Endocrine Clinical Assessment and Diagnostic Procedures
Assessment of the patient with endocrine dysfunction is a systematic process that incorporates history taking and physical examination. Most of the endocrine glands are deeply encased in the human body. Although the placement of the glands provides security for the glandular functions, their resulting inaccessibility limits clinical examination. Nevertheless, the endocrine glands may be assessed indirectly. The critical care nurse who understands the metabolic actions of the hormones produced by endocrine glands assesses the physiology of the gland by monitoring that gland’s target tissue as listed in Figure 31-1 in Chapter 31. This chapter describes clinical and diagnostic evaluation of the pancreas, the posterior pituitary, and the thyroid gland.
History
The initial presentation of the patient determines the rapidity and direction of the interview. For a patient in acute distress, the history is curtailed to only a few questions about the patient’s chief complaint and precipitating events. For the patient without obvious distress, the endocrine history focuses on four areas: 1) current health status, 2) description of the current illness, 3) medical history and general endocrine status, and 4) family history. Data collection in the endocrine history for diabetes complications is outlined in Box 32-1.
Pancreas
Physical Assessment
Insulin, which is produced by the pancreas, is responsible for glucose metabolism. The clinical assessment provides information about pancreatic functioning. Clinical manifestations of abnormal glucose metabolism often include hyperglycemia, which is the initial assessment priority for the patient with pancreatic dysfunction.1,2 Patients with hyperglycemia may ultimately be diagnosed with type 1 or type 2 diabetes or be hyperglycemic in association with a severe critical illness.1,2 All of these conditions have specific identifying features. More information on the specific pathophysiology and management of each condition is available in Chapter 33.
Laboratory Studies
Blood Glucose
The fasting plasma glucose (FPG) level is assessed by a simple blood test after the person has not eaten for 8 hours. A normal FPG level is between 70 and 100 milligrams per deciliter (mg/dL).1 A fasting glucose level between 100 and 125 mg/dL identifies a person who is prediabetic.1 Even these individuals are at increased risk for complications of diabetes, such as coronary heart disease and stroke. A FPG level of 126 mg/dL (7 millimoles per liter [mmol/L]) or higher is diagnostic of diabetes (Table 32-1). In nonurgent settings, the test is repeated on another day to ensure that the result is accurate. After a meal, the concentration of glucose increases in the bloodstream. Recommended postprandial blood glucose levels should not exceed 180 mg/dL (10 mmol/L).1
TABLE 32-1
| PATIENT STATUS | FASTING BG (mg/dL) | FASTING BG (mmol/L) |
| Hypoglycemia | <70 | < 3.9 |
| Normal | 70-100 | >3.9-5.6 |
| Prediabetes | 100-125 | 5.6-6.9 |
| Diabetes | ≥126 | ≥7.0 |
BG, Blood glucose; mg/dL, milligram per deciliter; mmol/L, millimole per liter.
Data from American Diabetes Association. Standards of medical care in diabetes—2012, Diabetes Care. 2012;35(suppl 1):S11.
All critically ill patients must have their blood glucose levels monitored frequently while in the hospital. Clinical practice guidelines from the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA) recommend instituting insulin therapy when the blood glucose is greater than 180 mg/dL in critical illness.3 A target blood glucose range of 140 to 180 mg/dL is recommended.3
When a continuous insulin infusion is administered, point-of-care blood glucose testing is performed hourly or according to hospital protocol by the critical care nurse to achieve and maintain the blood glucose within the target range.3
Hypoglycemia is defined as a blood glucose level below 70 mg/dL (3.9 mmol/L).1,3 A complication of intensive glucose control is that hypoglycemic episodes may occur more frequently both in the hospital and with self-management of glucose levels in diabetes.3
Before discharge to home, patients with diabetes should be taught to monitor their blood glucose levels.4 Maintaining blood glucose within the normal range is associated with fewer long-term diabetes related complications.4 Laboratory blood tests and point-of-care or self-monitoring of blood glucose represent the standard of care for management of diabetes. Unfortunately, home monitoring of blood glucose is not the norm despite research evidence that maintaining blood glucose levels as close to normal as possible prolongs life and reduces complications.
Glycated Hemoglobin
Blood testing of glucose is useful for daily management of diabetes. However, a different blood test is used to achieve an objective measure of blood glucose over an extended period. The glycated hemoglobin test, also known as glycosylated hemoglobin (HbA1C or A1C) provides information about the average amount of glucose that has been present in the patient’s bloodstream over the previous 3 to 4 months. During the 120-day life span of red blood cells (RBCs; erythrocytes), the hemoglobin within each cell binds to the available blood glucose through a process known as glycosylation. Typically, 4% to 6% of hemoglobin contains the glucose group A1C. A normal A1C value is less than 5.4%, with an acceptable target level for diabetic patients below 6.5%.1,2,5 The A1C value correlates with specific blood glucose levels as shown in Table 32-2.1,2 The American Diabetes Association recommends use of the A1C value both during initial assessment of diabetes mellitus, and for follow-up to monitor treatment effectiveness.1
TABLE 32-2
CORRELATION BETWEEN HEMOGLOBIN A1c CONCENTRATION AND PLASMA GLUCOSE LEVEL
| HbA1c (%) | MEAN PLASMA GLUCOSE LEVEL (mg/dL) | MEAN PLASMA GLUCOSE LEVEL (mmol/L) |
| 6 | 126 | 7.0 |
| 7 | 154 | 8.6 |
| 8 | 183 | 10.2 |
| 9 | 212 | 11.8 |
| 10 | 240 | 13.4 |
| 11 | 269 | 14.9 |
| 12 | 298 | 16.5 |
HbA1c, Glycosylated hemoglobin; mg/dL, milligram per deciliter; mmol/L, millimole per liter.
Data from American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11.
Blood Ketones
Ketone bodies are a byproduct of rapid fat breakdown. Ketone blood levels rise in acute illness, fasting, and with sustained elevation of blood glucose in type 1 diabetes in the absence of insulin. In diabetic ketoacidosis (DKA), fat breakdown (lipolysis) occurs so rapidly that fat metabolism is incomplete, and the ketone bodies (acetone, beta-hydroxybutyric acid, and acetoacetic acid) accumulate in the blood (ketonemia) and are excreted in the urine (ketonuria). It is recommended that all patients with diabetes perform self-test, or have their blood or urine tested, for the presence of ketones during any alteration in level of consciousness or acute illness with an elevated blood glucose.6 A blood test that measures beta-hydroxybutyrate, the primary metabolite of ketoacidosis, provides the most accurate measurement.6,7 Self-test meters to measure blood ketones from a fingerstick are now available.6
Pituitary Gland
Laboratory Assessment
Serum Antidiuretic Hormone
Serum ADH levels are then compared with the blood and urine osmolality to differentiate syndrome of inappropriate antidiuretic hormone (SIADH) from central diabetes insipidus (DI). Increased ADH levels in the bloodstream compared with a low serum osmolality and elevated urine osmolality confirms the diagnosis of SIADH. Reduced levels of serum ADH in a patient with high serum osmolality, hypernatremia, and reduced urine concentration signal central DI. Chapter 33 provides more information about SIADH and DI.
Diagnostic Procedures
Computed Tomography
CT of the base of the skull identifies pituitary tumors, blood clots, cysts, nodules, or other soft tissue masses. This rapid procedure causes no discomfort except that it requires the patient to lie perfectly still. CT studies can be performed with radiopaque contrast or without contrast. The contrast dye is given intravenously to highlight the hypothalamus, infundibular stalk, and pituitary gland. This dye may cause allergic reactions in iodine-sensitive persons, and the patient must be carefully questioned about iodine allergy before the test. The size and shape of the sella turcica and the position of the hypothalamus, infundibular stalk, and pituitary are identified.8
Magnetic Resonance Imaging
MRI enables the radiologist to visualize internal organs and cellular characteristics of specific tissues. MRI uses a magnetic field rather than radiation to produce high-resolution, cross-sectional images. The soft brain tissue and surrounding cerebrospinal fluid (CSF) make the brain especially suited to MRI especially in cases of DI or SIADH.8
Thyroid Gland
Clinical Assessment
History
The history of a patient in the critical care unit should be as detailed as possible. Information regarding the clinical manifestations of hypothyroidism or hyperthyroidism must be obtained from the patient, family, or others with knowledge about the patient. Sample questions considered pertinent to detection of thyroid disease are provided in Box 32-2.
Physical Examination
The thyroid is palpated for tenderness, nodules, and enlargement and is auscultated for bruits. The normal-size thyroid gland usually is neither visible nor palpable in the anterior neck.9 Palpation may be done from an anterior or posterior approach. Auscultation of the thyroid is accomplished by use of the bell portion of the stethoscope to identify a bruit or blowing noise from the circulation through the thyroid gland. The presence of a bruit indicates enlargement of the thyroid, as evidenced by increased blood flow through the glandular tissue.
Laboratory Studies
Controversy exists about routine measurement of thyroid function in adults without clinical symptoms. The American Thyroid Association recommends thyroid function measurement in all adults beginning at age 35 years and every 5 years thereafter; more frequent screening is recommended for high-risk or symptomatic individuals. The U.S. Preventive Services Task Force (USPSTF) found the research evidence insufficient to either recommend or recommend against routine screening for thyroid disease in asymptomatic adults.10 No specific recommendations have been made with regard to thyroid function screening for critically ill patients.
Thyroid hormone blood tests measure the levels of circulating thyroid hormone and assess the integrity of the hormonal negative feedback response within the hypothalamic–pituitary axis. An inverse, linear relationship exists between TSH and T4.11 When the hypothalamic–pituitary axis is normal, TSH production is inhibited by the presence of normal thyroid hormone and the TSH value will be normal.11 Laboratory diagnosis of hyperthyroidism and hypothyroidism usually is based on the simultaneous measurement of thyroid-stimulating hormone (TSH) and free thyroxine (T4).12
• Hypothyroidism: high TSH and low serum T4
• Hyperthyroidism (thyrotoxicosis): very low TSH, high serum T4, and an increased T3:T4 ratio13
Thyroid Stimulating Hormone
Over the past decade, laboratory tests developed to analyze TSH have become much more sensitive, allowing more accurate measurement of low levels of thyroid hormone. Thyroid hormone reference ranges in adults are listed in Table 32-3.14 Because serum values vary slightly among laboratory methods, it is imperative to know the normal reference values used by the hospital laboratory.
TABLE 32-3
| NAME OF TEST | ABBREVIATION | REFERENCE VALUE* | REFERENCE VALUE* |
| Total serum thyroxine | TT4 | 58-160 nmol/L | 4.5-12.6 mcg/dL |
| Free thyroxine | T4 | 9-23 pmol/L | 0.7-1.8 ng/dL |
| Total serum triiodothyronine | TT3 | 1.2-2.7 nmol/L | 80-180 ng/dL |
| Free triiodothyronine | T3 | 3.5-7.7 pmol/L | 0.2-0.5 ng/dL |
| Thyroid-stimulating hormone (thyrotropin) | TSH | 0.4-4.0 mIU/L | |
| Thyroglobulin† | Tg | 3.0-40 mcg/L |
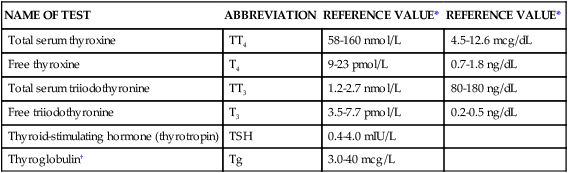
*Some tests are reported with more than one reference value because different laboratories report results using several reference scales.
†Thyroglobulin (Tg) reference values should be determined locally because serum Tg concentrations are influenced by iodide intake.
Reference values from Demers LM, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3.
The upper limit of normal for some of these values is under scrutiny. A U.S. national survey found the average level of TSH in the population in all ages to be only 1.49 milli-international unit per liter (mIU/L), considerably lower than most cited laboratory norms.11 The serum level of TSH increases as people grow older, which may signal declining thyroid function as more stimulation of the thyroid gland is required.11 The average TSH level varies by age:14
Because this age-adjusted increase is predictable, it is not recommended that laboratories use age-adjusted reference values when assessing thyroid function.11 Most asymptomatic people with a normal thyroid gland have a TSH level between 0.4 and 2.5 mIU/L.15
Medications and Thyroid Testing
Additional measurement difficulties involve concomitant use of certain medications that interfere with thyroid function and lower serum levels.17
TSH secretion is affected by several medications routinely administered in critical care units. Glucocorticoids in large doses may lower the serum level of T3 and inhibit TSH secretion.11 Dopamine infusions at greater than 1 mcg/kg/min directly blocks TSH release.17 Amiodarone, an antidysrhythmic medication is an iodine-rich compound that is structurally similar to T3 and T4. At usual doses, amiodarone contains 35 to 140 times the iodine recommended daily allowance (RDA) of 150 mcg per day.18
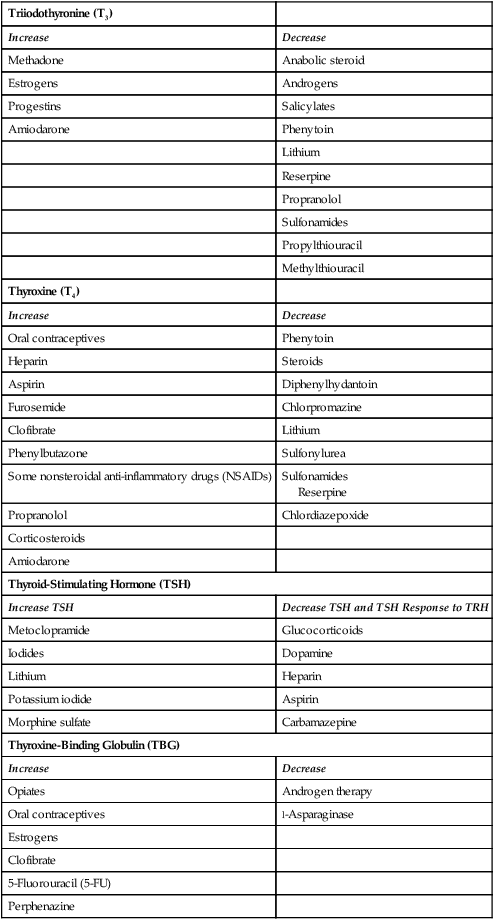
Several medications increase the serum level of T4 by displacing protein-bound T4.17 Medications that displace T4, including unfractionated and low-molecular-weight heparins, cause an increase in serum T4 levels.17 Salicylates (aspirin) and furosemide (Lasix) raise T4 serum levels by the same mechanism.17 A more complete list of medications that alter thyroid hormone serum levels is provided in Table 32-4. It is not clear whether it is necessary to adjust management of the critically ill patient in response to these laboratory findings.
TABLE 32-4
MEDICATIONS THAT INFLUENCE DIAGNOSTIC THYROID LEVELS
| Triiodothyronine (T3) | |
| Increase | Decrease |
| Methadone | Anabolic steroid |
| Estrogens | Androgens |
| Progestins | Salicylates |
| Amiodarone | Phenytoin |
| Lithium | |
| Reserpine | |
| Propranolol | |
| Sulfonamides | |
| Propylthiouracil | |
| Methylthiouracil | |
| Thyroxine (T4) | |
| Increase | Decrease |
| Oral contraceptives | Phenytoin |
| Heparin | Steroids |
| Aspirin | Diphenylhydantoin |
| Furosemide | Chlorpromazine |
| Clofibrate | Lithium |
| Phenylbutazone | Sulfonylurea |
| Some nonsteroidal anti-inflammatory drugs (NSAIDs) | Sulfonamides Reserpine |
| Propranolol | Chlordiazepoxide |
| Corticosteroids | |
| Amiodarone | |
| Thyroid-Stimulating Hormone (TSH) | |
| Increase TSH | Decrease TSH and TSH Response to TRH |
| Metoclopramide | Glucocorticoids |
| Iodides | Dopamine |
| Lithium | Heparin |
| Potassium iodide | Aspirin |
| Morphine sulfate | Carbamazepine |
| Thyroxine-Binding Globulin (TBG) | |
| Increase | Decrease |
| Opiates | Androgen therapy |
| Oral contraceptives | l-Asparaginase |
| Estrogens | |
| Clofibrate | |
| 5-Fluorouracil (5-FU) | |
| Perphenazine | |
Diagnostic Procedures
Diagnostic tests often begin with ultrasonography to visualize a nodule or tumor.19,20 To diagnose hypothyroidism, a nuclear medicine scan using an oral iodine radioactive isotope may be requested.21 The thyroid-scanning procedure may also detect the presence of ectopic thyroid tissue, thyroid carcinomas, and the amount of viable thyroid glandular tissue after therapeutic irradiation.
Adrenal Gland
Primary Adrenal Disorders
The adrenal gland is actually two glands in one, as described in Chapter 31, and this may make the history and presentation complex. The adrenal cortex (outer layer) secretes two classes of hormones, and if deficient or released in excess, they may cause clinical symptoms. Two hormones relevant to care of the critically ill are: 1) the glucocorticoid hormone cortisol and 2) the mineralocorticoid hormone aldosterone. Cortisol is secreted in response to physiologic stress as a result of infection, trauma, and hypoglycemia. Aldosterone is secreted in response to intravascular hypovolemia. It is the final step in renin–angiotensin–aldosterone system (RAAS) pathway.
Clinical Assessment
History
Adrenal Cortex
Primary Cushing Syndrome.
Cushing syndrome is caused by the excess release of the glucocorticoid hormone cortisol. Excess cortisol produces the classic signs and symptoms listed in Box 32-3. Primary Cushing disease is rare, but if a patient is not taking exogenous steroids, it becomes a diagnosis of exclusion when the relevant constellation of signs and symptoms are present.22
Secondary Cushing Syndrome.
Symptoms identical to those of primary Cushing syndrome (see Box 32-3) occur in patients with the secondary form who chronically take pharmacologic doses of glucocorticoids, for example, transplant recipients who take steroids to prevent solid organ rejection; patients with COPD, or those with chronic inflammatory conditions. When patients are admitted to the critical care unit, it is important to ascertain whether they are steroid dependent to avoid the deleterious effects of abrupt steroid withdrawal.
Primary Aldosteronism
In patients with primary aldosteronism, the adrenal cortex secretes excess mineralocorticoid (aldosterone) unrelated to the renin–angiotensin system.23 Several subtypes of this rare condition exist, but all may cause the patient to present emergently with severe hypertension and critically low serum level of potassium (hypokalemia), which can be lethal if not identified and effectively treated.
Critical Illness-Related Corticosteroid Insufficiency
The adrenal gland is designed to respond to acute physiologic stress. However, evidence suggests that the adrenal gland may become exhausted and no longer secrete adequate amounts of stress hormones in prolonged critical illness and in septic shock. The Society of Critical Care Medicine (SCCM) recommends that this condition be called critical illness–related corticosteroid insufficiency (CIRCI).24 Differentiating the signs and symptoms of adrenal insufficiency caused by shock from other causes by clinical examination alone is almost impossible in the critically ill, and CIRCI was probably an overlooked diagnosis in the past. The routine use of the corticotropin stimulation test in the presence of refractory vasopressor-dependent hypotension helps to make this diagnosis.24
Adrenal Medulla
Pheochromocytoma.
Pheochromocytomas are rare tumors that arise from the catecholamine-producing chromaffin cells of the adrenal medulla.25,26 Most produce norepinephrine, but some produce both norepinephrine and epinephrine.26 These tumors produce a far greater quantity of catechols than normal adrenal medullary tissue. The concentrations of catecholamines are so high within the tumor that it has have been likened to a volcano that can erupt at any time.26 When huge amounts of norepinephrine or epinephrine are released into the bloodstream, it creates a catecholamine storm that could be life threatening. The body responds as if to a severe fight-or-flight threat by hypertension, tachycardia, increased respiratory rate, and hyperglycemia. The patient may describe symptoms of headache, dizziness, palpitations, chest pain, anxiety, nervousness, and fatigue (Box 32-4).25,26 Because the body perceives the catecholamine onslaught to be a signal for the person to be ready to escape a threatening situation, it slows down the GI tract, and constipation is another symptom. Most pheochromocytomas are detected on autopsy rather than during life.27 The patients at greatest risk are those who are admitted to a critical care unit for another reason or are admitted in shock with an undiagnosed adrenal tumor.
Laboratory Studies
Adrenal Insufficiency and Critical Illness
For critically ill patients with refractory hypotension, especially if septic and hypotensive while on vasopressors after adequate fluid resuscitation, a diagnosis of CIRCI should be considered. To determine whether the adrenal gland can to respond to stress, the diagnosis is facilitated by the cosyntropin stimulation test (CST). Cosyntropin is the name for synthetic adrenocorticotropic hormone (ACTH). An initial measurement of total serum cortisol is obtained to establish a baseline value. If the baseline value is below 10 mcg/dL (in the presence of the clinical signs described previously), the patient is considered to have adrenal insufficiency. If after intravenous administration of a stimulation dose of 250 mcg of cosyntropin, the rise from baseline is less than 9 mcg/dL, the patient is considered to have adrenal insufficiency related to critical illness.24 This test is invalid if the patient has recently received steroids.24
Summary
Pituitary
• Normal serum ADH range is 1 to 5 pg/mL. Prior to ADH measurement, all medications that may alter ADH release are withheld for a minimum of 8 hours.
• Serum osmolality ranges from 275 to 295 mOsm/kg H2O. Urine osmolality ranges from 50 to 1400 mOsm/kg.
• Serum ADH levels are compared with blood and urine osmolality to differentiate SIADH from central DI.