CHAPTER 70 Endoscopic and Radiologic Treatment of Biliary Disease
IMAGING OF THE BILIARY TRACT
ULTRASONOGRAPHY
Noninvasive imaging of the biliary tree frequently begins with transabdominal ultrasound, which provides a global picture of the liver and is nearly universally available. There is no radiation exposure, and contrast agents are not required. Intrahepatic ductal dilatation can be visualized easily and the size of the bile duct can be documented. Ultrasound also provides imaging of the gallbladder and detects gallstones. For detection of choledocholithiasis, ultrasound has a high specificity, but the sensitivity does not exceed 68% and is often lower than 50%.1,2 The sensitivity decreases if the stones are small and the bile ducts are not dilated. Ultrasound is highly accurate (78% to 98%) for detecting extrahepatic biliary obstruction.2 When used in conjunction with the clinical evaluation, ultrasound allows differentiation between liver parenchymal disease and extrahepatic biliary obstruction with a reasonable sensitivity and high specificity.2 Ultrasound is less accurate, however, at defining the level and cause of obstruction, with accuracy rates ranging from 27% to 95% and 23% to 88%, respectively.2 In addition, ultrasound is limited in the ability to distinguish malignant from benign causes of obstruction.2
MAGNETIC RESONANCE CHOLANGIOPANCREATOGRAPHY AND MULTIDETECTOR COMPUTED TOMOGRAPHY CHOLANGIOGRAPHY
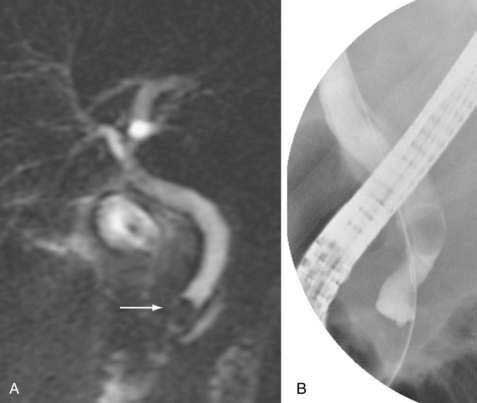
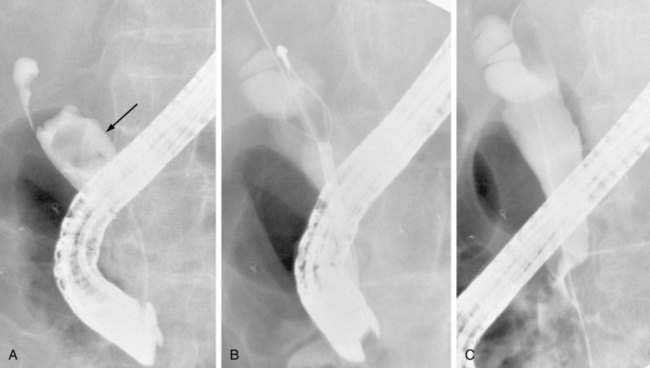
Magnetic resonance cholangiopancreatography (MRCP) is a magnetic resonance imaging (MRI) study (and thus noninvasive) that is dependent on the high T2-signal characteristics of bile. It does not require administration of oral or intravenous contrast material. For the detection of choledocholithiasis, MRCP has a sensitivity ranging from 81% to 100%, a specificity ranging from 96% to 100%, and high overall diagnostic accuracy (Fig. 70-1).3 In addition, MRCP is highly accurate in demonstrating the presence of benign and malignant strictures4 and allows a thorough evaluation of the intrahepatic bile ducts. In patients suspected of having post-liver transplant biliary complications, intravenous administration of mangafodipir trisodium (Teslascan, Amersham Health, Princeton, NJ) may be used. This agent is excreted primarily in the bile and may improve imaging sensitivity for post-liver transplant biliary leaks and strictures.5 An MRI can be performed as well with an intravenous contrast agent, such as gadodiamide (Omniscan, GE Healthcare, United Kingdom) or gadopentetate dimeglumine (Magnevist, Bayer Healthcare, Leverkusen, Germany or Multihance, Bracco, Princeton, NJ), to detect and characterize mass lesions in the liver, porta hepatis, or pancreas. Contraindications to MRI include a cardiac pacemaker, automatic implantable cardioverter defibrillator, and some types of cerebral aneurysm clips. A particular concern about gadolinium-based intravenous contrast agents is that they may precipitate nephrogenic systemic fibrosis, a rare scleroderma-like disease manifested by hardening of the skin and fibrotic changes that affect multiple organs. The cause remains unclear, but reports suggest that patients with preexisting kidney disease (renal failure) are at greatest risk.6,7
Multidetector computed tomography cholangiography (MDCT) with multiplanar reformation is a computed tomography (CT)-based imaging study. MDCT is a combination of rapid volume acquisition and thin-slice imaging. Water is used as an oral contrast agent for the biliary tree, and intravenous iodinated contrast is also administered. Images acquired in the axial plane can be reconstructed sagittally or coronally and reformatted three dimensionally. The intravenous contrast dye is not excreted in bile but enhances adjacent surrounding visceral structures such as the liver, pancreas, and other soft tissues. Bile ducts thus appear as low attenuation structures that are best visualized if dilated. The sensitivity and specificity of MDCT for bile duct strictures have been reported to be 85.7% and 100%, respectively.8 MDCT also has a high sensitivity and specificity for the detection of bile duct stones.9
PERCUTANEOUS TRANSHEPATIC CHOLANGIOGRAPHY
Percutaneous transhepatic cholangiography (THC) is an invasive diagnostic test and can be therapeutic if necessary. In light of the array of noninvasive imaging studies, percutaneous THC is rarely performed purely for diagnostic purposes. Subsequent decompression of biliary obstruction, removal of a stone, balloon dilation of a stricture, and placement of a stent for a stricture can be performed. The procedure is generally reserved for patients for whom ERCP is precluded because of difficult endoscopic access across a biliary-enteric (Roux-en-Y) anastomosis, gastric bypass, or extrahepatic biliary stricture that cannot be traversed endoscopically. Serious procedure-related complications such as bleeding, sepsis, or bile leakage occur in approximately 2% to 4% of cases.10 The procedure generally can be performed with monitored moderate (“conscious”) sedation.11 Broad-spectrum intravenous antibiotics are usually administered prophylactically.
TECHNIQUE
Review of CT and MR imaging of the liver prior to percutaneous THC can help determine the best approach (i.e., from the right or left side) and the location of the dominant dilated ducts and help avoid traversing adjacent structures, such as the colon, unintentionally. Dilated bile ducts on the left side may be easily accessible with a minimal number of needle passes with use of ultrasound guidance from a subxiphoid approach.12 A standard right-sided approach is used most frequently, however, and is performed from an intracostal approach, usually via the mid-axillary region below the 10th intercostal space. Higher punctures increase the risk of pneumothorax or biliary pleural effusion.
From either side, the procedure is initiated by advancing a 22-gauge needle under fluoroscopic guidance centrally toward the liver hilum and gently injecting contrast as the needle is withdrawn slowly. The initial use of such a small needle reduces hepatic trauma as well as the likelihood of bleeding despite the potential need for multiple needle passes to cannulate a bile duct, particularly when the bile ducts are not dilated. Ultrasound guidance and CT guidance can be used to access nondilated bile ducts.13,14
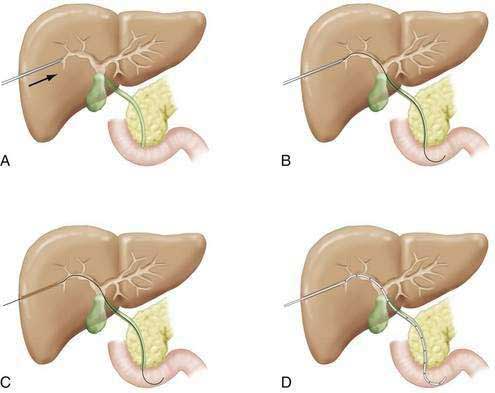
When a bile duct is cannulated, a diagnostic cholangiogram can be performed. Isolated ducts, because of strictures or stones that do not communicate with the rest of the biliary tree, may need to be opacified via additional needle passes. If the procedure is only diagnostic, and biliary obstruction is not evident, the needle is simply withdrawn. If the biliary system is obstructed, however, serious consideration should be given to traversing the obstruction and leaving a decompressive “external-internal” tube in place; abandoning an obstructed biliary system may lead to bile leakage from the puncture site. The risk of hepatic arterial injury is reduced by using a peripheral intrahepatic bile duct for final access. If the duct cannulated initially is too central (the larger branches of the hepatic artery tend to be more central), a more peripheral duct should be chosen for access into the biliary tree. Frequently, use of a second needle to puncture a more peripheral duct is required, and the initial needle is used to opacify and visualize this new and safer access duct. A 0.018-inch “micro” wire is then advanced via the needle into the biliary tree and the access system “upsized” by the passage of catheters of increasing diameter over the wire. When access is gained, the obstruction can be traversed and an external-internal biliary tube can be placed (Fig. 70-2). These tubes provide drainage holes positioned above the level of obstruction; the distal pigtail is configured within the small intestine. The size of the tube usually ranges from 8 to 12 French. A larger tube may yield better decompression, but care must be taken not to place a tube in which the size may actually obstruct drainage of smaller ducts, particularly in the setting of primary sclerosing cholangitis (PSC), in which many of the obstructed ducts are not dilated. If the obstruction cannot be traversed during the initial attempt, a drainage catheter can be left proximal to the obstruction in the biliary tree (external drainage), and subsequent attempts can be made via this access after several days of drainage. This delay often allows inflammation to decrease and increases the likelihood of subsequent internalization of a catheter. Generally, the external-internal drainage tube is left to external drainage until fever or blood in the biliary tree resolves. Capping of the external end of the tube to permit internal drainage only decreases biliary fluid losses, which can be more than 1 liter per day, and prevents associated dehydration or electrolyte abnormalities. Bile samples obtained during the initial procedure can be sent for culture or cytology.
Following initial biliary decompression, further intervention should be avoided until fever and sepsis have resolved. Patients need to be monitored closely for the first 24 to 48 hours following the procedure. Brisk bleeding around the catheter site, through the catheter itself, or from the gastrointestinal tract suggests the possibility of hepatic arterial injury.15 Presentation of a hepatic artery pseudoaneurysm can be delayed, sometimes for a week or two after the initial procedure. If bleeding persists, the hemoglobin level drops substantially or the patient becomes hemodynamically unstable, hepatic angiography should be considered, and, if an injured arterial branch is demonstrated, embolization should be performed. A small amount of blood in the biliary tube or bile ducts following the original procedure, or during subsequent manipulations, is frequently self-limited and clears within one or two days.
POSTOPERATIVE BILIARY STRICTURES
Postoperative strictures may occur following laparoscopic cholecystectomy, major hepatic resection, and liver transplantation at a choledochocholedochal anastomosis or within an intrahepatic duct as a result of ischemia or recurrent PSC (Table 70-1) (see Chapters 66, 68, and 95). Dilation of a postoperative or other benign biliary stricture can be performed via percutaneous THC or through a mature, surgically-placed T-tube tract. Maturation of the T-tube tract usually requires six weeks. Percutaneous THC and biliary balloon dilation may be performed at the same session in the absence of clinical signs of cholangitis or sepsis. An 8-French or 10-French transhepatic tube is left in place, and the patient returns for repeat cholangiography six weeks later, at which time further stricture dilation is performed if bile duct narrowing of 30% or greater persists. The tube is then repeatedly upsized to a 12-French tube to facilitate healing of the stricture at a larger diameter. If the stricture resolves on follow-up, the biliary tube can be removed; otherwise, a similar procedure should be performed after six to eight weeks.
Table 70-1 Principal Causes of Benign Biliary Strictures
In one of the largest series published with long-term follow-up, percutaneous biliary balloon dilation was performed in 85 patients with a benign biliary stricture.16 In the 75 patients with follow-up, 205 percutaneous procedures were performed during 112 treatments of 84 biliary strictures. Stricture balloon dilation from 8 to 12 mm was performed. Procedures were repeated at 2- to 14-day intervals until cholangiography demonstrated free drainage of contrast material to the small intestine and no residual stenosis. An internal-external biliary drain was left in place for a mean of 14 to 22 days and removed if the patient did well when the catheter was clamped and had a normal cholangiogram. All procedures were technically successful. A total of 52, 11, 10, and 2 patients underwent a total of one, two, three, and four dilations, respectively. Major complications occurred in 2% of procedures: two subphrenic abscesses, one hepatic arterial pseudoaneurysm, and one case of hemobilia. The probability that clinically significant restenosis did not develop at 5, 10, 15, 20, and 25 years was 0.52, 0.49, 0.49, 0.41, and 0.41, respectively, after the first treatment, and 0.43, 0.30, 0.20, 0.20, and 0.20, respectively, after the second treatment. No significant difference was found in the rate of restenosis for strictures at anastomotic and nonanastomotic sites. Overall, 56 of 75 patients (75%) had successful management with percutaneous therapy.
Following liver transplantation, percutaneous THC is used for treating complications in patients with a duct-to-duct anastomosis and especially in patients in whom hepaticojejunostomy has been performed; these latter anastomoses frequently cannot be accessed via an endoscopic approach. Hepaticojejunal anastomosis is performed at the time of liver transplantation in children, persons with PSC, persons who undergo reoperation for a complication of a duct-to-duct (choledococholedochal) anastomosis, and living-related donors. For treatment of both duct-to-duct and hepaticojejunal anastomotic strictures, percutaneous therapy provides a high nonoperative success rate.17–19 In addition, in those patients in whom the bile duct is approachable via ERCP but who fail an endoscopic approach, a percutaneous approach is often successful.20
PRIMARY SCLEROSING CHOLANGITIS
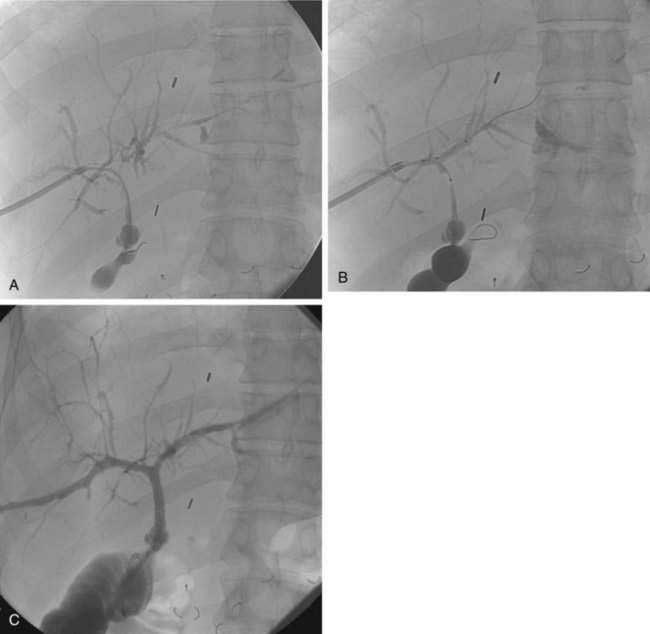
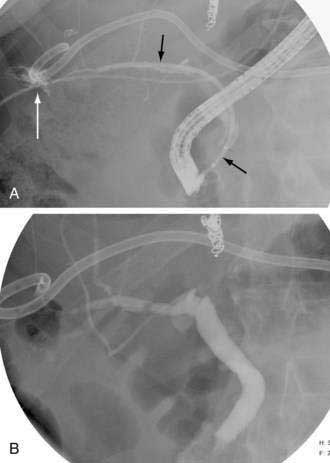
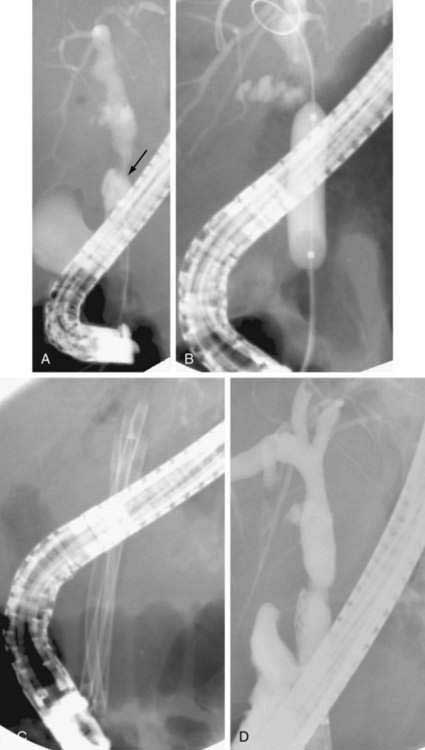
Most nonsurgical therapeutic interventions for PSC are now performed via ERCP (see Chapter 68). In the past, percutaneous therapy for a dominant stricture using balloon dilation followed by biliary drain placement for two to three months was found to be highly effective for treating obstructive biliary symptoms in patients with PSC21 but less effective in patients with jaundice for more than six months because of liver parenchymal dysfunction. More recently, only case reports of percutaneous therapy for PSC have appeared in the literature.22 In our experience, percutaneous therapy is useful for patients with a dominant stricture that cannot be accessed endoscopically (Fig. 70-3). In these cases, a guidewire or catheter passed percutaneously can be left in the duodenum to facilitate future endoscopic access (see later).
BILE LEAKS
Bile leaks are almost always postsurgical in etiology and arise from anastomotic (e.g., post-liver transplant) and nonanastomotic sites. The latter include cut surfaces of the liver and bile ducts following hepatectomy and laparoscopic injury. Percutaneous management may include drainage of free bile from the peritoneal cavity and of localized bile collections (bilomas) as well as placement of a biliary catheter above or across the leaking site to allow successful closure in the majority of cases.23–25
BILE DUCT INJURY
Widespread performance of laparoscopic cholecystectomy has led to an increased frequency of major bile duct injuries (see Chapter 66). Other causes of bile duct injury include bile duct exploration or biliary injury resulting from abdominal surgery or trauma. Percutaneous transhepatic biliary drain placement can be used as primary treatment of the injury or to augment surgical repair. Misra and colleagues26 retrospectively evaluated 51 patients who underwent percutaneous biliary management following laparoscopic cholecystectomy-related bile duct injuries over a 10-year period; 45 had operative repair prior to referral. Overall, 46 of the 51 were initially managed percutaneously, and 5 were managed percutaneously following failed hepaticojejunostomy. Nonoperative percutaneous management with balloon dilation resulted in an overall success rate of 58.8% at a mean follow-up of 76 months.
BILE DUCT STONES
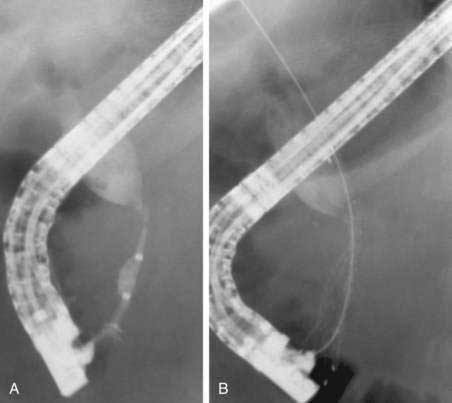
Bile duct stones can be managed percutaneously via cholecystostomy tubes, percutaneous placed drains, or surgical T-tubes. Gallbladder tube or T-tube tracts require approximately six weeks to mature prior to use. In many cases, bile duct stones can be cleared percutaneously by dilating the papilla from an antegrade approach.27,28 The stones, which may require mechanical fragmentation, are flushed into the duodenum. The percutaneous catheter is replaced for several days and then removed. With this approach, in one study27 stones were removed in 95 of 100 patients. In some cases, particularly in the setting of complex intrahepatic stones, a small-caliber choledochoscope (cholangioscope) can be passed through a mature percutaneous tract. Stones are then fragmented using a variety of techniques, with a high rate of success (see Chapter 65).29,30
MALIGNANT BILIARY OBSTRUCTION
Distal bile duct strictures (e.g., caused by pancreatic head cancer) are preferably managed via ERCP (see later) because endoscopic stent placement is less painful than percutaneous stent placement and is associated with fewer complications. This conclusion is based on a randomized trial of endoscopic and percutaneous approaches using plastic stents.31 Percutaneous stent placement can easily be achieved in these patients, however. Multiple interventions are often needed to place plastic stents prior to final internalization of the stent because large-bore (≥10-French) stents require dilation of a tract through the liver, which often cannot be accomplished in one stage. Bleeding, which occurs with such aggressive dilation, often requires maintenance of an external catheter to drain blood within the biliary tree. More recently, SEMS have been used. In a classic study, percutaneous placement of SEMS was associated with significantly longer stent patency, reduction in the need for re-intervention, and shorter hospital stays.32 In addition, advantages of SEMS when placed percutaneously are the availability of small-diameter pre-deployment delivery systems, so that the percutaneous tract does not require dilation, and the capability for stent insertion in one step.32,33 In a randomized trial34 of endoscopic versus percutaneous palliation of malignant bile duct obstruction in which metal biliary stents were placed percutaneously in one step and plastic stents were placed endoscopically, percutaneous placement of SEMS was associated with a 34% lower rate of recurrent biliary obstruction.
Relief of hilar biliary obstruction (e.g., caused by hilar cholangiocarcinomas, or Klatskin tumors) is more difficult to achieve endoscopically than relief of distal bile duct obstruction. Several studies have suggested that the percutaneous approach to these tumors is superior to the endoscopic approach, with a lower rate of post-procedure cholangitis.34,35
Covered SEMS were designed to improve stent patency by reducing the frequency of occlusion resulting from tumor ingrowth and tissue hyperplasia. Studies have shown promising results,36,37 although no randomized trials of covered versus uncovered stents placed via the percutaneous approach have been published.
PERCUTANEOUS CHOLECYSTOSTOMY TUBE PLACEMENT
The standard treatment of acute calculous cholecystitis is cholecystectomy (see Chapters 65 and 66). Even with the advent of laparoscopic cholecystectomy, some patients are still not surgical candidates. Percutaneous cholecystostomy tube placement is a minimally invasive way to treat these patients and can be performed with a local anesthetic or with moderate sedation. Tube placement enables immediate decompression of the gallbladder. Bile samples obtained during tube placement can be used to guide antimicrobial therapy, and the tube can be used for cholangiography to confirm cystic duct obstruction or, if the cystic duct becomes patent, bile duct obstruction. Percutaneous gallbladder therapy is useful for the management of severe acute calculous cholecystitis as a nonoperative approach in elderly patients or persons who are poor candidates for surgery and as a way to avoid emergency surgery.38 In the last situation, an elective cholecystectomy can be performed subsequently, often laparoscopically.39,40
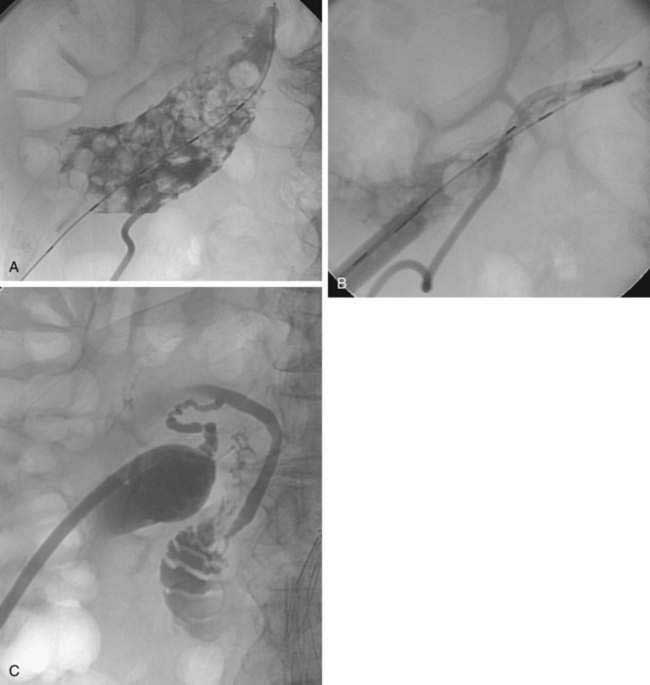
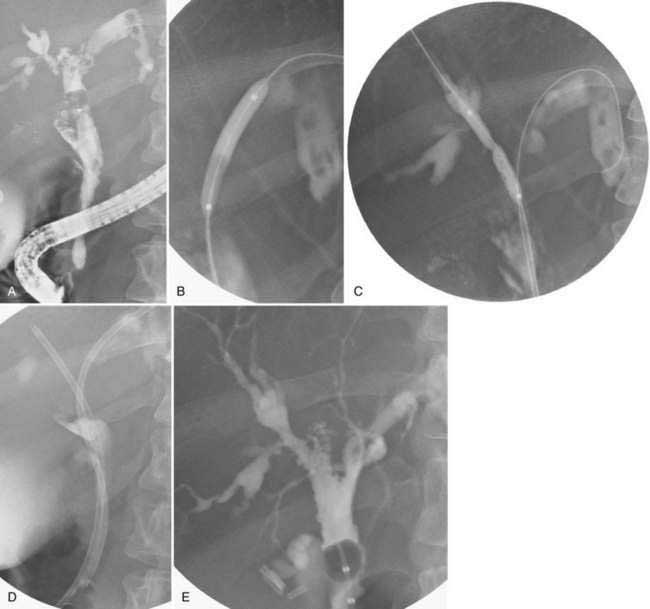
If the patient remains a poor surgical candidate, the cholecystostomy tube can remain in place long term. Alternatively, stones in the gallbladder, cystic duct, or bile duct can be managed percutaneously. The mature percutaneous tract can be dilated, after which the stones can be extracted (Fig. 70-4). Patients with intrahepatic gallbladders and small, shrunken, thick-walled gallbladders are not candidates for this approach. Despite the high success rate of percutaneous stone removal, stones can recur.41
An additional challenge in patients in an intensive care unit is the management of suspected acute acalculous cholecystitis (see Chapter 67). A Murphy sign can be difficult, if not impossible, to demonstrate, particularly in intubated or unresponsive patients. Delayed diagnosis and treatment can lead to gallbladder gangrene and perforation and to mortality. In patients in whom clinical suspicion for acute acalculous cholecystitis is high, a gallbladder tube should be placed percutaneously. If the gallbladder is not the source of the patient’s clinical problem, the cholecystostomy tube remains in place for six weeks. In one study of 55 critically ill patients with suspected acute acalculous cholecystitis who underwent percutaneous gallbladder tube placement, clinical improvement was seen in 58.7% within 24 hours and 95.7% within 72 hours.42
ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY
Endoscopic retrograde cholangiopancreatography has evolved from a purely diagnostic to an almost exclusively therapeutic procedure. ERCP is commonly performed using moderate sedation,43 although in severely ill patients and in cases anticipated to be complex, an anesthesiologist is often needed. ERCP is performed with a side-viewing duodenoscope that allows identification of the major papilla. The bile duct is cannulated under endoscopic and fluoroscopic guidance. A variety of catheters, guidewires, and stents are available to allow therapeutic interventions to be performed. Diagnostic ERCP is still used for facilitating manometry in patients with suspected sphincter of Oddi dysfunction (see Chapter 63) and for establishing the diagnosis of PSC when other imaging techniques have been nondiagnostic (see Chapter 68).44 A variety of biliary indications for ERCP45 will each be discussed.
BILE DUCT STONES
ERCP is usually performed in patients with known choledocholithiasis or in those patients with at least a moderate clinical suspicion of choledocholithiasis (see Chapter 65). In patients with gallbladder stones and a low clinical suspicion of choledocholithiasis, noninvasive imaging studies (MRCP, MDCT) or EUS are preferred to minimize the potential for complications of ERCP.46 In patients with a low clinical suspicion of choledocholithiasis in whom cholecystectomy is planned, intraoperative cholangiography can be performed, and, if stones are identified, laparoscopic exploration and stone removal can be undertaken. ERCP can then be reserved for patients in whom the stones are not extracted.46
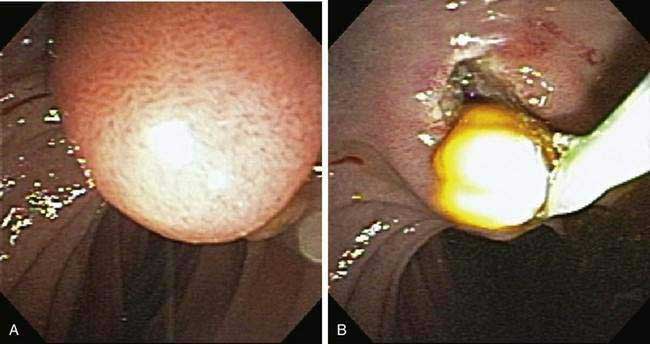
The standard method for stone removal is endoscopic biliary sphincterotomy to allow enlargement of the papilla and subsequent extraction of stones with a balloon or basket (Fig. 70-5). With this approach, more than 80% of all stones can be removed successfully.47 Larger stones may require additional removal techniques (discussed later).
An alternative to biliary sphincterotomy is balloon dilation of the papilla (balloon sphincteroplasty), which can be performed using small-diameter balloons (4 to 8 mm). The technique was introduced as a way to preserve sphincter of Oddi function, especially in young patients. The stones are removed using balloon or basket techniques. Most of the literature on balloon sphincteroplasty comes from outside the United States. Two meta-analyses of randomized trials of balloon sphincteroplasty versus sphincterotomy have shown that the rates of pancreatitis and need for mechanical lithotripsy are significantly higher, but the risk of bleeding is significantly lower, with balloon sphincteroplasty than with sphincterotomy.48,49 In the United States, the only randomized trial that compared balloon sphincteroplasty and sphincterotomy was closed prematurely because of two deaths in young patients from post-ERCP pancreatitis after sphincteroplasty.50 Sphincteroplasty still remains, however, an alternative approach in patients with coagulopathy,48 persons with underlying cirrhosis (particularly Child’s class C48 [see Chapter 90]), and those with altered anatomy (e.g., Billroth II gastrojejunostomy [see Chapter 53]), in which sphincterotomy is technically difficult.48
Removal of large bile duct stones (defined arbitrarily as ≥1.5 cm in diameter) may require additional techniques than those described earlier to be removed successfully. One such technique is lithotripsy. One form of lithotripsy is mechanical lithotripsy, in which the stone is captured in a specialized large basket and crushed51 (Fig. 70-6). The fragments are removed using standard extraction techniques. Another form of lithotripsy is intraductal lithotripsy, which is performed by passing laser or electrohydraulic catheters into the bile duct. The stones are fragmented under direct endoscopic visualization using a transpapillary choledochoscope.52 Direct visualization is necessary to ensure that the lithotripsy device is directed at the stone and not the bile duct wall. More recently, the combination of biliary sphincterotomy and large-diameter (>12 mm) balloon dilation has been used to remove large stones and decrease the need for mechanical lithotripsy.53 This large-diameter dilation method appears to be safe and not associated with an increased risk of post-ERCP pancreatitis.54 If large stones cannot be removed, a biliary stent is placed to relieve the obstruction.55 Additional procedures can then be undertaken electively to remove residual stones.
BILE LEAKS
As discussed previously, bile leaks arise as a result of post-surgical complications and trauma. Most commonly, post-cholecystectomy leaks arise from either the cystic duct or duct of Luschka (see Chapter 62). These smaller leaks can usually be managed with biliary sphincterotomy alone or placement of a small-caliber (7-French) plastic biliary stent (or both).56 This approach diverts bile away from the leak into the duodenum and negates the effect of the otherwise high-pressure biliary sphincter. More complex leaks usually require placement of one or more large-caliber plastic biliary stents in combination with biliary sphincterotomy (Fig. 70-7).57 The use of removable, covered SEMS for treatment of refractory leaks has also been described.58
PRIMARY SCLEROSING CHOLANGITIS
Patients with PSC may benefit from endoscopic intervention to treat a dominant stricture or biliary lithiasis.59 Patients with a dominant stricture usually present with progressive biliary obstruction. Cholangiocarcinoma must be considered in these patients. Routine brush cytology has a low sensitivity in these patients, but fluorescence in situ hybridization (FISH) has been shown to have a high sensitivity for the detection of cholangiocarcinoma (see Chapter 69).60 Choledochoscopy also may improve detection of malignancy in these patients.61
Endoscopic treatment of a dominant stricture involves balloon dilation, often in combination with short-term (<8 weeks) placement of a large-bore (10-French) stent (Fig. 70-8). Endpoints following endoscopic therapy have included clinical, biochemical, and radiologic improvement, with success rates ranging from 65% to 100%.62
BENIGN BILIARY STRICTURES
Benign biliary strictures are caused by a variety of disorders (see Table 70-1), and the response to therapy varies with the cause. Endoscopic therapy consists of balloon dilation followed by placement of plastic biliary stents. Data in the 2000s suggest that, for most causes of benign strictures, placement of multiple side-by-side large-bore plastic stents over the course of several endoscopic sessions (Fig. 70-9), with stents remaining in place for up to one year, allows a higher rate of successful stricture resolution than placement of only one or two stents.63,64
Chronic pancreatitis produces distal bile duct strictures that are usually refractory to endoscopic therapy with a single plastic stent, particularly in patients with calcific chronic pancreatitis.65 Multiple plastic stents can be placed. Covered SEMS also have been used for the treatment of chronic pancreatitis–induced bile duct strictures.66 The large diameter (10 mm) of the SEMS results in dilation of the stricture over time. The stent is removed after an interval of three to six months. Results using this approach have been encouraging, although these devices are not yet approved for use in benign diseases.
INDETERMINATE BILIARY STRICTURES
Some biliary strictures cannot be readily classified as benign or malignant on the basis of imaging studies and tissue sampling. Tissue sampling techniques at ERCP consist of wire-guided biliary brush cytology and intraductal forceps biopsy.67 Additional techniques that can be used to assess indeterminate strictures include intraductal ultrasonography68 and direct choledochoscopy with or without directed biopsy.69 In a small percentage of patients, the diagnosis still remains unclear. In some patients, the final diagnosis can only be established during long-term follow-up or at surgical exploration and resection.
MALIGNANT BILIARY STRICTURES
Distal Bile Duct Strictures
Pancreatic head cancer is the most common cause of distal bile duct obstruction (see Chapters 60 and 61). In patients with known pancreatic cancer in whom surgical resection (pancreaticoduodenectomy) is planned, routine preoperative ERCP for biliary decompression is discouraged. Several studies have shown that this approach does not improve surgical outcome and may cause postoperative morbidity and that complications from ERCP may delay or prevent surgical resection.70 The indications for preoperative ERCP include acute cholangitis and severe pruritus.71 In addition, stent placement is indicated when neoadjuvant chemoradiation is administered because the time to surgical resection is usually prolonged. In this situation, the use of a short-length SEMS (covered or uncovered) appears to be the best option; one study showed a high rate of stent occlusion in patients with pancreatic cancer who underwent preoperative chemoradiation and in whom plastic stents had been placed compared with those in whom a SEMS was placed.72 Uncovered SEMS are removed along with the tumor at the time of surgical resection. Indeed, in one study, a covered SEMS was placed in all patients with pancreatic cancer regardless of resectability, and this approach was found to be cost-effective when compared with placement of plastic stents.73
ERCP with biliary stent placement has been shown in randomized trials to be an acceptable alternative to palliative surgical bypass.74 Biliary stents can be placed safely in an outpatient setting.75 The comparative studies of endoscopy and surgery for palliation of distal biliary obstruction were performed using plastic stents, prior to the advent of SEMS. The main limitation to plastic stent placement is stent occlusion as a result of bacterial biofilm or reflux of vegetable matter.76 Therefore, in the comparative trials of surgery and endoscopy, the shorter length of initial hospital stay in the endoscopy group was offset by the need for subsequent hospitalizations and need for repeat ERCP to manage stent occlusion. The median time until stent occlusion for a standard large-bore stent is approximately three months. Stent occlusion results in recurrent jaundice, usually with cholangitis. SEMS have overcome the problem of bacterial biofilm, and randomized, controlled trials have shown superior patency rates for SEMS when compared with plastic stents (Fig. 70-10).77 Because the cost of SEMS is much greater than that of plastic stents, placement of a SEMS is cost-effective only if the patient survives longer than three to six months. Therefore, projected life expectancy should be taken into consideration when choosing between plastic and metal stents.78 Other factors to be considered include the patient’s adherence and ability to return for care.77 Uncovered SEMS occlusion is generally managed easily with placement of a plastic stent or a new SEMS within the existing one.79 More recently, covered metal stents have been developed in an attempt to overcome occlusion caused by tumor overgrowth and tissue hyperplasia. Early comparative studies have demonstrated prolonged patency with covered SEMS compared with uncovered SEMS80, although this advantage has not been firmly established.81 Covered SEMS are associated with higher migration rates82 and possibly an increased risk of acute cholecystitis.83
Hilar Biliary Obstruction
Hilar strictures may be caused by cholangiocarcinoma or metastatic disease. The clinical success rates for achieving adequate palliation for hilar tumors is less than that for distal bile duct tumors.84 Furthermore, technical success rates for bilateral endoscopic stent placement (right and left hepatic ducts) are also lower. Most patients with hilar obstruction will be adequately palliated when only one side of the liver is drained (unilateral drainage)—in other words, when only one side has been accessed and therefore contaminated.85 Patients who have had contrast instilled in both the left and right biliary systems require stenting of both to prevent progressive cholangitis.86 Not as firmly established is that metal stents offer superior prolongation of palliation compared with plastic stents for hilar tumors, as is the case for distal strictures. In a prospective, single-arm, pilot study of metal stent placement in 17 patients with Bismuth type II and III hilar cholangiocarcinoma (see Chapter 69), median stent patency was 12 months.87 A noncomparative, single-arm study showed that insertion of a Wallstent (a type of metal stent) is safe and feasible and achieves successful palliation without the need for further biliary re-intervention in the majority (69%) of patients with nonresectable hilar cholangiocarcinoma.88 In a more recent prospective, observational, cohort study of patients with hilar tumors treated with plastic or metal stents, patients in whom a metal stent was placed had significantly lower rates of post-procedural complications and need for percutaneous drainage.89 This finding suggests that metal stents may offer the same benefits for hilar biliary obstruction as they do for distal biliary obstruction.
Few comparative studies of endoscopic and percutaneous approaches to hilar tumors have been published. One study of the outcome following endoscopic and percutaneous treatment of hilar cholangiocarcinoma with use of only SEMS found that the rate of successful biliary decompression was significantly higher in the group in whom a SEMS was placed percutaneously than in the group in whom it was placed endoscopically.90 The median survival of patients in whom biliary drainage was successful initially, regardless of which procedure was performed, was much longer than that of patients in whom biliary drainage was not achieved. In addition, after successful biliary decompression had been achieved, the durations of median survival and stent patency were similar in the two groups.
In summary, in patients with unresectable hilar cholangiocarcinoma who undergo ERCP for palliation, unilateral stent placement should be performed with contrast injection confined to one side. A pre-ERCP abdominal CT may reveal atrophy of one lobe of the liver, and this lobe should specifically be avoided at ERCP because contamination will require drainage to prevent cholangitis but will not likely aid palliation. Alternatively, MRI can define the biliary anatomy prior to ERCP. One stent—plastic or metal—is usually adequate to achieve palliation.91 SEMS appear to offer superior palliation. Finally, from an endoscopic perspective, achieving successful drainage is more difficult technically for hilar tumors than for nonhilar tumors.
Photodynamic therapy (PDT) has been used for palliation of patients with unresectable hilar cholangiocarcinoma in whom jaundice does not resolve after endoscopic placement of plastic stents.92 Significant improvements in cholestasis, quality of life, and survival (as compared with historical controls) has been demonstrated with PDT93 and can be maintained for an extended period.94 The relevant studies were performed outside the United States, in countries in which smaller, more flexible laser fibers are available. At the present time, passage of laser fibers used for the treatment of esophageal cancer into the biliary system is difficult, but feasible. Nonetheless, PDT trials in the United States have shown promising results.95–97 PDT for cholangiocarcinoma is generally confined to selected centers with expertise in this procedure.
SPHINCTER OF ODDI DYSFUNCTION
Biliary sphincter of Oddi dysfunction (SOD) is classified as types I, II, and III (see Chapter 63).98,99 All types are characterized by intermittent biliary-type abdominal pain. Sphincter of Oddi manometry (SOM) is traditionally performed during ERCP by passing a water-perfused catheter into the bile duct or pancreatic duct to measure the biliary or pancreatic sphincter pressure, respectively. More recently, solid-state catheters have been used. SOD type I is associated with objective abnormalities in laboratory test results, often during attacks, and an abnormally dilated extrahepatic bile duct on an imaging study. SOD type II is associated with either abnormal laboratory test results or a dilated bile duct. SOD type III is characterized by pain alone. Patients with SOD type I invariably respond to endoscopic biliary sphincterotomy and do not require SOM for diagnosis.98 Patients with SOD type II who have a high basal sphincter pressure on SOM generally respond to biliary sphincterotomy.99 Patients with SOD type III do not appear to respond to biliary sphincterotomy at a greater rate than they do to placebo, and the role of ERCP in these patients is unclear. Indeed, one study has suggested that postcholecystectomy pain may be explained by persistent hyperexcitability of the nociceptive neurons in the central nervous system and may be unrelated to objective motility disorders of the sphincter of Oddi.100
COMPLICATIONS
Five major types of complications of ERCP may occur: sedation-related, pancreatitis, bleeding, perforation, and infection. Rates of post-ERCP pancreatitis vary because of differences in patient selection and operator technique and experience. Patients at highest risk are young, otherwise healthy women, especially those with known or suspected SOD.101 Elderly patients and those with chronic pancreatitis or pancreatic cancer have lower rates of pancreatitis. Prophylactic placement of a stent into the main pancreatic duct reduces the risk of pancreatitis in high-risk patients and nearly eliminates the risk of severe pancreatitis.102 Bleeding may occur after biliary sphincterotomy. Risk factors for post-sphincterotomy bleeding include coagulopathy and institution of anticoagulation within 72 hours of the sphincterotomy.103 Perforation of the duodenum occurs in less than 1% of patients and may require surgical management.104 Infection occurs primarily in patients in whom drainage of the biliary tree after ERCP is inadequate. Such patients include those with extensive intrahepatic PSC or advanced hilar tumors and those who have undergone failed stent placement for biliary obstruction. Data are accruing that a lower ERCP volume by an endoscopist is associated with a lower success rate and higher complication rate (see also Chapter 40).105
COMBINED PERCUTANEOUS AND ENDOSCOPIC APPROACHES
In some situations in which ERCP is unsuccessful but the bile duct still needs to be accessed endoscopically, a combined percutaneous-endoscopic approach can be undertaken, a so-called rendezvous procedure106 (Fig. 70-11). An example is a patient with a large duodenal diverticulum and a bile duct stone. If the diverticulum prevents endoscopic biliary access, a guidewire is passed percutaneously into the duodenum; the patient is then brought to the ERCP suite, and an ERCP is repeated. The wire is grasped by a forceps, and accessories are passed over the wire, thereby allowing sphincterotomy and stone extraction. The technique is not needed for most malignant strictures, which can be managed entirely with a percutaneous approach if the endoscopic approach fails.
ENDOSCOPIC ULTRASONOGRAPHY
DIAGNOSTIC ROLE
EUS has expanded the diagnostic role of endoscopy. EUS combines endoscopy and ultrasound to provide high-resolution images of the biliary system and is highly accurate for determining the cause of extrahepatic biliary obstruction, with a sensitivity of 97% and a specificity of 88%.2 The sensitivity, specificity, and accuracy rates of EUS for the diagnosis of bile duct stones are 95%, 98%, and 96%, respectively.2 EUS has the ability to distinguish different causes of malignant obstruction. In particular, EUS is more sensitive (93% to 100%) than CT (53% to 77%), transabdominal ultrasound (50% to 67%), MRI (50% to 67%), and ERCP (90%) for the detection of pancreatic tumors (see Chapter 61).2 EUS is less invasive than ERCP and has no associated radiation or contrast exposure. EUS combined with fine-needle aspiration provides tissue diagnosis of masses and lymph nodes.
THERAPEUTIC ROLE
Like ERCP, EUS is evolving into a therapeutic procedure, but the current therapeutic applications of biliary EUS are limited.107 EUS-guided biliary drainage has been described in which a needle followed by catheters or guidewires is passed into the biliary system, either through the left lobe of the liver108–111 into the intrahepatic ducts or through the duodenal wall directly into the bile duct.112 Another use of EUS is the drainage of bilomas.113
Adler DG, Baron TH, Davila RE, et al. Standards of Practice Committee of American Society for Gastrointestinal Endoscopy. ASGE guideline: The role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc. 2005;62:1-8. (Ref 45.)
Aytekin C, Boyvat F, Harman A, et al. Percutaneous management of anastomotic bile leaks following liver transplantation. Diagn Interv Radiol. 2007;13:101-4. (Ref 24.)
Cantwell CP, Pena CS, Gervais DA, et al. Thirty years’ experience with balloon dilation of benign postoperative biliary strictures: Long-term outcomes. Radiology. 2008;249:1050-7. (Ref 16.)
Costamagna G, Bulajic M, Tringali A, et al. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: Long-term results. Endoscopy. 2006;38:254-9. (Ref 65.)
De Palma GD, Galloro G, Siciliano S, et al. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: Results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547-53. (Ref 85.)
Freeman ML. Understanding risk factors and avoiding complications with endoscopic retrograde cholangiopancreatography. Curr Gastroenterol Rep. 2003;5:145-53. (Ref 101.)
Gluck M, Cantone NR, Brandabur JJ, et al. A twenty-year experience with endoscopic therapy for symptomatic primary sclerosing cholangitis. J Clin Gastroenterol. 2008;42:1032-9. (Ref 59.)
Lammer J, Hausegger KA, Flückiger F, et al. Common bile duct obstruction due to malignancy: Treatment with plastic versus metal stents. Radiology. 1996;201:167-72. (Ref 32.)
Leveau P, Andersson E, Carlgren I, et al. Percutaneous cholecystostomy: A bridge to surgery or definite management of acute cholecystitis in high-risk patients? Scand J Gastroenterol. 2008;43:593-6. (Ref 38.)
Matlock J, Freeman ML. Endoscopic therapy of benign biliary strictures. Rev Gastroenterol Disord. 2005;5:206-14. (Ref 63.)
Misra S, Melton GB, Geschwind JF, et al. Percutaneous management of bile duct strictures and injuries associated with laparoscopic cholecystectomy: A decade of experience. J Am Coll Surg. 2004;198:218-26. (Ref 26.)
Shah RJ, Langer DA, Antillon MR, Chen YK. Cholangioscopy and cholangioscopic forceps biopsy in patients with indeterminate pancreaticobiliary pathology. Clin Gastroenterol Hepatol. 2006;4:219-25. (Ref 69.)
Weinberg BM, Shindy W, Lo S. Endoscopic balloon sphincter dilation (sphincteroplasty) versus sphincterotomy for common bile duct stones. Cochrane Database Syst Rev 2006; 4:CD004890. (Ref 49.)
Williams EJ, Green J, Beckingham I, et al. British Society of Gastroenterology. Guidelines on the management of common bile duct stones (CBDS). Gut. 2008;57:1004-21. (Ref 46.)
Yoon WJ, Lee JK, Lee KH, et al. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gastrointest Endosc. 2006;63:996-1000. (Ref 81.)
1. Gandolfi L, Torresan F, Solmi L, Puccetti A. The role of ultrasound in biliary and pancreatic diseases. Eur J Ultrasound. 2003;16:141-59.
2. Tse F, Barkun JS, Romagnuolo J, et al. Nonoperative imaging techniques in suspected biliary tract obstruction. HPB (Oxford). 2006;8:409-25.
3. Fulcher AS. MRCP and ERCP in the diagnosis of common bile duct stones. Gastrointest Endosc. 2002;56(6 Suppl):S178-82.
4. Kim JY, Lee JM, Han JK, et al. Contrast-enhanced MRI combined with MR cholangiopancreatography for the evaluation of patients with biliary strictures: Differentiation of malignant from benign bile duct strictures. J Magn Reson Imaging. 2007;26:304-12.
5. Bridges MD, May GR, Harnois DM. Diagnosing biliary complications of orthotopic liver transplantation with mangafodipir trisodium-enhanced MR cholangiography: Comparison with conventional MR cholangiography. AJR Am J Roentgenol. 2004;182:1497-504.
6. Grobner T, Prischl FC. Patient characteristics and risk factors for nephrogenic systemic fibrosis following gadolinium exposure. Semin Dial. 2008;21:135-9.
7. Marckmann P, Skov L, Rossen K, Thomsen HS. Clinical manifestation of gadodiamide-related nephrogenic systemic fibrosis. Clin Nephrol. 2008;69:161-8.
8. Kim HJ, Park DI, Park JH, et al. Multidetector computed tomography cholangiography with multiplanar reformation for the assessment of patients with biliary obstruction. J Gastroenterol Hepatol. 2007;22:400-5.
9. Okada M, Fukada J, Toya K, et al. The value of drip infusion cholangiography using multidetector-row helical CT in patients with choledocholithiasis. Eur Radiol. 2005;15:2140-5.
10. Burke DR, Lewis CA, Cardella JF, et al. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003;14(9 Pt 2):S243-6.
11. Hatzidakis AA, Charonitakis E, Athanasiou A, et al. Sedations and analgesia in patients undergoing percutaneous transhepatic biliary drainage. Clin Radiol. 2003;58:121-7.
12. Hayashi N, Sakai T, Kitagawa M, et al. US-guided left-sided biliary drainage: Nine-year experience. Radiology. 1997;204:119-22.
13. Lee W, Kim GC, Kim JY, et al. Ultrasound and fluoroscopy guided percutaneous transhepatic biliary drainage in patients with nondilated bile ducts. Abdom Imaging. 2008;33:555-9.
14. Laufer U, Kirchner J, Kickuth R, et al. A comparative study of CT fluoroscopy combined with fluoroscopy versus fluoroscopy alone for percutaneous transhepatic biliary drainage. Cardiovasc Intervent Radiol. 2001;24:240-4.
15. Fidelman N, Bloom AI, Kerlan RKJr, et al. Hepatic arterial injuries after percutaneous biliary interventions in the era of laparoscopic surgery and liver transplantation: Experience with 930 patients. Radiology. 2008;247:880-6.
16. Cantwell CP, Pena CS, Gervais DA, et al. Thirty years’ experience with balloon dilation of benign postoperative biliary strictures: Long-term outcomes. Radiology. 2008;249:1050-7.
17. Ko GY, Sung KB, Yoon HK, et al. Percutaneous transhepatic treatment of hepaticojejunal anastomotic biliary strictures after living donor liver transplantation. Liver Transpl. 2008;14:1323-32.
18. Miraglia R, Maruzzelli L, Caruso S, et al. Percutaneous management of biliary strictures after pediatric liver transplantation. Cardiovasc Intervent Radiol. 2008;31:993-8.
19. Sung RS, Campbell DAJr, Rudich SM, et al. Long-term follow-up of percutaneous transhepatic balloon cholangioplasty in the management of biliary strictures after liver transplantation. Transplantation. 2004;77:110-15.
20. Kim ES, Lee B, Won JY, et al. Percutaneous transhepatic biliary drainage may serve as a successful rescue procedure in failed cases of endoscopic therapy for a post-living donor liver transplantation biliary stricture. Gastrointest Endosc. 2009;69:38-46.
21. May GR, Bender CE, LaRusso NF, Wiesner RH. Nonoperative dilatation of dominant strictures in primary sclerosing cholangitis. AJR Am J Roentgenol. 1985;145:1061-4.
22. Tritto G, Iaccarino V, De Martino S, D’Agostino L. A case of sclerosing cholangitis managed by a percutaneous approach. J Clin Gastroenterol. 2000;30:205-9.
23. Righi D, Franchello A, Ricchiuti A, et al. Safety and efficacy of the percutaneous treatment of bile leaks in hepaticojejunostomy or split-liver transplantation without dilatation of the biliary tree. Liver Transpl. 2008;14:611-15.
24. Aytekin C, Boyvat F, Harman A, et al. Percutaneous management of anastomotic bile leaks following liver transplantation. Diagn Interv Radiol. 2007;13:101-4.
25. Link BC, Yekebas EF, Bogoevski D, et al. Percutaneous transhepatic cholangiodrainage as rescue therapy for symptomatic biliary leakage without biliary tract dilation after major surgery. J Gastrointest Surg. 2007;11:166-70.
26. Misra S, Melton GB, Geschwind JF, et al. Percutaneous management of bile duct strictures and injuries associated with laparoscopic cholecystectomy: A decade of experience. J Am Coll Surg. 2004;198:218-26.
27. García-García L, Lanciego C. Percutaneous treatment of biliary stones: Sphincteroplasty and occlusion balloon for the clearance of bile duct calculi. AJR Am J Roentgenol. 2004;182:663-70.
28. Park YS, Kim JH, Choi YW, et al. Percutaneous treatment of extrahepatic bile duct stones assisted by balloon sphincteroplasty and occlusion balloon. Korean J Radiol. 2005;6:235-40.
29. Cheung MT, Wai SH, Kwok PC. Percutaneous transhepatic choledochoscopic removal of intrahepatic stones. Br J Surg. 2003;90:1409-15.
30. Gamal EM, Szabó A, Szüle E, et al. Percutaneous video choledochoscopic treatment of retained biliary stones via dilated T-tube tract. Surg Endosc. 2001;15:473-6.
31. Speer AG, Cotton PB, Russell RC, et al. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2:57-62.
32. Lammer J, Hausegger KA, Flückiger F, et al. Common bile duct obstruction due to malignancy: Treatment with plastic versus metal stents. Radiology. 1996;201:167-72.
33. Yoshida H, Mamada Y, Taniai N, et al. One-step palliative treatment method for obstructive jaundice caused by unresectable malignancies by percutaneous transhepatic insertion of an expandable metallic stent. World J Gastroenterol. 2006;12:2423-6.
34. Saluja SS, Gulati M, Garg PK, et al. Endoscopic or percutaneous biliary drainage for gallbladder cancer: A randomized trial and quality of life assessment. Clin Gastroenterol Hepatol. 2008;6:944-50.
35. Paik WH, Park YS, Hwang JH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: A percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55-62.
36. Hatzidakis A, Krokidis M, Kalbakis K, et al. ePTFE/FEP-covered metallic stents for palliation of malignant biliary disease: Can tumor ingrowth be prevented? Cardiovasc Intervent Radiol. 2007;30:950-8.
37. Han YM, Kwak HS, Jin GY, et al. Treatment of malignant biliary obstruction with a PTFE-covered self-expandable nitinol stent. Korean J Radiol. 2007;8:410-17.
38. Leveau P, Andersson E, Carlgren I, et al. Percutaneous cholecystostomy: A bridge to surgery or definite management of acute cholecystitis in high-risk patients? Scand J Gastroenterol. 2008;43:593-6.
39. Macrì A, Scuderi G, Saladino E, et al. Acute gallstone cholecystitis in the elderly: Treatment with emergency ultrasonographic percutaneous cholecystostomy and interval laparoscopic cholecystectomy. Surg Endosc. 2006;20:88-91.
40. Byrne MF, Suhocki P, Mitchell RM, et al. Percutaneous cholecystostomy in patients with acute cholecystitis: Experience of 45 patients at a US referral center. J Am Coll Surg. 2003;197:206-11.
41. Zou YP, Du JD, Li WM, et al. Gallstone recurrence after successful percutaneous cholecystolithotomy: A 10-year follow-up of 439 cases. Hepatobiliary Pancreat Dis Int. 2007;6:199-203.
42. Spira RM, Nissan A, Zamir O, et al. Percutaneous transhepatic cholecystostomy and delayed laparoscopic cholecystectomy in critically ill patients with acute calculus cholecystitis. Am J Surg. 2002;183:62-6.
43. Papachristou GI, Gleeson FC, Papachristou DJ, et al. Endoscopist administered sedation during ERCP: Impact of chronic narcotic/benzodiazepine use and predictive risk of reversal agent utilization. Am J Gastroenterol. 2007;102:738-43.
44. Weber C, Kuhlencordt R, Grotelueschen R, et al. Magnetic resonance cholangiopancreatography in the diagnosis of primary sclerosing cholangitis. Endoscopy. 2008;40:739-45.
45. Adler DG, Baron TH, Davila RE, et al. Standards of Practice Committee of American Society for Gastrointestinal Endoscopy. ASGE guideline: The role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc. 2005;62:1-8.
46. Williams EJ, Green J, Beckingham I, et al. British Society of Gastroenterology. Guidelines on the management of common bile duct stones (CBDS). Gut. 2008;57:1004-21.
47. Carr-Locke DL. Therapeutic role of ERCP in the management of suspected common bile duct stones. Gastrointest Endosc. 2002;56(6 Suppl):S170-4.
48. Baron TH, Harewood GC. Endoscopic balloon dilation of the biliary sphincter compared to endoscopic biliary sphincterotomy for removal of common bile duct stones during ERCP: A meta-analysis of randomized, controlled trials. Am J Gastroenterol. 2004;99:1455-60.
49. Weinberg BM, Shindy W, Lo S. Endoscopic balloon sphincter dilation (sphincteroplasty) versus sphincterotomy for common bile duct stones. Cochrane Database Syst Rev 2006; 4:CD004890.
50. Disario JA, Freeman ML, Bjorkman DJ, et al. Endoscopic balloon dilation compared with sphincterotomy for extraction of bile duct stones. Gastroenterology. 2004;127:1291-9.
51. Baron TH. Endoscopy for bile duct stones. Minerva Gastroenterol Dietol. 2005;51:289-301.
52. Piraka C, Shah RJ, Awadallah NS, et al. Transpapillary cholangioscopy-directed lithotripsy in patients with difficult bile duct stones. Clin Gastroenterol Hepatol. 2007;5:1333-8.
53. Misra SP, Dwivedi M. Large-diameter balloon dilation after endoscopic sphincterotomy for removal of difficult bile duct stones. Endoscopy. 2008;40:209-13.
54. Attasaranya S, Cheon YK, Vittal H, et al. Large-diameter biliary orifice balloon dilation to aid in endoscopic bile duct stone removal: A multicenter series. Gastrointest Endosc. 2008;67:1046-52.
55. Katsinelos P, Kountouras J, Paroutoglou G, et al. Combination of endoprostheses and oral ursodeoxycholic acid or placebo in the treatment of difficult to extract common bile duct stones. Dig Liver Dis. 2008;40:453-9.
56. Katsinelos P, Kountouras J, Paroutoglou G, et al. A comparative study of 10-Fr vs. 7-Fr straight plastic stents in the treatment of postcholecystectomy bile leak. Surg Endosc. 2008;22:101-6.
57. Sandha GS, Bourke MJ, Haber GB, Kortan PP. Endoscopic therapy for bile leak based on a new classification: Results in 207 patients. Gastrointest Endosc. 2004;60:567-74.
58. Baron TH, Poterucha JJ. Insertion and removal of covered expandable metal stents for closure of complex biliary leaks. Clin Gastroenterol Hepatol. 2006;4:381-6.
59. Gluck M, Cantone NR, Brandabur JJ, et al. A twenty-year experience with endoscopic therapy for symptomatic primary sclerosing cholangitis. J Clin Gastroenterol. 2008;42:1032-9.
60. Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106-17.
61. Tischendorf JJ, Krüger M, Trautwein C, et al. Cholangioscopic characterization of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Endoscopy. 2006;38:665-9.
62. McLoughlin M, Enns R. Endoscopy in the management of primary sclerosing cholangitis. Curr Gastroenterol Rep. 2008;10:177-85.
63. Matlock J, Freeman ML. Endoscopic therapy of benign biliary strictures. Rev Gastroenterol Disord. 2005;5:206-14.
64. Morelli G, Fazel A, Judah J, et al. Rapid-sequence endoscopic management of posttransplant anastomotic biliary strictures. Gastrointest Endosc. 2008;67:879-85.
65. Costamagna G, Bulajic M, Tringali A, et al. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: Long-term results. Endoscopy. 2006;38:254-9.
66. Kahaleh M, Behm B, Clarke BW, et al. Temporary placement of covered self-expandable metal stents in benign biliary strictures: A new paradigm? (with video). Gastrointest Endosc. 2008;67:446-54.
67. Papachristou GI, Smyrk TC, Baron TH. Endoscopic retrograde cholangiopancreatography tissue sampling: When and how? Clin Gastroenterol Hepatol. 2007;5:783-90.
68. Varadarajulu S, Eloubeidi MA, Wilcox CM. Prospective evaluation of indeterminate ERCP findings by intraductal ultrasound. J Gastroenterol Hepatol. 2007;22:2086-92.
69. Shah RJ, Langer DA, Antillon MR, Chen YK. Cholangioscopy and cholangioscopic forceps biopsy in patients with indeterminate pancreaticobiliary pathology. Clin Gastroenterol Hepatol. 2006;4:219-25.
70. Sewnath ME, Karsten TM, Prins MH, et al. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236:17-27.
71. Lermite E, Pessaux P, Teyssedou C, et al. Effect of preoperative endoscopic biliary drainage on infectious morbidity after pancreatoduodenectomy: A case-control study. Am J Surg. 2008;195:442-6.
72. Wasan SM, Ross WA, Staerkel GA, Lee JH. Use of expandable metallic biliary stents in resectable pancreatic cancer. Am J Gastroenterol. 2005;100:2056-61.
73. Kahaleh M, Brock A, Conaway MR, et al. Covered self-expandable metal stents in pancreatic malignancy regardless of resectability: A new concept validated by a decision analysis. Endoscopy. 2007;39:319-24.
74. Taylor MC, McLeod RS, Langer B. Biliary stenting versus bypass surgery for the palliation of malignant distal bile duct obstruction: A meta-analysis. Liver Transpl. 2000;6:302-8.
75. Cvetkovski B, Gerdes H, Kurtz RC. Outpatient therapeutic ERCP with endobiliary stent placement for malignant common bile duct obstruction. Gastrointest Endosc. 1999;50:63-6.
76. Weickert U, Venzke T, König J, et al. Why do bilioduodenal plastic stents become occluded? A clinical and pathological investigation on 100 consecutive patients. Endoscopy. 2001;33:786-90.
77. Levy MJ, Baron TH, Gostout CJ, et al. Palliation of malignant extrahepatic biliary obstruction with plastic versus expandable metal stents: An evidence-based approach. Clin Gastroenterol Hepatol. 2004;2:273-85.
78. Arguedas MR, Heudebert GH, Stinnett AA, Wilcox CM. Biliary stents in malignant obstructive jaundice due to pancreatic carcinoma: A cost-effectiveness analysis. Am J Gastroenterol. 2002;97:898-904.
79. Bueno JT, Gerdes H, Kurtz RC. Endoscopic management of occluded biliary Wallstents: A cancer center experience. Gastrointest Endosc. 2003;58:879-84.
80. Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729-34.
81. Yoon WJ, Lee JK, Lee KH, et al. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gastrointest Endosc. 2006;63:996-1000.
82. Nakai Y, Isayama H, Komatsu Y, et al. Efficacy and safety of the covered Wallstent in patients with distal malignant biliary obstruction. Gastrointest Endosc. 2005;62:742-8.
83. Fumex F, Coumaros D, Napoleon B, et al. Société Française d’Endoscopie Digestive. Similar performance but higher cholecystitis rate with covered biliary stents: Results from a prospective multicenter evaluation. Endoscopy. 2006;38:787-92.
84. Liu CL, Lo CM, Lai EC, Fan ST. Endoscopic retrograde cholangiopancreatography and endoscopic endoprosthesis insertion in patients with Klatskin tumors. Arch Surg. 1998;133:293-6.
85. De Palma GD, Galloro G, Siciliano S, et al. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: Results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547-53.
86. Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47:354-62.
87. Peters RA, Williams SG, Lombard M, et al. The management of high-grade hilar strictures by endoscopic insertion of self-expanding metal endoprostheses. Endoscopy. 1997;29:10-16.
88. Cheng JLS, Bruno MJ, Bergman JJ, Rauws EA, et al. Endoscopic palliation of patients with biliary obstruction caused by nonresectable hilar cholangiocarcinoma: Efficacy of self-expandable metallic Wallstents. Gastrointest Endosc. 2002;56:33-9.
89. Perdue DG, Freeman ML, Disario JA, et al. The ERCP Outcome Study (ERCOST) group. Plastic versus self-expanding metallic stents for malignant hilar biliary obstruction: A prospective multicenter observational cohort study. J Clin Gastroenterol. 2008;42:1040-6.
90. Paik WH, Park YS, Hwang JH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: A percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55-62.
91. Costamagna G, Tringali A, Petruzziello L, Spada C. Hilar tumours. Can J Gastroenterol. 2004;18:451-4.
92. Ortner MA, Liebetruth J, Schreiber S, et al. Photodynamic therapy of nonresectable cholangiocarcinoma. Gastroenterology. 1998;114:536-42.
93. Ortner M. Photodynamic therapy for cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2001;8:137-9.
94. Wiedmann M, Berr F, Schiefke I, et al. Photodynamic therapy in patients with non-resectable hilar cholangiocarcinoma: 5-Year follow-up of a prospective phase II study. Gastrointest Endosc. 2004;60:68-75.
95. Prasad GA, Wang KK, Baron TH, et al. Factors associated with increased survival after photodynamic therapy for cholangiocarcinoma. Clin Gastroenterol Hepatol. 2007;5:743-8.
96. Kahaleh M, Mishra R, Shami VM, et al. Unresectable cholangiocarcinoma: Comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin Gastroenterol Hepatol. 2008;6:290-7.
97. Baron TH. Photodynamic therapy: Standard of care for palliation of cholangiocarcinoma? Clin Gastroenterol Hepatol. 2008;6:266-7.
98. Petersen BT. An evidence-based review of sphincter of Oddi dysfunction: Part I, presentations with “objective” biliary findings (types I and II). Gastrointest Endosc. 2004;59:525-34.
99. Petersen BT. Sphincter of Oddi dysfunction, Part 2: Evidence-based review of the presentations, with “objective” pancreatic findings (types I and II) and of presumptive type III. Gastrointest Endosc. 2004;59:670-87.
100. Kurucsai G, Joó I, Fejes R, et al. Somatosensory hypersensitivity in the referred pain area in patients with chronic biliary pain and a sphincter of Oddi dysfunction: New aspects of an almost forgotten pathogenetic mechanism. Am J Gastroenterol. 2008;103:2717-25.
101. Freeman ML. Understanding risk factors and avoiding complications with endoscopic retrograde cholangiopancreatography. Curr Gastroenterol Rep. 2003;5:145-53.
102. Elta GH. Temporary prophylactic pancreatic stents: Which patients need them? Gastrointest Endosc. 2008;67:262-4.
103. Ferreira LE, Baron TH. Post-sphincterotomy bleeding: Who, what, when, and how. Am J Gastroenterol. 2007;102:2850-8.
104. Fatima J, Baron TH, Topazian MD, et al. Pancreaticobiliary and duodenal perforations after periampullary endoscopic procedures: Diagnosis and management. Arch Surg. 2007;142:448-54.
105. Kapral C, Duller C, Wewalka F, et al. Case volume and outcome of endoscopic retrograde cholangiopancreatography: Results of a nationwide Austrian benchmarking project. Endoscopy. 2008;40:625-30.
106. Mönkemüller KE, Linder JD, Fry LC. Modified rendezvous technique for biliary cannulation. Endoscopy. 2002;34:936.
107. Gleeson FC, Levy MJ. Endoscopic ultrasound (EUS) guided access and therapy of pancreatico-biliary disorders. Minerva Gastroenterol Dietol. 2008;54:151-60.
108. Will U, Thieme A, Fueldner F, et al. Treatment of biliary obstruction in selected patients by endoscopic ultrasonography (EUS)-guided transluminal biliary drainage. Endoscopy. 2007;39:292-5.
109. Shami VM, Kahaleh M. Endoscopic ultrasonography (EUS)-guided access and therapy of pancreatico-biliary disorders: EUS-guided cholangio and pancreatic drainage. Gastrointest Endosc Clin N Am. 2007;17:581-93.
110. Ang TL, Teo EK, Fock KM. EUS-guided transduodenal biliary drainage in unresectable pancreatic cancer with obstructive jaundice. JOP. 2007;8:438-43.
111. Kahaleh M, Hernandez AJ, Tokar J, et al. Interventional EUS-guided cholangiography: Evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52-9.
112. Yamao K, Bhatia V, Mizuno N, et al. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: Results of long-term follow-up. Endoscopy. 2008;40:340-2.
113. Shami VM, Talreja JP, Mahajan A, et al. EUS-guided drainage of bilomas: A new alternative? Gastrointest Endosc. 2008;67:136-40.