Chapter 86 Endovascular Treatment of Cerebral Arteriovenous Malformations
Pretherapeutic Evaluation
Noninvasive Techniques
Cerebral AVMs can be visualized with a variety of diagnostic modalities, including computed tomography (CT), computed tomography angiography (CTA), magnetic resonance imaging (MRI), and magnetic resonance angiography (MRA). Noncontrast CT scans have a low sensitivity but enable evaluation of acute hemorrhage. Prominent draining veins are often seen on noncontrast scans. CTA provides important vascular detail within the AVM, including the enlargement of feeding arteries, engorged cortical veins, or the presence of associated aneurysms. However, it provides lower definition of surrounding cerebral structures, which MRI and MRA can better show. MRI characterizes AVMs by exhibiting flow voids on T1 and T2 imaging, with occasional hemosiderin deposits suggesting prior hemorrhage.1 Additional modalities have been used in preoperative evaluation of AVMs in efforts to understand the anatomic, as well as functional, relationship of the brain to the vascular malformation.
Functional MRI
Functional MRI quantifies the local increase in oxyhemoglobin concentration secondary to increased blood flow and volume that occurs after stimulation of eloquent cortex. The images enable the clinician to recognize the relationship of AVMs to eloquent brain structures, as well as elucidate cortical reorganization secondary to the AVM.2–4 This information may help during preoperative planning and during patient counseling, because it could, in some measure, predict the development of post-therapy deficits in patients.
Diffusion-Tensor Imaging
Diffusion-tensor imaging is a developing technology that enables in vivo visualization of multiple white matter tracts and their relationship to an AVM. The ability to use this information can help minimize treatment risks and determine the best therapeutic modality for the patient.2,5
Invasive Techniques
Provocative Injection Testing (Wada’s Test)
Prior to embolization of an AVM, Wada’s testing of selective feeding vessels can be performed. Amobarbital sodium or propofol injections have been performed on vessels in or remote from the lesion.6–8 This pharmacologic test helps predict the effect of occluding a feeding vessel. Due to their short half-life, their effect disappears rapidly. Such evaluation helps prevent embolization of pedicles that may result in focal neurologic deficit. This preoperative functional evaluation is helpful when supraselective intranidal microcatheter placement is not achieved and there is concern that vessels to adjacent normal brain may be compromised.
Angiography
Angiography remains the gold standard for defining arterial and venous anatomy of an AVM.2 Such analysis of the angioarchitecture helps in evaluating the risk of hemorrhage and selecting the best therapeutic management of the AVM.9 Angiographic characterization of the AVM includes identification of the main arterial feeders, size of the AVM, shape of the nidus (compact vs. diffuse, without clear borders that separate it from adjacent brain), drainage patterns (superficial drainage into cortical veins, deep drainage through deep venous system, or proximity to dural sinuses), and characteristics of outflow (presence of restriction from stenosis or sinus thrombosis). Anatomic characteristics of AVMs such as deep location, deep venous drainage pattern, presence of a single draining vein, venous stenosis, eloquent location, and small diameter are all related to increased risk of hemorrhage or neurologic deficit.10–19
Angiography helps elucidate the presence of associated aneurysms. These aneurysms are categorized by their location (i.e., feeding artery, intranidal, circle of Willis, or venous). Associated aneurysms are found in roughly 7% to 41% of patients with AVMs.20–25 Studies evaluating hemorrhage risks for AVMs with associated aneurysms have shown conflicting results. A 2007 study from the Columbia study group described prospectively collected hemorrhage risk factors limited to the period between the initial AVM diagnosis and the start of treatment, calculating annual hemorrhage rates in 622 patients.26 In the group’s model, the presence of associated intranidal or feeding artery aneurysms did not have a significant effect on hemorrhage rates.11 Other studies, however, have included intranidal aneurysms as important risk factors for hemorrhage in AVMs,1,13–16,21,27,28 with increased size of the aneurysm related to increased risk of hemorrhage.21,29 These aneurysms are exposed to the same intraluminal pressures as the arterial components of the AVM. Therefore, any pressure changes following AVM embolization could lead to associated aneurysm rupture. Thus, vessels that supply intranidal aneurysms should be recognized and treated early to reduce the risk of hemorrhage.30
Angiography further evaluates for the presence of pseudoaneurysms (false aneurysms), which may form in previously ruptured AVMs. These irregularly shaped cavities have abnormally structured vessel walls and are often located at the point where artery and vein make contact due to inherent weakness.31 Previous rupture of an AVM is an independent predictor of future hemorrhage,26 and recognizing the presence of posthemorrhage pseudoaneurysm formation can help reduce the risk of future hemorrhage.
AVM-associated arteriovenous fistulas are sometimes discovered in the nidus during angiographic evaluation. The presence of these high-flow AVFs is associated with increased risk of intra- and postoperative complications, including hemorrhage.32,33 Prior endovascular treatment of these AVFs helps decrease the chance of this complication. Reducing the flow through the AVF also helps decrease the chances of undesired embolization of the AVMs’ venous drainage, cerebral veins, dural sinuses, or pulmonary circulation.33,34
Timing of imaging after a hemorrhage must be considered. Performing an angiogram immediately after hemorrhage can result in a false negative secondary to compression of the nidus by the hematoma.35–38 Therefore, a late angiogram, performed 3 months after the hemorrhage, remains the gold standard for detection of an AVM.35
Endovascular Technique
Anesthetic and Perioperative Considerations
The type of anesthesia is selected depending on the angioarchitecture of the AVM. If the target vessel is terminal, ending directly into the AVM, the procedure can be done under general anesthesia. This facilitates patient comfort and ensures a motionless patient during the procedure, enabling improved visualization of the AVM structures and minimizing patient risk associated with mobility during the procedure.1 Because there is no functional intervening tissue within the AVM, embolization of appropriate vessels should theoretically not cause any functional deficit. In our institution, general anesthesia and endotracheal intubation are utilized for all AVM embolizations. A brief period of apnea helps decrease the motion caused by the respiratory cycle in certain cases when rapid injection of embolic material and removal of microcatheter is necessary. Intraoperatively, the patient’s neurologic status is monitored continuously via somatosensory evoked potentials and electroencephalography.
In some cases, it is necessary to assess the patient’s functional status with provocative tests intraoperatively. This requires intravenous anesthesia with short-acting agents such as propofol and midazolam39 and avoiding paralytic agents so that the patient’s neurologic function can be assessed quickly during the procedure. Authors that favor this approach point to the wide variability and cortical reorganization described in patients with AVMs.40,41
Foley catheters and continuous transduction of arterial pressure are standard. Blood pressure transduction can be obtained through the femoral sheath or existing arterial line. This is especially important when vasoactive drugs must be given intraprocedurally.1 Deliberate systemic hypotension through vasoactive agents, general anesthesia, or adenosine-induced cardiac pause may also be used to slow flow through the AVM and allow for more controlled deposition of embolic material.42 In addition, a pulse oximeter is applied to the foot ipsilateral to the femoral sheath to monitor signs of early vessel obstruction, distal thromboemboli, or overcompression following sheath removal.1 Foley catheters are typically used for fluid management, and supplemental oxygen or nasopharyngeal airways can be considered, especially in those patients under sedative–hypnotic agent.1
Standard Procedure
The goals, expectations, risks, and benefits of endovascular treatment must be discussed with the patient and family prior to the procedure. Vascular access is obtained by inserting a No. 7 French gauge (Fr) sheath into the femoral artery via a Seldinger technique. Anticoagulation algorithms to prevent thromboembolic complications during and after the procedure remain controversial.43–45 In our institution, continuous heparinized flush is used for the femoral sheath, guide catheter, and microcatheter. However, no additional heparin bolus is used during the procedure. A 6-Fr guiding catheter is advanced into the internal carotid or vertebral artery. Flow-directed microcatheters are used to reach the intranidal target. Their selection is based on the size of the vessel. The catheter is drawn through the vessels primarily through blood flow and contains a flexible distal tip to enable navigation through distant and tortuous neurovasculature. Some flow-directed catheters have a steam-formed distal tip to enable more selective tracking of the catheter into the desired vessels. Variations in microcatheters, including those in Prowler (Cordis Endovascular, Miami Lakes, FL), Spinnaker (Boston Scientific/Target Therapeutics, Fremont, CA), Marathon (ev3 Neurovascular, Irvine, CA), and Echelon (ev3 Neurovascular), allow for differences in flexibility, torque, maneuverability, and responsiveness.
Identification of the Embolization Point
The clinician requires a full understanding of the AVM’s anatomy and architecture to identify pedicles that do not share supply with the adjacent normal brain. It is critical to identify en passage vessels that share supply with normal brain when en route to the nidus. In such a scenario, either a pretherapeutic or an intraoperative (Wada’s test) functional evaluation must demonstrate lack of functional significance to avoid neurologic deficit from embolization.34
Injection of Embolic Material
n-Butyl Cyanoacrylate
n-BCA is a liquid monomer that permanently polymerizes to a solid compound after contact with solutions containing anions like blood.46 This compound must be combined with Ethiodol, an iodine-based oil that serves as a radiopaque contrast agent. Tantalum powder can also be added to increase its opacity, although its use is not common currently. Embolization with n-BCA initiates an inflammatory endothelial response and subsequent occlusion of target vessels.47 A variety of factors influence the effectiveness of n-BCA in treating AVMs, namely, viscosity and polymerization time. Increasing the concentration of Ethiodol in the mixture increases the viscosity, which delays the transit within the lesion. Increasing the concentration of Ethiodol delays the polymerization time, which is useful in lesions that exhibit slow flow. Temperature, homogeneity of the mixture, and changes in the pH of the solution also play a role in polymerization time.48,49 The distance between the microcatheter and the nidus, tortuosity of the vessels, and intranidal flow are factors that influence the choice of n-BCA viscosity and polymerization time. Manipulation of viscosity and polymerization time allows for control of injection speed. Diluted glue permits prolonged injection times and dense casting of the nidus with less microcatheter retention risk.34 In general, a 1:1 dilution of n-BCA to Ethiodol is used when the hemodynamic flow of the AVM is very fast and the catheter is close to the nidus. Different concentrations, such as 1:2 or 1:3, are used when the flow is slower. We frequently use a 1:2 or 1:3 n-BCA-to-Ethiodol ratio. Acid compounds such as glacial acetic acid have been used to decrease the pH, which delays polymerization time without compromising viscosity.50 Dextrose 5% can also be used to improve distal progression of the n-BCA through small, tortuous feeders. In this technique, dextrose is injected through the guide catheter and n-BCA is simultaneously injected through the microcatheter. The dextrose injection delays the contact of the blood with the n-BCA and, in this way, delays its polymerization.51 Sometimes, as the microcatheter tip reaches smaller distal vessels, it can potentially occlude blood flow. This technique is known as “flow control” and can be utilized to prevent blood from contacting the n-BCA, thus allowing for its prolonged distal penetration.34 Injection of n-BCA is stopped when glue reaches the venous system through the nidus or if there is backflow toward the catheter. The microcatheter must be removed quickly to prevent catheter retention.47–4952
Onyx
Onyx is a newer liquid embolic agent composed of ethylene–vinyl alcohol copolymer (EVOH) mixed in a dimethyl sulfoxide (DMSO) solvent. This nonadhesive, cohesive solution is mixed with micronized tantalum for radiopacity and must be shaken together for at least 20 minutes53 to achieve homogeneity of the mixture. It is available in different concentrations of the EVOH polymer, with lower concentrations being associated with decreased viscosity and thus more distal penetration of the AVM.54 When the mixture comes into contact with aqueous solutions, the polymer begins to precipitate on the surface while the core remains liquid. This produces what has been described as a “lavalike flow pattern” within blood vessels of a soft and nonadherent mass that does not fragment during the injection.54 Similar to n-BCA, Onyx is a nonabsorbable agent. Therefore, theoretical permanent occlusion of the AVM can be achieved.55 However, recent evidence suggests that there exists a risk of recanalization or reperfusion of the AVM following Onyx embolization.56,57 Its nonadhesive nature enables longer, slower, and more controlled injections, which allow for the embolization of a larger percentage of the AVM from a single catheter position. However, only partial reflux around the catheter is permissible, because catheter withdrawal can become difficult. Its nonadhesive nature also enables cerebral angiography between injections to monitor the progress of embolization,54,55,58,59 a big advantage over the use of n-BCA. Care must be taken to use slow infusion rates and thus avoid the angiotoxicity associated with DMSO.60 Chaloupka et al., using a swine rete mirabilis embolization model, report that a DMSO injection of 0.5 to 0.8 ml over 30 to 90 seconds, while causing mild transient acute vasospasm, results in no adverse clinical sequelae and less angiotoxicity than reported in prior studies.60 Separation of tantalum from the Onyx is another pitfall that can increase the difficulty of viewing smaller feeding pedicles.59,61 Onyx injection is limited to DMSO-compatible microcatheters only, such as the Marathon and the Echelon.
Polyvinyl Alcohol
PVA consists of particles available in different diameters depending on the size of target vessels.52,62–64 PVA produces slower occlusion than liquid embolic agents. PVA particles occlude low pressure shunts first, causing an increase in intranidal vessel pressure and subsequent immediate risk of hemorrhage prior to nidal obliteration.65 In addition, PVA is associated with high recanalization rates, ranging from 12% to 43%.66,67 The development of collateral feeders secondary to proximal occlusion of the AVM with large size particles may contribute to such high recanalization rates.52,62–64 Given these characteristics of PVA, it has been used with success preoperatively to reduce AVM flow prior to open or radiosurgical treatment.
Goals of Treatment and Outcomes
There are four possible objectives of endovascular therapy.
Embolization for Definitive Cure
Liquid embolic agents, including n-BCA and Onyx, have been used for curative AVM embolization (Table 86-1). Varying rates of complete occlusion with n-BCA have been noted in the literature. Deruty et al. reported complete obliteration in 5% after embolization alone, done mostly in patients with high-grade AVMs.68 Vinuela et al. reported 9.9% rate for embolization alone in small to medium AVMs with less than four pedicles among 405 patients treated.69 Lundqvist et al. reported a 13% rate of occlusion.70 Valavanis and Yasargil reported a rate of 40% angiographic cure in a study of 387 consecutive patients.58 Gobin et al. reported a complete occlusion rate of 11.2% in patients after embolization prior to radiosurgery in a study of 125 patients who were poor surgical candidates.71 Newer studies using Onyx or a combination of Onyx and n-BCA have shown variable cure rates from 15% to 53.9%.54,56,57,59,72–83 Other studies have also shown evidence that embolization for cure of small, deep-seated, nonresectable AVMs offers immediate protection from new or recurrent hemorrhage.32,58,84–86
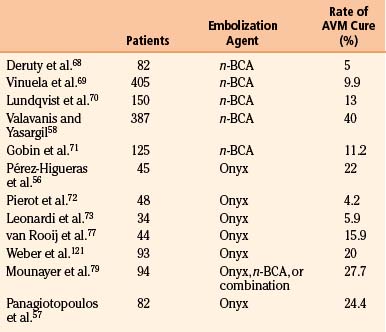
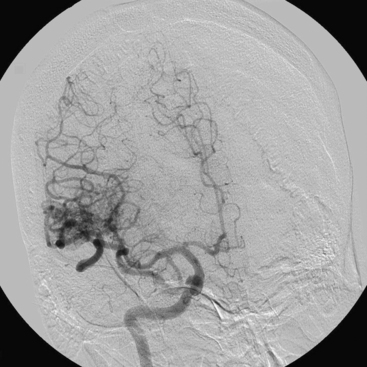
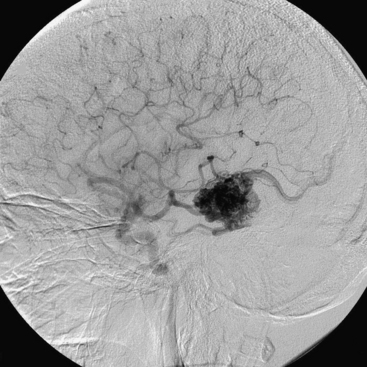
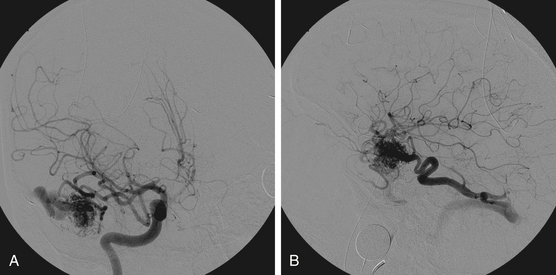
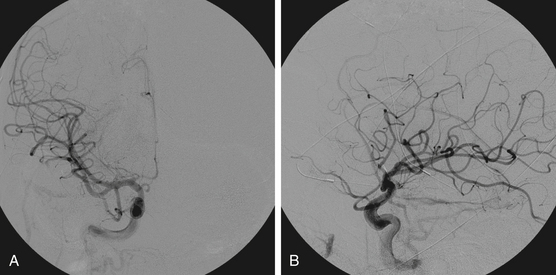
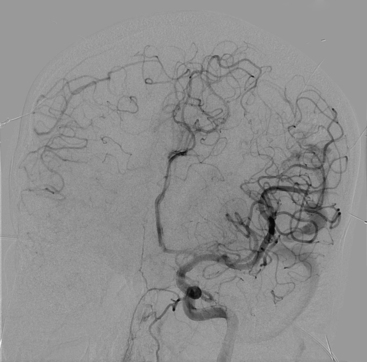
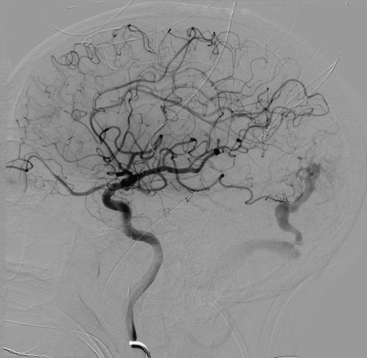
Figures 86-1 to 86-3 show a 26-year-old female with a past medical history of migraines who presented with seizures. Angiography reveal a right temporal AVM fed by multiple branches of the MCA and PCA, with superficial draining veins. Embolization using n-BCA over three sessions resulted in complete occlusion of the AVM.
Embolization as a Precursor for Definitive Operative Resection
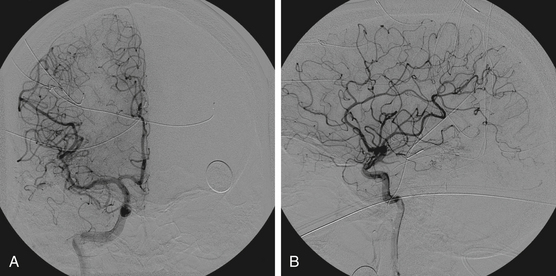
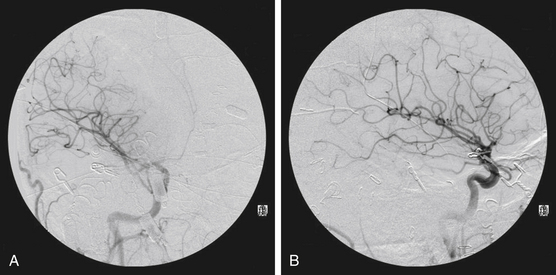
The goals of presurgical embolization are the reduction of the size of the nidus and the occlusion of deep arterial feeding vessels, which may be surgically inaccessible. Draining veins should be preserved, because restricting the venous outflow could result in increased risk of hemorrhage.59,77,79,87 In addition, preoperative embolization can be used to treat intranidal aneurysms and high-flow fistulas to promote progressive thrombosis of the nidus.1 Preoperative embolization may help reduce blood loss, improve operative time, and convert high-grade Spetzler-Martin AVMs to lower-grade lesions, and thereby reduce associated morbidity and mortality.88–92 However, proximal embolization of feeding vessels may result in the development of collaterals93 and thus result in a more treacherous open resection. Figure 86-4 reveals effective use of embolization prior to open surgical resection. In this example, a 32-year-old male with no past medical history presented with a new onset of seizure. Embolization of the right M4 and anterior temporal pedicles of the AVM with Onyx allowed for nearly 95% obliteration (Fig. 86-5) prior to the complete removal with craniotomy (Fig. 86-6). From a surgical perspective, lesions embolized with n-BCA intraoperatively exhibit a harder, less compliant mass in contrast to lesions embolized with Onyx, which is a softer agent.
Embolization as a Precursor to Radiosurgery
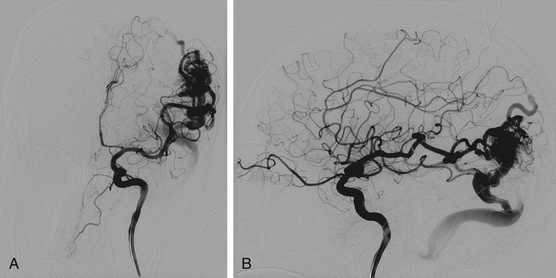
There are a few primary objectives of endovascular embolization prior to radiosurgery. Embolization decreases the target size of the AVM, which allows for a higher dose of radiation to be delivered to a smaller volume. This practice is associated with less morbidity (radiation necrosis) and higher cure rates following radiosurgery. Embolization can also reduce weakness in the AVM angioarchitecture by eliminating intranidal or venous aneurysms. This aims to reduce hemorrhage risk during the radiosurgery latency period. Embolization may also reduce symptoms related to venous hypertension.71,94,95 However, delayed recanalization related to the use of PVA, as well as n-BCA, has been noted in AVMs treated with embolization prior to radiosurgery.71,82,96,97 In addition, flow reduction alone without reduction of AVM volume has not been shown to improve radiosurgical success. It may even challenge conformal dose planning for radiosurgery.97 Recent studies with small series of patients have noted angiographic occlusion of AVMs with embolization followed by radiosurgery in some patients; however, more long-term clinical data are necessary for definitive analysis.59,77,79 Figure 86-7 shows a 40 × 40-mm left parietal AVM fed by the left MCA and PCA. Staged embolizations over four sessions with n-BCA enabled reduction of AVM volume prior to radiosurgery (Figs. 86-8 and 86-9). The patient is currently undergoing linear accelerator treatment for the residual AVM.
Embolization as Palliation for Progressive Debilitating Symptoms and Target Embolization of Bleeding or High-Risk Lesions
Palliative embolization is an alternative treatment for patients with large, nonresectable AVMs, medically intractable seizures, or progressive debilitating neurologic deficits secondary to arterial steal (due to preferential blood flow through the low-resistance AVM, causing ischemia of surrounding normal tissue), or venous hypertension that could cause headaches or local mass effect from engorged veins.98–101 It can also be used in a targeted approach or staged treatment to reduce the hemorrhagic risk from associated aneurysms or from increased pressure in veins that have an outflow restriction.102 While partial embolization can be helpful in reversing neurologic deficit, the development of rapidly forming collaterals following embolization reduces the long-term effectiveness of this strategy.
Complications
Embolization of AVMs carries with it inherent risks, primarily hemorrhage, edema, infarction, and ischemia. The complications that occur can result in temporary or permanent neurologic morbidity of variable severity or even death.
Hemorrhage and Edema
Causes of embolization-associated hemorrhage include arterial perforation, intranidal aneurysm rupture, or disturbances in AVM drainage through occlusion of major draining veins.45,88,103–105 Venous disturbances in areas of brain adjacent to the AVM result in passive engorgement of vessels, arterial stagnation, and subsequent cerebral edema and/or hemorrhage. This phenomenon may occur even with complete obliteration of the AVM and has been referred to as venous overload106 or occlusive hyperemia.107 Guglielmi in 2008 used a simple electric model to highlight the hemorrhagic risk. His study showed that occlusion of the venular part of the AVM nidus results in a 68% increase in blood pressure to the remaining proximal portion of the nidus, while occlusion of the drainage vein itself results in a 284% increase in blood pressure through the nidus.108 Hemorrhage and edema may also occur secondary to loss of autoregulation in surrounding ischemic tissue following embolization. The loss of autoregulation around the AVM results in disruption of normal capillary beds. This is known as the normal perfusion pressure breakthrough theory.109 Inflammatory reactions, mural necrosis induced by embolic material, and ischemic softening of tissue surrounding an abnormal blood vessel that bled under pressure are also potential causes of postembolization hemorrhage.57,110 If periprocedural hemorrhage occurs, protamine must be administered immediately for reversal of anticoagulation.111 Administration of steroids has been used to combat vasogenic edema related to AVM treatment.107
Ischemia and Infarction
Inadvertent embolization of normal blood vessels, thrombotic emboli, and retention of the microcatheter can result in ischemia and infarction. Immediate treatment with deliberate hypertension to open collateral circulation is indicated to help mitigate neurologic deficit.111 In addition, intra-arterial administration of platelet glycoprotein IIb/IIIa antagonists can be used to combat the development of periprocedural thrombi.57 Other specific hemodynamic complications can occur from the procedure itself and depend on the location and extension of the lesion. Angiographic data, as well as preprocedural noninvasive testing, are used to help minimize this risk.
Current literature estimates the morbidity rate associated with AVM embolization to be 10% for temporary neurologic deficits and 8% for permanent deficits. Mortality rates are documented as around 1%.112 These complications occurred mainly in high-grade AVMs. Newer studies documenting embolization prior to the use of Onyx report rates ranging between 5.6% and 14% for overall morbidity and 2% to 12.8% for permanent morbidity. Mortality rates during this period vary from 0.6% to 3.7%.23,32,71,113–119 Single- and multicenter studies have been performed to look at risks associated with embolization with Onyx. Overall morbidity rates have ranged from 7.1% to 10%, with 2% to 3% related to temporary morbidity and 1% to 15.5% related to permanent morbidity. Mortality rates have been quoted from 0% to 3%.54,56,59,73,74,76–79,120,121 These studies have shown that curative embolization of AVMs in multiple sessions can achieve high rates of total or near-total occlusion with acceptable rates of morbidity and mortality.101 A retrospective review of 153 patients by Kim et al. in 2006, looked at a variety of factors, including age, gender, AVM grade, location of lesion, number and location of embolized arteries, and number of embolization sessions. Analysis of these variables with respect to neurologic complications revealed that only the number of branches embolized was found to be related to neurologic deficit with statistical significance.117 Risk of embolization by Spetzler-Martin grade was also evaluated. Immediate and long-term neurologic deficits were found to increase with increasing grade of AVM, demonstrating a trend—although it was not statistically significant. Long-term rates of deficit were 0%, 5%, 7%, 10%, and 18% for AVM grades I, II, III, IV, and V, respectively.117
Berenstein A., Lasjaunias P., Surgical Neuroangiography, Vol 4;New York, NY, Springer-Verlag, 1987.
Brown R.D.Jr., et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68(3):352-357.
Friedlander R.M. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007;356(26):2704-2712.
Hartmann A., et al. Determinants of staged endovascular and surgical treatment outcome of brain arteriovenous malformations. Stroke. 2005;36(11):2431-2435.
Hartmann A., et al. Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002;33(7):1816-1820.
Haw C.S., et al. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006;104(2):226-232.
Linfante I., Wakhloo A.K. Brain aneurysms and arteriovenous malformations: advancements and emerging treatments in endovascular embolization. Stroke. 2007;38(4):1411-1417.
Lundqvist C., Wikholm G., Svendsen P. Embolization of cerebral arteriovenous malformations: part II—Aspects of complications and late outcome. Neurosurgery. 1996;39(3):460-467. discussion 467-469
Muller-Forell W., Valavanis A. How angioarchitecture of cerebral arteriovenous malformations should influence the therapeutic considerations. Minim Invasive Neurosurg. 1995;38(1):32-40.
Ogilvy C.S., et al. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Circulation. 2001;103(21):2644-2657.
Pérez-Higueras A., López R.R., Tapia D.Q.. Endovascular treatment of cerebral AVM: our experience with Onyx. Interventional Neuroradiology, 2005;11:141-157
Pierot L., et al. Endovascular treatment of brain arteriovenous malformations using onyx: results of a prospective, multicenter study. J Neuroradiol. 2009;36(3):147-152.
Stapf C., et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66(9):1350-1355.
Redekop G., et al. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89(4):539-546.
Rosenwasser R.H., Thomas J.E., Gannon P.M., et al. Current Strategies for the Management of Cerebral Arteriovenous Malformations. Rolling Meadows, Illinois: American Association of Neurological Surgeons; 1998.
Schaller C., Schramm J. Microsurgical results for small arteriovenous malformations accessible for radiosurgical or embolization treatment. Neurosurgery. 1997;40(4):664-672. discussion 672-674
Schmidek H.H., Roberts D.W. Schmidek & Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results, 5th ed., Philadelphia: Elsevier, 2006. 2 v. (xxxix, 2337, p.67 )
Spetzler R.F., Wilson C.B., Weinstein P., et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1977;25:651-672.
Spetzler R.F., et al. Surgical management of large AVM’s by staged embolization and operative excision. J Neurosurg. 1987;67(1):17-28.
Spetzler R.F., et al. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76(6):918-923.
Valavanis A., Christoforidis G.. Endovascular management of cerebral arteriovenous malformations. Neurointerventionist, 1999;1:34-40
Valavanis A., Yasargil M.G. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg. 1998;24:131-214.
Vinuela F., Duckwiler G., Guglielmi G. Contribution of interventional neuroradiology in the therapeutic management of brain arteriovenous malformations. J Stroke Cerebrovasc Dis. 1997;6(4):268-271.
Vinuela F., et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg. 1991;75(6):856-864.
Wikholm G., Lundqvist C., Svendsen P. Embolization of cerebral arteriovenous malformations: part I—Technique, morphology, and complications. Neurosurgery. 1996;39(3):448-457. discussion 457-459
1. Ogilvy C.S., et al. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Circulation. 2001;103(21):2644-2657.
2. Friedlander R.M. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007;356(26):2704-2712.
3. Latchaw R.E., et al. Functional magnetic resonance imaging as a management tool for cerebral arteriovenous malformations. Neurosurgery. 1995;37(4):619-625. discussion 625-626
4. Maldjian J., et al. Functional magnetic resonance imaging of regional brain activity in patients with intracerebral arteriovenous malformations before surgical or endovascular therapy. J Neurosurg. 1996;84(3):477-483.
5. Berube J., et al. Diffusion tensor imaging analysis of long association bundles in the presence of an arteriovenous malformation. J Neurosurg. 2007;107(3):509-514.
6. Feliciano C.E., et al. Provocative Test with Propofol: experience in Patients with Cerebral Arteriovenous Malformations Who Underwent Neuroendovascular Procedures. AJNR Am J Neuroradiol. 2010;31(3):470-475.
7. Lazar R.M., et al. Anterior translocation of language in patients with left cerebral arteriovenous malformation. Neurology. 1997;49(3):802-808.
8. Moo L.R., et al. Tailored cognitive testing with provocative amobarbital injection preceding AVM embolization. AJNR Am J Neuroradiol. 2002;23(3):416-421.
9. Muller-Forell W., Valavanis A. How angioarchitecture of cerebral arteriovenous malformations should influence the therapeutic considerations. Minim Invasive Neurosurg. 1995;38(1):32-40.
10. Crawford P.M., et al. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49(1):1-10.
11. Stapf C., et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66(9):1350-1355.
12. Graf C.J., Perret G.E., Torner J.C. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58(3):331-337.
13. Al-Shahi R., Warlow C. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain. 2001;124(Pt 10):1900-1926.
14. Pritz M.B. Ruptured supratentorial arteriovenous malformations associated with venous aneurysms. Acta Neurochir (Wien). 1994;128(1-4):150-162.
15. Redekop G., et al. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89(4):539-546.
16. Spetzler R.F., et al. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76(6):918-923.
17. Hartmann A., et al. Determinants of staged endovascular and surgical treatment outcome of brain arteriovenous malformations. Stroke. 2005;36(11):2431-2435.
18. Schaller C., Schramm J. Microsurgical results for small arteriovenous malformations accessible for radiosurgical or embolization treatment. Neurosurgery. 1997;40(4):664-672. discussion 672-674
19. Spears J., et al. A discriminative prediction model of neurological outcome for patients undergoing surgery of brain arteriovenous malformations. Stroke. 2006;37(6):1457-1464.
20. Brown R.D.Jr, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68(3):352-357.
21. Brown R.D.Jr, Wiebers D.O., Forbes G.S. Unruptured intracranial aneurysms and arteriovenous malformations: frequency of intracranial hemorrhage and relationship of lesions. J Neurosurg. 1990;73(6):859-863.
22. Cunha e Sa M.J., et al. The treatment of associated intracranial aneurysms and arteriovenous malformations. J Neurosurg. 1992;77(6):853-859.
23. Hartmann A., et al. Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002;33(7):1816-1820.
24. Starke R.M., et al. Treatment guidelines for cerebral arteriovenous malformation microsurgery. Br J Neurosurg. 2009;23(4):376-386.
25. Turjman F., et al. Aneurysms related to cerebral arteriovenous malformations: superselective angiographic assessment in 58 patients. AJNR Am J Neuroradiol. 1994;15(9):1601-1605.
26. Hartmann A., et al. Treatment of arteriovenous malformations of the brain. Curr Neurol Neurosci Rep. 2007;7(1):28-34.
27. Forster D.M., Steiner L., Hakanson S. Arteriovenous malformations of the brain. A long-term clinical study. J Neurosurg. 1972;37(5):562-570.
28. Pollock B.E., et al. Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke. 1996;27(1):1-6.
29. Lasjaunias P., et al. Cerebral arteriovenous malformations (C. AVM) and associated arterial aneurysms (AA). Analysis of 101 C. AVM cases, with 37 AA in 23 patients. Acta Neurochir (Wien). 1988;91(1-2):29-36.
30. Marks M.P., et al. Intranidal aneurysms in cerebral arteriovenous malformations: evaluation and endovascular treatment. Radiology. 1992;183(2):355-360.
31. Khayata M.H., et al. False aneurysm associated with rupture of an arteriovenous malformation—implications for treatment: case report. Neurosurgery. 1993;33(4):753-756.
32. Haw C.S., et al. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006;104(2):226-232.
33. Yuki I., et al. Treatment of brain arteriovenous malformations with high-flow arteriovenous fistulas: risk and complications associated with endovascular embolization in multimodality treatment. J Neurosurg. 2010;113(4):715-722.
34. Schmidek H.H., Roberts D.W. Schmidek & Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results, 5th ed., Philadelphia: Saunders Elsevier, 2006. 2 v. (xxxix, 2337, p.67)
35. Soderman M., et al. Management of patients with brain arteriovenous malformations. Eur J Radiol. 2003;46(3):195-205.
36. Willinsky R.A., et al. Delayed angiography in the investigation of intracerebral hematomas caused by small arteriovenous malformations. Neuroradiology. 1993;35(4):307-311.
37. Griffiths P.D., Beveridge C.J., Gholkar A. Angiography in non-traumatic brain haematoma. An analysis of 100 cases. Acta Radiol. 1997;38(5):797-802.
38. Hino A., et al. Value of repeat angiography in patients with spontaneous subcortical hemorrhage. Stroke. 1998;29(12):2517-2521.
39. Heros R.C., Morcos J., Korosue K. Arteriovenous malformations of the brain. Surgical management. Clin Neurosurg. 1993;40:139-173.
40. Pikus H.J., Beach M.L., Harbaugh R.E. Microsurgical treatment of arteriovenous malformations: analysis and comparison with stereotactic radiosurgery. J Neurosurg. 1998;88(4):641-646.
41. Pik J.H., Morgan M.K. Microsurgery for small arteriovenous malformations of the brain: results in 110 consecutive patients. Neurosurgery. 2000;47(3):571-575. discussion 575-577
42. Pile-Spellman J., et al. Adenosine-induced cardiac pause for endovascular embolization of cerebral arteriovenous malformations: technical case report. Neurosurgery. 1999;44(4):881-886. discussion 886-887
43. Eskridge J.M. Interventional neuroradiology. Radiology. 1989;172(3 Pt 2):991-1006.
44. Purdy P.D., Batjer H.H., Samson D. Management of hemorrhagic complications from preoperative embolization of arteriovenous malformations. J Neurosurg. 1991;74(2):205-211.
45. Vinuela F., Dion J., Halbach V. Interventional Neuroradiology: endovascular Therapy of the Central Nervous System. New York, NY: Raven Press; 1992.
46. Debrun G.M., et al. Embolization of the nidus of brain arteriovenous malformations with n-butyl cyanoacrylate. Neurosurgery. 1997;40(1):112-120. discussion 120-121
47. Wikholm G., Lundqvist C., Svendsen P. The Goteborg cohort of embolized cerebral arteriovenous malformations: a 6-year follow-up. Neurosurgery. 2001;49(4):799-805. discussion 805-806
48. Wakhloo A.K., et al. Transvenous n-butyl-cyanoacrylate infusion for complex dural carotid cavernous fistulas: technical considerations and clinical outcome. AJNR Am J Neuroradiol. 2005;26(8):1888-1897.
49. Lieber B.B., et al. Acute and chronic swine rete arteriovenous malformation models: effect of Ethiodol and glacial acetic acid on penetration, dispersion, and injection force of n-butyl 2-cyanoacrylate. AJNR Am J Neuroradiol. 2005;26(7):1707-1714.
50. Gounis M.J., et al. Effect of glacial acetic acid and ethiodized oil concentration on embolization with n-butyl 2-cyanoacrylate: an in vivo investigation. AJNR Am J Neuroradiol. 2002;23(6):938-944.
51. Moore C., Murphy K., Gailloud P. Improved distal distribution of n-butyl cyanoacrylate glue by simultaneous injection of dextrose 5% through the guiding catheter: technical note. Neuroradiology. 2006;48(5):327-332.
52. n-BCA Trail Investigators. n-Butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol. 2002;23(5):748-755.
53. Molyneux A.J., Coley S.C. Embolization of spinal cord arteriovenous malformations with an ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide (Onyx liquid embolic system). Report of two cases. J Neurosurg. 2000;93(Suppl 2):304-308.
54. Weber W., et al. Endovascular treatment of intracranial arteriovenous malformations with onyx: technical aspects. AJNR Am J Neuroradiol. 2007;28(2):371-377.
55. Murayama Y., et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery. 1998;43(5):1164-1175.
56. Pérez-Higueras A., López R.R., Taria D.Q.. Endovascular treatment of cerebral AVM: our experience with Onyx. Interventional Neuroradiology, 2005;11:141-157
57. Panagiotopoulos V., et al. Embolization of intracranial arteriovenous malformations with ethylene–vinyl alcohol copolymer (Onyx). AJNR Am J Neuroradiol. 2009;30(1):99-106.
58. Valavanis A., Yasargil M.G. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg. 1998;24:131-214.
59. Jahan R., et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48(5):984-995. discussion 995-997
60. Chaloupka J.C., et al. A reexamination of the angiotoxicity of superselective injection of DMSO in the swine rete embolization model. AJNR Am J Neuroradiol. 1999;20(3):401-410.
61. Taki W., et al. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol. 1990;11(1):163-168.
62. Davidson G.S., Terbrugge K.G. Histologic long-term follow-up after embolization with polyvinyl alcohol particles. AJNR Am J Neuroradiol. 1995;16(Suppl 4):843-846.
63. Purdy P.D., et al. Preoperative embolization of cerebral arteriovenous malformations with polyvinyl alcohol particles: experience in 51 adults. AJNR Am J Neuroradiol. 1990;11(3):501-510.
64. Wallace R.C., et al. The safety and effectiveness of brain arteriovenous malformation embolization using acrylic and particles: the experiences of a single institution. Neurosurgery. 1995;37(4):606-615. discussion 615-618
65. Linfante I., Wakhloo A.K. Brain aneurysms and arteriovenous malformations: advancements and emerging treatments in endovascular embolization. Stroke. 2007;38(4):1411-1417.
66. Sorimachi T., et al. Embolization of cerebral arteriovenous malformations achieved with polyvinyl alcohol particles: angiographic reappearance and complications. AJNR Am J Neuroradiol. 1999;20(7):1323-1328.
67. Mathis J.A., et al. The efficacy of particulate embolization combined with stereotactic radiosurgery for treatment of large arteriovenous malformations of the brain. AJNR Am J Neuroradiol. 1995;16(2):299-306.
68. Deruty R., et al. The combined management of cerebral arteriovenous malformations. Experience with 100 cases and review of the literature. Acta Neurochir (Wien). 1993;123(3-4):101-112.
69. Vinuela F., Duckwiler G., Guglielmi G. Contribution of interventional neuroradiology in the therapeutic management of brain arteriovenous malformations. J Stroke Cerebrovasc Dis. 1997;6(4):268-271.
70. Lundqvist C., Wikholm G., Svendsen P. Embolization of cerebral arteriovenous malformations: part II—Aspects of complications and late outcome. Neurosurgery. 1996;39(3):460-467. discussion 467-469
71. Gobin Y.P., et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85(1):19-28.
72. Pierot L., et al. Endovascular treatment of brain arteriovenous malformations using onyx: results of a prospective, multicenter study. J Neuroradiol. 2009;36(3):147-152.
73. Leonardi M., S.L, Cenni P., et al. Brain AVM embolization with Onyx: analysis of treatment in 34 patients. Interventional Neuroradiology, 2005;11:185-204
74. Tevah J., Huete I.. Endovascular treatment of cerebral AVMs with a new material: onyx. Interventional Neuroradiology, 2005;11:165-170
75. Joseph S., Chadaga H.C., Murali K.. Endovascular treatment of cerebral AVMs with Onyx: initial experience. Interventional Neuroradiology, 2005;11:171-178
76. Florio F., et al. Endovascular treatment of intracranial arterio-venous malformations with Onyx embolization: preliminary experience. Radiol Med. 2003;106(5-6):512-520.
77. van Rooij W.J., Sluzewski M., Beute G.N. Brain AVM embolization with Onyx. AJNR Am J Neuroradiol. 2007;28(1):172-177. discussion 178
78. Song D.L., et al. Clinical experience of 70 cases of cerebral arteriovenous malformations embolization with Onyx, a novel liquid embolic agent. Zhonghua Wai Ke Za Zhi. 2007;45(4):223-225.
79. Mounayer C., et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol. 2007;28(3):518-523.
80. Wikholm G., Lundqvist C., Svendsen P. Embolization of cerebral arteriovenous malformations: part I—Technique, morphology, and complications. Neurosurgery. 1996;39(3):448-457. discussion 457-9
81. Valavanis A., Christoforidis G.. Endovascular management of cerebral arteriovenous malformations. Neurointerventionist, 1999;1:34-40
82. Fournier D., et al. Revascularization of brain arteriovenous malformations after embolization with bucrylate. Neuroradiology. 1990;32(6):497-501.
83. Katsaridis V., Papagiannaki C., Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50(7):589-597.
84. Yu S.C., et al. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. AJNR Am J Neuroradiol. 2004;25(7):1139-1143.
85. Oran I., Parildar M., Derbent A. Ventricular/paraventricular small arteriovenous malformations: role of embolisation with cyanoacrylate. Neuroradiology. 2005;47(4):287-294.
86. Paulsen R.D., et al. Embolization of basal ganglia and thalamic arteriovenous malformations. Neurosurgery. 1999;44(5):991-996. discussion 996-997
87. Hamada J., et al. A mixture of ethylene vinyl alcohol copolymer and ethanol yielding a nonadhesive liquid embolic agent to treat cerebral arteriovenous malformations: initial clinical experience. J Neurosurg. 2002;97(4):881-888.
88. Spetzler R.F., et al. Surgical management of large AVM’s by staged embolization and operative excision. J Neurosurg. 1987;67(1):17-28.
89. Jafar J.J., et al. The effect of embolization with n-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg. 1993;78(1):60-69.
90. DeMeritt J.S., et al. Outcome analysis of preoperative embolization with n-butyl cyanoacrylate in cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 1995;16(9):1801-1807.
91. Cromwell L.D., Harris A.B. Treatment of cerebral arteriovenous malformations: a combined neurosurgical and neuroradiological approach. J Neurosurg. 1980;52(5):705-708.
92. Debrun G., et al. Embolization of cerebral arteriovenous malformations with bucrylate. J Neurosurg. 1982;56(5):615-627.
93. Liebman K.M., Rosenwasser R.H.. The hemodynamic changes measured in cerebral arterio-venous malformations following endovascular treatment. J Neurovasc Dis, 1997;2:112-116
94. Dawson R.C.3rd, et al. Treatment of arteriovenous malformations of the brain with combined embolization and stereotactic radiosurgery: results after 1 and 2 years. AJNR Am J Neuroradiol. 1990;11(5):857-864.
95. Dion J.E., Mathis J.M. Cranial arteriovenous malformations. The role of embolization and stereotactic surgery. Neurosurg Clin N Am. 1994;5(3):459-474.
96. Rao V.R., et al. Dissolution of isobutyl 2-cyanoacrylate on long-term follow-up. AJNR Am J Neuroradiol. 1989;10(1):135-141.
97. Pollock B.E., et al. Repeat stereotactic radiosurgery of arteriovenous malformations: factors associated with incomplete obliteration. Neurosurgery. 1996;38(2):318-324.
98. Berenstein A., Lasjaunias P., Surgical Neuroangiography, Vol. 4;New York, NY, Springer-Verlag, 1987.
99. Fox A.J., et al. Rolandic arteriovenous malformations: improvement in limb function by IBC embolization. AJNR Am J Neuroradiol. 1985;6(4):575-582.
100. Vinuela F.V., et al. Dominant-hemisphere arteriovenous malformations: therapeutic embolization with isobutyl-2-cyanoacrylate. AJNR Am J Neuroradiol. 1983;4(4):959-966.
101. Grzyska U., Fiehler J. Pathophysiology and treatment of brain AVMs. Klin Neuroradiol. 2009;19(1):82-90.
102. Meisel H.J., et al. Effect of partial targeted n-butyl-cyano-acrylate embolization in brain AVM. Acta Neurochir (Wien). 2002;144(9):879-887. discussion 888
103. Rosenwasser R.H., Thomas J.E., Gannon P.M., et al. Current Strategies for the Management of Cerebral Arteriovenous Malformations. Rolling Meadows, Illinois: American Association of Neurological Surgeons; 1998.
104. Purdy P.D., et al. Intraarterial sodium amytal administration to guide preoperative embolization of cerebral arteriovenous malformations. J Neurosurg Anesthesiol. 1991;3(2):103-106.
105. Purdy P.D., et al. Arteriovenous malformations of the brain: choosing embolic materials to enhance safety and ease of excision. J Neurosurg. 1992;77(2):217-222.
106. Wilson C.B., Hieshima G. Occlusive hyperemia: a new way to think about an old problem. J Neurosurg. 1993;78(2):165-166.
107. al-Rodhan N.R., et al. Occlusive hyperemia: a theory for the hemodynamic complications following resection of intracerebral arteriovenous malformations. J Neurosurg. 1993;78(2):167-175.
108. Guglielmi G. Analysis of the hemodynamic characteristics of brain arteriovenous malformations using electrical models: baseline settings, surgical extirpation, endovascular embolization, and surgical bypass. Neurosurgery. 2008;63(1):1-10. discussion 11
109. Spetzler R.F., Wilson C.B., Weinstein P., et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1977;25:651-672.
110. Picard L., et al. Acute spontaneous hemorrhage after embolization of brain arteriovenous malformation with n-butyl cyanoacrylate. J Neuroradiol. 2001;28(3):147-165.
111. Young W.L., Pile-Spellman J. Anesthetic considerations for interventional neuroradiology. Anesthesiology. 1994;80(2):427-456.
112. Frizzel R.T., Fisher W.S.3rd. Cure, morbidity, and mortality associated with embolization of brain arteriovenous malformations: a review of 1246 patients in 32 series over a 35-year period. Neurosurgery. 1995;37(6):1031-1039. discussion 1039-1040
113. Luessenhop A.J., Spence W.T. Artificial embolization of cerebral arteries. Report of use in a case of arteriovenous malformation. J Am Med Assoc. 1960;172:1153-1155.
114. Fiorella D., et al. The role of neuroendovascular therapy for the treatment of brain arteriovenous malformations. Neurosurgery. 2006;59(5 suppl 3):S163-S177. discussion S3-13
115. Taylor C.L., et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100(5):810-812.
116. Vinuela F., et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg. 1991;75(6):856-864.
117. Kim L.J., et al. Postembolization neurological deficits in cerebral arteriovenous malformations: stratification by arteriovenous malformation grade. Neurosurgery. 2006;59(1):53-59. discussion 53-59
118. Ledezma C.J., et al. Complications of cerebral arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery. 2006;58(4):602-611. discussion 602-611
119. Jayaraman M.V., et al. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. AJNR Am J Neuroradiol. 2008;29(2):242-246.
120. Pierot L., Januel A.C., Herbreteau D., et al. Endovascular treatment of brain arteriovenous malformations using Onyx: preliminary results of a prospective multicenter study. Interventional Neuroradiology. 2005;11:159-164.
121. Kis B., Siekmann R., Jans P., Laumer R., Kühne D. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery. 2007;61(2):244-252. discussion 252-254