Chapter 14 Endovenous Thrombectomy and Thrombolysis
Historical Background
After the first episode of deep venous thrombosis (DVT), affected patients may develop pulmonary embolism (PE), recurrent DVT, or postthrombotic syndrome (PTS), and all carry significant morbidity and negative socioeconomic impact.1 Important concepts in the natural history of the disease are recurrence, propagation, and recanalization with anatomic and/or hemodynamic changes following an episode of DVT. Spontaneous resolution of the DVT leaving intact the affected segment is rarely found, occurring only in one-third of patients.2 Frequently, the segment affected by thrombosis develops reflux due to venous valve injury or some degree of residual obstruction.3 Patients who develop a combination of reflux and venous obstruction following an episode of DVT have the highest risk of developing PTS.3,4 A combination of reflux and obstruction is found in up to 86% of the patients, while occlusion alone is found in less than 10%.5
Patients with extensive proximal DVT, such as iliocaval or iliofemoral occlusions, have a worse prognosis compared with patients with infrainguinal thrombosis.6,7 The resolution of venous obstruction with iliac thrombosis is often incomplete, and the incidence of PTS is higher.4 Recurrent DVT is a very important factor in the natural history of the disease because other than exposing the patient to PE, it also increases significantly the risk for developing PTS.8 In fact, it has been shown that a recurrent ipsilateral DVT is the strongest predictor for developing skin damage.9 The risk of recurrent DVT was 40% (95% confidence interval [CI], 35.4% to 44.4%) after 10 years, being 53% (95% CI, 45.6% to 59.5%) in patients with unprovoked DVT and 22% in those with secondary DVT (95% CI, 17.2% to 27.8%).10 Other important risk factors to develop recurrent DVT are age older than 65 years, incomplete thrombus resolution, and iliofemoral thrombosis.6,9,11–13
Treatment Options
To improve the long-term natural history of DVT, it is important to preserve venous function and outflow. Overall, the first line of treatment for DVT remains anticoagulation using either low-weight-molecular heparin (LMWH) or unfractionated heparin (UFH). Nonetheless, a review of 13 studies of proximal DVT showed total and partial resolution in only 4% and 14% of patients, respectively, who were treated with anticoagulation.14
Venous thrombectomy was first attempted in extreme cases of extensive DVT with impending venous gangrene aiming for limb salvage.15,16 Indications for surgical thrombectomy are extensive proximal (iliofemoral) DVT when thrombolytic agents are contraindicated.17 Therefore surgical thrombectomy has still not gained popularity, being performed in only a few specialized centers.18 More recently, local thrombolysis and venous thrombectomy have been used together with very good outcomes.19 Conversely, development of dedicated devises and safer use of thrombolytic agents has expanded percutaneous procedures. The goal with thrombolysis is to provide early thrombus removal with a minimally invasive procedure and low complications. By restoring venous flow, the objective is to prevent valve damage, venous hypertension, and recurrent thrombosis in an attempt to prevent PTS.
Patient Selection
Recommendations and patient’s eligibility criteria for endovenous thrombectomy and thrombolysis via catheter-directed thrombolysis (CDT) or pharmacomechanical thrombectomy (PMT) have been defined as follows17,20:
Modalities of Thrombolysis and Thrombectomy
Systemic Thrombolysis
Over the past 50 years, variable outcomes were reported with systemic thrombolysis. Analysis of 13 contemporary studies including 591 patients found that the success rate of anticoagulation with heparin only showed total dissolution, partial lysis, and no improvement of the thrombus in 4%, 14%, and 82%, respectively.14 Improvement with systemic thrombolysis was found in 45% of the patients in the same review.
Advantages of systemic infusion of thrombolytic agents were faster resolution of the clot load and less venous valve dysfunction compared with anticoagulation alone.14 Nonetheless, the disadvantages of the technique include variable outcomes with potential serious complications such as increased risk of intracranial hemorrhage due to higher doses required to dissolve a distant thrombus.21 In light of the variable success rate, systemic complications, and current advances in minimally invasive endovenous techniques such as CDT and PMT, systemic thrombolysis has been abandoned and now belongs to historical reports.
Catheter-Directed Thrombolysis
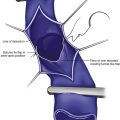
The higher risk of bleeding complications of systemic thrombolysis secondary to administration of high doses of thrombolytic agents via peripheral veins led to studies on devices to deliver intrathrombus thrombolytic agents.21–23 In general, the technique consists of a multi–side-hole catheter that is positioned in the thrombus and remains parked in position to deliver localized thrombolytics and lysing the thrombus over time (Fig. 14-1).
Initial experience using CDT was reported by Semba and Dake using urokinase in 21 consecutive patients with iliofemoral DVT.24 Total thrombus lysis was achieved in 72% of the limbs with overall angioplasty or angioplasty with stents required in 64%.24 Subsequently, several series were published comparing anticoagulation alone with CDT.14,23,25 The impact on quality of life (QoL) in 68 patients who underwent CDT and 30 patients treated with anticoagulation alone was assessed by Comerota and colleagues.22 Notably, patients who underwent CDT reported better overall physical functioning, less stigma, and less health distress. Furthermore, health distress and symptoms were reduced in patients who had a successful versus a failed CDT treatment.
The caveats of initial studies related to a small number of patients and single-center observation were addressed by a multicenter registry study with urokinase in 473 patients that showed complete lysis of the thrombus in 31% and partial lysis in 52% of the limbs with better results in patients with acute symptoms of DVT.26 Patients with iliofemoral DVT had higher patency rates at 1-year follow-up than those patients treated for femoropopliteal DVT (64% versus 47%, p < .01), and the mean intensive care unit stay for monitoring thrombolytic therapy was 48 hours.26 One patient had fatal intracranial bleeding and another required surgical evacuation of a subdural hematoma.
Two prospective randomized clinical trials (RCTs) have been reported. The first RCT enrolled 35 patients comparing CDT with streptokinase versus anticoagulation alone; it showed 72% of the limbs in the CDT group with no obstruction or reflux versus only 12% in the anticoagulant group at 6-month follow-up.25 No major bleeding or mortality was reported. The second RCT, the CaVEnT (Catheter-directed Venous Thrombolysis) trial, enrolled 118 patients to assess the long-term functional efficacy with frequency of PTS after 24 months and descriptive efficacy at 6 months’ evaluation for patency rates.23 Patency of the iliofemoral vein segment was 64% in the CDT group and 36% in the control group. In addition, lysis greater than50% was achieved in 88% of the patients who underwent CDT.23
The long interval of CDT treatment course remains problematic because of institutional requirements of an intensive care unit setting for monitoring. A longer infusion interval that is needed also raises concern of potential risk of major bleeding found in up to 11% of the patients.26 In addition, the use of fluoroscopy for multiple follow-up venograms has directed efforts to accomplish thrombus removal in a more expeditious fashion than has currently been performed by PMT devices.17,27
Percutaneous Mechanical Thrombectomy
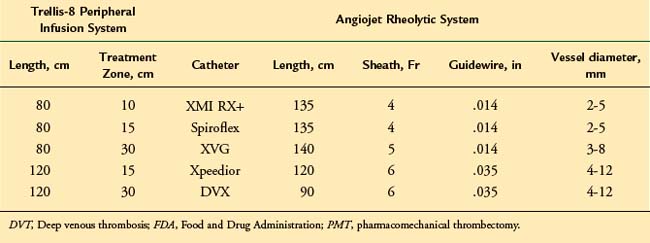
Two commercially available PMT devices are approved for treatment of DVT by the U.S. Food and Drug Administration (FDA): the Angiojet Thrombectomy system (Medrad/Possis Inc. Minneapolis, MN, and the Trellis-8 Peripheral Infusion System (Covidien, Mansfield, MA).28 In addition, a brief discussion about the Ekos Endowave system (Ekos Medical, Bothell, WA) is pertinent due to distinct mechanism combining ultrasound waves to facilitate chemical thrombolysis.
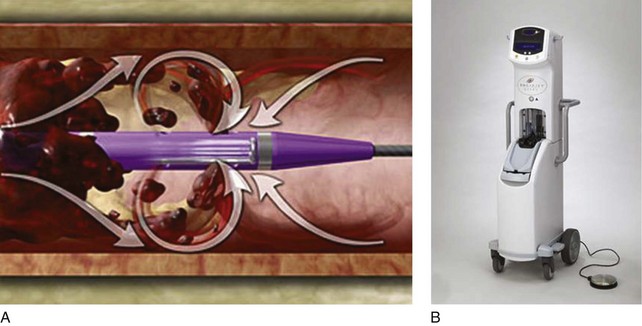
The mechanisms of PMT elicited by the Angiojet and Trellis device system are distinct. The Angiojet system is based on the Bernoulli-Venturi principle that states that high speed of a flow decreases the fluid pressure and creates a zone of vacuum (Fig. 14-2). Therefore the high-speed saline injections cause dissolution of the thrombus into small particles that are aspirated and eliminated through an effluent port. Theoretically, the advantages of the system are less endothelial damage and valve dysfunction. The device can also be used in combination with thrombolytics. Drug is delivered into the thrombus with the catheter, allowed to immerse in the thrombus, and then aspirated with the effluent port opened.
Initial experience was reported by Kasijaran et al. in 17 patients showing 59% of overall (>50%) thrombus removal.28 Bush et al. used the system in 23 limbs and reported complete and partial thrombus resolution in 65% and 35%, respectively.29 A comparison between the Angiojet versus CDT using only urokinase showed reduced treatment duration and lower costs with the Angiojet pulse system.30 Another study of 93 patients treated for symptomatic proximal DVT showed lower mean intensive care unit stays and overall length of hospital stays and lower total hospital costs with Angiojet compared with CDT only.27 One of the shortcomings of the system is a larger amount of fluid used to dissolve the thrombus, which can be impeditive in patients under fluid restriction, suggesting that precautions be used in cases of renal failure and decompensated congestive heart failure. Provoked hemolysis with increased bilirubin release in patients with liver failure may be another contraindication.
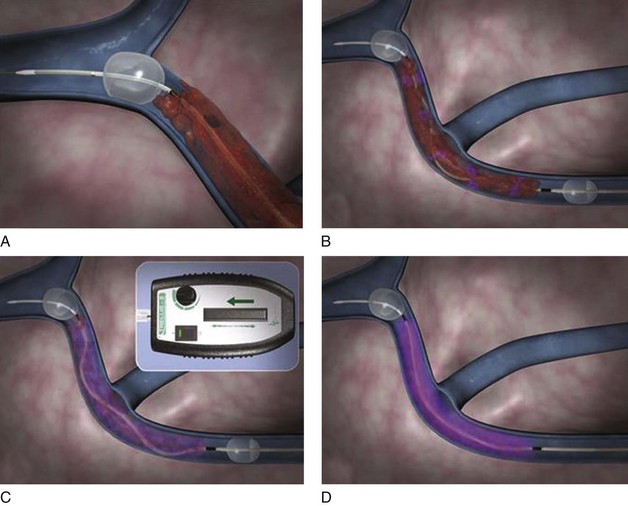
The Trellis-8 Peripheral Infusion System uses a dispersion spiral wire at higher spinning motion to macerated the thrombus in an isolated venous segment between a proximal and distal end balloon (Fig. 14-3). Initial experience with the device showed overall thrombus resolution in greater than 95% of limbs treated.31,32 No major complications including PE or major bleeding were reported. Trabal et al. reported a series of 25 limbs treated with the Trellis system showing greater than 50% thrombus resolution in 92% of the limbs with significant shorter treatment time and reduced thrombolytic agent used compared with CDT.33 In addition to thrombus burden resolution, the advantages of the Trellis system are its safety in patients with contraindications to thrombolytic agents and potentially less distal embolization due to isolation of a venous segment between two balloons.
The Ekos endowave functions by emitting high-frequency ultrasound waves that cause disaggregation of the fibrin in the thrombus, increasing permeability to the thrombolytic agents (Fig. 14-4).34 The catheter consists of four lumens from 6 to 50 cm of functional area containing an ultrasound wire and three other ports for thrombolytic agents and saline infusion. Initial experience in a study of 53 limbs with acute, chronic, and acute-on-chronic DVT showed complete thrombus removal in 70% and overall (complete and partial) lyses rate of 91%.35 There was no intracranial or retroperitoneal hematoma reported throughout the study. Further prospective, randomized studies on the use of the ultrasound-assisted thrombolysis are still expected.
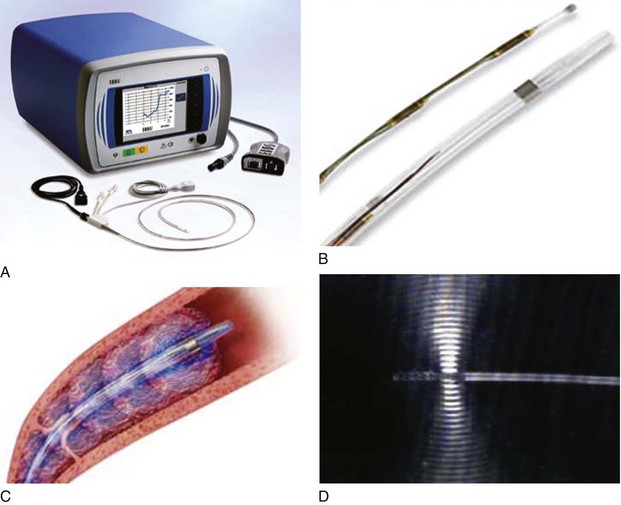
Fig 14–4 Ekos Endowave system. A, Ultrasound (US) source generator with monitor. B, Ultrasonic catheter
infusion. D, Detail of ultrasonic wave being emitted from distal probe.
An ongoing randomized multicenter trial, the ATTRACT trial, is designed to enroll 692 candidates to either PMT with anticoagulation versus anticoagulation alone in patients with symptomatic proximal DVT to assess the occurrence of PTS at 24-month follow-up.36 The impacts on QoL, safety, and cost analysis are expected to be addressed.
Operative Steps
Obtaining Access
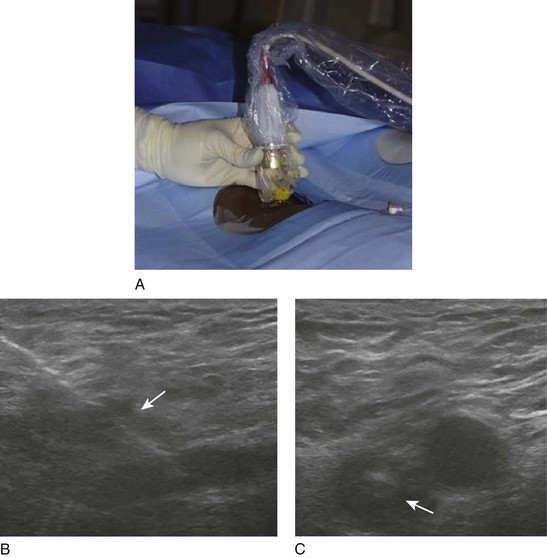
The patient is taken to the angiography or dedicated operating room suite, prepped, and draped in the prone position. Please note that local anesthetic with 1% lidocaine is used to infiltrate the skin in the popliteal region. A direct popliteal vein stick under ultrasound guidance is performed with a micropuncture kit (Fig. 14-5). A 3- or 4-Fr micropuncture catheter is inserted and its position is checked with gentle hand injections of dilute contrast. Often, the injection reveals filling defects throughout the proximal popliteal, distal, and middle femoral vein. The 3-Fr catheter is exchanged over a wire to a 5-Fr sheath. One may choose access of posterior tibial vein for cases where extensive infrapopliteal involvement is of concern in providing adequate inflow, or for isolated iliac vein thrombosis the patient can be placed in the supine position and access can be obtained in the ipsilateral femoral vein. Other access sites described include the contralateral femoral vein and jugular vein. Our preferred access site is ipsilateral popliteal vein.
Inferior Vena Cava Filter Placement
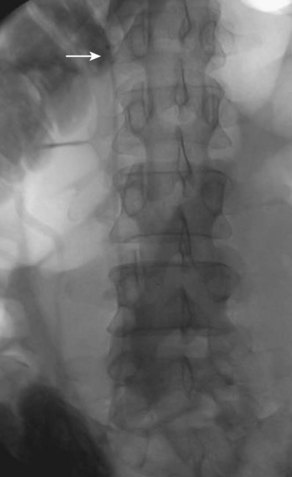
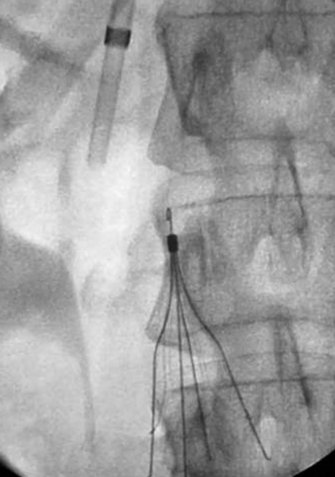
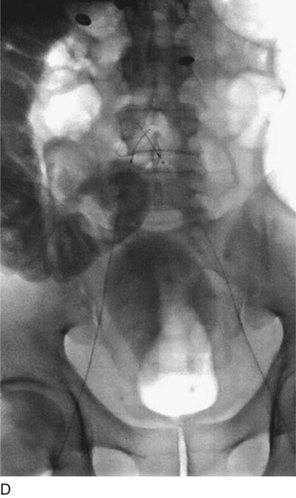
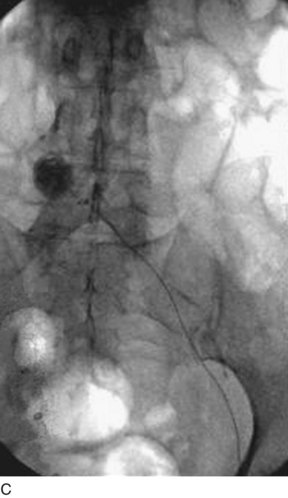
A retrievable filter when considered is placed via the right internal jugular (Fig. 14-6). Use of an IVC filter during thrombolysis is controversial. The risks and benefits of the filter placement must be explained to the patient. The right side of the neck is prepped and draped in the usual sterile fashion. Under continuous ultrasound guidance the right internal jugular vein is cannulated with the 22-gauge needle using a micropuncture kit. Subsequently, the 3-Fr sheath is exchanged for a 5-Fr sheath over a wire, which is passed into the infrarenal vena cava. Through the sheath and over the wire a pigtail marking catheter is positioned in the distal IVC, its position is confirmed with gentle hand injections with dilute contrast media, and then a cavogram is obtained with a power injection. Measurements are taken and the IVC filter is chosen based on operator’s preference. After IVC filter placement, hand injections through the sheath confirm the position of the filter and the opposition to the wall of the cava.
Catheter-Directed Thrombolysis
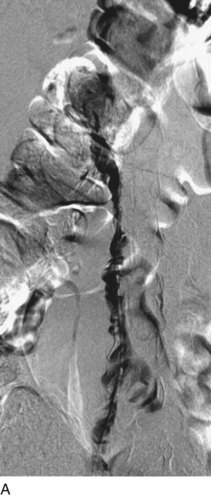
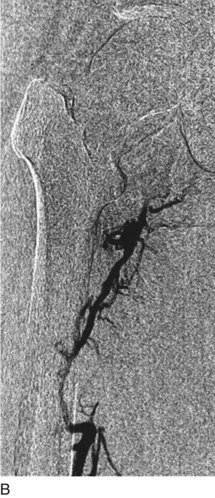
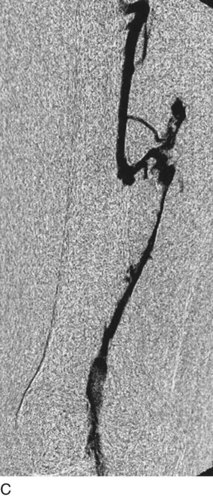
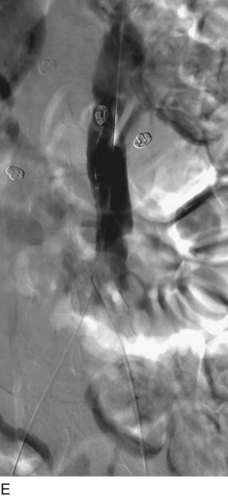
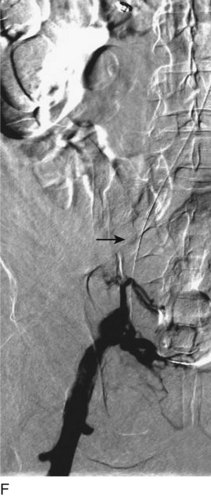
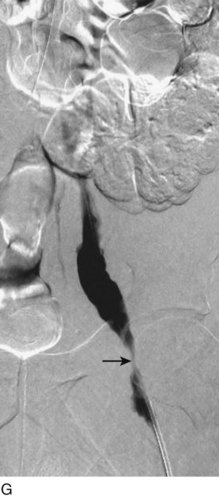
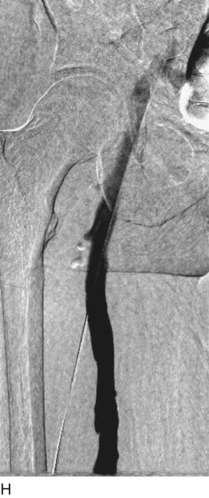
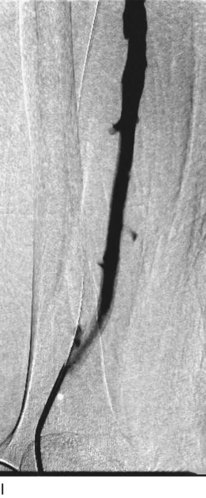
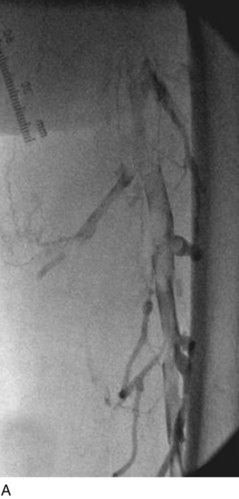
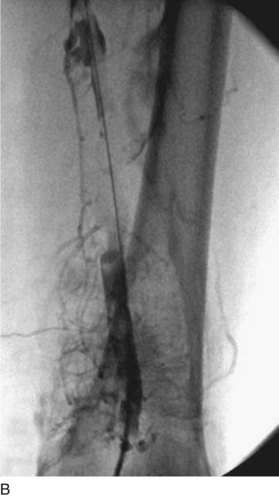
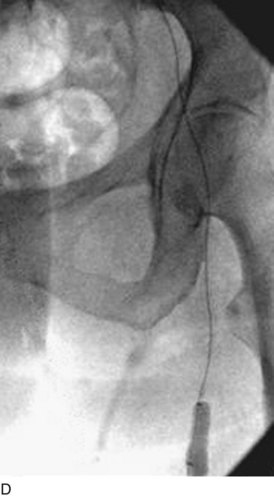
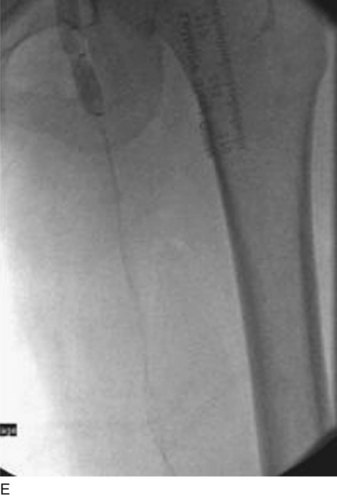
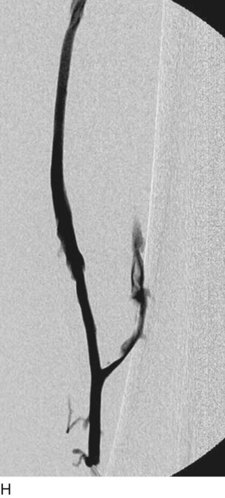
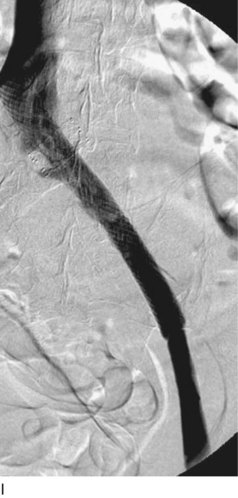
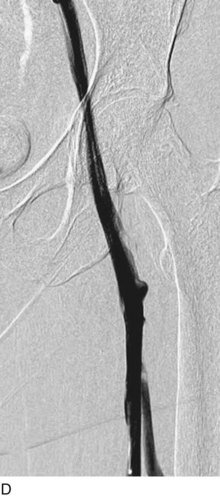
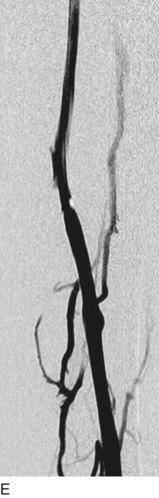
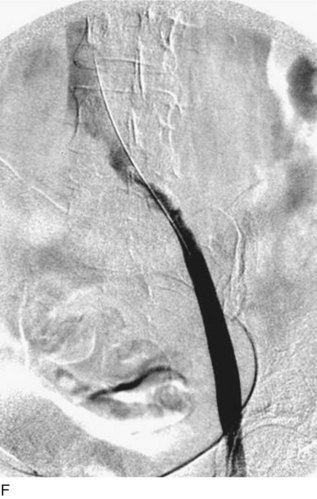
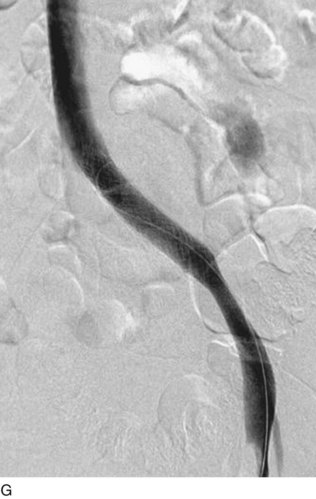
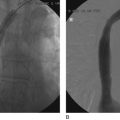
After access is obtained in the popliteal vein, a wire is advanced using a combination of an angled glidewire and glide catheter negotiating the iliofemoral segment to reach the IVC. Subsequently, the position of the catheter and patency of the IVC are confirmed with a venogram (Fig. 14-7, A–D).
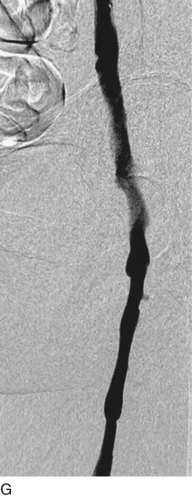
A 5-Fr, 40- or 50-cm-long infusion catheter and the ball-tipped occluding wire are then placed through the catheter. This occludes the end-hole of the catheter and directs delivery of the thrombolytics through the infusion holes. In the case when a valved infusion catheter is used, the occlusion wire is not necessary because there is a occluding one-way valve at the end of the catheter. The infusion length of catheter is chosen based on the length of thrombus present that needs to be treated. The infusion portion of the multi–side-hole catheter is parked in the lowest part of the thrombus and positioned to cover the entire length of thrombus. The catheter is then connected to the infusion pump with an initial dose of 1 mg/hr of rt-PA, and 400 to 600 U of heparin runs through the sheath. Our preference is to use larger volumes of diluted rt-PA to improve lytic therapy if it is not contraindicated. This is theoretical and has never been studied. The catheter and sheath are both wrapped sterilely in the usual manner. The venogram of the more proximal iliac system is not necessarily performed if it is significant filling defect throughout the iliofemoral vein is present in a preprocedure imaging. At the end of the procedure, the patient is transferred to an intensive care unit for close monitoring throughout the thrombolysis course. A sequential treatment of an extensive iliofemoral and caval DVT using CDT is illustrated in Figure 14-7, E–I.
Pharmacomechanical Thrombectomy
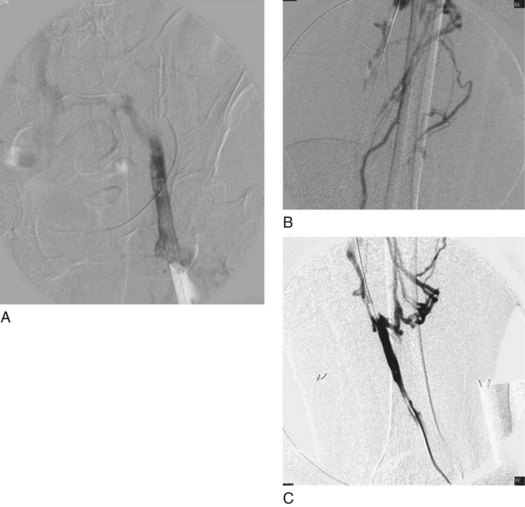
Trellis-8 Peripheral Infusion System. Access is obtained via popliteal vein as described earlier. An initial venogram is obtained as shown in Figure 14-8, A.
Angiojet Rheolytic System (MedRad; Warrendale, PA). After micropuncture access is obtained in the popliteal vein and changed to a 6-Fr sheath, a glidewire is advanced with an angled glide catheter crossing the thrombosed area into the IVC. A preintervention venogram is obtained. Fig. 14-9, A–C, depicts an extensive ileofemoropopliteal DVT.
Hemostasis and Anticoagulation
During thrombolysis, low-dose anticoagulation is achieved with UFH. Following completion of thrombolysis, therapeutic anticoagulation is accomplished with UFH or LMWH. The patient is subsequently bridged to warfarin sodium that is continued and tailored to the patient’s DVT presentation based on current guidelines.17
Puncture site hemostasis is effectively accomplished with manual compression or mechanical devices following completion of venography in the last lytic recheck.37 In our experience, manual compression is appropriate with no need to use external mechanical compression devices.
Complications
Comparative Effectiveness of Existing Treatments
Surgical thrombectomy was the first intervention attempted for limb salvage in cases of phlegmasia in patients with severe ischemia with impending venous gangrene. Contemporary results in a specialized center showed long-term patency of 80% at 5 years with no mortality.38 Meticulous and technical refined operations require considerable efforts with a long learning curve. In light of advances in catheter technology and very good initial results with PMT devices, the surgical treatment is limited to cases of rapidly evolving massive DVT in patients with contraindications to thrombolytic agents and with impending limb loss.
Pearls and Pitfalls
Choosing the Access
The right choice of access site for CDT or PMT is critical for an appropriate treatment. In the majority of the series, the popliteal vein is the most commonly used puncture site followed by the contralateral femoral vein.39
An additional access is often not necessary for CDT or PMT unless an intervention such as stenting is required in selected cases of bilateral iliac vein stenosis.40 Regardless of the preference of access, the needs of each patient should be considered, tailoring the best approach to a specific scenario of DVT.
Angioplasty and Stenting Post-CDT and PMT Therapy
Often, an iliac vein stenosis is found after CDT or PMT, compromising venous outflow. Angioplasty and stenting are always recommended when underlying venous lesions are present following a successful thrombolysis and thrombectomy.17 We advocate the use of self-expanding stents over balloon-expandable stents. The stent diameter for the common iliac should be 16 to 18 mm; for the external iliac, 14 to 16 mm; and for the common femoral vein, 12 to 14 mm. Covering all venous segments with residual disease with stents and achieving good inflow and good outflow are very important to maintain patency. To accomplish this, one may need to use longer stents (60 to 90 mm) and extend the stents into the IVC or below the inguinal ligament. When stents are extended into the common femoral vein, patency of the femoral or deep femoral veins is essential. The use of IVUS appears attractive to better define anatomy and size the stents to attain precise apposition to the venous wall. However, the role of IVUS in procedures to treat acute DVT is not well defined.
Inferior Vena Cava Filter
The use of devices for thrombectomy ultimately may cause distal embolization that eventually dissolves through the use of thrombolytics or the intrinsic fibrinolytic system. Up to now, no fatal PE has been reported following CDT or PMT. However, five patients presented with symptomatic PE in a multicenter registry after CDT.21 In our unreported experience, 30% of patients undergoing thrombolysis (PMT or CDT) experienced distal embolization (either PE or thrombus entrapped in the filter). Kolbel and colleagues analyzed data on 40 patients and found visible emboli within the filter in 45%.41
The role of IVC placement during CDT and PMT is not clear. Those who advocate the IVC filter placement based their decision on low morbidity and mortality of IVC placement, availability of temporary/retrievable filters, and potential complications such as PE.41,42 The drawbacks of the IVC filter include the additional costs of the device, risks related to the procedure, and the need for IVC filter retrieval in the future. Some specialists suggest selective use of filters in patients with free-floating thrombus in the IVC.43 Current guidelines recommend against routinely placed IVC filters aiming at prophylaxis17 but do not specify the use during CDT or PMT.31
1 Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S.
2 Mewissen MW, Seabrook GR, Meissner MH, et al. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211:39-49.
3 Comerota AJ, Throm RC, Mathias SD, et al. Catheter-directed thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. J Vasc Surg. 2000;32:130-137.
4 Lin PH, Zhou W, Dardik A, et al. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg. 2006;192:782-788.
5 Vedantham S, Vesely TM, Sicard GA, et al. Pharmacomechanical thrombolysis and early stent placement for iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2004;15:565-574.
1 Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370-372.
2 Meissner MH, Zierler BK, Bergelin RO, et al. Coagulation, fibrinolysis, and recanalization after acute deep venous thrombosis. J Vasc Surg. 2002;35:278-285.
3 Labropoulos N, Patel PJ, Tiongson JE, et al. Patterns of venous reflux and obstruction in patients with skin damage due to chronic venous disease. Vasc Endovasc Surg. 2007;41:33-40.
4 Prandoni P. Long-term clinical course of proximal deep venous thrombosis and detection of recurrent thrombosis. Semin Thromb Hemost. 2001;27:9-13.
5 Labropoulos N, Gasparis AP, Pefanis D, et al. Secondary chronic venous disease progresses faster than primary. J Vasc Surg. 2009;49:704-710.
6 Prandoni P, Villalta S, Bagatella P, et al. The clinical course of deep-vein thrombosis. Prospective long-term follow-up of 528 symptomatic patients. Haematologica. 1997;82:423-428.
7 Labropoulos N, Waggoner T, Sammis W, et al. The effect of venous thrombus location and extent on the development of post-thrombotic signs and symptoms. J Vasc Surg. 2008;48:407-412.
8 Agnelli G, Becattini C, Prandoni P. Recurrent venous thromboembolism in men and women. N Engl J Med. 2004;351:2015-2018. author reply 2015-2018
9 Labropoulos N, Jen J, Jen H, et al. Recurrent deep vein thrombosis: long-term incidence and natural history. Ann Surg. 2010;251:749-753.
10 Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199-205.
11 Labropoulos N, Spentzouris G, Gasparis AP, et al. Impact and clinical significance of recurrent venous thromboembolism. Br J Surg. 2010;97:989-999.
12 Prandoni P, Kahn SR. Post-thrombotic syndrome: prevalence, prognostication and need for progress. Br J Haematol. 2009;145:286-295.
13 Douketis JD, Crowther MA, Foster GA, et al. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med. 2001;110:515-519.
14 Comerota AJ, Aldridge SC. Thrombolytic therapy for deep venous thrombosis: a clinical review. Can J Surg. 1993;36:359-364.
15 Mahorner H. A new method of management for thrombosis of deep veins of the extremities: thrombectomy, restoration of the lumen and heparinization. Am Surg. 1954;20:487-498.
16 Fontaine R, Briot B, Vujadinovic B, et al. [Results, after five months, of bilateral thrombectomy in the legs for alterating venous thrombosis of a type called blue phlebitis (phlebitis with arteriospasm).]. Strasbourg Med. 1955;6:172-178.
17 Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(Suppl):454S-545S.
18 Juhan C, Alimi Y, Di Mauro P, et al. Surgical venous thrombectomy. Cardiovasc Surg (Lond Engl). 1999;7:586-590.
19 Blattler W, Heller G, Largiader J, et al. Combined regional thrombolysis and surgical thrombectomy for treatment of iliofemoral vein thrombosis. J Vasc Surg. 2004;40:620-625.
20 Klein SJ, Gasparis AP, Virvilis D, et al. Prospective determination of candidates for thrombolysis in patients with acute proximal deep vein thrombosis. J Vasc Surg. 2010;51:908-912.
21 Mewissen MW. Catheter-directed thrombolysis for lower extremity deep vein thrombosis. Techniques in vascular and interventional radiology. 2001;4:111-114.
22 Comerota AJ, Throm RC, Mathias SD, et al. Catheter-directed thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. J Vasc Surg. 2000;32:130-137.
23 Enden T, Klow NE, Sandvik L, et al. Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. J Thromb Haemost. 2009;7:1268-1275.
24 Semba CP, Dake MD. Iliofemoral deep venous thrombosis: aggressive therapy with catheter-directed thrombolysis. Radiology. 1994;191:487-494.
25 Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg. 2002;24:209-214.
26 Mewissen MW, Seabrook GR, Meissner MH, et al. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211:39-49.
27 Lin PH, Zhou W, Dardik A, et al. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg. 2006;192:782-788.
28 Kasirajan K, Gray B, Ouriel K. Percutaneous AngioJet thrombectomy in the management of extensive deep venous thrombosis. J Vasc Interv Radiol. 2001;12:179-185.
29 Bush RL, Lin PH, Bates JT, et al. Pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis: safety and feasibility study. J Vasc Surg. 2004;40:965-970.
30 Kim HS, Patra A, Paxton BE, et al. Adjunctive percutaneous mechanical thrombectomy for lower-extremity deep vein thrombosis: clinical and economic outcomes. J Vasc Interv Radiol. 2006;17:1099-1104.
31 Rao AS, Konig G, Leers SA, et al. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis: an alternative in patients with contraindications to thrombolysis. J Vasc Surg. 2009;50:1092-1098.
32 O’Sullivan GJ, Lohan DG, Gough N, et al. Pharmacomechanical thrombectomy of acute deep vein thrombosis with the Trellis-8 isolated thrombolysis catheter. J Vasc Interv Radiol. 2007;18:715-724.
33 Martinez Trabal JL, Comerota AJ, et al. The quantitative benefit of isolated, segmental, pharmacomechanical thrombolysis (ISPMT) for iliofemoral venous thrombosis. J Vasc Surg. 2008;48:1532-1537.
34 Braaten JV, Goss RA, Francis CW. Ultrasound reversibly disaggregates fibrin fibers. Thromb Haemost. 1997;78:1063-1068.
35 Parikh S, Motarjeme A, McNamara T, et al. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: initial clinical experience. J Vasc Interv Radiol. 2008;19:521-528.
36 Comerota AJ. The ATTRACT trial: rationale for early intervention for iliofemoral DVT. Perspect Vasc Surg Endovasc Ther. 2009;21:221-224. quiz 224-225
37 Coto HA. Closure of the femoral vein puncture site after transcatheter procedures using Angio-Seal. Catheter Cardiovasc Interv. 2002;55:16-19.
38 Hartung O, Benmiloud F, Barthelemy P, et al. Late results of surgical venous thrombectomy with iliocaval stenting. J Vasc Surg. 2008;47:381-387.
39 Comerota AJ, Aldridge SC, Cohen G, et al. A strategy of aggressive regional therapy for acute iliofemoral venous thrombosis with contemporary venous thrombectomy or catheter-directed thrombolysis. J Vasc Surg. 1994;20:244-254.
40 Vedantham S, Vesely TM, Sicard GA, et al. Pharmacomechanical thrombolysis and early stent placement for iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2004;15:565-574.
41 Kolbel T, Alhadad A, Acosta S, et al. Thrombus embolization into IVC filters during catheter-directed thrombolysis for proximal deep venous thrombosis. J Endovasc Ther. 2008;15:605-613.
42 Kwon SH, Oh JH, Seo TS, et al. Percutaneous aspiration thrombectomy for the treatment of acute lower extremity deep vein thrombosis: is thrombolysis needed? Clin Radiol. 2009;64:484-490.
43 Comerota AJ, Gravett MH. Iliofemoral venous thrombosis. J Vasc Surg. 2007;46:1065-1076.