Chapter 85 Endovascular Treatment of Stroke
Stroke is the third leading cause of death in the United States, accounting for more than 143,000 deaths each year. The Centers for Disease Control and Prevention estimates approximately 795,000 strokes occur annually in the United States. Of these, 610,000 are sentinel events, with the remainder occurring in those who have suffered a prior stroke.1 Stroke is a leading cause of long-term disability due to loss of independence, language, motor skills, and cognitive abilities.1 The American Heart Association has estimated the financial burden of stroke to be nearly $70 billion in 2009 due to both direct and indirect costs.2 Although acute stroke is dichotomized into both ischemic and hemorrhagic etiologies, the vast majority of strokes are secondary to ischemia. Roughly 80% to 85% of acute strokes are secondary to thromboembolic vessel occlusion, typically arising from cardiac, carotid, or intracranial artery pathology.3
Timely arterial recanalization and prevention of reocclusion after treatment have become integral foci of the modern management of acute stroke. Current therapeutic modalities include intravenous chemical thrombolysis, intra-arterial mechanical and chemical thrombolysis, and stenting. Rapid diagnosis and treatment of acute stroke have been shown to improve patient outcomes in multiple studies.4–10 However, multiple barriers exist that may prevent patients from receiving timely revascularization, including delay in diagnosis, resource constraints, and lack of physicians trained in stroke management. Regionalization of stroke care has been popularized in recent years in the United States to combat such barriers. Despite these efforts, it is estimated that less than 1% of patients suffering an acute stroke receive intravenous thrombolytic therapy.11 The recent results of the third European Cooperative Acute Stroke Study (ECASS), which showed benefit in patients who received intravenous recombinant tissue plasminogen activator (rtPA) within 4.5 hours of symptom onset,12 may increase the number of patients eligible to receive intravenous thrombolytic therapy.
Occlusion and Recanalization
Arterial recanalization is widely accepted as a surrogate marker of efficacy for stroke therapies. The Thrombolysis in Myocardial Infarction (TIMI) scale is accepted among neurointerventionalists as a semiquantitative measure of vessel recanalization (Table 85-1).13 Transcranial Doppler ultrasonography has also shown promise in evaluating vessel recanalization.14–16 Successful recanalization has been linked to improved clinical outcomes in many series.17 Pretreatment residual flow has been shown to be predictive of both time to achieve recanalization and likelihood of a successful intervention.15 In a meta-analysis of recanalization and outcome, Rha and Saver demonstrated a strong correlation between arterial recanalization and improved functional outcomes with reduced mortality.17 Successful arterial recanalization at the time of intervention does not always correlate with lasting benefit, because some vessels may reocclude. Reocclusion rates as high as 34% have been reported in some studies following intra-arterial administration of rtPA.18–20 In addition, distal propagation of thrombus has been reported in approximately 16% of patients undergoing pharmacologic or mechanical thrombolysis.20 Potential reperfusion injury may occur when the blood–brain barrier becomes destabilized. Secondary cerebral edema and/or intracranial hemorrhage may result, possibly necessitating operative intervention.
| TIMI Grade | Classification | Criteria |
|---|---|---|
| 0 | No perfusion | No recanalization of the primary occlusive lesion |
| 1 | Penetration without perfusion | Incomplete or partial recanalization with flow past the initial occlusion, but no distal branch filling |
| 2 | Partial perfusion | Partial recanalization with incomplete or slow distal branch filling |
| 3 | Complete perfusion | Full recanalization with filling of all distal branches |
Presentation
Patients suffering an acute ischemic stroke most commonly present with sudden-onset neurologic deficit(s) that localize to the brain territory supplied by the affected vessel(s). It is imperative to perform a thorough but succinct history and neurologic examination to assess the extent of stroke. Physicians must gather information regarding the progression of neurologic deficit over time and any improvements that have occurred since onset, as spontaneous improvement may indicate a transient ischemic attack or point to an alternative diagnosis, such as seizure. The National Institute of Health Stroke Scale (NIHSS) quantitatively rates the severity of the stroke and has demonstrated excellent reliability and clinical utility.21–23 The scale ranges from 0 (no symptoms) to 42 and grades patient deficits in multiple functions, including motor, sensory, language, and alertness. Generally, scores of 1 to 5 represent mild stroke, 5 to 20 moderate to severe stroke, and greater than 20 very severe stroke. The NIHSS and its booklet are accessible online at the National Institute of Neurological Disorders and Stroke (NINDS) Web site (http://www.ninds.nih.gov/).
Imaging
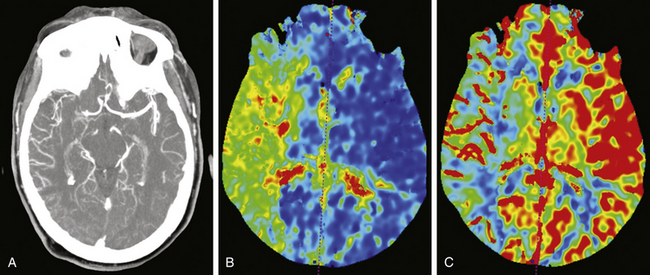
All patients presenting with stroke symptoms should undergo brain imaging. Computed tomography (CT) is the most readily available and efficient imaging modality, although magnetic resonance imaging (MRI) may also be performed. Early noncontrasted CT scanning is critical for evaluation of intracranial hemorrhage and serves as the primary differentiator for standard of care therapies. In addition, noncontrasted CT scanning of the head may reveal hypodensities consistent with evolving territories of infarction, cerebral edema or herniation, loss of gray–white matter differentiation, or arterial occlusion referred to as the “hyperdense sign.” At the University of Florida, computed tomography angiography (CTA) of the head and neck and cerebral CT perfusion are performed on all patients who present with acute stroke symptoms. It is imperative the aortic arch and major arteries of the head and neck be visualized to evaluate for arterial occlusion, flow-limiting stenosis, dissection, or other vessel anomalies. CT perfusion is used to assess the extent of stroke and the amount of potentially salvageable brain parenchyma. Tissue with preserved cerebral blood volume, despite reduced cerebral blood flow and increased mean transit time, represents the stroke penumbra (Fig. 85-1).24,25 CT perfusion may be limited at visualizing the posterior fossa due to significant bone artifact and beam scatter; however, it is hoped that newer imaging technologies and techniques will ameliorate this difficulty. Alternatively, some institutions rely on diffusion-weighted MRI to evaluate the brain’s physiologic state. Whichever modality is used, timely evaluation of actual brain physiology is rapidly becoming a new standard in the cutting-edge treatment of stroke.
Patient Selection
Indications and contraindications for intravenous thrombolysis are listed in Table 85-2. Intracranial hemorrhage is a direct contraindication for revascularization therapy. The therapeutic window for intravenous rtPA has been extended to 4.5 hours from the onset of symptoms based on results of the recent ECASS III trial.12,26 Patients eligible for intravenous rtPA should undergo this treatment prior to attempts at endovascular therapy. If patients fail to improve neurologically with intravenous rtPA, present outside of the therapeutic window, or have a contraindication to intravenous rtPA, endovascular therapy may be considered, including the use of intra-arterial rtPA, mechanical disruption using the microwire, the use of mechanical thrombectomy/aspiration devices, angioplasty, stenting, or a combination of procedures in those with persistent occlusive thrombi.
TABLE 85-2 Indications and Contraindications to rtPA Administration
| Indications | Contraindications |
|---|---|
• Minor or rapidly improving symptoms
• Stroke or serious head trauma within the past 3 months
• Major surgery within the last 2 weeks
• Known history of intracranial hemorrhage
• Sustained systolic blood pressure >185 mm Hg
• Sustained diastolic blood pressure >110 mm Hg
• Aggressive treatment needed to lower the patient’s blood pressure
• Symptoms suggestive of subarachnoid hemorrhage
• Gastrointestinal or urinary tract hemorrhage within the last 3 weeks
• Arterial puncture at noncompressible site within the last 7 days
• Heparin received within the last 48 hours and elevated partial thromboplastin time
The relative amount of ischemic parenchyma should be considered strongly when deciding to pursue endovascular treatment. Specifically, unsalvageable tissue (defined by regions with decreased cerebral blood volume in the setting of increased mean transit time and decreased cerebral blood flow on perfusion imaging) comprising greater than one third of the overall brain territory at risk of ischemia is a relative contraindication to endovascular therapy. In our experience, these patients are at high risk of hemorrhagic transformation following intra-arterial pharmacologic and/or mechanical thrombolysis or stenting, particularly if the nonsalvageable region involves the basal ganglia. Other factors linked to poor clinical outcomes in patients undergoing endovascular therapy include advanced age (greater than 75 years), presentation NIHSS score greater than 18, and admission glucose greater than 150 mg/dl.9 While the conventional ideal therapeutic window for endovascular therapy is within 6 to 8 hours of symptom onset,10,26,27 patients are increasingly being successfully treated outside of this time frame guided by physiologic imaging (CT perfusion or MRI perfusion).28,29
Intracerebral Hemorrhage
The major complication of endovascular therapies for stroke is intracranial hemorrhage, which is estimated to occur in roughly 5% to 10% of patients undergoing intravenous tissue plasminogen activator therapy.7,30,31 Clinically significant intracerebral hematomas, so-called symptomatic intracerebral hemorrhage (SICH), develops in around 3% of patients. The frequency of SICH increases with the concomitant presence of early signs of infarction on CT scan prior to administration.31,32 Furthermore, symptom severity, diabetes mellitus, and elevated blood glucose are well-demonstrated independent risk factors for SICH.32,33 Other possible risk factors include high systolic blood pressure, low platelets, advanced age, and delay in treatment. Intra-arterial tissue plasminogen activator, in contrast to venous, has a slightly higher risk of SICH. Estimates of SICH in those receiving intra-arterial thrombolysis are approximately 6% to 12%, while those receiving both intravenous and intra-arterial administration are estimated to be roughly 9% to 20%.10,31
The pathophysiology of post-treatment SICH appears to be primarily the result of reperfusion injury with hemorrhagic transformation at ischemic sites.34 Ischemia results in disruption of the blood–brain barrier, causing leakage from vessels, tissue edema, and loss of autoregulation secondary to increased free radical and matrix metalloproteinase activity.35,36 Reperfusion of damaged vessels results in small ruptures with microhemorrhages that may ultimately enlarge into a hematoma due to disruption of the normal clotting cascade by the presence of thrombolytic agents.
Intravenous Thrombolytic Therapy
Intravenous thrombolytic therapy with rtPA was the first FDA-approved intervention for acute stroke. The NINDS trial, published in 1995, was a large, randomized, placebo-controlled study consisting of more than 600 patients randomized to rtPA versus placebo within 3 hours of stroke onset.4 At 24 hours, no difference was seen in NIHSS improvement between those receiving rtPA and those receiving placebo. At 3 months, patients receiving rtPA had 1.7 times greater odds of a favorable outcome than did those receiving placebo. SICH occurred in 34 of the 312 patients receiving rtPA (20 were symptomatic, of which 9 were fatalities), compared to 11 of the 312 patients receiving placebo (2 symptomatic, 1 fatality). Two additional trials, ECASS I and II, demonstrated comparable results with improved functional outcomes but increased risk of hemorrhage.7,8 These findings led to approval by the FDA of rtPA, at a dose of 0.9 mg/kg, in acute stroke patients presenting within 3 hours of symptom onset.
Recently, trials have focused on the benefits of rtPA beyond the 3-hour window. The Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke (ATLANTIS) trial, published in 1999, evaluated the benefits of rtPA administration between 3 and 5 hours after symptom onset.5 This trial demonstrated no benefit to the administration of rtPA beyond 3 hours. In 2004, published pooled data from the ATLANTIS, ECASS, and NINDS trials suggested a potential benefit in administration beyond the 3-hour window.37 In 2008, Hacke et al. demonstrated significant benefit in clinical outcome at 90 days in patients receiving rtPA within 180 to 270 minutes after symptom onset.12 In 2009, the FDA approved the use of rtPA in acute ischemic stroke in patients 3 to 4.5 hours after symptom onset.
Despite the recent temporal expansion, intravenous rtPA faces several important limitations. Research has demonstrated ineffectiveness in lysing thromboembolic obstruction of large proximal vessels as compared to more distal, smaller vessels.16 One study of 349 patients undergoing intravenous rtPA for stroke revealed nearly one third with no recanalization, one third with partial recanalization, and only one third with complete recanalization.38 In addition, early reocclusion has been demonstrated in up to 34% of patients receiving intravenous rtPA.14,19 To combat these limitations, new endovascular therapies have been developed to provide better targeting of thrombolytic agents with improved recanalization and prevention of reocclusion. These therapies are described in the remaining sections of this chapter.
General Endovascular Procedure
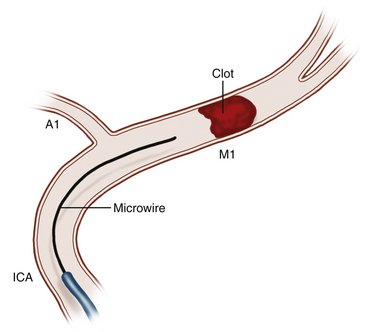
The access area, typically the common femoral artery (the radial artery remains a secondary option), is prepped and draped sterilely. A 5- to 8-French (Fr) sheath is then placed depending on the anticipated device to be utilized. After confirmed, uncomplicated arterial access, a heparin bolus may be administered to maintain an activated coagulation time between 250 and 300 seconds. Some interventionalists aim for a lower activated coagulation time to compensate for planned dosing of intra-arterial thrombolytics and antiplatelet agents and thus potentially reduce the theoretical risk of hemorrhagic transformation. If prior CTA or magnetic resonance angiography (MRA) has been performed (recommended), then selective catheterization of the target vessel can be performed. If CTA or MRA has not been completed, a diagnostic cerebral angiogram guided by patient symptomatology should be performed to localize the occlusion. The interventionalist then assesses the location of arterial occlusion and evaluates for additional arterial pathology (i.e., vessel dissection) prior to intracranial intervention. A microcatheter is then introduced over a microwire and is navigated to the site of arterial occlusion (Fig. 85-2). The microwire is then gently navigated across the occlusion with a twisting motion. Once this is done, the microcatheter is advanced over the microwire to a position beyond the occlusion. A microcatheter angiographic run should then be performed to define the distal aspect of the occlusion. At this juncture, the interventionalist must make a decision regarding the type of intra-arterial intervention that will be undertaken. There are no data published to definitively support the choice of one intervention modality over another. This decision should be guided by the comfort level and experience of the interventionalist with each technique and device, as well as the patient-specific anatomy. Following each endovascular intervention, angiography is performed to observe for vessel recanalization. All catheters and wires are removed upon conclusion of the intervention, and the arteriotomy puncture is sealed using a percutaneous closure device. Alternatively, a flexible sheath may be left in place until serum thrombolytic levels normalize. The patient should be monitored in an intensive care unit with frequent neurologic examinations and strict blood pressure and glucose control.
Intra-Arterial Thrombolytic Therapy
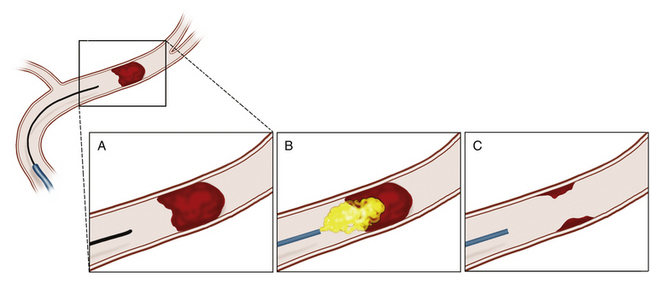
Intra-arterial thrombolysis utilizes precise localization for administration of thrombolytic agents while minimizing systemic exposure. The agent is administered through an endovascular microcatheter placed at the site of the occlusion. When the region of occlusion is approached, thrombolytics are administered in immediate proximity to the clot, maximizing the effect of the agent at the target while reducing the systemic consequences of circulating thrombolytic agent (Fig. 85-3). Intuitively, early series suggested improved recanalization rates.39,40 These findings prompted the first randomized, controlled trial in intra-arterial thrombolysis, the Prolyse in Acute Cerebral Thromboembolism (PROACT) II trial.6 Patients with middle cerebral artery (MCA) occlusion were randomized to 6 mg of intra-arterial recombinant prourokinase (rpro-UK) versus intra-arterial placebo. All patients received intravenous heparin. This trial demonstrated a significant improvement, following administration up to 6 hours after symptom onset, in both recanalization (66% vs. 18%) and functional independence following MCA occlusion. However, intra-arterial rpro-UK was associated with a twofold risk of SICH. Mortality was equivalent between groups. Unfortunately, at this time rpro-UK is unavailable in the United States. Therefore, while providing an exciting proof of concept, these data have not resulted in an available therapy for acute stroke therapy in the United States.
The Interventional Management of Stroke II (IMS II) study, published in 2007, was a pilot trial with a planned comparison of the 3-month outcomes of patients treated with a combined algorithm of intra-arterial and intravenous rtPA versus the NINDS intravenous rtPA trial cohorts as historical controls, placebo, and intravenous rtPA groups.10 The IMS II protocol involved administration of 0.6 mg/kg of intravenous rtPA followed by up to 22 mg of intra-arterial rtPA administered at the site of thrombus by the EKOS microinfusion or standard microcatheters. Outcomes and mortality were significantly better in the intra-arterial group compared to the NINDS placebo group in all categories and trended toward significance compared to the intravenous rtPA cohort in most categories (achieving significance in two global measures). The IMS III trial, a prospective, randomized, controlled trial to further investigate intra-arterial plus intravenous rtPA, is under way.
Mechanical Thrombectomy
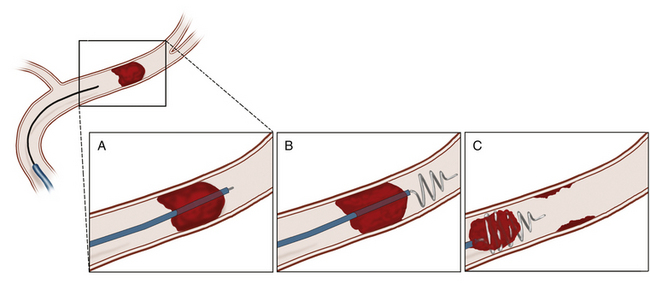
The phase 1 results of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) 1 study were published in 2004,41 demonstrating safety and efficacy of a new mechanical thrombectomy device. The Merci Retrieval System (Concentric Medical), a catheter-based retrieval device that physically ensnares the clot and then withdraws it from the site of occlusion, was further evaluated in the MERCI and Multi-MERCI trials that followed.42 These studies were single arm and therefore did not allow direct comparison to other therapeutic modalities; however, they suggested improved recanalization rates over either intravenous or intra-arterial thrombolysis. The first-generation MERCI devices, such as the Merci Retrieval System, demonstrated recanalization rates of approximately 50% with clot retrieval alone and 60% when paired with intra-arterial thrombolysis. In addition, benefit was seen up to 8 hours after symptom onset, compared to the 4.5- or 6-hour windows of intravenous or intra-arterial rtPA, respectively. Unlike intravenous or intra-arterial rtPA, mechanical thrombectomy devices do not use potent thrombolytics and therefore represent a useful alternative treatment modality in those patients with thromboembolic stroke who have contraindications to systemic thrombolytic therapy. SICH was comparable to other treatment modalities and has been reported in roughly 7% to 10% of patients. Given its documented efficacy and relative safety, the FDA approved the use of the Merci Retriever in 2004 for revascularization in acute ischemic stroke.
The Merci Retrieval System, the prototype for mechanical thrombectomy devices, is composed of five essential subunits: the balloon guide catheter, the distal access catheter, the microcatheter, the guidewire, and the retrieval device (Fig. 85-4). First, the balloon guide catheter is navigated into the distal cervical arterial vessel. The distal access catheter is then advanced over the microcatheter to a position of stability, preferably at least as distal as the origin of the target vessel. The microcatheter and guidewire are then advanced to the clot. The guidewire is then exchanged for the retriever device, and the retriever is deployed just beyond and within the clot. The retriever device has a natural coiled shape and, when deployed, acquires its coiled shape both distal and within the clot. When the clot is engaged by the retriever catheter, the balloon is inflated and the interventionalist then performs a slow pull and holds while maintaining the position of the distal access catheter relative to the clot. Next, the distal access catheter is secured relative to the microcatheter, and then all units are pulled proximally to the balloon catheter. While this maneuver is performed, aspiration through the balloon guide catheter is performed to minimize the likelihood of reembolization while the clot is withdrawn. Once all units and the clot have entered the balloon catheter, the elements are then removed from the system and angiography is performed to examine for adequacy of recanalization and distal perfusion.
Newer-generation MERCI thrombectomy devices utilize additional suture elements to improve clot retrieval. Such improvements have been demonstrated in the Multi-MERCI trial to provide roughly 60% recanalization rates when used alone and recanalization rates of nearly 70% when used with intra-arterial thrombolysis.27,42 Trends of improved recanalization, lower mortality, and improved outcomes were demonstrated by the newer devices compared to the prototype device, although these were not statistically significant. No differences in SICH were seen among devices, with all devices having a roughly 7% to 10% risk of clinically significant hemorrhage.
Thromboaspiration
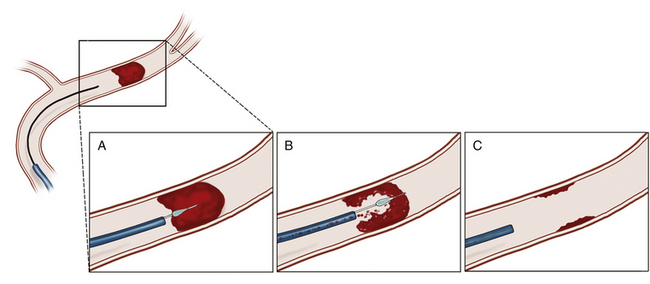
A novel device utilizing a suction catheter was introduced several years after the success of the mechanical thrombectomy devices. The first thromboaspiration device tested, the Penumbra System, was designed for large-vessel intracranial occlusive thrombi. The Penumbra System utilizes a suction catheter and separator to debulk and remove the thrombus piecemeal (Fig. 85-5).
The initial phase I study of the Penumbra System thromboaspiration catheter demonstrated 100% efficacy in achieving revascularization to TIMI grade 2 or 3 in the 21 patients tested.43 The phase III trial that followed, involving 125 patients from 24 centers, demonstrated an 82% revascularization rate, with 58% achieving an improvement in greater than or equal to 4 points on the NIHSS.44 SICH occurred in 11% of patients. After review of the efficacy of this system, the Penumbra System achieved FDA approval in 2008 for revascularization in acute ischemic stroke.
Angioplasty
Angioplasty, alone or with other endovascular therapies, has also demonstrated effectiveness in patients presenting with acute stroke. A pilot study by Nakano et al. in 1998 demonstrated successful recanalization with percutaneous angioplasty (PTA) in 8 of 10 patients with acute MCA occlusion.45 These findings led to a trial comparing PTA versus intra-arterial thrombolysis in patients with acute MCA trunk occlusion, in which 36 patients were treated with intra-arterial thrombolytic therapy alone (control group) and 34 patients underwent PTA.46 Recanalization rates were 91% in the PTA group versus 64% in the intra-arterial group. In addition, the incidence of symptomatic intracranial hemorrhage was only 2.9% in the PTA group versus 19% in controls.46 Other studies have since supported these findings, demonstrating high rates of recanalization, low rates of SICH or systemic bleeding, and significant improvement in neurologic function with combined angioplasty and thrombolysis.47,48 In addition, many investigations have shown the technical feasibility and high efficacy of PTA in acute ischemic stroke,46,49,50 and it seems to be particularly useful in cases of atherothrombotic disease, in which the residual stenosis may reduce flow sufficiently to lead to rethrombosis.51
Stenting
Stent placement as a treatment for acute ischemic stroke was introduced primarily as a rescue therapy in combination with other treatments. Investigations into the application of stents in this patient group focused on achieving the high recanalization rate of stenting seen in other physiologic settings while minimizing the risk of SICH through traumatic vessel injury. The development of self-expanding stents and innovations in delivery catheter design for neurointerventional applications has improved the overall safety and deliverability of stent deployment for stroke. One prospective study, the Stent-Assisted Recanalization in Acute Ischemic Stroke trial, analyzed outcomes for patients who underwent primary stenting for stroke.52 Of 20 patients presenting within 8 hours of stroke onset, 100% achieved recanalization rates of TIMI 2 or 3. In addition, 60% of patients achieved a modified Rankin score of 3 or less and nearly 50% achieved a score of 0 or 1 at 1-month follow-up.
Currently, no phase III trials have been published in either primary stenting or stent-assisted therapy in acute ischemic stroke. However, several small series have been published documenting anywhere from 68% to 92% recanalization rates.52–55 In addition, low SICH and in-stent stenosis rates have been reported. The theoretical benefits of stenting are well described but to this point have only been demonstrated in several small series. These benefits include immediate recanalization, reduced reocclusion rates compared to other techniques, and reduction of distal embolization through displacement of fragments against the vessel wall.
Future of Endovascular Techniques in Stroke
A prospective, randomized trial is under way to further evaluate the use of intra-arterial thrombolytics in conjunction with other modalities. In addition, numerous innovations to mechanical devices are actively being evaluated for efficacy. PROACT, the IMS trials, and the multiple mechanical device series all indicate that recanalization provides improved outcomes following acute stroke. As technological and pharmacologic therapies continue to improve and physician experience leads to increasingly refined technical abilities, an inflection point of maximal safety and efficacy must be reached. When this occurs, the endovascular treatment of acute stroke will rapidly become one of the most frequent and profound interventions performed by interventionalists. High-quality randomized trials are needed to demonstrate the successful achievement of this goal. Excitingly, such trials are under way.
Bose A., Henkes H., Alfke K., et al. The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol. 2008;29:1409-1413.
Clark W.M., Wissman S., Albers G.W., et al. Recombinant tissue-type plasminogen activator (alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282:2019-2026.
Del Zoppo G.J., Saver J.L., Jauch E.C., et al. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945-2948.
Flint A.C., Duckwiler G.R., Budzick R.F., et al. Mechanical thrombectomy of intracranial internal carotid occlusion: pooled results of the MERCI and Multi MERCI Part I trials. Stroke. 2007;38:1274-1280.
Furlan A., Higashida R., Wechsler L., et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003-2011.
Gobin Y.P., Starkman S., Duckwiler G.R., et al. MERCI 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke. 2004;35:2848-2854.
Hacke W., Donnan G., Fieschi C., et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768-774.
Hacke W., Kaste M., Bluhmki E., et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
Hacke W., Kaste M., Fieschi C., et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
Hacke W., Kaste M., Fieschi C., et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245-1251.
Hallevi H., Barreto A.D., Liebeskind D.S., et al. Identifying patients at high risk for poor outcome after intra-arterial therapy for acute ischemic stroke. Stroke. 2009;40:1780-1785.
IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke. 2007;38:2127-2135.
Janjua N., El-Gengaihy A., Pile-Spellman J., et al. Late endovascular revascularization in acute ischemic stroke based on clinical-diffusion mismatch. AJNR Am J Neuroradiol. 2009;30:1024-1027.
Levy E.I., Siddiqui A.H., Crumlish A., et al. First Food and Drug Administration–approved prospective trial of primary intracranial stenting for acute stroke: SARIS (Stent-Assisted Recanalization in Acute Ischemic Stroke). Stroke. 2009;40:3552-3556.
Lloyd-Jones D., Adams R., Carnethon M., et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21-181.
Murphy B.D., Fox A.J., Lee D.H., et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771-1777.
Nakano S., Iseda T., Yoneyama T., et al. Direct percutaneous transluminal angioplasty for acute middle cerebral artery trunk occlusion: an alternative option to intra-arterial thrombolysis. Stroke. 2002;33:2872-2876.
Nakano S., Yokogami K., Ohta H., et al. Direct percutaneous transluminal angioplasty for acute middle cerebral artery occlusion. AJNR Am J Neuroradiol. 1998;19:767-772.
Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761-2768.
Rha J.H., Saver J.L. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967-973.
Saqqur M., Molina C.A., Salam A., et al. Clinical deterioration after intravenous recombinant tissue plasminogen activator treatment: a multicenter transcranial Doppler study. Stroke. 2007;38:69-74.
Saqqur M., Tsivgoulis G., Molina C.A., et al. Symptomatic intracerebral hemorrhage and recanalization after IV rt-PA: a multicenter study. Neurology. 2008;71:1304-1312.
Smith W.S., Sung G., Saver J., et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205-1212.
TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med. 1985;312:932-936.
Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581-1587.
1. Centers for Disease Control and Prevention. Stroke facts and statistics. http://www.cdc.gov/stroke. [online] Available at
2. Lloyd-Jones D., Adams R., Carnethon M., et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21-181.
3. Alexandrov A.V., Alagona P. Stroke and atherthrombosis: an update on the role of antiplatelet therapy. Int J Stroke. 2008;3:175-181.
4. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581-1587.
5. Clark W.M., Wissman S., Albers G.W., et al. Recombinant tissue-type plasminogen activator (alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282:2019-2026.
6. Furlan A., Higashida R., Wechsler L., et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003-2011.
7. Hacke W., Kaste M., Fieschi C., et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
8. Hacke W., Kaste M., Fieschi C., et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245-1251.
9. Hallevi H., Barreto A.D., Liebeskind D.S., et al. Identifying patients at high risk for poor outcome after intra-arterial therapy for acute ischemic stroke. Stroke. 2009;40:1780-1785.
10. IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke. 2007;38:2127-2135.
11. Barber P.A., Zhang J., Demchuk A.M., et al. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001;56:1015-1020.
12. Hacke W., Kaste M., Bluhmki E., et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
13. TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med. 1985;312:932-936.
14. Saqqur M., Molina C.A., Salam A., et al. Clinical deterioration after intravenous recombinant tissue plasminogen activator treatment: a multicenter transcranial Doppler study. Stroke. 2007;38:69-74.
15. Saqqur M., Tsivgoulis G., Molina C.A., et al. Residual flow at the site of intracranial occlusion on transcranial Doppler predicts response to intravenous thrombolysis: a multi-center study. Cerebrovasc Dis. 2009;27:5-12.
16. Saqqur M., Uchino K., Demchuk A.M., et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948-954.
17. Rha J.H., Saver J.L. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967-973.
18. Zaidat O.O., Suarez J.I., Sunshine J.L., et al. Thrombolytic therapy of acute ischemic stroke: correlation of angiographic recanalization with clinical outcome. AJNR Am J Neuroradiol. 2005;26:880-884.
19. Alexandrov A.V., Grotta J.C. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59:862-867.
20. Janjua N., Alkawi A., Suri M.F., et al. Impact of arterial reocclusion and distal fragmentation during thrombolysis among patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2008;29:253-258.
21. Brott T., Adams H.P.Jr, Olinger C.P., et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864-870.
22. Goldstein L.B., Bertels C., Davis J.N. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46:660-662.
23. Muir K.W., Weir C.J., Murray G.D., et al. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke. 1996;27:1817-1820.
24. Murphy B.D., Fox A.J., Lee D.H., et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771-1777.
25. Wintermark M., Flanders A.E., Velthuis B., et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979-985.
26. Del Zoppo G.J., Saver J.L., Jauch E.C., et al. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945-2948.
27. Smith W.S., Sung G., Saver J., et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205-1212.
28. Ionita C.C., Yamamoto J., Tummala R.P., et al. MRI assessment followed by successful mechanical recanalization of a complete tandem (internal carotid/middle cerebral artery) occlusion and reversal of a 10-hour fixed deficit. J Neuroimaging. 2008;18:93-95.
29. Janjua N., El-Gengaihy A., Pile-Spellman J., et al. Late endovascular revascularization in acute ischemic stroke based on clinical-diffusion mismatch. AJNR Am J Neuroradiol. 2009;30:1024-1027.
30. Marti-Fabregas J., Bravo Y., Cocho D., et al. Frequency and predictors of symptomatic intracerebral hemorrhage in patients with ischemic stroke treated with recombinant tissue plasminogen activator outside clinical trials. Cerebrovasc Dis. 2007;23:85-90.
31. Singer O.C., Berkefeld J., Lorenz M.W., et al. Risk of symptomatic intracerebral hemorrhage in patients treated with intra-arterial thrombolysis. Cerebrovasc Dis. 2009;27:368-374.
32. Lansberg M.G., Albers G.W., Wijman C.A. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24:1-10.
33. Paciaroni M., Agnelli G., Caso V., et al. Acute hyperglycemia and early hemorrhagic transformation in ischemic stroke. Cerebrovasc Dis. 2009;28:119-123.
34. Rosell A., Foerch C., Murata Y., et al. Mechanisms and markers for hemorrhagic transformation after stroke. Acta Neurochir Suppl. 2008;105:173-178.
35. Rosell A., Cuadrado E., Ortega-Aznar A., et al. MMP-9-positive neutrophil infiltration is associated to blood–brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121-1126.
36. Wang X., Tsuji K., Lee S.R., et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35(11 Suppl 1):2726-2730.
37. Hacke W., Donnan G., Fieschi C., et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768-774.
38. Saqqur M., Tsivgoulis G., Molina C.A., et al. Symptomatic intracerebral hemorrhage and recanalization after IV rt-PA: a multicenter study. Neurology. 2008;71:1304-1312.
39. Barr J.D., Mathis J.M., Wildenhain S.L., et al. Acute stroke intervention with intraarterial urokinase infusion. J Vasc Interv Radiol. 1994;5:705-713.
40. Brott T., Broderick J., Kothari R. Thrombolytic therapy for stroke. Curr Opin Neurol. 1994;7:25-35.
41. Gobin Y.P., Starkman S., Duckwiler G.R., et al. MERCI 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke. 2004;35:2848-2854.
42. Flint A.C., Duckwiler G.R., Budzick R.F., et al. Mechanical thrombectomy of intracranial internal carotid occlusion: pooled results of the MERCI and Multi MERCI part I trials. Stroke. 2007;38:1274-1280.
43. Bose A., Henkes H., Alfke K., et al. The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol. 2008;29:1409-1413.
44. Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761-2768.
45. Nakano S., Yokogami K., Ohta H., et al. Direct percutaneous transluminal angioplasty for acute middle cerebral artery occlusion. AJNR Am J Neuroradiol. 1998;19:767-772.
46. Nakano S., Iseda T., Yoneyama T., et al. Direct percutaneous transluminal angioplasty for acute middle cerebral artery trunk occlusion: an alternative option to intra-arterial thrombolysis. Stroke. 2002;33:2872-2876.
47. Qureshi A., Siddiqui A.M., Suri M.F., et al. Aggressive mechanical clot disruption and low-dose intra-arterial third-generation thrombolytic agent for ischemic stroke: a prospective study. Neurosurgery. 2002;51:1319-1327. discussion 1327-1329
48. Yoneyama T., Nakano S., Kawano H., et al. Combined direct percutaneous transluminal angioplasty and low-dose native tissue plasminogen activator therapy for acute embolic middle cerebral artery trunk occlusion. AJNR Am J Neuroradiol. 2002;23:277-281.
49. Abou-Chebl A., Bajzer C.T., Krieger D.W., et al. Multimodal therapy for the treatment of severe ischemic stroke combining GPIIb/IIIa antagonists and angioplasty after failure of thrombolysis. Stroke. 2005;36:2286-2288.
50. Ueda T., Sakaki S., Nochide I., et al. Angioplasty after intra-arterial thrombolysis for acute occlusion of intracranial arteries. Stroke. 1998;29:2568-2574.
51. Qureshi A.I., Siddiqui A.M., Kim S.H., et al. Reocclusion of recanalized arteries during intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2004;25:322-328.
52. Levy E.I., Siddiqui A.H., Crumlish A., et al. First Food and Drug Administration–approved prospective trial of primary intracranial stenting for acute stroke: SARIS (Stent-Assisted Recanalization in Acute Ischemic Stroke). Stroke. 2009;40:3552-3556.
53. Levy E.I., Ecker R.D., Horowitz M.B., et al. Stent-assisted intracranial recanalization for acute stroke: early results. Neurosurgery. 2006;58:458-463. discussion 458-463
54. Levy E.I., Mehta R., Gupta R., et al. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol. 2007;28:816-822.
55. Brekenfeld C., Schroth G., Mattle H.P., et al. Stent placement in acute cerebral artery occlusion: use of a self-expandable intracranial stent for acute stroke treatment. Stroke. 2009;40:847-852.