CHAPTER 119 Endoscopic Approaches to Brain Tumors
History
Perhaps because of the similarities of working within the ventricle to working within the fluid-filled space of the urinary bladder, the first surgeon to perform intraventricular endoscopic surgery was Victor Lespinasse, a Chicago urologist.1,2 Using a primitive cystoscope, he fulgurated the choroid plexus in two infants to treat hydrocephalus. One infant died and the other survived 5 years. At this time, lighting, which relied on an external bulb, was poor. Furthermore, the endoscope was large and the view was provided by peering down its shaft. From the perspective of contemporary endoscopy, this early technique would not be considered “true” endoscopy because the view was under direct vision, not through a lens system. However, the parallels make this technique the forerunner of the contemporary approach. Walter Dandy was the first neurosurgeon to explore use of the endoscope extensively and to report on its use.3
Persistent limitations in instrumentation, lighting, and optics relegated intracranial endoscopy to a relatively minor position for the next several decades. The frustrations of working in such a small area with restricted access led surgeons to favor open approaches. With the advent of the view provided by the operating microscope, endoscopy was further eclipsed. In the 1980s, neuroendoscopy re-emerged with the advent of rigid rod lenses, fiberoptics, high-intensity light sources, charge-coupled devices, and customized instruments.4–8 As experience with all forms of endoscopic procedures increased, endoscopic tumor removal became technically feasible. Experience with removal of colloid cysts, in many ways an ideal intraventricular mass for neuroendoscopy, provided the surgical foundations for more complicated work. Today, endoscopic colloid cyst surgery achieves results similar to those obtained with microsurgery and with a better risk profile.9–15 The neuro-oncologic applications of neuroendoscopy are growing rapidly. Both within and outside the ventricles, the endoscope provides minimally invasive options for treating tumors and managing their side effects. As these techniques propagate throughout neuro-oncology, neuroendoscopy is assuming its place among the standard techniques with which all neurosurgeons must be familiar.
Overview of Endoscopy in the Approach to Brain Tumors
As a tool for visualization, the endoscope offers neurosurgeons several advantages. It delivers illumination directly to the point of interest, offers an extremely high degree of magnification, and provides a wide field of view. With the use of angled endoscopes, surgeons can see around corners.16 As familiarity with these attributes has grown, neurosurgeons have developed ways to use the endoscope both as a substitute for the microscope and for altogether new procedures.
Intraventricular Endoscopy for Tumors
Endoscopic applications for the management of intraventricular tumors include tumor biopsy, tumor resection, and treatment of tumor-associated hydrocephalus.17–19 Endoscopic biopsy is relatively easy to perform and should be considered for any tumor whose treatment strategy does not begin primarily with resection. If complete tumor resection is the initial goal, microsurgical removal remains the first choice for most tumors. A select minority are amenable to endoscopic removal. When feasible, endoscopic resection is performed through a transcortical route, usually with low neurological impact. In general, neuroendoscopic treatment of tumors is a high-level endoscopic skill that requires considerable familiarity with the endoscope, skill in its use, and experience. Detailed knowledge of anatomy is essential.20,21
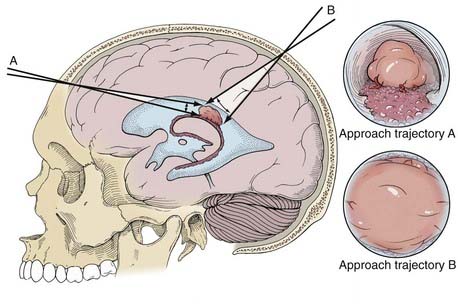
There is no absolute limit to the size of a tumor, but 2 cm is often cited.22 Cystic tumors can be larger because decompression of the cyst reduces the effective size of the tumor. High vascularity is a relative contraindication. Patience, irrigation, and cautery allow even bloody tumors to be treated, but as the length of surgery increases, the relative advantage of the minimally invasive approach may be lost. Hydrocephalus enlarges the working space, which is advantageous for an intraventricular approach. However, the tumor itself usually creates a working space, so hydrocephalus is not mandatory. The normal ventricle is large enough to provide access to a tumor for biopsy, and smaller tumors can even be safely resected.23–25 A major key in the surgical approach to intraventricular lesions is choosing the appropriate working trajectory. The brain must be transited to reach the ventricles. Therefore, a single working angle that minimizes yaw and pitch movement of the endoscope should be chosen.
Most ventricular endoscopic procedures are performed through a single portal, although the use of two portals has been described.26–29 Because normally only a single portal is used, the choice of trajectory is extremely important.
The selected approach trajectory (Fig. 119-1) should do the following: (1) enter the ventricle with some normal ventricle between the entry point and the mass; (2) allow access to the blood supply, if vascular; (3) allow access to the point of attachment to the ventricular wall or choroid plexus; (4) originate outside or avoid eloquent structures; and (5) when possible, allow any other necessary endoscopic procedures (e.g., septum pellucidotomy, endoscopic third ventriculostomy) to be performed through the same approach.
Image guidance is particularly valuable for this planning and for achieving a good approach. It is worthwhile even if it is used only for this step of the procedure.30–32 The trajectory should allow the edges of the tumor to be reached with excursions of the endoscope that subtend the minimal amount of normal brain tissue (see Fig. 119-1). The elastic modulus of the brain does not allow much stretching. Rather, it should be assumed that retraction has some impact on the brain with every movement of the endoscope. By limiting the amount of movement necessary to reach the entire target, the effects of the endoscope can be mitigated.
Image guidance is strongly recommended as an adjunct to neuroendoscopic approaches to overcome many of these obstacles.33 It is useful for intraventricular, intraparenchymal, and extra-axial approaches. For intraventricular neuroendoscopy, image guidance plays a role in selecting the appropriate entry point and optimal trajectory. It is a useful source of reorientation, especially when blood clouds the view or the anatomy is altered. It can be used successfully in both children and adults.34 Most rigid neuroendoscopes can support the mounting of a fiducial array for integration into a frameless stereotactic system. When such a system is used, the location of the tip of the endoscope can be updated continuously. With a rigid endoscope, the option of pulling the endoscope directly back out the way that it entered is almost always safe. With a flexible endoscope, the endoscope must be in its straight configuration to avoid hooking tissues as it is removed. Image guidance can also facilitate replacement of the endoscope should the ventricle be lost when the endoscope is removed.
Purely Endoscopic Approaches to Intraventricular Tumors
Details of the diagnosis, pathology, and nonsurgical treatment of intraventricular tumors are discussed in Chapter 138. As noted, certain tumors are more amenable than others to endoscopic removal.35 Tumors with low vascularity are preferred for purely endoscopic removal. Examples are colloid cysts, subependymomas, some ependymomas, subependymal giant cell astrocytomas associated with tuberous sclerosis, selected neurocytomas, exophytic gliomas (primarily pilocytic or low grade), cavernous hemangiomas, and hypothalamic hamartomas.36 Some choroid plexus tumors and pedunculated tumors can also be approached endoscopically because although they may be vascular, their blood supply is well defined.
Most tumors are approached by taking initial biopsy specimens with a cup forceps. Coagulation must be minimized to maintain the quality of the tissue for histopathologic analysis.35,37 For many tumors, this is sufficient management. If more extensive debulking and complete removal are intended, vessels on the surface of the tumor are coagulated with bipolar or monopolar electrocautery or a laser. Electrocautery (especially monopolar) is capable of generating high CSF temperatures and must therefore be used with caution. Copious irrigation with body-temperature lactated Ringer’s solution or a spinal fluid substitute solution is used to dissipate this heat.38 Normal saline is not used because it lacks electrolytes and is significantly acidotic. Consequently, it can alter the electrolytic balance in the brain and lead to postoperative confusion.39 When irrigating, it is essential to have a secure path of egress for the fluid to prevent trapping the fluid in the brain and resulting in increases in intracranial pressure.
Once the bleeding is controlled, cautery and blunt dissection are used to separate the tumor from normal tissue. The best tumors for neuroendoscopy have a distinct margin and can be retracted gently from the surrounding tissue. Ideally, a perimeter can be created, the tumor can be isolated as a mass, and it can be removed in one or more large pieces. If the tumor is soft, a stainless steel cannula or a hand-trimmed pediatric endotracheal suction catheter placed down the working channel can be used to remove significant portions of the tumor by suction.40 The gelatinous contents of colloid cysts and some other cystic tumors respond particularly well to this technique. An attempt is made to not spread tumor tissue around the ventricle. If care is not taken, tumor may fall with gravity toward dependent areas of the ventricles.
Location plays an important role in choice of trajectory and whether to approach a lesion endoscopically at all. A list of standard approaches and entry sites is presented in Table 119-1. Tumors of the third ventricle are generally good targets for biopsy and reasonable for resection, depending on their size and vascularity. Almost all approaches to the third ventricle are performed through the foramen of Monro. The entry points on the surface are chosen with the foramen as the pivot point. The fornix, which constitutes the anterior-superior margin of the foramen, must be respected in both choice and execution of approach to prevent potentially catastrophic memory loss.
| LESION LOCATION | USUAL ENTRY POINT | SUITABILITY FOR ENDOSCOPIC BIOPSY |
|---|---|---|
| Anterior third ventricle | 1 cm posterior to the coronal suture 2-3 cm lateral to the midline |
+++ |
| Floor of the third ventricle | 1 cm anterior to the coronal suture 2-3 cm lateral to the midline |
+++ |
| Posterior third ventricle | 7 cm posterior to the nasion 2 cm lateral to the midline |
+++ |
| Anterior lateral ventricle | 8 cm posterior to the nasion 4-6 cm lateral to the midline |
+++ |
| Atrium of the lateral ventricle | 8 cm posterior to the midline 1 cm lateral to the midline vs. the superior parietal lobule |
++ |
| Temporal horn | Superior parietal lobule | + |
| Occipital horn | 8 cm posterior to the midline 1 cm lateral to the midline |
+ |
| Fourth ventricle | 10 cm posterior to the nasion 2 cm lateral to the midline vs. suboccipital |
+ / 0 |
Complications of tumor biopsy and removal include intraventricular hemorrhage, neurological deficit, tension pneumocephalus, hydrocephalus, and injury to the basilar artery.41–43 In one series the hemorrhage rate was 3.5%.44 Intraventricular hemorrhage is avoided by respect for vascular structures and prophylactic coagulation. Neurological deficit is avoided by choosing an ideal working trajectory, respecting key areas of anatomy, and not working where visualization is poor. Tension pneumocephalus is caused by air entering the ventricles during the procedure. The ventricles should be refilled with lactated Ringer’s solution as much as possible. If large quantities of air are left, high inspired oxygen tension helps speed its absorption.45
Neuroendoscopic Management of Tumor-Associated Hydrocephalus
Obstructive hydrocephalus frequently complicates the course of patients with various intracranial tumors. Such hydrocephalus can be caused by direct blockage of the ventricle by tumor, by compression of the aqueduct or foramen of Monro, or by the formation of cysts or membranes. Endoscopic third ventriculostomy, septum pellucidotomy, or cyst fenestration should always be considered as the first-line management of this type of hydrocephalus.46–51 Endoscopic procedures are often sufficient management of tumor-associated obstructive hydrocephalus. Doing so avoids the complications intrinsic to shunting: infection and blockage and the rare cases of abdominal spread.52 Success rates are frequently 80% or higher.46 Common examples of tumor-associated obstruction are aqueductal obstruction by tectal gliomas, pineal tumors, and fourth ventricular tumors.47,48,53 The techniques of endoscopic third ventriculostomy and septum pellucidotomy are discussed elsewhere. Many tumors, despite their daunting appearance on computed tomography (CT) or MRI, will present a smooth surface at the ventricle, thereby allowing successful cannulation and opening of alternative CSF pathways. Equalization of pressure between the two ventricles, as afforded by septum pellucidotomy, or between the ventricles and the extraventricular subarachnoid space can prevent shift, herniation, and extreme ventricular dilation.
Endoscopic aqueductoplasty and stenting should be considered in the context of an isolated fourth ventricle, for example, when tumor fills the basal cisterns of the posterior fossa and obstructs the foramina of Luschka and Magendie or when removal of a fourth ventricular tumor has scarred these avenues closed. The consequences of fourth ventricular dilation can be severe but can be relieved by aqueductoplasty when combined with endoscopic third ventriculostomy or ventricular shunting. The technique of aqueductoplasty is described elsewhere.54 Aqueductoplasty may need to be combined with shunting or third ventriculostomy to be effective. Endoscopic third ventriculostomy has also been used successfully to manage hydrocephalus associated with tumors of the cerebellopontine angle and brainstem.47,55
Role of Endoscopic Biopsy in the Management of Intracranial Tumors
Endoscopic tumor biopsy solely for histopathologic diagnosis is an appropriate alternative to complete resection (whether endoscopic or microscopic) whenever a tumor appears to be amenable to adjuvant therapy. Indeed, a wide variety of tumors are accessible endoscopically with minimal morbidity. Issues of sampling error are the same with endoscopic biopsy as with other forms of biopsy.56 Lesions considered for management with endoscopic biopsy include pineal region tumors, tumors with ependymal spread in which a diagnosis is unclear (such as metastatic tumors and lymphoma), and even otherwise unresectable intrinsic tumors with an exophytic portion in the ventricle. Intrinsic tumors that are primarily intrinsic but reach the ependymal surface must be approached with caution because the ependymal surface may appear normal and thus complicate biopsy. Furthermore, if hemorrhage occurs, it drains into the ventricle with little to resist it.
Endoscopic Removal of Colloid Cysts of the Third Ventricle
Options for treatment include shunting (usually of both ventricles), stereotactic drainage, transcallosal or transcortical craniotomy, and endoscopic removal.57 Shunting does not prevent the cyst from growing. In the event of shunt blockage, the risk for sudden death persists. Stereotactic drainage is associated with a high rate of failure or recurrence.58,59 Microsurgical removal is effective, but more morbid than the endoscopic approach.11,13,60 Therefore, endoscopic removal is recommended for most cases.9,15,61,62
The approach is through a single bur hole located about 8 cm behind the nasion and as far lateral as the anatomy permits without transiting the caudate head, which is usually 5 to 7 cm from the midline.10,63 A 1.5-cm incision and 8-mm bur hole usually suffice. Image guidance is recommended for the initial ventricular entry. A peel-away sheath is optional. The landmarks of the colloid cyst and foramen of Monro are identified. The overlying choroid plexus is coagulated while taking particular care to prevent heat damage or direct trauma to the fornix.
The face of the cyst is opened, and the contents are aspirated with suction or removed with forceps. The wall of the cyst is then dissected free of the roof of the third ventricle. The cyst seldom adheres to the fornix itself. The entire cyst wall can usually be removed in gross fashion. Occasionally, the cyst is so adherent to the walls of the third ventricle or internal cerebral veins that it must be truncated and a small fragment left. Under these circumstances, the rate of recurrence appears to be low,12,64,65 and relief of obstructive hydrocephalus is the rule. Postoperatively, however, the decrease in size of the ventricles is sometimes modest.64
Neuroendoscopic Management of Pineal Region Tumors
Neuroendoscopy has taken a prominent role in the management of pineal region tumors.66 The pineal region is almost centrally located in the head. It is a common site for a wide range of pathologies, and a number of surgical corridors are available for access.67–72 Frequent pineal tumors include germinoma, nongerminomatous germ cell tumors, pineal parenchymal tumors, glial tumors, and a wide range of miscellaneous tumors, including meningioma. Tumors in the pineal region frequently obstruct the aqueduct of Sylvius and create a setting of hydrocephalus that makes anterior transventricular endoscopic approaches even more attractive. Much pathology in this region responds to primary or initial adjunctive radiation therapy and chemotherapy. Consequently, there is a strong rationale for primary use of neuroendoscopy to obtain a biopsy specimen for histologic analysis in the most minimally invasive way possible.73–76
In general, the initial work-up for a tumor in the pineal region involves serum and CSF analysis for markers (α-fetoprotein, β-human chorionic gonadotropin, lactate dehydrogenase, placental alkaline phosphatase) that could indicate a malignant tumor of germ cell lineage.77 Germinomas, in particular, are highly responsive to radiation and may be treated by radiation therapy alone if the diagnosis can be made endoscopically. Other nongerminomatous germ cell tumors may respond well to preoperative chemotherapy and thereby decrease operative complexity and blood loss. Blind stereotactic needle biopsy may be considered as an alternative. A simple scheme for choosing among the alternatives is presented in Table 119-2.
| GOAL | TUMOR EXTENDS INTO VENTRICLE | APPROACH |
|---|---|---|
| Biopsy | Yes | Anterior endoscopic biopsy |
| Biopsy | No | Stereotactic biopsy |
| Resect | Yes | Endoscopic vs. transchoroidal vs. posterior* |
| Resect | No | Posterior* |
* Includes the supracerebellar infratentorial and occipital transtentorial approaches.
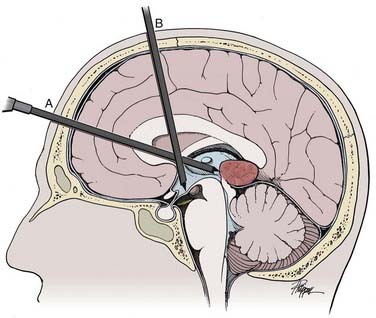
Pineal region tumors manifested as hydrocephalus should be considered for both endoscopic tumor biopsy and simultaneous third ventriculostomy (Fig. 119-2).78 They can be performed in either order. The rationale for performing third ventriculostomy first is to do so before potential bleeding and clouding of the ventricle occur as a result of the biopsy procedure. The rationale for performing a biopsy first is that it is the main goal and should be completed before potential bleeding occurs. If the tumor is small, it may be possible to open the CSF pathways endoscopically, thereby obviating the need for third ventriculostomy ![]() (Fig. 119-E1). In fact, there is little chance that either procedure will affect the other.
(Fig. 119-E1). In fact, there is little chance that either procedure will affect the other.
Endoscopic pineal tumor biopsy and third ventriculostomy with a rigid endoscope have been performed successfully through a single bur hole.79 Although technically possible, a single approach requires transit through more brain than occurs with two trajectories and may place the fornix at more risk. Therefore, it may not be truly minimally invasive (see Fig. 119-2). The need for simultaneous biopsy of a pineal region tumor and endoscopic third ventriculostomy is the most common application for flexible neuroendoscopy (Figs. 119-3).80 More often the two parts of the operation are performed through separate bur holes and trajectories with the use of rigid endoscopes (see Fig. 119-2).81,82
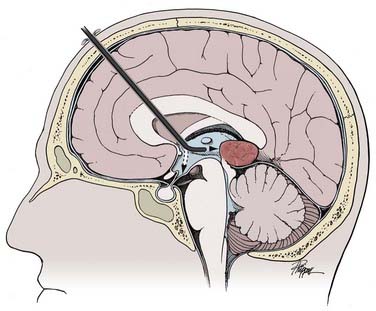
FIGURE 119-3 A flexible endoscope can accomplish both pineal tumor biopsy and third ventriculostomy from one starting point.
(Used with permission from Barrow Neurological Institute.)
An alternative endoscopic approach to the pineal region is via the supracerebellar infratentorial approach, in a manner analogous to the open approach.45,83 The patient is placed in the sitting position with a lumbar drain to create a working space without retraction. The procedure is performed in air. A purely endoscopic or an endoscope-assisted approach can be used via this route. The microsurgical route to this region is well established; however, it is a long corridor that keeps the pathology at some distance from the surgeon. The walls of the third ventricle, seen from behind, form a narrow corridor where residual tumor is often perpendicular to the direction of view and hence evades direct inspection. The endoscope overcomes all these limitations and thereby allows effective endoscopically controlled surgery. With a primarily microsurgical approach to the pineal region, angled endoscopes should be used to check the walls of the resection cavity for tumor and to inspect the third ventricle for residual blood that might obstruct the aqueduct.
Intraventricular Extension of Sellar Tumors
Endoscopic management of craniopharyngiomas can consist of biopsy, debulking, cyst fenestration with or without placement of intracystic catheters when the tumor cannot be completely removed, or any combination of these procedures.84 Biopsy is reserved for situations in which the diagnosis is unclear and definitive removal is impractical. A synchronous approach can be used for suprasellar craniopharyngiomas with a prominent third ventricular extension in which removal of the primary tumor is performed microsurgically through a pterional or orbitozygomatic approach. An endoscope is then introduced through a precoronal bur hole into the lateral ventricle to allow reduction of the cyst and separation of the tumor from the walls of the hypothalamus under direct vision.85 A similar approach can be used for giant pituitary macroadenomas.86
An endoscopic approach to the temporal lobe can be useful when tumor curves around the atrium in a C-shaped fashion, thus making it difficult to remove from one direction alone. In these cases a purely endoscopic approach is used to free the tumor and to document the completeness of resection, and the bulk of the tumor is removed through a traditional microsurgical approach.85
Endoscopically Assisted Microneurosurgery
Typical corridors that are particularly amenable to endoscopic assistance are the retrosigmoid, subfrontal/pterional, and interhemispheric transcallosal.87–93 In fact, any surgical corridor in the subarachnoid, intraparenchymal, or intraventricular space that is accessed by the microscope can benefit from endoscopic assistance.
Technique of Trans-Eyebrow Supraorbital Craniotomy
The minimally invasive trans-eyebrow craniotomy is a versatile point of access that provides much of the same viewing and working angles as the pterional and orbitozygomatic approaches.94–96 In combination with endoscope assistance, it can extend the surgeon’s reach to regions where the microscope cannot easily see. In craniopharyngioma surgery, for example, the use of angled endoscopes and instruments can allow visualization and surgical manipulation of tumor from the walls of the third ventricle from below.84 In these cases, traditional microsurgical instruments are used alongside the endoscope, much as they are in microsurgery. Therefore, the absolute minimum size of craniotomy can be determined by the size of the opening that will allow the bipolar forceps to be opened alongside the endoscope.
Neuroendoscopy for Intra-Axial Tumors
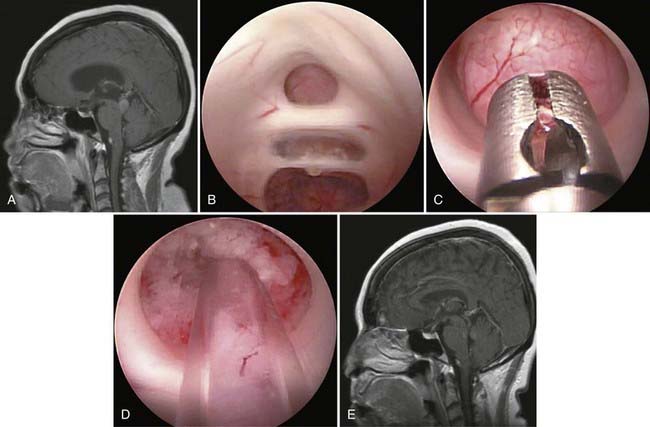
The use of neuroendoscopy as an adjunct for the surgical removal of intra-axial tumors is still relatively uncommon. Selected examples of anecdotal use illustrate its possibilities, which still wait to be fully explored. The applications for which it has both rationale and practical advantage are those in which the endoscope can help limit retraction and improve an otherwise restricted view. For example, during removal of an insular glioma, where the overlying opercula are extremely eloquent and cannot and should not be retracted, the endoscope can improve visualization of residual tumor![]() (Fig. 119-E2).97 For microsurgical removal of subcortical intrinsic tumors, the endoscope can be used through a small cortical portal to view the walls of the tumor cavity and to inspect the overhanging areas that are out of the line of sight of the microscope. This technique can allow the surgeon to avoid expanding the corticotomy solely to improve microscopic visualization.
(Fig. 119-E2).97 For microsurgical removal of subcortical intrinsic tumors, the endoscope can be used through a small cortical portal to view the walls of the tumor cavity and to inspect the overhanging areas that are out of the line of sight of the microscope. This technique can allow the surgeon to avoid expanding the corticotomy solely to improve microscopic visualization.
Experience with endoscopic removal is limited to small case series.98,99 Most techniques involve the use of neuronavigation to direct the placement of a sheath onto the tumor. Removal then proceeds under endoscopic control. Therefore, the sheath must be wide enough to accommodate both the endoscope and microinstruments.
Endonasal Transsphenoidal and Extended Transsphenoidal Approaches
In recent years, no area of neuroendoscopy has attracted as much interest and controversy as endoscopic endonasal approaches to the anterior skull base. Introduced by otorhinolaryngologists to treat sinus disease,100 endonasal endoscopy was initially applied in neurosurgery primarily as an adjunct to the microsurgical transsphenoidal approach to the sella turcica for pituitary pathology.101–105 However, in the past 2 decades, entirely endoscopic techniques have been brought to bear on a wide range of pathologies involving structures from the frontal fossa to the second cervical vertebra, all through a nostril approach without external incisions. A wide range of pathologies have proved accessible from this corridor, including sinonasal tumors (esthesioneuroblastomas, juvenile nasal angiofibromas), sellar pathologies (pituitary tumors, craniopharyngiomas), clival tumors (chordomas, chondrosarcomas), anterior cranial fossa dural-based tumors (meningiomas), and selected intradural tumors.106–112 Even vascular pathologies have been accessed and treated successfully. Most midline and paramedian masses are highly accessible through the endonasal route in both adults and children (Fig. 119-4).110,113–115
Endoscopic Endonasal Approach: Applications and Limitations
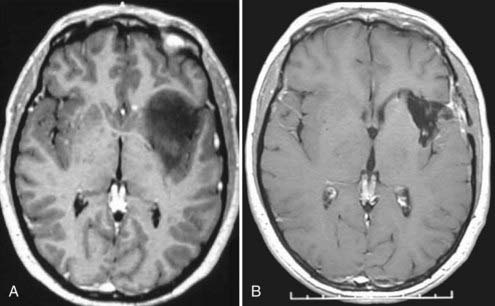
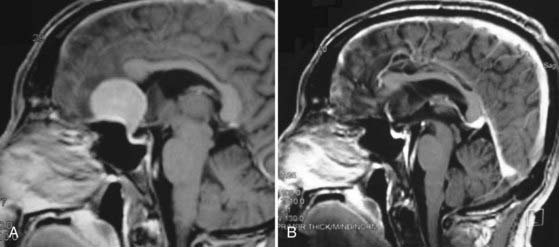
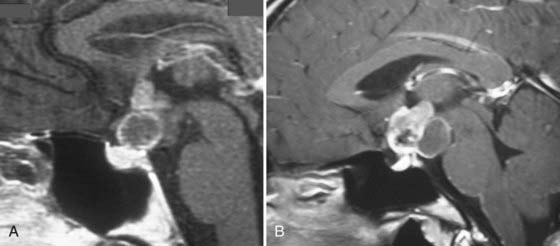
Specific tumors have their own limitations for the endonasal approach. Anterior cranial fossa meningiomas can be removed extremely effectively both transcranially and endonasally. If midsagittal MRI shows an anatomic gap between the posterior margin of the tumor and the anterior circulation vascular complex (i.e., the origin of the tumor is primarily from the olfactory groove), the endonasal approach is ideal (see Fig. 119-4). Conversely, if there is anatomic juxtaposition of the posterior margin of the tumor and large vessels or if the vessels are involuted into the tumor (as is often the case with tumors that are primarily of tuberculum sellae origin), a transcranial approach is preferred (Fig. 119-5).116
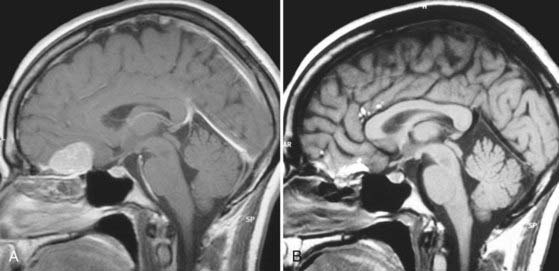
Craniopharyngiomas represent a challenging problem for neurosurgeons in many ways, particularly when a large suprasellar component adheres to the walls of the hypothalamus (Fig. 119-6A).84 If the optic chiasm is prefixed, the subfrontal approach is relatively contraindicated. However, this anatomic variation creates an excellent corridor to these tumors when operating from below (Fig. 119-6B). Expanded endonasal endoscopic techniques have been used to aid the surgeon in seeing these attachments of the tumor, thereby allowing it to be mobilized under direct vision.117–119 The pituitary gland can be mobilized and transposed to allow retrosellar exposure when needed.120 A second endoscopic option for craniopharyngiomas with third ventricular extension is to combine a craniotomy with a transforaminal endoscopic approach from above through a precoronal bur hole. This strategy allows the ventricular extension of the tumor to be freed before it is resected from below.
The principles of reconstruction are to make the smallest dural defect possible, open the dura sharply, preserve the arachnoid, leave a shelf of bone on which bone or a bone substitute can rest, and cover the defect with a watertight layer, of which a vascularized mucosal flap is the best option. The addition of fat grafts, fascia lata grafts, dural substitutes, dural sealants, acellular dermis, and other adjuncts is at the discretion and experience of the treating surgeon.121–123 Many applications require no nasal packing. In other cases, an inflated Foley catheter may be used to provide a bolster for the tissue repair.122 A lumbar drain is sometimes used to further improve the chance of healing.
Published rates of CSF leakage vary widely among series, depending largely on the pathology treated and the experience of the reporting group. With pituitary tumors, for instance, the typical leak rate is 2% to 10%.116,124 With dural-based or intradural masses, it may range as high as 16% to 58%.117,118,125,126
Cappabianca P, Cinalli G, Gangemi M, et al. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008;62(suppl 2):575-597.
Cappabianca P, de Divitiis E. Endoscopy and transsphenoidal surgery. Neurosurgery. 2004;54:1043-1050.
Doglietto F, Prevedello DM, Jane JAJr, et al. Brief history of endoscopic transsphenoidal surgery—from Philipp Bozzini to the First World Congress of Endoscopic Skull Base Surgery. Neurosurg Focus. 2005;19(6):E3.
Fries G, Perneczky A. Endoscope-assisted brain surgery: part 2—analysis of 380 procedures. Neurosurgery. 1998;42:226-231.
Jimenez DF, editor. Intracranial Endoscopic Neurosurgery. Park Ridge, IL: American Association of Neurological Surgeons, 1998.
Ono M, Rhoton ALJr, Peace D, et al. Microsurgical anatomy of the deep venous system of the brain. Neurosurgery. 1984;15:621-657.
Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery. 1998;42:219-224.
Rhoton ALJr, Yamamoto I, Peace DA. Microsurgery of the third ventricle: part 2. Operative approaches. Neurosurgery. 1981;8:357-373.
Teo C, Nakaji P. Neuro-oncologic applications of endoscopy. Neurosurg Clin N Am. 2004;15:89-103.
Yamamoto I, Rhoton ALJr, Peace DA. Microsurgery of the third ventricle: part I. Microsurgical anatomy. Neurosurgery. 1981;8:334-356.
1 Doglietto F, Prevedello DM, Jane JAJr, et al. Brief history of endoscopic transsphenoidal surgery—from Philipp Bozzini to the First World Congress of Endoscopic Skull Base Surgery. Neurosurg Focus. 2005;19(6):E3.
2 Grant JA. Victor Darwin Lespinasse: a biographical sketch. Neurosurgery. 1996;39:1232-1233.
3 Dandy W. Cerebral ventriculoscopy. Johns Hopkins Hosp Bull. 1922;33:189.
4 Berci G, Forde KA. History of endoscopy: what lessons have we learned from the past? Surg Endosc. 2000;14:5-15.
5 Cockett WS, Cockett AT. The Hopkins rod-lens system and the Storz cold light illumination system. Urology. 1998;51:1-2.
6 Fukushima T, Ishijima B, Hirakawa K, et al. Ventriculofiberscope: a new technique for endoscopic diagnosis and operation. Technical note. J Neurosurg. 1973;38:251-256.
7 Fukushima T. Endoscopic biopsy of intraventricular tumors with the use of a ventriculofiberscope. Neurosurgery. 1978;2:110-113.
8 Litynski GS. Endoscopic surgery: the history, the pioneers. World J Surg. 1999;23:745-753.
9 Abdou MS, Cohen AR. Endoscopic treatment of colloid cysts of the third ventricle. Technical note and review of the literature. J Neurosurg. 1998;89:1062-1068.
10 Greenlee JD, Teo C, Ghahreman A, et al. Purely endoscopic resection of colloid cysts. Neurosurgery. 2008;62:51-55.
11 Horn EM, Feiz-Erfan I, Bristol RE, et al. Treatment options for third ventricular colloid cysts: comparison of open microsurgical versus endoscopic resection. Neurosurgery. 2007;60:613-618.
12 Levine NB, Miller MN, Crone KR. Endoscopic resection of colloid cysts: indications, technique, and results during a 13-year period. Minim Invasive Neurosurg. 2007;50:313-317.
13 Lewis AI, Crone KR, Taha J, et al. Surgical resection of third ventricle colloid cysts. Preliminary results comparing transcallosal microsurgery with endoscopy. J Neurosurg. 1994;81:174-178.
14 Schroeder HW, Gaab MR. Endoscopic resection of colloid cysts. Neurosurgery. 2002;51:1441-1444.
15 Teo C. Complete endoscopic removal of colloid cysts: issues of safety and efficacy. Neurosurg Focus. 1999;6(4):e9.
16 Apuzzo ML, Heifetz MD, Weiss MH, et al. Neurosurgical endoscopy using the side-viewing telescope. J Neurosurg. 1977;46:398-400.
17 Gaab MR, Schroeder HW. Neuroendoscopic approach to intraventricular lesions. J Neurosurg. 1998;88:496-505.
18 Macarthur DC, Buxton N, Punt J, et al. The role of neuroendoscopy in the management of brain tumours. Br J Neurosurg. 2002;16:465-470.
19 Tirakotai W, Hellwig D, Bertalanffy H, et al. The role of neuroendoscopy in the management of solid or solid-cystic intra- and periventricular tumours. Childs Nerv Syst. 2007;23:653-658.
20 Ono M, Rhoton ALJr, Peace D, et al. Microsurgical anatomy of the deep venous system of the brain. Neurosurgery. 1984;15:621-657.
21 Yamamoto I, Rhoton ALJr, Peace DA. Microsurgery of the third ventricle: part I. Microsurgical anatomy. Neurosurgery. 1981;8:334-356.
22 Gaab M. Intracranial Endoscopic Neurosurgery. Park Ridge, IL: American Association of Neurological Surgeons; 1998.
23 Abdullah SH, Irie K, Oi S. Rapidly growing giant suprasellar tumor in a high-risk child: treatment strategy and role of neuroendoscopic surgery in slit-like ventricles. Childs Nerv Syst. 2006;22:403-408.
24 Souweidane MM. Endoscopic surgery for intraventricular brain tumors in patients without hydrocephalus. Neurosurgery. 2008;62:1042-1048.
25 Yamamoto M, Oka K, Takasugi S, et al. Flexible neuroendoscopy for percutaneous treatment of intraventricular lesions in the absence of hydrocephalus. Minim Invasive Neurosurg. 1997;40:139-143.
26 Bergsneider M. Complete microsurgical resection of colloid cysts with a dual-port endoscopic technique. Neurosurgery. 2007;60:ONS33-ONS42.
27 Cohen AR. Endoscopic ventricular surgery. Pediatr Neurosurg. 1993;19:127-134.
28 Jallo GI, Morota N, Abbott R. Introduction of a second working portal for neuroendoscopy. A technical note. Pediatr Neurosurg. 1996;24:56-60.
29 Veto F, Horvath Z, Doczi T. Biportal endoscopic management of third ventricle tumors in patients with occlusive hydrocephalus: technical note. Neurosurgery. 1997;40:871-875.
30 Harris AE, Hadjipanayis CG, Lunsford LD, et al. Microsurgical removal of intraventricular lesions using endoscopic visualization and stereotactic guidance. Neurosurgery. 2008;62(suppl 2):622-629.
31 Schroeder HW, Wagner W, Tschiltschke W, et al. Frameless neuronavigation in intracranial endoscopic neurosurgery. J Neurosurg. 2001;94:72-79.
32 Tirakotai W, Bozinov O, Sure U, et al. The evolution of stereotactic guidance in neuroendoscopy. Childs Nerv Syst. 2004;20:790-795.
33 Grunert P, Hopf N, Perneczky A. Frame-based and frameless endoscopic procedures in the third ventricle. Stereotact Funct Neurosurg. 1997;68:80-89.
34 Wagner W, Gaab MR, Schroeder HW, et al. Experiences with cranial neuronavigation in pediatric neurosurgery. Pediatr Neurosurg. 1999;31:231-236.
35 Cappabianca P, Cinalli G, Gangemi M, et al. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008;62(suppl 2):575-597.
36 Ng YT, Rekate HL, Prenger EC, et al. Endoscopic resection of hypothalamic hamartomas for refractory symptomatic epilepsy. Neurology. 2008;70:1543-1548.
37 Yurtseven T, Ersahin Y, Demirtas E, et al. Neuroendoscopic biopsy for intraventricular tumors. Minim Invasive Neurosurg. 2003;46:293-299.
38 Oka K, Yamamoto M, Nonaka T, et al. The significance of artificial cerebrospinal fluid as perfusate and endoneurosurgery. Neurosurgery. 1996;38:733-736.
39 Salvador L, Valero R, Carrero E, et al. Cerebrospinal fluid composition modifications after neuroendoscopic procedures. Minim Invasive Neurosurg. 2007;50:51-55.
40 Souweidane MM, Luther N. Endoscopic resection of solid intraventricular brain tumors. J Neurosurg. 2006;105:271-278.
41 Abtin K, Thompson BG, Walker ML. Basilar artery perforation as a complication of endoscopic third ventriculostomy. Pediatr Neurosurg. 1998;28:35-41.
42 Hamada H, Hayashi N, Kurimoto M, et al. Tension pneumocephalus after a neuroendoscopic procedure—case report. Neurol Med Chir (Tokyo). 2004;44:205-208.
43 Saxena S, Ambesh SP, Saxena HN, et al. Pneumoencephalus and convulsions after ventriculoscopy: a potentially catastrophic complication. J Neurosurg Anesthesiol. 1999;11:200-202.
44 Luther N, Cohen A, Souweidane MM. Hemorrhagic sequelae from intracranial neuroendoscopic procedures for intraventricular tumors. Neurosurg Focus. 2005;19(1):E9.
45 Gore PA, Maan H, Chang S, et al. Normobaric oxygen therapy strategies in the treatment of postcraniotomy pneumocephalus. J Neurosurg. 2008;108:926-929.
46 Fritsch MJ, Doerner L, Kienke S, et al. Hydrocephalus in children with posterior fossa tumors: role of endoscopic third ventriculostomy. J Neurosurg. 2005;103:40-42.
47 Klimo PJr, Goumnerova LC. Endoscopic third ventriculocisternostomy for brainstem tumors. J Neurosurg. 2006;105:271-274.
48 Li KW, Roonprapunt C, Lawson HC, et al. Endoscopic third ventriculostomy for hydrocephalus associated with tectal gliomas. Neurosurg Focus. 2005;18(6A):E2.
49 Oka K, Yamamoto M, Nagasaka S, et al. Endoneurosurgical treatment for hydrocephalus caused by intraventricular tumors. Childs Nerv Syst. 1994;10:162-166.
50 Ray P, Jallo GI, Kim RY, et al. Endoscopic third ventriculostomy for tumor-related hydrocephalus in a pediatric population. Neurosurg Focus. 2005;19(6):E8.
51 Schroeder HW, Oertel J, Gaab MR. Endoscopic treatment of cerebrospinal fluid pathway obstructions. Neurosurgery. 2008;62:1084-1092.
52 Wilson ER, Takei Y, Bikoff WT, et al. Abdominal metastases of primary intracranial yolk sac tumors through ventriculoperitoneal shunts: report of three cases. Neurosurgery. 1979;5:356-364.
53 Javadpour M, Mallucci C. The role of neuroendoscopy in the management of tectal gliomas. Childs Nerv Syst. 2004;20:852-857.
54 Schroeder HW, Gaab MR. Endoscopic aqueductoplasty: technique and results. Neurosurgery. 1999;45:508-515.
55 Hayhurst C, Javadpour M, O’Brien DF, et al. The role of endoscopic third ventriculostomy in the management of hydrocephalus associated with cerebellopontine angle tumours. Acta Neurochir (Wien). 2006;148:1147-1150.
56 Peltier J, Vinchon M, Baroncini M, et al. Bifocal mixed germ-cell tumor with growing teratoma syndrome and metachronous mature metastases: case report. J Neurooncol. 2008;90:111-115.
57 Desai KI, Nadkarni TD, Muzumdar DP, et al. Surgical management of colloid cyst of the third ventricle—a study of 105 cases. Surg Neurol. 2002;57:295-302.
58 Kondziolka D, Lunsford LD. Stereotactic techniques for colloid cysts: roles of aspiration, endoscopy, and microsurgery. Acta Neurochir Suppl. 1994;61:76-78.
59 Mathiesen T, Grane P, Lindquist C, et al. High recurrence rate following aspiration of colloid cysts in the third ventricle. J Neurosurg. 1993;78:748-752.
60 Grondin RT, Hader W, MacRae ME, et al. Endoscopic versus microsurgical resection of third ventricle colloid cysts. Can J Neurol Sci. 2007;34:197-207.
61 Decq P, Le Guerinel C, Brugières P, et al. Endoscopic management of colloid cysts. Neurosurgery. 1998;42:1288-1294.
62 King WA, Ullman JS, Frazee JG, et al. Endoscopic resection of colloid cysts: surgical considerations using the rigid endoscope. Neurosurgery. 1999;44:1103-1109.
63 Bristol R, Nakaji P, Smith K. Endoscopic management of colloid cysts. Oper Tech Neurosurg. 2005;8:176-178.
64 Hellwig D, Bauer BL, Schulte M, et al. Neuroendoscopic treatment for colloid cysts of the third ventricle: the experience of a decade. Neurosurgery. 2003;52:525-533.
65 Longatti P, Godano U, Gangemi M, et al. Cooperative study by the Italian neuroendoscopy group on the treatment of 61 colloid cysts. Childs Nerv Syst. 2006;22:1263-1267.
66 Oi S, Shibata M, Tominaga J, et al. Efficacy of neuroendoscopic procedures in minimally invasive preferential management of pineal region tumors: a prospective study. J Neurosurg. 2000;93:245-253.
67 Apuzzo ML, Chikovani OK, Gott PS, et al. Transcallosal, interforniceal approaches for lesions affecting the third ventricle: surgical considerations and consequences. Neurosurgery. 1982;10:547-554.
68 Carmel PW. Tumours of the third ventricle. Acta Neurochir (Wien). 1985;75:136-146.
69 Lozier AP, Bruce JN. Surgical approaches to posterior third ventricular tumors. Neurosurg Clin N Am. 2003;14:527-545.
70 Rhoton ALJr, Yamamoto I, Peace DA. Microsurgery of the third ventricle: Part 2. Operative approaches. Neurosurgery. 1981;8:357-373.
71 Stein BM. Supracerebellar-infratentorial approach to pineal tumors. Surg Neurol. 1979;11:331-337.
72 Wara WM, Jenkin RD, Evans A, et al. Tumors of the pineal and suprasellar region: Children’s Cancer Study Group treatment results 1960-1975: a report from Children’s Cancer Study Group. Cancer. 1979;43:698-701.
73 Buckner JC, Peethambaram PP, Smithson WA, et al. Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol. 1999;17:933-940.
74 Diez B, Balmaceda C, Matsutani M, et al. Germ cell tumors of the CNS in children: recent advances in therapy. Childs Nerv Syst. 1999;15:578-585.
75 Patel SR, Buckner JC, Smithson WA, et al. Cisplatin-based chemotherapy in primary central nervous system germ cell tumors. J Neurooncol. 1992;12:47-52.
76 Sano K. Diagnosis and treatment of tumours in the pineal region. Acta Neurochir (Wien). 1976;34:153-157.
77 Kim A, Ji L, Balmaceda C, et al. The prognostic value of tumor markers in newly diagnosed patients with primary central nervous system germ cell tumors. Pediatr Blood Cancer. 2008;51:768-773.
78 Pople IK, Athanasiou TC, Sandeman DR, et al. The role of endoscopic biopsy and third ventriculostomy in the management of pineal region tumours. Br J Neurosurg. 2001;15:305-311.
79 O’Brien DF, Hayhurst C, Pizer B, et al. Outcomes in patients undergoing single-trajectory endoscopic third ventriculostomy and endoscopic biopsy for midline tumors presenting with obstructive hydrocephalus. J Neurosurg. 2006;105:219-226.
80 Chernov MF, Kamikawa S, Yamane F, et al. Neurofiberscopic biopsy of tumors of the pineal region and posterior third ventricle: indications, technique, complications, and results. Neurosurgery. 2006;59:267-277.
81 Gangemi M, Maiuri F, Colella G, et al. Endoscopic surgery for pineal region tumors. Minim Invasive Neurosurg. 2001;44:70-73.
82 Robinson S, Cohen AR. The role of neuroendoscopy in the treatment of pineal region tumors. Surg Neurol. 1997;48:360-365.
83 Gonzales L, Nakaji P, Rekate H. Endoscopic approach to the pineal region. Oper Tech Neurosurg. 2005;8:172-175.
84 Teo C. Application of endoscopy to the surgical management of craniopharyngiomas. Childs Nerv Syst. 2005;21:696-700.
85 Gore PA, Nakaji P, Deshmukh V, et al. Synchronous endoscopy and microsurgery: a novel strategy to approach complex ventricular lesions. Report of three cases. J Neurosurg. 2006;105:485-489.
86 Greenfield JP, Leng LZ, Chaudhry U, et al. Combined simultaneous endoscopic transsphenoidal and endoscopic transventricular resection of a giant pituitary macroadenoma. Minim Invasive Neurosurg. 2008;51:306-309.
87 Cao ZW, Shi KS, Jin H, et al. [Endoscope-assisted supraorbital keyhole approach for excision of suprasellar region tumor.]. Zhonghua Wai Ke Za Zhi. 2003;41:414-416.
88 Chang SW, Gore PA, Nakaji P, et al. Juvenile intradural chordoma: case report. Neurosurgery. 2008;62:E525-E526.
89 Fratzoglou M, Leite dos Santos AR, Gawish I, et al. Endoscope-assisted microsurgery for tumors of the septum pellucidum: surgical considerations and benefits of the method in the treatment of four serial cases. Neurosurg Rev. 2005;28:39-43.
90 Gerganov VM, Romansky KV, Bussarsky VA, et al. Endoscope-assisted microsurgery of large vestibular schwannomas. Minim Invasive Neurosurg. 2005;48:39-43.
91 Schroeder HW, Oertel J, Gaab MR. Endoscope-assisted microsurgical resection of epidermoid tumors of the cerebellopontine angle. J Neurosurg. 2004;101:227-232.
92 Fries G, Perneczky A. Endoscope-assisted brain surgery: part 2—analysis of 380 procedures. Neurosurgery. 1998;42:226-231.
93 Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery. 1998;42:219-224.
94 Day JD. Surgical approaches to suprasellar and parasellar tumors. Neurosurg Clin N Am. 2003;14:109-122.
95 Menovsky T, Grotenhuis JA, de Vries J, et al. Endoscope-assisted supraorbital craniotomy for lesions of the interpeduncular fossa. Neurosurgery. 1999;44:106-110.
96 Reisch R, Perneczky A. Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery. 2005;57:242-255.
97 Teo C, Nakaji P. Application of endoscopy to the resection of intra-axial tumors. Oper Tech Neurosurg. 2005;8:179-185.
98 Akai T, Shiraga S, Sasagawa Y, et al. Intra-parenchymal tumor biopsy using neuroendoscopy with navigation. Minim Invasive Neurosurg. 2008;51:83-86.
99 Di X. Multiple brain tumor nodule resections under direct visualization of a neuronavigated endoscope. Minim Invasive Neurosurg. 2007;50:227-232.
100 Buiter C. Endoscopy of the Upper Airways. Amsterdam: Excerpta Medica; 1976. 41-49
101 Cappabianca P, de Divitiis E. Endoscopy and transsphenoidal surgery. Neurosurgery. 2004;54:1043-1048.
102 Cappabianca P, Alfieri A, Colao A, et al. Endoscopic endonasal transsphenoidal surgery in recurrent and residual pituitary adenomas: technical note. Minim Invasive Neurosurg. 2000;43:38-43.
103 Cappabianca P, Alfieri A, de Divitiis E. Endoscopic endonasal transsphenoidal approach to the sella: towards functional endoscopic pituitary surgery (FEPS). Minim Invasive Neurosurg. 1998;41:66-73.
104 Carrau RL, Jho HD, Ko Y. Transnasal-transsphenoidal endoscopic surgery of the pituitary gland. Laryngoscope. 1996;106:914-918.
105 Jho HD, Carrau RL. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87:44-51.
106 Al-Mefty O, Kadri PA, Hasan DM, et al. Anterior clivectomy: surgical technique and clinical applications. J Neurosurg. 2008;109:783-793.
107 de Divitiis E, Cappabianca P. Microscopic and endoscopic transsphenoidal surgery. Neurosurgery. 2002;51:1527-1529.
108 de Divitiis E, Cappabianca P, Cavallo LM. Endoscopic transsphenoidal approach: adaptability of the procedure to different sellar lesions. Neurosurgery. 2002;51:699-705.
109 Jho HD, Carrau RL, McLaughlin MR, et al. Endoscopic transsphenoidal resection of a large chordoma in the posterior fossa. Acta Neurochir (Wien). 1997;139:343-347.
110 Kassam A, Thomas AJ, Snyderman C, et al. Fully endoscopic expanded endonasal approach treating skull base lesions in pediatric patients. J Neurosurg. 2007;106:75-86.
111 Rudnik A, Zawadzki T, Wojtacha M, et al. Endoscopic transnasal transsphenoidal treatment of pathology of the sellar region. Minim Invasive Neurosurg. 2005;48:101-107.
112 Sciarretta V, Pasquini E, Frank G, et al. Endoscopic treatment of benign tumors of the nose and paranasal sinuses: a report of 33 cases. Am J Rhinol. 2006;20:64-71.
113 de Divitiis E, Cappabianca P, Gangemi M, et al. The role of the endoscopic transsphenoidal approach in pediatric neurosurgery. Childs Nerv Syst. 2000;16:692-696.
114 Kaptain GJ, Vincent DA, Sheehan JP, et al. Transsphenoidal approaches for the extracapsular resection of midline suprasellar and anterior cranial base lesions. Neurosurgery. 2001;49:94-100.
115 Locatelli D, Castelnuovo P, Santi L, et al. Endoscopic approaches to the cranial base: perspectives and realities. Childs Nerv Syst. 2000;16:686-691.
116 de Divitiis E, Cavallo LM, Esposito F, et al. Extended endoscopic transsphenoidal approach for tuberculum sellae meningiomas. Neurosurgery. 2008;62:1192-1201.
117 de Divitiis E, Cappabianca P, Cavallo LM, et al. Extended endoscopic transsphenoidal approach for extrasellar craniopharyngiomas. Neurosurgery. 2007;61:219-227.
118 Gardner PA, Kassam AB, Snyderman CH, et al. Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: a case series. J Neurosurg. 2008;109:6-16.
119 Halves E, Bushe KA. Transsphenoidal operation on craniopharyngiomas with extrasellar extensions. The advantage of the operating endoscope [proceedings]. Acta Neurochir Suppl. 1979;28:362.
120 Kassam AB, Prevedello DM, Thomas A, et al. Endoscopic endonasal pituitary transposition for a transdorsum sellae approach to the interpeduncular cistern. Neurosurgery. 2008;62:57-72.
121 Cavallo LM, Messina A, Esposito F, et al. Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. J Neurosurg. 2007;107:713-720.
122 Germani RM, Vivero R, Herzallah IR, et al. Endoscopic reconstruction of large anterior skull base defects using acellular dermal allograft. Am J Rhinol. 2007;21:615-618.
123 Leng LZ, Brown S, Anand VK, et al. “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery. 2008;62:ONSE342-ONSE343.
124 Fatemi N, Dusick JR, de Paiva Neto MA, et al. The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: a 10-year experience. Neurosurgery. 2008;63:244-256.
125 Senior BA, Ebert CS, Bednarski KK, et al. Minimally invasive pituitary surgery. Laryngoscope. 2008;118:1842-1855.
126 Stamm AC, Vellutini E, Harvey RJ, et al. Endoscopic transnasal craniotomy and the resection of craniopharyngioma. Laryngoscope. 2008;118:1142-1148.