CHAPTER 184 Endoscopic Treatment of Craniosynostosis
Definitive treatment of infants with craniosynostosis continues to be surgical in nature. The ultimate corrective goal remains achievement of proper cranial and facial anatomic relationships and normalization of overall craniofacial morphology. As described in previous chapters, many procedures have been devised in modern times since Lannalogue introduced strip craniectomies in 1890.1 These procedures have ranged from simple suturectomies and strip craniectomies1,2 to very extensive surgical procedures after the development of craniofacial surgery as a distinct surgical discipline in the 1970s.3–9 Surgical treatment of sagittal synostosis has varied from linear craniectomies to extensive calvarial vault remodeling.9–15 Management of metopic synostosis has also varied from linear craniectomies to standard bifrontal craniotomies and orbitofrontal advancement and forehead recontouring.16–26 Likewise, coronal synostosis has been treated with simple suturectomies, unilateral canthal advancements, or bifrontal craniotomies and forehead recontouring.6,27–43 Finally, lambdoid synostosis is most commonly treated with occipital craniectomies and some sort of occipital reconstructive technique.44–49
Because of the inconsistent nature of long-term results and the significant trauma associated with these operations, we began to develop and use minimally invasive, endoscopically assisted techniques to treat infants with craniosynostosis more than a decade ago.10,15,47–54 The underlying principles of our approach include (1) operating on very young infants (preferably 3 months of age or younger), (2) using the very rapid brain growth that occurs in this age group, (3) allowing brain growth to correct the presurgical deformities, and (4) applying postoperative helmets to aid in and achieve correction and normalization of craniofacial asymmetry. Our original goal was to perform a very simple and rapid operation to halt progressive craniofacial deformation and later perform a definitive procedure on a less severe deformity. However, our early results demonstrated excellent and persistent correction, and thus we began to use these procedures as definitive treatment. Presented in this chapter are the techniques and results of our protocols for treating patients with craniosynostosis by successfully using minimally invasive endoscopic techniques.
Instrumentation
Surgical
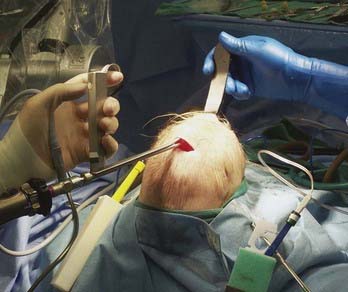
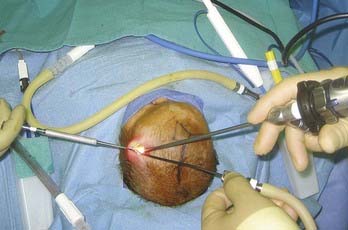
Although a comprehensive surgical tray is currently being developed and will soon be available (Karl Storz Endoskope, Tuttlingen, Germany), these procedures can be performed with a relatively simple tray of instruments that are readily available in most operating rooms. The endoscopic equipment consists of two rigid rod lens endoscopes. A 30-degree angled scope is used to visualize and separate the dura from the overlying stenosed suture and skull. A 0-degree angled endoscope is used to view the dura and bone during coagulation and hemostasis of these two structures (Fig. 184-1). Flexible and fiberoptic scopes are not used because of the lower image resolution that they provide. Standard three-chip cameras and high-definition monitors provide superb visualization of the surgical field. Additional visualization and exposure can be obtained with the use of a rhinoplasty lighted retractor. The retractor connected to a standard light source can be used to elevate the scalp while developing the dissection plane between the galea and pericranium. Bloodless dissection is undertaken with the use of monopolar electrocautery and the needle tip set at 15 W, blend 1 (Valley Lab, Valley Forge, PA). Insulated handheld malleable retractors are used to protect the dura and galea during dissection of the subgaleal plane and coagulation of the diploë after osteotomy and bone resection. To minimize intraoperative and postoperative blood loss from the diploë after the osteotomies are performed, a disposable suction electrocautery handpiece (Valley Lab, Valley Forge, PA) is used and set at 60 W. The malleable tip can be adjusted to reach the desired areas for further coagulation, and final hemostasis is achieved with Surgiflo (Johnson & Johnson, Cincinnati, OH) and liquid thrombin (GenTrac, Inc., Middleton, WI) and Gelfoam (Pfizer, New York). In most patients the osteotomies can be made with a pair of sharp curved Mayo scissors, but when the bone is thick (older patients), bone-cutting scissors can be used. When removing the bone in piecemeal fashion (metopic or coronal), curved bone rongeurs (DePuy Spine, Raynham, MA) or side-biting rongeurs (Jansen Middleton, C-Med Surgical, Pompton Lakes, NJ) can be used to resect the bone near the skull base. When treating a patient with significant trigonocephaly, the sharp bony edges of the osteotomies can be contoured with an electric rasp (Stryker, Kalamazoo, MI). Scalp closure is achieved with 4-0 Monocryl (Ethicon, Cincinnati, OH), and Dermabond is used for final skin closure. Of note, no drains are left in place, and Foley catheters, central lines, and arterial lines are not routinely used during any of these procedures.
Helmet
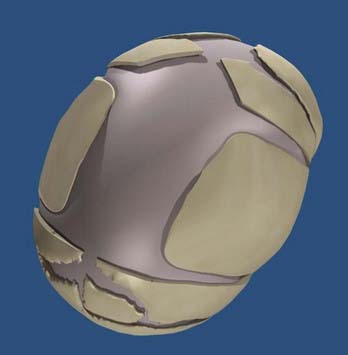
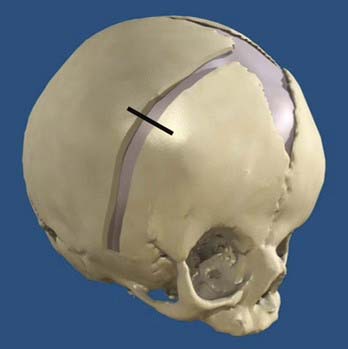
The use of helmets, albeit time- and effort-consuming, plays a critical role in achieving excellent results after the osteotomies and cranial release. Typically, on the third postoperative day the patient’s head is scanned with a proprietary infrared beam Star scanner (Orthomerica Products, Inc., Orlando, FL), and a custom-made helmet is manufactured and delivered on the fifth postoperative day. The helmets are manufactured with the commercially available resin Surlyn (Dupont, Wilmington, DE), which is a thermoplastic ionomer resin and is the random copolymer poly(ethylene-co-methacrylic acid). Its physical properties make it an ideal material for molding cranial helmets. The overall helmet shell is made of Surlyn and the inner padding is made of an ethylene acetate lining and Reston foam stabilizer pads (3M, St. Paul, MN). To standardize the manufacturing process, the patient’s head is scanned locally and the digital data sent to a central manufacturing center (Orlando, FL), where a computer-assisted process allows the creation of a custom-made helmet set to the practitioner’s specifications. The overall goal of the helmets is to assist the brain in reshaping the deformed cranium by allowing cranial expansion in recessed areas and compression in areas of overcompensated growth (Figs. 184-2 and 184-3). These helmets do not restrict cranial growth but rather redirect it, as evidenced by normal progression of head circumference growth. The younger the child, the more helmets will be worn, with up to three helmets usually required to complete treatment and the duration of treatment ranging between 6 months and 1 year. Parents can be reassured that the prosthetics are well tolerated.
Sagittal
Surgical Procedure
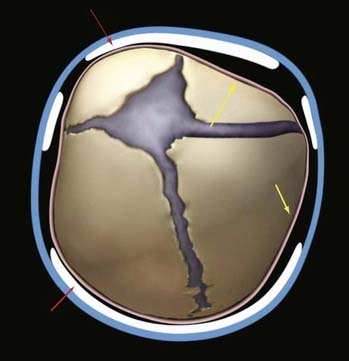
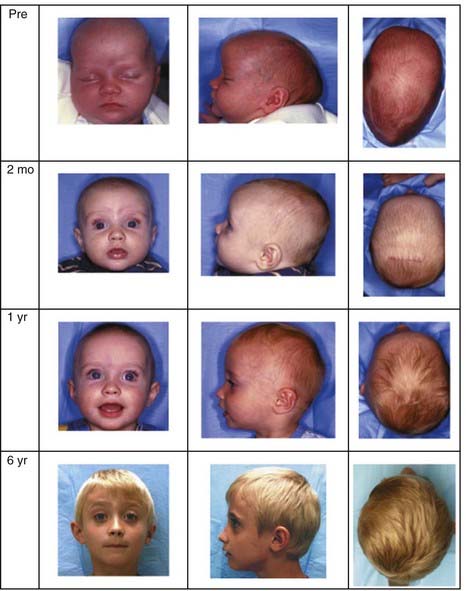
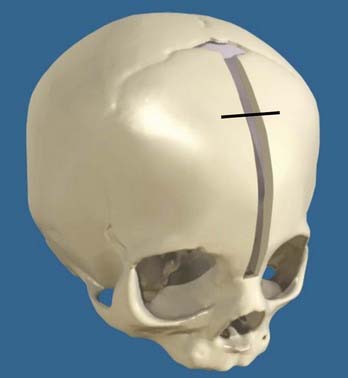
Patients are placed in the modified prone position (sphinx) in a viscoelastic pad. Two incisions are made across the midline, each measuring about 3 cm. The first incision is located approximately 2 to 3 cm behind the anterior fontanelle and the second incision is placed immediately in front of the lambda. The scalp incision is taken through the galea aponeurotica but with preservation of the pericranium. After applying insulated scalp retractors, a needle-tipped monopolar cautery is used to develop the subgaleal plane in a bloodless fashion from the anterior fontanelle to the lambda and about 3 cm from the midline bilaterally. A pediatric craniotome is used to make a bur hole at the edge of each incision. Once the dura is freed from the midline bone with a No. 1 Penfield dissector, a 6-mm osteotomy is made at each incision with 6-mm Kerrison rongeurs. The bone is dissected anteriorly toward the anterior fontanelle and then cut with Mayo scissors in a triangular shape. A 30-degree rigid endoscope is inserted under the bone at the anterior incision and advanced posteriorly toward the lambda with the aid of a 10 French suction tip. Suction is used to dissect, as well as keep the endoscopic field dry and free of blood. A second posterior osteotomy is made at the lambda in similar fashion. Once the dura has been freed from the overlying bone, bone-cutting or Mayo scissors are used to make the lateral osteotomies. Hemostasis is achieved with a large piece of Gelfoam (Pfizer, New York) on the dura along with gentle pressure. Wedge (barrel stave) osteotomies are also created with scissors after the dura is freed from the bone directly behind the coronal suture and then directly in front of the lambdoid sutures (Fig. 184-4). The wounds are closed with absorbable 4-0 Monocryl sutures and Ethibond. The patient is observed overnight, and pain control is maintained with alternating acetaminophen and ibuprofen and intermittent intravenous morphine as needed. The majority of patients are discharged on the morning after surgery.
Results
Approximately 200 consecutive patients have been treated over a period of 10+ years. Seventy-four percent were boys and 26% were girls. The mean and median ages at the time of surgery were 3.7 and 3.2 months, respectively. The length of the craniectomy varied between 4.5 and 13 cm, with a mean length of 10.5 cm. The width ranged between 1.5 and 7 cm, with a mean width of 5.4 cm. Two patients required intraoperative blood transfusion (1%), and 14 patients received postoperative blood transfusions (7.4%). The mean estimated blood loss was 27 mL with a range of 5 to 150 mL. All but 8 patients were discharged on the morning after surgery (96%). The mean surgical time was 57 minutes, with a range of 30 to 150 minutes. Postoperative swelling was minimal and confined to the scalp. The patients did not experience fever as is commonly seen with the traditional operations. Complications were minimal and included three small dural tears, which were repaired primarily without lasting effects; 5 patients experienced superficial scalp irritation, which was treated successfully by temporary (<1 week) removal of the helmet and local care. There were two deaths in the series. In both cases, the procedures went well and without long complications. During the postoperative period, massive hemolysis and diffuse cerebral ischemia developed in the first child. Systemic arterial and venous thrombosis of unknown etiology developed in the second child. In both cases, life support was withdrawn at the request of the parents. There were no infections, air embolism, seizures, postoperative hematomas, or need for conversion to an open procedure. Using a cephalic index (CI) of greater than 75 as the criterion for excellent results, 87% of the patients achieved excellent outcomes. A good result was classified as a final CI between 71 and 75. Eight percent of the patients had good results and 4.3% had poor results (CI <70) (Fig. 184-5).
Coronal
Surgical Procedure
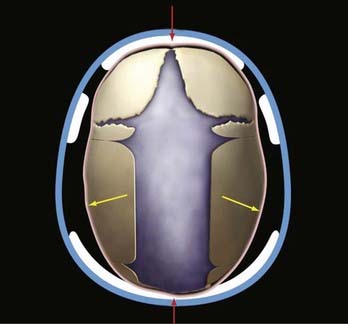
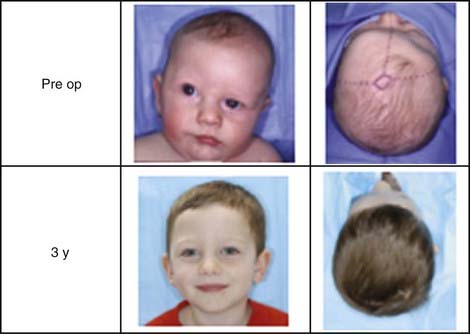
Access to the suture is gained through a 2- to 3-cm incision located behind the hairline at the level of the stephanion, and a subgaleal dissection plane is developed between the anterior fontanelle and the squamosal suture (Fig. 184-6). A 7-mm craniotome is used to make a bur hole, which is then enlarged to allow passage of the endoscopes under the bone. Once the dura is safely dissected away from the bone, a 6-mm osteotomy is made with a combination of Mayo scissors and bone rongeurs. The osteotomy extends from the anterior fontanelle to the squamosal suture behind the pterion (Fig. 184-7). Hemostasis is achieved as previously described and the incision closed with Monocryl and Dermabond.
Results
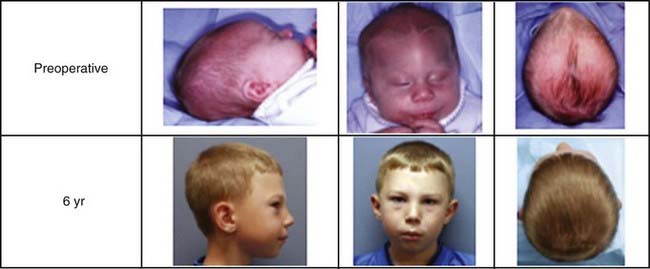
The mean surgical time was 56 minutes. Ninety-seven percent of the infants were discharged the morning after surgery. Mean estimated blood loss was about 20 mL. Overall, the blood transfusion rate was about 1%. Correction of preoperative deformities occurs sequentially and in the following order: nasal deviation, vertical dystopia, sagittal imbalance, and finally, frontal plagiocephaly. Continued improvement is seen over the subsequent years (Fig. 184-8). Review of risk factors showed that no patients experienced infections, venous air embolism, or sagittal sinus injury. No mortality, postoperative hematomas, or visual or ocular injuries occurred. Two patients had small dural tears. A minor scalp irritation developed in one patient but resolved with local care.
Metopic
Surgical Procedure
A small 2- to 3-cm incision is made behind the hairline and across the metopic suture. A rhinoplasty lighted retractor is used to develop the subgaleal plane from the anterior fontanelle to the nasofrontal suture. A bur hole is made with a 7-mm craniotome and enlarged with Kerrison rongeurs. A 30-degree endoscope is used to develop a plane of dissection between the dura and the overlying stenosed suture. Using scissors and bone rongeurs, a 6-mm osteotomy is made between the anterior fontanelle and the nasion (Fig. 184-9). Bridging veins from the sagittal sinus to the stenosed suture are often encountered and directly cauterized with bipolar forceps. After hemostasis, the incision is closed with Monocryl and Dermabond.
Results
Mean estimated blood loss was about 40 mL, and the overall transfusion rate was about 8%. All patients were discharged the day after surgery. Mean surgical time was just under 1 hour. Like the sagittal and coronal cohorts, metopic patients do not experience postoperative pyrexia, irritability, or major facial swelling or bruising. No conversions to open procedures were necessary. Progressive correction of trigonocephaly has been seen in all our patients with forward advancement of the recessed frontal bones. Additionally, correction of hypotelorism was achieved in all cases and without the need for extensive bifrontal craniectomies, orbital repositioning, or ocular manipulation. As with the coronal group, continued improvement was noted with the passage of years after the original surgery (Fig. 184-10).
Lambdoid
Surgical Procedure
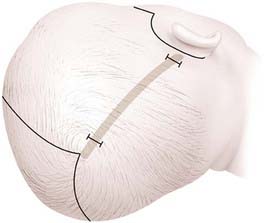
The patient is placed on a cerebellar horseshoe with the head turned so that the ipsilateral stenosed suture is on the superior horizontal plane. The first incision is made immediately lateral to the lambda on the side of the stenosis, and the second incision is made medial to the asterion. Each incision is about 2 to 3 cm. A rhinoplasty lighted retractor or a 0-degree endoscope can be used to develop the subgaleal plane between the incisions. Two bur holes are made with a pediatric craniotome, each at an incision. The bur holes are enlarged with Kerrison rongeurs. A 30-degree endoscope is then inserted under the bone, along the stenosed suture, to help develop the epidural space. Once freed, the bone can be cut with Mayo or bone-cutting scissors. The osteotomy is about 5 to 8 mm in width (Fig. 184-11). After bone hemostasis is achieved, the skin is closed in a similar fashion as for the other sutures described earlier.
Results
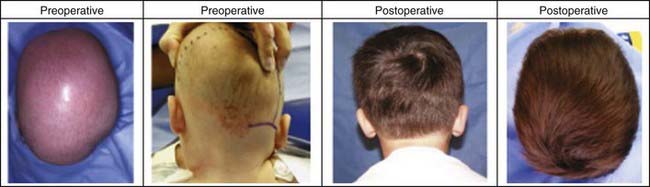
The cranial skull bone and calvarial deformities seen with lambdoid synostosis have been the most difficult to treat and correct with endoscopic techniques. Nevertheless, patients do show marked improvement, particularly those with moderate to mild deformities. However, even marked asymmetries can be corrected over time (Fig. 184-12). Blood loss is minimal with an average of 25 mL. No patient has needed blood transfusions, and mean surgical time was less than 1 hour. There have been no injuries to the transverse sinus or dura in the region of the lambda or asterion.
Anderson F. Treatment of coronal and metopic synostosis: 107 cases. Neurosurgery. 1981;8:143.
Bartlett S, Whitaker L, Marchac D. The operative treatment of isolated craniofacial dysostosis (plagiocephaly): a comparison of the unilateral and bilateral techniques. Plast Reconstr Surg. 1990;85:677.
Cohen SR, Kawamotto HJSr, Burstein F, et al. Advancement-onlay: an improved technique of fronto-orbital remodeling in craniosynostosis. Childs Nerv Syst. 1991;7:264.
Cohen SR, Maher H, Wagner JD, et al. Metopic synostosis: evaluation of aesthetic results. Plast Reconstr Surg. 1994;94:759.
Delashaw JB, Persing JA, Park TS, et al. Surgical approaches for the correction of metopic synostosis. Neurosurgery. 1986;19:228.
Di Rocco C, Velardi F, Ferrario A, et al. Metopic synostosis: in favor of a “simplified” surgical treatment. Childs Nerv Syst. 1996;12:654.
Hoffman HJ, Mohr G. Lateral canthal advancement of the superorbital margin. J Neurosurg. 1976;45:376.
Jane JA, Park TS, Zide BM, et al. Alternative techniques in the treatment of unilateral coronal synostosis. J Neurosurg. 1984;61:550.
Jimenez DF, Barone CM. Metopic synostosis. In: Benzel EC, Rengachary SS, editors. Calvarial and Dural Reconstruction. Park Ridge, IL: American Association of Neurological Surgeons; 1998:135.
Jimenez DF, Barone CM. Endoscopic craniectomy for early surgical correction of sagittal craniosynostosis. J Neurosurg. 1998;88:77.
Jimenez D, Barone C. The sunrise technique: the correction of occipital plagiocephaly using bandeau occipital plate and radial osteotomies. Pediatr Neurosurg. 1995;22:162.
Johnson JO, Jimenez DF, Barone CM. Blood loss after endoscopic strip craniectomy for craniosynostosis. J Neurosurg Anesthesiol. 1999;12:60.
Marchac D. Radical forehead remodeling for craniostenosis. Plast Reconstr Surg. 1978;61:823.
McCarthy JG, Bradley JP, Longacre MT. Step expansion of the frontal bar: correction for trigonocephaly. J Craniofac Surg. 1996;7:333.
Mohr G, Hoffman HJ, Munro IR, et al. Surgical management of unilateral and bilateral coronal craniosynostosis; 21 years of experience. Neurosurgery. 1978;2:83.
Moriyama E, Beck H, Iseda K, et al. Surgical correction of trigonocephaly: theoretical basis and operative procedures. Neurol Med Chir. 1998;38:110.
Persing J, Delashaw J, Jane J, et al. Lambdoid synostosis: surgical considerations. Plast Reconstr Surg. 1988:81.
Persing JA, Jane JA, Park TS, et al. Floating C shaped orbital osteotomy for orbital rim advancement in craniosynostosis: preliminary report. J Neurosurg. 1990;72:22.
Rosnick JC. Unilateral coronal synostosis (anterior plagiocephaly): current clinical perspectives. Ann Plast Surg. 1996;36:430.
Thaller S, Hoyt J, Boggan J. Surgical correction of unilateral lambdoid synostosis: occipital rotation flap. J Craniofac Surg. 1992;3:12.
1 Lannelongue M. De la craniectomie dans la mircocephalie. Compt Rend Seances Acad Sci. 1890;50:1382.
2 Lane LC. Pioneer craniectomy for relief of mental imbecility due to premature sutural closure and microcephalus. JAMA. 1892;18:49.
3 Rougerie J, Derome P, Angnez L. Craniostenosis et dysmorphies craniofaciales. Principles d’un nouvelle technique de traitement et ses resultats. Neurochirurgie. 1972;18:429.
4 Hoffman HJ, Mohr G. Lateral canthal advancement of the superorbital margin. J Neurosurg. 1976;45:376.
5 Whitaker LA, Schut L, Kerr LP. Early surgery for isolated craniofacial dysostosis. Plast Reconstr Surg. 1997;91:977.
6 Marchac D. Radical forehead remodeling for craniostenosis. Plast Reconstr Surg. 1978;61:823.
7 Marchac D, Remier D. “Le front flottanz”: treatment precoce des facio-craniostenoses. Ann Chir Plast. 1979;24:121.
8 Rosnick JC. Craniosynostosis: surgical management in infancy. In: Bell WH, editor. Orthognathic and Reconstructive Surgery. Philadelphia: Saunders; 1992:1839.
9 Rosnick JC. Unilateral coronal synostosis (anterior plagiocephaly): current clinical perspectives. Ann Plast Surg. 1996;36:430.
10 Jimenez DF, Barone CM. Metopic synostosis. In: Benzel EC, Rengachary SS, editors. Calvarial and Dural Reconstruction. Rolling Meadows, IL: American Association of Neurological Surgeons; 1998:135.
11 Jimenez DF, Barone CM. Endoscopic assisted craniectomies for the management of craniosynostosis. In: Jimenez DF, editor. Neurosurgical Topics: Intracranial Endoscopic Neurosurgery. Park Ridge, Ill.: American Association of Neurological Surgeons; 1998:209.
12 Jimenez DF, Barone CM. Endoscopic craniectomy for early surgical correction of sagittal craniosynostosis. J Neurosurg. 1998;88:77.
13 Barone CM, Jimenez DF. Endoscopic craniectomy for early correction of craniosynostosis. Plast Reconstr Surg. 1999;104:1965.
14 Johnson JO, Jimenez DF, Barone CM. Blood loss after endoscopic strip craniectomy for craniosynostosis. J Neurosurg Anesthesiol. 1999;12:60.
15 Jimenez DF, Barone CM. Endoscopic-assisted wide-vertex craniectomy, “barrel-stave” osteotomies and postoperative helmet molding therapy in the early management of sagittal suture craniosynostosis. Neurosurg Focus. 2000;9(3):e2.
16 Koskinen-Moffet L, Moffett BC. Sutures and intrauterine deformation. In: Persing JA, Edgerton MT, Jane JA, editors. Scientific Foundation and Surgical Treatments of Craniosynostosis. Baltimore: Williams & Wilkins; 1989:96.
17 Di Rocco C, Velardi F, Ferrario A, et al. Metopic synostosis: in favor of a “simplified” surgical treatment. Childs Nerv Syst. 1996;12:654.
18 Dhellemmes P, Pellerin P, Lejeune JP, et al. Surgical treatment of trigonocephaly, experience with 30 cases. Childs Nerv Syst. 1986;2:228.
19 Delashaw JB, Persing JA, Park TS, et al. Surgical approaches for the correction of metopic synostosis. Neurosurgery. 1986;19:228.
20 Salkind G, Sutton LN, Bruce DA, et al. Management of trigonocephaly. Surg Neurol. 1986;25:159.
21 McCarthy JG, Bradley JP, Longacre MT. Step expansion of the frontal bar: correction for trigonocephaly. J Craniofac Surg. 1996;7:333.
22 Moriyama E, Beck H, Iseda K, et al. Surgical correction of trigonocephaly: theoretical basis and operative procedures. Neurol Med Chir. 1998;38:110.
23 Mommaerts MY, Staels P. Coronal suture transplantation to correct fronto-orbital constriction in metopic synostosis. Int Surg. 1996;81:210.
24 Anderson F. Treatment of coronal and metopic synostosis: 107 cases. Neurosurgery. 1981;8:143.
25 Havlick RJ, Azurin DJ, Bartlett SP, et al. Analysis and treatment of severe trigonocephaly. Plast Reconstr Surg. 1999;103:381.
26 Cohen SR, Maher H, Wagner JD, et al. Metopic synostosis: evaluation of aesthetic results. Plast Reconstr Surg. 1994;94:759.
27 Hoffman HJ, Mohr G. Lateral canthal advancement of the superorbital margin. J Neurosurg. 1976;45:376.
28 Persing JA, Jane JA, Park TS, et al. Floating C shaped orbital osteotomy for orbital rim advancement in craniosynostosis: preliminary report. J Neurosurg. 1990;72:22.
29 Cohen SR, Kawamotto HJSr, Burstein F, et al. Advancement-onlay: an improved technique of fronto-orbital remodeling in craniosynostosis. Childs Nerv Syst. 1991;7:264.
30 Elisevich K, Bite V, Colcleugh RG. Orbital rim and malar advancement for unilateral corornal synostosis in older pediatric age group. J Neurosurg. 1991;74:219.
31 Jane JA, Park TS, Zide BM, et al. Alternative techniques in the treatment of unilateral coronal synostosis. J Neurosurg. 1984;61:550.
32 Whitaker LA, Schut L, Kerr LP. Early surgery for isolated craniofacial dysostosis. Plast Reconstr Surg. 1997;91:977.
33 Marchac D, Renier D. Craniofacial surgery for craniosynostosis. Scand J Plast Reconstr Surg. 1981;15:235.
34 Mohr G, Hoffman HJ, Munro IR, et al. Surgical management of unilateral and bilateral coronal craniosynostosis; 21 years of experience. Neurosurgery. 1978;2:83.
35 Hansen M, Mulliken JB. Frontal plagiocephaly: diagnosis and treatment. Craniofac Surg. 1994;21:543.
36 Cohen MM. The etiology of craniosynostosis. In: Cohen MM, editor. Craniosynostosis: Diagnosis, Evaluation and Management. New York: Raven; 1986:59.
37 Ingraham FD, Alexander E, Matson DD. Clinical studies in craniosynostosis: analysis of fifty cases and description of a method of surgical treatment. Surgery. 1948;24:518.
38 Anderson FM, Johnson FC. Craniosynostosis: a modification and surgical treatment. Surgery. 1956;40:961.
39 Kaiser G, Bittel M. Results of extended craniectomy including supraorbital advancement in premature coronal and frontal craniosynostosis. Eur J Pediatr Surg. 1991;1:227.
40 Laurent JP, Balasubramaniam C, Stal S, et al. Early surgical management of coronal synostosis. Clin Plast Surg. 1990;17:183.
41 Bartlett S, Whitaker L, Marchac D. The operative treatment of isolated craniofacial dyostosis (plagiocephaly): a comparison of the unilateral and bilateral techniques. Plast Reconstr Surg. 1990;85:677.
42 Sgouros S, Goldin JH, Hockely AD, et al. Surgery for unilateral coronal synostosis (plagiocephaly): unilateral or bilateral correction? J Craniofac Surg. 1996;7:284.
43 Polley JW, Charbel FT, Kim D, et al. Nonsyndromal craniosynostosis: longitudinal outcome following cranio-orbital reconstruction in infancy. Plast Reconstr Surg. 1992;102:619.
44 Persing J, Delashaw J, Jane J, et al. Lambdoid synostosis: surgical considerations. Plast Reconstr Surg. 1988;81:852.
45 McComb J. Treatment of functional lambdoid synostosis. Neurosurg Clin N Am. 1991;2:655.
46 Thaller S, Hoyt J, Boggan J. surgical correction of unilateral lambdoid synostosis: occipital rotation flap. J Craniofac Surg. 1992;3:12.
47 Jimenez D, Barone C, Argamaso R, et al. Asterion region synostosis. Cleft Palate J. 1994;31:36.
48 Jimenez D, Barone C. The sunrise technique: the correction of occipital plagiocephaly using bandeau occipital plate and radial osteotomies. Pediatr Neurosurg. 1995;22:162.
49 Kaiser G. The clinical significance of bilateral synostosis of the lambdoid suture and the usefulness of its treatment. Childs Brain. 1984;11:87.
50 Tobias JD, Johnson JO, Jimenez DF, et al. Venous air embolism during endoscopic strip craniectomy for repair of craniosynostosis in infants. Anesthesiology. 2001;95:340.
51 Jimenez DF, Barone CM, Cartwright C, et al. Early management of craniosynostosis using endoscopic assisted strip craniectomies and cranial orthotic molding therapy. Pediatrics. 2002;110:97.
52 Cartwright CC, Jimenez DF, Barone CM, et al. Endoscopic strip craniectomy: a minimally invasive treatment for early correction of craniosynostosis. J Neurosci Nurs. 2003;35:130.
53 Jimenez DF, Barone CM, McGee ME, et al. Endoscopy-assisted wide-vertex craniectomy, barrel stave osteotomies, and postoperative helmet molding therapy in the management of sagittal suture craniosynostosis. J Neurosurg. 2004;100(suppl 5):407.
54 Jimenez DF, Barone CM. Early treatment of anterior calvarial craniosynostosis using endoscopic-assisted minimally invasive techniques. Childs Nerv Syst. 2007;23:1411.