CHAPTER 6 Endocrine Disorders and Adrenal Support
THYROID DISORDERS: HYPOTHYROIDISM AND HYPERTHYROIDISM
Thyroid disease is a disorder in women that, left untreated, can exact pronounced consequences on health and quality of life.1,2 Thyroid dysfunction in women can alter menstrual regularity, affect reproduction, and lead to infertility, miscarriage, and affect intelligence in children born to women with untreated thyroid disorders during pregnancy. 3 4 5 6 7 Long-term, untreated thyroid conditions significantly increase the risk of cardiovascular disease, osteoporosis, reproductive cancer, and multisystem failure. Approximately 5% of Americans report having thyroid disease or taking thyroid medication, and numerous individuals have undiagnosed thyroid disorders.2 The most common thyroid disorders are hypothyroidism, both clinical and subclinical, and hyperthyroidism. Detection and treatment of most thyroid disorders is straightforward and can prevent long-term and potentially disastrous sequelae that may occur in the absence of appropriate care.
HYPOTHYROIDISM
Hypothyroidism is a persistent insufficiency in thyroid hormone production leading to a generalized decrease in metabolic functions (Box 6-1). It is the most prevalent of the pathologic hormone deficiencies, and can reduce physical and mental functional ability, quality of life, and long-term health.2,8 Hypothyroidism is classified on the basis of onset (congenital or acquired), endocrine dysfunction level (primary, secondary, or tertiary), and severity, which is classified as overt (clinical) or mild (subclinical) hypothyroidism.8 The total frequency of hypothyroidism, including subclinical cases, among adult females from all age groups, ranges from 3.0% to 7.5%, with significantly higher rates in women over 60 years old.1 Hypothyroidism occurs at a rate approximately 10 times higher in women than men.9
Pathophysiology
Hypothyroidism is classified as primary, secondary, or tertiary. Primary hypothyroidism is significantly more common than secondary, occurring at a rate of approximately 1000:1, and tertiary hypothyroidism, resulting from disease in the hypothalamus, is rare.8,10 Myxoedema refers to severe or complicated cases of overt hypothyroidism with cretinism syndrome, and is extremely rare.8 Those at increased risk of developing hypothyroidism include:9
The following biological activities are particularly impaired by hypothyroidism:8
The clinical manifestations of hypothyroidism (see Symptoms) are the result of effects occurring at the molecular level because of the impact of thyroid hormone insufficiency.8
Primary Hypothyroidism
Primary hypothyroidism is the most common form of hypothyroid disorder. It may be either congenital or acquired. Globally, the most common cause of congenital hypothyroidism is endemic iodine deficiency; however, it may also result from thyroid gland agenesis, defective thyroid hormone biosynthesis, or rarely, hemangiomas, which also may occur in young children.8,9 (Congenital hypothyroidism is not discussed in the remainder of this section.)
The most common form of primary hypothyroidism in areas of normal iodine intake is acquired primary hypothyroidism. It is most frequently a result of autoimmunity and is referred to as autoimmune thyroid disease (AITD) or autoimmune thyroiditis (Hashimoto’s disease).1,7,8 Antibodies are formed that bind to the thyroid (specifically against the thyroid peroxidase [TPO] enzyme, thyroglobulin, and TSH receptors) and prevent the manufacture of sufficient levels of thyroid hormone. In addition to binding to thyroid tissue, these antibodies also may bind to the adrenal glands, pancreas, and parietal cells of the stomach. Autoimmunity as an etiologic factor is supported by the presence of lymphatic infiltration of the thyroid gland and the presence of circulating thyroid autoantibodies in nearly all affected patients.8 In fact, the most common risk factor for both hypothyroidism and hyperthyroidism is the presence of TPO autoantibodies.1 Genetic predisposition (autosomal dominant inheritance) is a major factor in the etiology of AITD, accounting for as much as 79% of susceptibility to autoimmunity.1,8 Hormonal and environmental factors appear to account for the remaining etiologies.1 Autoimmune thyroiditis is increased in areas of high iodine intake, for example, in Iceland, suggesting an antigenic response.1,8
Other causes of hypothyroidism include iatrogenesis secondary to radiation or medications that interfere with thyroid function, genetic defects of the T3 hormone receptors and excessive consumption of goitrogens (substances that interfere with thyroid hormone production and release). Postpartum hypothyroidism is a transient form of hypothyroidism that affects 5% to 10% of postpartum women in the United States.11 Transient hypothyroidism may occur secondary to subacute thyroiditis caused by infection. Primary hypothyroidism is often idiopathic, with no definable cause.7
The long-term consequences of untreated overt hypothyroidism are significant, and include elevated cholesterol and atherosclerosis, cardiac, renal, and neurologic diseases, increased susceptibility to infectious diseases, possibly increased rates of reproductive cancers, and ultimately, multiple organ failure if the disease progresses.7,12,13 Hypothyroidism is readily detectable and treatable; therefore, these consequences should be almost entirely avoidable with screening and early treatment.
Subclinical Hypothyroidism
Subclinical hypothyroidism refers to patients with primary hypothyroidism with normal serum free thyroxine (free T4) and elevated thyroid-stimulating hormone (TSH).2 These individuals may or may not be symptomatic. Low Dog suggests that symptomatic euthyroid state is a more appropriate label for these patients.10
The prevalence of subclinical hypothyroidism is highest in the United States among white women (5.8%), and is 5.3% and 1.2% among Hispanic-American and African-American women, respectively. Rates tend to increase significantly with age, reaching as high as 8% to 10% in women ages 45 to 74 years, and 17.4% in women over 75 years.14
There is strong evidence from high-quality longitudinal studies that subclinical hypothyroidism places women at significant risk for the later development of overt hypothyroidism, yet it frequently goes undetected and untreated.2 Untreated subclinical hypothyroidism can lead to daily interference with optimal physical, neurologic, psychological, and emotional functioning, and can cause a diminished quality of life. Controversy exists regarding the routine screening and treatment of subclinical hypothyroidism for all women, a practice that has not been well studied or determined to be conclusively beneficial. 7 8 9 Its proponents argue that preventative treatment with thyroxine is relatively safe, effective, and inexpensive, and can prevent the development of overt hypothyroidism and its consequences.7,8 Further, women who have been treated for subclinical hypothyroidism have retrospectively reported improvements in their physical and mental wellness.7 Patients with subclinical hypothyroidism and abnormal lipid profiles may experience improvement within 1 month of thyroxine treatment.7 Subtle and reversible changes in myocardial performance also have been reported in women with mild hypothyroidism.8 Careful follow-up is essential, with periodic re-evaluation of relevant laboratory markers and symptoms. Because of the frequency of hypothyroidism in older women, routine screening and treatment may be justified in this population. Routine screening also may be prudent during pregnancy, because of the serious consequences of long-term cognitive dysfunction and decreased intelligence in the offspring of women with untreated prenatal hypothyroidism.6
Secondary Hypothyroidism
Secondary hypothyroidism can result from diseases that interfere with thyrotropin-releasing hormone (TRH) production by the hypothalamus, its delivery by the pituitary stalk, or with problems of pituitary thyrotropin production (e.g., pituitary adenomas, hypothalamic tumors, or their treatments such as surgery or radiation therapy). Head trauma, metastatic disease, and infection can also lead to secondary hypotyroidism.8 Iatrogenic hypothyroidism is the second most common cause and is the result of radioactive iodine therapy or ablation treatment for Graves’ disease and other forms of hyperthyroidism.
Signs and Symptoms
Any of the symptoms listed in Box 6-2 may be present in degrees ranging from mild (requiring careful discernment of the clinical picture) to severe. Hypothyroidism may also be asymptomatic, detectable only by laboratory screening. Hypothyroidism is commonly overlooked clinically because of the presence of these symptoms in any number of other diseases.
Diagnosis
TSH measurement is commonly accepted as the most significant and sensitive measurement for hypothyroidism diagnosis. Elevated TSH identifies patients with primary hypothyroidism regardless of the cause or severity.8 Primary hypothyroidism presents with a low serum T4 with attendant elevation of serum TSH. Subclinical hypothyroidism is marked by normal serum T4 levels with slight to moderately increased TSH levels and a normal FTI (Table 6-1). Laboratory tests are considered generally unnecessary to determine the underlying cause of primary hypothyroidism. Factors such as previous neck/thyroid irradiation or surgery, or other exposure to radiation (e.g., pharmaceutical exposure) postpartum status, or other known contributing factors is adequate. Autoimmune causes can be assumed on the basis of ruling out other possible etiologies.8 An important note is that serum TSH levels may rise in the recovery phase of illness, mimicking values associated with hypothyroidism. Therefore, measurement of TSH after complete recovery is appropriate. Free T4 is required to give an accurate measurement of thyroid hormone activity, given that only 0.03% of total T4 hormone is unbound and reflects the thyroid hormone activity of T4. The remaining 99.97% of total T4 is bound to carrier proteins and is metabolically inactive. The fT4 or FTI in conjunction with a TSH can be used to categorize most cases of thyroid dysfunction. The exception occurs when FT4 remains normal but FT3 is abnormal, as may occur when there is a deficient conversion of T4 to T3.
| THYROID DISORDER | TSH LEVEL | THYROID HORMONE LEVEL |
|---|---|---|
| Overt hypothyroidism | >5 mU/L | Low FT4 |
| Subclinical hypothyroidism | >5 mU/L | Normal FT4 |
| Overt hyperthyroidism | Low or undetectable | Elevated FT4 or FT3 |
| Subclinical hyperthyroidism | Low or undetectable | Normal FT4 or FT3 |
Data from Helfand M: Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the U.S. Preventive Services Task Force, Ann Int Med 140(2):128-141, 2004.
Conventional Treatment
Treatment of hypothyroidism with thyroid extract has been practiced since 1891, when Murray first reported the use of sheep thyroid extract. Thyroid hormone was first crystallized in 1914, and initial testing with thyroxine began in 1927.8 Exogenous thyroid hormone replacement remains the standard treatment, with thyroxine (T4) considered the treatment of choice based on its general efficacy and relatively small risk of adverse effects when given at the proper dose.7,8 Conventional practice advocates the use of thyroxine alone over T3 and T4 combinations, the latter of which may provide T3 in excess of normal thyroid secretion.7 However, many physicians find that the addition of T3 can be beneficial for patients not responding optimally to T4 alone.
Dosing of thyroid replacement therapies should be carefully monitored because of the narrow toxic-to-therapeutic ratio of thyroid hormone, with the patient maintaining on the lowest possible effective dose, which will be individually determined. The typical required daily dose is 1.5 µg/lb body weight, with doses for older adults at approximately 70% of that required for younger women.7 It has been estimated by some researchers that as many as 20% of hypothyroid patients are receiving excessive doses. Adverse reactions to thyroxine are usually related to excessive dosing or increased thyroid hormone activity.9 T3 supplementation may be implemented for patients unresponsive to T4 treatment alone.
No studies of controlled treatment of subclinical hypothyroidism have been conducted.2
Botanical Treatment
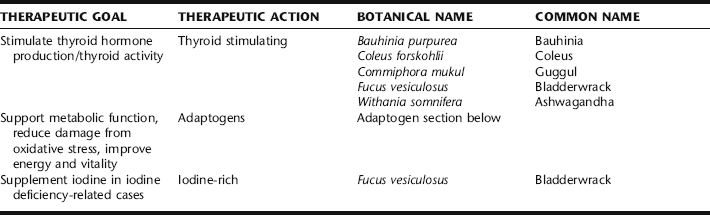
Traditionally, hypothyroidism would have been recognized and treated by herbal practitioners on the basis of its presenting metabolic deficiency symptoms, rather than as a discrete disease entity. The botanical practitioner recognized the patient picture as one of overall depletion. Herbalists today also view hypothyroidism with the goal of improving overall metabolism and the general integrity of the endocrine system. Many consider primary thyroid dysfunction to be a treatable condition with herbs and specific nutritional supplements (Table 6-2). Symptoms of hypothyroidism (e.g., constipation) may be treated with a symptom-specific protocol.
Adaptogens
Ashwagandha (Withania somnifera) is the only adaptogen for which a thyroid-related study was identified. In one study, the effects of daily administration of Ashwagandha root extract (1.4 g/kg body wt.) and Bauhinia purpurea bark extract (2.5 mg/kg body wt.) for 20 days on thyroid function in female mice were investigated. T3 and T4 concentrations were increased significantly by Bauhinia, and serum T4 concentration was enhanced by Withania. Both the plant extracts showed an increase in hepatic glucose-6-phosphatase (G-6-Pase) activity and antiperoxidative effects as indicated either by a decrease in hepatic lipid peroxidation (LPO) and/or by an increase in the activity of antioxidant enzyme(s). It appears that these plant extracts are capable of stimulating thyroid function in female mice.15 The importance of Withania somnifera root extract in the regulation of thyroid function with special reference to type-I iodothyronine 5′-monodeiodinase activity in mouse liver was investigated. Although the extract (1.4 g/kg, p.o. for 20 days) increased serum T3 and T4 concentrations and hepatic glucose-6-phosphatase activity, hepatic iodothyronine 5′-monodeiodinase activity did not change significantly. Furthermore, the extract significantly reduced hepatic lipid peroxidation, whereas the activities of antioxidant enzymes such as superoxide dismutase and catalase were increased. It was concluded that the extract stimulates thyroid activity and also reduces lipid peroxidation of hepatic tissue.16
Bladderwrack
Many herbalists and naturopathic physicians have relied on seaweed species in the treatment of hypothyroidism predicated on their iodine content. Fucus vesiculosus, or bladderwrack, for example, contains variable amounts of iodine, up to 600 mg/g. Much of the iodine content is organically bound, a more potent thyroid stimulating form than mineral bound iodine.17 There are case reports of seaweed, especially bladderwrack, causing both hypothyroidism and hyperthyroidism, and evidence suggests thyroid activity. However, there are no studies of efficacy, dosing, or safety to support its use, and no standardization of iodine content.18,19 Using seaweeds with the rationale that its iodine content is what is affecting treatment may be erroneous, as most thyroid insufficiency in the United States is not attributable to iodine deficiency. Further, excess iodine, as discussed, can contribute to or worsen hypothyroidism. Bladderwrack may interfere with thyroid replacement therapies such as thyroxine.17 Bladderwrack also contains organically bound arsenic, which although rapidly excreted, should suggest caution when using large amounts.19
Coleus
Coleus spp. has been used for centuries in Ayurvedic medicine.20 Forskolin stimulated thyroid function with increased thyroid hormone production in the isolated gland. However, in vitro, low forskolin concentrations inhibited thyroid function.19 No other research on the use of this herb for thyroid conditions was identified.
Guggul
Guggul has shown thyroid stimulating activity, but not via the pituitary-TSH mechanism. It is thought to have a direct action on the thyroid gland. It acts on the peripheral conversion of T4 to T3 increasing T3 levels without changing T4 levels. By increasing thyroid metabolism and activity, guggul reduces LDL cholesterol in individuals with functional hypothyroidism, which may be related to the stimulation of T3 by guggulsterones.21 The effect of a petroleum ether extract of Commiphora mukul was tested on mice thyroid gland grown in organotype of culture using modified Dulbecco’s eagle medium. There was significant increase in the structure and function of thyroid cultivated explants using media containing the guggul extract with raised media T3 resin uptake, PBI, and free thyroxine index. It is inferred that extract of Commiphora mukul augment thyroid hormone synthesis and release.22
Nutritional Considerations
| Coleus | (Coleus forskohlii) | 20 mL |
| Ashwagandha | (Withania somnifera) | 20 mL |
| Bladderwrack | (Fucus vesiculosus) | 15 mL |
| Licorice | (Glycyrrhiza glabra) | 10 mL |
| Guggul | (Commiphora mukul) | 10 mL |
| Nettles | (Urtica dioica) | 10 mL |
| Reishi mushroom | (Ganoderma japonica) | 10 mL |
| Ginger | (Zingiber officinalis) | 5 mL |
| Total: 100 mL |
treatment of autoimmune hypothyroidism. The ingestion of goitrogens—foods that block iodine utilization—are best limited in those patents with goiter. These include such foods as turnips, cabbage, mustard, cassava root, soybean, peanuts, pine nuts, and millet. Cooking usually inactivates goitrogens.23 Rich sources of iodine include ocean fish, sea vegetables (kelp, dulse, arame, hijiki, nori, wakame, kombu), and iodized salt, and should be included when there is iodine deficiency, but reduced when there is iodine excess.
Thyroid function may be supported nutritionally, even with the use of thyroid replacement therapy. Nutrients that may be beneficial supplements include selenium, zinc, tyrosine, and vitamins A, D, E, and C.23 Good sources of zinc include seafood (especially oysters), beef, oatmeal, chicken, liver, spinach, nuts, and seeds. The richest food source of selenium is Brazil nuts, especially those that are unshelled.
Selenium is a cofactor in normal thyroid hormone production. Selenium deficiency decreases conversion of T4 to T3. People with selenium deficiency have elevated T4 and TSH. Patients with normal circulating hormone levels who display clinical hypothyroid symptoms may be selenium deficient; thus, selenium levels should be evaluated and supplementation provided if deficiency is present. In a double-blind, placebo-controlled trial of selenium supplementation of 100 µg/day for 3 months among older subjects showed an improvement in selenium indices, a decrease in T4, and a trend toward normalization of T3:T4 ratio.10
Zinc is involved with synthesis of hypothalamic thyrotropin-releasing hormone (TRH); a zinc deficiency may lower 5′-deiodinase function, thereby contributing to a lower conversion of T4 to T3. Supplementation with zinc acts to normalize the TRH-induced TSH reaction and increase conversion of T4 to T3. The recommended dose is zinc picolinate, 30 mg/day.23
Tyrosine is an amino acid used as a precursor for making thyroid hormone. Tyrosine deficiency can contribute to low thyroid function. Low protein diets may provide insufficient tyrosine for normal thyroid hormone production. Supplementation of tyrosine at a dose of 500 to 1500 mg daily has therapeutic benefits in hypothyroidism.
Exercise
Regular daily exercise stimulates thyroid gland function and increases tissue sensitivity to thyroid hormone.23 Exercise is especially important for dieting overweight hypothyroid patients, as dieting can often put the body into a lower metabolic rate as the body tries to conserve fuel. Adjunctive regular exercise prevents the metabolic rate from dropping with the decrease in caloric intake.
CASE HISTORY: HYPOTHYROIDISM
| Coleus | (Coleus forskohlii) | 20 mL |
| Ashwagandha | (Withania somnifera) | 20 mL |
| Bladderwrack | (Fucus vesiculosus) | 15 mL |
| Licorice | (Glycyrrhiza glabra) | 10 mL |
| Guggul | (Commiphora mukul) | 10 mL |
| Nettles | (Urtica dioica) | 10 mL |
| Reishi mushroom | (Ganoderma japonica) | 10 mL |
| Ginger | (Zingiber officinalis) | 5 mL |
| Total: 100 mL |
HYPERTHYROIDISM
Pathophysiology
Hyperthyroidism, or thyrotoxicosis, is the result of excessive levels of circulating thyroid hormones. It is characterized by elevated total T4, free T4, free T4 index, and/or T3 and T3 resin uptake. Low TSH and normal levels of T3 and T4 characterize subclinical hyperthyroidism, and it has the same causes as overt hyperthyroidism.2 Graves’ disease, an autoimmune disorder in which stimulatory anti-TSH receptor antibodies are formed, comprises the majority of hyperthyroid cases. In fact, the strongest risk factor for both hypothyroidism and hyperthyroidism is the presence of TPO antibodies.1 These antibodies are directed toward the receptors in the cell membrane of the thyroid gland, causing the gland to increase growth, size, and function. Graves’ disease is characterized by several common features, including thyrotoxicosis, goiter, exophthalmos, and pretibial myxedema. Graves’ disease is eight times more common in women than men, typically presents between the ages of 20 and 40 years old, and the most common presentation is a diffuse nonpainful goiter. It may be more prevalent in some genetic HLA haplotypes.24
There are several types of thyroiditis that can cause hyperthyroidism, including Hashimoto’s thyroiditis, subacute thyroiditis, painless thyroiditis, postpartum thyroiditis, and radiation thyroiditis. Other contributing factors include stress, smoking, and iodine supplements/excessive iodine intake, drug-induced hypothyroidism, higher pregnancy frequency, being postpartum, and microbial infections. Hyperthyroid patients have a significantly lower exposure to exogenous estrogens than euthyroid patients.1
Toxic adenoma is a solitary nodule within the thyroid that produces excessive amounts of thyroid hormones. It typically occurs in the middle-aged and older populations.10
Thyroid storm, or thyrotoxic crisis, can occur as a result of a serious stressor, such as surgery, infection, or trauma in a poorly managed case. The mortality rate is approximately 25% even with proper medical treatment.10
Hyperthyroidism and subclinical hyperthyroidism affect quality of life, producing symptoms mimicking adrenergic overactivity. Subclinical hyperthyroidism exerts significant effects on the cardiovascular system. It is associated with a higher heart rate and increased risk of supraventricular arrhythmias, and with an increased left ventricular mass, often accompanied by impaired diastolic function and sometimes by reduced systolic performance on effort and decreased exercise tolerance. These changes usually precede the onset of more severe cardiovascular disease, thus potentially contributing to increased cardiovascular morbidity and mortality. Subclinical hyperthyroidism may accelerate the development of osteoporosis and hence increased bone vulnerability to trauma, particularly in postmenopausal women with a pre-existing predisposition. Fortunately, subclinical hyperthyroidism and its symptoms are readily preventable, and reversible with timely treatment.25
Signs and Symptoms
Symptoms of hyperthyroidism are listed in Box 6-3. Menstrual symptoms associated with hyperthyroidism can vary, and may range from amenorrhea to oligomenorrhea, but menstrual cycles also may appear normal. Anxiety, nervousness, and depression rates are higher in hyperthyroid patients than in euthyroid controls.2 Graves’ disease is characterized by a triad of hyperthyroidism, exophthalmos, and pretibial myxedema. Hyperthyroidism symptoms in postmenopausal women present differently than in younger women. Symptoms are usually confined to a single organ system, particularly the cardiovascular or central nervous system. Goiter is usually absent in 40% of cases, and in older women, a co-occurring disease such as infection of coronary heart disease is usually predominant. The triad of weight loss, constipation, and appetite loss occurs in about 15% of older patients, whereas ophthalmic disease is rare. Practitioners may notice failure to thrive in older patients, with signs of heart disease, unexplained weight loss, and mental or psychological changes signaling possible hyperthyroidism.7
Diagnosis
Definitive laboratory diagnosis is based on elevated serum free T4, total T4, free thyroxine index, and T3 resin uptake. If these are borderline elevated, the T3 should be checked as it is often elevated out of proportion to the T4. TSH is typically decreased. Test for Graves’ disease using the serum TSH receptor antibodies (TSH-R-Ab) test. If nodular goiter presents, a thyroid scan to rule out cancer is recommended.26 As with hypothyroidism, controversy exists as to whether to routinely screen for subclinical hyperthyroidism. Proponents of screening advocate for the potential benefit via prevention of atrial fibrillation, osteoporotic fractures, and other complications of overt hyperthyroidism. Controlled studies of the treatment of subclinical disease have not been conducted.2
Conventional Treatment
The primary goal of conventional medicine is to limit the amount of thyroid hormone production by the thyroid gland.11 Three main treatment methods are available: (1) antithyroid drug therapy, (2) surgery, or (3) radioactive iodine therapy. Although Graves’ disease is an autoimmune disorder, conventional treatment of the disorder is aimed at managing the hyperthyroidism.
Antithyroid drug therapy seems to be most useful in young patients with mild disease. The drugs propylthiouracil, carbimazole, and methimazole may be given until spontaneous remission occurs. Twenty to forty percent of patients have spontaneous remission within 6 months to 15 years duration. There is a fifty to sixty percent relapse rate in patients treated with this method of therapy.10,24
In radioactive iodine therapy, radioactive iodine is given in one dose, following which the gland shrinks and the patient becomes euthyroid over a period of 6 to 12 weeks. The major complication of this method of therapy is hypothyroidism, which develops in 80% of patients treated.27
In mild cases of hyperthyroidism, beta-blockers may be given to provide symptomatic relief of adrenergic symptoms, including arrhythmia, tremor, tachycardia, and anxiety. They also provide minimal prevention of peripheral conversion of T4 to T3. As beta-blockers have no effect on inhibition on the production or release of thyroid hormone, they are an adjunctive therapy alongside of one of the more invasive therapies described in the preceding.10
Botanical Treatment
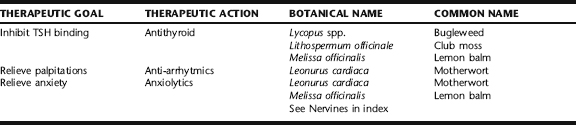
Traditional Western botanical medicine practitioners have found several herbs effective in the treatment of hyperthyroidism, a number of which have demonstrated antithyroid activity, inhibiting the binding of TSH to thyroid tissue (Table 6-3). Additionally, a number of herbs are effective in the treatment of heart palpitations, anxiety, and adrenergic symptoms associated with hyperthyroidism. Note the treatment of mild hyperthyroidism only with botanical medicines is recommended.10
Herbs that increase thyroid activity, as discussed under hypothyroidism, should be avoided in the hypothyroid patient. Additionally, the use of ephedra is contraindicated in patients with hyperthyroidism, and herbs with high caffeine content should be avoided.28 Increased consumption of goitrogens (leafy greens, cabbage, broccoli, and Brussels sprouts, as well as soy) can be part of a treatment strategy to reduce thyroid hormone.10
Bugleweed
Bugleweed (Fig. 6-1) has a long history of use by herbalists for the treatment of palpitations and anxiety.10 It is widely recommended in medical herbalism texts: Priest describe it for the treatment of palpations, tachycardia, and dysregulation of the autonomic nervous system. Weiss refers to bugleweed as having thyrostatic effects and suggests its use for the treatment of hyperthyroidism, whereas the British Herbal Pharmacopoeia (BHP) calls it a thyroxine antagonist. 29 30 31 Hoffmann reports it to be indicated for mild forms of hyperthyroidism, especially when symptoms include tightness of breathing, palpitations, and shaking.32 Priest and Priest recommend combining bugleweed with motherwort for hyperthyroid cardiac reactions, a common practice among herbalists.31
In vivo and in vitro evidence has demonstrated that Lycopus spp. can be beneficial in the treatment of hyperthyroid symptoms.10 Rosmarinic acid, ellagic acid, chlorogenic acid, and luteolin-7-beta-glucoside appear to be the active constituents leading to blocking of TSH receptors and inhibition of peripheral conversion of T4 to T3.10,33 Aqueous, freeze-dried extracts of Lycopus spp., Lithospermum officinale, and Melissa officinalis have been studied in vivo and in vitro; preliminary results support their use in Graves’ disease. This combination was shown to inhibit TSH effects on TSH receptor sites on thyroid cell membranes, block effects of antithyroid immunoglobulins on TSH receptors, and inhibit peripheral deiodination of T4 to T3.34 No human clinical trials have been
| Motherwort | (Leonurus cardiaca) | 25 mL |
| Bugleweed | (Lycopus spp.) | 25 mL |
| Lemon balm | (Melissa officinalis) | 25 mL |
| Nettles | (Urtica dioica) | 25 mL |
| Total: 100 mL |
conducted using bugleweed.10 The German Commission E recognizes the use of bugleweed for mild hyperthyroid conditions with neuroanatomic dysfunction based on pharmacologic studies only, and states that in rare cases high doses have resulted in thyroid enlargement, whereas sudden discontinuation of use has increased disease symptoms.35
Club Moss, Gromwell
Like bugleweed, Lithospermum has a long history of use for the treatment of hyperthyroid conditions. It was used by the Eclectic physicians for this purpose, and although less widely used than Lycopus, has been equally well studied.10,36 Animal studies using Lithospermum officinale have demonstrated its ability to block TSH activity at the receptor level, block the release of TSH from the thyroid, and suppress the iodide pump. It also inhibits peripheral T4-deiodination and conversion to T3.34,37 An in vitro study using freeze-dried extract demonstrated the ability to decrease antibody binding to thyroid tissue on Graves’ disease.34 No human clinical trials have evaluated the use of Lithospermum for hyperthyroid diseases.10
Lemon Balm
Lemon balm (Fig. 6-2), historically referred to as the “gladdening herb,” is calming to the nervous system and has been used since ancient times for this purpose. In vitro studies have confirmed this herb’s ability to block TSH receptors and inhibit both binding of bovine TSH to human thyroid tissue, and binding of autoantibodies in Graves’ disease.34 The herb has a high safety profile and is appropriate for the treatment of mild hyperthyroidism, as well as associated anxiety and depression.10
Motherwort
Motherwort (Fig. 6-3) is classically used for the treatment of anxiety, depression, heart palpitations, and tachycardia, making it highly appropriate for symptomatic relief in hyperthyroid disease.10,29,32 Chemical analytical and animal studies confirm the herbs sedative, anxiolytic, anti-arrhythmic, and antispasmodic effects.10 The German Commission E supports the use of motherwort for the treatment of cardiac disorders associated with anxiety and for the symptomatic relief of hyperthyroidism.36
Nutritional Considerations
The risk of oxidative damage is increased in the hyperthyroid patient because of a higher metabolic rate. Lipid peroxidation is increased and activities of antioxidant enzymes are altered. Dietary changes involve an emphasis on goitrogens, foods that naturally block thyroid hormone synthesis, and the avoidance of certain foods, particularly those high in iodine content such as seaweeds. Dietary goitrogens include broccoli, cauliflower, Brussels sprouts, cabbage, kohlrabi, sweet potatoes, almonds, pine nuts, millet, peaches, and peanuts.8
Additional Therapies
Stress reduction methods include biofeedback, meditation, tai chi, yoga and prayer therapy, and should be included in a plan for hyperthyroid treatment.
She was diagnosed with hyperthyroidism (Graves’ disease).
| Bugleweed | (Lycopus spp.) | 20 mL |
| Club moss | (Lithospermum officinale) | 20 mL |
| Lemon balm | (Melissa officinalis) | 20 mL |
| Motherwort | (Leonurus cardiaca) | 10 mL |
| Skullcap | (Scutellaria lateriflora) | 10 mL |
| Valerian | (Valeriana officinalis) | 10 mL |
| Eleuthero | (Eleutherococcus senticosus) | 10 mL |
| Total: 100 mL |
Evaluation after 7 months of treatment shows TSH <0.01, T3: 110, fT4: 0.72, and FTI: 2.9.
STRESS, ADAPTATION, THE HYPOTHALAMIC-PITUITARY-ADRENAL-AXIS (HPA) AND WOMEN’S HEALTH
Jillian E. Stansbury, Aviva Romm
Stress is a fact of life. However, for most of our biological history, stress was a short-term crisis, after which, according to Robert Sapolsky, author of Why Zebras Don’t Get Ulcers, “it’s either over with or you’re over with.”43 Modern society, with its 24/7 work requirements and global Internet access, high level of stimulation and demand, and chronic (daily) repeated stresses, has opened us to a whole new realm of chronic, debilitating diseases. Western medicine is beginning to understand what has long been recognized by traditional medicine systems: that stress, or more traditionally viewed, one’s relationship with and response to the world, has an impact on health.
What we now know scientifically is that the challenge of a small amount of stress, whether from positive or negative stressors (eustress/distress), can actually increase the overall health and performance of the individual organism, but that prolonged or repeated stress leads to wear and tear on the body—allostatic load—part of a deleterious picture leading to numerous health consequences. These may include reproductive disorders, endocrine dysregulation, insulin resistance (Syndrome X), obesity, chronic fatigue syndrome (CFS), cardiovascular disease, osteoporosis, impaired immunity and autoimmune disorders, cognitive impairment, thyroid disorders, chronic anxiety, postpartum depression, and major depression, to name a few of the big players. 43 44 45 46 47 48 It might not surprise readers that women are experiencing these conditions in increasing and significant numbers. Although stress is not the sole cause of these illnesses—as most illnesses have multifactorial etiologies—stress appears to be an underlying factor in many conditions. Unlike exposure to environmental toxins and radiation, or traffic patterns, and other factors over which we have little control, it may be one factor whose effects we have the ability to minimize.
STRESS, HEALTH, AND DISEASE: THE PHYSIOLOGY AND PATHOPHYSIOLOGY OF STRESS AND THE STRESS RESPONSE
The groundwork for the scientific understanding of the physiology of mind–body interactions was first established in the 1930s by the work of Walter Cannon, and followed in the 1940s by the extensive work of Hans Selye, who first formally elaborated the concept of stress and its effects on physiology.44, 49 50 51 52 53 Selye is also credited with introducing the terms corticoids, glucocorticoids, and mineralocorticoids, and through his work demonstrated the “triad of stress”: adrenal enlargement, GI ulcers, and thymus gland atrophy, in response to exposure to chronic stressors.51 George Chrousos summarizes stress and the stress response as follows:
Life exists by maintaining a complex dynamic equilibrium, or homeostasis, that is constantly challenged by intrinsic or extrinsic adverse forces or stressors. Stress is, thus, defined as a state of threatened homeostasis, which is reestablished by a complex repertoire of physiologic and behavioral adaptive responses of the organism. The adaptive responses may be inadequate for the reestablishment of homeostasis or excessive and prolonged; in either case a healthy steady state is not attained, and pathology may ensue.49
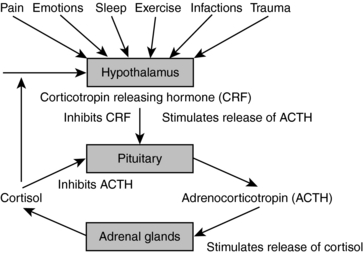
Stressors are threats to homeostasis and the adaptive responses are the counteracting forces intended to re-establish it.43,48,52 Selye termed the adaptive stress response general adaptation syndrome, and demonstrated that it consisted of a consistent set of physiologic responses that included initial response to the stressor followed by an exhaustion phase, and eventually a recovery phase (Fig. 6-4).
More recently the stress response has been renamed allostasis, the ability of the organism to maintain stability, or homeostasis, through change.44,52 McEwen elaborates:
The terms, “allostasis” and “allostatic overload,” allow for a more accurate definition of the overused word “stress” and provide a view of how the essential protective and adaptive effects of physiological mediators that maintain homeostasis—the body’s optimal set points for important factors such as blood pressure, fluid balance, pH, glucose levels, oxygen levels, temperature, etc.—are also involved in the cumulative effects of daily life when they are mismanaged or overused. When mediators of allostasis, like cortisol and adrenaline, are released in response to stressors or to lifestyle factors such as diet, sleep, and exercise, they promote adaptation and are generally beneficial. However, when these mediators are not turned off when the stress is over, or when they are not turned on adequately during stress, or when they are overused by many stressors, there are cumulative changes that lead to a wear-and-tear, called “allostatic load or overload,” on the body and brain. The concept of allostasis refers to the network of interacting mediators by which stability, that is, homeostasis, is achieved through change. There are primary mediators of allostasis, such as, but not confined to, hormones of the hypothalamic-pituitary-adrenal (HPA) axis, catecholamines, and cytokines. These mediators interact with each other to create a network of reciprocal effects.44
Our bodies possess complex and elegant mechanisms for responding to and recovering from acute exposure to stressors. The neuroendocrine system has evolved two primary pathways responsible for responding and adapting to potentially harmful or life-threatening encounters: the sympathoadrenal system (SAS) and hypothalamic-pituitary-adrenal axis (HPA). Both mediate a two-way brain-body communication that sets in motion a series of hormonal and neuroendocrine responses that “switch on” and “switch off” what has been commonly referred to as the “fight or flight” response.43,52 In response to the alert system being switched on, the body’s resources are mobilized for protective action: The heart rate increases and blood is diverted from digestion (who needs to digest when being chased by the proverbial saber tooth tiger?) into the periphery, especially the legs (yup, you want to be able to run away from the tiger!), the respiratory rate increases, blood pressure increases and urinary output decreases, the pupils dilate to increase sight, and other senses such as hearing and smell become keener, the mind becomes sharp and alert and vigilance is enhanced, appetite decreases, immunity is suppressed, and large amounts of sugar are delivered to the bloodstream via lypolysis and gluconeogenesis to fuel the energy needed for a massive response. Growth, reproduction, and sexual response are inhibited—resources are instead diverted to immediate life-saving needs, rather than toward what Sapolsky refers to as optimistic activities. In the recovery phase, interestingly, the body responds to the need for repair by increasing appetite and storing fat (primarily in the abdomen).43
Hormonal and neuroendocrine mediators and messengers from the sympathetic nervous system and HPA axis orchestrate all of these responses. At the first sign of threat, or even a perceived threat, the sympathetic nervous system goes into action. Epinephrine (adrenaline) and norepinephrine (noradrenalin) are released from the nerve endings of the adrenal glands and the rest of the body, respectively, and begin to stimulate the body to further reaction in a matter of seconds. The hypothalamus releases a substance called corticotrophin-releasing hormone (CRH), which triggers the release of ACTH (corticotrophin), which within a few minutes reaches the adrenal glands, where it causes the release of glucocorticoids (cortisol, corticosteroids). The pancreas releases glucagons, which with the glucocorticoids increase the levels of circulating glucose. Glucocorticoids do this via the promotion of protein and lipid degradation from muscle, skin, and fat. Energy is mobilized at the expense of storage. Other hormones are released as well. Prolactin is secreted by the pituitary, and plays a role in the suppression of reproduction during stress. Endorphins and enkephalins are released, blunting pain perception, while vasopressin (antidiuretic hormone) is released from the pituitary, and maintains blood pressure, forestalling for example, hypovolemic shock in the event of massive blood loss. Prostaglandins, PAF, and NO are all stimulated. The allostatic response also inhibits the release of numerous hormones, for example, estrogen, progesterone, and testosterone, as well as growth hormone and insulin.
Prolonged and repeated exposure to aversive events—as well as anticipation of aversive events (e.g., worry and anxiety about future or impending events such as an interview, an exam, paying the bills)—may lead to a sustained activation and dysregulation of the HPA axis.43 In time, allostatic states place wear and tear on the regulatory systems of the brain and body, which can lead to HPA hyperfunctioning or hypofunctioning (respectively, the inability to turn off, or turn on the adaptive response) maladaptive responses thought to be causally linked to a number of disorders via glucocorticoid and other effects on the cardiovascular system, mobilization of bone stores of calcium, effects on weight and fat distribution, hormonal effects on the reproductive system, thyroid effects, and so forth.43,44,47,52
Women are more likely to experience menstrual irregularities, anovulation, infertility, osteoporosis, chronic fatigue, autoimmunity, and cardiovascular disease, Syndrome X, and diabetes, for example. Further, there is significant evidence that a history of childhood sexual or psychological abuse predisposes to HPA dysfunction later in life.54 Excessive exercise (overtraining) also can lead to HPA dysfunction with increased fatigue and decreased immune response.55 Irregular and inadequate food intake, regular hypoglycemic episodes, and yo-yo dieting also cause excessive stress and cause blood sugar imbalances that can lead to allostatic overload and eventually HPA dysregulation.
The medical profession does not define a category of illnesses that encompasses the effects of allostatic load. Prevention and treatment of stress and its effects on the HPA axis are not considered a part of preventative care or treatment for the numerous and serious stress-related diseases mentioned earlier, with limited exception. Adrenal disease is recognized only in its severest forms: Addison’s disease and Shy-Drager syndrome. Yet the symptoms of HPA axis dysregulation are rampant in modern society. Aside from overt dysfunction and disease [reproductive disorders and endocrine dysregulation, insulin resistance (syndrome X) and obesity, chronic fatigue syndrome (CFS), cardiovascular disease, osteoporosis, impaired immunity and autoimmune disorders, thyroid disorders, cognitive impairment, chronic anxiety, and major depression], Americans are plagued by fatigue and exhaustion, insomnia, emotional frustration, digestive problems, weight problems, menstrual problems, infertility, menopausal problems, headaches, susceptibility to colds, musculoskeletal tension, allergies and asthma, atopic conditions, and numerous other problems that can be related to stress and chronic HPA hyperfunctioning or hypofunctioing. 43 44 45 46 47 The need to address chronic stress as part of the prevention and treatment of chronic illness is more than a lip service to a holistic approach—it is a significant part of a comprehensive medical and public health approach to reducing chronic health problems affecting all populations in the United States. It is also an integral aspect of herbal medicine care, as discussed in the following.
EUSTRESS AND DISTRESS
Interestingly, pleasant and pleasurable activities (sex, numerous mind-altering and energy-altering substances that are commonly used and abused, heavy exercise) also trigger the stress response. The primary factors distinguishing distress from eustress appear to be the quality and intensity of the stressor, termination of the stress response after a particular stressor has ceased, and the return of homeostasis.52 Genetic predisposition, life history, and age also seem to play significant roles in the perception of stress and in the stress response.43,52
CONVENTIONAL THERAPIES FOR HPA AXIS DYSFUNCTION
Although Selye’s work was accepted as logical and well supported, and was validated by numerous subsequent researchers, conventional medicine does not recognize generalized HPA dysfunction as a discrete entity, much less propose methods for prevention and treatment. Patients presenting with weakness, fatigue, insomnia, anxiety, susceptibility to colds, stress symptoms and stress intolerance are typically prescribed antidepressants and anxiolytics. If hypertension and heart palpitations are most prominent, beta-blockers or other cardiovascular medications may be prescribed. If low adrenal function has affected progesterone or reproductive function, hormone replacement is typically offered. If dysglycemia and episodic blood sugar difficulties are the presenting symptoms, the case is often misunderstood. Vital signs and routine blood work, including blood glucose, may often be normal, and the individual is typically told all is fine, or perhaps offered psychiatric medications.
BOTANICAL APPROACHES TO STRESS AND HPA AXIS DYSREGULATION
The primary class of herbs used to support and restore adrenal health and optimal HPA axis functioning is known as the adaptogens. Many are considered “tonics” in traditional medicine systems (e.g., ginseng in TCM, ashwagandha in Ayurveda). Lazarev, a Russian pharmacologist, researching the resistance of organisms to stress in experimental studies and initially testing pharmacologic drugs, first coined the term “adaptogen” in 1947. To be considered an adaptogen, the substance had to demonstrate the following:53
Lazarev, Brekhman, and other Russian researchers were using the terms stress and stressors in the classical sense as defined by the work of Selye, and were seeking to develop medications able to mobilize the intrinsic adaptive mechanisms to help individuals cope with and survive in situations of intense or prolonged stress while maintaining physical and mental work capacities. Adaptogens were considered to constitute a new class of metabolic regulators, of natural origin, which increased the organism’s adaptive abilities to environmental factors, and prevented damage from these factors. Most studies of adaptogens were originally conducted in Russia and focused on Eleutherococcus senticosus, Rhodiola rosea, Schisandra chinensis, and Bryonia alba. These herbs were incorporated into official medical practice in the USSR and produced as standardized extracts in various forms. Having been found quite safe, they are still used in Russia in both self-care and physician-prescribed regimens.48 By 1984, there were over 1500 studies in Russia alone on just three adaptogenic herbs (Eleutherococcus, Rhodiola, and Schisandra). Interest in adaptogens has also spread worldwide; the term adaptogen, for example, is recognized by the FDA as a functional term.53
A substance that reduces the state and severity of stress and counteracts the effect of stressor is an adaptogen.56 Adaptogens contain phenolic compounds with a structural resemblance to catecholamines, suggesting an effect on the SAS, tetracyclic triterpenes similar to the corticosteroids that inactivate the stress system; and oxylipins, unsaturated trihydroxy or epoxy fatty acids resembling leukotrienes and lipoxines.48 Adaptogens have a wide range of effects, and appear to act broadly on tissue involved in homeostatic regulatory systems (immune, endocrine, central nervous system [CNS]) rather than having specific targets. There is significant evidence that adaptogens increase exercise capacity, endurance, stamina, cognitive function, and mental alertness, and that they increase nonspecific immunity, stress resistance, relieve fatigue, and improve energy metabolism and tissue repair. Evidence also indicates that administration of adaptogens modulates ACTH and corticosteroid formation and normalizes levels of stress hormones.53 They may be considered substances that allow the organism to resist stress at higher levels of challenge.
Adaptogens have been used historically as general tonic medicines, thought to gently strengthen the CNS in cases of fatigue, physical exertion, aging, weakness from disease and injury, and prolonged stress. They are considered to induce “states of non-specifically increased resistance” (SNIR). Many cultures have embraced the widespread use of such herbs in older adults and infirm; Panax in China and Asia, Eleutherococcus in Russia, and Withania in India. They have been historically and clinically reported to improve diabetes, blood pressure, and cardiac action, and relieve mental confusion, headache, and weakness among older adults. These plants are also credited with an affinity for the nervous system and an ability to relieve mental stress in cases of insomnia and anxiety disorders. Muscle fatigue, physical weakness, and immune deficiency were all thought to improve with the use of such herbs. Athletes, for example, sometimes benefit from the use of adaptogens, noting improved stamina and endurance. They also may be used to improve postsurgical healing and convalescence.48
It is generally recommended to give a course of adaptogens over a prolonged period of time, a minimum of 3 months generally, or on an ongoing basis for up to several years for severely depleted patients. Adaptogens may be used singly; however, it is preferable to combine them with other herbs to support, direct, and moderate their individual effects. Although adaptogens, by definition, lack serious side effects, their specific qualities may best be tempered by combination with other herbs. For example, Rhodiola or Schisandra taken alone are both quite drying and astringent, and can be tempered by combining them with herbs that are moistening and sweet, for example, licorice. Ginseng, particularly red ginseng, can be heating and stimulating. Looking at TCM formulas, one quickly notices that herbs such as ginseng are one of only several of many herbs included in the formula for this very reason. Additionally, although adaptogens share many similar qualities, each individual herb possesses unique characteristics that distinguish it from the others; thus, prescribing should still be based on the individual patient. Adaptogens may be used prophylactically prior to times of physical, emotional, or mental stress, or restoratively, such as after a long illness or prolonged period of debility or stress.
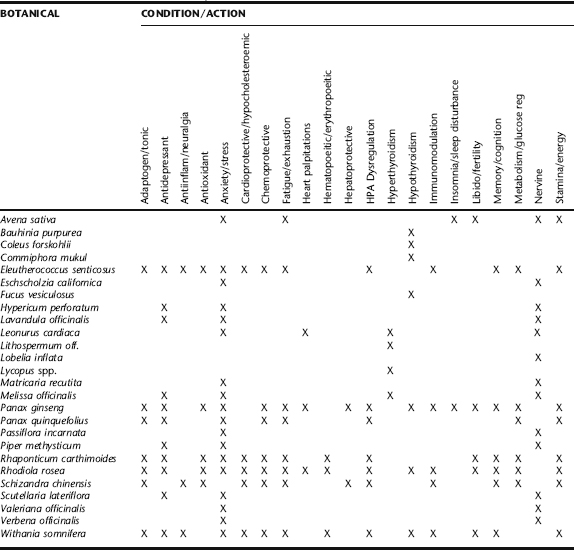
The discussion that follows presents evidence on the most commonly used herbal adaptogens. Licorice, which may not be truly classified as an adaptogen, is included because of its marked cortisol-sparing, adrenal tonic effects. Calmative nervines that act as sympathetic relaxing agents such as Matricaria recutita, Scutellaria lateriflora, Avena sativa, and Passiflora incarnata, for example (Table 6-4), are indicated, as these may reduce stress-induced CRH stimulation of the adrenal output of cortisol and adrenaline. Parasympathomimetics directly or indirectly increase parasympathetic function, reducing sympathetic dominance. They are indicated for anxiety and stress, and to relieve symptoms that result from adrenergic stress.57 Evidence for nervines and other herbs listed in Table 6-4 are found elsewhere throughout this text. For insomnia, sedatives may be included, as for chronic musculoskeletal problems, antispasmodics incorporated into formulae. Adaptogens also may be effectively combined with herbs for individual systems, for example, hawthorn for the cardiovascular system, or chaste berry for the reproductive system.
TABLE 6-4 Botanical Treatments for Improving the Stress Response
| HERBAL ACTION | BOTANICAL NAME | COMMON NAME |
|---|---|---|
| Adaptogens |
Ashwagandha
The roots of ashwagandha have long been used as “rasayana” drugs in Ayurvedic medicine to prevent or treat disease through the restoration of a healthy balance of life.56 Ashwagandha is used in Ayurvedic medicine as a general restorative medicine, and to improve general health, longevity, and prevent disease. Ashwagandha is much less stimulating than ginseng, making it preferable for patients with irritability, anxiety, and insomnia, and as a gentle tonic herb for the nervous system.58 The species name, somnifera, indicates the plant’s traditional use for sleep induction. Ashwagandha is immunomodulatory and improves energy in patients experiencing stress-induced illness or exhaustion. It is indicated in inflammatory conditions, such as arthritis or other musculoskeletal disorders, and it is combined with other herbs in the treatment of cancer. Ashwagandha is used in Ayurveda and Unani systems of medicine for the treatment of pain, skin diseases, infection, inflammation, gastrointestinal disorders, rheumatism, and epilepsy. It is also used as a general tonic for the improvement of libido, liver health, mental state, cancer, heart disease, and the immune system.59 In vivo studies support its use for anti-inflammatory, immunomodulatory, antioxidant, thyroid stimulating, anxiolytic, stress-reduction, memory enhancing, and antineoplastic effects (Table 6-5).56, 59 60 61 62 63 64 65 66 67 68 69 Ashwagandha is also reported to be hematopoietic, making it useful in the treatment of anemia.70 Ashwagandha is combined with levodopa, tropane alkaloid–containing plants, and other herbs as a therapy for Parkinsonism.71 Ashwagandha and other herbs may take the place of benzodiazepines and have a calming effect on the nervous system. Applications for ashwagandha based on traditional use, animal studies, and clinical evidence are listed in Box 6-4.
TABLE 6-5 Effects and Supposed Mechanisms of Some Actions of Ashwagandha
| EFFECTS | SUPPOSED MECHANISM OF ACTION |
|---|---|
| Adaptogenic and immunomodulatory activity | Steroidal lactones 5,20(R)-dihydroxy-6,7 alpha-epoxy-1-oxo-(5 alpha)-with a-2,24-dienolide and solasodine are known to possess adaptogenic and immunomodulating activity.56,59,60,65 |
| Anticonvulsant activity | May be mediated via a GABA-ergic mechanism, likely through the barbiturate site on the macromolecule ionophore complex.59,73 |
| Anti-inflammatory effect | May be mediated by decreased glycosaminoglycan synthesis. Ashwagandha extract increased phagocytosis and intracellular killing of peritoneal macrophages but did not increase the number of peripheral leukocytes.63 |
| Antistress/antianxiety effects | Ashwagandha has demonstrated inhibition of stress-induced increases in dopaminergic receptor population in the corpus striatum.74 Ashwagandha may have a GABA-mimetic action.67,73,75,76 |
| Anticancer effects | Withaferin A is the primary antineoplastic agent. Its mechanisms of action are still unclear.60 It has been found to arrest cellular division at metaphase, has shown inhibition of protein and nucleic acid syntheses in P388 cells in vitro, and RNA synthesis in Sarcoma 180 cells was inhibited. Withaferin A induces a G2/M block.59,60 An immunostimulatory effect may be partially responsible for antineoplastic activity but this is uncertain, because in animal experiments withaferin A has shown both immunostimulatory and immunosuppressive activity.60 Some studies have focused on the ability to reduce stress-induced oxidative damage.63 Rat models have observed condensation and fragmentation of chromatin as quantitative markers, and other observable cellular changes to assess the cytoprotective properties of Withania.77 Such researchers propose that Withania extracts may help prevent free radical induced cleavage of DNA. Withania somnifera has been shown to increase the percentage of cells containing neurites in human neuroblastoma cells, promote the growth of new dendrites, may aid in the repair of damaged neuronal circuits.62 Withaferin is reported to have a radiosensitizing effect that has been noted in animal studies to reduce the toxicity of irradiation therapy, while improving the effects.60 Antitumor effects of Withania have also been investigated.78,79 |
| Memory-enhancing effects | Withania appears to mediate stress-induced disruption of memory formation and retention. Neuroelectric, physical stress, and scopolamine induced disruption of acquisition and retention of memory consolidation all appear to be significantly reduced with the administration of Withania extracts.64 Animal experiments have noted Withania to provide protection to neuronal cell bodies when animals are subjected to stressful conditions.80 Effects may also be due to increased cholinergic signal transduction cascade in the brain. |
| Sedative effects | May be due to the alkaloid somniferum |
| Cardiovascular effects | The steroidal lactones have a mild ionotropic and chronotropic effect on the heart. |
Overall, toxicity studies have demonstrated a high level of safety of ashwagandha and its extracts.56,70 Safety is discussed further in Plant Profiles: Ashwagandha. The American Herbal Products Association has rated it a class 2b herb (not to be used during pregnancy); however, the evidence contraindicating its use during pregnancy is limited and questionable, and Ayurvedic practitioners have used it traditionally during pregnancy.70,72 Because ashwagandha reverses cyclophosphamide-induced neutropenia, it may be prudent to avoid its use in patients with leukemia who are being treated with cyclophosphamide.60
Eleuthero
Eleuthero, a native of northeast Asia, is used in TCM for general weakness and debility, lassitude, anorexia, insomnia, and dream-disturbed sleep.81 Its use as an adaptogen originated in the former Soviet Union, in the latter half of the twentieth century, when it was researched and promoted by scientists as a substitute for Panax ginseng, which was more expensive and less accessible. Pharmacologic studies have suggested that its effects are at least equal to, and perhaps superior to those of Panax ginseng.81
Until recently referred to as Siberian ginseng, the herb is now properly referred to as Eleuthero, because of recognition that although the plants are from the same family, their actions arise from very different chemical constituents.72 Eleuthero’s actions much like ginseng, are considered immunomodulating, stress reducing, performance and energy enhancing, anabolic, and adaptogenic, hence the original misnomer.81 The herb has demonstrated the ability to improve adrenal function, stress tolerance, enhance immune function and resistance to infection including influenza, and enhance selective memory.18,28,53,76,82 The plant contains phenylpropionates (e.g., syringin, caffeic acid, sinapyl alcohol, coniferyl aldehyde), lignins (e.g., sesamin, syringoresinol and its glucoside), saponins (e.g., daucosterol, β-sitosterol, hederasaponin B), coumarins (e.g., isofraxidin and its glucoside), vitamins (e.g., vitamin E), and provitamins (provitamin A, i.e., beta-carotene). These molecules have demonstrated a wide range of pharmacologic activities and are associated with an extensive literature base.83 Six secondary compounds found in Eleutherococcus have been shown to have various levels of activity, as shown in Table 6-6. Previously, many of these compounds were referred to as eleutherosides; however, as these compounds are not unique to this plant, and had been previously identified from other sources, the term eleutherosides is not properly applied to these constituents.81,83
| EFFECTS | CONSTITUENT(S) |
|---|---|
| Antioxidant | Syringin, caffeic acid, caffeic acid ethyl aldehyde, coniferyl aldehyde |
| Anticancer | Sesamin, sitosterol, isofraxidin |
| Hypocholesterolemic | Sesamin, sitosterol, beta-sitosterol and beta-sitosterol 3-D-glucoside |
| Immunostimulatory | Sesamin, syringin |
| Choleretic | Isofraxidin |
| Reduce moderate insulin levels | Beta-sitosterol and its glucoside |
| Radioprotectant | Syringin |
| Anti-inflammatory and antipyretic activities | Beta-sitosterol |
| Antibacterial agent | Caffeic acid |
Data from Wichtl M: Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis, ed 4, Stuttgart, 2004, Medpharm; Davydov M, Krikorian AD: Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an adaptogen: a closer look, J Ethnopharmacol 72(3):345-393, 2000; Brekhman II, Dardymov IV: New substances of plant origin which increase nonspecific resistance, Annu Rev Pharmacol 9:419-430, 1969.
Animal experiments have confirmed not only these actions but also the reduction of NK activity and the inhibition of corticosterone elevation induced by swimming stress in animal models.84 Stress-induced gastric ulcers have been prevented in animal models, and positive results have been shown with reduction of serum lipid-peroxide levels and improved lipid metabolism.81,85 In healthy volunteers, ingestion of fluid extracts led to markedly increased T-lymphocyte counts and studies have demonstrated overall improvements in cellular defense.35,85 Studies on athletic performance and stress response have shown that Eleuthero improves the testosterone:cortisol ratio by over 28%, a marker of reduced stress response in athletes.86 Clinical findings also have suggested that patients with moderate fatigue in chronic fatigue syndrome may benefit from use of Eleuthero, and that older adults may safely experience improvement in some aspects of mental health and social functioning after 4 weeks of therapy, although these differences attenuate with continued use.87 Eleuthero also has been shown to cause reductions in cardiovascular stress response in healthy patients. Eleuthero is considered to have a high safety profile. Russian studies have noted a general absence of side effects and adverse reactions, and there are no expected significant herb–drug interactions; however, its use is not recommended for patients with hypertension or during acute phase of infection, although it may be combined with antibiotics for treatment of dysentery.18,28,35,88 The German Commission E considers it an invigorating tonic to be used in cases of fatigue, decreased work capacity and concentration, and for convalescence.35,81 It is not heating or stimulating to the degree of ginseng or Schisandra. In fact, herbalists consider it a generally neutral herb that can be used by anyone.89,90
Ginseng (Panax ginseng; Panax quinquefolius)
Ginseng species include Panax ginseng and Panax quinquefolius, Asian and American ginseng, respectively (Fig.6-5). Panax notoginseng and Panax pseudoginseng are also ginsengs but are not discussed here. Eleutherococcus senticosus, formerly referred to as Siberian ginseng, is not, in fact, a ginseng. White and red ginsengs are both forms of Panax ginseng, white being unprocessed, and the red having been steam prepared.91 In TCM, white and red ginseng are considered to have different actions, the former being much less stimulating, and the latter being used for deep deficiencies and to move the qi. Western herbalists consider American ginseng to be less heating and gentler than either Asian ginseng, especially compared with red ginseng. The word Panax is derived from the word panacea in deference to wide-ranging uses from immune support to energy enhancement to promotion of longevity. Ginsenosides are considered to be the pharmacologically active components of ginseng; however, as stated in Wichtl, “the theory for its use in traditional medicine cannot be explained based on the criteria of western rational medicine.”81,91
Chinese medicine has included ginseng in its pharmacopoeias for as much has 5000 years. It is not considered a medication so much for specific conditions; rather, it is a tonic for improving overall energy and sense of well-being. It is, however, included in formulae for specific conditions, especially those associated with debility, fatigue, immunodeficiency, irritability and insomnia, decreased cognitive and memory functions, impotence or loss of libido, calming the nerves, and promoting the production of moisture in the body, and other conditions. The German Commission E approved its use as a tonic to combat feelings of lassitude and debility, lack of energy and ability to concentrate, and during convalescence.35 Ginseng is one of the most extensively researched botanical medicines in the world.91 This review of ginseng is by no means comprehensive, and primarily is intended to convey its overall effects in the context of this section. The actions most ascribed to ginseng are tonic and adaptogenic, demonstrating the ability to enhance nonspecific immunity, inhibit fatigue, and have antiaging effects (Box 6-5).* Randomized, double-blind, controlled trials have shown that (Korean) ginseng significantly improves quality of life and well-being measures while under stress, including alertness, relaxation, appetite, fatigue levels, sleep quality, recovery from the common cold and bronchitis, and significantly decreases systolic blood pressure compared with controls.19
Ginseng’s adaptogenic effects are notable in the HPA axis. Ginseng improves recovery from chronic stress by improving corticoid response from the adrenal gland, and the corticotropin feedback loops with the HPA.76,82 Animal studies have noted ginseng administration to enhance energy metabolism during exercise.92 Panax ginseng has been noted to elevate testosterone when low, but not elevate it excessively when within the normal range. Panax ginseng has been investigated for immune modulating and anticancer activities.93 Animal and human investigations have shown ginseng to possibly reduce the occurrence of cancer. Mice exposed to carcinogens have fared better when treated with ginseng than untreated controls.94 In one human trial, Panax was shown to be an effective therapy in the treatment of acute infectious bronchitis.95 Ginseng may improve immune function, as evidenced by increased blood levels of basic immune cells including natural killer cells, lymphocytes, and macrophages, seen after the administration of ginseng preparations. 96 97 98
Enhancing gonadotropin activity may be added to the list of the many uses for Panax. Gonadotropin levels have been shown to increase in men with low sperm counts taking ginseng extracts but not in men with normal sperm counts.99 After 3 months of daily ginseng consumption, testosterone, dihydrotestosterone, and related sperm counts and sperm motility were noted to improve in the infertile men, whereas normal controls displayed only slight increases, with none of the controls developing abnormally high or excessive levels of hormones. Human clinical studies also observed an increase in libido and erectile function. The Chinese species, Panax ginseng, is also reported to be a sexual tonic and aphrodisiac useful in maintaining the reproductive organs and sexual desire into old age. Low sperm counts are a symptom of hypogonadism and/or hormonal imbalance. There are case reports of acute life-threatening hypopituitarism (postpartum Sheehan’s syndrome) being successfully treated with Panax and Glycyrrhiza.100
Ginseng may improve the stress response by reducing the excessive sympathetic response that promotes a fight or flight cascade. Adrenal cortisol production and activity may be improved, along with corticotropin feedback loops with the use of adrenal tonic herbs such as Panax ginseng.76 Blood sugar reductions have been demonstrated, with potential benefit for patients with type 2 diabetes mellitus.101,102 Several human trials have shown clinically useful antianxiety effects with the use of ginseng preparations, without any adverse side effects reported.103 Side effects, and drug interactions are not expected with proper use.35 However, reported side effects in noncontrolled studies in which subjects have been using high doses of caffeine and taking products with additional ingredients, have included sleeplessness, anxiety, diarrhea, and skin problems.
Concerns about ginseng abuse syndrome (GAS) occurring with regular use have been entirely debunked, the progenitor of the concept himself retracting his conclusions.91 Pregnancy use is contraindicated in the British Herbal Compendium to the British Herbal Pharmacopeia; however, ginseng is traditionally used during pregnancy in China, and studies have shown no teratogenicity, mutagenicity, or other adverse effects, and in fact one study demonstrated a reduction in pre-eclampsia compared with a control group.91 Neither the American Herbal Products Association nor the German Commission E suggest restricted use during pregnancy; however, given that long-term safety studies are lacking, it is best to avoid except in TCM formulae specifically for pregnancy-related problems and under the supervision of a qualified TCM provider or herbalist skilled in obstetric herbal medicine.28 Diabetic patients may need to adjust insulin doses; patients taking anticoagulant drugs are recommended to speak with their health care providers before taking ginseng.28 Herbalists generally discourage the use of stimulants with ginseng, and also may contraindicate ginseng use in patients with hypertension, hyperthyroidism, or other “excess” states.
Licorice
Although licorice is sometimes categorized as an adaptogen, it does not strictly meet the criteria of one: Its actions are specific rather than nonspecific, and its use in certain patients in high doses or over a prolonged period is not always benign, and in fact can pose serious consequences. However, because of licorice’s action on the adrenal glands, as well as on several conditions associated with HPA dysfunction, it raises questions about the potential role of licorice in the prevention and treatment of HPA dysfunction, and merits mention in this section. Peptic ulcer was one of the first conditions ever to be associated with an overactive stress response. Interestingly, licorice extract has demonstrated efficacy against Helicobacter pylori, including against clarithromycin-resistant strains.91,111
Licorice studies have demonstrated its positive effects in treating viral infection, particularly those caused by herpes simplex virus, an active infection associated with increased stress. A recent study demonstrated that licorice root extract might even interfere with the latency of the herpes virus.112 Licorice components also have demonstrated the ability to modulate bone disorders in menopausal women because of affinity to estradiol-17 beta. This potential exists with or without the presence of vitamin D.91,113 (Licorice has also demonstrated estrogen-inhibitory effects.)91 Licorice hydrophobic flavonoids have evidenced abdominal fat-lowering and hypoglycemic effects, possibly mediated via activation of peroxisome proliferator-activated receptor-gamma (PPAR-gamma).114
Researchers examined the effects of licorice on memory and learning in a mouse model and found promise as a memory enhancer in both exteroceptive and interoceptive behavioral models of memory. The anti-inflammatory and antioxidant properties of licorice may be contributing favorably to the memory enhancement effect. Because scopolamine-induced amnesia was reversed by licorice, it is possible that the beneficial effect on learning and memory may result from facilitation of cholinergic transmission in the brain.73 In the treatment of postpartum anterior pituitary insufficiency, 10 patients demonstrated complete recovery with a decoction of licorice and ginseng.91
Licorice inhibits corticoid dehydrogenases, prolonging the half-life of cortisol in the body. The British Herbal Compendium cites licorice as having adrenocorticotropic activity, indicating it for adrenocorticoid insufficiency.58 Thus, it is sometimes described by herbalists as an adrenal tonic, and cortisol sparing.18,58,115
Licorice is contraindicated by the German Commission E in patients with cholestatic liver disorders, liver cirrhosis, hypertension, hypokalemia, severe kidney insufficiency, and pregnancy.35 It is also contraindicated in congestive heart failure and edema.19 Licorice safety issues are discussed further in Plant Profiles and discussions of pregnancy and herb safety.
Rhaponticum
R. carthamoides has been used for centuries in Siberia as a folk medicine for the treatment of fatigue, anemia, and impotence, as well as for convalescence after illness.116 In 1961 the liquid extract (1:1) was officially recognized and included in the Soviet Pharmacopoeia as a natural agent for overcoming fatigue, improving physical and mental productivity and stamina, and shortening recovery time after illness.117 The roots and rhizomes are considered the plant’s medicinal parts; the active ingredients are primarily phytoecdysterones (especially ecdysterone), although the plant also contains a number of other biologically active compounds, including flavonoids, sesquiterpene lactones, and polyines.118,119 R. carthamoides extract standardized to 5% ecdysterone is considered the most potent form.117,120 Several decades of research have demonstrated numerous pharmacologic effects in animal models and human studies.117,121,122 It is a classic adaptogen with a wide range of activities, including normalizing effect on the central nervous and cardiovascular systems, sleep, appetite, moods (neurotic, asthenic, depressive, hypochondriac), mental and physical state, and the ability to function well under stress. It has marked anabolic activities, building lean muscle, reducing body fat, and improving work and athletic capacity and performance, improving mental acuteness, alleviating depression; is a tonic for the vital organ systems, and is erythropoietic and antioxidant, delaying the effects of aging.117 Rhaponticum improves stress response and adaptability to physical and mental challenges, enhances mental and physical capacity for work under stressful conditions, inhibits disorders of energetic metabolism, maintains stable glycogen levels in the skeletal muscles, increases the blood supply to the muscles and brain, and shortens the recovery period after prolonged muscular workloads.117,123 Rhaponticum favorably affects heart rate, improving arterial pressure, and hastening recovery after work load.124,125 In trained athletes, Rhaponticum improves endurance, speed, recovery, and physiologic markers, and allows anabolic processes to outpace catabolic processes, leading to greater fitness, endurance, and performance.117,124,, 126 127 128 129 Rhaponticum has also shown some preliminary beneficial outcomes in childbearing women, shortening the duration of labor and improving postpartum recovery.117 A comprehensive list of rhaponticum’s effects appears in Box 6-6.116,117,122,, 129 130 131 132 133 134 135 136 137 138
Rhodiola
Rhodiola rosea, also called golden root, Arctic root, and rose root, grows in arctic and mountain regions throughout Europe, Asia, and America. 139 140 141 Its use was first recorded by the Greek physician Dioscorides in 77 ce in De Materia Medica.141 It has been used for centuries as a traditional medicine in Russia, Scandinavia, and other countries for the treatment of fatigue, depression, anemia, impotence, GI ailments, infections, and nervous system disorders, and to promote physical endurance, longevity, and work productivity.141,142 Rhodiola appeared in the scientific literature of Sweden, Norway, France, Germany, the Soviet Union, and Iceland as early as 1725.141 Because most of the identified literature on this herb is from foreign language sources, I have relied largely upon secondary sources for this review. 140 141 142 R. rosea has been an accepted medicine in Russia since 1969 for the treatment of fatigue, somatic and infectious illness, psychiatric and neurologic conditions, and as a psychostimulant to increase memory, attention span, and productivity in healthy individuals. It is also officially registered in Sweden and Denmark and is widely used in Scandinavia as a general tonic and to increase mental work ability under stress.141 Rhodiola is classified as an adaptogen. 139 140 141 142 It contains a range of antioxidant compounds, and its adaptogenic activities are attributed to its unique phenylpropanoids rosavin, rosarin, and rosidirin, and to phenylethanol derivatives p-tyrosyl and salidroside (also called rhodioloside), as well as to flavonoids, triterpenes, monoterpenes, and phenolic acids. Rosavins are the accepted marker compounds for water and alcohol extracts.140,141 Research both from animal models and human clinical trials indicates a number of favorable effects associated with its use, including CNS stimulation, pronounced antistress effects, enhanced physical work and exercise performance, increased muscle strength, reduction in mental fatigue, and prevention of high altitude sickness.139, 142 143 144 145 Cardioprotective and anticancer effects also have been attributed to its intake.139,142 Although research on Rhodiola has been extensive, it has also been described as “fragmentary,” with methods, statistics, and controls poorly defined.139,145 Further, not all studies have yielded positive outcomes for efficacy, although this may be related to product, dose, and duration of administration. Rhodiola rosea extract exhibited an anti-inflammatory effect and protected muscle tissue during exercise.146 Studies have demonstrated its ability to induce a general sense of well-being and reduce situational anxiety.141,142 It has demonstrated improvement in depressive syndromes, mental and physical fatigues secondary to medical conditions, sexual dysfunction, thyroid hypofunction (without causing hyperthyroidism), thymus gland functioning, adrenal functioning, and menopause-related conditions.141,142 Its mechanism of action is partly attributed to the herb’s ability to influence levels of monoamines, including serotonin, dopamine, and norepinephrine in the cerebral cortex, brainstem, and hypothalamus through inhibition of degradation enzymes and facilitation of neurotransmitter support in the brain. It also appears to prevent catecholamine release and camp elevation in the myocardium, to prevent depletion of adrenal catecholamines by acute stress, and to induce opioid peptide biosynthesis and activation of central and peripheral opioid receptors.140,147,148 Enhanced antitumor and antimetastatic activity has been demonstrated when R. rosea extract is combined with cyclophosphamide (an antitumor agent).140,149
Schisandra
This herb (spelled schisandra or schizandra) has an ancient history of use in China, where it is called wu wei zi, or five flavored fruit, because of it is said to possess the five flavors of classical Chinese medicine: sour, bitter, sweet, salty, and pungent. Because of this, it is held in high regard in the Chinese materia medica and is still widely used in TCM today.91,150 In the first century classic herbal compendium, the Divine Husbandman’s Classic of the Materia Medica (Shen Nong Ben Cao Jing), schisandra is classified among the superior medicines, purported to “prolong the years of life without aging,” increase energy (qi), treat fatigue, emaciation and langor, act as a male sexual tonic, and treat asthma.91,150,151 It was also considered antihepatotoxic, antidiabetic, antitussive, and is a sedative, tonic, and treatment for cholera. In combination with other herbs, its applications become much broader.91,151 The fruit is considered highly astringent, and is therefore used for a variety of secretory excesses, including night sweats, chronic diarrhea, and in males, spermatorrhea.150 Official indications for the fruit include diabetes, frequent urination, night sweats, chronic cough, and dyspnea.19,91 Schisandra was introduced from Eastern Russia into Europe in the 1850s.91 Since the 1950s, research in the former Soviet Union has focused on its potential uses as an adaptogen, primarily to enhance concentration and increase endurance, and its use is integrated into conventional medical and pharmacy practice in Russia.53,150 The primary active ingredients are lignans.91,150,152 It is official in the pharmacopoeias of China, Japan, Korea, and Russia. Current research has focused on its effects in treating diabetes and liver damage related to hepatic disease, for example, hepatitis.91,150 Hepatoprotective, antioxidant, antiproliferative and chemopreventive, anti-inflammatory, cardioprotective, and antimicrobial effects have all been demonstrated in in vivo and in vitro studies.91,150,153 Hepatoprotective and performance/endurance enhancing effects have been demonstrated in human clinical studies.91,154 Research into Schisandra’s use as an adaptogen was inspired by its TCM use as a tonic for debility. Numerous clinical trials conducted in the 1950s showed improvement in activities requiring concentration, coordination, and endurance, a reduction in fatigue, and an increase in accuracy and work quality.150 Uncontrolled trials suggest general improvements in mental efficiency in humans, with associated improvement in vision and hearing and skin receptor sensitivity.155 Animal models have demonstrated adaptogenic effects, including increased renal and gonadal RNA, glycogen and enzyme levels in older models (rabbits) compared with younger animals.19 In a double-blind, placebo-controlled RCT involving race and show-jump horses, treatment with schisandra reduced heart rate, respiratory frequency, and lactate levels, and increased plasma glucose and performance.19,150 Treated horses also completed the race faster than controls.150 Similar results demonstrating enhanced performance and recovery after exercise have been found in other studies involving racehorses.150 In an in vivo study with phenobarbital, ethanol, and ether intraperitoneal administration of schisandra reduced sleeping time, suggesting antidepressant activity.19,150 Several other studies have also suggested antidepressant activity, both in animal and human clinical models. One rat study showed a significant increase in dopamine and its metabolites in the rat brain. Stimulating effects on the CNS have been reported, including restlessness, increased aggressiveness, and insomnia at higher doses.150 Increased resistance to heat and frostbite have both been demonstrated, suggesting the ability of the herb to increase response to environmental stressors.150 Schisandra is considered to have a high safety profile, and appears to be entirely free of toxicity when used in the recommended dosage range.150 No clinical reports of overdosage in humans have been identified.91 According to TCM, schisandra should not be used in cases of excess heat, or in the early stages of a rash or cough.151 Patients with high gastric acidity or peptic ulcers may experience exacerbation.150 It is also considered contraindicated in patients with epilepsy, hypertension, and intracranial pressure.156 Side effects may include restlessness, insomnia, and dyspnea.91,151 Schisandra may increase uterine contractility, and is used in TCM to induce or promote prolonged labor; therefore, its use is not recommended during pregnancy.91,150,151
ADDITIONAL THERAPIES
Dehydroepiandrosterone
DHEA is active in the CNS, and is taken up by the amygdala, hippocampus, thalamus, midbrain, and frontal cortex.157 DHEA and DHEAS also appear to have neurotrophic effects, increasing the number of neurofilament-positive neurons and regulating the motility and growth of corticothalamic projections in cultured mouse embryo brain cells.158 DHEA and DHEAS output is maximal between the ages of 20 and 30 years and then declines with age at a rate of approximately 2% per year, leaving a residual of 10% to 20% of the peak production by the eighth or ninth decade of life. The exact mechanism of action and clinical role of DHEA and DHEAS remain unclear. Epidemiologic data indicate an inverse relationship between serum DHEA and DHEAS levels and the frequency of cancer, cardiovascular disease (in men only), Alzheimer’s disease and other age-related disorders, immune function, and progression of HIV infection.158
Beneficial effects of DHEA, based on animal studies, include improved immune function and memory and prevention of atherosclerosis, cancer, diabetes, and obesity. Clinically validated uses of DHEA include replacement therapy in patients with low serum DHEA levels secondary to chronic disease, adrenal exhaustion, or corticosteroid therapy; treating systemic lupus erythematosus (SLE), improving bone density in postmenopausal women; improving symptoms of severe depression; improving depressed mood and fatigue in patients with HIV infection; and increasing the rate of reepithelialization in patients undergoing autologous skin grafting for burns; however, such uses remain controversial.158 Supporting clinical studies also suggest possible benefit in enhancing immune response and sense of well-being in older adults, and a reduction in some cardiovascular risk factors. Other uses for DHEA, for example, for retarding the aging process, improving cognition, promoting weight loss, increasing lean muscle mass, and slowing the progression of Parkinson’s disease and Alzheimer’s disease are clinically unsubstantiated.158
DHEA supplementation has been found to elevate serum testosterone, estrone, and estriol levels in postmenopausal women. DHEA may lead to an increase in progesterone production indirectly, because both DHEA and progesterone require pregnenolone as a precursor. If the body does not have to manufacture DHEA, more pregnenolone may be shunted to progesterone synthesis. Physiologic replacement dosages of oral DHEA in healthy women over 40 range from 5 to 30 mg/day, and are usually given once in the morning. This is generally adequate to raise serum DHEAS to the levels found in adults 20 to 30 years of age and impart the documented benefits of heightened sense of well-being and increased bone mineral density in postmenopausal women.158 Higher doses may be necessary for increasing suppressed DHEA and DHEAS levels secondary to chronic disease, adrenal exhaustion, and corticosteroid therapy.158 Pharmacologic dosages of 200 mg/day have been successfully used in patients with SLE. Dosages of 200 to 500 mg/day have been used in HIV-positive patients with depressed mood and fatigue.158 With physiologic and supraphysiologic doses, the most common side effects include acne and mild hirsutism.158,159 Rakel suggests that dosing not exceed 50 mg/day of an oral dose regimen in non–adrenal deficient patients.23 As OTC sources of DHEA are not standardized, inconsistent dosing with insufficient daily dosing may be a problem.23
The long-term effects of significantly raised androgen levels in women using DHEA are unknown. A case control study of postmenopausal women not taking DHEA or hormone replacement therapy whose levels of endogenous DHEAS were in the highest quartile had a significantly higher risk of breast cancer than women whose levels of endogenous DHEAS were in the lowest quartile. The effects of long-term physiologic or supraphysiologic doses of DHEA on suppression of adrenal cortex are unknown; however, there does not appear to be feedback inhibition of DHEA or DHEAS secretion by the HPA axis.158 Baseline DHEAS should be checked prior to
initiating therapy and the serum DHEAS level should be checked at least annually to ensure that it is in the normal range.158 DHEA can affect (raising some, lowering others) serum levels of: calcium channel blockers, metformin, corticosteroids, insulin, and triazolam. Diet and exercise also can affect DHEA and DHEAS levels. DHEA supplementation is contraindicated in patients with a history (personal or family) of sex hormone–responsive cancers. DHEA supplementation should be avoided during pregnancy and lactation.158
Lifestyle and Reducing the Effects of Stress
Stress is an unavoidable fact of life. Its effects, however, can be mitigated. The effects of lifestyle on preventing and reducing stress, and consequently stress-related illness, cannot be overstated. The following are simple suggestions for managing stress:43