Chapter 57 Endometrial Cancer
Endometrial carcinoma is the most frequent gynecologic malignancy diagnosed in the United States and European countries, and it is the fourth most common cancer in women. Because most patients present with early-stage disease, their prognosis is usually favorable. The relative 5-year survival rate is 84% for all patients. Patients with disease confined to the uterus have a 5-year relative survival rate of 96%, compared with 67% for those with regional involvement.1
Epidemiology and Etiology
Epidemiology
The incidence of endometrial cancer in the United States is 23.5 cases per 100,000 women, and it has remained more or less stable since 1995.1 The U.S. incidence is among the highest in the world. European countries report incidence rates ranging from 15 to 20 cases per 100,000 women.2 The American Cancer Society estimates that a total of 43,470 new cancers of the uterus will be diagnosed in 2010.1 This represents 5.8% of the estimated 739,940 new female malignancies, and it is the most common gynecologic malignancy and the fourth most common cancer in women. The mortality rate for uterine cancer in the United States is 4.1 deaths per 100,000, and the estimate for 2010 is 7,950 deaths, which is 2.5% of all cancer deaths among women and 25% of all deaths from gynecologic malignancies. The mortality rate decreased from 5.3 to 4.1 deaths per 100,000 women between 1973 and 1995 and has remained stable since 1995. On January 1, 2007, in the United States there were approximately 575,108 women alive who had a history of cancer of the corpus uteri.
Uterine cancer is typically a cancer of postmenopausal women between 55 and 85 years old, with incidence rates exceeding 80 per 100,000 women aged 60 to 85 and a peak incidence of 90 per 100,000 women aged 65 to 69.1 Less than 5% of the patients are younger than age 40 years. Uterine sarcomas also manifest primarily in the postmenopausal population. Leiomyosarcomas occur at an earlier age than carcinosarcomas.3
Etiology
The cause of endometrial carcinoma is related to exposure of the endometrium to unopposed estrogens. Many studies have documented the association of endometrial cancer with increased exogenous or endogenous estrogen exposure.4 Early menarche, late menopause, obesity, nulliparity, infertility, and estrogen-producing ovarian tumors are classically associated with the development of endometrial cancer. In obese women, elevated estrogen levels caused by increased peripheral conversion of androstenedione may be the underlying mechanism for the increased risk. Endometrial cancer has also been associated with conditions such as hypertension and diabetes, but it is not clear whether these are true independent risk factors or related to obesity.5 Unopposed exogenous estrogen levels have strong association with the risk of endometrial cancer.6 The use of tamoxifen for the prevention or treatment of breast cancer has been documented to statistically increase the risk of subsequent endometrial cancer.7–10 Despite its antiestrogen effects on breast tissue, tamoxifen has some weak estrogenic effects on other organs of the body, including the uterus, which accounts for this risk.
Patients who have endometrial biopsies confirming complex hyperplasia with atypia have a 30% to 40% risk of subsequent development of endometrial cancer. Hyperplasia with atypia is considered a premalignant phase of endometrioid carcinoma and has a similar origin.11
An increased risk associated with a family history of endometrial cancer has been observed, especially in women younger than 50 years, but less than 1% of all endometrial cancers were attributable to familial and potentially genetic factors.12–14 Familial clustering of ovarian and endometrial cancers specific to the endometrioid morphology has been reported.15,16 The risk of developing other malignancies, especially of the colon and breast, is increased after the diagnosis of endometrial carcinoma.15,16 Women with mutations in the MLH1, MSH2, or MSH6 genes, which are responsible for the hereditary nonpolyposis colorectal cancer (HNPCC) syndrome, are at increased risk for developing endometrial cancer.17–19 HNPCC, or Lynch syndrome, is classified based on the presence or absence of tumors other than colorectal cancers. HNPCC syndrome type II patients have a high risk of endometrial cancer, second only to that of colorectal cancers.20,21 These women have a 20% risk of developing endometrial cancer before 50 years of age, which increases to 60% by age 60 years.
Prevention and Early Detection
No measures that may contribute to prevention of endometrial cancer other than avoiding unopposed estrogen use and avoiding obesity have been identified. Prophylactic hysterectomy is recommended in patients with known hyperplasia with atypia,11,22,23 and prophylactic hysterectomy and oophorectomy is advised in HNPCC type II gene carriers, for whom the risk of concurrent or subsequent endometrial cancer is substantial.20,21,24
Screening for endometrial cancer has not been performed in the general population. The early symptoms and favorable prognosis of this disease preclude efficacy of population screening.25 Vaginal ultrasound studies alone have not been found effective for screening purposes.26 Prompt analysis of every patient with postmenopausal or abnormal vaginal bleeding with vaginal ultrasound studies and endometrial biopsy is indicated.
Screening of patients using tamoxifen has been suggested. However, the characteristic hyperplastic aspect of the endometrium at ultrasound studies leads to high false-positive rates and frequent invasive diagnostic procedures in asymptomatic tamoxifen users.27,28 In a prospective ultrasound screening study of 247 tamoxifen users,27 it was shown that, of 52 asymptomatic patients with a thickened endometrium, most had an atrophic endometrium and only 1 had cancer, whereas in 2 of 20 patients with vaginal bleeding, endometrial cancer was diagnosed. The study authors concluded that routine ultrasound screening is not indicated in asymptomatic women using tamoxifen but that all women with abnormal bleeding should be evaluated.
Screening programs of gene carriers for the HNPCC syndrome not electing prophylactic surgery have shown increased efficacy when using annual endometrial biopsy in addition to vaginal ultrasonography. An evaluation of gynecologic surveillance among 175 mutation carriers (503 surveillance visits during 759 person-years at risk) using vaginal ultrasonography and endometrial biopsy at 94% and 74%, respectively, of the visits showed that 11 of 14 endometrial cancer cases were diagnosed by surveillance and 8 by endometrial biopsies. Vaginal ultrasonography indicated only four patients with endometrial cancer and missed 6 other cases. Endometrial biopsy detected 14 additional cases of potentially premalignant hyperplasia. The stage distribution and survival tended to be more favorable, although not statistically so, in the 14 cases of endometrial cancer in the surveillance group (no deaths) than in a group of 83 symptomatic mutation carriers of whom 6 died of endometrial cancer.29
Pathology and Pathways Of Spread
Pathology
Almost all uterine epithelial cancers are adenocarcinomas. The World Health Organization (WHO) has described several subtypes (Table 57-1). The most common type of endometrial adenocarcinoma is the endometrioid type, which accounts for 75% of cases.30,31 Other histologic types, also referred to as nonendometrioid endometrial carcinomas, include serous (5% to 10%), mucinous (1% to 3%), and clear cell (1% to 5%) carcinomas. Uterine mesenchymal and mixed tumors, usually called the uterine sarcomas, include leiomyosarcoma, endometrial stromal sarcoma, and carcinosarcoma (or malignant mixed müllerian tumor). The WHO classification of mesenchymal and mixed tumors of the uterus is summarized in Table 57-2. Carcinosarcoma is the most common type of uterine mesenchymal and mixed tumor (45%), followed by leiomyosarcoma (40%) and endometrial stromal sarcoma (10% to 15%). Carcinosarcomas are composed of malignant epithelial and stromal components; and because of this biphasic appearance, their origin has long been debated. Based on molecular genetic analysis, the current opinion is that these cancers should be considered metaplastic carcinomas. Clinical data support this view that carcinosarcomas should be considered high-risk carcinomas, particularly because the epithelial component is usually of high grade.31–33
TABLE 57-1 World Health Organization Histologic Classification of Epithelial Tumors of the Uterus
TABLE 57-2 World Health Organization Histologic Classification of Mesenchymal and Mixed Tumors of the Uterus
| Mesenchymal Tumors |
Nonendometrioid histologic types have a poorer prognosis. Serous and clear cell carcinomas are uniformly identified in this category. Serous adenocarcinoma was previously called uterine papillary serous carcinoma. It is histologically similar to its ovarian counterpart. Uterine papillary serous carcinoma was first described in 1982 and was found to have a higher rate of failure within the abdomen, as in ovarian cancer.34,35 This entity can be confused with the papillary villoglandular subtype of endometrioid adenocarcinoma, which carries a significantly more favorable prognosis. Serous carcinoma is frequently diagnosed at a higher stage than endometrioid adenocarcinomas. Clear cell carcinoma has been reported to have the same poor prognosis, with more advanced disease at diagnosis.36,37 Serous and clear cell carcinomas have been shown to have outcomes similar to grade 3 endometrioid carcinoma.38,39
Adenosquamous cell carcinoma has been suggested by some investigators to be another poor histologic subtype,36,37 but others think the prognosis of these patients is no different from that for the typical endometrioid adenocarcinoma.40,41 Zaino and colleagues41 evaluated a large population of patients who were part of a Gynecologic Oncology Group (GOG) study and found a parallel between the glandular grade and the degree of differentiation in the squamous component. It was subsequently suggested that the name of this histologic subtype should reflect the lack of importance of this feature by calling it adenocarcinoma with squamous differentiation.
Endometrial hyperplasia frequently precedes endometrial carcinoma. Endometrial hyperplasia is considered a precursor of endometrioid carcinoma, and endometrial intraepithelial carcinoma has been associated with the development of nonendometrioid carcinoma. The International Society of Gynecological Pathologists has identified two architectural forms of endometrial hyperplasia, simple and complex. Endometrial hyperplasia displaying marked architectural abnormalities is designated complex hyperplasia, whereas lesions with a lesser degree of architectural abnormalities are designated simple hyperplasia. Atypical nuclear changes can be associated with the simple or complex types and are regarded separately from hyperplasia that is not displaying atypia. Progression of simple hyperplasia to endometrioid carcinoma is rare (<2%), whereas progression to carcinoma occurs in 30% to 40% of the patients with simple and complex hyperplasia with atypia.11,22,23,42,43 The risk of subsequent endometrial cancer warrants hysterectomy in patients with hyperplasia displaying atypia.
Not all endometrial cancers arise in a setting of atypical hyperplasia. Nonendometrioid cancers, especially serous carcinomas, are related to endometrial intraepithelial carcinoma, which appears to represent malignant transformation of atrophic endometrium. It is found in the adjacent endometrium of up to 90% of serous carcinomas.44
Pathways of Spread
Most endometrial cancers remain confined to the uterine body for a long time. The initial spread occurs by local extension along the endometrial surface. Subsequent growth continues in the radial and longitudinal directions. Longitudinal growth may result in involvement of the lower uterine segment and the cervix, initially involving the endocervical glands and later spreading by cervical stromal invasion. The tumor can also extend along the cornua to the fallopian tubes. Radial growth results in myometrial invasion, initially superficially and later penetrating to the subserosa and the serosa. Two patterns of myometrial invasion have been described: an expansive growth pattern with pushing borders and an infiltrating growth pattern with cancer cells and nests penetrating the myometrium haphazardly.45,46 The infiltrating growth pattern is associated with frequent lymphovascular space invasion (LVSI) and early lymphatic spread. After the tumor breaches the serosa, transperitoneal dissemination can occur, with a pattern resembling that of ovarian cancer. Occasionally, after extensive penetration of the myometrium or the cervix, direct invasion of the bladder or the rectum may occur, or the tumor may involve the pelvic soft tissues and continue to reach the pelvic sidewall. Peritoneal seeding can result from growth through the serosa or from transtubal spillage of tumor cells into the peritoneal cavity.
As the tumor invades the myometrium more extensively, the risk of lymph node metastasis is higher.47,48 The endometrium has few lymphatics, but after the tumor penetrates the myometrium and especially when it reaches the lymphatic-rich subserosa, spread by lymphatic invasion is common. Lymphatics from the uterine fundus can drain directly to the para-aortic lymph nodes. Typically, the internal and external iliac lymph node groups are the first echelon of spread although isolated para aortic nodal involvement also occurs. Sentinel node detection studies49–51 have shown the sentinel nodes to be located in the obturator, external iliac, and para-aortic regions. Lymphatic spread is also believed to be responsible for involvement of the vagina and for isolated adnexal involvement.
Overall, about 11% of patients with clinical stage I and occult stage II endometrial carcinoma and 5% to 7% of patients with tumors confined to the uterus have pelvic or para-aortic lymph node involvement.47,52 The extent of myometrial penetration correlates strongly with the histologic grade of the tumor, and the depth of invasion and tumor grade correlate with the risk of lymphatic involvement. The risk of intra-abdominal dissemination is higher in patients with nonendometrioid carcinoma. Abdominal involvement of endometrioid carcinoma is associated with other risk factors, such as lymph node involvement, adnexal involvement, and LVSI.
Biologic Characteristics and Prognostic Factors
Biologic Features
Two different types of endometrial carcinoma have been described.53 Type I tumors are estrogen related, are often preceded by hyperplasia, and are typically low-grade endometrioid carcinomas. They usually develop in an estrogen-rich environment (e.g., obesity, premenopausal and perimenopausal phases), and they have a good prognosis. Type II tumors are unrelated to estrogen and develop in atrophic endometrium, presumably preceded by endometrial intraepithelial carcinoma, and they are more often serous and clear cell carcinomas. Patients with type II tumors are older postmenopausal patients; have high-grade, deeply invasive tumors; and have an unfavorable prognosis.31,53 A summary of the differences between these two groups is shown in Table 57-3. The genetic abnormalities involved in the carcinogenesis of endometrial cancer are different for type I and II carcinomas.
TABLE 57-3 Predominant Features of Type I and II Endometrial Cancer
| Characteristic | Type I | Type II |
|---|---|---|
| Clinicopathologic Features | ||
| Estrogen relation | Yes | No |
| Precursor lesion | Hyperplasia | Intraepithelial carcinoma |
| Age | Younger | Older |
| Histologic type | Endometrioid | Nonendometrioid |
| Grade | 1 or 2 | 3 |
| Stage | 1 | More advanced |
| Prognosis | Good | Poor |
| Genetic Features | ||
| Ploidy | Diploid | Aneuploid |
| TP53 mutation | 10-20% (late event) | 60-90% (early event) |
| PTEN inactivation | 35-50% | 5-10% |
| ERBB2 protein overexpression | 10-15% | 20-25% (serous: 60%) |
| EGFR overexpression | 10-30% | 60-80% |
| KRAS mutation | 15-30% | 0-5% |
| Microsatellite instability | 20-30% | 0-5% |
Specific molecular alterations have been found in type I and II endometrial cancers. In type II cancers, TP53 mutations have been found in up to 90% of cases (invasive and intraepithelial carcinomas), suggesting it to be an early event in carcinogenesis. ERBB2 protein overexpression has been reported in a significant proportion (up to 80%) of serous carcinomas. About 20% to 30% of serous carcinomas are found to have amplification of the ERBB2 gene. The ERBB2 gene product, similar to the epidermal growth factor receptor, is a transmembrane receptor protein that plays an important role in the ERBB signaling network that is responsible for regulating cell growth and differentiation. ERBB2 overexpression is associated with aggressive biologic behavior and poor survival. Targeted therapy using trastuzumab (Herceptin), a monoclonal antibody to ERBB2, is a potentially attractive treatment strategy.54,55 However, the one prospective clinical trial testing single-agent trastuzumab in endometrial carcinomas with ERBB2 overexpression or amplification reported no major responses.56 Epidermal growth factor receptor (EGFR) overexpression occurs in 60% to 80% of type II cancers, and it correlates with advanced-stage disease and poor prognosis.57 Targeted therapy with anti-EGFR agents including tyrosine kinase inhibitors such as gefitinib, lapatinib, and erlotinib as well as monoclonal antibodies such as cetuximab is being investigated.58,59 Results thus far have been modest. The National Cancer Institute of Canada (NCI Canada) reported a 12.5% response rate to single-agent erlotinib for chemotherapy-naive tumors. There was no correlation found between response and EGFR gene mutations or amplification.58
No specific abnormalities have been associated with type I cancers in general, which suggests that type I is a heterogeneous group of tumors with different combinations of abnormalities. TP53 mutations have been found in 10% to 20% of type I cancers, are associated with grade 3 tumors, and may be related to dedifferentiation. Mutant TP53 protein expression has been associated with advanced stage and other adverse factors, such as poor differentiation, deep invasion, and poor survival.60,61 More general markers of genetic damage such as DNA aneuploidy have consistently been associated with an inferior outcome.60,62–65
PTEN is frequently altered in endometrioid endometrial carcinomas (37% to 61%) and is considered an early event in carcinogenesis. The loss of PTEN, with consequent activation of the PI3K (phosphatidylinositol-3-kinase)-AKT (serine/threonine-specific protein kinase)-mTOR (mammalian target of rapamycin) signaling pathway, has been found in 32% to 83% of endometrioid-type endometrial carcinomas.66 TOR is the central component of a complex signaling network that regulates cell growth and proliferation and is interconnected with the PI3K/Akt signaling pathway. Studies have shown that PIK3CA mutations are frequently found in association with myometrial invasion, advanced stage, and adverse prognostic factors.60,67,68,69 This suggests a role for mTOR inhibition, and the mTOR pathway is currently regarded an important target for therapy. Phosphorylated mTOR overexpression has been found in both type I and II endometrial carcinomas.70 Phase II trials using mTOR inhibitors including deforolimus, temsirolimus, and everolimus have been performed. The most promising preliminary results thus far have been reported by the NCI Canada in women with chemotherapy-naive disease, in whom a 21% response rate to single-agent therapy with temsirolimus was observed.71 To date no markers of activation of the PI3K/Akt signaling pathway have proven to be predictive of response to mTOR inhibitors in patients.
As listed in Table 57-4, some molecular abnormalities such as PTEN and KRAS mutations, which are considered early events in the development of endometrioid carcinomas, are associated with a favorable prognosis, whereas others predict an unfavorable outcome. In most multivariate analyses, however, the prognostic significance of these molecular markers is lost in the presence of the traditional major prognostic factors: stage, grade, depth of invasion, and histologic subtype. The major promise of the molecular markers is the identification of specific targets for therapy and development of individual, effective therapeutic strategies for patients with advanced and/or metastatic disease.
TABLE 57-4 Prognostic Value of Genetic Abnormalities
| Genetic Abnormality | Prognostic Value |
|---|---|
| Aneuploidy | Decreased survival* |
| TP53 mutation | Decreased survival |
| PTEN inactivation | Conflicting data |
| ERBB2 overexpression | Decreased survival |
| EGFR overexpression | Decreased survival |
| KRAS mutation | Conflicting data |
| Microsatellite instability | No prognostic significance |
* Remains significant prognostic factor in multivariate analysis.
Prognostic Factors
Numerous studies have identified the major prognostic factors in endometrial carcinoma. Comprehensive retrospective analyses and prospective, randomized studies have established the major prognostic factors for survival and relapse to be stage, patient age, histologic cell type, tumor grade, depth of myometrial invasion, and presence of LVSI.48,72,73 Randomized trials have confirmed the prognostic value of these factors.74–76 Among stage I patients, grade has been found to be a major factor, with grade 3 tumors associated with a threefold to fivefold increased risk of relapse and cancer death.77,78 In most studies, grade 2 tumors do not have significantly different outcomes compared with grade 1, and two-tiered grading systems have been proposed to overcome the limited clinical value and poor reproducibility of the intermediate grade.45,79 The binary grading system proposed by Lax and co-workers45 is based on the proportion of solid growth, the pattern of myometrial invasion, and the presence of tumor cell necrosis. In a comparative analysis of the prognostic significance and the interobserver variability of these grading systems in a series of 800 stage I endometrial cancers, the reproducibility of the binary system was not found to be greater than that of the International Federation of Gynecology and Obstetrics (FIGO) grading system, but the prognostic power of the systems was equally strong. A simple two-tiered system, dividing tumors into low or high risk based on the proportion of solid growth (<50% vs. >50%), was shown to have superior prognostic power and greater reproducibility.80 Alternatively, the FIGO grading system may be used as a binary system by dividing tumors into grades 1 and 2 versus grade 3. Such a binary FIGO system has been shown to have strong prognostic significance, and it has the additional advantages of being highly reproducible and familiar to practicing pathologists worldwide.80,81
In most studies, depth of myometrial invasion had less strong prognostic value than tumor grade. Deep invasion, particularly into the outer third of the myometrial wall, has been associated with increased risk of relapse and inferior outcome.72 A diffusely infiltrating pattern of myometrial invasion, as opposed to an expansive growth pattern with pushing borders, has been suggested to be a stronger adverse prognostic factor than the depth of invasion.77,82 Because the effluent lymphatics and capillaries are mainly located in the subserosa, the tumor-free distance from the serosa may prove to be the strongest prognostic factor. In a multivariate analysis of 153 patients, tumor-free distance was found to be a highly significant predictor of relapse and death from disease, stronger than depth of invasion, and to have greater reproducibility.83
LVSI has been found to be a major prognostic factor that significantly and independently increases the risk of relapse, especially distant relapse.76,78,84 In an analysis of 609 patients with stage I to III endometrial cancer, those with LVSI were found to have a 5-year relapse rate of 39%, in contrast to 19% for patients without LVSI (p <.0001). Even in otherwise low-risk stage I disease, the presence of LVSI significantly increased the risk of relapse (28% with vs. 14% without LVSI). In stage I patients with high-risk features, those with LVSI had a 43% relapse rate.84
Clinical Manifestations/Patient Evaluation/Staging
Patient Evaluation
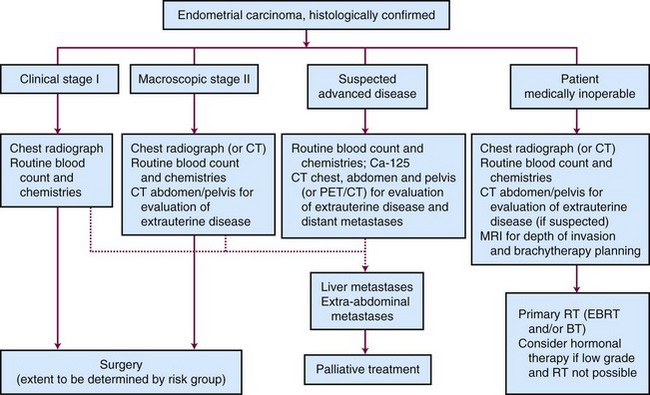
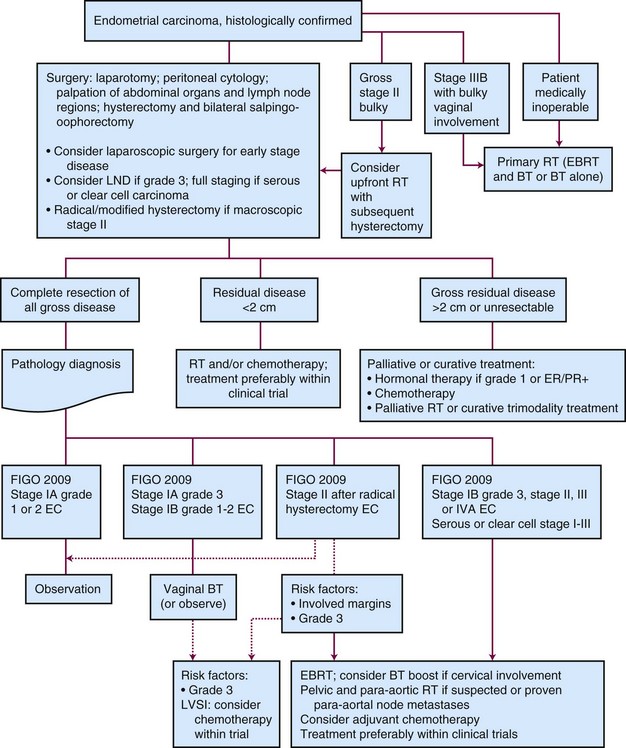
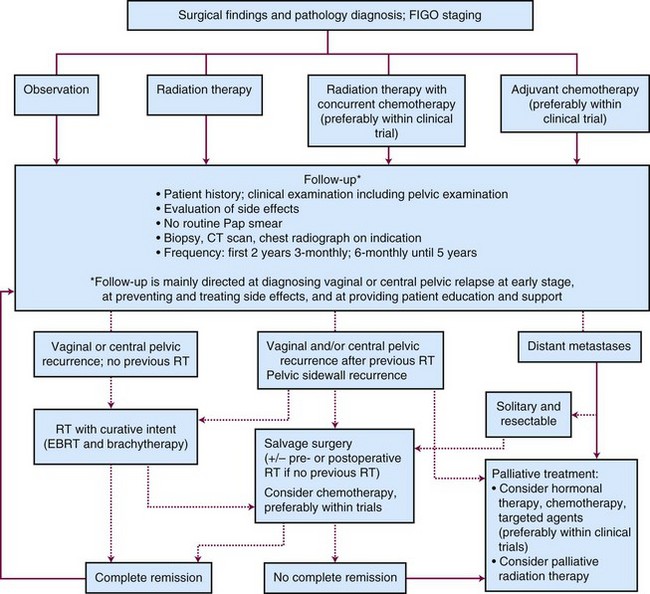
Outpatient procedures, such as transvaginal ultrasonography and endometrial biopsy or aspiration curettage, establish the diagnosis in more than 90% of patients suspected to have endometrial cancer. Various endometrial biopsy tools are available, such as a small Novak curette, Pipelle instrument, or Vabra aspirator, that permit the procedure to be performed without general anesthesia. If these procedures are not conclusive, a formal dilation and fractional curettage (D&C) should be performed, with or without hysteroscopy. Hysteroscopy allows direct visual assessment of the endocervix and the endometrial cavity and can be useful in guiding biopsy of any visible abnormality in symptomatic patients when other procedures have been nondiagnostic. Figure 57-1 presents a diagnostic algorithm for patient workup and evaluation.
Staging
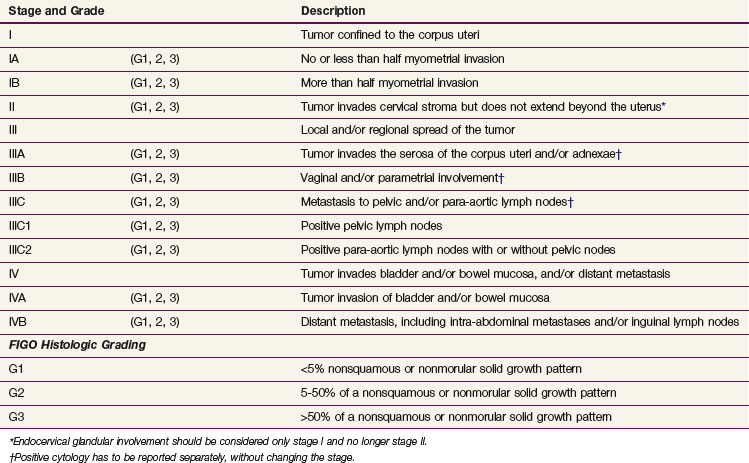
After a diagnosis of malignancy is established, the preoperative evaluation and staging workup are done. Definitive staging according to the FIGO system is based on the surgical and pathology findings. In 2009, a revised FIGO staging system was published, and this has replaced the 1988 FIGO staging system.85 Table 57-5 lists the 2009 FIGO staging and the differences between the 1988 and 2009 systems. (The differences between the 1988 and 2009 systems are seen in a web-only addition to the table, available on the Expert Consult website![]() for this chapter.) The changes in stage IA, IB and IC especially should be kept in mind when evaluating literature data, because FIGO 1988 stage IA and IB have been grouped together in FIGO 2009 as stage IA and because FIGO 1988 stage IC is IB in FIGO 2009. The previous nonsurgical (clinical) FIGO staging system continues to be used for patients not undergoing surgery.
for this chapter.) The changes in stage IA, IB and IC especially should be kept in mind when evaluating literature data, because FIGO 1988 stage IA and IB have been grouped together in FIGO 2009 as stage IA and because FIGO 1988 stage IC is IB in FIGO 2009. The previous nonsurgical (clinical) FIGO staging system continues to be used for patients not undergoing surgery.
TABLE 57-5 Federation of Gynecology and Obstetrics (FIGO) Surgical Staging System for Endometrial Carcinoma: 2009
| Stage and Grade | Description | |
|---|---|---|
| I | Tumor confined to the corpus uteri | |
| IA | (G1, 2, 3) | No or less than half myometrial invasion |
| IB | (G1, 2, 3) | More than half myometrial invasion |
| II | (G1, 2, 3) | Tumor invades cervical stroma but does not extend beyond the uterus* |
| III | Local and/or regional spread of the tumor | |
| IIIA | (G1, 2, 3) | Tumor invades the serosa of the corpus uteri and/or adnexae† |
| IIIB | (G1, 2, 3) | Vaginal and/or parametrial involvement† |
| IIIC | (G1, 2, 3) | Metastasis to pelvic and/or para-aortic lymph nodes† |
| IIIC1 | (G1, 2, 3) | Positive pelvic lymph nodes |
| IIIC2 | (G1, 2, 3) | Positive para-aortic lymph nodes with or without pelvic nodes |
| IV | Tumor invades bladder and/or bowel mucosa, and/or distant metastasis | |
| IVA | (G1, 2, 3) | Tumor invasion of bladder and/or bowel mucosa |
| IVB | (G1, 2, 3) | Distant metastasis, including intra-abdominal metastases and/or inguinal lymph nodes |
| FIGO Histologic Grading | ||
| G1 | <5% nonsquamous or nonmorular solid growth pattern | |
| G2 | 5-50% of a nonsquamous or nonmorular solid growth pattern | |
| G3 | >50% of a nonsquamous or nonmorular solid growth pattern | |
* Endocervical glandular involvement should be considered only stage I and no longer stage II.
† Positive cytology has to be reported separately, without changing the stage.
TABLE 57-5 Federation of Gynecology and Obstetrics (FIGO) Surgical Staging System for Endometrial Carcinoma: 2009
The patient’s general evaluation should include standard blood tests and a chest radiograph. Endometrial carcinoma patients are often elderly and frail and may have a number of concurrent medical problems that influence selection of the appropriate treatment. Computed tomography (CT) and magnetic resonance imaging (MRI) are not routinely performed for patients with clinical stage I disease who will be undergoing surgery, but imaging should be done if locally advanced disease is suspected. The major limitations of CT are its unreliability in assessing myometrial invasion, especially in atrophic uteri, and the failure to detect minimal parametrial, lymph nodal, or local extrauterine invasion.86 The greatest value of CT may be for staging in patients with clinical stage III or IV disease and in medically inoperable patients, for whom CT is useful for evaluating the size and extent of the tumor, for excluding gross extrauterine disease, and for planning of appropriate target volumes for radiation therapy. MRI using an intravenously administered contrast agent is superior to CT in determining myometrial invasion and diagnosing stage II disease.87
Initial studies of the potential role of FDG-PET/CT suggest a promising role for evaluation of relapse during follow-up and for presurgical evaluation of pelvic node metastases. In an analysis of PET/CT in 25 women with suspected relapse during follow-up,88 lesion site–based sensitivity, specificity, positive (PPV) and negative (NPV) predictive values, and accuracy of PET/CT were 94.7%, 99.5%, 94.7%, 99.5%, and 99.0%, respectively. Diagnostic accuracy of PET/CT was evaluated among 37 patients with high-risk endometrial cancer, of whom 9 (24%) had pelvic node metastases on histopathologic analysis. Patient-based sensitivity, specificity, PPV, NPV, and accuracy of PET/CT for detection of nodal disease were 77.8%, 100.0%, 100.0%, 93.1%, and 94.4%, respectively. Nodal lesion site–based sensitivity, specificity, PPV, NPV, and accuracy of FDG-PET/CT were 66.7%, 99.4%, 90.9%, 97.2%, and 96.8%, respectively, showing that PET/CT has a high NPV and may be useful in selecting patients for lymphadenectomy.91
Initial studies of the potential role of FDG-PET/CT suggest a promising role for evaluation of relapse during follow-up and for presurgical evaluation of pelvic node metastases. In an analysis of PET/CT in 25 women with suspected relapse during follow-up,88 lesion site–based sensitivity, specificity, positive (PPV) and negative (NPV) predictive values, and accuracy of PET/CT were 94.7%, 99.5%, 94.7%, 99.5%, and 99.0%, respectively. Other follow-up studies confirmed the efficacy of PET and/or PET/CT in detecting relapse in both asymptomatic and symptomatic patients, with sensitivity of 100% and specificity, PPV, NPV, and accuracy of 83% to 100%.89,90 PET/CT detected relapse in 3 patients with elevated tumor markers but negative CT findings, and altered treatment in 22% of patients.89 Diagnostic accuracy of PET/CT was evaluated among 37 patients with high-risk endometrial cancer, of whom 9 (24%) had pelvic node metastases on histopathologic analysis. Patient-based sensitivity, specificity, PPV, NPV, and accuracy of PET/CT for detection of nodal disease were 77.8%, 100.0%, 100.0%, 93.1%, and 94.4%, respectively. Nodal lesion site–based sensitivity, specificity, PPV, NPV, and accuracy of FDG-PET/CT were 66.7%, 99.4%, 90.9%, 97.2%, and 96.8%, respectively, showing that PET/CT has a high NPV and may be useful in selecting patients for lymphadenectomy.91
Primary Therapy
Surgery
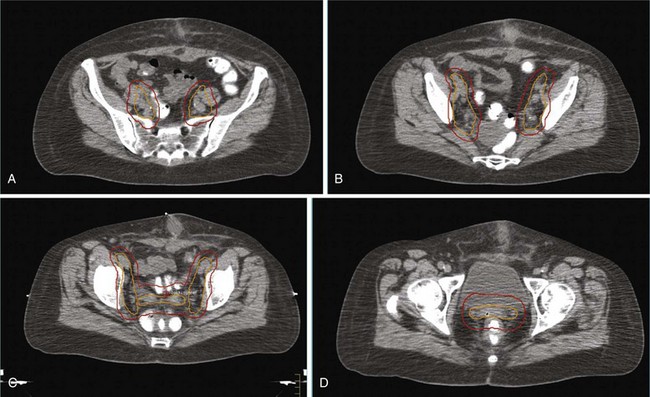
Surgery is the mainstay of the initial treatment of endometrial carcinoma. Surgical evaluation should start with exploration and collection of ascites or peritoneal lavage fluid for cytologic evaluation (although its utility for adding additional prognostic value is questioned). Thorough examination and palpation of the pelvic and abdominal organs and lymph node regions should be performed, and any suspicious sites should be sampled. After initial assessment, the standard surgical procedure is a total abdominal hysterectomy with bilateral salpingo-oophorectomy (TAH-BSO). In situations with gross cervical involvement (macroscopic stage II disease), a radical hysterectomy should be considered. Literature data support the use of radical hysterectomy in macroscopic stage II disease to clear potential parametrial disease, although a survival advantage over TAH-BSO and pelvic radiation therapy has not been proved.92,93
The standard surgical approach has traditionally been laparotomy through a midline incision, which allows full exposure of the abdomen, pelvic areas, and lymphatic sites. However, laparoscopic techniques for staging and treatment have been developed, and a number of institutions have evaluated laparoscopic staging and laparoscopic-assisted vaginal hysterectomy, especially for early-stage disease.94–96 The advantages of laparoscopy are the shorter hospitalization and recovery time and decrease of surgical morbidity. Disadvantages are the increased length of the operation and the learning curve involved with laparoscopic techniques. Increased risks of malignant cells in the peritoneal cytology specimen97 and vaginal cuff recurrence or port-site metastases98 have been reported, especially when this technique was first adopted. The importance of early occlusion of the fallopian tubes and uterine artery and avoidance of intrauterine manipulators to prevent such peritoneal spread and vaginal cuff recurrence has been stressed.94,98–100 Retrospective evaluations and initial randomized trials have shown the overall and relapse-free survival rates to be similar to those of laparotomy, with fewer complications and earlier recovery.101,102,103 Other randomized trials are ongoing.104 Laparotomy remains preferable to laparoscopy in very obese patients and in patients with intra-abdominal adhesions.
The role of pelvic and para-aortic lymphadenectomy or lymph node sampling has been widely debated. Determination of nodal involvement has prognostic implications, and in patients with nodal involvement it directs further therapy. The potential therapeutic implications of lymphadenectomy are directly related to the a priori risk of nodal disease in the population studied. Prospective and retrospective studies of lymphadenectomy in patients with clinical stage I or II endometrial carcinoma without extrauterine spread identified at surgery have shown the rates of pelvic and aortic nodal involvement to be 7% to 9% and 2% to 3%, respectively.47,52,105–108 The risk of lymph node involvement varies with the major risk factors, as was demonstrated in the landmark GOG surgical pathologic staging study47 (Table 57-6). Some of these features can be identified at the time of hysterectomy and used to evaluate the indication for lymph node dissection. The addition of lymphadenectomy, especially if pelvic and aortic lymphadenectomies are performed, prolongs operation time and has side effects, such as leg edema (5% to 10%), lymphocysts (symptomatic in 5% to 7%), increased rates of deep vein thrombosis (2%) and small bowel obstruction (up to 5%), and increased blood loss and higher transfusion rates (5% to 10%).85,86,87 Studies suggesting a survival advantage were small, single-center retrospective analyses that were flawed by patient selection and stage migration.107–109 The larger National Cancer Institute and Duke University analyses reported a survival benefit with multiple-site lymphadenectomy for grade 3 cancers, whereas no benefit was found for grade 1 or 2 disease.105,106 Analysis of data from the SEER program did not show benefit from lymphadenectomy for patients with stage I grade 1 or 2 disease but suggested improved disease-specific survival (DSS) for those with stage I grade 3 or more advanced-stage disease.110 Lymphadenectomy should therefore be considered for patients with grade 3 disease, cervical involvement, and high-risk histologic findings.105,106,110,111 It has been shown that if pelvic lymphadenectomy is performed a minimum of 11 nodes should be removed from multiple sites.109 Retrospective analyses are, however, flawed by elimination of patients from earlier-stage categories (i.e., stage migration) and by exclusion of patients at increased surgical risk due to advanced age or concurrent morbidities (i.e., selection bias). For the majority of patients with clinical stage I or occult stage II disease, the risk of nodal involvement is extremely low; therefore it is difficult to justify staging in all patients.112
| Risk Level | Lymph Node Metastasis | |
|---|---|---|
| Pelvic Nodes (%) | Aortic Nodes (%) | |
| All Clinical Stage I | ||
| Low risk | ||
| Grade 1, endometrium only, no intraperitoneal disease | 0 | 0 |
| Moderate risk | ||
| No intraperitoneal disease | ||
| Inner-middle invasion or grade 2 or 3 | 3 | 2 |
| Both factors | 6 | 2 |
| High risk | ||
| Deep invasion | 18 | 15 |
| Intraperitoneal disease | 33 | 8 |
| No Gross Extrauterine Disease | ||
| Low risk | ||
| No invasion or grade 1 with invasion* | <5 | <2 |
| Moderate risk | ||
| All other | 5-10 | <5 |
| High risk | ||
| Grade 3, outer 33% invasion | >10 | >10 |
*Excluding serous or clear cell histology.
Data from Creasman WT, Morrow CP, Bundy BN, et al: Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 60:2035-2041, 1987.
Two randomized trials investigating the role of lymphadenectomy in clinical stage I endometrial cancer have recently been published. In the U.K. Medical Research Council ASTEC trial 1408 patients were randomized, 704 to TAH-BSO with lymphadenectomy and 704 to TAH-BSO alone.113 The baseline characteristics were well balanced between the groups: 9% had any nodal involvement. The results showed no benefit of lymphadenectomy: 3-year overall survival (OS) rates were 89% (TAH-BSO alone) and 88% (TAH-BSO plus lymphadenectomy), and 3-year relapse-free survival (RFS) was even better in the TAH-BSO alone arm (hazard ratio [HR] 1.35, p = .017; HR 1.25 after adjustment, p = .14). An Italian randomized trial comparing TAH-BSO with lymphadenectomy with TAH-BSO alone for stage I endometrial carcinoma confirmed these results, with a median of 30 nodes removed in the patients in the lymphadenectomy study arm.114 Although the rate of nodal involvement was 13% in the lymphadenectomy arm as compared with 3% in the standard arm, rates of disease-free survival (DFS), OS, and relapses were the same in both arms. Even the pattern and sites were very similar, with vaginal recurrences in 2.6% versus 2.4% and lymph node recurrences in 1.5% versus 1.6% of patients in the lymphadenectomy versus standard study arms.114 Although many U.S.-based gynecologic oncologists continue to advocate for nodal staging in low-risk patients despite these results from randomized trials, the data do not support the routine use of lymphadenectomy for patients with stage I endometrial carcinoma.
Two randomized trials investigating the role of lymphadenectomy in clinical stage I endometrial cancer have recently been published. In the U.K. Medical Research Council ASTEC trial 1408 patients were randomized, 704 to TAH-BSO with lymphadenectomy and 704 to TAH-BSO alone.113 The baseline characteristics were well balanced between the groups: 9% had any nodal involvement. The results showed no benefit of lymphadenectomy: 3-year overall survival (OS) rates were 89% (TAH-BSO alone) and 88% (TAH-BSO plus lymphadenectomy), and 3-year relapse-free survival (RFS) was even better in the TAH-BSO alone arm (hazard ratio [HR] 1.35, p = .017; HR 1.25 after adjustment, p = .14). Subgroup analysis did not reveal any subgroup benefiting from lymphadenectomy. Analysis of the impact of the number of nodes removed was done by comparing centers with median node counts greater than 10 versus those with 10 or less and those with counts greater than 15 versus those with 15 or less. In both of these comparisons there was no difference between the study arms, with a nonsignificant trend in regression-free survival favoring the TAH-BSO alone arm. More women in the lymphadenectomy study arm had moderate or severe complications (17% vs. 12%; moderate to severe lymphedema 3.5% vs. 0.003%).113 An Italian randomized trial comparing TAH-BSO with lymphadenectomy with TAH-BSO alone for stage I endometrial carcinoma confirmed these results, with a median of 30 nodes removed in the patients in the lymphadenectomy study arm.114 Although the rate of nodal involvement was 13% in the lymphadenectomy arm as compared with 3% in the standard arm, rates of disease-free survival (DFS), OS, and relapses were the same in both arms. Even the pattern and sites were very similar, with vaginal recurrences in 2.6% versus 2.4% and lymph node recurrences in 1.5% versus 1.6% of patients in the lymphadenectomy versus standard study arms.114 Although many U.S.-based gynecologic oncologists continue to advocate for nodal staging in low-risk patients despite these results from randomized trials, the data do not support the routine use of lymphadenectomy for patients with stage I endometrial carcinoma. Subsequent therapy in the ASTEC trial was not determined by the lymph node status found at time of surgery, and the trial therefore tested the therapeutic value of lymphadenectomy per se, not whether any subsequent treatment based on lymph node status could affect outcomes. In the Italian trial, the use of adjuvant therapy was not significantly different between the study arms; the rates of patients receiving radiation therapy, chemotherapy, or both were 17%, 9%, and 6% in the lymphadenectomy study arm and 25%, 6%, and 4% in those who did not have lymphadenectomy. Ten of 59 patients received extended-field radiation therapy (EFRT) in the lymphadenectomy group versus 5 of 74 who did not have lymph nodes removed. There was, however, no difference in rates of total (12.8% vs. 13.2%), intraperitoneal (3% vs. 2.8%), or other relapses.114
Sentinel node detection may be an alternative way to identify patients requiring more intensive therapy. Initial results from sentinel node studies in endometrial carcinoma have shown that the combined use of radiocolloid labeling and patent blue dye results in sentinel node detection rates of 82% to 94%, with identification of two or three sentinel nodes per patient.50,51,115 The sentinel nodes were located in the obturator, external iliac, or para-aortic regions. In the study by Barranger and associates,49 macrometastases were identified in three sentinel nodes from two patients with routine staining, and immunohistochemical analysis identified six additional micrometastatic sentinel nodes in three other patients and one sentinel node containing isolated tumor cells. No false-negative sentinel nodes were found. Increasing surgical volume (and operator experience) has been shown to be associated with increased detection rates (77% vs. 94% after at least 30 cases).116 Additional larger studies are needed to further explore this concept, establish optimal techniques, and develop reliable accuracy data.111,117
Sentinel node detection may be an alternative way to identify patients requiring more intensive therapy. In breast cancer it has been shown that removal and meticulous analysis of sentinel nodes with serial sectioning and immunohistochemistry more adequately identifies occult nodal involvement while sparing most patients the risks and added toxicities of more extensive procedures. Initial results from sentinel node studies in endometrial carcinoma have shown that the combined use of radiocolloid labeling and patent blue dye results in sentinel node detection rates of 82% to 94%, with identification of two or three sentinel nodes per patient.50,51,115 The sentinel nodes were located in the obturator, external iliac, or para-aortic regions. In the study by Barranger and associates,49 macrometastases were identified in three sentinel nodes from two patients with routine staining, and immunohistochemical analysis identified six additional micrometastatic sentinel nodes in three other patients and one sentinel node containing isolated tumor cells. No false-negative sentinel nodes were found. Increasing surgical volume (and operator experience) has been shown to be associated with increased detection rates (77% vs. 94% after at least 30 cases).116 Additional larger studies are needed to further explore this concept, establish optimal techniques, and develop reliable accuracy data.111,117
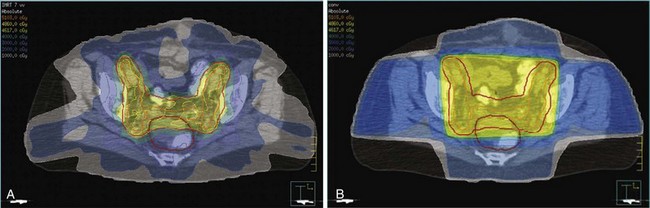
Primary Radiation Therapy: Indications and Results
Several studies have demonstrated the efficacy of primary RT for clinical stage I disease. With the use of uterine brachytherapy with or without EBRT, 5-year uterine control rates of 70% to 90% and disease-free survival rates ranging from 50% to 80% have been reported.118–124 Grade 3 tumors had significantly lower pelvic control and survival rates than grade 1 or 2 disease. Studies that included patients with stage II disease reported 5-year pelvic control rates of 40% to 60% and DSS of 50% to 60%.120,124
Surgical Results: Early-Stage Disease
Many retrospective studies have reported invariably good outcomes for stage IA and IB grade 1 and 2 endometrioid carcinoma treated with TAH-BSO alone, with a 5-year RFS of 95% and 5-year vaginal relapse rates of 2% to 5%.125–128 Several investigators stressed that lymphadenectomy is not indicated in these low-risk patients.127,128 In some studies, vaginal brachytherapy was used.128 The addition of brachytherapy to TAH-BSO may reduce vaginal relapse rates from between 2% and 5% to between 0% and 2% but without a survival difference and at the cost of (albeit minimal) morbidity and procedural cost. Effective salvage treatment is available for the occasional patient with vaginal relapse.
Adjuvant Therapy: Stages I and II
Four prospective, randomized trials have been published that evaluated the role of postoperative pelvic irradiation in intermediate-risk endometrial carcinoma,74–76129 and they are summarized in Table 57-7. The Norwegian trial, published in 1980, included 540 women with clinical stage I endometrial carcinoma. After hysterectomy and postoperative vaginal brachytherapy (60 Gy to the mucosal surface), patients were randomly assigned to additional pelvic irradiation (40 Gy in 2-Gy fractions, with a midline block after 20 Gy) or observation. Although additional pelvic irradiation reduced vaginal and pelvic relapse rates (2% at 5 years vs. 7% in the control group), more distant metastases were found in the pelvic irradiation group (10% vs. 5%), and survival was not improved (89% vs. 91% at 5 years).74 The subgroup with grade 3 tumors and deep (>50%) myometrial invasion showed improved local control and survival after pelvic irradiation (18% vs. 27% cancer-related deaths); however, there were too few patients in this category to reach significance.
TABLE 57-7 Comparison of the Randomized Trials of Adjuvant Radiation Therapy for Stage I Endometrial Carcinoma
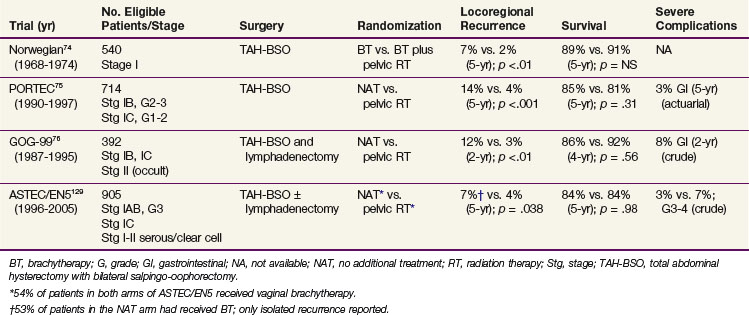
In the Postoperative Radiation Therapy in Endometrial Carcinoma (PORTEC) trial, 715 patients with stage I endometrial carcinoma, grade 1 or 2 with deep (>50%) myometrial invasion or grade 2 or 3 with superficial (≤50%) invasion were randomized after TAH-BSO to receive pelvic radiation therapy (46 Gy in 2-Gy fractions) or no further treatment.75 The 10-year locoregional relapse rates were 5% in the radiation therapy group and 14% in the control group (p <.0001). There was no significant survival difference between the treatment arms with 10-year OS of 66% (irradiation) and 73% (controls; p = .09).130 Endometrial cancer-related death rates were 10% in the RT group and 8% in the control group (p = .47). The patients younger than age 60 years (both study arms) had a 5-year locoregional relapse rate of 3%, compared with 9% for patients 60 to 70 years old and 10% for those older than 70 years. Patients with grade 2 tumors with superficial invasion had a 5-year locoregional relapse rate of 5%. Risk criteria for relapse were grade 3, age older than 60 years, and outer 50% invasion.
The 5-year survival rate after any relapse was 13% in the RT group and 48% in the control group (p <.001). After vaginal relapse, 5-year actuarial survival rates were 38% in the RT group and 70% in the control group, which shows the high salvage rates of vaginal relapse in patients not previously irradiated. Most (87%) of the 39 patients with isolated vaginal relapse could be treated with curative intent, usually with pelvic EBRT and brachytherapy (BT) and with surgery in some. A complete remission was obtained in 89%, and 77% remained in complete remission after further follow-up.131 In contrast, only 4 of 10 patients who were treated for pelvic relapse reached a complete remission, and the outcome after pelvic and distant relapse was poor, with only 6% and 11% of patients, respectively, surviving 5 years.
The GOG-99 trial included 392 evaluable patients with stage IB, IC, or IIA endometrial carcinoma of any histologic grade who were randomized after TAH-BSO and lymphadenectomy to receive pelvic irradiation (50.4 Gy in 1.8-Gy fractions) or no additional treatment.76 A high-intermediate risk group was defined based on the prognostic factors of age, histologic grade, myometrial invasion, and the presence of LVSI. This group (33% of the study population) had a 2-year incidence of relapse in the no additional treatment arm of 27%, in contrast to 6% for the low-intermediate risk group (67% of patients). Radiation therapy resulted in similar hazard reductions for the high- and low-intermediate risk subgroups (58% and 54%), but in absolute terms the differences were greater for the high-intermediate risk patients, with a reduction of 4-year cumulative relapse from 27% (in the group with no additional treatment) to 13% (those with irradiation). There was even a slight, although nonsignificant survival benefit: 4-year OS was 86% for the no additional treatment group and 92% for those who had RT. The 2-year estimated vaginal and pelvic failure rate was 12% in the no additional treatment group and 3% in the RT group, for a 58% hazard reduction by irradiation. These results are strikingly similar to those obtained in the PORTEC study without lymphadenectomy. However, the 4-year crude rate of severe complications in GOG-99 was 13% for patients who had received irradiation, compared with a 5-year actuarial rate of 3% in the PORTEC trial, which underlines the increased risk of toxicity when combining extended surgery with nodal sampling or dissection with pelvic RT.
The multicenter randomized ASTEC/EN5 trial included 905 patients with stage I-IIA endometrial carcinoma with risk features (deep invasion or grade 3 with superficial invasion or serous histology), who were randomly allocated to EBRT or observation. Brachytherapy was permitted if used in both study arms, and 53% of the patients received BT. Again, there was no difference in OS (84% at 5 years in both groups).129
A meta-analysis of pooled data from the ASTEC/EN5, GOG-99, and PORTEC-1 trials, which updated the 2007 Cochrane review and meta-analysis132 with the ASTEC/EN5 data, reliably excluded an absolute survival benefit of pelvic RT of more than 3%. The hazard ratio for isolated vaginal or pelvic RFS was 0.46 (p = .02), with 5-year cumulative incidence rates of 6.1% (observation only) versus 3.2% (RT). The relatively low rate of isolated vaginal or pelvic recurrence can probably be explained by the fact that 53% of the patients in the observation study arm received vaginal BT.129
Conclusions that can be drawn from these randomized trials of pelvic RT in stage I endometrial carcinoma are that pelvic irradiation provides a highly significant improvement of local control but offers no survival advantage. A large proportion of endometrial cancer patients has a very favorable prognosis, and these patients should be observed after TAH-BSO. Radiation therapy is a very effective salvage treatment for vaginal relapse in patients not previously irradiated. The data suggest that the use of postoperative RT should be limited to the group of patients at sufficiently high risk for locoregional relapse to accept the risk of treatment-associated morbidity to maximize initial local control and RFS. This has led to a reduction of the use of pelvic EBRT and use of high-intermediate risk criteria to determine the indication for radiation therapy. In the PORTEC study, patients with two of the three major risk factors (grade 3, age 60 or older, and outer 50% myometrial invasion) were found to have the highest absolute benefit from pelvic irradiation. The 10-year locoregional relapse rates in this “high-intermediate risk” category were 5% in the radiation therapy group and 20% in the control group. In the GOG-99 trial, similar high-intermediate risk criteria were identified, with reduction of isolated 4-year local relapse in the high-intermediate risk group from 13% to 5%. The risk criteria as defined in the PORTEC and GOG-99 trials and the risk reduction with radiation therapy in the high-risk groups are listed in Table 57-8.
TABLE 57-8 Comparison of the Risk Groups in the PORTEC and GOG-99 Trials and Risk Reduction with Radiation Therapy
| Risk Levels | Risk Groups | |
|---|---|---|
| PORTEC75 | GOG-9976 | |
| Risk factors | ||
| Age | ≤60 vs. >60 | ≤50 vs. ≤70 vs. >70 |
| Grade | Grade 1-2 vs. 3 | Grade 1 vs. 2-3 |
| Deep invasion | ≤50% vs. >50% | ≤66% vs. >66% |
| Lymphovascular space invasion | Absent vs. present | |
| High-risk group | At least two of the three factors | Any age and three factors |
| Age ≥50 and two factors | ||
| Age ≥70 and one factor | ||
| Results for the high-risk group | 10-yr locoregional relapse | 4-yr relapse (any) |
| RT: 5% | RT: 13% | |
| NAT: 23% | NAT: 27% | |
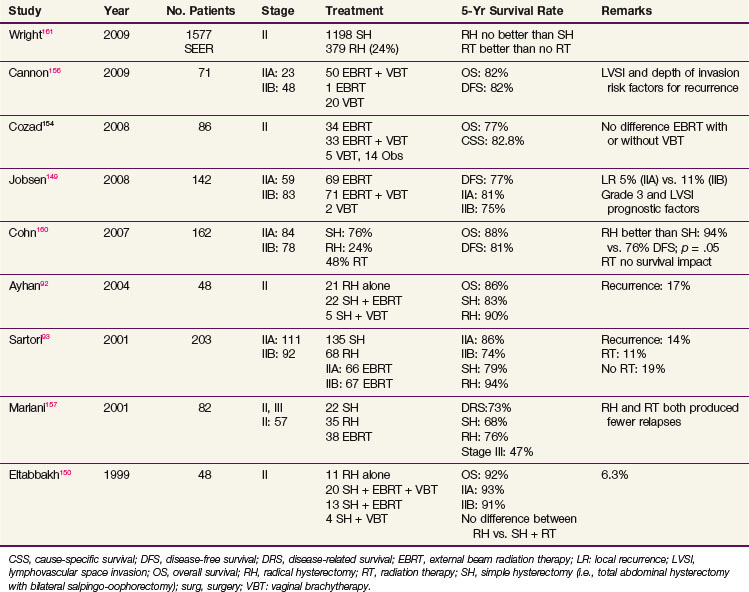
| Rel. risk: 0.22 | Rel. risk: 0.48 | |
| With GOG high-risk criteria | 4-yr isolated local relapse | |
| RT: 8% | RT: 5% | |
| NAT: 22% | NAT: 13% | |
| Rel. risk: 0.36 | Rel. risk: 0.38 | |
GOG, Gynecologic Oncology Group; NAT, no additional treatment; PORTEC, Postoperative Radiation Therapy in Endometrial Carcinoma; Rel. risk, relapse with RT compared with NAT; RT, radiation therapy.
Because most relapses occur in the vagina, the use of vaginal BT alone has been advocated, especially after extensive surgical staging. Data from mostly retrospective studies that used vaginal brachytherapy alone for stage I endometrial cancer have shown the 5-year risk of vaginal relapse to be 0% to 7%.133–141 Pelvic and distant failure rates, however, remain similar to those of patients treated with surgery alone, which is the reason that most studies included only or mainly low-risk patients (i.e., grade 1 to 2 disease with no or superficial invasion).
Vaginal control and complication rates for high-dose-rate (HDR) vaginal BT are comparable to those of low-dose-rate (LDR) therapy.137 Petereit and Pearcey142 reviewed the results of HDR BT for stage I endometrial cancer patients and concluded that local control rates of 98% and higher were obtained with modest doses (e.g., 30-35 Gy HDR to the surface or 21 Gy to 5-mm depth in three fractions). The use of higher doses did not further increase local control, but complication rates were higher. Several HDR studies using surgical staging included patients with high-risk stage I or stage II disease and reported vaginal control rates of 98% to 100%. Pelvic and distant relapses were found mainly in the stage I or II patients with high-risk features.138–141
The results of the randomized trials for intermediate-risk endometrial carcinoma suggested that, in view of the absence of survival benefit with EBRT and of the fact that most recurrences were located in the vagina, vaginal BT might also be effective for patients with high-intermediate risk features to obtain local control with fewer side effects than EBRT and better quality of life. This was the rationale for the randomized PORTEC-2 trial (2002-2006), which compared EBRT and vaginal BT with regard to both efficacy and health-related quality of life. In the PORTEC-2 trial, 427 patients with stage FIGO 1988 stage I-IIA endometrial carcinoma with high-intermediate risk features (i.e., age of at least 60 years, grade 1 or 2 tumors with outer 50% invasion or grade 3 with inner 50% invasion) were randomly assigned after surgery (TAH-BSO without lymphadenectomy) to EBRT (n = 214) or vaginal brachytherapy (n = 213). Quality of life was significantly better in the patients in the vaginal BT arm. Patients who had brachytherapy reported better social functioning (p <.002) and lower symptom scores for diarrhea, fecal leakage, the need to stay close to the toilet, and limitation in daily activities due to bowel symptoms (p <.001). At baseline, 15% of patients were sexually active; this increased significantly to 39% during the first year (p <.001). Sexual functioning and symptoms did not differ between the treatment arms.143 Final results of the PORTEC-2 trial confirmed the efficacy of vaginal brachytherapy. At median follow-up of 45 months, estimated 5-year rates of vaginal recurrence were 1.8% for VBT and 1.6% for EBRT (p = .74). Five-year rates of locoregional relapse (vaginal recurrence and/or pelvic recurrence) were 5.1% and 2.1% (p = .17). Only 1.5% versus 0.5% (p = .30) presented with isolated pelvic recurrence; other pelvic recurrences were part of widespread disease relapse, whereas rates of distant metastases were similar (8.3 vs. 5.7%, p = .46). There were no differences in OS (84.8% vs. 79.6%, p = .57) and DFS (82.7% vs. 78.1%, p = .74). Rates of grade 1 to 2 gastrointestinal toxicity were significantly lower in the vaginal brachytherapy group.144 In view of the efficacy of vaginal brachytherapy with fewer side effects and better quality of life, many groups have started using this modality alone for patients with high-intermediate risk disease, both with and without lymphadenectomy.
FIGO 2009 Stage IB, Grade 3 Disease
Stage I endometrial carcinoma, grade 3 with outer 50% myometrial invasion is usually regarded as a separate group among patients with stage I disease, because this subgroup is at increased risk for pelvic relapse and distant metastases and has lower survival rates.78,145,146 In an analysis of the outcome of 220 patients with stage IC endometrial cancer who had surgical staging, including pelvic and para-aortic lymphadenectomy, 99 (45%) patients treated with RT (pelvic EBRT or BT alone; selection criteria not specified) were compared with 121 (55%) who did not receive RT. The 5-year DFS were significantly lower for the observation group (75% vs. 93%), but OS were similar (90% vs. 92%). Among the 47 patients with grade 3 and outer 50% myometrial invasion, 5-year DFS were 90% after RT and 59% for the observation group.146
During the inclusion period of the PORTEC trial, 99 evaluable patients with grade 3 tumors with deep myometrial invasion were registered and received RT. The 5-year actuarial vaginal and pelvic relapse rate was 13% in this group, significantly higher than for other patients with stage I disease, who had excellent pelvic control after RT (97% to 99%). The 5-year rates of distant metastases were 20% for grade 3 disease with less than 50% invasion and 31% for grade 3 disease with more than 50% invasion, compared with 3% to 8% for grade 1 and 2 disease. OS at 5 years was 58% for those with grade 3 disease and outer 50% invasion and 74% for those with grade 3 disease and inner 50% invasion compared with 83% to 86% for the patients with grade 1 to 2 tumors (p <.001).78 In multivariate analyses, grade 3 disease was the most important adverse prognostic factor, with hazard ratios for any relapse and for endometrial carcinoma-related death of 5.4 (p = .0001) and 5.5 (p = .0004), respectively.
Whether surgical staging has been performed or not, pelvic irradiation is usually recommended for grade 3 tumors with deep myometrial invasion.145,147,148 An increasing number of authors, however, recommend vaginal brachytherapy alone for patients with fully staged IC grade 3 disease.140,141 In view of the small numbers, with patients with grade 3 disease always being a small minority in clinical trials, firm data are lacking. In view of the increased risk of abdominal and distant relapse and cancer-related death, adjuvant chemotherapy is being investigated by several groups (see section on adjuvant chemotherapy).
Stage II Disease
Stage II endometrial carcinoma includes patients with minimal microscopic involvement of the cervix (often called stage II occult) and those with macroscopic cervical involvement, even though these two groups have different prognoses. Cervical involvement has been associated with a poorer prognosis owing to the increased risk of LVSI and pelvic lymph node metastases.73 Patients with minimal extension to the endocervix used to be in stage IIA; however, in the FIGO 2009 update endocervical glandular involvement is included in stage I, because outcomes are identical to those of patients with stage I disease and treatment should be the same as for stage I disease. Patients with true stage II (former stage IIB) disease have a less favorable outcome.149
Patients with occult stage II disease have already undergone TAH-BSO. Pelvic irradiation with vaginal cuff brachytherapy boost is usually recommended for stage II disease treated with TAH-BSO because cervical involvement increases the risk of parametrial lymphatic disease.150 For stage I disease, the addition of vaginal BT does not significantly add to local control when pelvic EBRT is used but it does increase toxicity.151–153 Although not extensively evaluated, this also may apply to occult stage II disease,153 but because a vaginal cuff is usually not taken at TAH-BSO, most investigators continue to recommend a cuff boost for stage II disease. Two retrospective analyses, however, did not find any difference in recurrence with or without brachytherapy.149,154 Reported adverse prognostic factors among patients with stage II disease are grade 3, lymphovascular space invasion, and advanced age.149,155,156 Vaginal BT alone has been advocated for stage II disease after full surgical staging and in absence of risk factors such as deep invasion, high grade, and LVSI. This analysis, however, had 30% patients with FIGO 1988 stage IIA disease whose disease would now not be considered stage I.156
Patients with macroscopic stage II disease should be considered for radical hysterectomy, if surgically suitable, with adjuvant pelvic irradiation depending on the surgical findings (e.g., parametrial or vaginal extension, lymph node involvement, surgical margin involvement). Retrospective studies have shown that radical hysterectomy alone for macroscopic stage II endometrial cancer has local control and survival rates similar to those obtained with TAH-BSO plus pelvic irradiation.92,93,150,157–159 Some studies reported improved survival after radical hysterectomy compared with TAH-BSO alone, but the selection criteria for radical hysterectomy and for radiation therapy were unclear and might have influenced the results.93,157,158,160 Pelvic irradiation improved local control for patients treated with TAH-BSO.155,157 A SEER analysis of 1577 women with stage II endometrioid type endometrial carcinoma included 1198 women who underwent simple hysterectomy (76%) and 379 who underwent radical hysterectomy (24%). Radical hysterectomy had no significant effect on survival (HR 0.86; 95% confidence interval [CI], 0.61 to 1.23). Patients who did not receive radiation were 48% (HR, 1.48; 95% CI, 1.14 to 1.93) more likely to die than those who underwent adjuvant RT. The survival benefit from irradiation was most pronounced in women who underwent radical hysterectomy.161 Although the selection criteria are unclear, patients with high-risk stage II tumors appeared to benefit from RT, even after radical hysterectomy. An overview of the results for stage II disease is presented in Table 57-9.
Unfavorable Histologic Types
Serous and clear cell cancers, approximately 10% and 5% of endometrial carcinomas, respectively, have been identified as histologic types with an inferior prognosis owing to aggressive growth and spread patterns with frequent diffuse intra-abdominal dissemination. These histologic types often manifest as advanced disease (46% stage II to IV, compared with 21% for all endometrial cancers). Different treatment approaches (e.g., extended surgery, surgery with whole-abdomen irradiation, surgery with adjuvant chemotherapy) have been suggested. Several studies have shown that stage I serous and clear cell carcinomas have similar relapse and survival rates compared with stage I grade 3 endometrioid carcinomas.38,39 In an analysis of 5694 surgically staged endometrial cancer patients in the 25th annual report of FIGO, 3996 were stage I. Serous and clear cell cancers represented 5.2% of stage I cancers, and grade 3 carcinomas accounted for 8.1%. There were more stage I cancers among serous and clear cell cancers than among grade 3 carcinomas (54% and 49% vs. 42%). Five-year survival rates were 72% and 81%, respectively, for serous and clear cell cancers, compared with 76% for grade 3 disease. Postoperative radiation therapy improved survival by about 8% for these histologic types (76% vs. 68% for grade 3; 74% vs. 66% for serous cancers; and 83% vs. 77% for clear cell carcinoma), but these differences were not significant.39 Results from an analysis of 68 stage I and II serous cancers showed adjuvant treatment with chemotherapy, radiation therapy, or both, to significantly improve survival.162 More recent analyses of outcomes of patients with serous163 or clear cell cancers164 showed better survival with radiation therapy for patients with additional risk factors.
Adjuvant Hormonal Therapy
Adjuvant hormonal therapy for endometrial cancer has been extensively studied in view of the high incidence of progesterone receptor positivity and the 18% to 34% response rates to progesterone therapy seen in metastatic grade 1 or 2 disease. However, a meta-analysis of six randomized trials with a total of 3544 patients did not show a survival benefit for adjuvant progesterone treatment.165 A randomized trial enrolling 1148 patients showed a higher intercurrent death rate in the progesterone group owing to an increased risk of thromboembolic disease.166 Among the 461 high-risk patients, a tendency toward fewer cancer-related deaths in the progesterone group was observed, but overall survival was unchanged. The COSA-NZ-UK trial showed a decrease in the rate of relapse with 3 years of adjuvant progestins, but it did not make a difference in disease-specific survival.167
Adjuvant Chemotherapy
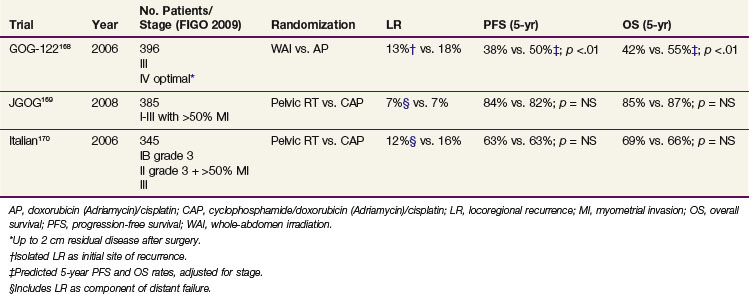
Three randomized trials have evaluated the efficacy of adjuvant chemotherapy as compared with radiation therapy (Table 57-10). The results of GOG-122, a randomized trial comparing whole-abdominal irradiation to combination doxorubicin-cisplatin chemotherapy in advanced endometrial carcinoma (i.e., stages III-IV; up to 2-cm residual disease after surgery allowed) showed combination chemotherapy to improve PFS and OS, with a predicted difference in DFS of 12% at 5 years (50% vs. 38%) and 5-year OS difference of 13% (55% vs. 42%).168 There appeared to be no significant differences in relapse rates in the pelvis or abdomen, although chemotherapy appeared to reduce distant metastases. Benefit from chemotherapy was observed for both those with stage III and those with stage IV disease. However, relapses remained common (55%). Twenty-five percent of initial recurrences on the whole-abdomen irradiation study arm and 35% on the chemotherapy arm were limited to the pelvis; 30% and 28%, respectively, of recurrences were intra-abdominal. Adverse effects were substantial, especially in the doxorubicin/cisplatin study arm, although current antiemetic therapy and use of granulocyte colony-stimulating factors might have mitigated many of the observed toxicities. Because residual disease was allowed, this trial did not study true adjuvant treatment for microscopic disease, and it can be debated whether the radiation dose used would be appropriate for macroscopic residual disease. The chemotherapy used was the most aggressive of the regimens in adjuvant trials to date, with seven cycles of doxorubicin 60 mg/m2 plus cisplatin 50 mg/m2 every 3 weeks followed by an eighth cycle of single-agent cisplatin.
The Japanese multicenter randomized JGOG-2033 trial compared whole-pelvic irradiation (at least 40 Gy) with three or more cycles every 4 weeks of cyclophosphamide (333 mg/m2), doxorubicin (40 mg/m2), and cisplatin (50 mg/m2) (CAP) chemotherapy in 385 evaluable patients with FIGO 1988 stage IC to IIIC endometrioid adenocarcinoma (“intermediate risk”; 60% stage IC, median age 59; 55% grade 1). At a median follow-up of 5 years, no differences in PFS (whole-pelvis irradiation 83.5% vs. CAP 81.8%) and OS (85.3% vs. 86.7%) were seen. Relapse rates were similar: 15.5% vs. 17.2% of the patients had a relapse, with 6.7% and 7.3% being pelvic and 13.5% and 16.1% extrapelvic, respectively.169 In a post hoc subset analysis the subgroup of “high to intermediate risk” cases (stage IC >70 yr, IC grade 3, stage II or stage IIIA [cytology], n = 120) a survival benefit for CAP was suggested, whereas no progression-free survival or overall survival difference was found among 75 “high-risk” cases (stage IIIA to IIIC). Grade 3 to 4 toxicity rates were 1.6% (whole-pelvis irradiation) and 4.7% (CAP), p = .08. Bowel obstructions were the main toxicity in the group undergoing whole-pelvis irradiation, and myelosuppression was the most common toxicity in the chemotherapy group.169
An Italian randomized trial used a design similar to that of the JGOG trial but in a somewhat higher-risk group of patients.170 The Italian study compared whole-pelvis irradiation (45 to 50 Gy) with five cycles every 4 weeks of cyclophosphamide (600 mg/m2), doxorubicin (45 mg/m2), and cisplatin (50 mg/m2) chemotherapy and included 345 evaluable patients with stage IC to IIIC endometrioid adenocarcinoma.170 In this trial most patients had stage III disease (64% stage III, 36% stage IC to II, grade 3). After a median follow-up of 95.5 months there were no significant differences in PFS and OS, with 5-year OS of 69 (radiation therapy) versus 66% (CAP regimen) and 5-year PFS of 63% versus 63%. Radiation therapy delayed pelvic relapses and chemotherapy delayed metastases (both nonsignificant trends).
Increased pelvic relapse rates have also been reported in retrospective series when using adjuvant chemotherapy alone in patients with high-risk or advanced-stage endometrial carcinoma.171 Of the 67% who experienced relapsed, 40% had pelvic recurrence and 56% had distant relapse. The 3-year pelvic failure rate was 47%, and in 31% the pelvis was the first or only site of relapse.
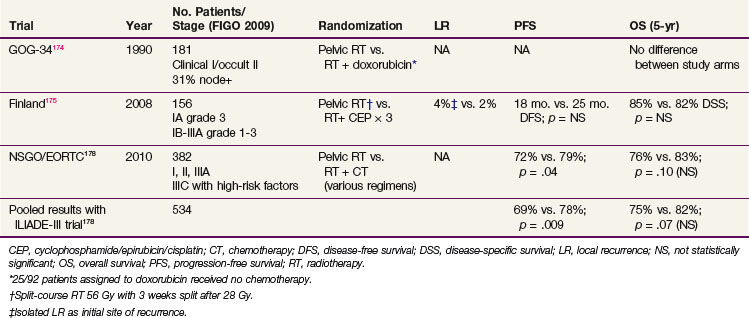
Because these data support the use of pelvic RT in high-risk and advanced-stage patients undergoing adjuvant chemotherapy, the use of combined chemoradiation has been explored (Table 57-11). Initial pilot studies have indicated combined therapy to be tolerable.172,173 The first early randomized trial, using single-agent doxorubicin after completion of radiotherapy, did not show any benefit.174 This trial, with only 181 patients, was underpowered and plagued by a particularly high rate of refusal of assigned therapy: 25 of the 92 patients assigned to doxorubicin received no chemotherapy. A small Finnish trial randomly assigned 156 patients to pelvic radiation therapy alone (split-course irradiation, 56 Gy in 2-Gy fractions with 3-week interval after 28 Gy) versus pelvic radiation therapy plus three cycles of cisplatin 50 mg/m2, epirubicin 60 mg/m2, and cyclophosphamide 500 mg/m2.175 The first cycle of chemotherapy was given 1 to 2 weeks after surgery, the second during the pause in radiation therapy, and the last within 2 weeks after completion of the second course of irradiation. Women with FIGO 1988 stages IA/B grade 3 and IC to IIIA grades 1 to 3 cancers were enrolled; the overall prognosis of the group was better than anticipated with 4-year DSS of 84.7% and 82.1% in the group with RT alone versus combined chemoradiation groups. There were no differences in the rates of local recurrence. The Radiation Therapy Oncology Group (RTOG) has reported a nonrandomized phase II trial of concurrent pelvic radiotherapy (45 Gy in 1.8-Gy fractions and a brachytherapy boost) and cisplatin (two cycles of 50 mg/m2 on days 1 and 28), followed by four cycles of cisplatin (50 mg/m2) and paclitaxel (175 mg/m2 as a 24-hour infusion) every 28 days after RT for high-risk or advanced-stage endometrial carcinoma.176 A total of 46 patients were included: 44 were evaluable, of whom 15 had stage I to II disease with high-risk features and 29 (66%) had stage III disease. Surgery consisted of TAH-BSO with or without additional surgical staging. The protocol completion rate was 98%. During the adjuvant phase, 35 of the 44 patients received all four cycles. Acute toxicities during chemoradiation were grade 3 in 27% and grade 4 in 2%. During adjuvant chemotherapy, 21% grade 3 and 62% grade 4 toxicity was observed, with severe toxicity being primarily hematologic. Late toxicity was grade 3 in 16% and grade 4 in 5%. Four-year DFS and OS were 81% and 85% for the whole group and 72% and 77% for patients with stage III disease. The 4-year rates of pelvic, regional, and distant relapse were 2%, 2%, and 19%, respectively. There were no relapses among patients with stage IC and II disease, suggesting additive effects of chemotherapy and irradiation.
At the American Society of Clinical Oncology 2007 annual meeting, preliminary results were reported for the randomized NSGO-EC-9501/European Organization for Research and Treatment of Cancer (EORTC)-55991 trial comparing irradiation alone with irradiation and adjuvant chemotherapy (given before or after irradiation) in 382 patients with stage I to III disease who had risk factors for relapse (one or more of grade 3 tumor, deep myometrial invasion, nondiploid DNA or serous/clear cell/anaplastic histology).177 Chemotherapy was not standardized and could be doxorubicin/cisplatin, paclitaxel/doxorubicin/cisplatin, paclitaxel/platinum, or paclitaxel/cisplatin/epirubicin. Results showed a 7% improvement in PFS in the chemotherapy arm (at 5 years, 79% vs. 72%, p = .03) and a nonsignificant 8% increase in OS (82% vs. 74% p = .08). Final results of this trial were published in a pooled data analysis with the Italian MaNGO ILIADE-III trial (157 patients), for a combined total of 534 evaluable patients. Pooled results were similar, with a statistically significant difference in 5-year PFS favoring the combined arm (78% vs. 69%, HR 0.63, p = .009), but only a trend for improved 5-year OS (82% vs. 75%, HR 0.69, p = .07).178 Interestingly, for patients with serous and clear cell tumors, although they were few in number (n =140), there was no evidence of benefit from adjuvant chemotherapy (HR for PFS 0.83, p = .59 and for OS 0.94, p =.88).178
Since these first reports of efficacy of adjuvant chemotherapy, three trials are currently ongoing that further explore the role of concurrent chemoradiation and/or adjuvant chemotherapy. (Web-Only Table 57-1). The international randomized PORTEC-3 trial for patients with high-risk and advanced-stage disease compares pelvic radiation alone with concurrent chemoradiation (two cycles of cisplatin during radiation therapy followed by adjuvant chemotherapy (four cycles of carboplatin/paclitaxel) with regard to efficacy and toxicity and quality of life. The randomized GOG-249 trial for patients with stage I-II tumors with high-intermediate and high-risk factors compares pelvic radiotherapy alone with vaginal brachytherapy followed by adjuvant chemotherapy (three cycles of carboplatin/paclitaxel). The randomized GOG-258 trial includes women with stage III-IVA disease and randomly assigns them to six cycles of carboplatin/paclitaxel with no radiotherapy versus radiotherapy with concomitant cisplatin (days 1 and 28) followed by four cycles of paclitaxel plus carboplatin. All three trials are better powered than their predecessors and should help resolve many of the uncertainties regarding which patients may benefit from chemotherapy as well as the role of radiotherapy.
Although advocated in small nonrandomized studies179 and widely practiced in view of their poorer prognosis, benefits of adjuvant chemotherapy in women with serous and clear cell carcinoma remain unproven.168,177,180 In both the randomized GOG-122 and the NSGO-EORTC trials, which showed a PFS benefit for the whole trial population, the subgroups with serous cancers did not appear to benefit from chemotherapy.168,177
Although advocated in small nonrandomized studies179 and widely practiced in view of their poorer prognosis, benefits of adjuvant chemotherapy in women with serous and clear cell carcinoma remain unproven.168,177,180 In both the randomized GOG-122 and the NSGO-EORTC trials, which showed a PFS benefit for the whole trial population, the subgroups with serous cancers did not appear to benefit from chemotherapy.168,177 This may be an artifact of retrospective subset analysis; in GOG trials for women with metastatic endometrial cancer, despite their different biology, serous carcinomas had response rates to doxorubicin/cisplatin or doxorubicin/cisplatin/paclitaxel chemotherapy no different from those of nonserous carcinomas.180 Women with early-stage serous carcinomas are eligible for both the PORTEC-3 and GOG-249 studies.
Locally Advanced Disease and Palliation
Locally Advanced Disease: Stages III and IV
The FIGO stage III category includes patients with a wide variation in tumor volume and local extension, and with a wide range of survival rates. In the FIGO 2009 revised staging system, positive peritoneal cytology as an isolated finding has been removed from stage IIIA based on data showing that patients with cytology as a sole adverse factor have outcomes similar to patients with stage I disease.181,182 To best assess treatment, stage III disease must be evaluated as several different entities rather than as a single stage or disease entity.
Most patients with stage III endometrial carcinoma have been treated with adjuvant pelvic irradiation.183–186 In view of the high rate of abdominal relapse, the use of whole-abdomen irradiation (WAI) has been supported, and several studies have demonstrated favorable results with this modality.187,188 However, because of the toxicity and limited efficacy of WAI, adjuvant chemotherapy has been studied in locally advanced disease. The results of GOG-122,168 a randomized trial comparing WAI with doxorubicin-cisplatin chemotherapy in advanced (stages III to IV) endometrial carcinoma were discussed previously (see Adjuvant Chemotherapy section). Current studies are focusing on the relative benefits of chemotherapy and pelvic or involved field (pelvic and para-aortic) irradiation, alone or combined.
Stage IIIA disease
FIGO stage IIIA disease varies from serosa breakthrough or isolated adnexal metastasis to extensive macroscopic involvement of the pelvic tissues and ovaries. Patients with microscopic stage IIIA disease have a better outcome than those with gross clinical stage III disease.184,189 Patients with stage IIIA disease due to involvement of the serosa have a better DFS (42% vs. 20%) than patients with multiple extrauterine disease sites.190 The number of extrauterine disease sites correlates with probability of relapse. Patients with one, two, and three or more extrauterine sites had 5-year DFS of 68%, 56%, and 0%, respectively.183 In a large series of 126 patients with stage III disease, ovarian or tubal involvement had a better outcome (60% 5-year survival) than pelvic peritoneal or parametrial involvement (44%). Local control and survival were improved with surgical resection.184
Stage IIIA disease should be treated with curative intention whenever possible. Complete debulking surgery with radiation and/or chemotherapy based on histologic findings is the preferred treatment approach, preferably within the scope of a randomized trial.191,192 For unresectable disease, depending on the tumor extent and patient condition, irradiation and/or chemotherapy could be considered followed by surgery if the tumor becomes resectable. Patients with very advanced disease and/or poor general condition should be considered for palliative hormonal therapy and/or radiation therapy in case of symptoms.192
Stage IIIB disease
Because stage IIIB disease is rare (<1% of all endometrial carcinomas), outcome data are scarce. Nicklin and Petersen193 reported inferior outcome for clinical stage IIIB disease compared with stage IIIC, but survival was not different from that of patients with stage IIIC or IV disease. FIGO 2009 staging has included tumors with parametrial extension in the IIIB category.85 Treatment of patients with stage IIIB disease should be individualized; and depending on the degree of vaginal extension, it may involve radical surgery with radiation therapy and brachytherapy depending on histologic findings or primary radiation therapy.
Stage IIIC Disease
Stage IIIC disease varies from microscopic involvement of a single pelvic node to extensive macroscopic disease involving the pelvic and para-aortic nodes. Patients with stage IIIC disease with nodal involvement only have a more favorable outcome compared with patients with both nodal and adnexal disease or extensive pelvic involvement. Women with only pelvic node metastases have a better outcome than those with metastases in pelvic and para-aortic nodes, and disease-free survival up to 80% has been reported.194–196
The most effective treatment approach to stage IIIC disease is generally agreed to be TAH-BSO with debulking of all macroscopic nodes (or complete pelvic and para-aortic lymphadenectomy) followed by pelvic and para-aortic radiotherapy.197,198,199 In a retrospective analysis of 71 women treated for stage IIIC endometrial cancer, 50 received definitive pelvic or extended field radiotherapy (EFRT) with or without systemic therapy (regional RT group), whereas 18 received adjuvant platinum-based chemotherapy or hormonal therapy without EBRT. Five-year pelvic-RFS (98% vs. 61%, p = .001), DSS (78% vs. 39%, p = .01), and OS (73% vs. 40%, p = .03) were significantly better for the regional RT group than the systemic therapy group. DSS was not significantly correlated with number of nodes removed in the regional radiation therapy group but was in patients treated without regional RT (p = .001). Relapses in patients treated with EBRT primarily occurred in patients with grade 3 cancer who may be most likely to benefit from combined chemoradiation.198
The risk of microscopic para-aortic disease increases with clinical stage. Microscopic para-aortic nodal metastases were diagnosed in 10%, 22%, and 71% of patients with clinical stage I with risk factors, stage II, and stage III disease, respectively.200 EFRT is indicated for documented para-aortic node involvement. A retrospective analysis reported 27% 5-year DFS for 11 patients with microscopic para-aortic nodal disease treated with EFRT, but none of 8 patients treated with pelvic RT combined with progestins survived.201 Complete removal of macroscopic or microscopic para-aortic nodal disease followed by EFRT resulted in a 5-year OS of 46% and 53% among 50 and 17 patients, respectively, whereas patients with residual macroscopic nodal disease or omission of para-aortic field radiation had significantly decreased survival rates (13% to 18%).200,202,203 The combination of extended surgery and EFRT has, however, a substantial complication rate, with reported intestinal obstruction rates of 12% to 27%.200,201 These results are reported from a time period when intensity-modulated radiation therapy (IMRT) was not available to potentially increase the dose delivered to the nodal area while giving better ability to spare organs at risk for radiation damage. This technique may improve the therapeutic ratio. EFRT therefore should be reserved for patients with suspected or proven para-aortic lymph node involvement. However, it has been suggested that EFRT should be used for patients with positive pelvic nodes in combination with other adverse factors such as grade 3 disease.194 The randomized GOG-184 trial confirmed that chemotherapy can be given after EFRT. Women (mostly with stage III disease) were treated with “involved-field” RT (pelvic RT in women with negative para-aortic nodes and pelvic RT plus EFRT in women with positive para-aortic nodes or no nodal sampling) followed by six cycles of doxorubicin/cisplatin or doxorubicin/cisplatin/paclitaxel chemotherapy.204 No survival differences were observed with the addition of paclitaxel to doxorubicin/cisplatin chemotherapy in this setting, with 3-year RFS of 64% and 62% with and without paclitaxel. Granulocyte colony-stimulating factor was required in both treatment groups in order to be able to deliver the chemotherapy. The cumulative probability of a late grade 3 or higher treatment-related gastrointestinal adverse event was 5% (all RT groups combined). As seen in other reports, grade 1 disease had a relatively good prognosis even in this more advanced disease setting: relative to grade 1 endometrioid adenocarcinoma, grade 3 endometrioid adenocarcinoma had a relapse-free survival hazard ratio of 3.12, similar to the hazard ratio of 3.45 for clear cell histology. Serous histology had the largest effect on RFS relative to grade 1 endometrioid adenocarcinoma, with a hazard ratio of 4.43.204
Stage IV Disease
Patients with stage IV disease have a poor prognosis with reported median survival of 12 to 15 months. Retrospective analyses have shown that maximal cytoreduction is an important prognostic factor, with median survival of 18 to 30 months for optimal (<2 cm) cytoreduction, in contrast to 3 to 7 months without debulking.205–207 However, if debulking did not result in optimal cytoreduction, survival was severely impaired owing to a 37% rate for major postoperative complications and a 13% rate for mortality.207 In an analysis of 45 stage III-IV patients treated after surgery (usually TAH-BSO) with chemotherapy (four courses of cisplatin, epirubicin, and cyclophosphamide) and radiation therapy, 9-year PFS and OS of 30% and 53%, respectively, were reported.208 Neoadjuvant chemotherapy (carboplatin/paclitaxel) followed by cytoreduction was used in a series of 30 patients with stage IV disease. Partial and complete response rates were 67% and 7%. Optimal debulking was achieved in 80%. Still, median PFS and OS were only 13 and 23 months.209 In an analysis of 41 cases, 28 patients had clinical diagnosis of stage IV endometrial carcinoma before surgery or without surgery, and PFS and OS were 10.4 and 21.3 months, respectively. Grade 1 or 2 endometrioid subtype and 0 or 1 sites of extraperitoneal metastasis were independent predictors of survival, whereas neither surgery as primary therapy nor optimal cytoreduction was significantly related to OS.210 The indication for surgery and decision whether maximal surgery is undertaken, or a palliative approach is chosen, should be carefully made.192
Radiation Therapy for Recurrent Disease
Management of locoregional recurrent disease requires consideration of patient, treatment and tumor-related factors, including prior surgical and radiation therapy, extent and location of the relapse, the presence of distant metastases, and the performance status of the patient. Factors determining the prognosis after treatment for relapse are the size and stage of the recurrence, the initial histology and grade, the relapse-free interval, and the initial treatment.211–216
Reported local control rates after radical treatment for isolated vaginal relapse in previously unirradiated patients range from 80% to 90% for patients with recurrences confined to the mucosa to 60% to 80% for those with more advanced disease. Five-year OS are 40% to 70%.131,211,214,217–219 For patients previously treated with pelvic irradiation, survival rates after vaginal relapse of 10% to 30% have been reported. In the PORTEC trial, the 5-year actuarial survival rate after vaginal relapse was 38% in the radiation therapy group and 70% in the control group.
Curative treatment for isolated vaginal recurrence in patients not previously irradiated consists of pelvic irradiation and vaginal brachytherapy. The use of the brachytherapy boost is essential.218 Distal vaginal recurrence in the suburethral region or more extensive vaginal recurrence requires careful individualized BT planning to encompass the target volume with vaginal cylinder or interstitial BT, or both.220
Pelvic and distant relapses generally have a poor prognosis. Complete remission rates of treatment for pelvic relapse range from less than 5% for patients who had previous pelvic RT and those with sidewall disease to 5% to 30% for patients not previously irradiated.221 To try to improved prognoses in this situation, the GOG has initiated a randomized trial (GOG-238) of pelvic RT with or without concurrent weekly cisplatin in patients with pelvic-only relapses.
The frequency of follow-up examinations and their value in detecting asymptomatic relapses have been subject of discussion.222–225,226 It has been argued that 50% to 75% of women with disease relapse were symptomatic and that regular follow-up is not cost effective because patients can only occasionally be salvaged after relapse. However, patients with early-stage disease treated with surgery alone should be observed closely, especially during the first 3 years, to diagnose vaginal relapse at an early, usually asymptomatic stage, because salvage rates are high. Follow-up examinations should include a careful history focusing on symptoms of recurrence or of side effects of treatment and visual inspection of the vagina and bimanual rectovaginal examinations. Papanicolaou smears or biopsy samples should be taken only if abnormalities are found. Because distant relapse only occasionally can be treated with a view to a long symptom-free interval, it cannot be recommended to actively screen for distant metastases outside the scope of clinical trials. In general, after the first 2 years less frequent and more patient-directed follow-up visits are preferred, after specific patient education with written information as to which symptoms would necessitate evaluation and with contact numbers they could use for rapid access.226
Radiation Therapy for Palliation
Radiation therapy has an important role in palliation of symptoms from locally advanced, recurrent, or metastatic endometrial cancer. Vaginal bleeding, vaginal discharge, or pain from advanced or recurrent disease or lymphedema from enlarged pelvic lymph nodes can be successfully palliated with RT. Commonly used schedules include a short course of fractionated EBRT using 3- to 4-Gy fractions to a dose of 20 to 30 Gy over 1 to 2 weeks or, depending on the general condition and symptoms, shorter schedules of 16 to 24 Gy in 8-Gy fractions can be used. Other sites of symptomatic metastases, such as brain metastases or distant lymph node disease, are uncommon but can also be effectively palliated with similar radiation doses. Rapid and effective palliation of pain from bone metastases can be obtained using a single 8-Gy dose.227
Systemic Therapy for Recurrent or Metastatic Disease
Hormonal Therapy
Progestational agents are the standard hormonal therapy for advanced or metastatic grade 1 or 2 disease, especially for progesterone receptor-positive tumors.228,229,230,231 Reported response rates range from 15% to 34%, with a median response duration of several months. For progesterone receptor and/or estrogen receptor positive tumors response rates up to 77% have been observed, in contrast to 9% for receptor-negative tumors.228,229 However, levels of progesterone receptor positivity (using current immunohistochemical assays) that predict response to hormonal therapy are not well established. The GOG has used alternating cycles of tamoxifen and progestin therapy in an attempt to use tamoxifen to up-regulate the progesterone receptor, reporting a response rate of 27%.232 Response rates to such therapy have not been compared with single agent progestin therapy in any randomized trials. For patients with disease progression after a response to progestins, tamoxifen and aromatase inhibitors may be considered.
Progestational agents are the standard hormonal therapy for advanced or metastatic grade 1 or 2 disease, especially for progesterone receptor-positive tumors.228,229,230,231 Reported response rates range from 15% to 34%, with a median response duration of several months. For progesterone receptor and/or estrogen receptor positive tumors response rates up to 77% have been observed, in contrast to 9% for receptor-negative tumors.228,229 However, levels of progesterone receptor positivity (using current immunohistochemical assays) that predict response to hormonal therapy are not well established. Many of the large trials of progestin therapy were completed years ago, and it is not certain that response rates would be as high using more stringent current response criteria. Toxicity reporting was also less complete in the older studies, and progestins cause weight gain and increase thrombosis risk in a population already prone to these complications. Nonetheless, for relatively asymptomatic patients with unknown receptor status and grade 1 or 2 disease, progestins should be considered. Grade 3 disease is unlikely to respond to hormonal therapy (<10%). High-dose therapy (800 mg/day of megestrol acetate or 1000 mg/day of medroxyprogesterone acetate [MPA]) was not found to be more effective than standard doses (160 mg/day of megestrol or 200 mg/day of MPA).230,231 The GOG has used alternating cycles of tamoxifen and progestin therapy in an attempt to use tamoxifen to up-regulate the progesterone receptor, reporting a response rate of 27%.232 Response rates to such therapy have not been compared with single agent progestin therapy in any randomized trials. For patients with disease progression after a response to progestins, tamoxifen and aromatase inhibitors may be considered.
Chemotherapy
Platinum drugs, anthracyclines, and taxanes are the most active agents tested to date for endometrial carcinoma, and multiagent chemotherapy has been shown to produce higher response rates than single-agent therapy.* Platinum- or taxane-containing multiagent therapy provides response rates of 30% to 75%, with median remission durations of 6 to 12 months.230,233,234,235 Median OS on most trials of chemotherapy for metastatic or recurrent endometrial carcinoma is only in the range of 12 months. The best published results in a randomized trial come from the GOG-177 trial comparing doxorubicin/cisplatin therapy with paclitaxel/doxorubicin/cisplatin with filgrastim support. The triple-drug regimen showed an improved response rate (57% vs. 34%) and somewhat longer survival (58% vs. 50% 1-year survival) for paclitaxel/doxorubicin/cisplatin, but at the cost of significant paclitaxel neurotoxicity.235 Carboplatin/paclitaxel therapy, which is the standard for ovarian cancer and is used commonly in the community, has less toxicity than doxorubicin/cisplatin or paclitaxel/doxorubicin/cisplatin, and it may be equally effective.236,237
Platinum drugs, anthracyclines, and taxanes are the most active agents tested to date for endometrial carcinoma, and multiagent chemotherapy has been shown to produce higher response rates than single-agent therapy.* Platinum- or taxane-containing multiagent therapy provides response rates of 30% to 75%, with median remission durations of 6 to 12 months.230,233,234,235 Median OS on most trials of chemotherapy for metastatic or recurrent endometrial carcinoma is only in the range of 12 months. The best published results in a randomized trial come from the GOG-177 trial comparing doxorubicin/cisplatin therapy with paclitaxel/doxorubicin/cisplatin with filgrastim support. The triple-drug regimen showed an improved response rate (57% vs. 34%) and somewhat longer survival (58% vs. 50% 1-year survival) for paclitaxel/doxorubicin/cisplatin, but at the cost of significant paclitaxel neurotoxicity.235 Carboplatin/paclitaxel therapy, which is the standard for ovarian cancer and is used commonly in the community, has less toxicity than doxorubicin/cisplatin or paclitaxel/doxorubicin/cisplatin, and it may be equally effective.236,237 A large randomized GOG noninferiority trial comparing carboplatin/paclitaxel with paclitaxel/doxorubicin/cisplatin (GOG-209) has completed accrual, and results should be available soon. The epothilone agent ixabepilone is a newer cytotoxic drug that produced a 12% response rate in a GOG trial as second-line therapy for endometrial carcinoma.238 It is currently being compared in an industry-sponsored randomized phase III trial to either doxorubicin or paclitaxel (depending on which agent the patient has not already received) in women with endometrial carcinoma that has progressed on front-line therapy.
Current interest has focused on developing and evaluating molecular targeted therapies and integrating them with cytotoxic chemotherapy.239,240 A preliminary report of a GOG phase II trial of the anti-VEGF monoclonal antibody bevacizumab in women with endometrial carcinoma already treated with one to two prior chemotherapy regimens noted a promising 15% major response rate.239 Trials with the anti-VEGF agent aflibercept and VEGF receptor tyrosine kinase inhibitors such as sorafenib and sunitinib are ongoing or have been completed, and results should be available soon. Preliminary reports of a trial of sorafenib in women with one prior chemotherapy regimen noted a 5% major response rate.240 The current front-line GOG trial for women with advanced or recurrent disease is a randomized phase II study testing paclitaxel/carboplatin/bevacizumab versus paclitaxel/carboplatin/temsirolimus versus ixabepilone/carboplatin/bevacizumab.
Current interest has focused on developing and evaluating molecular targeted therapies and integrating them with cytotoxic chemotherapy.239,240 Anti-ERBB2 and anti-EGFR agents (discussed earlier) have not yet provided much promise in clinical trials, but trials with newer agents and attempts to develop better targeting are ongoing. Inhibitors of mTOR have reproducible but modest activity as single agents. Preclinical data suggest that they should enhance the activity of cytotoxic chemotherapy, and a variety of early-stage trials are underway to develop tolerable combination regimens. Angiogenesis plays a part in growth and spread of endometrial carcinoma, as with most solid tumors, and anti–vascular endothelial growth factor (VEGF) agents are of considerable interest. A preliminary report of a GOG phase II trial of the anti-VEGF monoclonal antibody bevacizumab in women with endometrial carcinoma already treated with one to two prior chemotherapy regimens noted a promising 15% major response rate.239 Trials with the anti-VEGF agent aflibercept and VEGF receptor tyrosine kinase inhibitors such as sorafenib and sunitinib are ongoing or have been completed, and results should be available soon. Preliminary reports of a trial of sorafenib in women with one prior chemotherapy regimen noted a 5% major response rate.240 The current front-line GOG trial for women with advanced or recurrent disease is a randomized phase II study testing paclitaxel/carboplatin/bevacizumab versus paclitaxel/carboplatin/temsirolimus versus ixabepilone/carboplatin/bevacizumab.
Significant challenges remain in selecting from the many available new agents and choosing the (sometimes uncommon) subset of patients likely to have significant benefit. For one example, activating mutations of the fibroblast growth factor receptor-2 gene (FGFR2) have been identified in 10% to 12% of endometrial carcinomas, and may respond to targeted therapy.241
Significant challenges remain in selecting from the many available new agents and choosing the (sometimes uncommon) subset of patients likely to have significant benefit. For one example, activating mutations of the fibroblast growth factor receptor-2 gene (FGFR2) have been identified in 10% to 12% of endometrial carcinomas, and may respond to targeted therapy.241 Brivanib is an oral multitargeted tyrosine kinase inhibitor whose spectrum includes the VEGF and FGF receptor families. It is currently being tested in a GOG trial that will also seek to determine whether activating FGFR2 mutations predict for response.
Treatment Of Uterine Sarcomas
Uterine sarcomas are rare, and management therefore varies because data from randomized studies are lacking. Most patients present with vaginal bleeding, much like patients with adenocarcinomas, but the tumor is typically larger before this occurs. Uterine sarcoma may also be an unexpected diagnosis in a uterus resected for presumed symptomatic leiomyoma.242 Other patients can present with local discomfort from uterine enlargement.
Undifferentiated uterine sarcoma, formerly called high-grade endometrial stromal sarcoma, should be treated with more extensive surgery. Carcinosarcomas and undifferentiated uterine sarcomas warrant lymph node evaluation, and most current authors favor full surgical staging, including TAH-BSO, peritoneal cytology, full pelvic lymph node dissection, and omentectomy. Clinical data support the view that carcinosarcomas should be considered high-risk carcinomas and should be treated accordingly.243 In leiomyosarcoma, the risk of lymph node involvement is very low, and lymphadenectomy is therefore not indicated.
Retrospective studies of adjuvant radiation therapy have shown improved pelvic control, especially for carcinosarcomas, but no survival benefit.243–247 The EORTC-55874 prospective, randomized trial compared pelvic RT versus no further therapy in 224 patients with uterine sarcomas stage I and II (103 leiomyosarcomas, 91 carcinosarcomas, 28 endometrial stroma sarcomas). Radiation therapy provided a significant reduction of pelvic recurrence for carcinosarcomas, with 5-year pelvic recurrence rates of 22% with irradiation versus 40% for the control group (p = .0004). However, survival was not improved (5-year OS of 58% and 56%, p = .92). This trial showed the need to separate the different sarcoma types. Carcinosarcomas were found to benefit from pelvic irradiation in terms of pelvic control. Leiomyosarcomas had no benefit from radiation therapy (20% vs. 24% pelvic recurrence) and were most likely to relapse at distant sites. There were too few endometrial stromal sarcomas included to allow specific analysis.248
Chemotherapy for recurrent or metastatic sarcomas has limited efficacy. Agents with activity against carcinosarcoma include ifosfamide, platinum drugs, and taxanes.249–251,252,253–257 Combination therapy with cisplatin, doxorubicin, and ifosfamide was found to produce an overall response rate of 56%, albeit with considerable toxicity.250 Two randomized trials of first-line treatment for advanced disease have compared single-agent therapy with ifosfamide to ifosfamide plus cisplatin251 and to ifosfamide plus paclitaxel,252 respectively; in each case the combination produced higher response rates at the cost of added toxicity; the addition of paclitaxel also improved survival, with response rates of 29% and 45% and median overall survival of 8.4 and 13.5 months for single-agent and combination therapy, respectively.
Chemotherapy for recurrent or metastatic sarcomas has limited efficacy. Agents with activity against carcinosarcoma include ifosfamide, platinum drugs, and taxanes.249–251,252,253–257 Combination therapy with cisplatin, doxorubicin, and ifosfamide was found to produce an overall response rate of 56%, albeit with considerable toxicity.250 Two randomized trials of first-line treatment for advanced disease have compared single-agent therapy with ifosfamide to ifosfamide plus cisplatin251 and to ifosfamide plus paclitaxel,252 respectively; in each case the combination produced higher response rates at the cost of added toxicity; the addition of paclitaxel also improved survival, with response rates of 29% and 45% and median overall survival of 8.4 and 13.5 months for single-agent and combination therapy, respectively. A prospective phase II trial of carboplatin plus paclitaxel in chemotherapy-naive patients produced a response rate of 52%, and would appear to be better tolerated than ifosfamide-based regimens.253 An ongoing phase III trial is testing the combination of carboplatin/paclitaxel versus the combination of ifosfamide/paclitaxel in women with newly diagnosed stage I to IV persistent or recurrent uterine carcinosarcoma.
Ifosfamide and doxorubicin have single-agent activity in the treatment of advanced/metastatic uterine leiomyosarcoma, and the combination of the two has produced a response rate of 30%.254 More recently, a prospective trial of single-agent gemcitabine has been reported to produce a 20.5% response rate as second-line therapy,255 and the combination of docetaxel and gemcitabine produced a 28% response rate as second-line chemotherapy256 and a 35.8% response rate as first-line chemotherapy257 for metastatic disease; an ongoing phase III study is testing the addition of bevacizumab to the gemcitabine/docetaxel combination.
Adjuvant chemotherapy has been investigated in uterine sarcomas in view of their increased risk of distant metastases. A small older (n = 156 total, 48 leiomyosarcoma, 93 carcinosarcoma, 15 other sarcoma) randomized study evaluating adjuvant doxorubicin showed no benefit.258 A retrospective study among 49 patients with stage I to IV carcinosarcoma showed nonsignificantly better survival after chemotherapy (carboplatin and paclitaxel) with or without RT than after RT alone (3-year OS of 66% vs. 34%, p = .15).259 Another retrospective study, again among 49 patients, suggested the combination of RT and chemotherapy (sequential) to be more effective than chemotherapy or RT alone.260 A prospective GOG study using adjuvant ifosfamide and cisplatin (without radiation therapy) among 65 patients with stage I to II carcinosarcoma showed the combination to be tolerable, but impact on PFS (69%) and OS (82% at 2 years) was unclear owing to absence of controls, and the pelvic recurrence rate was high.261 A randomized GOG trial among 232 patients with stage I to IV (45% stage III) carcinosarcoma compared chemotherapy (three cycles of cisplatinum, ifosfamide, and mesna) with WAI (30 Gy, with pelvic boost to 50 Gy). The recurrence rate was nonsignificantly lower for chemotherapy than for WAI (52 vs. 58%, HR = 0.789, p = .24), and survival was nonsignificantly better (47% vs. 34%, p = .08). After chemotherapy alone, more vaginal recurrences were found.262 As discussed earlier, carboplatin/paclitaxel is currently being compared with ifosfamide/paclitaxel in this same setting. Further trials investigating platinum-based regimens with sequential RT are needed to determine optimal treatment of patients with uterine carcinosarcoma.
A prospective phase II trial of adjuvant gemcitabine plus docetaxel for completely resected high-grade uterine leiomyosarcoma reported 59% 2-year PFS among the 18 patients with stage I or II disease263; much larger randomized trials will be needed to demonstrate any benefit from adjuvant chemotherapy in this setting.
Irradiation Techniques and Tolerance
External Beam Pelvic Irradiation
Most patients receive pelvic radiation therapy in the postoperative setting. The clinical target volume for pelvic radiation therapy consists of the parametrial tissues, the proximal half of the vagina, and the pelvic lymph node areas (i.e., internal and external iliac and the lower common iliac lymph node regions). The planning target volume is derived by adding an appropriate margin around the clinical target to allow for the beam penumbra, geometric uncertainties, and variability in daily patient setup. The main organs at risk for complications are the rectosigmoid, small bowel, and bladder. Most acute and late complications are of the gastrointestinal tract, and it is essential to reduce the volume of small bowel within the treatment fields as much as possible. A four-field box technique has traditionally been employed, which had a smaller volume of small bowel in the high-dose region compared with anterior and posterior parallel-opposed ports. The prone treatment position has been found to reduce the volume of small bowel within the pelvis, and special belly board devices have been designed that are more effective in pushing the small bowel out of the treatment fields and that ensure reproducible positioning by acting as a positioning device.264 The use of a belly board device has been shown to be more effective in the postoperative setting than for primary treatment. In thin patients with firm abdominal walls, there may be less benefit. High-energy photons from a linear accelerator are preferred to ensure a homogeneous dose distribution.
Most radiation therapy departments are using three-dimensional CT planning, and multileaf collimators are used in all fields to shield organs at risk and reduce the treated volume. The use of CT planning has the clear advantage of delineating the actual nodal sites, instead of using standard field borders, which risk insufficiently or too generously encompassing the lymphatic chains, and determining field borders based on bony landmarks should be considered obsolete. Excellent contouring atlases have been published265,266 that guide the radiation oncologist to ensure adequate delineation of the areas at risk (Fig. 57-2, A to C).
A total dose of 45 to 46 Gy is usually prescribed in the postoperative setting, with daily fractions of 1.8 to 2.0 Gy for five fractions per week. For cases with involved margins or residual macroscopic disease, the target dose is 50.4 Gy with a boost dose to the areas of involvement using CT planning to a cumulative dose of 60 to 70 Gy, depending on the situation. Ideally, these risk areas should be marked with clips during surgery and an omentoplasty should be used to move the small bowel out of the high-dose area.267
Intensity-Modulated Radiation Therapy
IMRT uses multiple beams of various intensities that conform the high-dose region to the shape of the target tissues in three dimensions, minimizing the dose to the surrounding organs. Treatment planning studies268–270 have shown that a substantial reduction of the doses to the small bowel, rectum, and the pelvic bone marrow can be realized (Fig. 57-3). The volume of small bowel irradiated to the prescription dose is reduced by a factor of 2, compared with a CT-based, conventional, three-dimensional, four-field box technique.268,270 Initial results of clinical studies have shown a modest reduction of acute gastrointestinal toxicity in patients treated with pelvic IMRT268 and of acute hematologic toxicity in gynecologic cancer patients receiving pelvic IMRT combined with chemotherapy.271 In an analysis of 36 gynecologic cancer patients treated with pelvic irradiation with or without chemotherapy or brachytherapy (or both), IMRT also resulted in a significant reduction of patients experiencing chronic gastrointestinal toxicity (11% mild symptoms compared with 50% in a historical series of patients receiving pelvic irradiation) and less severe gastrointestinal complications (0% vs. 3%).272 Especially for the increasing number of high-risk endometrial carcinoma patients treated with surgery, radiation therapy, and chemotherapy, IMRT may be an essential tool in reducing the chronic toxicities of multimodality treatment.
Complications of Pelvic Irradiation
Side effects of radiation therapy for gynecologic malignancies have been well documented. Complication rates are dose and volume dependent and are higher for the combination of pelvic EBRT and vaginal brachytherapy than for EBRT or vaginal brachytherapy alone.125,151,152,273,274 Complication rates after EBRT are also increased if a full lymphadenectomy is added to the hysterectomy.274–278 Other treatment-related factors associated with the risk of complications are treatment volume, daily fractionation, and irradiation technique.276,279,280 Patient-related risk factors are prior abdominal surgery, young age, low weight, concurrent diabetes, hypertension, inflammatory bowel disease, or other pelvic inflammatory conditions, although literature data show conflicting results regarding several of these factors. The most significant complications are obstruction of the small bowel, chronic diarrhea, proctitis, fistula formation, vaginal stenosis, and insufficiency fractures of bone. The use of EBRT is associated with the risk of small bowel complications.278 The addition of vaginal brachytherapy to EBRT especially increases the incidence of vaginal and rectal side effects, such as fibrosis, stenosis, ulcers, and fistula.151,152,274,275 Reported rates of severe complications after TAH-BSO plus EBRT range from 2% to 6%; after surgery followed by EBRT and vaginal brachytherapy, from 4% to 13%; after vaginal brachytherapy alone, from 0% to 7% (dose dependent); and after EBRT and surgery, including lymphadenectomy, from 7% to 18%.
The rates of mild to moderate complications are less well established. Acute side effects of EBRT, such as diarrhea, urgency, abdominal cramps, and urinary frequency are usually not reported if they do not cause an interruption or discontinuation of treatment.281 Mild late side effects (including increased bowel movements, episodes of diarrhea, abdominal cramps, frequent urination, and minor incontinence) are underreported.282,283
In the PORTEC trial, 63% of the patients were treated during radiation therapy with medication or dietary measures, or both, for mild, acute symptoms. Discontinuation of irradiation due to acute symptoms occurred in 2% of cases.284 In the ASTEC trial, any acute toxicity was reported in 57% of radiation therapy patients versus 27% for controls and were mostly mild (32%) or moderate symptoms. Severe acute toxicity was reported in 3% versus less than 1% of patients.129
The 5-year actuarial rates of any late complication in the PORTEC trial were 26% in the RT group and 4% in the control group (p <.0001). Most complications (67%) were mild (grade 1), and almost 50% of symptoms resolved in the course of 2 to 3 years. Gastrointestinal symptoms were the predominant side effects, with a 5-year actuarial rate of (mostly mild) gastrointestinal complications in the radiation therapy group of 20%. The 5-year actuarial rates of severe (grade 3 or 4) late complications were 3% in the radiation therapy group and 0% in the control group. No severe late genitourinary or vaginal complications occurred.284 In the ASTEC trial, slightly higher late toxicity rates were reported: grade 1, 2, 3, and 4 predominantly gastrointestinal (GI) or urogenital late toxicity was reported in 30%, 22%, 7%, and 1%, respectively, of EBRT patients, compared with 24%, 16%, 35, and 0% among control subjects.129
The presence of acute treatment-related symptoms is one of the most important risk factors for late complications.284 The association between acute and late treatment complications has become a topic of interest and research.285–288 The fact that a subset of patients with acute toxicity have no symptom-free interval between acute and late complications supports the theory that late injury occurs as a consequence of persisting acute injury of the intestinal mucosa.285 Pelvic RT is associated with short-term and long-term effects on GI function, most notably increased frequency of bowel actions, less bile acid absorption, and faster intestinal transit.283 Most of these changes improve with time, but some long-term effects persist.
Results from the PORTEC-2 trial showed that rates of complications after vaginal brachytherapy alone were very low. During irradiation there was a significantly increased rate of acute RTOG grade 1 and 2 acute gastrointestinal symptoms among EBRT patients as compared with patients who had vaginal brachytherapy (53% vs. 12%, p <.001). This difference decreased with further follow-up and lost significance after 24 months. Late grade 3 gastrointestinal toxicity was reported in four (1.9%) EBRT patients and in one (0.5%) vaginal brachytherapy patient who required surgery for bowel obstruction owing to adhesions or fibrosis. Late grade 2 moderate mucosal atrophy (without narrowing or shortening) was significantly more frequent after vaginal brachytherapy than after EBRT (17% vs. 4% after 2 years). Grade 3 atrophy (marked atrophy with or without shortening or narrowing) was reported in only one (0.5%) EBRT patient and in four (1.9%) vaginal brachytherapy patients. Patient-reported rates of sexual activity increased during the first 6 months after treatment and remained stable thereafter, without significant differences between the treatment groups.144
Brachytherapy Techniques
In primary treatment of endometrial carcinoma, brachytherapy has traditionally been administered with LDR techniques such as Heyman packing or Simon capsules or with a single intrauterine tube, depending on the size of the uterus. The technical aspects of intracavitary placement of a single tube are similar to those for cervical carcinoma, but different dose specification points have been used (e.g., point My for myometrium, 2 cm laterally and caudally to the tip of the most advanced applicator, or the A-line, 2 cm laterally from the tip). A small uterus can be treated with a single tube, using a stepping source and increasing the dwell times at the fundus to obtain a pear-shaped isodose distribution (i.e., inverted pear). However, an individualized approach is preferable, with dose specification at the serosa at the site of the tumor. MRI or CT is used, ideally with the intrauterine tubes in place, to determine the depth of tumor invasion and the myometrial width in relation to the tubes. CT or MRI planning ensures adequate coverage of the tumor while avoiding overdosing in the bowel surrounding the uterus. Specific uterine applicators for high- and pulsed-dose-rate intracavitary brachytherapy are currently available. An intermediate-size uterus can be treated with a two-channel applicator (one applicator in each uterine cornu), which results in a pear-shaped isodose distribution at the fundus. For treatment of a large uterus with a tumor deeply infiltrating the myometrium, Heyman packing using Norman-Simon applicators is preferred. The American Brachytherapy Society has published treatment guidelines.289
Vaginal Brachytherapy
In patients with vaginal recurrence the most common site is the upper vagina, and vaginal cuff brachytherapy has long been used to decrease the risk of vaginal cuff recurrence after hysterectomy. Since the mid-1990s, HDR fractionated vaginal brachytherapy alone has been increasingly used as adjuvant therapy after hysterectomy in selected patients. There are institutional variations in the target volume for vaginal brachytherapy, especially the length of the vagina to be treated—the vaginal cuff, proximal half or two thirds of the vagina, or the entire vagina. Vaginal recurrences are mainly located in the vaginal vault and upper vagina.131 It is recommended to treat only the upper half of the vagina, because this is associated with lower complication rates (i.e., vaginal stenosis and fistula) than treating the entire length of the vagina. Although most radiation oncologists use vaginal cylinders treating the upper half to two thirds of the vagina, others claim that vaginal colpostats have the advantage of providing a better dose distribution around the vaginal vault with lower doses to the bladder and rectum.135
Convenient, moderate-dose fractionation schedules of vaginal brachytherapy provide very high vaginal control rates (>95%) and very low morbidity rates.142 The use of higher doses increases the risk of side effects and does not further increase the vaginal control rates. Based on these data, optimal LDR and HDR schedules seem to be those that give an equivalent of 45 to 50 Gy to the mucosal surface of the upper half of the vagina. Typical examples are LDR 30 Gy, specified at a 5-mm depth at a dose rate of 60 to 65 cGy per hour and given in one session of 2 to 3 days, or HDR 21 Gy, specified at a 5-mm depth and given in three fractions of 7 Gy each, 1 week apart.
Usually, the dose is specified at 5 mm from the surface and 5 mm from the apex of the vagina. Because of the distention of the vagina after placement of the vaginal cylinder, it has been argued that the wall becomes less than 5 mm thick and that the reference isodose should be chosen at a 3-, 4-, or 5-mm depth, depending on a clinical estimation of the mucosal thickness. By individualizing the depth and avoiding applicators with small diameters, the rate of vaginal side effects was significantly reduced (17% and 1% for grade 1 and 2, compared with 26% and 8%), without a difference in vaginal control.290 In individual cases, the target depth can be adjusted according to the clinical situation. For example, in case of a thick scar at the cuff the dose can be specified at 10 mm from the apex and 5 mm along the wall. CT or MRI planning should be utilized for treatment of such thicker targets and especially for brachytherapy treatment of vaginal recurrence. CT- and MRI-compatible cylinders and colpostats are available for this purpose.
Treatment Algorithm, Conclusions, and Challenges
Management of each endometrial cancer patient must consider the risk factors for tumor relapse and tolerance of the treatment that is being considered. Ideally, patient evaluation and surgery should be performed by a gynecologic oncologist or by a gynecologist with specific oncology expertise and dedication. Deciding on the indication for and extent of surgery and adjuvant therapy requires a multidisciplinary team of gynecologic oncologists, radiation oncologists, and medical oncologists. The approach to the management of endometrial cancer patients is summarized in the treatment algorithm (Fig. 57-4) and the follow-up algorithm (Fig. 57-5).
29 Renkonen-Sinisalo L, Butzow R, Leminen A, et al. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer. 2007;120:821-824.
39 Creasman WT, Kohler MF, Odicino F, et al. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol. 2004;95:593-596.
47 Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group study. Cancer. 1987;60:2035-2041.
56 Fleming GF, Sill MW, Darcy KM, et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma. A Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:15-20.
58 Oza AM, Eisenhauer EA, Elit L, et al. Phase II study of erlotinib in recurrent or metastatic endometrial cancer. NCIC IND-148. J Clin Oncol. 2008;26:4319-4325.
68 Catasus L, Gallardo A, Cuatrecasas M, et al. Concomitant PI3K-AKT and p53 alterations in endometrial carcinomas are associated with poor prognosis. Mod Pathol. 2009;22:522-529.
74 Aalders J, Abeler V, Kolstad P, et al. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma. Clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56:419-427.
75 Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma. Multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404-1411.
76 Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma. A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744-751.
78 Creutzberg CL, van Putten WL, Warlam-Rodenhuis CC, et al. Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients. The Postoperative Radiation Therapy in Endometrial Carcinoma Trial. J Clin Oncol. 2004;22:1234-1241.
80 Scholten AN, Smit VTHB, Beerman H, et al. Prognostic significance and interobserver variability of histologic grading systems for endometrial carcinoma. Cancer. 2004;100:764-772.
84 Briët JM, Hollema H, Reesink N, et al. Lymphovascular space involvement. An independent prognostic factor in endometrial cancer. Gynecol Oncol. 2005;96:799-804.
85 Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103-104.
91 Signorelli M, Guerra L, Buda A, et al. Role of the integrated FDG PET/CT in the surgical management of patients with high risk clinical early stage endometrial cancer. Detection of pelvic nodal metastases. Gynecol Oncol. 2009;115:231-235.
101 Malzoni M, Tinelli R, Cosentino F, et al. Total laparoscopic hysterectomy versus abdominal hysterectomy with lymphadenectomy for early-stage endometrial cancer. A prospective randomized study. Gynecol Oncol. 2009;112:126-133.
112 Aalders JG, Thomas G. Endometrial cancer—revisiting the importance of pelvic and para-aortic lymph nodes. Gynecol Oncol. 2007;104:222-231.
113 Kitchener H, Swart AM, Qian Q, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial). A randomised study. Lancet. 2009;373:125-136.
114 Benedetti PP, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma. Randomized clinical trial. J Natl Cancer Inst. 2008;100:1707-1716.
116 Khoury-Collado F, Glaser GE, Zivanovic O, et al. Improving sentinel lymph node detection rates in endometrial cancer. How many cases are needed? Gynecol Oncol. 2009;115:453-455.
129 Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials). Pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137-146.
130 Scholten AN, van Putten WL, Beerman H, et al. Postoperative radiotherapy for stage 1 endometrial carcinoma. Long-term outcome of the randomized PORTEC trial with central pathology review. Int J Radiat Oncol Biol Phys. 2005;63:834-838.
132 Kong A, Simera I, Collingwood M, et al. Adjuvant radiotherapy for stage I endometrial cancer. Systematic review and meta-analysis. Ann Oncol. 2007;18:1595-1604.
142 Petereit DG, Pearcey R. Literature analysis of high dose rate brachytherapy fractionation schedules in the treatment of cervical cancer. Is there an optimal fractionation schedule? Int J Radiat Oncol Biol Phys. 1999;43:359-366.
143 Nout RA, Putter H, Jurgenliemk-Schulz IM, et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer. First results of the randomized PORTEC-2 Trial. J Clin Oncol. 2010;375:816-823.
144 Nout RA, Smit VTHB, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial carcinoma of high-intermediate risk (PORTEC-2). An open-label, non-inferiority, randomised trial. Lancet. 2010;375:816-823.
154 Cozad SC. Stage II adenocarcinoma of the endometrium. Adjuvant radiotherapy and recurrence patterns. Int J Radiat Oncol Biol Phys. 2008;71:205-212.
165 Martin-Hirsch PL, Lilford RJ, Jarvis GJ. Adjuvant progestagen therapy for the treatment of endometrial cancer. Review and meta-analyses of published randomised controlled trials. Eur J Obstet Gynecol Reprod Biol. 1996;65:201-207.
168 Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma. A Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36-44.
169 Susumu N, Sagae S, Udagawa Y, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer. A Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
170 Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma. Results of a randomised trial. Br J Cancer. 2006;95:266-271.
171 Mundt AJ, McBride R, Rotmensch J, et al. Significant pelvic recurrence in high-risk pathologic stage I-IV endometrial carcinoma patients after adjuvant chemotherapy alone. Implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:1145-1153.
176 Greven K, Winter K, Underhill K, et al. Final analysis of RTOG 9708. Adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 2006;103:155-159.
178 Hogberg T, Signorelli M, De Oliveira CF, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer: Results from two randomised studies. Eur J Cancer. 2010;46:2422-2431.
192 Van Wijk FH, Van Der Burg ME, Burger CW, et al. Management of surgical stage III and IV endometrioid endometrial carcinoma. An overview. Int J Gynecol Cancer. 2009;19:431-446.
197 Mariani A, Webb MJ, Keeney GL, et al. Stage IIIC endometrioid corpus cancer includes distinct subgroups. Gynecol Oncol. 2002;87:112-117.
198 Klopp AH, Jhingran A, Ramondetta L, et al. Node-positive adenocarcinoma of the endometrium. Outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol. 2009;115:6-11.
204 Homesley HD, Filiaci V, Gibbons SK, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: A Gynecologic Oncology Group study. Gynecol Oncol. 2009;112:543-552.
226 Fung-Kee-Fung M, Dodge J, Elit L, et al. Follow-up after primary therapy for endometrial cancer. A systematic review. Gynecol Oncol. 2006;101:520-529.
230 Thigpen JT, Brady MF, Homesley HD, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma. A Gynecologic Oncology Group study. J Clin Oncol. 2004;22:3902-3908.
235 Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma. A Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159-2166.
239 Aghajanian CA, Sill MW, Darcy KM, et al. A phase II evaluation of bevacizumab in the treatment of recurrent or persistent endometrial cancer. A Gynecologic Oncology Group (GOG) study. J Clin Oncol. 27(Suppl, abstract 5531), 2009.
248 Reed NS, Mangioni C, Malmstrom H, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II. A European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group study (protocol 55874). Eur J Cancer. 2008;44:808-818.
252 Homesley HD, Filiaci V, Markman M, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma. A Gynecologic Oncology Group study. J Clin Oncol. 2007;25:526-531.
262 Wolfson AH, Brady MF, Rocereto T, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecol Oncol. 2007;107:177-185.
265 Taylor A, Rockall AG, Powell ME. An atlas of the pelvic lymph node regions to aid radiotherapy target volume definition. Clin Oncol (R Coll Radiol). 2007;19:542-550.
266 Small WJr, Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:428-434.
272 Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56:1354-1360.
284 Creutzberg CL, van Putten WL, Koper PC, et al. The morbidity of treatment for patients with stage I endometrial cancer. Results from a randomized trial. Int J Radiat Oncol Biol Phys. 2001;51:1246-1255.
289 Nag S, Erickson B, Parikh S, et al. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the endometrium. Int J Radiat Oncol Biol Phys. 2000;48:779-790.
1 Altekruse SF, Kosary CL, Krapcho M, et al, editors. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute, 1975-2007. Available online at seer.cancer.gov/csr/1975_2007/
2 Dutch Cancer Registry, www.ikcnet.nl/cijfers.
3 Harlow BL, Weiss NS, Lofton S. The epidemiology of sarcomas of the uterus. J Natl Cancer Inst. 1986;76:399-402.
4 Gambrell RDJr, Bagnell CA, Greenblatt RB. Role of estrogens and progesterone in the etiology and prevention of endometrial cancer. Review. Am J Obstet Gynecol. 1983;146:696-707.
5 Purdie DM, Green AC. Epidemiology of endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2001;15:341-354.
6 Shapiro S, Kelly JP, Rosenberg L, et al. Risk of localized and widespread endometrial cancer in relation to recent and discontinued use of conjugated estrogens. N Engl J Med. 1985;313:969-972.
7 Bergman L, Beelen ML, Gallee MP, et al. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of Liver and Endometrial cancer Risk following Tamoxifen. Lancet. 2000;356:881-887.
8 Fisher B, Costantino JP, Redmond CK, et al. Endometrial cancer in tamoxifen-treated breast cancer patients. Findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527-537.
9 Magriples U, Naftolin F, Schwartz PE, et al. High-grade endometrial carcinoma in tamoxifen-treated breast cancer patients. J Clin Oncol. 1993;11:485-490.
10 van Leeuwen FE, Benraadt J, Coebergh JW, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet. 1994;343:448-452.
11 Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403-412.
12 Olson JE, Sellers TA, Anderson KE, et al. Does a family history of cancer increase the risk for postmenopausal endometrial carcinoma? A prospective cohort study and a nested case-control family study of older women. Cancer. 1999;85:2444-2449.
13 Parazzini F, La Vecchia C, Moroni S, et al. Family history and the risk of endometrial cancer. Int J Cancer. 1994;59:460-462.
14 Parslov M, Lidegaard O, Klintorp S, et al. Risk factors among young women with endometrial cancer. A Danish case-control study. Am J Obstet Gynecol. 2000;182:23-29.
15 Hemminki K, Aaltonen L, Li X. Subsequent primary malignancies after endometrial carcinoma and ovarian carcinoma. Cancer. 2003;97:2432-2439.
16 Re A, Taylor TH, DiSaia PJ, et al. Risk for breast and colorectal cancers subsequent to cancer of the endometrium in a population-based case series. Gynecol Oncol. 1997;66:255-257.
17 Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214-218.
18 Baglietto L, Lindor NM, Dowty JG, et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst. 2010;102:193-201.
19 Wijnen J, de Leeuw W, Vasen H, et al. Familial endometrial cancer in female carriers of MSH6 germline mutations. Nat Genet. 1999;23:142-144.
20 Lynch HT, Smyrk TC, Watson P, et al. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer. An updated review. Gastroenterology. 1993;104:1535-1549.
21 Vasen HF, Wijnen JT, Menko FH, et al. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology. 1996;110:1020-1027.
22 Janicek MF, Rosenshein NB. Invasive endometrial cancer in uteri resected for atypical endometrial hyperplasia. Gynecol Oncol. 1994;52:373-378.
23 Lindahl B, Willen R. Spontaneous endometrial hyperplasia. A prospective, 5 year follow-up of 246 patients after abrasio only, including 380 patients followed-up for 2 years. Anticancer Res. 1994;14:2141-2146.
24 Vasen HF, Moslein G, Alonso A, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer). J Med Genet. 2007;44:353-362.
25 Gerber B, Krause A, Muller H, et al. Ultrasonographic detection of asymptomatic endometrial cancer in postmenopausal patients offers no prognostic advantage over symptomatic disease discovered by uterine bleeding. Eur J Cancer. 2001;37:64-71.
26 Dove-Edwin I, Boks D, Goff S, et al. The outcome of endometrial carcinoma surveillance by ultrasound scan in women at risk of hereditary nonpolyposis colorectal carcinoma and familial colorectal carcinoma. Cancer. 2002;94:1708-1712.
27 Gerber B, Krause A, Muller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer. A prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000;18:3464-3470.
28 Mourits MJ, Van der Zee AG, Willemse PH, et al. Discrepancy between ultrasonography and hysteroscopy and histology of endometrium in postmenopausal breast cancer patients using tamoxifen. Gynecol Oncol. 1999;73:21-26.
29 Renkonen-Sinisalo L, Butzow R, Leminen A, et al. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer. 2007;120:821-824.
30 Christopherson WM, Connelly PJ, Alberhasky RC. Carcinoma of the endometrium: V. An analysis of prognosticators in patients with favorable subtypes and stage I disease. Cancer. 1983;51:1705-1709.
31 Kurman RJ, editor. Blaustein’s Pathology of the Female Genital Tract: Endometrial Carcinoma, ed 4. Springer-Verslag, New York, 1994;439-486.
32 Abeln EC, Smit VT, Wessels JW, et al. Molecular genetic evidence for the conversion hypothesis of the origin of malignant mixed müllerian tumours. J Pathol. 1997;183:424-431.
33 Bitterman P, Chun B, Kurman RJ. The significance of epithelial differentiation in mixed mesodermal tumors of the uterus. A clinicopathologic and immunohistochemical study. Am J Surg Pathol. 1990;14:317-328.
34 Eifel PJ, Ross J, Hendrickson M, et al. Adenocarcinoma of the endometrium. Analysis of 256 cases with disease limited to the uterine corpus. Treatment comparisons. Cancer. 1983;52:1026-1031.
35 Hendrickson M, Ross J, Eifel P, et al. Uterine papillary serous carcinoma. A highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93-108.
36 Fanning J, Evans MC, Peters AJ, et al. Endometrial adenocarcinoma histologic subtypes. Clinical and pathologic profile. Gynecol Oncol. 1989;32:288-291.
37 Wilson TO, Podratz KC, Gaffey TA, et al. Evaluation of unfavorable histologic subtypes in endometrial adenocarcinoma. Am J Obstet Gynecol. 1990;162:418-423.
38 Alektiar KM, McKee A, Lin O, et al. Is there a difference in outcome between stage I-II endometrial cancer of papillary serous/clear cell and endometrioid FIGO Grade 3 cancer? Int J Radiat Oncol Biol Phys. 2002;54:79-85.
39 Creasman WT, Kohler MF, Odicino F, et al. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol. 2004;95:593-596.
40 Abeler VM, Kjorstad KE. Endometrial adenocarcinoma with squamous cell differentiation. Cancer. 1992;69:488-495.
41 Zaino RJ, Kurman R, Herbold D, et al. The significance of squamous differentiation in endometrial carcinoma. Data from a Gynecologic Oncology Group study. Cancer. 1991;68:2293-2302.
42 Baloglu H, Cannizzaro LA, Jones J, et al. Atypical endometrial hyperplasia shares genomic abnormalities with endometrioid carcinoma by comparative genomic hybridization. Hum Pathol. 2001;32:615-622.
43 Hunter JE, Tritz DE, Howell MG, et al. The prognostic and therapeutic implications of cytologic atypia in patients with endometrial hyperplasia. Gynecol Oncol. 1994;55:66-71.
44 Ambros RA, Sherman ME, Zahn CM, et al. Endometrial intraepithelial carcinoma. A distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol. 1995;26:1260-1267.
45 Lax SF, Kurman RJ, Pizer ES, et al. A binary architectural grading system for uterine endometrial endometrioid carcinoma has superior reproducibility compared with FIGO grading and identifies subsets of advance-stage tumors with favorable and unfavorable prognosis. Am J Surg Pathol. 2000;24:1201-1208.
46 Mittal KR, Barwick KW. Diffusely infiltrating adenocarcinoma of the endometrium. A subtype with poor prognosis. Am J Surg Pathol. 1988;12:754-758.
47 Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60:2035-2041.
48 Morrow CP, Bundy BN, Kurman RJ, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium. A Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55-65.
49 Barranger E, Cortez A, Grahek D, et al. Laparoscopic sentinel node procedure using a combination of patent blue and radiocolloid in women with endometrial cancer. Ann Surg Oncol. 2004;11:344-349.
50 Burke TW, Levenback C, Tornos C, et al. Intraabdominal lymphatic mapping to direct selective pelvic and paraaortic lymphadenectomy in women with high-risk endometrial cancer. Results of a pilot study. Gynecol Oncol. 1996;62:169-173.
51 Niikura H, Okamura C, Utsunomiya H, et al. Sentinel lymph node detection in patients with endometrial cancer. Gynecol Oncol. 2004;92:669-674.
52 COSA-NZ-UK endometrial cancer study group. Pelvic lymphadenectomy in high risk endometrial cancer. Int J Gynecol Cancer. 1996;6:102-107.
53 Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10-17.
54 Santin AD, Bellone S, Gokden M, et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clin Cancer Res. 2002;8:1271-1279.
55 Santin AD. HER2/neu overexpression. Has the Achilles’ heel of uterine serous papillary carcinoma been exposed? Gynecol Oncol. 2003;88:263-265.
56 Fleming GF, Sill MW, Darcy KM, et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma. A Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:15-20.
57 Khalifa MA, Mannel RS, Haraway SD, et al. Expression of EGFR, HER-2/neu, p53, and PCNA in endometrioid, serous papillary, and clear cell endometrial adenocarcinomas. Gynecol Oncol. 1994;53:84-92.
58 Oza AM, Eisenhauer EA, Elit L, et al. Phase II study of erlotinib in recurrent or metastatic endometrial cancer. NCIC IND-148. J Clin Oncol. 2008;26:4319-4325.
59 Konecny GE, Venkatesan N, Yang G, et al. Activity of lapatinib a novel HER2 and EGFR dual kinase inhibitor in human endometrial cancer cells. Br J Cancer. 2008;98:1076-1084.
60 Jeon YT, Kang S, Kang DH, et al. Cyclooxygenase-2 and p53 expressions in endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1538-1542.
61 Athanassiadou P, Athanassiades P, Grapsa D, et al. The prognostic value of PTEN, p53, and beta-catenin in endometrial carcinoma. A prospective immunocytochemical study. Int J Gynecol Cancer. 2007;17:697-704.
62 Baak JPA, Snijders W, van Diermen B, et al. Prospective multicenter validation confirms the prognostic superiority of the endometrial carcinoma prognostic index in International Federation of Gynecology and Obstetrics stage 1 and 2 endometrial carcinoma. J Clin Oncol. 2003;21:4214-4221.
63 Lukes AS, Kohler MF, Pieper CF, et al. Multivariable analysis of DNA ploidy, p53, and HER-2/neu as prognostic factors in endometrial cancer. Cancer. 1994;73:2380-2385.
64 Mariani A, Sebo TJ, Katzmann JA, et al. Pretreatment assessment of prognostic indicators in endometrial cancer. Am J Obstet Gynecol. 2000;182:1535-1544.
65 Suehiro Y, Okada T, Okada T, et al. Aneuploidy predicts outcome in patients with endometrial carcinoma and is related to lack of CDH13 hypermethylation. Clin Cancer Res. 2008;14:3354-3361.
66 Oda K, Stokoe D, Taketani Y, et al. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65:10669-10673.
67 Catasus L, Gallardo A, Cuatrecasas M, et al. PIK3CA mutations in the kinase domain (exon 20) of uterine endometrial adenocarcinomas are associated with adverse prognostic parameters. Mod Pathol. 2008;21:131-139.
68 Catasus L, Gallardo A, Cuatrecasas M, et al. Concomitant PI3K-AKT and p53 alterations in endometrial carcinomas are associated with poor prognosis. Mod Pathol. 2009;22:522-529.
69 Darb-Esfahani S, Faggad A, Noske A, et al. Phospho-mTOR and phospho-4EBP1 in endometrial adenocarcinoma. Association with stage and grade in vivo and link with response to rapamycin treatment in vitro. J Cancer Res Clin Oncol. 2009;135:933-941.
70 Wahl H, Daudi S, Kshirsagar M, et al. Expression of metabolically targeted biomarkers in endometrial carcinoma. Gynecol Oncol. 2010;116:21-27.
71 Oza AM, Elit L, Provencher D, et al. and NCIC Clinical Trials Group: A phase II study of temsirolimus (CCI-779) in patients with metastatic and/or locally advance recurrent endometrial cancer previously treated with chemotherapy. NCIC CTG IND 160b. J Clin Oncol. 26(Suppl [abstract 5516]), 2008.
72 Zaino RJ, Kurman RJ, Diana KL, et al. Pathologic models to predict outcome for women with endometrial adenocarcinoma. The importance of the distinction between surgical stage and clinical stage—a Gynecologic Oncology Group study [published erratum appears in Cancer 1997 Jan 15,79(2):422]. Cancer. 1996;77:1115-1121.
73 Greven KM, Corn BW, Case D, et al. Which prognostic factors influence the outcome of patients with surgically staged endometrial cancer treated with adjuvant radiation? Int J Radiat Oncol Biol Phys. 1997;39:413-418.
74 Aalders J, Abeler V, Kolstad P, et al. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma. Clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56:419-427.
75 Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma. Multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404-1411.
76 Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma. A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744-751.
77 Scholten AN, Creutzberg CL, Noordijk EM, et al. Long-term outcome in endometrial carcinoma favors a two- instead of a three-tiered grading system. Int J Radiat Oncol Biol Phys. 2002;52:1067-1074.
78 Creutzberg CL, van Putten WL, Warlam-Rodenhuis CC, et al. Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients. The Postoperative Radiation Therapy in Endometrial Carcinoma Trial. J Clin Oncol. 2004;22:1234-1241.
79 Taylor RR, Zeller J, Lieberman RW, et al. An analysis of two versus three grades for endometrial carcinoma. Gynecol Oncol. 1999;74:3-6.
80 Scholten AN, Smit VTHB, Beerman H, et al. Prognostic significance and interobserver variability of histologic grading systems for endometrial carcinoma. Cancer. 2004;100:764-772.
81 Alkushi A, Abdul-Rahman ZH, Lim P, et al. Description of a novel system for grading of endometrial carcinoma and comparison with existing grading systems. Am J Surg Pathol. 2005;29:295-304.
82 Suzuki C, Matsumoto T, Sonoue H, et al. Prognostic significance of the infiltrative pattern invasion in endometrioid adenocarcinoma of the endometrium. Pathol Int. 2003;53:495-500.
83 Lindauer J, Fowler JM, Manolitsas TP, et al. Is there a prognostic difference between depth of myometrial invasion and the tumor-free distance from the uterine serosa in endometrial cancer? Gynecol Oncol. 2003;91:547-551.
84 Briët JM, Hollema H, Reesink N, et al. Lymphovascular space involvement. An independent prognostic factor in endometrial cancer. Gynecol Oncol. 2005;96:799-804.
85 Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103-104.
86 Dore R, Moro G, D’Andrea F, et al. CT evaluation of myometrium invasion in endometrial carcinoma. J Comput Assist Tomogr. 1987;11:282-289.
87 Manfredi R, Mirk P, Maresca G, et al. Local-regional staging of endometrial carcinoma. Role of MR imaging in surgical planning. Radiology. 2004;231:372-378.
88 Sironi S, Picchio M, Landoni C, et al. Post-therapy surveillance of patients with uterine cancers. Value of integrated FDG PET/CT in the detection of recurrence. Eur J Nucl Med Mol Imaging. 2007;34:472-479.
89 Park JY, Kim EN, Kim DY, et al. Clinical impact of positron emission tomography or positron emission tomography/computed tomography in the post-therapy surveillance of endometrial carcinoma. Evaluation of 88 patients. Int J Gynecol Cancer. 2008;18:1332-1338.
90 Chung HH, Kang WJ, Kim JW, et al. The clinical impact of [(18)F]FDG PET/CT for the management of recurrent endometrial cancer. Correlation with clinical and histological findings. Eur J Nucl Med Mol Imaging. 2008;35:1081-1088.
91 Signorelli M, Guerra L, Buda A, et al. Role of the integrated FDG PET/CT in the surgical management of patients with high risk clinical early stage endometrial cancer. Detection of pelvic nodal metastases. Gynecol Oncol. 2009;115:231-235.
92 Ayhan A, Taskiran C, Celik C, et al. The long-term survival of women with surgical stage II endometrioid type endometrial cancer. Gynecol Oncol. 2004;93:9-13.
93 Sartori E, Gadducci A, Landoni F, et al. Clinical behavior of 203 stage II endometrial cancer cases. The impact of primary surgical approach and of adjuvant radiation therapy. Int J Gynecol Cancer. 2001;11:430-437.
94 Malur S, Possover M, Michels W, et al. Laparoscopic-assisted vaginal versus abdominal surgery in patients with endometrial cancer—a prospective randomized trial. Gynecol Oncol. 2001;80:239-244.
95 Eltabbakh GH, Shamonki MI, Moody JM, et al. Laparoscopy as the primary modality for the treatment of women with endometrial carcinoma. Cancer. 2001;91:378-387.
96 Gemignani ML, Curtin JP, Zelmanovich J, et al. Laparoscopic-assisted vaginal hysterectomy for endometrial cancer. Clinical outcomes and hospital charges. Gynecol Oncol. 1999;73:5-11.
97 Sonoda Y, Zerbe M, Smith A, et al. High incidence of positive peritoneal cytology in low-risk endometrial cancer treated by laparoscopically assisted vaginal hysterectomy. Gynecol Oncol. 2001;80:378-382.
98 Chu CS, Randall TC, Bandera CA, et al. Vaginal cuff recurrence of endometrial cancer treated by laparoscopic-assisted vaginal hysterectomy. Gynecol Oncol. 2003;88:62-65.
99 Schneider A. Vaginal cuff recurrence of endometrial cancer treated by laparoscopic-assisted vaginal hysterectomy. Gynecol Oncol. 2004;94:861-2004.
100 Holub Z. Vaginal cuff recurrence of endometrial cancer treated by laparoscopic-assisted vaginal hysterectomy. Gynecol Oncol. 2003;90:495-497.
101 Malzoni M, Tinelli R, Cosentino F, et al. Total laparoscopic hysterectomy versus abdominal hysterectomy with lymphadenectomy for early-stage endometrial cancer. A prospective randomized study. Gynecol Oncol. 2009;112:126-133.
102 Bijen C, de Bock GH, Arts HJG, et al. J. Preliminary results of a randomised multicenter trial. Laparoscopy versus laparotomy in the treatment of early stage endometrial cancer. Int J Gynecol Cancer. 19(Suppl 2, abstract 833), 2009.
103 Walker JL, Piedmonte M, Spirtos NM, et al. Surgical staging of uterine cancer. Randomized phase III trial of laparoscopy vs. laparotomy—a Gynecologic Oncology Group study (GOG). Preliminary results. J Clin Oncol. 2006;24:5010.
104 Janda M, Gebski V, Forder P, et al. Total laparoscopic versus open surgery for stage 1 endometrial cancer. The LACE randomized controlled trial. Contemp Clin Trials. 2006;27:353-363.
105 Trimble EL, Kosary C, Park RC. Lymph node sampling and survival in endometrial cancer. Gynecol Oncol. 1998;71:340-343.
106 Cragun JM, Havrilesky LJ, Calingaert B, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005;23:3668-3675.
107 Mohan DS, Samuels MA, Selim MA, et al. Long-term outcomes of therapeutic pelvic lymphadenectomy for stage I endometrial adenocarcinoma [see comments]. Gynecol Oncol. 1998;70:165-171.
108 Fanning J, Nanavati PJ, Hilgers RD. Surgical staging and high dose rate brachytherapy for endometrial cancer. Limiting external radiotherapy to node-positive tumors. Obstet Gynecol. 1996;87:1041-1044.
109 Kilgore LC, Partridge EE, Alvarez RD, et al. Adenocarcinoma of the endometrium. Survival comparisons of patients with and without pelvic node sampling. Gynecol Oncol. 1995;56:29-33.
110 Chan JK, Wu H, Cheung MK, et al. The outcomes of 27,063 women with unstaged endometrioid uterine cancer. Gynecol Oncol. 2007;106:282-288.
111 Bernardini MQ, Murphy JK. Issues surrounding lymphadenectomy in the management of endometrial cancer. J Surg Oncol. 2009;99:232-241.
112 Aalders JG, Thomas G. Endometrial cancer—revisiting the importance of pelvic and para aortic lymph nodes. Gynecol Oncol. 2007;104:222-231.
113 Kitchener H, Swart AM, Qian Q, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial). A randomised study. Lancet. 2009;373:125-136.
114 Benedetti PP, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma. Randomized clinical trial. J Natl Cancer Inst. 2008;100:1707-1716.
115 Barranger E, Delpech Y, Coutant C, et al. Laparoscopic sentinel node mapping using combined detection for endometrial cancer. A study of 33 cases—is it a promising technique? Am J Surg. 2009;197:1-7.
116 Khoury-Collado F, Glaser GE, Zivanovic O, et al. Improving sentinel lymph node detection rates in endometrial cancer. How many cases are needed? Gynecol Oncol. 2009;115:453-455.
117 Abu-Rustum NR, Khoury-Collado F, Pandit-Taskar N, et al. Sentinel lymph node mapping for grade 1 endometrial cancer. Is it the answer to the surgical staging dilemma? Gynecol Oncol. 2009;113:163-169.
118 Nguyen TV, Petereit DG. High-dose-rate brachytherapy for medically inoperable stage I endometrial cancer. Gynecol Oncol. 1998;71:196-203.
119 Fishman DA, Roberts KB, Chambers JT, et al. Radiation therapy as exclusive treatment for medically inoperable patients with stage I and II endometrioid carcinoma with endometrium. Gynecol Oncol. 1996;61:189-196.
120 Rouanet P, Dubois JB, Gely S, et al. Exclusive radiation therapy in endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1993;26:223-228.
121 Lehoczky O, Bosze P, Ungar L, et al. Stage I endometrial carcinoma. Treatment of nonoperable patients with intracavitary radiation therapy alone. Gynecol Oncol. 1991;43:211-216.
122 Kucera H, Knocke TH, Kucera E, et al. Treatment of endometrial carcinoma with high-dose-rate brachytherapy alone in medically inoperable stage I patients. Acta Obstet Gynecol Scand. 1998;77:1008-1012.
123 Grigsby PW, Kuske RR, Perez CA, et al. Medically inoperable stage I adenocarcinoma of the endometrium treated with radiotherapy alone. Int J Radiat Oncol Biol Phys. 1987;13:483-488.
124 Knocke TH, Kucera H, Weidinger B, et al. Primary treatment of endometrial carcinoma with high-dose-rate brachytherapy. Results of 12 years of experience with 280 patients. Int J Radiat Oncol Biol Phys. 1997;37:359-365.
125 Carey MS, O’Connell GJ, Johanson CR, et al. Good outcome associated with a standardized treatment protocol using selective postoperative radiation in patients with clinical stage I adenocarcinoma of the endometrium [see comments]. Gynecol Oncol. 1995;57:138-144.
126 Poulsen HK, Jacobsen M, Bertelsen K, et al. Adjuvant radiation therapy is not necessary in the management of endometrial carcinoma stage I, low-risk cases. Int J Gynecol Cancer. 1996;6:38-43.
127 Mariani A, Webb MJ, Keeney GL, et al. Low-risk corpus cancer. Is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506-1516.
128 Alektiar KM, McKee A, Venkatraman E, et al. Intravaginal high-dose-rate brachytherapy for stage IB (FIGO grade 1, 2) endometrial cancer. Int J Radiat Oncol Biol Phys. 2002;53:707-713.
129 Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials). Pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137-146.
130 Scholten AN, van Putten WL, Beerman H, et al. Postoperative radiotherapy for stage 1 endometrial carcinoma. Long-term outcome of the randomized PORTEC trial with central pathology review. Int J Radiat Oncol Biol Phys. 2005;63:834-838.
131 Creutzberg CL, van Putten WLJ, Koper PC, et al. Survival after relapse in patients with endometrial cancer. Results from a randomized trial. Gynecol Oncol. 2003;89:201-209.
132 Kong A, Simera I, Collingwood M, et al. Adjuvant radiotherapy for stage I endometrial cancer. Systematic review and meta-analysis. Ann Oncol. 2007;18:1595-1604.
133 Eltabbakh GH, Piver MS, Hempling RE, et al. Excellent long-term survival and absence of vaginal recurrences in 332 patients with low-risk stage I endometrial adenocarcinoma treated with hysterectomy and vaginal brachytherapy without formal staging lymph node sampling. Report of a prospective trial. Int J Radiat Oncol Biol Phys. 1997;38:373-380.
134 Rose PG, Tak WK, Fitzgerald TJ, et al. Brachytherapy for early endometrial carcinoma. A comparative study with long-term follow-up. Int J Gynecol Cancer. 1999;9:105-109.
135 Petereit DG, Tannehill SP, Grosen EA, et al. Outpatient vaginal cuff brachytherapy for endometrial cancer. Int J Gynecol Cancer. 1999;9:456-462.
136 Elliott P, Green D, Coates A, et al. The efficacy of postoperative vaginal irradiation in preventing vaginal recurrence in endometrial cancer. Int J Gynecol Cancer. 1994;4:84-93.
137 Pearcey RG, Petereit DG. Post-operative high dose rate brachytherapy in patients with low to intermediate risk endometrial cancer. Radiother Oncol. 2000;56:17-22.
138 Chadha M, Nanavati PJ, Liu P, et al. Patterns of failure in endometrial carcinoma stage IB grade 3 and IC patients treated with postoperative vaginal vault brachytherapy. Gynecol Oncol. 1999;75:103-107.
139 Weiss E, Hirnle P, Arnold-Bofinger H, et al. Adjuvant vaginal high-dose-rate afterloading alone in endometrial carcinoma. Patterns of relapse and side effects following low-dose therapy. Gynecol Oncol. 1998;71:72-76.
140 Anderson JM, Stea B, Hallum AV, et al. High-dose-rate postoperative vaginal cuff irradiation alone for stage IB and IC endometrial cancer. Int J Radiat Oncol Biol Phys. 2000;46:417-425.
141 Rittenberg PV, Lotocki RJ, Heywood MS, et al. High-risk surgical stage 1 endometrial cancer: outcomes with vault brachytherapy alone. Gynecol Oncol. 2003;89:288-294.
142 Petereit DG, Pearcey R. Literature analysis of high dose rate brachytherapy fractionation schedules in the treatment of cervical cancer. Is there an optimal fractionation schedule? Int J Radiat Oncol Biol Phys. 1999;43:359-366.
143 Nout RA, Putter H, Jurgenliemk-Schulz IM, et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: First results of the randomized PORTEC-2 Trial. J Clin Oncol. 2010;375:816-823.
144 Nout RA, Smit VTHB, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial carcinoma of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816-823.
145 Greven KM, Randall M, Fanning J, et al. Patterns of failure in patients with stage I, grade 3 carcinoma of the endometrium. Int J Radiat Oncol Biol Phys. 1990;19:529-534.
146 Straughn JM, Huh WK, Orr JW, et al. Stage IC adenocarcinoma of the endometrium. Survival comparisons of surgically staged patients with and without adjuvant radiation therapy small star, filled. Gynecol Oncol. 2003;89:295-300.
147 Koh WJ, Tran AB, Douglas JG, et al. Radiation therapy in endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2001;15:417-432.
148 Jereczek-Fossa BA. Postoperative irradiation in endometrial cancer. Still a matter of controversy. Cancer Treat Rev. 2001;27:19-33.
149 Jobsen JJ, Lybeert ML, van der Steen-Banasik EM, et al. Multicenter cohort study on treatment results and risk factors in stage II endometrial carcinoma. Int J Gynecol Cancer. 2008;18:1071-1078.
150 Eltabbakh GH, Moore AD. Survival of women with surgical stage II endometrial cancer. Gynecol Oncol. 1999;74:80-85.
151 Irwin C, Levin W, Fyles A, et al. The role of adjuvant radiotherapy in carcinoma of the endometrium. Results in 550 patients with pathologic stage I disease. Gynecol Oncol. 1998;70:247-254.
152 Randall ME, Wilder J, Greven K, et al. Role of intracavitary cuff boost after adjuvant external irradiation in early endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:49-54.
153 Greven KM, D’Agostino RBJr, Lanciano RM, et al. Is there a role for a brachytherapy vaginal cuff boost in the adjuvant management of patients with uterine-confined endometrial cancer? Int J Radiat Oncol Biol Phys. 1998;42:101-104.
154 Cozad SC. Stage II adenocarcinoma of the endometrium. Adjuvant radiotherapy and recurrence patterns. Int J Radiat Oncol Biol Phys. 2008;71:205-212.
155 Pitson G, Colgan T, Levin W, et al. Stage II endometrial carcinoma. Prognostic factors and risk classification in 170 patients. Int J Radiat Oncol Biol Phys. 2002;53:862-867.
156 Cannon GM, Geye H, Terakedis BE, et al. Outcomes following surgery and adjuvant radiation in stage II endometrial adenocarcinoma. Gynecol Oncol. 2009;113:176-180.
157 Mariani A, Webb MJ, Keeney GL, et al. Role of wide/radical hysterectomy and pelvic lymph node dissection in endometrial cancer with cervical involvement. Gynecol Oncol. 2001;83:72-80.
158 Cornelison TL, Trimble EL, Kosary CL. SEER data, corpus uteri cancer. Treatment trends versus survival for FIGO stage II, 1988-1994. Gynecol Oncol. 1999;74:350-355.
159 Leminen A, Forss M, Lehtovirta P. Endometrial adenocarcinoma with clinical evidence of cervical involvement. Accuracy of diagnostic procedures, clinical course, and prognostic factors. Acta Obstet Gynecol Scand. 1995;74:61-66.
160 Cohn DE, Woeste EM, Cacchio S, et al. Clinical and pathologic correlates in surgical stage II endometrial carcinoma. Obstet Gynecol. 2007;109:1062-1067.
161 Wright JD, Fiorelli J, Kansler AL, et al. Optimizing the management of stage II endometrial cancer. The role of radical hysterectomy and radiation. Am J Obstet Gynecol. 2009;200:419.
162 Hamilton CA, Liu L, Osann KN, et al. Observation versus adjuvant treatment in early stage uterine serous carcinoma. Proc Int Gynecol Cancer Soc. 2004(abstract 57), 2005.
163 Thomas MB, Mariani A, Cliby WA, et al. Role of systematic lymphadenectomy and adjuvant therapy in stage I uterine papillary serous carcinoma. Gynecol Oncol. 2007;107:186-189.
164 Thomas M, Mariani A, Wright JD, et al. Surgical management and adjuvant therapy for patients with uterine clear cell carcinoma. A multi-institutional review. Gynecol Oncol. 2008;108:293-297.
165 Martin-Hirsch PL, Lilford RJ, Jarvis GJ. Adjuvant progestagen therapy for the treatment of endometrial cancer. Review and meta-analyses of published randomised controlled trials. Eur J Obstet Gynecol Reprod Biol. 1996;65:201-207.
166 Vergote I, Kjorstad K, Abeler V, et al. A randomized trial of adjuvant progestagen in early endometrial cancer. Cancer. 1989;64:1011-1016.
167 COSA-NZ-UK Endometrial Cancer Study Groups. Adjuvant medroxyprogesterone acetate in high-risk endometrial cancer. Int J Gynecol Cancer. 1998;8:387-391.
168 Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma. A Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36-44.
169 Susumu N, Sagae S, Udagawa Y, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer. A Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
170 Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma. Results of a randomised trial. Br J Cancer. 2006;95:266-271.
171 Mundt AJ, McBride R, Rotmensch J, et al. Significant pelvic recurrence in high-risk pathologic stage I-IV endometrial carcinoma patients after adjuvant chemotherapy alone. Implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:1145-1153.
172 Frigerio L, Mangili G, Aletti G, et al. Concomitant radiotherapy and paclitaxel for high-risk endometrial cancer. First feasibility study. Gynecol Oncol. 2001;81:53-57.
173 Reisinger SA, Asbury R, Liao SY, et al. A phase I study of weekly cisplatin and whole abdominal radiation for the treatment of stage III and IV endometrial carcinoma. A Gynecologic Oncology Group pilot study. Gynecol Oncol. 1996;63:299-303.
174 Morrow CP, Bundy BN, Homesley HD, et al. Doxorubicin as an adjuvant following surgery and radiation therapy in patients with high-risk endometrial carcinoma, stage I and occult stage II. A Gynecologic Oncology Group Study. Gynecol Oncol. 1990;36:166-171.
175 Kuoppala T, Maenpaa J, Tomas E, et al. Surgically staged high-risk endometrial cancer. Randomized study of adjuvant radiotherapy alone vs. sequential chemo-radiotherapy. Gynecol Oncol. 2008;110:190-195.
176 Greven K, Winter K, Underhill K, et al. Final analysis of RTOG 9708. Adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 2006;103:155-159.
177 Hogberg T, Rosenberg P, Kristensen G, et al. A randomized phase-III study on adjuvant treatment with radiation (RT) ± chemotherapy (CT) in early-stage high-risk endometrial cancer (NSGO-EC-9501/EORTC 55991). Proceedings of the 2007 ASCO annual meeting. J Clin Oncol. 2007;25:5503.
178 Hogberg T, Signorelli M, De Oliveira CF, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer. Results from two randomised studies. Eur J Cancer. 2010;46:2422-2431.
179 Kelly MG, O’Malley DM, Hui P, et al. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol Oncol. 2005;98:353-359.
180 McMeekin DS, Filiaci VL, Thigpen JT, et al. The relationship between histology and outcome in advanced and recurrent endometrial cancer patients participating in first-line chemotherapy trials. A Gynecologic Oncology Group study. Gynecol Oncol. 2007;106:16-22.
181 Tebeu PM, Popowski Y, Verkooijen HM, et al. Positive peritoneal cytology in early-stage endometrial cancer does not influence prognosis. Br J Cancer. 2004;91:720-724.
182 Kasamatsu T, Onda T, Katsumata N, et al. Prognostic significance of positive peritoneal cytology in endometrial carcinoma confined to the uterus. Br J Cancer. 2003;88:245-250.
183 Greven KM, Lanciano RM, Corn B, et al. Pathologic stage III endometrial carcinoma. Prognostic factors and patterns of recurrence. Cancer. 1993;71:3697-3702.
184 Greven KM, Curran WJJr, Whittington R, et al. Analysis of failure patterns in stage III endometrial carcinoma and therapeutic implications. Int J Radiat Oncol Biol Phys. 1989;17:35-39.
185 Grigsby PW, Perez CA, Kuske RR, et al. Results of therapy, analysis of failures, and prognostic factors for clinical and pathologic stage III adenocarcinoma of the endometrium. Gynecol Oncol. 1987;27:44-57.
186 Schorge JO, Molpus KL, Goodman A, et al. The effect of postsurgical therapy on stage III endometrial carcinoma. Gynecol Oncol. 1996;63:34-39.
187 Gibbons S, Martinez A, Schray M, et al. Adjuvant whole abdominopelvic irradiation for high risk endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1991;21:1019-1025.
188 Potish RA, Twiggs LB, Adcock LL, et al. Role of whole abdominal radiation therapy in the management of endometrial cancer. Prognostic importance of factors indicating peritoneal metastases. Gynecol Oncol. 1985;21:80-86.
189 Aalders JG, Abeler V, Kolstad P. Clinical (stage III) as compared to subclinical intrapelvic extrauterine tumor spread in endometrial carcinoma. A clinical and histopathological study of 175 patients. Gynecol Oncol. 1984;17:64-74.
190 Ashman JB, Connell PP, Yamada D, et al. Outcome of endometrial carcinoma patients with involvement of the uterine serosa. Gynecol Oncol. 2001;82:338-343.
191 Thomas MB, Mariani A, Cliby WA, et al. Role of cytoreduction in stage III and IV uterine papillary serous carcinoma. Gynecol Oncol. 2007;107:190-193.
192 Van Wijk FH, Van Der Burg ME, Burger CW, et al. Management of surgical stage III and IV endometrioid endometrial carcinoma. An overview. Int J Gynecol Cancer. 2009;19:431-446.
193 Nicklin JL, Petersen RW. Stage 3B adenocarcinoma of the endometrium. A clinicopathologic study. Gynecol Oncol. 2000;78:203-207.
194 Mundt AJ, Murphy KT, Rotmensch J, et al. Surgery and postoperative radiation therapy in FIGO stage IIIC endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2001;50:1154-1160.
195 Nelson G, Randall M, Sutton G, et al. FIGO stage IIIC endometrial carcinoma with metastases confined to pelvic lymph nodes. Analysis of treatment outcomes, prognostic variables, and failure patterns following adjuvant radiation therapy. Gynecol Oncol. 1999;75:211-214.
196 Ayhan A, Taskiran C, Celik C, et al. Surgical stage III endometrial cancer. Analysis of treatment outcomes, prognostic factors and failure patterns. Eur J Gynaecol Oncol. 2002;23:553-556.
197 Mariani A, Webb MJ, Keeney GL, et al. Stage IIIC endometrioid corpus cancer includes distinct subgroups. Gynecol Oncol. 2002;87:112-117.
198 Klopp AH, Jhingran A, Ramondetta L, et al. Node-positive adenocarcinoma of the endometrium. Outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol. 2009;115:6-11.
199 Patel S, Portelance L, Gilbert L, et al. Analysis of prognostic factors and patterns of recurrence in patients with pathologic stage III endometrial cancer. Int J Radiat Oncol Biol Phys. 2007;68:1438-1445.
200 Rose PG, Cha SD, Tak WK, et al. Radiation therapy for surgically proven paraaortic node metastasis in endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1992;24:229-233.
201 Hicks ML, Piver MS, Puretz JL, et al. Survival in patients with paraaortic lymph-node metastases from endometrial adenocarcinoma clinically limited to the uterus. Int J Radiat Oncol Biol Phys. 1993;26:607-611.
202 Corn BW, Lanciano RM, Greven KM, et al. Endometrial cancer with paraaortic adenopathy—patterns of failure and opportunities for cure. Int J Radiat Oncol Biol Phys. 1992;24:223-227.
203 Bristow RE, Zahurak ML, Alexander CJ, et al. FIGO stage IIIC endometrial carcinoma. Resection of macroscopic nodal disease and other determinants of survival. Int J Gynecol Cancer. 2003;13:664-672.
204 Homesley HD, Filiaci V, Gibbons SK, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel. A Gynecologic Oncology Group study. Gynecol Oncol. 2009;112:543-552.
205 Chi DS, Welshinger M, Venkatraman ES, et al. The role of surgical cytoreduction in stage IV endometrial carcinoma. Gynecol Oncol. 1997;67:56-60.
206 Cook AM, Lodge N, Blake P. Stage IV endometrial carcinoma. A 10 year review of patients. Br J Radiol. 1999;72:485-488.
207 Lambrou NC, Gomez-Marin O, Mirhashemi R, et al. Optimal surgical cytoreduction in patients with stage III and stage IV endometrial carcinoma. A study of morbidity and survival. Gynecol Oncol. 2004;93:653-658.
208 Bruzzone M, Miglietta L, Franzone P, et al. Combined treatment with chemotherapy and radiotherapy in high-risk FIGO stage III-IV endometrial cancer patients. Gynecol Oncol. 2004;93:345-352.
209 Vandenput I, Van CB, Capoen A, et al. Neoadjuvant chemotherapy followed by interval debulking surgery in patients with serous endometrial cancer with transperitoneal spread (stage IV): a new preferred treatment? Br J Cancer. 2009;101:244-249.
210 Tanioka M, Katsumata N, Sasajima Y, et al. Clinical characteristics and outcomes of women with stage IV endometrial cancer. Med Oncol. 2010;27:1371-1377.
211 Wylie J, Irwin C, Pintilie M, et al. Results of radical radiotherapy for recurrent endometrial cancer. Gynecol Oncol. 2000;77:66-72.
212 Jereczek-Fossa B, Badzio A, Jassem J. Recurrent endometrial cancer after surgery alone. Results of salvage radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:405-413.
213 Curran WJJr, Whittington R, Peters AJ, et al. Vaginal recurrences of endometrial carcinoma. The prognostic value of staging by a primary vaginal carcinoma system. Int J Radiat Oncol Biol Phys. 1988;15:803-808.
214 Sears JD, Greven KM, Hoen HM, et al. Prognostic factors and treatment outcome for patients with locally recurrent endometrial cancer. Cancer. 1994;74:1303-1308.
215 Podczaski E, Kaminski P, Gurski K, et al. Detection and patterns of treatment failure in 300 consecutive cases of “early” endometrial cancer after primary surgery. Gynecol Oncol. 1992;47:323-327.
216 Poulsen MG, Roberts SJ. The salvage of recurrent endometrial carcinoma in the vagina and pelvis. Int J Radiat Oncol Biol Phys. 1988;15:809-813.
217 Ackerman I, Malone S, Thomas G, et al. Endometrial carcinoma—relative effectiveness of adjuvant irradiation vs therapy reserved for relapse [see comments]. Gynecol Oncol. 1996;60:177-183.
218 Pai HH, Souhami L, Clark BG, et al. Isolated vaginal recurrences in endometrial carcinoma. Treatment results using high-dose-rate intracavitary brachytherapy and external beam radiotherapy. Gynecol Oncol. 1997;66:300-307.
219 Hart KB, Han I, Shamsa F, et al. Radiation therapy for endometrial cancer in patients treated for postoperative recurrence. Int J Radiat Oncol Biol Phys. 1998;41:7-11.
220 Nag S, Yacoub S, Copeland LJ, et al. Interstitial brachytherapy for salvage treatment of vaginal recurrences in previously unirradiated endometrial cancer patients. Int J Radiat Oncol Biol Phys. 2002;54:1153-1159.
221 Burke TW, Heller PB, Woodward JE, et al. Treatment failure in endometrial carcinoma. Obstet Gynecol. 1990;75:96-101.
222 Burke TW. How should we monitor women treated for endometrial carcinoma? Gynecol Oncol. 1997;65:377-378.
223 Berchuck A, Anspach C, Evans AC, et al. Postsurgical surveillance of patients with FIGO stage I/II endometrial adenocarcinoma. Gynecol Oncol. 1995;59:20-24.
224 Reddoch JM, Burke TW, Morris M, et al. Surveillance for recurrent endometrial carcinoma. Development of a follow-up scheme. Gynecol Oncol. 1995;59:221-225.
225 Shumsky AG, Brasher PM, Stuart GC, et al. Risk-specific follow-up for endometrial carcinoma patients. Gynecol Oncol. 1997;65:379-382.
226 Fung-Kee-Fung M, Dodge J, Elit L, et al. Follow-up after primary therapy for endometrial cancer: a systematic review. Gynecol Oncol. 2006;101:520-529.
227 van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases. Cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95:222-229.
228 Thigpen JT, Brady MF, Alvarez RD, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma. A dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736-1744.
229 Moore TD, Phillips PH, Nerenstone SR, et al. Systemic treatment of advanced and recurrent endometrial carcinoma. Current status and future directions. J Clin Oncol. 1991;9:1071-1088.
230 Thigpen JT, Brady MF, Homesley HD, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma. A Gynecologic Oncology Group study. J Clin Oncol. 2004;22:3902-3908.
231 Lentz SS, Brady MF, Major FJ, et al. High-dose megestrol acetate in advanced or recurrent endometrial carcinoma. A Gynecologic Oncology Group study. J Clin Oncol. 1996;14:357-361.
232 Fiorica JV, Brunetto VL, Hanjani P, et al. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma. A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:10-14.
233 Aapro MS, Van Wijk FH, Bolis G, et al. Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma. Definitive results of a randomised study (55872) by the EORTC Gynaecological Cancer Group. Ann Oncol. 2003;14:441-448.
234 Dimopoulos MA, Papadimitriou CA, Georgoulias V, et al. Paclitaxel and cisplatin in advanced or recurrent carcinoma of the endometrium. Long-term results of a phase II multicenter study. Gynecol Oncol. 2000;78:52-57.
235 Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma. A Gynecologic Oncology Group study. J Clin Oncol. 2004;22:2159-2166.
236 Santin AD, Bellone S, O’Brien TJ, et al. Current treatment options for endometrial cancer. Expert Rev Anticancer Ther. 2004;4:679-689.
237 Hoskins PJ, Swenerton KD, Pike JA, et al. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer. A phase II study. J Clin Oncol. 2001;19:4048-4053.
238 Dizon DS, Blessing JA, McMeekin DS, et al. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer. Gynecologic Oncology Group trial 129-P. J Clin Oncol. 2009;27:3104-3108.
239 Aghajanian CA, Sill MW, Darcy KM, et al. A phase II evaluation of bevacizumab in the treatment of recurrent or persistent endometrial cancer. A Gynecologic Oncology Group (GOG) study. J Clin Oncol. 27(Suppl [abstract 5531]), 2009.
240 Nimeiri HS, Oza AM, Morgan RJ, et al. Sorafenib (SOR) in patients (pts) with advanced/recurrent uterine carcinoma (UCA) or carcinosarcoma (CS). A phase II trial of the University of Chicago, PMH, and California Phase II Consortia. J Clin Oncol. 26(Suppl [abstract 5585]), 2010.
241 Pollock PM, Gartside MG, Dejeza LC, et al. Frequent activating FGFR2 mutations in endometrial carcinomas parallel germline mutations associated with craniosynostosis and skeletal dysplasia syndromes. Oncogene. 2007;26:7158-7162.
242 Sagae S, Yamashita K, Ishioka S, et al. Preoperative diagnosis and treatment results in 106 patients with uterine sarcoma in Hokkaido, Japan. Oncology. 2004;67:33-39.
243 Gerszten K, Faul C, Kounelis S, et al. The impact of adjuvant radiotherapy on carcinosarcoma of the uterus. Gynecol Oncol. 1998;68:8-13.
244 Salazar OM, Bonfiglio TA, Patten SF, et al. Uterine sarcomas. Analysis of failures with special emphasis on the use of adjuvant radiation therapy. Cancer. 1978;42:1161-1170.
245 Perez CA, Askin F, Baglan RJ, et al. Effects of irradiation on mixed mullerian tumors of the uterus. Cancer. 1979;43:1274-1284.
246 Livi L, Andreopoulou E, Shah N, et al. Treatment of uterine sarcoma at the Royal Marsden Hospital from 1974 to 1998. Clin Oncol (R Coll Radiol). 2004;16:261-268.
247 Le T. Adjuvant pelvic radiotherapy for uterine carcinosarcoma in a high risk population. Eur J Surg Oncol. 2001;27:282-285.
248 Reed NS, Mangioni C, Malmstrom H, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II. A European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group study (protocol 55874). Eur J Cancer. 2008;44:808-818.
249 Curtin JP, Blessing JA, Soper JT, et al. Paclitaxel in the treatment of carcinosarcoma of the uterus. A Gynecologic Oncology Group study. Gynecol Oncol. 2001;83:268-270.
250 van Rijswijk RE, Vermorken JB, Reed N, et al. Cisplatin, doxorubicin and ifosfamide in carcinosarcoma of the female genital tract. A phase II study of the European Organization for Research and Treatment of Cancer Gynaecological Cancer Group (EORTC 55923). Eur J Cancer. 2003;39:481-487.
251 Sutton G, Brunetto VL, Kilgore L, et al. A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus. A Gynecologic Oncology Group study. Gynecol Oncol. 2000;79:147-153.
252 Homesley HD, Filiaci V, Markman M, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma. A Gynecologic Oncology Group study. J Clin Oncol. 2007;25:526-531.
253 Powell MA, Filiaci VL, Rose PG, et al. A phase II evaluation of paclitaxel and carboplatin in the treatment of carcinosarcoma of the uterus. A Gynecologic Oncology Group (GOG) study. J Clin Oncol. 27(abstract 5515), 2009.
254 Sutton G, Blessing JA, Malfetano JH. Ifosfamide and doxorubicin in the treatment of advanced leiomyosarcomas of the uterus. A Gynecologic Oncology Group study. Gynecol Oncol. 1996;62:226-229.
255 Look KY, Sandler A, Blessing JA, et al. Phase II trial of gemcitabine as second-line chemotherapy of uterine leiomyosarcoma. A Gynecologic Oncology Group (GOG) study. Gynecol Oncol. 2004;92:644-647.
256 Hensley ML, Blessing JA, DeGeest K, et al. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma. A Gynecologic Oncology Group phase II study. Gynecol Oncol. 2008;109:323-328.
257 Hensley ML, Blessing JA, Mannel R, et al. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma. A Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2008;109:329-334.
258 Omura GA, Blessing JA, Major F, et al. A randomized clinical trial of adjuvant adriamycin in uterine sarcomas. A Gynecologic Oncology Group study. J Clin Oncol. 1985;3:1240-1245.
259 Makker V, bu-Rustum NR, Alektiar KM, et al. A retrospective assessment of outcomes of chemotherapy-based versus radiation-only adjuvant treatment for completely resected stage I-IV uterine carcinosarcoma. Gynecol Oncol. 2008;111:249-254.
260 Menczer J, Levy T, Piura B, et al. A comparison between different postoperative treatment modalities of uterine carcinosarcoma. Gynecol Oncol. 2005;97:166-170.
261 Sutton G, Kauderer J, Carson LF, et al. Adjuvant ifosfamide and cisplatin in patients with completely resected stage I or II carcinosarcomas (mixed mesodermal tumors) of the uterus. A Gynecologic Oncology Group study. Gynecol Oncol. 2005;96:630-634.
262 Wolfson AH, Brady MF, Rocereto T, et al. A Gynecologic Oncology Group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecol Oncol. 2007;107:177-185.
263 Hensley ML, Ishill N, Soslow R, et al. Adjuvant gemcitabine plus docetaxel for completely resected stages I-IV high grade uterine leiomyosarcoma. Results of a prospective study. Gynecol Oncol. 2009;112:563-567.
264 Olofsen-Van Acht M, van den BH, Quint S, et al. Reduction of irradiated small bowel volume and accurate patient positioning by use of a bellyboard device in pelvic radiotherapy of gynecological cancer patients. Radiother Oncol. 2001;59:87-93.
265 Taylor A, Rockall AG, Powell ME. An atlas of the pelvic lymph node regions to aid radiotherapy target volume definition. Clin Oncol (R Coll Radiol). 2007;19:542-550.
266 Small WJr, Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:428-434.
267 Logmans A, van Lent M, van Geel AN, et al. The pedicled omentoplasty, a simple and effective surgical technique to acquire a safe pelvic radiation field. Theoretical and practical aspects. Radiother Oncol. 1994;33:269-271.
268 Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52:1330-1337.
269 Lujan AE, Mundt AJ, Yamada SD, et al. Intensity-modulated radiotherapy as a means of reducing dose to bone marrow in gynecologic patients receiving whole pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:516-521.
270 Heron DE, Gerszten K, Selvaraj RN, et al. Conventional 3D conformal versus intensity-modulated radiotherapy for the adjuvant treatment of gynecologic malignancies. A comparative dosimetric study of dose-volume histograms. Gynecol Oncol. 2003;91:39-45.
271 Brixey CJ, Roeske JC, Lujan AE, et al. Impact of intensity-modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;54:1388-1396.
272 Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56:1354-1360.
273 Greven KM, Corn BW. Endometrial cancer. Curr Probl Cancer. 1997;21:65-127.
274 MacLeod C, Fowler A, Duval P, et al. Adjuvant high-dose rate brachytherapy with or without external beam radiotherapy post hysterectomy for endometrial cancer. Int J Gynecol Cancer. 1999;9:247-255.
275 Greven KM, Lanciano RM, Herbert SH, et al. Analysis of complications in patients with endometrial carcinoma receiving adjuvant irradiation. Int J Radiat Oncol Biol Phys. 1991;21:919-923.
276 Corn BW, Lanciano RM, Greven KM, et al. Impact of improved irradiation technique, age, and lymph node sampling on the severe complication rate of surgically staged endometrial cancer patients. A multivariate analysis. J Clin Oncol. 1994;12:510-515.
277 Lewandowski G, Torrisi J, Potkul RK, et al. Hysterectomy with extended surgical staging and radiotherapy versus hysterectomy alone and radiotherapy in stage I endometrial cancer. A comparison of complication rates. Gynecol Oncol. 1990;36:401-404.
278 Potish RA, Dusenbery KE. Enteric morbidity of postoperative pelvic external beam and brachytherapy for uterine cancer. Int J Radiat Oncol Biol Phys. 1990;18:1005-1010.
279 Letschert JG, Lebesque JV, Aleman BM, et al. The volume effect in radiation-related late small bowel complications. Results of a clinical study of the EORTC Radiotherapy Cooperative Group in patients treated for rectal carcinoma. Radiother Oncol. 1994;32:116-123.
280 Jereczek-Fossa B, Jassem J, Nowak R, et al. Late complications after postoperative radiotherapy in endometrial cancer. Analysis of 317 consecutive cases with application of linear-quadratic model. Int J Radiat Oncol Biol Phys. 1998;41:329-338.
281 Jereczek-Fossa BA, Badzio A, Jassem J. Factors determining acute normal tissue reactions during postoperative radiotherapy in endometrial cancer. Analysis of 317 consecutive cases. Radiother Oncol. 2003;68:33-39.
282 Pedersen D, Bentzen SM, Overgaard J. Reporting radiotherapeutic complications in patients with uterine cervical cancer. The importance of latency and classification system. Radiother Oncol. 1993;28:134-141.
283 Yeoh E, Horowitz M, Russo A, et al. Effect of pelvic irradiation on gastrointestinal function. A prospective longitudinal study. Am J Med. 1993;95:397-406.
284 Creutzberg CL, van Putten WL, Koper PC, et al. The morbidity of treatment for patients with stage I endometrial cancer. Results from a randomized trial. Int J Radiat Oncol Biol Phys. 2001;51:1246-1255.
285 Wang CJ, Leung SW, Chen HC, et al. The correlation of acute toxicity and late rectal injury in radiotherapy for cervical carcinoma. Evidence suggestive of consequential late effect (CQLE). Int J Radiat Oncol Biol Phys. 1998;40:85-91.
286 Schultheiss TE, Lee WR, Hunt MA, et al. Late GI and GU complications in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 1997;37:3-11.
287 Bourne RG, Kearsley JH, Grove WD, et al. The relationship between early and late gastrointestinal complications of radiation therapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 1983;9:1445-1450.
288 Weiss E, Hirnle P, Arnold-Bofinger H, et al. Therapeutic outcome and relation of acute and late side effects in the adjuvant radiotherapy of endometrial carcinoma stage I and II. Radiother Oncol. 1999;53:37-44.
289 Nag S, Erickson B, Parikh S, et al. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the endometrium. Int J Radiat Oncol Biol Phys. 2000;48:779-790.
290 Onsrud M, Strickert T, Marthinsen AB. Late reactions after postoperative high-dose-rate intravaginal brachytherapy for endometrial cancer. A comparison of standardized and individualized target volumes. Int J Radiat Oncol Biol Phys. 2001;49:749-755.