Chapter 21 Endocrinologically Silent Pituitary Tumors
Epidemiology and Nomenclature
Accounting for approximately 15% to 20% of all intracranial neoplasms and being found in up to 27% of hypophyseal glands in autopsy studies and 22.5% of radiologic studies, pituitary adenomas are among the most frequent intracranial tumors. Most are pituitary incidentalomas and only a relatively small portion of such tumors become clinically evident.1–6
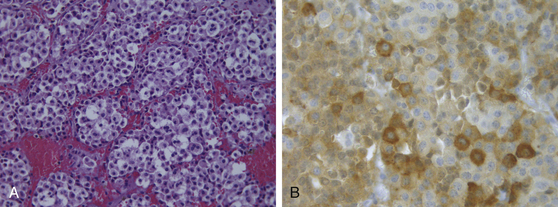
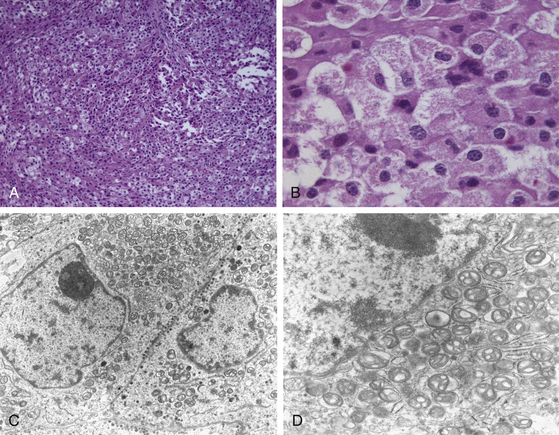
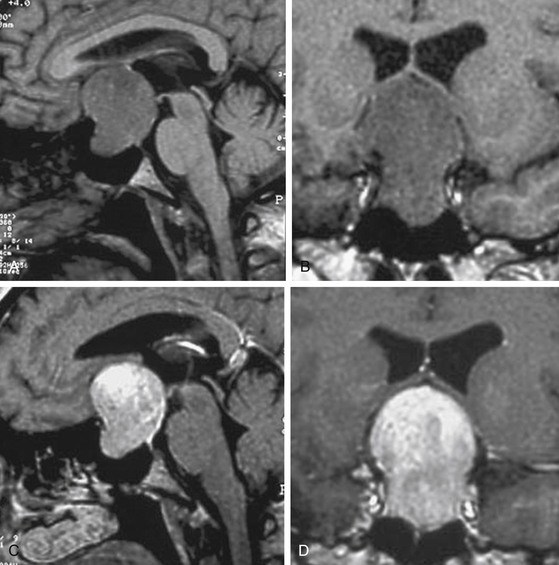
Once primarily classified according to their maximum diameter (microadenomas, <1 cm; macroadenomas, ≥1 cm), these tumors are further classified according to immunohistochemistry and functional status, since they display an array of hormonal and proliferative activity. Clinically silent pituitary adenomas (nonfunctioning, silent, or endocrine-inactive adenomas), which constitute the most frequent subtype of such pathologic entities, are pituitary adenomas that are not associated with syndromes of hormonal excess. They should not be preoperatively classified as nonsecretory adenomas, as it is known that after immunocytochemical studies, most produce one or more molecules, such as the α- or the β-subunits of the pituitary sexual glycoproteins (luteinizing hormone [LH], follicle-stimulating hormone [FSH]), in addition to the intact hormone; however, serum hormone levels may be not elevated, given that the secretion of such adenomas is often insufficient, incomplete, or inefficient.7–14 Such gonadotroph adenomas account for 40% to 50% of all macroadenomas and up to 80% of clinically endocrinologically silent pituitary tumors (Fig. 21-1). The rest of these tumors is constituted by the true nonsecreting adenomas (“null-cell adenomas” in immunohistochemical and ultrastructural classifications) (Fig. 21-2) and adenomas that secrete an incomplete or mutated form of other pituitary hormones (“silent ACTH-omas,” “silent GH-omas,” and so on according to immunohistochemical and ultrastructural classifications).
Clinical and Anatomic Features
By definition, endocrinologically silent pituitary tumors do not cause symptoms related to hormone hypersecretion. Rather, their clinical presentation is dominated by mass effect symptoms. Consequently, the vast majority of them are not recognized until they become sizable macroadenomas. As a matter of fact, according to recent reports,15,16 almost all such tumors are macroadenomas (96.5%) and the main presenting symptoms are visual defects (67.8%) and headache (41.4%), and the most frequent pituitary deficit is hypogonadism (43.3%).
Visual Symptoms
Among the most common of such symptoms are the visual deficits, either qualitative and/or quantitative. Such symptoms are related to the suprasellar expansion of the pituitary tumor, with compression and distortion of the optic chiasm and consequent chiasmatic syndrome. That is characterized by visual field defect (classically, incomplete to complete bitemporal hemianopia) due to the compression of the nasal retinal fibers (Fig. 21-3). The deficit of one or of both the temporal hemi-fields begins in such a subdolous manner that it is not appraisable by the patient, in the superior temporal quadrants, progressing towards the bottom in a counterclockwise direction, for the left eye and in a clockwise sense for the right eye. Other perimetric defects are seldom present, such as binasal perimetric defects, resulting from a lateral compression of the chiasm; bilateral altitudinal hemianopia, from a lesion to the superior fibers or to the inferior ones, the latter being involved with greater frequency; homonymous hemianopia, because of the involvement of the posterior portion of the chiasm; and monocular perimetric defects.
Besides the visual field deficits, loss of vision of various degrees, even to blindness, may be present. The visus, especially in the initial stages, when the tumor does not compress the fibers originating from the macula (which are situated in the posterior-median portion of the chiasm), may be preserved. The reduction of visual acuity is often unilateral or is not the same in both eyes. More rare is palsy of the eye musculature, palpebral ptosis, ophthalmoplegia, and diplopia due to the adenomatous mass growing laterally, compressing and/or invading the cavernous sinus and therefore the oculomotor nerves. Another impairment of the ocular motility that could be encountered is a dissociated vertical-pendular nistagmus (see-saw nystagmus), sometimes associated with bitemporal hemianopia, and thought to be related to a lesion of Cajal’s interstitial nucleus. A splitting of the images could also be present in the absence of any muscular palsy, and this symptom is due to the fact that for the patient with bitemporal hemianopia, vision is missing in the outer (temporal or lateral) half of both the right and left visual fields.
Headache
Another common symptom caused by endocrinologically silent pituitary tumors is headache, which does not seem to be correlated with the size of the adenoma.17 Such symptom is due to the stretching of the diaphragma sellae and/or the medial wall of the cavernous sinuses, caused by an even mild or moderate intrasellar hypertension.18
Pituitary Apoplexy
Pituitary apoplexy is a relatively rare condition presenting with sudden headache, acute visual loss, ophthalmoplegia, altered level of consciousness, lethargy, and even collapse from acute adrenal insufficiency. It is caused by a hemorrhage into the tumor and/or its acute necrosis, with subsequent swelling and frequent spreading into the subarachnoid space, leading to other signs of meningeal irritation (Fig. 21-4). Clinical conditions or precipitating factors that are associated with an increased risk for such occurrence are coronary artery surgery and other major surgery, pregnancy, anticoagulant or antiaggregant therapy, and coagulopathies.19 The related acute and severe clinical syndrome demands glucocorticoid replacement and surgical decompression, usually trans-sphenoidal, if visual loss is severe and progressive. These patients typically require a full hormonal replacement therapy in the perioperative period. If the patient has a subclinical or a mild form of apoplexy and is clinically stable, it is prudent to measure the serum prolactin, since some patients with prolactinoma present in this fashion and may be successfully treated with medical therapy.
Preoperative Evaluation
Global management of pituitary masses usually involves a multidisciplinary team. Thus, an accurate, complete, balanced, and global preoperative evaluation of these patients is critical, including diagnostic and clinical tests and laboratory investigations, to minimize surgical risks and optimize the outcome.
Neuroradiologic Evaluation
• Identify the lesion, excluding various differential diagnoses
• Control effects of medical therapy
• Define spatial situation of the lesion (presurgical planning)
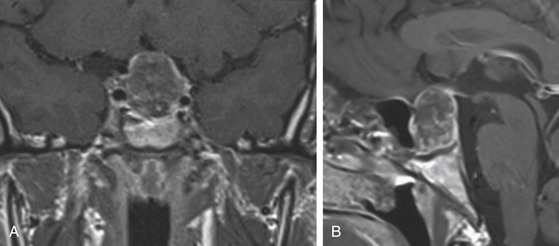
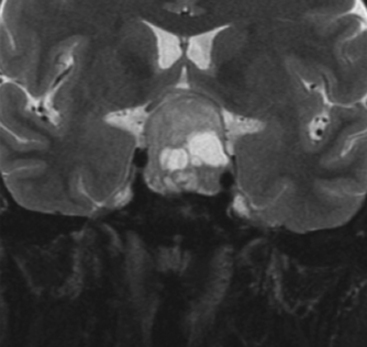
The gold standard among the radiologic studies available is the magnetic resonance imaging. A complete MR protocol should include, at least T1- and T2-weighted images and T1-weighted postcontrast (postgadolinium) images (Fig. 21-5) in the three orthogonal planes and 3-mm thick sections.20 Complementary MR sequences are also useful, such as dynamic sequences; MR angiography, namely when one suspects the vascular nature of a lesion; diffusion-weighted and/or perfusion-weighted MRI; and MR spectroscopy (Fig. 21-6).
FIGURE 21-6 Preoperative axial MRI, diffusion-weighted sequence, of clinically nonfunctioning pituitary macroadenoma.
Fibrous adenomas are encountered in approximately 10% of cases21 and can be difficult to remove via the conventional trans-sphenoidal approach. In such cases, preoperative MRI may be useful in anticipating tumor consistency and allow planning of the surgical strategy correctly. Tumors with a hypo- or iso-intense appearance on T2-weighted MRI may be indicative of greater collagen content21–23 and a more fibrous tumor consistency. In contrast, softer tumors are hyperintense on T2 (Fig. 21-7).
When a trans-sphenoidal approach is foreseen—especially the endonasal variant—axial and coronal CT scanning may be helpful in addition to an accurate assessment of the anatomy of the nasal cavities and the paranasal sinuses and for the definition of the best route from the nasal cavity toward the sella, since MR imaging alone does not provide the necessary detail of bone anatomy.20 Axial and coronal CT scanning allows the neurosurgeon to more easily assess the three-dimensional (3D) aspects of the nasal and paranasal cavities, particularly the sphenoid bone, and the relationships with the sellar floor and carotid canals. CT study is essential in order to reveal the presence of eventual anatomic variations or pathologic changes of the cavities. Moreover, CT remains the diagnostic imaging study of choice in patients who are unable to undergo an MR study.
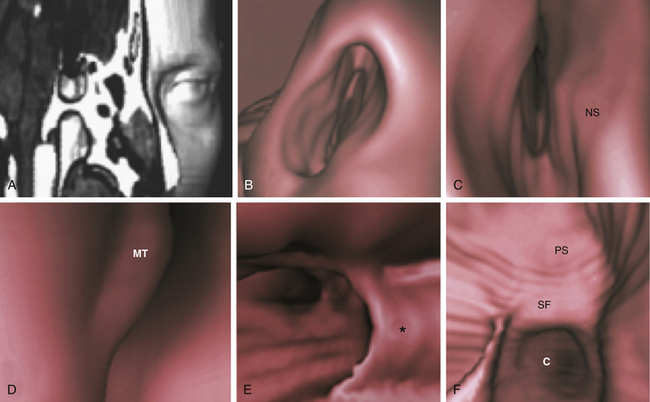
Although analysis of axial and coronal scans gives a detailed true depiction of rhino-sinusal anatomy, the 3D virtual endoscopy yields images similar to those provided by actual endoscopy, giving the surgeon a view before the operation and avoiding the complex task of mental reconstruction in the space of images obtained on traditional scan planes20,24 (Fig. 21-8).
Ophthalmologic Evaluation
The ophthalmologist’s evaluation is of utmost importance when a suprasellar lesion is suspected, in all the stages of the disease from the diagnosis to the pre- and post-operative evaluation.25 The visual field evaluation, by means of either the manual kinetic technique or the computerized threshold static perimetry (Fig. 21-3) and the study of visual evoked potentials (VEPs) are the most suitable exams to show eventual functional deficits due to chiasmatic involvement, as well as to monitor the preservation or vision recovery after surgery. VEPs are useful in the evaluation of the pharmacologic or surgical treatment, rather than for routine diagnostic purposes, where the basic test was and still remains the field exam. The examination of chromatic sensibility is not considered a routine investigation, but it should be performed in cases where the field evaluation and VEPs are normal, but there is still suspicion of chiasmatic compression based on neuroradiologic studies.
Management
Medical Management
It is now verified, by immunocytochemistry and ultrastructural studies, that the majority of such tumors are glycoprotein-producing, followed by nonfunctioning somatotroph, lactotroph, or corticotroph adenomas.16 As a matter of facts, a large proportion of nonfunctioning adenomas (up to 90%) is shown to secrete low amounts of either intact FSH and LH and/or their α- and β-subunits and the vast majority of nonfunctioning adenomas also express on cell membranes different subtypes of somatostatin and dopamine receptors (respectively, sst1-sst5 and D1-D5) in a variable amount. Based on this feature, both dopamine agonists and somatostatin analogues have a rationale in the treatment of such tumors and such fact has opened a new perspective of medical treatment for these lesions.
Somatostatin Receptors and Their Role
Somatostatin (growth hormone-inhibiting hormone [GHIH] or somatotropin release–inhibiting factor [SRIF]) is a peptide hormone that regulates the endocrine system and affects neurotransmission and cell proliferation via interaction with G-protein–coupled somatostatin receptors and inhibition of the release of numerous secondary hormones. It is classified as an inhibitory hormone. Somatostatin analogs (SSAs) have been used in clinical practice over the past 20 years. They act through the somatostatin receptors, which have been demonstrated to be highly expressed in all pituitary adenomas with a predominance of sst2 and sst5 and infrequent expression of sst416,26–28 Another analogue, the slow-release lanreotide (LAN), showing a similar receptor affinity for sst to that of OCT, was made available for intramuscular injections every 7 to 14 days. More recently, OCT and LAN have been made available for injections either intramuscular or subcutaneous every 28 to 56 days, respectively. The response to octreotide and lanreotide is predicted by the expression of sst216,29 In nonfunctioning adenomas the receptor subtype expressed in a higher amount was found to be the sst3 followed by the sst2, also correlated with sst5 expression.30 The expression of sst2 and sst5 in nonfunctioning adenomas has been found to be associated with reduced cell viability by 20% to 80% (in 8 of 13 sst2-positive tumors) and by 15% to 80% (in 10 of 13 tumors, all but three sst5 positive).31 More recently, pasireotide (SOM230), a somatostatin analogue binding sst1-3 and sst5, completely abrogated the promoting effects of vascular endothelial growth factor (VEGF) on nonfunctioning adenoma cell viability.32
Only few clinical trials have been conducted to evaluate potential effects of SSA in patients with nonfunctioning adenomas. However, a meta-analysis of such data revealed that tumor reduction was reported only in 12% of cases, while the vast majority of patients had stable remnant tumors.16 A still unexplained finding reported by some authors33,34 is that OCT treatment was followed by a rapid improvement in headache and visual disturbances, without any change in tumor volume. This effect was likely not owing to a direct effect on tumor size but more likely to a direct effect on the retina and the optic nerve.35 However, approximately in one third of the patients there was an improvement in visual field defects.16
Dopamine Receptors and Their Role
Dopamine receptors, namely the D2 subtype, in the normal pituitary mediate the tonic inhibitory control of PRL secretion36 by dopamine.37 A meta-analysis of several studies16 has shown the presence of dopamine binding sites (most likely D2) in nonfunctioning adenomas. Furthermore, in gonadotropin-immunopositive adenomas, the D2 were mainly localized in LH- and FSH-immunopositive cells.38 Anyway, the D2 receptor is not the only dopamine receptor expressed in the pituitary gland. Indeed, the D4 receptor, in particular its D4.4 variant, is also expressed, although its role in the physiology of the pituitary gland is not known.39 Cabergoline is the actual most commonly used dopamine agonist, able to inhibit in vitro the α-subunit concentration in 56% of cases and such result was associated with D2 expression,40 while the tumor shrinkage after DA therapy is 27.6%.16
A recent study of our group40 demonstrated that 1-year treatment with cabergoline at the dose of 3 mg/wk induced a more than 25% tumor shrinkage in 56% of patients with histologically proven nonfunctioning adenomas and such shrinkage was significantly correlated with the expression of the D2 isoform. The counterpart of such evidence was the finding of tumor regrowth after stopping the dopamine-agonist treatment,41 thus supporting the use of such treatment in these subjects.
Cumulative Role of Somatostatin and Dopamine Receptors
It is known that D2 and sst5 physically interact through hetero-oligomerization to create novel receptor with enhanced functional activity.16,42 This fact constitutes the rationale of whether the combination treatment with selective ligands of these two individual receptors, as well as new molecules binding both receptors simultaneously, could be effective in nonfunctioning adenomas.16 Anyway, such approach is still under investigation.43
Gonadotropin-Releasing Hormone Analogues and Their Role
Since a large percentage of nonfunctioning pituitary adenomas produce intact gonadotropins or, at least, the α-subunit, attempts were made to inhibit gonadotropin secretion by using GnRH analogues.16 Unfortunately, the results of the different attempts were not conclusive and no data are available concerning the tumor shrinkage or the visual effects. Though, such treatment is not anymore proposed in patients with nonfunctioning adenomas.
Other Medical Approaches
Type I interferon (IFN) molecules routinely used in the treatment of chronic hepatitis B and C, multiple sclerosis, and a few tumors44 have been investigated in recent years for their possible regulatory role in the hypothalamic-pituitary axis. As a matter of facts, incubation with IFN-α resulted in 24% to 62% inhibition of the secretion of gonadotropins and/or alpha-subunit in a recent study of Hofland et al.45 However, in healthy volunteers or in treated patients for hepatitis or multiple sclerosis, changes of testosterone or gonadotropin levels were found to be temporary.44
Ongoing experimental studies for other possible medical approaches concern the folate receptor (FR-α), which is significantly overexpressed in clinically nonfunctional adenomas but not in functional adenomas.46,47 Such evidence may hold significant promise for medical treatment by enabling novel molecular imaging and targeted therapy.
Surgical Management
Surgical management of pituitary masses and, therefore, endocrinologically silent pituitary tumors, remains a distinct subspecialty of neurosurgery. It requires a precise knowledge and skills of basic neurosurgical techniques, together with specific knowledge, interest, and appreciation of pituitary-hypothalamic pathophysiology, allowing the surgeon to choose among different approaches based upon the specific features of the patient. The neurosurgeon must know the detailed surgical anatomy, must be experienced in neuroimaging, must know pathophysiology and the natural history of pituitary disease, and must be familiar with the various therapeutic options and the related pros and cons. Furthermore, surgery of the pituitary tumors yields the best outcomes when performed in dedicated centers where the entire range of pituitary specialties is offered in an environment of effective teamwork, which requires a “teamwork attitude” that is not just the addition of the expertise of the single contributors, but rather a cultural and psychological attitude, with the single units working with a goal of true exchange and sincere collaboration, thus allowing cooperative effort for the benefit of the patient and positive feedback for physicians and surgeons.48 Pituitary surgery is not only choosing the correct surgical route and performing and uneventful operation but, rather, it also requires careful and specific postoperative management and long-term patient follow-up, with a continuous information exchange among the different specialists of the teamwork, i.e., the pathologist, the ophthalmologist, the neuroradiologist, the endocrinologist, the radiation oncologist and, of course, the neurosurgeon.
Large invasive pituitary tumors and those with a frank cavernous sinus invasion are thought to be difficult to cure regardless of the approach because the gross total removal of the tumor is often not achievable. Recently introduced extended trans-sphenoidal approaches sometimes can represent a valid alternative to transcranial options.49
The surgical approach, with respect to the basic principles for resecting pituitary adenomas, can be performed by two main approaches, each of them with several subcategories (Table 21-1). This chapter will focus on the trans-sphenoidal approaches only. Transcranial approaches will be described in a chapter dedicated to this issue.
| Trans-Sphenoidal Approach |
|---|
| Microscopic |
| Sublabial |
| Trans-septal |
| Endonasal |
| Endoscopic |
| Combined or endoscope-assisted microsurgery |
| Transcranial Approach |
| Pterional or frontolateral |
| Subfrontal unilateral |
| Subfrontal bilateral interhemispheric |
Trans-Sphenoidal Approaches
1. It is the least traumatic route to the sella.
2. It does not leave visible scars.
3. It provides excellent visualization of the surgical field.
4. It offers excellent outcomes with a lower morbidity and mortality rate compared with transcranial procedures.
Indications for trans-sphenoidal surgery today include more than 95% of the surgical indications in the sellar area and approximately 96% of all pituitary adenomas.50,51 Furthermore, absolute indications for such approaches include any condition that poses an elevated surgical risk via the transcranial route; the elderly patient; longstanding compression of the chiasm, and thus inability to tolerate additional trauma; acute endosellar hypertension, such as in pituitary apoplexy; paninvasive and not otherwise radically removable adenomas; adenomas with downward growth (into the sphenoid sinus); and microadenomas.
In case of exceptionally big adenomas or paninvasive tumors, an intentionally two-staged trans-sphenoidal operation may be scheduled. Such option is designed to encourage the descent of a suprasellar remnant of the adenoma incompletely removed in the first step, to limit the risks of a brisk decompression of huge lesions, and to manage the residual mass with a second surgery.52
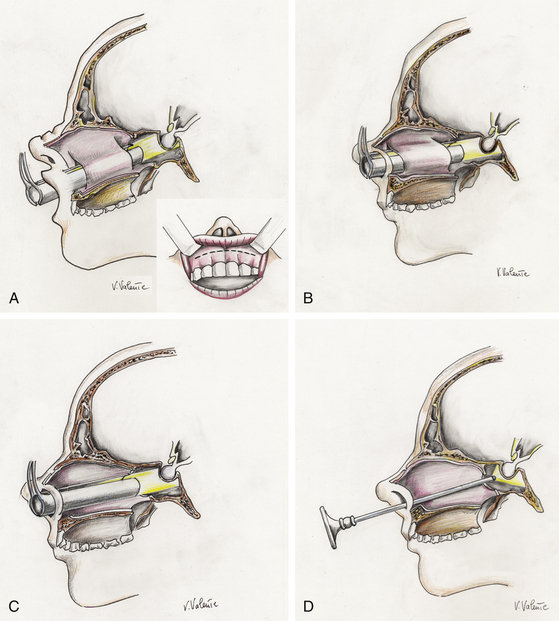
The trans-sphenoidal approach represents a midline extracranial approach that has been performed since the 1960s by means of the operating microscope as visualizing tool, through transnasal trans-septal, sublabial trans-septal, or endonasal procedures (microsurgical trans-sphenoidal procedures) (Fig. 21-9A, B, and C). The trans-sphenoidal approach also can be performed by means of the endoscope as the sole visualizing tool during the entire surgical procedure, realizing a “pure” endoscopic endonasal trans-sphenoidal approach. The combined use of the microscope and the endoscope during the same approach defines the condition of endoscope-assisted microsurgery48 (see Table 21-1).
Microsurgical Trans-Sphenoidal Approaches
Although many different trans-sphenoidal procedures and variations have been described, currently there are three basic microsurgical trans-sphenoidal approaches to pituitary tumors: the transnasal trans-septal trans-sphenoidal approach, the sublabial trans-septal trans-sphenoidal approach, and the endonasal trans-sphenoidal approach (Table 21-1). The procedure is performed with an operating microscope for visualization, illumination, and magnification of the surgical field. Intermittent fluoroscopy is used for trajectory guidance, or, more recently, neuronavigational systems permit the surgeon to gather information about the current position of anatomic structures or instruments during the procedure iself.49,53–57 Intraoperative MRI (iMRI) may provide additional knowledge about the completeness of lesion removal.58–60 The three main microscopic trans-sphenoidal methods differ slightly primarily in the initial phase up to the exposure of the sphenoid sinus; they then follow the same surgical sphenoidal and sellar steps.
Endoscopic Endonasal Trans-Sphenoidal Approach
Endoscopic endonasal trans-sphenoidal surgery is a more recent, minimally invasive trans-sphenoidal approach performed by means of the endoscope as a stand-alone visualizing and operating instrument, without the need of the trans-sphenoidal retractor48,49,61,62 (Fig. 21-9D). It has the same indications as the conventional microsurgical technique and since the 1990s has enjoyed progressive acceptance among surgeons and patients for its minimal invasiveness and for the excellent surgical view it provides.63–70 Based on these advantages of trans-sphenoidal surgery in combination with the continuous search for less invasive procedures and the development of endoscopic equipment, endoscopic endonasal trans-sphenoidal surgery has slowly and inexorably become an accepted technique in the field of pituitary surgery during the last decade of the 20th century.62,63,71,72
It requires detailed knowledge of the sinonasal anatomy and specific endoscopic skills, both favored by a close cooperation with an ENT/otolaryngologist with specific competences in endoscopy, and is based on a different concept because the endoscopic view that the surgeon receives on the video monitor is not the transposition of the real image, as it would be looking through the eyepiece of a microscope, but is the result of a microprocessor’s elaboration.48 Nevertheless, other factors have contributed to determine the success of the endoscopic skull base and pituitary surgery. As a matter of facts, if the operation itself has become increasingly simply, safe and elegant, that is unimaginable without an appropriate instrumentation.73 Every operation and even every technique for each type of operation, requires a dedicated set of instruments—endoscope, camera, light source, monitor, video-recording systems, drills, and so on—which should be of the best quality possible and be dedicated to this type of operation. The instruments form a sort of chain, where each link must to be strong enough to perform its role in the operation otherwise the chain will break and the surgery will not be successful.49,73,74
As for other techniques of skull base surgery, the endoscopic endonasal procedure consists of three main aspects: exposure of the lesion, management of the relevant pathology, and reconstruction of the sella.49
Because trans-sphenoidal endoscopy is a more recent contribution to pituitary surgery, some advantages, pitfalls, and peculiar aspects related to this technique must be highlighted. The main advantages of the endoscopic procedure compared with the microsurgical procedures are related to the properties of the endoscope itself and to the absence of the nasal speculum.62,68 As a matter of facts, the nasal speculum creates a “fixed tunnel” and an almost coaxial restriction of the microinstruments and, with its absence, the endoscope discloses its superior properties, permitting a wider vision of the surgical field, with a close-up “look” inside the anatomy. The angled lens endoscopes enable the surgeon to work on tumors located in suprasellar and parasellar regions under direct visual control. The endoscopic endonasal procedure seems to be less traumatic, and the percentage of many complications is reduced compared with the traditional microsurgical approach.75 Since the actual procedure starts from the natural ostium of the sphenoid sinus and the submucosal nasal phase is almost avoided, damage to the nasal spine and orodental complications due to the incision in the buccogingival junction are prevented. As a consequence, in almost all cases, no nasal packing is employed, and postoperative breathing difficulties are reduced. So, the endoscopic endonasal approach can be employed in cases of intentionally two-staged trans-sphenoidal operations52 because of its excellent ability in reaching the sellar region during the second operation.
Furthermore, the endoscopic approach is particularly advantageous in the case of recurrent or sresidual tumors already treated with a trans-sphenoidal operation,76 in which the surgeon usually finds distorted anatomy and may encounter nasal synechiae, septal perforations, mucoceles, and intrasellar scarring. With the endoscopic procedure, thanks to the avoidance of the submucosal nasal phase of the microsurgical operation, the real beginning of the operation is at the sphenoid sinus, already enlarged by the former approach, rendering the procedure faster and more straightforward, compared with the microsurgical trans-sphenoidal method. The wide anatomic view of the surgical field the endoscope offers in the sphenoid and sellar area minimizes the chance of a misdirected orientation, when the midline anatomic landmarks are not recognizable or absent, reducing the possibility of injury to the intrasellar and parasellar structures, particularly if a neuronavigation system is used.
Disadvantages of the endoscopic approach include a learning curve to become confident with the unfamiliar anatomy of the nasal cavities and with the specific endoscopic dexterity. Nevertheless, after adequate experience, the operating time becomes the same or shorter than that required for trans-sphenoidal microsurgery, especially in case of recurrences. The endoscope offers only bidimensional vision on the video monitor. The sense of depth can be gained with the surgeon’s experience, making the endoscope execute in and out movements, looking for many useful different anatomic landmarks and referring to the many protuberances and depressions in the sphenoid sinus, representing reflections and shadows corresponding to different structures. Dedicated microsurgical endoscopic instruments with secure grip, straight and not bayonet shaped, provided with different and variably angled tips are necessary to reach the surgical targets, particularly the targets that the angled endoscopes are able to show.74,77
Extended Endoscopic Endonasal Approach to the Suprasellar Area
First introduced by Weiss in 1987,78 the extended trans-sphenoidal approach is a trans-sphenoidal approach with the removal of additional bone along the tuberculum sellae and the posterior planum sphenoidale between the optic canals. As a matter of fact, the dural opening was completely above the diaphragma sellae. More recently, the widespread use of the endoscope in trans-sphenoidal surgery has shed new interest on this surgical technique, affording the extension of the trans-sphenoidal approach.49,79–86 Today, pituitary macroadenomas with suprasellar symmetric and/or asymmetric extension even inside the cavernous sinus, once considered all amenable to open transcranial surgery only are successfully treated by means of such approaches.49,81,87
• Detailed, complete preoperative planning, integrated by 3D reconstructions, in order to tailor the skull base opening with the 3D volume of the lesion
• An image-guided system (neuronavigator) to intraoperatively identify the limits of the lesion, the midline, and the trajectory, offering more precision in defining the boundaries of the bone removal and neurovascular relationships
• Dedicated instruments—that is, high-speed, low-profile microdrills, micro-Doppler probes (especially when approaching the cavernous sinus), bipolar forceps with angled tips, low-profile ultrasonic aspirator or radiofrequency cavitron-like coagulation—to properly manage lesions in such a delicate environment
It is interesting to note that with the endoscopic extended approaches, the surgeon could proceed performing a bimanual dissection while a “tuned” coworker holds the endoscope moving it dynamically and, as requested, insert another surgical instrument. This so-called “3-4 hands technique” requires a good collaboration between two surgeons that should be perfectly tuned, one holding the endoscope and another handling two surgical instruments inside the surgical field.88 The two surgeons have therefore the possibility to continuously pass between the close-up view, as during the dissecting maneuvers, and a panoramic view of the neurovascular structures. However, it is possible to fix the endoscope to an autostatic holder that can be handled by a single surgeon.
Due to the conspicuous intraoperative cerebrospinal fluid (CSF) leakage resulting from the usually large osteodural opening required to approach the suprasellar area, an accurate reconstruction of the skull base defect is needed after lesion removal. The reconstruction has to be watertight in order to prevent postoperative CSF leak, which could occur even more frequently large openings of the arachnoid cisterns or of the third ventricle.89,90 The reconstruction is performed using various techniques, including intradural, extradural, and intra-extradural, and the recent adoption of the pedicled nasoseptal flap,91–93 even though the most effective seems to be the extradural one.90 No lumbar drainage is used by our group at the end of the procedure; nevertheless, we advise our patients to have bed rest for 3 to 5 days, depending also on the grade of the pneumoencephalus, while medical therapy with acetazolamyde, laxatives, and broad-spectrum antibiotics is administered.
When compared to the transcranial routes, the extended trans-sphenoidal approach provides a direct view of the neurovascular structures of the suprasellar region from below and no brain and optic nerve manipulation is required, with reduced risk of postoperative vision worsening.94
On the other hand, it should be minded that the extended endoscopic endonasal approach to the suprasellar area is more technically demanding and requires some additional skills, both related to the endoscope itself and the opposite anatomic point of view.84 Besides, it is crucial to focus on some parameters, concerning either the lesion and the anatomy, that could affect lesion management via such a route.6 Indeed, a well-pneumatized sphenoid sinus allows better visualization of all important landmarks in the posterior wall that, again, are necessary for maintaining surgical orientation while performing the bone removal.49 On the contrary, a conchal-type sphenoid sinus could hinder the extended endonasal approach, whilst a small sella, with two close intracavernous carotids, could determine a narrower approach.
Postoperative Evaluation and Management After Uncomplicated Trans-Sphenoidal Operation
Regarding the antibiotic therapy, it is our policy to adopt a short-term prophylaxis with intravenous third-generation cephalosporins (ceftriaxone or ceftazidime): 30 minutes before starting the procedure and 6 hours after the first injection, with no increased risk for infectious complication. Only in the case of intraoperative CSF leak, where a large communication exists with the subarachnoid compartment, we use prophylaxis for 3 days after the operation.
The patient is usually discharged from the hospital on postoperative day 2 or 3, and is expected to contact the endocrinologist for hormonal follow-up. Furthermore, the patient will be instructed to have serum sodium checked on postoperative days 4 to 6 in order to control for incipient hyponatremia17 that may be caused by a SIADH (syndrome of inappropriate antidiuretic hormone secretion). (Such hyponatremia is typically subclinical, although on occasion it is severe enough to require rehospitalization.) The patient will then be seen in the outpatient clinic 3 months later for a postoperative sellar-contrast–enhanced MRI. If grade-2 or -3 CSF has occurred during the operation, or in the case of large lesions, the patient is scheduled as an outpatient for an endoscopic evaluation of the nasal and sphenoid sinus cavities to ensure that the mucosa is healing well and that CSF leakage is not present.
Black P.M., Hsu D.W., Klibanski A., et al. Hormone production in clinically nonfunctioning pituitary adenomas. J Neurosurg. 1987;66(2):244-250.
Cappabianca P., Cavallo L.M., de Divitiis E. Endoscopic endonasal transsphenoidal surgery. Neurosurgery. 2004;55(4):933-940. discussion 940–931
Cappabianca P., Cavallo L.M., de Divitiis O.. Pituitary surgery, DeGroot L., Jameson J.L., editors. Endocrinology, 5th ed, vol 1. Philadelphia: Elsevier, 2006;511-535.
Cappabianca P., Cavallo L.M., Esposito F., de Divitiis E. Endonasal approaches to the Cavernous sinus. In: Anand V.K., Schwartz T.H. Practical endoscopic skull base surgery. San Diego, Oxford, Brisbane: Plural Publishing; 2007:187-199.
Cappabianca P., Esposito F., Cavallo L.M., Corriero O.V. Instruments. In: Cappabianca P., Califano L., Iaconetta G. Cranial, Craniofacial and Skull Base Surgery. New York: Springer-Verlag; 2010:7-16.
Cavallo L.M., Messina A., Esposito F., et al. Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. J Neurosurg. 2007;107(4):713-720.
Colao A., Di Somma C., Pivonello R., et al. Medical therapy for clinically non-functioning pituitary adenomas. Endocr Relat Cancer. 2008;15(4):905-915.
de Bruin T.W., Kwekkeboom D.J., Van’t Verlaat J.W., et al. Clinically nonfunctioning pituitary adenoma and octreotide response to long term high dose treatment, and studies in vitro. J Clin Endocrinol Metab. 1992;75(5):1310-1317.
Elias W.J., Chadduck J.B., Alden T.D., Laws E.R.Jr. Frameless stereotaxy for transsphenoidal surgery. Neurosurgery. 1999;45(2):271-275. discussion 275–277
Esposito F., Dusick J.R., Cohan P., et al. Clinical review: early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab. 2006;91(1):7-13.
Ezzat S., Asa S.L., Couldwell W.T., et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(3):613-619. 1
Frank G., Pasquini E. Endoscopic endonasal cavernous sinus surgery, with special reference to pituitary adenomas. Front Horm Res. 2006;34:64-82.
Hofland L.J., Lamberts S.W. Somatostatin receptor subtype expression in human tumors. Ann Oncol. 2001;12(suppl 2):S31-S36.
Iuchi T., Saeki N., Tanaka M., et al. MRI prediction of fibrous pituitary adenomas. Acta Neurochir (Wien). 1998;140(8):779-786.
Kassam A., Snyderman C.H., Mintz A., et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(1):E3. 15
Laws E.R.J. Foreword. In: De Divitiis E., Cappabianca P. Endoscopic endonasal transsphenoidal surgery. Wien, New York: Springer, 2003. v-vii
Levy M.J., Jager H.R., Powell M., et al. Pituitary volume and headache: size is not everything. Arch Neurol. 2004;61(5):721-725.
Minniti G., Gilbert D.C., Brada M. Modern techniques for pituitary radiotherapy. Rev Endocr Metab Disord. 2009;10(2):135-144.
Pivonello R., Matrone C., Filippella M., et al. Dopamine receptor expression and function in clinically nonfunctioning pituitary tumors: comparison with the effectiveness of cabergoline treatment. J Clin Endocrinol Metab. 2004;89(4):1674-1683.
Prevedello D.M., Doglietto F., Jane J.A.Jr., et al. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg. 2007;107(1):206-213.
Semple P.L., Jane J.A.Jr., Laws E.R.Jr. Clinical relevance of precipitating factors in pituitary apoplexy. Neurosurgery. 2007;61(5):956-961. discussion 961–952
Shirodkar M., Jabbour S.A. Endocrine incidentalomas. Int J Clin Pract. 2008;62(9):1423-1431.
Snyder P.J.. Gonadotroph and other clinically nonfunctioning pituitary adenomas, DeGroot L., Jameson J.L., editors. Endocrinology, 5th ed, vol 1. Philadelphia: Elsevier, 2006;465-484.
Visot A., Pencalet P., Boulin A., Gaillard S.. Surgical management of endocrinologically silent pituitary tumors, Schmideck H.H., Sweet D.W., editors. Operative Neurosurgical Techniques. Indications, Methods and Results, 5th ed, vol 1. Philadelphia: Elsevier, 2006;355-373.
1. Ezzat S., Asa S.L., Couldwell W.T., et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(3):613-619. 1
2. Buurman H., Saeger W. Subclinical adenomas in postmortem pituitaries: classification and correlations to clinical data. Eur J Endocrinol. 2006;154(5):753-758.
3. Shirodkar M., Jabbour S.A. Endocrine incidentalomas. Int J Clin Pract. 2008;62(9):1423-1431.
4. Koch Devin J., Blevings L.S.Jr.. Endocrinologic approach to the evaluation and management of the patient undergoing surgery for a pituitary tumor, Schmideck H.H., Sweet D.W., editors. Operative Neurosurgical Techniques. Indications, Methods and Results, 5th ed, vol 1. Philadelphia: Elsevier, 2006;300-320.
5. Blevins L.S., Shore D., Weinstein J.. Clinical presentation of pituitary tumors, Krisht A.F., Tindall G.T., editors, Pituitary disorders: comprehensive management. Baltinore, Lippincott Williams & Wilkins, 1999:145-161
6. Golden S.H., Robinson K.A., Saldanha I., et al. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853-1878.
7. Chaidarun S.S., Klibanski A. Gonadotropinomas. Semin Reprod Med. 2002;20(4):339-348.
8. Asa S.L. Atlas of Tumor Pathology: Tumors of the Pituitary Gland. Washington, DC: Armed Forces Institute of Pathology; 1998.
9. Asa S.L. Tumors of the Pituitary Gland. Bethesda: Universities Associated for Research and Education in Pathology; 1997.
10. Thapar K., Kovacs K., Laws E.R. The classification and molecular biology of pituitary adenomas. Adv Tech Stand Neurosurg. 1995;22:3-53.
11. Black P.M., Hsu D.W., Klibanski A., et al. Hormone production in clinically nonfunctioning pituitary adenomas. J Neurosurg. 1987;66(2):244-250.
12. Ho D.M., Hsu C.Y., Ting L.T., Chiang H. The clinicopathological characteristics of gonadotroph cell adenoma: a study of 118 cases. Hum Pathol. 1997;28(8):905-911.
13. Visot A., Pencalet P., Boulin A., Gaillard S.. Surgical management of endocrinologically silent pituitary tumors, Schmideck H.H., Sweet D.W., editors. Operative Neurosurgical Techniques. Indications, Methods and Results, 5th ed, vol 1. Philadelphia: Elsevier, 2006;355-373.
14. Snyder P.J.. Gonadotroph and other clinically nonfunctioning pituitary adenomas, DeGroot L., Jameson J.L., editors. Endocrinology, 5th ed, vol 1. Philadelphia: Elsevier, 2006;465-484.
15. Ferrante E., Ferraroni M., Castrignano T., et al. Non-functioning pituitary adenoma database: a useful resource to improve the clinical management of pituitary tumors. Eur J Endocrinol. 2006;155(6):823-829.
16. Colao A., Di Somma C., Pivonello R., et al. Medical therapy for clinically non-functioning pituitary adenomas. Endocr Relat Cancer. 2008;15(4):905-915.
17. Levy M.J., Jager H.R., Powell M., et al. Pituitary volume and headache: size is not everything. Arch Neurol. 2004;61(5):721-725.
18. Levy M.J., Matharu M.S., Meeran K., et al. The clinical characteristics of headache in patients with pituitary tumours. Brain. 2005;128(Pt 8):1921-1930.
19. Semple P.L., Jane J.A.Jr., Laws E.R.Jr. Clinical relevance of precipitating factors in pituitary apoplexy. Neurosurgery. 2007;61(5):956-961. discussion 961-962
20. Briganti F., Caranci F., Cirillo S., Elefante R. The role of the neuroradiologist. In: de Divitiis E., Cappabianca P. Endoscopic Endonasal Skull Base Surgery. Wien, New York: Springer-Verlag; 2003:61-81.
21. Naganuma H., Satoh E., Nukui H. Technical considerations of transsphenoidal removal of fibrous pituitary adenomas and evaluation of collagen content and subtype in the adenomas. Neurol Med Chir (Tokyo). 2002;42(5):202-212. discussion 213
22. Iuchi T., Saeki N., Tanaka M., et al. MRI prediction of fibrous pituitary adenomas. Acta Neurochir (Wien). 1998;140(8):779-786.
23. Snow R.B., Johnson C.E., Morgello S., et al. Is magnetic resonance imaging useful in guiding the operative approach to large pituitary tumors? Neurosurgery. 1990;26(5):801-803.
24. Wolfsberger S., Neubauer A., Buhler K., et al. Advanced virtual endoscopy for endoscopic transsphenoidal pituitary surgery. Neurosurgery. 2006;59(5):1001-1009. discussion 1009-1010
25. Fusco R., Cennamo G., Bonavolontà G. The role of the ophthalmologist. In: de Divitiis E., Cappabianca P. Endoscopic endonasal skull base surgery. Wien, New York: Springer-Verlag, 2003.
26. Hofland L.J., Lamberts S.W. Somatostatin receptor subtype expression in human tumors. Ann Oncol. 2001;12(suppl 2):S31-S36.
27. Greenman Y., Melmed S. Expression of three somatostatin receptor subtypes in pituitary adenomas: evidence for preferential SSTR5 expression in the mammosomatotroph lineage. J Clin Endocrinol Metab. 1994;79(3):724-729.
28. Greenman Y., Melmed S. Heterogeneous expression of two somatostatin receptor subtypes in pituitary tumors. J Clin Endocrinol Metab. 1994;78(2):398-403.
29. van der Hoek J., Lamberts S.W., Hofland L.J. Preclinical and clinical experiences with the role of somatostatin receptors in the treatment of pituitary adenomas. Eur J Endocrinol. 2007;156(suppl 1):S45-S51.
30. Pawlikowski M., Lawnicka H., Pisarek H., et al. Effects of somatostatin-14 and the receptor-specific somatostatin analogs on chromogranin A and alpha-subunit (alpha-SU) release from “clinically nonfunctioning” pituitary adenoma cells incubated in vitro. J Physiol Pharmacol. 2007;58(1):179-188.
31. Padova H., Rubinfeld H., Hadani M., et al. Effects of selective somatostatin analogs and cortistatin on cell viability in cultured human non-functioning pituitary adenomas. Mol Cell Endocrinol. 2008;286(1-2):214-218. 14
32. Zatelli M.C., Piccin D., Vignali C., et al. Pasireotide, a multiple somatostatin receptor subtypes ligand, reduces cell viability in non-functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Endocr Relat Cancer. 2007;14(1):91-102.
33. Warnet A., Timsit J., Chanson P., et al. The effect of somatostatin analogue on chiasmal dysfunction from pituitary macroadenomas. J Neurosurg. 1989;71(5 Pt 1):687-690.
34. de Bruin T.W., Kwekkeboom D.J., Van’t Verlaat J.W., et al. Clinically nonfunctioning pituitary adenoma and octreotide response to long term high dose treatment, and studies in vitro. J Clin Endocrinol Metab. 1992;75(5):1310-1317.
35. Lamberts S., de Herder W., van der Lely A., Hofland L. Imaging and medical management of clinically nonfunctioning pituitary tumours. Endocrinologist. 1995;5:448-451.
36. Lamberts S.W., Macleod R.M. Regulation of prolactin secretion at the level of the lactotroph. Physiol Rev. 1990;70(2):279-318.
37. Duet M., Mundler O., Ajzenberg C., et al. Somatostatin receptor imaging in non-functioning pituitary adenomas: value of an uptake index. Eur J Nucl Med. 1994;21(7):647-650.
38. Renner U., Arzberger T., Pagotto U., et al. Heterogeneous dopamine D2 receptor subtype messenger ribonucleic acid expression in clinically nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 1998;83(4):1368-1375.
39. Van Tol H.H., Wu C.M., Guan H.C., et al. Multiple dopamine D4 receptor variants in the human population. Nature. 1992;358(6382):149-152. 9
40. Pivonello R., Matrone C., Filippella M., et al. Dopamine receptor expression and function in clinically nonfunctioning pituitary tumors: comparison with the effectiveness of cabergoline treatment. J Clin Endocrinol Metab. 2004;89(4):1674-1683.
41. Clark J.D., Wheatley T., Edwards O.M. Rapid enlargement of non-functioning pituitary tumour following withdrawal of bromocriptine. J Neurol Neurosurg Psychiatry. 1985;48(3):287.
42. Rocheville M., Lange D.C., Kumar U., et al. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288(5463):154-157. 7
43. Colao A., Filippella M., Di Somma C., et al. Somatostatin analogs in treatment of non-growth hormone-secreting pituitary adenomas. Endocrine. 2003;20(3):279-283.
44. Vitale G., Caraglia M., van Koetsveld P.M., et al. Potential role of type I interferons in the treatment of pituitary adenomas. Rev Endocr Metab Disord. 2009;10(2):125-133.
45. Hofland L.J., de Herder W.W., Waaijers M., et al. Interferon-alpha-2a is a potent inhibitor of hormone secretion by cultured human pituitary adenomas. J Clin Endocrinol Metab. 1999;84(9):3336-3343.
46. Evans C.O., Reddy P., Brat D.J., et al. Differential expression of folate receptor in pituitary adenomas. Cancer Res. 2003;63(14):4218-4224. 15
47. Evans C.O., Yao C., Laborde D., Oyesiku N.M. Folate receptor expression in pituitary adenomas cellular and molecular analysis. Vitam Horm. 2008;79:235-266.
48. Cappabianca P., Cavallo L.M., de Divitiis O.. Pituitary surgery, DeGroot L., Jameson J.L., editors. Endocrinology, 5th ed, vol. 1. Philadelphia: Elsevier, 2006;511-535.
49. Cappabianca P., Cavallo L.M., Esposito F., et al. Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg. 2008;33:151-199.
50. Thapar K., Laws E.R.J. Pituitary tumors. In: Kaye A.W.Jr., LER. Brain Tumors. London: Churchill Livingstone; 2001:804-854.
51. Laws E.R.J. Foreword. In: De Divitiis E., Cappabianca P. Endoscopic Endonasal Transsphenoidal Surgery. Wien, New York: Springer, 2003. v-vii
52. Saito K., Kuwayama A., Yamamoto N., Sugita K. The transsphenoidal removal of nonfunctioning pituitary adenomas with suprasellar extensions: the open sella method and intentionally staged operation. Neurosurgery. 1995;36(4):668-675. discussion 675-666
53. Elias W.J., Chadduck J.B., Alden T.D., Laws E.R.Jr. Frameless stereotaxy for transsphenoidal surgery. Neurosurgery. 1999;45(2):271-275. discussion 275-277
54. Lasio G., Ferroli P., Felisati G., Broggi G. Image-guided endoscopic transnasal removal of recurrent pituitary adenomas. Neurosurgery. 2002;51(1):132-136. discussion 136-137
55. Jane J.A.Jr., Thapar K., Alden T.D., Laws E.R.Jr. Fluoroscopic frameless stereotaxy for transsphenoidal surgery. Neurosurgery. 2001;48(6):1302-1307. discussion 1307-1308
56. Kajiwara K., Nishizaki T., Ohmoto Y., et al. Image-guided transsphenoidal surgery for pituitary lesions using Mehrkoordinaten Manipulator (MKM) navigation system. Minim Invasive Neurosurg. 2003;46(2):78-81.
57. Ohhashi G., Kamio M., Abe T., et al. Endoscopic transnasal approach to the pituitary lesions using a navigation system (InstaTrak system): technical note. Minim Invasive Neurosurg. 2002;45(2):120-123.
58. Cappabianca P., Esposito F., Cavallo L.M., Corriero O.V. Instruments. In: Cappabianca P., Califano L., Iaconetta G. Cranial, Craniofacial and Skull Base Surgery. Springer-Verlag; 2010:7-16.
59. Steinmeier R., Fahlbusch R., Ganslandt O., et al. Intraoperative magnetic resonance imaging with the magnetom open scanner: concepts, neurosurgical indications, and procedures: a preliminary report. Neurosurgery. 1998;43(4):739-747. discussion 747-738
60. Nimsky C., von Keller B., Ganslandt O., Fahlbusch R. Intraoperative high-field magnetic resonance imaging in transsphenoidal surgery of hormonally inactive pituitary macroadenomas. Neurosurgery. 2006;59(1):105-114. discussion 105-114
61. Cappabianca P., de Divitiis E. Back to the Egyptians: neurosurgery via the nose. A five-thousand year history and the recent contribution of the endoscope. Neurosurg Rev. 2007;30(1):1-7. discussion 7
62. Cappabianca P., Cavallo L.M., de Divitiis E. Endoscopic endonasal transsphenoidal surgery. Neurosurgery. 2004;55(4):933-940. discussion 940-941
63. Carrau R.L., Jho H.D., Ko Y. Transnasal-transsphenoidal endoscopic surgery of the pituitary gland. Laryngoscope. 1996;106(7):914-918.
64. Jho H.D., Carrau R.L., Ko Y.. Endoscopic pituitary surgery, Wilkins H., Rengachary S., editors, Neurosurgical Operative Atlas, Park Ridge, IL, American Association of Neurological Surgeons, 1996;vol. 5:1-12.
65. Cappabianca P., Alfieri A., de Divitiis E. Endoscopic endonasal transsphenoidal approach to the sella: towards functional endoscopic pituitary surgery (FEPS). Minim Invasive Neurosurg. 1998;41(2):66-73.
66. de Divitiis E., Cappabianca P., Cavallo L.M. Endoscopic endonasal transsphenoidal approach to the sellar region. In: De Divitiis E., Cappabianca P. Endoscopic Endonasal Transsphenoidal Surgery. Wien, New York: Springer; 2003:91-130.
67. Cappabianca P., Cavallo L.M., Colao A., et al. Endoscopic endonasal transsphenoidal approach: outcome analysis of 100 consecutive procedures. Minim Invasive Neurosurg. 2002;45(4):193-200.
68. de Divitiis E., Cappabianca P.. Endoscopic endonasal transsphenoidal surgery, Pickard J.D., editor, Advances and Technical Standards in Neurosurgery, Wien, New York, Springer-Verlag, 2002;vol. 27:137-177.
69. Jho H.D., Carrau R.L. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87(1):44-51.
70. Jho H.D. Endoscopic transsphenoidal surgery. In: Schmidek H.H., editor. Schmidek & Sweet Operative Neurosurgical Techniques. Philadelphia: W.B. Saunders; 2000:385-397.
71. Cappabianca P., Cavallo L.M., Esposito F., de Divitiis E. Endoscopic endonasal transsphenoidal surgery: procedure, endoscopic equipment and instrumentation. Childs Nerv Syst. 2004;20(11-12):796-801.
72. Cappabianca P., de Divitiis O., Maiuri F. Evolution of transsphenoidal surgery. In: De Divitiis E., Cappabianca P. Endoscopic Endonasal Transsphenoidal Surgery. Wien, New York: Springer; 2003:1-7.
73. Cinalli G., Cappabianca P., de Falco R., et al. Current state and future development of intracranial neuroendoscopic surgery. Expert Rev Med Devices. 2005;2(3):351-373.
74. Leonhard M., Cappabianca P., de Divitiis E. The endoscope, endoscopic equipment and instrumentation. In: de Divitiis E., Cappabianca P. Endoscopic Endonasal Transsphenoidal Surgery. Wien. NewYork: Springer-Verlag; 2003:9-19.
75. Cappabianca P., Cavallo L.M., Colao A., de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002;97(2):293-298.
76. Cappabianca P., Alfieri A., Colao A., et al. Endoscopic endonasal transsphenoidal surgery in recurrent and residual pituitary adenomas: technical note. Minim Invasive Neurosurg. 2000;43(1):38-43.
77. Cappabianca P., Alfieri A., Thermes S., et al. Instruments for endoscopic endonasal transsphenoidal surgery. Neurosurgery. 1999;45(2):392-395. discussion 395-396
78. Weiss M. The transnasal transsphenoidal approach. In: Apuzzo M.L.J., editor. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1987:476-494.
79. Cavallo L.M., de Divitiis O., Aydin S., et al. Extended endoscopic endonasal transsphenoidal approach to the suprasellar area: anatomic considerations—part 1. Neurosurgery. 61, 2007. ONS-24-ONS-34
80. Prevedello D.M., Kassam A.B., Gardner P., et al. “Q-tip” retractor in endoscopic cranial base surgery. Neurosurgery. 2010;66(2):363-366. discussion 366-367
81. Frank G., Pasquini E. Endoscopic endonasal cavernous sinus surgery, with special reference to pituitary adenomas. Front Horm Res. 2006;34:64-82.
82. Maroon J.C. Skull base surgery: past, present, and future trends. Neurosurg Focus. 2005;19(1):E1. 15
83. Prevedello D.M., Doglietto F., Jane J.A.Jr., et al. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg. 2007;107(1):206-213.
84. Kassam A., Snyderman C.H., Mintz A., et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(1):E3. 15
85. Kassam A., Snyderman C.H., Mintz A., et al. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19(1):E4. 15
86. de Divitiis E., Cavallo L.M., Cappabianca P., Esposito F. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: part 2. Neurosurgery. 2007;60(1):46-59.
87. Cappabianca P., Cavallo L.M., Esposito F., de Divitiis E. Endonasal approaches to the Cavernous sinus. In: Anand V.K., Schwartz T.H. Practical Endoscopic Skull Base Surgery. San Diego, Oxford, Brisbane: Plural Publishing; 2007:187-199.
88. Castelnuovo P., Pistochini A., Locatelli D. Different surgical approaches to the sellar region: focusing on the “two nostrils four hands technique”. Rhinology. 2006;44(1):2-7.
89. Esposito F., Dusick J.R., Fatemi N., Kelly D.F. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Neurosurgery. 2007;60(2):ONS1-ONS9.
90. Cavallo L.M., Messina A., Esposito F., et al. Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. J Neurosurg. 2007;107(4):713-720.
91. Hadad G., Bassagasteguy L., Carrau R.L., et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882-1886.
92. Kassam A.B., Thomas A., Carrau R.L., et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008;63(1 suppl 1):ONS44-ONS52. discussion ONS52-53
93. Fortes F.S., Carrau R.L., Snyderman C.H., et al. The posterior pedicle inferior turbinate flap: a new vascularized flap for skull base reconstruction. Laryngoscope. 2007;117(8):1329-1332.
94. de Divitiis E., Esposito F., Cappabianca P., et al. Tuberculum sellae meningiomas: high route or low route? A series of 51 consecutive cases. Neurosurgery. 2008;62(3):556-563. discussion 556-563
95. Esposito F., Dusick J.R., Cohan P., et al. Clinical review: early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab. 2006;91(1):7-13.
96. Krieger M.D., Couldwell W.T., Weiss M.H. Assessment of long-term remission of acromegaly following surgery. J Neurosurg. 2003;98(4):719-724.
97. Dumont A.S., Nemergut E.C.II, Jane J.A.Jr., Laws E.R.Jr. Postoperative care following pituitary surgery. J Intensive Care Med. 2005;20(3):127-140.