Endocrine surgery
Thyroid
1. Using the Bethesda System, list the possible results of fine-needle aspiration (FNA) of thyroid nodules, and describe the appropriate surgical intervention.
 Nondiagnostic: Repeat FNA with ultrasound guidance. Thyroid lobectomy is performed if FNA results are still nondiagnostic.
Nondiagnostic: Repeat FNA with ultrasound guidance. Thyroid lobectomy is performed if FNA results are still nondiagnostic.
 Benign: Risk of cancer is less than 5%. Clinical follow-up is safe.
Benign: Risk of cancer is less than 5%. Clinical follow-up is safe.
 Atypia of undetermined significance (ACUS) or follicular lesion of undetermined significance (FLUS): Risk of cancer is 5% to 15%. Options include repeat FNA with or without molecular testing or surgery.
Atypia of undetermined significance (ACUS) or follicular lesion of undetermined significance (FLUS): Risk of cancer is 5% to 15%. Options include repeat FNA with or without molecular testing or surgery.
 Follicular neoplasm: Risk of cancer is 15% to 30%. Options include repeat FNA for molecular testing or surgery, either thyroid lobectomy or thyroidectomy.
Follicular neoplasm: Risk of cancer is 15% to 30%. Options include repeat FNA for molecular testing or surgery, either thyroid lobectomy or thyroidectomy.
 Suspicious: Risk of cancer is 60% to 75%. Surgery consists of either thyroid lobectomy or thyroidectomy.
Suspicious: Risk of cancer is 60% to 75%. Surgery consists of either thyroid lobectomy or thyroidectomy.
 Malignant: Risk of cancer is greater than 97%. Surgery consists of thyroidectomy.
Malignant: Risk of cancer is greater than 97%. Surgery consists of thyroidectomy.
2. A patient underwent thyroid lobectomy for a suspicious thyroid nodule, and the final pathology report revealed papillary carcinoma. How do you decide whether completion thyroidectomy is necessary?
When electing to undergo lobectomy for an indeterminate or suspicious thyroid nodule, patients should be counseled that if the final pathologic determination is malignant, a second surgical procedure for completion thyroidectomy will be necessary in most cases. Lobectomy may be sufficient treatment for low-risk patients who meet the following criteria: young age (< 45 years), tumor less than 1 cm without invasion, no regional or distant metastases, no history of neck radiation exposure, and no family history of thyroid cancer.
3. Why not just do an intraoperative frozen section on indeterminate thyroid nodules to determine whether to perform lobectomy versus thyroidectomy?
Unfortunately, the accuracy of frozen section for thyroid nodules is not much better than FNA, and frozen section is therefore not routinely used. To distinguish a benign from malignant follicular thyroid lesion requires a detailed assessment for capsular and/or vascular invasion that cannot practically be accomplished intraoperatively. Frozen section can sometimes be useful for definitive diagnosis of cancer in nodules that are in the suspicious category by FNA.
4. What is the role for molecular testing of thyroid nodules?
Fifteen percent to 30% of thyroid nodules are cytologically indeterminate on FNA, and most of these patients undergo surgery to make a definitive diagnosis. However, most of these nodules turn out to be benign on final pathologic examination, in which case the surgery was unnecessary. The goal of molecular testing of thyroid nodules is to stratify the risk of malignancy further in those with indeterminate cytology to decrease the number of patients who undergo unnecessary surgery for benign disease. The most commonly analyzed mutations include those occurring in BRAF, RAS, and RET/PTC. There are commercially available tests that analyze for these and other mutations; however, it is very important to consider the positive and negative predictive values of these tests, as well as cost, when using them to guide clinical care.
5. What are the differences among total, near-total, and subtotal thyroidectomy?
Total thyroidectomy removes all grossly visible thyroid tissue. Near-total thyroidectomy removes all grossly visible thyroid tissue except for a small amount (< 1 g) adjacent to where the recurrent laryngeal nerve enters the larynx. Total thyroidectomy and near-total thyroidectomy have equivalent oncologic outcomes and are often considered synonymous. Subtotal thyroidectomy leaves more than 1 g of thyroid tissue and is not an appropriate cancer operation. It is used occasionally in patients with benign multinodular goiter or hyperthyroidism in an attempt to leave enough thyroid so that thyroid hormone replacement is not required. However, doing so increases the risk of recurrent disease compared with near-total thyroidectomy.
6. What is the appropriate extent of thyroidectomy for differentiated thyroid carcinoma?
Most patients with differentiated thyroid carcinoma (papillary, follicular, Hürthle cell) should undergo total or near-total thyroidectomy. Several studies have shown that for larger tumors, total or near-total thyroidectomy compared with lesser resections results in lower recurrence rates and improved survival. There appears to be no difference in outcome between patients who undergo lobectomy and those who have near-total or total thyroidectomy when tumor size is less than 1 cm. However, for tumors larger than 1 cm, patients who undergo near-total or total thyroidectomy have lower recurrence and improved survival compared with those who undergo lobectomy. This improved outcome is seen even in the subset of patients with tumors 1 to 2 cm in size. Therefore, most patients should undergo total or near-total thyroidectomy.
7. What is the incidence of lymph node metastasis in well-differentiated thyroid cancer, and when is neck dissection indicated?
Differentiated thyroid cancer (predominantly papillary) involves cervical lymph nodes in 30% to 80% of cases. In most cases, the metastatic lymph nodes are not clinically evident; therefore, all patients should undergo a preoperative full neck ultrasound scan to assess for abnormal nodes. Unlike in many other malignant diseases, the presence of occult lymph node metastases does not worsen the outcome for most patients with differentiated thyroid cancer, and routine neck dissection does not clearly improve outcome except for patients in the high-risk group. Moreover, neck dissection may increase the risk of complications. For these reasons, the decision to perform neck dissection for differentiated thyroid cancer is somewhat controversial. The following are some general guidelines:
 All patients with clinically palpable nodes require compartment (central and/or lateral) dissection at the same time as thyroidectomy.
All patients with clinically palpable nodes require compartment (central and/or lateral) dissection at the same time as thyroidectomy.
 Any suspicious nodes on ultrasound should undergo FNA and, if positive, should be removed via formal neck dissection as described earlier.
Any suspicious nodes on ultrasound should undergo FNA and, if positive, should be removed via formal neck dissection as described earlier.
 Physical examination, ultrasound, and intraoperative assessment are insensitive in determining nodal metastasis in the central neck. Whether prophylactic central neck dissection at the time of thyroidectomy is indicated for papillary carcinoma is debated. The current American Thyroid Association Guidelines Taskforce state that prophylactic central neck dissection may be indicated in patients with advanced tumors (> 4 cm and/or grossly invasive), and that thyroidectomy alone may be appropriate for noninvasive tumors less than 4 cm.
Physical examination, ultrasound, and intraoperative assessment are insensitive in determining nodal metastasis in the central neck. Whether prophylactic central neck dissection at the time of thyroidectomy is indicated for papillary carcinoma is debated. The current American Thyroid Association Guidelines Taskforce state that prophylactic central neck dissection may be indicated in patients with advanced tumors (> 4 cm and/or grossly invasive), and that thyroidectomy alone may be appropriate for noninvasive tumors less than 4 cm.
8. What is central and modified radical neck dissection?
Central neck dissection removes all the perithyroidal and tracheoesophageal groove nodes (level VI) from the hyoid bone superiorly down to the thoracic inlet. Laterally, the dissection extends from the carotid to the carotid artery. The lateral spread of disease usually involves the jugular lymph nodes (levels II–IV) and less commonly the posterior (level V) nodes. Modified radical neck dissection, sometimes referred to as “functional dissection,” removes all lymphatic tissue from levels II to IV (and sometimes V) and spares the internal jugular vein, sternocleidomastoid muscle, and spinal accessory nerve because sacrificing these structures (radical neck dissection) does not improve outcome.
9. Describe the appropriate surgical management of medullary thyroid carcinoma.
Medullary thyroid carcinoma accounts for less than 5% of thyroid cancers but occurs as part of an inherited syndrome in 20% to 25% of cases. Thus, all patients with medullary thyroid carcinoma should be considered for genetic testing. If the patient has multiple endocrine neoplasia type 2 (MEN-2) syndrome, then prophylactic thyroidectomy is indicated; the specific RET gene mutation can help determine at what age the surgical procedure should occur. Patients with MEN-2 should also be screened for pheochromocytoma and primary hyperparathyroidism (HPT) so that these conditions can be surgically corrected before or concomitant with the thyroidectomy, respectively. Because medullary thyroid cancer is not sensitive to radioiodine or thyroid-stimulating hormone (TSH) suppression, total thyroidectomy is indicated. Given the high incidence of regional lymph node involvement, central neck dissection is performed at the time of thyroidectomy. Some surgeons also advocate routine bilateral modified neck dissection at the initial surgery; however, despite this aggressive approach, biochemical cure (normalization of calcitonin) is rare in patients with positive lymph nodes. Current guidelines recommend that lateral neck dissection should be performed selectively, based on clinically or ultrasonographically abnormal nodes.
10. Discuss the role of surgery in anaplastic carcinoma of the thyroid.
Anaplastic carcinoma of the thyroid accounts for less than 1% of thyroid cancers but is one of the most aggressive solid tumors known and is rarely curable. At the time of diagnosis, 50% of patients harbor distant metastases, and 95% have local invasion precluding curative resection. Thus, surgery is usually restricted to a diagnostic or palliative role. Palliative surgical debulking and tracheostomy should be reserved for symptoms of dysphagia or airway compromise, respectively, because they do not prolong survival. An attempt at curative resection should be reserved for younger patients without distant disease and only when all gross cervical and mediastinal disease can be resected without excessive morbidity. In this select subgroup of patients, curative-intent surgery combined with adjuvant external beam radiation and/or chemotherapy has been shown to prolong survival compared with patients treated with adjuvant therapy alone.
11. When is surgery indicated for recurrent thyroid cancer?
Suspected recurrent disease in the neck should be evaluated by FNA. Confirmed nodal recurrence should be treated with formal dissection of the involved neck compartment. Recurrence in a neck compartment that has already been subjected to formal neck dissection can be challenging because of scarring of the tissue planes that renders repeat formal neck dissection virtually impossible. In these situations, the risks and benefits of additional surgery must be carefully considered because the risk of complications increases and the likelihood of cure decreases with each subsequent surgical procedure for disease recurrence. Observation may be the best option for patients with low-risk disease. When indicated, nodal recurrences that are palpable can be locally excised. If these recurrences are not palpable, intraoperative ultrasound can be used to guide the excision. For patients who are poor surgical candidates or have had multiple neck operations, percutaneous ethanol injection of nodal metastases is an alternative. Radioiodine is the standard therapy for distant metastatic disease, but isolated metastases can occasionally be surgically resected or treated with external beam radiation.
12. How many times should a thyroid cyst be aspirated if it reaccumulates fluid?
Thyroid cysts are most often benign, and the initial diagnostic and therapeutic procedure is aspiration. Fluid cytology results are typically nondiagnostic. If the nodule does not completely disappear after aspiration, it may be a complex cyst, which is associated with higher malignant potential. Therefore, FNA of the solid component should be performed. Recurrence of thyroid cysts occurs in more than 50%, and controlled studies have shown that aspiration followed by ethanol injection has a higher success rate compared with aspiration alone. If the cyst recurs after a second aspiration, it should be considered for surgical excision.
13. List the indications for thyroidectomy in hyperthyroidism.
In the United States, thyroidectomy is not commonly performed for hyperthyroidism unless the condition is secondary to a single hyperfunctioning adenoma or to a toxic multinodular goiter that is associated with compressive symptoms or contains a suspicious nodule. Despite the excellent success rate, low recurrence rate, safety, and more rapid return to a euthyroid state, fewer than 10% of patients with hyperthyroidism undergo thyroidectomy. Possible indications for thyroidectomy in patients with hyperthyroidism include:
 Failure of antithyroid medications
Failure of antithyroid medications
 Large goiter and low iodine uptake
Large goiter and low iodine uptake
 Compression symptoms, such as dysphagia, stridor, or hoarseness
Compression symptoms, such as dysphagia, stridor, or hoarseness
 Pregnant patients who are difficult to treat medically
Pregnant patients who are difficult to treat medically
 Young female patients who want to become pregnant in the near future
Young female patients who want to become pregnant in the near future
14. How should patients with hyperthyroidism be prepared for surgery?
It is important to render patients euthyroid before surgery for hyperthyroidism, to avoid perioperative thyroid storm. Antithyroid medications administered for 4 weeks preoperatively are usually adequate. Some surgeons use saturated solution of potassium iodide (SSKI) or Lugol’s solution, 3 to 5 drops three times a day for 3 to 5 days before surgery, to decrease the vascularity of the goiter and reduce the risk of bleeding. Patients who are very symptomatic may benefit from preoperative beta-blockade. For more rapid induction of a euthyroid state, patients may also be given dexamethasone, which can return thyroxine (T4) and triiodothyronine (T3) levels to within the normal range in less than 7 days. In cases of severe, refractory hyperthyroidism, plasmapheresis may occasionally be indicated.
15. What are the complications of thyroidectomy?
Thyroidectomy is a safe procedure with a mean length of hospitalization in large series of less than 1.5 days. The incidence rates of specific complications after thyroidectomy include the following:
 Recurrent laryngeal nerve injury: 1%
Recurrent laryngeal nerve injury: 1%
 Superior laryngeal nerve injury: 1%
Superior laryngeal nerve injury: 1%
 Temporary hypocalcemia: 10% to 15%
Temporary hypocalcemia: 10% to 15%
16. What is the significance of a “hot” thyroid nodule incidentally discovered on a positron emission tomography (PET) scan?
Fluorodeoxyglucose (FDG) whole body PET scan is increasingly used in the evaluation and surveillance of patients with various types of cancers. A focal area of increased FDG uptake within the thyroid is incidentally noted in up to 4% of PET scans. The risk of malignancy in these lesions is about 33%. Thus, thyroid incidentalomas noted on PET scans have a high risk of malignancy and warrant appropriate diagnostic evaluation. Diffuse FDG uptake is usually related to underlying thyroiditis and in most cases is not indicative of malignancy.
17. What is the appropriate therapy for an intrathoracic (substernal) goiter?
Intrathoracic goiters are typically cervical goiters with mediastinal extension. Although they are commonly asymptomatic, up to 40% of patients present with compressive symptoms resulting from impingement on the airway, esophagus, vascular structures, or nerves. There is general agreement that medical therapy (thyroid hormone suppression and/or radioiodine) is ineffective for intrathoracic goiters. Whether there is an increased risk of malignancy in intrathoracic compared with cervical goiters is controversial; however, when cases of microcarcinoma are excluded, there does not appear to be an increased risk of malignancy in intrathoracic goiters. Even so, the presence of an intrathoracic goiter is considered by many as an indication for thyroidectomy. Because the arterial supply of intrathoracic goiters originates in the neck, most of these tumors can be resected through a cervical approach. Extension into the posterior mediastinum, malignancy, or compression of the vena cava may necessitate a combined cervical and sternotomy approach, although this is required in less than 5% of cases.
18. When should thyroglossal duct cysts be removed? Describe the operation.
During the embryologic development of the thyroid, a diverticulum forms from the foramen cecum at the base of the tongue and descends as the thyroglossal duct to the future anatomic position of the thyroid overlying the anterolateral surface of the upper tracheal rings. The thyroglossal duct normally disappears during further development but in rare cases persists as a patent duct or as a thyroglossal duct cyst. Patients may complain of infection, pain, or compressive symptoms, or they may have cosmetic concerns. Because of the risk of infection, thyroglossal duct cysts should be removed; this requires excision of the entire cyst and cyst tract from the origin at the foramen cecum down to the cyst itself. Because the tract nearly always passes through the hyoid bone, the center of the hyoid should be resected to lower the risk of recurrence; this causes no disability and requires no repair.
Parathyroid
19. Which patients with primary HPT should undergo parathyroidectomy?
Patients with classic symptoms of HPT (nephrolithiasis, severe bone disease or fractures, or overt neuromuscular syndrome) should undergo parathyroidectomy; however, most patients with HPT do not have the classic symptoms. The National Institutes of Health (NIH) established criteria to assist clinicians in determining which patients with “asymptomatic” HPT should undergo surgery. In the absence of any of these criteria, continued surveillance is a reasonable option; however, if the patient meets any one of the criteria, then surgery is recommended:
 Calcium greater than 1.0 mg/dL above normal
Calcium greater than 1.0 mg/dL above normal
 Creatinine clearance reduced by more than 30%
Creatinine clearance reduced by more than 30%
 Bone mineral density reduced more than 2.5 standard deviations below mean peak adult value (T score)
Bone mineral density reduced more than 2.5 standard deviations below mean peak adult value (T score)
 Patients who do not desire or cannot undergo surveillance
Patients who do not desire or cannot undergo surveillance
Nonspecific symptoms, such as fatigue, mental slowing, musculoskeletal aches and pains, and depression, were not included in the NIH indications for surgery but are commonly reported by patients. Compared with controls (patients undergoing thyroid surgery), patients with HPT score significantly lower on preoperative quality of life questionnaires. Several studies indicate improvement in these patient-reported outcomes following parathyroidectomy.
20. When should preoperative parathyroid localization studies be performed?
An experienced parathyroid surgeon does not require preoperative localization before initial bilateral neck exploration. However, most patients with primary HPT have a single parathyroid adenoma, so preoperative localization is commonly performed and, when successful, enables minimally invasive parathyroidectomy. Patients with a prior history of neck surgery and certainly all patients with persistent or recurrent HPT should undergo preoperative localization studies before planned re-exploration. The best localization study available is the technetium-99m sestamibi scan, although ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and parathyroid venous sampling with or without arteriography may all be useful in certain situations, especially persistent or recurrent HPT.
21. What is the best treatment for a 45-year-old woman with primary HPT but negative preoperative localization studies?
Surgery. By the NIH criteria, her age alone warrants parathyroidectomy. Preoperative localization studies fail to localize an abnormal parathyroid approximately 15% of the time. Failure to localize has nothing to do with whether or not a patient has HPT or whether or not the patient should undergo surgery. Most patients with negative preoperative imaging still have a single adenoma as the cause of their HPT, and the success rate of the surgical procedure, if performed by an experienced surgeon, is still high (> 90%–95%).
22. Define minimally invasive parathyroidectomy.
Conventional parathyroidectomy entails bilateral neck exploration, identification of all four glands, and removal of the grossly enlarged gland or glands. The development of accurate preoperative localization studies and a rapid intraoperative parathyroid hormone (ioPTH) assay fostered the development of minimally invasive approaches to parathyroidectomy. A directed unilateral approach uses preoperative imaging to limit the dissection to one side. The abnormal gland is found and removed; after 10 to 15 minutes, a postexcision blood sample is drawn, and the PTH level is compared with a preexcision blood sample. A reduction of the PTH to 50% of the preoperative level and into the normal range predicts successful removal of all hyperfunctioning glands, and the surgical procedure is terminated. If the PTH does not drop appropriately, then all four glands must be identified because the patient likely has multiglandular disease.
23. What is minimally invasive radioguided parathyroidectomy (MIRP)?
MIRP is a second alternative to conventional parathyroidectomy and involves a technetium-99m sestamibi scan the morning of the surgery. An incision is made, either unilateral or bilateral neck exploration is performed, and the abnormal parathyroid glands are removed. A small, hand-held gamma probe is then used to measure the ex vivo radioactive counts of the excised parathyroid to determine whether the gland is hyperfunctioning. Biopsy of normal or borderline enlarged parathyroid glands can also be performed. The ex vivo radioactive counts can be used to confirm that the biopsy represents parathyroid tissue and to determine whether the gland is hyperfunctioning, in which case the rest of the parathyroid is resected. Contrary to common perception, the gamma probe is not used to localize the abnormal parathyroid. An ioPTH assay can also be used to exclude the possibility of multiglandular disease further (5%–10%).
24. Summarize the advantages of minimally invasive approaches.
Multiple studies have shown the minimally invasive approaches to be as safe and effective as conventional parathyroidectomy. However, there are also multiple studies showing that conventional parathyroidectomy (bilateral neck exploration) can similarly be performed through a small incision, on an outpatient basis, and with excellent results. Some studies have found the minimally invasive approaches to be more time and cost efficient because they limit the amount of dissection required and can be done without hospitalization. Because the minimally invasive approach is typically performed through a smaller incision, cosmesis may be improved.
25. How is the ioPTH assay used in parathyroid surgery?
The half-life of PTH is 3 to 5 minutes, and this allows for a rapid assay for ioPTH to be used intraoperatively to assess the functional success of the operation. This test is performed by drawing a sample of blood before the operation and 10 minutes after removal of the suspected abnormal gland or glands. A reduction of the ioPTH by 50% predicts successful removal of all hyperfunctioning glands, and the surgical procedure is terminated. The rate of residual multiglandular disease is approximately 5% when ioPTH is used to determine the completeness of resection, whereas the rate is 10% to 35% when conventional parathyroidectomy is performed (i.e., bilateral neck exploration and removal of grossly enlarged parathyroids). Therefore, the use of ioPTH may prevent the unnecessary removal of glands that appear enlarged but are not hyperfunctional.
26. What is the expected success of surgery for primary HPT?
Parathyroidectomy is highly successful for primary HPT. The procedure corrects hypercalcemia in more than 95% of patients when it is performed by an experienced surgeon. Bone density stabilizes or increases in most patients. Successful parathyroidectomy significantly decreases the risk of kidney stone recurrence. Following successful surgery, most patients experience improvement in the vague nonspecific symptoms of HPT.
27. Describe the appropriate management of a “missing” parathyroid.
Despite meticulous operative technique during conventional parathyroidectomy (identification of all four glands), the surgeon occasionally encounters a “missing gland.” Up to 20% of parathyroid glands are ectopic, with the most common locations within the thymus, retroesophageal, and intrathyroidal. A systematic search of the most common ectopic locations is required for successful outcome in these patients. When three normal glands have been identified and the fourth gland is not in a normal position, the most likely ectopic location depends on whether it is a missing upper or lower gland.
28. List the likely locations for an ectopic inferior parathyroid gland.
29. List the likely locations for an ectopic superior parathyroid gland.
30. What if a patient has multiglandular parathyroid disease?
A single adenoma is by far the most common cause of primary HPT. Depending on the method used to define multiglandular disease (i.e., ioPTH assay versus gross appearance or size), the reported rates range from 5% to 35%. Multiglandular disease can result from either multiple adenomas or four-gland hyperplasia. Hyperplasia may be sporadic or secondary to MEN syndrome, or it may be caused by secondary or tertiary HPT. When four glands are hyperplastic, the patient must undergo either subtotal (removal of 3½ glands [SPTx]) or total parathyroidectomy with autotransplantation of parathyroid tissue (TPTx + AT). The success of either approach depends on finding all four glands. Most patients (95%) have normal calcium and low or normal PTH levels in the early postoperative period; however, recurrent HPT occurs in 10% to 30% of patients.
31. Discuss the advantages and disadvantages of SPTx versus TPTx + AT.
It is generally thought that SPTx has a lower incidence of temporary postoperative hypocalcemia; however, the rate of permanent hypoparathyroidism is similar with either approach (10%–20%). The advantage of TPTx + AT is that persistent or recurrent hypercalcemia can be treated by partially or completely removing the grafts (usually placed in a forearm muscle) with the use of local anesthesia, whereas the same complication occurring after SPTx requires repeat neck operation with higher morbidity. One very small prospective, randomized trial demonstrated a clear benefit of TPTx + AT for secondary HPT in patients with renal failure; in those patients, TPTx + AT resulted in a more rapid return of normal calcium homeostasis and relief of symptoms.
32. List the complications of parathyroidectomy for primary HPT and their prevalence.
 Persistent or recurrent HPT: 1% to 12%
Persistent or recurrent HPT: 1% to 12%
 Transient hypocalcemia: 10% to 25%
Transient hypocalcemia: 10% to 25%
 Permanent HPT: 2% to 5% (< 1% for solitary adenoma)
Permanent HPT: 2% to 5% (< 1% for solitary adenoma)
 Temporary recurrent laryngeal nerve injury: 3%
Temporary recurrent laryngeal nerve injury: 3%
33. Define persistent or recurrent HPT.
Persistent HPT is defined as failure of calcium and PTH levels to normalize or remain normal in the initial 6 months after operation, whereas recurrent HPT is defined by recurrence of hypercalcemia after 6 months.
34. What is the most common cause of elevated PTH but normal calcium levels following parathyroidectomy?
Persistent PTH elevation with normal serum calcium can be observed in up to 30% of patients following parathyroidectomy. This can be disconcerting to the patient and surgeon but in most cases is not the result of persistent or recurrent HPT. The origin of this phenomenon is likely multifactorial, but vitamin D deficiency, rapid bone turnover (hungry bone syndrome), and inadequate calcium intake are thought to be the main causes. Postoperative supplementation with calcium and vitamin D decreases this phenomenon. Long-term studies have shown that the PTH eventually returns to normal in most patients, and the long-term recurrence rate is not increased in this subset of patients.
35. Discuss the approach to patients with persistent or recurrent HPT.
The approach to patients with persistent or recurrent HPT requires confirmation of the diagnosis (e.g., exclude familial hypocalciuric hypercalcemia, vitamin D deficiency), estimation of disease severity, careful review of the operative and pathology reports, and preoperative localization. Causes of failure include missed adenoma in a normal location, ectopic glands, inadequate resection in multiglandular disease, and supernumerary glands.
36. Discuss the treatment options for persistent or recurrent HPT.
Although preoperative localization is optional before initial surgery for HPT, it is essential in cases of persistent or recurrent disease because the success rate of the surgery is much higher when the abnormal gland has been accurately localized. Technetium-99m sestamibi scan is the best test, but when negative, ultrasound, CT, MRI, and parathyroid venous sampling with or without arteriography may all be useful. Repeat cervical exploration is successful in normalizing PTH levels in about 85% of patients and may be aided by intraoperative ultrasound and ioPTH assay. Mediastinal parathyroid tissue is most often removed via the transcervical approach, but thoracoscopy or median sternotomy may be required 1% to 2% of the time. Angiographic ablation of mediastinal parathyroid tissue using high doses of ionic contrast may be successful in selected patients with high surgical risk.
37. How does one recognize parathyroid cancer?
Parathyroid cancer is the rarest of all endocrine tumors, with a reported incidence of less than 1% in patients with primary HPT. It is difficult to distinguish parathyroid cancer from the more common benign causes of HPT, and the diagnosis is frequently not suspected preoperatively. Parathyroid cancer should be suspected preoperatively when patients present with rapid onset, severe, symptomatic hypercalcemia (> 14 mg/dL), very high PTH levels (more than five times normal), a palpable neck mass, or hoarseness. It should be suspected intraoperatively when the tumor is large, firm, fibrotic, or invasive to the thyroid or other surrounding structures. Successful outcome requires early recognition and complete resection of the tumor and any involved structures.
38. Describe the management of parathyroid cancer.
Surgery is the mainstay of treatment for parathyroid cancer given that radiation and chemotherapy have shown little benefit. Local invasion and pathologic nodes should be assumed to represent cancer. Any suspicious parathyroid lesions should be carefully removed without disrupting the parathyroid capsule because such disruption may result in tumor spillage and local recurrence. If a parathyroid gland is obviously abnormal and infiltrating other tissues, those tissues should be resected en bloc with the tumor whenever possible, including the ipsilateral thyroid lobe when necessary. Removal of the central nodes on the side of the tumor is indicated at the initial operation. Any obviously enlarged lateral nodes should be resected by formal neck dissection. Prophylactic neck dissections have shown no benefit. The histopathologic diagnosis of this cancer is also difficult; thus, intraoperative frozen section is rarely useful other than to confirm parathyroid tissue.
39. Give the recurrence and survival rates for parathyroid cancer.
Recurrence rates are high and depend on whether the patient underwent routine parathyroidectomy for presumed benign disease (> 50% recurrence) or en bloc resection for suspicion of cancer (10%–33%). Despite this high recurrence rate, prolonged survival is still possible. The National Cancer Database reports 5- and 10-year survival rates of 85.5% and 49.1%, respectively.
Adrenal glands
40. Should all incidentally discovered adrenal masses be resected?
No. Clinically inapparent adrenal masses are common (up to 6% in autopsy series and 4% in abdominal CT series) and most are benign, hormonally inactive adenomas that require no treatment. The decision to remove an adrenal incidentaloma surgically is based on tumor size, imaging characteristics, and biochemical activity.
41. Summarize the appropriate laboratory evaluation of an adrenal mass.
Hormonally active adrenal tumors should be resected, and up to 20% of adrenal incidentalomas are found to have subclinical hormonal dysfunction. Therefore, patients should be screened for hypercortisolism, “silent” pheochromocytoma, and, if hypertensive, hyperaldosteronism by the following tests:
 1-mg overnight dexamethasone suppression test
1-mg overnight dexamethasone suppression test
 24-hour urinary and/or plasma fractionated metanephrines and catecholamines
24-hour urinary and/or plasma fractionated metanephrines and catecholamines
 In hypertensive patients: a morning plasma aldosterone–plasma renin activity ratio
In hypertensive patients: a morning plasma aldosterone–plasma renin activity ratio
Routine screening for excess androgens or estrogens is not warranted because sex hormone–secreting adrenal tumors are rare and typically occur in the presence of clinical manifestations.
42. What imaging studies are available for evaluating adrenal disorders?
The appropriate imaging study for adrenal lesions depends on the presumed diagnosis. For incidentally discovered, hormonally inactive adrenal tumors, an “adrenal protocol” CT scan is an appropriate choice. This involves thin-cut imaging through the adrenals with and without intravenous contrast and delayed images to assess how quickly the contrast washes out. For cortisol-producing adenomas and most pheochromocytomas, CT scans are very accurate because the tumors are usually larger than 2 cm by the time they are diagnosed. MRI is essentially equivalent to CT for adrenal tumors; however, it may be superior in recurrent or metastatic disease and for pheochromocytomas. Meta-iodobenzylguanidine (MIBG) scans are best used for recurrent, familial, or nonadrenal pheochromocytomas. Aldosteronomas are typically less than 2 cm in diameter, and therefore the sensitivity of CT scans is only 85%. Adrenal venous sampling should be used in most patients with hyperaldosteronism to exclude bilateral hyperplasia and confirm the correct side in patients with unilateral aldosterone excess.
43. What findings on CT help to distinguish between benign and malignant tumors?
Although most adrenal incidentalomas are benign, a series of more than 2000 patients found that adrenocortical carcinoma accounted for 4.7% of tumors and metastatic cancer another 2.5%. The size of the mass and its appearance on imaging are the two major predictors of malignancy. Adrenocortical carcinoma accounts for 2% of tumors less than 4 cm but up to 25% of tumors greater than 6 cm. The lipid content of the adrenal mass and rapidity of the washout of contrast are also important CT characteristics in differentiating benign tumors from adrenal cancer, pheochromocytoma, and metastatic disease. Benign adenomas typically have high lipid content (low attenuation) and rapid contrast washout (> 50% washout at 10 minutes after contrast). The following imaging characteristics are used to estimate malignant potential of adrenal incidentalomas.
 Benign tumors are typically smaller than 4 cm, are homogeneous with smooth borders, and have low attenuation (< 10 Hounsfield units [HU]) and rapid contrast washout.
Benign tumors are typically smaller than 4 cm, are homogeneous with smooth borders, and have low attenuation (< 10 Hounsfield units [HU]) and rapid contrast washout.
 Malignant tumors are typically larger than 6 cm, are heterogeneous with irregular borders, and have increased attenuation (> 10 HU) and slower contrast washout.
Malignant tumors are typically larger than 6 cm, are heterogeneous with irregular borders, and have increased attenuation (> 10 HU) and slower contrast washout.
44. Discuss the role of percutaneous biopsy in the evaluation of an adrenal mass.
Percutaneous biopsy cannot differentiate an adrenal adenoma from a carcinoma and is rarely indicated in the evaluation of an adrenal mass. However, metastases are the cause of adrenal incidentaloma in approximately half of patients who have a prior history of malignant disease. Therefore, percutaneous biopsy is typically reserved for patients with a history of cancer to evaluate for metastasis and is performed only if the result will influence therapy. It is always necessary to exclude pheochromocytoma first, to avoid the potential for precipitating a hypertensive crisis. The complication rate is 3% with bleeding, pain, infection, and malignant seeding of the biopsy tract most commonly reported.
45. List the indications for surgery.
 Size larger than 4 cm (some sources recommend a threshold of > 6 cm)
Size larger than 4 cm (some sources recommend a threshold of > 6 cm)
 Any size with worrisome radiographic signs (rapid growth, heterogeneous appearance, irregular borders, high attenuation [> 10–20 HU], or delayed washout of contrast)
Any size with worrisome radiographic signs (rapid growth, heterogeneous appearance, irregular borders, high attenuation [> 10–20 HU], or delayed washout of contrast)
 Unilateral tumor with signs or symptoms of hormonal dysfunction
Unilateral tumor with signs or symptoms of hormonal dysfunction
 Subclinical Cushing syndrome: Whether these patients are best treated medically or surgically is debated. Current guidelines suggest that adrenalectomy should be reserved for younger patients (< 40 years) with worsening hypertension, abnormal glucose tolerance, dyslipidemia, or osteoporosis.
Subclinical Cushing syndrome: Whether these patients are best treated medically or surgically is debated. Current guidelines suggest that adrenalectomy should be reserved for younger patients (< 40 years) with worsening hypertension, abnormal glucose tolerance, dyslipidemia, or osteoporosis.
46. Is adrenalectomy best performed laparoscopically or by the open technique?
Advances in laparoscopic surgical techniques have been applied to adrenalectomy, and most endocrine surgeons agree that laparoscopic adrenalectomy is the procedure of choice for benign adrenal tumors. Laparoscopic adrenalectomy has been associated with decreased hospital stay, less postoperative pain, less blood loss, shorter recovery, and overall increased patient satisfaction compared with the open techniques. However, open adrenalectomy should be performed when malignancy is suspected.
47. What approaches are used for laparoscopic surgery?
The most common technique is via an anterolateral approach in which the patient is positioned on the side. This provides excellent exposure but does not allow removal of both glands without repositioning the patient. An anterior approach provides access to both adrenal glands, but exposure is more difficult. A posterior endoscopic retroperitoneal approach avoids entering the peritoneal cavity altogether. This may be advantageous if the patient has had extensive prior abdominal surgery or needs bilateral adrenalectomy; however, this approach provides a limited working space and may hinder removal of larger lesions. The various laparoscopic approaches are believed to be equivalent in terms of safety and recovery.
48. Summarize the long-term success of adrenalectomy for functional tumors.
Following adrenalectomy for aldosteronomas, blood pressure is improved in 60% to 70% of patients; however, only 33% will require no antihypertensive therapy. The aldosterone level normalizes and hypokalemia is corrected in at least 95%; however, the long-term effect on hypertension is variable. The factors that predict postoperative normotension are younger age (< 40 years), short duration of hypertension (< 6 years), two or fewer antihypertensives, and no family history of hypertension. In older patients with severe, long-standing hypertension associated with renal dysfunction, adrenalectomy may not normalize the blood pressure, but it often results in easier control of hypertension with fewer or lower-dose medications.
Unilateral adrenalectomy is 95% effective in treating cortisol-producing adenomas. Bilateral adrenalectomy, in patients in whom hypophysectomy for adrenocorticotropic hormone–dependent Cushing syndrome fails, is slightly less effective, with approximately 25% of patients having persistent symptoms, hypertension, or diabetes. For patients undergoing unilateral adrenalectomy for Cushing syndrome, the hypothalamic-pituitary-adrenal axis recovers in a mean time of 9 months. Patients who undergo bilateral adrenalectomy require lifelong hormone replacement.
Adrenalectomy for nonfamilial, benign pheochromocytomas is curative in most cases. However, a long-term recurrence rate as high as 15% has been reported. Thus, patients should undergo lifelong surveillance with annual 24-hour urinary catecholamine and metanephrine measurement.
49. Describe the appropriate management of adrenal malignant disease.
Adrenocortical carcinoma is a rare (1–2 per million) and aggressive cancer with a poor prognosis. At the time of diagnosis, approximately 25% of patients will have nodal involvement and 20% will have distant metastases. Approximately 60% of adrenocortical carcinomas are functioning tumors, and the mean size of tumors at the time of diagnosis is greater than 10 cm. The overall 5-year survival rate is around 25% and depends largely on the stage at diagnosis. Patients undergoing complete resection of small tumors (< 5 cm) without local invasion (stage 1) have a 5-year survival of 60%, whereas patients with metastases or invasion into other organs (stage 4) have a median survival less than 12 months. The only chance for cure is surgery, which should be offered to all patients without metastases who have a reasonable surgical risk. Surgery should also be considered for young patients with an isolated, easily resectable metastasis. Despite limited response rates, patients with stage 3 or 4 disease are frequently offered adjuvant therapy with mitotane (with or without cytotoxic chemotherapy) and/or radiotherapy because of the high recurrence rate (up to 85%).
50. Describe the appropriate management of pheochromocytoma.
Most pheochromocytomas are benign, sporadic tumors, and adrenalectomy is curative in nearly all such patients; however, late recurrences have been reported in up to 15% of patients. Therefore, all patients should undergo long-term surveillance with annual biochemical screening. Studies have found that up to 25% of pheochromocytomas are associated with a familial syndrome. It is recommended that most patients, especially younger patients or those with an extraadrenal pheochromocytoma, undergo genetic testing for MEN-2, von Hippel–Lindau syndrome, neurofibromatosis type 1, or defects in the succinate dehydrogenase genes.
The differentiation of benign and malignant pheochromocytoma is difficult histopathologically; however, approximately 10% of pheochromocytomas are malignant. Surgical resection offers the only chance for cure. Therefore, care must be taken not to disrupt the tumor during resection, and en bloc resection of any structure invaded by the tumor should be performed when feasible. The 5-year survival for patients with malignant pheochromocytoma is approximately 40% and depends on the completeness of the resection and on whether distant metastases are present.
51. What is a cortical-sparing adrenalectomy, and when is it indicated?
Approximately 20% to 30% of pheochromocytomas occur in patients with a hereditary predisposition, such as MEN-2, von Hippel–Lindau syndrome, neurofibromatosis type 1, or defects in the succinate dehydrogenase genes. Patients with pheochromocytoma associated with a familial syndrome are at increased risk of developing bilateral and/or recurrent pheochromocytoma. To prevent adrenocortical insufficiency, these patients may undergo a cortical-sparing adrenalectomy. This is actually just a partial adrenalectomy in which the tumor and a margin of normal adrenal are resected. This approach balances the benefit of avoiding the need for lifelong hormone replacement with a slightly higher risk of recurrent pheochromocytoma in the adrenal remnant.
52. How should patients with pheochromocytoma be prepared for surgery?
The stress of anesthesia and/or manipulation of the tumor during surgery can result in a rapid increase in circulating catecholamine levels and can precipitate a hypertensive crisis or arrhythmia even in patients who have not had significant preoperative hypertension. Thus, all patients should undergo preoperative alpha-adrenergic blockade using either phenoxybenzamine or another selective alpha-antagonist. The addition of a beta-blocker can be used to control tachycardia if needed but only after initiation of an alpha-blocker. Beta-blockade should never be started first because the unopposed alpha-adrenergic effect can cause a hypertensive crisis. Calcium channel blockers have been shown to be a safe alternative to adrenergic antagonists. Because of the hyperadrenergic state, patients with pheochromocytoma are typically volume contracted and can develop orthostatic hypotension on initiation of alpha-blockade. Volume expansion is accomplished by instructing patients to increase their fluid and salt intake (> 5 g/day) after starting alpha-blockers. Intraoperatively, the patient’s blood pressure can change dramatically during manipulation of the tumor and ligation of the adrenal vein. An experienced anesthesiologist who is prepared for these hemodynamic changes is critical to a safe operation.
Neuroendocrine tumors of the pancreas and gastrointestinal tract
53. How common are pancreatic neuroendocrine tumors (PNETs)?
PNETs are the most common neuroendocrine tumors occurring in the abdomen, but overall they account for less than 2% of pancreatic tumors. The incidence in the United States is estimated to be 1 to 2 per 1,000,000.
54. Are most PNETs functional?
Although most PNETs secrete biologically inactive peptides such as chromogranins, neuron-specific enolase, and pancreatic polypeptide, 90% of PNETs are considered nonfunctional. PNETs that cause symptoms resulting from overproduction of hormones such as insulin, gastrin, glucagon, vasoactive intestinal peptide (VIP), and somatostatin are considered functional. Nonfunctional PNETs typically manifest similarly to pancreatic adenocarcinoma, with abdominal pain or biliopancreatic duct obstruction, or they are found incidentally. Thus, the definitive diagnosis of a nonfunctional PNET is often not made until final histopathologic examination is performed. Compared with pancreatic adenocarcinoma, patients undergoing resection for malignant PNETs have improved median survival (13 months versus 30 months). In addition, patients with functional tumors tend to have improved survival compared with those with nonfunctional PNETs (50 months versus 25 months).
55. What are the types of functional PNETs?
Insulinoma is the most common functional PNET (60%–70%), and more than 90% are benign. Gastrinoma is the second most common functional PNET (20%–30%), and approximately 50% are malignant. Glucagonoma is the next most common type, and 80% of these tumors are malignant. VIP-secreting tumors (VIPoma) and somatostatinomas are even rarer.
56. How should functional PNETs be imaged?
When a hormonally active tumor is suspected, the diagnosis should be confirmed biochemically before any imaging is performed. This is important not only for reasons of cost effectiveness, but also for patient safety because some localization studies are invasive. Given the small size of many PNETs, preoperative localization can be difficult, and the extent of preoperative imaging needed is controversial. Ultrasound, CT, MRI, and angiography have reported sensitivities around 60%. Octreotide scans are highly sensitive (85%) in locating most PNETs, especially for finding metastases, but they are less sensitive for insulinomas (50% sensitivity). Provocative arterial stimulation (secretin for gastrinomas and calcium for insulinomas) and hepatic venous sampling have higher sensitivity and have replaced portal vein sampling, but their invasiveness and their ability only to regionalize a tumor make them less desirable. Reports have shown endoscopic ultrasound to be the most sensitive preoperative test for localizing PNETs, although it is invasive and highly operator dependent.
57. How important is it to localize functional PNETs before surgery?
When performed by an experienced surgeon, intraoperative palpation with intraoperative ultrasound localizes nearly 100% of PNETs. Therefore, many surgeons believe that exhaustive efforts to localize the tumor preoperatively are unwarranted. They prefer to obtain a preoperative CT scan to identify obviously invasive or metastatic tumors and then rely on intraoperative palpation and ultrasound for tumor localization. However, all patients undergoing re-exploration for PNETs should have thorough preoperative localization studies.
58. What is the appropriate surgical approach for insulinomas?
Insulinomas account for the majority of nonfamilial functional PNETs. The small size of these tumors and the rarity of malignancy allow simple enucleation (60% of cases) or distal pancreatectomy (35% of cases) in most cases. Rarely, formal pancreaticoduodenectomy is required (< 5% of cases), most typically for malignant tumors. Laparoscopy for enucleation or distal pancreatectomy is increasingly used for insulinomas.
59. Describe the surgical approach to gastrinomas.
The surgical approach to gastrinomas is more complex because these tumors are more frequently malignant and occur outside the pancreas in up to 50% of cases. Tumors occurring distal to the pancreatic neck should be removed by formal pancreatic resection because of the high incidence of malignancy. Tumors in the pancreatic head can often be enucleated, reserving formal pancreaticoduodenectomy for more invasive tumors or those in close proximity to the pancreatic duct or superior mesenteric vessels. Careful evaluation of the duodenum by palpation, endoscopic transillumination, or duodenotomy is necessary to identify tumors within the duodenal wall, which occur commonly and can be quite small. Small submucosal lesions can be enucleated, but full-thickness resection of the duodenal wall may be necessary. Routine duodenotomy has been shown in some studies to improve early and long-term cure rates. The propensity for these tumors to metastasize to lymph nodes necessitates regional lymph node dissection in all patients.
60. Should PNETs occurring in patients with MEN-1 be approached differently from PNETs occurring sporadically?
Yes. Approximately 70% of patients with MEN-1 develop PNETs, and gastrinomas are most common. Because of the multifocal nature of tumors in these patients, aggressive surgery rarely results in biochemical cure. The morbidity and mortality rates of aggressive surgical resection combined with low cure rates and the availability of effective palliative treatment options for symptomatic patients sway many clinicians to treat patients medically unless there is suspicion of malignancy. Other surgeons take a more aggressive approach and cite studies that demonstrate decreased development of liver metastases and improved survival in patients undergoing surgery. Further, the larger tumors seen on preoperative imaging (> 2 cm) often account for symptoms, and therefore surgical extirpation of these tumors may be beneficial. Formal resection of the distal pancreas accompanied by enucleation of tumors in the pancreatic head is necessary. A careful search for duodenal tumors and regional lymph node dissection must accompany resection of the pancreatic tumors.
61. Discuss the role of surgery for liver metastases from neuroendocrine tumors.
Patients who undergo resection of isolated liver metastases from neuroendocrine tumors experience symptomatic improvement in 95% of cases and have prolonged survival (60%–75% versus 25%–30% 5-year survival rates) compared with patients with similar tumor burdens who are not undergoing hepatic resection. Patients with unresectable liver metastases or those with prohibitive surgical risks may benefit from cryosurgical or radiofrequency thermal ablation and/or transarterial chemoembolization.
62. Describe the presentation of nonpancreatic neuroendocrine tumors (carcinoid tumors).
Bronchial carcinoids may manifest with hemoptysis, asthma-like symptoms, or carcinoid syndrome. Gastric carcinoids are frequently found incidentally on endoscopy but may also cause symptoms such as pain or bleeding. Neuroendocrine tumors of the small intestine are the most likely to result in carcinoid syndrome, which typically does not occur until the patient has developed metastases to the liver. These tumors frequently result in a desmoplastic (fibrotic) reaction of the adjacent mesentery that causes bowel obstruction. Hindgut carcinoids do not usually produce active hormones and are typically found incidentally during endoscopy performed for other reasons.
63. Describe the carcinoid syndrome.
Carcinoid syndrome results from the production and release of serotonin from neuroendocrine tumors, most commonly tumors of the small intestine. The liver metabolizes serotonin to inactive products, so most patients do not develop carcinoid syndrome until they have developed liver metastases, which permit serotonin to enter the systemic circulation. Patients frequently experience intermittent abdominal pain, brief flushing episodes, and diarrhea. Asthma-like symptoms, hypotension, and right-sided heart failure (marantic endocarditis) can also occur.
64. Once a patient is diagnosed with carcinoid syndrome, what is the next step?
The tumor must then be localized. This goal may be difficult because of the small size of most carcinoid tumors. Tumors arise in the small bowel and appendix in nearly 70% of patients, and therefore a small bowel contrast study or abdominal CT scan is often the initial study performed. If these tests fail to localize the tumor, a chest radiograph and/or chest CT scan should be obtained to exclude a bronchial carcinoid. Metaiodobenzylguanidine or octreotide scintigraphy is sometimes able to localize tumors not found by conventional methods.
65. Describe the appropriate surgical management for nonpancreatic neuroendocrine tumors (carcinoid tumors).
Bronchial carcinoids tend to spread locoregionally and therefore should be resected by formal lobectomy when possible. Gastric carcinoids are classified into three types. Types 1 and 2 account for most (> 75%) gastric carcinoids and are associated with chronic hypergastrinemia resulting from pernicious anemia and Zollinger-Ellison syndrome, respectively. These tumors are most commonly small (< 1 cm) and multifocal and are typically treated by endoscopic resection and surveillance, with excellent outcomes. Type 3 gastric carcinoids occur sporadically and are usually larger, solitary, and invasive, with more than 50% metastatic at diagnosis. They are treated similarly to gastric adenocarcinoma with formal gastric resection and lymph node dissection. Small intestinal carcinoids without metastases should be excised by segmental resection and lymph node dissection. Appendiceal carcinoids are typically incidentally discovered and occur most commonly at the appendiceal tip. Distal lesions smaller than 2 cm are adequately treated by appendectomy. The presence of a carcinoid near the appendiceal base, size larger than 2 cm, or gross lymph node involvement requires formal right hemicolectomy. Rectal carcinoids often manifest with bleeding or are incidentally found on endoscopy. Extensive surgery for rectal carcinoids offers no survival advantage over local excision.
66. Discuss the role of surgery in carcinoid syndrome.
Patients with surgically resectable hepatic tumors experience improvement in symptoms and survival comparable to that for PNETs metastatic to the liver. The development of somatostatin analogs has allowed successful control of symptoms in most patients with carcinoid syndrome and diffuse hepatic metastases. Systemic chemotherapy and hepatic artery embolization have not been very effective in palliating these patients; however, selective hepatic artery chemoembolization has been successful in decreasing tumor burden and alleviating symptoms in up to 80% of patients. Patients who do not respond to medical palliation may benefit from aggressive tumor debulking by resecting the primary tumor and as many of the liver metastases as feasible.
Bariatric surgery
67. Define obesity. How common is it?
Obesity is simply defined as the excess of body fat. The degree of body fat relative to weight is calculated by the body mass index (BMI – kg/m2). Obesity is a BMI of 30 or greater. Morbid obesity is a BMI of 40 or greater. Increasing BMI correlates with increasing health issues including diabetes mellitus, hypertension, sleep apnea and pickwickian syndromes, asthma, coronary artery disease, cardiomyopathy, gastroesophageal reflux disease, degenerative joint disease, hyperlipidemia, fatty liver, gout, urinary incontinence, gallbladder disease, psychological disorders, menstrual irregularities, and certain cancers (endometrial, colon, postmenopausal breast, kidney). Most important, a BMI greater than 40 increases the risk of death from all causes by twofold. In the United States, 64% or 127 million adults are considered overweight, and 36% or 78 million adults are obese; 17% of children and adolescents are obese.
68. What are the limitations of BMI?
BMI interpretation is limited in those with a higher proportion of fat relative to muscle (elderly persons) or in those with an unusually high proportion of muscle (bodybuilders).
69. How successful is nonsurgical treatment of obesity?
Evidence suggests that nonsurgical treatment (diet and behavior modification, exercise programs, and psychological support) for morbid obesity has more than a 90% failure rate. Similarly, pharmacologic therapy for morbid obesity has been hampered by serious side effects and, overall, has shown disappointing results.
70. What are the indications for surgery for obesity?
An NIH Consensus Conference held in 1991 recommended that the following patients be considered for bariatric surgery:
 A BMI of 35 to 40 is associated with other severe obesity-related medical problems that are likely to improve with weight reduction.
A BMI of 35 to 40 is associated with other severe obesity-related medical problems that are likely to improve with weight reduction.
71. Have there been any more recent updates to the classic surgical indications listed earlier?
 The Food and Drug Administration (FDA) in 2011 expanded the use of the lap band to include obese individuals with a BMI of 30 to 34 who also have an existing obesity-related comorbidity.
The Food and Drug Administration (FDA) in 2011 expanded the use of the lap band to include obese individuals with a BMI of 30 to 34 who also have an existing obesity-related comorbidity.
 The International Diabetes Federation included the following recommendation in the 2011 position statement: Surgery should be considered as an alternative treatment option in patients with a BMI between 30 and 35 when diabetes cannot be adequately controlled by an optimal medical regimen.
The International Diabetes Federation included the following recommendation in the 2011 position statement: Surgery should be considered as an alternative treatment option in patients with a BMI between 30 and 35 when diabetes cannot be adequately controlled by an optimal medical regimen.
72. List the contraindications to bariatric operations.
73. Categorize the various surgical options for weight reduction.
74. List the options for restrictive surgery.
 Vertical-banded gastroplasty: A stapling device is used to divide the stomach vertically along the lesser curve starting at the angle of His to create a small (20-mL) pouch. A prosthetic device is then wrapped around the outlet of the pouch to prevent it from dilating over time. This operation is no longer performed because of poor long-term success.
Vertical-banded gastroplasty: A stapling device is used to divide the stomach vertically along the lesser curve starting at the angle of His to create a small (20-mL) pouch. A prosthetic device is then wrapped around the outlet of the pouch to prevent it from dilating over time. This operation is no longer performed because of poor long-term success.
 Gastric banding: This procedure is performed laparoscopically and involves placement of an adjustable band around the proximal stomach to create a small (15-mL) pouch. The band is connected to a reservoir placed in the subcutaneous tissue that enables band adjustment. Concerns about long-term success have led to a decline in popularity.
Gastric banding: This procedure is performed laparoscopically and involves placement of an adjustable band around the proximal stomach to create a small (15-mL) pouch. The band is connected to a reservoir placed in the subcutaneous tissue that enables band adjustment. Concerns about long-term success have led to a decline in popularity.
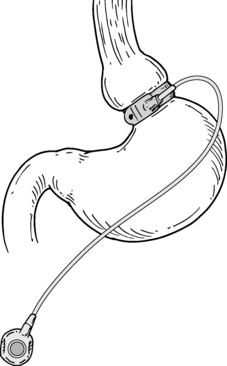
Figure 57-1. Gastric band.
 Sleeve gastrectomy: This procedure is gaining in popularity and involves stapling and removing a majority of the gastric body and fundus and leaving the lesser curvature and a small amount of antrum. The pylorus remains intact.
Sleeve gastrectomy: This procedure is gaining in popularity and involves stapling and removing a majority of the gastric body and fundus and leaving the lesser curvature and a small amount of antrum. The pylorus remains intact.
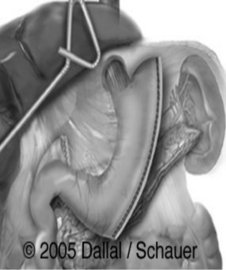
Figure 57-2. Sleeve gastrectomy.
75. What is the option for malabsorptive surgery?
Biliopancreatic diversion with and without a duodenal switch. Subtotal gastrectomy is performed, leaving a gastric remnant of 250 to 500 mL. The small bowel is divided 200 to 300 cm proximal to the ileocecal valve, and the ileum is anastomosed to the stomach. The jejunum is connected to the side of the ileum approximately 50 to 100 cm from the ileocecal valve, the “common channel.” This procedure results in malabsorption by creating a short common channel for digestion and absorption of food. Similarly, a “distal” gastric bypass involves creating a short common channel that leads to considerable malabsorption.
76. Explain the combined option.
This procedure is known as the “proximal” Roux-en-Y gastric bypass. The proximal stomach is stapled to create a small, 15- to 30-mL proximal stomach pouch that is completely separated from the excluded remnant stomach. This small reservoir restricts the amount of food that can be ingested at one time. The jejunum is then divided just distal to the ligament of Treitz, and the distal end is anastomosed to the proximal stomach pouch (the Roux limb). The proximal end of the jejunum is then anastomosed to the side of the jejunum 75 to 150 cm distal to the gastrojejunostomy. The length of this Roux limb determines the degree of malabsorption and is typically made longer for patients with very high BMIs. This procedure is performed using laparoscopic technique.
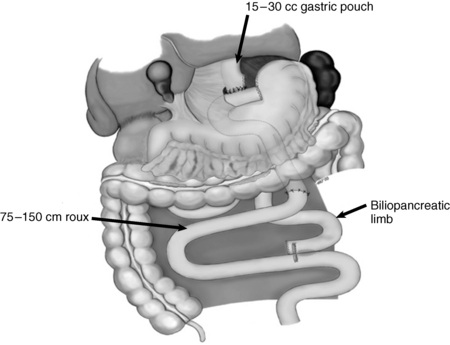
Figure 57-3. The Roux limb.
77. How much weight do patients lose following bariatric surgery?
Success following bariatric surgery is determined by both weight lost and improvement in obesity-related comorbidities. However, most surgical studies report outcome as percentage of excess weight lost (EWL) and consider loss of at least 50% of excess weight as a minimum criterion for success. The lap band typically produces 40% to 60% EWL gradually over 2 to 3 years, but it has a 20% failure rate. The gastric bypass typically produces 60% to 80% EWL rapidly over 2 years, but it has some recidivism and an estimated 10% failure rate. The biliopancreatic diversion is arguably the most effective weight loss procedure, resulting in 80% EWL, maintained over the long term. The sleeve gastrectomy is currently being studied for long-term success and so far mimics the gastric bypass in terms of weight loss efficacy.
78. What are the effects of bariatric surgery on obesity-related comorbidities?
Long-term weight loss following bariatric surgery has been shown to reduce obesity-related comorbidities significantly. Approximately 85% of patients with diabetes, hyperlipidemia, and obesity hypoventilation syndrome will be improved or cured at 2 years after surgery. In fact, the gastric bypass is now being studied as a surgical option for resolution of type 2 diabetes in patients without severe obesity. Hypertension also improves or resolves in more than two thirds of patients after successful weight loss. Salutary effects on other comorbidities, such as asthma, depression, and arthritic pain, as well as unemployment, are frequently observed following surgery.
79. What are the complications of bariatric surgery?
Perioperative mortality for the lap band is 0.1%; for the gastric bypass, it is 0.3% to 1%; and for the biliopancreatic diversion, it is 1% to 3%. The laparoscopic technique has changed the pattern of perioperative complications. Although wound complications and postoperative cardiopulmonary complications are less frequent, anastomotic stenosis, gastrointestinal bleeding, and bowel obstruction occur more frequently with laparoscopic compared with open techniques. Mean hospital stay following laparoscopic bariatric surgery is 2 to 3 days, significantly shorter than after open surgery (5–7 days). The lap band is usually performed as either an outpatient procedure or a 24-hour stay. Each procedure has its own unique risk of complications; the lap band has the least number of serious complications, and the biliopancreatic diversion has the greatest.
80. Give the incidence of complications following laparoscopic bariatric procedures in general.
Bilimoria, KY, Bentrem, DJ, Ko, CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–381.
Burnison, CM, Lim, S. Multimodal approach to anaplastic thyroid cancer. Oncology (Williston Park). 2012;26:378–398.
Cibas, ES, Ali, SZ. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132:658–665.
Cooper, DS, Doherty, GM, Haugen, BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214.
Kloos, RT, Eng, C, Evans, DB, et al, Medullary thyroid cancer. management guidelines of the American Thyroid Association. Thyroid 2009;19:565–612.
White, ML, Doherty, GM, Gauger, PG. Evidence-based surgical management of substernal goiter. World J Surg. 2008;32:1285–1300.
Allendorf, J, DiGorgi, M, Spanknebel, K, et al. 1112 consecutive bilateral neck explorations for primary hyperparathyroidism. World J Surg. 2007;31:2075–2080.
Bilezikian, JP, Khan, AA, Potts, JT, Jr., Guidelines for the management of asymptomatic primary hyperparathyroidism. summary statement from the third international workshop. J Clin Endocrinol Metab 2009;94:335–339.
Coker, LH, Rorie, K, Cantley, L, et al. Primary hyperparathyroidism, cognition, and health-related quality of life. Ann Surg. 2005;242:642–650.
Roy, M, Mazeh, H, Chen, H, et al, Incidence and localization of ectopic parathyroid adenomas in previously unexplored patients. (E-published ahead of print). World J Surg Sep 2012;12
Schulte, KM, Talat, N. Diagnosis and management of parathyroid cancer. Nat Rev Endocrinol. 2012;8:612–622.
Starker, LF, Fonseca, AL, Carling, T, et al. Minimally invasive parathyroidectomy. Int J Endocrinol. 2011;206:502.
LaFemina, J, Brennan, MF, Adrenocortical carcinoma. past, present, and future. J Surg Oncol 2012;106:586–594.
Palazzo, FF, Sebag, F, Sierra, M, et al. Long-term outcome following laparoscopic adrenalectomy for large solid adrenal cortex tumors. World J Surg. 2006;30:893–898.
Porterfield, JR, Thompson, GB, Young, WF, Jr., et al, Surgery for Cushing’s syndrome. an historical review and recent ten-year experience. World J Surg 2008;32:659–677.
Webb, R, Mathur, A, Chang, R, et al. What is the best criterion for the interpretation of adrenal vein sample results in patients with primary hyperaldosteronism? Ann Surg Oncol. 2012;19:1881–1886.
Young, WF, Jr., Clinical practice. the incidentally discovered adrenal mass. N Engl J Med 2007;356:601–610.
Zeiger, MA, Thompson, GB, Duh, Q, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(Suppl 1):1–20.
Neuroendocrine tumors of the pancreas and gastrointestinal tract
Burns, WR, Edil, BH, Neuroendocrine pancreatic tumors. guidelines for management and update. Curr Treat Options Oncol 2012;13:24–34.
Halfdanarson, TR, Rabe, KG, Rubin, J, et al, Pancreatic neuroendocrine tumors (PNETs). incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727–1733.
Huang, LC, Poultsides, GA, Norton, JA. Surgical management of neuroendocrine tumors of the gastrointestinal tract. Oncology. 2011;25:794.
Nikfarjam, M, Warshaw, AL, Axelrod, L, et al, Improved contemporary surgical management of insulinomas. a 25-year experience at the Massachusetts General Hospital. Ann Surg 2008;247:165–172.
Norton, JA, Fraker, DL, Alexander, HR, et al. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006;244:410–419.
Perez, EA, Koniaris, LG, Snell, SE, et al, 7201 carcinoids. increasing incidence overall and disproportionate mortality in the elderly. World J Surg 2007;31:1022–1030.
Anthone, GJ, Lord, RV, DeMeester, TR, et al. The duodenal switch operation for the treatment of morbid obesity. Ann Surg. 2003;238:618–627.
Biertho, L, Steffen, R, Ricklin, T, et al, Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding. a comparative study of 1,200 cases. J Am Coll Surg 2003;197:536–544.
Brolin, RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–2796.
Buchwald, H, et al, Bariatric surgery. a systematic review and meta-analysis. JAMA 2004;292:1724–1737.
Podnos, YD, Jimenez, JC, Wilson, SE, et al. Complications after laparoscopic gastric bypass. Arch Surg. 2003;138:957–961.
Schauer, PR, Burguera, B, Ikramuddin, S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484.
Sjöström, L, Narbro, K, Sjöström, CD, et al, Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med: 2007;357:741–752.








































