Chapter 33 Entrapment Neuropathies
• Entrapment neuropathy occurs when a peripheral nerve is subjected to repetitive movement in a constricted space. Conservative treatment involves reducing the movement; surgical treatment enlarges the space.
• Carpal tunnel syndrome is by far the most common entrapment neuropathy and the commonest cause of neurological symptoms in the hand. The symptoms are not always confined to the hand and can spread proximally.
• In cubital tunnel surgery, when comparing simple ulnar nerve decompression versus anterior subcutaneous transposition techniques, one can expect similar outcomes but fewer complications with simple ulnar decompression.
• Diagnosis of entrapment neuropathy can usually be made from the clinical history, and can then be confirmed by physical signs, electromyography, nerve conduction studies, and magnetic resonance imaging (MRI).
• Surgery for entrapment neuropathy is indicated if conservative measures fail or if the clinical picture is one of severe compression with significant pain and muscular weakness and wasting.
Some peripheral nerves, irrespective of whether they are motor, sensory, or of mixed type, pass through narrow, constricted areas in the arms or legs. Under certain circumstances, these nerves are susceptible to compression at these sites,1–3 and this compression can eventually clinically manifest as entrapment neuropathy.4–6 Entrapment of nerves usually occurs as they pass beside a joint, such as the elbow, wrist, or hip and only very occasionally elsewhere in the limbs. This, along with the fact that entrapment neuropathy seldom occurs in the head or trunk, suggests that repetitive motion is a major factor that precipitates entrapment in an anatomically constricted segment.
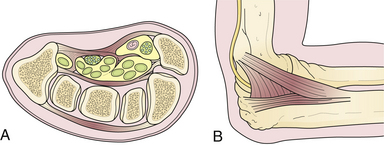
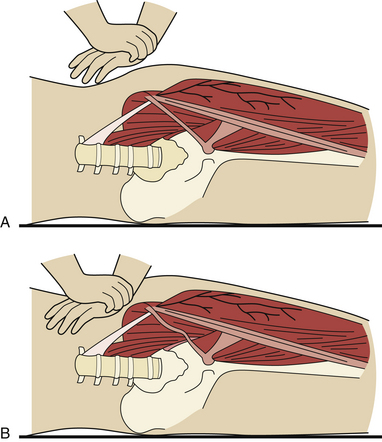
Two types of physical constrictions predispose to entrapment neuropathy. The first type (Fig. 33.1A) is a fibro-osseous tunnel. The space available for the nerve within the tunnel becomes constricted either because the contents of the tunnel become larger or hypertrophic, as when a patient with tenosynovitis has carpal tunnel syndrome, or because the walls of the tunnel encroach upon the tunnel’s lumen, as when fractured fragments of a carpal bone displace into the carpal canal. Compression of a nerve in a tunnel is an example of static compression. The second type (Fig. 33.1B) involves dynamic compression of the nerve as it passes through a fibrotendinous arcade. The nerve is flanked by two bellies of a muscle that under static conditions do not compress the nerve. When they contract, however, they cause a shutter-like closure of the arcade, compressing the nerve. For example, this can occur at the arcade of Frohse in the supinator muscle, the two heads of the flexor carpi ulnaris at the entrance to the cubital tunnel, or the two heads of the flexor digitorum sublimis forming the “sublimis bridge.”
Pathology of Nerve Compression7
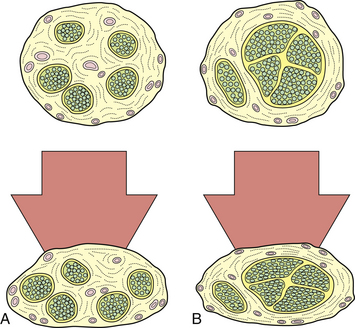
The pathophysiological changes following nerve compression8–21 are dependent on the degree, rate, and duration of compression. Loss of function of the nerve as a result of compression is manifested clinically by motor paralysis, paresthesia, or numbness. In physiological terms, mild and brief compression produces a transient and reversible conduction block within the nerve. Sustained compression over a long period causes structural changes. Not all components of the nerve are equally susceptible to a given degree of compression. Nerve fibers that have a greater amount of epineurium compared to the nerve fascicles are less susceptible to compression than those with larger fascicles and scanty epineurium (Fig. 33.2). Also, within a given nerve, not all fibers undergo degenerative changes to the same extent. The superficially located fibers tend to bear the brunt of the compression, while the central fibers are relatively spared. Large, heavily myelinated fibers subserving light touch and motor function are more sensitive to compressive changes than unmyelinated fibers subserving pain sensation.
Impediment to microvascular flow appears to be a major factor in the pathophysiology of nerve impingement.7 Capillary blanching and venular obstruction herald progressive compression. This leads to nerve ischemia, which in turn leads to endothelial impairment and progressive edema; the edema compounds the ischemia and swelling of the nerve (Fig. 33.3). Critical swelling of a nerve within the constraints of its surroundings may lead to further nerve compression, a phenomenon that can be called a mini-compartmental syndrome.
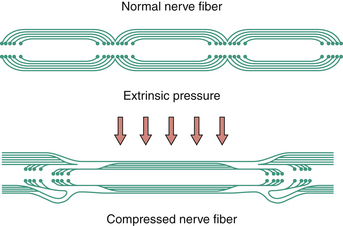
With acute and severe compression one observes a characteristic sequential invagination or telescoping of the myelin sheath (Fig. 33.4). The polarity of invagination is reversed at the edges of the compression. With chronic compression, segmental demyelination occurs within the compressed segments, accounting for the slowing of conduction velocity of the nerve. In the early phases, the nerve fibers distal to the compression show normal morphology. With sustained compression, axolysis occurs within the compressed segment, leading to distal wallerian degeneration.
Double Crush Syndrome
If a nerve is compressed proximally, its distal part is more susceptible to compression than a normal nerve would be, because the antegrade axonal flow is blocked by the first compression. In a similar manner, if there is distal compression, the nerve cell body undergoes degeneration more quickly if a second compression is present proximally, because of impediment of retrograde flow. This latter syndrome is called a reverse double crush syndrome.22
The Entrapment Syndromes
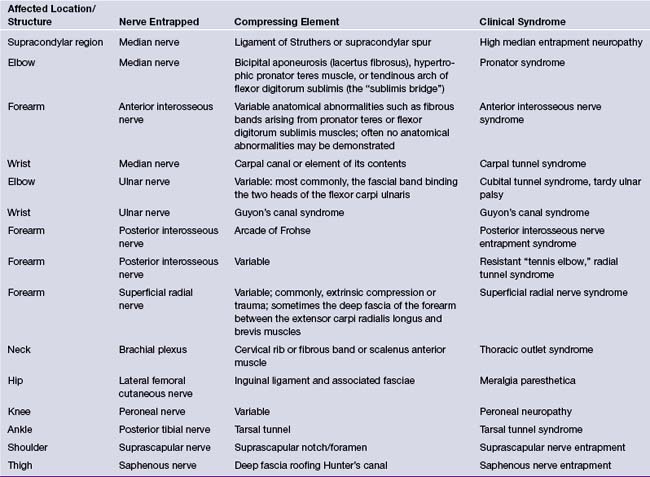
The entrapment sites, the nerves involved at each site, and the corresponding syndromes are listed in Table 33.1. This chapter will cover the most common entrapment syndromes: carpal tunnel syndrome, cubital tunnel syndrome, meralgia paresthetica, suprascapular nerve entrapment, and tarsal tunnel syndrome. Some patients can develop multiple entrapment neuropathies.23
Carpal Tunnel Syndrome24
The carpal tunnel syndrome25–27 is the most common entrapment neuropathy encountered in clinical practice. It results from compression of the distal median nerve within the carpal tunnel, located in the proximal part of the palm of the hand.28 The carpal tunnel is bounded dorsally by the carpal bones and ventrally by the transverse carpal ligament. The carpal bones form a shallow trough that is converted into a tunnel by the carpal ligament. The contents of the tunnel are the median nerve and tendons of the long flexor muscles (see Fig. 33.1A). Any lesion affecting the synovial sheath tends to compromise the cross-sectional diameter of the carpal canal and may induce compressive neuropathy.29 Recent studies that include magnetic resonance imaging (MRI) and computed tomography (CT) scans show that patients with carpal tunnel syndrome tend to have small carpal canals. The small size of the carpal canal, measured by the decrease in its cross-sectional diameter, is a congenital or developmental phenomenon.30 Its small size in women may account for their higher incidence of carpal tunnel syndrome.
Clinical Features31
Women are more commonly affected than men, by a ratio of 7:3. Most patients are middle-aged at the onset of symptoms. The predominant symptom is an aching, burning, tingling, numb sensation in the hand, ordinarily in the lateral half of the hand and the outer three or four digits.32 Frequently there may be an aching pain in the proximal forearm or even in the arm up to the shoulder and it can lead to confusion with cervical radiculopathy. Patients typically wake up at night with increased pain, and they may shake their hand to obtain relief. The symptoms are often bilateral. With severe or advanced compression patients complain of weakness of grip and a tendency to drop things.
In the early stages of the syndrome, at which time most patients are seen in contemporary practice, there are few objective findings. Two mechanical tests can be performed. Tinel’s sign may be elicited by lightly tapping over the median nerve at the wrist crease, which results in a tingling in the distribution of the median nerve if positive. Phalen’s test consists of asking the patient to flex the wrist to 90 degrees for about 60 seconds, which will precipitate paresthesia in the distribution of the median nerve if positive.33–35 Neither test is conclusive and both results are often absent. Perception of light touch, pinprick, and two-point discrimination in the tips of the fingers in the median nerve distribution may be impaired. In advanced cases there may be atrophy of the thenar muscles, especially in the abductor pollicis brevis. A recently proposed scratch collapse test for evaluation of carpal and cubital canal syndrome may be a significant addition for clinicians but it needs to be evaluated by independent reviewers.36
The clinical history, especially of nocturnal pain, is usually the most reliable diagnostic clue. There are several local and systemic risk factors that precipitate the symptoms of carpal tunnel syndrome (Table 33.2).37–44
TABLE 33.2 Risk Factors in the Pathogenesis of Carpal Tunnel Syndrome
| Local Factors | Systemic Factors |
|---|---|
| Increased volume of the contents of the carpal canal
Masses: neurofibroma, hemangioma, lipoma, ganglion cyst, gouty tophus, xanthoma Anomalous muscles and tendons40 Persistent median artery with or without thrombosis, aneurysm, arteriovenous malformation Reduction in the capacity of the carpal canal Congenitally small carpal canal30 Idiopathic or familial thickening of the transverse carpal ligament39 Malunion or callus following Colles’ fracture or fracture of the carpal bones Unreduced dislocations of the wrist or intercarpal joints Improper immobilization of the wrist (“cotton loader position”) Other local factors |
Alcoholic or diabetic polyneuropathy
Hereditary neuropathy with liability to pressure palsies
Proximal lesions of the median nerve (“double crush” syndrome)
Factors unique to women
Inflammatory and autoimmune disorders
Metabolic disorders
Diagnosis
The most important diagnostic tests are electromyography and study of nerve conduction velocity.45 The earliest and most significant finding is the prolongation of sensory latency due to demyelination. The sensory evoked response will show diminution of amplitude and may even be absent. Motor latency abnormalities occur late in the course of the disease. Needle electromyography may show loss of motor unit potentials and the presence of denervation potentials in the median-innervated muscles in the thenar eminence due to axonal loss. Clinically it corresponds to the impairment of two-point discrimination.
Treatment
Current management strategies based on the evidence-based medicine approach were summarized in the American Academy of Orthopaedic Surgeons practice guidelines published in 200946 and are summarized in Table 33.3.47–51
TABLE 33.3 Management of Entrapment Syndromes: Evidence-Based Medicine Results
| Study/Review | Conclusions |
|---|---|
| Carpal Tunnel Syndrome | |
| Ono S, et al. Optimal management of carpal tunnel syndrome.47 (A review of RCTs and systematic reviews.) Jarvik JG, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomized parallel-group trial.51 |
A trend toward recommending early surgery for cases with and without median nerve denervation. Symptoms in both groups improved, but surgical treatment led to better outcome than that with nonsurgical treatment. However, the clinical relevance of this difference was moderate. |
| Cubital Canal Syndrome | |
| Bartels RHMA, et al. Prospective randomized controlled study comparing simple decompression versus anterior subcutaneous transposition for idiopathic neuropathy of the ulnar nerve at the elbow.48 Chung K. Treatment of ulnar nerve compression at the elbow.49 (A review of RCTs and systematic reviews.) |
The outcomes were equivalent, but simple decompression was associated with fewer complications. Use of this approach is advised even in the presence of (sub)luxation. Different transposition techniques (subcutaneous and submuscular) yielded results similar to those with simple decompression. All mentioned randomized controlled trials had relatively small samples. |
| Tarsal Tunnel Syndrome | |
| Patel AT, et al. Usefulness of electrodiagnostic techniques in the evaluation of suspected tarsal tunnel syndrome: an evidence-based review.50 | Nerve conduction studies may be useful for confirming the diagnosis of tibial neuropathy at the ankle (recommendation level C). |
In early cases with minimal symptoms or in individuals in whom the syndrome is expected to be transient, conservative treatment should be instituted. This consists of a wrist splint in neutral position at night (initial relief in approximately 50%), a course of vitamin B6, and anti-inflammatory drugs. Injection of local anesthesia and steroids around the median nerve may be beneficial,52,53 but accidental injection directly into the nerve may result in annoying paresthesias in the distribution of the median nerve.
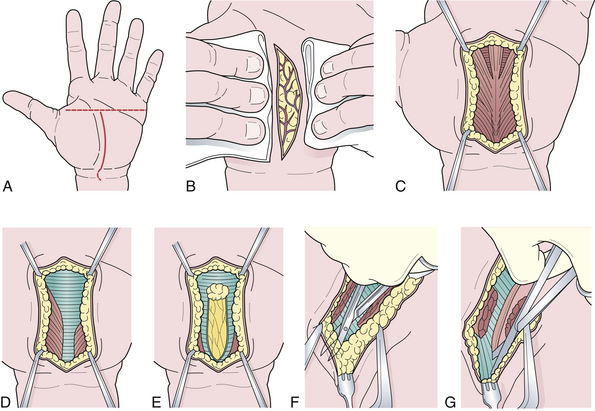
Surgical therapy is indicated when conservative measures fail.51 The surgical procedure can be performed by either the open method54 or an endoscopic technique.55 The steps in the surgical sectioning of the transverse carpal ligament are shown in Figure 33.5. Usually local or regional anesthesia (Bier block) is used. General anesthesia may be used if the patient is extremely nervous.
Endoscopic section of the carpal ligament has recently been introduced.55 The advantages are that the postoperative recovery period is shorter, a sensitive scar in the palm of the hand is avoided, and the structural integrity of the carpal tunnel mechanism is minimally disturbed. However, there is a greater risk of injury to the ulnar artery and to the sensory branch of the median nerve serving the middle and ring fingers. With any technique the patient should be instructed to move the fingers postoperatively to minimize scar formation.
Good outcome is to be expected in at least 85% to 90% of the cases but if there is significant weakness and wasting at the time of presentation the results are less satisfactory. Relief of typical nocturnal pain is usually immediate.56
Cubital Tunnel Syndrome
The cubital tunnel syndrome57–80 results from entrapment of the ulnar nerve at the elbow. The cubital tunnel is located on the medial side of the elbow joint. It is a fibro-osseous tunnel that is roofed by the aponeurotic attachment of the two heads of the flexor carpi ulnaris and a tough fascial band that bridges these two heads, also known as the Osborne’s ligament74,75 (see Fig. 33.1B). The floor is formed by the medial ligament of the elbow joint. During flexion of the elbow, the volume of the cubital tunnel decreases; the reverse happens in extension. This is because the points of attachment of the flexor carpi ulnaris, that is, the medial epicondyle and the olecranon process, are farthest apart during flexion. Thus, there is more tension on the fascial band between these two heads, which increases the pressure on the cubital tunnel.
Diagnosis
The characteristic electrodiagnostic finding is a delay in the conduction velocity in the ulnar nerve across the elbow. The sensory latency is prolonged, and the amplitude of the motor response in the abductor digiti minimi is decreased. A needle examination of the ulnar-innervated muscles may show denervation potentials. A special inching technique as well as MRI help to detect ulnar nerve entrapment across the elbow. A three-grade scale of the severity of ulnar entrapment at the elbow was proposed by McGowan.71
Tardy ulnar paralysis should be differentiated from lesions in the spinal cord affecting the C8, T1 segments, such as syringomyelia, spinal cord tumor, or amyotrophic lateral sclerosis, and extradural spinal lesions, such as cervical disk disease or spondylosis, neurofibroma, or meningioma. Lesions of the brachial plexus involving the lower trunk or the medial cord (Pancoast tumor), entrapment of the ulnar nerve distally at the wrist (Guyon’s canal), and polyneuropathy should also be ruled out.
Treatment
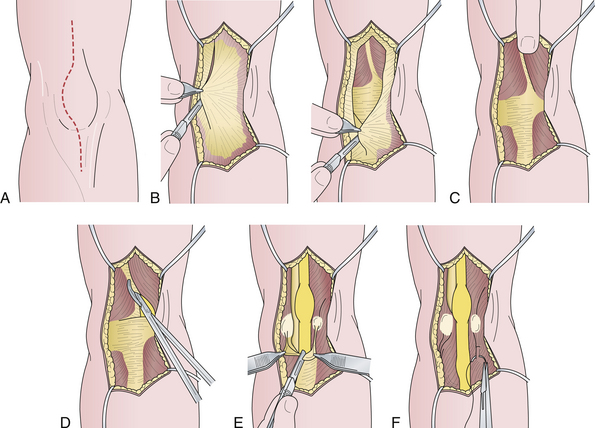
In early, minimally symptomatic cases, a conservative approach is recommended. The patient should wear an elbow pad for protection against direct pressure to the nerve and avoid excessive flexion of the elbow and strenuous exercise for some time, especially sports with maneuvers that involve vigorous throwing, such as baseball. In persistent or highly symptomatic cases, surgical options should be considered. There is no other entrapment neuropathy for which the surgical options are more controversial than cubital tunnel syndrome. The available surgical methods are listed in Box 33.1. The simplest and most satisfactory procedure for uncomplicated cases is cubital tunnel release.81 Excellent to good pain relief has been demonstrated in 75% to 90% of patients. Recovery of muscle weakness usually takes many months and does not always occur in severe cases. (The steps of the procedure are shown in Fig. 33.6.) In more involved cases complicated by elbow-joint abnormality, malunited fractures, or other abnormalities, the nerve may be transposed anterior to the elbow joint into the subcutaneous, intramuscular, or submuscular planes. Results of the recent prospective randomized controlled study comparing simple decompression with anterior subcutaneous transposition showed similar outcomes but fewer complications with decompression. It also was associated with lower cost.48
Meralgia Paresthetica
Meralgia paresthetica82–84 is a syndrome caused by the entrapment of the lateral femoral cutaneous nerve of the thigh in the inguinal region. The name refers to the burning sensation that affected individuals complain of in the anterolateral thigh (meros, thigh; algos, pain).
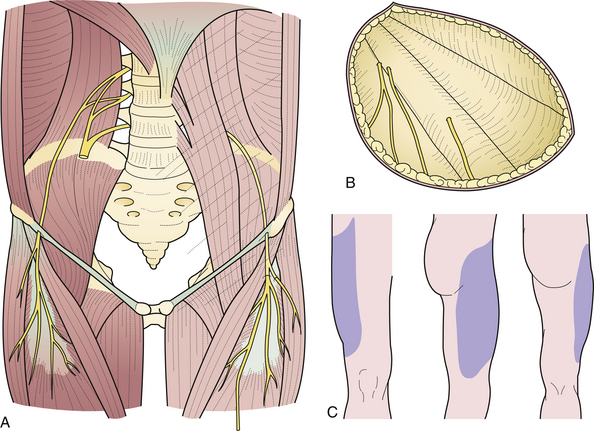
The lateral femoral cutaneous nerve of the thigh arises from the lumbar plexus, emerges at the lateral margin of the psoas major muscle, descends obliquely downward and forward under the iliac fascia, pierces the inguinal ligament near the anterior superior iliac spine, courses under the fascia lata for about 5 cm, and then becomes subcutaneous by piercing the fascia lata (Fig. 33.7A). It innervates the skin of the anterolateral aspect of the thigh and the gluteal region (Fig. 33.7B and C).
Entrapment of the nerve occurs in the inguinal region at the point where it pierces the inguinal ligament. Obese individuals with a pendulous, flabby anterior abdominal wall are more prone to this disorder. Persons who spend much of the day walking or standing, such as patrolmen, postal workers, and traveling salesmen, are also more susceptible. Patients complain of a tingling, crawling, pricking, “pins and needles” sensation in the anterolateral thigh. Varying degrees of sensory loss may be present in the anterolateral thigh. Because the affected nerve is a purely cutaneous nerve, there are no motor abnormalities or reflex changes. Indeed, if they are present an alternative diagnosis should be entertained.
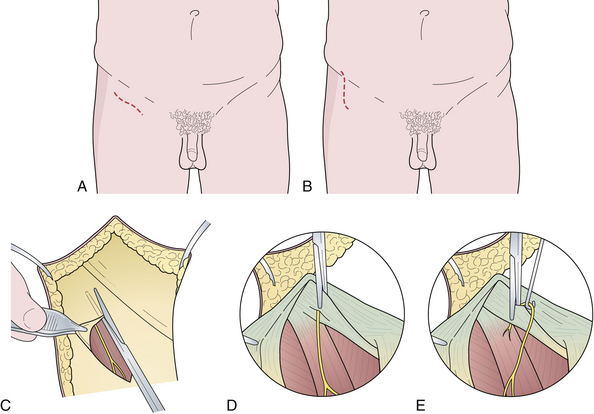
Electrodiagnostic tests are not helpful in establishing the diagnosis of meralgia paresthetica—they are used instead to exclude other disorders that involve the lumbosacral plexus or the cauda equina. The best test for confirmation of the clinical impression is a diagnostic nerve block, performed by injecting 5 mL of 0.5% lidocaine with epinephrine just medial to the anterior superior iliac spine. Complete relief of symptoms is generally predictive of a good operative result. The recently proposed pelvic compression test is supposed to release the nerve and provide relief of pain (Fig. 33.8).85 It will also predict the result of surgery. The technique of sectioning of the inguinal ligament and decompression is shown in Figure 33.9.
Suprascapular Nerve Entrapment
Suprascapular neuropathy86–88 results from injury to the suprascapular nerve and is typically due to direct compression or stretching that occurs with retraction of a large rotator cuff tear. The typical presentation includes deep and throbbing pain, located along the superior border of the scapula, and weakness of shoulder abduction despite a strong deltoid muscle (supraspinatus) and external rotation (infraspinatus). Muscle atrophy may be noticeable.
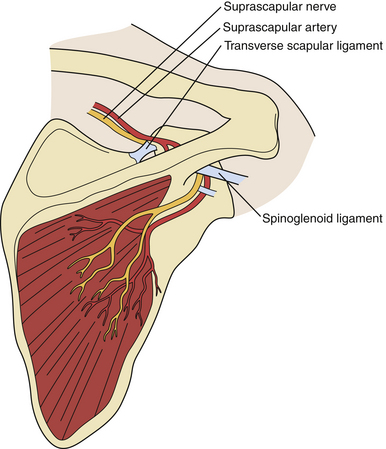
Of the two potential sites of constriction, the suprascapular notch is more common than the spinoglenoid notch (Fig. 33.10). The injury to the nerve at the suprascapular notch affects both supraspinatus and infraspinatus muscles but at the spinoglenoid notch only the infraspinatus.
Differential diagnosis includes various musculoskeletal conditions involving the shoulder as well as cervical radiculopathy affecting the C5 and the C6 roots, axillary neuropathy and neuralgic amyotrophy. Suprascapular entrapment neuropathy has often been overlooked as a source of shoulder pain.88
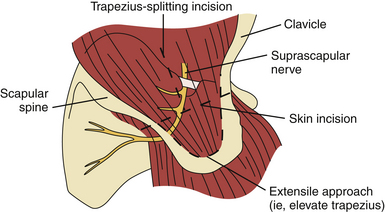
Operative treatment may include decompression of the suprascapular nerve with or without repair of associated shoulder abnormalities. Decompression of the suprascapular notch can be done in an open fashion (Fig. 33.11)87 or arthroscopically. At the time of this writing there are no prospective studies comparing operative and nonoperative treatment.88
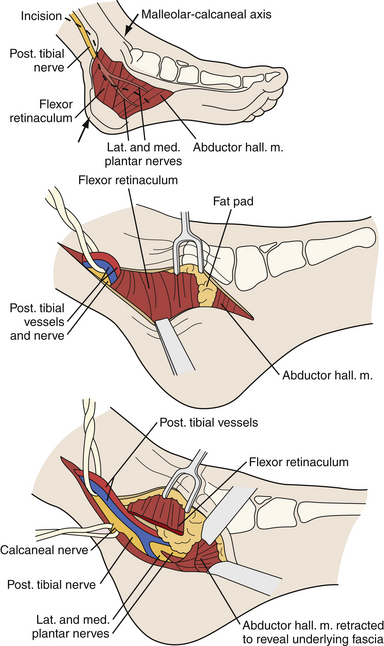
Tarsal Tunnel Syndrome
Entrapment neuropathy of the tibial nerve is relatively rare. Consequently, it is often misdiagnosed. The most common site of tibial mononeuropathy is at the level of the tarsal tunnel where the tibial nerve can be compressed posterior and inferior to the medial malleolus (Fig. 33.12). The typical constellation of symptoms includes pain and numbness in the sole of the foot and a sensation of tightness, cramping pain, and worsening of symptoms with prolonged standing or walking.50
The diagnosis is usually based on positive Tinel’s sign and objective sensory loss in the territory of any of the terminal branches of the tibial nerve. The value of electrodiagnostic studies was recently evaluated but it was concluded that it was only of level C (poor evidence).50
As with other entrapment neuropathies, surgery should be considered when conservative treatment has failed or when there is a mass lesion. The technique involves exploration of the tarsal tunnel and release of the flexor retinaculum. As well as decompressing the tarsal tunnel roof, any space-occupying lesion should be removed. It is extremely important to follow the medial and the lateral plantar nerves well into the plantar aspect of the foot to check for any compression of these nerves by the abductor hallucis muscle or other fibrous bands. If these compressed nerves are encountered, they should be also released. An endoscopic technique has also been described.89
American Academy of Orthopaedic Surgeons Work Group Panel. Clinical practice guidelines on the treatment of carpal tunnel syndrome. Available at www.aaos.org/research/guidelines/CTStreatmentguide.asp Accessed Dec. 6, 2010
Chung K. Treatment of ulnar nerve compression at the elbow. J Hand Surg Am. 2008;33:1625-1627.
Huang J.H., Zager E.L. Mini-open carpal tunnel decompression. Neurosurgery. 2004;54:397-400.
Jimenez D.F., Loftus T. Endoscopic carpal tunnel release. In: Midha R., Zager E., editors. Surgery of Peripheral Nerves. New York: Thieme; 2008:100-104.
Ono S., Clapham P.J., Chung K.C. Optimal management of carpal tunnel syndrome. Intern J Gen Med. 2010;3:255-261.
Please go to expertconsult.com to view the complete list of references.
1. Aguayo A., Nair C.P.V., Midgely R. Experimental progressive compression neuropathy in the rabbit. Arch Neurol. 1971;24:358.
2. Bentley F.H., Schlapp W. The effects of pressure on conduction in peripheral nerve. J Physiol. 1943;102:72.
3. Dahlin L.B., McLean W.G. Effects of graded experimental compression on slow and fast axonal transport in rabbit vagus nerve. J Neurol Sci. 1986;72:19-30.
4. Dahlin L.B., Lundborg G. The neuron and its response to peripheral nerve compression. J Hand Surg. 1990;15B:5-10.
5. Dahlin L.B., Rydevik B., McLean W.G., et al. Changes in fast axonal transport during experimental nerve compression at low pressures. Exp Neurol. 1984;84:29-36.
6. Dahlin L.B., Sjorstrand J., McLean W.G. Graded inhibition of retrograde axonal transport by compression of rabbit vagus nerve. J Neurol Sci. 1986;76:221-230.
7. Dahlin L.B., Nordborg C., Lundborg G. Morphologic changes in nerve cell bodies induced by experimental graded nerve compression. Exp Neurol. 1986;95:611-621.
8. Dahlin L.B., Shyu B.C., Danielsen N., et al. Effects of nerve compresson or ischemia on conduction properties of myelinated and non-myelinated nerve fibers: an experimental study in the rabbit peroneal nerve. Acta Physiol Scand. 1989;136:97-105.
9. Denny-Brown D., Brenner C. Paralysis of nerve induced by direct pressure and by tourniquet. Arch Neurol Psychiatr. 1944;51:1-26.
10. Duncan D. Alterations in the structure of nerves caused by restricting their growth with ligatures. J Neuropathol Exp Neurol. 1948;7:261-273.
11. Fowler T.J., Ochoa J. Unmyelinated fibers in normal and compressed peripheral nerves of the baboon: a quantitative electron microscopic study. Neuropathol Appl Neurobiol. 1975;1:247.
12. Fullerton P.M., Gilliatt R.W. Pressure neuropathy in the hindfoot of the guinea-pig. J Neurol Neurosurg Psychiatr. 1967;30:18-25.
13. Fullerton P.M., Gilliatt R.W. Median and ulnar neuropathy in the guinea-pig. J Neurol Neurosurg Psychiatr. 1967;30:393-402.
14. Lundborg G., Myers R., Powell H. Nerve compression injury and increase in endoneurial fluid pressure: a “miniature compartment syndrome.”. J Neurosurg Psychiatr. 1983;46:1119.
15. Neary D., Eames R.A. The pathology of ulnar nerve compression in man. Neuropathol Appl Neurobiol. 1975;1:69-88.
16. Ochoa J. Nerve fiber pathology in acute and chronic compression. In: Omer G.E.Jr., Spinner M., editors. Management of Peripheral Nerve Problems. Philadelphia: WB Saunders; 1980:487-501.
17. Ochoa J., Marotte L. The nature of the nerve lesion caused by chronic entrapment in the guinea-pig. J Neurol Sci. 1973;19:491.
18. Ochoa J., Fowler T.J., Gilliatt R.W. Anatomical changes in peripheral nerves compressed by a pneumatic tourniquet. J Anat. 1972;113:433.
19. Ogatta K., Naito M. Blood flow of peripheral nerve: effects of dissection, stretching and compression. J Hand Surg. 1986;11B:10.
20. Rydevik B., Lundborg G. Permeability of intraneural microvessels and perineurium following acute, graded experimental nerve compression. Scand J Plast Reconstr Surg. 1977;11:179.
21. Rydevik B., Nordborg C. Changes in nerve function and nerve fiber structure induced by acute, graded compression. J Neurol Neurosurg Psychiatr. 1980;43:1070.
22. Upton A.R.M., McComas A.J. The double crush in nerve entrapment syndromes. Lancet. 1973;2:359.
23. Karpati G., Carpenter S., Eisen A.A., et al. Multiple peripheral nerve entrapments: an unusual phenotypical variant of the Hunter syndrome (mucopolysaccharidosis II) in a family. Arch Neurol. 1974;31:418-422.
24. Spinner M., editor, Management of Peripheral Nerve Problems. Philadelphia: WB Saunders; 1980:487-501.
25. Bauman T.D., Gelbermann R.H., Mubarak S.J., et al. The acute carpal tunnel syndrome. Clin Orthop. 1981;156:151-156.
26. Bendler E.M., Greenspun B., Yu J., et al. The bilaterality of carpal tunnel syndrome. Arch Phys Med Rehabil. 1967;58:363-364.
27. Gelberman R.H., Hergenroeder P.T., Hargens A.R., et al. The carpal tunnel syndrome. J Bone Joint Surg. 1981;63A:380-383.
28. Spinner M., Spencer P.S. Nerve compression lesions of the upper extremity. Clin Orthop. 1974;104:46-67.
29. Smith E.M., Sonstegard D.A., Anderson W.H. Carpal tunnel syndrome: contribution of flexor tendons. Arch Phys Med Rehabil. 1977;58:379-385.
30. Dekel S., Papaioannou T., Rushworth G., et al. Idiopathic carpal tunnel syndrome caused by carpal stenosis. Br Med J. 1980;280:1297-1299.
31. Tanzer R.C. The carpal-tunnel syndrome: a clinical and anatomical study. J Bone Joint Surg. 1959;41A:626-634.
32. Kremer M., Gilliatt R.W., Golding J.S.R., et al. Acroparaesthesiae in the carpal-tunnel syndrome. Lancet. 1953;2:590-595.
33. Phalen G.S. Reflections on 21 years’ experience with the carpal-tunnel syndrome. JAMA. 1970;212:1365-1367.
34. Phalen G.S. The carpal-tunnel syndrome. J Bone Joint Surg. 1966;48A:211-228.
35. Phalen G.S., Kendrick J.I. Compression neuropathy of the median nerve in the carpal tunnel. JAMA. 1953;164:524-595.
36. Cheng C.J., Mackinnon-Patterson B., Beck J.L., Mackinnon S. Scratch collapse test for evaluation of carpal and cubital canal syndrome. J Hand Surg. 2008;33A:1518-1524.
37. Barnes C.G., Currey H.L.F. Carpal tunnel syndrome in rheumatoid arthritis: a clinical and electrodiagnostic survey. Ann Rheum Dis. 1967;26:226-233.
38. Bradish C.F. Carpal tunnel syndrome in patients on haemodialysis. J Bone Joint Surg. 1985;67B:130-132.
39. Brain W.R., Wright A.D., Wilkinson M. Spontaneous compression of both median nerves in the carpal tunnel. Lancet. 1947;1:277-282.
40. Carroll M.P., Montero C. Rare anomalous muscle cause of carpal tunnel syndrome. Orthop Rev. 1980;9:83-85.
41. Gould J.S., Wissinger H.A. Carpal tunnel syndrome in pregnancy. South Med J. 1978;71:144-145.
42. Halter S.K., DeLisa J.A., Stolov W.C., et al. Carpal tunnel syndrome in chronic renal dialysis patients. Muscle Nerve. 1980;3:438A.
43. Hartwell S.W., Kurtay M. Carpal tunnel compression caused by hematoma associated with anticoagulant therapy. Cleveland Clin. 1966;33:127-129.
44. Votik A.J., Mueller J.C., Farlinger D.E., Johnston R.U. Carpal tunnel syndrome in pregnancy. Can Med Assoc J. 1983;128:277-281.
45. Thomas J.E., Lambert E.H., Czeuz K.A. Electrodiagnostic aspects of the carpal tunnel syndrome. Arch Phys Med Rehabil. 1980;16:635-641.
46. American Academy of Orthopaedic Surgeons Work Group Panel. Clinical practice guidelines on the treatment of carpal tunnel syndrome. Available at www.aaos.org/research/guidelines/CTStreatmentguide.asp Accessed Dec. 6, 2010
47. Ono S., Clapham P.J., Chung K.C. Optimal management of carpal tunnel syndrome. Intern J Gen Med. 2010;3:255-261.
48. Bartels R.H.M.A., Verhagen W.I.M., der Wilt G.J., et al. Prospective randomized controlled study comparing simple decompression versus anterior subcutaneous transposition for idiopathic neuropathy of the ulnar nerve at the elbow (Parts 1 and 2). Neurosurgery. 2005;56:522-537.
49. Chung K. Treatment of ulnar nerve compression at the elbow. J Hand Surg Am. 2008;33:1625-1627.
50. Patel A.T., Gaines K., Malamut R., et al. Usefulness of electrodiagnostic techniques in the evaluation of suspected tarsal tunnel syndrome: an evidence-based review. Muscle Nerve. 2005;32:236-240.
51. Jarvik J.G., Comstock B.A., Kliot M., et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomized parallel-group trial. Lancet. 2009;374:1074-1081.
52. Goodman H.V., Foster J.B. Effect of local corticosteroid injection on median nerve conduction in carpal tunnel syndrome. Ann Phys Med. 1962;6:287-294.
53. Green D.P. Diagnostic and therapeutic value of carpal tunnel injection. J Hand Surg. 1984;9A:850-854.
54. Huang J.H., Zager E.L. Mini-open carpal tunnel decompression. Neurosurgery. 2004;54:397-400.
55. Jimenez D.F., Loftus T. Endoscopic carpal tunnel release. In: Midha R., Zager E., editors. Surgery of Peripheral Nerves. New York: Thieme; 2008:100-104.
56. Cseuz K.A., Thomas J.E., Lambert E.H., et al. Long-term results of operation for carpal tunnel syndrome. Mayo Clin Proc. 1966;41:232-241.
57. Adelarr R.S., Foster W.C., McDowell C. The treatment of the cubital tunnel syndrome. J Hand Surg. 1984;9A:90-95.
58. Apfelberg D.B., Larson S.J. Dynamic anatomy of the ulnar nerve at the elbow. Plast Reconstr Surg. 1973;51:76-81.
59. Broudy A.S., Leffert R.D., Smith R.J. Technical problems with ulnar nerves by Learmonth technique. J Hand Surg. 1982;7:147-155.
60. Chan R.C., Paine K.W.E., Varughese G. Ulnar neuropathy at the elbow: comparison of simple decompression and anterior transposition. Neurosurgery. 1980;7:545-550.
61. Craven P.R., Green D.P. Cubital tunnel syndrome: treatment by medial epicondylectomy. J Bone Joint Surg. 1980;62A:986-989.
62. Dahners L.E., Wood F.M. Aconeus epitrochlearis, a rare cause of cubital tunnel syndrome: a case report. J Hand Surg. 1984;9A:579-580.
63. Eisen A. Early diagnosis of ulnar nerve palsy. Neurology. 1974;24:256-262.
64. Eisen A., Danon J. The mild cubital tunnel syndrome: its natural history and indications for surgical intervention. Neurology. 1974;24:608-613.
65. Feindel W., Stratford J. The role of the cubital tunnel in tardy ulnar palsy. Can J Surg. 1958;1:287-300.
66. Harrison M.J.G., Nurick S. Results of anterior transposition of the ulnar nerve for ulnar neuritis. Br Med J. 1970;1:27-29.
67. Jabre J.F., Wilbourn A.J. The EMG findings in 100 consecutive ulnar neuropathies. Acta Neurol Scand. 1979;60(Suppl):73-91.
68. Laha R.K., Panchal P.D. Surgical treatment of ulnar neuropathy. Surg Neurol. 1979;11:393-398.
69. Leffert R.D. Anterior submuscular transposition of the ulnar nerves by the Learmonth technique. J Hand Surg. 1982;7:147-155.
70. Levy D.M., Apfelberg D.B. Results of anterior transposition for ulnar neuropathy at the elbow. Am J Surg. 1972;123:304-308.
71. McGowan A.J. The results of transposition of the ulnar nerve for traumatic ulnar neuritis. J Bone Joint Surg. 1950;32B:293-301.
72. Miller R.G. The cubital tunnel syndrome: diagnosis and precise localization. Ann Neurol. 1979;6:56-59.
73. Miller R.G., Hummel E.E. The cubital tunnel syndrome: treatment with simple decompression. Ann Neurol. 1980;7:567-569.
74. Osborne G. Compression neuritis of the ulnar nerve at the elbow. Hand. 1970;2:10-13.
75. Osborne G.V. The surgical treatment of the tardy ulnar neuritis. J Bone Joint Surg. 1957;39B:782.
76. Paine K.W.E. Tardy ulnar palsy. Can J Surg. 1970;13:255-261.
77. Payan J. Cubital tunnel syndrome. Br Med J. 1979;2:868.
78. Payan J. Electrophysiological localization of ulnar nerve lesions. J Neurol Neurosurg Psychiatr. 1960;32:208-220.
79. Wadsworth T.G., Williams J.R. Cubital tunnel external compression syndrome. Br Med J. 1973;1:662-666.
80. Wilson D.H., Krout R. Surgery of ulnar neuropathy at the elbow: 16 cases treated by decompression without transposition. J Neurosurg. 1973;38:780-785.
81. Huang J.H., Samadani U., Zager E.L. Ulnar nerve entrapment neuropathy at the elbow: simple decompression. Neurosurgery. 2004;55:1150-1153.
82. Ecker A.D., Woltman H.W. Meralgia paraesthetica. JAMA. 1938;110:1650-1652.
83. Stevens H. Meralgia paresthetica. Arch Neurol Psychiatr. 1957;77:557-574.
84. Stookey B. Meralgia paresthetica. JAMA. 1928;90:1705-1707.
85. Nouraei S.A.R., Anand B., Spink G., O’Neill K.S. A novel approach to the diagnosis and management of meralgia paresthetica. Neurosurgery. 2007;60:696-700.
86. Piasecki D.P., Romeo A.A., Bach B.R.Jr., Nicholson G.P. Suprascapular neuropathy. J Am Acad Orthop Surg. 2009;17:665-676.
87. Laxton A.W., Midha R. Suprascapular nerve palsy. In: Midha R., Zager E., editors. Surgery of Peripheral Nerves. New York: Thieme, 2008.
88. Boykin R.E., Friedman D.J., Higgins L.D., Warner J.J.P. Suprascapular neuropathy. J Bone Joint Surg Am. 2010;92:2348-2364.
89. Oh S.J., Meyer R.D. Entrapment neuropathies of the tibial (posterior tibial) nerve in entrapment and other focal neuropathies. Neurol Clin. 1999;17:593-615.