CHAPTER 55 Enucleation and evisceration
Indications for enucleation and evisceration
The third indication for enucleation in a blind eye is atrophy bulbi. The presence of phthisis bulbi, solely a histological term, must be inferred from the clinical findings of a hypotonic, atrophic, and opaque globe1. Phthisical eyes have an increased incidence of choroidal malignant melanoma, which has been estimated at 4–15%1,2. Melanomas have been found in 21% of enucleated eyes that had opaque media1, and 12% of these melanomas had been unsuspected prior to enucleation3. Because phthisical eyes with opaque media are difficult to examine clinically, enucleation may be a precautionary measure for the development of malignant disease. If not enucleated, an atrophic eye should be examined periodically with ultrasonography, MRI or CT scanning.
A fourth indication for enucleation in a blind or seeing eye is the presence of suspected intraocular malignancy. When enucleation is undertaken for malignant disease, careful attention should be paid to technique. Traumatic enucleation for malignant disease may actually hasten metastatic spread4,5.
A fifth indication for enucleation is aplasia or severe hypoplasia of a globe in childhood. Full orbital volume is essential for the development of the bony structure of the orbit6. This bony asymmetry can be minimized by early placement of a large orbital implant.
A sixth indication for enucleation is as a prophylactic measure against the development of sympathetic ophthalmia. Sympathetic ophthalmia occurs most commonly after penetrating ocular trauma. Ninety percent of cases occur between 2 weeks and 1 year after penetrating ocular injury7. The published incidence of sympathetic ophthalmia after penetrating ocular injury varies. It has been estimated to be as high as 3–5% of cases in one series1. These previous studies have been exclusively retrospective and may not reflect the benefits of modern microsurgical techniques and immunosuppression.
Evisceration is performed for some of the same reasons as enucleation. The first indication for evisceration is a blind eye with active, uncontrolled endophthalmitis. Evisceration may be less likely to spread infection to cerebral spinal fluid than would enucleation, in which severing of the optic nerve sheath is required8. If infection has already spread through the sclera, however, enucleation may be necessary to remove all the infected tissue. Often a secondary enucleation is performed after the infection has cleared.
Enucleation techniques
Authors’ technique
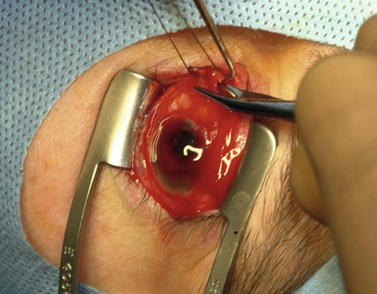
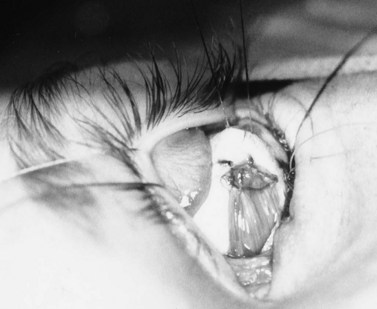
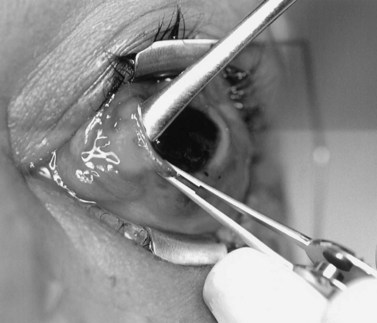
A doubled-armed 5-0 Vicryl suture is passed through the substance of the muscle near its insertion site with locking bites at each edge of the muscle for additional security. The muscle tendon is then transected from the globe (Fig. 55.1). A small stump of tendon may be left on the sclera to assist in globe manipulation. In a similar manner, the remaining three recti muscles are isolated, ligated, and disinserted from the globe. All needles are left on the sutures for later use.
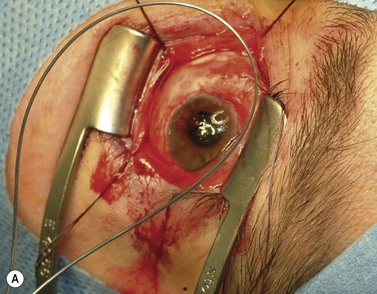
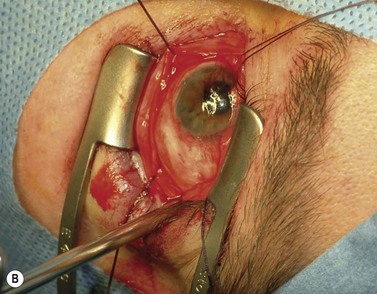
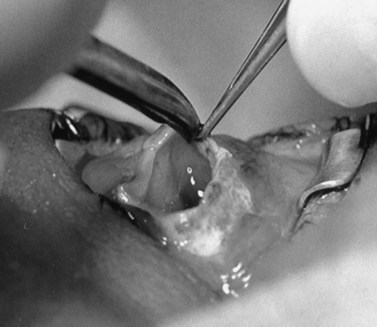
The nerve is transected with either an enucleation snare or a pair of enucleation scissors (Fig. 55.2A&B). If scissors are used, it may be helpful to crush the optic nerve with a hemostat prior to transection. In this technique, a curved hemostat is inserted behind the globe from a medial approach, with the concave angle facing anteriorly and the tip toward the optic nerve. The hemostat is used to feel the optic nerve with the tips closed. Once the nerve is located, the tips of the hemostat are slipped around the nerve and then slid posteriorly along the nerve toward the apex of the orbit. The nerve is then clamped for hemostasis. A curved enucleation scissors is placed just above the hemostat using the same maneuver. Next, the nerve is transected just anterior to the hemostat. Bipolar cautery may be used to coagulate the transected nerve end to seal off the central retinal artery.
The ‘no touch’ technique
The ‘no touch’ technique was advocated by Fraunfelder and Wilson4 for enucleation of eyes that harbor intraocular malignancy. Zimmerman, McLean and Foster5 have suggested that enucleation may precipitate metastatic spread of melanoma through the dissemination of tumor emboli.
Placement of orbital implant
Orbital implants
The silicone sphere, hydroxyapatite, and porous polyethylene implants are the most common synthetic implants in current use. Due to its low rate of complications and extrusions, our preference is the silicone implant. Alternatively, the hydroxyapatite implant may be used in selected cases where there is no microvascular disease, radiation exposure, or multiple surgeries, resulting in an increased extrusion or exposure rate12,13. Silicone is also considerably less expensive than hydroxyapatite or porous polyethylene. Multiple studies demonstrate no difference in motility between non-porous and porous implants14.
Silicone sphere
After enucleation, a silicone sphere of appropriate size is selected. A 20 mm spherical implant coupled with an average-sized ocular prosthesis will adequately fill orbital volume in an enucleated socket9. If the implant is less than 20 mm, orbital volume loss with secondary superior sulcus deformity may result.
Authors’ technique
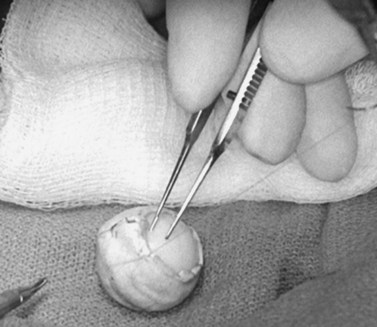
The implant is wrapped in autogenous sclera after thorough removal of uveal tissue. Banked sclera or autogenous fascia lata may be used as a substitute. A 5-0 Vicryl suture may be used to approximate the edges of the wrap (Fig. 55.3). If sclera is used, the implant will be exposed at the corneal opening, which will be placed facing the apex of the orbit. The uvea must be meticulously scrubbed off the sclera, which is then rinsed thoroughly with normal saline or antibiotic solution. Autogenous sclera is not used in a case involving suspected malignancy.
The wrapped implant is then placed deep within the muscle cone. The extraocular muscles are sewn to the wrapping material or directly to the implant in their approximate normal anatomical positions using the previously placed sutures. The needles are left on the sutures after the muscles are attached to the implant (Fig. 55.4). The muscles should not be imbricated over the anterior surface of the implant. This will not provide a barrier for extrusion, as the implant may easily extrude through the area between the recti muscles. Furthermore, the imbricating technique encourages migration of the implant, usually in the superior temporal direction10,11.
Dermis-fat grafting
Posterior Tenon’s fascia is opened in all four quadrants allowing orbital fat to come into contact with the grafted fat tissue. The dermal-fat graft is placed directly into the anophthalmic socket with the fat facing posteriorly. The recti muscles are sutured to the margins of the dermis. Tenon’s fascia and the conjunctiva are then sutured directly overlapping the margin of the dermal fat graft (Fig. 55.5). The desired orbital volume is over-corrected by approximately 20% to allow for reabsorption of the fatty graft.
Autogenous composite dermal fat grafting provides the advantage of no likelihood of implant extrusion or migration. Dermal-fat grafted sockets frequently have good motility and are usually comfortable after prosthetic fitting15,16. Unfortunately, the rate of reabsorption of transplanted fatty tissue is unpredictable. Fat reabsorption may lead to superior sulcus deformity and clinically significant eyelid retraction, which occurs when recti muscles and lid retractor muscles become adherent to the fat graft (Fig. 55.6).
In the authors’ experience, reoperations within the first 3 years of dermal fat grafting occur in approximately 60% of cases17. Two of every three patients require major refabrication of the ocular prosthesis within 1 year of surgery, to compensate for changes in socket anatomy from fat reabsorption.
Orbital implantation in pediatric patients
A normally growing eye stimulates expansion and development of the orbit until bony growth is complete. Pediatric orbits without normal-sized eyes become hypoplastic and may cause ipsilateral facial hypoplasia6,18,19. Effect of orbital volume deficit on bony growth in childhood is greater in cases of congenital anophthalmia or severe microphthalmia when compared with acquired anophthalmia in later childhood. Children with normal sized globes during embryonic development and early childhood are more likely to retain a relatively normal cosmetic appearance than to those with congenital anophthalmia. Hintschich et al. demonstrated loss of orbital bony volume in both pediatric and adult patients undergoing enucleation20. The loss of orbital bony volume increased with the passage of time from enucleation, with the greatest volume loss occurring in children enucleated in the first 2 years of life.
Treatment of the pediatric anophthalmic socket should begin as early as possible. Cepela, Nunery and Martin demonstrated that bony stimulation was proportional to the volume of the implant placed into the orbit. Therefore, the largest implant possible (up to 20 mm in diameter) should be placed. Heinz, Nunery, and Cepela also showed that the amount of achievable orbital expansion was inversely proportional to the age at which expansion begins. Treatment may include placing sequential orbital expanders21. We prefer to initially place the implant into the orbit via a lateral orbital approach without disturbing the conjunctiva. An expandable orbital implant that enlarges with saline injections is an alternative to sequential replacement of orbital sphere implants.
Evisceration technique
Historically, evisceration has been considered to be a technique that provides better motility of the prosthesis and a better cosmetic result than does enucleation22. This may be true if enucleation techniques such as release of the recti muscles or imbrication over the anterior surface of the implant are employed.
Evisceration may also have a higher incidence of implant extrusion. One series demonstrated the incidence of extrusion as being four times (6% vs. 1.4%) greater with evisceration than with enucleation23.
Authors’ evisceration technique
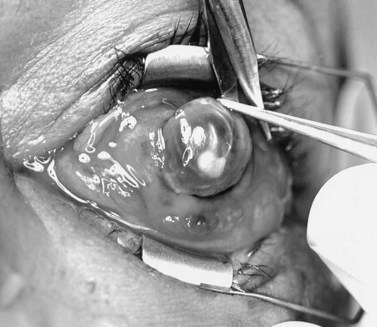
Under general or monitored anesthesia, a 360° limbal peritomy is performed. A No. 11 or No. 15 Bard–Parker blade is used to enter the eye at the limbus and a full-thickness incision around the entire limbus is completed (Fig. 55.7). The intraocular contents are removed with an evisceration spoon or periosteal elevator (Fig. 55.8). The intraocular surface of the sclera is vigorously scrubbed free of uveal tissue. The gauze may be soaked with 100% ethanol or 10% phenol to denature any remaining uveal protein. Care must be taken to avoid spilling either on the conjunctival surface. The sclera should then be irrigated with a copious amount of normal saline solution.
If evisceration is performed for intraocular infection, the sclera should be irrigated with antibiotic solution and packed with antibiotic gauze until the infection subsides. The packing is usually removed after 24–48 hours. Once the infection is cleared, the remaining sclera may be excised in preparation for placement of an orbital implant. Alternatively, an orbital implant may be placed into the eviscerated scleral shell. Placement may be facilitated by incising the limbal scleral edge in an anterior–posterior direction to create a larger anterior opening (Fig. 55.9). Additionally, posterior radial incisions may be made in the sclera to allow placement of a larger implant. Anterior sclera, Tenon’s fascia, and conjunctiva are closed separately with 5-0 Vicryl sutures. We avoid using hydroxyapatite implants in evisceration patients because we believe a higher exposure rate occurs, compared with non-porous implants. Once the implantation is completed, a conformer is placed behind the lids and a temporary 6-0 mild chromic suture tarsorrhaphy is placed.
Complications of enucleation and evisceration
Late enucleation complications
Implant migration and extrusion
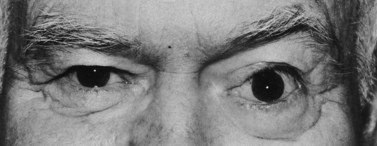
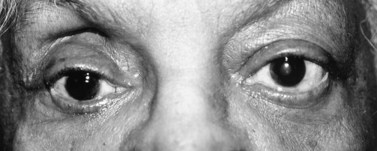
Superior–temporal implant migration may cause ptosis by impeding the superior rectus and levator (Fig. 55.10). The prosthesis may therefore be custom-designed with minimal superior volume to eliminate further restriction on the levator. Ptosis buckles on the prosthesis are ineffective when ptosis is caused by a migrated implant.
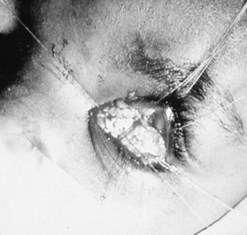
Patching the early implant extrusion defect with eyebank sclera may prevent complete extrusion in some cases24,25. However, this may only delay the final extrusion and thus may not represent a long-term solution to the problem.
Volume deficit
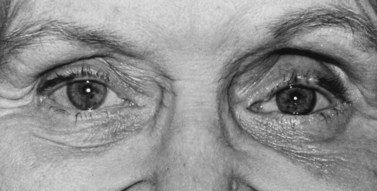
Volume deficit and superior sulcus deformity seem to be an almost inevitable consequence of anophthalmic socket reconstruction when using small implants (Fig. 55.11). The use of a 20 mm sphere in an adult socket usually prevents volume loss and superior sulcus deformity.
1 Hogan MJ, Zimmerman LE. Ophthalmic Pathology: An Atlas and Textbook. Philadelphia: WB Saunders; 1962.
2 Duke-Elder S. Cysts and tumors of the uveal tract: diagnosis of malignant melanoma, editor. System of Ophthalmology. St. Louis: CV Mosby; 1966:896.
3 Yanoff M, Fine B. Ocular Pathology. Hagerstown, MD: Harper & Row; 1975.
4 Fraunfelder FT, Wilson RS. A new approach for intraocular malignancy: the ‘no touch’ technique. In: Jakobiec FA, editor. Ocular and Adnexal Tumors. Birmingham, UK: Aesculapius; 1978:39-45.
5 Zimmerman LE, McLean IW, Foster WD. Does enucleation of the eye containing malignant melanoma prevent or accelerate the dissemination of tumor cells? An unanswered question!. In: Jakobiec FA, editor. Ocular and Adnexal Tumors. Birmingham, UK: Aesculapius, 1978.
6 Kennedy RE. Effects of early enucleation on the orbit in animals and humans. Trans Am Ophthalmol Soc. 1964;62:459.
7 Duke-Elder S. Uveitis of unknown origin: sympathetic ophthalmitis – aetiological theories, editor. Systems of Ophthalmology. St. Louis: CV Mosby; 1966:566.
8 Berkmann LW, Bennett DR. Meningoencephalitis following enucleation for cryptococcal endophthalmitis. Ann Neurol. 1978;4:476-477.
9 Perry A. Volume of anophthalmic implants. Address to American Society of Ocularists, San Francisco, 1983.
10 Fox SA. Ophthalmic Plastic Surgery. New York: Grune & Stratton; 1976. p. 543
11 Allen L. The argument against imbricating the rectus muscles over spherical orbital implants after enucleation. Trans Am Acad Ophthalmol. 1983;90:1116-1120.
12 Nunery WR, Cepela MA, Heinz GW, et al. Extrusion rate of silicone spherical anophthalmic socket implants. Ophthalmic Plast Reconstr Surg. 1993;9:90-95.
13 Nunery WR, Heinz GW, Bonnin JM, et al. Exposure rate of hydroxyapatite spheres in the anophthalmic socket: histopathologic correlation and comparison with silicon sphere implants. Ophthalmic Plast Reconstr Surg. 1993;9:96-104.
14 Custer PL, Kennedy RH, Woog JJ, et al. Orbital Implants in Enucleation Surgery: A Report by The American Academy of Ophthalmology. Ophthalmology. 2003;110:2054-2061.
15 Guberina C, Hornblass A, Meltzer MA, et al. Autogenous dermis-fat implantations. Arch Ophthalmol. 1983;101:1587-1590.
16 Smith B, Petrelli R. Dermis-fat graft on a moveable implant within the muscle cone. Am J Ophthalmol. 1978;85:62-66.
17 Nunery WR, Hetzler KJ. Dermal-fat graft as a primary enucleation technique. Ophthalmology. 1985;92:1256-1261.
18 Osborne D, Hadden OB, Deeming LW. Orbital growth after childhood enucleation. Am J Ophthalmol. 1974;77:756-759.
19 Schaffer DP. Evaluation and management of the anophthalmic socket and socket reconstruction. In: Nesi FA, Lisman RD, Levine MR, editors. Smith’s Ophthalmic Plastic and Reconstructive Surgery. St. Louis: CV Mosby; 1988:1079-1124.
20 Hintschich C, Zonneveld F, Baldeschi L, et al. Bony orbital development after early enucleation in humans. Br J Ophthalmol. 2001;85:205-208.
21 Heinz GW, Nunery WR, Cepela MA. The effect of maturation on the ability to stimulate orbital growth using tissue expanders in the anophthalmic cat orbit. Ophthalmic Plast Reconstruct Surg. 1997;13:115-128.
22 Ruedemann ADJr. Modified Burch-type evisceration with scleral implant. Am J Ophthalmol. 1960;49:41.
23 Zolli CL. Implant extrusion in eviscerations. Paper presented at American Society of Ocularist Meeting, 1984, San Francisco.
24 Helveston EM. Human bank scleral patch for repair of exposed or extruded orbital implants. Arch Ophthalmol. 1969;82:83.
25 Zolli C, Shannon GM. Experience with donor sclera for extruding orbital implants. Ophthalmic Surg. 1977;8:63-70.