CHAPTER 396 Endovascular Treatment of Spinal Vascular Malformations
Vascular lesions of the spinal axis have engendered many classification schemes in the past century.1 Although these conditions were first recognized in the late 19th century by Hebold2 and Gaupp,3 for the first half of the 20th century our knowledge of spinal vascular malformations was limited to a variety of case reports and autopsy findings. The first recorded successful operation on a dural arteriovenous fistula (AVF) was performed in 1914 by Charles Elsberg.4 It was not until the 1960s that angiographic diagnosis and endovascular treatment were first reported by Djindjian and colleagues5 and Doppman and associates.6–9 Since then, advances in imaging modalities, angiographic techniques, and endovascular material have contributed significantly to a better understanding of the pathology of these diseases, as well as better outcomes for patients.
Vascular Anatomy
Crucial to understanding the pathophysiology behind spinal vascular malformations is a thorough comprehension of the spinal cord’s vascular architecture. The spinal cord’s blood supply is provided by one anterior spinal artery (ASA) and paired posterior spinal arteries (PSAs). The ASA runs in the anterior median sulcus and supplies the anterior two thirds of the spinal cord, including the anterior horn cells, the anterior and lateral corticospinal tracts, and the spinothalamic tract. It is formed at the craniovertebral junction by the convergence of two smaller paired ASAs that arise from the vertebral arteries distal to the origin of the posterior inferior cerebellar arteries and ends at the conus medullaris. In the cervical spine, blood flow to the ASA is augmented by radiculomedullary feeders from the vertebral and subclavian arteries, most notably, by the artery of cervical enlargement at C5 or C6. A total of 6 to 10 additional radiculomedullary feeders that derive from the aorta and iliac arteries reinforce the ASA at the thoracic and lumbar levels. The most prominent of these is the great anterior segmental medullary artery, referred to eponymously as the artery of Adamkiewicz, which usually arises from the left between T8 and L2. On an anteroposterior projection, this artery is 0.5 to 1 mm in diameter and has an ascending segment that makes a classic “hairpin” bend into the descending ASA in the midline. Angiographic identification of this artery is crucial before the embolization of middle thoracic to upper lumbar malformations. The main watershed area of the spinal cord is at the upper thoracic levels, and any ischemia of the ASA risks paralysis.10–16
The PSAs arise from the vertebral arteries and descend along while supplying the posterolateral cord, including the posterior columns. They are augmented by radiculomedullary vessels from the vertebral, cervical, intercostal, and lumbar arteries, which also make the “hairpin” turn as they join the PSAs, albeit lateral to the midline and thinner in diameter. At the conus medullaris, the paired PSAs merge with the ASA to form the cruciate anastomosis.10–17
The non-neural elements of the spinal axis are also supplied by branches of the vertebral, intercostal, and lumbar arteries. The blood supply to the anterior and lateral aspects of the vertebral bodies of the cervical spine are provided by branches of the vertebral arteries and the thyrocervical trunk. In the thoracic and lumbar region, however, the bodies are predominantly fed by branches of the intercostal and lumbar radicular arteries, respectively. More distal branches of the radicular arteries circumscribe the vertebrae, thereby forming a vascular arcade anterior to the posterior longitudinal ligament, which in turn supplies the posterior aspect of the vertebral bodies. Even further radicular branches form an arcade in the posterior epidural space that supplies the lamina and a portion of the posterior spinous process, as well as the dorsospinal artery, which supplies the outer surface of the lamina and the posterior spinous process.10,12,15,16
It is important to note that the transition of a median vein into a radicular vein has the same “hairpin” turns as the arteries described earlier. Drainage of blood from the spine occurs through the valveless internal and external venous vertebral plexus, which is connected to the azygos and hemiazygos venous systems.11,13,15–17
Diagnostic Imaging of Spinal Vascular Malformations
Magnetic Resonance Imaging
Digital subtraction angiography remains the “gold standard” as the most reliable and accurate method for detecting spinal vascular malformations. Spinal angiography, however, is invasive, has associated risks, and should therefore be reserved for patients who are likely to have spinal vascular malformations and for those who require endovascular therapy. This is particularly true since the advent of magnetic resonance imaging (MRI), which is currently the best modality for noninvasive evaluation.11,18
MRI findings of spinal cord edema coupled with the demonstration of flow voids secondary to dilated arteries or veins within or on the surface of the spinal cord are characteristic of spinal vascular malformations and suggest the diagnosis. In addition, MRI is useful in evaluating associated findings such as cord atrophy, hematomyelia, thrombosis, and cavitation.11,19 Neither the location of pathologic vessels nor the intramedullary imaging findings seem to be related to the level of the fistula.11 Localizing the fistula, therefore, may be very difficult and often necessitates spinal angiography. Newer noninvasive diagnostic techniques such as contrast-enhanced magnetic resonance angiography (MRA) with relative fast acquisition protocols and larger fields of view have been developed to assist in fistula localization.11,19
Computed Tomography
Recently, there have been several small case series investigating the value of multidetector computed tomographic (CT) spinal angiography in diagnosing spinal vascular malformations. The benefits of this modality over contrast-enhanced MRA include a shorter acquisition time with increased scanning range and spatial resolution.20 In addition, there is limited experience in using immediate postembolization CT scans as a tool for predicting permanent fistula occlusion and the prognosis for outcome.21
Digital Subtraction Angiography
Angiography confirms the diagnosis and determines the feasibility of either surgical or endovascular treatment. Preferably, spinal angiography is performed with the patient under general anesthesia and with controlled respiration to avoid motion artifact. This increases the resolution of images, an advantage important in avoiding complications, such as missing a small spinal cord artery, which can lead to disastrous results during embolization.12,22
If a spinal dural AVF is not discovered despite bilateral angiography from the supreme intercostal artery to the internal iliac artery, the external carotid arteries and the vertebral arteries should be studied to rule out an intracranial pial or dural arteriovenous malformation (AVM) draining to the spinal cord.23
Principles of Endovascular Therapy
The remainder of this chapter focuses on endovascular therapy for arteriovenous lesions using the most recent classification described by Spetzler and colleagues (see Table 396-3 and Chapter 395).24 Because cavernous malformations are angiographically occult, endovascular therapy has no role in their treatment.24a
Monitoring
Many authors advocate the adjunctive use of physiologic monitoring with somatosensory evoked potentials (SEPs) and motor evoked potentials (MEPs) during embolization of spinal cord vascular malformations.25–30 In these situations, a continuous infusion of propofol and fentanyl is used rather than halogenated agents or muscle relaxants. This allows the detection of any iatrogenic abnormal spinal cord function that may be related to the embolization; if an abnormality is detected, the physician can either abandon the procedure or change the position of the catheter. Because the changes detected by physiologic monitoring may occur in a delayed fashion after the insult, chemical provocative testing can be performed under SEP and MEP monitoring. The chemical provocative test is performed by injecting 50 to 75 mg of amobarbital sodium (Amytal Sodium), followed by 20 to 40 mg of lidocaine (Xylocaine).26,28,29 Amobarbital is used to test functional changes in neurons, and lidocaine is used to test axonal function. If a decrease in amplitude (of 50%) or an increase in latency (>10%) of SEPs or disappearance of MEPs is induced by the chemical provocative test, embolization from this catheter position is aborted.26,28,29 Recovery of SEPs and MEPs to baseline values usually occurs within 5 to 10 minutes. Further superselective catheterization of the same pedicle after advancing the catheter closer to the nidus—or another pedicle if the former approach still fails—is performed after searching for a safer catheter position.
Niimi and colleagues have recently reported their experience in monitoring embolization of spinal arteriovenous lesions. In their experience of 60 provocative tests during 84 angiographic procedures in 52 patients with intended endovascular embolization, they found only one false-negative result (negative predictive value of 97.6%), in which the patient had a transient increase in spasticity after embolization with n-butyl cyanoacrylate (NBCA) through an ASA feeder despite a negative provocative test.28
Embolic Material
Different embolic agents are available to treat spinal cord vascular malformations, and there are different opinions regarding the best embolic agent. The choices include polyvinyl alcohol (PVA) and microspheres (of polyacrylamide and gelatin), liquid adhesives, and a variety of coils. The advantage of PVA and microspheres is their ease of use; however, the occlusive effect tends to be temporary and is frequently associated with recanalization.10,12,22,31–33 Liquid adhesives such as NBCA are currently the best agents in terms of a long-lasting effect, but they are cumbersome to use and require that the microcatheter be advanced up to the nidus or fistula.10,12,22,31–33 Moreover, the use of liquid adhesives requires a greater level of expertise because of their complex properties. Coil embolization is often used only for large fistulas and aneurysms. Onyx is a relatively new liquid embolic agent that is licensed in the United States for presurgical endovascular treatment of cerebral AVMs, but it is not yet approved by the Food and Drug Administration for the treatment of spinal AVMs. It is a mixture of ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide (DMSO) that contains micronized tantalum powder for fluoroscopic visualization. Onyx is delivered in liquid phase via a microcatheter to the target lesion, where it transforms into a solid polymer as the DMSO diffuses away. In the largest patient series (n = 17) of spinal intramedullary AVMs treated exclusively with Onyx, Corkill and coauthors reported promising results in which total AVM obliteration was achieved in 6 patients (35.3%), subtotal obliteration (tiny and insignificant remnant) in 5 patients (29.4%), partial obliteration (substantial residual nidus) in 5 patients (29.4%), and procedure abortion in 1 patient (5.9%) secondary to intraprocedural rupture.34 Improvement in neurological or functional status, or both, was noted in 14 patients for an 82% rate of overall good clinical outcome. No long-term studies exist, however, in which persistent obliteration of the lesion is monitored.
Endovascular Treatment of Spinal Arteriovenous Malformations
Extradural-Intradural Arteriovenous Malformations
Extradural-intradural AVMs are uncommon and have previously been referred to as juvenile, metameric, or type III AVMs. Treatment is typically palliative because complete obliteration of these extensive lesions is rarely achieved as a result of the unacceptable risk for neurological morbidity.10,12,13 Embolization of multiple feeding arteries with the delivery of PVA, NBCA, and coils is required, often in combination. Staged embolization may be necessary to decrease the arteriovenous shunt if the symptoms are related to steal phenomena. Compression symptoms secondary to the lesion may be reduced or even eliminated by embolizing the enlarged vascular structures. Staged resection may follow the embolization.10,12,13,35–39
Intradural Arteriovenous Malformations
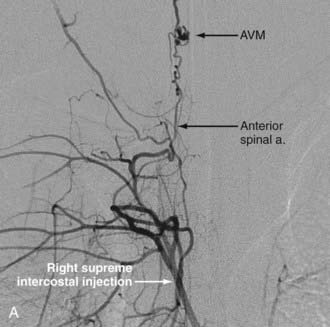
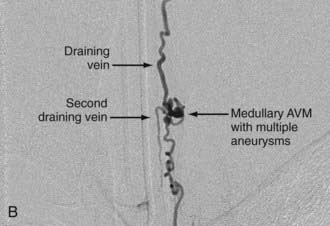
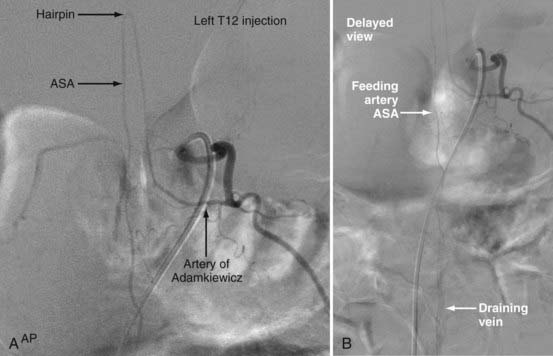
Analogous to brain AVMs, intradural intramedullary spinal AVMs are located entirely within the cord parenchyma. These lesions were previously referred to as glomus-type lesions, type II AVMs. There is a slight male preponderance, and they can occur at any level of the spinal cord. Aneurysms associated with this type of AVM are common (Fig. 396-1).
In the majority of patients, symptoms occur before 16 years of age. MRI has significantly improved the diagnosis, with subarachnoid hemorrhage or hematomyelia being the usual initial clinical findings. Recurrent hemorrhage is seen more commonly with spinal AVMs than with cerebral malformations, and 40% of these AVMs rebleed within the first year after the initial hemorrhage. The hemorrhage is frequently accompanied by significant neurological deficits, and each ictus is associated with significant mortality of 10% to 20%. Less frequently, a patient will have acute or progressive neurological deterioration related to an ischemic cause without evidence of hemorrhage. This is usually secondary to a mass effect or venous hypertension arising from arterialization of the draining veins and resulting in their impaired function.25,40 Some authors believe that a progressive myelopathy can occur as a result of vascular steal,41 but others disagree.25 Rarely, the diagnosis is made in patients with no neurological signs or symptoms and is based on an evaluation for unrelated symptoms or associated metameric or skin lesions.
The poor prognosis of patients with untreated spinal cord AVMs justifies aggressive intervention, especially in young patients, even if significant neurological deficits are present.10,24,25,40 In some paraplegic patients, embolization may suppress their severe radicular pain and diminish their spasticity.10,42 When the neurological deficits are due to recent hemorrhage, treatment is usually delayed to permit maximal recovery before the initiation of treatment.
The diagnosis can be made with MRI, but angiography is necessary to define the exact angioarchitecture of the lesion and identify potential associated aneurysms. This allows a clear understanding of the lesion, which is vital for planning and executing treatment. At the cervical level, these AVMs can have both anterior and posterior suppliers, with multiple feeders arising from the deep cervical, upper intercostal, and vertebrobasilar arterial systems.10 The largest feeder is frequently the artery of cervical enlargement at C5 or C6.10,42 Attempted catheterization of small anterior feeders may produce spasm, which can be dangerous because emboli may then stop in the ASA and cause inadvertent paralysis. The collateral blood supply, however, is extensive in the cervical spine and may obviate any neurological insult. Posterior pedicle spasm is less dangerous but may preclude adequate nidal occlusion. At the thoracolumbar levels, embolization is often possible via a pathologically enlarged artery of Adamkiewicz, with collateral flow supplied by the cruciate anastomosis.10,42 Because of a more limited collateral blood supply than in the cervical neck and in situations in which the blood supply to this region arises from the AVM feeders themselves, catheterization at these levels cannot be attempted without demonstrating an alternative arterial supply to the conus medullaris.
Some authors believe that surgical resection remains the mainstay of treatment.43 In Spetzler and associates’ series, complete resection was not possible in only 8% of cases.24,44 Many other authors believe that embolization should be considered the treatment of choice, and others have demonstrated that in select cases, embolization can be the definitive treatment.25 Many centers advocate a combined technique, with presurgical embolization aimed at facilitating surgical resection. Embolization to cure the lesion requires catheterization of the primary feeder close to the nidus, with obliteration of the nidus being achieved with either particulate matter or liquid adhesives. Care must be taken to not occlude the ASA or open up collaterals, which can lead to filling of the ASA and inadvertent stroke.10
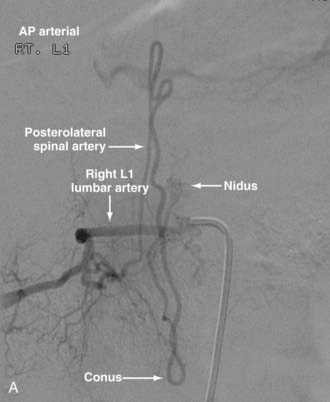
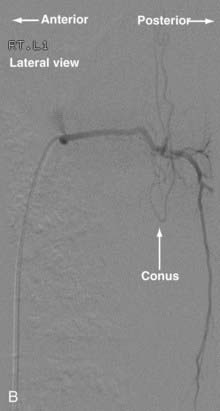
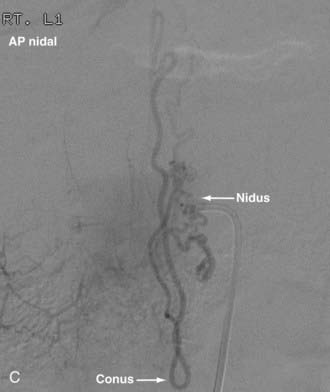
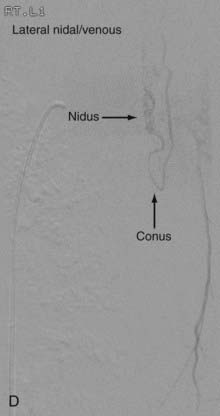
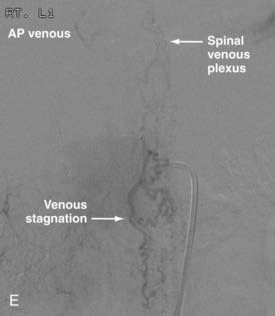
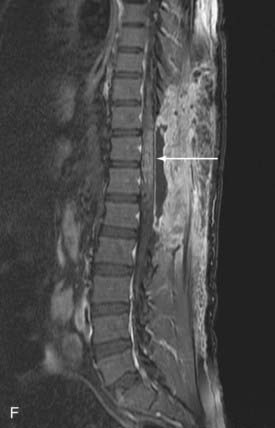
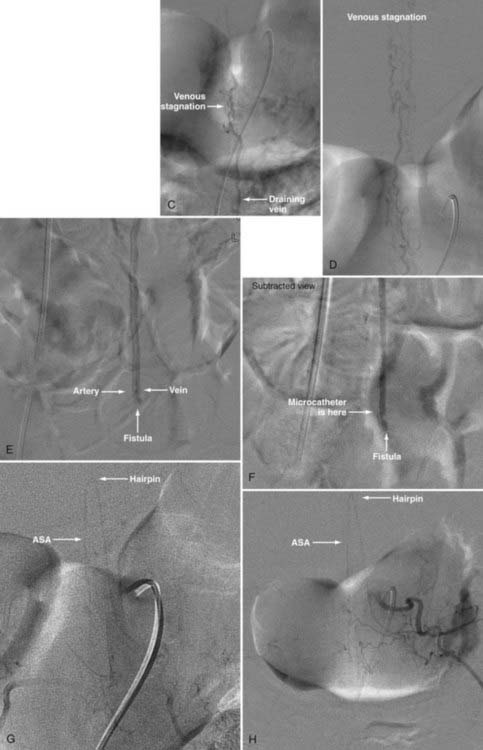
Conus Medullaris Arteriovenous Malformations
First described by Hurst and coworkers,45 Spetzler and colleagues’ classification lists conus AVMs as a separate category characterized by multiple feeding arteries, multiple nidi, and complex venous drainage.12,24 They have multiple direct arteriovenous shunts that derive their blood supply from the ASA and PSA and have glomus-type nidi that are usually extramedullary and pial based, but they may also have an intramedullary component. They are always located in the conus medullaris and cauda equina but can extend along the entire filum terminale (Fig. 396-2).12,24,45 Conus medullaris AVMs are likely to be a consequence of abnormal neurulation and may be associated with a tethered cord. Symptoms are similar to those of other cauda equina lesions and include bowel or bladder symptoms (or both), myelopathy, or radiculopathy, and they may be associated with upper or lower motor neuron signs (or both).12,24,45 These lesions can be treated by both surgery and embolization, and the experience of Spetzler and colleagues is that this type of lesion is preferentially treated by aggressive embolization with PVA or NBCA and subsequent microsurgical resection, with careful attention paid to identification of the ASA and PSA feeders.12,24 Elimination of mass effect on the descending nerve roots of the cauda equina can be associated with striking clinical improvement.12,24
Endovascular Treatment of Spinal Arteriovenous Fistulas
Extradural Arteriovenous Fistulas
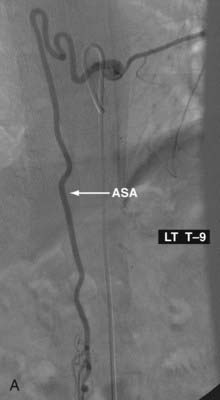
Also known as epidural AVFs, these lesions are characterized by a direct connection between an extradural artery and vein that leads to the development of a high-flow fistula and engorgement of the epidural venous system.24 The location of the fistula may be epidural or between the two layers of the dura mater. The epidural venous engorgement is due to exclusive drainage to the epidural vein.40,46–48 In some cases, however, drainage into both the intradural and epidural veins has been reported.49,50 Patients can have myelopathy,51,52 radiculopathy,52–54 subarachnoid hemorrhage,53 and rarely, a spontaneous epidural hematoma.55 Myelopathy can be caused by venous hypertension and congestion of the spinal cord or by a mass effect by the dilated epidural vein. The radiculopathy is most likely due to a mass effect, but disturbance of venous drainage of the nerve root may be a contributing factor. Subarachnoid hemorrhage occurs in AVFs that have intradural venous drainage. Symptoms can rarely be attributed to the shunting of large quantities of arterial blood into the venous system or to steal of blood flow from the spinal cord.12,24,56
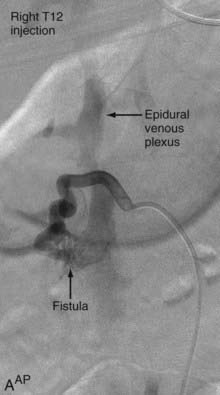
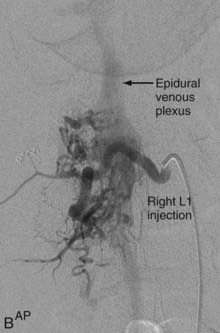
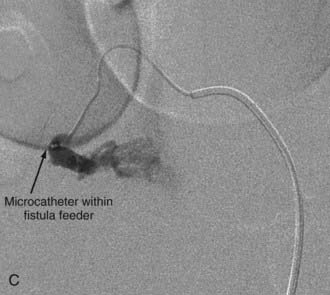
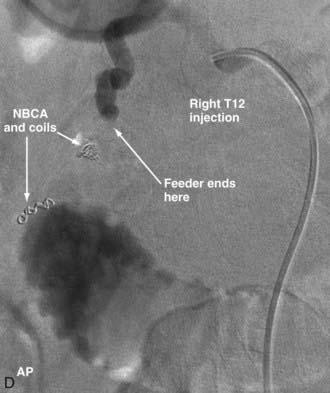
Extradural AVFs are treated almost exclusively by endovascular procedures and only rarely require open surgery.12,22,24 Endovascular treatment usually involves a transarterial approach, but occasionally a transvenous approach provides better access to the fistula.12 Closure of the fistula requires elimination of a small portion of the feeding artery proximal to the fistula, the fistula itself, and a limited portion of the proximal draining vein as well. Some authors advocate the use of fibered detachable coils rather than embolic agents for the treatment of these lesions because there is less risk of the embolic material traveling beyond the target zone with the former material12 (Fig. 396-3).
Intradural Dorsal Arteriovenous Fistulas
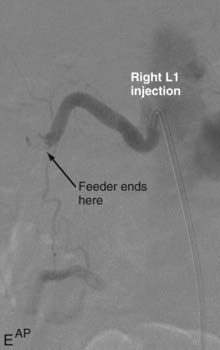
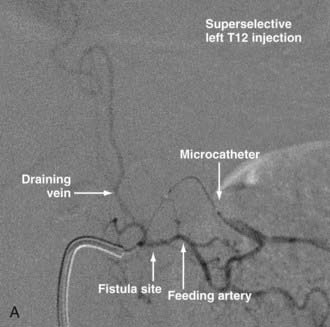
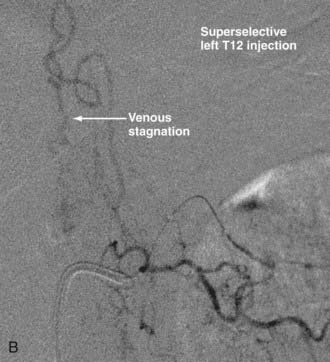
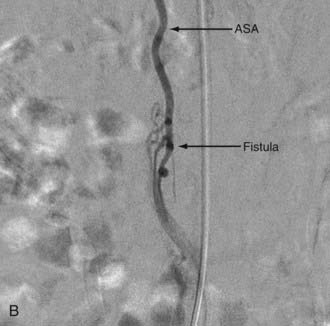
Intradural dorsal AVFs are the most common type of spinal AVF. They are also known as type I malformations,12,24,57 spinal dural AVFs,25,40 long dorsal AVFs,58 dorsal extramedullary AVFs,59 and angioma venosum racemosum.59,60 The primary pathophysiology of intradural dorsal AVFs is venous hypertension leading to hypoperfusion of the spinal cord. This is demonstrated on angiography by the slow-flow shunt, with delayed clearance of contrast material from the draining perimedullary veins and prolonged circulation time in the ASA.40 The venous drainage is usually cranial on the posterior surface of the spinal cord. It has been postulated that venous drainage on the anterior surface of the spinal cord or in the caudal direction indicates more severely compromised spinal cord venous drainage and correlates with severity of the clinical symptoms.40
Clinically, patients usually exhibit progressive myelopathy.12,24,40,61 It is common for patients to have a history of pain in the back or legs. By the time of diagnosis, many patients also have bladder or bowel and sexual dysfunction. The mode of progression of the myelopathy is variable; it can be continuous, stepladder-like, or waxing and waning with gradual progression. Symptoms can be aggravated by activities causing increased intra-abdominal pressure, such as bending or straining. Approximately 10% to 20% of patients experience acute exacerbation of myelopathy with or without pain but without hemorrhage. The cause of this acute exacerbation is probably acute decompensation of the already damaged drainage of the spinal cord as a result of thrombosis or other additional hemodynamic changes. In rare cases, patients may experience clinical deterioration secondary to hemorrhage.62,63 Because of the natural history of this lesion, the present standard of care is to eliminate the fistula to arrest disease progression.
Spinal dural AVFs can be treated by embolization, surgery, or both. Institutions with a surgical bias cite the high rates of recurrence and progressive myelopathy associated with embolization,64–66 but these studies are somewhat dated. In addition, in surgical series the reported morbidity rate is extremely low and the success rate is high.24,44,67–70 Institutions that favor embolization as the treatment of choice cite its less invasive nature than surgery, the ability to perform embolization in the same sitting as diagnostic angiography, the possibility of early rehabilitation (started the day after embolization), and the capability of performing surgery if embolization fails. Recent studies advocate endovascular therapy if technically feasible, with surgery being reserved for failure of embolization or recurrence after embolization.12,70 Most recently, data from the University of California at San Francisco revealed slightly higher obliteration rates for the surgical approach (83% versus 75%), shorter length of stay for the endovascular approach (9.8 days versus 3.1 days), but comparable clinical response to either intervention.71
Techniques for catheterization and embolization are similar to those used for other spinal vascular abnormalities, except that a flow-guided catheter may not be successful in these slow-flow lesions; therefore, a wire-assisted microcatheter is preferred. Provocative testing is not necessary.25 Superselective angiography from the microcatheter should be performed before embolization because a spinal cord artery may not be visualized as a result of its slow flow on angiography performed at the orifice of the segmental artery. Spinal dural AVFs that have arterial feeders arising from the same pedicle as the spinal cord artery should be treated by surgery.72
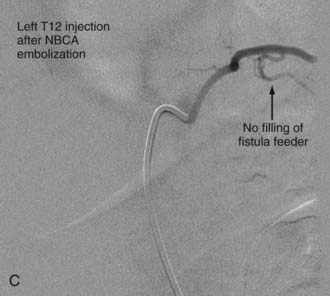
With regard to the choice of embolic agent, NBCA provides a more permanent result because PVA particles are known to recanalize.10,12,64–66 Coils and particles are occasionally used to protect normal territories before embolization of the direct feeder with NBCA. The goal of embolization is to have a column of NBCA penetrate to the proximal portion of the draining vein through the fistula. Both underpenetration and overpenetration of NBCA should be avoided. Underpenetration causes proximal occlusion and recurrence of the AVF as a result of collateralization. Overpenetration and occlusion of perimedullary veins can cause aggravation of venous hypertension and the associated myelopathy.12,72 Control angiography after embolization should be performed on the contralateral segmental artery at the same level as the feeding pedicle and on segmental arteries two levels above and below on both sides to rule out collateral circulation reconstituting the fistula. An angiogram of the ASA may show immediate improvement of the spinal cord circulation (Fig. 396-4).25,40
Intradural Ventral Arteriovenous Fistulas
In 1977, Djindjian and coworkers73 described six perimedullary or intradural ventral AVFs that originate directly from the ASA and have a direct fistula to an enlarged venous network with no intervening capillary system.74,75 They are located ventrally in the subarachnoid space in the midline. Blood flow through the lesions is rapid and can produce flow-related aneurysms and venous hypertension. They are more common in the pediatric population and in patients with Rendu-Osler-Weber syndrome; other syndromes known to be associated with them are neurofibromatosis and Klippel-Trénaunay and Parkes Weber syndromes (Table 396-1).35,76
TABLE 396-1 Classification of Spinal and Spinal Cord Vascular Lesions
From Niimi Y, Berenstein A. Endovascular treatment of spinal vascular malformations. Neurosurg Clin N Am. 1999;10:47-71.
Heros and associates introduced the designation type IV lesion in 1986,77 and Gueguen and coworkers divided ventral fistulas into three subtypes: 1, 2, and 3.78 Anson and Spetzler reclassified the subtypes as IV-A, IV-B, and IV-C57 (Table 396-2); in Spetzler and colleagues’ most recent classification, these subtypes have been maintained (Table 396-3).24 Type A, B, and C fistulas are progressively larger shunts, with the last characterized by a giant fistula and a markedly distended venous network. As the size and flow of the fistula increase, the signs and symptoms attributable to progressive vascular steal and spinal cord compression become more pronounced.
TABLE 396-2 Classification of Spinal Arteriovenous Malformations
| TYPE | DESCRIPTION |
|---|---|
| I | Arteriovenous fistula located between a dural branch of the spinal ramus of a radicular artery and an intradural medullary vein |
| II | Intramedullary glomus malformation with a compact nidus within the substance of the spinal cord |
| III | Extensive arteriovenous malformation often extending into the vertebral body and paraspinal tissues |
| IV | Intradural perimedullary arteriovenous fistula |
| IV-A | Simple perimedullary fistula fed by a single arterial branch |
| IV-B | Intermediate-sized fistula with multiple dilated arterial feeders |
| IV-C | Large perimedullary fistula with multiple giant arterial feeders |
From Anson JA, Spetzler RF. Classification of spinal arteriovenous malformations and implications for treatment. BNI Q. 1992;8:2-8.
TABLE 396-3 Classification of Spinal Cord Vascular Malformations
From Spetzler RF, Detwiler PW, Riina H, et al. Modified classification of spinal cord vascular lesions. J Neurosurg Spine. 2002;96:145-156.
Treatment of these AVFs differs depending on their subtype, with embolization therapy being more indicated with increasing subtype.10,12,79 Regardless of the subtype of the lesion, the patency of the ASA must be maintained. In smaller lesions with smaller fistulas (type IV-A), the ASA is minimally dilated and there is slow flow through it. The emboli consequently cannot reach the fistula, and PVA embolization risks occluding the ASA. Treatment is usually surgical for these subtypes.22,24,44,57,78
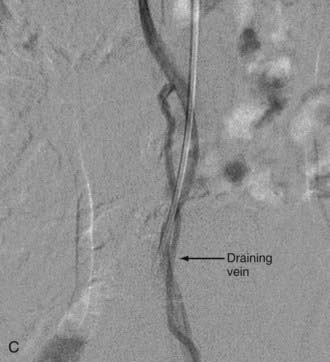
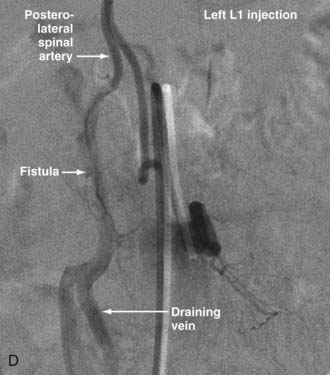
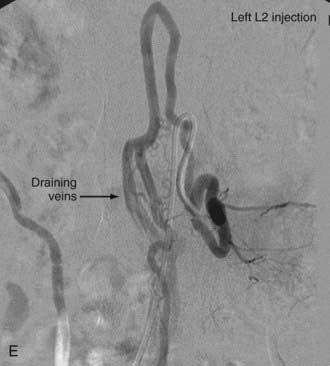
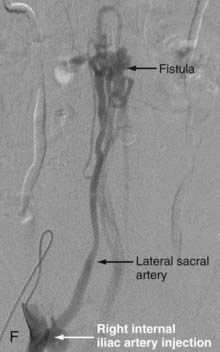
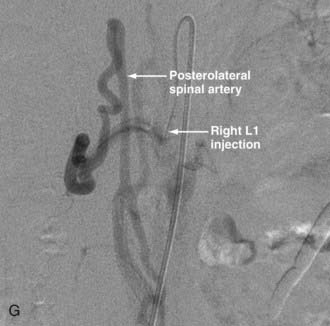
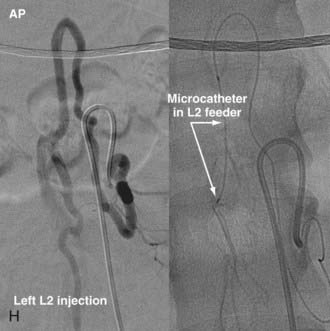
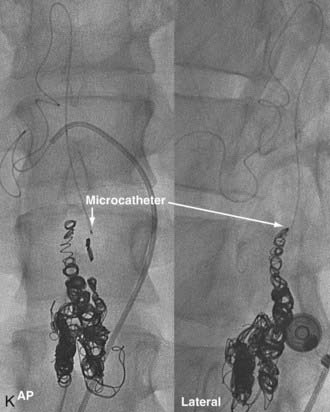
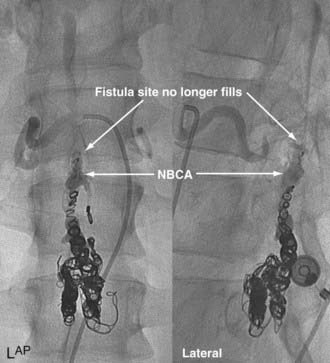
In type IV-B lesions, the size and flow of the fistula are increased and superselective catheterization is usually possible.10,22 Obliteration of the entire fistula is required; if only the feeder but not the fistula is embolized, the fistula will recruit collateral supply later. NBCA is the embolic agent of choice if the microcatheter can achieve a wedged-flow position.13 The fistula may be closed with coils. Embolization with PVA particles may facilitate subsequent surgical resection or stabilize the neurological deficits (Fig. 396-5).22
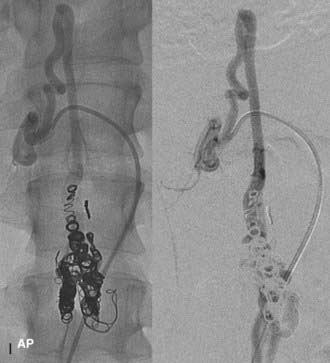
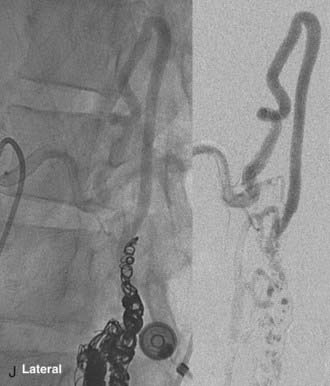
In type IV-C lesions, the shunt is largest and has an associated venous pouch at the fistula site.10,22 A transarterial or transvenous approach may be used to access the venous pouch. The venous pouch may be packed with coils, which act as a meshwork preventing venous embolization.10,13,22 The coiling may then be followed by transarterial injection of NBCA for permanent closure (Fig. 396-6).
Complications
The neurological and clinical complications of the endovascular treatment of spinal malformations include those that are direct consequences of the embolization technique, those that develop as a result of failure to obliterate the lesion initially, and those that arise as the lesion recanalizes. Periprocedural complications include neurological deterioration secondary to accidental occlusion of important branches or an extraordinary length of feeding arteries or occlusion of penetrating arteries to normal spinal cord parenchyma. These infarcts may arise from improper placement or accidental displacement of the catheter, incorrect particle size or choice of embolic material, overembolization of a nidus, unexpected reflux of liquid embolic material into the proximal artery or into newly opened unexpected collateral supply to the spinal cord, and fundamentally incomplete understanding of the lesion’s angioarchitecture.10 The adjunctive use of SEP and MEP monitoring has significantly decreased rates of neurological insult.28–30
Too distal occlusion of the venous drainage system of intradural dorsal AVFs aggravates the existing venous hypertension, thereby impeding blood flow to the surrounding cord, possibly increasing pressure at the fistula, and thus triggering a hemorrhage. Complications of embolizing intradural or extradural-intradural AVMs, as well as spinal aneurysms, relate to occlusion of normal feeding vessels and subsequent paralysis. The risk for permanent deficit is greater with occlusion of the artery of Adamkiewicz than with occlusion of cervical feeders because the former has fewer collateral feeders, as mentioned earlier. Incomplete obliteration of intradural ventral AVFs may result in later recurrence of the lesion because of recruitment of collateral arterial feeders, which may be more difficult to deal with at a later time. Recanalization of AVMs—with concomitant recurrent clinical decline—within 6 months of initial obliteration with PVA particles is well documented. Embolization with NBCA is associated with much lower rates of recanalization.10,12,22,31–33
Anson JA, Khayata MH, Merland JJ. Spinal arteriovenous malformations: Intravascular technique. In: Spetzler RF, Carter LP, Hamilton MG, editors. Neurovascular Surgery. New York: McGraw-Hill; 1994:1213-1227.
Anson JA, Spetzler RF. Classification of spinal arteriovenous malformations and implications for treatment. BNI Q. 1992;8:2-8.
Barrow DL, Colohan AR, Dawson R. Intradural perimedullary arteriovenous fistulas (type IV spinal cord arteriovenous malformations). J Neurosurg. 1994;81:221-229.
Berenstein A, Lasjaunias P. Spine and spinal cord vascular lesions. In: Berenstein A, Lasjaunias P, editors. Surgical Neuroangiography: Endovascular Treatment of the Spine and Spinal Cord Lesions. Berlin: Springer-Verlag, 1992.
Corkill RA, Mitsos AP, Molyneux AJ. Embolization of spinal intramedullary arteriovenous malformations using the liquid embolic agent, Onyx: a single-center experience in a series of 17 patients. J Neurosurg Spine. 2007;7:478-485.
Cullen S, Krings T, Ozanne A, et al. Diagnosis and endovascular treatment of pediatric spinal arteriovenous shunts. Neuroimaging Clin N Am. 2007;17:207-221.
Djindjian M, Djindjian R, Rey A, et al. Intradural extramedullary spinal arterio-venous malformations fed by the anterior spinal artery. Surg Neurol. 1977;8:85-93.
Gueguen B, Merland JJ, Riché MC, et al. Vascular malformations of the spinal cord: intrathecal perimedullary arteriovenous fistulas fed by medullary arteries. Neurology. 1987;37:969-979.
Heros RC, Debrun GM, Ojemann RG, et al. Direct spinal arteriovenous fistula: a new type of spinal AVM. Case report. J Neurosurg. 1986;64:134-139.
Hurst RW, Bagley LJ, Marcotte R, et al. Spinal cord arteriovenous fistulas involving the conus medullaris: presentation, management, and embryologic considerations. Surg Neurol. 1999;52:95-99.
Krings T, Lasjanias PL, Rodesch G, et al. Imaging in spinal vascular disease. Neuroimaging Clin N Am. 2007;17:57-72.
Lasjaunias PL, Berenstein A, Ter Brugge KG. Surgical Neuroangiography: Clinical Vascular Anatomy and Variations, Vol 1. New York: Springer. 2001.
McDougall CG, Deshmukh VR, Fiorella DJ, et al. Endovascular techniques for vascular malformations of the spinal axis. Neurosurg Clin N Am. 2005;16:395-410. x-xi
Mull M, Nijenhuis RJ, Backes WH, et al. Value and limitations of contrast-enhanced MR angiography in spinal arteriovenous malformations and dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2007;28:1249-1258.
Niimi Y, Berenstein A. Endovascular treatment of spinal vascular malformations. Neurosurg Clin N Am. 1999;10:47-71.
Niimi Y, Sala F, Deletis V, et al. Neurophysiologic monitoring and pharmacologic provocative testing for embolization of spinal cord arteriovenous malformations. AJNR Am J Neuroradiol. 2004;25:1131-1138.
Rosenwasser RH, Armonda R. Spinal vascular malformations: normal anatomy, diagnostic angiography, and angiographic classification. In: Barrow DL, Awad IA, editors. Spinal Vascular Malformations. Park Ridge, Ill: Thieme; 1999:23-36.
Si-Jia G, Meng-wei Z, Xi-ping L, et al. The clinical application studies of CT spinal angiography with 64-detector row spiral CT in diagnosing spinal vascular malformations. Eur J Radiol. 2009;71:22-28.
Spetzler RF, Detwiler PW, Riina HA, et al. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002;96(2 suppl):145-156.
Thron AK. Vascular Anatomy of the Spinal Cord. New York: Springer-Verlag; 1988. 114
Veznedaroglu E, Nelson PK, Jabbour PM, et al. Endovascular treatment of spinal cord arteriovenous malformations. Neurosurgery. 2006;59(5 suppl 3):S202-S209.
1 Akopov SE, Schievink WI. History of spinal cord vascular malformations and their treatment. Semin Cerebrovasc Dis Stroke. 2002;2:178-185.
2 Hebold O. Aneurysmen der kleinsten rucken marksgefasse. Arch Psychiatr Nervnkr. 1885;16:813-823.
3 Gaupp J. Hamorrhoiden der pia matter spinalis im gebiet des lendenmarks. Beith Pathol. 1888;2:516-518.
4 Elsberg CA. Diagnosis and Treatment of Surgical Diseases of the Spinal Cord and Its Membranes. Philadelphia: Saunders; 1916.
5 Djindjian R, Dumesnil M, Faure C, et al. Etude angiographique d’un angiome intra-rachidien. Rev Neurol. 1962;106:278-285.
6 Di Chiro G, Doppman J, Ommaya AK. Selective arteriography of arteriovenous aneurysms of spinal cord. Radiology. 1967;88:1065-1077.
7 Doppman J, Di Chiro G. Subtraction-angiography of spinal cord vascular malformations. Report of a case. J Neurosurg. 1965;23:440-443.
8 Doppman JL, Di Chiro G, Ommaya A. Obliteration of spinal-cord arteriovenous malformation by percutaneous embolisation. Lancet. 1968;1:477.
9 Doppman JL, Di Chiro G, Ommaya AK. Percutaneous embolization of spinal cord arteriovenous malformations. J Neurosurg. 1971;34:48-55.
10 Anson JA, Khayata MH, Merland JJ. Spinal arteriovenous malformations: Intravascular technique. In: Carter LP, Spetzler RF, Hamilton MG, editors. Neurovascular Surgery. New York: McGraw-Hill; 1994:1213-1227.
11 Krings T, Lasjanias PL, Rodesch G, et al. Imaging in spinal vascular disease. Neuroimaging Clin N Am. 2007;17:57-72.
12 McDougall CG, Deshmukh VR, Fiorella DJ, et al. Endovascular techniques for vascular malformations of the spinal axis. Neurosurg Clin N Am. 2005;16:395-410. x-xi
13 Veznedaroglu E, Nelson PK, Jabbour PM, et al. Endovascular treatment of spinal cord arteriovenous malformations. Neurosurgery. 2006;59(5 suppl 3):S202-S209.
14 Wells-Roth D, Zonenshayn M. Vascular anatomy of the spine. Oper Tech Neurosurg. 2003;6:116-121.
15 Lasjaunias PL, Berenstein A, Ter Brugge KG. Surgical Neuroangiography: Clinical Vascular Anatomy and Variations. New York: Springer; 2001.
16 Thron AK. Vascular Anatomy of the Spinal Cord. New York: Springer-Verlag; 1988. 114
17 Rosenwasser RH, Armonda R. Spinal vascular malformations: normal anatomy, diagnostic angiography, and angiographic classification. In: Barrow DL, Awad IA, editors. Spinal Vascular Malformations. Park Ridge, Ill: Thieme; 1999:23-36.
18 Caragine LP, Halbach VV, Ng PP, et al. Endovascular treatment of spinal cord vascular malformations. Semin Cerebrovasc Dis Stroke. 2002;2:236-244.
19 Mull M, Nijenhuis RJ, Backes WH, et al. Value and limitations of contrast-enhanced MR angiography in spinal arteriovenous malformations and dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2007;28:1249-1258.
20 Si-Jia G, Meng-wei Z, Xi-ping L, et al. The clinical application studies of CT spinal angiography with 64-detector row spiral CT in diagnosing spinal vascular malformations. Eur J Radiol. 2009;71:22-28.
21 Guillevin R, Ballee JN, Cormier E, et al. N-Butyl 2-cyanoacrylate embolization of spinal dural arteriovenous fistulae: CT evaluation, technical features, and outcome prognosis in 26 cases. AJNR Am J Neuroradiol. 2005;26:929-935.
22 Liu A, Gobin P, Riina H. Endovascular surgery for vascular malformations of the spinal cord. Oper Tech Neurosurg. 2003;6:163-170.
23 Schaat TJ, Salzman KL, Stevens EA. Sacral origin of a spinal dural arteriovenous fistula: case report and review. Spine. 2002;27:893-897.
24 Spetzler RF, Detwiler PW, Riina HA, et al. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002;96(2 suppl):145-156.
24a Gonzalez LF, Spetzler RF. Treatment of spinal vascular malformations. In: McKhann GM, editor. Clinical Neurosurgery: A Publication of the Congress of Neurological Surgeons. Philadelphia: Lippincott Williams & Wilkins; 2005:119-123.
25 Niimi Y, Berenstein A. Endovascular treatment of spinal vascular malformations. Neurosurg Clin N Am. 1999;10:47-71.
26 Berenstein A, Young W, Ransohoff J, et al. Somatosensory evoked potentials during spinal angiography and therapeutic transvascular embolization. J Neurosurg. 1984;60:777-785.
27 Linden D, Berlit P. Spinal arteriovenous malformations: clinical and neurophysiological findings. J Neurol. 1996;243:9-12.
28 Niimi Y, Sala F, Deletis V, et al. Neurophysiologic monitoring and pharmacologic provocative testing for embolization of spinal cord arteriovenous malformations. AJNR Am J Neuroradiol. 2004;25:1131-1138.
29 Sala F, Beltramello A, Gerosa M. Neuroprotective role of neurophysiological monitoring during endovascular procedures in the brain and spinal cord. Neurophysiol Clin. 2007;37:415-421.
30 Sala F, Niimi Y, Berenstein A, et al. Neuroprotective role of neurophysiological monitoring during endovascular procedures in the spinal cord. Ann N Y Acad Sci. 2001;939:126-136.
31 Howington JU, Kerber CW, Hopkins LN. Liquid embolic agents in the treatment of intracranial arteriovenous malformations. Neurosurg Clin N Am. 2005;16:355-363. ix-x
32 Phadke RV, Venkatesh SK, Kumar S, et al. Embolization of cranial/spinal tumours and vascular malformations with hydrogel microspheres. An experience of 69 cases. Acta Radiol. 2002;43:15-20.
33 Scialfa G, Sciotti G, Biondi A, et al. Embolization of vascular malformations of the spinal cord. J Neurosurg Sci. 1985;29:1-9.
34 Corkill RA, Mitsos AP, Molyneux AJ. Embolization of spinal intramedullary arteriovenous malformations using the liquid embolic agent, Onyx: a single-center experience in a series of 17 patients. J Neurosurg Spine. 2007;7:478-485.
35 Cullen S, Krings T, Ozanne A, et al. Diagnosis and endovascular treatment of pediatric spinal arteriovenous shunts. Neuroimaging Clin N Am. 2007;17:207-221.
36 Miyatake S, Kikuchi H, Koide T, et al. Cobb’s syndrome and its treatment with embolization. Case report. J Neurosurg. 1990;72:497-499.
37 Nagashima T, Tamaki N, Fujiwara F, et al. Endovascular and surgical treatment of a metameric spinal arteriovenous malformation. J Clin Neurosci. 1998;5(suppl):1-4.
38 Spetzler RF, Zabramski JM, Flom RA. Management of juvenile spinal AVM’s by embolization and operative excision. Case report. J Neurosurg. 1989;70:628-632.
39 Touho H, Karasawa J, Shishido H, et al. Successful excision of a juvenile-type spinal arteriovenous malformation following intraoperative embolization. Case report. J Neurosurg. 1991;75:647-651.
40 Berenstein A, Lasjaunias P. Spine and spinal cord vascular lesions. In: Berenstein A, Lasjaunias P, editors. Surgical Neuroangiography: Endovascular Treatment of the Spine and Spinal Cord Lesions. Berlin: Springer-Verlag, 1992.
41 Djindjian M, Djindjian R, Hurth M, et al. Steal phenomena in spinal arteriovenous malformations. J Neuroradiol. 1978;5:187-201.
42 Djindjian R. Embolization of angiomas of the spinal cord. Surg Neurol. 1975;4:411-420.
43 Connolly ESJr, Zubay GP, McCormick PC, et al. The posterior approach to a series of glomus (type II) intramedullary spinal cord arteriovenous malformations. Neurosurgery. 1998;42:774-785.
44 Kim LJ, Spetzler RF. Classification and surgical management of spinal arteriovenous lesions: arteriovenous fistulae and arteriovenous malformations. Neurosurgery. 2006;59(5 suppl 3):S195-S201.
45 Hurst RW, Bagley LJ, Marcotte R, et al. Spinal cord arteriovenous fistulas involving the conus medullaris: presentation, management, and embryologic considerations. Surg Neurol. 1999;52:95-99.
46 Pirouzmand F, Wallace MC, Willinsky R. Spinal epidural arteriovenous fistula with intramedullary reflux. Case report. J Neurosurg. 1997;87:633-635.
47 Tanaka K, Waga S, Kojuma T, et al. Spinal dural arteriovenous malformation: report of an unusual case. Neurosurgery. 1989;24:915-918.
48 Heier LA, Lee BC. A dural spinal arteriovenous malformation with epidural venous drainage: a case report. AJNR Am J Neuroradiol. 1987;8:561-563.
49 Afshar JK, Doppman JL, Oldfield EH. Surgical interruption of intradural draining vein as curative treatment of spinal dural arteriovenous fistulas. J Neurosurg. 1995;82:196-200.
50 Arnaud O, Bille F, Pouget J, et al. Epidural arteriovenous fistula with perimedullary venous drainage: case report. Neuroradiology. 1994;36:490-491.
51 Bradac GB, Simon RS, Schramm J. Cervical epidural AVM. Report of a case of uncommon location. Neuroradiology. 1977;14:97-100.
52 Willinsky R, terBrugge K, Montanera W, et al. Spinal epidural arteriovenous fistulas: arterial and venous approaches to embolization. AJNR Am J Neuroradiol. 1993;14:812-817.
53 Cahan LD, Higashida RT, Halbach VV, et al. Variants of radiculomeningeal vascular malformations of the spine. J Neurosurg. 1987;66:333-337.
54 Han SS, Love MB, Simeone FA. Diagnosis and treatment of a lumbar extradural arteriovenous malformation. AJNR Am J Neuroradiol. 1987;8:1129-1130.
55 Miyagi Y, Miyazono M, Kamikaseda K. Spinal epidural vascular malformation presenting in association with a spontaneously resolved acute epidural hematoma. Case report. J Neurosurg. 1998;88:909-911.
56 Asai J, Hayashi T, Fujimoto T, et al. Exclusively epidural arteriovenous fistula in the cervical spine with spinal cord symptoms: case report. Neurosurgery. 2001;48:1372-1375.
57 Anson JA, Spetzler RF. Classification of spinal arteriovenous malformations and implications for treatment. BNI Q. 1992;8:2-8.
58 Malis LI. Microsurgery for spinal cord arteriovenous malformations. Clin Neurosurg. 1979;26:543-555.
59 Krayenbuhl H, Yasargil MG, McClintock HG. Treatment of spinal cord vascular malformations by surgical excision. J Neurosurg. 1969;30:427-435.
60 Newman MJ. Racemose angioma of the spinal cord. QJM. 1959;28:97-108.
61 Aminoff MJ, Logue V. The prognosis of patients with spinal vascular malformations. Brain. 1974;97:211-218.
62 Aminoff MJ, Barnard RO, Logue V. The pathophysiology of spinal vascular malformations. J Neurol Sci. 1974;23:255-263.
63 Bederson JB, Spetzler RF. Pathophysiology of type I spinal dural arteriovenous malformations. BNI Q. 1996;12:23-92.
64 Hall WA, Oldfield EH, Doppman JL. Recanalization of spinal arteriovenous malformations following embolization. J Neurosurg. 1989;70:714-720.
65 Morgan MK, Marsh WR. Management of spinal dural arteriovenous malformations. J Neurosurg. 1989;70:832-836.
66 Nichols DA, Rufenacht DA, Jack CRJr, et al. Embolization of spinal dural arteriovenous fistula with polyvinyl alcohol particles: experience in 14 patients. AJNR Am J Neuroradiol. 1992;13:933-940.
67 Huffmann BC, Gilsbach JM, Thron A. Spinal dural arteriovenous fistulas: a plea for neurosurgical treatment. Acta Neurochir (Wien). 1995;135:44-51.
68 Lee TT, Gromelski EB, Bowen BC, et al. Diagnostic and surgical management of spinal dural arteriovenous fistulas. Neurosurgery. 1998;43:242-246.
69 Symon L, Kuyama H, Kendall B. Dural arteriovenous malformations of the spine. Clinical features and surgical results in 55 cases. J Neurosurg. 1984;60:238-247.
70 Eskandar EN, Borges LF, Budzik RFJr, et al. Spinal dural arteriovenous fistulas: experience with endovascular and surgical therapy. J Neurosurg. 2002;96(2 suppl):162-167.
71 Narvid J, Hetls SW, Larsen D, et al. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery. 2008;62:159-166.
72 Niimi Y, Berenstein A, Setton A, et al. Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery. 1997;40:675-682.
73 Djindjian M, Djindjian R, Rey A, et al. Intradural extramedullary spinal arterio-venous malformations fed by the anterior spinal artery. Surg Neurol. 1977;8:85-93.
74 Barrow DL, Colohan AR, Dawson R. Intradural perimedullary arteriovenous fistulas (type IV spinal cord arteriovenous malformations). J Neurosurg. 1994;81:221-229.
75 Benhaiem-Sigaux N, Zerah H, Gherardi R, et al. A retromedullary arteriovenous fistula associated with the Klippel-Trenaunay-Weber syndrome. A clinicopathologic study. Acta Neuropathol. 1985;66:318-324.
76 Riché MC, Modenesi-Freitas J, Djindjian M, et al. Arteriovenous malformations (AVM) of the spinal cord in children. A review of 38 cases. Neuroradiology. 1982;22:171-180.
77 Heros RC, Debrun GM, Ojemann RG, et al. Direct spinal arteriovenous fistula: a new type of spinal AVM. Case report. J Neurosurg. 1986;64:134-139.
78 Gueguen B, Merland JJ, Riché MC, et al. Vascular malformations of the spinal cord: intrathecal perimedullary arteriovenous fistulas fed by medullary arteries. Neurology. 1987;37:969-979.
79 Halbach VV, Higashida RT, Dowd CF, et al. Treatment of giant intradural (perimedullary) arteriovenous fistulas. Neurosurgery. 1993;33:972-979.