62 Endocarditis
• Endocarditis is an inflammation of the endothelial lining of the heart. It is usually focal and commonly occurs at points of endocardial injury. The mitral and aortic valves are the most common sites of involvement.
• Most sites of endocardial injury become seeded with bacteria during episodes of transient bacteremia and thus develop into infective endocarditis.
• The initial symptoms are often vague—low-grade fever, malaise, and weakness.
• Manifestations can vary from direct structural cardiac injury to conduction system disturbances, embolic phenomena, and cardiogenic or septic shock.
• Suspicion of infective endocarditis should be raised by the presence of well-known risk factors, such as acquired or congenital valvular or structural heart disease, a prosthetic valve, implanted medical devices, injection drug use, and a previous history of endocarditis.
• Laboratory testing is often not useful for the emergency physician, but at least three sets of blood cultures performed over time are critical for the diagnosis of infective endocarditis, as well as for guiding subsequent therapy.
• The most useful initial diagnostic test is echocardiography.
• In an acutely ill patient, prompt resuscitation, antibiotics, and surgical consultation are imperative.
• In a stable patient with subacute disease, time until initiation of antibiotic therapy is less critical than performance of serial blood cultures.
• Nearly all patients with infective endocarditis require hospital admission. Only the most stable patients with no complications in whom the diagnosis of infective endocarditis is being entertained but not confirmed may be discharged with very close follow-up care.
• Despite medical advances, the overall mortality for both native valve and prosthetic valve infective endocarditis still ranges from 20% to 25%.1
• Prevention of disease is most important. In 2007 the American Heart Association issued revised guidelines for antibiotic prophylaxis in patients at risk for endocarditis.
Epidemiology
Over the last 30 years, published reports regarding the overall incidence of IE have conflictingly cited both a stable incidence and a rising incidence.1–3 Mortality ranges from 5% to 50% or higher. The reason for such variation in the statistics is that IE is a diverse and evolving disease entity—one that is strongly influenced by the characteristics of both the human and microbial populations being studied (Table 62.1).
Table 62.1 Statistics for Infective Endocarditis (IE) in the Developed World
| Median age of IE patients in the preantibiotic era | 30-40 yr |
| Median age of IE patients in the antibiotic era | 47-69 yr |
| Mean male-to-female ratio | 1.7-2.0:1 |
| Incidence of community-acquired native valve IE (western Europe/United States) | 1.7-6.2 cases per 100,000 person-years |
| Incidence of IE in persons with known mitral valve prolapse | 100 cases per 100,000 person-years |
| Incidence of IE in injection drug users | 150-2000 cases per 100,000 person-years |
| Prosthetic valve IE | 7-25% of all cases of IE |
| Overall mortality for both native and prosthetic valve IE | 20-25% |
| Mortality with viridans group streptococci and Streptococcus bovis IE | 4-16% |
| Mortality with enterococci IE | 15-25% |
| Mortality with Q fever IE | 5-37% |
| Mortality with Staphylococcus aureus IE | 25-47% |
| Mortality with Pseudomonas aeruginosa, Enterobacteriaceae, or fungal IE | >50% |
Adapted from Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med 2001;345:1318-30.
In the developed world, IE has undergone a remarkable transformation over the last century. In the developing world, however, it has remained rather unchanged. Much of this difference is a result of the influence of advances in health care (e.g., antibiotics, disease prevention, medical devices, the resulting longevity of populations), as well as the complications that arise from these advances (e.g., nosocomial infections and resistant organisms).4,5
Unfortunately, the tremendous advances made in health care have not translated into the gains that we have seen in other infectious diseases in the last 50 to 80 years. Untreated, IE has a mortality of nearly 100%. When treated, however, IE is still associated with a mortality rate of 20% to 25%.5 The overall incidence of IE in the developed world has remained unchanged.1 Why has the advent of antibiotics, advanced critical care and surgical techniques, and medical devices such as prosthetic valves not made a difference in this statistic? There are several reasons.
First, with a low prevalence, no pathognomonic signs or symptoms, and no single diagnostic front-line test, IE remains difficult to diagnose. Therefore, many cases are missed or diagnosed only when the disease is advanced. Second, despite the effective control of rheumatic heart disease in the developed world, new risk factors have arisen to fill the void. Degenerative heart disease in the growing elderly population has replaced rheumatic fever as the major cause of valvular disease. The same intravascular medical devices that have improved survival for patients (e.g., valvular prosthetics, cardiac pacemakers, long-term indwelling vascular catheters) predispose them to the development of IE (regardless of whether they have had IE in the past). Third, the number of patients at risk for IE has increased—the elderly, patients receiving critical care, and immunocompromised patients (because of acquired immunodeficiency syndrome, diabetes mellitus, end-stage renal disease, chemotherapy, and other reasons). Risky social behavior, such as body piercing and injection drug use, is practiced more today than in the early 20th century. Finally and most concerning of all, burgeoning antibiotic resistance is making treatment of IE more challenging and sometimes unachievable.6
Pathophysiology
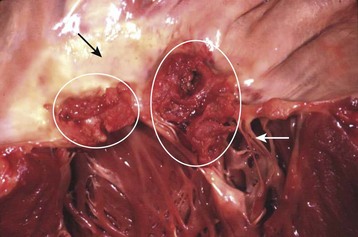
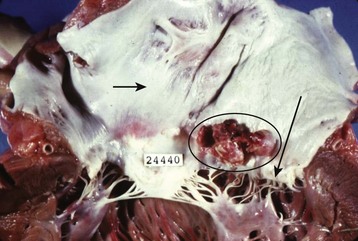
The term endocarditis literally means inflammation of the inner lining or endothelium of the heart or lining of heart valves (or both). Local or systemic stressors, such as trauma, blood-borne contaminants (e.g., talc from injection drug use), inflammation, and abnormal blood turbulence, induce injury to the endothelium. Clinically relevant endocarditis results from the formation of a fibrin and platelet cap on the area of altered surface endothelium. Most commonly, a sterile cap forms at a site of endothelial injury. IE occurs when microbes adhere to these sites of sterile endothelial injury during transient periods of bacteremia, fungemia, or viremia. Colonization occurs, followed by microbial multiplication and growth of each cap into a vegetation (Figs. 62.1 and 62.2). Because of their direct contact with the bloodstream, these infections cause a continuous, albeit low-level presence of microbes in the blood. The clinical manifestations of endocarditis are quite varied as a result of immunologic, infectious, and embolic processes. It is this variation in manifestations that often makes endocarditis difficult to identify.
Microbiology of Infective Endocarditis
Although the microbiology of IE can predict the course of a patient’s illness and guide therapy, the actual infecting organism is rarely known to the EP. The EP needs to know the microbes that cause IE (Box 62.1) and the local resistance patterns to make sound choices regarding empiric antibiotic treatment regimens. This section discusses the organisms most commonly associated with IE.6 Certain patient characteristics and clinical scenarios are associated with particular microorganisms (Table 62.2). These scenarios may guide the EP’s choice of empiric antibiotics; specific regimens are discussed later in this chapter (see Table 62.4).
Box 62.1
Microorganisms that Cause Infective Endocarditis (Approximate Percentage)
Adapted from Baddour LM, Wilson WR, Bayer AS, et al, for the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394-434; and Murdoch DR, Corey GR, Hoen B, et al, for the International Collaboration on Endocarditis—Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch Intern Med 2009;169:463-73.
Table 62.2 Characteristics of Patients with Infectious Endocarditis and Associated Microorganisms
| CHARACTERISTIC | ORGANISM | COURSE/FACTS* |
|---|---|---|
| Community-acquired IE involving a native valve | Viridans group streptococci |
GI, Gastrointestinal; IE, infective endocarditis.
* Data from Baddour LM, Wilson WR, Bayer AS, et al, for the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394-434.
Bacteria
Viridans Group Streptococci
Streptococcus viridans, formerly a species name, is actually a group of gram-positive cocci. This group has been the most common cause of IE, although more recent case series have shown that Staphylococcus aureus may now be more common.6,7 These streptococci usually seed previously damaged cardiac tissue. The clinical findings are usually more insidious, however, with patients experiencing malaise, weakness, and low-grade fever.
Staphylococcus aureus
Studies have now identified S. aureus rather than the viridans group of streptococci as the most common cause of IE.1,7 S. aureus can infect normal valvular endothelium—that is, endothelium without antecedent damage or disease—and usually causes aggressive valve destruction. It is associated with injection drug use, as well as with prosthetic valve endocarditis that occurs more than 1 month after surgery.
Over the last decade, the story of S. aureus and S. aureus IE has become increasingly complicated with the emergence of methicillin-resistant S. aureus (MRSA), as well as the subsequent identification of community-associated (CA-MRSA) and hospital-associated (HA-MRSA) subtypes. CA-MRSA has a tendency to affect previously healthy individuals but has a drug sensitivity pattern more favorable than that of HA-MRSA. HA-MRSA tends to affect the infirm (hospitalized, nursing home, elderly, preterm, and immunocompromised patients) and has a limited sensitivity pattern. A review of cases of native valve IE caused by these organisms reveals a higher mortality rate with HA-MRSA (37%) than with methicillin-sensitive S. aureus and CA-MRSA (23% and 13%, respectively).8
Culture-Negative Bacteria
The culture-negative bacteria group infrequently causes IE. These bacteria are characterized as culture negative because they either grow slowly in routine media, require special media to grow, or cannot be cultured. If clinical suspicion exists, the clinician must ask that blood cultures be held for a prolonged incubation period (14 to 21 days), request special culture media, or use the serologic and polymerase chain reaction assays available for some of these bacteria. A list of culture-negative bacteria is provided in Box 62.1.
Fungi
Fungi are rarely a cause of endocarditis, but fungal IE has high mortality. Candida species are responsible for most cases of fungal IE. Aspergillus species are also seen. Fungal IE tends to occur in patients with cardiac abnormalities, medical devices (prosthetic valves, long-term indwelling vascular catheters), some level of compromised immunity (human immunodeficiency virus, malignancy, organ transplantation), and injection drug use.9 Fungal IE usually produces large vegetations and is an indication for surgical intervention.
Presenting Signs and Symptoms
IE can vary greatly in the severity of its manifestations. Depending on the extent of the injury, location of the injury, microorganism involved, and comorbid conditions in the patient, IE can be an insidious chronic or subacute disease or an aggressive, rapidly debilitating process. Recent prospective cohort data from an international multicenter study have revealed that the acute manifestation is becoming more common—perhaps because of the increasing prevalence of S. aureus IE.7
EPs must maintain high clinical suspicion in situations associated with IE. Patients at high risk for IE are listed in Box 62.2. In such patients, sepsis, embolization, or cardiac failure or shock should warrant an evaluation for endocarditis. By understanding the pathophysiology of this disease, the clinician can predict the signs and symptoms that might be seen with IE.
Box 62.2
Risk Factors for Infective Endocarditis
Acquired or congenital valvular and structural heart disease, including mitral valve prolapse, rheumatic heart disease, and hypertrophic cardiomyopathy
Prosthetic valves, including bioprosthetic devices
Implantable medical devices (cardiac pacemakers, long-term indwelling vascular catheters, implantable defibrillators)
Adapted from Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med 2001;345:1318-30.
Classic Triad
The triad consisting of fever, heart murmur, and anemia has classically been ascribed to the diagnosis of IE. Unfortunately, the sensitivity and specificity of these findings for endocarditis are poor. The clinician must combine these findings with high-risk patient characteristics (see Box 62.2).
Organ-Specific Clinical Findings
Vascular Signs and Symptoms
The signs and symptoms that may be seen with involvement of specific vascular sites are as follows:
• Central nervous system (CNS) arteries—headache, focal neurologic deficits, confusion
• Sinus of Valsalva—pleuritic chest pain, muffled heart tones
• Hepatic artery—right upper quadrant pain, hematemesis
• Splenic artery—abdominal pain, intraabdominal hemorrhage
• Renal arteries—flank pain, hematuria
• Intestinal arteries—abdominal pain, intraabdominal hemorrhage, melena, hematochezia.
Ophthalmologic Signs and Symptoms
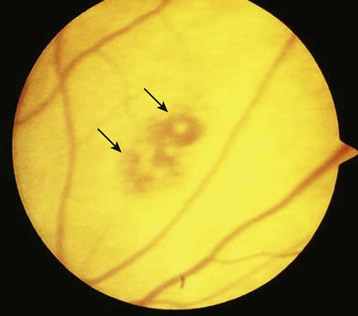
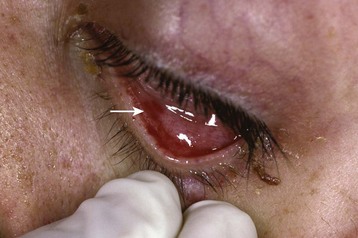
The eye is not immune to endocarditis. Both embolic and immune phenomena can affect the optic nerves, ophthalmic vessels, conjunctivae, and retina. Initial complaints may include painless conjunctival hemorrhages, visual field cuts, and even monocular blindness. Emboli can cause infarction of the ophthalmic or retinal vessels and lead to loss of vision. Hemorrhages with pale cotton-wool centers known as Roth spots can be visualized on the retina (Fig. 62.3). EPs should be aware that these spots are not pathognomonic for IE but rather, if seen, should raise suspicion for this disease. Painless subconjunctival hemorrhages (Fig. 62.4), or petechiae involving the conjunctivae, can also be present and again are not specific.
Dermatologic Signs and Symptoms
Janeway lesions are small, painless hemorrhages with a macular or slightly nodular character (Fig. 62.5). They are found on the thenar and hypothenar eminences of the palms and soles. The histologic findings are generally consistent with septic microemboli. Bacteria have been cultured from these lesions. Janeway lesions are usually present for days to weeks before healing. They are most often associated with acute IE from S. aureus.
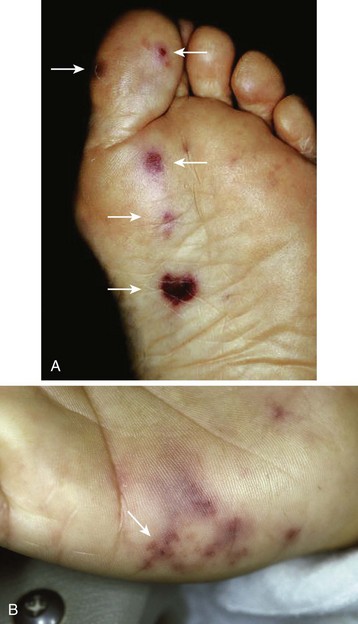
Fig. 62.5 Janeway lesions.
(From Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005; B, courtesy Marc E. Grossman, MD, FACP.)
Osler nodes are small, tender, red to purple nodules. They are most often found on the pulp of the distal phalanges of the fingers and toes, the soles, and the thenar and hypothenar eminences of the palms (Fig. 62.6). Preceding the appearance of the nodes, patients often experience neuropathic pain. Although these nodes were initially believed to be purely immunologic in nature, reports have now isolated bacteria from them. It has been postulated that early microembolization with microabscess formation is followed by an immune-mediated hypersensitivity vasculitis. Osler nodes can appear at any point in the course of IE and can last a few hours to several days. They tend to be associated with subacute IE.
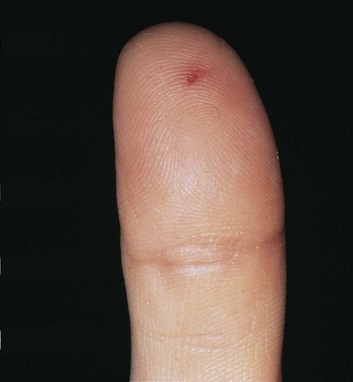
Fig. 62.6 Osler nodes.
(From Cohen J, Powderly WG, editors. Infectious diseases. 2nd ed. Philadelphia: Mosby; 2004.)
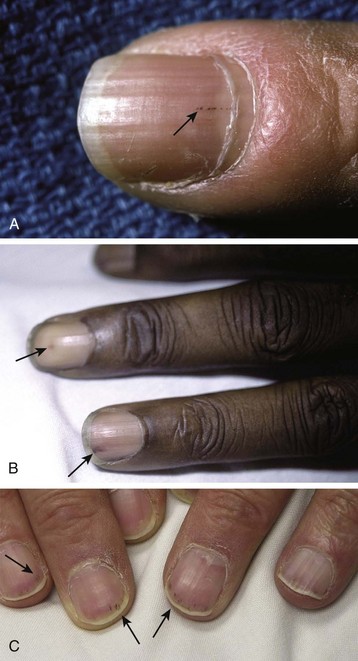
Splinter hemorrhages are linear petechiae visible on the nail beds of affected patients (Fig. 62.7). They do not blanch when pressure is applied to the nail and are seen better if a bright light is shone directly onto the distal tip of the digit.
Differential Diagnosis and Medical Decision Making
Differential Diagnosis
Because of the many clinical manifestations of IE, the list of differential diagnoses is overwhelmingly large. Broad areas include infectious and febrile illnesses, as well as cardiovascular, neurologic, psychiatric, GI, renal, dermatologic, and immunologic disorders (Box 62.3). Most interestingly, IE can lead to manifestations that are diagnoses; the EP must think past that initial diagnosis and identify IE as the underlying cause. For example, a cerebrovascular accident or myocardial infarction may be secondary to the embolization or mycotic aneurysms of IE; this fact is important because therapy must also focus on treating the IE.
Box 62.3 Partial Differential Diagnosis for Infective Endocarditis
As mentioned frequently in this chapter, high clinical suspicion guided by knowledge of risk factors (see Box 62.2) is the key to including IE in this long, complex list of differential diagnoses.
Medical Decision Making
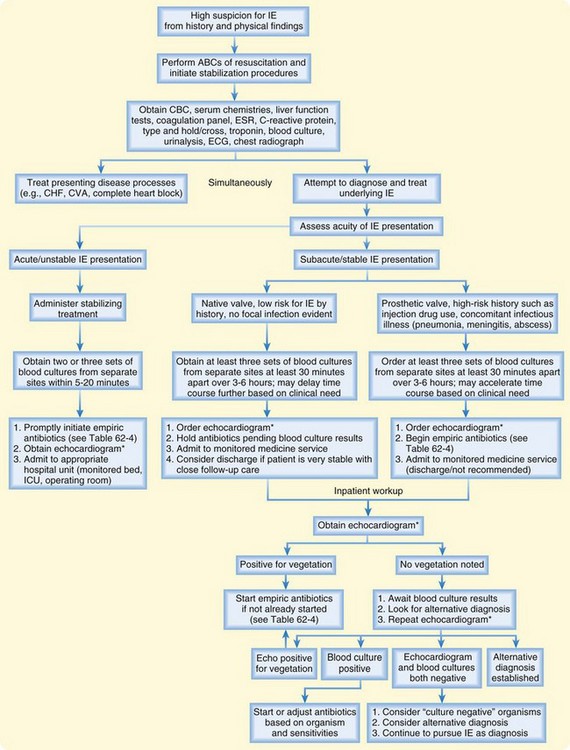
Contributing to the complexity of IE is the fact that there is neither a single rapid test to diagnose the disorder nor any routine ancillary test that hints at the diagnosis. Some physical findings might clue the EP to this diagnosis, but they are nonspecific. For the EP, the most important tool for diagnosing IE is clinical suspicion. Elements of the history known to identify patients at high risk for endocarditis must trigger this suspicion and lead the EP to focus on physical findings typical of endocarditis. When suspicious for IE based on the history and physical findings, the EP must order serial blood cultures and an echocardiogram to confirm the diagnosis. A diagnostic algorithm is presented in Figure 62.8.
The most accepted diagnostic schema for IE is the modified Duke criteria (Box 62.4).10 Unfortunately for the EP, this approach relies heavily on blood culture and echocardiography, the results of which are rarely available to EPs at the outset of care. With recent efforts to bring ultrasonography skills to the ED bedside, echocardiography may become more readily available as an initial evaluation tool, but for now, the procedure requires cardiology consultation and is often not available during the first few hours of care.
Box 62.4
Modified Duke Diagnostic Criteria for Infective Endocarditis
Stratification of Patients
A diagnosis of “definite” endocarditis is made in a patient with one of the following:
• Histologic and/or microbiologic evidence of infection at surgery or autopsy
A diagnosis of “possible” endocarditis is made in a patient with one of the following:
A diagnosis of endocarditis is “rejected” in a patient with one of the following:
Major Criteria
Blood Culture Results Positive for Infective Endocarditis
1. Typical microorganisms causing IE from two separate blood cultures in the absence of a primary focus:
2. Persistently positive blood culture results, defined as recovery of a microorganism consistent with IE from one of the following:
3. Single positive blood culture result for Coxiella burnetii or anti–phase 1 immunoglobulin G antibody titer > 1 : 800.
Evidence of Endocardial Involvement
1. Positive echocardiographic results for IE:
2. New valvular regurgitation. An increase or change in a preexisting murmur is not sufficient evidence.
Minor Criteria
Predisposition: Predisposing heart condition or intravenous drug use
Fever: Body temperature > 38.0° C (100.4° F)
Vascular phenomena: Major arterial emboli, septic pulmonary infarcts, mycotic aneurysms, intracranial hemorrhage, conjunctival hemorrhages, Janeway lesions
Immunologic phenomena: Glomerulonephritis, Osler nodes, Roth spots, rheumatoid factor
Microbiologic evidence: Positive blood culture result but not meeting the major criteria as noted above (excluding a single positive culture result for coagulase-negative staphylococci and organisms that do not cause endocarditis) OR serologic evidence of active infection with an organism consistent with IE.
Data from Li IS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633-8.
According to the modified Duke criteria (see Box 62.4), an echocardiogram positive for IE along with presence of three minor criteria would allow the EP to actually make a “definite diagnosis” of IE in the ED. In all other cases, an echocardiogram positive for IE enables the diagnosis to be “possible IE” as the clinician awaits the results of blood cultures or serologic analysis. It must be emphasized, however, that normal echocardiographic findings would not eliminate the diagnosis of IE, especially in the setting of high clinical suspicion (high-risk patient, strongly suggestive findings). Evaluation should continue until the diagnosis of IE is “rejected” according to the modified Duke criteria. This often means establishment of an alternative diagnosis or the finding of sterile blood cultures after appropriate incubation procedures (proper media and incubation times) and resolution of the illness.
Diagnostic Testing
Laboratory Tests
Less useful for the EP but part of the modified Duke criteria for IE are serologic tests. Rheumatoid factor may occasionally be found in patients with IE, particularly those with long-standing, indolent cases. Serologic assays can detect the presence of bacteria such as Coxiella, Brucella, Bartonella, Legionella, and Chlamydia. Polymerase chain reaction testing for specific DNA or RNA from blood, urine, or surgically excised tissue can be performed when the potential pathogen is slow growing or cannot be cultured by conventional methods.11
Microbiology
The EP must remember that antecedent antibiotic treatment can also result in negative blood culture results, so the history should include questions about such treatment.11 In a stable patient with a history of antecedent antibiotic therapy, serial blood culture specimens should be collected over a longer time (even days) before initiation of antibiotic therapy for IE.
Echocardiography
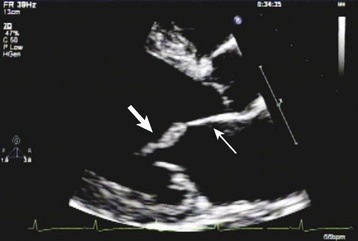
Echocardiography is the most important diagnostic tool available to the EP for the early identification of endocarditis and must be performed rapidly in all cases of suspected IE.6,12 It is one of the major criteria for the diagnosis of IE according to the modified Duke criteria (see Box 62.4 for echocardiographic findings that are diagnostic of IE10) (Fig. 62.9). In addition, echocardiography allows assessment of disease severity, complications, prognosis, and the need for surgical therapy. It is therefore a critical component in the initial assessment of a patient suspected of having IE.12,13
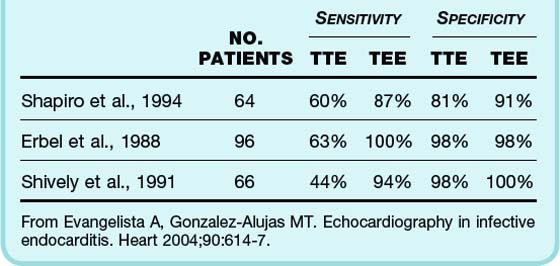
Transesophageal Echocardiography
Although it requires more preparation and is more invasive than TTE, transesophageal echocardiography (TEE) has greater sensitivity and specificity (Table 62.3). It can visualize smaller vegetations, as well as myocardial involvement such as abscesses and prosthetic valve vegetations. TEE is recommended for patients with the following findings:
• The TTE result is negative, but clinical suspicion for IE is high.
• The TTE result is positive, but there is concern for the presence of a high-risk complication of IE, such as large or mobile vegetations, significant valvular insufficiency, or a suggestion of perivalvular extension.
• The TTE result is suboptimal because of, for example, morbid obesity, mechanical ventilation, emphysema, or chest wall deformity.
Table 62.3 Comparison of the Sensitivity and Specificity of Transthoracic and Transesophageal Echocardiography for the Diagnosis of Cardiac Vegetations
In patients with high clinical suspicion for IE, a normal echocardiographic result is not sufficient to discontinue diagnostic assessment or treatment. TTE or TEE should be repeated within 7 to 10 days. TTE or TEE can be used subsequently to assess the response to treatment or progression of the disease. Please see Figure 62.10 for the echocardiography algorithm.6,12
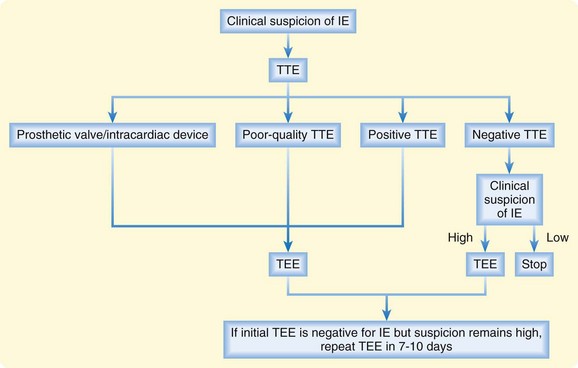
Fig. 62.10 Algorithm for the role of echocardiography in the diagnosis of infective endocarditis (IE).
TEE, Transesophageal endocardiography; TTE, transthoracic echocardiography.
(Modified from Habib, G, Hoen B, Tornos P, et al, for the ESC Committee for Practice Guidelines [CPG]. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis [new version 2009]: the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology [ESC]. Eur Heart J 2009;30:2369-413.)
Radiography
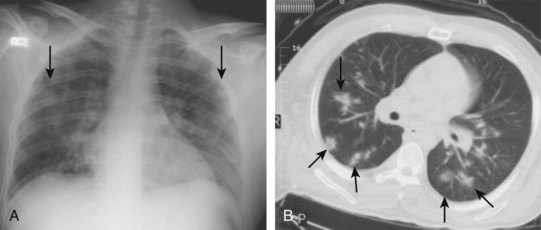
Chest radiographs are obtained routinely in most patients with cardiopulmonary symptoms, fever, or both. No specific finding on the chest radiograph is pathognomonic for IE. Multiple bilateral pulmonary infiltrates might be a clue that septic emboli may be present (Fig. 62.11, A).
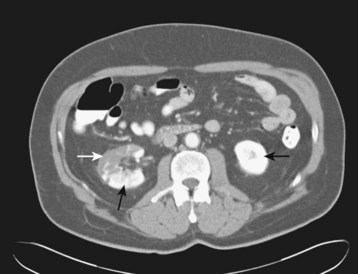
Chest CT may better identify multiple septic emboli, effusions, or pulmonary abscesses (see Fig. 62.11, B). Brain CT for the evaluation of patients with neurologic or neuropsychiatric symptoms may demonstrate ischemic or infectious foci. Contrast-enhanced abdominopelvic CT might identify ischemia or infarction of the intestines, liver, spleen, or kidneys (Fig. 62.12).
Treatment
Empiric Antibiotic Therapy
Unfortunately for the EP, the causative organism is rarely known because culture results are often not available yet. As with many infections, the EP must choose an empiric regimen. So how does the EP choose the empiric regimen? A group of organisms are known to cause IE (see Box 62.1). Certain of these organisms are more common, depending on the clinical scenario (see Table 62.2). Armed with this knowledge, the EP can tailor the empiric antibiotic regimen to the clinical scenario presented. Examples of such scenarios are native valve involvement, prosthetic valve involvement, injection drug use, history of allergy to antibiotics, prolonged hospitalization, admission to the intensive care unit, and a high prevalence of resistant organisms (Box 62.5). All these factors would influence the antibiotic regimen chosen.
Box 62.5 Clinical Scenarios to Help Tailor an Empiric Antibiotic Regimen for a Patient with Suspected Infective Endocarditis
Initial antibiotic therapy should always be parenteral and bactericidal.1 Because of growing antibiotic resistance, combination therapy with two or more agents should be used. Recommended empiric antibiotic regimens are listed in Table 62.4.
Table 62.4 Empiric Antibiotic Regimen for Presumed Infectious Endocarditis*†‡
IE, Infectious endocarditis; IM, intramuscularly; IV, intravascularly; PO, orally.
* Empiric therapy must be designed on the basis of clinical and epidemiologic clues.
† The duration of therapy varies with the microorganism and its drug sensitivities, the presence of prosthetic devices, and the response to therapy.
‡ The pediatric dose should not exceed the normal adult dose.
§ Penicillin G or ampicillin is added to this regimen because nafcillin/oxacillin and gentamicin may not be adequate coverage of enterococci.
 Aminoglycosides such as gentamicin should not be given as single daily doses.
Aminoglycosides such as gentamicin should not be given as single daily doses.
¶ Doses of vancomycin and gentamicin should be adjusted for reduced renal function, as well as measured serum concentration values.
** Rifampin increases the warfarin requirement for anticoagulation.
Data from Baddour LM, Wilson WR, Bayer AS, et al, for the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394-434.
Anticoagulation
Understanding the risks associated with anticoagulation in the setting of IE, what does the EP do with patients who would normally require anticoagulation? An excellent example would be a patient with IE and an anticoagulation-requiring prosthetic valve. The recommendation is that anticoagulation should be continued, barring the presence of acute hemorrhage, CNS infarction or hemorrhage, or a mycotic aneurysm. Any complaint that may result from these issues should be fully investigated to best inform decisions regarding anticoagulation. CT of the brain should be performed in any patient with possible CNS involvement. Any patient with unexplained abdominal or flank pain should undergo contrast-enhanced abdominal CT to evaluate for the presence of a mycotic aneurysm. Visual complaints warrant a complete funduscopic examination. If contraindications are identified, temporary discontinuation of anticoagulation is appropriate even in patients with a prosthetic valve.1,13,14 In these circumstances, close consultation with the appropriate specialty services is recommended.
Aspirin has not been shown to prevent embolic events but is probably associated with an increased risk for bleeding. It therefore has no role in early management of IE.15
Surgical Therapy
Other potential indications for surgery in patients with IE are failure of antibiotic therapy, vegetations larger than 10 mm on echocardiography, fungal endocarditis, early prosthetic valve endocarditis (within the first 2 months after surgery), and recurrent embolization despite medical therapy.14
Follow-Up, Next Steps in Care, and Patient Education
Nonbacterial Thrombotic Endocarditis
Endocarditis is not always associated with infection. In some disease states, vegetations composed of bland, platelet-fibrin aggregates may adhere to the endocardium. Eventually, fibrosis of these lesions occurs. These vegetations are usually sterile but may become seeded with infectious organisms. Illnesses associated with nonbacterial thrombotic endocarditis (NBTE) include malignancies, severe burns, hypercoagulable states (e.g., antiphospholipid syndrome and disseminated intravascular coagulopathy), uremia, and connective tissue diseases such as systemic lupus erythematosus.16
Endocarditis Prophylaxis
For more than 50 years, the American Heart Association (AHA) has set forth recommendations in an effort to prevent IE. Antimicrobial regimens were recommended for planned, potentially contaminated procedures (dental, respiratory, GI, and genitourinary) in an effort to prevent IE in at-risk populations. Unfortunately, these guidelines lacked good evidence to support their efficacy and were so complex that their implementation was difficult. In April 2007, the AHA released new guidelines in an effort to address these issues.17
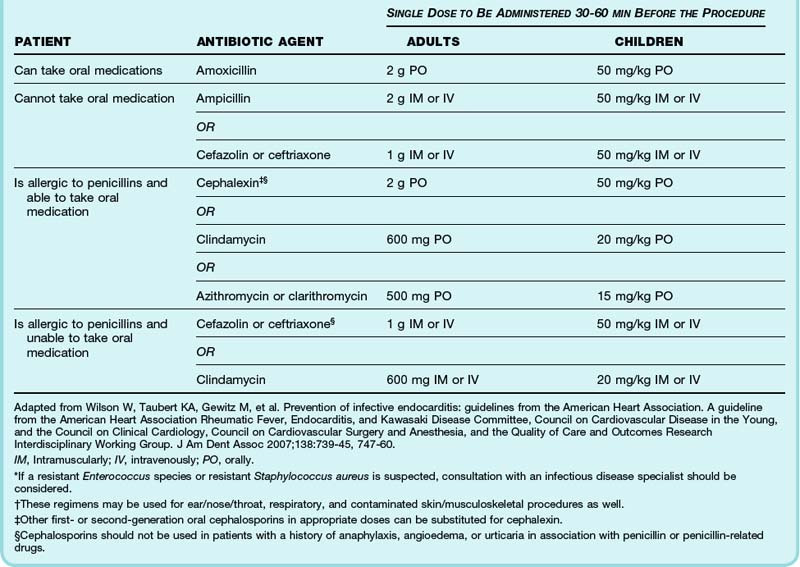
Rather than provide prophylaxis to patients at risk for the development of IE, the 2007 guidelines have changed to recommend that antimicrobial prophylaxis be administered only to patients at highest risk for an adverse outcome from IE (Box 62.6), thereby greatly reducing the group of eligible patients. The procedures for which prophylaxis is recommended are listed in Box 62.7 and antimicrobial regimens in Table 62.5.
Box 62.6
AHA Recommendations for Prevention of Infective Endocarditis
• Previous infective endocarditis
• Congenital heart disease (CHD)*
• Cardiac valvulopathy in a cardiac transplantation recipient
Adapted from Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc 2007;138:739-45, 747-60.
Box 62.7
Emergency Department Procedures Requiring Antimicrobial Prophylaxis Against Infective Endocarditis in High-Risk Patients
Patients at high risk for an adverse outcome from infective endocarditis (see Box 62.6) should receive antimicrobial prophylaxis (see Table 62.4) for the following procedures:
Dental Procedures
Minor Surgical Procedures
Modified from Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc 2007;138:739-45, 747-60.
EPs may encounter difficulty managing the expectations of patients who were previously recommended to receive IE prophylaxis but now are not. The rationale for the changes is the lack of scientific evidence to support the claim that IE prophylaxis is actually effective. In fact, the bacteremia resulting from daily activities such as toothbrushing and eating is more likely to result in IE than is the bacteremia associated with dental procedures. Therefore, even if 100% effective, prophylaxis probably prevents only an extremely small number of cases of IE. The cost of prophylaxis is borne financially18 and through adverse drug reactions. As a result, the AHA is now emphasizing maintenance of optimal oral health and hygiene as the means to reduce the incidence of IE related to daily activities, as well as dental procedures.17
Acknowledgment
We are indebted to Dr. Ahmet R. Sayan (cardiology) for his critical appraisal of this manuscript.
Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. for the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Circulation. 2005;111:e394–e434.
Habib G, Hoen B, Tornos P, et al. for the ESC Committee for Practice Guidelines (CPG). Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J. 2009;30:2369–2413.
Li IS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638.
Murdoch DR, Corey GR, Hoen B, et al. for the International Collaboration on Endocarditis—Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch Intern Med. 2009;169:463–473.
Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–1330.
Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc. 2007;138:739–745. 747–760
1 Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–1330.
2 Tleyjeh IM, Steckelberg J, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmstead County, Minnesota. JAMA. 2005;293:3022–3028.
3 Fowler VG, Jr., Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–3021.
4 Verheul HA, van der Brink RB, van Vreeland T, et al. Effects of change in management of active infective endocarditis on outcome in a 25-year period. Am J Cardiol. 1993;72:682–687.
5 Habib G. Management of infective endocarditis. Heart. 2006;92:124–130.
6 Baddour LM, Wilson WR, Bayer AS, et al. for the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394–e434.
7 Murdoch DR, Corey GR, Hoen B, et al. for the International Collaboration on Endocarditis—Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch Intern Med. 2009;169:463–473.
8 Millar BC, Prendergast BD, Moore JE. Community-associated MRSA (CA-MRSA): an emerging pathogen in infective endocarditis. J Antimicrob Chemother. 2008;61:1–7.
9 Pierrotti LC, Baddour LM. Fungal endocarditis, 1995–2000. Chest. 2002;122:302–310.
10 Li IS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638.
11 Habib G, Hoen B, Tornos P, et al. for the ESC Committee for Practice Guidelines (CPG). Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J. 2009;30:2369–2413.
12 Habib G, Badano L, Tribouilloy C, et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr. 2010;11:202–219.
13 Bayer AS, Bolger AF, Taubert KA, et al. Diagnosis and management of infective endocarditis and its complications. Circulation. 1998;98:2936–2948.
14 Bonow RO, Carabello B, De Leon AC, et al. ACC/AHA guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). J Am Coll Cardiol. 1998;32:1486–1588.
15 Chan KL, Dumesnil JG, Cujec B, et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol. 2003;42:775–780.
16 Eiken PW, Edwards WD, Tazelaar HD, et al. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985–2000. Mayo Clin Proc. 2001;76:1204–1212.
17 Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc. 2007;138:739–745. 747–60
18 Agha Z. Is antibiotic prophylaxis for bacterial endocarditis cost-effective? Med Decis Making. 2005;25:308–320.