Chapter 40 Endovascular Therapy for Abdominal Aortic Aneurysms
Surgical treatment of abdominal aortic aneurysms (AAAs) dates back centuries. Some of the initial approaches involved techniques similar in some fashion to modern endovascular techniques. In 1684, Moore reported on the use of large quantities of wire placed intraluminally into the aneurysm sac to induce thrombosis of an AAA.1 Later, electric currents were passed through the wire to further promote thrombosis. A self-expanding endoluminally placed umbrella device was reported by Colt in the early 1900s to treat AAA.2 In the mid-1900s, the use of endoluminally placed wire with the passage of electricity through it was revived and remained the procedure of choice until conventional operative therapy of AAA was introduced.3 Operative repair of AAA evolved during the second half of the 20th century. Early techniques ranged from simple aortic ligation to aortic wrapping with cellophane.4,5 Neither was successful. In 1951, the first replacement of an aortic aneurysm with an aortic homograft was described by Dubost et al.6 Homografts, however, became aneurysmal over time, and the procedure evolved to the use of prosthetic material to reconstruct the aorta.7,8 This technique was later modified by Creech, who reported on endoaneurysmorrhaphy with intraluminal graft placement, leaving the aneurysm sac in situ9; this has become the mainstay of treatment.
Although excellent results have been obtained with conventional aneurysm repair, it remains a complex, challenging operation that initiates great physiological stress for patients. The pursuit of a less invasive approach to AAA repair has subsequently evolved. Parodi et al.10 reported the first use of endovascular AAA repair (EVAR). This approach allowed for intraluminal exclusion of an aneurysm with placement, through the femoral arteries, of an endograft. The hope was that this would decrease the morbidity and mortality of aneurysm repair and allow repairs to be performed in patients with significant comorbidities. The original endograft was constructed of a Dacron tube sutured to a Palmaz stent. Several generations of endografts have since been developed, tested, and put into general clinical use. With the evolution of aortic endografting, our knowledge about the pathophysiology of AAA has changed. Our understanding of the complexities of this mode of treatment is only just being realized and examined. This chapter reviews what is currently understood about endograft repairs of abdominal aortic aneurysms.
Indications
The indications for endovascular repair of an AAA remain the same as conventional repair with regard to the size of the aneurysm and its rate of growth. The classic teaching is that rupture rates for aneurysms depend on the size of the aneurysm. Rupture rates of 5% to 7% per year are estimated for aneurysms between 5 and 7 cm in diameter, and a greater than 20% rupture rate per year is estimated for larger aneurysms.11 Compared with observation, surgical treatment for patients with these larger aneurysms significantly improves mortality.12 Although it is known that small aneurysms do have the potential to rupture, the U.K. Small Aneurysm Trial, which randomized patients with AAAs between 4.5 and 5.5 cm to either surgery or observation, suggested that early repair did not improve survival.13 The operative arm of this study, however, had a mortality rate of 5.8%, which is high compared with other large series of elective open AAA repair.14 Perhaps with lower mortality rates in the operative arm, the conclusions of the study would have been reversed.15
Endovascular AAA repair is a less invasive technique than open surgery and offers several potential benefits over conventional AAA repair. It requires small femoral incisions instead of a large abdominal incision, which may decrease the incidence of postoperative pulmonary complications. Avoidance of extensive retroperitoneal dissection decreases the risk for perioperative bleeding. The period of aortic occlusion is minimal and accounts for the lower incidence of intraoperative hemodynamic and metabolic stress compared with patients undergoing open surgery.16 Given these differences, endovascular aneurysm repair may be reasonable in patients who are “unfit” for conventional AAA surgery.17 Proving its durability as a replacement for conventional surgery in relatively healthy patients is the aim of many clinical trials, the results of which will be discussed in more detail later.
Anatomical Requirements
Imaging
Historically, contrast aortography was used as a routine adjunct to axial imaging because it was felt to allow for a more accurate determination of vessel length and angulation before computed tomography (CT) reformatting was widely available. Preoperative angiography is now rarely employed and reserved for cases where an adjunctive therapeutic intervention (i.e., coil embolization) is necessary.18 Recently the advent of flat panel technology has allowed for development of rotational angiography techniques that facilitate construction of three-dimensional (3D) images found to be comparable to standard multidetector computed tomographic angiography (CTA). This technique has been termed C-arm cone-beam CT or fluoro-CT. Fluoro-CT uses a modified C-arm with specialized software and allows for precise measurements to be performed without using standard CT imaging.19 Its routine use in preoperative planning prior to EVAR is currently under investigation.20
Spiral CT of the abdomen and pelvis is the mainstay of aortoiliac imaging. The imaging protocol is different from the standard protocol for most abdominal CT scans. Acquisition should use a 1:1.5 helical pitch and 3- to 5-mm collimation.21 Two- to 3-mm slices are ideal for providing adequate information for stent graft planning. The two-dimensional (2D) images, however, can often be misinterpreted. The axial images may “cut” vessels at an angle, particularly iliac arteries that have some degree of tortuosity, thus creating an ellipse as opposed to visualization of the true lumen diameter. Due to this problem, some physicians recommend 3D image processing as a better method to evaluate aortoiliac anatomy for endograft therapy.22 Although it was common initially to perform an angiogram in addition to a CT scan, recent evaluations suggest that high-quality 3D CT scans alone may provide sufficient data for endovascular graft planning.18,23 Currently, proprietary products for CT postprocessing provide the ability to evaluate the 3D reconstruction of the aortoiliac system rapidly, rotate the images on the screen to obtain better vessel diameter measurements, and provide “virtual endograft” simulation.
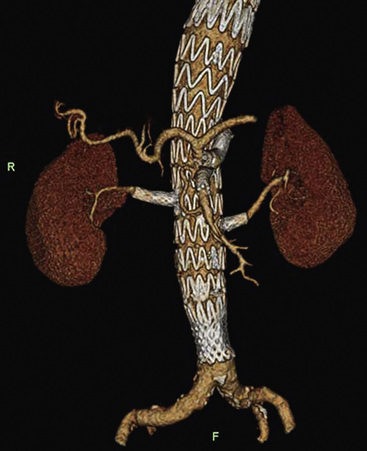
Aortic Neck
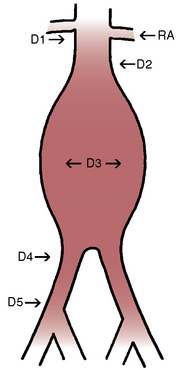
The aortic neck is defined as the area of the aorta cephalad to the aneurysm in which the aortic endograft is placed (Fig. 40-1). This zone of the aorta is important for two reasons during aortic endografting. First, it is the site of proximal fixation that will prevent the device from migrating distally. Second, a circumferential seal must be obtained between the graft and the aorta in this area to prevent leakage of blood into the aneurysm sac. The exact length of aortic neck required is somewhat device dependent, but most commercially available devices require a 10- to 15-mm length of aortic neck below the level of the most caudal renal artery. Some investigational devices may allow for shorter necks. Several devices employ the use of a suprarenal uncovered (or bare) stent to provide additional protection against graft migration. Suprarenal stent fixation may be useful, particularly in patients who have a shorter aortic neck, because it transfers protection against migration to a more normal segment of aorta. The suprarenal stent, however, does not provide any function with regard to creating a circumferential seal.
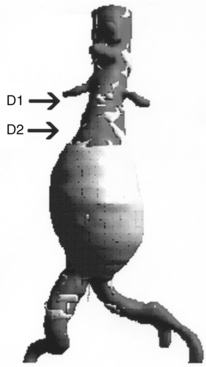
In addition to the length of the neck, other anatomical characteristics are important when determining whether patients are suitable candidates for endovascular aneurysm repair. These include aortic neck angulation, the shape of the neck, and the quality of the neck. Neck angulation refers to an alteration in the direction the aorta takes with regard to the centerline pathway. Acute angulation of the aortic neck can greatly affect the endograft’s ability to obtain a proximal seal. Aortic neck angulation of greater than 60 degrees compared to the centerline is often considered prohibitive for endovascular aneurysm repair. The shape of the aortic neck also affects the ability of the graft to obtain a seal as well as fixation. A conical-shaped neck (Fig. 40-2) is generally felt to be unstable and predisposes to distal migration.24 An increase in diameter from the top of the neck to the bottom of greater than 10% is often believed to be a contraindication to routine aortic endografting. Presence of circumferential thrombus or aortic calcification can also negatively affect an endograft’s ability to obtain a proximal seal.
Iliac Arteries
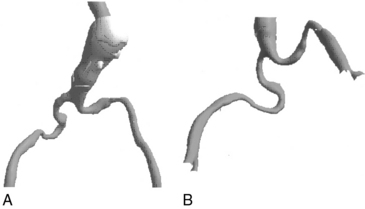
The iliofemoral arterial system is important in endograft placement for two reasons. First, most endografts are placed through the common femoral artery (CFA) and must traverse the iliofemoral system to reach the aorta. Iliac artery diameter and tortuosity can adversely affect the ease with which the endograft traverses this course. This topic is covered in more detail below. Certainly the presence of significant atherosclerotic disease can cause arterial narrowing that inhibits placement of the device. In addition, tortuosity of the iliac arteries can hinder placement of the grafts (Fig. 40-3). Second, the iliofemoral system is important because it is the site of the distal seal between the endograft and the iliac artery, preventing retrograde flow of blood into the aneurysm sac. Many of the features necessary for an adequate aortic neck are also necessary for the distal landing zone. Presence of thrombus, calcification, and tortuosity can significantly hinder the iliac limb seal. Ectatic or aneurysmal iliac arteries obviously affect the ability of the graft to seal against the iliac limb. Most available endograft systems require at least a 15-mm segment of iliac artery to be of adequate caliber and free of significant disease to obtain a distal seal. If this is not present, adjunct interventions can be performed to assist in placing the device (i.e., iliac artery conduit placement, coil embolization of the internal iliac artery [IIA]). Management of these complicated situations is discussed in more detail later.
Endograft Design
Delivery System
Standard endograft insertion involves placement of the device through an arteriotomy in the common femoral artery, from where the graft traverses the external iliac and common iliac arteries (CIAs). The ability to deliver the endograft safely and effectively in this fashion is a prerequisite for effective repair. Three factors are important determinants of device delivery.25
Flexibility
Tortuosity, another anatomical variant, affects the ability to adequately deliver the endograft system. Tortuous iliac vessels can be “straightened” with the use of stiff guidewires, but this is not always possible or desirable. The ideal delivery system easily traverses these arteries on the basis of an intrinsic degree of flexibility. Again, different delivery systems have different abilities to track through tortuous iliac arteries, and some may be more successfully placed than others in this anatomical variant. Delivery systems composed of long, flexible, tapered tips pass more easily than those with short, stiff, blunt tips. In addition, other aspects of device construction, such as metallic struts that provide columnar strength, increase device rigidity and limit use in tortuous vessels.25
Deliverability
A number of features have been noted to affect the deliverability of endograft devices. As stated previously, long, flexible, tapered tips pass more easily than short, blunt, stiff ones. This allows for easier maneuverability through tortuous vessels, as well as past sites of narrowing. Larger-caliber devices are also more difficult to deliver, particularly in patients with smaller-diameter arteries.25 Some delivery systems allow for placement of the endograft system through alternate sheaths, whereas other systems necessitate use of the manufacturer’s own delivery system. This can greatly affect placement of specific endografts in specific anatomical variants. A thorough understanding of the patient’s arterial anatomy and the limitations of different endograft systems are important. The complexity of the delivery system also affects the ease with which it is placed. Some devices generally provide a simple maneuver to deploy the graft, whereas others have several complicated steps.
Specific Grafts
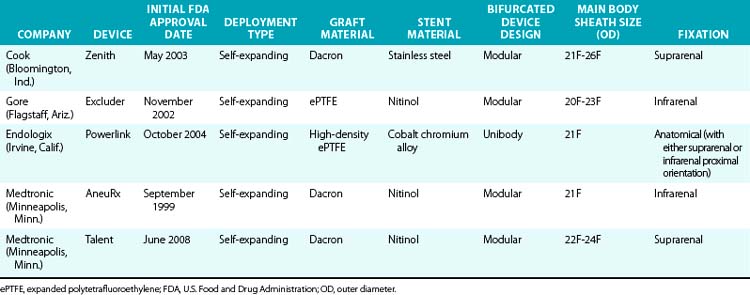
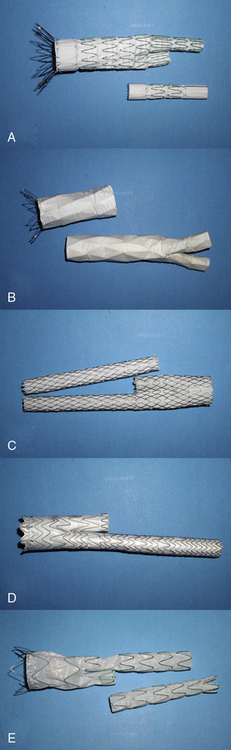
Various endografts are currently commercially available or in clinical trials in the United States. A brief description of the currently commercially available endograft systems (in the United States) is outlined in Table 40-1 and depicted in Figure 40-4.
Graft Placement and Postoperative Management
Once the patient is deemed an endograft candidate, the best graft has been chosen, and the device properly sized, the patient can undergo implantation. The majority of endografts are placed through the femoral arteries that have been operatively exposed. The majority of surgeons prefer the use of the transverse incision as it associated with a lower rate of wound complications (12.7% in transverse incision vs. 47.5% in vertical incisions).26 Percutaneous access for EVAR is growing in popularity, and its use will become even more widespread with the further development of low- profile devices. Suture-mediated closure devices facilitate this process, and using a “preclose” technique has been described to allow closure of sheaths as large as 24 F.27 Use of this procedure has been associated with 70% to 100% technical success, and immediate failures mandate surgical exploration of the femoral artery. Prospective analysis has demonstrated that use of a percutaneous approach may shorten operating times and reduce the rate of wound-related complications, without a significant increase in overall procedural cost.27–29 The aorta is then cannulated with a guidewire and catheter. Small boluses of contrast agent are delivered to further define the anatomy and localize the renal arteries. With an angulated aorta, it is important to remember that the best view of the renal arteries and visualization of the fixation zone may not be in a direct anterior-posterior plane but at a more cranial-caudal angle. The device is then generally advanced over a stiff guidewire and correctly positioned to allow the most extensive coverage within the aortic neck without intruding on the orifice of the renal arteries. Each device has its own unique instruction for actual deployment. Once the main body and ipsilateral limb have been placed, the contralateral limb has to be placed. The sequence of events for this varies depending on graft design—whether unibody or modular.
Recovery following EVAR is generally rapid and uncomplicated, and most patients are discharged home on the first or second postoperative day. Return to activities of daily living has been shown to be quicker following endovascular repair than open surgery. In addition, most patients report less postoperative pain. Aortic remodeling following EVAR, however, is a slow process that continues for several years. Anatomical changes in the native vessel, particularly at the proximal neck, can cause conformational changes in the implanted device that mandate close follow-up. In addition, late failures have been identified that have required reintervention.30 Given these facts, routine surveillance following EVAR is universally recommended, although there are no standard regimens, and the requirements of a standard intensive regimen are debated. Nordon et al. performed a meta-analysis evaluating secondary intervention rates based on contemporary graft implants.31 Their findings demonstrated that surveillance imaging alone initiated the secondary intervention in 1.4% to 9% of cases. Over 90% of EVAR cases, however, received no benefit from surveillance scans. Based on these findings, the group recommended that surveillance should be directed toward those patients identified as having a high risk for postoperative complications. Identification of this group, however, is not obvious but may be necessary in patients with complicated aortic neck anatomy or in patients in whom the device was used outside of the indications for use (IFU).
Contrast-enhanced CT is the most widely used modality for follow-up after EVAR. It is widely available, has rapid data acquisition, reproducibility, and is uniform across institutions. The major concerns associated with this modality are use of a contrast agent and the potential associated nephrotoxicity, radiation exposure, and cost. It is considered the gold standard for assessing aortic diameter, with nearly 100% sensitivity and specificity. Sensitivity and specificity rates for endoleak detection with CT are better than those with conventional angiography: 92% and 90% for CT vs. 63% and 77% for angiography, respectively.32–34 Triphasic CT (noncontrasted phase, arterial phase, and delayed phase) results in the greatest amount of information but at the cost of increased radiation exposure. Unenhanced CT imaging is useful for differentiation of endoleaks from calcifications from the metallic portion of a stent graft, and can help detect small perigraft leaks better than arterial-phase images. Use of arterial phase alone has a lower diagnostic value than combined arterial and delayed-phase scanning.35
Repeated CT scanning subjects the patient to potential carcinogenic risks associated with ionizing radiation exposure. The estimated lifetime attributable risk of death from cancer following an abdominal CT scan in a patient older than 50 years of age is 0.02%.31,36 Although this effect in itself is small, the cumulative effects over time with repeat imaging can be significant. Repetitive use of iodinated contrast can have a cumulative deleterious effect on renal function, especially in the elderly and those patients with preexisting renal impairment.37 Given this, as well as the expense, the use of alternate modes of surveillance has been evaluated.
Magnetic resonance imaging (MRI) and MRA provide much of the same imaging information that can be acquired by CT scanning. Multiple format MR images (T1, T2, gadolinium-enhanced) can be viewed in 2D and be reformatted into 3D volumes, allowing dimensional measurements, assessment of luminal patency, device positioning, and detecting the presence of an endoleak. Limitations of MRA/MRI include potential magnet-induced in vivo metallic heating or motion, which may prevent imaging in the immediate postimplant time period. In addition, postimplant artifacts, in particular with ferromagnetic metallic stents, will limit morphological assessments. Furthermore, there is a risk of nephrogenic systemic fibrosis associated with gadolinium contrast use in patients with renal insufficiency.38 Benefits of MRI are related to the lack of exposure to ionizing radiation and low nephrotoxicity of MR contrast medium. Disadvantages of MRI are its lack of wide availability, longer procedure time than CT, patient claustrophobia, and contraindications for patients with cardiac pacemakers.
Color duplex ultrasonography (US) is a convenient, noninvasive, inexpensive portable means of postimplant surveillance. Its reliability as a useful surveillance tool, however, is still debated. Grayscale US is accurate for measurement of aortic aneurysm diameter. Endoleak detection by US requires color duplex. The reported specificity rates of color duplex US for endoleak detection are high (89%-97%), but the sensitivity and diagnostic power of color duplex US for endoleak detection compared with CT is still debated.39 The use of duplex US to detect an endoleak has a sensitivity of approximately 77%, with a specificity approaching 94%.40 The addition of US contrast agents increases sensitivity to 98%, with no significant change in specificity.40 Contrast agents are useful in identifying slow leaks that are not readily discernible on CT.41,42 Detection of flow direction of the endoleak is also an advantage of US that is not easily discernable with CT.43 Presence of a “to-and-fro” flow pattern is associated with spontaneous closure of the endoleak, whereas a monophasic or biphasic waveform is consistent with endoleak persistence.43 Limitations of color duplex US include its operator dependence, variation based on patient physical size, and the need for optimal patient preparation. The substitution of duplex US imaging for CT, however, may result in long-term cost savings.44
Problems with Endografting and Management
Iliac Artery Disease
When iliac artery disease is present, whether it be aneurysmal disease, atherosclerotic disease, or severe tortuosity, the use of an iliac conduit can provide a safe route to deliver the endograft.45 In cases of iliac artery lumen narrowing resulting from atherosclerotic disease or increased vessel tortuosity, advancement of the device, despite the presence of resistance, can result in rupture of the iliac artery. Iliac artery rupture has been reported in 1% to 2% of cases.46,47 To circumvent prohibitive iliac artery anatomy, an iliac conduit can be used. An iliac conduit involves suturing a prosthetic graft (generally 8-10 mm in diameter) to the mid–CIA even if it is aneurysmal. This can be done in an end-to-end or end-to-side fashion, although the latter often provides a greater lumen for passage of the device. The device is placed through the prosthetic graft, and the iliac limb of the endovascular graft traverses the CIA and anastomosis and seals within the conduit. The distal end of the graft is tunneled along the natural course of the iliac artery and anastomosed to the femoral artery. The distal end of the CIA is oversewn to allow retrograde flow through the EIA to supply the ipsilateral hypogastric artery. Alternatively, the hypogastric artery can be anastomosed directly to the conduit.
Iliac artery ectasia or aneurysms can present a problem in obtaining a distal seal. Enlarged CIAs are present in up to 30% of patients presenting for endovascular aneurysm repair.48–51 Many available endografts do not allow for ectatic or aneurysmal common iliac arteries, so the distal seal may have to be obtained within the external iliac artery, which is often of normal caliber. If the distal seal occurs in the external iliac artery, the hypogastric artery is generally sacrificed using coil embolization. Presence of a hypogastric artery aneurysm would necessitate the same approach. Rarely, bilateral hypogastric artery embolization is required. Hypogastric artery embolization can occur before aneurysm repair or concurrently. If bilateral embolization is planned, it is generally performed in a staged fashion, although its occlusion is not always planned. Hypogastric artery embolization is not without risk, and side effects can occur in up to 50% of patients.49 Buttock claudication is the predominant complaint after hypogastric artery occlusion. This occurs in 12% to 50% of patients, but in most it generally resolves after several months.48–53 Some 5% to 25% of men complain of new-onset erectile dysfunction (ED).51,52 Buttock ischemia and bowel ischemia requiring resection are of theoretical concern, but they have not been described in any of the larger series. Patients requiring embolization in the more distal branches of the hypogastric artery (as might be done with the presence of an IIA aneurysm) and those in whom coil placement was not adequately controlled are at higher risk of developing pelvic symptoms.53 Bilateral hypogastric artery embolization has not been associated with increased symptoms when compared with unilateral occlusion.49,50,52 Coil embolization of the IIA is not necessary if it is not aneurysmal. In the face of CIA aneurysms, Wyers et al.54 have shown that if there is a 5-mm neck of iliac artery proximal to the hypogastric artery in addition to a 15-mm neck in the external iliac artery, coil embolization of the hypogastric artery is not necessary to obtain a distal seal. This may be possible in up to two thirds of patients requiring coverage of the hypogastric artery.
Endoleaks
An endoleak is the persistence of blood flow outside the endograft, but in the aneurysm sac. Endoleaks are classified according to their etiology, and currently five types have been described47,48 (Table 40-2). A type 1 endoleak (Fig. 40-5) arises from inadequate sealing at either the proximal aortic (allowing antegrade flow) or distal iliac (allowing retrograde flow) attachment sites. Type 2 endoleaks (Fig. 40-6) arise from patent branch vessels off of the aortic sac that allow for retrograde flow into the aneurysm. Such branches may include a patent lumbar or inferior mesenteric artery (IMA). Type 3 endoleaks develop from defects in the fabric of the graft or at the junction zone between modular components. Type 4 endoleaks develop secondary to diffuse “leaking” of blood between the interstices of the fabric or where the graft is sutured to a stent. Type 5 endoleaks describe a scenario in which the aneurysm sac remains pressurized and the aneurysm enlarges, but no demonstrable flow of blood into the sac can be visualized on current imaging modalities. These may be due to imaging that is not sophisticated enough to discern these leaks or due to intermittent episodes of leakage.49 The pressure applied to the aneurysm sac causing it to continue to expand in this situation, has been termed endotension.50,51 Controversy with regard to this concept exists, in particular with the ability of the thrombus to transmit pressure to the aneurysm wall. It is argued that these merely represent a type 1, 2, or 3 endoleak in which the defect is large enough to allow blood to flow into the sac and transmit pressure to the sac, but the exit site is not present or too small to be detected.
| Endoleak | Cause | Blood Flow Into Sac |
|---|---|---|
| Type 1 | Inadequate seal at aortic or iliac attachment sites | Antegrade or retrograde |
| Type 2 | Patent branches off aneurysm sac | Retrograde |
| Type 3 | Fabric defects or component junctions | Antegrade |
| Type 4 | Leak at fabric interstices | Antegrade |
| Type 5 | Endotension | No clear leak |
Type 1 and type 3 endoleaks are associated with significant risks of aneurysm enlargement and possible rupture, and these should be treated.55,56 This may be accomplished with placement of an extension cuff limb over the site of the leak. If the leak is a type 1 and the graft is juxtaposed to the inferior border of the renal arteries, a large balloon-expandable stent can be placed in the proximal aspect of the endograft. This provides increased radial force, causing better juxtaposition of the graft and aortic wall, thus ameliorating the leakage. If this is unsuccessful, open repair and graft explantation are generally indicated. Fabric tears are easily managed if the site of the leak is localized. In these situations, the tear can be covered with a cuff or extension. When it is more diffuse, the entire endograft can be relined with a second endograft, or the device can be removed and the aneurysm repaired in an open fashion.
Type 2 endoleaks are rarely associated with aneurysm rupture.57 At least 10% to 15% of patients are identified with a type 2 endoleak during follow-up.58–61 Warfarin treatment is not associated with an increased incidence of early or delayed postoperative endoleak, but type 2 endoleaks are less likely to undergo spontaneous resolution in these patients.62 Type 2 endoleaks are generally observed unless they are associated with an increase in aneurysm size or aortic pulsatility on physical examination. In these situations, arteriography is the next step to identify the source of the endoleak. Superior mesenteric artery injection reveals retrograde IMA flow as the source, whereas selective hypogastric artery injection demonstrates a lumbar artery filling the aneurysm. Super-selective arterial canalization can then be performed with embolization of the feeding vessels. Another approach is through direct aneurysm sac puncture.63 With direct sac puncture, one can measure sac pressure and inject the sac directly with contrast agent to precisely identify the leak. The systolic sac pressure is related to the size of the leak, and the pulse amplitude is related to the resistance of the outflow vessels and sac compliance.64 After localization of the leak, feeding vessels can be directly accessed and embolized.65 In addition, the sac can be filled with substances such as coils, glue, or gel foam to further prevent flow. Differences in outcomes between these two different approaches has not been realized.66
Measurement of intrasac pressures may help determine whether an endoleak is present at the time of the original surgery or if an endoleak has been adequately treated if it has been approached through direct sac puncture. In an ex vivo model of endoleaks, Parodi et al.67 evaluated pressure changes in the aortic sac with various types and sizes of endoleaks. In this model, sac pressures were significantly higher than systemic pressures in the presence of all endoleaks. This obviously places the aneurysm at significant risk for rupture. Presence of patent side branches significantly reduced the pressure within the sac, particularly the mean pressure and diastolic pressure. Clinically, persistent side branches augment the development of type 2 endoleaks and influence early sac behavior.68,69 Gawenda et al.70 evaluated the use of sac pressure monitoring and found it helpful in the detection and treatment of endoleaks. They noted, however, that intrasac pressure measurements did not correlate with AAA size change over ensuing follow-up. This may be an effective modality for monitoring aneurysms after endograft exclusion once less invasive methods of pressure measurement are developed.
Structural Failure
Material failure represents one of the most concerning problems for potential failure of endograft placement. This is a difficult event to identify because patients are often asymptomatic and may not present with any acute changes in their endograft evaluation. Three modes of structural failure have been described in aortic endografting and involve fabric erosion, suture disruption, and metal fracture.71
Development of endoleaks secondary to graft erosion has been documented with some first-generation endograft devices72,73 (Fig. 40-7). It has been speculated that the areas of graft erosion are secondary to friction between the stent material and the fabric, which can be confounded by pulsation of the aorta. Predicting the incidence of fabric fatigue is difficult, and although this does occur in grafts placed by conventional open aneurysm repair, it occurs much more rapidly and more commonly in the endograft systems.74,75 In several device designs, the graft fabric is attached to the metal skeleton through the use of sutures. Disruption of these sutures is believed to explain graft failure in some instances.76–78 The mechanism for suture failure is believed to be the same as for fabric erosion—namely, motion of the stents with aortic pulsations causes friction and wear of the sutures, with subsequent suture fracture.
The most common structural problem identified in aortic endograft systems has been metallic stent fractures.75 Stent and hook fractures in the phase 1 trial of the Endovascular Technologie’s graft resulted in suspension of the program and redesign of the metallic attachment system.79 In a review of 686 patients who underwent endovascular aneurysm repair, Jacobs et al. identified 60 patients who had material failure.75 Forty-three (72%) of these failures were due to metallic stent fractures and occurred in various different endografts with different stent composition. The cause of metal failure has been attributed to stress fatigue and metal corrosion, particularly in nitinol stents.80 Corrosion has not been seen in next-generation endografts and may reflect improved nitinol processing.81–83 Tortuosity of the arterial system can also stress the stent graft system and lead to metal fracture. This has been reported in the longitudinal bar of the Talent and Gore stent graft devices.75
Limb Thrombosis
Endograft limb thrombosis after endovascular repair of infrarenal AAA is a recognized complication occurring in up to 11% of patients.84–90 Various underlying factors have been purported to place patients at increased risk for limb thrombosis. One reported risk factor is the lack of device support. Although Carroccio et al.85 reported on the results of 351 bifurcated grafts with no significant association between use of unsupported devices and graft thrombosis, others have suggested there is a significant relationship. Baum et al.91 specifically evaluated the rates of graft limb kinking and thrombosis between supported and unsupported abdominal aortic stent grafts. In total, 12% of the limbs in their series required an intervention for kinking. In the supported limbs, 5% required subsequent placement of arterial stents; 2% required these for evidence of kinking at the time of the initial operation, and 3% required stenting in the postoperative period after the patients presented with limb thrombosis. In the unsupported grafts, there was an intervention rate of 44%. About half of these had an additional stent placed at the time of the initial procedure, and the remainder had a subsequent stent placed in the postoperative follow-up period, owing to limb thrombosis or severe stenosis.
Another factor increasing the risk of limb thrombosis is oversizing of the iliac limb. Oversizing causes the graft material to have a significant amount of infolding, reducing the intraluminal diameter.87 Along these lines, significant intraluminal vessel narrowing from underlying atherosclerotic disease or tortuosity can result in flow abnormalities and eventually cause graft limb thrombosis.91 Extension of the graft limb into the EIA has also been described as a risk factor for developing limb thrombosis.85 It was believed that transition into the EIA caused both a significant reduction in arterial diameter and a kink in the graft due to acute angulation of the limb as it passed through the pelvis. Damage to the distal iliac or femoral artery, such as dissection during graft placement, can subsequently cause outflow obstruction and graft limb thrombosis.84
Management of patients presenting with limb thrombosis depends on the severity of the patient’s symptoms. In the series by Carroccio et al.,85 nearly a third of the patients presenting with symptoms had such mild symptoms that no intervention was required. Most patients, however, underwent a femoral-femoral bypass to restore flow to the affected extremity. Few patients are successfully treated with thrombolysis or graft thrombectomy followed by endovascular repair of the underlying problem. In most series, patients with limb problems generally present early, within the first 6 months following endograft repair.84,91–95 In fact, Sampram et al.93 reported that no limb occlusions presented after 30 months of follow-up.
Migration
Distal stent graft migration after abdominal aortic endografting has been reported to occur in 9% to 45% of patients.61,96–99 Migration certainly has been identified as a risk for development of a type 1 endoleak and delayed aneurysm rupture or late conversion to open repair. The pathophysiology behind aortic endograft migration is complex, and various factors contribute to its occurrence.100 A number of forces are at play within the aortic endograft, but blood flow acts as the main displacing force. As the tube of the aortic graft curves, there is a change in the velocity of the blood, resulting in an increased displacement force. For many endografts, the forces providing protection against migration are friction forces of the graft against the aortic wall and the columnar strength of the graft. The friction forces depend on the apposition of the graft fabric and the aortic wall and obviously can be affected by aortic wall composition (thrombus, calcifications), size of the aorta, radial force of the stent, and the nature of the graft fabric. It has been suggested that the presence of barbs or hooks in the proximal portion of the stent graft may provide additional protection.101
The infrarenal aortic neck length and its maximum diameter, shape, and angulation have all been implicated as causes of stent graft migration.96,102,103 All of these work to decrease the friction between the stent graft and aortic wall. Albertini et al.102 evaluated the development of proximal perigraft endograft leak and device migration following endovascular aneurysm repair. Fifteen patients had graft migration, and 31 of 184 repairs developed a proximal endoleak. Neck angulation was the only factor found to be significant in the development of device migration, whereas neck angulation and neck diameter were the two factors important in developing a proximal perigraft endoleak.102 Lee et al.,104 however, were unable to identify any specific anatomical correlate and device migration. They did observe that any device that migrated distally by more than 1 cm subsequently required an intervention.
Other hypotheses as to the cause of device migration have focused on morphological changes in the aneurysm and aortic neck after endovascular AAA repair. Specifically, aortic neck dilation, longitudinal sac shrinkage, and graft shortening have been described.96,97,105,106 One of the more widely accepted hypotheses is aortic neck dilation following aortic endografting. After endovascular aneurysm repair, the aneurysm neck has been documented to dilate significantly, mostly in the first 2 years after graft placement.107 In a review by Cao et al.,99 17 (15%) of 148 patients had an episode of device migration. The only two independent risk factors for device migration were neck dilation postoperatively and an AAA diameter of greater than 55 mm. Others have argued that neck dilation is not a significant event, provided adequate graft oversizing was performed at initial endograft placement.104 The amount the aortic neck dilated did not exceed the size of the original aortic endograft placed. Larger aneurysms have also been noted to have increased risks of developing type 1 endoleak, graft migration, and the subsequent need for open surgical conversion compared with larger aneurysms.108
Outcomes
Results of Aortic Endografting
Endovascular AAA repair generally has a low mortality rate (1%-3%) compared to open repair, and subsequent rates of aneurysm rupture after endovascular repair are reduced to 1% per year.56,61,93,109 Endograft placement is not free of adverse events, however, and there is frequent need for secondary interventions. Naslund et al.110 reported technical complications in 26% of 34 endografts placed. Fairman et al. evaluated the occurrence of critical events during deployment of their initial 75 endografts, and patients were divided into three groups corresponding to the time period in which the graft was placed.94 Critical events were defined as unanticipated technical difficulties that occurred during the course of operation that threatened the success of the procedure. Difficulty in obtaining access occurred in nearly one quarter of all patients. Although it would be expected that the latter 25 patients should not have experienced as great a difficulty in obtaining access, these patients had increased complexity of their aortoiliac anatomy compared with endograft patients earlier in their experience. This group had a greater frequency of iliac artery balloon angioplasty, as well as the use of iliac artery conduits. Deployment difficulties existed and were composed mostly of graft foreshortening, necessitating the placement of additional distal covered extensions. Other deployment issues encountered included suprarenal graft displacement, infrarenal graft displacement, and device-related issues such as iliac limb kinking or twisting. Malplacement of the graft did not correlate with anatomical complexity.
The need for subsequent secondary procedures has been evaluated by several large series of patients who had an abdominal aortic endograft placed.88,93,111,112 The Eurostar registry reported the results of 1023 patients with a follow-up of 12 months or longer.111 Overall, 186 (18%) patients required a secondary intervention. The majority of these interventions (76%) involved a transfemoral procedure, whereas the remaining patients required transabdominal (12%) or extra-anatomical (11%) surgery. The rates of freedom from intervention at 1, 3, and 4 years were 89%, 67%, and 62%, respectively. The transfemoral procedures performed most frequently were aortic or iliac limb extension for graft migration or endoleak. Late death was more frequent in those patients requiring a secondary intervention resulting in a 3-year cumulative survival of 85%, which is lower than the 90% rate (P <0.05) observed in those that did not require reintervention. In addition, death was more frequently associated with those requiring a transabdominal procedure.
The Montefiore Medical Center and the Cleveland Clinic Foundation have published their single-institution results on the durability of aortic endografting. Montefiore reported on 239 endografts placed over 9 years, with a technical success rate of 88.7%.88 The 5-year survival rate in this group was only 37%. Secondary interventions were required in 10% of the patients, with more than half of the secondary procedures being performed for presence of an endoleak. Sampram et al.93 reported the results from the Cleveland Clinic Foundation on 703 patients undergoing endovascular aortic endografting, with follow-up averaging 1 year. Survival in this group was 90% at 1 year and declined to 70% at 3 years. Overall, 128 secondary interventions were performed in 105 patients (15%). Freedom from intervention mirrored that of the Eurostar registry, with freedom from intervention rates of 88%, 76%, and 65% at 1, 2, and 3 years, respectively. Mortality related to the secondary procedure was 8% but rose to 18% in those requiring a transabdominal procedure. Univariate analysis revealed that secondary procedures were more common in patients with larger major and minor sac axes, in patients who received a large aortic stent because of a proximal endoleak present at initial aneurysm repair, and in patients who received treatment later in the course of the review. This last finding is felt to be secondary to the increased complexity of cases approached in an endovascular fashion.
The Cleveland Clinic Foundation review included the use of six different devices, which included two Zenith grafts—one that was part of the multicenter national trial and one group that was part of a sponsor-investigator investigational device exemption trial.25 The overall freedom from risk of rupture was 98.7% at 2 years. Results of this review reveal that there are significant differences in outcomes between groups with different endovascular devices, in particular with regard to limb occlusion and rate of endoleak. Limb occlusion occurred most frequently with the Ancure device, at a rate of 11% at 2 years. Endoleak of any kind was most common with the Excluder device, at a rate of 64% at 1 year. Modular separations were the most frequent with the Zenith graft at 3.5%. Aneurysm sac shrinkage correlated inversely with the frequency of endoleaks, and aneurysm sac shrinkage was most common in the Zenith and Talent groups but least common in the Excluder group. There were no differences with regard to rate of secondary procedures, conversion to open repair, or migration. Sternbergh et al.113 have reported similar findings. Outcomes were compared between the Zenith device and the AneuRx device, and it was determined that the Zenith graft was associated with fewer endoleaks and a higher rate and amount of aneurysm sac shrinkage. Bertges et al.114 also reported similar findings in their evaluation. Regression of AAA size after endograft placement was more significant after placement of the Talent and Ancure endografts than with the AneuRx or Excluder devices. During the first 2 years of follow-up, the initial size of the AAA, presence of an endoleak, and type of graft used were significant predictors of sac shrinkage. After 2 years, however, only graft type was significant. Ouriel et al.108 have additionally concluded that the outcome after endovascular AAA repair depends on the initial size of the aneurysm.
Comparison with Open Surgery
Endovascular AAA repair has been shown to be associated with lower postoperative morbidity, shorter length of hospital stay, and quicker return to normal function.115 Direct comparison to open surgery utilizing randomized prospective trials, however, has only recently been available for evaluation. There have been three randomized prospective trials evaluating the use of EVAR compared with open surgery. The EVAR-1 trial enrolled 1082 patients with AAA who were healthy enough to be suitable candidates for surgery.115 They were randomized to either EVAR or open repair. Results from this trial demonstrated that the 30-day mortality rate was lower after EVAR (1.7%) than open surgery (4.7%, P <0.0001). At 4-years follow-up, the aneurysm-related mortality rate in the EVAR group was half that in the open group (P = 0.04), but there was no difference in all-cause mortality (26% for the EVAR group and 29% for the open group). The Dutch Randomized Endovascular Aneurysm Management (DREAM) trial was a prospective randomized trial that enrolled 351 patients. As in EVAR-1, the 30-day mortality rate was lower in patients who underwent endovascular repair than in those who underwent open surgical reconstruction, but the 2-year outcomes were similar between the two groups. In addition, the results of the Open versus Endovascular Repair (OVER) Veterans Affairs Cooperative Study Group results have been reported.116 In this trial, 881 patients suitable for open or EVAR were randomized to one of the two surgical techniques. As with the other two trials, 30-day mortality was lower in the EVAR arm (0.5%) than the open surgery arm (3.0%, P = 0.004). This difference, however, resolved by 2-year follow-up time points (7.0% vs. 9.8%, respectively). Patients undergoing EVAR had shorter hospital stays, shorter operative durations, and required fewer blood transfusions, but they had increased exposure to fluoroscopy and contrast. Given its promising initial results, it is not surprising EVAR has become increasingly popular with both patients and providers over the past decade.
One of the most controversial aspects of AAA repair, however, is when to perform EVAR and when to perform conventional open surgery. Open surgical repair of AAA has long been considered the gold standard, and there is evidence this option provides good long-term durability.117,118 Endovascular AAA repair however, given its young age, does not have similar time-tested outcomes data. Recently, longer-term outcomes from both EVAR-1 and DREAM have been reported.30,119 For EVAR-1,30 the median follow-up was 6 years (range, 5-10 years), and at follow-up, the overall aneurysm-related mortality was 1.0 deaths per 100 person-years in the EVAR group and 1.2 deaths per 100 person-years in the open repair group (P = 0.73). All-cause mortality was 7.2 deaths per 100 person-years (EVAR) and 7.1 deaths per 100 person-years (open surgery). Graft-related complication rates were higher in the EVAR group (12.6 per 100 person-years) compared to the open surgical arm (2.5 per 100 person-years; P < 0.001), and significantly more patients in the EVAR group required reintervention (5.1 per 100 person-years vs. 1.7 per 100 person-years; P <0.001). In fact, new graft-related complications and reinterventions were reported for as long as 8 years following EVAR. For DREAM,119 at a median follow-up of 6.4 years (5.1-8.2 years), cumulative survival rates were 69.9% for open repair and 68.9% for EVAR. The cumulative rates of freedom from secondary interventions were 81.9% for the open repair group and 70.4% for EVAR (P = 0.03). Based on these data, it is clear that EVAR is not without its drawbacks. These factors may change as the technology improves and we gain a better understanding of the long-term implications of placing an endovascular graft in the aorta.
Initial applications of EVAR were geared toward patients considered high risk for conventional surgery, but this concept has come under some scrutiny after the results of the EVAR-2 trial.120 In this trial, the outcomes of 404 patients with large AAAs (≥5.5 cm in diameter) who were considered to be physically ineligible for open repair were evaluated. Of this cohort, 197 patients were assigned to undergo endovascular repair, while 207 were assigned to have no intervention. The 30-day operative mortality rate for the EVAR group was 7.3%, and the overall rate of aneurysm rupture in the observation group was 12.4 per 100 person years. Aneurysm-related mortality was lower in the endovascular repair group, but this did not provide an advantage when evaluating all-cause mortality, and during follow-up, EVAR required a considerable increase in expense. These results called into question the appropriateness of using EVAR in high-risk patients. The results, however, have been refuted by others demonstrating lower rates of perioperative mortality and better long-term survivals in these high-risk patients.121 These improved outcomes are likely due to an aggressive multidisciplinary approach to managing these patients’ comorbidities.
Other Considerations
EVAR for Small Abdominal Aortic Aneurysms
Randomized prospective trials have demonstrated that there is no benefit to open repair of AAA for aneurysms that are less than 5.5 cm in diameter.122,123 Operative mortality rates of 2.7% and 5.8% in these two trials raised the question of whether a procedure with lower operative mortality might provide benefit compared with observation in patients with smaller AAA. The Positive Impact of Endovascular Options for Treating Aneurysms Early (PIVOTAL) trial sought to evaluate whether endovascular repair of small AAA (4-5 cm) might provide a survival advantage compared with surveillance.124 In this trial, 728 patients were randomly assigned to either EVAR (n = 366) or ultrasound surveillance (n = 362). Of the patients randomized to EVAR, 89% underwent repair, and of those assigned to surveillance, 31% subsequently underwent repair during the course of the study (mean follow-up 20 ± 12 months, range 0-41 months). The unadjusted hazard ratio (95% confidence interval [CI]) for mortality after EVAR was 1.01 (0.49-2.07; P = 0.98), with 15 deaths (4.1%) occurring in each group. No survival advantage was demonstrated with EVAR.
Endovascular Treatment of Ruptured Abdominal Aortic Aneurysms
With the widespread application of EVAR for repair of AAA, its use in the repair of ruptured AAA has similarly expanded. Initially there were several limitations to the application of this technology to treat the devastating problem of ruptured AAA: (1) unavailability of preoperative CT in patients with ruptured AAA, (2) unavailability of a dedicated operating room and ancillary staff equipped to perform emergent EVAR at all times, (3) unavailability of off-the-shelf stent grafts; and (4) lack of data from multicenter randomized trials.125 Many large-volume centers, however, have developed protocols that allow treatment teams to overcome these hurdles and provide emergent care for ruptured AAA with endovascular devices.125–128 Gerassimidis et al.126 reported on the treatment of 69 patients with a ruptured AAA. Of these, 42 patients (63%) were suitable for EVAR. The in-hospital and 30-day mortality rates were 36% and 41%, respectively. Veith et al.128 reported the worldwide experience with treating ruptured AAA with endovascular grafts. Data were collected from 49 centers at which 1037 patients were treated with EVAR for ruptured AAA. Overall 30-day mortality was 21%, which was significantly lower than the 30-day mortality rate for the 763 patients undergoing open repair (36%, P < 0.001) for ruptured AAA at these same institutions during the same time period. Given these experiences, there is a trend toward even more centers instituting a program of EVAR for ruptured AAA. Certainly, longer-term follow-up and larger series will be required to assess whether EVAR has ultimately affected outcomes from ruptured AAA.
Fenestrated and Branched Aortic Endografts
The most common reason for excluding patients from EVAR is lack of a suitable proximal implantation site between the renal arteries and the aneurysm. Although commercially available devices provide a mechanism for supplementing fixation within the suprarenal aorta without detrimentally affecting renal function,129 such a practice has not been advocated to treat juxtarenal aneurysms. Despite evidence of short-term success with treatment of short necks with devices intended to treat infrarenal aneurysms,130 the risk of later failure remains high.131 To overcome this, fenestrated stent graft technology was developed. The devices used currently are hybrids of original abdominal devices. The primary goal of treating an aneurysm with a fenestrated graft is to move the sealing and fixation region of the repair into healthy aorta with a parallel neck and without wall defects. A fenestration (hole in the graft) allows the stent graft to occupy this more proximal location while providing for transgraft flow to the renal arteries (or other significant branches) (Fig. 40-8). It is designed to incorporate the minimum number of visceral vessels required to achieve fixation and seal within healthy aorta. The fenestrations are constructed to match the ostial diameter of the visceral vessels and maximize the sealing zone. Several large series of fenestrated endograft deployments have been reported, demonstrating the midterm safety and efficacy of fenestrated stent grafting.132–134 One of the largest series is reported by O’Neill et al.132 They outline a series of 119 patients with mean follow-up of 19 months. There was only one perioperative death, and survival at 12, 24, and 36 months was 92%, 83%, and 79%, respectively. The 30-day endoleak rate was 10%, and all endoleaks were type II in nature. Regression of the aneurysm sac was noted in 79% of the patients by 12 months. Complications related to the renal arteries was noted in 10 of the 231 stented renal arteries, and only one patient who did not have significant renal dysfunction preoperatively went on to require dialysis. Incorporation of the renal arteries raises questions about the effect of fenestrated stent graft repair on long-term renal function.
Application of fenestrated technology has advanced to allow for the treatment of thoracoabdominal aortic aneurysms (TAAA) in which the aneurysm involves the renal and visceral vessels. When treating TAAA with an endograft, however, use of a simple fenestration is inadequate. Unlike fenestrated grafts where a hole in the graft suffices, in more complex aneurysms such as TAAA, the branch arteries arise from the aneurysm. In this scenario, blood flow has to be carried from the endograft, across the aneurysm, and to the target vessel, without extravasation into the aneurysm (Fig. 40-9). There are two modes by which this can be assured. The first is the fenestrated branched stent graft135 or reinforced fenestrated graft136 (see Fig. 40-8). In this style, the addition of a covered bridging stents converts a fenestrated stent graft into a form of branched stent graft.137 Sealing between the covered stent and the fenestration is tenuous because there is very limited overlap of material. A nitinol ring is added to the fenestration to reinforce the site of interaction between the covered stent and the fenestration. These are termed reinforced fenestrations. The second mode of branched graft design is the cuffed branched stent graft138 or directional branched stent graft136 (see Fig. 40-8). The cuff or branch creates an overlap zone between the stent graft and the branch artery. It provides a segment of overlap that can be used to provide better sealing and fixation than the thin joint between a reinforced fenestration and mating visceral stent graft. A longer overlap affords one the ability to use a self-expanding stent graft rather than a balloon-expandable stent graft. This may provide a means to better accommodate tortuosity and diameter discrepancies and may limit type 1 endoleaks and component separation from this region.
Investigators tend to pool results of fenestrated branch grafts and cuffed branched grafts, with few series containing significant numbers of patients.139–145 The largest single-center experience has been reported by the Cleveland Clinic. Greenberg et al.143 performed a retrospective analysis on patients who underwent elective open surgical repair (N = 372) or endovascular repair (N = 352) of descending thoracic or thoracoabdominal aortic aneurysms. The group of patients treated with endovascular repair was older and had more comorbid conditions than those undergoing open repair. Open repair, however, was more frequently applied to patients with type II or type III aneurysms and those that were associated with a chronic dissection. Despite the differences in patient age and comorbid conditions, mortality rates at 30 days (5.7% vs. 8.3%) and 12 months (15.6% vs. 15.9%) were not different between endovascular repair and surgical repair, nor was there a difference in the development of spinal cord ischemia (4% vs. 8%, respectively; P = 0.08). Bakoyiannis et al. performed a meta-analysis of all English language literature published between 2000 and 2009 on endovascular procedures using fenestrated and/or branched technology.145 The results of this analysis demonstrated that complex endografting can be performed with a technical success rate of 94%, with a 30-day mortality of 7%. Typically these procedures were performed in patients who were deemed high risk for conventional surgery. The 1-year mortality was 1.3%. The application of fenestrated and branched technology is very much in its infancy. As the technology progresses, we will be able to better discern who will best benefit from these procedures, and ultimately replace open surgery with this less invasive option.
1 Criado FJ, Barnatan MF, Lingelbach JM, et al. Abdominal aortic aneurysm: overview of stent-graft devices. J Am Coll Surg. 2002;194(1 Suppl):S88.
2 Power D. Palliative treatment of aneurysms by wiring with Colt’s apparatus. Br J Surg. 1927;9:27.
3 Blakemore A, King B. Electrothermic coagulation of aortic aneurysms. JAMA. 1938;111:1821.
4 Matas R. Ligation of the abdominal aorta: report of the ultimate result. 1 year, 5 months and 9 days after the ligation of the abdominal aorta for aneurysm of the bifurcation. Ann Surg. 1925;81:457.
5 Rea C. Surgical treatment of aneurysm of the abdominal aorta. Minn Med. 1948;31:153.
6 Dubost C, Allary M, Oeconomos N. Resection of an aneurysm of the abdominal aorta: resection of the continuity by a preserved arterial graft, with result after five months. Arch Surg. 1952;64:405.
7 Voorhees A, Jaretski A, Blakemore A. Use of tubes constructed from Vinyon “N” cloth in bridging arterial defects: a preliminary report. Ann Surg. 1952;135:322.
8 Edwards W, Tapp J. Chemically treated nylon tubes as arterial grafts. Surgery. 1955;38:61.
9 Creech O. Endo-aneurysmorrhaphy and treatment of aortic aneurysm. Ann Surg. 1966;164:935.
10 Parodi J, Palmaz J, Barone H. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491.
11 Ernst C. Abdominal aortic aneurysm. N Engl J Med. 1993;1993:1167.
12 Szilagyi D, Smith R, DeRusso F, et al. Contributions of abdominal aortic aneurysmectomy to prolongation of life. Ann Surg. 1966;164:678.
13 United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445.
14 Hertzer NR, Mascha EJ, Karafa MT, et al. Open infrarenal abdominal aortic aneurysm repair: the Cleveland Clinic experience from 1989 to 1998. J Vasc Surg. 2002;35:1145.
15 Cronenwett J, Johnston K. The United Kingdom Small Aneurysm Trial: implications for surgical treatment of abdominal aortic aneurysms. J Vasc Surg. 1999;29:191.
16 Baxendale B, Baker D, Hutchinson A, et al. Haemodynamic and metabolic response to endovascular repair of infra-renal aortic aneurysms. Br J Anaesth. 1996;77:581.
17 Laheij RJ, Van Marrewijk CJ. Endovascular stenting of abdominal aortic aneurysm in patients unfit for elective open surgery. Eurostar group. EUROpean collaborators registry on Stent-graft Techniques for abdominal aortic Aneurysm Repair. Lancet. 2000;356:832.
18 Beebe H, Kritpracha B, Serres S, et al. Endograft planning without preoperative arteriography: a clinical feasibility study. J Endovasc Ther. 2000;7:8.
19 Eide K, Odegard A, Myhre H, et al. DynaCT during EVAR – a comparison with multidetector CT. Eur J Endovasc Surg. 2009;37:23.
20 Nordon I, Hinchcliffe R, Malkawi A, et al. Validation of DynaCT in the morphological assessment of abdominal aortic aneurysm for endovascular repair. J Endovasc Ther. 2010;17:183.
21 Beebe H, Kritpracha B. Imaging of abdominal aortic aneurysm: current status. Ann Vasc Surg. 2003;17:111.
22 Beebe H, Jackson T, Pigott J. Aortic aneurysm morphology for planning endovascular aortic grafts: limitations of conventional imaging methods. J Endovasc Surg. 1995;2:139.
23 Truijers M, Resch T, Van Den Berg JC, et al. Endovascular aneurysm repair: state-of-art imaging techniques for preoperative planning and surveillance. J Cardiovasc Surg (Torino). 2009;50:423.
24 Resch T, Ivancev K, Brunkwall J, et al. Distal migration of stent-grafts after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 1999;10:257–264.
25 Ouriel K. Endovascular repair of abdominal aortic aneurysms: the Cleveland Clinic experience with five different devices. Semin Vasc Surg. 2003;16(2):88–94.
26 Swinnen J, Chao A, Tiwari A, et al. Vertical or transverse incisions for access to the femoral artery: a randomized control study. Ann Vasc Surg. 2010;24:336–341.
27 Lee W, Brown M, Nelson P, et al. Total percutaneous access for endovascular aortic aneurysm repair (“preclose” technique). J Vasc Surg. 2007;45:1095–1101.
28 McDonnell C, Forlee M, Dowdall J, et al. Percutaneous endovascular abdominal aortic aneurysm repair leads to a reduction in wound complications. Ir J Med Sci. 2008;177:49–52.
29 Smith S, Timaran C, Valentine R, et al. Percutaneous access for endovascular abdominal aortic aneurysm repair: can selection criteria be expanded? Ann Vasc Surg. 2009;23:621–626.
30 The United Kingdom EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1863–1871.
31 Nordon I, Karthikesalingam R, Hinchliffe R, et al. Secondary interventions following endovascular aneurysm repair (EVAR) and the enduring value of graft surveillance. Eur J Endovasc Surg. 2010;39:547–554.
32 Armerding M, Rubin G, Beaulieu C, et al. Aortic aneurysmal disease: assessment of stent-graft treatment – CT versus conventional angiography. Radiology. 2000;215:138–146.
33 Rozenblit A, Marin M, Veith F, et al. Endovascular repair of abdominal aortic aneurysm: value of postoperative follow-up with helical CT. Am J Roentgenol. 1995;165:1473–1479.
34 Gorich J, Rlinger N, Sokirnaski R, et al. Leakages after endovascular repair of aortic aneurysms: classification based on findings at CT, angiography, and radiography. Radiology. 1999;213:767–772.
35 Buth J, Harris P, Marrewijk C. Causes and outcomes of open conversion and aneurysm rupture after endovascular abdominal aortic aneurysm repair: can type II endoleaks be dangerous? J Am Coll Surg. 2002;194:S98–S102.
36 Brenner D, Hall E. Computed tomography – an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284.
37 Solomon R, DuMouchel W. Contrast media and nephropathy: findings from systematic analysis and food and drug administration reports of adverse effects. Invest Radiol. 2006;41:651–660.
38 Broome D, Giguis M, Baron P, et al. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. Am J Roentgenol. 2007;189:586–592.
39 Sun Z. Diagnostic value of color duplex ultrasonography in the follow-up of endovascular repair of abdominal aortic aneurysm. J Vasc Interv Radiol. 2006;17:759–764.
40 Mirza T, Karthikesalingam A, Jackson D, et al. Duplex ultrasound and contrast-enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: systematic review and bivariate meta-analysis. Eur J Vasc Endovasc Surg. 2010;39:418–428.
41 Napoli V, Bargellini I, Sardella S, et al. Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology. 2004;233:217–225.
42 Henao E, Hodge M, Felkai D, et al. Contrast-enhanced duplex surveillance after endovascular abdominal aortic aneurysm repair: improved efficacy using a continuous infusion technique. J Vasc Surg. 2006;43:259–264.
43 Parent F, Meier G, Godziachvili V, et al. The incidence and natural history of type I and II endoleak: a 5-year follow-up assessment with 1 color duplex ultrasound scan. J Vasc Surg. 2002;35:474–481.
44 Beeman B, Doctor L, Doerr K, et al. Duplex ultrasound imaging alone is sufficient for midterm endovascular aneurysm repair surveillance: a cost analysis study and prospective comparison with computed tomography scan. J Vasc Surg. 2009;50:1019–1024.
45 Yao O, Faries P, Morrissey N, et al. Ancillary techniques to facilitate endovascular repair of aortic aneurysms. J Vasc Surg. 2001;34:69.
46 Zarins CK. The US AneuRx Clinical Trial: 6-year clinical update 2002. J Vasc Surg. 2003;37:904–908.
47 May J, White G, Waugh R, et al. Improved survival after endoluminal repair with second-generation prostheses compared with open repair in the treatment of abdominal aortic aneurysms: a 5-year concurrent comparison using life table method. J Vasc Surg. 2001;33:S21–S26.
48 Lee WA, O’Dorisio J, Wolf YG, et al. Outcome after unilateral hypogastric artery occlusion during endovascular aneurysm repair. J Vasc Surg. 2001;33:921–926.
49 Wolpert LM, Dittrich KP, Hallisey MJ, et al. Hypogastric artery embolization in endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2001;33:1193–1198.
50 Criado FJ, Wilson EP, Velazquez OC, et al. Safety of coil embolization of the internal iliac artery in endovascular grafting of abdominal aortic aneurysms. J Vasc Surg. 2000;32:684–688.
51 Schoder M, Zaunbauer L, Holzenbein T, et al. Internal iliac artery embolization before endovascular repair of abdominal aortic aneurysms: frequency, efficacy, and clinical results. AJR Am J Roentgenol. 2001;177:599–605.
52 Mehta M, Veith F, Ohki T, et al. Unilateral and bilateral hypogastric artery interruption during aortoiliac aneurysm repair in 154 patients: a relatively innocuous procedure. J Vasc Surg. 2001;33:S27–S32.
53 Kritpracha B, Pigott JP, Price CI, et al. Distal internal iliac artery embolization: a procedure to avoid. J Vasc Surg. 2003;37:943–948.
54 Wyers MC, Schermerhorn ML, Fillinger MF, et al. Internal iliac occlusion without coil embolization during endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2002;36:1138–1145.
55 Zarins CK, White RA, Hodgson KJ, et al. Endoleak as a predictor of outcome after endovascular aneurysm repair: AneuRx multicenter clinical trial. J Vasc Surg. 2000;32:90–107.
56 Holzenbein T, Kretschmer G, Thurnher S, et al. Midterm durability of abdominal aortic aneurysm endograft repair: a word of caution. J Vasc Surg. 2001;33:S46–S54.
57 Buth J, Harris PL, Van MC, et al. The significance and management of different types of endoleaks. Semin Vasc Surg. 2003;16:95–102.
58 Chuter TA, Faruqi RM, Sawhney R, et al. Endoleak after endovascular repair of abdominal aortic aneurysm. J Vasc Surg. 2001;34:98–105.
59 Buth J, Laheji R. Early complications and endoleaks after endovascular abdominal aortic aneurysm repair: report of a multicenter study. J Vasc Surg. 2000;31:134–146.
60 Dattilo JB, Brewster DC, Fan CM, et al. Clinical failures of endovascular abdominal aortic aneurysm repair: incidence, causes, and management. J Vasc Surg. 2002;35:1137–1144.
61 Zarins C. The US AneuRx clinical trial: 6-year clinical update 2002. J Vasc Surg. 2003;37:904–908.
62 Fairman R, Carpenter J, Baum R, et al. Potential impact of therapeutic warfarin treatment on type II endoleaks and sac shrinkage rates on midterm follow-up examination. J Vasc Surg. 2002;35:679.
63 Baum RA, Carpenter JP, Cope C, et al. Aneurysm sac pressure measurements after endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2001;33:32–41.
64 Marty B, Sanchez LA, Ohki T, et al. Endoleak after endovascular graft repair of experimental aortic aneurysms: does coil embolization with angiographic “seal” lower intraaneurysmal pressure? J Vasc Surg. 1998;27:454–461.
65 Baum R, Cope C, Fairman R, et al. Translumbar embolization of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2001;12:111–116.
66 Stavropoulos S, Park J, Fairman R, et al. Type 2 endoleak embolization comparison: translumbar embolization versus modified transarterial embolization. J Vasc Interv Radiol. 2009;20:1299–1302.
67 Parodi J, Berguer R, Ferreira L, et al. Intra-aneurysmal pressure after incomplete endovascular exclusion. J Vasc Surg. 2001;33:909.
68 Back MR, Bowser AN, Johnson BL, et al. Patency of infrarenal aortic side branches determines early aneurysm sac behavior after endovascular repair. Ann Vasc Surg. 2003;17:27–34.
69 Fan CM, Rafferty EA, Geller SC, et al. Endovascular stent-graft in abdominal aortic aneurysms: the relationship between patent vessels that arise from the aneurysmal sac and early endoleak. Radiology. 2001;218:176–182.
70 Gawenda M, Heckenkamp J, Zaehringer M, et al. Intra-aneurysm sac pressure–the holy grail of endoluminal grafting of AAA. Eur J Vasc Endovasc Surg. 2002;24:139–145.
71 Jacobs T, Teodorescu V, Morrissey N, et al. The endovascular repair of abdominal aortic aneurysm: an update analysis of structural failure modes of endovascular stent grafts. Semin Vasc Surg. 2003;16:103–112.
72 Stelter W, Umscheid T, Ziegler P. Three-year experience with modular stent-graft devices for endovascular AAA treatment. J Endovasc Surg. 1997;4:362–369.
73 Beebe HG. Lessons learned from aortic aneurysm stent graft failure; observations from several perspectives. Semin Vasc Surg. 2003;16:129–138.
74 Riepe G, Loos J, Imig H, et al. Long-term in vivo alterations of polyester vascular grafts in humans. Endovasc Surg. 1997;13:540.
75 Jacobs TS, Won J, Gravereaux EC, et al. Mechanical failure of prosthetic human implants: a 10-year experience with aortic stent graft devices. J Vasc Surg. 2003;37:16–26.
76 Alimi YS, Chakfe N, Rivoal E, et al. Rupture of an abdominal aortic aneurysm after endovascular graft placement and aneurysm size reduction. J Vasc Surg. 1998;28:178–183.
77 Riepe G, Heilberger P, Umscheid T, et al. Frame dislocation of body middle rings in endovascular stent tube grafts. Eur J Vasc Endovasc Surg. 1999;17:28–34.
78 Krajcer Z, Howell M, Dougherty K. Unusual case of AneuRx stent-graft failure two years after AAA exclusion. J Endovasc Ther. 2001;8:465–471.
79 Moore W, Rutherford R. Transfemoral endovascular repair of abdominal aortic aneurysm: results of the North American EVT phase I trial. J Vasc Surg. 1996;34:353.
80 Heintz C, Riepe G, Birken L, et al. Corroded nitinol wires in explanted aortic endografts: an important mechanism of failure? J Endovasc Ther. 2001;8:248–253.
81 Trepanier C, Tabrizian M, Yahia L, et al. Effect of modification of oxide layer on NiTi stent corrosion resistance. J Biomed Mater Res B Appl Biomater. 1998;43:433.
82 Duerig T, Pelton A, Stockel D. An overview of nitinol medical applications. Mater Sci Eng. 1999;A273–275:149.
83 Starosvetsky E, Gotman I. Corrosion behavior of titanium nitride coated Ni-Ti shape memory surgical alloy. Biomaterials. 2001;22:1853.
84 Fairman R, Baum R, Carpenter J, et al. Limb intervention in patients undergoing treatment with an unsupported bifurcated aortic endograft system: a review of the phase II EVT trial. J Vasc Surg. 2002;36:118–126.
85 Carroccio A, Faries P, Morrissey N, et al. Predicting iliac limb occlusion after bifurcated aortic stent grafting: anatomic and device-related causes. J Vasc Surg. 2002;36:679–684.
86 Baum R, Shetty S, Carpenter J, et al. Limb kinking in supported and unsupported abdominal aortic stent-grafts. J Vasc Interv Radiol. 2000;11:1165–1171.
87 Amesur NB, Zajko AB, Orons PD, et al. Endovascular treatment of iliac limb stenoses or occlusions in 31 patients treated with the Ancure endograft. J Vasc Interv Radiol. 2000;11:421–428.
88 Ohki T, Veith FJ, Shaw P, et al. Increasing incidence of midterm and long-term complications after endovascular graft repair of abdominal aortic aneurysms: a note of caution based on a 9-year experience. Ann Surg. 2001;234:323–334.
89 Carpenter JP, Neschis DG, Fairman RM, et al. Failure of endovascular abdominal aortic aneurysm graft limbs. J Vasc Surg. 2001;33:296–302.
90 Cao P, De RP, Verzini F, et al. Endoleak after endovascular aortic repair: classification, diagnosis and management following endovascular thoracic and abdominal aortic repair. J Cardiovasc Surg (Torino). 2010;51:53–69.
91 Baum RA, Shetty SK, Carpenter JP, et al. Limb kinking in supported and unsupported abdominal aortic stent-grafts. J Vasc Interv Radiol. 2000;11:1165–1171.
92 Carroccio A, Faries PL, Morrissey NJ, et al. Predicting iliac limb occlusions after bifurcated aortic stent grafting: anatomic and device-related causes. J Vasc Surg. 2002;36:679–684.
93 Sampram ES, Karafa MT, Mascha EJ, et al. Nature, frequency, and predictors of secondary procedures after endovascular repair of abdominal aortic aneurysm. J Vasc Surg. 2003;37:930–937.
94 Fairman RM, Velazquez O, Baum R, et al. Endovascular repair of aortic aneurysms: critical events and adjunctive procedures. J Vasc Surg. 2001;33:1226–1232.
95 Conners MIII, Sternbergh WIII, Carter G, et al. Secondary procedures after endovascular aortic aneurysm repair. J Vasc Surg. 2002;36:992.
96 Resch T, Ivancev K, Brunkwall J, et al. Distal migration of stent-grafts after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 1997;10:257–264.
97 Harris P, Vallavhaneni S, Desgranges P, et al. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. J Vasc Surg. 2000;32:739–749.
98 Albertini N, Kalliafas S, Travis S, et al. Anatomical risk factors for proximal perigraft endoleak and graft migration following endovascular repair of abdominal aortic aneurysms. Eur J Endovasc Surg. 2000;19:308–312.
99 Cao P, Verzini F, Zannetti S, et al. Device migration after endoluminal abdominal aortic aneurysm repair: analysis of 113 cases with a minimum follow-up period of 2 years. J Vasc Surg. 2002;35:229–235.
100 Lawrence-Brown M, Semmens J, Hartley D, et al. How is durability related to patient selection and graft design with endoluminal grafting for abdominal aortic aneurysm? In: Greenhalgh R, ed. Durability of vascular and endovascular surgery. London: WB Saunders; 1999:375–385.
101 Resch T, Malina M, Lindblad B, et al. The impact of stent-graft development on outcome of AAA repair–a 7-year experience. Eur J Vasc Endovasc Surg. 2001;22:57–61.
102 Albertini J, Kalliafas S, Travis S, et al. Anatomical risk factors for proximal perigraft endoleak and graft migration following endovascular repair of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2000;19:308–312.
103 Malina M, Lindblad B, Ivancev K, et al. Endovascular AAA exclusion: will stents with hooks and barbs prevent stent-graft migration? J Endovasc Surg. 1998;5:310–317.
104 Lee J, Lee J, Aziz I, et al. Stent-graft migration following endovascular repair of aneurysms with large proximal necks: anatomical risk factors and long-term sequelae. J Endovasc Ther. 2002;9:652–664.
105 White G, May J, Waugh R, et al. Shortening of endografts during deployment in endovascular AAA repair. J Endovasc Ther. 1999;6:4.
106 Prinssen M, Wever J, Mali W, et al. Concerns for the durability of the proximal abdominal aortic aneurysm endograft fixation from a 2-year and 3-year longitudinal tomography angiography study. J Vasc Surg. 2001;33:S64–S69.
107 Badran MF, Gould DA, Raza I, et al. Aneurysm neck diameter after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2002;13(9 Pt 1):887–892.
108 Ouriel K, Srivastava SD, Sarac TP, et al. Disparate outcome after endovascular treatment of small versus large abdominal aortic aneurysm. J Vasc Surg. 2003;37:1206–1212.
109 Becker G, Kovacs M, Mathison M, et al. Risk stratification and outcomes of transluminal endografting for abdominal aortic aneurysm: 7-year experience and long-term follow-up. J Vasc Interv Radiol. 2003;12:1033–1046.
110 Naslund TC, Edwards WHJr, Neuzil DF, et al. Technical complications of endovascular abdominal aortic aneurysm repair. J Vasc Surg. 1997;26:502–509.
111 Laheji R, Buth J, Harris P, et al. Need for secondary interventions after endovascular repair of abdominal aortic aneurysms. Intermediate-term follow-up results of a European collaborative registry (EUROSTAR). Br J Surg. 2000;87:1666.
112 May J, White G, Waugh R, et al. Life-table analysis and assisted success following endoluminal repair of abdominal aortic aneurysms: the role of supplementary endovascular intervention in improving outcome. Eur J Vasc Endovasc Surg. 2000;19:648.
113 Sternbergh WIII, Conners M, Tonnessen B, et al. Aortic aneurysm sac shrinkage after endovascular repair is device-dependent: a comparison of Zenith and AneuRx endografts. Ann Vasc Surg. 2003;17:49.
114 Bertges D, Chow K, Wyers M, et al. Abdominal aortic aneurysm size regression after endovascular repair is endograft dependent. J Vasc Surg. 2003;37:716.
115 EVAR Trial Participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179.
116 Lederle F, Freischlag J, Kyriakides T, et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm. A randomized trial. J Am Med Assoc. 2009;302:1535–1542.
117 Hallet JJr, Marshall D, Petterson T, et al. Graft-related complications after abdominal aortic aneurysm repair: reassurance from a 36-year population-based experience. J Vasc Surg. 1997;25:277–284.
118 Conrad M, Crawford R, Pedraza J, et al. Long-term durability of open abdominal aortic aneurysm repair. J Vasc Surg. 2007;46:669–675.
119 De Bruin J, Baas A, Buth J, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1881–1889.
120 The United Kingdom EVAR Trial Investigators. Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med. 2010;362:1872–1880.
121 Sobocinski J, Maurel B, Delsart P, et al. Should we modify our indications after the EVAR-2 trial conclusions? Ann Vasc Surg. 2011;25:590–597.
122 Lederle F, Wilson S, Johnson G, et al. Immediate repair compared with surveillance of small abdominal aneurysms. N Engl J Med. 2002;346:1437–1444.
123 Powell J, Brown L, Forbes J, et al. Final 12-year follow-up of surgery versus surveillance in the UK small aneurysm trial. Br J Surg. 2007;94:702–708.
124 Ouriel K, Clair D, Kent C, et al. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081–1087.
125 Mehta M, Taggert J, Darling RCIII, et al. Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysms: outcomes of a prospective analysis. J Vasc Surg. 2006;44:1–8.
126 Gerassimidis TS, Karkos CD, Karamanos DG, et al. Endovascular management of ruptured abdominal aortic aneurysms: an 8-year single-centre experience. Cardiovasc Intervent Radiol. 2009;32:241–249.
127 Starnes B, Quiroga E, Hutter C, et al. Management of ruptured abdominal aortic aneurysm in the endovascular era. J Vasc Surg. 2010;51:9–18.
128 Veith F, Lachat M, Mayer D, et al. Collected world and single center experience with endovascular treatment of ruptured abdominal aortic aneurysms. Ann Vasc Surg. 2009;250:818–824.
129 Greenberg RK, Chuter TA, Sternbergh WCIII, et al. Zenith AAA endovascular graft: intermediate-term results of the US multicenter trial. J Vasc Surg. 2004;39:1209–1218.
130 Greenberg R, Fairman R, Srivastava S, et al. Endovascular grafting in patients with short proximal necks: an analysis of short-term results. Cardiovasc Surg. 2000;8:350–354.
131 Waasdorp EJ, de Vries JP, Hobo R, et al. Aneurysm diameter and proximal aortic neck diameter influence clinical outcome of endovascular abdominal aortic repair: a 4-year EUROSTAR experience. Ann Vasc Surg. 2005;19:755–761.
132 O’Neill S, Greenberg RK, Haddad F, et al. A prospective analysis of fenestrated endovascular grafting: intermediate-term outcomes. Eur J Vasc Endovasc Surg. 2006;32:115–123.
133 Greenberg RK, Sternbergh WCIII, et al. Intermediate results of a United States multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2009;50:730–737.
134 Greenberg RK, Haulon S, O’Neill S, et al. Primary endovascular repair of juxtarenal aneurysms with fenestrated endovascular grafting. Eur J Vasc Endovasc Surg. 2004;27:484–491.
135 Chuter TA. Fenestrated and branched stent-grafts for thoracoabdominal, pararenal and juxtarenal aortic aneurysm repair. Semin Vasc Surg. 2007;20:90–96.
136 Greenberg R. Aortic aneurysm, thoracoabdominal aneurysm, juxtarenal aneurysm, fenestrated endografts, branched endografts, and endovascular aneurysm repair. Ann N Y Acad Sci. 2006;1085:187–196.
137 Anderson J, Adam D, Berce M, et al. Repair of thoracoabdominal aortic aneurysms with fenestrated and branched endovascular stent grafts. J Vasc Surg. 2005;42:600–607.
138 Chuter TA. Fenestrated and branched stent-grafts for thoracoabdominal, pararenal and juxtarenal aortic aneurysm repair. Semin Vasc Surg. 2007;20:90–96.
139 Anderson JL, Adam DJ, Berce M, et al. Repair of thoracoabdominal aortic aneurysms with fenestrated and branched endovascular stent grafts. J Vasc Surg. 2005;42:600–607.
140 Chuter TA. Fenestrated and branched stent-grafts for thoracoabdominal, pararenal and juxtarenal aortic aneurysm repair. Semin Vasc Surg. 2007;20:90–96.
141 Chuter TA, Rapp JH, Hiramoto JS, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg. 2008;47:6–16.
142 Roselli EE, Greenberg RK, Pfaff K, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2007;133:1474–1482.
143 Greenberg R, Lu Q, Roselli E, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair. A comparison of endovascular and open techniques. Circulation. 2008;118:808–817.
144 Vourliotakis G, Bos WT, Beck AW, et al. Fenestrated stent-grafting after previous endovascular abdominal aortic aneurysm repair. J Cardiovasc Surg (Torino). 2010;51:383–389.
145 Bakoyiannis CN, Economopoulos KP, Georgopoulos S, et al. Fenestrated and branched endografts for the treatment of thoracoabdominal aortic aneurysms: a systematic review. J Endovasc Ther. 2010;17:201–209.