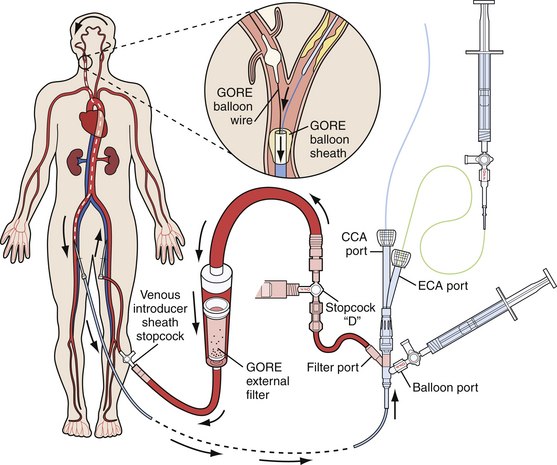
Chapter 17 Endovascular Neurosurgery
• Endovascular neurosurgery has undergone tremendous expansion in the past two decades and is becoming a mainstay of treatment of cerebrovascular disease and other vascular disease of the head and neck as a primary or alternative treatment to open microvascular treatment.
• Endovascular techniques are used to treat a wide variety of cerebrovascular conditions that include severe symptomatic vasospasm after subarachnoid hemorrhage (SAH), arteriovenous malformation (AVM) and tumor embolization, cerebral aneurysm occlusion, and temporary or permanent balloon occlusion during parent vessel sacrifice.
• Endovascular therapy is expanding the time-window after stroke symptom onset for revascularization in patients with acute ischemic stroke. Clinical trials have established a benefit of intra-arterial thrombolysis (IAT) up to 6 hours after stroke symptom onset, with an increase in recanalization rates. The mechanical device trials show effectiveness of mechanical revascularization therapy up to 8 hours after stroke symptom onset.
• Endovascular treatment of intracranial aneurysms has undergone and continues to undergo improvements since the introduction of Guglielmi detachable coils for endosaccular occlusion of cerebral aneurysms in 1994. Current adjunctive techniques include balloon-assisted and stent-assisted coiling to improve the success rate of long-term occlusion of the aneurysm. Flow diversion for giant aneurysms may be a promising technology.
• Preoperative endovascular embolization of feeding arteries has rendered many previously difficult AVMs much easier to remove surgically. Only a minority of AVMs can be cured by endovascular embolization despite the significant advances in embolic material such as Onyx. Most intracranial tumor embolization procedures have been performed on neoplasms with robust vascular pedicles in order to decrease the blood loss during resection.
• Endovascular therapy is evolving and adds a plethora of tools and opens up multiple options with which to treat complex vascular lesions, in addition to microsurgical approaches. Currently, neurosurgeons who desire to be trained in treating the entire spectrum of cerebrovascular disease may pursue both microsurgery and endovascular neurosurgery training.
Introduction (Box 17.1)
The treatment of neurovascular diseases has been completely revolutionized with the introduction and growth of endovascular neurosurgery. Endovascular neurosurgery is becoming a primary treatment alternative to conventional open surgery for multiple neurovascular pathological conditions, including carotid and vertebral atherosclerotic stenosis, cervical vessel dissections, acute and chronic cerebral ischemia, intracranial aneurysms, arteriovenous malformations (AVMs), and dural arteriovenous fistulas (dAVFs). Endovascular revascularization has resulted in recanalization rates as high as 70% to 100% for acute large vessel occlusions responsible for ischemic stroke in patients in whom intravenous (IV) tissue plasminogen activator (t-PA) therapy has failed or was contraindicated. Endovascular techniques are used to treat severe symptomatic vasospasm after subarachnoid hemorrhage (SAH), for tumor embolization, and for temporary or permanent balloon occlusion during parent vessel sacrifice. Neuroendovascular surgery is being increasingly integrated into the neurosurgical training curriculum. It is essential for future neurosurgeons to have a basic understanding of endovascular techniques and tools and have them as an option for treating cerebrovascular pathological conditions. In this chapter, we discuss the basics of arterial access, catheters for supra-aortic and intracranial access, access site management, and the rationale and techniques for the treatment of various cerebrovascular diseases.
BOX 17.1 Abbreviations Used
ACAS, Asymptomatic Carotid Atherosclerosis Study
ACST, Asymptomatic Carotid Surgery Trial
AVM, arteriovenous malformation
CAS, carotid angioplasty with stenting
COCOA, Complete Occlusion of Coilable Intracranial Aneurysms Study
DAC, distal access catheter(s)
dAVF, dural arteriovenous fistula
DSMB, Data and Safety Monitoring Board
ECST, European Carotid Surgery Trial
EVIDENCE, Endovascular Treatment of Intracranial Aneurysms with Pipeline versus Coils with or without Stents
FDA, Food and Drug Administration
GESICA Groupe d’Etude des Sténoses Intra-Crâniennes Athéromateuses symptomatiques
IAT, intra-arterial thrombolysis
IDE, Investigational Device Exemption
MERCI, Mechanical Embolus Removal in Cerebral Ischemia
NASCET, North American Symptomatic Carotid Endarterectomy Trial
NIH, National Institutes of Health
NIHSS, National Institutes of Health Stroke Scale
NINDS, National Institute of Neurological Disorders and Stroke
PED, Pipeline Embolization Device
PGLA, polyglycolic-polylactic acid
PITA, Pipeline Embolization Device in the Intracranial Treatment of Aneurysms
PROACT, Prolyse in Acute Cerebral Thromboembolism
PUFS, Pipeline for Uncoilable or Failed Aneurysms Study
SAMMPRIS, Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis
SAPPHIRE, Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy
SARIS, Stent-Assisted Recanalization in Acute Ischemic Stroke
SWIFT, Solitaire FR with the Intention for Thrombectomy
TIA, transient ischemic attack
TIMI, Thrombolysis in Myocardial Infarction
TLR, target lesion revascularization
t-PA, tissue plasminogen activator
WASID, Warfarin vs. Aspirin for Symptomatic Intracranial Disease
General Principles
Contrast Agents
Iohexol, a nonionic contrast agent, is the agent most commonly used for cerebral angiography. Patients with normal renal function can tolerate 400 to 800 mL of iohexol administered at a concentration of 300 mg/mL without adverse effects. Nonionic, low-osmolality contrast agents, such as iodixanol and iopromide, have been shown to be less renal-toxic when compared to iohexol.
Femoral Artery Puncture
Radial or brachial artery access can be attempted if femoral artery access is not feasible.
Carotid and Vertebral Artery Catheterization
For carotid artery catheterization, the innominate artery is engaged, and the wire is advanced superiorly into the right CCA, followed by the catheter. To engage the left CCA, the wire is brought inside the catheter, and the catheter is withdrawn and pointed to the left. If selective ICA catheterization is planned, angiography of the cervical carotid arterial system should be done to check for ICA stenosis. Once the CCA is catheterized, turning the head away from the side being catheterized facilitates catheterization of the ICA and turning toward the ipsilateral side facilitates ECA catheterization.
Ischemic Stroke Intervention
Stroke remains the third most common cause of death in industrialized nations and the single most common reason for permanent adult disability.1 Each year, approximately 795,000 Americans experience a new or recurrent stroke.2 The estimated direct and indirect cost of stroke for 2010 is $73.7 billion.2 The incidence of new or recurrent strokes per year is projected to rise to 1.2 million per year by 2025.3
The only medical therapy approved by the Food and Drug Administration (FDA) for acute ischemic stroke treatment until recently was IV recombinant t-PA (rt-PA) administered within 3 hours of symptom onset to patients eligible for thrombolysis.4,5 The American Heart Association/American Stroke Association guidelines6 recommend evaluating patients for consideration of IV t-PA therapy 3 to 4.5 hours after stroke symptom onset (on the basis of data from the European Cooperative Acute Stroke Study III).7 Unfortunately, less than 1% of acute ischemic stroke patients in the United States receive t-PA, primarily because of a delay in presentation for treatment.8 Early reocclusion following thrombolysis has been demonstrated by transcranial Doppler imaging to occur in 34% of patients receiving IV t-PA and may result in neurological worsening in many of these patients.9–11 Recanalization rates after IV t-PA therapy for proximal, large vessel arterial occlusions are poor and range from only 10% for ICA occlusion to 30% for middle cerebral artery (MCA) occlusion.12 IV thrombolysis (IVT) is not as effective in thromboembolic obstruction of these large, proximal vessels, as compared with more distal smaller vessels.13 The outcome after large intracranial vessel thromboembolic occlusion currently remains dismal and is associated with high rates of morbidity and mortality.14–17 Patients who do not meet the eligibility criteria for thrombolytic therapy, who fail to improve neurologically after thrombolytic therapy, or who improve and then worsen (patients with reocclusion) are currently candidates for endovascular revascularization therapies.
Endovascular Stroke Revascularization Techniques
Time Window for Endovascular Stroke Therapy
Endovascular therapy is expanding the time window after stroke symptom onset for revascularization in patients with acute ischemic stroke The Prolyse in Acute Cerebral Thromboembolism (PROACT) trials18,19 established a benefit of intra-arterial thrombolysis (IAT) up to 6 hours after stroke symptom onset, with an increase in recanalization rates. Flow is reestablished faster with mechanical revascularization strategies than with thrombolytics; thus, such strategies may increase the benefit of treatment, even when there is a delay in presentation for treatment. The Mechanical Embolus Removal in Cerebral Ischemia (MERCI),20,21 Multi-MERCI,22 and Penumbra23 trials show effectiveness of mechanical revascularization therapy up to 8 hours after stroke symptom onset. There is increasing evidence that identification of potentially salvageable brain tissue with advanced magnetic resonance (MR) and computed tomography (CT) imaging may allow the selection of patients who can be effectively and safely treated more than 8 hours post ictus.7,24–29
Higher Recanalization Rate and Improved Outcome after Stroke Intervention
In MERCI,20,21 Multi-MERCI,22 and the combined analysis of data from the Interventional Management of Stroke I and II studies,30 functional outcome (measured by modified Rankin Scale [mRS] score of ≤2 at 3 months) was significantly better and the 3-month mortality rate was significantly lower in patients who had thrombolysis in myocardial infarction (TIMI) scores 2 or 3 recanalization than in patients in whom vessels failed to recanalize after endovascular therapy. In a review of 53 studies that included 2066 patients, good functional outcome (mRS score ≤2) at 3 months was identified more frequently in patients with vessel recanalization than without vessel recanalization.31 The 3-month mortality rate was reduced in patients in whom vessels were recanalized. Higher rates of recanalization were achieved with endovascular methods, particularly mechanical therapies, and consequently were associated with better outcomes.
Merci Device (Fig. 17.1 and Video 17.1)
The Merci retriever system has a flexible nitinol wire that assumes a helical shape once it emerges from the tip of the microcatheter. A microcatheter containing this wire is passed distal to the thrombus, the catheter is withdrawn, and the wire, in its helical configuration, ensnares the clot for removal from the vasculature. Vessels amenable to embolectomy with the Merci device include the ICA, M1 and M2 segments of the MCA, VA, basilar artery, and the posterior cerebral artery. The retriever is then retracted into the guide catheter under proximal flow arrest. FDA approval of the Merci device in 2004 was based on a review of data obtained in the multicenter MERCI trial that involved 141 patients (mean age, 60 years; mean National Institutes of Health Stroke Scale [NIHSS] score, 20) ineligible for standard thrombolytic therapy.20,21 The Multi-MERCI trial22 is a prospective, multicenter, single-arm registry that included 164 patients who were treated with different Merci retrieval systems (X5, X6, and L5).
Penumbra Device (Fig. 17.2 and Video 17.2)
The Penumbra System (Penumbra, Inc., San Leandro, CA) is the second FDA-approved mechanical treatment for acute stroke. The system primarily involves clot aspiration using a microcatheter attached to a powered aspiration pump that is capable of producing 25 mm Hg of suction. The separator is a soft wire with a 6-mm teardrop-shaped enlargement proximal to its tip and is inserted via the microcatheter to fragment the clot and keep the microcatheter tip from clogging. A major advantage over the Merci system is that the Penumbra System works at the proximal face of the clot, eliminating the need to blindly position the microcatheter distal to the occlusion. A prospective, single-arm, independently monitored trial was performed to assess the efficiency and safety of the Penumbra System.23 In a prospective multicenter single-arm trial of 125 patients with acute stroke who underwent revascularization with the Penumbra device, TIMI 2 or 3 recanalization was achieved in 81.6% patients, with a symptomatic intracranial hemorrhage (ICH) rate of 11.2% and only 25% of patients achieving an mRS score of 2 or less.32
Stent-Assisted Thrombolysis
Self-expanding stents (SES) designed specifically for the cerebrovasculature are available. These stents can be delivered to intracranial target areas with a greater than 95% technical success rate with an increased safety profile because they are deployed at significantly lower pressures than balloon-mounted coronary stents.33 With the stent-for-stroke technique (using Wingspan [Boston Scientific] and Enterprise [Codman Neurovascular] SES), vessel recanalization is instantaneous, and the chance of early reocclusion after treatment is decreased. Reocclusion after IVT (34%) and pharmacological IAT (17%) has been shown and is associated with poor outcome.34
For stent-assisted thrombolysis, standard femoral access is obtained, and a 6F (or larger) guide catheter is placed in the target vessel proximal to the occlusion. To minimize the release of distal emboli, the occlusion is crossed in a fashion similar to that used for the Merci clot retriever. First, a .014-inch steerable wire is softly advanced through the clot. A low-profile catheter is then advanced over the wire distal to the occlusion. Following microangiographic confirmation that the microcatheter is distal to the occlusion, an exchange wire is brought through the microcatheter and anchored distal to the occlusion. The microcatheter is removed, and the stent delivery catheter is delivered over the exchange wire. To minimize the release of debris, the stent is deployed first distal to the occlusion (thus trapping any debris that may be later released between the stent and the vessel wall), then through the occlusion, and finally just proximal to the occlusion.
Several retrospective case series reported successful use of SES for acute stroke treatment, with higher rates of recanalization than other recanalization modalities.35–37 Presently, SES are used only off-label under a humanitarian device exemption as a salvage therapy when current FDA-approved thrombectomy devices fail. On the basis of these preliminary data, we received FDA approval for a pilot study, Stent-Assisted Recanalization in Acute Ischemic Stroke (SARIS),38 to evaluate the Wingspan stent for revascularization in patients who did not improve after IVT or had a contraindication to IVT. Average presenting NIHSS score was 14. Seventeen patients presented with a TIMI score of 0 and three patients with a TIMI score of 1. Intracranial SES were placed in 19 of 20 enrolled patients. One patient experienced recanalization of the occluded vessel during positioning of the Wingspan stent delivery system, prior to stent deployment. In two patients, the tortuous vessel did not allow tracking of the Wingspan stent. The more navigable Enterprise stent was used in both these cases. Twelve patients had other adjunctive therapies. TIMI 2 or 3 recanalization was achieved in 100% of patients; 65% of patients improved more than 4 points in NIHSS score after treatment. One patient (5%) had symptomatic ICH, and two had asymptomatic ICH. At the time of the 1-month follow-up evaluation, 12 of 20 (60%) patients had mRS scores of 2 or less and nine (45%) had mRS scores of 1 or less. Mortality rate at 1 month was 25%. None of these patients died because of stent-placement-related causes; all deaths were due to the severity of the initial stroke and associated comorbid conditions.
Stent-Platform-Based Thrombectomy Devices (Stentrievers)
The Solitaire FR Revascularization Device (ev3) (Figs. 17.3 and 17.4) and Trevo device (Concentric Medical Inc.) are recoverable self-expanding thrombectomy devices that are being tested clinically in the United States and Europe, respectively, for acute ischemic stroke revascularization. The advantage of these devices is that they are fully recoverable, SES-platform-based devices that can be used for both temporary endovascular bypass and thrombectomy. The devices restore flow immediately and avoid the placement of a permanent stent, thus obviating the need for antithrombotic therapy and the risk of in-stent stenosis. The Solitaire can be electrolytically detached like a coil in case a permanent stent is necessary, such as in the setting of an atherothrombotic lesion. The Solitaire FR With the Intention for Thrombectomy (SWIFT) trial is an ongoing multicenter randomized controlled trial that is testing the ability of the Solitaire FR device versus the Merci device to achieve TIMI 2 or 3 recanalization without symptomatic ICH. Two small preliminary studies with this device have shown promising results.39,40
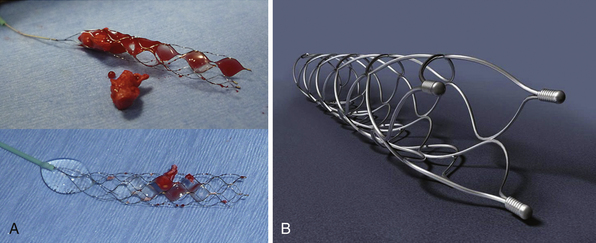
FIGURE 17.4 A, Solitaire FR device with retrieved clot (actual size). B, Solitaire FR.
(A from Natarajan SK, Siddiqui AH, Hopkins LN, Levy EI. Retrievable, detachable stent-platform-based clot-retrieval device (Solitaire FR) for acute stroke revascularization: first demonstration of feasibility in a canine stroke model. Vasc Dis Management 2010;7:E120-E125; B courtesy of ev3, Irvine, CA.)
Perfusion Imaging for Complication Avoidance and Patient Selection
Endovascular revascularization is an invasive procedure and is associated with acceptable complications in light of the morbidity and mortality risks associated with a major stroke if recanalization is not achieved. Despite aggressive revascularization with mechanical therapies, only up to 45% of patients recover to an mRS score of 0 to 2 at 3 months, and there is approximately an 8% to 10% procedure-related risk of symptomatic ICH, a potentially detrimental complication.19–23,32,38 CT and MR perfusion imaging are utilized in attempts to differentiate the three zones of postischemia brain tissue that are critical for patient selection and complication avoidance, and thus the ability to discriminate among them with these modalities is envisioned to lead to an improvement in outcomes after endovascular stroke therapy in the future. The three zones are core (tissue that is dead), penumbra (tissue at risk of death if not revascularized/reperfused), and benign oligemia (tissue that will tolerate the reduced blood flow and will survive even without reperfusion, mainly dependent on collaterals). Currently, no imaging modality is capable of differentiating between penumbra and benign oligemia. MR-diffusion-weighted imaging and cerebral blood flow maps obtained with CT perfusion imaging are able to identify core tissue with acceptable false positive and false negative rates. At our center, we have found that core tissue in the basal ganglia region or a core occupying more than 30% of occluded territory presages a higher risk of symptomatic ICH after endovascular stroke intervention. Sophisticated and precise imaging is needed to identify these three territories after cerebral ischemia.
Intracranial Atherosclerosis Treatment
Approximately 8% to 10% of ischemic strokes are attributable to intracranial atherosclerosis.41,42 An estimated 40,000 to 60,000 new strokes per year in the United States are due to intracranial atherosclerosis.43 In a study of patients with intracranial stenosis undergoing repeat angiography at an average interval of 26.7 months, 40% of lesions had stabilized, 20% had regressed, and 40% had progressed.44 Stenosis progression, as detected by transcranial Doppler imaging, was an independent predictor of stroke recurrence.45
Need for Treatment
The most definitive study of symptomatic intracranial stenosis thus far is the prospective Warfarin versus Aspirin for Symptomatic Intracranial Disease (WASID) trial, which found an 11% to 12% first-year risk of ischemic stroke in territory attributable to the patient symptoms.46 The majority of strokes (73%) in WASID patients were in the territory of the stenotic artery.47 Patients in the Extracranial-Intracranial Bypass Study with MCA stenosis who were randomized to medical therapy had an annual ipsilateral ischemic stroke rate of 7.8%.48,49 In the prospective, nonrandomized Groupe d’Etude des Sténoses Intra-Crâniennes Athéromateuses symptomatiques (GESICA), 102 patients with more than 50% symptomatic intracranial stenosis had “optimal” medical therapy, with a follow-up period of 23.4 months.50 Among these patients, ipsilateral transient ischemic attack (TIA) occurred in 12.6% and ipsilateral stroke in 7.0%.
Endovascular Tools and Technique
The Wingspan Stent System with the Gateway percutaneous transluminal angioplasty balloon catheter was designed for the treatment of intracranial atherosclerotic stenosis and received FDA approval for this indication under the humanitarian exemption device provision in August 2005. Prestent dilation of the stenotic lesion is done with the angioplasty balloon and the stent, a self-expanding nitinol device, is then deployed (Fig. 17.5 and Video 17.3).
Wingspan Studies
U.S. Wingspan Registry
In the U.S. Wingspan registry,51–53 treatment with the stent system was attempted in 158 patients with 168 intracranial atheromatous lesions. Of these, 161 lesions were successfully treated (96.0%) during the first treatment session. Of the 168 lesions in which treatment was attempted, there were 9 (5.4%) major periprocedural neurological complications, 4 of which ultimately led to the death of the patient within 30 days of the procedure. The total periprocedural event rate was 12.5% (21 of 168 cases). Most postprocedure events (18 of 21) were related to definable (and potentially controllable) issues: early antiplatelet interruption (n = 6) and in-stent restenosis (ISR) (n = 13). Imaging follow-up was available for 129 treated lesions (75 anterior circulation, 54 posterior circulation). Thirty-six of 129 (27.9%) patients with imaging follow-up of treated lesions experienced ISR. Of these 36, 29 (80.6%) underwent target lesion revascularization (TLR) with angioplasty alone (n = 26) or angioplasty with restenting (n = 3). Post-Wingspan ISR was more common in patients younger than 55 years old (odds ratio = 2.6). This increased risk can be accounted for by a high prevalence of anterior circulation lesions in this population, specifically those affecting the supraclinoid segment. When patients of all ages were considered, much higher rates of both ISR (66.6% vs. 24.4%) and symptomatic ISR (40% vs. 3.9%) were associated with supraclinoid segment lesions, by comparison with all other locations. Of the 29 patients undergoing primary TLR, 9 required one intervention for recurrent ISR, for a total of 42 TLR interventions. Only one major complication, a postprocedural reperfusion hemorrhage, was encountered during TLR (complication rates: 2.4% per procedure; 3.5% per patient). Angiographic follow-up was available for 22 of 29 patients after primary TLR. Eleven of 22 (50%) patients demonstrated recurrent ISR at follow-up angiography. Subsequently, 9 of these patients have undergone multiple re-treatments (6 patients had two re-treatments each, 2 had three re-treatments each, and 1 had four re-treatments) for recurrent ISR.
The 12-month follow-up results were recently published.54 The average follow-up duration was 14.2 months with 110 patients having at least 12 months of follow-up. The cumulative rate of the primary endpoint (stroke or death within 30 days or ipsilateral stroke after 30 days) was 15.7% for all patients and 13.9% for patients with high-grade stenosis (70-99%). Thirteen ipsilateral strokes occurred after 30 days, and 3 of these patients died. Ten of 13 (76.9%) strokes occurred within the first 6 months of the stenting procedure, and no events occurred after 12 months. An additional 9 patients had ipsilateral TIA after 30 days. Most postprocedure events (86%) were related to in-stent restenosis (n = 12), interruption of antiplatelet therapy (n = 6), or both (n = 1).
Wingspan NIH Registry
In the Wingspan National Institutes of Health (NIH) registry,37 129 patients with symptomatic 70% to 99% intracranial stenosis were enrolled from 16 medical centers. The rate of technical success (stent placement across the target lesion with <50% residual stenosis) was 97%. The rate of any stroke, ICH, or death within 30 days or ipsilateral stroke beyond 30 days was 14% at 6 months. The rate of 50% or greater restenosis on follow-up angiography was 25% among 52 patients with follow-up. The NIH registry investigators published a posthoc analysis report of 158 of 160 patients who had successful placement for intracranial atherosclerotic lesions with 50% to 99% stenosis.55 The primary endpoint (any stroke or death within 30 days or stroke in the territory of the stented artery beyond 30 days and up to 6 months) occurred in 13.9%. In multivariable analysis, the primary endpoint was associated with posterior circulation stenosis (vs. anterior circulation) (hazard ratio [HR] 3.4, p = 0.018), stenting at low enrollment sites (<10 patients each) (vs. high enrollment site) (HR 2.8, p = 0.038), 10 or fewer days from qualifying event to stenting (vs. ≥10 days) (HR 2.7, p = 0.058), and stroke as a qualifying event (vs. TIA or other cerebral ischemic event, such as vertebrobasilar insufficiency) (HR 3.2, p = 0.064).
SAMMPRIS Study
In 2007, the NIH approved funding for the multicenter, prospective, randomized Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) study.56 The hypothesis of the study is that “Compared with intensive medical therapy alone, intracranial angioplasty and stenting combined with intensive medical therapy will decrease the risk of the primary endpoint by 35% over a mean follow-up of 2 years in high-risk patients (patients with 70-99% intracranial stenosis who had a TIA or stroke within 30 days prior to enrollment) with symptomatic stenosis of a major intracranial artery.” A total of 764 patients will be recruited within 30 days of TIA or stroke due to 70% to 99% stenosis of a major intracranial artery. The patients will be randomized to either stent treatment with the Wingspan intracranial stent and Gateway balloon system plus intensive medical therapy with management of blood pressure, lipids, and other risk factors for vascular events or to this intensive medical therapy alone. Each patient will be followed for a minimum of 1 year and a maximum of 4 years after randomization. On April 11, 2011, the National Institute of Neurological Disorders and Stroke (NINDS) issued an alert57 to the SAMMPRIS investigators to stop enrollment in this trial because the last data and safety monitoring board (DSMB) review found that 14% of patients treated with angioplasty combined with stenting experienced a stroke or died within the first 30 days after enrollment compared with 5.8% of patients treated with medical therapy alone, a highly significant difference. The 30-day rate of stroke or death in the intensive medical treatment arm was substantially lower than the estimated rate of 10.7% based on historical control subjects, most of whom received standard medical care. In addition, the 30-day rate in the stent group was substantially higher than the estimated rate of 5.2% to 9.6%, based on registry data. The SAMMPRIS Executive Committee was in agreement with NINDS and the DSMB that enrollment in the study should be stopped and that the trial data currently available indicate that aggressive medical management alone is superior to angioplasty combined with stenting in patients with recent symptoms and high-grade intracranial arterial stenosis. Follow-up of currently enrolled patients and a comprehensive analysis of the total trial dataset will be important in the final interpretation of this study.
Carotid Angioplasty and Stenting
Atherosclerotic disease in the carotid arteries is thought to be the cause in up to 30% of ischemic strokes.41 The North American Symptomatic Carotid Endarterectomy Trial (NASCET),58,59 Asymptomatic Carotid Atherosclerosis Study (ACAS),60 Asymptomatic Carotid Surgery Trial (ACST),61 and the European Carotid Surgery Trial (ECST)62,63 established carotid endarterectomy (CEA) as an effective means of future stroke prevention in at-risk populations with significant carotid artery disease. Carotid angioplasty with stenting (CAS) was introduced as a less-invasive alternative to conventional CEA. With the subsequent development of distal embolic protection devices and stents for the carotid system, CAS became a promising and viable option for patients who were poor candidates for CEA.64–66
The major impetus for the advancement of CAS came with the publication of the results of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial,67 which demonstrated effectively that patients considered high risk for CEA were less likely to have complications if treated with CAS. This resulted in the approval of CAS by the FDA, Centers for Medicare & Medicaid Services, and Medicare as a viable option for such patients. More recently, an entirely new method of embolic protection achieved through flow reversal from the ICA into the arterial guide sheath, classified as proximal embolic protection, is being tested.68
Technique (Video 17.4) and Tools
After completion of the diagnostic angiogram and positioning of the catheter in the CCA, roadmapping of the cervical carotid artery is performed. An exchange-length 0.035-inch wire is positioned in the ECA. The diagnostic catheter is exchanged over the wire for a 90-cm, 6F to 10F sheath that is then advanced into the CCA below the bifurcation. For patients who have undergone complete diagnostic cerebral angiography before the stenting procedure, a combination of a 6F, 90-cm shuttle over a 6.5F Headhunter 125-cm slip-catheter (Cook) or a 5F, 125-cm Vitek catheter (Cook) can be used. In these cases, the shuttle is introduced primarily in the femoral artery over a 0.35-inch wire and is parked in the descending aorta. The inner obturator and wire are removed. The 125-cm catheter is then advanced into the shuttle, and the target vessel is catheterized. The shuttle is brought over the wire and the catheter in the CCA. The size of the shuttle is usually dictated by the profile of the embolic protection device and compatibility with the stent system. An optimal angiographic view that maximizes the opening of the bifurcation and facilitates crossing of the stenosis should be sought. The lesion is crossed with the protection device. Predilation of the stenotic vessel segment is performed at the operator’s discretion. We avoid predilation of the lesion whenever possible; however, if predilation is necessary, we prefer to undersize the balloon to simply facilitate crossing of the stent, usually using a 2- to 3-mm-diameter balloon. A 3- to 4-mm coaxial angioplasty balloon is advanced to the lesion over the 0.014-inch wire holding the protection device. On rare occasions, predilation needs to be performed before the introduction of an embolic protection device.
Embolic Protection Devices
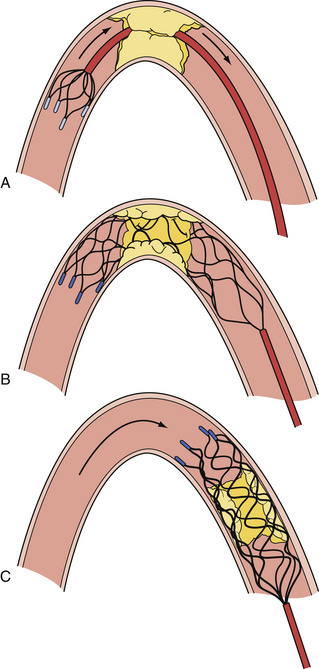
All embolic protection systems currently on the market can be classified under three main groups, each with its own working principle: (1) distal occlusion balloons, (2) distal filters, and (3) proximal occlusion devices. With the distal occlusion devices, a balloon is inflated in the ICA between the lesion and the brain to block the blood flow toward the cerebrum. Consequently, debris cannot enter the cerebral vasculature during the procedure. The debris is aspirated and flushed, forcing it either into the ECA or out of the body, through a sheath in the CCA. Filtration systems function in the manner of an umbrella- or windsock-like filter hose, which are opened in between the carotid lesion and the brain to capture all debris during the CAS procedure. Together with the distal filter, the debris is removed at the end of the procedure. A distal filter can be mounted on a guidewire, on which it is directly brought in place and retrieved, or alternatively, can come with its own specific delivery and retrieval system. Proximal occlusion systems (Fig. 17.6) are characterized by two compliant balloons to be inflated, one in the proximal CCA and one in the ECA. This double-balloon inflation creates either a no-flow or a reversed-flow pattern within the ICA, thereby preventing embolization of debris in the cerebral circulation. Proximal occlusion devices are especially attractive because complete cerebral protection is established before the device is passed across the lesion. The concerns associated with proximal protection devices are their large size (currently requiring 9F access to the femoral artery) and the concurrent delivery of a 9F system into tortuous CCAs.
Intracranial Aneurysm Treatment
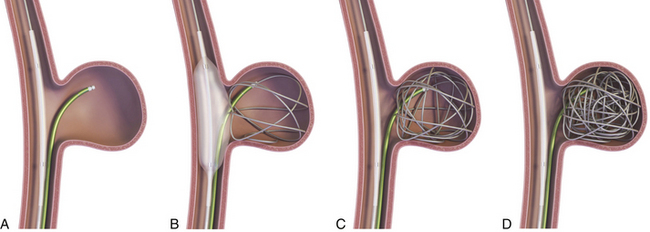
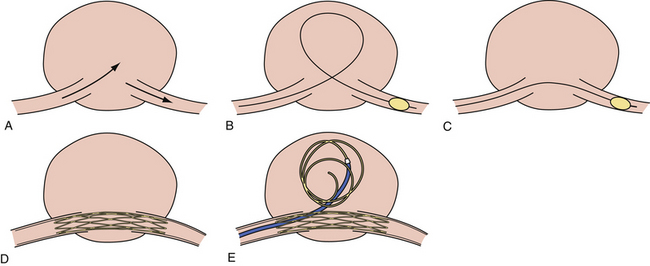
Balloon-Assisted Coil Embolization (Fig. 17.7)
The use of balloons to occlude the aneurysm neck during coiling of wide-necked aneurysms was first described in 1994 by Moret and associates.69 A 6F or larger guide catheter is required to accommodate both a balloon catheter and a microcatheter. A microcatheter is placed into the aneurysm fundus, and a balloon catheter is centered over the neck of the aneurysm. The balloon is subsequently inflated during placement of a coil and then deflated intermittently in between coils to allow antegrade flow. Sequential inflations and deflations are performed as additional coils are placed until the aneurysm is completely coiled, at which point the balloon is removed. The rationale behind this technique is that the presence of the balloon prevents distal embolization and conforms the coil mass to the shape of the balloon and that the coil mass shape becomes stable, thereby protecting the parent artery as the individual coils interlock. During the initial insertion of a coil, one should be careful to form a loop that directs the distal end of the coil away from the aneurysm fundus in order to limit the risk of aneurysm perforation during balloon inflation.
At our center, balloon assistance is used for cases of ruptured wide-necked giant aneurysms in which the use of antiplatelet agents is contraindicated and in which the deployment of a stent is not feasible. The number of patients in the second category is decreasing as more deliverable intracranial SES become available. We currently use the HyperGlide and HyperForm balloons (Micro Therapeutics, Irvine, CA) in such cases.
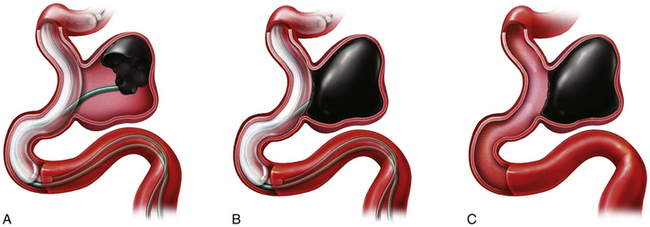
Balloon-Assisted Onyx Embolization (Fig. 17.8)
At the completion of the embolization process, with the balloon deflated, the microcatheter syringe is decompressed by the aspiration of 0.2 mL of Onyx left in the microcatheter, which prevents dribbling of Onyx material during removal of the microcatheter. An interval of 10 minutes is allowed to elapse prior to microcatheter removal, in order to permit the Onyx material to set within the aneurysm. As the microcatheter is withdrawn, the balloon is inflated a final time to stabilize the Onyx mass. The patient should be kept on an antiplatelet regimen for 1 month after the procedure.
In a multicenter investigation of cerebral aneurysm treatment with Onyx, the rate of permanent neurological morbidity was 8.3% (8/97 patients), and there were two procedural deaths.70 The procedures for large and giant aneurysms were lengthy (up to 6 hours). Delayed occlusion of the carotid artery occurred in 9 (9%) of 100 patients. At the 12-month follow-up evaluation of 53 patients, 38 (72%) large and giant aneurysms were completely occluded. Re-treatment was performed in 9 (11%) of 79. Although some single-center studies show slightly better results,71 in our opinion, the relatively high complication rate and high rate of delayed carotid artery occlusion do not justify this treatment in patients with unruptured aneurysms who cannot tolerate carotid artery occlusion. Currently, short-term results of Onyx occlusion for large aneurysms are not better than for selective coil occlusion, and the immediate and delayed complication rates are probably higher.
Self-Expanding Intracranial Microstents
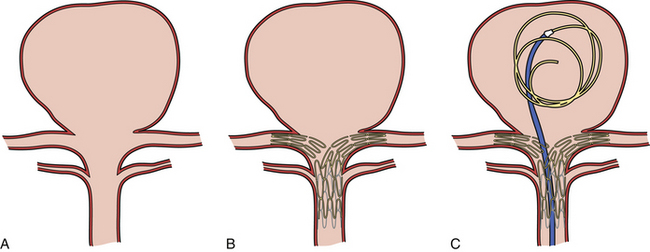
Neuroform Stent (Fig. 17.9)
The Neuroform stent comes from the manufacturer preloaded in a 3F microdelivery catheter. A “stabilizer” catheter (also preloaded in the delivery microcatheter) is then used to stabilize and deploy the stent as the microdelivery catheter is withdrawn. The stent consists of a fine wire mesh that cannot be seen on standard fluoroscopy; however, the four platinum marker bands at each end can be seen. The devices come in sizes ranging between 2.5 and 4.5 mm in diameter and 10 and 30 mm in length. The recommended diameter for placement is 0.5 mm greater than the largest diameter of the parent artery to be stented. The length is chosen such that the stent extends for at least 5 mm proximal and distal to the aneurysm neck. The struts composing the stent measure approximately 60 µm in thickness. The interstices of the fully expanded stent are large enough to accommodate a microcatheter tip size of 2.5F or less (realistically, <2.0F) for coiling.
Indications for Self-Expanding Intracranial Microstents
Stent-Assisted Coil Embolization
The other technique used is a jailing technique in which a microcatheter is placed inside the aneurysm before stent deployment.72 The jailing technique is not preferred because (a) there is a chance of displacement of the microcatheter during stent placement and (b) there is a higher chance of coil stretching or breakage because the coil can get caught between the stent and the vessel wall.
Rescue During Coil Embolization
If detached coils or entire coil mass prolapse into the parent vessel during the procedure, the stent is placed in the vessel either to replace the coils back in the aneurysm lumen or tack up the coils against the vessel wall and thus prevent further migration and distal emboli.73,74
Advanced Self-Expanding Intracranial Microstent Techniques Used in Complex Aneurysms
Balloon Anchor Technique for Difficult Distal Vessel Access (Fig. 17.11 and Video 17.7)
Y-Stent (Fig. 17.12)
This technique is most commonly used for bifurcation aneurysms arising from the basilar tip or carotid terminus. Two stents can be placed, the first extending out one limb of the bifurcation and the second introduced through the interstices of the first stent and extending into the other limb of the bifurcation. This configuration forms a Y-shaped construct at the bifurcation and provides very robust support for the coil embolization of terminal aneurysms.75–77 This Y-stent technique has also been applied to treat middle cerebral artery aneurysms76 and anterior communicating artery aneurysms.
Waffle-Cone Technique (Fig. 17.13)
In cases in which a Y-stent is not feasible, a stent may be deployed from the parent artery directly into the aneurysm (i.e., “intra-extra” aneurysmal stent placement or waffle-cone technique) to achieve parent artery protection. Using this technique, a single stent can be used to stabilize an intra-aneurysmal coil mass.78 However, the final construct sets up a vector of flow redirection directly into the terminal aneurysm sac and actually disrupts flow into the bifurcation branches. One might expect that such a construct could lead to high rates of recanalization. In addition, if this technique fails or leads to recanalization, other available means of treatment (surgical clipping, endovascular therapy with balloon remodeling or Y-stent reconstruction) are made considerably more difficult, if not impossible.
Trans-Circle of Willis Stenting (Video 17.8A and B)
In some cases when Y-stenting is not feasible for terminal aneurysms, a stent can be placed across the circle of Willis from P1 to P1 via the posterior communicating artery, from A1 to M1 via the anterior communicating artery, or from the ipsilateral A1 to contralateral A1 via the anterior communicating artery.79 This technique provides a means by which to achieve protection of both limbs of the bifurcation with a single self-expanding intracranial microstent.
Flow-Diverting Devices
The concept of parent vessel reconstruction is quickly advancing with the recent development of dedicated flow-diverting endovascular constructs designed for intracranial use. These devices primarily target parent vessel reconstruction, rather than endosaccular occlusion, as the means by which to achieve definitive aneurysm treatment. The current flow-diverting devices are high-metal surface area coverage, stent-like constructs that are designed to provide enough flow redirection, and endovascular remodeling to induce aneurysm thrombosis without the use of additional endosaccular occlusive devices (i.e., coils). At the same time, the pore size of the constructs is large enough to allow for the continued perfusion of branch vessels and perforators arising from the reconstructed segment of the parent vessel.80 Large or giant size or the presence of intra-aneurysmal thrombus, which are both factors typically associated with coil compaction and aneurysm recurrence, are not an issue with the flow-diverting devices, because no endosaccular coils are placed. In addition, as a purely “extrasaccular” treatment strategy, no direct catheterization or manipulation of the aneurysm sac is required with the flow-diverting devices, possibly reducing the likelihood of procedural rupture and potentially improving the safety of endovascular aneurysm treatment. The Pipeline Embolization Device (PED) (ev3) represents the first flow-diversion device used in humans. We have recently used the SILK flow-diverting device (BALT, Montmorency, France) in a patient (on a compassionate basis). Multiple other flow-diverting devices are currently in development and testing.
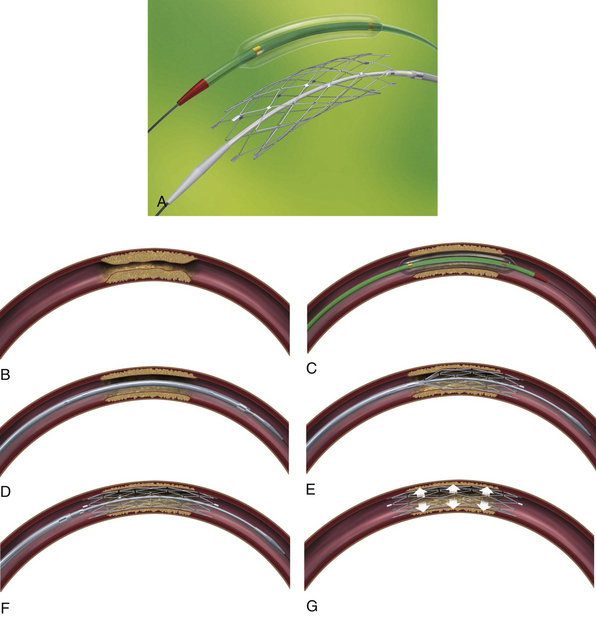
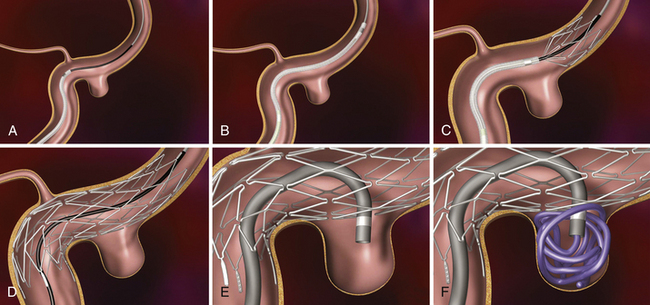
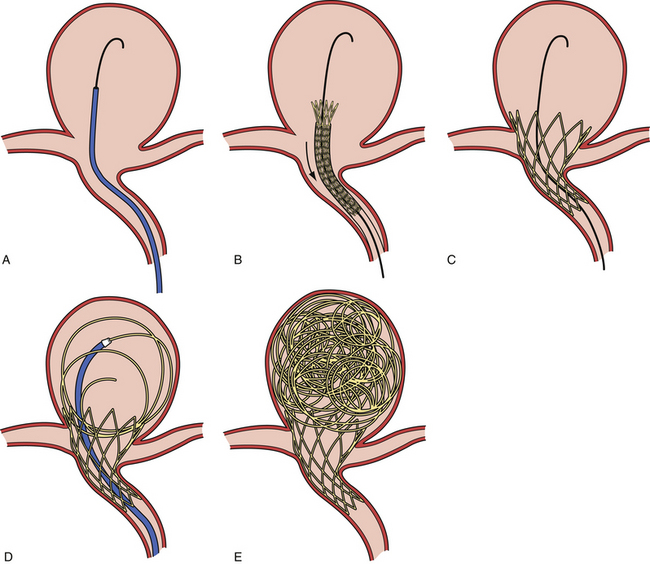
Pipeline Embolization Device (Fig. 17.14 and Video 17.9)
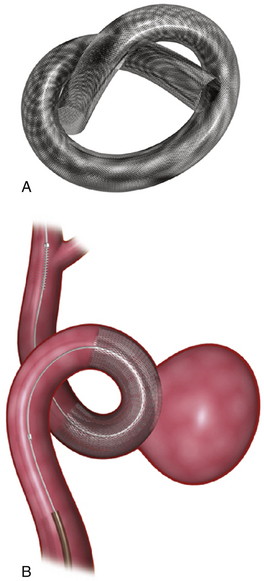
FIGURE 17.14 Pipeline Embolization Device (PED). A, The PED (ev3). B, The PED deployed across a wide-necked aneurysm.
(Courtesy of ev3, Irvine, CA.)
When fully expanded, the PED provides approximately 30% metal surface area coverage. When deployed in a parent artery smaller than the nominal diameter of the device, the PED cannot fully expand, and as such, it deploys longer than its nominal length and yields a lesser metal surface area coverage. To augment surface area coverage, several devices can be overlapped, or an individual device can be deployed with forward pressure on the microcatheter. To achieve coverage of vessel defects measuring longer than 20 mm, multiple devices can be telescoped to reconstruct longer segments of the cerebrovascular anatomy. The tremendous versatility of the device essentially allows the operator to achieve reconstructions of most any segment of the cerebrovascular anatomy and allows some control of the metal surface coverage of different regions of the conglomerate construct. This control over the length, shape, and porosity of the final reconstructed vessel allows the operator to build a “customized” implant for each patient treated.
By August 2010, 1178 aneurysms had been treated with the PED.81 The Pipeline Embolization Device in the Intracranial Treatment of Aneurysms (PITA) trial was an industry-sponsored safety trial for the Conformité Européenne (CE) mark of approval. The trial comprised a prospective four-center, single-arm study with core laboratory image analysis. Thirty-one patients with unruptured wide-necked intracranial aneurysms in whom treatment with coil embolization had failed were enrolled. The mean aneurysm neck diameter was 5.8 mm, and the mean aneurysm diameter was 11.5 mm. The PED was used alone in 48% of patients and with coils in 52% of patients. Six-month imaging follow-up was conducted in 96% of patients. Complete occlusion at 6 months was achieved in 93.3% of patients. At 6 months, the mortality and permanent morbidity rates were 0% and 6.5%, respectively.82 This unprecedented rate of complete angiographic occlusion at follow-up surpasses any of the reported occlusion rates for aneurysms after endovascular therapy and far exceeds those rates reported for large or wide-necked lesions.
The Budapest single-center study, which was a continuation of the PITA study, confirmed the findings of that study.83 A total of 19 large or giant wide-necked aneurysms were treated in 18 patients. Angiography at 6 months demonstrated complete occlusion in 17 aneurysms. Four neurological complications resulted in one patient (5.5%) with permanent morbidity and one (5.5%) death. Of the 17 ophthalmic arteries that were covered by a PED, one (5.9%) was occluded acutely, with visual deficit, and two (11.8%) were occluded in a delayed fashion, with no clinically detectable deficit. No other side branch occlusions were documented.
The Buenos Aires study,84 a prospective single-center registry that comprised 53 patients with 63 intracranial aneurysms, also expanded on the PITA study and confirmed the findings of the aforementioned two studies. Thirty-three (52.4%) aneurysms in the Buenos Aires study were small wide-necked aneurysms. Complete angiographic occlusion was achieved in 56%, 93%, and 95% of aneurysms at 3 months (n = 42), 6 months (n = 28), and 12 months (n = 18), respectively. There were no deaths; three patients (5%) with giant aneurysms experienced transient exacerbation of preexisting cranial neuropathies or headache. Five patients developed hematomas at the femoral puncture site.
The experience of the group at Hacettepe University (Ankara, Turkey) with the PED was presented recently.85 The study comprised 129 patients with intracranial aneurysms treated with the PED. The 12-month occlusion rate was 95%. There was one (0.8%) symptomatic parent artery stenosis, four (3.2%) permanent morbidities, and one (0.8%) death. Again, the outcomes were similar to the other three reported studies.
The PED has also been used to successfully treat nonsaccular (fusiform or circumferential) aneurysms. Three such cases have been performed in North America under an FDA exemption.86 All three lesions, which were judged to be untreatable with existing endovascular or open surgical technologies, were angiographically cured with PED treatment, without technical or neurological complications. In two cases, eloquent perforator or side-branch vessels were covered by the PED construct; and in both cases, vessels remained patent at angiographic follow-up. Two of these patients now have more than 1 year of clinical and angiographic follow-up and remain angiographically cured of their lesions and are without neurological symptoms.85
The Pipeline for Uncoilable or Failed Aneurysms Study (PUFS) was a U.S. investigational device exemption (IDE), nonrandomized, single-arm, multicenter premarket approval study using a historical control group.87 PUFS enrolled 120 patients with large or giant (paraclinoid or cavernous) ICA aneurysms and 6-month data were available in 107 (89%) patients. After reviewing these data, the FDA on April 6, 2011, approved the PED “for the endovascular treatment of adults (22 years of age or older) with large or giant wide-necked intracranial aneurysms in the ICA from the petrous to the superior hypophyseal segments.”88,89
SILK Flow-Diverting Device
The SILK device is similar to the PED and has 48 braided wires (44 nitinol and 4 platinum). It is available in 2.0- to 5.5-mm diameters (0.5-mm increments) and 15- to 40-mm lengths (5-mm increments). The SILK device shortens by at least 50% when deployed. It comes prepackaged with a delivery system comprising a delivery wire and an introducer and a reinforced microcatheter for placement (Vasco + 21 2.4F for devices 2-4.5 mm in diameter and 3F for devices 5-5.5 mm in diameter; preshaped with a multipurpose distal curve). The SILK device is preloaded on the delivery wire inside the introducer. After microcatheter access to the aneurysm has been obtained, working projections and vessel measurements are taken for device sizing. The SILK device is transferred from the introducer into the hub of the microcatheter using a Y-connector and by gently advancing the delivery wire. The device is positioned past the aneurysm neck at least 1.5 times the diameter of the parent vessel. The delivery wire is gently pushed until the distal radiopaque end is out of the distal marker on the microcatheter. Withdrawing the microcatheter to deploy the SILK device to approximately 1 cm creates a fix-point on the delivery wire. This manipulation ensures that SILK does not move distally, which can result in the wire struts of the SILK damaging the vessel wall. Once the SILK is deployed by approximately 1 cm, the distal tip is positioned by simultaneously pulling on the catheter and delivery wire. The SILK device may be moved by pulling the delivery wire, as long as the distal ring of the catheter does not superimpose the marker on the delivery wire. If repositioning of the device is required, the catheter is gently advanced over the deployed SILK, the system is repositioned, and the device is re-deployed in the new location. The SILK is fully deployed when the marker of the delivery wire lines up with the distal ring of the catheter. It is then impossible to resheath the SILK. The delivery wire is pushed until its marker passes the catheter’s marker by a minimum of 2 mm. The microcatheter is positioned distal to the stent to maintain access through the stent before removal of the delivery wire. The main advantages of the SILK device over the PED are the better translatability of the one-to-one movement of the microcatheter and the delivery system and the ability to recapture and reposition the system even up to 90% deployment. In our experience, the ability to recapture a flow-diverting device in our experience is very important. These devices frequently require repositioning because they foreshorten during deployment, and we often realize the final position of the stent only after it has been partially deployed.
Parent Vessel Sacrifice
Parent vessel sacrifice is still used as a last resort in giant aneurysms that are high risk for open surgical methods and cannot be safely excluded by currently available endovascular reconstructive techniques. Balloon test occlusion is performed concurrently if permanent vessel occlusion (endovascular or surgical) is considered as a treatment option or as a bail-out maneuver. Currently, deconstructive strategies without a bypass are used only as a bail-out when other treatment options are not available because all the temporary occlusion tests for the presence of collateral supply have false negative results; and there is a 16% to 20% chance of developing an ischemic event after carotid sacrifice even if balloon occlusion tests were negative.90,91 If surgical bypass is planned, endovascular sacrifice should be performed promptly after the surgical procedure to minimize the risk of graft thrombosis due to low flow.
Detachable Balloon Embolization
A large guide catheter is required, often 7F or 8F (alternatively, a 6F or 7F, 90-cm sheath may be used). A balloon is chosen that is slightly larger than the diameter of the vessel to be occluded. The balloons are attached to their recommended delivery catheters. If the guide catheter is large enough, it is preferable to advance the two balloons simultaneously through the guide catheter and into the vessel in order to limit the risk of premature detachment. Ideally, the balloons should be positioned in a relatively straight segment of the vessel. When the balloons are in proper position, they are inflated with contrast material. If they are properly sized, they will flatten out and elongate as they are inflated. When the position and stability of the balloon appear to be satisfactory, the distal balloon is detached by slowly, gently pulling back on the balloon catheter. The proximal balloon is for flow arrest and is deflated and removed after the distal balloon is deployed.
Intracranial Arteriovenous Malformation Embolization
Endovascular Embolization
Preoperative endovascular embolization of feeding arteries has rendered many previously difficult AVMs much easier to remove surgically. In general, endovascular embolization is most useful for Spetzler-Martin grade III AVMs.92 If endovascular access to the feeding vessels can be achieved without placing normal vessels at substantial risk, it can be used for lower grade AVMs as well.
Technique of Embolization
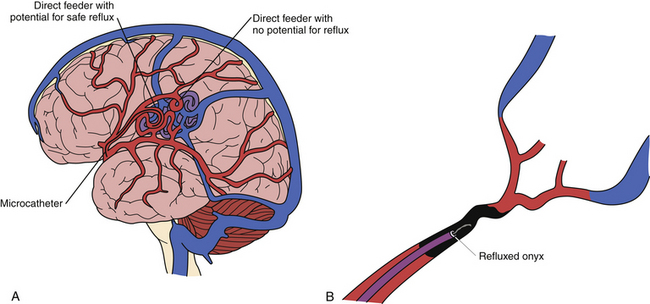
At our center, most AVM embolizations are performed under conscious sedation to allow neurological examinations to be performed throughout the procedure. In addition, we routinely perform Wada testing in the arterial pedicle to be embolized to determine whether the AVM is located in an eloquent region or there is en passant supply from the selected pedicle. We most commonly use Onyx as the embolic agent during embolization procedures. After microcatheterization, often with a flow-directed microcatheter, we initially perform microruns to study the angioarchitecture in detail. The endovascular neurosurgeon must be aware of en passant feeders and critical feeders taking off proximal to the point of injection in order to judge the safest distance for allowable reflux of the embolic agent (Fig. 17.15A). We initially use Onyx-34 to create a plug around the microcatheter (Fig. 17.15B), and this facilitates the forward injection of Onyx-18. Oblique views are obtained to see the tip of the catheter clearly, so that the any reflux of the Onyx can be appreciated readily.
Once the microcatheter tip is in the desired position, the injection of the Onyx is carried out as follows: the microcatheter is flushed with 10 mL of normal saline; 0.23 mL DMSO is injected into a Marathon flow-directed microcatheter (ev3) to fill the dead space. DMSO is also used to wash the hub of the syringe to avoid the polymerization of Onyx (when it comes in contact with water). Onyx is aspirated into a 1-mL syringe. Meniscus-to-meniscus connection is made between the Onyx (in the syringe) and the DMSO (in the catheter hub). Onyx is then injected slowly at a flow rate of 0.1 mL/second to fill the microcatheter and replace the DMSO in the dead space. The embolic agent is released at the tip of the microcatheter under free-flow conditions and fills the nidus compartment directly in an antegrade fashion, with subsequent reflux into the feeding artery beyond the tip of the microcatheter (first penetration). The goal is to form a cast of Onyx around the tip of the microcatheter over a short distance, so that when the Onyx is injected, it flows forward into the AVM and not retrogradely into the feeding vessel. The injection procedure is then interrupted for up to 1 minute to allow the cast to form, and small volumes of the Onyx are injected per cycle until there is enough reflux to form an attenuated cast for a second penetration of the nidus. The maximum safe distance of reflux is usually approximately 2 cm or at least 1 cm distal to a cortical branch of the feeding artery. Careful repeated subtraction roadmaps allow better visualization of the regions of the AVM that are being embolized. We are currently using a triple coaxial guide system containing an Outreach DAC or a Neuron (Penumbra Inc.) catheter to allow more distal placement in order to perform better selective angiography and further selective MCA, posterior cerebral artery, or anterior cerebral artery catheterization to evaluate further details of the AVM with the remainder of the circulation subtracted. Further, in our experience, the use of a triple coaxial guide system greatly facilitates removal of the microcatheter from the Onyx plug after completion of the embolization procedure.
Intracranial Dural Arteriovenous Fistula Embolization
dAVFs account for 10% to 15% of all intracranial AVMs.93 The venous drainage pattern is the most important predictor of the clinical behavior, and dAVFs with cortical venous reflux exhibit a much higher incidence of ICH or venous infarction. The annual mortality rate for dAVFs with cortical venous reflux may be as high as 10.4%, whereas the annual risk for hemorrhage or nonhemorrhagic neurological deficits during follow-up of nontreated lesions are 8.1% and 6.9%, respectively, resulting in an annual event rate of 15%.94 Recent studies demonstrate evidence that the risk of bleeding for a dAVF with cortical venous reflux is less when the patient does not present with a hemorrhage or a nonhemorrhagic neurological deficit.95,96 Strom and colleagues96 report that asymptomatic versus symptomatic dAVFs (in patients presenting with hemorrhage or neurological deficit) have annual hemorrhage rates of 1.4% versus 19%, respectively. Although small, these studies make the important point that the natural history may depend on the type of presentation.
Transarterial Embolization
A 6F guiding catheter (MPD; Codman Neurovascular) or a triple coaxial guiding system that allows more distal intracranial placement of the guide (Neuron-2, Penumbra, Inc.) is navigated into the target vessel. A DMSO-compatible catheter (Marathon or Echelon, ev3) with a microwire (Mirage or X-Pedion, ev3) is navigated into the feeding vessel of the dAVF as close to the nidus as possible and allowing for an adequate distance for reflux of the embolic agent. When there are multiple feeders, the feeder with maximum reflux distance from the skull base is chosen to decrease the chances of cranial nerve palsies. Embolization of the dAVF is then performed with Onyx-34 or Onyx-18 in the manner previously described for AVMs. The endpoint of the embolization is the angiographic obliteration of the fistula, with some filling of the draining veins. When the fistula is very complex, more than one session of Onyx embolization through multiple feeders is necessary. The average interval between such sittings is 2 to 4 weeks.
Endovascular Therapy for Vasospasm
Symptomatic cerebral vasospasm (also known as clinical vasospasm or delayed ischemic neurological deficit) is the leading cause of death and disability in patients with SAH. Symptomatic vasospasm occurs in some 20% to 25% of patients.97,98 Balloon angioplasty is an option for symptomatic vasospasm affecting intracranial arteries larger than 1.5 mm in diameter, such as the intracranial ICA, the M1, A1, and the VA and basilar arteries and P1 segments. The internal elastic lamina and smooth muscle cells are stretched and thinned during angioplasty. Dilation of the vessel segments is essentially “permanent” for the duration of the clinical vasospasm; vasospasm generally does not recur after angioplasty. Reversal of neurological deficits with angioplasty has been reported in 30% to 70% of patients who fail hypervolemic, hypertensive, and hemodilution (triple H) therapy.99–101 Clinical improvement appears to be strongly dependent on the timing of the procedure, with significantly better results reported with angioplasty done within 24 hours99 and within 2 hours102 of the neurological change. An IA infusion of antispasmodic medications (verapamil, nimodipine, or nicardipine) can supplement angioplasty and can be used for the endovascular treatment of spasm of vessels distal to the circle of Willis that are too small for balloon angioplasty.
Tumor Embolization
Potential Complications
With the variety of agents used for embolization of meningiomas, complication rates range from 0% to 9%. An uncommon but potentially devastating neurological complication is peritumoral or intraluminal hemorrhage from acute changes in the tumor blood supply. Often, a partially embolized tumor may hemorrhage into the tumor capsule or into portions of necrotic tumor. Other complications include the formation of iatrogenic fistulas, such as carotid-cavernous fistulas following vessel perforations. Infarctions to the retina or parenchyma tend to occur when anastomoses between the external carotid and internal carotid artery are poorly appreciated. Such anastomoses occur between the middle meningeal artery and the ophthalmic artery, between the ascending pharyngeal artery and the VA, between the occipital artery and the VA, between the accessory meningeal branches to the inferolateral trunk, and finally through persistent fetal anastomoses. It should be noted that parasagittal or convexity meningiomas may receive bilateral supply from the middle meningeal artery, and thus retinal infarctions may occur bilaterally via aberrant anastomoses.
Conclusion
Endovascular neurosurgery has undergone tremendous expansion in the past two decades and is becoming the mainstay of treatment of cerebrovascular diseases. The use of endovascular therapy for acute ischemic stroke revascularization has tremendously multiplied the endovascular neurosurgeons’ patient base just by the sheer number of cases of acute and chronic ischemic stroke per year when compared with other cerebrovascular disease conditions that were traditionally treated by neurosurgeons. Endovascular therapy has also increased the disease entities that fall under the purview of neurosurgery including head and neck vascular lesions and disease of supra-aortic vessels. Endovascular neurosurgical therapy adds a plethora of tools and opens up multiple options with which to treat these complex lesions in addition to microsurgical approaches. It is important for a neurosurgeon to be “completely” trained (both in microsurgery and endovascular neurosurgery) if he or she wishes to specialize in treating cerebrovascular diseases. At present, multiple trials are attempting to establish the superiority of one of these treatment options over the other. However, we strongly believe that these are complementary therapeutic modalities that require evaluation by treating physicians with the intent to achieve the treatment goals with the least morbidity. We are excited and fortunate to be a part of and to contribute to this dynamic era of cerebrovascular neurosurgery.
del Zoppo G.J., Higashida R.T., Furlan A.J., et al. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in acute cerebral thromboembolism. Stroke. 1998;29:4-11.
Levy E.I., Siddiqui A.H., Crumlish A., et al. First Food and Drug Administration-approved prospective trial of primary intracranial stenting for acute stroke: SARIS (Stent-Assisted Recanalization in acute Ischemic Stroke). Stroke. 2009;40:3552-3556.
Lloyd-Jones D., Adams R.J., Brown T.M., et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46-e215.
National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581-1587.
Yadav J.S., Wholey M.H., Kuntz R.E., et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493-1501.
Please go to expertconsult.com to view the complete list of references.
1. Report of the WHO Task Force on Stroke and Other Cerebrovascular Disorders, 1989. Recommendations on stroke prevention, diagnosis, and therapy. Stroke. 1989;20:1407-1431.
2. Lloyd-Jones D., Adams R.J., Brown T.M., et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46-e215.
3. Broderick J.P., William M. Feinberg Lecture: stroke therapy in the year 2025: burden, breakthroughs, and barriers to progress. Stroke. 2004;35:205-211.
4. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581-1587.
5. Adams H.P.Jr., del Zoppo G., Alberts M.J., et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:e478-e534.
6. del Zoppo G.J., Saver J.L., Jauch E.C., et al. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator. A science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945-2948.
7. Hacke W., Kaste M., Bluhmki E., et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
8. Barber P.A., Zhang J., Demchuk A.M., et al. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001;56:1015-1020.
9. Alexandrov A.V., Grotta J.C. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59:862-867.
10. Janjua N., Alkawi A., Suri M.F., et al. Impact of arterial reocclusion and distal fragmentation during thrombolysis among patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2008;29:253-258.
11. Saqqur M., Molina C.A., Salam A., et al. Clinical deterioration after intravenous recombinant tissue plasminogen activator treatment: a multicenter transcranial Doppler study. Stroke. 2007;38:69-74.
12. Wolpert S.M., Bruckmann H., Greenlee R., et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-PA Acute Stroke Study Group. AJNR Am J Neuroradiol. 1993;14:3-13.
13. Saqqur M., Uchino K., Demchuk A.M., et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948-954.
14. Arnold M., Nedeltchev K., Mattle H.P., et al. Intra-arterial thrombolysis in 24 consecutive patients with internal carotid artery T occlusions. J Neurol Neurosurg Psychiatry. 2003;74:739-742.
15. Jansen O., von Kummer R., Forsting M., et al. Thrombolytic therapy in acute occlusion of the intracranial internal carotid artery bifurcation. AJNR Am J Neuroradiol. 1995;16:1977-1986.
16. Zaidat O.O., Suarez J.I., Santillan C., et al. Response to intra-arterial and combined intravenous and intra-arterial thrombolytic therapy in patients with distal internal carotid artery occlusion. Stroke. 2002;33:1821-1826.
17. Sorimachi T., Fujii Y., Tsuchiya N., et al. Recanalization by mechanical embolus disruption during intra-arterial thrombolysis in the carotid territory. AJNR Am J Neuroradiol. 2004;25:1391-1402.
18. del Zoppo G.J., Higashida R.T., Furlan A.J., et al. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in acute cerebral thromboembolism. Stroke. 1998;29:4-11.
19. Furlan A., Higashida R., Wechsler L., et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA. 1999;282:2003-2011.
20. Gobin Y.P., Starkman S., Duckwiler G.R., et al. MERCI 1: a phase 1 study of mechanical embolus removal in cerebral ischemia. Stroke. 2004;35:2848-2854.
21. Smith W.S., Sung G., Starkman S., et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432-1438.
22. Smith W.S., Sung G., Saver J., et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205-1212.
23. Bose A., Henkes H., Alfke K., et al. The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol. 2008;29:1409-1413.
24. Hacke W., Albers G., Al-Rawi Y., et al. The desmoteplase in acute ischemic stroke trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66-73.
25. Furlan A.J., Eyding D., Albers G.W., et al. Dose escalation of desmoteplase for acute ischemic stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37:1227-1231.
26. Thomalla G., Schwark C., Sobesky J., et al. Outcome and symptomatic bleeding complications of intravenous thrombolysis within 6 hours in MRI-selected stroke patients: comparison of a German multicenter study with the pooled data of ATLANTIS, ECASS, and NINDS tPA trials. Stroke. 2006;37:852-858.
27. Kohrmann M., Juttler E., Fiebach J.B., et al. MRI versus CT-based thrombolysis treatment within and beyond the 3 h time window after stroke onset: a cohort study. Lancet Neurol. 2006;5:661-667.
28. Davis S.M., Donnan G.A., Parsons M.W., et al. Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299-309.
29. Hacke W., Furlan A.J., Al-Rawi Y., et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8:141-150.
30. Tomsick T., Broderick J., Carrozella J., et al. Revascularization results in the interventional management of stroke II trial. AJNR Am J Neuroradiol. 2008;29:582-587.
31. Rha J.H., Saver J.L. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967-973.
32. Penumbra Pivotal Stroke Trial Investigators. The Penumbra Pivotal Stroke Trial: Safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761-2768.
33. Henkes H., Miloslavski E., Lowens S., et al. Treatment of intracranial atherosclerotic stenoses with balloon dilatation and self-expanding stent deployment (WingSpan). Neuroradiology. 2005;47:222-228.
34. Qureshi A.I., Siddiqui A.M., Kim S.H., et al. Reocclusion of recanalized arteries during intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2004;25:322-328.
35. Brekenfeld C., Schroth G., Mattle H.P., et al. Stent placement in acute cerebral artery occlusion: use of a self-expandable intracranial stent for acute stroke treatment. Stroke. 2009;40:847-852.
36. Levy E.I., Mehta R., Gupta R., et al. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol. 2007;28:816-822.
37. Zaidat O.O., Klucznik R., Alexander M.J., et al. The NIH registry on use of the Wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology. 2008;70:1518-1524.
38. Levy E.I., Siddiqui A.H., Crumlish A., et al. First Food and Drug Administration-approved prospective trial of primary intracranial stenting for acute stroke: SARIS (Stent-Assisted Recanalization in acute Ischemic Stroke). Stroke. 2009;40:3552-3556.
39. Cohen J.E., Gomori J.M., Leker R.R., et al. Preliminary experience with the use of self-expanding stent as a thrombectomy device in ischemic stroke. Neurol Res. 2011;33:439-443.
40. Miteff F., Faulder K.C., Goh A.C., et al. Mechanical thrombectomy with a self-expanding retrievable intracranial stent (Solitaire AB): experience in 26 patients with acute cerebral artery occlusion. AJNR Am J Neuroradiol. 2011;32(6):1078-1081. (epub April 14, 2011)
41. Sacco R.L., Roberts J.K., Boden-Albala B., et al. Race-ethnicity and determinants of carotid atherosclerosis in a multiethnic population. The Northern Manhattan Stroke Study. Stroke. 1997;28:929-935.
42. Wityk R.J., Lehman D., Klag M., et al. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27:1974-1980.
43. Higashida R.T., Meyers P.M., Connors J.J.3rd, et al. Intracranial angioplasty and stenting for cerebral atherosclerosis: a position statement of the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, and the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2005;26:2323-2327.
44. Akins P.T., Pilgram T.K., Cross D.T.3rd, et al. Natural history of stenosis from intracranial atherosclerosis by serial angiography. Stroke. 1998;29:433-438.
45. Arenillas J.F., Molina C.A., Montaner J., et al. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke. 2001;32:2898-2904.
46. Chimowitz M.I., Lynn M.J., Howlett-Smith H., et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305-1316.
47. Kasner S.E., Chimowitz M.I., Lynn M.J., et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555-563.
48. EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. 1985;313:1191-1200.
49. Bogousslavsky J., Barnett H.J., Fox A.J., et al. Atherosclerotic disease of the middle cerebral artery. Stroke. 1986;17:1112-1120.
50. Mazighi M., Tanasescu R., Ducrocq X., et al. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology. 2006;66:1187-1191.
51. Albuquerque F.C., Levy E.I., Turk A.S., et al. Angiographic patterns of Wingspan in-stent restenosis. Neurosurgery. 2008;63:23-28.
52. Fiorella D.J., Levy E.I., Turk A.S., et al. Target lesion revascularization after Wingspan: assessment of safety and durability. Stroke. 2009;40:106-110.
53. Turk A.S., Levy E.I., Albuquerque F.C., et al. Influence of patient age and stenosis location on wingspan in-stent restenosis. AJNR Am J Neuroradiol. 2008;29:23-27.
54. Fiorella D.J., Turk A.S., Levy E.I., et al. US Wingspan registry: 12-month follow-up results. Stroke. 2011;42(7):1976-1981. (epub June 2, 2011)
55. Nahab F., Lynn M.J., Kasner S.E., et al. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology. 2009;72:2014-2019.
56. Derdeyn C.P., Chimowitz M.I. Angioplasty and stenting for atherosclerotic intracranial stenosis: rationale for a randomized clinical trial. Neuroimaging Clin North Am. 2007;17:355-363. viii-ix
57. National Institute of Neurological Disorders and Stroke (NINDS). Alert issued. April 11, 2011. Available at http://www.heart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_425679.pdf
58. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445-453.
59. Barnett H.J., Taylor D.W., Eliasziw M., et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415-1425.
60. Asymptomatic Carotid Atherosclerosis Study Group. Study design for randomized prospective trial of carotid endarterectomy for asymptomatic atherosclerosis. Stroke. 1989;20:844-849.
61. Halliday A., Mansfield A., Marro J., et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491-1502.
62. European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet. 1991;337:1235-1243.
63. European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379-1387.
64. Diethrich E.B., Ndiaye M., Reid D.B. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg. 1996;3:42-62.
65. Jordan W.D.Jr., Voellinger D.C., Fisher W.S., et al. A comparison of carotid angioplasty with stenting versus endarterectomy with regional anesthesia. J Vasc Surg. 1998;28:397-403.
66. Theron J., Courtheoux P., Alachkar F., et al. Intravascular technics of cerebral revascularization. J Mal Vasc. 1990;15:245-256.
67. Yadav J.S., Wholey M.H., Kuntz R.E., et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493-1501.
68. Ansel G.M., Hopkins L.N., Jaff M.R., et al. Investigators for the ARMOUR Pivotal Trial. Safety and effectiveness of the INVATEC MO.MA(R) proximal cerebral protection device during carotid artery stenting: results from the ARMOUR pivotal trial. Catheter Cardiovasc Interv. 2010;76:1-8.
69. Moret J., Pierot L., Boulin A., et al. Remodeling of the arterial wall of the parent vessel in the endovascular treatment of intracranial aneurysms (abstr S83). Proceedings of the 20th Congress of the European Society of Neuroradiology. Neuroradiology. 1994;36(Suppl 1):S83.
70. Molyneux A.J., Cekirge S., Saatci I., et al. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol. 2004;25:39-51.
71. Weber W., Siekmann R., Kis B., et al. Treatment and follow-up of 22 unruptured wide-necked intracranial aneurysms of the internal carotid artery with Onyx HD 500. AJNR Am J Neuroradiol. 2005;26:1909-1915.
72. Hanel R.A., Boulos A.S., Sauvageau E.G., et al. Stent placement for the treatment of nonsaccular aneurysms of the vertebrobasilar system. Neurosurg Focus. 2005;18:E8.
73. Fiorella D., Albuquerque F.C., Han P., et al. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery. 2004;54:6-17.
74. Lavine S.D., Larsen D.W., Giannotta S.L., et al. Parent vessel Guglielmi detachable coil herniation during wide-necked aneurysm embolization: treatment with intracranial stent placement: two technical case reports. Neurosurgery. 2000;46:1013-1017.
75. Chow M.M., Woo H.H., Masaryk T.J., et al. A novel endovascular treatment of a wide-necked basilar apex aneurysm by using a Y-configuration, double-stent technique. AJNR Am J Neuroradiol. 2004;25:509-512.
76. Sani S., Lopes D.K. Treatment of a middle cerebral artery bifurcation aneurysm using a double Neuroform stent “Y” configuration and coil embolization: technical case report. Neurosurgery. 2005;57:E209.
77. Thorell W.E., Chow M.M., Woo H.H., et al. Y-configured dual intracranial stent-assisted coil embolization for the treatment of wide-necked basilar tip aneurysms. Neurosurgery. 2005;56:1035-1040.
78. Horowitz M., Levy E., Sauvageau E., et al. Intra/extra-aneurysmal stent placement for management of complex and wide-necked bifurcation aneurysms: eight cases using the waffle cone technique. Neurosurgery. 58, 2006. ONS-258–262
79. Kelly M.E., Turner R., Gonugunta V., et al. Stent reconstruction of wide-necked aneurysms across the circle of Willis. Neurosurgery. 2007;61:249-255.
80. Kallmes D.F., Ding Y.H., Dai D., et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38:2346-2352.
81. Wong G.K., Kwan M.C., Ng R.Y., et al. Flow diverters for treatment of intracranial aneurysms: current status and ongoing clinical trials. J Clin Neurosci. 2011;18:737-740.
82. Nelson P.K., Lylyk P., Szikora I., et al. The Pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011;32:34-40.
83. Szikora I., Berentei Z., Kulcsar Z., et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. AJNR Am J Neuroradiol. 2010;31:1139-1147.
84. Lylyk P., Miranda C., Ceratto R., et al. Curative endovascular reconstruction of cerebral aneurysms with the Pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64:632-643.
85. Cekirge S. FD or “homemade” FD with multiple stents? Presented at the 2nd European Society of Minimally Invasive Neurological Therapy (ESMINT) Congress, Nice, France, Sept. 10, 2010.
86. Fiorella D., Woo H.H., Albuquerque F.C., et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the Pipeline embolization device. Neurosurgery. 2008;62:1115-1121.
87. Szikora I. Results using flow diverter devices: ongoing or reported studies. Presented at the 2nd European Society of Minimally Invasive Neurological Therapy (ESMINT) Congress, Nice, France, Sept. 10, 2010.
88. U.S. Food and Drug Administration. Instructions for use (IFU) Pipeline embolization device. Available at http://www.accessdata.fda.gov/cdrh_docs/pdf10/P100018c.pdf. Accessed May 18, 2011.
89. U.S. Food and Drug Administration. Pipeline embolization device—P100018. Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cftopic/pma/pma.cfm?num=p100018 Accessed May 18, 2011
90. Larson J.J., Tew J.M.Jr., Tomsick T.A., et al. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery. 1995;36:26-30.
91. Origitano T.C., al-Mefty O., Leonetti J.P., et al. Vascular considerations and complications in cranial base surgery. Neurosurgery. 1994;35:351-363.
92. Lawton M.T. Spetzler-Martin grade III arteriovenous malformations: surgical results and a modification of the grading scale. Neurosurgery. 2003;52:740-749.
93. Newton T.H., Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969;93:1071-1078.
94. van Dijk J.M., terBrugge K.G., Willinsky R.A., et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002;33:1233-1236.
95. Soderman M., Pavic L., Edner G., et al. Natural history of dural arteriovenous shunts. Stroke. 2008;39:1735-1739.
96. Strom R.G., Botros J.A., Refai D., et al. Cranial dural arteriovenous fistulae: asymptomatic cortical venous drainage portends less aggressive clinical course. Neurosurgery. 2009;64:241-248.
97. Charpentier C., Audibert G., Guillemin F., et al. Multivariate analysis of predictors of cerebral vasospasm occurrence after aneurysmal subarachnoid hemorrhage. Stroke. 1999;30:1402-1408.
98. Murayama Y., Malisch T., Guglielmi G., et al. Incidence of cerebral vasospasm after endovascular treatment of acutely ruptured aneurysms: report on 69 cases. J Neurosurg. 1997;87:830-835.
99. Bejjani G.K., Bank W.O., Olan W.J., et al. The efficacy and safety of angioplasty for cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1998;42:979-987.
100. Fujii Y., Takahashi A., Yoshimoto T. Effect of balloon angioplasty on high grade symptomatic vasospasm after subarachnoid hemorrhage. Neurosurg Rev. 1995;18:7-13.
101. Haque R., Kellner C.P., Komotar R.J., et al. Mechanical treatment of vasospasm. Neurol Res. 2009;31:638-643.
102. Rosenwasser R.H., Armonda R.A., Thomas J.E., et al. Therapeutic modalities for the management of cerebral vasospasm: timing of endovascular options. Neurosurgery. 1999;44:975-980.