Chapter 29 Endoscopic Approach to Intraventricular Brain Tumors
Endoscopic surgery for intraventricular brain tumors is a logical application of endoscopic technology. Because of the central and deep location of intraventricular brain tumors, conventional neurosurgical approaches have a relative increase in potential morbidity. Auspiciously, the location of intraventricular tumors being within a cerebrospinal fluid (CSF) compartment affords excellent light and image transmission. The fact that most intraventricular tumors cause hydrocephalus makes endoscopic surgery particularly attractive since simultaneous procedures can be employed both for CSF diversion and tumor management. In addition, the inherent benefits of minimally invasive techniques including reduced surgical time, improved cosmetic results, shortened hospital stay, and reduced cost also factor into the appeal of neurosurgical endoscopy for managing intraventricular tumors.1 Five commonly employed endoscopic procedures are highlighted including endoscopic fenestration, endoscopic tumor biopsy, simultaneous tumor biopsy with endoscopic third ventriculostomy (ETV), endoscopic removal of solid tumors and endoscopic removal of colloid cysts.
Patient Selection
Patient selection is critical in optimizing the desired surgical goal, avoiding unnecessary procedures, and minimizing surgical morbidity. The intended surgical goal must be carefully established prior to surgery. Many patients that can undergo endoscopic surgery may not be logical candidates since they will ultimately require conventional surgical tumor removal or no surgery. Examples that serve to highlight this point are the patient with a large intraventricular tumor of the ventricular atrium or the patient with a biochemically proven malignant germ cell tumor. In selecting patients, the less experienced surgeon should begin with less demanding cases (septal fenestration and endoscopic third ventriculostomy) and eventually incorporate more complex cases (colloid cyst and solid tumor resection). Although endoscopic surgery can be accomplished in patients with normal sized ventricles, concomitant hydrocephalus affords easier ventricular cannulation and intraventricular navigation.2
Equipment
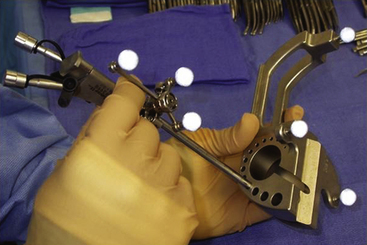
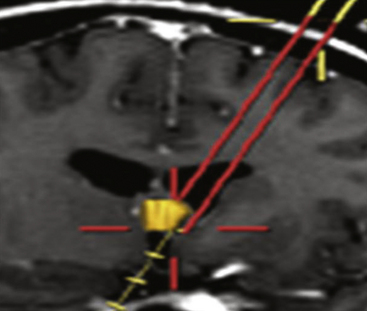
Endoscopes vary in the type of optics (fiberoptic or solid lens), diameter of the scope, and the dimension and number of working portals. While the fiberoptic systems are appealing for their light weight and reduced cost, the greater image resolution with a sold lens system is preferred by the author for most endoscopic tumor surgery. For all procedures featured in this article, scopes should have at the very least one working channel, one irrigation port, and a dedicated egress channel. Navigational guidance is strongly encouraged not so much for ventricular cannulation, but for selecting an optimal trajectory (Figs. 29-1 and 29-2). This simple integration greatly reduces the tendency to torque once in the ventricular compartment thus reducing potential hemorrhage and neurological injury. Endoscopic courses are a useful means for becoming familiar with the requisite instrumentation and surgical techniques.
Endoscopic Tumor Procedures
Endoscopic Fenestration
Septal fenestration and tumor cyst fenestration are two of the simplest endoscopic procedures owing the relative avascular nature of these membranes. Fenestration of the septum pellucidum should be considered in any patient in which a tumor mass is situated in the anterior third ventricle or within the lateral ventricle at the foramen of Monro resulting in compartmentalized hydrocephalus (Fig. 29-3). Shunt burden can thus be reduced in the former situation or eliminated in the latter by simple endoscopic septal fenestration. Endoscopic fenestration of the septum pellucidum generally is performed via an entry site that lies more lateral than the conventional coronal burr hole. The entry site is thus positioned at least 4 cm from the midsagittal plane. It is recommended that the site of septal fenestration be positioned between the larger tributaries of the septal veins and as superior as possible from the fornix. Generous fenestrations are made with cautery and confirmation of effective communication is established with identification of contralateral ventricular landmarks including the choroid plexus and ependymal veins.
Tumor cyst fenestration is an appealing therapeutic option when a patient’s symptoms can be relieved by cyst decompression and when aggressive tumor resection may be avoided (Fig. 29-4). Craniopharyngiomas, hypothalamic/chiasmatic astrocytomas, and suprasellar germ cell tumors are examples of such tumors.3 Transventricular endoscopic cyst decompression is a minimally invasive method for temporarily or permanently alleviating obstructive hydrocephalus or visual loss. For most cystic tumors causing obstructive hydrocephalus at the level of the third ventricle, a standard coronal approach is an ideal trajectory. The transcavum interforniceal endoscopic approach4 to the third ventricle is a further refinement of the technique for biopsy or fenestration of lesions within the third ventricle in those patients a large cavum vergae.
Tumor Biopsy
Endoscopic biopsy is a well-established method for sampling intraventricular brain tumors.5–7 The procedure should always be considered in situations in which surgical tumor removal may not be necessary or when the diagnosis would significantly alter the therapeutic approach. Primary examples of these situations include marker-negative germ cell tumors, Langerhans cell histiocytosis, and infiltrative hypothalamic gliomas. Candidates should have overt intraventricular extension of their tumor mass rather than a lesion that is entirely subependymal in location. The diagnostic yield is high and the risk is low.8 In the authors’ current series of 65 patients who have undergone endoscopic tumor biopsy the diagnostic yield was 98%. To maintain diagnostic accuracy it is imperative to avoid cauterizing the tumor prior to sampling. The samples are small and histologic interpretation can be challenging without superimposed artifact from cautery. If bleeding is encountered, continuous irrigation through the endoscope or an external catheter is recommended until the efflux clears.
Simultaneous Tumor Biopsy and Endoscopic Third Ventriculostomy
The prominence of pineal region tumors in children coupled with the high frequency of tumors that may not necessitate aggressive surgical resection converges nicely with endoscopic applications. Notably, primary central nervous system germ cell tumors (CNS GCT), both pure germinomas and nongerminomatous germ cell tumors, can be effectively treated without radical resection. Thus, children who present with noncommunicating hydrocephalus with a pineal region tumor should always be considered for primary endoscopic management by way of ETV and tumor biopsy. Serum biochemical analysis for alfafetoprotein (AFP) and human chorionic gonadotropin (HCG) should always precede endoscopic biopsy since marker-positive GCTs should be initially managed with neoadjuvant chemotherapy.9–12 When performing simultaneous ETV and tumor biopsy, the CSF diversion should always be performed first. This recommendation is based on the fact that the patient’s hydrocephalus is the more emergent clinical condition requiring treatment. Further, when tumor biopsy is performed some intraventricular hemorrhage is expected that may obscure vision. Given that the trajectory for ETV and pineal region tumor biopsy are different one must select between using one or two entry sites for performing these simultaneous procedures. The typical entry site for performing ETV is at the coronal suture 2 cm from midline while that for accomplishing a pineal region tumor biopsy is 4 to 6 cm precoronal. While two separate entry sites can be used one single entry site that is midway between these has been shown to be successful. It is the authors’ preference to use a single precoronal approach that lies between the ideal entry sites for either separate procedure. This site is routinely dictated based upon stereotactic guidance. With the use of a 30-degree angled endoscope, it is rare that both procedures will not be possible simultaneously.
Solid Tumor Resection
Solid tumor removal, although a logical application of endoscopic techniques, is somewhat limited due to the inadequacy of compatible instrumentation and the small caliber of current endoscopic portals. Thus far, most descriptions for tumor removal have focused on the colloid cyst. The colloid cyst, unlike solid tumors, wonderfully lends itself to endoscopic removal given the primarily cystic nature of that mass. The success of endoscopic tumor removal is dependent upon the tumor characteristics including size, density, and vascularity. Tumors larger than 2 cm, those that have appreciable calcification on computed tomography (CT), and those that have significant subependymal infiltration are not currently amenable to endoscopic removal.2,13 The resection of solid tumors is principally achieved through the use of aspiration with a variable, self-regulated suction catheter alternating with generous bipolar diathermy. It is critical to note that aspiration is only applied once the catheter tip is firmly and completely imbedded within the tumor tissue so as to avoid rapid evacuation of CSF. The feasibility of endoscopic removal of solid tumors within the ventricular system is expected to improve with the advent of compatible instrumentation designed for tissue ablation such as an ultrasonic aspirator.
Colloid Cyst Extirpation
Patient Selection
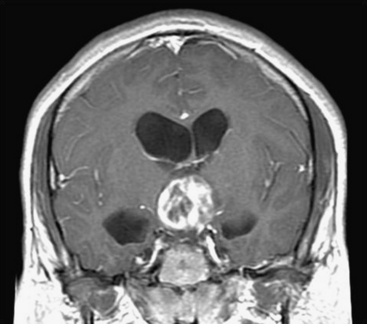
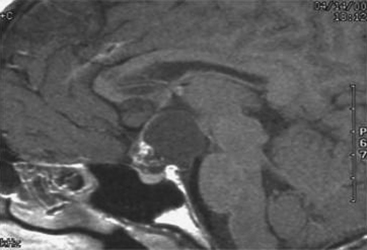
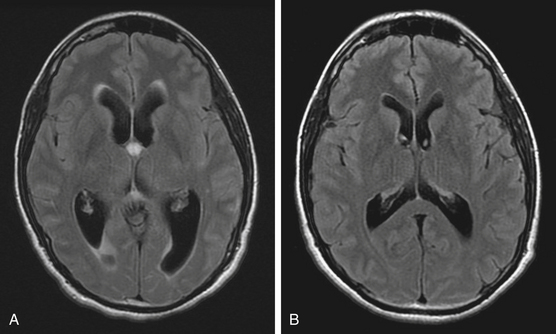
The colloid cyst of the third ventricle is an ideal tumor for endoscopic removal (Fig. 29-5A and B). This appeal is principally governed by the cystic nature of the mass. The deep central location within the ventricular compartment along with the associated complexity of standard microsurgical removal further enhances the desire for endoscopic management. Careful patient selection is critical given the frequency with which asymptomatic patients are diagnosed. Rationales for surgical intervention appropriately include symptoms of raised intracranial pressure, ventriculomegaly in the absence of symptoms, and radiographic evidence of progression (ventricular size or tumor mass). Less-defined indicators include prophylaxis against clinical progression or sudden death and young age at the time of diagnosis. The ability to predict clinical progression is poorly defined. The natural history is not clear but is estimated that clinical or radiographic progression occurs in about 8% of patients over 10 years. The expectation of progression during a patient’s lifetime is thus greater for younger patients. Variables that may precede clinical deterioration are ventriculomegaly, chronic headache, and cyst size (>1 cm diameter). Offering surgery to avoid the possibility of rapid deterioration must be balanced by a true estimation of operative risk to the patient, bearing in mind that many of the patients will have normal sized ventricles.
Surgical Technique
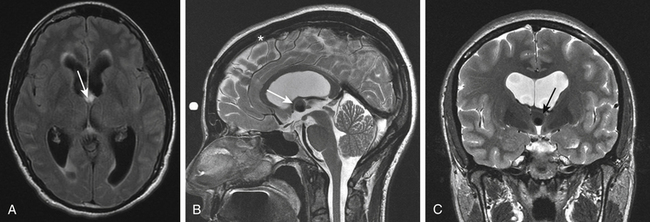
Because of variability in each patient’s anatomy, surgical planning is critical in optimizing the surgical goal and reducing potential morbidity. Integrated stereotatic navigation is always used for surgical planning and optimizing ventricular cannulation (Fig. 29-6A through C). Laterality should be determined based on a preoperative MRI with emphasis on the relative size of the frontal horns of the lateral ventricles and the dimensions of the foraminae of Monro. Because the intent is to work below the ipsilateral column of the fornix, an entry site is used that is far forward of the coronal suture. In a sagittal dimension, the ideal trajectory passes just above the floor of the anterior horn, through the foramen of Monro, and below the roof of the third ventricle. In an axial and coronal plane, a trajectory is selected that passes between the head of the caudate and the column of the fornix into the foramen of Monro. The entry site that is selected with stereotactic guidance is marked on the skin surface prior to the surgical prep. Regardless of the entry site, a curvilinear incision is planned on the hair line with a variable length depending upon the anterior location of the planned burr hole. A longer incision is needed to allow more anterior retraction of the scalp. In patients with a receding hairline the incision is alternatively placed within a forehead crease.
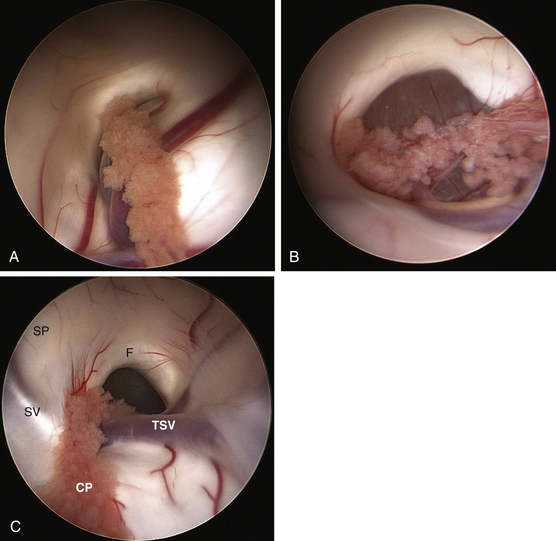
Once the frontal horn of the lateral ventricle is entered, the anatomical landmarks of the frontal horn are identified. Using a 30-degree angled lens the endoscopic is then rotated to offer a superiorly directed view toward the roof of the third ventricle; the site of attachment of the colloid cyst. The endoscope is then navigated toward the foramen of Monro where typically the wall of the colloid cyst is in clear sight obstructing the foramen of Monro (Fig. 29-7A and B). Generous bipolar coagulation of the choroid plexus is used to gain better visualization of the lateral cyst surface. Bipolar forceps are utilized to bluntly dissect the cyst wall away from the walls of the third ventricle. This coagulation device without current is used to push the cyst wall away from ependymal surfaces. When sufficient space exists between the cyst and the walls of the third ventricle power is then switched on to coagulate the cyst surface. Perforation of the cyst wall by either sharp dissection or electrocautery is utilized followed by aspiration of the cyst contents. A graduated 6-French endotracheal suction catheter that has had the distal fenestrations removed is preferred. This clear cannula allows direct visualization of the contents being suctioned. This ability is very helpful in gauging the strength of suction applied and in discontinuing aspiration if choroid plexus gets inadvertently aspirated. It is critical that aspiration only be applied once the tip of the device is placed within the cyst wall, thus avoiding rapid evacuation of CSF from the ventricular compartment. Utilizing suction aspiration the contents of the cyst can usually be fully evacuated. The viscosity of some contents may require repeated clearing of the cannula due to frequent clogging. With partial evacuation the cyst can commonly be drawn into the foramen with grasping forceps. This maneuver positions the lesion for continued aspiration and further coagulation. The superior aspect of the cyst can be dissected away from the roof of the third ventricle by using a rotary motion with grasping forceps in effect pulling the cyst in an inferior direction toward the floor of the ventricle. When visualized, adherent potions of choroid plexus should be coagulated and sharply divided.
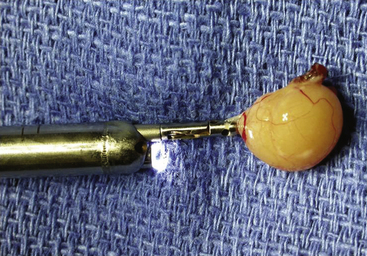
Repeatedly using these techniques one of two situations usually occur. First, the cyst may freely separate from the confines of the third ventricular roof. If this situation occurs then the cyst should be removed by extracting the entire endoscope (Fig. 29-8). Because of the difference in size between the cyst and the working portal (1 to 2 mm) any attempt to extract the cyst through the sheath runs the risk of dislodging the cyst into the ventricular compartment. The second frequent scenario that occurs during endoscopic colloid cyst removal is that the cyst is entirely evacuated of contents leaving only adherent membrane. That membrane is then generously coagulated followed by sharp dissection. Some portions of the cyst wall may not be amenable to further removal due to adherence of venous structures. Any membrane remnants should be generously coagulated. Once the removal is complete, inspection of the third ventricle for residual clot is performed. If recognized small hematomas within the third ventricle are removed using aspiration applied directly to the clot. The placement of an externalized ventricular drain is advocated on an individual basis depending upon the degree of intraventricular hemorrhage. The overwhelming numbers of drains are discontinued the day after surgery based on intracranial pressures within a normal range and postoperative imaging failing to indicate any appreciable hematoma within the third ventricle.
Cappabianca P., Cinalli G., Gangemi M., et al. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008;62(suppl 2):575-597. discussion 597–598
Delitala A., Brunori A., Chiappetta F. Purely neuroendoscopic transventricular management of cystic craniopharyngiomas. Childs Nerv Syst. 2004;20:858-862.
Depreitere B., Dasi N., Rutka J., et al. Endoscopic biopsy for intraventricular tumors in children. J Neurosurg. 2007;106:340-346.
Ellenbogen R.G., Moores L.E. Endoscopic management of a pineal and suprasellar germinoma with associated hydrocephalus: technical case report. Minim Invasive Neurosurg. 1997;40:13-15. discussion 16
Luther N., Cohen A., Souweidane M.M. Hemorrhagic sequelae from intracranial neuroendoscopic procedures for intraventricular tumors. Neurosurg Focus. 2005;19:E9.
Luther N., Edgar M.A., Dunkel I.J., Souweidane M.M. Correlation of endoscopic biopsy with tumor marker status in primary intracranial germ cell tumors. J Neuro-Oncol. 2006;79:45-50.
Pople I.K., Athanasiou T.C., Sandeman D.R., Coakham H.B. The role of endoscopic biopsy and third ventriculostomy in the management of pineal region tumours. Br J Neurosurg. 2001;15:305-311.
Schwartz T.H., Ho B., Prestagiacomo C.J., et al. Ventricular volume following third ventriculostomy. J Neurosurg. 1999;91:20-25.
Shono T., Natori Y., Morioka T., et al. Results of a long-term follow-up after neuroendoscopic biopsy procedure and third ventriculostomy in patients with intracranial germinomas. J Neurosurg. 2007;107:193-198.
Souweidane M.M. Endoscopic management of pediatric brain tumors. Neurosurg Focus. 2005;18:E1.
Souweidane M.M. Endoscopic surgery for intraventricular brain tumors in patients without hydrocephalus. Neurosurgery. 2005;57:312-318. discussion 312-318
Souweidane M.M., Luther N. Endoscopic resection of solid intraventricular brain tumors. J Neurosurg. 2006;105:271-278.
Souweidane M.M., Sandberg D.I., Bilsky M.H., Gutin P.H. Endoscopic biopsy for tumors of the third ventricle. Pediatr Neurosurg. 2000;33:132-137.
Souweidane M.M., Hoffman C.E., Schwartz T.H. Transcavum interforniceal endoscopic surgery of the third ventricle. J Neurosurg Pediatr. 2008 Oct;2(4):231-236. PubMed PMID: 18831654
1. Cappabianca P., Cinalli G., Gangemi M., et al. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008;62(Suppl 2):575-597. discussion 597-598
2. Souweidane M.M. Endoscopic surgery for intraventricular brain tumors in patients without hydrocephalus. Neurosurgery. 2005;57:312-318. discussion 312-318
3. Delitala A., Brunori A., Chiappetta F. Purely neuroendoscopic transventricular management of cystic craniopharyngiomas. Childs Nerv Syst. 2004;20:858-862.
4. Souweidane M.M., Hoffman C.E., Schwartz T.H. Transcavum interforniceal endoscopic surgery of the third ventricle. J Neurosurg Pediatr. 2008;2(4):231-236.
5. Depreitere B., Dasi N., Rutka J., et al. Endoscopic biopsy for intraventricular tumors in children. J Neurosurg. 2007;106:340-346.
6. Souweidane M.M. Endoscopic management of pediatric brain tumors. Neurosurg Focus. 2005;18:E1.
7. Souweidane M.M., Sandberg D.I., Bilsky M.H., et al. Endoscopic biopsy for tumors of the third ventricle. Pediatr Neurosurg. 2000;33:132-137.
8. Luther N., Cohen A., Souweidane M.M. Hemorrhagic sequelae from intracranial neuroendoscopic procedures for intraventricular tumors. Neurosurg Focus. 2005;19:E9.
9. Ellenbogen R.G., Moores L.E. Endoscopic management of a pineal and suprasellar germinoma with associated hydrocephalus: technical case report. Minim Invasive Neurosurg. 1997;40:13-15. discussion 16
10. Luther N., Edgar M.A., Dunkel I.J., et al. Correlation of endoscopic biopsy with tumor marker status in primary intracranial germ cell tumors. J Neuro-Oncol. 2006;79:45-50.
11. Pople I.K., Athanasiou T.C., Sandeman D.R., et al. The role of endoscopic biopsy and third ventriculostomy in the management of pineal region tumours. Br J Neurosurg. 2001;15:305-311.
12. Shono T., Natori Y., Morioka T., et al. Results of a long-term follow-up after neuroendoscopic biopsy procedure and third ventriculostomy in patients with intracranial germinomas. J Neurosurg. 2007;107:193-198.
13. Souweidane M.M., Luther N. Endoscopic resection of solid intraventricular brain tumors. J Neurosurg. 2006;105:271-278.