Chapter 18 Endovenous Management of Central and Upper Extremity Veins
Primary Upper Extremity Deep Venous Thrombosis: Paget Schroetter Syndrome
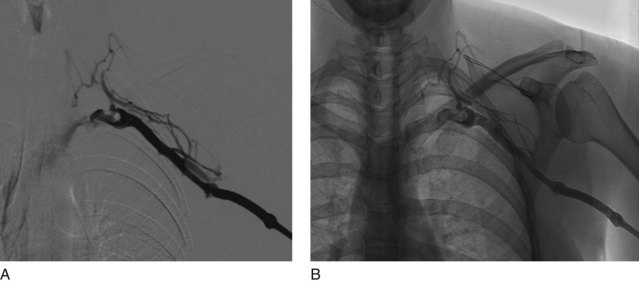
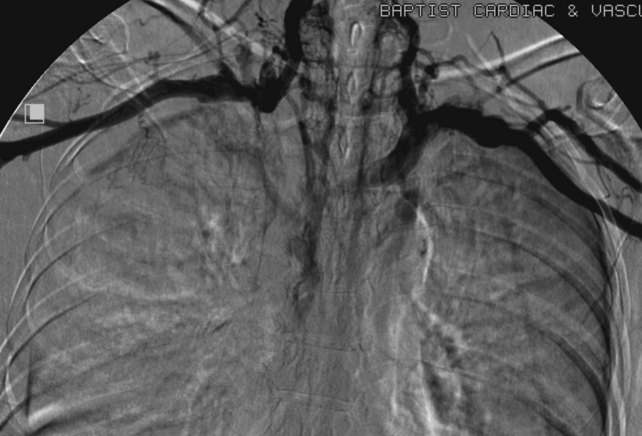
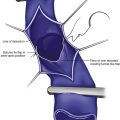
UEDVT can be classified as primary, when it occurs without an inciting cause, and secondary, when it is related to an underlying catheter or previous catheter. Primary UEDVT, also known as effort thrombosis, usually occurs in healthy young individuals (third decade of life) who present with sudden, severe swelling of the upper extremity that may be associated with cyanosis and arm paresthesias. The condition is usually seen in patients after a repetitive activity and is known by the eponym Paget-Schroetter syndrome after a British and a German physician who simultaneously reported it. These patients usually have a compressive phenomenon at the thoracic outlet. It is traditionally seen at the level of the clavicular head and first rib. The scalenus anterior muscle and tendon, along with the bony structures, may compress the subclavian vein at this level, particularly at some stressed positions of the arm. The process can also be seen with the cervical ribs (Figs. 18-1 and 18-2). The combination of the compression of the costoclavicular portion of the axillosubclavian vein and repetitive stress leads to intimal damage and subsequent fibrotic reactions of the underlying vein. This intimal damage then promotes thrombosis. Patients with effort thrombosis must be identified because if this condition is not treated, it can progress and patients may experience chronic disability (25%-74%).
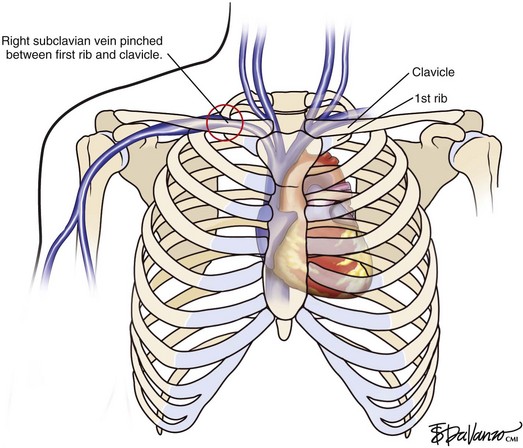
Fig 18–1 Diagram demonstrating the junction of both brachiocephalic veins into the superior vena cava.
Treatment
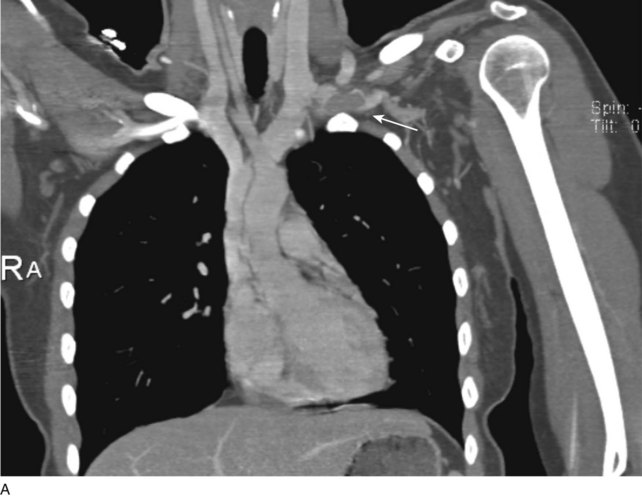
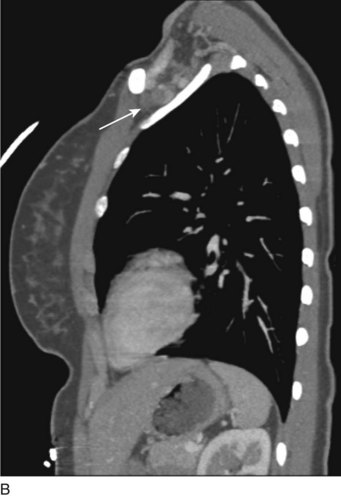
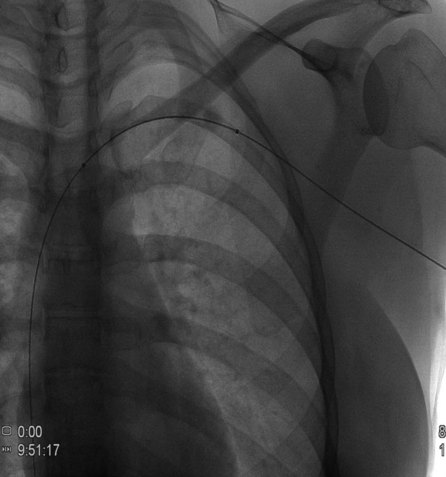
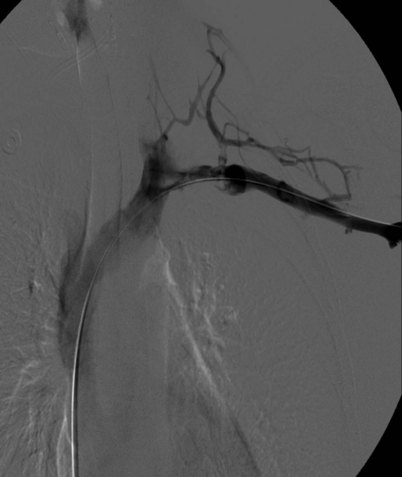
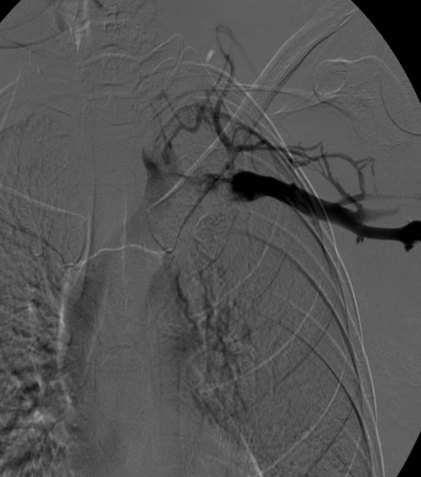
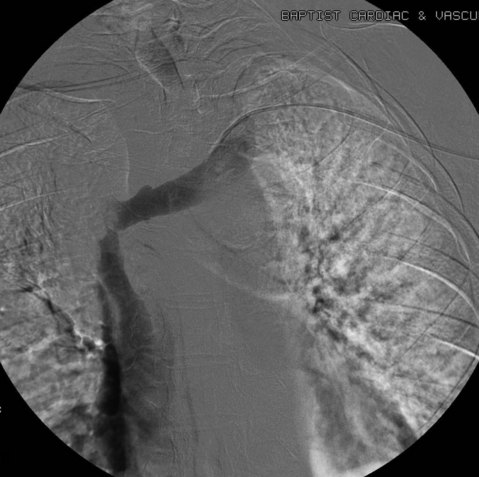
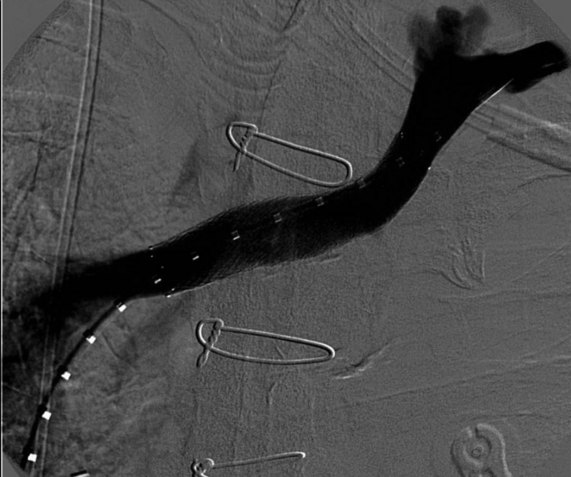
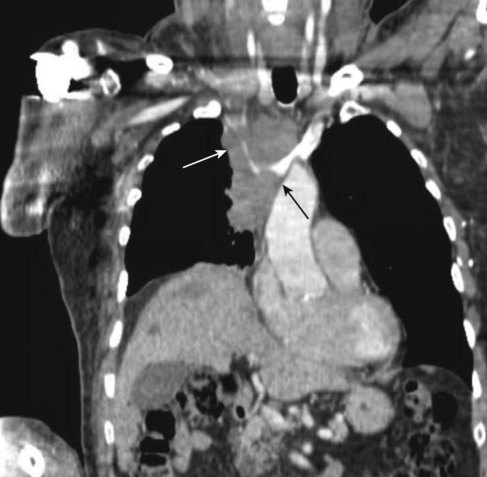
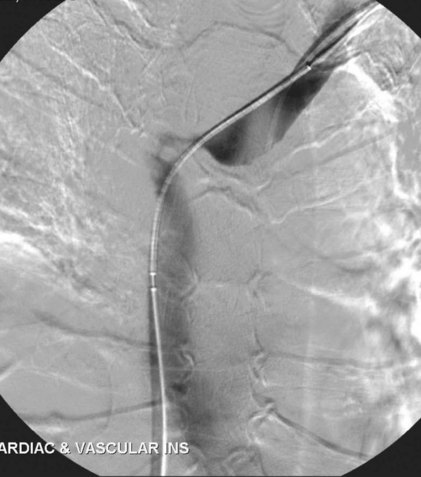
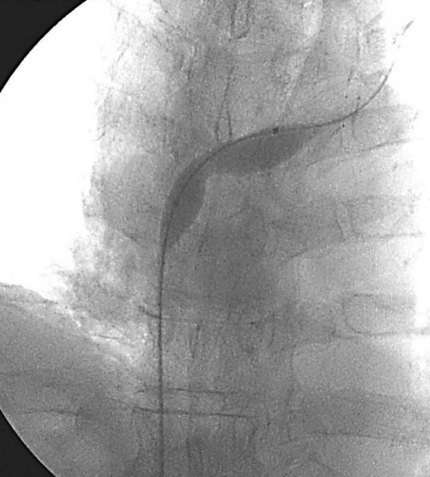
Prior to treatment, it is common for patients to have a venous duplex ultrasound demonstrating thrombosis. A computed tomography scan or magnetic resonance image highlighting the venous phase may be helpful in demonstrating the compression and excluding other sources of central occlusion (Fig. 18-3). Ultrasound-guided vascular access is achieved traditionally into the basilic or brachial vein. It is important to establish that the vein being punctured is patent. A 4-Fr or 5-Fr mini-stick microcatheter system is used to perform an initial venogram and document the extent of thrombosis (Fig. 18-4). Once the entry vein is found to be satisfactory, a 5-Fr or 6-Fr vascular sheath is placed. The axillosubclavian thrombosis is crossed with a soft tip guidewire (Bentson wire; Cook Medical, Bloomington, IN) and an angled catheter (Kumpe or multipurpose). The catheter is exchanged for a thrombolysis catheter that is placed across the area of thrombosis (Fig. 18-5). The catheter infusion length is matched to the length of thrombosis, and the patient is treated overnight with thrombolytics. The choice and dose are usually dependent on the operator’s choice, amount of thrombus, and patient risk factors. We usually coadminister peripheral low-dose heparin during the infusion, typically 300 units of heparin per hour (Fig. 18-6). In the setting of an acute decompensation requiring rapid thrombectomy or a patient who may not be a candidate for chemical thrombolysis, mechanical thrombectomy may be performed.
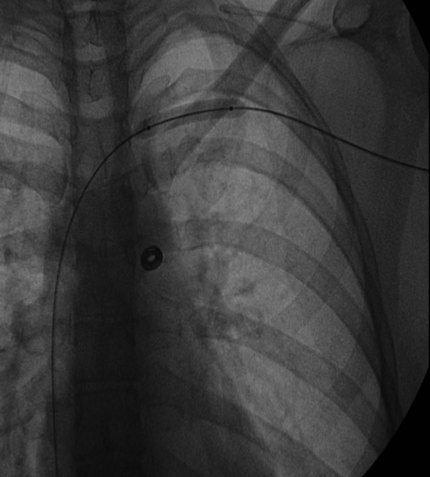
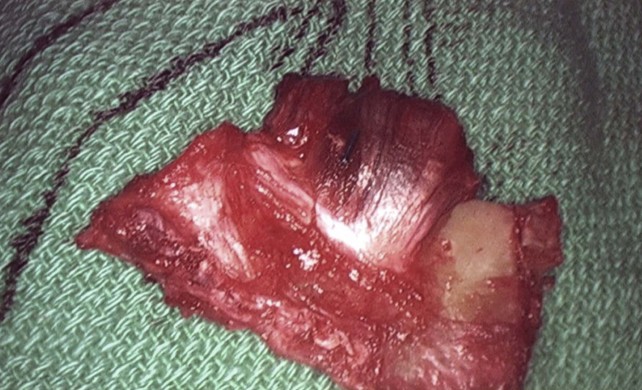
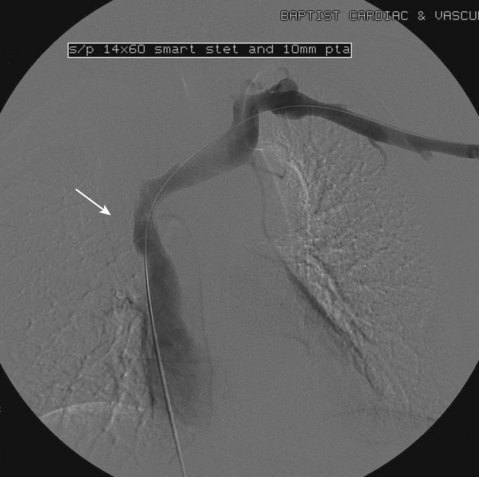
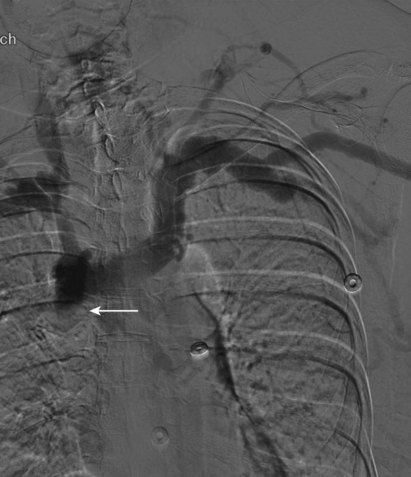
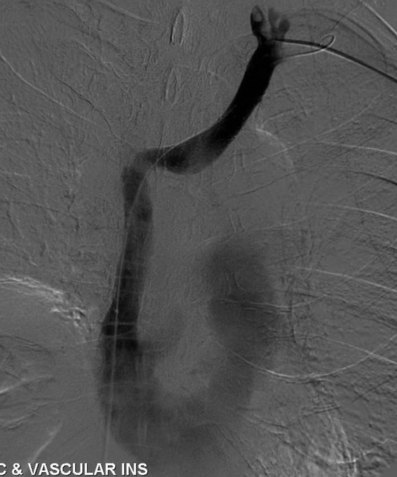
After overnight catheter-directed thrombolysis, a venogram is performed to assess the patency of the axillosubclavian vein (Fig. 18-7). Usually, overnight thrombolysis is sufficient to clear the associated thrombus, but in certain patients a second day of thrombolysis may be necessary. As with any thrombolysis procedure, the patient is informed of the potential risk of bleeding and monitored carefully as well as with fibrinogen levels. A venogram is then performed in a provocative position to assess the amount of compression and secure the diagnosis. We usually abduct and externally rotate the arm over the patient’s head. The area of compression on the vein is usually still present after thrombolysis (Figs. 18-8 and 18-9). Angioplasty can then be performed if there is a persistent high-grade stenosis (Fig. 18-10). A low-pressure balloon is used not solely to treat the venous obstruction but also to assess the amount of fibrosis and neointimal damage because a severely damaged vein may require a venous patch at the time of surgical decompression. The surgical decompression may be performed from a transaxillary or supraclavicular approach. A portion of the first rib along with the tendinous attachment of the transected anterior scalene muscle is usually removed (Fig. 18-11).
Dialysis Access–Related Central Venous Stenosis
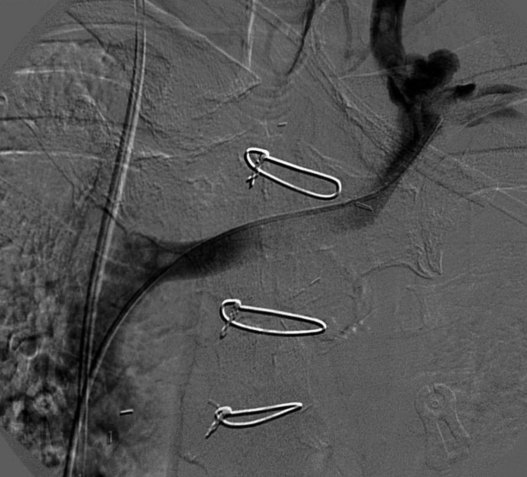
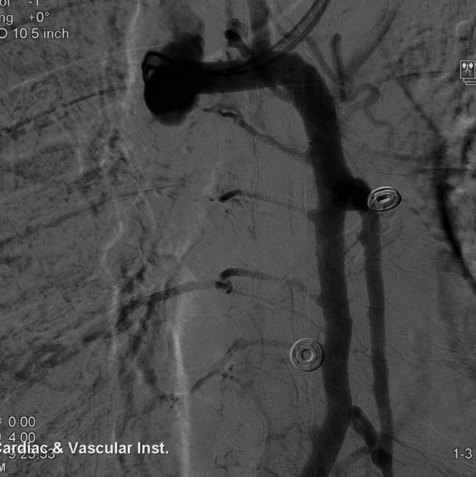
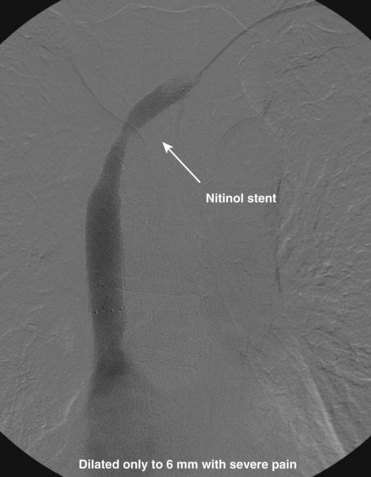
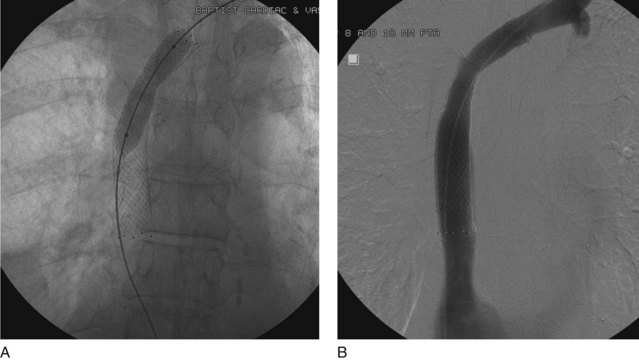
The central venous stenosis not only can be symptomatic with severe arm swelling but also may limit the function of a hemodialysis access. The treatment of central vein stenosis associated with hemodialysis is traditionally angioplasty. Unfortunately, the 1-year patency of these treatments has been reported to be between 10% and 30%, and the use of multiple additional procedures to maintain secondary patency is the rule. The initial use of stents to treat central venous stenosis has been discouraged because of a similar patency rate to that of angioplasty and the possibility of stent compression in the thoracic outlet. In addition, there are no U.S. Food and Drug Administration (FDA)–approved uncovered stents for the venous system. Even though the results from angioplasty are limited, endovascular dilatation of these stenoses is preferred over surgical options because of its availability, noninvasiveness, low risk of morbidity, and ability to repeat as needed. DOQI guidelines recommend stent placement for central lesions that recur within 3 months after angioplasty and demonstrate immediate vessel recoil greater than 50% after angioplasty and vessel perforation. The patency of a stent in central venous stenosis is similar to that of angioplasty, but the ability of retreatment becomes limited (Figs. 18-12 and 18-13). The use of covered self-expandable stents has shown promise, particularly in the setting of peripheral dialysis graft anastomotic stenoses. Their use will likely increase in the central veins; however, the operator needs to be careful in not excluding other draining veins with a central covered stent.
Superior Vena Cava Syndrome
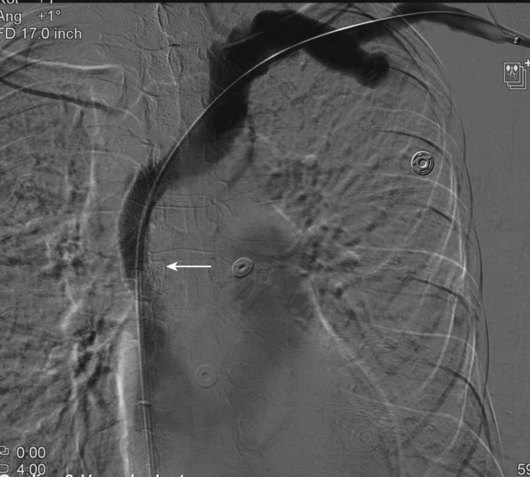
Even though there are no FDA-approved stents for the venous system, stents are used “off-label” in patients with SVC syndrome with improvement in 70% to 90% of patients’ symptoms. It is important to remember that the goal of treatment in patients with SVC syndrome is palliation. The long-term effects and patency of stents may not be relevant in patients with malignant obstruction or life-threatening symptoms. There is controversy surrounding stenting in the setting of SVC obstruction because a percentage of patients develop collateral pathways and may not become symptomatic. It is important to evaluate every patient individually, assessing symptoms and all available treatment options (Figs. 18-14 through 18-16). It is common for patients on hemodialysis to present with SVC syndrome due to central occlusions and stenosis (Figs. 18-17 through 18-19).
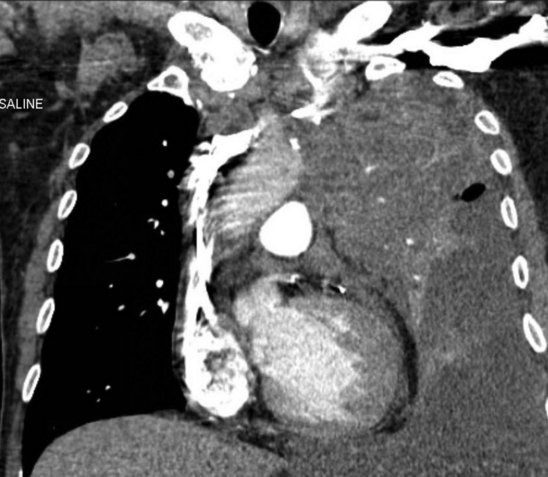
Patients with SVC syndrome are initially studied using venography. A complete ultrasound examination including pulsed-wave Doppler and gray-scale imaging may be helpful to establish the number and degree of stenoses or occlusions prior to the venogram. If possible, a cross-sectional study such as a computed tomography angiogram or magnetic resonance angiogram with a venous phase may be obtained to confirm the level of stenosis and occlusion as well as highlight the amount of underlying mass effect or malignancy (Fig. 18-20).
Although it may not be necessary to treat both innominate veins to achieve a clinical outcome, bilateral central venous stenting may be needed to reestablish optimal flow. The goal is to restore flow into the right atrium (Figs. 18-21 through 18-23). The patency of the jugular veins is important because its intact drainage centrally is usually sufficient to relieve face and head symptoms. At the time of venography, brachial vein access is complemented by femoral and possibly jugular access. In the setting of acute thrombosis, chemical or mechanical thrombolysis may be necessary to uncover the underlying stenosis and minimize embolization. At our institution, we usually attempt to place the central stents first and then extend additional stents peripherally to help anchor the stents in place as needed. Stent migration is a complication and may be limited by the use of proper oversizing of the stents. The SVC is a thin vessel, and aggressive dilatation of stents should be avoided because caval perforation may occur, especially in patients after radiation therapy (Figs. 18-24 through 18-26).
Bashit B, Parisi A, Frager DH, Suster B. Abdominal CT findings when the superior vena cava, brachiocephalic vein or subclavian vein is obstructed. AJR Am J Roentgenol. 1996;167:1457-1463.
Fassiadis N, Roidl M, South M. Are we managing primary upper limb deep venous thrombosis aggressively enough in the district? Int Angiol. 2005;24:255-257.
Kapur S, Paik E, Rezaei A, Vu D. Where there is blood there is a way: Unusual collateral vessels in superior and inferior vena cava obstruction. Radiographics. 2010;30:67-78.
Kucher N. Deep-vein thrombosis of the upper extremities. N Engl J Med. 2011;364:861-869.
Lee WA, Hill BB, Harris EJ, et al. Surgical intervention is not required for all patients with subclavian vein thrombosis. J Vasc Surg. 2000;32:57-67.
Lindblad B, Bornmyr S, Kullendorff B, Bergqvist D. Venous haemodynamics of the upper extremity after subclavian vein thrombosis. Vasa. 1990;19:218-222.
Madan AK, Allmon JC, Harding M, et al. Dialysis access-induced superior vena cava syndrome. Am Surg. 2002;68:904-906.
Owens CA, Bui JT, Knuttinen MG, et al. Pulmonary embolism from upper extremity deep vein thrombosis and the role of superior vena cava filters: a review of the literature. J Vasc Interv Radiol. 2010;21:779-787.
Thomas IH, Zierler BK. An integrative review of outcomes in patients with acute primary upper extremity deep venous thrombosis following no treatment or treatment with anticoagulation, thrombolysis or surgical algorithms. Vasc Endovasc Surg. 2005;39:163-174.
Tilney ML, Griffiths HJ, Edwards EA. Natural history of major venous thrombosis of the upper extremity. Arch Surg. 1970;101:792-796.
Vendantham S, Benenati JF, Kundu S, et al. Interventional endovascular management of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis: a position statement by the Society of Interventional Radiology, endorsed by the Canadian Interventional Radiology Association. J Vasc Interv Radiol. 2010;21:1335-1337.
Zamboni P, Galeotti R, Menegatti E, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:392-399.
Zamboni P, Galeotti R, Menegatti E, et al. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J Vasc Surg. 2009;50:1348-1358.