Chapter 20 Endovascular Treatment of Peripheral Artery Disease
The concept of nonsurgical catheter-based peripheral vascular revascularization was first described by Charles Dotter1 and further advanced with the development of balloon dilation catheters by Andreas Gruentzig.2 Catheter-based revascularization has largely replaced conventional open surgery as the treatment of first choice in selected patients treated for lower-extremity ischemia.3
No single specialty program (cardiology, radiology, or surgery) offered training that satisfied the entire skill set needed to perform peripheral endovascular intervention (Table 20-1). Recognition of this unmet need for a trained cadre of clinicians to care for patients with peripheral artery disease (PAD) prompted the development of a core cardiology training symposium (COCATS-11) to codify the necessary cardiology fellowship training.4
Table 20-1 Required Skill Elements for Optimal Peripheral Vascular Intervention
| Skill Element | Description |
|---|---|
| Cognitive | Extensive knowledge of vascular disease, including natural history, pathophysiology, diagnostic methods, and treatment alternatives |
| Technical | Competence in both diagnostic angiography and interventional techniques, such as use and selection of balloons, guidewires, stents, and emboli protection devices |
| Clinical | Ability to manage inpatients, interpret laboratory tests, obtain informed consent, assess risk/benefit ratio, and admitting privileges |
Patient and Lesion Selection Criteria
Indications
Anatomical and Functional Criteria
Patient selection for catheter-based vascular intervention depends upon both anatomical and functional criteria (Table 20-2). Anatomical lesion criteria include ability to gain vascular access, a reasonable likelihood of crossing the lesion with a guidewire, and the expectation that a therapeutic catheter can be advanced across the target lesion (Fig. 20-1). A strategy of “provisional” (bailout) stenting, or use of a stent for a failed balloon dilation attempt (in contrast to “primary” stenting, in which stents are placed with or without balloon predilation), has become the standard of practice for shorter, more discrete lesions. Longer lesions and occlusions are better treated with primary stent placement.3,5–8
Table 20-2 Classification of Peripheral Arterial Disease: Fontaine’s Stages and Rutherford’s Categories
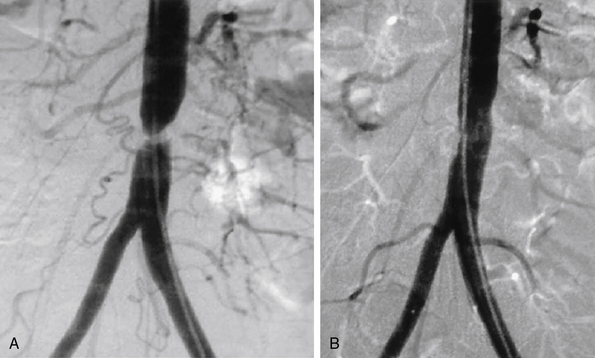
Figure 20-1 A, Tight stenosis of infrarenal aorta amenable to angioplasty. B, Final postangioplasty result.
Availability of endovascular stents (balloon expandable and self-expanding) has significantly extended the anatomical subset of patients who may be considered candidates for peripheral vascular intervention, particularly for longer stenotic lesions and occlusions. The rate-limiting step for nonsurgical revascularization of the aortoiliac vessels is the ability to pass a guidewire across the lesion. Regardless of the balloon dilation result, the option of stent placement offers a reliable and reproducible method to recanalize these large vessels.9
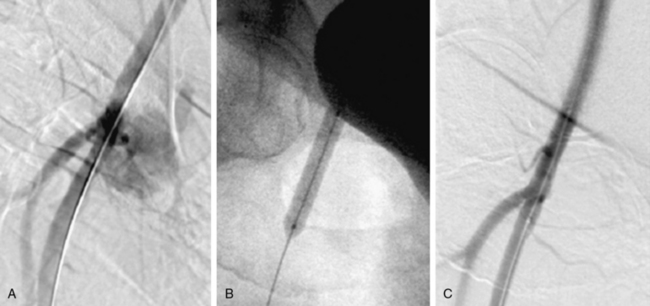
Vascular access site complications following catheter-based procedures often can be treated with percutaneous therapy10 (Fig. 20-2). Patients with hypotension and a high suspicion of bleeding after common femoral artery (CFA) access require urgent diagnostic angiography from the contralateral femoral artery to determine the bleeding site. Rapid identification of the bleeding site may provide an opportunity for lifesaving hemostasis with balloon tamponade.
Patients with CLI or limb-threatening ischemia (gangrene, nonhealing ulcer, or rest pain) are candidates for urgent revascularization. When considering a patient with CLI for revascularization, it is important to remember that multilevel disease (iliac, femoral, and tibial) is likely to be present and that simply improving “inflow” without addressing the more distal vascular lesions or runoff vessels may fail to solve the clinical problem. Patients with CLI (rest pain, nonhealing ulcers, or gangrene) typically have more extensive disease than claudicants and require urgent revascularization to prevent tissue loss3,11 (see Table 20-2).
Prognosis for patients presenting with CLI is poor.12 Those with tobacco abuse and/or diabetes are 10 times more likely to require amputation. Patients with CLI tend to be older, with almost 50% of patients older than 80 years undergoing amputations. Within 3 months of presentation, 12% will require an amputation, and 9% will die; 1-year mortality rate is 22%. Anatomy suitable for endovascular therapy is often present in one or more below-knee vessels. Therapy should be designed to restore pulsatile straight-line flow to the distal part of the limb, with as low a procedural morbidity as possible. The guiding principle is that less blood flow is required to maintain tissue integrity than to heal a wound, so restenosis does not usually result in recurrent CLI unless there has been repeated injury to the limb. Therefore, the emphasis is less on long-term vessel patency and more on amputation-free survival.
The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial was a multicenter randomized trial comparing an initial strategy of balloon angioplasty to open surgery in 452 patients with CLI.13 The primary outcome was time to amputation or death (amputation-free survival). After 6 months, the two treatment strategies did not differ significantly in amputation-free survival. There was no difference between the groups for quality-of-life outcomes, but for the first year of follow-up, costs associated with a surgery-first strategy were higher than for angioplasty. For this reason, the authors concluded that a percutaneous-first strategy was the treatment of choice in patients who are candidates for either surgery or endovascular intervention.
Technical and Procedural Considerations
Preprocedure
General Measures
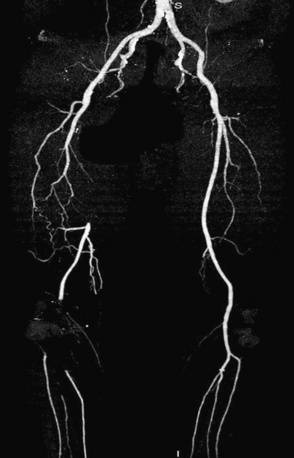
Prior to performing lower-extremity endovascular intervention, it is necessary to objectively determine the patient’s functional status. A history, physical examination, and appropriate noninvasive testing should be obtained prior to planning peripheral endovascular revascularization. If the patient is ambulatory, a rest and exercise ankle-brachial index (ABI) should be measured, and pulse volume recordings (PVR) should be performed. Other noninvasive modalities, such as vascular ultrasound, or alternative imaging modalities, such as magnetic resonance angiography (MRA) or computed tomographic angiography (CTA), may be helpful to resolve conflicting data and are used at the discretion of the physician (Fig. 20-3). When planning lower-extremity revascularization, status of the inflow and outflow vessels relative to the target lesion must be visualized angiographically. This is usually done with invasive diagnostic angiography, but in selected patients, MRA or CTA may be very useful.
Equipment Choices
Stents
The two categories of stents are balloon expandable and self-expanding. Both types may be covered with material. Balloon expandable stents are intended for use within the axial skeleton to protect them from external compression. This generally limits their use to the iliac arteries, but coronary balloon expandable stents are used to salvage failed angioplasty results in below-knee vessels.14,15
Balloon expandable stents can be deployed with more precision than self-expanding stents, although there is some shortening associated with their expansion. Self-expanding stents resist permanent deformation and are elastic. Their flexible nature allows them to delivered in longer lengths, and they will fit themselves to a tapering artery. Self-expanding stents may be made of nitinol or a stainless steel alloy. At this time, there is no evidence that either material is associated with any safety or efficacy advantage.16,17
Clinical Outcomes
Aortoiliac Vessels
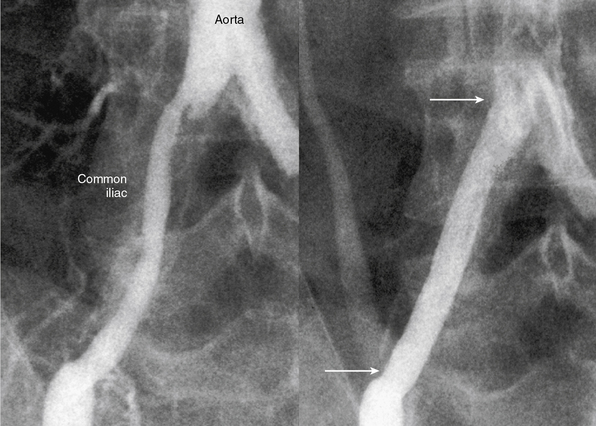
The current best practice, in experienced hands, for aorto-iliac lesions favors an endovascular strategy (Fig. 20-4). This recommendation is based upon the morbidity and mortality associated with major vascular surgery in patients with significant comorbidity, and the excellent outcomes available with current endovascular techniques. In a large single-center registry of 505 iliac stent procedures, the technical success rate was 98%, 8-year primary stent patency rate was 74%, and secondary patency rate was 84%.18 In a 10-year iliac stent patency study, there was no effect of age, diabetes, tobacco smoking, or hypertension on patency.19 Common iliac artery (CIA) lesions had greater long-term patency than external iliac artery (EIA) lesions. Outcomes from another series of 89 consecutive patients with symptomatic occluded iliac arteries demonstrated a 92% success rate for endovascular treatment.20 Increasing severity and complexity of lesions did not significantly alter iliac artery patency rates.
An observational study compared nonrandomized results of iliac artery stenting with surgery in patients with moderately complex lesions.21 There was no difference regarding limb salvage or patient survival out to 5 years, but vessel patency was reduced in limbs treated with stents compared to surgery. A nonrandomized retrospective comparison of endovascular intervention compared to open surgery for complex aortoiliac occlusive lesions found a shorter hospital stay, fewer postprocedural complications, and lower primary patency rates but equivalent secondary patency rates for the endovascular arm.22
There is debate regarding the most efficacious method of endovascular therapy between “provisional stent placement,” which is selective use of stents only when balloon dilation has failed or is suboptimal, and “primary stent placement,” which is the practice of deploying a stent regardless of the balloon result. The Dutch Iliac Stent Trial demonstrated that selective iliac artery stenting achieved an equivalent hemodynamic result compared to primary stenting. Translesional pressure gradients after primary stent placement (5.8 ± 4.7 mmHg) were significantly lower than after balloon angioplasty alone (8.9 ± 6.8 mmHg), but not after provisional stenting (5.9 ± 3.6 mmHg) in the percutaneous transluminal angioplasty (PTA) group.23 The procedural success rate, defined as a postprocedural gradient less than 10 mmHg, revealed no difference between the two treatment strategies, (primary stenting = 81% vs. PTA plus provisional stenting = 89%). By employing a provisional stenting strategy in the iliac artery, stent placement was avoided in 63% of lesions. After 5 years of follow-up, the selective stent placement strategy had greater symptomatic improvement compared with primary stent placement, but there was no difference in patency rates, ABI, and quality of life between groups.24,25
Preferred clinical practice, however, is primary stent placement, and this is supported by a meta-analysis which looked at more than 2000 patients.26 Procedural success was higher in the primary stent group, and there was a 43% reduction in 4-year failures for aortoiliac stent placement compared to balloon angioplasty alone. Advantages of primary stent placement include efficient and reliable vascular reconstruction, minimizing concern over abrupt occlusion. Direct stenting minimizes the technical challenges of determining translesional pressure gradients and the need to administer vasodilator medications. The current American College of Cardiology/American Heart Association (ACC/AHA) guideline document supports primary stenting of the common and EIAs with a class I recommendation (Level of Evidence B).3
Femoral-Popliteal Vessels
Angioplasty and Stent Placement
A meta-analysis comparing angioplasty to exercise therapy in patients with intermittent claudication reported that ABI improved more with endovascular therapy than with exercise, but quality-of-life outcomes at 3 and 6 months were similar.27 A cost-effectiveness study suggested that cost-effectiveness for quality-adjusted life-year was greater with percutaneous therapy than exercise alone.28
A matched cohort study of 526 patients with intermittent claudication found significant advantages for a revascularization strategy (surgery or PTA) compared to medical therapy.29 Revascularization was more effective than medical therapy for improvement in physical function, bodily pain, and walking distance. Patients with the greatest improvement in their ABI results had the most clinical improvement.
A recently published trial from the United Kingdom randomized 178 patients with claudication into three groups: supervised exercise, balloon angioplasty, and both if they had suitable lesions for angioplasty of the femoral-popliteal arteries.30 The study demonstrated that combining supervised exercise with angioplasty produced superior clinical outcomes, but that there was no significant difference among the three groups for quality-of-life outcomes. Two main limitations of this trial were use of balloon-only treatment and the fact that the exercise time was capped at 207 meters, which may have underestimated the degree of improvement from revascularization (Fig. 20-5).
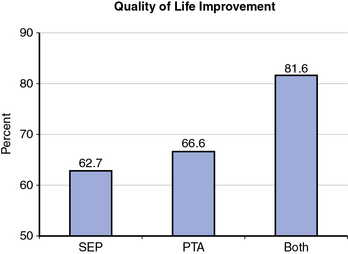
(From Mazari FA, Gulati S, Rahman MN, et al: Early outcomes from a randomized, controlled trial of supervised exercise, angioplasty, and combined therapy in intermittent claudication. Ann Vasc Surg 24:69–79, 2010.)30
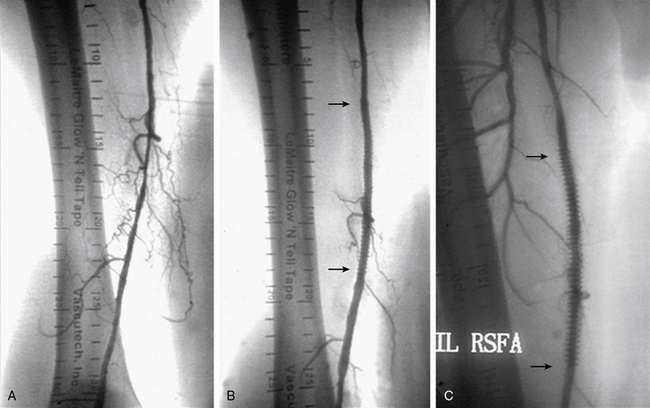
Clinical success in patients with femoral artery lesions depends upon a durable long-lasting procedure. A meta-analysis demonstrated superior patency at 3 years for stents compared to PTA in the most severely affected patients, who were those with occlusions and CLI.31 There have been three randomized controlled trials comparing primary stenting to balloon angioplasty with bailout stenting (provisional stenting).6–8 Lesion length and complexity accounted for restenosis rates for balloon angioplasty, but not for stent placement (Fig. 20-6). Synthesizing these results, the data suggest that longer femoral-popliteal lesions (≥ 7 cm) are better approached with a strategy of primary stenting, whereas more discrete lesions (< 4 cm) do well with a provisional stenting strategy in which balloon angioplasty is given an opportunity to stand alone (Table 20-3).
Table 20-3 Comparison of Primary and Provisional Femoral-Popliteal Stenting
| Schillinger | Krankenberg | |
|---|---|---|
| Stent | Guidant | Bard |
| Lesion length | 101 mm | 45 mm |
| Stent restenosis | 36.7% | 31.7% |
| PTA restenosis | 63.5% | 38.6%* |
PTA, percutaneous transluminal angioplasty.
From Schillinger M, Sabeti S, Loewe C, et al: Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 354:1879–1888, 2006; and Krankenberg H, Schluter M, Steinkamp HJ, et al: Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the Femoral Artery Stenting Trial (FAST). Circulation 116:285–292, 2007.5,6
Stent fractures have been associated with restenosis of femoral artery lesions.32 There are differences regarding stent fracture among femoral artery stents, presumably related to their composition and architecture. Fracture rates are 28% for the SMART stent (Cordis, Miami Lakes, Fla.), 19% for the Wallstent (Boston Scientific, Natick, Mass.), and 2% for the Dynalink/Absolute (Abbott Vascular, Santa Clara, Calif.).33
Covered Stents
Covered stents are used effectively to treat vascular perforations and exclude aneurysms in the peripheral vascular circulation. The Viabahn stent graft (W.L. Gore, Flagstaff, Ariz.) has a 1-year femoral artery patency rate of 62%. In one study, patients randomized to the Viabahn stent graft had about twice as many major adverse events (8.2%) as patients randomized to PTA (4.0%). The theoretical benefit for the Viabahn stent graft is that ingrowth of tissue between the stent struts, which plagues femoral stents, is prevented. However, edge restenosis may not be avoided, and concerns about stent thrombosis must be addressed. In one study of 60 limbs treated with a covered stent, two patients had major procedural complications requiring surgical correction. Thrombotic occlusion of the covered stent occurred in 10% of patients within 30 days, and the 1-year primary patency rate was 67%.34 Late occlusion was presumed to represent edge restenosis and thrombosis. In another study, 86 patients were randomized to bypass surgery or endovascular therapy with a covered stent graft. No significant difference was observed for primary or secondary patency, but there was a trend favoring surgery for more complex lesions.35,36
Drug-Eluting Balloons and Stents
A clinical trial of a small number of patients randomized to either a bare metal self-expanding stent or a drug-eluting (sirolimus) self-expanding stent (Smart, Cordis, Miami Lakes, Fla.) found no difference in severity of restenosis.17,37 A recently completed Cook Zilver PTX trial randomized 420 patients has not reported results; however, the registry data have shown some promise.38 There have been two small randomized trials of drug-coated (paclitaxel) balloons for femoral disease that have shown positive results.39,40 These trials found that lumen loss, restenosis, and target lesion revascularization were less after treatment with coated balloons than with uncoated balloons (Fig. 20-7). Reasons for failure of drug-eluting stents (DES) and early success for balloons in the femoral artery may be related to stent fracture and the ongoing irritant related to the metal stent against the vessel wall.41
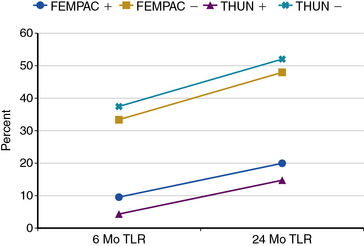
TLR, target lesion revascularization.
(From Tepe G, Zeller T, Albrecht T, et al: Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 358:689–699, 2008; and Werk M, Langner S, Reinkensmeier B, et al: Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: Femoral Paclitaxel Randomized Pilot Trial. Circulation 118:1358–1365, 2008.)39,40
Brachytherapy
Adjunctive endovascular brachytherapy (EBT) with an iridium-192 source, with a prescribed dose of 12 to 14 Gy, compared to PTA alone for treatment of de novo long-segment stenoses of the femoral artery has a delaying effect on the occurrence of restenosis.42,43 In one study, there appeared to be an early restenosis benefit for the EBT plus PTA group; however, at 5-year follow-up, there was “catch-up,” and the recurrence rate was equal (72.5%) in both groups.43 Brachytherapy has greater efficacy in restenotic lesions compared with de novo lesions.44,45 A novel approach has been to deliver external beam irradiation to de novo femoral artery lesions after PTA. At the 1-year follow-up of one trial, there was a significant benefit for patients treated with 14 Gy in a single treatment session, compared with a control group and a group who received lower Gy doses.46
Atherectomy
There had been expectation that by “debulking” atherosclerotic plaque, primary patency of femoral artery interventions could be improved.47 Yet, successive generations of atherectomy catheters have failed to demonstrate any benefit over less-expensive conventional therapies.48,49 Comparative studies are lacking, and the data supporting the use of devices are derived from self-reported registries and subject to bias. Also, there are safety concerns regarding the incidence of distal embolization and perforation.50–52
Laser-Assisted Angioplasty
Laser-assisted angioplasty does not appear to add any benefit to conventional endovascular therapy for peripheral arterial recanalization.53,54 The Peripheral Excimer Laser Angioplasty (PELA) trial randomized 251 patients to either PTA or laser-assisted PTA in patients with claudication and a total femoral occlusion. There was no difference in clinical events or patency rates at 1 year of follow-up.
Cutting Balloon
The cutting balloon is used in coronary arteries for “undilatable” lesions. There is limited evidence that would support extending the indications for this device beyond peripheral arteries,55 and there is no evidence that a cutting balloon is an efficacious treatment for in-stent restenosis.
Cryoplasty
Clinical trials have failed to demonstrate any advantage for cryoplasty over conventional angioplasty in peripheral arterial intervention.56 In a diabetic population with femoral-popliteal artery lesions, cryoplasty was associated with lower primary patency rates and more clinically driven repeat procedures after long-term follow-up than conventional balloon angioplasty.57
Infrapopliteal Intervention
Tibioperoneal Angioplasty
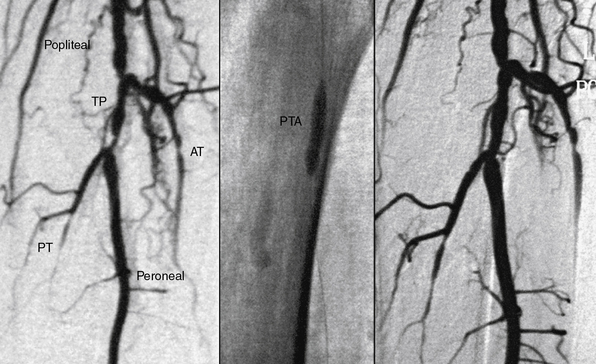
Below-knee angioplasty has generally been reserved for treatment of CLI and threatened limbs because of the technical difficulty of using conventional peripheral angioplasty equipment in these vessels and the fear of limb loss should a complication occur (Fig. 20-8). Experience with PTA demonstrated the feasibility of a percutaneous approach with procedural success rates better than 80% for tibioperoneal angioplasty.58,59 Replacement of bulkier 0.035-inch equipment with the 0.014-inch devices used in coronary angioplasty improved results of below-knee intervention. In 111 patients treated with tibioperoneal angioplasty for treatment of claudication, tissue loss, or rest pain, Dorros et al.60 reported a primary success rate of 90% for all lesions, including a 99% success rate for stenoses and a 65% success rate for occlusions. At the time of hospital discharge, 95% of patients were symptomatically improved.
Two clinical trials have demonstrated the efficacy and attractiveness of an initial percutaneous approach to selected patients with CLI and below-knee vascular disease.58,61 The limb salvage rate in these patients treated with PTA ranged from 85% to 91% after 2 to 5 years. This evidence supports the contention that angioplasty of the tibioperoneal vessels should not necessarily be reserved for limb salvage situations. However, caution is still advised in patient selection, since the surgical options are limited if angioplasty fails.
Optimal treatment of infrapopliteal disease requires appropriate patient and lesion selection for treatment. Focal stenoses have the best outcomes, and vessels with fewer than five separate lesions are associated with a higher success rate. Anatomically, the goal is to open as many tibial artery stenoses as possible to increase the degree of revascularization and improve clinical outcomes.62 Treatment success is measured by relief of rest pain, healing of ulcers, and avoiding amputation, not necessarily by long-term vessel patency. When trying to heal ischemic ulcers, the basic principle is that it takes more oxygenated blood flow to heal a wound than to maintain tissue integrity.58 Percutaneous therapy can result in long-term limb salvage in more than 80% of patients and should be considered the current standard of treatment in patients with limb-threatening ischemia who are candidates for endovascular intervention.
Tibioperoneal Stents
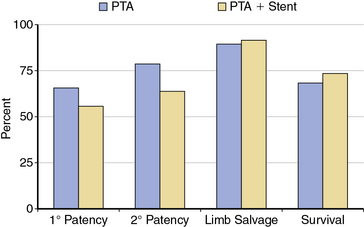
The role of provisional versus primary stent placement in tibial arteries is unsettled.63 A recent small randomized trial of PTA compared to PTA with a bare metal stent (BMS) demonstrated no significant differences for 1-year outcomes, including patency rates, limb salvage, or survival64 (Fig. 20-9). Preliminary results of the use of balloon expandable coronary DES in tibial vessels have been reported.14,15,65,66 Smaller series have shown excellent patency when comparing below-knee BMS to DES.67,68 The largest published series is a nonrandomized report of 106 patients (118 limbs) with CLI treated with below-knee DES.15 The 3-year cumulative incidence of amputation was only 6%, and amputation-free survival was 68%.
Angiogenesis
Not infrequently, the severity of infrapopliteal disease abolishes most if not all of the named vasculature, and percutaneous mechanical revascularization is not possible. Therapeutic angiogenesis using growth factors as agents (e.g., vascular endothelial growth factor [VEGF], fibroblast growth factor [FGF]), genes, and cellular therapies have been proposed as a means of maintaining limb viability.69–71 At present, there have been no clinical breakthroughs, but much ongoing basic science activity appears to be promising.72
1 Dotter C.T., Judkins M.P. Transluminal treatment of arteriosclerotic obstruction. Description of a new technique and a preliminary report of its application. Circulation. 1964;30:654–670.
2 Gruntzig A., Hopff H. Percutaneous recanalization after chronic arterial occlusion with new dilator-catheter (modification of the Dotter technique) (author’s transl). Dtsch Med Wochenschr. 1974;99:2502–2510.
3 Hirsch A.T., Haskal Z.J., Hertzer N.R., et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary of a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–1312.
4 Creager M.A., Cooke J.P., Olin J.W., et al. Task force 11: training in vascular medicine and peripheral vascular catheter-based interventions endorsed by the Society for Cardiovascular Angiography and Interventions and the Society for Vascular Medicine. J Am Coll Cardiol. 2008;51:398–404.
5 AbuRahma A.F., Hayes J.D., Flaherty S.K., et al. Primary iliac stenting versus transluminal angioplasty with selective stenting. J Vasc Surg. 2007;46:965–970.
6 Schillinger M., Sabeti S., Loewe C., et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888.
7 Krankenberg H., Schluter M., Steinkamp H.J., et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: The Femoral Artery Stenting Trial (FAST). Circulation. 2007;116:285–292.
8 Laird J.R., Katzen B.T., Scheinert D., et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv. 2010;3:267–276.
9 Scheinert D., Schroder M., Ludwig J., et al. Stent-supported recanalization of chronic iliac artery occlusions. Am J Med. 2001;110:708–715.
10 Samal A.K., White C.J. Percutaneous management of access site complications. Catheter Cardiovasc Interv. 2002;57:12–23.
11 Norgren L., Hiatt W.R., Dormandy J.A., et al. Inter-Society Consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–S67.
12 Jamsen T., Manninen H., Tulla H., et al. The final outcome of primary infrainguinal percutaneous transluminal angioplasty in 100 consecutive patients with chronic critical limb ischemia. J Vasc Interv Radiol. 2002;13:455–463.
13 Adam D.J., Beard J.D., Cleveland T., et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934.
14 Grant A.G., White C.J., Collins T.J., et al. Infrapopliteal drug-eluting stents for chronic limb ischemia. Catheter Cardiovasc Interv. 2008;71:108–111.
15 Feiring A.J., Krahn M., Nelson L., et al. Preventing leg amputations in critical limb ischemia with below-the-knee drug-eluting stents: the PaRADISE (PReventing Amputations using Drug eluting StEnts) trial. J Am Coll Cardiol. 2010;55:1580–1589.
16 Ponec D., Jaff M.R., Swischuk J., et al. The Nitinol SMART stent vs. Wallstent for suboptimal iliac artery angioplasty: CRISP-US trial results. J Vasc Interv Radiol. 2004;15:911–918.
17 Duda S.H., Bosiers M., Lammer J., et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther. 2006;13:701–710.
18 Murphy T.P., Ariaratnam N.S., Carney W.I.Jr., et al. Aortoiliac insufficiency: long-term experience with stent placement for treatment. Radiology. 2004;231:243–249.
19 Park K.B., Do Y.S., Kim J.H., et al. Stent placement for chronic iliac arterial occlusive disease: the results of 10 years experience in a single institution. Korean J Radiol. 2005;6:256–266.
20 Leville C.D., Kashyap V.S., Clair D.G., et al. Endovascular management of iliac artery occlusions: extending treatment to TransAtlantic Inter-Society Consensus class C and D patients. J Vasc Surg. 2006;43:32–39.
21 Timaran C.H., Prault T.L., Stevens S.L., et al. Iliac artery stenting versus surgical reconstruction for TASC (TransAtlantic Inter-Society Consensus) type B and type C iliac lesions. J Vasc Surg. 2003;38:272–278.
22 Hans S.S., DeSantis D., Siddiqui R., et al. Results of endovascular therapy and aortobifemoral grafting for Transatlantic Inter-Society type C and D aortoiliac occlusive disease. Surgery. 2008;144:583–589. discussion 589–590
23 Tetteroo E., Haaring C., van der Graaf Y., et al. Intraarterial pressure gradients after randomized angioplasty or stenting of iliac artery lesions. Dutch Iliac Stent Trial Study Group. Cardiovasc Intervent Radiol. 1996;19:411–417.
24 Klein W.M., van der Graaf Y., Seegers J., et al. Long-term cardiovascular morbidity, mortality, and reintervention after endovascular treatment in patients with iliac artery disease: the Dutch Iliac Stent Trial Study. Radiology. 2004;232:491–498.
25 Klein W.M., van der Graaf Y., Seegers J., et al. Dutch iliac stent trial: long-term results in patients randomized for primary or selective stent placement. Radiology. 2006;238:734–744.
26 Bosch J.L., Hunink M.G. Meta-analysis of the results of percutaneous transluminal angioplasty and stent placement for aortoiliac occlusive disease. Radiology. 1997;204:87–96.
27 Spronk S., Bosch J.L., Veen H.F., et al. Intermittent claudication: functional capacity and quality of life after exercise training or percutaneous transluminal angioplasty–systematic review. Radiology. 2005;235:833–842.
28 de Vries S.O., Visser K., de Vries J.A., et al. Intermittent claudication: cost-effectiveness of revascularization versus exercise therapy. Radiology. 2002;222:25–36.
29 Feinglass J., McCarthy W.J., Slavensky R., et al. Functional status and walking ability after lower extremity bypass grafting or angioplasty for intermittent claudication: results from a prospective outcomes study. J Vasc Surg. 2000;31:93–103.
30 Mazari F.AK., Gulati S., Rahman M.NA., et al. Early outcomes from a randomized, controlled trial of supervised exercise, angioplasty, and combined therapy in intermittent claudication. Ann Vasc Surg. 2010;24:69–79.
31 Muradin G.S., Bosch J.L., Stijnen T., et al. Balloon dilation and stent implantation for treatment of femoropopliteal arterial disease: meta-analysis. Radiology. 2001;221:137–145.
32 Scheinert D., Scheinert S., Sax J., et al. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol. 2005;45:312–315.
33 Schlager O., Dick P., Sabeti S., et al. Long-segment SFA stenting–the dark sides: in-stent restenosis, clinical deterioration, and stent fractures. J Endovasc Ther. 2005;12:676–684.
34 Fischer M., Schwabe C., Schulte K.L. Value of the Hemobahn/Viabahn endoprosthesis in the treatment of long chronic lesions of the superficial femoral artery: 6 years of experience. J Endovasc Ther. 2006;13:281–290.
35 McQuade K., Gable D., Hohman S., et al. Randomized comparison of ePTFE/nitinol self-expanding stent graft vs. prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive disease. J Vasc Surg. 2009;49:109–115. 116 e1–e9, discussion 116
36 McQuade K., Gable D., Pearl G., et al. Four-year randomized prospective comparison of percutaneous ePTFE/nitinol self-expanding stent graft versus prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive disease. J Vasc Surg. 2010;52:584–590.
37 Duda S.H., Pusich B., Richter G., et al. Sirolimus-eluting stents for the treatment of obstructive superficial femoral artery disease: six-month results. Circulation. 2002;106:1505–1509.
38 Dake M.D., Van Alstine W.G., Zhou Q., et al. Polymer-free paclitaxel-coated Zilver PTX Stents–evaluation of pharmacokinetics and comparative safety in porcine arteries. J Vasc Interv Radiol. 2011;22:603–610.
39 Tepe G., Zeller T., Albrecht T., et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:689–699.
40 Werk M., Langner S., Reinkensmeier B., et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation. 2008;118:1358–1365.
41 Gray W.A., Granada J.F. Drug-coated balloons for the prevention of vascular restenosis. Circulation. 2010;121:2672–2680.
42 Diehm N., Silvestro A., Do D.D., et al. Endovascular brachytherapy after femoropopliteal balloon angioplasty fails to show robust clinical benefit over time. J Endovasc Ther. 2005;12:723–730.
43 Wolfram R.M., Budinsky A.C., Pokrajac B., et al. Endovascular brachytherapy for prophylaxis of restenosis after femoropopliteal angioplasty: five-year follow-up–prospective randomized study. Radiology. 2006;240:878–884.
44 Wolfram R.M., Budinsky A.C., Pokrajac B., et al. Endovascular brachytherapy: restenosis in de novo versus recurrent lesions of femoropopliteal artery–the Vienna experience. Radiology. 2005;236:338–342.
45 Pokrajac B., Kirisits C., Rainer S., et al. Beta endovascular brachytherapy using CO2-filled centering catheter for treatment of recurrent superficial femoropopliteal artery disease. Cardiovasc Revasc Med. 2009;10:162–165.
46 Therasse E., Donath D., Lesperance J., et al. External beam radiation to prevent restenosis after superficial femoral artery balloon angioplasty. Circulation. 2005;111:3310–3315.
47 Reekers J. Challenging a myth: directional atherectomy. Cardiovasc Intervent Radiol. 2009;32:203–204.
48 Vroegindeweij D., Tielbeek A.V., Buth J., et al. Directional atherectomy versus balloon angioplasty in segmental femoropopliteal artery disease: two-year follow-up with color-flow duplex scanning. J Vasc Surg. 1995;21:255–268. discussion 268–9
49 Indes J.E., Shah H.J., Jonker F.HW., et al. Subintimal angioplasty is superior to Silverhawk atherectomy for the treatment of occlusive lesions of the lower extremities. J Endovasc Ther. 2010;17:243–250.
50 Zeller T., Rastan A., Schwarzwalder U., et al. Percutaneous peripheral atherectomy of femoropopliteal stenoses using a new-generation device: six-month results from a single-center experience. J Endovasc Ther. 2004;11:676–685.
51 Suri R., Wholey M.H., Postoak D., et al. Distal embolic protection during femoropopliteal atherectomy. Catheter Cardiovasc Interv. 2006;67:417–422.
52 Zeller T., Rastan A., Sixt S., et al. Long-term results after directional atherectomy of femoro-popliteal lesions. J Am Coll Cardiol. 2006;48:1573–1578.
53 Scheinert D., Laird J.R.Jr., Schroder M., et al. Excimer laser-assisted recanalization of long, chronic superficial femoral artery occlusions. J Endovasc Ther. 2001;8:156–166.
54 Steinkamp H.J., Rademaker J., Wissgott C., et al. Percutaneous transluminal laser angioplasty versus balloon dilation for treatment of popliteal artery occlusions. J Endovasc Ther. 2002;9:882–888.
55 Engelke C., Sandhu C., Morgan R.A., et al. Using 6-mm cutting balloon angioplasty in patients with resistant peripheral artery stenosis: preliminary results. AJR Am J Roentgenol. 2002;179:619–623.
56 Wildgruber M., Berger H. Cryoplasty for the prevention of arterial restenosis. Cardiovasc Intervent Radiol. 2008;31:1050–1058.
57 Spiliopoulos S., Katsanos K., Karnabatidis D., et al. Cryoplasty versus conventional balloon angioplasty of the femoropopliteal artery in diabetic patients: long-term results from a prospective randomized single-center controlled trial. Cardiovasc Intervent Radiol. 33, 2010. 929–938
58 Dorros G., Jaff M.R., Dorros A.M., et al. Tibioperoneal (outflow lesion) angioplasty can be used as primary treatment in 235 patients with critical limb ischemia: five-year follow-up. Circulation. 2001;104:2057–2062.
59 Krankenberg H., Sorge I., Zeller T., et al. Percutaneous transluminal angioplasty of infrapopliteal arteries in patients with intermittent claudication: acute and one-year results. Catheter Cardiovasc Interv. 2005;64:12–17.
60 Dorros G., Lewin R.F., Jamnadas P., et al. Below-the-knee angioplasty: tibioperoneal vessels, the acute outcome. Cathet Cardiovasc Diagn. 1990;19:170–178.
61 Soder H.K., Manninen H.I., Jaakkola P., et al. Prospective trial of infrapopliteal artery balloon angioplasty for critical limb ischemia: angiographic and clinical results. J Vasc Interv Radiol. 2000;11:1021–1031.
62 Peregrin J., Kožnar B., Kováč J., et al. PTA of infrapopliteal arteries: long-term clinical follow-up and analysis of factors influencing clinical outcome. Cardiovasc Intervent Radiol. 2010;33:720–725.
63 Feiring A.J., Wesolowski A.A., Lade S. Primary stent-supported angioplasty for treatment of below-knee critical limb ischemia and severe claudication: early and one-year outcomes. J Am Coll Cardiol. 2004;44:2307–2314.
64 Randon C., Jacobs B., De Ryck F., et al. Angioplasty or primary stenting for infrapopliteal lesions: results of a prospective randomized trial. Cardiovasc Intervent Radiol. 2010;33:260–269.
65 Siablis D., Karnabatidis D., Katsanos K., et al. Infrapopliteal application of sirolimus-eluting versus bare metal stents for critical limb ischemia: analysis of long-term angiographic and clinical outcome. J Vasc Interv Radiol. 2009;20:1141–1150.
66 Biondi-Zoccai G.G., Sangiorgi G., Lotrionte M., et al. Infragenicular stent implantation for below-the-knee atherosclerotic disease: clinical evidence from an international collaborative meta-analysis on 640 patients. J Endovasc Ther. 2009;16:251–260.
67 Scheinert D., Ulrich M., Scheinert S., et al. Comparison of sirolimus-eluting vs. bare-metal stents for the treatment of infrapopliteal obstructions. EuroIntervention. 2006;2:169–174.
68 Siablis D., Kraniotis P., Karnabatidis D., et al. Sirolimus-eluting versus bare stents for bailout after suboptimal infrapopliteal angioplasty for critical limb ischemia: 6-month angiographic results from a nonrandomized prospective single-center study. J Endovasc Ther. 2005;12:685–695.
69 Lederman R.J., Mendelsohn F.O., Anderson R.D., et al. Therapeutic Angiogenesis with Recombinant Fibroblast Growth Factor-2 for Intermittent Claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–2058.
70 Rajagopalan S., Mohler E.R.3rd, Lederman R.J., et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938.
71 Henry T.D., Annex B.H., McKendall G.R., et al. The VIVA Trial: Vascular Endothelial Growth Factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365.
72 Tongers J., Roncalli J.G., Losordo D.W. Therapeutic angiogenesis for critical limb ischemia: microvascular therapies coming of age. Circulation. 2008;118:9–16.