Chapter 87 Endovascular Treatment of Intracranial Occlusive Disease
Intracranial Atherosclerotic Disease
Approximately 8% to 10% of ischemic strokes are attributable to intracranial atherosclerosis.1,2 In the United States, it is estimated that 40,000 to 60,000 new strokes per year are due to intracranial atherosclerosis.3 The most common intracranial location for stenosis is the middle cerebral artery (MCA) (33.9%), followed by the internal carotid artery (ICA) (20.3%), basilar artery (20.3%), vertebral arteries (VAs) (19.6%), and a combination of these arteries (5.9%).4 Ischemic symptoms due to intracranial stenosis are believed to arise from (1) hypoperfusion5; (2) thrombosis at the site of stenosis due to plaque rupture, hemorrhage within the plaque, or occlusive growth of the plaque5; (3) thromboembolism distal to the stenosis; and (4) occlusion of small perforating arteries at the site of the plaque.6,7
Risk Factors
African Americans with transient ischemic attack (TIA) or stroke are more likely than Caucasian Americans to have intracranial stenosis, whereas the latter are more likely to have extracranial carotid atherosclerotic stenosis.2 Asian Americans have a higher proportion of MCA stenosis compared with Caucasian and African Americans.6 Hypertension is present in up to 75% of individuals with intracranial atherosclerosis.8 Diabetes, coronary artery disease, cigarette smoking, hypercholesterolemia, and peripheral arterial occlusive disease are also strongly associated. Individuals without carotid bifurcation disease are more likely to demonstrate progression of intracranial stenosis than those with carotid bifurcation disease.9 Metabolic syndrome is present in approximately 50% of individuals who have symptomatic intracranial atherosclerotic disease and is associated with a substantially higher risk of major vascular events.10
Natural History
In a study of patients with intracranial stenosis undergoing repeat angiography at an average interval of 26.7 months, 40% of lesions had stabilized, 20% had regressed, and 40% had progressed.9 Stenosis progression, as detected by transcranial Doppler imaging, was an independent predictor of stroke recurrence.11 Extracranial–intracranial (EC-IC) bypass surgery appears to promote progression of the lesion and occlusion of the MCA in patients with nonoccluded MCA stenosis.12
Asymptomatic intracranial stenosis is believed by some to be benign. In a series of 50 patients with asymptomatic MCA stenosis followed for a mean of 351 days, no patient had an ischemic stroke in the corresponding territory.8
The most definitive study of symptomatic intracranial stenosis thus far is the prospective Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) trial, which found an 11% to 12% first-year risk of ischemic stroke in territory attributable to the patient’s symptoms.4 The majority of strokes (73%) in WASID patients were in the territory of the stenotic artery.13 The risk of stroke in the territory of the stenotic artery was greatest in patients with severe (70%) stenosis (p = 0.0025) and among patients enrolled early (17 days) (p = 0.028). At 1 year, the stroke risk for patients with 50% to 69% stenosis was 6%, compared with 19% for those with 70% to 99% stenosis. The recent results of the WASID trial suggest that perhaps an indication for endovascular therapy can be extended to those patients who present with stroke (regardless of previous medical therapy) and have stenosis of a major intracranial artery exceeding 70%.13
The subset of patients in the EC-IC Bypass Study with MCA stenosis randomized to medical therapy had an annual ipsilateral ischemic stroke rate of 7.8% per year.6,14 In the prospective, nonrandomized Groupe d’Etude des Sténoses Intra-Crâniennes Athéromateuses Symptomatiques study, 102 patients with more than 50% symptomatic intracranial stenosis had “optimal” medical therapy, with a follow-up of 23.4 months.15 Risks in the territory of the affected artery were 12.6% for TIA and 7.0% for stroke.
Evolution and Results of Endovascular Therapy
Primary Angioplasty
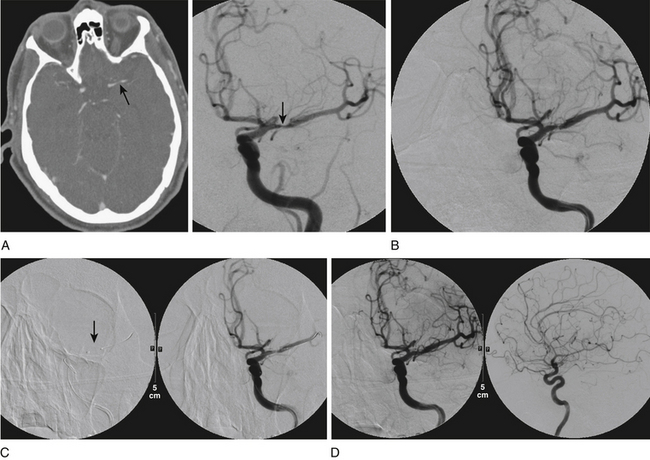
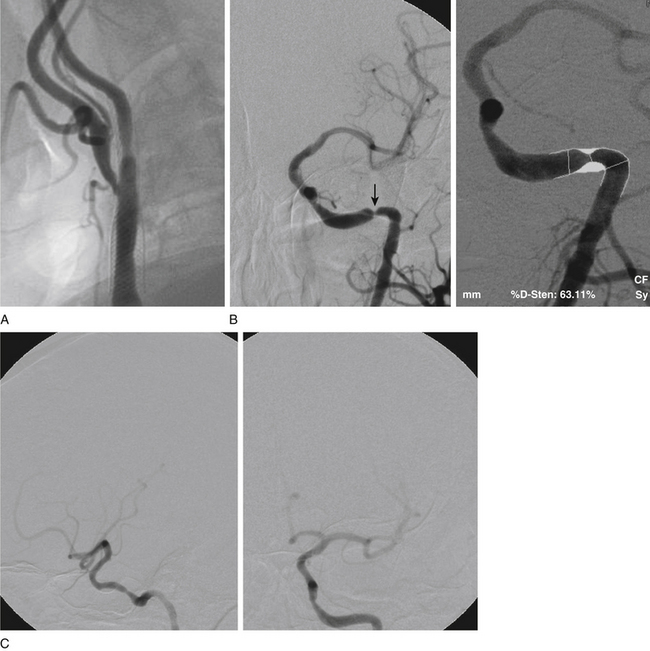
Initially, coronary angioplasty balloons were used off-label to perform intracranial percutaneous transluminal angioplasty (PTA) (Fig. 87-1). In 1999, Connors and Wojak16 published the report of their learning curve with a series of 70 patients who underwent angioplasty for intracranial atherosclerotic disease. Their technique progressed from directly sizing the balloon to the artery caliber with rapid balloon inflation to undersizing the balloon with slow inflation. The occurrence of acute vessel occlusion and dissection dropped from 75% to 14% with this technique. Currently, we utilize a technique of slow inflation (1 atmosphere, or atm, per minute) when performing angioplasty. In addition, we tend to undersize the balloon relative to the parent vessel.
Mori et al.17,18 reported on angioplasty without stenting in 42 patients with more than 70% intracranial stenosis. The risk of recurrent stenosis was strongly associated with lesion length and complexity. Lesion types were categorized as follows: type A as 5 mm or less in length, concentric or moderately eccentric lesions that were less than totally occlusive; type B as tubular, 5 to 10 mm in length, extremely eccentric or totally occluded lesions that were less than 3 months old; and type C as diffuse, more than 10 mm in length, extremely angulated (approximately 90 degrees) lesions with excessive tortuosity of the proximal segment or totally occluded lesions that were 3 months old. At the 1-year follow-up evaluation, restenosis rates associated with these lesion types were 0%, 33%, and 100%, respectively; the risk of major stroke or death was 8%, 26%, and 87%, respectively.
In 2006, Marks et al.19 reported on 20 patients with 50% intracranial stenosis who were treated with angioplasty without stenting. A total of 116 patients were available for a mean follow-up duration of 42.3 months. The degree of stenosis was reduced by angioplasty from a mean of 82.2% to a mean of 36.0%. The combined 30-day periprocedural stroke and death rate was 5.8%. The annual postprocedure stroke rate in the territory of the treatment was 3.2%, and the annual overall stroke rate was 4.4%.
Balloon-Mounted Coronary Stents
Angioplasty without stenting was associated with a significant risk of restenosis, which led to interest in angioplasty with stenting. Balloon-mounted coronary stents, however, are limited by the high inflation pressures needed for deployment in fragile intracranial vessels and the risk of shearing the stent from the balloon while navigating to the target lesion. These stents were associated with relatively high rates of technical failure due to the tortuosity of the intracranial circulation and the relative stiffness of coronary platforms; for successful procedures, the rates of periprocedural morbidity and mortality were acceptable.20–22 Jiang et al.22 reported a technical success rate (defined as equal to 20% residual stenosis) of 97.6%, with a 10% major complication rate in 40 patients with 42 symptomatic M1 stenotic lesions treated with angioplasty and balloon-mounted coronary stents.
Balloon-Mounted Intracranial Stents
The Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA) trial was a multicenter, prospective, nonrandomized feasibility study involving the balloon-mounted Neurolink intracranial stent system (a product of the Guidant Corporation, which is now part of Boston Scientific, Natick, MA).23 A total of 43 patients with symptomatic intracranial stenosis and 18 patients with extracranial VA stenosis were enrolled. Successful stent placement was achieved in 95% of cases. The 30-day periprocedural stroke rate was 6.6%. No deaths occurred. Two strokes occurred during the procedure. At the 6-month angiographic follow-up, more than 50% restenosis occurred in 32.4% of intracranial vessels and 42.9% of extracranial VAs; 39% of the recurrent stenoses were symptomatic. Strokes in the distribution of the target lesion occurring after 30 days but within 12 months were seen in 7.3% of patients. On the basis of the results of this study, the U.S. Food and Drug Administration (FDA) granted a humanitarian device exemption (HDE) to treat patients with significant intracranial and extracranial atherosclerotic disease via balloon angioplasty and stent placement. Boston Scientific is not currently marketing the Neurolink device in favor of its Wingspan system (discussed later).
The Pharos Vitesse intracranial stent (Micrus Endovascular, San Jose, CA) is a relatively new balloon-expandable stent developed for the treatment of intracranial stenosis. The first clinical experience of 21 patients with symptomatic intracranial stenoses (more than 70%) who were treated with the Pharos stent reported one stroke due to restenosis and one death during a median follow-up period of 7.3 months.24 This pilot study was limited by the small sample size and severe morbidity of the included patients. The Pharos stent received Conformité Européen mark of approval in June 2008. The Vitesse Intracranial Stent Study for Ischemic Therapy clinical trial is currently under way to evaluate the efficacy of this stent. In our experience, this balloon-mounted stent navigates the intracranial circulation with relative ease.
Staged Submaximal Angioplasty with Delayed Stenting
Because blood flow is directly proportional to the fourth power of the vessel radius (according to Poiseuille’s law), small increases in luminal diameter increase blood flow significantly, thereby alleviating hemodynamic insufficiency and changing the milieu such that an embolism is less likely to form. A technique of submaximal angioplasty followed by delayed repeat angioplasty and, if necessary, stenting was developed for intracranial symptomatic atherosclerotic disease.25 In this staged treatment approach, the patient returned for angiography approximately 4 to 6 weeks after angioplasty. If there was evidence of binary stenosis in the lesion (50% luminal-diameter stenosis), stenting was performed. The rationale for this approach is that during the weeks of delay, neointimal proliferation and scar formation after angioplasty result in a thickened fibrous lesion,26,27 which may incur a lower risk for “snow-plowing” (stent struts pushing plaque into branch vessels), plaque embolization, and vessel dissection during a subsequent stenting procedure. With this strategy, the composite rate of mortality and permanent neurologic morbidity for the procedure dropped to below 5%, and 20% to 30% of patients did not require further intervention at follow-up.28
Self-expanding Stents
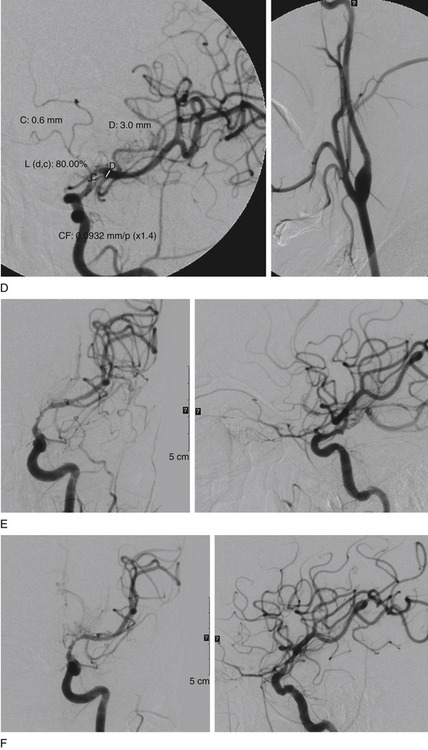
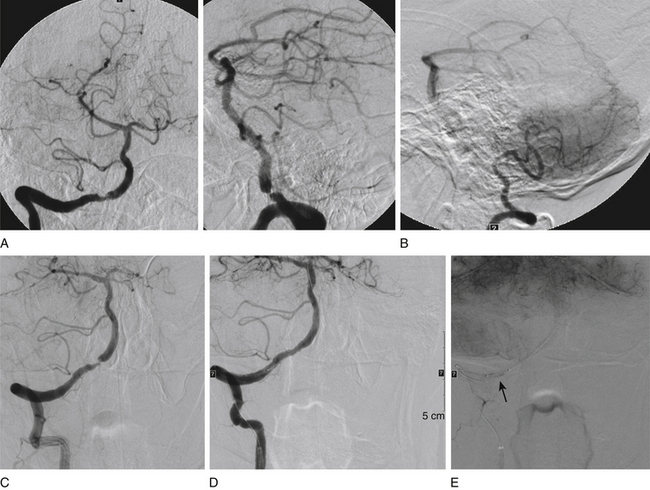
The Wingspan stent system with a Gateway PTA balloon catheter (Boston Scientific) was designed for the treatment of intracranial atherosclerotic stenosis. Prestent dilation of the lesion is done with the angioplasty balloon; the stent, a self-expanding nitinol device, is then deployed (Fig. 87-2). The device received FDA approval as a new HDE device in August 2005. The approval was based on a 45-patient Wingspan HDE safety study that was conducted at 12 sites in Europe and Asia.29 Patients who had a stroke caused by an intracranial lesion (stenosis of 50% or more) and for whom medical treatment was ineffective were enrolled in the study. Among the 45 patients, 44 patients subsequently underwent stent placement; in 1 patient, the lesion could not be traversed by the microwire. The procedural success rate was 98%, and a 4.4% incidence (n = 2) of death or ipsilateral stroke was observed 30 days after the procedure. The mean severity of angiographic stenosis decreased after the procedure from 75% to 32%. Among the 43 patients who completed the 6-month follow-up, the incidence of death or ipsilateral stroke was 7.0% (n = 3). Further lesion reduction was observed in 24 of 40 patients (mean severity 28%) who underwent follow-up angiography at 6 months. Angiograms in 3 patients (6.8%) showed more than 50% restenosis; all patients were asymptomatic. In contrast to SSYLVIA, which reported a rate of restenosis of more than 50% in 32.4% of patients at 6 months, the mean degree of stenosis at 6 months in the Wingspan study was not significantly different from the degree of stenosis immediately after the procedure.
In the U.S. Wingspan registry,30–32 treatment with the stent system was attempted in 158 patients with 168 intracranial atheromatous lesions (updated statistics, Fiorella DJ, personal communication, November 2009). Of these, 161 lesions (96.0%) were successfully treated during the first treatment session. Of the 168 lesions in which treatment was attempted, there were 9 (5.4%) major periprocedural neurologic complications, 4 of which ultimately led to the death of the patient within 30 days of the procedure. The total periprocedural event rate was 12.5% (21 of 168 cases). Most postprocedure events (18 of 21) were related to definable (and potentially controllable) issues: early antiplatelet interruption (n = 6) and in-stent restenosis (ISR) (n = 13). There were 10 patients with strokes and 12 patients with TIAs that occurred 30 days after the procedure. Imaging follow-up was available for 129 treated lesions (75 anterior circulation and 54 posterior circulation). Post-Wingspan ISR was more common in patients younger than 55 years (2.6 odds ratio). This increased risk can be accounted for by a high prevalence of anterior circulation lesions in this population, specifically those affecting the supraclinoid segment. When patients of all ages were considered, much higher rates of both ISR (66.6% vs. 24.4%) and symptomatic ISR (40% vs. 3.9%) were associated with supraclinoid segment lesions, in comparison with all other locations. Of 129 patients with imaging follow-up of treated lesions, 36 patients (27.9%) experienced ISR. Of these 36, 29 patients (80.6%) underwent target lesion revascularization (TLR) with angioplasty alone (n = 26) or angioplasty with restenting (n = 3). Restenting was performed for in-stent dissections that occurred after the initial PTA. Among the retreated lesions, 23 were located within the anterior circulation (79.3%) versus 6 in the posterior circulation. ISR lesions selected for retreatment were often either symptomatic (n = 4), angiographically more severe (longer segment involved or greater percentage of stenosis) than the presenting stenosis (n = 8), or both (n = 9). In some cases (n = 8), however, retreatment was performed in the absence of any of these factors. When symptomatic (n = 13), approximately two thirds of patients presented with TIA (n = 9) and one third presented with ipsilateral stroke (n = 4). Of the 29 patients undergoing primary TLR, 9 required 1 intervention for recurrent ISR, for a total of 42 TLR interventions. Only 1 major complication, a postprocedural reperfusion hemorrhage, was encountered during TLR (complication rates: 2.4% per procedure, 3.5% per patient). Angiographic follow-up was available for 22 of 29 patients after primary TLR. Of the 22 patients, 11 patients (50%) demonstrated recurrent ISR at follow-up angiography. Subsequently, 9 of these patients have undergone multiple retreatments (6 patients had two retreatments each, 2 had three retreatments each, and 1 had four retreatments) for recurrent ISR.
In the Wingspan National Institutes of Health (NIH) registry,33 129 patients with symptomatic 70% to 99% intracranial stenosis were enrolled from 16 medical centers. The rate of technical success rate (stent placement across the target lesion with less than 50% residual stenosis) was 97%. The rate of any stroke, intracerebral hemorrhage, or death within 30 days or ipsilateral stroke beyond 30 days was 14% at 6 months. The rate of 50% restenosis on follow-up angiography was 25% among 52 patients with follow-up. The NIH registry investigators published a post hoc analysis report of 158 of 160 patients who had successful placement for intracranial atherosclerotic lesion with 50% to 99% stenosis.34 The primary endpoint(any stroke or death within 30 days or stroke in the territory of the stented artery beyond 30 days) at 6 months occurred in 13.9%. In multivariable analysis, the primary endpoint was associated with posterior circulation stenosis (vs. anterior circulation), with a hazard ratio (HR) of 3.4 (p = 0.018); stenting at low enrollment sites with fewer than 10 patients each (vs. high enrollment sites) and an HR of 2.8 (p = 0.038); 10 days from the qualifying event to stenting (vs. 10 days), with an HR of 2.7 (p = 0.058); and stroke as a qualifying event (vs. TIA or another cerebral ischemic event, e.g., vertebrobasilar insufficiency), with an HR of 3.2 (p = 0.064).
Drug-Eluting Stents
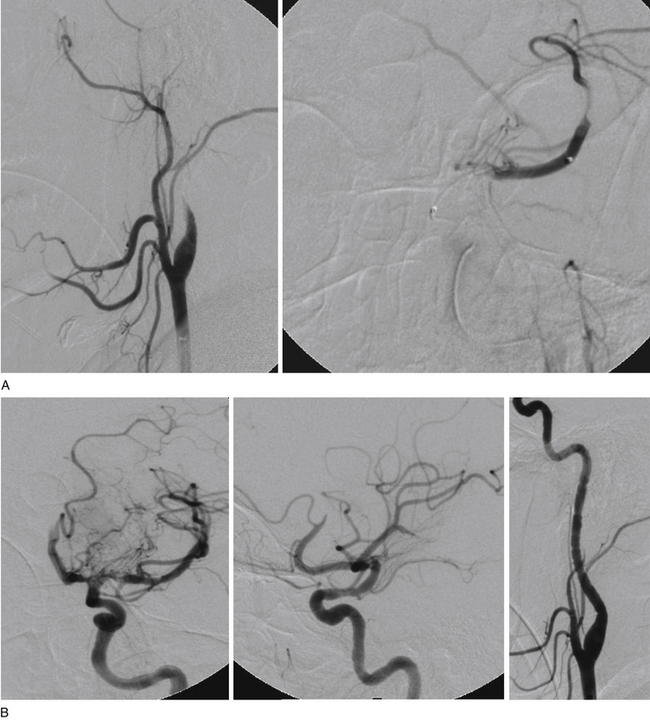
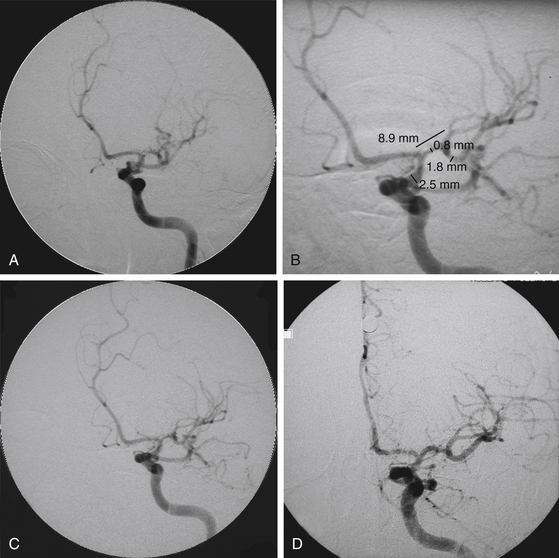
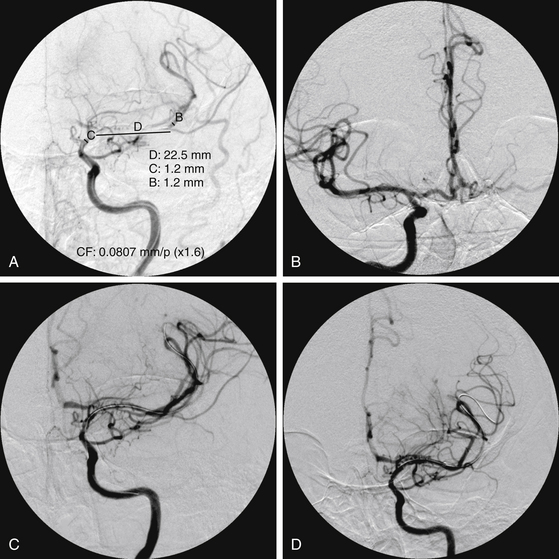
In a recent systematic review of 31 studies including 1177 procedures for symptomatic high-grade intracranial atheromatous disease, ISR occurred more frequently after the use of a self-expandable stent (16 of 92 cases, or 17.4%; mean follow-up period 5.4 months) than after use of a balloon-mounted stent (61 of 443 cases, or 13.8%; mean follow-up period 8.7 months; p < 0.001).35 Thus, ISR is a major potential downfall of the predicate technologies that might be overcome with the implementation of drug-eluting stents (DESs) (Figs. 87-3 and 87-4). First-generation balloon-mounted coronary DESs coated with sirolimus or paclitaxel have reduced the coronary ISR rate to 10% from the 30% observed with bare-metal stents.36 There have been a few reports of the use of the first-generation DESs in patients with intracranial arterial stenosis,37–41 and the safety of implanting these devices has been studied in the intracranial vasculature of canine models.42,43 The Xience V everolimus-eluting stent (EES) (Abbott Vascular, Abbott Park, IL) is a new second-generation DES that was approved by the FDA for coronary stenting in July 2008. This stent has a thin cobalt-chromium strut stent platform that allows greater deliverability in tortuous vasculature44 and is coated with an antirestenotic drug, everolimus, that has proven ability to suppress ISR to a degree equivalent to that with sirolimus and more than that for paclitaxel-eluting stents.45,46 The DESs may reduce the overall ISR rate if they can be used selectively in patients with lesions who have been identified to have a higher incidence of ISR. We have reported our initial experience with EES in patients with intracranial arterial stenosis.47 These devices are used off-label in the intracranial vasculature.
Endovascular Therapy versus Best Medical Therapy
A comparison between 254 patients recruited in the WASID trial and 158 patients entered in the NIH Wingspan multicenter stenting registry matched by patient and lesion characteristics was performed to determine the differential rates of primary outcome of stroke or death within 30 days or ipsilateral stroke beyond 30 days.48 The frequency of the primary outcome at 6 months in patients with 70% to 99% stenosis was 16% for WASID patients (treated with best medical therapy) and 13% for stent-treated Wingspan registry patients. The study concluded that stent placement may offer benefit in patients with 70% to 99% stenosis, but the benefit may not be seen in patients with 50% to 69% stenosis due to the low event rates in the medically treated patients.
In 2007, the NIH approved funding for the multicenter, prospective, randomized Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis study.49 The hypothesis of the study is that “Compared with intensive medical therapy alone, intracranial angioplasty and stenting combined with intensive medical therapy will decrease the risk of the primary endpoint by 35% over a mean follow-up of 2 years in high-risk patients (patients with 70% to 99% intracranial stenosis who had a TIA or stroke within 30 days prior to enrollment) with symptomatic stenosis of a major intracranial artery.”49 A total of 764 patients will be recruited within 30 days of TIA or stroke due to 70% to 99% stenosis of a major intracranial artery. The patients will be randomized to receive either (1) stent treatment with the Wingspan intracranial stent and Gateway balloon system plus intensive medical therapy with management of blood pressure, lipids, and other risk factors for vascular events or (2) intensive medical therapy with management of blood pressure, lipids, and other risk factors for vascular events. Each patient will be followed up for a minimum of 1 year and a maximum of 4 years after randomization.
Primary Angioplasty versus Stenting
Some controversy exists as to whether primary angioplasty is equivalent to intracranial stent placement for intracranial atherosclerotic disease. In a systematic review50 of 69 studies (33 primary angioplasty studies with a total of 1027 patients and 36 stent-placement studies with a total of 1291 patients), the incidence of technical success (defined as 50% or less residual stenosis of the target vessel after angioplasty or stent treatment) was 80% in the angioplasty group and 95% in the stent-treated group (relative risk, or RR, of 0.84, p < 0.0001). A total of 91 strokes and deaths were reported in the angioplasty-treated group (9%), compared with 104 in the stent-treated group (8%) during the 1-month postprocedure period (RR 1.1, p = 0.5). The pooled incidence of 1-year stroke and death in the angioplasty group was 20%, compared with 14% in the stent-placement group (RR 1.39, p = 0.009). The pooled restenosis rate was 14% in the angioplasty group, compared with 11% in the stent-placement group (RR 1.28, p = 0.04).
Technique of Angioplasty or Stenting
Anesthesia
At our center, we prefer to perform these procedures in conscious patients after the administration of mild sedatives and local anesthetic agents so that we can perform intermittent neurologic examinations during the procedure. Avoidance of general anesthesia also reduces the cardiovascular risk of the overall procedure. However, if a complication does occur, the potential exists for further harm until the patient’s airway can be secured and additional maneuvers (e.g., ventriculostomy and further embolization) can be performed. We must be prepared to intubate the patient on a moment’s notice. Not all patients are candidates for conscious sedation because of poor neurologic status, young age, excessive anxiety, or inability to lie still. General anesthesia is routinely used at most centers and offers the advantages of control of the airway as well as reduction or elimination of patient movement during the procedure. In a report of 37 intracranial angioplasty and stenting cases performed with conscious sedation, technical success was achieved in all patients.51 Approximately 61% of patients experienced intraprocedural symptoms that led to some alteration of the interventional technique. Headache was the most common symptom and, when persistent, signaled the occurrence of intracranial hemorrhage.
Access Site
For each case, the iliofemoral anatomy, arch anatomy, and supra-aortic vessel need to be assessed to decide on the access site and the devices that will facilitate safe access and provide a stable platform for intracranial therapies. (The brachial or radial artery may be chosen as the access site if there is a disease in the iliofemoral segments or descending aorta.) Patients with intracranial atherosclerotic disease have a higher chance of having an atherosclerotic arch or tortuous and elongated supra-aortic vessels. In such cases, special devices to access difficult arch anatomy, a carotid cut-down in the neck, or a cut-down of the extracranial VA as it traverses over the posterior arch of the C1 vertebra may be used to access the intracranial vasculature.
Wingspan Stent (Video)
The Wingspan is a 3.5-Fr, nitinol, over-the-wire self-expanding stent. The design of this stent is similar to that of the Neuroform2 stent (Boston Scientific); the Wingspan has four platinum markers at each end for visualization and is deployed from the delivery microcatheter (called the “outer body”) with the “inner body”; the inner body is analogous to the “stabilizer” device that is used to deploy Neuroform stents. Available sizes include stent diameters of 2.5 to 4.5 mm in 0.5-mm increments, and stent lengths include 9, 15, and 20 mm. The stent length should extend a minimum of 3 mm on both sides of the lesion. If the target vessel has different diameters proximal and distal to the stenotic lesion, the stent is sized to the larger of the two.
Postprocedure Management
Patients are admitted to the intensive care unit or a step-down unit. Neurologic status and access site are evaluated hourly. Most patients can be discharged from the hospital 1 or 2 days after the procedure. Aspirin is continued for life, and we prefer to continue clopidogrel (75 mg by mouth daily) for 3 to 6 months. Cardiologists are recently moving toward longer periods of dual antiplatelet therapy (3, 6, or 12 months) after coronary angioplasty and stenting.52 It can be argued that atherosclerotic intracranial arteries are similar in size and pathology to similarly diseased coronary arteries.
Moyamoya Disease
MMD is a rare, progressive cerebrovascular disease with spontaneous occlusion or stenosis at the circle of Willis and its principal branches, along with the development of an aberrant network of collateral circulation that leads to hemorrhagic and ischemic strokes. Patients with unilateral disease are included in the cohort of moyamoya syndrome (MMS), even though they do not have any of the described associated risk factors for MMS.53 MMD primarily affects Asians, with a prevalence of 3 to 6 per 100,000 people in Japan.54–56 A less severe or less progressive form of the disease has been identified in the United States57–59 with an incidence of 0.086 cases per 100,000 people. This form of the disease assumes a more benign course, predominates in adults between the third and fifth decade, and is associated with more ischemic than hemorrhagic strokes.
Traditionally, patients with MMD and ischemic symptoms are treated with direct (superficial temporal artery–MCA bypass) or indirect (encephaloduroarteriosynangiosis) surgical revascularization procedures. The largest surgical series, which is from Stanford University School of Medicine, reported on 233 adult patients, of whom 95.1% underwent direct revascularization procedures.60 The cumulative 5-year risk of perioperative or subsequent stroke or death in this series was 5.5%.
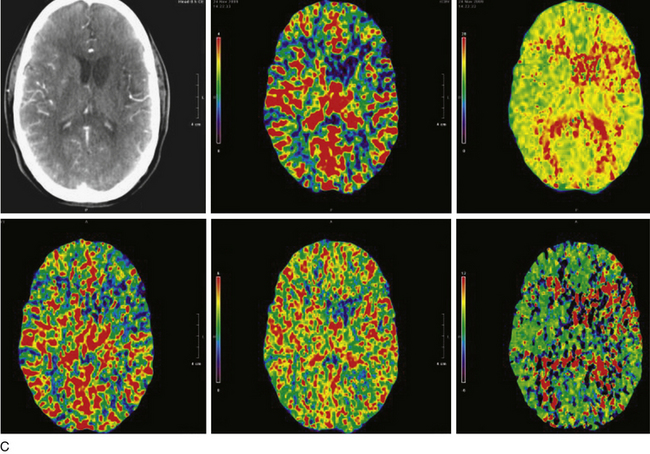
There have been four single-case reports of treatment of endovascular therapy for MMD in the literature.61–64 At our center, we treated six MMS patients (Figs. 87-5 to 87-7) with endovascular therapy between August 2005 and February 2009. All patients were women who were symptomatic, had a previous stroke or TIA, and were young (mean age 36.8 years). Stenotic lesion locations included a unilateral M1-segment lesion in five patients and, in the remaining patient, ipsilateral ICA-terminus, M1, and A1 lesions, in addition to contralateral supraclinoid carotid artery stenosis. The mean M1 stenosis was 77.3 ± 14.3%. Five patients had balloon angioplasty for M1 stenosis. In the remaining patient, balloon angioplasty failed to relieve the M1 stenosis, and a Wingspan stent was deployed successfully. Among the six patients in our series, mean post-treatment M1 stenosis was 39.3 ± 35%. Vessel rupture that occurred during angioplasty in one patient with near-complete M1 occlusion caused severe disability (modified Rankin score of 5). Of the five successfully treated patients, two were asymptomatic for 4 years and 6 months, respectively, after the primary treatment. Three of the remaining patients had retreatment 1, 2, and 5 months, respectively, after primary treatment. The first had repeat balloon angioplasty and has been asymptomatic for 6 months. The second underwent encephaloduroarteriosynangiosis after a failed balloon angioplasty and has been asymptomatic for 10 months. The third patient was asymptomatic but had angiographic restenosis (81%) and underwent Wingspan stent placement. She developed subsequent asymptomatic, severe in-stent restenosis that was treated with angioplasty at 3 months and has been asymptomatic for 4 months postangioplasty.
As we built our experience with these six patients, we realized that endovascular therapy was dangerous and was associated with a high rate of TLR when performed in patients with MMS who had severe (80%-100%) intracranial stenosis. The results were more sustainable in patients who had a single lesion that was forme fruste, minimally stenotic, and shorter. The goal of angioplasty in these patients was to achieve adequate distal perfusion with the use of a submaximal, slow inflation technique. We chose the shortest angioplasty balloon that would fully cover the lesion and inflated this balloon to approximately 50% to 75% of the parent vessel lumen. A slow inflation was used, as has been described by others.16 The lesion need not be completely dilated because of the higher chance of reperfusion hemorrhage associated with a significant increase of flow in these patients. Excessive dilatation and the use of maximal balloon dilatation, especially for a tight stenosis, may be dangerous and lead to vessel rupture.
Intracranial stents were used only when the lesion narrowed or occluded after balloon angioplasty. The Wingspan intracranial self-expanding stent is our choice for these lesions. The stents were sized to exactly match the largest dimensions of both the proximal and the distal parts of the vessel. Prestent angioplasty with a Gateway balloon was always used, and poststent angioplasty was avoided, unless a flow-limiting lesion remained. Patients who had stent placement were placed on aspirin and clopidogrel for at least 1 year because of the high rate of reported asymptomatic ISR after stenting in intracranial atherosclerotic stenosis (especially in patients younger than 55 years in the anterior circulation and more so with ICA lesions).32,33,65 For most patients with symptomatic MMD, we favor a surgical option over endovascular treatment.
Conclusions
Intracranial angioplasty with or without stent placement is an emerging technique with evolving technology. New data will provide additional information on appropriate patient selection, optimal timing of interventional therapy, and surgical and endovascular treatment options for intracranial occlusive disease. Development of more trackable, noncompliant balloons and stent systems and technology to avoid ISR and accelerate stent endothelialization may make endovascular therapy the primary and a safe treatment option for intracranial occlusive disease. Identification of patients who will benefit from therapy by physiologic imaging may increase the benefit of revascularization for these patients against the risk involved in the procedure.
Albuquerque F.C., Levy E.I., Turk A.S., et al. Angiographic patterns of Wingspan in-stent restenosis. Neurosurgery. 2008;63:23-28.
Bose A., Hartmann M., Henkes H., et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007;38:1531-1537.
Chimowitz M.I., Lynn M.J., Howlett-Smith H., et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305-1316.
Derdeyn C.P., Chimowitz M.I. Angioplasty and stenting for atherosclerotic intracranial stenosis: rationale for a randomized clinical trial. Neuroimaging Clin N Am. 2007;17:355-363. viii-ix
EC/IC Bypass Study Group. Failure of extracranial–intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. 1985;313:1191-1200.
Fiorella D., Chow M.M., Anderson M., et al. A 7-year experience with balloon-mounted coronary stents for the treatment of symptomatic vertebrobasilar intracranial atheromatous disease. Neurosurgery. 2007;61:236-243.
Fiorella D.J., Levy E.I., Turk A.S., et al. Target lesion revascularization after wingspan: assessment of safety and durability. Stroke. 2009;40:106-110.
Guzman R., Lee M., Achrol A., et al. Clinical outcome after 450 revascularization procedures for moyamoya disease. J Neurosurg. 2009;111:927-935.
Higashida R.T., Meyers P.M., Connors J.J.3rd, et al. Intracranial angioplasty & stenting for cerebral atherosclerosis: a position statement of the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, and the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2005;26:2323-2327.
Kasner S.E., Chimowitz M.I., Lynn M.J., et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555-563.
Levy E.I., Hanel R.A., Bendok B.R., et al. Staged stent-assisted angioplasty for symptomatic intracranial vertebrobasilar artery stenosis. J Neurosurg. 2002;97:1294-1301.
Levy E.I., Hanel R.A., Boulos A.S., et al. Comparison of periprocedure complications resulting from direct stent placement compared with those due to conventional and staged stent placement in the basilar artery. J Neurosurg. 2003;99:653-660.
Levy E.I., Turk A.S., Albuquerque F.C., et al. Wingspan in-stent restenosis and thrombosis: incidence, clinical presentation, and management. Neurosurgery. 2007;61:644-651.
Marks M.P., Wojak J.C., Al-Ali F., et al. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke. 2006;37:1016-1020.
Mori T., Fukuoka M., Kazita K., et al. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol. 1998;19:1525.
Mori T., Mori K., Fukuoka M., et al. Percutaneous transluminal cerebral angioplasty: serial angiographic follow-up after successful dilatation. Neuroradiology. 1997;39:111-116.
Natarajan S.K., Ogilvy C.S., Hopkins L.N., Siddiqui A.H., Levy E.I. Initial Experience with an Everolimus-Eluting, Second-Generation Drug-Eluting Stent for Treatment of Intracranial Atherosclerosis. J Neurointervent Surg. 2010;2:104-109.
Scott R.M., Smith E.R. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360:1226-1237.
SSYLVIA Study Investigators. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004;35:1388-1392.
Turk A.S., Levy E.I., Albuquerque F.C., et al. Influence of patient age and stenosis location on Wingspan in-stent restenosis. AJNR Am J Neuroradiol. 2008;29:23-27.
Zaidat O.O., Klucznik R., Alexander M.J., et al. The NIH registry on use of the Wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology. 2008;70:1518-1524.
1. Sacco R.L., Roberts J.K., Boden-Albala B., et al. Race–ethnicity and determinants of carotid atherosclerosis in a multiethnic population. The Northern Manhattan Stroke Study. Stroke. 1997;28:929-935.
2. Wityk R.J., Lehman D., Klag M., et al. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27:1974-1980.
3. Higashida R.T., Meyers P.M., Connors J.J.3rd, et al. Intracranial angioplasty & stenting for cerebral atherosclerosis: a position statement of the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, and the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2005;26:2323-2327.
4. Chimowitz M.I., Lynn M.J., Howlett-Smith H., et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305-1316.
5. Craig D.R., Meguro K., Watridge C., et al. Intracranial internal carotid artery stenosis. Stroke. 1982;13:825-828.
6. Bogousslavsky J., Barnett H.J., Fox A.J., et al. Atherosclerotic disease of the middle cerebral artery. Stroke. 1986;17:1112-1120.
7. Caplan L.R. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. 1989;39:1246-1250.
8. Kremer C., Schaettin T., Georgiadis D., et al. Prognosis of asymptomatic stenosis of the middle cerebral artery. J Neurol Neurosurg Psychiatry. 2004;75:1300-1303.
9. Akins P.T., Pilgram T.K., Cross D.T.3rd, et al. Natural history of stenosis from intracranial atherosclerosis by serial angiography. Stroke. 1998;29:433-438.
10. Wilson P.W. Estimating cardiovascular disease risk and the metabolic syndrome: a Framingham view. Endocrinol Metab Clin North Am. 2004;33:467-481. v
11. Arenillas J.F., Molina C.A., Montaner J., et al. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke. 2001;32:2898-2904.
12. Awad I., Furlan A.J., Little J.R. Changes in intracranial stenotic lesions after extracranial–intracranial bypass surgery. J Neurosurg. 1984;60:771-776.
13. Kasner S.E., Chimowitz M.I., Lynn M.J., et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555-563.
14. EC/IC Bypass Study Group. Failure of extracranial–intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. 1985;313:1191-1200.
15. Mazighi M., Tanasescu R., Ducrocq X., et al. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology. 2006;66:1187-1191.
16. Connors J.J.3rd, Wojak J.C. Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: evolution of technique and short-term results. J Neurosurg. 1999;91:415-423.
17. Mori T., Mori K., Fukuoka M., et al. Percutaneous transluminal cerebral angioplasty: serial angiographic follow-up after successful dilatation. Neuroradiology. 1997;39:111-116.
18. Mori T., Fukuoka M., Kazita K., et al. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol. 1998;19:1525-1533.
19. Marks M.P., Wojak J.C., Al-Ali F., et al. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke. 2006;37:1016-1020.
20. Fiorella D., Chow M.M., Anderson M., et al. A 7-year experience with balloon-mounted coronary stents for the treatment of symptomatic vertebrobasilar intracranial atheromatous disease. Neurosurgery. 2007;61:236-243.
21. Siddiq F., Vazquez G., Memon M.Z., et al. Comparison of primary angioplasty with stent placement for treating symptomatic intracranial atherosclerotic diseases: a multicenter study. Stroke. 2008;39:2505-2510.
22. Jiang W.J., Wang Y.J., Du B., et al. Stenting of symptomatic M1 stenosis of middle cerebral artery: an initial experience of 40 patients. Stroke. 2004;35:1375-1380.
23. SSYLVIA Study Investigators. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004;35:1388-1392.
24. Kurre W., Berkefeld J., Sitzer M., et al. Treatment of symptomatic high-grade intracranial stenoses with the balloon-expandable Pharos stent: initial experience. Neuroradiology. 2008;50:701-708.
25. Levy E.I., Hanel R.A., Bendok B.R., et al. Staged stent-assisted angioplasty for symptomatic intracranial vertebrobasilar artery stenosis. J Neurosurg. 2002;97:1294-1301.
26. Anderson P.G., Boerth N.J., Liu M., et al. Cyclic GMP-dependent protein kinase expression in coronary arterial smooth muscle in response to balloon catheter injury. Arterioscler Thromb Vasc Biol. 2000;20:2192-2197.
27. Tanaka H., Sukhova G.K., Swanson S.J., et al. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993;88:1788-1803.
28. Levy E.I., Hanel R.A., Boulos A.S., et al. Comparison of periprocedure complications resulting from direct stent placement compared with those due to conventional and staged stent placement in the basilar artery. J Neurosurg. 2003;99:653-660.
29. Bose A., Hartmann M., Henkes H., et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007;38:1531-1537.
30. Fiorella D.J., Levy E.I., Turk A.S., et al. Target lesion revascularization after wingspan: assessment of safety and durability. Stroke. 2009;40:106-110.
31. Albuquerque F.C., Levy E.I., Turk A.S., et al. Angiographic patterns of Wingspan in-stent restenosis. Neurosurgery. 2008;63:23-28.
32. Turk A.S., Levy E.I., Albuquerque F.C., et al. Influence of patient age and stenosis location on Wingspan in-stent restenosis. AJNR Am J Neuroradiol. 2008;29:23-27.
33. Zaidat O.O., Klucznik R., Alexander M.J., et al. The NIH registry on use of the Wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology. 2008;70:1518-1524.
34. Nahab F., Lynn M.J., Kasner S.E., et al. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology. 2009;72:2014-2019.
35. Groschel K., Schnaudigel S., Pilgram S.M., et al. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke. 2009;40:e340-347.
36. Serruys P.W., Kutryk M.J., Ong A.T. Coronary-artery stents. N Engl J Med. 2006;354:483-495.
37. Steinfort B., Ng P.P., Faulder K., et al. Midterm outcomes of paclitaxel-eluting stents for the treatment of intracranial posterior circulation stenoses. J Neurosurg. 2007;106:222-225.
38. Qureshi A.I., Kirmani J.F., Hussein H.M., et al. Early and intermediate-term outcomes with drug-eluting stents in high-risk patients with symptomatic intracranial stenosis. Neurosurgery. 2006;59:1044-1051.
39. Abou-Chebl A., Bashir Q., Yadav J.S. Drug-eluting stents for the treatment of intracranial atherosclerosis: initial experience and midterm angiographic follow-up. Stroke. 2005;36:e165-168.
40. Lee S.H., Jung J.H., Shim W.H. Endovascular treatment of basilar artery stenosis with drug-eluting stent: a case report and literature review. Catheter Cardiovasc Interv. 2005;64:296-300.
41. Gupta R., Al-Ali F., Thomas A.J., et al. Safety, feasibility, and short-term follow-up of drug-eluting stent placement in the intracranial and extracranial circulation. Stroke. 2006;37:2562-2566.
42. Levy E.I., Hanel R.A., Tio F.O., et al. Safety and pharmacokinetics of sirolimus-eluting stents in the canine cerebral vasculature: 180 day assessment. Neurosurgery. 2006;59:925-934.
43. Levy E.I., Hanel R.A., Howington J.U., et al. Sirolimus-eluting stents in the canine cerebral vasculature: a prospective, randomized, blinded assessment of safety and vessel response. J Neurosurg. 2004;100:688-694.
44. Kukreja N., Onuma Y., Serruys P.W. Xience V everolimus-eluting coronary stent. Expert Rev Med Devices. 2009;6:219-229.
45. Carter A.J., Brodeur A., Collingwood R., et al. Experimental efficacy of an everolimus eluting cobalt chromium stent. Catheter Cardiovasc Interv. 2006;68:97-103.
46. Joner M., Nakazawa G., Finn A.V., et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333-342.
47. Natarajan S.K., Ogilvy C.S., Hopkins L.N., Siddiqui A.H., Levy E.I. Initial Experience with an Everolimus-Eluting, Second-Generation Drug-Eluting Stent for Treatment of Intracranial Atherosclerosis. J Neurointervent Surg. 2010;2:104-109.
48. Jarvis A.L., Lynn M.J., Alexander M.J., et al. NIH Multicenter Wingspan Intracranial Stent Study Group. Outcome of patients with 50% to 99% intracranial stenosis and TIA or stroke on antithrombotic therapy treated medically vs. stenting. Chicago, IL: Presented at the 60th Annual Meeting of the American Academy of Neurology; April 12-19, 2008.
49. Derdeyn C.P., Chimowitz M.I. Angioplasty and stenting for atherosclerotic intracranial stenosis: rationale for a randomized clinical trial. Neuroimaging Clin N Am. 2007;17:355-363. viii-ix
50. Siddiq F., Memon M.Z., Vazquez G., et al. Comparison between primary angioplasty and stent placement for symptomatic intracranial atherosclerotic disease: meta-analysis of case series. Neurosurgery. 2009;65:1024-1034.
51. Abou-Chebl A., Krieger D.W., Bajzer C.T., et al. Intracranial angioplasty and stenting in the awake patient. J Neuroimaging. 2006;16:216-223.
52. Coolong A., Mauri L. Clopidogrel treatment surrounding percutaneous coronary intervention: when should it be started and stopped? Curr Cardiol Rep. 2006;8:267-271.
53. Scott R.M., Smith E.R. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360:1226-1237.
54. Kuriyama S., Kusaka Y., Fujimura M., et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. 2008;39:42-47.
55. Baba T., Houkin K., Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. 2008;79:900-904.
56. Wakai K., Tamakoshi A., Ikezaki K., et al. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg. 1997;99(Suppl 2):S1-5.
57. Uchino K., Johnston S.C., Becker K.J., et al. Moyamoya disease in Washington State and California. Neurology. 2005;65:956-958.
58. Chiu D., Shedden P., Bratina P., et al. Clinical features of moyamoya disease in the United States. Stroke. 1998;29:1347-1351.
59. Yilmaz E.Y., Pritz M.B., Bruno A., et al. Moyamoya: Indiana University Medical Center experience. Arch Neurol. 2001;58:1274-1278.
60. Guzman R., Lee M., Achrol A., et al. Clinical outcome after 450 revascularization procedures for moyamoya disease. J Neurosurg. 2009;111:927-935.
61. Kornblihtt L.I., Cocorullo S., Miranda C., et al. Moyamoya syndrome in an adolescent with essential thrombocythemia: successful intracranial carotid stent placement. Stroke. 2005;36:E71-73.
62. Lee S.J., Ahn J.Y. Stenosis of the proximal external carotid artery in an adult with moyamoya disease: moyamoya or atherosclerotic change? Neurol Med Chir (Tokyo). 2007;47:356-359.
63. Rodriguez G.J., Kirmani J.F., Ezzeddine M.A., et al. Primary percutaneous transluminal angioplasty for early moyamoya disease. J Neuroimaging. 2007;17:48-53.
64. Drazin D., Calayag M., Gifford E., et al. Endovascular treatment for moyamoya disease in a Caucasian twin with angioplasty and Wingspan stent. Clin Neurol Neurosurg. 2009;111(10):913-917.
65. Levy E.I., Turk A.S., Albuquerque F.C., et al. Wingspan in-stent restenosis and thrombosis: incidence, clinical presentation, and management. Neurosurgery. 2007;61:644-651.