CHAPTER 200 Ependymoma
Clinical Overview
Roughly 10% of pediatric brain tumors are classified as ependymoma, and the mean age at diagnosis is 4 to 6 years.1–5 About 5% of patients with ependymoma present with evidence of leptomeningeal dissemination at the time of diagnosis, as determined by a combination of magnetic resonance imaging (MRI) with contrast enhancement and cerebrospinal fluid (CSF) sampling.6 Historical 5-year survival estimates range from 50% to 64%; progression-free survival estimates are lower at 23% to 45%.2,3,7–9 Tumor typically recurs at the site of the original tumor, but in roughly 20% of cases there is isolated metastatic recurrence. The median time to recurrence is 13 to 25 months, and most children who are disease free at 5 years are cured (although late recurrences have been described).2,3,7–9
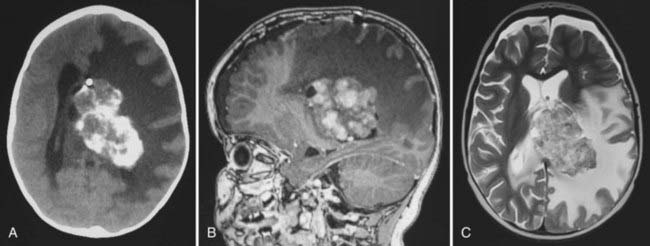
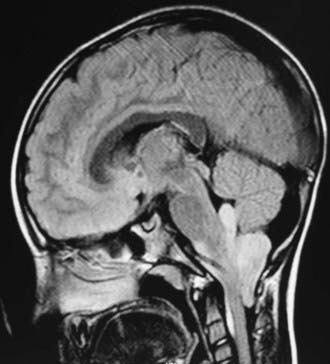
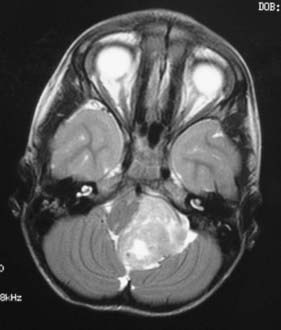
In childhood, 90% of ependymomas are located intracranially: one third supratentorially, and two thirds infratentorially.10 Sixty percent of supratentorial ependymomas arise contiguous with or adjacent to the ventricular system (lateral or third ventricle); the other 40% arise in areas remote to the ventricles and their ependymal surface (Fig. 200-1). Infratentorial (posterior fossa) ependymomas arise from the floor (60%), lateral aspect (30%), or roof (10%) of the fourth ventricle (Fig. 200-2).11,12
Prognostic Factors
Extent of Resection
The single most important determinant of outcome in pediatric ependymoma is the extent of surgical resection, emphasizing the role of the pediatric neurosurgeon. The 5-year survival rate in children who receive gross total resection is 67% to 80%, and the 5-year progression-free survival rate is 51% to 75%.2–5,7–9,13–21 In striking comparison, the 5-year survival rate of children who undergo subtotal resection ranges from 22% to 47%, with a 5-year progression-free survival ranging from 0% to 26%. Many authors have advocated repeated resection when residual tumor is found on postoperative imaging.12,15,22
MRI with contrast is recommended within 72 hours of resection to avoid confusing postoperative artifacts. Ependymoma resections are classified as gross total, near total, subtotal, and biopsy. Gross total resection is defined as the absence of tumor on postoperative MRI, even if microscopic disease (visualized with the operating microscope during surgery) is still present. Near total resection is arbitrarily defined as less than 1.5 cm3 residual tumor on postoperative imaging (similar to the values quoted for medulloblastoma) or 0.5 cm residual thickness of tumor bed enhancement.9 Subtotal resection is defined as remaining gross residual tumor that is visible to the surgeon during surgery or on postoperative neuroimaging. Because minimal residual disease can be cured with radiation, it is not necessary to perform extensive, aggressive resections that put the patient at risk.
Histologic Grade
The value of histologic grading in determining the prognosis of ependymoma patients is controversial.4,9,16,18,19,23–26 Compared with classic ependymoma, anaplastic ependymoma exhibits histologic evidence of advanced anaplasia, including nuclear atypia, marked mitotic activity, high cellularity, and microvascular proliferation or necrosis. Analysis of a group of 50 contemporary ependymoma patients from St. Jude Children’s Research Hospital showed that tumor grade was significantly related to progression-free survival after irradiation (P < .001).15 In this group of patients, the 2-year event-free survival estimate was 32% ± 14% for children with anaplastic ependymoma, compared with 84% ± 7% for children with classic ependymoma. This association remained significant after adjusting for age younger than 3 years, chemotherapy, and extent of resection. Of note, supratentorial ependymoma is more likely to show anaplastic histology than is posterior fossa ependymoma.
Age at Diagnosis
Children younger than 3 years at the time of ependymoma diagnosis have a poorer prognosis than older children. Younger children are less resilient and more likely to suffer complications from surgery, radiation, and chemotherapy. As a rule, radiation is not given to children younger than 3, or it is given in lower doses supplemented by chemotherapy. A study by the Pediatric Oncology Group showed a 63% 5-year survival for children aged 24 to 35 months (radiation delayed 1 year), but only a 26% 5-year survival for children aged 0 to 23 months (radiation delayed 2 years).27 These data emphasize the importance of radiation treatment for increasing the survival rates in children and that the poor prognosis in very young children relates at least in part to our inability or unwillingness to irradiate the developing brain.28,29 Although it has been suggested that higher doses of radiotherapy may improve the outcome for ependymoma patients, this has not been shown in a randomized trial.30
Pathology
In the World Health Organization (WHO) classification of brain tumors, a grade II ependymoma can be diagnosed only when mitoses are rare or absent; occasional foci of palisading necrosis are allowed, as are nodules with increased cellularity and mitotic activity.31 Anaplastic ependymoma is a grade III lesion in the WHO classification.32 It is diagnosed in tumors with increased cellularity, brisk mitotic activity, vascular proliferation, pseudopalisading necrosis, clear ependymal differentiation, perivascular pseudorosettes, cytologic atypia, and microvascular atypia.31 Areas of hypercellularity may be diffuse or focal and may form well-circumscribed regions abutting those of lower cellularity. Additionally, areas of cytologic atypia, including increased nuclear-to-cytoplasmic ratios and cellular pleomorphism, may be seen. Anaplastic regions often have a higher mitotic rate, although no specific threshold for a diagnosis of anaplastic ependymoma is widely accepted. Neither focal areas of atypia nor brisk mitotic activity is sufficient to make a diagnosis of anaplastic ependymoma. It is unclear whether anaplastic ependymoma arises from the progression or malignant degeneration of classic ependymoma or whether it occurs de novo.
Histologic grading and its clinical significance are difficult to assess, and agreement among pathologists when assigning a tumor grade to ependymoma is poor. Failure to find true ependymal rosettes or perivascular pseudorosettes is associated with a poor prognosis in children with ependymoma.13 Two separate groups have reported that the combination of necrosis, endothelial proliferation, and a mitotic index greater than 5 is a negative predictive factor for both overall and progression-free survival.13,33 Others have shown an inverse correlation between mitotic rate and survival in ependymoma.34 Ki-67 and MIB-1 immunolabeling methods to measure mitotic activity have also been used to show an inverse correlation between mitotic rate and outcome in two series of pediatric ependymoma.13,21,33,35 Another group of investigators found that mitotic rate was important only in determining the prognosis of supratentorial ependymoma.36
Hereditary Tumor Syndromes
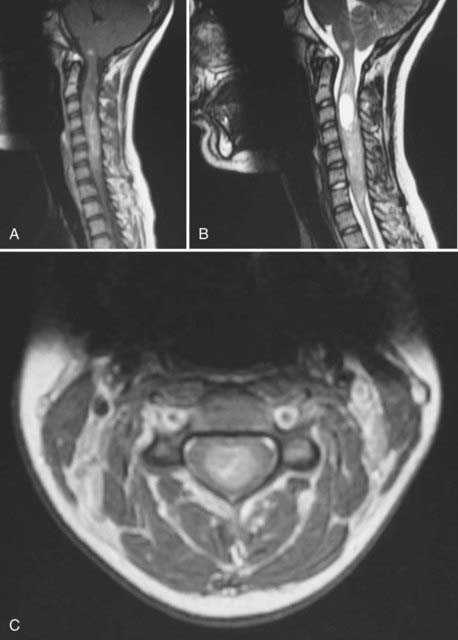
The vast majority of ependymomas occur sporadically, although some well-documented associations with hereditary tumor syndromes have been described. Intramedullary spinal ependymoma is well known to occur in patients with neurofibromatosis type 2 (NF2) (Fig. 200-3). In contrast, patients with NF2 are probably not at increased risk of developing intracranial ependymomas. The same biology is found in patients with sporadic (nonfamilial) intramedullary ependymomas who have somatic mutations of the NF2 gene. Mutations of the NF2 gene are not found in intracranial ependymomas, myxopapillary ependymomas, or tanycytic ependymomas.37,38 The NF2 gene is located on chromosome 22q. Although intracranial ependymomas are well known to show a loss of genetic material on 22q, they do not harbor NF2 mutations, suggesting the existence of another distinct ependymoma tumor suppressor gene in this region of the genome.
Ependymoma has also been reported in patients with the Li-Fraumeni familial cancer syndrome (due to a germline mutation in the p53 tumor suppressor gene).39,40 However, somatic mutations of p53 are not commonly found in sporadic ependymomas and likely do not play a large role in their pathogenesis.41,42 Scattered reports of patients with ependymoma and Turcot’s syndrome (brain tumors plus colonic neoplasia) are found in the literature, with some patients developing more than one ependymoma.43–45 These patients have germline mutations in the APC gene on chromosome 5 and an overactive Wnt signaling pathway. The role of the APC gene and Wnt signaling in sporadic ependymomas has not been elucidated. Spinal ependymomas have also been reported in the context of multiple endocrine neoplasia type I due to mutation of a tumor suppressor gene on chromosome 11q13.46,47 Other families with an increased incidence of ependymoma but no currently recognized familial tumor syndrome have also been reported.48–50 Epidemiology studies have shown an increased incidence of breast cancer in the family members of children with ependymoma.
Cancer Genetics
There is a large body of literature on the karyotypic changes found in adult and pediatric ependymomas. As a group, ependymomas studied by G-banding karyotype show a frequent loss of genetic material on chromosomes 22q, 6q, 9q, 17p, and 11q and show a gain of genetic material on chromosome 1q.38,40,51–56 Loss of heterozygosity on chromosome 22q is more common in adult ependymomas and intramedullary spinal ependymomas and less common in pediatric ependymomas.40 There is some evidence that a gain of genetic material on chromosome 1q may be an early event in the initiation of ependymoma.51
Ependymomas from different regions of the central nervous system (CNS) have distinct genetic profiles. There appear to be three genetic groups of childhood posterior fossa ependymomas. Ependymomas from very young children frequently show a balanced karyotype, with few or no regions of chromosomal gain or loss (no observed gains or losses).57,58 A second subset of posterior fossa ependymomas has few regions of gain or loss other than gain of chromosome 1q. A third group of posterior fossa tumors shows recurrent regions of copy number gain across several autosomes. It is unclear whether there is any clinical or prognostic significance to these three groups. Supratentorial ependymomas have different regions of gain and loss, and many show homozygous deletion of the INK4A locus on chromosome 9, which is also frequently deleted in glioblastoma multiforme.58 This key tumor suppressor locus plays a role in both TP53 and RB signaling. Recurrent tumors show more extensive karyotypic abnormalities than the original tumors; whether this is due to biologic progression or secondary to random DNA damage from radiation or chemotherapy is not clear.59 Myxopapillary ependymomas show a high number of cytogenetic abnormalities (averaging nine per tumor) compared with other ependymomas.60 The most common changes seen in cases of classic ependymoma are gains of chromosomal material on chromosome arms 1q or 9 and losses of 6q, 22, and the X chromosome.61 Overall, our knowledge of the genetics of ependymoma is limited by the resolution of the techniques used and the small number of tumors examined. Identification of specific genes and pathways for the development of targeted therapies will require larger studies done at higher resolution to differentiate driver genes from passenger genes.
Stem Cell Biology
It has been suggested that in many tumors only a small subset of tumor cells is capable of “regrowing” or recapitulating the tumor. These so-called cancer stem cells (CSCs) have been found in a number of tumor types, including ependymoma.58 Ependymoma CSCs grow as neurospheres, can differentiate along several different lineages, and can recapitulate ependymoma when transplanted into immune-deficient mice. Conversely, the non-CSCs from an ependymoma do not grow when xenografted. CSCs from ependymoma samples, but not medulloblastoma CSCs, express markers of radial glial cells (i.e., Blbp, RC2, Glast).58 These data suggest that ependymomas arise from radial glial cells, a type of CNS progenitor cell that is in fact the cell of origin of ependyma.62 Indeed, ependymomas from different regions of the nervous system have expression profiles similar to those of radial glial cells from those same regions, supporting a radial glial cell origin. A radial cell origin for ependymoma would certainly help explain the extraventricular location of many supratentorial ependymomas. Knowledge of the cell of origin may allow the creation of genetically modified mouse models of ependymoma by driving oncogene expression or the deletion of tumor suppressor genes in the radial glial cell compartment. These models would be welcome, because the shortage of model systems for ependymoma is undoubtedly responsible for the lack of research on the subject. There are no well-characterized ependymoma cell lines and no genetically modified mice that develop ependymoma; ependymoma xenografts have only recently been described.63
Ependymoma Variants
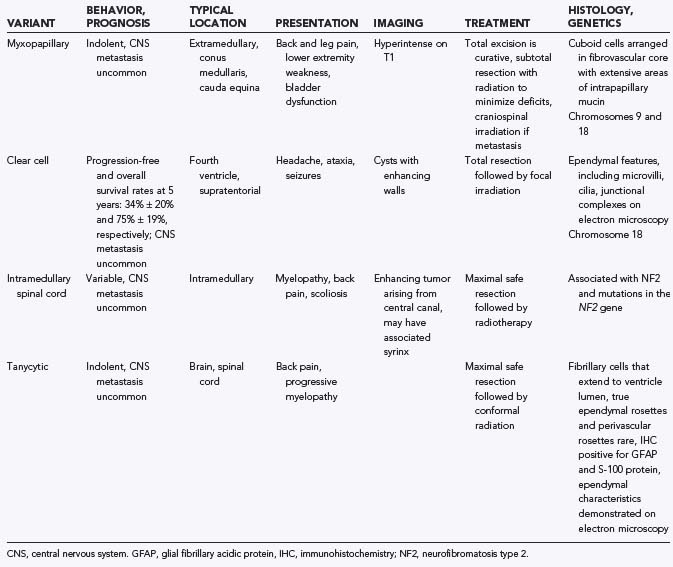
There are a number of histologic variants of ependymoma that have a distinct appearance under the light microscope and are likely separate entities from classic ependymoma, each with its own unique biology (Table 200-1).
Neuroimaging
Although most ependymomas enhance, they often contain areas that do not enhance; thus, careful scrutiny of the preoperative films is warranted to avoid missing tumor at the time of resection. It is important to compare the pre- and postoperative films side by side to determine the location and extent of residual tumor. Most children with recurrence or progression of tumor present between 12 and 24 months after the initiation of radiation therapy. Although there is no definitive proof, it is well accepted that early detection of recurrence maximizes the opportunity for salvage therapies. Some retrospective studies have even suggested that the early detection of asymptomatic recurrence of ependymoma on surveillance imaging leads to better outcomes than diagnosis when the recurrence becomes symptomatic.64,65
Initial Surgery
Children with ependymoma most often present with localized, invasive disease. Most recurrences are local, but subarachnoid dissemination occurs rarely and is usually fatal. The surgeon plays the most important role in the treatment of ependymoma, because surgery has the greatest impact on the quality and quantity of the patient’s future life. The overriding value of gross total resection of ependymoma has been demonstrated in several institutional retrospective reviews and two prospective phase III trials.2–5,7–9,16,19,27 Although complete resection is of paramount importance in the treatment of ependymoma, it is achieved in only 42% to 62% of patients.3,5,8,19 Complete resection is more readily achieved in supratentorial tumors and in those that arise from the roof of the fourth ventricle. Gross total resection of tumors that invade the floor of the fourth ventricle or pass out the foramen of Luschka to involve the lower cranial nerves is much more difficult, and surgical complication rates are higher.
Sutton and colleagues5 retrospectively analyzed 45 patients with ependymoma and found that the 5-year survival after gross total resection or near total resection was 60%, but with subtotal resection (defined in this chapter as <90% resection) it fell to 21%. Pollack and associates8 reported a 5-year survival of 80% after gross total resection, compared with 22% after less than total resection. Perilongo and coworkers4 retrospectively evaluated 92 children with ependymoma: the 10-year survival after gross total resection was 70%, and the progression-free survival estimate was 57%. With subtotal resection the 10-year survival was 32% and the 10-year progression-free survival was 11%. Robertson and colleagues9 prospectively treated 32 patients on the Children’s Cancer Group Protocol 921 and found that the 5-year progression-free survival was 66% for patients with less than 1.5 cm2 of residual tumor and 11% for those with more tumor. Although no randomized trial of complete versus partial resection for ependymoma has been (or will be) carried out, there is overwhelming evidence that cytoreductive surgery is beneficial to children with ependymoma. Children whose tumor burden can be decreased to minimal residual disease have the best chance at long-term survival.
Surgery without adjuvant treatment (radiation or chemotherapy) for children with ependymoma has been studied by two groups.17,66 The results of these studies demonstrate that surgery alone is probably a reasonable therapeutic option for children with supratentorial ependymoma in the absence of pathologic features of anaplastic ependymoma and when the surgeon is able to take a rim of white matter around the tumor. Biopsies of the walls of the resection cavity should be performed if surgery is contemplated as the sole treatment modality.
Surgery for Residual or Recurrent Tumor
Except in those rare cases in which resection of the residual tumor may injure a critical CNS structure (e.g., brainstem), most children who initially receive less than a near-total resection should have additional surgery to reduce the tumor load to minimal residual disease (gross total or near-total resection). Chemotherapy before resection of residual ependymoma may decrease the vascularity of the tumor and allow surgery to be delayed, providing very young children an opportunity to grow.22,67
Second resections for ependymoma have an acceptable morbidity.22,67 In a series of 40 pediatric ependymoma patients referred over a 3-year period to St. Jude Children’s Research Hospital, 24 (60%) had undergone complete resection, and 16 (40%) had residual tumor at the time of referral. Of those 16 patients with residual tumor, 12 were selected to receive additional surgery based on the extent and location of disease and the child’s neurological status at the time of referral. Of those 12 patients, gross total resection was achieved in 10 and near-total resection was achieved in the other 2 during the second surgery. The overall gross or near-total resection rate for the entire cohort was 36 of 40 (90%). Successful gross total resection at second-look surgery may prolong survival time as well as allow a lower dose of radiation to be used, leading to a lower incidence of neurocognitive deficits.67 Significant morbidity occurred in only one child at the time of second resection. Children who had a second operation within 30 days of the initial procedure had an improved performance level at 4 and 24 weeks after the second surgery and a trend toward a lower complication rate than those who had second-look surgery more than 30 days after the initial procedure.67
Surgical Technique
Preoperative planning should consider the different surgical risks and strategies associated with posterior fossa ependymomas arising from the cerebellar hemisphere, the roof of the fourth ventricle, the floor of the fourth ventricle, or the CPA (Fig. 200-4). Fourth ventricular ependymomas are generally supplied by branches from the posterior inferior cerebellar artery. Preoperative MRI is important so that the main artery feeding the tumor can be identified ahead of time.
The patient is positioned prone with maximal tolerated flexion so that the floor of the fourth ventricle is sloping away from the surgeon. If there is considerable hydrocephalus, a ventriculostomy may be placed before opening the posterior fossa. Care should be taken to release CSF slowly so as not to precipitate upward herniation or shifts in the tumor, with consequent intratumoral hemorrhage. Precipitous release of CSF and consequent lowering of intracranial pressure can also lead to retinal hypoperfusion with retinal infarct. Craniotomy is preferred to craniectomy at our institution because it decreases the incidence of CSF leak and pseudomeningocele as well as the incidence of chronic postoperative pain.68 The presence of a bone flap also facilitates surgery for residual or recurrent ependymoma.
CPA ependymomas typically originate from the lateral wall of the brainstem at the junction of the pons and the medulla (see Fig. 200-4). Their slow growth results in the encasement of multiple cranial nerves by the time of diagnosis (cranial nerves V to X are all at risk). Unilateral growth rotates the brainstem, distorting the normal anatomy and disorienting the surgeon. CPA ependymomas can grow back up the foramen of Luschka into the fourth ventricle, filling the ventricle with tumor. Like all posterior fossa ependymomas, they derive their blood supply from the posterior inferior cerebellar artery; however, they may also receive significant blood supply from the anterior inferior cerebellar artery, which runs through the CPA cistern. CPA ependymomas are often large by the time they come to clinical attention, and they occur predominantly in infants, making blood loss a major issue. In these cases, it is vital to have washed red blood cells available for transfusion to avoid high levels of potassium from the excessive transfusion of unwashed red blood cells in a very young child.
Treatment of Hydrocephalus
Postoperative hydrocephalus usually develops slowly over days or even weeks and has an incidence of 25% to 50%. The typical presentation is a pseudomeningocele at the site of the posterior fossa incision, but hydrocephalus may also present in a more traditional manner with headache, nausea, vomiting, and lethargy. Ventriculomegaly that is stable and asymptomatic after fourth ventricular tumor excision should be observed rather than treated. Patients undergoing a second surgery have a statistically significantly higher incidence of requiring a shunt compared with those treated by one surgery.69 When a child has progressive and symptomatic hydrocephalus, treatment should involve either a CSF diversionary device (ventriculoperitoneal shunt) or an endoscopic third ventriculostomy.70
Radiation Therapy
Postoperative radiation is part of the standard of care for ependymoma patients. Mork and Loken28 demonstrated that survival was roughly 17% for patients who underwent resection alone, compared with 40% for those who underwent resection and radiation. It is acceptable to withhold radiation therapy in children with supratentorial ependymomas if gross total resection with adequate margins has been achieved and the pathology is classic ependymoma (not anaplastic ependymoma).17,66 Additionally, radiation may not be necessary in some children after resection of an intramedullary spinal cord ependymoma.
A great deal of literature has focused on the danger of radiation therapy in ependymoma patients younger than 3 years. Numerous strategies to avoid or delay radiation have been proposed in this patient cohort in an attempt to minimize neurological, endocrinologic, and cognitive morbidity. Although the side effects associated with irradiating very young patients with ependymoma have not been well documented, they are assumed to resemble those seen in infants with medulloblastoma. A Pediatric Oncology Group study showed that young children with completely resected ependymoma in whom radiation was delayed for 2 years had a worse outcome (5-year survival 38%) than those in whom radiation was delayed only 1 year (5-year survival 88%).27 These results suggest that delaying radiation for the treatment of focal ependymoma of infancy is not advisable.
Less than 5% of children with ependymoma have disseminated (leptomeningeal) disease at the time of diagnosis. Risk factors for dissemination include younger age, less than gross total resection, high proliferative index, and high-grade histology.71 Most children with ependymoma who relapse do so at the primary site, regardless of the location of the primary tumor or its grade.2,4,5 Multiple retrospective trials have failed to show the benefit of prophylactic craniospinal irradiation in the treatment of localized ependymoma.3,12,30,72 In light of these facts, the current recommendation for nondisseminated ependymoma is localized radiotherapy. Craniospinal radiotherapy is reserved for those children with leptomeningeal dissemination.
The radiation dose response level for ependymoma ranges from 54 to 59.4 Gy, but the optimal dose remains unclear.73,74 Children with subtotal resection (who cannot benefit from further surgery) may benefit from dose escalation or hyperfractionation, or both, although recent reports have shown that hyperfractionation does not improve outcome. Conformal radiation therapy (CRT) limits the highest dose of radiation to the primary tumor site (as opposed to irradiating the entire posterior fossa, for example) and decreases the dose received by normal tissues (especially the inferior portion of the temporal lobes).15 The role of CRT in the treatment of children with ependymoma was tested in a phase II trial from 1997 to 2003 at St. Jude.15 Patients with localized ependymoma were evaluated before and after CRT to determine its neurological, endocrine, and cognitive effects. A total of 88 children (median age, 2.8 years) received irradiation therapy: 73 patients received 59.4 Gy, and 15 patients (age younger than 18 months and with gross total resection) received 54.0 Gy. Gross total resection was achieved through one or more craniotomies in 74 patients before irradiation; near-total resection was obtained in 6 patients, and subtotal resection in 8. This study showed excellent results at 38 months, with a 3-year progression-free survival estimate of 74.7% ± 5.7%. These impressive results show the value of gross total resection of ependymoma and that effective tumor targeting can be achieved using CRT techniques, without an increase in the relapse rate at the margin of the treatment volume. Children treated with CRT had their IQs evaluated before and after radiation; surprisingly, their IQs were normal and did not change significantly during the study period (there was trend toward improvement). The study included 48 children younger than 3 years at the time of irradiation. These results suggest that CRT is the preferred radiotherapy modality in children with localized ependymoma.15
Radiosurgery has been used to treat a number of children with ependymoma, with good local control and no excessive neurotoxicity.75–77 It is unclear when stereotactic radiosurgery should be used instead of open resection, but it is probably appropriate for children at high risk for anesthesia and for very small tumors in locations associated with high surgical risks. Children who present with recurrent ependymoma within a previously irradiated field are treated with maximal safe surgical resection of the recurrence, followed by repeat irradiation in the form of fractionated external beam irradiation or radiosurgery as some of their options. Disease control in patients with recurrent ependymoma treated with surgery and a second course of radiation therapy has recently been reported.78
Chemotherapy
The role of chemotherapy in the treatment of childhood ependymoma is unclear; it is not part of standard care. The response rate of ependymoma to single agents is roughly 11%, with less than 5% showing a complete response.79 Of the chemotherapeutic agents tried in the treatment of ependymoma, cisplatin seems to be the most active.79 In those rare children with ependymoma that responds to chemotherapy, there is no clear increase in survival. Several retrospective reviews failed to show a benefit of cytotoxic chemotherapy in the treatment of newly diagnosed ependymoma.2–5,8,30,73 The Children’s Cancer Group conducted a randomized controlled trial comparing children with ependymoma who received irradiation alone with those who received radiation and chemotherapy: there was no survival benefit in the arm that received chemotherapy.1 Similarly, a Pediatric Oncology Group trial did not show any overall survival benefit for infants with ependymoma treated with dose-intensive chemotherapy. A Societé Internationale d’Oncologie Pediatrique study treated 73 children with chemotherapy for 16 to 18 months after maximal resection; the children did not receive radiotherapy. Progression-free survival estimates at 2 and 4 years were 33% and 22%, respectively.80 These results are clearly worse than historical controls treated with radiotherapy. Despite the best efforts of the neuro-oncology community, there is no clear role for chemotherapy in the treatment of children with localized ependymoma.
Chemotherapy may have a role in avoiding or delaying radiotherapy in children younger than 3 years with intracranial ependymoma. The United Kingdom Children’s Cancer Study Group–Societé Internationale d’Oncologie Pediatrique prospective trial demonstrated that the use of adjuvant chemotherapy to delay or avoid radiation in children younger than 3 years was feasible, without affecting survival in a negative manner.81 Chemotherapy may also have a role in the treatment of children with residual tumor after initial tumor resection. For example, some authors suggest that the administration of chemotherapy between the first and second craniotomies makes the tumor borders better defined and easier to dissect.22 Chemotherapy may also have a role in the treatment of children who need to delay surgery or radiation treatment because of illness or neurological complications of the disease or its treatment.
Carter M, Nicholson J, Ross F, et al. Genetic abnormalities detected in ependymomas by comparative genomic hybridisation. Br J Cancer. 2002;86:929-939.
Dyer S, Prebble E, Davison V, et al. Genomic imbalances in pediatric intracranial ependymomas define clinically relevant groups. Am J Pathol. 2002;161:2133-2141.
Gnanalingham KK, Lafuente J, Thompson D, et al. Surgical procedures for posterior fossa tumors in children: does craniotomy lead to fewer complications than craniectomy? J Neurosurg. 2002;97:821-826.
Good CD, Wade AM, Hayward RD, et al. Surveillance neuroimaging in childhood intracranial ependymoma: how effective, how often, and for how long? J Neurosurg. 2001;94:27-32.
Granzow M, Popp S, Weber S, et al. Isochromosome 1q as an early genetic event in a child with intracranial ependymoma characterized by molecular cytogenetics. Cancer Genet Cytogenet. 2001;130:79-83.
Grill J, Le Deley MC, Gambarelli D, et al. Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: a multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol. 2001;19:1288-1296.
Grundy RG, Wilne SA, Weston CL, et al. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol. 2007;8:696-705.
Hodgson DC, Goumnerova LC, Loeffler JS, et al. Radiosurgery in the management of pediatric brain tumors. Int J Radiat Oncol Biol Phys. 2001;50:929-935.
Huang B, Starostik P, Schraut H, et al. Human ependymomas reveal frequent deletions on chromosomes 6 and 9. Acta Neuropathol. 2003;106:357-362.
Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215-225.
Merchant TE, Boop FA, Kun LE, et al. A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys. 2008;71:87-97.
Merchant TE, Lee H, Zhu J, et al. The effects of hydrocephalus on intelligence quotient in children with localized infratentorial ependymoma before and after focal radiation therapy. J Neurosurg. 2004;101:159-168.
Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22:3156-3162.
Neale G, Su X, Morton CL, et al. Molecular characterization of the pediatric preclinical testing panel. Clin Cancer Res. 2008;14:4572-4583.
Spassky N, Merkle FT, Flames N, et al. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10-18.
Taylor MD, Poppleton H, Fuller C, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323-335.
Yokota T, Tachizawa T, Fukino K, et al. A family with spinal anaplastic ependymoma: evidence of loss of chromosome 22q in tumor. J Hum Genet. 2003;48:598-602.
Zamecnik J, Snuderl M, Eckschlager T, et al. Pediatric intracranial ependymomas: prognostic relevance of histological, immunohistochemical, and flow cytometric factors. Mod Pathol. 2003;16:980-991.
1 Evans AE, Anderson JR, Lefkowitz-Boudreaux IB, et al. Adjuvant chemotherapy of childhood posterior fossa ependymoma: cranio-spinal irradiation with or without adjuvant CCNU, vincristine, and prednisone: a Children’s Cancer Group study. Med Pediatr Oncol. 1996;27:8-14.
2 Foreman NK, Love S, Thorne R. Intracranial ependymomas: analysis of prognostic factors in a population-based series. Pediatr Neurosurg. 1996;24:119-125.
3 Horn B, Heideman R, Geyer R, et al. A multi-institutional retrospective study of intracranial ependymoma in children: identification of risk factors. J Pediatr Hematol Oncol. 1999;21:203-211.
4 Perilongo G, Massimino M, Sotti G, et al. Analyses of prognostic factors in a retrospective review of 92 children with ependymoma: Italian Pediatric Neuro-oncology Group. Med Pediatr Oncol. 1997;29:79-85.
5 Sutton LN, Goldwein J, Perilongo G, et al. Prognostic factors in childhood ependymomas. Pediatr Neurosurg. 1990;16:57-65.
6 Gajjar A, Fouladi M, Walter AW, et al. Comparison of lumbar and shunt cerebrospinal fluid specimens for cytologic detection of leptomeningeal disease in pediatric patients with brain tumors. J Clin Oncol. 1999;17:1825-1828.
7 Needle MN, Goldwein JW, Grass J, et al. Adjuvant chemotherapy for the treatment of intracranial ependymoma of childhood. Cancer. 1997;80:341-347.
8 Pollack IF, Gerszten PC, Martinez AJ, et al. Intracranial ependymomas of childhood: long-term outcome and prognostic factors. Neurosurgery. 1995;37:655-666.
9 Robertson PL, Zeltzer PM, Boyett JM, et al. Survival and prognostic factors following radiation therapy and chemotherapy for ependymomas in children: a report of the Children’s Cancer Group. J Neurosurg. 1998;88:695-703.
10 Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000;88:2398-2424.
11 Ikezaki K, Matsushima T, Inoue T, et al. Correlation of microanatomical localization with postoperative survival in posterior fossa ependymomas. Neurosurgery. 1993;32:38-44.
12 Sanford RA, Gajjar A. Ependymomas. Clin Neurosurg. 1997;44:559-570.
13 Figarella-Branger D, Civatte M, Bouvier-Labit C, et al. Prognostic factors in intracranial ependymomas in children. J Neurosurg. 2000;93:605-613.
14 Healey EA, Barnes PD, Kupsky WJ, et al. The prognostic significance of postoperative residual tumor in ependymoma. Neurosurgery. 1991;28:666-671.
15 Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22:3156-3162.
16 Nazar GB, Hoffman HJ, Becker LE, et al. Infratentorial ependymomas in childhood: prognostic factors and treatment. J Neurosurg. 1990;72:408-417.
17 Palma L, Celli P, Mariottini A, et al. The importance of surgery in supratentorial ependymomas. Long-term survival in a series of 23 cases. Childs Nerv Syst. 2000;16:170-175.
18 Paulino AC, Wen BC, Buatti JM, et al. Intracranial ependymomas: an analysis of prognostic factors and patterns of failure. Am J Clin Oncol. 2002;25:117-122.
19 Rousseau P, Habrand JL, Sarrazin D, et al. Treatment of intracranial ependymomas of children: review of a 15-year experience. Int J Radiat Oncol Biol Phys. 1994;28:381-386.
20 van Veelen-Vincent ML, Pierre-Kahn A, Kalifa C, et al. Ependymoma in childhood: prognostic factors, extent of surgery, and adjuvant therapy. J Neurosurg. 2002;97:827-835.
21 Zamecnik J, Snuderl M, Eckschlager T, et al. Pediatric intracranial ependymomas: prognostic relevance of histological, immunohistochemical, and flow cytometric factors. Mod Pathol. 2003;16:980-991.
22 Foreman NK, Love S, Gill SS, et al. Second-look surgery for incompletely resected fourth ventricle ependymomas: technical case report. Neurosurgery. 1997;40:856-860.
23 Kovalic JJ, Flaris N, Grigsby PW, et al. Intracranial ependymoma long term outcome, patterns of failure. J Neurooncol. 1993;15:125-131.
24 Pierre-Kahn A, Hirsch JF, Roux FX, et al. Intracranial ependymomas in childhood. Survival and functional results of 47 cases. Childs Brain. 1983;10:145-156.
25 Shaw EG, Evans RG, Scheithauer BW, et al. Postoperative radiotherapy of intracranial ependymoma in pediatric and adult patients. Int J Radiat Oncol Biol Phys. 1987;13:1457-1462.
26 Timmermann B, Kortmann RD, Kuhl J, et al. Combined postoperative irradiation and chemotherapy for anaplastic ependymomas in childhood: results of the German prospective trials HIT 88/89 and HIT 91. Int J Radiat Oncol Biol Phys. 2000;46:287-295.
27 Duffner PK, Krischer JP, Sanford RA, et al. Prognostic factors in infants and very young children with intracranial ependymomas. Pediatr Neurosurg. 1998;28:215-222.
28 Mork SJ, Loken AC. Ependymoma: a follow-up study of 101 cases. Cancer. 1977;40:907-915.
29 Shuman RM, Alvord ECJr, Leech RW. The biology of childhood ependymomas. Arch Neurol. 1975;32:731-739.
30 Merchant TE, Haida T, Wang MH, et al. Anaplastic ependymoma: treatment of pediatric patients with or without craniospinal radiation therapy. J Neurosurg. 1997;86:943-949.
31 Kleihues P, Sobin LH. World Health Organization classification of tumors. Cancer. 2000;88:2887.
32 Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215-225.
33 Ritter AM, Hess KR, McLendon RE, et al. Ependymomas: MIB-1 proliferation index and survival. J Neurooncol. 1998;40:51-57.
34 Schiffer D, Chio A, Giordana MT, et al. Proliferating cell nuclear antigen expression in brain tumors, and its prognostic role in ependymomas: an immunohistochemical study. Acta Neuropathol. 1993;85:495-502.
35 Bennetto L, Foreman N, Harding B, et al. Ki-67 immunolabelling index is a prognostic indicator in childhood posterior fossa ependymomas. Neuropathol Appl Neurobiol. 1998;24:434-440.
36 Schiffer D, Chio A, Giordana MT, et al. Histologic prognostic factors in ependymoma. Childs Nerv Syst. 1991;7:177-182.
37 Ebert C, von Haken M, Meyer-Puttlitz B, et al. Molecular genetic analysis of ependymal tumors. NF2 mutations and chromosome 22q loss occur preferentially in intramedullary spinal ependymomas. Am J Pathol. 1999;155:627-632.
38 Lamszus K, Lachenmayer L, Heinemann U, et al. Molecular genetic alterations on chromosomes 11 and 22 in ependymomas. Int J Cancer. 2001;91:803-808.
39 Metzger AK, Sheffield VC, Duyk G, et al. Identification of a germ-line mutation in the p53 gene in a patient with an intracranial ependymoma. Proc Natl Acad Sci U S A. 1991;88:7825-7829.
40 von Haken MS, White EC, Daneshvar-Shyesther L, et al. Molecular genetic analysis of chromosome arm 17p and chromosome arm 22q DNA sequences in sporadic pediatric ependymomas. Genes Chromosomes Cancer. 1996;17:37-44.
41 Fink KL, Rushing EJ, Schold SCJr, et al. Infrequency of p53 gene mutations in ependymomas. J Neurooncol. 1996;27:111-115.
42 Tong CY, Ng HK, Pang JC, et al. Molecular genetic analysis of non-astrocytic gliomas. Histopathology. 1999;34:331-341.
43 Hamilton SR, Liu B, Parsons RE, et al. The molecular basis of Turcot’s syndrome. N Engl J Med. 1995;332:839-847.
44 Mullins KJ, Rubio A, Myers SP, et al. Malignant ependymomas in a patient with Turcot’s syndrome: case report and management guidelines. Surg Neurol. 1998;49:290-294.
45 Torres CF, Korones DN, Pilcher W. Multiple ependymomas in a patient with Turcot’s syndrome. Med Pediatr Oncol. 1997;28:59-61.
46 Giraud S, Choplin H, Teh BT, et al. A large multiple endocrine neoplasia type 1 family with clinical expression suggestive of anticipation. J Clin Endocrinol Metab. 1997;82:3487-3492.
47 Kato H, Uchimura I, Morohoshi M, et al. Multiple endocrine neoplasia type 1 associated with spinal ependymoma. Intern Med. 1996;35:285-289.
48 Nijssen PC, Deprez RH, Tijssen CC, et al. Familial anaplastic ependymoma: evidence of loss of chromosome 22 in tumour cells. J Neurol Neurosurg Psychiatry. 1994;57:1245-1248.
49 Savard ML, Gilchrist DM. Ependymomas in two sisters and a maternal male cousin with mosaicism with monosomy 22 in tumour. Pediatr Neurosci. 1989;15:80-84.
50 Yokota T, Tachizawa T, Fukino K, et al. A family with spinal anaplastic ependymoma: evidence of loss of chromosome 22q in tumor. J Hum Genet. 2003;48:598-602.
51 Granzow M, Popp S, Weber S, et al. Isochromosome 1q as an early genetic event in a child with intracranial ependymoma characterized by molecular cytogenetics. Cancer Genet Cytogenet. 2001;130:79-83.
52 Huang B, Starostik P, Schraut H, et al. Human ependymomas reveal frequent deletions on chromosomes 6 and 9. Acta Neuropathol. 2003;106:357-362.
53 Kramer DL, Parmiter AH, Rorke LB, et al. Molecular cytogenetic studies of pediatric ependymomas. J Neurooncol. 1998;37:25-33.
54 Mazewski C, Soukup S, Ballard E, et al. Karyotype studies in 18 ependymomas with literature review of 107 cases. Cancer Genet Cytogenet. 1999;113:1-8.
55 Vagner-Capodano AM, Zattara-Cannoni H, Gambarelli D, et al. Cytogenetic study of 33 ependymomas. Cancer Genet Cytogenet. 1999;115:96-99.
56 Wernicke C, Thiel G, Lozanova T, et al. Involvement of chromosome 22 in ependymomas. Cancer Genet Cytogenet. 1995;79:173-176.
57 Carter M, Nicholson J, Ross F, et al. Genetic abnormalities detected in ependymomas by comparative genomic hybridisation. Br J Cancer. 2002;86:929-939.
58 Taylor MD, Poppleton H, Fuller C, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323-335.
59 Dyer S, Prebble E, Davison V, et al. Genomic imbalances in pediatric intracranial ependymomas define clinically relevant groups. Am J Pathol. 2002;161:2133-2141.
60 Scheil S, Bruderlein S, Eicker M, et al. Low frequency of chromosomal imbalances in anaplastic ependymomas as detected by comparative genomic hybridization. Brain Pathol. 2001;11:133-143.
61 Reardon DA, Entrekin RE, Sublett J, et al. Chromosome arm 6q loss is the most common recurrent autosomal alteration detected in primary pediatric ependymoma. Genes Chromosomes Cancer. 1999;24:230-237.
62 Spassky N, Merkle FT, Flames N, et al. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10-18.
63 Neale G, Su X, Morton CL, et al. Molecular characterization of the pediatric preclinical testing panel. Clin Cancer Res. 2008;14:4572-4583.
64 Good CD, Wade AM, Hayward RD, et al. Surveillance neuroimaging in childhood intracranial ependymoma: how effective, how often, and for how long? J Neurosurg. 2001;94:27-32.
65 Steinbok P, Hentschel S, Cochrane DD, et al. Value of postoperative surveillance imaging in the management of children with some common brain tumors. J Neurosurg. 1996;84:726-732.
66 Hukin J, Epstein F, Lefton D, et al. Treatment of intracranial ependymoma by surgery alone. Pediatr Neurosurg. 1998;29:40-45.
67 Khan RB, Sanford RA, Kun LE, et al. Morbidity of second-look surgery in pediatric central nervous system tumors. Pediatr Neurosurg. 2001;35:225-229.
68 Gnanalingham KK, Lafuente J, Thompson D, et al. Surgical procedures for posterior fossa tumors in children: does craniotomy lead to fewer complications than craniectomy? J Neurosurg. 2002;97:821-826.
69 Merchant TE, Lee H, Zhu J, et al. The effects of hydrocephalus on intelligence quotient in children with localized infratentorial ependymoma before and after focal radiation therapy. J Neurosurg. 2004;101:159-168.
70 Sainte-Rose C, Cinalli G, Roux FE, et al. Management of hydrocephalus in pediatric patients with posterior fossa tumors: the role of endoscopic third ventriculostomy. J Neurosurg. 2001;95:791-797.
71 Rezai AR, Woo HH, Lee M, et al. Disseminated ependymomas of the central nervous system. J Neurosurg. 1996;85:618-624.
72 Vanuytsel LJ, Bessell EM, Ashley SE, et al. Intracranial ependymoma: long-term results of a policy of surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 1992;23:313-319.
73 Chiu JK, Woo SY, Ater J, et al. Intracranial ependymoma in children: analysis of prognostic factors. J Neurooncol. 1992;13:283-290.
74 Goldwein JW, Leahy JM, Packer RJ, et al. Intracranial ependymomas in children. Int J Radiat Oncol Biol Phys. 1990;19:1497-1502.
75 Aggarwal R, Yeung D, Kumar P, et al. Efficacy and feasibility of stereotactic radiosurgery in the primary management of unfavorable pediatric ependymoma. Radiother Oncol. 1997;43:269-273.
76 Hodgson DC, Goumnerova LC, Loeffler JS, et al. Radiosurgery in the management of pediatric brain tumors. Int J Radiat Oncol Biol Phys. 2001;50:929-935.
77 Jawahar A, Kondziolka D, Flickinger JC, et al. Adjuvant stereotactic radiosurgery for anaplastic ependymoma. Stereotact Funct Neurosurg. 1999;73:23-30.
78 Merchant TE, Boop FA, Kun LE, et al. A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys. 2008;71:87-97.
79 Bouffet E, Foreman N. Chemotherapy for intracranial ependymomas. Childs Nerv Syst. 1999;15:563-570.
80 Grill J, Le Deley MC, Gambarelli D, et al. Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: a multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol. 2001;19:1288-1296.
81 Grundy RG, Wilne SA, Weston CL, et al. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol. 2007;8:696-705.