Chapter 36 Endovascular Therapy for Aortic Dissection
Acute aortic dissection (AAD) is a precipitous event associated with a wide range of outcomes from uncomplicated to catastrophic. Current endovascular strategies are based on identifying features that portend increased risk of death or other poor outcome and applying interventional techniques to prevent the life-threatening complications of the dissection.1–3
During the last 2 decades, there has been increasing interest in exploring endovascular procedures for management of aortic dissection.4–13 Initially, endovascular approaches focused on addressing branch vessel involvement and ischemic complications associated with the dissection process8,9 (Fig. 36-1). Subsequently, endovascular aortic stent grafts (initially developed to repair aortic aneurysms) were applied in type B aortic dissection to cover the primary entry tear of the dissection and promote thrombosis of the thoracic aortic false lumen4,5 (Fig. 36-2). These basic endovascular tactics are now routine in the contemporary armamentarium for treatment of aortic dissection and its myriad manifestations.
Branch Vessel Interventions
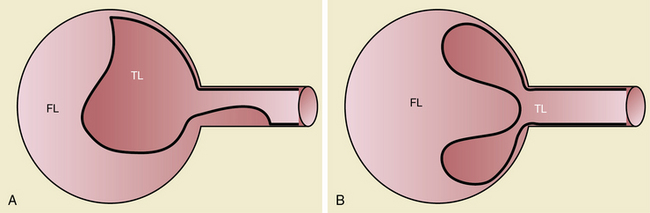
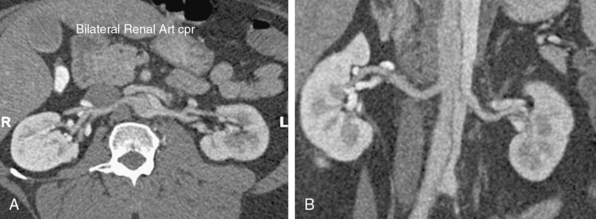
Branch vessel involvement accompanying aortic dissection is a well-recognized complication occurring in over 30% of cases.7,8,14 For appropriate intervention selection, the pathoanatomical concepts of static and dynamic branch involvement are crucial to selection of the endovascular option for reperfusion of an affected vascular bed.15–17 As the dissection process extends distally from the primary entry tear, the dissection septum may engage the ostia of branch vessels. If the aortic flap, which consists of the intima and portion of the media shorn away from the wall, engages a branch orifice as it extends, two pathophysiological situations referred to respectively as static and dynamic branch involvement may occur (Fig. 36-3).
Static Branch Involvement
One manifestation that may arise when the advancing dissection septum intersects an aortic branch is static branch vessel involvement (Fig. 36-4). In static involvement, the aortic dissection flap extends directly into the branch for a variable distance. In contrast to the geometry described earlier, orientation of the septal trajectory is such that the branch ostium is incompletely engaged by the edge of the dissection plane. Rather than being circumferentially shorn by the septum, there is only partial circumferential involvement of the branch by the dissection. The aortic flap extends into the branch, creating a false lumen within the artery. As a result, the individual branch has both a true and false lumen like the aorta.
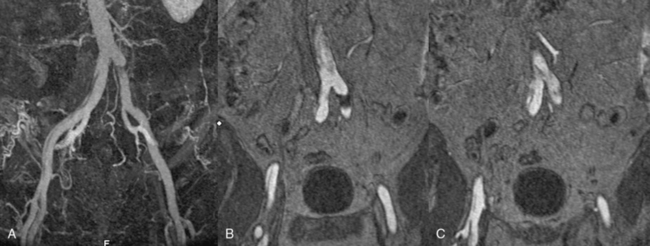
In most cases, the preferred strategy involves increasing branch flow by decreasing the resistance to true lumen blood flow. This is performed by placing a stent in the true lumen of the branch through catheterization from the aortic true lumen. The stent is typically placed from beyond the end of the false lumen in the branch back to the aortic true lumen. A self-expanding nitinol stent is commonly employed because this distance is frequently greater than 2 cm and because there is a risk of squeezing any existing clot out of the false lumen with a balloon-expandable stent. These stents are sized to the total transarterial diameter of the branch and allowed to progressively expand on their own (post deployment) without supplemental balloon dilation. There are many successful reports of this approach in mesenteric, renal, and iliac arteries affected by no-reentry or static involvement.8,9,18,19
Occasionally, static branch vessel involvement with reentry anatomy and double-barrel flow may require endovascular intervention. The most common indication for stent placement in this setting occurs with involvement of a renal artery (Fig. 36-5). The kidney supplied by a dissected renal artery may be affected by the physical presence of a flap within the branch. The variable flow reduction caused by the flap, and resultant disrupted pattern of true and false lumen perfusion, may contribute to an exacerbation of hypertension. In cases where high blood pressure is sustained and recalcitrant to numerous intravenous (IV) medications, endovascular intervention may be warranted to restore a single lumen without flap. The approach to treatment involves placement of a balloon-expandable renal stent within the true lumen of the renal artery through the aortic true lumen. In most cases, this type of reentry involvement does not extend into the branch as far as the no-reentry extension. Thus, stents less than 2-cm long are typically implanted. This technique is well established at most centers that manage cases of aortic dissection frequently.
Dynamic Branch Involvement
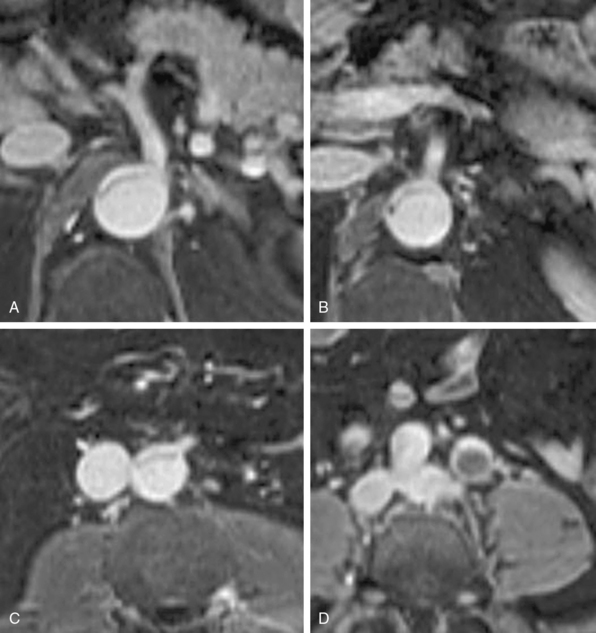
In addition to primary branch pathology that occurs as a complication of aortic dissection, another mechanism, dynamic branch vessel involvement, may be responsible for organ ischemia. Dynamic branch involvement is a phenomenon associated with obstruction to branch vessel flow by an aortic septum that has prolapsed over the branch ostia like a curtain. In contrast to static involvement, where the aortic flap extends directly into a branch, dynamic obstruction occurs as an aortic process exclusively without an associated branch lesion. Propagation of the aortic flap may create a circumferential cleavage of the aortic wall surrounding the branch ostium (Fig. 36-6). Factors associated with this event include the flap trajectory, the resultant orientation of the septal plane proximal to the branch, and the inclusion of the ostium by the cleaved flap as it extends past. In this situation, the dissection septum surrounds the branch ostium as it tears distally. The cleavage plane extends 1 to 2 mm into the branch, and then circumferentially reenters, creating a cylindrical tear, coring out a short segment of the intimal/medial lining of the most proximal aspect of the branch. The septum retracts into the aortic lumen with a fenestration corresponding to the branch orifice. This gives the flap a stencil-like appearance when viewed en face, with the number of holes related to the number of branch vessels involved by this phenomenon. When imaged in an axial plane, the affected artery appears to originate exclusively from the aortic false lumen. Closer inspection usually allows identification of a tear in the flap at the level or adjacent to the level of the branch. The flap often displays small projections angled from the edge of the tear, giving its outline on axial imaging an appearance similar to the contour of a metal rivet, the short-legged extensions corresponding to the amputated proximal lining of the branch.
Aortic Interventions
Endovascular aortic stent grafting is a less invasive alternative to open surgery for selected patients with both thoracic and abdominal aneurysms. Recently, the application of similar technology for management of acute aortic syndromes, including aortic dissection, has emerged as a focus of interest and study.7,10–13,20,21 As with any new procedure, the key question is the determination of specific patient populations who may benefit from the new technique. In this regard, the use of traditional classification parameters for risk stratification of aortic dissection patients has advanced evaluation of the possible benefits and risks of endograft management.
Medical management is the traditional treatment strategy for uncomplicated acute aortic dissection. Current reports cite a 30-day mortality rate of approximately 10%.22,23 Use of stent grafting for stable uncomplicated patients with type B aortic dissection has yet to realize any improvement in survival compared to traditional medical therapy. Indeed, current conservative noninterventional management of uncomplicated cases is associated with 1-year survival rates of around 80%. Such results may be hard to improve upon with endograft therapy.23,33
Stent Grafts for Uncomplicated Type B Dissection
The Investigation of Stent Grafts in Patients with Type B Aortic Dissection (INSTEAD) trial observed that elective stent graft placement in survivors of uncomplicated chronic type B dissection does not improve 1-year survival and adverse event rates compared with medical therapy. Among the 140 patients randomized in this prospective trial, 1-year survival was 91% compared with 97% in patients randomized to medical therapy.34,35 Moreover, aorta-related mortality was not different, and the risk for the combined endpoint of aorta-related death (rupture) and progression (including conversion or additional endovascular or open surgical intervention) was similar.
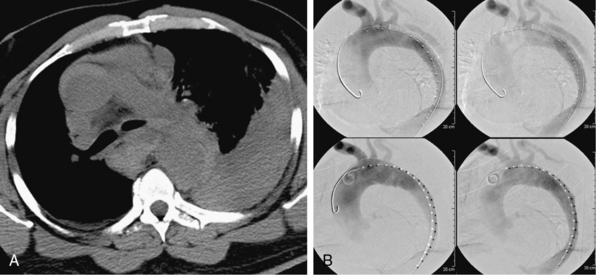
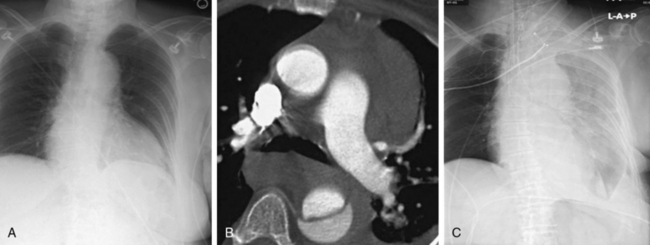
In the setting of complicated aortic dissection, medical management is associated with a high mortality rate, such that most patients will undergo surgery to address life-threatening complications.2,4 Depending on the patient’s underlying medical conditions and the nature of the complication(s), surgical mortality rates range from between 30% and 60% or higher.24,25 It is in these high-risk scenarios that an opportunity exists to establish a role for interventional management. Thus the question becomes, What constitutes complicated type B aortic dissection? There is no strict definition for this category of disease, but traditionally it is relegated to two unambiguous disease manifestations: aortic rupture (Fig. 36-7) and symptomatic branch vessel involvement. These conditions are clear and their diagnosis unequivocal. Other adverse effects of the dissection process, such as uncontrollable hypertension, unrelenting pain, and increasing pleural fluid, defy easy classification and do not have uniform criteria for comparative assessment. These so-called softer indications for intervention are commonly included as a surgical indication in most published series of acute complicated dissection.8,26
Endograft Treatment of Complicated Type B Dissection
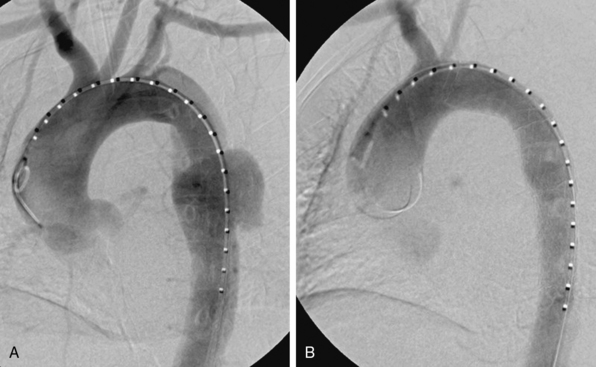
The procedural goal for endovascular stent grafting in patients with complicated acute type B aortic dissection is endograft elimination of blood flow entry into the proximal entry tear. Obliterating the primary communication between the true lumen and the false redirects pulsatile flow into the true lumen, promotes false lumen thrombosis, and ultimately improves remodeling of the aorta by increasing the dimensions of the true lumen while shrinking the false lumen (Fig. 36-8).
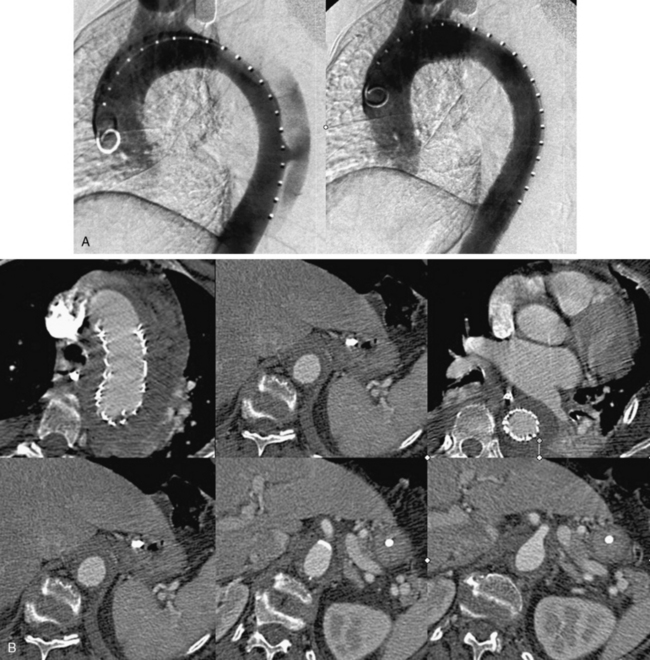
Figure 36-8 Treatment and follow-up imaging of type B aortic dissection with rupture.
A, Aortograms pre- and post placement of a thoracic endograft across mid-descending aorta entry tear of a type B dissection in the 68-year-old woman described in Figure 36-7. B, Series of axial computed tomography (CT) images obtained 1 week postendograft management of a type B dissection with rupture. Stent graft is in good position, and false lumen is thrombosed. Residual extravascular blood and hematoma are evident.
Endovascular Treatment of Branch Vessel Involvement
The outcomes of stent graft therapy for reversal of dynamic branch vessel involvement are excellent, with procedural success in up to 95% of cases and complete false lumen thrombosis in 85% of patients.7–9 These procedures are associated with 67.7% 5-year freedom from aortic rupture and open repair.9 Additionally, static branch involvement remote from the covered proximal aortic entry tear may require separate targeted intervention to manage residual ischemic compromise. This is especially important in cases with no-reentry anatomy complicating static branch involvement. In these situations, endovascular branch intervention should be provided emergently.
An alternative to endograft placement in dynamic branch compromise is distal flap fenestration.9,27 Percutaneous balloon fenestration of the aortic septum has replaced the operative procedure. Balloon fenestration of the septum is designed to unload the aortic false lumen by decreasing the resistance to outflow. Technically, initial transgression of the aortic flap with a small cardiac transseptal TIPS needle and cannula usually is performed from the small true lumen into the larger target of the false channel. The site of the needle puncture commonly lies within the infrarenal aorta at the level of the aortic bifurcation. Once successful transgression of the septum is confirmed, a wire is advanced across the flap and well into the targeted lumen. Sequentially larger balloon dilation of the flap is performed until a final size of between 20 and 25 mm is obtained.
Aortic Rupture
Rupture that complicates aortic dissection is an interventional imperative.28,29 The procedural considerations for aortic rupture focus on preventing exsanguination. Both open surgical and endovascular therapies are associated with high mortality and morbidity rates in the presence of aortic rupture. Recent reports suggest that endovascular approaches permit treatment of more patients, including older and less fit individuals whose operative risk in this setting is prohibitive.21,28,29
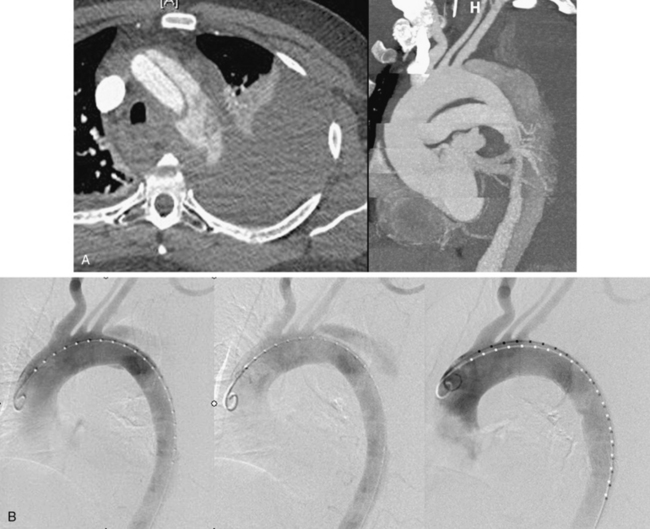
Localizing the precise site of rupture noninvasively is not always possible. The point of rupture through the false lumen wall may be evident by the presence of contrast enhancement beyond the anticipated aortic border, though this occurs typically in the setting of severe hemodynamic instability or shock (Fig. 36-9). More commonly, a periaortic, mediastinal, and/or pleural collection is evident on CT imaging, which has an appearance and attenuation value consistent with hematoma or complex fluid. This abnormality may be most prominent around a focal aortic segment or extend diffusely over a wider zone.
The goal of endograft management for aortic rupture is coverage of the proximal entry tear, with isolation of the false lumen, to ensure false lumen obliteration and expeditious thrombosis. It is thrombosis of the false lumen that prevents aortic leakage of blood. To facilitate rapid false lumen thrombosis, the overall endograft coverage of the aorta is often longer than that used for other thoracic pathologies. By extending the length of coverage (20-30 cm) to at least the level of the diaphragm or celiac trunk, the aortic septum is braced by the stent in the true lumen, and the thoracic false lumen is converted to a long, inverted cul-de-sac, or blind pouch. Then with flap pulsation limited by the buttressing stent, blood in the false lumen becomes stagnant and prone to thrombosis.30–32
Other Indications for Aortic Endografts
Marui et al. proposed that patients with uncomplicated aortic dissection and transaortic diameter greater than 40 mm were at high risk of rapid aortic expansion.36 When applied to larger groups of patients with dissection, this benchmark provided modest prognostic value. The poor results encouraged others to focus on the issues and pursue more in-depth imaging analysis. Thereafter, Marui et al. offered an improved prognostic factor that was based on the extent of proximal descending aorta dilation at the time of initial diagnosis37: the fusiform index. This index is defined as the maximum transaortic diameter of the distal aortic arch divided by the sum of the minimum diameter of the proximal aortic arch plus the aortic diameter at the level of the pulmonary artery. A value greater than 0.64 anticipates late aortic events in patients with uncomplicated type B aortic dissection. The investigators recommended that patients with these predictors should undergo early intervention with open surgery or stent graft implantation.
Immer et al. analyzed imaging studies (CT or MRI) over the initial 18 months after diagnosis in 84 patients with acute type A aortic dissection.38 They concluded that a large false lumen at the time of the initial diagnostic scan is the strongest predictor of subsequent downstream aortic enlargement. This was especially true if the true lumen was less than 30% of the overall transaortic area 6 months after aortic surgery for repair of type A dissection.
This concept of the initial false lumen diameter as a determinant of late clinical deterioration was evaluated for type B disease in 2007 by Song et al.39 These authors studied 100 consecutive patients with acute aortic dissection, including 51 with type A dissection and 49 with type B dissection. Over half of the patients underwent CT imaging follow-up through 24 months. Of these, an aneurysm (diameter > 60 mm) was diagnosed in 28%, with the maximal aortic diameter located in the proximal descending segment. A greater than 22-mm initial false lumen diameter of the upper thoracic segment of the descending aorta predicted late aneurysm formation with a sensitivity of 100% and a sensitivity of 76%. The 42 patients with an initial false lumen diameter greater than 22 mm had a higher event rate than the 58 with smaller false lumen aortic diameters (aneurysm, 42% vs. 5%; or death, 12% vs. 5%).
More recently, another predictive feature for early complication and clinical deterioration was described by Tsai et al. after reviewing data from the International Registry of Aortic Dissection (IRAD).40 They reviewed 201 cases of type B acute aortic dissection. During the index hospitalization, 114 patients (56.7%) had a patent false lumen, 68 patients (33.8%) had partial thrombosis of the false lumen, and 19 (9.5%) had complete thrombosis of the false lumen. The mean 3-year mortality rate for patients with a patent false lumen was 13.7%, for those with partial thrombosis was 31.6%, and for those with complete thrombosis was 22.6%. Although postdischarge mortality was high among patients with acute type B aortic dissection, partial thrombosis, as compared with complete patency, is a significant independent predictor of postdischarge mortality (relative risk, 2.69; 95% confidence interval [CI], 1.45-4.98; P = 0.002).
1 Erbel R., Alfonso F., Boileau C., et al. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22:1642–1681.
2 Glower D.D., Speier R.H., White W.D., et al. Management and long-term outcome of aortic dissection. Ann Surg. 1991;214:21–41.
3 Wong D.R., Lemaire S.A., Coselli J.S. Managing dissections of the thoracic aorta. Am Surg. 2008;74:364–380.
4 Dake M.D., Kato N., Mitchell R.S., et al. Endovascular stent graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340:1546–1552.
5 Neinaber C.A., Fattori R., Lund G., et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539–1545.
6 Mukherjee D., Eafle K.A. Aortic dissection–an update. Curr Probl Cardiol. 2005;30:287–325.
7 Parker J.D., Golledge J. Outcome of endovascular treatment of acute type B aortic dissection. Ann Thorac Surg. 2008;86:1707–1712.
8 Fattori R., Botta L., Lovato L., et al. Malperfusion syndrome in type B aortic dissection: role of the endovascular procedures. Acta Chir Belg. 2008;108:192–197.
9 Patel H.J., Williams D.M., Meekov M., et al. Long-term results of percutaneous management of malperfusion in acute type B aortic dissection: implications for thoracic aortic endovascular repair. J Thorac Cardiovasc Surg. 2009;138:300–308.
10 Czermak B.V., Waldenberger P., Fraedrich G., et al. Treatment of Stanford type B aortic dissection with stent grafts: preliminary results. Radiology. 2000;217:544–550.
11 Feezor R.J., Martin T.D., Hess P.J., et al. Early outcomes after endovascular management of acute, complicated type B aortic dissection. J Vasc Surg. 2009;49:561–566.
12 Pearce B.J., Passman M.A., Patterson M.A., et al. Early outcomes of thoracic endovascular stent-graft repair for acute complicated type B dissections using the gore TAG endoprosthesis. Ann Vasc Surg. 2008;22:742–749.
13 Parsa C.J., Schroder J.N., Daneshmand M.A., et al. Midterm results for endovascular repair of complicated acute and chronic type B aortic dissection. Ann Thorac Surg. 2010;89:97–104.
14 Oderich G.S., Panneton J.M., Bower T.C., et al. Aortic dissection with aortic side branch compromise: impact of malperfusion on patient outcome. Perspect Vasc Surg Endovasc Ther. 2008;20:190–200.
15 Apostolakis E., Baikoussis N.G., Georgiopoulos M. Acute type-B aortic dissection: the treatment strategy. Hellenic J Cardiol. 2010;51:338–347.
16 Williams D.M., Lee D.Y., Hamilton B.H., et al. The dissected aorta: part III. Anatomy and radiological diagnosis of branch-vessel compromise. Radiology. 1997;203:37–44.
17 Williams D.M., Lee D.Y., Hamilton B.H., et al. The dissected aorta: percutaneous treatment of ischemic complications-principles and results. J Vasc Interv Radiol. 1997;8:605–625.
18 Shiiya N., Matsuzaki K., Kunihara T., et al. Management of vital organ malperfusion in acute aortic dissection: proposal of a mechanism-specific approach. Gen Thorac Cardiovasc Surg. 2007;55:85–90.
19 Deeb G.M., Patel H.J., Williams D.M. Treatment for malperfusion syndrome in acute type A and B aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg. 2010;140:98–100.
20 Kische S., Ehrlich M.P., Nienaber C.A., et al. Endovascular treatment of acute and chronic aortic dissection: midterm results from the talent thoracic retrospective registry. J Thorac Cardiovasc Surg. 2009;138:115–124.
21 Fattori R., Tsai T.T., Myrmel T., et al. Complicated acute type B dissection: is surgery still the best option?: a report from the international registry of acute aortic dissection. JACC Cardiovasc Interv. 2008;1:395–402.
22 Tefera G., Acher C.W., Hoch J.R., et al. Effectiveness of intensive medical therapy in type B aortic dissection: a single-center experience. J Vasc Surg. 2007;45:1114–1118.
23 Estrera A.L., Miller C.C., Safi H.J., et al. Outcomes of medical management of acute type B aortic dissection. Circulation. 2006;114:384–389.
24 Giersson A., Szeto W.Y., Pochettino A., et al. Eur J Cardiothorac Surg. 2007;32:255–262.
25 Hagan P.G., Nienaber C.A., Isselbacher E.M., et al. The international registry of acute aortic dissection (IRAD): new insight into an old disease. JAMA. 2000;283:897–903.
26 Trimarchi S., Eagle K.A., Neinaber C.A., et al. Importance of refractory pain and hypertension in acute type B aortic dissection: insights from the international registry of acute aortic dissection (IRAD). Circulation. 2010;122:1283–1289.
27 Slonim S.M., Miller D.C., Mitchell R.S., et al. Percutaneous balloon fenestration and stenting for life-threatening ischemic complications in patients with acute aortic dissections. J Thorac Cardiovasc Surg. 1999;117:1118–1127.
28 Xenos E.S., Minion D.J., Davenport D.L., et al. Endovascular versus open repair for descending thoracic aortic rupture: institutional experience and meta-analysis. Eur J Cardiothorac Surg. 2009;35:282–286.
29 Patel H.J., Williams D.M., Upchurch G.R., et al. A comparative analysis of open and endovascular repair for the ruptured descending thoracic aorta. J Vasc Surg. 2009;50:1265–1270.
30 Resch T.A., Delle M., Falkenberg M., et al. Remodeling of the thoracic aorta after stent grafting of type B dissection: a Swedish multicenter study. J Cardiovasc Surg (Torino). 2006;47:503–508.
31 Kusagawa H., Shimono T., Ishida M., et al. Changes in false lumen after transluminal stent-graft placement in aortic dissections: six years’ experience. Circulation. 2005;111:2951–2957.
32 Sayer D., Bratby M., Brooks M., et al. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur J Vasc Endovasc Surg. 2008;36:522–529.
33 Tsai T.T., Fattori R., Trimarchi S., et al. Long-term survival in patients presenting with type B acute aortic dissection: insights from the international registry of acute aortic dissections. Circulation. 2006;114:2226–2231.
34 Nienaber C.A., Rousseau H., Eggebrecht H., et al. Randomized comparison of strategies for type B aortic dissection. The Investigation of Stent Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120:2519–2528.
35 Nienaber C.A., Kische S., Akin I., et al. Strategies for subacute/chronic type B aortic dissection: the Investigation of Stent Grafts in Patients with Type B Aortic Dissection (INSTEAD) trial 1-year outcome. J Thorac Cardiovasc Surg. 2010;140:101–108.
36 Marui A., Mochizuki T., Mitsui N., et al. Toward the best treatment for uncomplicated patients with type B acute aortic dissection: a consideration for sound surgical indication. Circulation. 1999;100(Suppl II):II-275–II-280.
37 Marui A., Mochizuki T., Koyama T., et al. Degree of fusiform dilation of the proximal descending aorta in type B acute aortic dissection can predict late events. J Thorac Cardiovasc Surg. 2007;134:1163–1170.
38 Immer F., Krahenbuhl E., Hagan U., et al. Large area of false lumen favors secondary dilation of the aorta after acute type A aortic dissection. Circulation. 2005;112(Suppl I):I-249–I-252.
39 Song J.M., Kim J.H., Kang D.H., et al. Long-term predictors of descending aorta aneurismal change in patients with aortic dissection. J Am Coll Cardiol. 2007;50:799–804.
40 Tsai T.T., Evangelista A., Nienaber C.A., et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med. 2007;357:349–359.