Chapter 19 Endogenous Growth Factors as Cosmeceuticals
BIOCHEMICAL PATHWAYS OF SKIN AGING
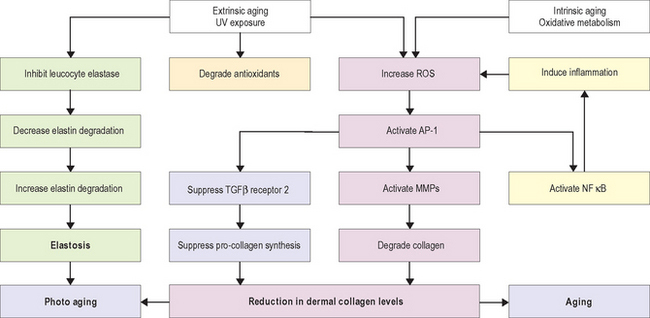
Research in the area of photoaging over the past decade has resulted in an improved understanding of the molecular mechanism of the aging process. Figure 19.1 shows a summary of major pathways involved in the aging process. Absorption of UV radiation by chromophores in the skin as well as cellular oxidative metabolism results in formation of reactive oxygen species (ROS). ROS increase oxidative phosphorylation of cell-surface receptors causing activation of the transcription factor activator protein-1 (AP-1) and nuclear factor kappa B (NF-κB).
AP-1 stimulates transcription of matrix metalloproteinase (MMP) growth factor genes in fibroblasts and keratinocytes, and inhibits type 1 procollagen gene expression in fibroblasts. MMPs produce degradation of fibrillar type I and type III collagen. Activity of MMP is decreased by binding with tissue inhibitors of metalloproteinase (TIMPs). ROS inactivate TIMPs, thereby increasing MMP activity. AP-1 mediated reduction in synthesis of procollagen appears to result from two mechanisms—interference of AP-1 with type 1 and type 3 procollagen gene transcription and blocking the profibrotic effects of transforming growth factor-beta (TGF-β) by impairment of the TGF-β type 2 receptor/Smad pathway.
GROWTH FACTORS IN WOUND HEALING
Hundreds of growth factors have been identified. Those that are important in wound healing include cytokines involved in immune response and phagocytosis, and growth factors that induce the synthesis of new collagen, elastin, and GAGs (components of the dermal extracellular matrix) that are abnormally affected by UV radiation. Table 19.1 lists the functions of the most important growth factors in wound healing.
| Growth factors and cytokines | Properties/actions |
| Vascular endothelial growth factor (VEGF) | Mediates angiogenesis |
| Chemotactic for endothelial cells | |
| Mitogenic for endothelial cells and keratinocytes | |
| Hepatocyte growth factor (HGF) | Mediates tissue organization and regeneration |
| Platelet-derived growth factor (PDGF) | Chemotactic for fibroblasts and macrophages |
| Mitogenic for fibroblasts, smooth muscle cells, and endothelial cells | |
| Epidermal growth factor (EGF) | Mediates angiogenesis |
| Chemotactic for endothelial cells | |
| Mitogenic for fibroblasts, endothelial cells, and keratinocytes | |
| Granulocyte colony stimulating factor (G-CSF) | Mediates angiogenesis |
| Mitogenic for hematopoietic cells | |
| Transforming growth factor-beta (TGF-β) | Mediates angiogenesis |
| Chemotactic for fibroblasts, keratinocytes, and macrophages | |
| Mitogenic for fibroblasts and smooth muscle cells | |
| Inhibits endothelial cells, keratinocytes, and lymphocytes | |
| Regulates matrix proteins including collagen, proteoglycans, fibronectin, and matrix-degrading proteins | |
| Keratinocyte growth factor | Mediates tissue organization and regeneration |
| Interleukins (IL-6 and IL-8) | Chemotactic for inflammatory cells and keratinocytes |
| Mitogenic for lymphocytes and keratinocytes |
Sources: Fitzpatrick RE, Rostan EF 2003 Reversal of photodamage with topical growth factors: a pilot study. Journal of Cosmetic and Laser Therapy 5:25–34; Moulin V 1995 Growth factors in skin wound healing. European Journal of Cell Biology 68:1–7.
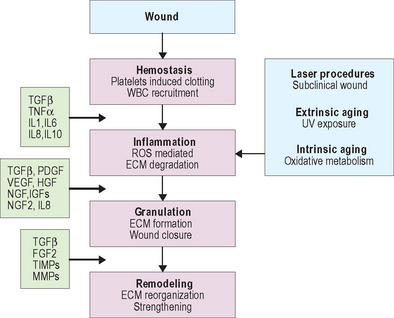
Wound healing is dependent on the synergistic interaction of many growth factors. After injury, cytokines and other growth factors flood the wound site to mediate the inflammatory response, promote new cell growth, and decrease wound contraction and scarring. The process of wound healing is commonly divided into four overlapping phases that describe physiologic responses to injury. These phases include hemostasis, inflammation, proliferation, and remodeling. Figure 19.2 summarizes each phase of wound healing along with dominant growth factors and cytokines. During hemostasis, platelets release various cytokines and other growth factors at the wound site to promote chemotaxis and mitogenesis. In the inflammatory stage, neutrophils and monocytes migrate to the wound site in response to specific cytokines and growth factors to initiate phagocytosis and to release additional growth factors that will attract fibroblasts. The proliferation phase is marked by epithelialization, angiogenesis, granular tissue formation, and collagen deposition. During proliferation, keratinocytes restore barrier function to the skin and secrete additional growth factors that stimulate the expression of new keratin proteins. Fibroblasts produce collagen that is deposited in the wound bed. This cycle of collagen production and growth factor secretion continues in a type of autocrine feedback loop of continuous wound repair.
The remodeling phase is the final step in the wound repair process and typically lasts several months. It is during remodeling that the extracellular matrix is reorganized, scar tissue is formed, and the wound is strengthened. Type III collagen deposited during the proliferation phase is gradually replaced by type I collagen, which is more tightly cross-linked and provides more tensile strength to the matrix than type III collagen. Cells at the wound site secrete several growth factors with specific functions related to remodeling and matrix formation. For example, collagen and fibronectin synthesis are initiated by TGF-β, while PDGF and TGF-β stimulate fibroblasts to produce GAGs and modulate the proliferation of smooth muscle cells. Other growth factors modify the vasculature. Over time, cell density increases and skin tissue is strengthened.
Specific growth factors directly initiate activity that promotes wound healing as well as modifying the activities of matrix cells and other growth factors. Growth factors are capable of both stimulating and inhibiting specific actions. The activity of growth factors is modulated through other growth factors and through various intrinsic factors that interact to achieve homeostasis and balance during wound healing. Research continues to uncover more information about the functions of individual growth factors during wound healing and, importantly, the synergistic interaction of growth factors with each other and with other components of wound healing. Whether the presence or absence of a single growth factor is significant in wound healing is not yet known. Current understanding suggests that it is the interaction of many growth factors that is significant, with no single growth factor being solely determinant in the outcome of wound healing.
TOPICAL APPLICATION OF GROWTH FACTORS
Individual growth factors (e.g. TGF-β, EGF, PDGF, etc.) have been shown to accelerate wound healing in both acute and chronic wounds. Photodamaged skin is similar to a chronic wound that may not progress to complete tissue remodeling. Total healing of photodamaged skin is unlikely because the surface area of the injury is too large to be completely repaired, and ongoing cumulative damage continues to occur daily. It is estimated that only 15 minutes of sun exposure can induce enough damage to collagen and elastic fibers to require remodeling activity. Table 19.1 lists some important growth factors and cytokines that affect the proliferation of dermal fibroblasts and extracellular matrix production. Providing some of these agents to cells responsible for extracellular matrix production and remodeling may result in rejuvenation of aging skin. Several cosmeceutical products containing a combination of multiple human growth factors and cytokines are currently marketed for skin rejuvenation. Clinical results are now available for some of these products and the results show that human growth factors when applied topically provide beneficial effects in reducing the signs of facial skin aging.
COMBINATION APPROACHES: LASER PLUS TOPICAL GROWTH FACTORS
Laser resurfacing and topical application of growth factors have each been shown to improve clinical signs of photodamage and to stimulate the formation of dermal collagen. Depending on whether CO2 or nonablative resurfacing is used, some degree of erythema will occur, and the process of wound healing will take time. A human fibroblast-derived temporary skin substitute was approved in 1997 for use as a temporary wound covering for mid-dermal to indeterminate depth wounds. This skin substitute has been used extensively in patients with partial thickness burns. In addition to providing a protective barrier, this temporary skin substitute contains growth factors secreted by the tissue culture and allows fibroblasts to proliferate and secrete dermal collagen, matrix proteins, and growth factors. A study of the use of fibroblast-derived temporary skin after CO2 laser resurfacing demonstrated faster healing and less pain and inflammation than traditional postoperative measures.
RISKS ASSOCIATED WITH GROWTH FACTORS
Another potential concern about the application of growth factors is whether they could contribute to hypertrophic scarring. Specifically, it has been postulated that TGF-β may increase the risk for scarring during wound healing due to its function in activating fibroblasts, which synthesize collagen, and because elevated levels of TGF-β have been noted at the site of dermal injury. But these findings cannot be extrapolated to suggest a causative role for TGF-β in the development of hypertrophic scarring. It has been suggested that an abnormal response of proliferative scar fibroblasts to TGF-β stimulation may contribute to the development of keloid and burn scars, but evaluation of patients with a genetic proclivity for the development of keloid scars failed to demonstrate a relationship between TGF-β plasma levels and keloid formation. Increased levels of TGF-β have been noted at the site of dermal wounds, but whether this growth factor contributes to scarring or is produced in response to scar development is unclear. With regard to the topical application of growth factors, there is no clinical evidence that they induce abnormal scarring, and anecdotal observations have failed to reveal any activity of growth factors that would produce an abnormal wound-healing response. In fact, the current understanding of growth factor activity in the wound-healing environment reveals a balance of both stimulatory and inhibitory actions that are carefully modulated to achieve homeostasis.
CONCLUSIONS
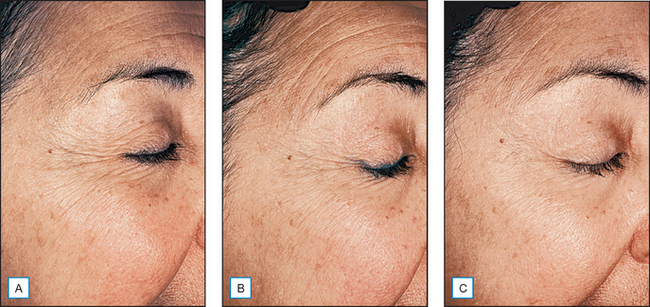
Studying the role of growth factors in cutaneous wound healing has led to research demonstrating positive cosmetic and clinical outcomes in photodamaged skin (Fig. 19.3). Although the topical use of growth factors is an emerging treatment approach, initial studies suggest that dermal collagen production and clinical improvement in photodamage appearance are substantial. Further, the increase in dermal collagen produced by topical growth factors can be measured quantitatively by biopsy. Although the functions of growth factors in the natural wound-healing process are complex and incompletely understood, it appears that wound healing is dependent on the synergistic interaction of many growth factors.
Alam M, Hsu TS, Dover JS, et al. Nonablative laser and light treatments: histology and tissue effects—a review. Lasers in Surgery and Medicine. 2003;33:30–39.
Bayat A, Bock O, Mrowietz U, et al. Genetic susceptibility to keloid disease and hypertrophic scarring: transforming growth factor beta 1 common polymorphisms and plasma levels. Plastic and Reconstructive Surgery. 2003;111:535–543.
Bernstein EF, Brown DB, Urbach F, et al. Ultraviolet radiation activates the human elastin promoter in transgenic mice: a novel in vivo and in vitro model of cutaneous photoaging. Journal of Investigative Dermatology. 1995;105:269-273.
Bernstein EF, Fisher LW, Li K, et al. Differential expression of the versican and decorin genes in photoaged and sun-protected skin. Comparison by immunohistochemical and northern analyses. Laboratory Investigation. 1995;72:662–669.
Bernstein EF, Underhill CB, Hahn PJ, et al. Chronic sun exposure alters both the content and distribution of dermal glycosaminoglycans. British Journal of Dermatology. 1996;135:255–262.
Bernstein EF, Andersen D, Zelickson BD. Laser resurfacing for dermal photoaging. Clinics in Plastic Surgery. 2000;27:221–240.
Bernstein EF, Ferreira M, Anderson D. A pilot investigation to subjectively measure treatment effect and side-effect profile of non-ablative skin remodeling using a 532 nm, 2 ms pulse-duration laser. Journal of Cosmetic Laser Therapy. 2001;3:137–141.
Brown GL, Nanney LB, Griffen J, et al. Enhancement of wound healing by topical treatment with epidermal growth factor. New England Journal of Medicine. 1989;321:76–79.
Cobb MH. MAP kinase pathways. Progress in Biophysics and Molecular Biology. 1999;71:479–500.
El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Experimental Dermatology. 2002;11:398–405.
Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. New England Journal of Medicine. 1997;337:1419–1428.
Fisher GJ, Talwar HS, Lin J, et al. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. Journal of Clinical Investigation. 1998;101:1432–1440.
Fitzpatrick RE 2000 TNS recovery complex aids in the healing of sun-damaged skin improving hydration, roughness, dispigmentation and wrinkles. Society for Investigative Dermatology 2001. May 11–14th. Chicago, IL, Poster
Fitzpatrick RE, Rostan EF. Reversal of photodamage with topical growth factors: a pilot study. Journal of Cosmetic Laser Therapy. 2003;5:25–34.
Fournier N, Dahan S, Barneon G, et al. Nonablative remodeling: a 14 month clinical ultrasound imaging and profilometric evaluation of a 1540 nm Er:glass laser. Dermatologic Surgery. 2002;28:926–931.
Gold MH, Goldman MP, Biron J. Efficacy of novel skin cream containing mixture of human growth factors and cytokines for skin rejuvenation. Journal of Drugs in Dermatology. 2007;6:197–201.
Goldberg DJ. New collagen formation after dermal remodeling with an intense pulsed light source. Journal of Cutaneous Laser Therapy. 2000;2:59–61.
Goldman R. Growth factors and chronic wound healing: past, present, and future. Advances in Skin and Wound Care. 2004;17:24–35.
Graeven U, Fiedler W, Karpinski S, et al. Melanoma-associated expression of vascular endothelial growth factor and its receptors FLT-1 and KDR. Journal of Cancer Research and Clinical Oncology. 1999;125:621–629.
Hardaway CA, Ross EV, Paithankar DY. Nonablative cutaneous remodeling with a 1.45 micron mid infrared diode laser; phase II. Journal of Cosmetic Laser Therapy. 2002;4:9–14.
Hensley K, Floyd R. Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Archives of Biochemistry and Biophysics. 2002;397:377–383.
Herold-Mende C, Steiner HH, Andl T, et al. Expression and functional significance of vascular endothelial growth factor receptors in human tumor cells. Laboratory Investigation. 1999;79:1573–1582.
Kao B, Kelly KM, Majaron B, et al. Novel model for evaluation of epidermal preservation and dermal collagen remodeling following photorejuvenation of human skin. Lasers in Surgery and Medicine. 2003;32:115–119.
Lavker RM, Kligman AM. Chronic heliodermatitis: a morphologic evaluation of chronic actinic dermal damage with emphasis on the role of mast cells. Journal of Investigative Dermatology. 1988;90:325–330.
Lazar-Molnar E, Hegyesi H, Toth S, Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine. 2000;12:547–554.
Lewis MP, Lygoe KA, Nystrom ML, et al. Tumour-derived TGF-beta 1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. British Journal of Cancer. 2004;90:822–832.
Liu B, Earl HM, Baban D, et al. Melanoma cell lines express VEGF receptor KDR and respond to exogenously added VEGF. Biochemical and Biophysical Research Communications. 1995;217:721–727.
Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81.
Mehta RC, Fitzpatrick RE. Endogenous growth factors as cosmeceuticals. Dermatologic Therapy. 2007;20:350–359.
Miyachi Y, Ishikawa O. Dermal connective tissue metabolism in photoageing. Australasian Journal of Dermatology. 1998;39:19–23.
Moulin V. Growth factors in skin wound healing. European Journal of Cell Biology. 1995;68:1–7.
Mustoe TA, Pierce GF, Thomason A, et al. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987;237:1333–1336.
Mustoe TA, Pierce GF, Morishima C, Deuel TF. Growth factor-induced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. Journal of Clinical Investigation. 1991;87:694–703.
Naughton GK, Pinney E, Mansbridge J, Fitzpatrick RE. Tissue-engineered derived growth factors as a topical treatment for rejuvenation of photodamaged skin. Poster: Society for Investigative Dermatology, 2001. 2001
Omi T, Kawana S, Sato S, et al. Ultrastructural changes elicited by a non-ablative wrinkle reduction laser. Lasers in Surgery and Medicine. 2003;32:46–49.
Polo M, Smith PD, Kim YJ, Wang X, Ko F, Robson MC. Effect of TGF-beta 2 on proliferative scar fibroblast cell kinetics. Annals of Plastic Surgery. 1999;43:185–190.
Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. American Journal of Pathology. 2004;165:741–751.
Ramont L, Pasco S, Hornebeck W, Maquart FX, Monboisse JC. Transforming growth factor-beta 1 inhibits tumor growth in a mouse melanoma model by down-regulating the plasminogen activation system. Experimental Cell Research. 2003;291:1–10.
Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proceedings of the National Academy of Sciences USA. 1986;83:4167–4171.
Rosenberg L, de la Torre J. Wound healing, growth factors. Online. Available: http://www.emedicine.com/plastic/topic457.htm, 2004.
Ross EV, Sajben FP, Hsia J, et al. Nonablative skin remodeling: selective dermal heating with a mid-infrared laser and contact cooling combination. Lasers in Surgery and Medicine. 2000;26:186–195.
Rostan E, Bowes LE, Iyer S, Fitzpatrick RE. A double-blind, side-by-side comparison study of low fluence long pulse dye laser to coolant treatment for wrinkling of the cheeks. Journal of Cosmetic Laser Therapy. 2001;3:129–136.
Schwartz E, Cruickshank FA, Christensen CC, Perlish JS, Lebwohl M. Collagen alterations in chronically sun-damaged human skin. Photochemistry and Photobiology. 1993;53:841-844.
Tanzi EL, Williams CM, Alster TS. Treatment of facial rhytides with a nonablative 1450–nm diode laser: a controlled clinical and histologic study. Dermatologic Surgery. 2003;29:124–128.
Uitto J. Collagen, Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, editors. Dermatology in general medicine, 4th edn., vol 1. New York: McGraw-Hill, 1993;299–314.
Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. Journal of Clinical Investigation. 2001;107:135–142.
Yu W, Naim JO, Lanzafame RJ. Expression of growth factors in early wound healing in rat skin. Lasers in Surgery and Medicine. 1994;15:281–289.