was not evident.80 However, the German component of the large INTERPHONE Study found no association.81
With conflicting findings across studies, the impact of dental radiographs on meningioma risk remains uncertain. Because radiation dose from a full-mouth radiograph series has diminished considerably: from 1000 to 3000 mGy in the 1940s and 1950s to less than 40 mGy by the 1990s,80 future studies must account for potential changes in radiation dose over time. These studies should also have adequate power to detect potential differences in risk among population subgroups by demographic and other characteristics and should explore the impact of newer dental and medical diagnostic procedures (e.g., CT).
Cellular Telephones
Unlike ionizing radiation that is known to damage DNA, the radiation emitted by cell phones that use radiofrequency (RF) energy does not cause ionization of molecules and atoms.82 RF energy in sufficient amounts can heat and potentially damage tissue, but whether and through which mechanism the low-level RF energy emitted by cell phones poses a health risk is not known.83 However, given widespread use of cells phones beginning in the mid-1990s,84 the potential for health risks from this new technology requires investigation.
In a meta-analysis85 that included 527 meningioma cases from eight studies published by December 1, 2005,83,84,86–91 Lahkola and colleagues did not observe an effect of cell phone use on meningioma development (pooled OR: 0.87; 95% CI: 0.72–1.05). Because tumors related to cell phone exposure would likely occur on the side of the head where the phone is most often used, these researchers conducted an additional analysis that examined cell phone use in relation to these ipsilateral meningiomas and found no association. Because cell phone technology has shifted from analogue to digital phone use, these investigators also examined risk of all intracranial tumors combined by cell phone type and again found no association.85
The meta-analysis of meningioma risk described in the preceding text included two reports from the INTERPHONE study,83,86 the largest case-control study conducted to date of the effects of self-reported cell phone use and related exposures on the development of intracranial and other tumors, conducted across 13 countries.92 Since publication of the meta-analysis, two additional reports from the INTERPHONE study, conducted in Germany93 and in Norway,82 have become available. Neither found an increased risk of meningioma with cell phone use. Further, a recent follow-up study on cell phone use in a Danish cohort of 420,095 cell phone subscribers also found no impact of cell phone use on meningioma formation.94
Although the results of these studies are quite consistent, they have some methodological shortcomings. With the exception of the Danish cohort study, most of the evidence regarding cell phone use is based on case control studies with self-reported exposure measurement. In the INTERPHONE study, for example, random and systematic errors in exposure reporting were found.95 In addition, even the most recent published INTERPHONE studies included a relatively small proportion of individuals with 10 or more years of cell phone use.83,86,93 We have learned from studies of ionizing radiation that time from exposure to meningioma formation is long relative to some other tumors. Thus, 20 years or more of follow-up may be needed to examine the effect of cell phones.2,84,85 In addition, exposure may vary depending on the type of device used82 (e.g., hands-free, Bluetooth), which needs to be taken into consideration in future work.
Occupational Exposures
Several hypothesis-generating studies have examined occupation as a proxy for exposure to potential risks of meningioma. As reviewed by Rajaraman and colleagues, these studies found significant associations between this tumor and a number of occupations including carpenters, cooks, chemists, computer specialists, gas station attendants, glassmakers, inspectors, insurance agents, technicians, and toolmakers. In their study, meningioma risk was associated with having ever worked as an autobody painter, designer/decorator, machine operator, motor vehicle driver, industrial production supervisor, teacher or manager, or having served in the military. These investigators suggested that this extensive list might include two categories: (1) individuals exposed to potential carcinogens (benzene, solvents, lead) and (2) individuals in occupations where there is a greater likelihood of diagnosis.96
There have been few studies that have endeavored to measure specific occupational exposures and meningioma risk. The German INTERPHONE study site recently reported no association between occupational exposure to RF energy and meningioma.97 A hospital-based case control study conducted in the United States found no relationship between meningioma and occupational exposure to pesticides in men or women, but did find increased risk among women exposed to herbicides.98 In addition, several studies have detected an elevated risk of meningioma with occupational lead exposure.99–101 The results of all of these occupational studies should be interpreted cautiously, however, owing to the potential for exposure measurement error and other methodological shortcomings. Additional work to examine the effects of lead exposure is warranted.
Other Medical Conditions
Head trauma
Descriptions of and debates about the association between head trauma and meningioma have been occurring for more than 100 years.80,102 Proposed biologic mechanisms, reviewed by Inskip and Bondy, to explain how head injury may lead to neoplastic changes include production of oxygen free radicals, increased cell proliferation, and release of autocoids that may contribute to breakdown of the blood–brain barrier, thereby exposing the brain to agents from which it is normally protected.51,103
During the past 30 years, several case control and cohort studies were conducted to examine this association. Some case control studies have not found an association,79,102 but others have found an increased risk. The latter include several studies that investigated this association among men and women in Los Angeles county,74–76 an international case control study published in 1998104 and additional case control studies conducted in China and in Washington state.99,105 However, because of a common belief among lay persons that head trauma can cause brain tumors,74 differential recall of head injuries between cases and controls that might artificially inflate meningioma risk is an oft sited concern.106 A case control study by Preston-Martin and colleagues that examined the effect of head trauma on both meningiomas and gliomas found that only the risk of meningioma was elevated, which argues against the distortion of study results attributable to recall bias.74 However, one study found a more pronounced effect of mild than severe brain injury on meningioma risk105 and another found that risk was no longer elevated when only serious injuries were included.104 These counterintuitive findings have been attributed to recall bias,106 as cases are more likely than controls to remember and report minor head injuries. Two cohort studies that were not prone to that methodological problem did not find increased risk of meningioma attributable to head trauma.103,107 The largest and most recent ascertained head injury via hospital discharge records and included a Danish cohort of 228,055, followed for an average of 8 years. Relative to population incidence rates, these investigators found an excess of brain tumors among patients with head trauma during the first year after those injuries, which they attributed to the detection of preexisting tumors. There was no excess of meningiomas more than 1 year after the injury (standardized incidence ratio [SIR]: 1.2; 95% CI: 0.8–1.7).106
Allergies
A hyperactive immune state and the anti-inflammatory effects of cytokines characteristic of allergic conditions have been postulated to decrease growth of abnormal cells and protect against the development of brain tumors2,108 Indeed, researchers have observed a protective effect against glioma in a number of studies.3 However, in a meta-analysis of several studies published through 2006,79,109–117 Linos and colleagues did not find that allergy protected against meningioma.108 A subsequent paper, published in 2007, that assessed the impact of allergy on more than 1200 meningioma cases from Denmark, Norway, Finland, Sweden, and southeast England also found no effect.
Breast cancer
There are multiple case reports of patients with breast carcinoma and meningioma in the literature, but relatively few studies have endeavored to quantify an association between these tumors.118 Custer and colleagues identified four studies that provided numeric estimates,119–122 three of which found significant associations.119,120,122 Their own study, based on data from the western Washington State cancer registry, found nonsignificant elevated risks of breast cancer after meningioma diagnosis and of meningioma after breast cancer.118 Since 2002, when those data were published, a study by Lee and colleagues found no association between breast cancer and subsequent meningioma.123 The largest study conducted to date, published in 2007, examined nearly 40 years of Swedish cancer registry data. That study identified 12,012 meningioma patients, 926 of whom developed a subsequent primary diagnosis of cancer. The investigators observed elevated risk of several cancers, including brain and thyroid cancers, across age groups. An increased risk of breast cancer was observed only among women ages 50–59 (SIR: 1.61; 95% CI: 1.23–2.08).124 In two studies of family cancer history, one found no association between these tumors within families,125 whereas a second found a strong association between family breast cancer history and meningioma in adults younger than 50 years of age (OR: 3.9; 95% CI: 1.4–11.0).126
Overall, these findings suggest a possible relationship between breast cancer and meningioma within individuals and across families. If these tumors are related, there may be a common genetic pathway.1,118 Although mutations in breast cancer susceptibility genes were not found among 60 meningioma brain specimens,127 exploration of other possible shared genetic factors would be of interest. There may also be common, hormonally-drive factors between these tumors such as HRT use or late age at menopause.1,118 Research in this area is detailed in the text that follows.
Hormones
The possible association between breast cancer and meningioma, the higher incidence of meningioma in women than men,5 reports that meningiomas may increase in size during pregnancy and the luteal phase of the menstrual cycle,128–130 and the presence of hormone receptors on some meningiomas point to a possible link between hormonal factors and meningioma risk. This area of study has generated considerable interest, as it holds promise for prevention and for development of hormonally based treatments to control tumor growth, although results from clinical studies remain uncertain.131,132 We review work on endogenous and exogenous steroid hormones and on tumor hormone receptors in meningioma.
Endogenous hormones
It is well established that breast cancer risk increases with higher serum estrogen levels and with menstrual and reproductive factors that may increase exposure to endogenous estrogens, including early age at menarche and late age at menopause. Lactation, which decreases the number of ovulatory cycles, and early age at first birth are protective.133 To explore the possibility of shared hormonal factors with breast cancer and to assess the potential impact of circulating levels of estrogen, progesterone, and other steroid hormones on meningioma formation and development, some epidemiologic studies have examined hormonally driven indicators in women.
With regard to age at menarche, two case control studies found no association with meningioma risk123,134 and older age at menarche was associated with higher risk in the large cohort of the Nurse’s Health Study, a finding contrary to the investigators’ expectations.135 A significant effect of parity was not observed in that study, nor in several others.134–136 However, researchers who conducted a hospital-based case control study that included 219 cases did find a strong protective effect of lower age at first pregnancy and of having three or more pregnancies.123 There was a nonsignificant protective trend of duration of breastfeeding in one study,134 and additional work with larger sample sizes is warranted. The impact of menopause was assessed by the Nurses Health Study, which found an increased risk of meningioma among premenopausal women compared with postmenopausal women who had never used hormone therapy (relative risk [RR], 2.48; 95% CI: 1.29–4.77), adjusted for age and body mass index (BMI).135
Higher BMI and adiposity have correlated with higher levels of estrogen and other steroid hormones137–139 and the hypothesis that higher BMI may increase risk of meningioma through such hormonal mechanisms140 has been investigated in both women and men, with conflicting results. In the Nurses Health Study, there was a trend toward increased risk of meningioma with higher BMI,135 although a retrospective study conducted in Germany found no such association.141 Among men, this hypothesis was recently investigated in a small retrospective study of patients who had undergone craniotomies. The investigators found that men with meningiomas were more likely to be obese than men with aneurysms or gliomas.140 Others have not found an association between obesity and meningioma in men,141,142 but additional study with larger samples is needed.
Although the potential for an association between endogenous hormonal factors and meningioma is intriguing, epidemiologic evidence is lacking. Inconsistent results across studies may be due to methodological factors such as small samples sizes and the potential for residual confounding.135 In men, the study of the relationship between meningioma and hormonally driven factors is in an embryonic stage. The impact of age at puberty and of hair loss, among others factors in men, has not to our knowledge been reported and should be assessed. Examination of serum levels in both sexes would help to clarify which steroid hormones may be involved.
Exogenous hormones
There is a modest increased risk of breast cancer among users of combined oral contraceptives (OCs) and hormone replacement therapy (HRT).143 Among six studies of meningioma that examined the influence of OCs,118,123,134,135,144,145 there was no association in four and two found a protective effect. One of these, a population-based case control study that included only spinal meningioma cases, reported decreased estimated risk for those with greater than 3 years of OC use, compared with those who had never used OCs (OR: 0.2; 95% CI: 0.1–0.7).144 The second, a hospital-based case control study, included all 219 incident cases of meningioma recruited from three large medical centers. Compared with never-users, this study found a protective effect for those who had ever used OCs (OR: 0.5; 95% CI: 0.4–0.8) and an even greater effect for current users (OR: 0.2; 95% CI: 0.0–0.8). The Swedish site of the INTERPHONE study examined the impact of non-oral contraceptives including progesterone-only subdermal implants, injections, and intrauterine devices. This study found an elevated estimated risk of meningioma that approached significance (OR: 2.7; 95% CI: 0.9–0.5),145 signaling the need for larger studies that can separately examine the potential effect of estrogen/progestin and progestin-only contraceptives.
We identified seven studies that examined the impact of HRT on meningioma.118,123,134,135,144–146 Three case control studies found no effect,118,123,134 an early case control study restricted to spinal meningiomas found a decreased risk with current use of estrogen-only HRT,144 and three studies found increased risk.135,145,147 The latter include the large cohort of the Nurses Health Study that found a RR of 1.86 (95% CI: 1.07–3.24) for current versus never users of HRT.135 In addition, the Swedish INTERPHONE study showed marginally significant estimates of meningioma risk (in relation to those who had never used HRT), among those who had ever used HRT (OR: 1.7; 95% CI: 1.0–2.8) and among those with 10 or more years of use (OR: 1.9; 95% CI: 1.0–3.8), although a clear dose–response relationship was lacking.145 In the largest and most recently published study on this topic, a retrospective cohort study based on the Mayo Clinic Jacksonville database that included 1390 confirmed cases, the OR was 2.2 for HRT users versus nonusers (95% CI: 1.9–2.6).146
Based on current knowledge of exogenous hormones use, there is little evidence of increased risk of meningioma with OCs, although oral and non-oral preparations including only progesterone may be of concern. With HRT use, there is a suggestion of increased risk of meningioma. Most studies, however, had small sample sizes that precluded meaningful analyses of subgroups (e.g., by parity) and limited power to assess exposure measurement with detail and precision (e.g., dosage, timing, duration of use, and hormone[s] used [estrogen-only, estrogen/progestin, progesterone-only]). In addition, there is a lack of studies of other exogenous hormones. We located only one study that examined the effect of hormones used to treat gynecologic problems (e.g., irregular bleeding) where no association with meningioma was found,145 and we are not aware of any studies that have examined the influence of fertility medications. We were also unable to identify epidemiologic studies of exogenous hormones and meningioma in men. This an important area of inquiry, as widely used prostate cancer treatments such as luteinizing hormone-releasing hormone (LHRH) agonists may contribute to meningioma growth.148 Moreover, in addition to examining the impact of exogenous hormone use on meningioma occurrence, the impact of these hormones on tumor progression remains to be elucidated.131
Tumor hormone receptors
In 1979, Donnell described the presence of estrogen receptor (ER) protein in four of six meningioma specimens.149 Since that seminal work, the prevalence of progesterone, estrogen, and androgen receptors has been quantified by multiple investigators, their potential prognostic value has been explored, some antihormonal treatments have been tried, and epidemiologic research in this area has begun.
In 2004, Wolfsberger reported that 69% (range 10%–100%) of meningiomas were progesterone receptor positive (PR+) across 26 studies published since 1988.150 Although some investigators have found more PR+ meningiomas among women,151,152 others have not corroborated that finding.150,153–155 PR status does not appear to vary by age.156 Notwithstanding Donnell’s early work, ER+ meningiomas appear to be less common that PR+ tumors.157–159 Tumors harboring both PR+ and ER+ receptors were detected in 39% of meningiomas in one study.154 In addition, both identified isoforms of PR have been expressed in meningiomas,160 as have both isoforms of ER.157 Inoue and colleagues found PR-A in 40% and 42% of tumors in men and women respectively, while PR-B was observed in 65% and 53% of male and female tumors.160 Carroll and colleagues detected ER-α in 68% of meningiomas and ER-β in 44%.157 Though less studied, androgen receptors (ARs) have been detected in 29%-67% of meningiomas.154,161–163 In two studies, there were no significant differences in the distributions of ER+ and AR+ tumors by gender,154,164 but another study found AR+ tumors more common among women.
Nuclear localization of PRs in meningiomas suggests they are functional.165 In addition, some in vivo and in vitro studies have demonstrated effectiveness of anti-progesterones in reducing tumor growth, as in one study of nude mice implanted with human meningioma.166 These findings spurred exploration of the potential efficacy of anti-progesterone treatment in clinical settings. However, in long-term (median 35 months) mifepristone treatment of non-resectable meningiomas, only 8 of 28 cases showed minor improvements and adverse effects, including endometrial hyperplasia, were noted.167 Because PR positivity is more often found in WHO Grade I than higher grade tumors, Wolfsberger suggested that PR expression may decrease with tumor progression, potentially limiting the usefulness of anti-progesterone therapies.150 Indeed, low or no PR expression correlates with higher MIB-1 cell proliferation indices,150 apoptosis,164 higher tumor grade155 and other adverse prognostic factors. In addition, there is consistent evidence of worse prognosis measured by shorter disease-free interval158 and greater likelihood of recurrence153 among patients with PR- meningiomas, either as a single predictor or in combination with high proliferative and mitotic indices, and higher tumor grade.155,158 In contrast, ER+ meningiomas, though less prevalent, are evident in higher grade, more aggressive tumors.168 However, in two phase II trials, tamoxifen treatment of meningioma patients has yielded limited benefit,169,170 although a subsequent case report demonstrated effectiveness of the anti-estrogen mepitiostane.171 ARs are likely to be functional,161 but we are not aware of any clinical studies of anti-androgen treatments for meningioma.
Further work to identify potential subgroups of patients by receptor isoform, histologic subtype, and other parameters might boost antihormonal treatment efficacy. For example, in breast cancer, ER-β+ patients obtain less benefit from the antiestrogen therapy tamoxifen than ER-α+ patients. It is possible that the effectiveness of antiestrogen therapy in meningioma may also vary depending on ER isoform.1 The same may apply to the effectiveness of anti-progesterone treatment, given the existence of two PR isoforms. Treatment efficacy may also vary based on endogenous hormonal status, as was the case with mifepristone treatment that was far more effective in men and premenopausal women than in menopausal women.167
Genetic Epidemiology
Family aggregation and hereditary syndromes
Examining whether a disease affects multiple family members is a frequent starting point for assessing its potential genetic etiology. Researchers have conducted several such studies among families affected with meningioma. The results of case control studies have not been consistent, but the most recent did find that individuals with meningioma were more likely to report a family history of benign brain tumors (OR: 4.5; 95% CI: 1.0–21.0).126 The potential for errors in case control and other studies that rely on self-reported family history was overcome in two studies by Hemminki and colleagues that found a familial risk of meningioma using medically verified data from the Swedish Family-Cancer Database, a multigenerational registry with near-complete reporting.125,172 In their most recent analysis of concordant meningioma, the SIRs were 3.06 (95% CI: 1.84–4.79) for offspring with a parental history and 4.41 (95% CI: 2.10–8.14) among siblings.172
But whether familial aggregation is a function of genetic susceptibility, shared environmental factors, or both cannot be determined from these studies. In an effort to disentangle these effects, Malmer and co-investigators used Swedish databases to examine risk of primary brain tumors in first-degree relatives (FDRs) and spouses of affected individuals. They found no excess of meningiomas in spouses (SIR: 0.96; 95% CI: 0.35–2.10) but they did find that the observed number of meningiomas in FDRs was twice the expected number (SIR: 2.17; 95% CI: 1.44–3.14).173
One genetic explanation for family clustering is neurofibromatosis type 2 (NF2), a rare, highly penetrant autosomal dominant disease associated with multiple tumors, including meningiomas.174 NF2 is caused by mutations that inactivate the NF2 tumor suppressor gene, located on the long arm of chromosome 22.175 However, investigators have observed meningioma family clustering in the absence of NF2,172,174,176 which suggests that other genetic factors may be involved.174
Genetic polymorphisms and gene–environment interactions
Because most meningiomas are sporadic and only a small proportion are attributable to rare, highly penetrant mutations, attention is now directed at identifying the potential effects of variation in more common, less penetrant genes, and to the potential effects of these genetic polymorphisms in combination with environmental and other factors. For example, although ionizing radiation is strongly associated with meningioma, fewer than 1% of exposed individuals develop this tumor, which suggests that some individuals may be more genetically susceptible to this environmental risk.177 This was well illustrated in a study of the Tinea Capitis Cohort, augmented by data from the Israeli Cancer Registry. Flint-Richter and Sadetski examined 525 families that included an index member who (1) had radiation-associated meningioma; (2) had radiation but did not develop meningioma; (3) had meningioma with no history of radiation; or (4) had no history of radiation and did not develop meningioma. Compared with index cases in other groups, the index cases with radiation-associated meningioma had a much higher proportion of FDRs who also had radiation-associated meningiomas or cancers. None of the cases had NF2. These findings suggest a genetic susceptibility to the adverse effects of ionizing radiation and compel further study to identify specific genes that may be implicated.176
Likely candidates include DNA repair, cell cycle control, and apoptosis genes. However, three case control studies did not find an association between genetic polymorphisms in tumor suppressor gene TP53 and meningioma in the total samples under investigation.178–180 When the analysis was restricted to individuals with a family history of cancer in one of those studies, the risk of meningioma among those with the CC-CG-CC polymorphism combination was significantly increased (OR: 5.69; 95% CI: 1.81–17.96).178 In addition, Malmer and colleagues did find that haplotypes in ataxia telangiectasia mutated (ATM) were associated with meningioma risk. Specifically, the 1-1-1-2-1 haplotype was increased and the 2-1-2-1-1 haplotype was decreased in meningioma cases relative to controls.179 Further, Rajaraman and fellow investigators found that two variants in CASP8, Ex14-271A>T and Ex13+51G>C, were associated with decreased and increased risk of meningioma, respectively.180 The most recent report included a relatively large subset of subject from the INTERPHONE study (631 cases and 637 population controls) and examined 1127 single-nucleotide polymorphisms (SNPs) in 136 DNA repair genes.181 That study discovered a significant association between meningioma risk and rs4968451 that held after adjustment for multiple comparisons. This SNP maps to intron 4 of the breast cancer susceptibility gene 1-interacting protein 1, thus supporting epidemiologic observations of shared factors between these tumors.181
Sadetzki and colleagues conducted the only study to date that specifically examined the potential impact of DNA repair and cell cycle control genes among irradiated and nonirradiated groups. These investigators found an association between meningioma formation and variants in cell cycle control gene Ki-ras and DNA repair gene ERCC2 among all cases and controls in their study. In addition, they found differences between irradiated and nonirradiated groups. Compared with the CC genotype, the homozygote T genotype of cyclin D1 significantly increased risk of meningioma among nonirradiated individuals and nonsignificantly decreased risk among individuals who had received radiation therapy (P = .005 for interaction). A similar, thou weaker “inverse” effect for the irradiated and nonirradiated groups was shown for the TG compared with the GG genotype of p16 (P = .005 for interaction).177
Rajaraman and colleagues have examined the potential for genetic susceptibility to meningioma among those exposed to lead, a potential environmental risk. Their work focused on the ALAD gene that codes for the enzyme δ-aminolevulinic acid dehydratase (ALAD) which is involved in heme synthesis and is inhibited by lead. Their first study found that the ALAD2 allele of the G177C polymorphism increased risk of meningioma in the total sample and particularly among males.182 A follow-up study included an assessment of occupational lead exposure and found that this exposure increased meningioma risk most notably among those with the ALAD2 allele. They suggest that the association between the ALAD2 variant and meningioma among men observed in their earlier study may relate to greater lead exposure among men than women.183
Some studies have examined the relationship between meningioma and genes involved in metabolism of xenobiotics, steroids, and products of oxidative stress (e.g., S-transferases [GTS], cytochrome P450 [CYP] and NAD(P)H:quinine oxidoreductase 1 [NQ01]). In a meta-analysis of three of these studies,184–186 Lai and colleagues found that the GTST1 null genotype conferred elevated risk (pooled OR 1.95; 95% CI: 1.02–3.76), whereas GTSM1 did not.187 A subsequent study found increased risk of meningioma with the GSTM3 ⁎B/⁎B genotype (stronger among smokers than nonsmokers), and a decreased risk with the CYP1B1 V4321 homozygous variant.188 These findings were not confirmed, however, in the largest study conducted to date. That study, which included 546 meningioma cases, found no association between meningioma and GSTM3, GSTT1, GSTP1, GSTM1, CPY1A1, or NQ01 polymorphisms.189 In contrast to previous studies that relied on hospital-based controls, the controls in the Schwartzbaum study were population-based. This may, in part, account for the null findings in that study; hospital controls may not represent the general population and studies that use such controls may produce different estimates.187
As discussed, there is mounting evidence to support a role for progesterone receptors in meningioma. A recent pilot study of 31 meningioma samples reported that gene expression appeared more strongly associated with PR status than with ER status. Genes on the long arm of chromosome 22 and near the NF2 gene (22q12) were most frequently noted to have expression variation, with significant up-regulation in PR+ versus PR– lesions, suggesting a higher rate of 22q loss in PR– lesions. Pathway analyses indicated that genes in collagen and extracellular matrix pathways were most likely to be differentially expressed by PR status. These data, although preliminary, are the first to examine gene expression for meningioma cases by hormone receptor status and indicate a stronger association with progesterone than with estrogen receptors. PR status is related to the expression of genes near the NF2 gene, mutations in which have been identified as the initial event in many meningiomas. These findings suggest that PR status may be a clinical marker for genetic subgroups of meningioma and warrant further examination in a larger data set.190
As is the case with epidemiologic studies of environmental and other exposures, genetic epidemiology studies are subject to many potential methodological limitations. In the studies reviewed here, several methodological problems may have affected the results. Sample sizes tended to be small, thereby limiting power to detect potentially meaningful associations. In addition, cases that were most ill may have been less likely to participate; some participants were unwilling to donate blood for DNA testing;179 errors in genotyping may have occurred;191 and the hospital controls used in some studies may not have represent the source population. These factors may have introduced bias or limited the generalizability of the study findings. Moreover, polymorphisms in certain genes may have been missed as rare variants may not be examined.179 In addition, the common practice of testing many candidate genes in a given study raises the possibility of detecting a spurious positive association.178 In many cases, the first genetic epidemiology studies to report an association have not been replicated.191 Indeed, the findings presented here should be considered preliminary. Multiple, well-designed studies are need to further examine promising associations identified in exploratory work and to examine additional genetic variants, in combination with environmental and other putative risk factors.
[1] Claus E.B., Bondy M.L., Schildkraut J.M., Wiemels J.L., Wrensch M., Black P.M. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57(6):1088-1095. discussion 1088–1095
[2] Fisher J.L., Schwartzbaum J.A., Wrensch M., Berger M.S. Evaluation of epidemiologic evidence for primary adult brain tumor risk factors using evidence-based medicine. Prog Neurol Surg. 2006;19:54-79.
[3] Fisher J.L., Schwartzbaum J.A., Wrensch M.M., Wiemels J.L. Epidemiology of brain tumors. Neurol Clin. 2007;25(4):867-890. vii
[4] Wrensch M.M., Minn Y.Y., Chew T.T., Bondy M.M., Berger M.S. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-oncol. 2002;4(4):278-299.
[5] CBTRUS. Statistical report: Primary Brain Tumors in the United States, 1998–2002. Central Brain Tumor Registry of the United States, 2005. Published by the
[6] Jukich P.J., McCarthy B.J., Surawicz T.S., Freels S.S., Davis F.G. Trends in incidence of primary brain tumors in the United States, 1985–1994. Neuro-oncol. 2001;3(3):141-151.
[7] Radhakrishnan K., Mokri B., Parisi J.E., O’Fallon W.M., Sunku J., Kurland L.T. The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol. 1995;37(1):67-73.
[8] onlineChristensen H.C., Kosteljanetz M.M., Johansen C.C. Incidences of gliomas and meningiomas in Denmark, 1943 to 1997. Neurosurgery. 2003;52(6):1327-1333. discussion 1333
[9] Klaeboe L.L., Lonn S.S., Scheie D.D., et al. Incidence of intracranial meningiomas in Denmark, Finland, Norway and Sweden, 1968–1997. Int J Cancer. 2005;117(6):996-1001.
[10] Kaneko S.S., Nomura K.K., Yoshimura T.T., Yamaguchi N.N. Trend of brain tumor incidence by histological subtypes in Japan: estimation from the Brain Tumor Registry of Japan, 1973–1993. J Neurooncol. 2002;60(1):61-69.
[11] McCarthy B.J., Davis F.G., Freels S., et al. Factors associated with survival in patients with meningioma. J Neurosurg. 1998;88(5):831-839.
[12] Surawicz T.S., Davis F., Freels S., Laws E.R.Jr, Menck H.R. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40(2):151-160.
[13] Preston-Martin S., Staples M., Farrugia H., Giles G. Primary tumors of the brain, cranial nerves and cranial meninges in Victoria, Australia, 1982–1990: patterns of incidence and survival. Neuroepidemiology. 1993;12(5):270-279.
[14] Sankila R., Kallio M., Jaaskelainen J., Hakulinen T. Long-term survival of 1986 patients with intracranial meningioma diagnosed from 1953 to 1984 in Finland. Comparison of the observed and expected survival rates in a population-based series. Cancer. 1992;70(6):1568-1576.
[15] Talback M., Stenbeck M., Rosen M. Up-to-date long-term survival of cancer patients: an evaluation of period analysis on Swedish Cancer Registry data. Eur J Cancer. 2004;40(9):1361-1372.
[16] Davis F.G., Kupelian V., Freels S., McCarthy B., Surawicz T. Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. Neuro-oncol. 2001;3(3):152-158.
[17] Goldsmith B.B., McDermott M.W. Meningioma. Neurosurg Clin North Am. 2006;17(2):111-120. vi
[18] onlineNakamura M.M., Roser F.F., Michel J.J., Jacobs C.C., Samii M.M. The natural history of incidental meningiomas. Neurosurgery. 2003;53(1):62-70. discussion 70
[19] Go R.S., Taylor B.V., Kimmel D.W. The natural history of asymptomatic meningiomas in Olmsted County. Minnesota. Neurology. 1998;51(6):1718-1720.
[20] Olivero W.C., Lister J.R., Elwood P.W. The natural history and growth rate of asymptomatic meningiomas: a review of 60 patients. J Neurosurg. 1995;83(2):222-224.
[21] Yano S.S., Kuratsu JJ-i. Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg. 2006;105(4):538-543.
[22] Yoneoka Y.Y., Fujii Y.Y., Tanaka R.R. Growth of incidental meningiomas. Acta Neurochir. 2000;142(5):507-511.
[23] Firsching R.P., Fischer A.A., Peters R.R., Thun F.F., Klug N.N. Growth rate of incidental meningiomas. J Neurosurg. 1990;73(4):545-547.
[24] Herscovici Z.Z., Rappaport Z.Z., Sulkes J.J., Danaila L.L., Rubin G.G. Natural history of conservatively treated meningiomas. Neurology. 2004;63(6):1133-1134.
[25] Bindal R., Goodman J.M., Kawasaki A., Purvin V., Kuzma B. The natural history of untreated skull base meningiomas. Surg Neurol. 2003;59(2):87-92. discussion 92
[26] Van Havenbergh T., Carvalho G., Tatagiba M., Plets C., Samii M. Natural history of petroclival meningiomas. Neurosurgery. 2003;52(1):55-62. discussion 62–74
[27] onlineMathiesen T.T., Lindquist C.C., Kihlström L.L., Karlsson B.B. Recurrence of cranial base meningiomas. Neurosurgery. 1996;39(1):2-7. discussion 8
[28] Stafford S.L., Perry A.A., Suman V.J., et al. Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc. 1998;73(10):936-942.
[29] Condra K.S., Buatti J.M., Mendenhall W.M., Friedman W.A., Marcus R.B., Rhoton A.L. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39(2):427-436.
[30] Black P.M., Villavicencio A.T., Rhouddou C.C., Loeffler J.S. Aggressive surgery and focal radiation in the management of meningiomas of the skull base: preservation of function with maintenance of local control. Acta Neurochir. 2001;143(6):555-562.
[31] onlineLittle K.M., Friedman A.H., Sampson J.H., Wanibuchi M.M., Fukushima T.T. Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery. 2005;56(3):546-559. discussion 546
[32] Sindou M.M., Wydh E.E., Jouanneau E.E., Nebbal M.M., Lieutaud T.T. Long-term follow-up of meningiomas of the cavernous sinus after surgical treatment alone. J Neurosurg. 2007;107(5):937-944.
[33] Karnofsky D., Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod C., editor. Evaluation of Chemotherapeutic Agents. New York: Columbia University Press; 1949:191-205.
[34] De Jesus O., Sekhar L.N., Parikh H.K., Wright D.C., Wagner D.P. Long-term follow-up of patients with meningiomas involving the cavernous sinus: recurrence, progression, and quality of life. Neurosurgery. 1996;39(5):915-919. discussion 919–920
[35] Roser F., Ebner F.H., Ritz R., Samii M., Tatagiba M.S., Nakamura M. Management of skull based meningiomas in the elderly patient. J Clin Neurosci. 2007;14(3):224-228.
[36] Natarajan S.K., Sekhar L.N., Schessel D., Morita A. Petroclival meningiomas: multimodality treatment and outcomes at long-term follow-up. Neurosurgery. 2007;60(6):965-979. discussion 979–981
[37] McHorney C.A., Ware J.E., Lu J.F., Sherbourne C.D. The MOS 36–item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40-66.
[38] McHorney C.A., Ware J.E., Raczek A.E. The MOS 36–Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247-263.
[39] Ware J.E., Sherbourne C.D. The MOS 36–item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
[40] Lang D.A., Neil-Dwyer G., Garfield J. Outcome after complex neurosurgery: the caregiver’s burden is forgotten. J Neurosurg. 1999;91(3):359-363.
[41] Neil-Dwyer G., Lang D.A., Davis A. Outcome from complex neurosurgery: an evidence based approach. Acta Neurochir. 2000;142(4):367-371.
[42] Kalkanis S.N., Quinones-Hinojosa A., Buzney E., Ribaudo H.J., Black P.M. Quality of life following surgery for intracranial meningiomas at Brigham and Women’s Hospital: a study of 164 patients using a modification of the functional assessment of cancer therapy-brain questionnaire. J Neurooncol. 2000;48(3):233-241.
[43] Mohsenipour I., Deusch E., Gabl M., Hofer M., Twerdy K. Quality of life in patients after meningioma resection. Acta Neurochir. 2001;143(6):547-553.
[44] Goyal L.K., Suh J.H., Mohan D.S., Prayson R.A., Lee J.J., Barnett G.H. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys. 2000;46(1):57-61.
[45] Harris A.E., Lee J.Y.K., Omalu B.B., Flickinger J.C., Kondziolka D.D., Lunsford L.D. The effect of radiosurgery during management of aggressive meningiomas. Surg Neurol. 2003;60(4):298-305. discussion 305
[46] Liu Y.Y., Liu M.M., Li F.F., Wu C.C., Zhu S.S. Malignant meningiomas: a retrospective study of 22 cases. Bull Cancer. 2007;94(10):31.
[47] Palma L.L., Celli P.P., Franco C.C., Cervoni L.L., Cantore G.G. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg. 1997;86(5):793-800.
[48] Coke C.C., Corn B.W., Werner-Wasik M.M., Xie Y.Y., Curran W.J. Atypical and malignant meningiomas: an outcome report of seventeen cases. J Neurooncol. 1998;39(1):65-70.
[49] Pasquier D., Bijmolt S., Veninga T., et al. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the rare cancer network. Int J Radiat Oncol Biol Phys. 2008;71:1388-1393.
[50] DeAngelis L.M. Brain tumors. N Engl J Med. 2001;344(2):114-123.
[51] Bondy M.M., Ligon B.L. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29(3):197-205.
[52] Harrison M.J., Wolfe D.E., Lau T.S., Mitnick R.J., Sachdev V.P. Radiation-induced meningiomas: experience at the Mount Sinai Hospital and review of the literature. J Neurosurg. 1991;75(4):564-574.
[53] Yonehara S.S., Brenner A.V., Kishikawa M.M., et al. Clinical and epidemiologic characteristics of first primary tumors of the central nervous system and related organs among atomic bomb survivors in Hiroshima and Nagasaki, 1958–1995. Cancer. 2004;101(7):1644-1654.
[54] Sadamori N.N., Shibata S.S., Mine M.M., et al. Incidence of intracranial meningiomas in Nagasaki atomic-bomb survivors. Int J Cancer. 1996;67(3):318-322.
[55] Shintani T.T., Hayakawa N.N., Hoshi M.M., et al. High incidence of meningioma among Hiroshima atomic bomb survivors. J Radiat Res. 1999;40(1):49-57.
[56] Preston D.L., Ron E.E., Yonehara S.S., et al. Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst. 2002;94(20):1555-1563.
[57] Cahan W., Woodward H., Higinbotham N.L., et al. Sarcomas arising in irradiated bone. Report of eleven cases. Cancer. 1948;1:3-29.
[58] Al-Mefty O.O., Topsakal C.C., Pravdenkova S.S., Sawyer J.R., Harrison M.J. Radiation-induced meningiomas: clinical, pathological, cytokinetic, and cytogenetic characteristics. J Neurosurg. 2004;100(6):1002-1013.
[59] Musa B.S., Pople I.K., Cummins B.H. Intracranial meningiomas following irradiation–a growing problem? Br J Neurosurg. 1995;9(5):629-637.
[60] Sadetzki S.S., Flint-Richter P.P., Ben-Tal T.T., Nass D.D. Radiation-induced meningioma: a descriptive study of 253 cases. J Neurosurg. 2002;97(5):1078-1082.
[61] Caroli E.E., Russillo M.M., Ferrante L.L. Intracranial meningiomas in children: report of 27 new cases and critical analysis of 440 cases reported in the literature. J Child Neurol. 2006;21(1):31-36.
[62] Salvati M.M., Caroli E.E., Brogna C.C., Orlando E.R., Delfini R.R. High-dose radiation-induced meningiomas. Report of five cases and critical review of the literature. Tumori. 2003;89(4):443-447.
[63] Sadetzki S.S., Chetrit A.A., Freedman L.L., Stovall M.M., Modan B.B., Novikov I.I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163(4):424-432.
[64] Gold D.G., Neglia J.P., Dusenbery K.E. Second neoplasms after megavoltage radiation for pediatric tumors. Cancer. 2003;97(10):2588-2596.
[65] Goshen Y.Y., Stark B.B., Kornreich L.L., Michowiz S.S., Feinmesser M.M., Yaniv I.I. High incidence of meningioma in cranial irradiated survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49(3):294-297.
[66] Hijiya N.N., Hudson M.M., Lensing S.S., et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297(11):1207-1215.
[67] Mack E.E., Wilson C.B. Meningiomas induced by high-dose cranial irradiation. J Neurosurg. 1993;79(1):28-31.
[68] Neglia J.P., Robison L.L., Stovall M.M., et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98(21):1528-1537.
[69] Kawahara I.I., Masui K.K., Horie N.N., et al. Radiation-induced meningioma following prophylactic radiotherapy for acute lymphoblastic leukemia in childhood. Pediatr Neurosurg. 2007;43(1):36-41.
[70] Phillips L.E., Frankenfeld C.L., Drangsholt M.M., Koepsell T.D., van Belle G.G., Longstreth W.T. Intracranial meningioma and ionizing radiation in medical and occupational settings. Neurology. 2005;64(2):350-352.
[71] Yousaf I.I., Byrnes D.P., Choudhari K.A. Meningiomas induced by high dose cranial irradiation. Br J Neurosurg. 2003;17(3):219-225.
[72] Ron E.E., Modan B.B., Boice J.D., et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319(16):1033-1039.
[73] Preston-Martin S., Hendersen B., Bernstein L.L. Medical and dental x rays as risk factors for recently diagnosed tumors of the head. Natl Cancer Inst Monogra. 1985;69:175-179.
[74] Preston-Martin S.S., Mack W.W., Henderson B.E. Risk factors for gliomas and meningiomas in males in Los Angeles County. Cancer Res. 1989;49(21):6137-6143.
[75] Preston-Martin S.S., Paganini-Hill A.A., Henderson B.E., Pike M.C., Wood C.C. Case-control study of intracranial meningiomas in women in Los Angeles County, California. J Natl Cancer Inst. 1980;65(1):67-73.
[76] Preston-Martin S.S., Yu M.C., Henderson B.E., Roberts C.C. Risk factors for meningiomas in men in Los Angeles County. J Natl Cancer Inst. 1983;70(5):863-866.
[77] Rodvall Y.Y., Ahlbom A.A., Pershagen G.G., Nylander M.M., Spännare B.B. Dental radiography after age 25 years, amalgam fillings and tumours of the central nervous system. Oral Oncol. 1998;34(4):265-269.
[78] Ryan P.P., Lee M.W., North B.B., McMichael A.J. Amalgam fillings, diagnostic dental x-rays and tumours of the brain and meninges. Eur J Cancer B Oral Oncol. 1992;28B(2):91-95.
[79] Schlehofer B.B., Blettner M.M., Becker N.N., Martinsohn C.C., Wahrendorf J.J. Medical risk factors and the development of brain tumors. Cancer. 1992;69(10):2541-2547.
[80] Longstreth W.T., Phillips L.E., Drangsholt M.M., et al. Dental X-rays and the risk of intracranial meningioma: a population-based case-control study. Cancer. 2004;100(5):1026-1034.
[81] Blettner M.M., Schlehofer B.B., Samkange-Zeeb F.F., Berg G.G., Schlaefer K.K., Schüz J.J. Medical exposure to ionising radiation and the risk of brain tumours: Interphone study group, Germany. Eur J Cancer. 2007;43(13):1990-1998.
[82] Klaeboe L.L., Blaasaas K.G., Tynes T.T. Use of mobile phones in Norway and risk of intracranial tumours. Eur J Cancer Prev. 2007;16(2):158-164.
[83] Lönn S.S., Ahlbom A.A., Hall P.P., Feychting M.M. Long-term mobile phone use and brain tumor risk. Am J Epidemiol. 2005;161(6):526-535.
[84] Inskip P.D., Tarone R.E., Hatch E.E., et al. Cellular-telephone use and brain tumors. N Engl J Med. 2001;344(2):79-86.
[85] Lahkola A.A., Tokola K.K., Auvinen A.A. Meta-analysis of mobile phone use and intracranial tumors. Scand J Work Health Environ. 2006;32(3):171-177.
[86] Christensen H.C., Schüz J.J., Kosteljanetz M.M., et al. Cellular telephones and risk for brain tumors: a population-based, incident case-control study. Neurology. 2005;64(7):1189-1195.
[87] Hardell L.L., Hallquist A.A., Mild K.H., Carlberg M.M., Påhlson A.A., Lilja A.A. Cellular and cordless telephones and the risk for brain tumours. Eur J Cancer Prev. 2002;11(4):377-386.
[88] Hardell L.L., Näsman A.A., Påhlson A.A., Hallquist A.A., Hansson Mild K.K. Use of cellular telephones and the risk for brain tumours: a case-control study. Int J Oncol. 1999;15(1):113-116.
[89] Johansen C.C., Boice J.J., McLaughlin J.J., Olsen J.J. Cellular telephones and cancer–a nationwide cohort study in Denmark. J Natl Cancer Inst. 2001;93(3):203-207.
[90] Auvinen A.A., Hietanen M.M., Luukkonen R.R., Koskela RSR-S. Brain tumors and salivary gland cancers among cellular telephone users. Epidemiology. 2002;13(3):356-359.
[91] Hardell L.L., Carlberg M.M., Hansson Mild K.K. Case-control study on cellular and cordless telephones and the risk for acoustic neuroma or meningioma in patients diagnosed 2000–2003. Neuroepidemiology. 2005;25(3):120-128.
[92] Cardis E.E., Richardson L.L., Deltour I.I., et al. The INTERPHONE study: design, epidemiological methods, and description of the study population. Eur J Epidemiol. 2007;22(9):647-664.
[93] Schüz J.J., Böhler E.E., Berg G.G., et al. Cellular phones, cordless phones, and the risks of glioma and meningioma (Interphone Study Group, Germany). Am J Epidemiol. 2006;163(6):512-520.
[94] Schüz J.J., Jacobsen R.R., Olsen J.H., Boice J.D., McLaughlin J.K., Johansen C.C. Cellular telephone use and cancer risk: update of a nationwide Danish cohort. J Natl Cancer Inst. 2006;98(23):1707-1713.
[95] Vrijheid M.M., Cardis E.E., Armstrong B.K., et al. Validation of short term recall of mobile phone use for the Interphone study. Occup Environ Med. 2006;63(4):237-243.
[96] Rajaraman P.P., De Roos A.J., Stewart P.A., et al. Occupation and risk of meningioma and acoustic neuroma in the United States. Am J Indust Med. 2004;45(5):395-407.
[97] Berg G.G., Spallek J.J., Schüz J.J., et al. Occupational exposure to radio frequency/microwave radiation and the risk of brain tumors: Interphone Study Group, Germany. Am J Epidemiol. 2006;164(6):538-548.
[98] Samanic C.M., De Roos A.J., Stewart P.A., Rajaraman P.P., Waters M.A., Inskip P.D. Occupational exposure to pesticides and risk of adult brain tumors. Am J Epidemiol. 2008. Advanced Access published online Feburary 24
[99] Hu J.J., Little J.J., Xu T.T., et al. Risk factors for meningioma in adults: a case-control study in northeast China. Int J Cancer. 1999;83(3):299-304.
[100] Navas-Acién A.A., Pollán M.M., Gustavsson P.P., Plato N.N. Occupation, exposure to chemicals and risk of gliomas and meningiomas in Sweden. Am J Indust Med. 2002;42(3):214-227.
[101] Cocco P.P., Heineman E.F., Dosemeci M.M. Occupational risk factors for cancer of the central nervous system (CNS) among US women. Am J Indust Med. 1999;36(1):70-74.
[102] Monteiro G.T.R., Pereira R.A., Koifman R.J., Koifman S.S. Head injury and brain tumours in adults: a case-control study in Rio de Janeiro, Brazil. Eur J Cancer. 2006;42(7):917-921.
[103] Inskip P.D., Linet M.S., Heineman E.F. Etiology of brain tumors in adults. Epidemiol Rev. 1995;17(2):382-414.
[104] Preston-Martin S.S., Pogoda J.M., Schlehofer B.B., et al. An international case-control study of adult glioma and meningioma: the role of head trauma. Int J Epidemiol. 1998;27(4):579-586.
[105] Phillips L.E., Koepsell T.D., van Belle G.G., Kukull W.A., Gehrels J.A., Longstreth W.T. History of head trauma and risk of intracranial meningioma: population-based case-control study. Neurology. 2002;58(12):1849-1852.
[106] Inskip P.D., Mellemkjaer L.L., Gridley G.G., Olsen J.H. Incidence of intracranial tumors following hospitalization for head injuries (Denmark). Cancer Causes Control. 1998;9(1):109-116.
[107] Annegers J.F., Laws E.R., Kurland L.T., Grabow J.D. Head trauma and subsequent brain tumors. Neurosurgery. 1979;4(3):203-206. online
[108] Linos E.E., Raine T.T., Alonso A.A., Michaud D.D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99(20):1544-1550.
[109] Brenner A.V., Linet M.S., Fine H.A., et al. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int J Cancer. 2002;99(2):252-259.
[110] Cicuttini F.M., Hurley S.F., Forbes A.A., et al. Association of adult glioma with medical conditions, family and reproductive history. Int J Cancer. 1997;71(2):203-207.
[111] Hochberg F.F., Toniolo P.P., Cole P.P., Salcman M.M. Nonoccupational risk indicators of glioblastoma in adults. J Neurooncol. 1990;8(1):55-60.
[112] Ryan P.P., Lee M.W., North B.B., McMichael A.J. Risk factors for tumors of the brain and meninges: results from the Adelaide Adult Brain Tumor Study. Int J Cancer. 1992;51(1):20-27.
[113] Schlehofer B.B., Blettner M.M., Preston-Martin S.S., et al. Role of medical history in brain tumour development. Results from the international adult brain tumour study. Int J Cancer. 1999;82(2):155-160.
[114] Schoemaker M.J., Swerdlow A.J., Hepworth S.J., van Tongeren M.M., Muir K.R., McKinney P.A. History of allergic disease and risk of meningioma. Am J Epidemiol. 2007;165(5):477-485.
[115] Schwartzbaum J.J., Ahlbom A.A., Malmer B.B., et al. Polymorphisms associated with asthma are inversely related to glioblastoma multiforme. Cancer Res. 2005;65(14):6459-6465.
[116] Schwartzbaum J.J., Jonsson F.F., Ahlbom A.A., et al. Cohort studies of association between self-reported allergic conditions, immune-related diagnoses and glioma and meningioma risk. Int J Cancer. 2003;106(3):423-428.
[117] Wiemels J.L., Wiencke J.K., Sison J.D., Miike R.R., McMillan A.A., Wrensch M.M. History of allergies among adults with glioma and controls. Int J Cancer. 2002;98(4):609-615.
[118] Custer B.S., Koepsell T.D., Mueller B.A. The association between breast carcinoma and meningioma in women. Cancer. 2002;94(6):1626-1635.
[119] Emry J. The association between breast cancer and meningioma [master’s thesis]. University of Southern California, 1984.
[120] Helseth A.A., Mørk S.S.J., Glattre E.E. Neoplasms of the central nervous system in Norway. V. Meningioma and cancer of other sites. An analysis of the occurrence of multiple primary neoplasms in meningioma patients in Norway from 1955 through 1986. APMIS. 1989;97(8):738-744.
[121] Jacobs D.H., Holmes F.F., McFarlane M.J. Meningiomas are not significantly associated with breast cancer. Arch Neurol. 1992;49(7):753-756.
[122] Schoenberg B.S., Christine B.W., Whisnant J.P. Nervous system neoplasms and primary malignancies of other sites. The unique association between meningiomas and breast cancer. Neurology. 1975;25(8):705-712.
[123] Lee E.E., Grutsch J.J., Persky V.V., Glick R.R., Mendes J.J., Davis F.F. Association of meningioma with reproductive factors. Int J Cancer. 2006;119(5):1152-1157.
[124] Davis F.F., Tavelin B.B., Grutsch J.J., Malmer B.B. Second primary tumors following a diagnosis of meningioma in Sweden, 1958–1997. Neuroepidemiology. 2007;29(1–2):101-106.
[125] Hemminki K.K., Li X.X., Collins V.P. Parental cancer as a risk factor for brain tumors (Sweden). Cancer Causes Control. 2001;12(3):195-199.
[126] Hill D.A., Linet M.S., Black P.M., et al. Meningioma and schwannoma risk in adults in relation to family history of cancer. Neuro-oncol. 2004;6(4):274-280.
[127] Kirsch M.M., Zhu J.J., Black P.M. Analysis of the BRCA1 and BRCA2 genes in sporadic meningiomas. Genes Chromosomes Cancer. 1997;20(1):53-59.
[128] Bickerstaff E.R., Small J.M., Guest I.A. The relapsing course of certain meningiomas in relation to pregnancy and menstruation. J Neurol Neurosurg Psychiatry. 1958;21:89-91.
[129] Smith J.S., Quiñones-Hinojosa A.A., Harmon-Smith M.M., Bollen A.W., McDermott M.W. Sex steroid and growth factor profile of a meningioma associated with pregnancy. Can J Neurol Sci. 2005;32(1):122-127.
[130] Hatiboglu M.A., Cosar M., Iplikeioglu A.C., Ozcan D. Sex steroid and epidermal growth factor profile of giant meningiomas associated with pregnancy. Surg Neurol. 2008;69:356-362.
[131] Claus E.B., Black P.M., Bondy M.L., et al. Exogenous hormone use and meningioma risk: what do we tell our patients? Cancer. 2007;110(3):471-476.
[132] Sanson M.M., Cornu P.P. Biology of meningiomas. Acta Neurochir. 2000;142(5):493-505.
[133] Feigelson H.S. Breast cancer: epidemiology and molecular endocrinology. In: Henderson B.E., Ponder B., Ross R.K., editors. Hormones, Genes, and Cancer. New York: Oxford University Press; 2003:120-138.
[134] Hatch E.E., Linet M.S., Zhang J.J., et al. Reproductive and hormonal factors and risk of brain tumors in adult females. Int J Cancer. 2005;114(5):797-805.
[135] Jhawar B.S., Fuchs C.S., Colditz G.A., Stampfer M.J. Sex steroid hormone exposures and risk for meningioma. J Neurosurg. 2003;99(5):848-853.
[136] Lambe M.M., Coogan P.P., Baron J.J. Reproductive factors and the risk of brain tumors: a population-based study in Sweden. Int J Cancer. 1997;72(3):389-393.
[137] Hankinson S.E., Willett W.C., Manson J.E., et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87(17):1297-1302.
[138] Kaye S.A., Folsom A.R., Soler J.T., Prineas R.J., Potter J.D. Associations of body mass and fat distribution with sex hormone concentrations in postmenopausal women. Int J Epidemiol. 1991;20(1):151-156.
[139] Tworoger S.S., Eliassen A.H., Missmer S.A., et al. Birthweight and body size throughout life in relation to sex hormones and prolactin concentrations in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2494-2501.
[140] onlineAghi M.K., Eskandar E.N., Carter B.S., Curry W.T., Barker F.G. Increased prevalence of obesity and obesity-related postoperative complications in male patients with meningiomas. Neurosurgery. 2007;61(4):754-760. discussion 760
[141] Schneider B.B., Pülhorn H.H., Röhrig B.B., Rainov N.G. Predisposing conditions and risk factors for development of symptomatic meningioma in adults. Cancer Detect Prev. 2005;29(5):440-447.
[142] Jacobs D.H., McFarlane M.J., Holmes F.F. Meningiomas and obesity reconsidered. Ann Neurol. 1986;20(3):376.
[143] Reeves G.K., Banks E., Key J.A. The impact of exogenous hormone use on breast cancer risk. In: Henderson B.E., Ponder B., Ross R.K., editors. Hormones, Genes, and Cancer. New York: Oxford University Press; 2003:139-156.
[144] Preston-Martin S.S., Monroe K.K., Lee P.J., et al. Spinal meningiomas in women in Los Angeles County: investigation of an etiological hypothesis. Cancer Epidemiol Biomarkers Prev. 1995;4(4):333-339.
[145] Wigertz A.A., Lönn S.S., Mathiesen T.T., Ahlbom A.A., Hall P.P., Feychting M.M. Risk of brain tumors associated with exposure to exogenous female sex hormones. Am J Epidemiol. 2006;164(7):629-636.
[146] Blitshteyn S.S., Crook J.E., Jaeckle K.A. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26(2):279-282.
[147] Blitshteyn S., Crook J., Jaeckle K.A. Abstract EP-02. Is there an association between meningioma and hormone replacement therapy? A study of women at the Mayo Clinic Jacksonville. Ninth Annual Meeting of the Society for Neuro-Oncology, November 18–21, 2004. 2004
[148] Lee K.L., Terris M.K. Luteinizing hormone-releasing hormone agonists and meningioma: a treatment dilemma. Urology. 2003;62(2):351.
[149] Donnell M.S., Meyer G.A., Donegan W.L. Estrogen-receptor protein in intracranial meningiomas. J Neurosurg. 1979;50(4):499-502.
[150] Wolfsberger S.S., Doostkam S.S., Boecher-Schwarz HGH-G, et al. Progesterone-receptor index in meningiomas: correlation with clinico-pathological parameters and review of the literature. Neurosurg Rev. 2004;27(4):238-245.
[151] Blankenstein M.A., Verheijen F.M., Jacobs J.M., Donker T.H., van Duijnhoven M.W., Thijssen J.H. Occurrence, regulation, and significance of progesterone receptors in human meningioma. Steroids. 2000;65(10–11):795-800.
[152] Carroll R.S., Glowacka D.D., Dashner K.K., Black P.M. Progesterone receptor expression in meningiomas. Cancer Res. 1993;53(6):1312-1316.
[153] Fewings P.E., Battersby R.D., Timperley W.R. Long-term follow up of progesterone receptor status in benign meningioma: a prognostic indicator of recurrence? J Neurosurg. 2000;92(3):401-405.
[154] Korhonen K.K., Salminen T.T., Raitanen J.J., Auvinen A.A., Isola J.J., Haapasalo H.H. Female predominance in meningiomas can not be explained by differences in progesterone, estrogen, or androgen receptor expression. J Neurooncol. 2006;80(1):1-7.
[155] Roser F.F., Nakamura M.M., Bellinzona M.M., Rosahl S.K., Ostertag H.H., Samii M.M. The prognostic value of progesterone receptor status in meningiomas. J Clin Pathol. 2004;57(10):1033-1037.
[156] Roser F.F., Nakamura M.M., Ritz R.R., et al. Proliferation and progesterone receptor status in benign meningiomas are not age dependent. Cancer. 2005;104(3):598-601.
[157] Carroll R.S., Zhang J.J., Black P.M. Expression of estrogen receptors alpha and beta in human meningiomas. J Neurooncol. 1999;42(2):109-116.
[158] Hsu D.W., Efird J.T., Hedley-Whyte E.T. Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg. 1997;86(1):113-120.
[159] Maiuri F.F., De Caro M.B., Esposito F.F., et al. Recurrences of meningiomas: predictive value of pathological features and hormonal and growth factors. J Neurooncol. 2007;82(1):63-68.
[160] Inoue T.T., Akahira JJ-I, Suzuki T.T., et al. Progesterone production and actions in the human central nervous system and neurogenic tumors. J Clin Endocrinol Metab. 2002;87(11):5325-5331.
[161] Carroll R.S., Zhang J.J., Dashner K.K., Sar M.M., Wilson E.M., Black P.M. Androgen receptor expression in meningiomas. J Neurosurg. 1995;82(3):453-460.
[162] Chen J.J., Chen G.G. Expression of androgen receptor in meningiomas. J Tongji Med U. 2001;21(2):140-142.
[163] Maxwell M.M., Galanopoulos T.T., Neville-Golden J.J., Antoniades H.N. Expression of androgen and progesterone receptors in primary human meningiomas. J Neurosurg. 1993;78(3):456-462.
[164] Konstantinidou A.E., Korkolopoulou P.P., Mahera H.H., et al. Hormone receptors in non-malignant meningiomas correlate with apoptosis, cell proliferation and recurrence-free survival. Histopathology. 2003;43(3):280-290.
[165] onlineCarroll R.S., Zhang J.J., Dashner K.K., Black P.M. Progesterone and glucocorticoid receptor activation in meningiomas. Neurosurgery. 1995;37(1):92-97.
[166] Olson J.J., Beck D.W., Schlechte J.A., Loh P.M. Effect of the antiprogesterone RU-38486 on meningioma implanted into nude mice. J Neurosurg. 1987;66(4):584-587.
[167] Grunberg S.M., Weiss M.H., Russell C.A., et al. Long-term administration of mifepristone (RU486): clinical tolerance during extended treatment of meningioma. Cancer Invest. 2006;24(8):727-733.
[168] Pravdenkova S.S., Al-Mefty O.O., Sawyer J.J., Husain M.M. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. J Neurosurg. 2006;105(2):163-173.
[169] Flaherty L.E., Liu P.Y., Mitchell M.S., et al. The addition of tamoxifen to dacarbazine and cisplatin in metastatic malignant melanoma. A phase II trial of the Southwest Oncology Group, (SWOG-8921). Am J Clin Oncol Cancer Clin Trials. 1996;19(2):108-113.
[170] Goodwin J.W., Crowley J.J., Eyre H.J., Stafford B.B., Jaeckle K.A., Townsend J.J. A phase II evaluation of tamoxifen in unresectable or refractory meningiomas: a Southwest Oncology Group study. J Neurooncol. 1993;15(1):75-77.
[171] Oura S.S., Sakurai T.T., Yoshimura G.G., et al. Regression of a presumed meningioma with the antiestrogen agent mepitiostane. Case report. J Neurosurg. 2000;93(1):132-135.
[172] Hemminki K.K., Li X.X. Familial risks in nervous system tumors. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1137-1142.
[173] Malmer B.B., Henriksson R.R., Grönberg H.H. Familial brain tumours-genetics or environment? A nationwide cohort study of cancer risk in spouses and first-degree relatives of brain tumour patients. Int J Cancer. 2003;106(2):260-263.
[174] Maxwell M.M., Shih S.D., Galanopoulos T.T., Hedley-Whyte E.T., Cosgrove G.R. Familial meningioma: analysis of expression of neurofibromatosis 2 protein Merlin. Report of two cases. J Neurosurg. 1998;88(3):562-569.
[175] Antinheimo J., Sankila R., Carpen O., Pukkala E., Sainio M., Jaaskelainen J. Population-based analysis of sporadic and type 2 neurofibromatosis-associated meningiomas and schwannomas. Neurology. 2000;54(1):71-76.
[176] Flint-Richter P., Sadetzki S. Genetic predisposition for the development of radiation-associated meningioma: an epidemiological study. Lancet Oncol. May 2007;8(5):403-410.
[177] Sadetzki S.S., Flint-Richter P.P., Starinsky S.S., et al. Genotyping of patients with sporadic and radiation-associated meningiomas. Cancer Epidemiol Biomarkers Prev. 2005;14(4):969-976.
[178] Malmer B.B., Feychting M.M., Lönn S.S., Ahlbom A.A., Henriksson R.R. p53 Genotypes and risk of glioma and meningioma. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2220-2223.
[179] Malmer B.S., Feychting M.M., Lönn S.S., et al. Genetic variation in p53 and ATM haplotypes and risk of glioma and meningioma. J Neurooncol. 2007;82(3):229-237.
[180] Rajaraman P.P., Wang S.S., Rothman N.N., et al. Polymorphisms in apoptosis and cell cycle control genes and risk of brain tumors in adults. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1655-1661.
[181] Bethke L.L., Murray A.A., Webb E.E., et al. Comprehensive analysis of DNA repair gene variants and risk of meningioma. J Natl Cancer Inst. 2008;100(4):270-276.
[182] Rajaraman P.P., Schwartz B.S., Rothman N.N., et al. Delta-aminolevulinic acid dehydratase polymorphism and risk of brain tumors in adults. Environ Health Perspect. 2005;113(9):1209-1211.
[183] Rajaraman P.P., Stewart P.A., Samet J.M., et al. Lead, genetic susceptibility, and risk of adult brain tumors. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2514-2520.
[184] De Roos A.J., Rothman N.N., Inskip P.D., et al. Genetic polymorphisms in GSTM1, -P1, -T1, and CYP2E1 and the risk of adult brain tumors. Cancer Epidemiol Biomarkers Prev. 2003;12(1):14-22.
[185] Elexpuru-Camiruaga J.J., Buxton N.N., Kandula V.V., et al. Susceptibility to astrocytoma and meningioma: influence of allelism at glutathione S-transferase (GSTT1 and GSTM1) and cytochrome P-450 (CYP2D6) loci. Cancer Res. 1995;55(19):4237-4239.
[186] Pinarbasi H.H., Silig Y.Y., Gurelik M.M. Genetic polymorphisms of GSTs and their association with primary brain tumor incidence. Cancer Genet Cytogenet. 2005;156(2):144-149.
[187] Lai R.R., Crevier L.L., Thabane L.L. Genetic polymorphisms of glutathione S-transferases and the risk of adult brain tumors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1784-1790.
[188] De Roos A.J., Rothman N.N., Brown M.M., et al. Variation in genes relevant to aromatic hydrocarbon metabolism and the risk of adult brain tumors. Neuro-oncol. 2006;8(2):145-155.
[189] Schwartzbaum J.A., Ahlbom A.A., Lönn S.S., et al. An international case-control study of glutathione transferase and functionally related polymorphisms and risk of primary adult brain tumors. Cancer Epidemiol Biomarkers Prev. 2007;16(3):559-565.
[190] Claus E.B., Park P.J., Carroll R.R., Chan J.J., Black P.M. Specific genes expressed in association with progesterone receptors in meningioma. Cancer Res. 2008;68(1):314-322.
[191] Ioannidis J.P., Boffetta P.P., Little J.J., et al. Assessment of cumulative evidence on genetic associations: interim guidelines. Int J Epidemiol. 2008;37(1):120-132.