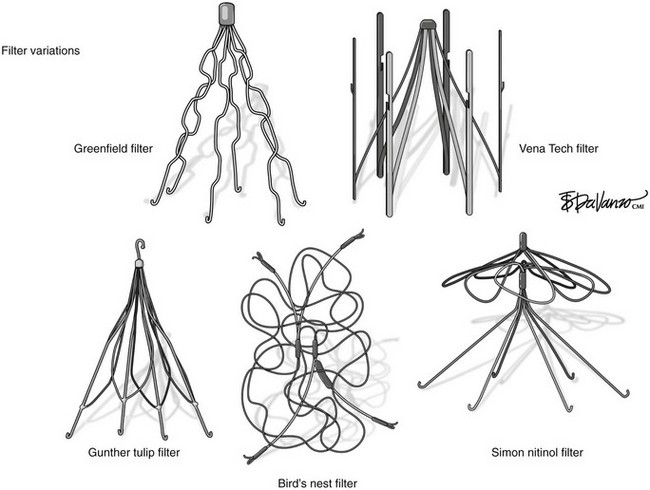
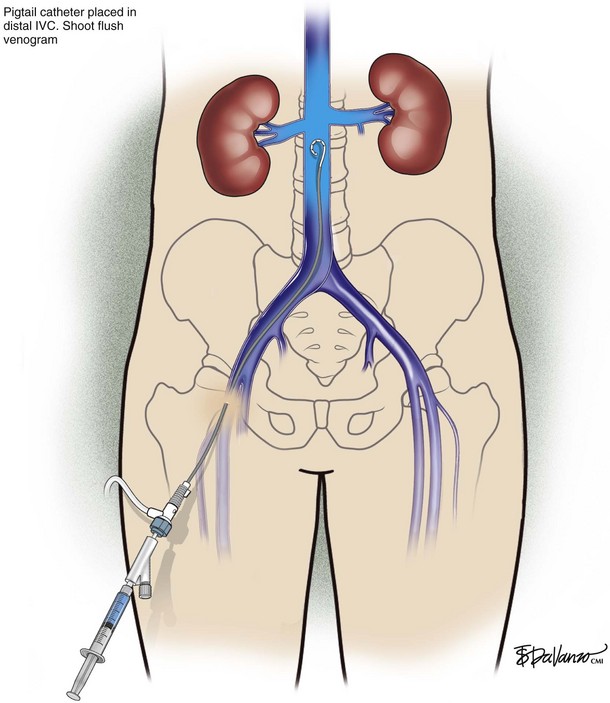
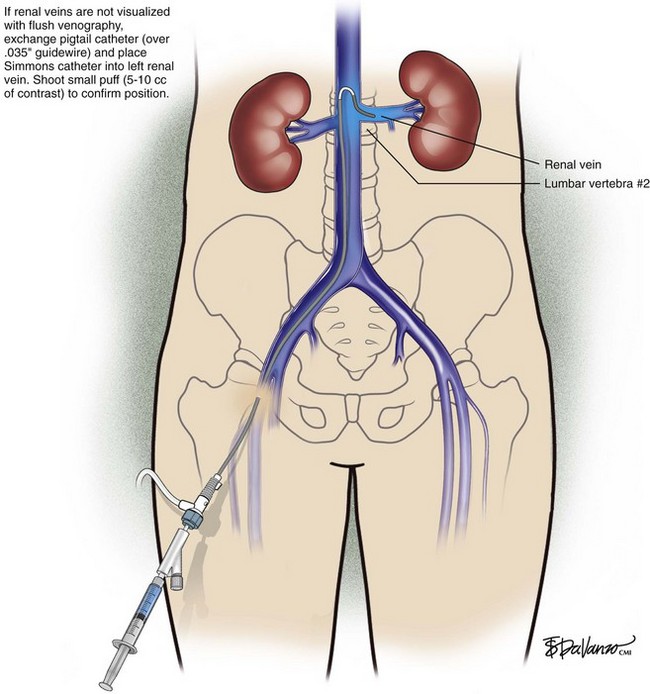
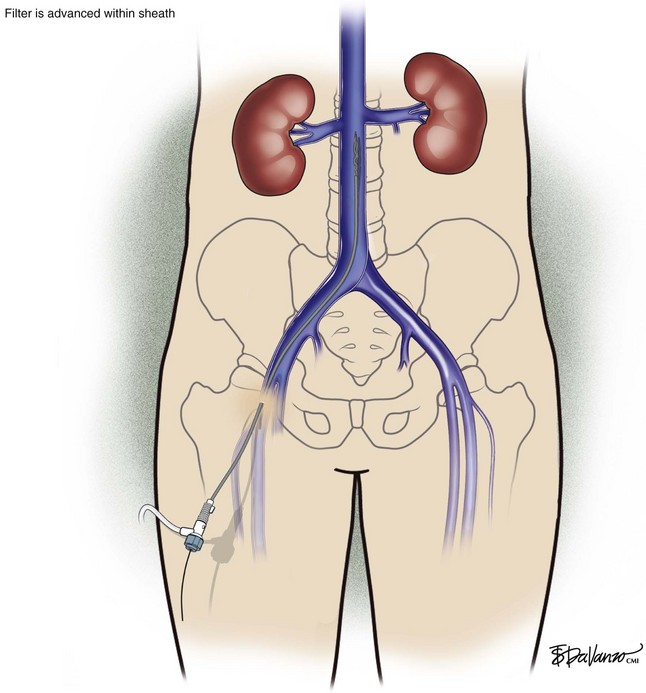
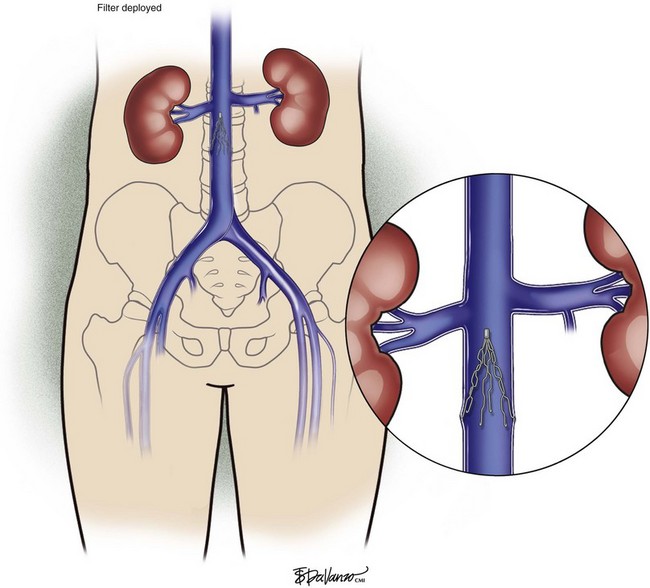
Chapter 13 Endovenous Placement of Inferior Vena Caval Filters
Patient Selection
The accepted and relative indications1 for placement of an IVC filter are shown in Table 13-1. Although anticoagulation is the mainstay of therapy in patients with acute deep venous thrombosis (DVT) or pulmonary embolism, it may be contraindicated for several reasons. Active internal bleeding is an absolute contraindication to therapeutic anticoagulation. However, an increased risk of bleeding due to recent trauma or major surgery (especially neurologic or ocular surgery) more often is a relative contraindication that is subject to clinical judgment. In the era when unfractionated heparin and vitamin K antagonists were the only available antithrombotic agents, nonhemorrhagic complications of anticoagulation (e.g., heparin-induced thrombocytopenia, warfarin-induced skin necrosis) were more common indications for IVC filter insertion. Currently, however, several alternative anticoagulants are available, and they should be considered prior to insertion of an IVC filter. Direct-thrombin inhibitors are very effective antithrombotic alternatives to heparin or low-molecular-weight heparin in patients who develop heparin-induced thrombocytopenia. Subcutaneously injected low-molecular-weight heparins and pentasaccharides, as well as oral direct-thrombin inhibitors, are potential alternatives in patients with warfarin-induced skin necrosis.
![]() TABLE 13–1 Indications for Placement of an Inferior Vena Cava Filter
TABLE 13–1 Indications for Placement of an Inferior Vena Cava Filter
| Common Indications for IVC Filter Placement |
DVT, Deep venous thrombosis; IVC, inferior vena cava; LE, lower extremity; VTE, venous thromboembolism.
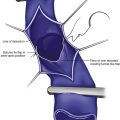
An often-cited indication for IVC filter insertion is “failure of anticoagulation.” Significant proximal DVT extension and pulmonary embolism may occur in up to 4% to 11% of patients who receive anticoagulation for acute lower extremity DVT. Over 70% of these failures occur in the first 3 weeks after initiation of therapy. However, there should be a distinction between patients who are receiving adequate versus those receiving inadequate antithrombotic therapy. Patients should be carefully questioned, and the anticoagulation records should be reviewed to determine whether dosages and frequency of antithrombotic medications were adequate (Table 13-2; Fig. 13-1; see also Table 13-1).
![]() TABLE 13–2 Guidewires and Catheters
TABLE 13–2 Guidewires and Catheters
| Name | Diameter | Length |
|---|---|---|
| Guidewires | ||
| Bentson/Rosen | .035 in | 150-180 cm |
| Angled glidewire | .035 in | 150-180 cm |
| Catheters | ||
| Pigtail (with 2-cm calibration) | 5-Fr | 65-90 cm |
| Kumpe (or other angled catheter) | 5-Fr | 65-90 cm |
| Ancillary Supplies | ||
| Heparinized saline | 1000 units/ 1000 mL of normal saline | |
| Syringes (2) | 20 mL, Luer Lock | |
| Dilators | 5-Fr and 6-Fr | |
| Injectable nonionic contrast, high-flow power injector | ||
Imaging
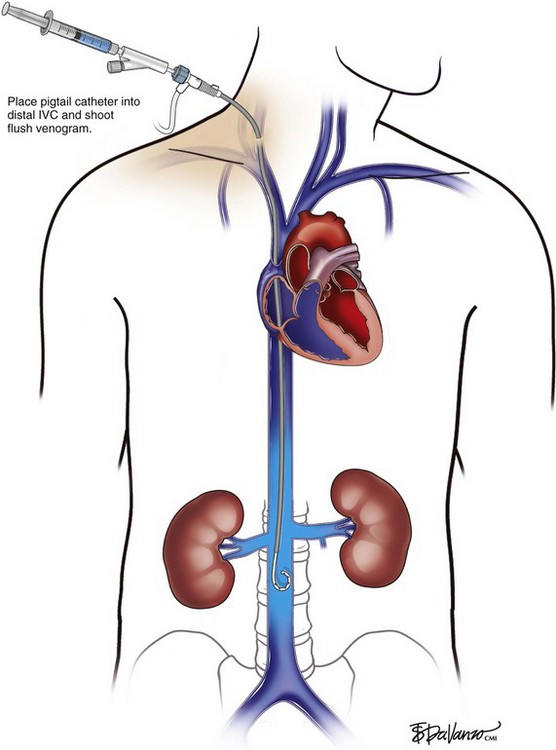
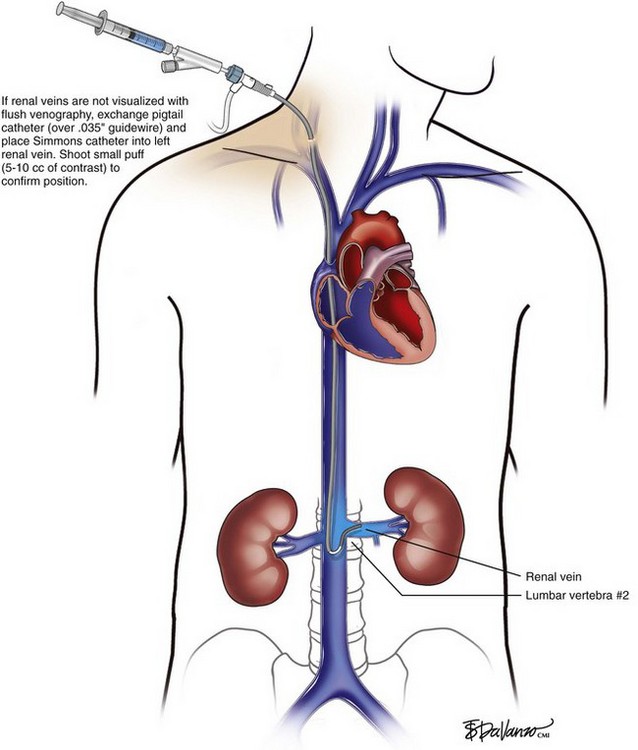
Venography
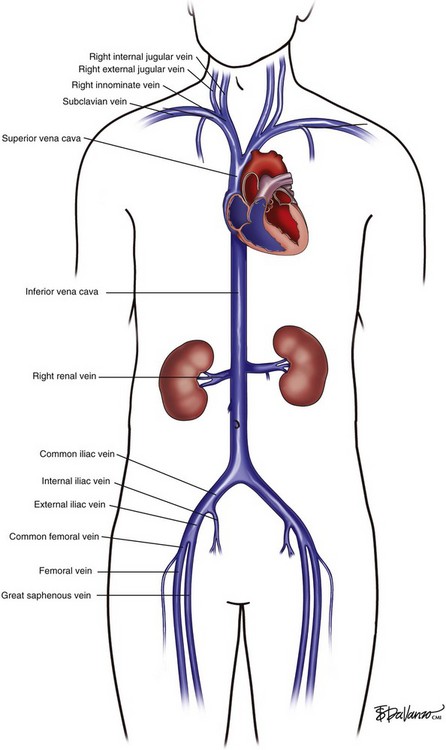
In patients with normal anatomy, the common iliac vein confluence occurs at the L4-L5 vertebral body, and the renal veins insert into the IVC at the L1 vertebral body. Kaufman et al.2 performed a detailed magnetic resonance imaging analysis of the anatomy of the IVC. The average length of the infrarenal IVC is 94 mm in females and 110 mm in males. However, these lengths vary significantly, especially in patients who have anomalous venous return. Retroaortic and circumaortic left renal veins are found in 7% and 5%, respectively, and multiple right renal veins are present in 8%. Prior literature indicates that the prevalence of duplicate IVCs ranges from 0.7% to 3% based on radiographic investigations and cadaver dissections. A left-sided vena cava is even less common (0.2% to 0.5%). In this latter setting, the infrarenal IVC ascends on the left lateral aspect of the aorta. After insertion in the left renal vein, it then crosses anterior to the aorta to assume its usual anatomic position.
Access and Operative Steps
Right Internal Jugular Approach
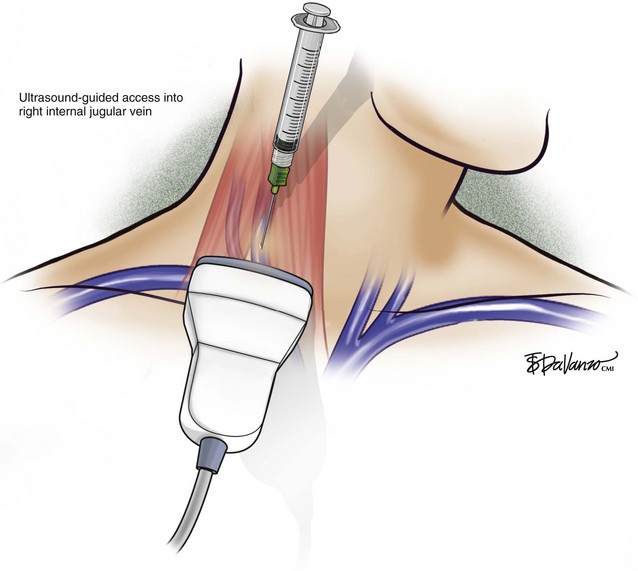
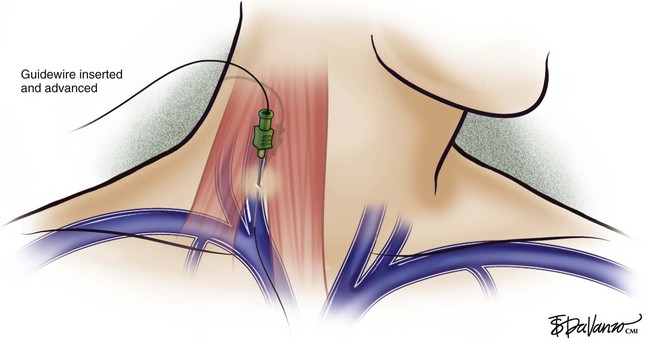
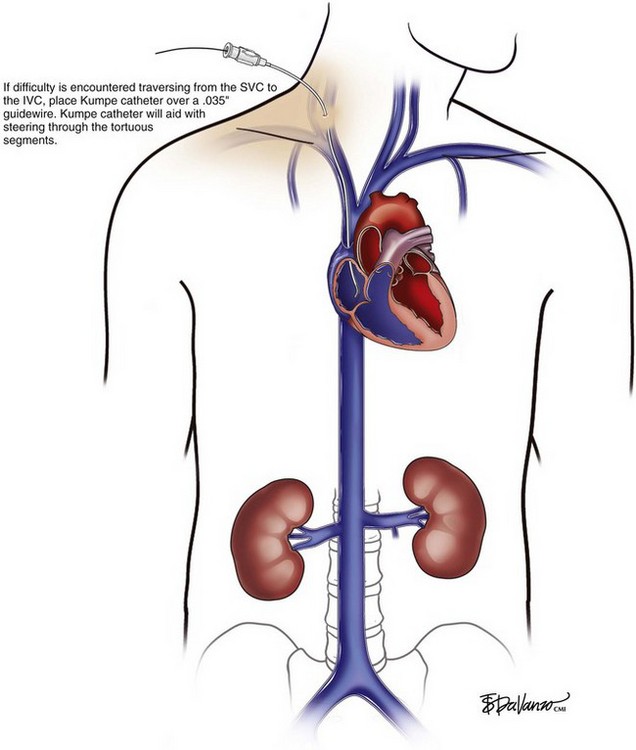
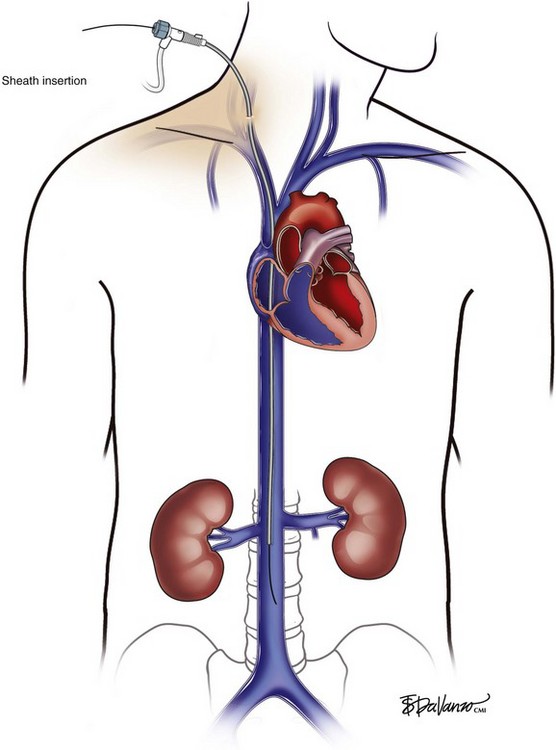
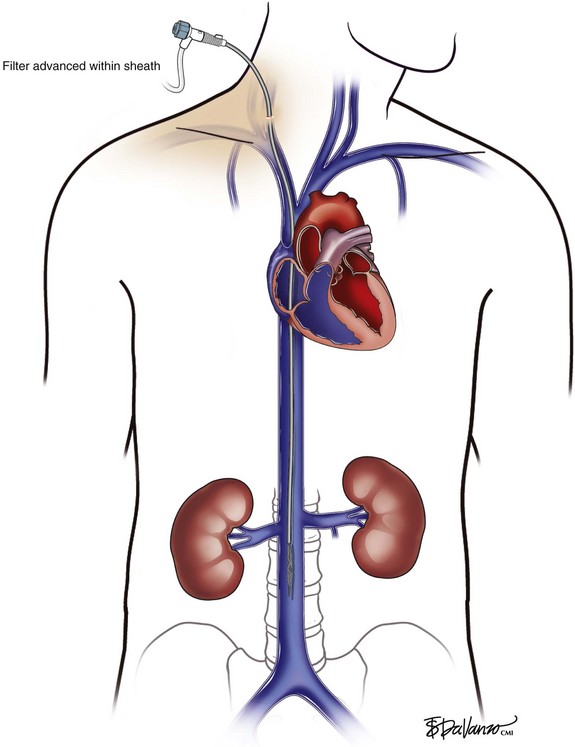
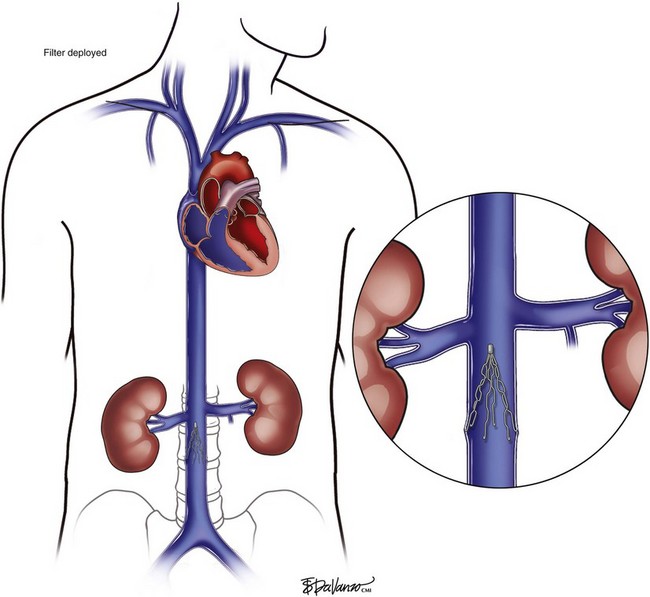
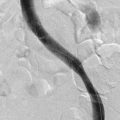
An 18-gauge single-wall puncture needle connected to a 5-mL or 10-mL syringe is used to access the vein with ultrasound guidance. The syringe should freely aspirate dark blood, and a .035-inch guidewire is passed into the IVC under fluoroscopic guidance. Occasionally, an angled catheter may be required to steer the wire into the IVC. Alternatively, a 21-gauge needle, .018-in guidewire, and 5-Fr catheter/dilator set (Micropuncture Introducer Set; Cook Medical, Bloomington, IN) may be used to avoid using the larger needle (Table 13-3; Figs. 13-2 through 13-10).
Common Femoral Vein Approach
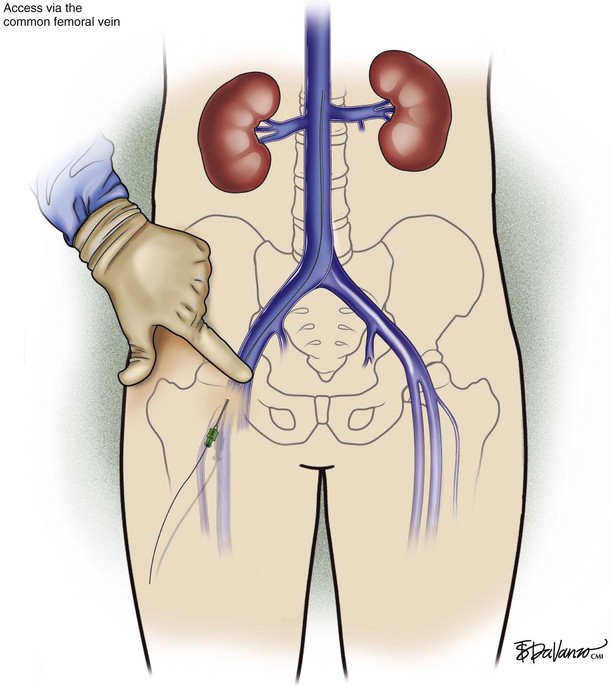
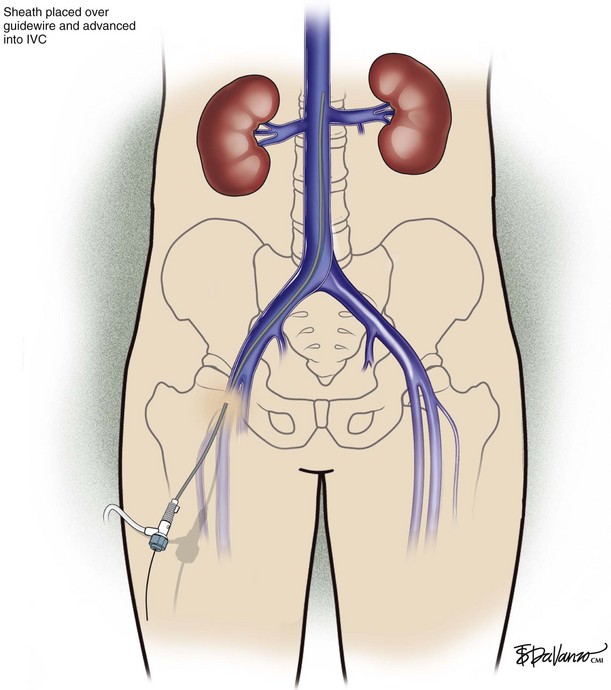
The common femoral vein usually is accessed using anatomic landmarks alone. The femoral pulse is palpated laterally. Ultrasound guidance may be easily applied in circumstances when the patient is obese or if the femoral pulse is absent. The common femoral vein cannulation techniques with an 18- or 21-gauge needle are similar to those that were described for internal jugular venous access (Figs. 13-11 through 13-16).
Anticoagulation Management
The management of anticoagulation around the time of IVC filter insertion will depend on the indication for the filter. If the filter is to be inserted because of a contraindication to anticoagulation (e.g., active internal bleeding, recent major surgery, trauma, anticoagulation-related bleeding), then the IVC filter can be placed without periprocedural anticoagulation. However, if the indication was venous thromboembolism recurrence despite adequate anticoagulation, then the anticoagulation should not be discontinued around the time of the procedure.3
Comparative Effectiveness
Success achieved with vena cava filters expanded the indications in some series to prophylaxis for free-floating thrombi longer than 5 cm,4 in cases where the risk of anticoagulation was thought to be prohibitive such as in older patients with DVT, after major trauma,5 and in high-risk situations such as orthopedic and bariatric operations.6 Statistical data to support these indications are lacking, and enthusiasm for permanent vena caval filtration has declined with case reports of caval occlusion and filter fractures.
The development of retrievable filters has rekindled interest in their use, particularly during mechanical or thrombolytic treatment of large iliofemoral thrombi.7 Unfortunately, data to date show equivalent complications, less secure fixation, and the added expense of multiple procedures. Improvements in techniques and devices should overcome some of these limitations but not the fundamental problem of knowing when embolic protection is no longer necessary.
1 Krishnamurthy VN, Greenfield LJ, Proctor MC, Rectenwald JE. Indications, techniques, and results of interior vena cava filters. In: Gloviczki P, editor. Handbook of Venous Disorders. 3 ed. London: Hodder Arnold; 2009:299-313.
2 Kaufman JA, Waltman AC, Rivitz SM, Geller SC. Anatomical observations on the renal veins and inferior vena cava at magnetic resonance angiography. Cardiovasc Intervent Radiol. 1995;18:153-157.
3 Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2008;133:454-545.
4 Berry R, George J, Shaver W. Free-floating deep venous thrombosis. A retrospective analysis. Ann Surg. 1990;211:719-723.
5 Langan EM3rd, Miller RS, Casey WJ3rd, et al. Prophylactic inferior vena cava filters in trauma patients at high risk: follow-up examination and risk/benefit assessment. J Vasc Surg. 1999;30:484-488.
6 Sugerman HJ, Sugerman EL, Wolfe L, et al. Risks and benefits of gastric bypass in morbidly obese patients with severe venous stasis disease. Ann Surg. 2001;234:41-46.
7 Thery C, Bauchart JJ, Lesenne M, et al. Predictive factors of effectiveness of streptokinase in deep venous thrombosis. Am J Cardiol. 1992;69:117-122.