CHAPTER 374 Endovascular Approaches to Intracranial Aneurysms
The past 30 years have witnessed landmark advances in the endovascular management of intracranial aneurysms.1,2 These advances include the invention of numerous embolic devices and major refinements in endovascular techniques and imaging.3,4 The mainstay of treatment remains the detachable coil, but other novel devices—such as flow diverters, stents, and liquid embolic agents—have broadened the spectrum of “coilable” aneurysms.5–8 Moreover, the results of randomized trials, the most important of which is the International Subarachnoid Aneurysm Trial (ISAT), have validated that coiling is safer than clipping aneurysms.9–15 This finding, coupled with the rapid pace of endovascular innovation, will lead to the proliferation of these techniques and their application to a wider range of patients.
Rather than review the numerous endovascular techniques used to treat aneurysms, we have chosen to describe approaches as they apply to specific aneurysm locations. The technical nuances, potential complications, and embolic devices employed differ from one aneurysm location to the other. For example, balloon remodeling is more often used to treat posterior communicating artery (PCoA) aneurysms than for anterior communicating artery (ACoA) aneurysms.16–18 Likewise, stents may be used in different configurations to treat aneurysms involving the basilar artery, terminus of the internal carotid artery (ICA), and dissecting aneurysms of the fourth segment of the vertebral artery (V4). Knowledge of these techniques and of how they apply to specific aneurysm locations is the single most important means of preventing complications.
Endovascular Approaches to ACoA Aneurysms
Coiling remains the endovascular treatment of choice for ACoA aneurysms.19,20 The acute angle of the A2 segments as they originate from the ACoA often renders balloon remodeling and stenting difficult. Several studies have validated the efficacy and safety of coiling these aneurysms. Nonetheless, they remain a challenging subgroup of lesions to treat21 because of a number of factors: difficulty catheterizing the A1 segment, tortuosity at the A1-2 junction, and suboptimal imaging caused by multiple arterial branches arising near the aneurysm site. Improvements in catheter and microwire technology and refinement of three-dimensional (3-D) angiography have reduced these limitations to a certain degree.22
For aneurysms arising from the ACoA segment itself, the direction that the aneurysm points is a good predictor of the success of treatment. Inferiorly and posteriorly projecting lesions are more difficult to coil because visualization is often suboptimal and catheter stability within the aneurysm can be tenuous. Steam shaping the catheter tip or using an appropriately angled catheter can reduce the tendency of catheter “kick back” while coiling. That ACoA aneurysms are typically small further complicates treatment.23,24 This factor, coupled with the tortuosity of the A1 and ACoA segments, often renders catheter stability precarious within the aneurysm sac. The operator may be forced to reposition the catheter and to recatheterize the aneurysm several times before a stable coil mass is achieved within the lesion. These repeated efforts increase the likelihood of these aneurysms rupturing during the procedure.
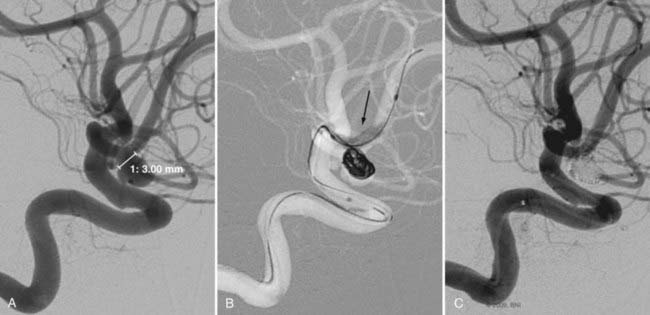
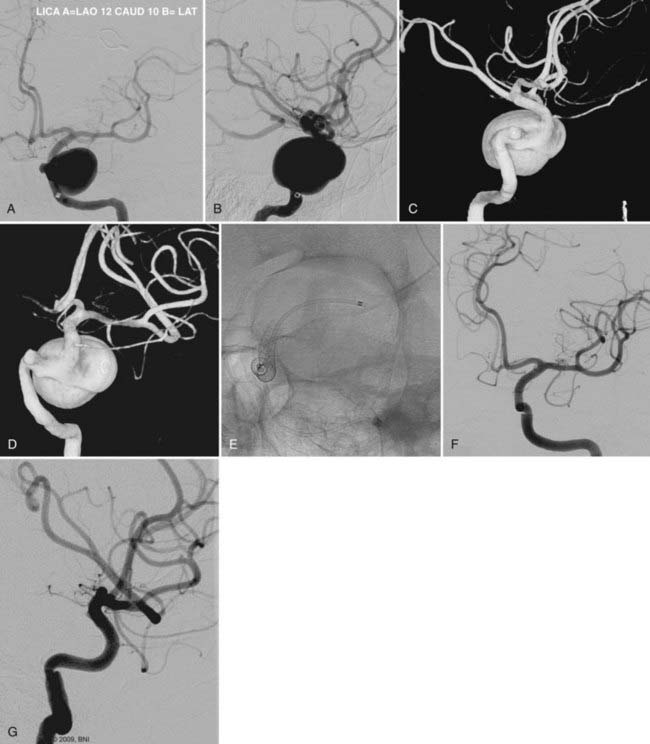
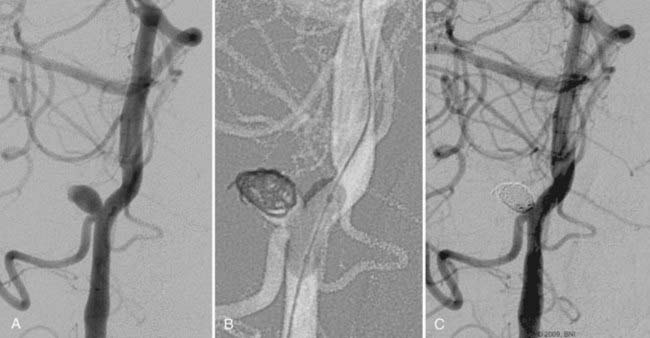
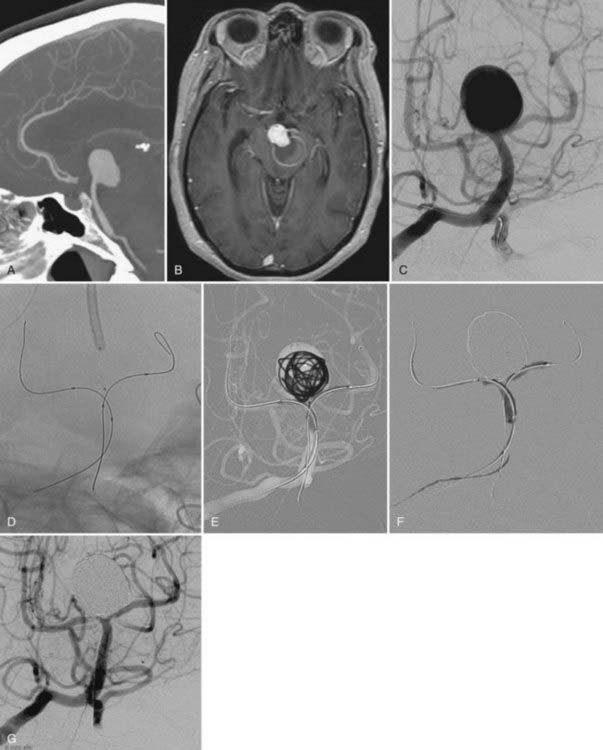
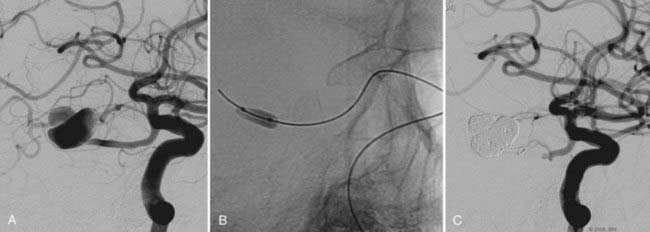
The use of balloon remodeling can serve three important purposes in the treatment of ACoA aneurysms.25 Specifically, this technique can improve the likelihood of coil retention within wide-necked lesions, increase the density of the coil pack, and provide a means of tamponade in the event of an intraprocedural rupture.26 Navigating a balloon, however, into a small or tortuous A1 segment can be difficult and often impossible (Fig. 374-1). The smaller caliber of the balloon catheter microwire and the relative stiffness of the device itself can preclude navigation along this segment. A microcatheter exchange, in which a more supple and navigable microcatheter is first directed into the anterior cerebral circulation and then exchanged for the balloon catheter over a long microwire, can be used when balloon remodeling is required. Nevertheless, this exchange technique is technically challenging and poses a high risk of devastating complications, such as vessel perforation and thrombosis.
Another elegant, but technically difficult, means of balloon remodeling is to navigate a microcatheter into the aneurysm from one A1 and the balloon catheter from the contralateral A1. This technique underscores the need for a clear understanding of the bilateral anatomy of A1 and ACoA. One trajectory may be better for navigating into the aneurysm while the other may be better for navigating the balloon across the ACoA segment and into the contralateral A2. Finally, the so-called kissing balloon technique, in which balloons are navigated from both sides and inflated while a third catheter is used to coil the aneurysm is perhaps the most challenging catheter approach used to treat these lesions.27 The high morbidity and mortality rates associated with these “transcirculation” techniques should first prompt the operator to consider clip ligation of these challenging aneurysms.
Stent-supported coil embolization can also be used to treat ACoA aneurysms. The recent evolution of easily navigable microstents permits catheterization of the distal anterior cerebral arteries (ACAs).28 Nonetheless, because antiplatelet medical prophylaxis is needed, this strategy is typically reserved for unruptured aneurysms and used only as a “salvage” technique in cases of subarachnoid hemorrhage (SAH). Salvage situations include herniation of coils into the parent artery or vessel thrombosis, in which a stent can be deployed to reestablish vessel patency. For unruptured wide-necked aneurysms of the ACoA region, stenting offers a potential alternative to microsurgical clipping. The risks of stenting, including the technical factors associated with catheterization and the need for long-term single and occasionally dual antiplatelet medical therapy, must be weighed against the comparative risks of clip ligation. For older patients or those with other medical comorbidities, stent-supported embolization may be the treatment of choice for wide-necked aneurysms in the region of the ACoA.
Endovascular Approaches to Pericallosal Aneurysms
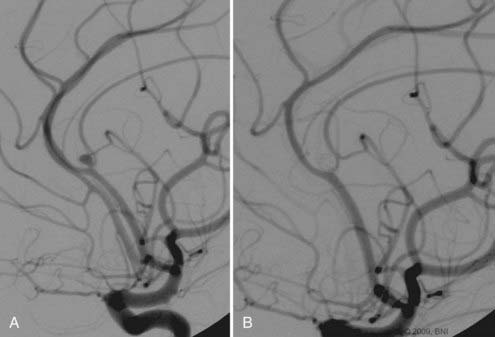
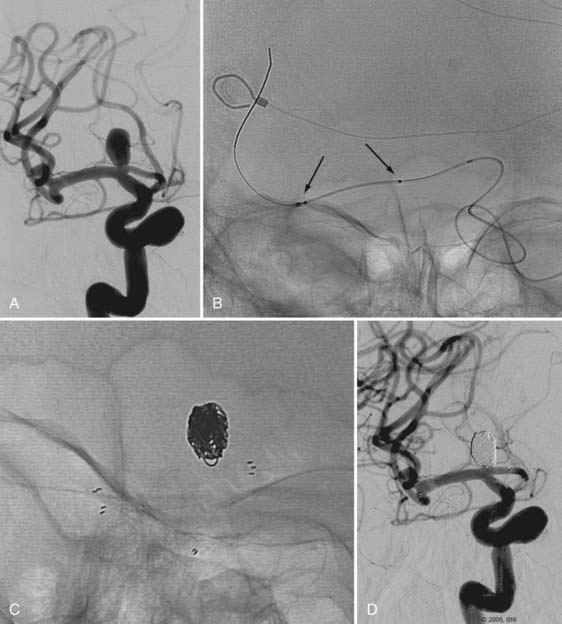
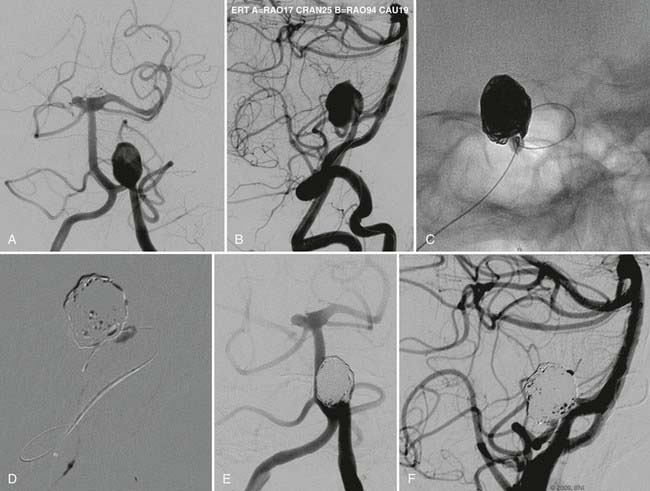
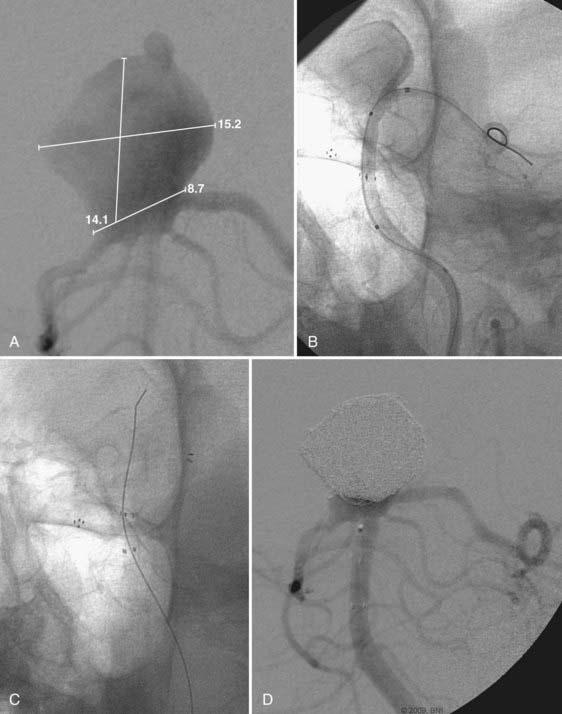
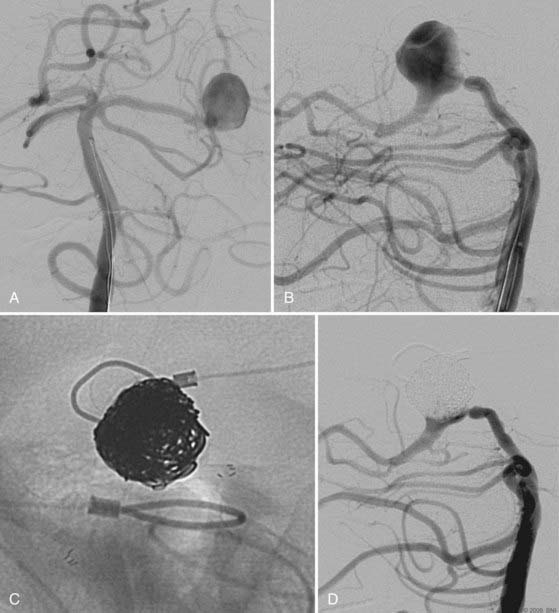
Pericallosal aneurysms are rare and frequently amenable to coil embolization. These lesions usually arise from the branch point of the pericallosal and callosomarginal arteries. As such, the most salient concern in their treatment is preservation of these parent arteries (Fig. 374-2). Wide-neck lesions of this region are best addressed through an interhemispheric craniotomy because both stent and balloon-assistance can be difficult to achieve in their distal location. However, in patients with large caliber ACAs and straightforward arterial anatomy, such adjuvant techniques can be employed. As with ACoA aneurysms, 3-D angiography is essential to delineate the aneurysm and its anatomy relative to the parent pericallosal and callosomarginal arteries. Bilateral carotid angiography is recommended to determine the easiest A1 route to the aneurysm. Navigation across the ACoA segment to the contralateral A2 may be required but is seldom difficult to accomplish.
Endovascular Approaches to PCoA Aneurysms
This variable group of lesions accounts for the second most common type of aneurysm after those of the ACoA. Both ruptured and unruptured PCoA aneurysms can manifest with acute palsy of the third cranial nerve.29 Which treatment improves function of the third cranial nerve in this setting is under debate.29 Surgical clip ligation offers rapid relief of aneurysmal mass effect, while progressive thrombosis and retraction of the aneurysm after coiling may be a relatively prolonged process. Regardless, this subgroup of aneurysms is readily treated through a variety of endovascular techniques.
Once again the use of 3-D angiography is paramount. This modality facilitates visualization of both the PCoA and parent ICA and delineates their relationship to the aneurysm neck.30 Another crucial point is determining whether the PCoA is fetal. Both computerized tomographic angiography (CTA) and magnetic resonance angiography (MRA) can adequately demonstrate the posterior circulation. In their absence, however, digital subtraction angiography (DSA) of the posterior circulation must be undertaken to determine the presence of the ipsilateral P1 artery. If a fetal PCoA is present, its sacrifice by a protruding coil mass within the aneurysm can produce an infarction of the distal territory of the posterior cerebral artery (PCA). Conversely, the operator can be more aggressive and allow coils to encroach on the PCoA when the vessel is not fetal.
Once the anatomic characteristics of the PCoA have been delineated and the optimal working angle for visualization of the neck achieved, coil embolization of these lesions is typically straightforward. Many operators prefer to use balloon remodeling for most PCoA aneurysms. In this setting, balloon remodeling may allow greater packing of the aneurysm and serve as a means of tamponade in the event of intraprocedural rupture.31–33 The highly compliant balloons currently available for use also partially herniate into aneurysms, potentially improving the chances of preserving a PCoA arising from the proximal neck of the aneurysm. Finally, in patients with a patent ACoA segment, the balloon can be left inflated for prolonged periods while multiple coils are placed into the aneurysm without subjecting the patient to an inordinate risk of stroke. Electrophysiologic monitoring is also useful for guiding the length of time the balloon remains inflated. A diminution of the somatosensory evoked potentials or a depression in the electroencephalogram (EEG) should prompt rapid deflation of the device.
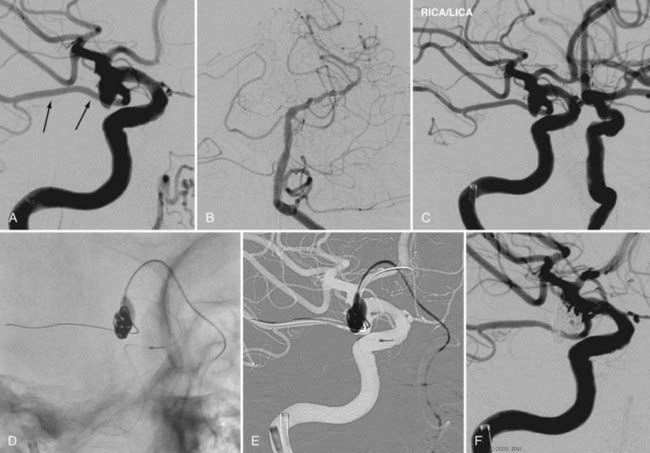
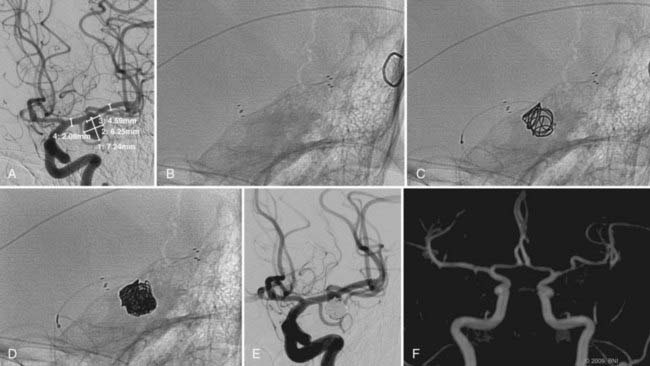
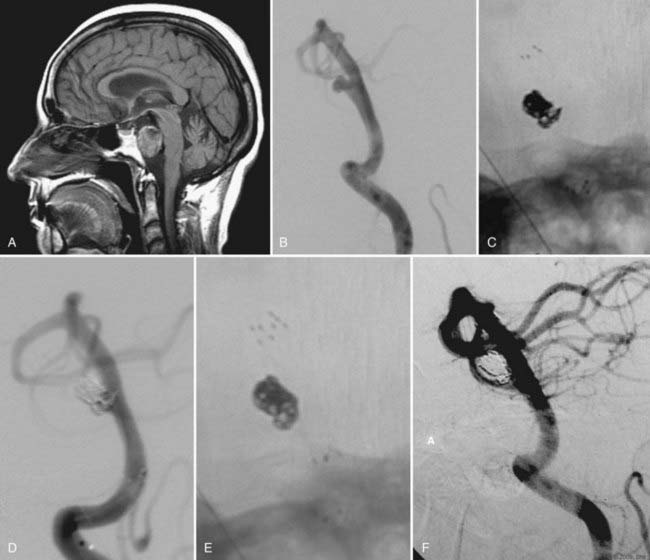
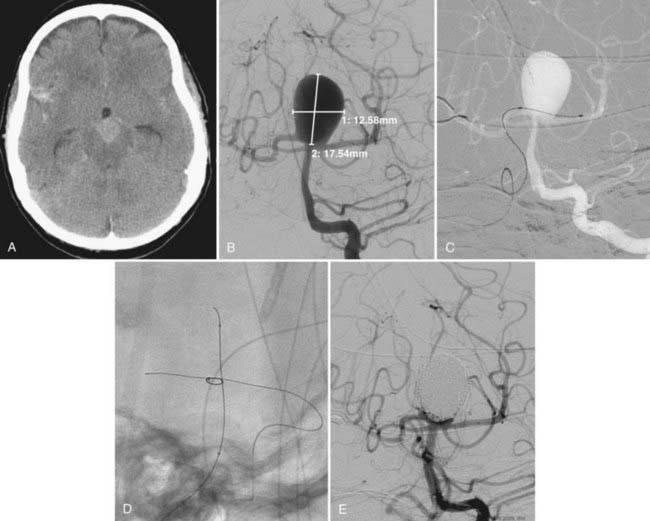
Every effort must be made to preserve the patency of a fetal PCoA. As described, herniation of a compliant balloon into the aneurysm, partially covering the orifice of the fetal artery, may provide adequate protection. When this configuration cannot be accomplished, navigation of the balloon directly into the PCoA can be considered. Often, however, the acute angulation of this arterial segment off the ipsilateral ICA precludes navigation of the balloon catheter. A more steerable microcatheter may take this arterial turn more easily and can then be exchanged for the balloon catheter over a 300-cm microwire. Transcirculation catheterization of the fetal PCoA via the contralateral ICA can also be undertaken but is more technically challenging and requires adequate patency of the ACoA segment (Fig. 374-3). These more demanding catheter techniques should first prompt the operator to consider the feasibility and safety of clip ligation for these challenging lesions.
Finally, balloon-assisted Onyx HD 500 (Onyx, Irvine, CA) infusion can be used to treat large and wide-necked PCoA aneurysms.34 This technique requires placement of a long compliant balloon across the neck of the aneurysm to achieve a “seal” and to prevent inadvertent propagation of the embolisate into the distal circulation. The durability of Onyx HD 500 has yet to be established.34 However, several preliminary studies suggest that this agent may be more effective than coiling for the treatment of large and wide-necked aneurysms.34 The adjunctive use of coils and stents with Onyx embolization has also been reported and may further decrease the likelihood of an aneurysmal recurrence.
Endovascular Approaches to Ophthalmic Aneurysms
The most important caveat for the endovascular treatment of ophthalmic aneurysms is to verify the location of the ophthalmic artery as it relates to the neck of the aneurysm. Frequently, the artery arises from the proximal portion of the neck rendering coiling a less than ideal means of treatment. Several studies have reported that proximal occlusion of the ophthalmic artery is well-tolerated by patients.10,16 However, the gravity of monocular visual loss should prompt surgical exploration as the treatment of choice in these select cases. Nevertheless, when the ophthalmic artery is distinct from the aneurysm, endovascular treatment is straightforward and consists of many of the techniques described earlier.
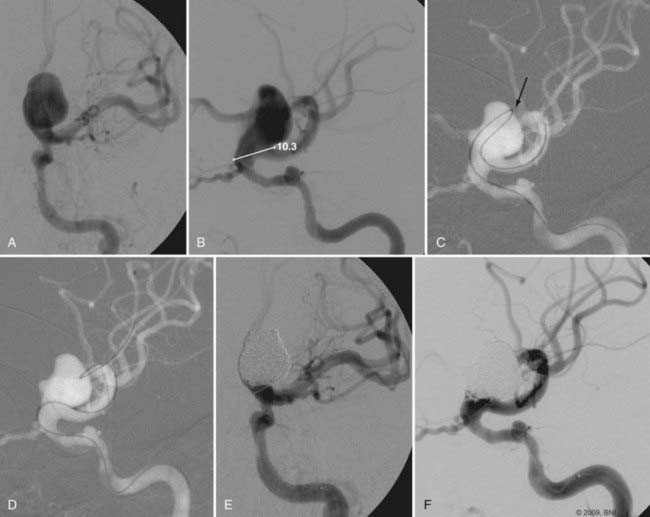
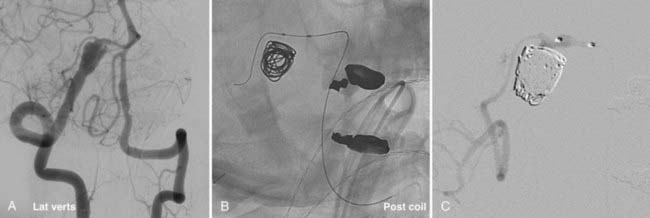
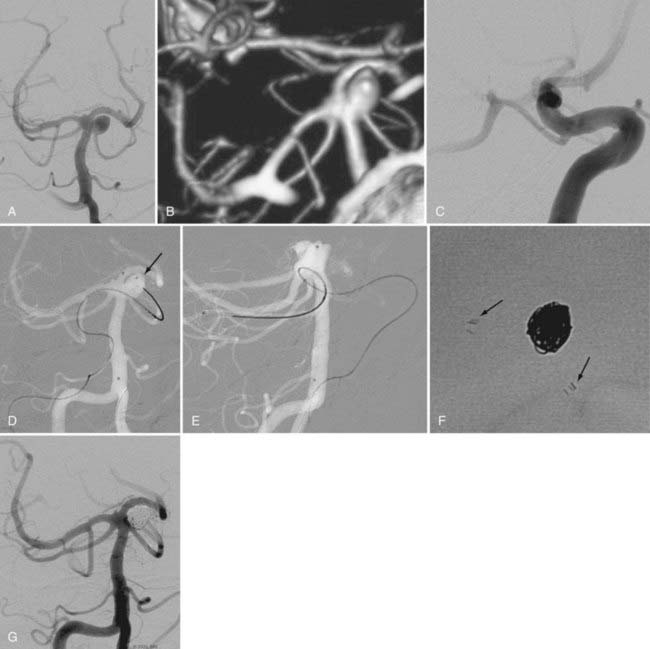
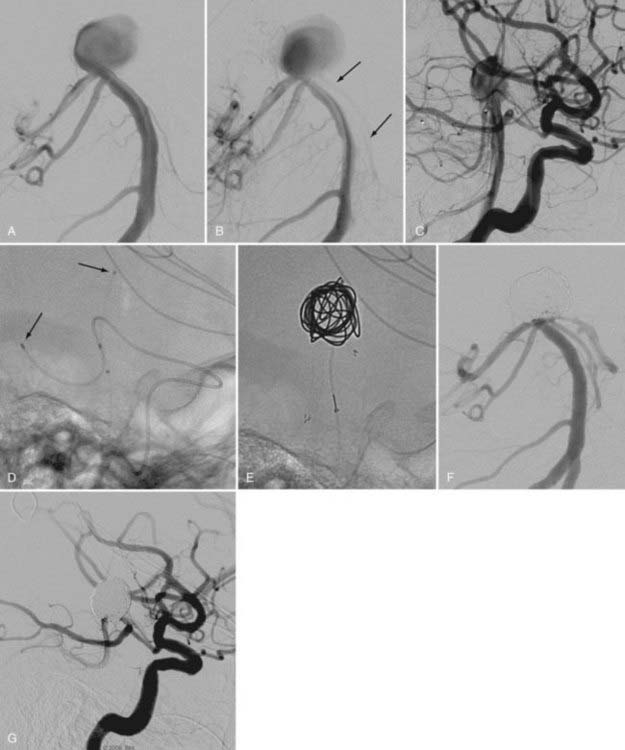
Catheter stability, especially in this location where the aneurysm typically arises just after the siphon turn, is the most challenging aspect of embolization. Steam shaping or employing a preshaped catheter may afford greater purchase of the catheter within the aneurysm during coiling. Similarly, in cases of wide-necked aneurysms, balloon remodeling or the jailing/stenting technique may provide additional support for the catheter as it enters the neck of the aneurysm, thereby facilitating embolization (Fig. 374-4). These adjuvant techniques are frequently required for the treatment of these aneurysms. The proximal location of the lesion and the relatively large caliber of the ICA along this segment simplify the use of these devices.
Approaches to Cavernous Aneurysms
This heterogeneous group of aneurysms has a variety of indications for treatment.35 These lesions have an exceedingly low propensity for life-threatening sequelae even after hemorrhage. Nonetheless, there are several compelling reasons to treat cavernous aneurysms: the risk of rupture with the creation of a carotid-cavernous fistula (CCF), compressive cranial neuropathy, progressively worsening headache, and erosion of the sphenoid sinus by the aneurysm. CCFs obviously threaten visual integrity and optic mobility and therefore mandate urgent treatment. Headache, especially ipsilateral retro-orbital pain, can be debilitating and treatable by embolization of the aneurysm. Compressive cranial neuropathies causing ptosis and paralysis of eye movement can be addressed through endovascular flow diversion techniques, in which progressive thrombosis and shrinkage of the aneurysm relieve the compressive forces. Finally, erosion by the aneurysm of the sphenoid sinus potentially exposes the patient to life-threatening epistaxis. These lesions are unsecured and should be treated urgently.
Cavernous aneurysms requiring treatment tend to be large or giant.36,37 Indeed, it is difficult to argue for the treatment of small cavernous aneurysms given their benign natural history. Treatment techniques have evolved considerably for this subgroup of aneurysms. Techniques of historical interest include hunterian parent artery ligation either surgically or through endovascular methods.38–40 Parent artery ligation was typically preceded by balloon test occlusion (BTO).41,42 In the event of BTO failure, sophisticated bypass techniques were used to augment cerebral blood flow before the parent artery was sacrificed.43 This deconstructive strategy was largely supplanted by detachable balloon embolization of the aneurysm and later by the advent of aneurysm coiling. Adjuvant techniques, such as balloon remodeling and stent-supported coiling, were often used to address the typically wide-necked anatomy of these aneurysms. The evolution of flow diversion technology, however, will likely supplant all of these modalities as the treatment of choice for large and giant cavernous aneurysms. Nevertheless, the current lack of widespread availability of these products will prolong the use of these other techniques.
BTO, followed by parent artery ligation, with or without surgical bypass, remains a commonly employed treatment strategy for this group of aneurysms.44,45 BTO is performed through bifemoral access in which the balloon catheter is inflated in the target artery while diagnostic angiography of the contralateral ICA and vertebral artery is performed. This technique allows assessment of the patient’s collateral circulation to the affected hemisphere. Nuclear medicine scans are also more readily obtained to improve quantification of the effect of balloon inflation on cerebral perfusion and to reduce the likelihood of false-negative results. Failure of BTO mandates revascularization, which usually involves a high-flow bypass with a radial artery or saphenous vein graft to anastomose the cervical ICA with the middle cerebral artery (MCA). The ICA can be ligated at the time of bypass or in a delayed fashioned through embolization. If the patient passes the BTO, the artery can be occluded with a coil anywhere from the cervical portion to the aneurysmal segment.
With the advent of flexible stents designed specifically for navigation in the intracranial vasculature, coil embolization of these aneurysms became substantially easier. Stent-supported coiling can be accomplished either through the jailing technique or with primary passage of the microcatheter through the sidewall tines of the stent. Again, movement of the stent during this process should prompt the operator to abort the procedure to allow the stent to scar in place. In cases of ultrawide-necked aneurysms, a balloon-in-stent technique improves assessment of the parent artery during coil embolization.46 In this technique, a balloon is inflated within the stent during coiling and then deflated on blank roadmap imaging. Coil migration toward the “ghost” of the balloon confirms herniation into the parent artery and should prompt repositioning of the coil.
Flow diversion will likely supplant these technically difficult endovascular procedures in the treatment of symptomatic cavernous aneurysms (Fig. 374-5). These devices, which are stents constructed with a higher density of metallic strands, allow flow to be diverted through the central lumen of the device and away from the aneurysm neck. Flow diversion produces stagnation and eventual thrombosis within the aneurysm sac. Thrombosis shrinks the size of the aneurysm and can ameliorate cranial nerve compression and potentially reduce the severity of headache. Recent multicenter experience documents a nearly 100% rate of aneurysm obliteration with the use of these devices. Nonetheless, because of the novelty of this technique, the long-term patency of the stented vessel is not yet known nor is it well-understood how and for how long the patient should be treated with antiplatelet medications. Preliminary results, however, suggest persistent patency and excellent efficacy of aneurysm obliteration.
Endovascular Approaches to Aneurysms of the Ica Terminus
Transcirculation techniques can also be used to assist the coiling process. Either a balloon catheter or a stent delivery device can be navigated from the contralateral ICA, across the ACoA segment to the neck of the aneurysm (Fig. 374-6). In the event of tortuous A1s, a catheter exchange may be required to deliver the balloon or stent catheter to the aneurysmal neck. Simultaneous angiography, with injection through both ICAs, easily delineates both A1s and the ACoA segment and facilitates transcirculation catheterization. Three-dimensional angiography is also critical for demonstrating the neck of the aneurysm as it relates to the ACA and MCA branches.
Endovascular Approaches to MCA Aneurysms
The proximity to and involvement of MCA branches renders this aneurysm subgroup particularly challenging for endovascular treatment.47,48 Conversely, clip ligation is relatively straightforward because most of these lesions reside within the distal aspect of the sylvian fissure. Compromise of any of the MCA branches by the coil mass risks a devastating ischemic or thromboembolic insult. Nevertheless, if 3-D angiography shows a favorable neck and anatomic distinction from the parent MCA branches, coil embolization can be undertaken.
Adjunctive techniques such as balloon remodeling and stent assistance are often required to treat this heterogeneous group of aneurysms (Fig. 374-7). Technical challenges include the distal location of the aneurysm, often at the MCA trifurcation, the caliber of the affected MCA branches, and the tortuosity of the catheterization. The relatively small caliber of the MCA compared with the proximal ICA increases the risk of complications associated with balloon-remodeling and stent-supported techniques. Regarding the former, inflation of a balloon in a vessel that is often less than 2 mm in diameter likely increases the risk of arterial rupture or dissection. Similarly, deposition of a stent in this small arterial segment may encourage in-stent stenosis and certainly impedes navigation of a second microcatheter across the side wall of the stent. Jailing the first microcatheter within the aneurysm is often the necessary first step when undertaking a stent-supported treatment strategy. Deploying a stent at the MCA trifurcation undoubtedly covers the ostia of one or more of the M2 branches. Although laboratory studies have demonstrated that these vessels remain patent in the acute phase, their rate of chronic patency has not yet been determined clinically. Finally, a tortuous ipsilateral ICA and distal M2 branches may complicate navigation of the balloon catheter and stent-delivery device to the target site.
Endovascular Approaches to Aneurysms of the Distal Vertebral Artery (V4)
The optimal management of this complex and dangerous subgroup of aneurysms is controversial.49,50 Endovascular approaches encompass a number of deconstructive and reconstructive techniques. What is well known, however, is that these lesions can manifest with a devastating clinical picture and are prone to early rerupture. Proximity to the brainstem can trigger cardiac arrhythmias and secondary myocardial infarction. Despite appearing moribund on presentation, however, many patients completely recover after these aneurysms rupture. These lesions are typically dissections involving the wall of the V4 segment circumferentially. That they occur where the vessel enters the cranial dura suggests that traumatic irritation of the artery is an etiologic factor.
The location of the posterior inferior cerebellar artery (PICA) and anterior spinal artery (ASA) are crucial in determining the optimal endovascular approach. Involvement of either or both of these vessels with the aneurysmal segment should prompt a reconstructive rather than deconstructive strategy. When these arteries arise opposite the aneurysmal segment of the vessel, balloon and stent assistance may be necessary. Balloon remodeling across the neck of the aneurysm may suffice to allow adequate coiling when a distinct saccular component is present. Dissecting fusiform aneurysms, however, are better treated through stent assistance. In these cases, the deposition of one or more stents has the added benefit of reinforcing the dissected arterial wall.51,52 Indeed, stenting alone can allow for vessel remodeling and complete regression of the aneurysmal segment.53 Despite these adjunctive techniques, these aneurysms can recur and mandate early angiographic follow-up.54
When either or both the PICA and ASA arise directly from the diseased arterial segment, flow diversion techniques or surgical reinforcement should be entertained. The latter includes wrapping the diseased aneurysmal segment, ligating the vessel after performing a PICA-to-PICA bypass, or both. Flow diversion may become the treatment of choice for all V4 aneurysms.7 Animal studies have confirmed that perforating branches through the stented segment remain patent despite coverage with these “heavy metal” devices.7 Curative reconstruction with these devices has already been documented in the treatment of these aneurysms. Again, the long-term patency of these treated arterial segments has yet to be established.
When the aneurysmal segment is distinct from the PICA and ASA, the contralateral vertebral artery should be assessed carefully before embarking on a deconstructive approach. If the contralateral artery is patent, large, and anastomoses with the basilar artery, the diseased segment of the ipsilateral vertebral artery can be safely occluded with coils.55 Occasionally, the transcirculation navigation of a balloon from the contralateral artery to a point just distal to the aneurysm may be necessary to ensure that coils do not migrate distally toward the vertebrobasilar junction or across a critical arterial branch (Fig. 374-8). This hunterian approach has proved effective in the treatment of V4 aneurysms and is associated with a very low rate of delayed ischemic complications. In the setting of an atretic or occluded contralateral vertebral artery or one that ends in PICA, the ipsilateral diseased artery cannot be sacrificed. Such cases are better managed with stenting, flow diversion, or surgical reconstruction.
Endovascular Approaches to Pica Aneurysms
Like MCA aneurysms, PICA aneurysms are amenable to a variety of microsurgical strategies.56 However, these lesions often incorporate part of the parent vessel wall, thereby rendering endovascular treatment quite challenging.57 Impingement of the PICA proximal to the choroidal point by a coil mass risks brainstem and cerebellar infarction. These fusiform lesions are best treated through surgical clipping, vessel ligation with PICA-to-PICA bypass, or both. Nonetheless, aneurysms with well-defined necks can be addressed via endovascular means. Similarly, aneurysms that arise from the vertebral artery at the origin of the PICA are often amenable to balloon-assisted or stent-supported coiling techniques (Fig. 374-9).
Balloon remodeling of the PICA itself is challenging and can require transcirculation navigation of the balloon from the contralateral vertebral artery. This process is necessary because the PICA often courses inferiorly in an acute angle off the ipsilateral vertebral artery. Navigating the balloon catheter down from the vertebrobasilar junction facilitates catheterization of the affected PICA (Fig. 374-10). The aneurysm can then be catheterized from an ipsilateral approach. The complexity of this strategy and the increased risk associated with balloon inflation in a small arterial branch such as the PICA should first prompt consideration of a direct surgical approach. In fact, this technique is best reserved for patients in whom the risks of surgery are inordinately high. This subgroup includes the elderly and patients with other significant comorbid medical factors.
Endovascular Approaches to Basilar Trunk Aneurysms
Stent-supported coil embolization has broadened the armamentarium for treating basilar trunk aneurysms (Fig. 374-11). The balloon-in-stent technique described earlier may be required in cases of ultrawide-necked aneurysms. The placement of multiple stents without coils along the course of fusiform or dissecting aneurysms has shown promise in select cases. The recent evolution of flow-diverting stents has advanced the treatment of these lesions.58 Of major concern with these methods, however, is the long-term patency of the stented segment and that of the myriad perforating arteries along the basilar trunk. Preliminary studies employing flow-diverting devices show promise in treating large fusiform aneurysms.58 Previously inoperable lesions have been cured through the deployment of multiple devices along the arterial course of the aneurysm. Perforator patency has also been documented, but the long-term sequelae are not yet known.
Endovascular Approaches to Superior Cerebellar Artery Aneurysms
Complex SCA aneurysms may require a transcirculation approach (Fig. 374-12). An example is the SCA aneurysm in which the parent artery originates from the proximal aneurysm neck. In the setting of a large PCoA artery, a balloon or stent may be navigated down the basilar artery and into the SCA. Because the SCA often arises in an acute angle from the basilar apex, this downward navigation is more anatomically favorable than making this acute turn from below. Nevertheless, the inability to steer the balloon and stent-deployment catheters often requires that a microcatheter exchange be performed. The cumulative risk of these complex treatment strategies must be weighed against the natural history of the aneurysm if left untreated.
Endovascular Approaches to Basilar Apex Aneurysms
The complexity of treating this group of aneurysms through open surgical approaches has spawned the development of several innovative endovascular techniques.59–61 Although coiling of narrow-necked basilar aneurysms is perhaps the easiest endovascular coiling procedure, wide-necked lesions pose unique challenges.62–64
A kissing balloon construct may be required to assist coiling of a wide-necked basilar apex aneurysm involving both PCAs (Fig. 374-13). In this technique, bilateral vertebral artery access must be obtained. A large guide catheter is necessary on one side to allow passage of the coiling catheter and one balloon. The contralateral guide can be smaller given that it accommodates only a single balloon. The first balloon spans the basilar apex and one of the P1 segments, while the second balloon is placed within the proximal contralateral P1. These lesions are typically large, and multiple balloon inflations may be required to complete coiling. Prolonged inflations subject the patient to a higher risk of ischemic and thromboembolic complications. Similarly, repetitive inflations increase the risk of vessel dissection or rupture. For these reasons, this complex technique should be reserved for the most difficult and highest risk scenarios.
In certain wide-necked basilar aneurysms, a single balloon can be navigated into the proximal neck of the aneurysm. After a coiling microcatheter is navigated to the dome of the aneurysm, the balloon is inflated. This “cork-in-the-bottle” technique may be sufficient to protect both PCAs during coil embolization. Another technique involves placing two microcatheters within the aneurysm. A large three-dimensional coil can be threaded out of one of the catheters but not deployed.65,66 Subsequent smaller coils are then deployed through the second catheter, with the initial three-dimensional coil serving as a scaffold to protect the PCAs. This first coil can be deployed after several additional coils are threaded into the aneurysm.
Stent assistance can be employed in much the same fashion as the balloon techniques described earlier. A Y-stent construct, in which one stent is driven through the side wall of a previously placed stent, provides a stable construct for coiling and ensuring preservation of both PCAs (Fig. 374-14). In such cases, the first stent deployed is typically of the open-cell variety to allow the subsequent passage of a closed-cell stent through the side wall of the first stent. The coiling catheter can be jailed or navigated through the stent construct, although the latter may damage or distort the stents. Similarly, when an aneurysm is eccentric to one PCA, placement of a single stent spanning the basilar apex and the affected artery may be sufficient to allow complete coiling. Obviously, antiplatelet therapy is mandatory before either stenting procedure is begun. The complex nature of these techniques is associated with greater risk to the patient, and, again, should be reserved for cases in which the natural history of the untreated lesion is poor.
Transcirculation techniques are also effective in treating certain basilar apex aneurysms (Fig. 374-15). In this setting, a large PCoA is used as a conduit to place either a balloon or stent across the neck of a basilar apex aneurysm (Fig. 374-16). These approaches require catheterization of both the vertebral and internal carotid arteries. Simultaneous angiography through these two vessels delineates the course of the PCoA and facilitates its catheterization. Because of the lack of navigability of both the balloon and stent delivery catheters, a microcatheter exchange may be necessary to ensure accurate placement of these devices across the neck of the aneurysm. When stenting through this transcirculation approach, the coiling catheter can either be jailed primarily or navigated secondarily across both walls of the stent. The latter is less optimal because the stent can be moved or disrupted during catheterization of the aneurysm.
Endovascular Approaches to PCA Aneurysms
These challenging aneurysms often require adjuvant techniques for their management.67,68 Typically, these lesions are large and often involve part of the PCA wall circumferentially. Therefore, in most cases, care must be taken to preserve patency of the PCA. In the event of a large PCA and a cooperative patient, a BTO of the arterial segment just proximal to the aneurysm can be performed to delineate whether the parent artery can be sacrificed during coiling of the aneurysm (Fig. 374-17). Patients who fail BTO should either undergo an occipital-to-PCA bypass before coiling or have the aneurysm addressed through microsurgical clipping. Patients who pass the BTO should tolerate coiling of the aneurysm with either partial or complete blockage of the affected PCA.
Because of the difficulty of directly approaching these aneurysms microsurgically, the treatment of choice is endovascular embolization. Balloon and stent-assisted techniques are effective and often mandatory for the treatment of PCA aneurysms. Navigation of these devices may require a microcatheter exchange. Given the small caliber of the PCA, jailing the microcatheter before stenting is preferable to crossing the side wall of the stent with the coiling catheter (Fig. 374-18). This is because the small PCA will constrain the stent, thus narrowing the distance between the tines. Navigating through the side wall risks damaging or moving the stent. Similarly, in cases of balloon assistance, great care must be taken to prevent overinflation and rupture of the small caliber PCA. These adjuvant techniques often require catheterization of both vertebral arteries. The complexity of these procedures should prompt a thorough assessment of the risks that the aneurysm, if left untreated, poses to the patient. Elderly or medically frail patients should probably be treated only if the aneurysm enlarges or ruptures.
Albuquerque FC, Fiorella DJ, Han PP, et al. Endovascular management of intracranial vertebral artery dissecting aneurysms. Neurosurg Focus. 2005;18:E3.
Albuquerque FC, Spetzler RF, Zabramski JM, et al. Effects of three-dimensional angiography on the coiling of cerebral aneurysms. Neurosurgery. 2002;51:597-605.
Chen PR, min-Hanjani S, Albuquerque FC, et al. Outcome of oculomotor nerve palsy from posterior communicating artery aneurysms: comparison of clipping and coiling. Neurosurgery. 2006;58:1040-1046.
Cognard C, Weill A, Castaings L, et al. Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology. 1998;206:499-510.
Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656-665.
Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery. 2006;59:291-300.
Fiorella D, Albuquerque FC, Masaryk TJ, et al. Balloon-in-stent technique for the constructive endovascular treatment of “ultra-wide necked” circumferential aneurysms. Neurosurgery. 2005;57:1218-1227.
Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62:1115-1120.
Guglielmi G, Vinuela F, Dion J, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: preliminary clinical experience. J Neurosurg. 1991;75:8-14.
Guglielmi G, Vinuela F, Sepetka I, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: electrochemical basis, technique, and experimental results. J Neurosurg. 1991;75:1-7.
Gupta V, Chugh M, Jha AN, et al. Coil embolization of very small (2 mm or smaller) berry aneurysms: feasibility and technical issues. AJNR Am J Neuroradiol. 2009;30:308-314.
Hacein-Bey L, Connolly ESJr, Mayer SA, et al. Complex intracranial aneurysms: combined operative and endovascular approaches. Neurosurgery. 1998;43:1304-1312.
Higashida RT, Halbach VV, Dowd C, et al. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg. 1990;72:857-863.
Im SH, Han MH, Kwon OK, et al. Endovascular coil embolization of 435 small asymptomatic unruptured intracranial aneurysms: procedural morbidity and patient outcome. AJNR Am J Neuroradiol. 2009;30:79-84.
Lefkowitz MA, Gobin YP, Akiba Y, et al. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysms: part II—clinical results. Neurosurgery. 1999;45:531-537.
McDougall CG, Halbach VV, Dowd CF, et al. Causes and management of aneurysmal hemorrhage occurring during embolization with Guglielmi detachable coils. J Neurosurg. 1998;89:87-92.
Molyneux A, Kerr R, Stratton I, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
Moret J, Cognard C, Weill A, et al. The “remodelling technique” in the treatment of wide neck intracranial aneurysms. Interv Neuroradiol. 1997;3:21-35.
Murayama Y, Vinuela F, Tateshima S, et al. Endovascular treatment of experimental aneurysms by use of a combination of liquid embolic agents and protective devices. AJNR Am J Neuroradiol. 2000;21:1726-1735.
Ponce FA, Albuquerque FC, McDougall CG, et al. Combined endovascular and microsurgical management of giant and complex unruptured aneurysms. Neurosurg Focus. 2004;17:E11.
Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery. 1997;41:1235-1245.
Suzuki S, Tateshima S, Jahan R, et al. Endovascular treatment of middle cerebral artery aneurysms with detachable coils: angiographic and clinical outcomes in 115 consecutive patients. Neurosurgery. 2009;64:876-888.
Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475-482.
Wakhloo AK, Lanzino G, Lieber BB, et al. Stents for intracranial aneurysms: the beginning of a new endovascular era? Neurosurgery. 1998;43:374-379.
Yamaura I, Tani E, Yokota M, et al. Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Guglielmi detachable coils. J Neurosurg. 1999;90:853-856.
1 Lanzino G, Guterman LR, Hopkins LN. Endovascular treatment of aneurysms. In: Winn HR, editor. Youmans Neurological Surgery. 5th ed. Philadelphia: Saunders; 2004:2057-2078.
2 Renowden SA, Benes V, Bradley M, et al. Detachable coil embolisation of ruptured intracranial aneurysms: a single center study, a decade experience. Clin Neurol Neurosurg. 2009;111:179-188.
3 Wakhloo AK, Lanzino G, Lieber BB, et al. Stents for intracranial aneurysms: the beginning of a new endovascular era? Neurosurgery. 1998;43:377-379.
4 Im SH, Han MH, Kwon OK, et al. Endovascular coil embolization of 435 small asymptomatic unruptured intracranial aneurysms: procedural morbidity and patient outcome. AJNR Am J Neuroradiol. 2009;30:79-84.
5 Lanzino G, Hopkins LN. Treatment of an intracranial aneurysm using a new three-dimensional shape Guglielmi detachable coil: technical case report. Neurosurgery. 1999;44:1145.
6 Gruber A, Killer M, Bavinzski G, et al. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery. 1999;45:793-803.
7 Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62:1115-1120.
8 Lubicz B, Francois O, Levivier M, et al. Preliminary experience with the enterprise stent for endovascular treatment of complex intracranial aneurysms: potential advantages and limiting characteristics. Neurosurgery. 2008;62:1063-1069.
9 Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery. 1997;41:1235-1245.
10 Byrne JV, Sohn MJ, Molyneux AJ, et al. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg. 1999;90:656-663.
11 Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475-482.
12 Malisch TW, Guglielmi G, Vinuela F, et al. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg. 1997;87:176-183.
13 Maud A, Lakshminarayan K, Suri MF, et al. Cost-effectiveness analysis of endovascular versus neurosurgical treatment for ruptured intracranial aneurysms in the United States. J Neurosurg. 2009;110:880-886.
14 Ryttlefors M, Enblad P, Kerr RS, et al. International subarachnoid aneurysm trial of neurosurgical clipping versus endovascular coiling: subgroup analysis of 278 elderly patients. Stroke. 2008;39:2720-2726.
15 Molyneux A, Kerr R, Stratton I, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
16 Akiba Y, Murayama Y, Vinuela F, et al. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysms: part I—experimental evaluation. Neurosurgery. 1999;45:519-527.
17 Lefkowitz MA, Gobin YP, Akiba Y, et al. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysms: part II—clinical results. Neurosurgery. 1999;45:531-537.
18 Moret J, Cognard C, Weill A, et al. The “remodelling technique” in the treatment of wide neck intracranial aneurysms. Interv Neuroradiol. 1997;3:21-35.
19 Guglielmi G, Vinuela F, Duckwiler G, et al. Endovascular treatment of 306 anterior communicating artery aneurysms: overall, perioperative results. J Neurosurg. 2009;110:874-879.
20 Gonzalez N, Sedrak M, Martin N, et al. Impact of anatomic features in the endovascular embolization of 181 anterior communicating artery aneurysms. Stroke. 2008;39:2776-2782.
21 Proust F, Martinaud O, Gerardin E, et al. Quality of life and brain damage after microsurgical clip occlusion or endovascular coil embolization for ruptured anterior communicating artery aneurysms: neuropsychological assessment. J Neurosurg. 2009;110:19-29.
22 Albuquerque FC, Spetzler RF, Zabramski JM, et al. Effects of three-dimensional angiography on the coiling of cerebral aneurysms. Neurosurgery. 2002;51:597-605.
23 Gupta V, Chugh M, Jha AN, et al. Coil embolization of very small (2 mm or smaller) berry aneurysms: feasibility and technical issues. AJNR Am J Neuroradiol. 2009;30:308-314.
24 Tsutsumi M, Aikawa H, Onizuka M, et al. Endovascular treatment of tiny ruptured anterior communicating artery aneurysms. Neuroradiology. 2008;50:509-515.
25 Pierot L, Spelle L, Leclerc X, et al. Endovascular treatment of unruptured intracranial aneurysms: comparison of safety of remodeling technique and standard treatment with coils. Radiology. 2009;251:846-855.
26 Kwon BJ, Chang HW, Youn SW, et al. Intracranial aneurysm perforation during endosaccular coiling: impact on clinical outcome, initial occlusion, and recanalization rates. Neurosurgery. 2008;63:676-682.
27 Kelly ME, Gonugunta V, Woo HH, et al. Double-balloon trapping technique for embolization of a large wide-necked superior cerebellar artery aneurysm: case report. Neurosurgery. 2008;63:291-292.
28 Lieber BB, Stancampiano AP, Wakhloo AK. Alteration of hemodynamics in aneurysm models by stenting: influence of stent porosity. Ann Biomed Eng. 1997;25:460-469.
29 Chen PR, min-Hanjani S, Albuquerque FC, et al. Outcome of oculomotor nerve palsy from posterior communicating artery aneurysms: comparison of clipping and coiling. Neurosurgery. 2006;58:1040-1046.
30 Zada G, Breault J, Liu CY, et al. Internal carotid artery aneurysms occurring at the origin of fetal variant posterior cerebral arteries: surgical and endovascular experience. Neurosurgery. 2008;63:ONS55-ONS61.
31 Fernandez ZA, Guglielmi G, Vinuela F, et al. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol. 1994;15:815-820.
32 Moret J, Cognard C, Weill A, et al. Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases. J Neuroradiol. 1997;24:30-44.
33 McDougall CG, Halbach VV, Dowd CF, et al. Causes and management of aneurysmal hemorrhage occurring during embolization with Guglielmi detachable coils. J Neurosurg. 1998;89:87-92.
34 Murayama Y, Vinuela F, Tateshima S, et al. Endovascular treatment of experimental aneurysms by use of a combination of liquid embolic agents and protective devices. AJNR Am J Neuroradiol. 2000;21:1726-1735.
35 International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-1733.
36 Bavinzski G, Killer M, Ferraz-Leite H, et al. Endovascular therapy of idiopathic cavernous aneurysms over 11 years. AJNR Am J Neuroradiol. 1998;19:559-565.
37 Jahromi BS, Mocco J, Bang JA, et al. Clinical and angiographic outcome after endovascular management of giant intracranial aneurysms. Neurosurgery. 2008;63:662-674.
38 Larson JJ, Tew JM, Tomsick TA, et al. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery. 1995;36:23-30.
39 Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656-665.
40 Higashida RT, Halbach VV, Dowd C, et al. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg. 1990;72:857-863.
41 Mathis JM, Barr JD, Jungreis CA, et al. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol. 1995;16:749-754.
42 Standard SC, Ahuja A, Guterman LR, et al. Balloon test occlusion of the internal carotid artery with hypotensive challenge. AJNR Am J Neuroradiol. 1995;16:1453-1458.
43 Ponce FA, Albuquerque FC, McDougall CG, et al. Combined endovascular and microsurgical management of giant and complex unruptured aneurysms. Neurosurg Focus. 2004;17:E11.
44 Sekhon LH, Morgan MK, Sorby W, et al. Combined endovascular stent implantation and endosaccular coil placement for the treatment of a wide-necked vertebral artery aneurysm: technical case report. Neurosurgery. 1998;43:380-383.
45 Hacein-Bey L, Connolly ESJr, Mayer SA, et al. Complex intracranial aneurysms: combined operative and endovascular approaches. Neurosurgery. 1998;43:1304-1312.
46 Fiorella D, Albuquerque FC, Masaryk TJ, et al. Balloon-in-stent technique for the constructive endovascular treatment of “ultra-wide necked” circumferential aneurysms. Neurosurgery. 2005;57:1218-1227.
47 Regli L, Uske A, de TN. Endovascular coil placement compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: a consecutive series. J Neurosurg. 1999;90:1025-1030.
48 Suzuki S, Tateshima S, Jahan R, et al. Endovascular treatment of middle cerebral artery aneurysms with detachable coils: angiographic and clinical outcomes in 115 consecutive patients. Neurosurgery. 2009;64:876-888.
49 Yamaura I, Tani E, Yokota M, et al. Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Guglielmi detachable coils. J Neurosurg. 1999;90:853-856.
50 Albuquerque FC, Fiorella DJ, Han PP, et al. Endovascular management of intracranial vertebral artery dissecting aneurysms. Neurosurg Focus. 2005;18:E3.
51 Suzuki S, Kurata A, Iwamoto K, et al. Endovascular surgery using stents for vertebral artery dissecting aneurysms and a review of the literature. Minim Invasive Neurosurg. 2008;51:193-198.
52 Yi AC, Palmer E, Luh GY, et al. Endovascular treatment of carotid and vertebral pseudoaneurysms with covered stents. AJNR Am J Neuroradiol. 2008;29:983-987.
53 Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery. 2006;59:291-300.
54 MacKay CI, Han PP, Albuquerque FC, et al. Recurrence of a vertebral artery dissecting pseudoaneurysm after successful stent-supported coil embolization: case report. Neurosurgery. 2003;53:754-759.
55 Aymard A, Gobin YP, Hodes JE, et al. Endovascular occlusion of vertebral arteries in the treatment of unclippable vertebrobasilar aneurysms. J Neurosurg. 1991;74:393-398.
56 Sanai N, Tarapore P, Lee AC, et al. The current role of microsurgery for posterior circulation aneurysms: a selective approach in the endovascular era. Neurosurgery. 2008;62:1236-1249.
57 Song HH, Won YD, Kim YJ, et al. The endovascular management of saccular posterior inferior cerebellar artery aneurysms. Korean J Radiol. 2008;9:396-400.
58 Fiorella D, Kelly ME, Albuquerque FC, et al. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009;64:212-217.
59 Gruber DP, Zimmerman GA, Tomsick TA, et al. A comparison between endovascular and surgical management of basilar artery apex aneurysms. J Neurosurg. 1999;90:868-874.
60 Raymond J, Roy D, Bojanowski M, et al. Endovascular treatment of acutely ruptured and unruptured aneurysms of the basilar bifurcation. J Neurosurg. 1997;86:211-219.
61 Pierot L, Boulin A, Castaings L, et al. Selective occlusion of basilar artery aneurysms using controlled detachable coils: report of 35 cases. Neurosurgery. 1996;38:948-953.
62 Eskridge JM, Song JK. Endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachable coils: results of the Food and Drug Administration multicenter clinical trial. J Neurosurg. 1998;89:81-86.
63 Bavinzski G, Killer M, Gruber A, et al. Treatment of basilar artery bifurcation aneurysms by using Guglielmi detachable coils: a 6-year experience. J Neurosurg. 1999;90:843-852.
64 McDougall CG, Halbach VV, Dowd CF, et al. Endovascular treatment of basilar tip aneurysms using electrolytically detachable coils. J Neurosurg. 1996;84:393-399.
65 Rosenwasser RH. Treatment of an intracranial aneurysm using a new three-dimensional-shape Guglielmi detachable coil: technical case report. Neurosurgery. 1999;44:1144.
66 Malek AM, Higashida RT, Phatouros CC, et al. Treatment of an intracranial aneurysm using a new three-dimensional-shape Guglielmi detachable coil: technical case report. Neurosurgery. 1999;44:1142-1144.
67 Guglielmi G, Vinuela F, Duckwiler G, et al. Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg. 1992;77:515-524.
68 Nakabayashi K, Negoro M, Itou Y, et al. Endovascular approach vs microsurgical approach for posterior circulation aneurysms. Interv Neuroradiol. 1997;3(Suppl 2):171-176.