Chapter 41 Endoscopic Approaches to Ventricular Tumors and Colloid Cysts
• It is essential to be knowledgeable about the equipment and practice on a cadaver or under the guidance of an experienced neuroendoscopist.
• Orientation and anatomy are everything. Knowledge of the anatomy will ensure that you know where you are, what you are looking at, and your orientation at all times. If you are not sure, abort the procedure.
• Use the endoscope for its advantages and don’t fight its disadvantages. Increase use of angled scopes and increase the size of the visual field.
• Become comfortable with the endoscopic management of hydrocephalus (endoscopic third ventriculostomy) prior to using the endoscope for neuro-oncological applications.
• Endoscopic ventricular applications are not “all or nothing.” Some lesions are more safely dealt with from an open microsurgical approach. In these cases use an endoscope-assisted approach to your advantage.
History
Six major advancements in the use of neuroendoscopy have occurred. A century ago, Lespinasse, a urologist, attempted a choroid plexus coagulation to treat hydrocephalus.1,2 In the early twentieth century Dandy and Mixter attempted endoscopic third ventriculostomy (ETV).3 In the 1970s technological advances allowed the production of flexible fiberscopes as well as rigid endoscopes. Throughout the late 1980s and 1990s ETV and decompartmentalization of the ventricular system were popularized, which allowed many neurosurgeons to become familiar with the tools of neuroendoscopy. This familiarity resulted in the fifth and sixth advances. The use of the endoscope for extra-axial lesions was popularized by pioneers such as Perneczky who championed the keyhole approach to aneurysms. The final and most recent wave of endoscopic enthusiasm has been its application to anterior skull base surgery, especially transsphenoidal and transtubercular surgery. The use of the endoscope for intraventricular neuro-oncological applications including lesion removal, tumor biopsy, and cyst management is also becoming more common, so much so that endoscopic colloid cyst surgery has results that are similar to those of microsurgery, and with a better risk profile.4–8
Equipment
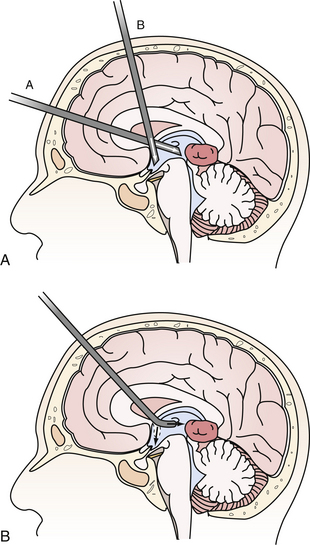
The flexible fiberscope is maneuverable in three directions with relatively simple controls. The advantage of the flexible scope is that its geometry is not fixed and it may be fashioned to proceed along a curved trajectory such as traversing the foramen of Monro into the posterior third ventricle to biopsy a pineal tumor. This allows a single trajectory to treat hydrocephalus via ETV and sample the tumor (Fig. 41.1A and B). The flexible scope may also be used down the working port of the rigid scope in a “scope-in-scope” technique.9 The main drawbacks of the flexible scope over the rigid scope are the poorer optical resolution and that the surgeon may inadvertently withdraw the scope in the “bent” configuration with devastating repercussions.
Frameless neuronavigation is helpful to guide insertion of the sheath, especially in the absence of ventriculomegaly, and for localizing lesions for biopsy under an intact ependyma.10 In the instance that the surgeon becomes disoriented or confused by distorted anatomy, stereotaxis can assist in reorientation.
Recently, several potentially important advances in instrumentation available to the endoscopic surgeon have been reported. The length of time required to aspirate a tumor using a “biopsy after biopsy” approach may be shortened by the use of traditional ultrasonic aspiration with a specialized handpiece down the working port of the endoscope. It has been used successfully on pituitary tumors, intraventricular clot removal, and craniopharyngioma cyst wall removal.11 Endoneurosonography has been used to supply additional information about the relationship of the tip of the probe and structures orthogonal to it.12 Water-jet dissection has been reported to be a useful adjunct in the safe perforation of a craniopharyngioma cyst wall, septum pellucidum, or floor of the third ventricle.13 This may help to decrease the risk of hemorrhage associated with blindly inserting an instrument through a thin structure such as the third ventricle floor.
Endoscopic Principles
There are two principal forms of endoscopy: coaxial and extra-axial. Coaxial endoscopic approaches, or “pure” endoscopy, are those in which all components of the endoscopic system (lighting, camera, working channels, irrigant channels, and instruments) are all parallel and enclosed in a single sheath. The instruments are introduced through working channels and are aimed by redirecting the endoscope. The impact to the surrounding brain from removing and reintroducing instruments is minimized because the entire working and visualization area is within the endoscopic sheath. Most intraventricular endoscopic procedures are performed in a coaxial manner.14
During “endoscope-controlled” surgery, the endoscope is the sole mode of visualization and surgery is performed using the same techniques and instrumentation as microsurgery, with the addition of curved instruments and suctions that allow the surgeon to operate around corners. In these forms of endoscopy, a substantial learning curve exists because of peripheral distortion, angled view when using non-0 degree endoscopes, and the close proximity of the surgical field to the tip of the endoscope. Once this is overcome, these same “problems” may be used to the surgeon’s advantage, resulting in better outcomes.
Intraventricular Endoscopy for Cysts
Colloid Cysts
Colloid cysts of the third ventricle are non-neoplastic masses that typically arise from the roof of the third ventricle. They can occlude the foramen of Monro, causing headache, hydrocephalus, memory disturbances, and sudden death. Colloid cysts have a variable consistency, from mucinous, that are easily aspirated, to a hard and cheesy consistency. Cysts 1 cm or larger and those causing symptoms or hydrocephalus are generally recommended for removal. Other options including shunting and stereotactic drainage are possible but not recommended owing to their poor durability. Microsurgical removal is effective but more invasive than the endoscopic approach.4,5,15 Therefore, endoscopic removal is recommended in the majority of cases.16–19 However, when imaging predicts the consistency of the cyst contents to be hard and cheesy the cyst is better removed microsurgically with bimanual dissection. Additionally, cysts larger than 2 cm may compromise or adhere to the fornix and may be more safely removed using microsurgical bimanual dissection.
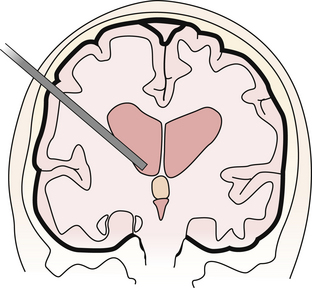
A single bur hole, approximately 8 cm behind the nasion and 5 to 7 cm lateral to the midline in the nondominant hemisphere (be careful not to injure the caudate head), is sufficient for removal7,20 (Fig. 41.2). Image guidance helps with the initial ventricular entry. A peel-away sheath is optional. The landmarks of the colloid cyst and foramen of Monro are identified and the overlying choroid plexus is coagulated, avoiding the fornix. The cyst is coagulated and opened and the contents are aspirated. A pediatric endotracheal suction catheter with the end cut to 45 degrees is particularly effective if the consistency is favorable. Alternatively, some endoscope manufacturers have designed rigid endoscopic suction that uses either blunt or beveled tips and works well to suction the mucinous material. One can twist the catheter and use the cut end as a dissector to “morselize” the contents of the cyst prior to aspiration. If the contents are too dense, forceps may be required to empty the cyst contents. The wall of the cyst is then dissected free of the roof of the third ventricle with generous coagulation. Generally the cyst wall is not densely attached to the fornix and can be removed completely. However, in the case that the wall is so adherent to the internal cerebral veins or the fornix that it cannot be separated using either sharp or blunt dissection it may be prudent to leave a thin “carpet.” Under these circumstances, the recurrence rate appears to be low, but we must await reports of long-term outcome.6,21,22 Symptomatic relief of obstructive hydrocephalus is generally obtained, though mild ventriculomegaly often persists.21
Neurocysticercotic Cysts
Neurocysticercosis (NCC) is the neurological manifestation of the parasite Taenia solium. This is commonly contracted in underdeveloped countries by hand-to-mouth contamination from unclean water or food. NCC most often presents with seizures but may also present with sudden hydrocephalus due to intraventricular cysts blocking normal cerebrospinal fluid (CSF) pathways. There is a growing body of literature suggesting that neuroendoscopic removal of cysts results in improved patient outcomes and lessens or avoids altogether the need for shunting.23–25 Recently, pediatric data regarding neuroendoscopic cyst evacuation has been reported. The shunting rate is lower (22%) in the neuroendoscopic group than the traditional medical treatment group (70%) and the Karnofsky performance scale was higher in the endoscopic group (90.0% vs. 85.5%, p = 0.003).23 Two other studies show complete resolution of cysts and no need for shunting with minimal transient morbidity.24,25
Miscellaneous Cysts
Other cysts, such as arachnoid cysts, occur within the ventricles. Often fenestration of these to normal CSF spaces is desirable. The ease of working in a fluid-filled space makes the endoscope ideal for fenestration. The anatomy is often distorted and the arachnoid surface is often thick and somewhat opaque. Stereotaxis is very helpful in these cases to know what is behind an opaque membrane prior to fenestration. Blunt perforation should be avoided at all costs as pushing against these flimsy membranes may damage important neurovascular structures that lie behind. Enlarging interhemispheric cysts can be fenestrated directly to the ventricle or via cystocisternoventricular fenestration.26 Fourth ventricular cysts obstructing outflow are also amenable to fenestration.22
Intraventricular Endoscopy for Tumors
Endoscopic applications for intraventricular tumors include tumor biopsy, tumor resection, and management of tumor-associated hydrocephalus.27,28 In general, neuroendoscopy for tumors is a step up in technical difficulty from the endoscopic management of hydrocephalus. The ideal conditions for endoscopic resection of an intraventricular tumor are that it should be small, avascular or with relatively low vascularity, partially or totally cystic, and located in enlarged ventricles. Hydrocephalus creates an ideal working space. However, a normal ventricle is adequate to gain access to a tumor and biopsy it and, for smaller tumors, to resect it safely.29,30 A recent report even suggests that in experienced hands, operating in a normal-sized ventricle yields the same success/complication rate as operating in large ventricles.
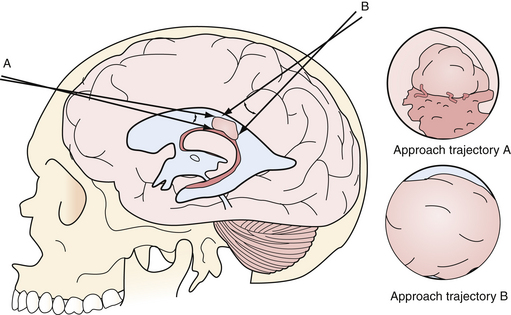
Considerations when selecting an approach trajectory are to select one which:
1. Enters the ventricle with some normal ventricle between the entry point and the mass
2. Allows access to the blood supply, if vascular
3. Allows access to the point of attachment to the ventricular wall or choroid plexus
4. Does not originate in or traverse eloquent structures
5. Allows management of associated hydrocephalus or trapped CSF spaces
Having some normal ventricle and CSF between the entry point into the ventricle and the tumor allows better visualization of the tumor margin and allows visualized normal structures to aid in orientation (Fig. 41.3). Access to the blood supply and point of attachment may turn a tedious piecemeal tumor resection into a disconnection and en bloc removal. As mentioned above, the use of transendoscope ultrasonic aspirators may speed up tumor removal if en bloc removal is not possible.11
Image guidance is particularly valuable for approach planning and execution, and is worthwhile even if it is only used for this step of the procedure.31–33 Third ventricular tumor resections are particularly dependant on proper approach angle because often the endoscope must traverse the foramen of Monro, putting the fornix, at the anterior border of the foramen, at some risk. Image guidance allows the surgeon to use trajectory views to draw a line from the anterior border of the tumor to the anterior-most border of the foramen of Monro. This line can then be extended to the surface to choose the appropriate entry point and angle. The fornix will not tolerate anterior “windshield wiping” movements of the endoscope while its tip lies in the third ventricle. Posterior movements are tolerated much better; however, the venous structures coalescing at the posterior margin of the foramen of Monro also limit scope excursion. Fortunately, many intraventricular tumors are associated with ventriculomegaly and an enlarged foramen of Monro that allows for larger excursions of the endoscope.
It is worth noting the major source of complications in intraventricular endoscopic approaches for brain tumors is disorientation. Complications can be minimized through appropriate entry point and trajectory choice, prior orientation of the camera, detailed examination of the equipment and video image prior to entry into the brain, a thorough knowledge of normal ventricular anatomy, and a constant self-inquiry into where the endoscope is, what all structures seen represent, and how the endoscope’s intrinsic optical distortion is affecting the scene. The use of frameless stereotaxis helps reduce disorientation.
Endoscopic Approaches to Intraventricular Tumors
Details of the diagnosis, pathology, and nonsurgical treatment of intraventricular tumors are discussed in Chapter 42. Certain tumors are more amenable than others for endoscopic removal.34 When working within the fluid-filled ventricle, bleeding may be difficult to control and obscures the operative field. Therefore, tumors of low vascularity are preferred for purely endoscopic removal. Examples are subependymomas, some ependymomas, the subependymal giant cell astrocytomas associated with tuberous sclerosis, selected neurocytomas, exophytic gliomas (primarily pilocytic or low grade), and hypothalamic hamartomas.35 Some vascular tumors such as choroid plexus and pedunculated tumors can also be approached endoscopically, because the blood supply is often well defined and easy to coagulate and divide.
Most tumors are approached by taking initial biopsy specimens with cup forceps, minimizing coagulation to maintain the quality of the tissue for analysis. Any vessels on the surface of the tumor are then coagulated. Electrocautery (especially monopolar) is capable of generating high CSF temperatures and must be used with caution. Irrigation with warmed lactated Ringer’s solution or a spinal fluid substitute solution is used to dissipate this heat.36 Normal saline is not used because it lacks electrolytes, is acidotic, alters the electrolyte balance in the brain, and leads to postoperative confusion.37Again, appropriate egress of irrigant will avoid a dangerous rise in ICP.
Once bleeding is controlled, cautery, blunt dissection, and bites with the forceps or scissors are used to separate the tumor from the normal tissue. The best tumors for neuroendoscopy have a distinct margin and can be gently retracted away from the surrounding tissue. Ideally, a perimeter can be created, the tumor can be isolated as a mass, and it can be removed in one or more large pieces. If the tumor is soft, multiple methods of tumor aspiration are possible. A stainless steel suction cannula or the previously mentioned pediatric endotracheal suction catheter placed down the working channel can be used to morselize and aspirate the tumor.38 Shortened endovascular catheters can also be used. They have the advantage of a stiffer, thinner wall that can be shaped. The catheter allows a larger inner lumen diameter for more efficient aspiration of appropriate consistency tumors.39 The gelatinous contents of colloid cysts and some other cystic tumors respond particularly well to this technique. As mentioned previously, the length of time required to aspirate a tumor using a “biopsy after biopsy” approach may be shortened by the use of ultrasonic aspiration with a specialized handpiece down the working port of the endoscope.11 Regardless of the removal technique, every attempt should be made to avoid dispersion of tumor remnants throughout the ventricles.
In some cases tumor biopsy rather than removal may be the goal. Central nervous system (CNS) lymphoma is often periventricular and amenable to endoscopic biopsy for diagnosis. “Nonoperative” gliomas may also be appropriate for this biopsy method. Stereotactic guidance is helpful in locating the tumor. However, identification of the tumor tissue through overlying normal ependyma may be problematic. Recently, the use of 5-ALA (5-aminolevulinic acid) fluorescence to identify and biopsy a midbrain glioma through an intact ependymal layer has been reported.40
Complications of tumor biopsy and removal include intraventricular hemorrhage, neurological deficit, tension pneumocephalus, hydrocephalus, and basilar artery injury.41–43 Tension pneumocephalus results from air being exchanged for CSF during the procedure. To avoid this, the ventricles should be refilled with lactated Ringer’s solution. If large quantities of air remain, 100% oxygen administered via face mask will help with dissolution.44
Peretta and associates reported an 8.8% complication rate for neuroendoscopic (nonhydrocephalus procedures) in pediatric patients.45 Hemorrhagic complications of tumor biopsy are reported at 3.5% per patient and 2.4% per procedure.46 To minimize the incidence of hemorrhagic complications one should never cut or pull any structure without being able to visualize the structure completely.
Hemorrhage is often the rate-limiting step during endoscopic tumor removal. Several techniques can be used to control hemorrhage with the endoscope. The first maneuver is to patiently irrigate. Most bleeding in the ventricle will stop with irrigation alone. The second maneuver is to attempt coagulating the bleeding source, but only if it can be directly visualized. Visualization may be improved by draining the CSF and working in an air-filled ventricle. A third maneuver is to gently tamponade the bleeding point using the endoscope itself or an instrument placed down the working channel. This maneuver is appropriate for larger veins that one is attempting to preserve such as the thalamostriate vein. An external ventricular drain can be left if necessary or as a “safety valve” in the case that tumor or hemorrhage occludes the foramen or aqueduct. The question of whether to leave a drain is not convincingly answered in the literature. We do not routinely leave drains after endoscopic procedures.
Endoscope-Assisted Microsurgical Approaches to Intraventricular Tumors
Many intraventricular tumors cannot be completely removed through a purely endoscopic approach, but endoscopy still maintains an important role. The concept of “endoscope-assisted” refers to a procedure whereby the approach is the traditional microsurgical one and the endoscope is used as an adjunct either through the same opening or through a separate bur hole for better overall visualization.47 The endoscope allows the surgeon to look around corners and to visualize structures that are not visible by microscopic imaging alone, thus expanding the operating field. Utilizing angled endoscopes can maximize the area of the ventricle visualized. Endoscope-assisted microsurgery has been shown to be useful in 35 patients with traditional microsurgical approaches to the ventricular system done through keyhole craniotomies; 31 of the 35 patients had no morbidity at 6 months and three of the patients with 6-month morbidity had preexisting Parinaud’s syndrome that persisted. Seventy-eight percent of patients had complete tumor resections. One procedure was aborted due to hemorrhage and was repeated successfully with gross total removal 2 days later.48 Another example of endoscope-assisted microsurgery is the transventricular management of craniopharyngioma. This includes gross total removal of intraventricular components, fenestration of cysts as a stand-alone procedure for symptomatic control, and cyst fenestration followed by collapse and subsequent craniotomy for definitive removal of the solid component.49
Abdou M.S., Cohen A.R. Endoscopic treatment of colloid cysts of the third ventricle. Technical note and review of the literature. J Neurosurg. 1998;89(6):1062-1068.
Bristol R.E., Nakaji P., Smith K.A. Endoscopic management of colloid cysts. Oper Tech Neurosurg. 2005;8(4):176-178.
Cinalli G., Peretta P., Spennato P., et al. Neuroendoscopic management of interhemispheric cysts in children. J Neurosurg. 2006;105(Suppl 3):194-202.
Gaab M.R., Schroeder H.W. Neuroendoscopic approach to intraventricular lesions. J Neurosurg. 1998;88(3):496-505.
Cappabianca P., Cinalli G., Gangemi M., et al. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008;62(Suppl 2):575-597. discussion 597-598
Charalampaki P., Filippi R., Welschehold S., et al. Tumors of the lateral and third ventricle: removal under endoscope-assisted keyhole conditions. Neurosurgery. 2005;57(Suppl 4):302-311. discussion 302-311
Please go to expertconsult.com to view the complete list of references.
1. Doglietto F., Prevedello D.M., Jane J.A.Jr., et al. Brief history of endoscopic transsphenoidal surgery—from Philipp Bozzini to the First World Congress of Endoscopic Skull Base Surgery. Neurosurg Focus. 2005;19(6):E3.
2. Grant J.A. Victor Darwin Lespinasse: a biographical sketch. Neurosurgery. 1996;39:1232-1233.
3. Dandy W.E. Cerebral ventriculoscopy. Johns Hopkins Hosp Bull. 1922;33:189.
4. Lewis A.I., Crone K.R., Taha J., et al. Surgical resection of third ventricle colloid cysts. Preliminary results comparing transcallosal microsurgery with endoscopy. J Neurosurg. 1994;81(2):174-178.
5. Horn E.M., Feiz-Erfan I., Bristol R.E., et al. Treatment options for third ventricular colloid cysts: comparison of open microsurgical versus endoscopic resection. Neurosurgery. 2007;60(4):613-618. discussion 618-620
6. Levine N.B., Miller M.N., Crone K.R. Endoscopic resection of colloid cysts: indications, technique, and results during a 13-year period. Minim Invasive Neurosurg. 2007;50(6):313-317.
7. Greenlee J.D., Teo C., Ghahreman A., Kwok B. Purely endoscopic resection of colloid cysts. Neurosurgery. 2008;62(3 Suppl 1):51-55. discussion 55-56
8. Schroeder H.W., Gaab M.R. Endoscopic resection of colloid cysts. Neurosurgery. 2002;51(6):1441-1444. discussion 1444-1445
9. Suri A., Goel R.K., Ahmad F.U., et al. Transventricular, transaqueductal scope-in-scope endoscopic excision of fourth ventricular neurocysticercosis: a series of 13 cases and a review. J Neurosurg Pediatr. 2008;1(1):35-39.
10. Prat R., Galeano I. Endoscopic biopsy of foramen of Monro and third ventricle lesions guided by frameless neuronavigation: usefulness and limitations. Clin Neurol Neurosurg. 2009;111(7):579-582. (Epub 2009 May 26)
11. Oertel J., Krauss J.K., Gaab M.R. Ultrasonic aspiration in neuroendoscopy: first results with a new tool. J Neurosurg. 2008;109(5):908-911.
12. Resch K.D. Transendoscopic ultrasound in ventricular lesions. Surg Neurol. 2008;69(4):375-382. discussion 382 (Epub 2008 Feb 20)
13. Oertel J., Gen M., Krauss J.K., et al. The use of waterjet dissection in endoscopic neurosurgery. Technical note. J Neurosurg. 2006;105(6):928-931.
14. Nakaji P., Teo C. Endoscopic approaches to brain tumors. Youmas Neurological Surgery. 2011;5:1300-1308.
15. Grondin R.T., Hader W., MacRae M.E., Hamilton M.G. Endoscopic versus microsurgical resection of third ventricle colloid cysts. Can J Neurol Sci. 2007;34(2):197-207.
16. Teo C. Complete endoscopic removal of colloid cysts: issues of safety and efficacy. Neurosurg Focus. 1999;6(4):e9.
17. Abdou M.S., Cohen A.R. Endoscopic treatment of colloid cysts of the third ventricle. Technical note and review of the literature. J Neurosurg. 1998;89(6):1062-1068.
18. King W.A., Ullman J.S., Frazee J.G., et al. Endoscopic resection of colloid cysts: surgical considerations using the rigid endoscope. Neurosurgery. 1999;44(5):1103-1109. discussion 1109-1111
19. Decq P., Le Guerinel C., Brugières P., et al. Endoscopic management of colloid cysts. Neurosurgery. 1998;42(6):1288-1294. discussion 1294-1296
20. Bristol R.E., Nakaji P., Smith K.A. Endoscopic management of colloid cysts. Oper Tech Neurosurg. 2005;8(4):176-178.
21. Hellwig D., Bauer B.L., Schulte M., et al. Neuroendoscopic treatment for colloid cysts of the third ventricle: the experience of a decade. Neurosurgery. 2003;52(3):525-533. discussion 532-533
22. Longatti P., Godano U., Gangemi M., et al. Italian neuroendoscopy group. Cooperative study by the Italian neuroendoscopy group on the treatment of 61 colloid cysts. Childs Nerv Syst. 2006;22(10):1263-1267. (Epub 2006 Apr 29). Erratum in Childs Nerv Syst. 2006;22(10):1375
23. Proana J.V., Torres-Corzo J., Guizar-Sahagun G., et al. Intraventricular and subarachnoid basal cisterns neurocysticercosis: a comparative study between traditional treatment versus neuroendoscopic surgery. Childs Nerv Syst. 2009;25(11):1467-1475. (Epub 2009 Jun 26)
24. Goel R.K., Ahmad F.U., Vellimana A.K., et al. Endoscopic management of intraventricular neurocysticercosis. J Clin Neurosci. 2008;15(10):1096-1101. (Epub 2008 Jul 23)
25. Suri A., Goel R.K., Ahmad F.U., et al. Endoscopic excision of intraventricular neurocysticercosis in children: a series of six cases and review. Childs Nerv Syst. 2008;24(2):281-285. (Epub 2007 Nov 10)
26. Cinalli G., Peretta P., Spennato P., et al. Neuroendoscopic management of interhemispheric cysts in children. J Neurosurg. 2006;105(Suppl 3):194-202.
27. Gaab M.R., Schroeder H.W. Neuroendoscopic approach to intraventricular lesions. J Neurosurg. 1998;88(3):496-505.
28. Macarthur D.C., Buxton N., Punt J., et al. The role of neuroendoscopy in the management of brain tumours. Br J Neurosurg. 2002;16(5):465-470.
29. Yamamoto M., Oka K., Takasugi S., et al. Flexible neuroendoscopy for percutaneous treatment of intraventricular lesions in the absence of hydrocephalus. Minim Invasive Neurosurg. 1997;40(4):139-143.
30. Souweidane M.M. Endoscopic surgery for intraventricular brain tumors in patients without hydrocephalus. Neurosurgery. 2008;62(6 Suppl 3):1042-1048.
31. Harris A.E., Hadjipanayis C.G., Lunsford L.D., et al. Microsurgical removal of intraventricular lesions using endoscopic visualization and stereotactic guidance. Neurosurgery. 2008;62(Suppl 2):622-629.
32. Tirakotai W., Bozinov O., Sure U., et al. The evolution of stereotactic guidance in neuroendoscopy. Childs Nerv Syst. 2004;20(11-12):790-795. (Epub 2004 Jul 17)
33. Schroeder H.W., Wagner W., Tschiltschke W., Gaab M.R. Frameless neuronavigation in intracranial endoscopic neurosurgery. J Neurosurg. 2001;94(1):72-79.
34. Cappabianca P., Cinalli G., Gangemi M., et al. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008;62(Suppl 2):575-597. discussion 597-598
35. Ng Y.T., Rekate H.L., Prenger E.C., et al. Endoscopic resection of hypothalamic hamartomas for refractory symptomatic epilepsy. Neurology. 2008;70(17):1543-1548.
36. Oka K., Yamamoto M., Nonaka T., Tomonaga M. The significance of artificial cerebrospinal fluid as perfusate and endoneurosurgery. Neurosurgery. 1996;38(4):733-736.
37. Salvador L., Valero R., Carrero E., et al. Cerebrospinal fluid composition modifications after neuroendoscopic procedures. Minim Invasive Neurosurg. 2007;50(1):51-55.
38. Souweidane M.M., Luther N. Endoscopic resection of solid intraventricular brain tumors. J Neurosurg. 2006;105(2):271-278.
39. Husain M., Jha D.K., Rastogi M. Angiographic catheter: unique tool for neuroendoscopic surgery. Surg Neurol. 2005;64(6):546-549.
40. Tamura Y., Kuroiwa T., Kajimoto Y., et al. Endoscopic identification and biopsy sampling of an intraventricular malignant glioma using a 5-aminolevulinic acid-induced protoporphyrin IX fluorescence imaging system. Technical note. J Neurosurg. 2007;106(3):507-510.
41. Hamada H., Hayashi N., Kurimoto M., et al. Tension pneumocephalus after a neuroendoscopic procedure—case report. Neurol Med Chir (Tokyo). 2004;44(4):205-208.
42. Saxena S., Ambesh S.P., Saxena H.N., Kumar R. Pneumoencephalus and convulsions after ventriculoscopy: a potentially catastrophic complication. J Neurosurg Anesthesiol. 1999;11(3):200-202.
43. Abtin K., Thompson B.G., Walker M.L. Basilar artery perforation as a complication of endoscopic third ventriculostomy. Pediatr Neurosurg. 1998;28(1):35-41.
44. Gore P.A., Maan H., Chang S., et al. Normobaric oxygen therapy strategies in the treatment of postcraniotomy pneumocephalus. J Neurosurg. 2008;108(5):926-929.
45. Peretta P., Ragazzi P., Galarza M., et al. Complications and pitfalls of neuroendoscopic surgery in children. J Neurosurg. 2006;105(Suppl 3):187-193.
46. Luther N., Cohen A., Souweidane M.M. Hemorrhagic sequelae from intracranial neuroendoscopic procedures for intraventricular tumors. Neurosurg Focus. 2005;19(1):E9.
47. Gore P.A., Nakaji P., Deshmukh V., Rekate H.L. Synchronous endoscopy and microsurgery: a novel strategy to approach complex ventricular lesions. Report of three cases. J Neurosurg. 2006;105(Suppl 6):485-489.
48. Charalampaki P., Filippi R., Welschehold S., et al. Tumors of the lateral and third ventricle: removal under endoscope-assisted keyhole conditions. Neurosurgery. 2005;57(Suppl 4):302-311. discussion 302-311
49. Cinalli G., Spennato P., Cianciulli E., et al. The role of transventricular neuroendoscopy in the management of craniopharyngiomas: three patient reports and review of the literature. J Pediatr Endocrinol Metab. 2006;19(Suppl 1):341-354.