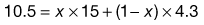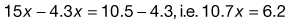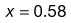Emulsions and creams
Gillian M. Eccleston
Chapter contents
Development of pharmaceutical emulsions
Emulsion theory related to pharmaceutical emulsions and creams
Selection of the emulsifying agent (emulsifier)
Emulsifying agents (emulsifiers)
Function of emulsifying agents
Classification of emulsifying agents
Natural macromolecular materials
Manufacture and processing of emulsions and creams
Identification of emulsion type
Stabilization by use of mixed emulsifiers
Key points
Introduction
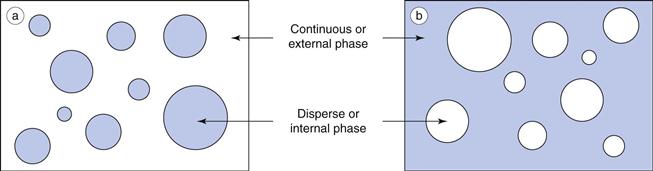
An emulsion is a dispersion of two immiscible (or partially miscible) liquids, one of which is distributed uniformly in the form of fine droplets (the dispersed phase) throughout the other (the continuous phase). The immiscible liquids are by convention described as ‘oil’ and ‘water’, as invariably one liquid is non-polar (e.g. an oil, wax or lipid) and the other is polar (e.g. water or aqueous solution). For simplicity and consistency, the terms ‘oil’ and ‘water’ are used in this context throughout this chapter.
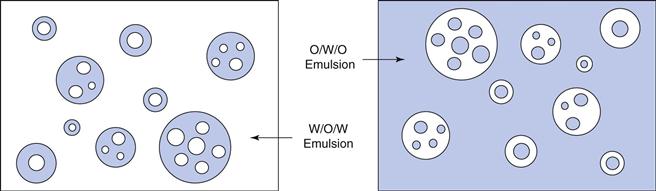
Oil-in-water emulsions (o/w) contain oil droplets dispersed in water, and water-in-oil emulsions (w/o) contain water droplets dispersed in oil (Fig. 27.1). Multiple emulsions can also be formed from oil and water by the re-emulsification of an existing emulsion to form two disperse phases. For example, multiple emulsions can be described as oil-in-water-in-oil (o/w/o). These are o/w emulsions which are further dispersed in an oil continuum. Conversely water-in-oil-in-water (w/o/w) type multiple emulsions can be prepared by further emulsification of a w/o emulsion in water (Fig. 27.2).
Emulsion formation
When two immiscible liquids are placed together in a container, they will form distinct layers with a minimum area of contact (interfacial area) between the two liquids. In this state, the surface free energy, G, is at a minimum. On mixing or mechanical agitation (i.e. input of energy) both liquids will form droplets of various sizes, thereby increasing the interfacial area between the liquids with a corresponding increase in the surface free energy of the system. Emulsions are therefore thermodynamically unstable.
The increase in surface free energy ΔG, brought about by the formation of droplets and the corresponding increase in surface area ΔA is given in Eqn 27.1 in which γ is the surface (or interfacial) tension.
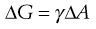 (27.1)
(27.1)
In order to reduce this surface free energy, the droplets assume a spherical shape; this gives a minimum surface area per unit volume. On contact droplets will coalesce (merge and re-combine) in an attempt to reduce the total interfacial area (and thus total surface energy, as indicated by Eqn 27.1).
Thus, emulsification can be considered to be the result of two competing processes that occur simultaneously. The first process requires energy input to disrupt the bulk liquids and form fine droplets thereby increasing the free energy of the system. The second process, which involves the coalescence of droplets, occurs spontaneously to reduce the interfacial area and minimize the free energy. If agitation ceases altogether, coalescence will continue until complete phase separation is obtained, the state of minimum free energy.
Droplet diameters vary enormously in pharmaceutical emulsions, but typically cover the range 0.1 µm (100 nm) to 25 µm. The visual appearance of an emulsion reflects the influence of droplet size on light scattering, and varies from transparent or translucent for emulsions composed of small nano-sized droplets (less than approximately 200 nm) to milky white and opaque for emulsions containing larger droplets.
Since emulsions are thermodynamically unstable, they will revert back to separate oil and water continuous phases unless kinetically stabilized by the addition of emulsifying agents (see sections below on ‘Emulsifying agents (emulsifiers)’ and ‘Emulsion stability’).
Partially miscible liquids
It should be noted, that when oil and water phases are partially miscible, droplet growth with eventual phase separation may occur by Ostwald ripening (see later) rather than coalescence. Ostwald ripening is an irreversible process which involves the growth of large droplets at the expense of smaller ones; it is considered later in this chapter in the section on ‘Emulsion stability’. Ostwald ripening does not require any contact between droplets and is an important mechanism of instability in sub-micrometre pharmaceutical emulsions.
Emulsions in pharmacy
Emulsions can be formulated for virtually all the major routes of administration, although most commercial products are developed for the oral, parenteral and topical routes. Oral and intravenous emulsions are almost exclusively of the o/w type, whereas dermatological emulsions, and emulsions for subcutaneous or intramuscular injection may also be formulated as w/o emulsions.
Medicinal o/w emulsions for oral administration have a long tradition of use to deliver medicinal oils for the local treatment of constipation (e.g. mineral oil, castor oil) and as oral food supplements (e.g. fish liver oils and vegetable oils) in a more palatable and acceptable form. The unpleasant taste of the oil is masked by the aqueous phase and any odour is suppressed when it is administered as the internal phase of an o/w emulsion.
Oil-in-water emulsions containing vegetable oils are also used for the oral delivery of drugs and vitamins of low aqueous solubility. Intestinal absorption is generally enhanced when an oily solution of drug is presented in the form of small sub-micrometre oil droplets, because of the larger interfacial area available for contact at the absorption site. Absorption is also generally faster and more complete than from suspension or tablet forms, because the drug in oral emulsions is already solubilized in the oil, thus eliminating the dissolution step prior to absorption.
Oral drug delivery using emulsions can be unpredictable because emulsions may become unstable in the low pH environment of the stomach. Emulsion concentrates, described as self-emulsifying drug delivery systems (SEDDS), are available commercially to minimize instability. SEDDS are composed of drug, oil(s), surfactants and sometimes co-solvents. They are not themselves emulsions, but form an emulsion on mild agitation in the aqueous environment of the stomach.
Sterile intravenous lipid o/w emulsions are used clinically as a source of calories and essential fatty acids for debilitated patients. Such emulsions (e.g Intralipid®) are also used as intravenous drug carriers for drugs of limited water solubility; marketed products are available for drugs such as diazepam (Diamuls®), propofol (Diprovan®), vitamin K (Phytonadione®) and docetaxol (Aventrix®). The advantages of such intravenous emulsions over solution formulations (in which the drug is solubilized by various co-solvents, and/or surfactants and/or pH control) include, a higher drug payload, lower toxicity, less pain on injection and protection of labile drugs by the oily environment.
Emulsions incorporating contrast agents (iodized oils, bromized perfluorocarbon oils) are used in diagnostic imaging including X-ray examinations of body organs, computed tomography and magnetic resonance imaging.
Water-in-oil emulsions administered by the subcutaneous or intramuscular routes can be used to prolong the delivery of water-soluble antigens and thus provide a longer lasting immunity. The antigen or drug must first diffuse from the aqueous droplets through the oily external phase before it reaches the tissues. Such emulsions are sometimes difficult to inject because of the high viscosity of the oily continuous phase. Multiple w/o/w emulsions, which are less viscous, have also been investigated for the prolonged release of drugs and vaccines incorporated in the innermost aqueous phase (see Chapter 36).
Dermatological emulsions are the largest class of emulsions used in pharmacy, and range in consistency from structured fluids (lotions, liniments) to semisolids (creams). Both oil-in-water and water-in-oil emulsions are extensively used as vehicles to deliver drugs to the skin, and for their therapeutic properties. Patient acceptance of such formulations is based on sensory attributes such as appearance, texture and ‘skin feel’. Water-in-oil emulsions tend to be greasy, and although this conveys a greater feeling of richness, w/o emulsions do not mix well with aqueous wound exudates and are also sometimes difficult to wash off the skin. They do however hydrate the skin by occlusion, an important factor in drug permeation. In contrast o/w lotions and creams readily mix with tissue exudates and are more easily removed by washing.
Dermatological emulsions (Chapter 39) facilitate drug permeation into and through the skin by occlusion, by the incorporation of penetration enhancing components and/or by evaporation on the skin surface. As most o/w creams are applied and rubbed onto the skin as a thin film, the drug delivery system is not the bulk emulsion, but rather a dynamic evaporating film in which the dissolution and partitioning environment alters as the relative concentrations of the volatile ingredients change. Rapid evaporation may temporarily supersaturate the film increasing thermodynamic activity and drug permeation.
Whilst dermatological emulsions and creams are two-phase systems, single-phase systems, including ointments and gels, are also available for topical application. These are described in Chapter 39.
Development of pharmaceutical emulsions
Although emulsions have many distinct advantages over other dosage forms, often improving bioavailability and reducing side effects, there are relatively few commercial oral or parenteral emulsions available. This comparative lack of usage is due to the fundamental problems of maintaining emulsion stability. Unstable emulsions are unsightly, give unpredictable drug-release profiles and may be toxic, for example droplet size increases in parenteral emulsions may cause thrombosis following injection. However, there is currently a large increase in research into all aspects of emulsions, although as yet there are few new products. This resurgence of interest, which is mainly focused on lipid emulsions for local or intravenous delivery, combines nano-science with the drive for cell-selective drug targeting and delivery.
Nanoemulsions
Nomenclature relating to nanoemulsions
It is necessary to spend a little time here considering the nomenclature of nanoemulsions as unfortunately there is some confusion in the literature and definitions may change.
Conventional emulsions (macroemulsions) and nanoemulsions.
According to the convention for nanoscale materials, nanoemulsions are defined in the wider literature as clear or transluscent emulsions containing droplet sizes typically below ~200 nm (0.2 µm). In the pharmaceutical literature however, confusion arises because the term nanoemulsion is sometimes used to include milky white emulsions containing droplets of up to 500 nm (0.5 µm) in diameter. In this chapter, milky white emulsions containing sub-microscopical droplets of less than a micrometre will be called colloidal, submicron (sub-micrometre) or ultrafine emulsions, whilst the term nanoemulsion will be reserved here for transparent emulsions containing droplet diameters less than ~200 nm.
Microemulsions and nanoemulsions.
The interchangeable use of the terms microemulsion and nanoemulsion is a more serious error that is becoming increasingly common in the pharmaceutical literature, causing confusion and inaccurate reporting. Although both microemulsions and nanoemulsions are clear and transparent, they are structurally quite different. Nanoemulsions are thermodynamically unstable dispersions of oil and water that contain individual small droplets. In contrast, so-called microemulsions are not emulsions. They are thermodynamically stable, single-phase systems that form spontaneously and have a number of different microstructures depending on the nature and concentration of the components (see also Chapter 5).
Properties of nanoemulsions
Nanoemulsions are relatively stable physically, as the droplets do not collide as frequently as in ordinary emulsions and their small droplet sizes enable them to penetrate deep into the tissues through fine capillaries. Thus, such emulsions are being investigated extensively as drug carriers and for their ability to target specific sites in the body including the liver and the brain. The surface properties of emulsions can be modified by controlling the charged nature of the interfacial film or by incorporating homing devices into the film to target specific tissues and organs after injection.
Negatively charged droplets are cleared more rapidly from the blood than neutral or positively charged ones. Lipid emulsions modified with apo-E specifically target the paranchymal cells of the liver and cationic emulsions complexed with plasmid DNA show promise in gene delivery.
Positively charged (cationic) nanoemulsions have also been shown to improve skin permeation of poorly soluble antifungal drugs and ceramides due to their interaction with the negatively charged skin epithelia cells. Water-in-oil nanoemulsion formulations are under investigation in cancer chemotherapy for prolonging drug release after intramuscular or intratumoral injection, and as a means of enhancing the transport of anticancer agents via the lymphatic system.
Emulsion theory related to pharmaceutical emulsions and creams
The classical theories of emulsification for simple two-phase oil and water model emulsions based on droplet interactions and interfacial films are considered in Chapter 5. However commercial pharmaceutical emulsions (even dilute mobile fluids for intravenous administration) are rarely such simple oil and water systems. They are more often complex multiphase emulsions containing additional phases (e.g. liquid crystalline) to oil and water. A unified theory of emulsification cannot be applied quantitatively to such multiphase emulsions, which range in consistency from mobile or structured fluids to soft or stiff semisolids.
Formulation of emulsions
When formulating a pharmaceutical emulsion, the choice of oil, emulsifier and emulsion type (o/w, w/o or multiple emulsion) will depend on the route of administration and its ultimate clinical use. The formulator must optimize processing conditions as these control droplet size distributions and rheology, which in turn influence emulsion stability and therapeutic response. The potential toxicity of all the excipients, their cost and possible chemical incompatibilities in the final formulation must also be identified. It is sometimes difficult to isolate these effects in practical emulsions as each is dependent on, and influenced by, the other. Thus ingredient selection is made often by trial and error and is dependent on the experience of the formulator.
Selection of the oil phase
The oil used in the preparation of pharmaceutical emulsions may be the medicament itself or it may function as a carrier for a lipid-soluble drug. The selection of the oil phase will depend on many factors including the desired physical properties of the emulsion, the miscibility of the oil and aqueous phases, the solubility of the drug (if present) in the oil and the desired consistency of the final emulsion. Some oils, in particular unsaturated oils of vegetable origin, are liable to auto-oxidation and become rancid, and so antioxidants or preservatives must be incorporated into the emulsion to inhibit this degradation process.
For externally applied emulsions, oils based on hydrocarbons are widely used. Liquid paraffin, either alone or combined with soft or hard paraffin, is used in numerous dermatological lotions and creams, both as a vehicle for the drug, and for the occlusive and sensory characteristics imparted when the emulsion is spread onto the skin. Turpentine oil, benzyl benzoate and various silicone oils are examples of other externally applied oils that are formulated as emulsions.
In oral emulsions, the most widely used medicinal oils are castor oil and liquid paraffin, which are non-biodegradable and provide a local laxative effect in the gastrointestinal tract, fish liver oils (e.g. cod or halibut) that are high in vitamins A and D or various fixed oils of vegetable origin (e.g. arachis oil) as nutritional supplements. Vegetable oils are also used as drug carriers as they are readily absorbed in the gastrointestinal tract. The oil phase is rarely inert, as it may influence bioavailability by its influence on gastric emptying time.
The choice of oil is severely limited in emulsions for parenteral administration, as many are inherently toxic. Although purified mineral oil is used in some water-in-oil depot preparations for intramuscular injection, where its potential toxicity (e.g. abscess formation at the injection site) is balanced against efficacy, it is too toxic to be incorporated into intravenous emulsions. A range of purified vegetable oils have been used, almost exclusively over many years in lipid emulsions for parenteral nutrition and as intravenous carriers for drugs of limited aqueous solubility.
The purified vegetable oils used in parenteral products comprise mixtures of long-chain triglycerides (LCTs) containing C12-C18 saturated and unsaturated fatty acid moieties, mainly oleic, linoleic, palmitic and stearic acids. Although a large number of vegetable oils have been investigated as possible stable, non-toxic oils for use in lipid emulsions, most commercial products contain soya bean or safflower oils because of their high content of the essential fatty acid, linoleic acid. Medium chain triglycerides (MCTs) which contain shorter fatty acid moieties (~C6-C10) are obtained by the re-esterification of fractionated coconut oil fatty acids (mainly capric and caprylic) with glycerol. These provide a more rapidly available source of energy, as well as enhancing the solubilizing capacity for lipid soluble drugs, including ciclosporin.
Mixtures containing both long and medium chain triglycerides have been adopted in some commercial preparations (see Table 27.1). Structured triglycerides, formed by modifying the oil enzymatically to produce 1,3-specific triglycerides with a mixture of long chain and medium chain fatty acids within the same molecule are under investigation as possible alternatives to physical mixtures of LCTs and MCTs.
Table 27.1
Selected commercial lipid emulsions for parenteral nutrition
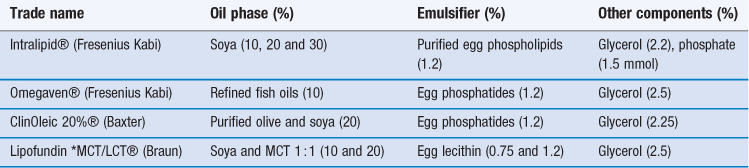
*MCT = medium chain triglyceride; LCT = long chain triglyceride
Emulsified perfluorochemicals are also considered acceptable for intravenous use provided that they are excreted relatively quickly. A major problem in the formulation of the early perfluorocarbon emulsions as blood substitutes was that the oils that formed the most stable emulsions were not cleared rapidly from the body.
Selection of the emulsifying agent (emulsifier)
Emulsifiers are used to control emulsion stability during a shelf-life that can vary from days for extemporaneously prepared emulsions, to months or years for commercial preparations. In practice, combinations of emulsifiers rather than single agents are generally used. The choice of emulsifier depends on the type of emulsion to be prepared, emulsifier toxicity (or irritancy if applied to the skin) and potential cost and availability. The final clinical use of the emulsion is also an important consideration, as emulsifiers control the in-vivo fate of emulsions by their influence on droplet size distribution, and the charge and surface properties of the individual droplets.
The functionality and types of emulsifying agent are of such importance to the properties of the emulsion that emulsifiers are considered in a separate major section below headed ‘Emulsifying agents (emulsifiers)’.
Other excipients
Preservatives
The aqueous continuous phase of an oil-in-water emulsion can produce ideal conditions for the growth of bacteria, moulds and fungi. The potential sources of contamination may be from the water used, the raw materials (especially if these are natural products), the manufacturing and packaging equipment, or introduced by the patient during use. Such contamination, which may constitute a health hazard, can also affect the physicochemical properties of the formulation, causing colour, odour or pH changes and even phase separation. Water-in-oil emulsions are less susceptible to such contamination because the aqueous phase is essentially enclosed and protected by the oil.
An ideal preservative should exhibit a wide spectrum of activity against bacteria, yeasts and moulds; it should be free from toxic, irritant or sensitizing activity (Chapter 50). Large-volume injectable fat emulsions do not contain preservatives and sterilization is achieved by autoclaving without a preservative. Phenoxyethanol, benzoic acid, and the parabenzoates are used as preservatives in oral and topical emulsions. The preservative will partition between the oil and aqueous phases, with the oil phase acting as a reservoir. Aqueous pH is an additional factor to be considered, as a sufficient concentration of the unionized form must be present to ensure proper preservation. Compatibility problems can occur between emulsifiers and preservatives, for example polyoxyethylene non-ionic surfactants emulsifiers and phenolic preservatives, not only destroying their microbial activity but also the emulsification properties of the surfactant.
Antioxidants and humectants
Antioxidants are added to some emulsions to prevent oxidative deterioration of the oil, emulsifier or the drug itself during storage. Such deterioration imparts an unpleasant, rancid odour and taste. Some oils are supplied containing suitable antioxidants. Antioxidants commonly used in pharmacy include butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) at concentrations up to 0.2%, and the alkyl gallates, which are effective at very low concentrations (0.001-0.1%). Alpha-tocopherol is added to some commercial lipid emulsions to prevent peroxidation of unsaturated fatty acids.
Humectants, such as propylene glycol, glycerol and sorbitol at concentrations up to 5%, are often added to dermatological preparations to reduce the evaporation of the water from the emulsion during storage and use. However, high concentrations may also remove moisture from the skin, causing dryness.
Emulsifying agents (emulsifiers)
Function of emulsifying agents
The function of an emulsifying agent (emulsifier) is to maintain the dispersion state of the emulsion for an extended period of time after the cessation of agitation, i.e. to impart kinetic stability to the emulsion. The dispersed droplets do not retain their initial character because the emulsion becomes thermodynamically stable (for the free energy is still high) but rather because the added emulsifiers inhibit or delay the processes of coalescence and Ostwald ripening (described below).
Emulsifiers generally impart time-dependent stability by the formation of a mechanical or electrostatic barrier at the droplet interface (an interfacial film) or in the external phase (a rheological barrier). The formation of interfacial films by adsorption of the emulsifier at the oil/water interface has been discussed in Chapter 5.
The interfacial film may increase droplet-droplet repulsion by the introduction of electrostatic or steric repulsive forces to counteract the van der Waals forces of attraction. Electrostatic repulsions are important in o/w emulsions stabilized by ionic emulsifiers, whereas steric repulsive forces, which arise when hydrated polymer chains approach one another, dominate with non-ionic emulsifiers and in w/o emulsions. The interfacial film may also provide a mechanical barrier to prevent droplet coalescence, particularly if it is close packed and elastic. Generally, mixtures of emulsifiers provide stronger interfacial films. Surfactant emulsifiers lower the interfacial tension between the oil and water. Although this facilitates the formation of droplets during emulsification and reduces the thermodynamic tendency for coalescence, interfacial tension reduction is not a major factor in maintaining long-term stability.
Interfacial films do not have the dominant role in maintaining stability in many practical emulsions in which the external phase is thickened by the emulsifier, i.e. in which the emulsifier significantly increases the viscosity of the continuous phase. In these, the structured continuous phase forms a rheological barrier which prevents the movement and hence the close approach of droplets. Emulsifiers that thicken the external phase but do not form an interfacial film are variously described as auxiliary emulsifiers, co-emulsifiers or viscosity enhancers. Many pharmaceutically important mixed emulsifiers, including lecithin and the emulsifying waxes, form interfacial films at low concentration and also structure the external phase at higher concentrations by the formation of additional lamellar liquid crystalline (with lecithins) or crystalline gel network phases (with emulsifying waxes).
Emulsion type
The type of emulsion that forms (whether o/w or w/o or multiple emulsion) and droplet size distribution depend on a number of interrelated factors, including the method of preparation (energy input), the relative volumes of the oil and water phases and the chemical nature of the emulsifying agent. When oil and water are mixed vigorously in the absence of an emulsifier, droplets of both liquids are produced initially, with the more rapidly coalescing droplets forming the continuous phase. Generally this is the liquid present in the greater amount because the greater number of droplets formed increases the probability of droplet collision and subsequent coalescence. With the inclusion of an emulsifier, the type of emulsion that forms is no longer a function of phase volume alone, but also depends on the relative solubility of the emulsifier in the oil and water phases. In general, the phase in which the emulsifying agent is more soluble (or in the case of solids, more easily wetted by) will form the continuous phase. Thus, hydrophilic surfactants and polymers promote o/w emulsions and lipophilic emulsifiers (with low HLB, see ‘Emulsifier selection’ section below) promote w/o systems.
Theoretically, the disperse phase of an emulsion can occupy up to a maximum of 74% of the phase volume. Whilst such high internal phase o/w emulsions stabilized by suitable emulsifiers have been produced, it is more difficult to form w/o emulsions with greater than 50% disperse phase because of the steric mechanisms involved in their stabilization. In practice, pharmaceutical emulsions usually contain 10-30% disperse phase.
Classification of emulsifying agents
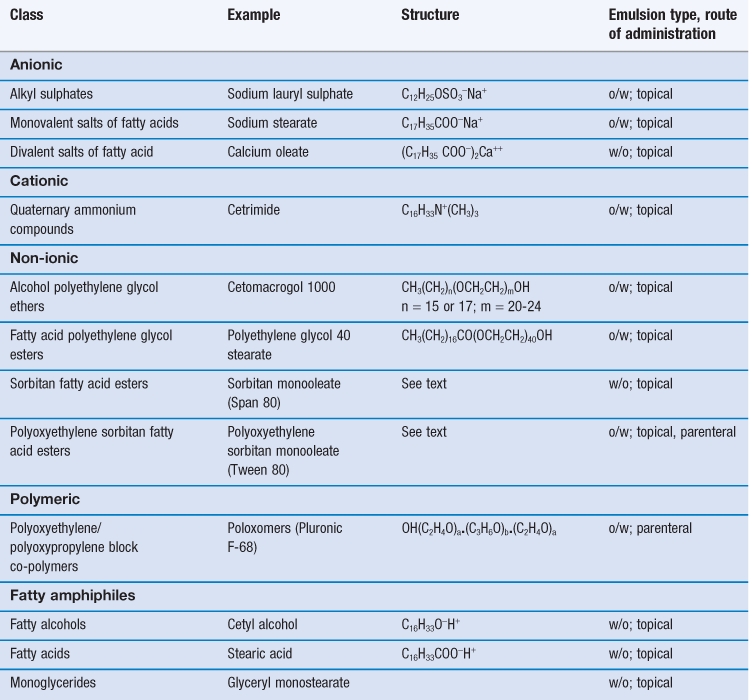
Emulsifying agents may be classified into two groups (i) synthetic or semi-synthetic surface active agents and polymers and (ii) naturally occurring materials and their derivatives. Examples of typical pharmaceutical emulsifying agents are shown in Tables 27.2 and 27.3.
Table 27.3
| Class | Example | Emulsion type; route of administration |
| Polysaccharide | Acacia | o/w; oral |
| Methylcellulose | o/w; oral | |
| Phospholipid | Purified lecithins | o/w; oral, parenteral |
| Sterol | Wool fat | w/o; topical |
| Cholesterol and its esters | w/o; topical | |
| Finely divided solid | Bentonite | o/w and w/o; topical |
| Aluminium hydroxide | o/w, oral |
Surface active agents and polymers
Surface active agents (surfactants for short) are further classified as ionic (i.e. anionic or cationic) or non-ionic according to their characteristics on dissociation. Most are mixtures of long chain homologues having hydrocarbon chain lengths between 12-18 carbon atoms with a hydrophilic head group. Their emulsifying power is influenced by batch variations in the homologue composition, with pure homologue surfactants proving to be very poor emulsifiers.
There are an enormous number of synthetic surfactants available commercially, and they form by far the largest group of emulsifiers studied in the general scientific literature. Unfortunately, the majority of the synthetic surfactants are toxic (many are haemolytic) and irritant to the skin and the mucous membranes of the gastrointestinal tract. In general, cationic surfactants are the most toxic and irritant and non-ionic surfactants the least. Thus, for pharmaceutical emulsions, ionic synthetic surfactants are used only in external topical preparations where they are present at relatively low concentration. Both ionic and non-ionic surfactants are combined with fatty alcohols to produce anionic, cationic or non-ionic emulsifying waxes, which are used to both stabilize and structure aqueous lotions and creams. A limited number of non-ionic surfactants (e.g. the polysorbates, discussed below) are also used internally in oral and parenteral emulsions, although lecithin (a mixture of anionic and neutral phospholipids) is the main emulsifier in commercial lipid emulsions. The non-ionic block co-polymer poloxomer 188 (Pluronic® F68) has been used in perfluorochemical emulsions for intravenous infusion, although some patients are sensitive to this emulsifier.
Anionic surfactants
Anionic surfactants dissociate at high pH to form a long-chain anion with surface activity. Emulsifying properties are lost and emulsions are unstable in acid conditions and in the presence of cationic materials, such as cationic surfactants and polymers. Examples of anionic surfactants include:
Alkyl sulphates.
Sodium lauryl (dodecyl) sulphate is the most widely used surfactant in topical products. The commercial sulphate is actually a mixture containing predominantly the C12 homologue, but also contains some C14 and C16 homologues. Sodium lauryl sulphate alone is a weak emulsifier of the o/w type, but forms a powerful o/w blend when it is used in conjunction with cetostearyl alcohol.
Monovalent salts of fatty acids.
Emulsifiers in this group consist mainly of the alkali salts of long chain fatty acids, e.g. C17H35COO−X+, where X may be Na, K, NH4 or triethanolamine (TEA). Alone, these ‘soaps’ promote rather unstable, mobile o/w emulsions, but when combined with fatty acids they form powerful o/w emulsifying blends that stabilize a number of dermatological products.
In many formulations, the ‘nascent soap’ method of preparation is used, in which soap is formed in situ from the partial neutralization of a fatty acid (which may be a component of the oil phase) with the appropriate alkali. For example, in white liniment, ammonium oleate is formed in situ from the reaction between ammonia solution and oleic acid. Triethanolamine soaps, formed in situ by the partial neutralization of fatty acid (generally stearic acid) by TEA have a long history of use in the formulation of cosmetic and pharmaceutical o/w vanishing creams.
Divalent salts of fatty acids.
Calcium salts of fatty acids containing two hydrocarbon chains form w/o emulsions due to their limited solubility in water. These are generally formed in situ by the interaction of calcium hydroxide with a fatty acid. In zinc cream, calcium oleate is formed in situ from the interaction between oleic acid and calcium hydroxide. This approach is also used in some formulations of oily calamine cream, in which oleic acid and some of the free fatty acid component of arachis oil are partially neutralized by calcium hydroxide to form a calcium oleate/oleic acid mixed emulsifier.
Cationic surfactants
Cationic surfactants dissociate at low pH to form a long-chain surface-active cation. Emulsions containing cationic surfactant as emulsifier are unstable at high pH and in the presence of anionic materials including anionic surfactants and polymers.
Quaternary ammonium compounds.
These constitute an important group of cationic emulsifiers in dermatological preparations because they also have antimicrobial properties. Cetrimide (cetyltrimethyl ammonium bromide) is blended with cetostearyl alcohol to form cationic emulsifying wax which is the mixed emulsifier used in cetrimide cream.
Non-ionic surfactants
There are an enormous number of non-ionic surfactants available commercially with different oil and water solubility producing either o/w emulsions or w/o emulsions. Non-ionic surfactants are particularly useful as emulsifiers because they are less toxic and irritant than ionic surfactants, and therefore a limited number (e.g. polysorbate 80; Tween® 80) are used in parenteral and oral products. In addition, non-ionic surfactants do not ionize to any extent and thus are more resistant than ionic surfactants to changes in pH and the presence of electrolytes and polyvalent ions. Most non-ionic surfactants are based on:
• a hydrophobic moiety with 12-18 carbon atoms. The starting material may be a fatty acid or sorbitan.
For each starting material, the polyoxyethylene chain can be modified and water solubility increased by the systematic addition of ethylene oxide.
Polyoxyethylene glycol ethers (macrogols).
These are a series of non-ionic surfactant condensation products of fatty alcohols with hydrocarbon chain lengths from C12-C18 and polyethylene glycol. They are used as both o/w and w/o emulsifiers as their oil and water solubility can be controlled by altering both the length of the hydrocarbon chain and the length of the polyoxyethylene (POE) chain. The most widely used emulsifier in this class is cetomacrogol 1000 (Table 27.2) which is used combined with cetostearyl alcohol to stabilize o/w lotions and creams, including the official Cetomacrogol Cream.
Sorbitan esters.
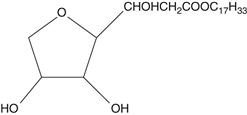
The sorbitan esters are a series of surfactants, widely known as the Spans®, that are produced by the esterification of one or more of the hydroxyl groups of sorbitan with a fatty acid (hence the synonym sorbitan fatty acid esters). Various fatty acids are combined resulting in a range of commercial products, e.g. sorbitan monolaurate (Span 20), sorbitan monopalmitate (Span 40), sorbitan monostearate (Span 60), sorbitan monooleate (Span 80). The structure of sorbitan monooleate (Span 80) is shown below. The series of sorbitan esters are hydrophobic in nature and by themselves produce w/o emulsions.
Polyoxyethylene sorbitan esters (polysorbates).
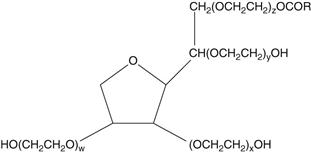
The polysorbates are more hydrophilic polyoxyethylene derivatives of the sorbitan esters above (full name – polyoxyethylene sorbitan fatty acid esters). The following grades are used in pharmacy: polyethylene 20 sorbitan monolaurate (polysorbate 20), polyethylene 20 sorbitan monopalmitate (polysorbate 40), polyethylene 20 sorbitan monostearate (polysorbate 60) and polyethylene 20 sorbitan monooleate (polysorbate 80). Commercially these are known as the Tweens®. The 20 in the name refers to the number of POE groups in the molecule. The formula for polyoxyethylene 20 sorbitan monooleate (Tween 80) is shown below. In this molecule, the subscripts w, x, y and z add up to 20. The group R is the fatty acid chain – in this case —CH2COOC17H33.
An enormous range of polysorbate surfactants of differing oil and water solubility are available by controlling the fatty acid and the length of the polyethylene glycol chains in the molecule. Thus, the polysorbates are able to stabilize both w/o and o/w emulsions, depending on their HLB value (see ‘Emulsifier selection’ section below). Mixtures of sorbitan esters and their POE derivatives are used to form stable emulsions.
Fatty amphiphiles
Fatty alcohols and fatty acids.
These are sometimes described in older texts as auxiliary emulsifiers. When used alone, they are weak w/o emulsifiers. However, in the presence of ionic or non-ionic surfactants, for example when formulated as emulsifying waxes, they are very powerful o/w blends.
Glycerol monoesters.
Glyceryl monostearate and glyceryl monooleate are the most common monoesters used in dermatological formulations. The amphiphilic glycerol monoesters, described as non-emulsifying grades in the various pharmacopoeias, are poor w/o emulsifiers. Self-emulsifying grades, which are similar to the emulsifying waxes, are produced either by the partial neutralization of some of the free fatty acid component of the monoester using alkali, or alternatively by the addition of ~ 5% of ionic or non-ionic surfactant.
Most fatty amphiphiles are not pure homologues. For example, cetostearyl alcohol is a mixture of C16 cetyl alcohol (20-35%) and C18 stearyl alcohol (50–70%). Similarly, stearic acid is generally composed of approximately 55% palmitic acid (C16) and 45% stearic acid (C18) homologues. Such mixes show considerable inter-manufacturer and inter-batch variations. In creams, the homologue composition of the fatty amphiphile markedly influences structure and stability (see later in this chapter).
Polymeric surfactants
The poloxamers (also known as Pluronics®, e.g. poloxamer 188 is Pluronic® F68) are a series of neutral synthetic polyoxyethylene-polyoxypropylene block co-polymers which are used either alone, or as auxiliary emulsifiers with lecithin in small-volume parenteral injections. Poloxamer 188 is resistant to breakdown during autoclave sterilization and its combination with lecithin may stabilize the emulsion by giving a more close packed interfacial film.
Natural macromolecular materials
Many traditional emulsifying agents are derived from natural plant or animal sources and show considerable batch-to-batch variation in their composition which may result in variable emulsifying properties. Many are also susceptible to microbial contamination and degradation by oxidation or hydrolysis of components. In order to reduce such instabilities and extend product shelf-life, purified and semi-synthetic derivatives are generally used in commercial preparations.
Phospholipids
Purified lecithins are natural surfactants derived from egg yolk or soya bean oil. They are used extensively as o/w emulsifiers in parenteral and oral lipid emulsions (Table 27.3). Lecithins are composed of complex mixtures of neutral and negatively charged phospholipids of which the major components are phosphatidylcholine and phosphatidylethanolamine (~90%) (which are uncharged at physiological pH) with smaller quantities of phosphatidylserine (PS), phosphatidylglycerol (PG) and phosphatidic acid (PA) (which are negatively charged). The lecithins stabilize lipid emulsions by increasing the surface charge of the droplets, and by the formation of interfacial liquid crystalline phases. The emulsifying properties are related to the relative proportions of neutral and anionic lipids which vary with phospholipid source and degree of purification. Currently, egg-yolk lecithin is the emulsifier of choice (see Table 27.1).
Hydrophilic colloids; polysaccharides
Polysaccharides, including gums, such as acacia and tragacanth, and alginate and cellulose derivatives are hydrophilic colloids that are predominantly used as emulsifying agents in oral preparations. They render an unpleasant feel to topical emulsions. They are susceptible to degradation, in particular by depolymerization. Polysaccharides provide a good growth medium for microorganisms so that preservation of emulsions containing them is essential. Whilst they do not lower interfacial tension, some polysaccharides, including acacia and purified and semi-synthetic derivatives of methylcellulose, stabilize o/w emulsions by the formation of thick multi-layered films which are highly resistant to film rupture. As an example, methylcellulose 20 is used at a concentration of 2% to stabilize Liquid Paraffin Oral Emulsion BP. Other polysaccharides form poor interfacial films and are relatively inefficient emulsifiers when used alone. They act mainly as viscosity modifiers as they increase the consistency of the external phase and thereby inhibit creaming and coalescence (see section on ‘Emulsion stability’).
Steroidal emulsifiers
Examples of steroidal emulsifying agents derived from animal sources include wool fat (lanolin), wool alcohols (lanolin alcohols), beeswax and cholesterol. They are generally complex mixtures of cholesterol, long-chain alcohols and related sterols. Purified derivatives are still widely used in traditional dermatological emulsions, such as creams, as w/o emulsifiers, and for their emollient properties. They are prone to oxidation and hydrolysis, and antioxidants may need to be incorporated into the emulsion. Wool fat is used in combination with calcium oleate in oily calamine lotion, with beeswax in proflavin cream and with cetostearyl alcohol in zinc and ichthammol cream.
A large number of purified and chemically modified derivatives are available commercially that produce more stable w/o emulsions and retain desirable emollient properties. They are sometimes modified to produce o/w emulsions. For example, a series of non-ionic water soluble lanolin derivatives that promote the formation of o/w emulsions have been produced commercially by reacting lanolin with ethylene oxide.
Solid particles
Finely divided solid particles may stabilize emulsions if they are partially wetted by both the oil and water phases and possess sufficient adhesion for one another to form a coherent interfacial film to give a mechanical barrier against droplet coalescence. If the particles are preferentially wetted by the aqueous phase, an o/w emulsion forms whereas if the solid is preferentially wetted by oil, a w/o emulsion is produced. The particles must be orders of magnitude smaller than the droplets and their effectiveness in stabilizing the emulsion will depend on particle size, shape, wettability, inter-particle interactions and the emulsion medium. Emulsions stabilized by solid particles are sometimes described as Pickering emulsions or surfactant-free emulsions. Magnesium hydroxide is used as the emulsifier in liquid paraffin and magnesium hydroxide oral emulsion.
Solid particles may also act as viscosity modifiers. Clays, such as bentonite and aluminium magnesium silicate, are often incorporated into cosmetic creams. There is a resurgence in interest in Pickering emulsions, especially for the topical delivery of drugs, and a number of new types of hydrophobically modified colloidal silica particles are being investigated.
Emulsifier selection
The hydrophile-lipophile balance (HLB) method
As previously discussed, pharmaceutical emulsions generally contain mixtures of emulsifiers, as these form more stable emulsions. The hydrophile-lipophile balance (HLB) method provides a systematic method of selecting mixtures of emulsifying agents to produce physically stable emulsions. Although originally applied to non-ionic surfactants, its use has now been extended to ionic surfactants.
Each surfactant is allocated an HLB number between 0 and 20 which expresses numerically the size and strength of the polar portion relative to the non-polar portion of the molecule. Thus, the higher the HLB number, the more hydrophilic or water soluble the surfactant, and the lower the number the more lipophilic or oil soluble the surfactant. The HLB values of ionic surfactants are much higher (up to 50) as they are based on ionization properties. Table 27.4 gives HLB values for commonly used surfactant emulsifiers. The theoretical concept of HLB values is discussed more fully in Chapter 5.
Table 27.4
| Commercial name | Pharmacopoeia name | HLB value |
| Span 85 | Sorbitan trioleate | 1.8 |
| Span 80 | Sorbitan oleate | 4.3 |
| Span 60 | Sorbitan monostearate | 4.7 |
| Span 20 | Sorbitan monolaurate | 8.6 |
| Brij 98 | Polyoxyethylene 10 stearyl ether | 12 |
| Tween 60 | Polysorbate 60 (polyoxyethylene 20 sorbitan monostearate) | 14.9 |
| Tween 80 | Polysorbate 80 (polyoxyethylene 20 sorbitan oleate) | 15 |
| Cetomacrogol 1000 | Macrogol cetostearyl ether | 15.7 |
| Tween 20 | Polysorbate 20 (polyoxyethylene 20 sorbitan monolaurate) | 16.7 |
| Potassium oleate | 20 | |
| Sodium dodecyl (lauryl) sulphate | 40 |
Determination of ‘required HLB’ value
The HLB value of the emulsifier blend giving the most stable emulsion is known as the ‘required HLB value’ for that oil phase. The HLB value required to most effectively form an emulsion for a range of individual oils, fats and waxes may be obtained from the literature. If a mixed emulsifier system is used in a formulation and the HLB values of the individual components in an oily mixture are known, the required HLB can be calculated theoretically from the proportions of each component in the oil phase. Alternatively, if the required HLB of the oil is not available, it can be found by experimentation. To perform such experiments, a series of emulsions using blends of a given pair of non-ionic emulsifiers covering a range of HLB numbers is made. These HLB values can then be used to assess the suitability of other blends that may give a better emulsion.
The method of selection of emulsifying agents is based on the observation that different oils require emulsifying agents of different HLB numbers to produce close-packed interfacial films and stable emulsions. Thus, individual oils are often given two ‘required’ HLB numbers – a high value to form an o/w emulsions and a low one to form w/o emulsions, respectively (Table 27.5). A number of non-ionic emulsifiers and their blends, chemically different but all with HLB values similar to the required HLB of the oil, are then examined to find which emulsifying system forms the most stable emulsion. Assessments are based on physical properties, such as droplet size distribution.
Table 27.5
The ‘required’ HLB values for oils and oil phase ingredients
| Oil | O/W emulsion | W/O emulsion |
| Petrolatum | 7-8 | 4 |
| Liquid paraffin | 10.5 | 4 |
| Mineral oil, light | 12 | 4 |
| Castor oil | 14 | – |
| Lanolin, anhydrous | 12 | 8 |
| Beeswax | 9 | 5 |
| Cottonseed oil | 6 | – |
| Pine oil | 16 | – |
Calculation of ratio of emulsifier to produce a particular required HLB value
One of the most important aspects of the HLB system is that the HLB values are additive if the amount of each in a blend is taken into account. Thus, blends of high and low HLB surfactants can be used to obtain the required HLB of an oil. The HLB of the mixture of surfactants, consisting of fraction x of A and (1 − x) of B, is assumed to be the algebraic mean of the two HLB numbers, i.e.:
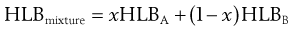 (27.2)
(27.2)
Limitations of the HLB method
Although the HLB system narrows the range of emulsifiers to select, and provides some sort of order to a seemingly endless choice of emulsifiers, it is limited by its strict relation to the molecular structure of individual surfactants. The system is insensitive to the affinity of the emulsifier components for the aqueous and oily phases. For example, when surfactants with widely different HLB numbers are mixed to give the optimum theoretical HLB, then unstable emulsions sometimes result because of the high solubility of the surfactants in the disperse and continuous phases which may change the balance of molecules at the interface, giving a weak interfacial film. In addition, the HLB method does not take into account the influence of additional components in the formulation or the profound influence of temperature changes on surfactant HLB.
The HLB-phase inversion temperature system (PIT)
This method extends the HLB method to include a characteristic property of the emulsion, the phase inversion temperature (PIT) of the system. If an o/w emulsion stabilized by a mixture of non-ionic polyether surfactants is heated, phase inversion from an o/w to a w/o emulsion will occur at a specific temperature unique to the particular emulsion. Phase inversion can be seen visually.
Phase inversion is based on the fact that the stabilities of o/w emulsions containing non-ionic surfactants are closely related to the degree of hydration of the interfacial films. Oil/water emulsions will form if the surfactant is predominantly hydrophilic, whereas w/o emulsions are produced when the lipophilic part of the molecule dominates. As temperature increases, the HLB value of a non-ionic surfactant will decrease as it becomes more hydrophobic. At the temperature at which its hydrophobic tendency just exceeds its hydrophilic tendency, the PIT, the emulsion will invert to form a w/o emulsion. Therefore conditions, for example added salts or an increase in temperature, which decrease the degree of hydration of the interfacial film also decrease the stability of the emulsion. As a general rule, relatively stable o/w emulsions are obtained when the proposed storage temperatures are between 20-60 °C below the PIT, because the interfacial films are then sufficiently hydrated.
Creams
Creams are white, semisolid preparations, often medicated, intended for external application to the skin and mucous membranes. Pharmaceutical and cosmetic creams are generally o/w emulsions (aqueous creams), although the term is also used occasionally to describe semisolid w/o emulsions (oily creams). In addition other non-emulsion bases, such as the oil-free aqueous mixed emulsifier systems (described below), are referred to as creams as they are also white and semisolid. The aqueous continuous phase of an o/w cream may be structured either i) directly, by the addition of the appropriate amount of structuring agent (often described as a rheological modifier) such as clay particles or polymeric materials and/or ii) indirectly from interactions between various emulsifier components and water to form lamellar gel network phases.
Although the consistencies of some complex cream formulations are controlled by both mechanisms, the stability and rheological properties of most aqueous creams are due mainly to the presence of lamellar gel networks in the continuous phases.
Formulation of aqueous creams
In the preparation of o/w creams, sparingly soluble fatty amphiphiles combined with more water-soluble ionic or ionic surfactants are widely used (Table 27.6). The components of the emulsifier may be added separately during the preparation of the cream, or alternatively in the form of a pre-blended emulsifying wax (Table 27.7). Some emulsifier combinations contain the same components (e.g. fatty alcohols and ionic surfactants) as those investigated originally by Schulman and Cockbain in their classical work on interfacial films (discussed in Chapter 5). The properties of such films, although important, are not the main mechanisms in controlling shelf-life stability of practical o/w creams.
Table 27.6
Selection of commonly used fatty amphiphiles and surfactants
| Fatty amphiphile | Surfactant |
| Cetostearyl alcohol | Cetomacrogol 1000 |
| Commercial cetyl alcohol | Sodium lauryl sulphate |
| Commercial stearyl alcohol | Cetrimide |
| Triple pressed stearic acid | Triethanolamine stearate |
| Glyceryl monostearate | Sodium stearate |
Table 27.7
| Emulsifying wax | Components | Weight ratio (and ~ molar ratio) of alcohol to surfactant |
| Emulsifying Wax BP | Cetostearyl alcohol, sodium lauryl sulphate | 9 : 1 (~12 : 1) |
| Cationic Emulsifying Wax BP | Cetostearyl alcohol, cetimide | 9 : 1 (~12 : 1) |
| Cetomacrogol Emulsifying Wax BP | Cetostearyl alcohol, cetomacrogol 1000 | 4 : 1 (~20 : 1) |
| Glyceryl Stearate SE (self-emulsifying) | Glyceryl monostearate, anionic soap | – |
In creams, long-term stability is due to the formation of viscoelastic gel network phases which trap oil droplets, preventing their movement and interaction. However, it is emphasized that interfacial films are still important because even complex multiphase emulsions are sometimes fluid during their lifetime, so that droplets are then free to interact. For example, the existence of a strong interfacial film is particularly important at the high temperatures of preparation before networks consolidate, and in non-ionic creams in which networks consolidate only slowly on storage. During the emulsification process, surfactant emulsifiers reduce interfacial tension making droplets easier to break up, and the interfacial film then reduces the tendency for freshly formed droplets to re-combine.
The gel-network theory of emulsion stability
The gel-network theory of emulsion stability gives a coherent explanation for the manner in which fatty amphiphiles and surfactant combined as mixed emulsifiers not only stabilize o/w lotions and creams, but also control their consistencies between wide limits, from mobile lotions at low concentrations of emulsifying wax to soft or stiff semisolid creams at higher concentrations (self-bodying action). Although most early work was performed using long chain alcohols, the same general principles apply whichever amphiphile or surfactant (ionic or non-ionic) is used. The gel network theory established that the structure and stability of o/w creams are dominated by the swelling properties of an α-crystalline gel-network phase formed when the mixed emulsifier, in excess of that required to form an interfacial film at the oil/ water interface, interacts with continuous phase water.
A valuable method of approach when developing the theory was to investigate the interaction of mixed emulsifiers and their components in water over the ranges of concentration and temperature relevant to the manufacture, storage and use of the emulsion. This protocol is now generally adopted to develop new formulations. Oil-free ternary systems, containing similar concentrations of mixed emulsifier as used to stabilize emulsions, represent useful structural models for the continuous phases of the corresponding emulsions.
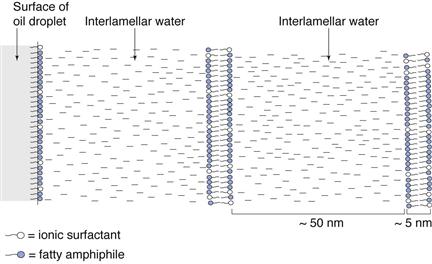
Interaction of mixed emulsifiers in water
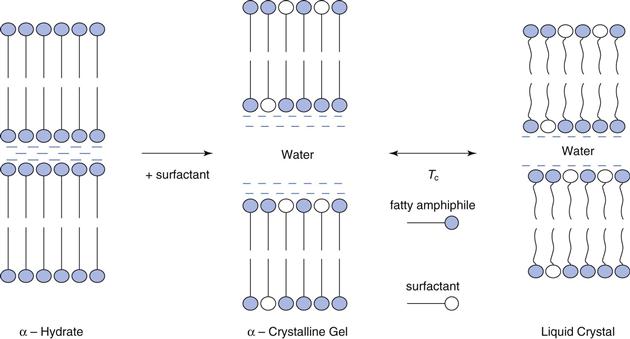
Figure 27.3 illustrates the phases that form spontaneously when a fatty alcohol such as cetostearyl alcohol is dispersed in water alone, and when it is dispersed in the presence of small quantities of surfactant at low and high temperature. Other fatty amphiphiles show similar phase behaviour, although the terminology used to describe the polymorphs may differ.
Pure long-chain alcohols exist in three polymorphic forms. The high temperature α-form separates first from the melt and is stable over a narrow temperature range. At lower temperatures, the β-form and γ-form can co-exist. Transition temperatures are lowered and polymorphic temperature ranges extended with homologue admixtures such as cetostearyl alcohol, and in the presence of water. Thus, at room temperature, cetostearyl alcohol may be in the α-form whilst pure cetyl or stearyl alcohols may exist as β-and γ-crystalline polymorphs. Crystallization in the α-form is generally a pre-requisite for the formation of the swollen crystalline and liquid crystalline phases described below.
In excess water, the α-crystals show limited swelling to form waxy crystalline hydrates (Fig. 27.3). However, in the presence of very small quantities of ionic or non-ionic surfactant (molar ratios of alcohol to surfactant in the region of 10-30 : 1 which are the proportions present in commercial emulsifying waxes, see Table 27.7), the swelling in excess water increases spontaneously to give a viscoelastic, swollen α-crystalline gel phase. On heating the gel phase transforms to lamellar liquid crystals at a specific temperature, the crystalline gel-liquid transition temperature, TC. The liquid crystalline phase in which the hydrocarbon chains are in a dynamic disordered state is fluid as it does not swell as extensively as the low temperature gel phase (Fig. 27.3).
With cetostearyl alcohol and other amphiphiles used in pharmaceutical emulsions, the gel-liquid crystalline transition temperature is around 40-50 °C so that although fluid liquid-crystalline phases are present at the high temperatures of emulsion manufacture, when the emulsion cools they convert to a semisolid gel-network phase composed of swollen-crystalline gel phase in equilibrium with hydrated α-crystals and bulk free water. Many creams thicken at the transition temperature during the cooling process of manufacture and this temperature is sometimes described as the ‘setting temperature’.
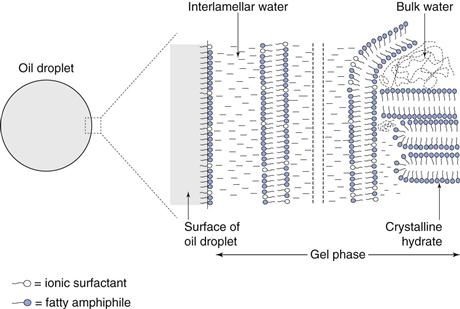
Microstructure of creams
Figure 27.4 shows a schematic diagram of a typical multiple phase o/w cream. The emulsion is composed of four phases:
• dispersed oil phase stabilized by a mixed monomolecular film
• α-crystalline hydrates that show limited swelling in water
This multi-component continuous phase is viscoelastic, so that the oil droplets are essentially immobilized in the structured continuous phase, preventing flocculation and coalescence.
Figure 27.4 is a general schematic representation. The overall consistency of the product (whether it is a structured liquid or a semisolid cream), its cosmetic appearance (shiny, pearly or matt), its rheological properties (fluid or semisolid) and its rheological stability on storage (thinning or thickening) is related to:
• the mechanisms and kinetics involved in the formation of the phases
• the thickness of the interlamellar water layers
• the proportion of the added water that is incorporated between the lamellae
The gel-network theory explains the manner in which formulation factors such as the nature of the fatty amphiphile and its homologue purity, the ionic or non-ionic nature of the surfactant, the molar ratios of amphiphile to surfactant, and the total concentration of the mixed emulsifier will influence microstructure and properties.
Self-bodying action
The ability to control the consistency of o/w creams and the corresponding oil-free systems between wide limits by altering the mixed emulsifier concentration (self-bodying action) is related to the swelling ability of the lamellar gel-network phase and its volume fraction. At low concentrations of mixed emulsifier, structured liquids form as the proportion of bulk (continuous phase) free water is relatively high. At higher concentrations of mixed emulsifier, the proportion of swollen lamellar phase is increased with a corresponding decrease in the amount of free bulk water, and emulsions become thicker or semisolid.
Fatty alcohol mixed emulsifiers
Ionic surfactants.
Combinations of fatty alcohols and ionic surfactants exhibit a phenomenal swelling in the aqueous continuous phase of o/w emulsions, for the thickness of the water layers is over 10 times the thickness of the hydrocarbon bilayers, as shown schematically in Figure 27.5. The extensive swelling is electrostatic in nature. The surfactant molecules interposition among the fatty alcohol molecules and electrical double layers arise from the dissociation and diffusion of counter-ions from the surfactant head groups at the surface of the bilayers into the surrounding water. Electrostatic repulsion between adjacent bilayers arises from the overlap of the electrical double layers, and is described by the DLVO theory of colloid stability (Chapter 5).
The addition of electrolytes, such as sodium chloride, to creams stabilized by ionic mixed emulsifiers reduces electrostatic swelling between the bilayers thereby decreasing the gel-network phase volume with a corresponding increase in the quantity of bulk free water. Thus, emulsions containing additional electrolyte are thinner with lower apparent viscosities than their electrolyte-free counterparts.
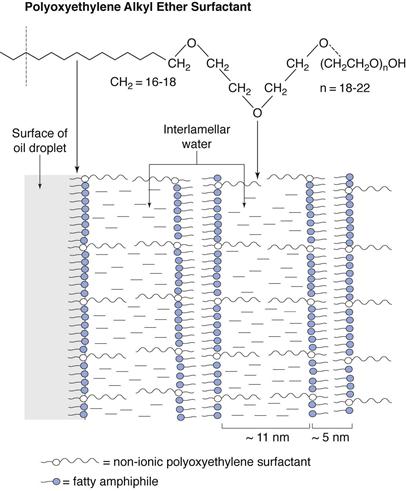
Non-ionic polyoxyethylene surfactants.
The fatty alcohols also swell in the presence of non-ionic surfactants, although both the mechanism and timescale are different from the ionic systems described above. With non-ionic surfactants, the swelling of the α-crystals of fatty alcohol waxes is due to the hydration of the polyoxyethylene (POE) chains of the surfactant. These are orientated and extended into the interlamellar water, hydrated by this layer and stabilized by steric repulsions. With straight chain POE surfactants, such as cetomacrogol 1000 containing a straight chain of 20-24 POE groups, the interlamellar thickness is approximately twice that of the extended chain length (Fig. 27.6).
Creams containing non-ionic emulsifying waxes often show considerable structural changes on storage, sometimes changing from a milky liquid when first prepared to a semisolid on storage. Such changes are undesirable not only from a cosmetic point of view, but also because variable bioavailability profiles may result. These changes can be explained by considering the relationship between temperature and hydration of POE chains. At the high temperature of preparation, the POE groups do not hydrate significantly. On cooling to below the transition temperature, the POE chains become increasingly soluble and bilayers form as the chains extend into, and are hydrated by water. This means that the lamellar gel phase may only be partially formed after the cooling process, so that the emulsion is thin immediately after preparation. On storage the increased solubility of the POE chains allows additional gel phase to form, although this occurs very slowly because of the crystalline nature of the hydrocarbon chains. Thus, emulsions thicken and gradually become semisolid on storage.
Fatty acid mixed emulsifiers
Fatty acids exhibit marked polymorphism, and form lamellar gel network phases. Stearic acid is widely used as a component of ‘vanishing’ creams. Such creams are extensively used in cosmetics because they usually have an attractive pearlescent sheen, and appear to vanish during application leaving a matt, non-greasy residue on the skin. Stearate creams are not traditional emulsions, but rather oil-free ternary systems composed of stearic acid, a stearate soap (i.e. an ionic surfactant) and water. The acid soap is formed in situ during the manufacture of the product from the partial neutralization (10-40%) of some of the fatty acid with alkali, traditionally triethanolamine, although sodium hydroxide and potassium hydroxide are also used in some formulations to produce potassium or sodium soaps. If an oil phase is included in the formulation, the stearic acid and its soap function as a mixed emulsifier to stabilize and control the consistency of the emulsion.
Stearate creams containing partially neutralized fatty acids show a more complicated phase behaviour than those prepared with alcohols, and they are extremely sensitive to mechanical disruption. Creams formed by the partial neutralization (35%) of stearic acid in situ by triethanolamine contain swollen lamellar gel phase with interlamellar water thickness ranging from 14-16 nm. This phase exists in equilibrium with crystals of stearic acid and bulk continuous phase water. The translucent nature of stearic acid crystals imparts a translucent sheen to the cream. In contrast, ordered swollen lamellar structures are not apparent in creams in which sodium or potassium hydroxides are used to partially neutralize stearic acid. In these, the structure is a result of highly disordered interlinking bilayers of mixed emulsifier (twisted ribbons) holding vast amounts of water by capillary forces.
Self-emulsifying glyceryl monoesters
Glycerol monoesters are poor w/o emulsifiers. The self-emulsifying grades containing small quantities of either ionic or non-ionic surfactant are essentially emulsifying waxes. When dispersed in water the self-emulsifying grades form swollen lamellar gel-network phases, and exhibit a self-bodying action in which mobile emulsions are obtained at low concentration and semisolid products at higher concentrations. In common with fatty acids, the network phases formed from self-emulsifying grades are very sensitive to mechanical disruption.
Molar ratio of fatty amphiphile to surfactant
For semisolid products structured by gel networks, a large excess of alcohol, in the region of at least 10-30 molecules of alcohol to one molecule of surfactant is essential. Commercial emulsifying waxes and those of the various pharmacopoeias contain such an excess of alcohol (Table 27.7). With higher surfactant concentrations, micellar phases rather than gel networks form. With excess alcohol, there is a broad range of molar ratios of alcohol to surfactant (from ~10 : 1 to 100 : 1) over which gel networks form. The ratio controls the relative proportions of the swollen gel, crystalline and water phases in the gel networks, and hence the appearance and rheology of the product. As the ratio of alcohol to surfactant increases, the proportion of crystals increases at the expense of swollen lamellar phase, and systems become progressively less structured and eventually fluid.
Source and batch variations of components
Source and batch variations of the components may cause undesirable changes in rheological behaviour, such as emulsion thinning or thickening during the shelf-life. A thinner product may allow droplet interaction leading to flocculation and coalescence on storage; whereas a thicker product may be cosmetically unacceptable.
Surfactants.
The homologue composition of the hydrocarbon chains of the surfactant has little influence on the consistency of the product, due to the low concentrations of surfactant chains in the bilayers. Batch variations of the POE chain length in non-ionic surfactants can influence the consistency of the product, for there is a linear relationship between POE chain length, inter-lamellar water thickness and apparent viscosity. Batches with a higher proportion of long POE chains will produce thicker products, as more water is trapped between lamellae and the opposite will occur with batches containing a higher proportion of shorter POE chain lengths. Ionic impurities in charged surfactants may also cause minor variations in consistency by their influence in suppressing electrostatic swelling.
Fatty amphiphiles.
In contrast, mixed homologue fatty alcohols and acids are essential to the formation of stable swollen gel network phases. Creams prepared from pure C16 or C18 alcohols, although initially semisolid, are rheologically unstable and break down on storage to form mobile crystalline fluids. This is because, the gel-network phase formed after a heating and cooling cycle of manufacture is unstable at low temperature. On storage, the swollen α-crystalline gel networks convert to non-swollen β- and γ- polymorphs, and the system becomes fluid.
Similarly, pure homologue fatty acids do not form stable structured creams. Mixed homologue triple-pressed stearic acid, composed of approximately 45% stearic acid (C18) and 55% palmitic acid (C16) is generally used in cosmetic products.
Manufacture and processing of emulsions and creams
Fluid emulsions
When oil and water are mixed, energy in the form of agitation is necessary to produce the required droplet size distribution. Emulsifiers have an important role in the process of emulsification. Surfactant emulsifiers reduce interfacial tensions making droplets easier to break up during mixing and reducing the tendency to recombine. Other emulsifiers, such as the polymeric macromolecules, alter the hydrodynamic forces generated during emulsification by their influence on rheological properties.
Emulsions are generally prepared experimentally on a small-scale in the laboratory before they are scaled-up and manufactured in much larger quantities. Each scale of preparation involves similar generic steps. First, the emulsifying agents and other oil- or water-soluble components are dissolved separately in the phase in which they are most soluble. When heat is required, for example to melt waxes in the oil phase during the preparation of lotions and creams, both the oil and water phases are heated separately to the same temperature (a few degrees above the highest melting point of the wax) and the elevated temperature maintained as they are brought together and mixed. Prior heating of each phase to the same temperature before blending is important to avoid the formation of a granular or lumpy product by the premature solidification of the oil phase when it is mixed with colder aqueous phase. High temperature also has the advantage of reducing the consistency of the system, making it easier to mix. Generally the disperse phase is added to the external continuous phase with constant agitation to produce the required droplet size distribution. The emulsion is finally cooled (if necessary) to the storage temperature whilst mixing is continued. Volatile or heat-sensitive components are incorporated at the appropriate temperature as the emulsion cools.
A variation of the above procedure is when o/w emulsions containing non-ionic surfactant emulsifiers with phase inversion temperatures (PIT) within the temperature range of normal processing (60–80 °C) are prepared by the phase inversion method. In this method, the external phase is added to the dispersed phase at temperatures above the phase inversion temperature, temporarily forming a w/o emulsion. On cooling, the emulsion will revert to an o/w emulsion at a specific narrow temperature range, the PIT. Enormous forces are generated by phase inversion, and very fine nano-sized droplets may be produced. This method is sometimes described as a low (applied) energy method as it utilizes the chemical energy of the system with minimal heat, rather than providing external energy from extreme agitation.
On an industrial scale, the oil and water phases are often heated separately in large tanks, and then combined by pumping each phase into the mixing vessel fitted with suitable emulsification agitator, such as a mechanical mixer or homogenizer. Proprietary mixing vessels are available in a variety of sizes to accommodate a few hundred litres of emulsion in initial scale up, to several thousand litres for manufacture. The mixing vessel is usually made from stainless steel, jacketed so that heating or cooling can be applied, and sometimes fitted with baffles to modify circulation of the emulsion during mixing.
A wide range of agitation techniques are available for dispersing the internal phase into droplets. These include simple hand mixers, various stirrers and propeller or turbine mixers, homogenizers, microfluidizers, colloid mills and ultrasonic devices. Most disrupt droplets either by shear forces in laminar flow, by inertial forces in turbulent flow or by cavitation during ultrasound. Extensional flow, where there is a velocity gradient in the direction of flow, also has a very powerful droplet-breaking effect.
The choice of emulsification equipment for a particular emulsion depends on a number of interrelated factors including:
• the volume of emulsion to be prepared, i.e. whether laboratory or production scale
• the range of droplet sizes required
• the flow properties of the emulsion during the emulsification and cooling processes.
For the extemporaneous preparation of small quantities of a fluid emulsion, blending the oil and water phases in the presence of a suitable emulsifier in a mortar and pestle or by manual shaking or stirring is often sufficient to produce a coarse emulsion with droplets sizes in the region of 1-50 µm. Mechanical hand stirrers with the stirring rod held or placed directly into the system to be emulsified may also be used. Mechanical mixers, fitted with various impellers and paddles are also available in various sizes and motor speeds to prepare both small and large scale batches of emulsion.
When smaller droplets with narrower droplet size distributions are required, stronger agitation techniques are necessary. Nanoemulsion formulations that are unsuitable for preparation by the phase inversion method require extreme forces to overcome the large interfacial tension and form nano-sized droplets. Parenteral emulsions also require a large input of energy to produce droplet sizes considerably less than 1 µm; thus lipid and perfluorochemical emulsions are usually prepared aseptically by homogenization at high temperature and pressure, or by microfluidization (see below).
Homogenizers are available for processing quantities of emulsions from a few mL in the laboratory up to several thousand litres for manufacture. Homogenizers function essentially by forcing the crude mixture of liquids through a small orifice under pressure. In some, the liquid impacts on a solid surface set at right angles to the direction of flow and, depending on the pressure applied, the intense extensional, shear and turbulent flow patterns develop to produce fine droplets of less than 1 µm. Membrane homogenizers produce emulsions with uniform fine droplet sizes on a laboratory scale by forcing the internal phase to flow through specific glass membranes into the external phase under high external pressure.
Microfluidizers are also commonly used to prepare parenteral emulsions in both the laboratory and on scale-up. Separate oil and water phases are pumped into a chamber under high pressure causing the liquids to accelerate at high velocity and interact with each other as they impinge on a hard surface. The shear and turbulent forces induced lead to the breakup of droplets to form an emulsion. Very small droplets are produced by recycling the system a number of times through the microfluidizer.
Although both ultrasound and colloid mills also produce sub-microscopical droplet sizes, they are usually confined to laboratory scale batches. Colloid mills generate considerable heat and so need extremely efficient cooling which is expensive with large scale batches. Ultrasound produces alternate regions of cavitation and compression in the emulsion and very fine droplets form when the cavities collapse with extreme force. However, the energy density is highly localized giving poorer reproducibility on a large scale.
Multiple emulsions
Multiple emulsions are generally prepared in two steps. In the first step a w/o or o/w emulsion (the primary emulsion) is prepared using a suitable emulsifier. The primary emulsion is then re-emulsified during the second step to form a w/o/w or o/w/o multiple emulsion. The primary emulsion is prepared under high shear conditions to obtain small inner droplets whilst the secondary emulsification step is carried out at lower shear to avoid rupture of the internal droplets.
Creams
The processing and manufacture of commercial creams is more complex than for fluid emulsions because structure is formed in addition to droplet phases during their preparation. Scale-up, based on a previously developed laboratory procedure is particularly challenging due to the difficulties in matching the exact laboratory conditions of preparation. Every type of emulsification equipment introduces energy into the system in a different way. On scale-up, energy input and hence emulsion microstructure can be affected by a change in the settings of a specific type of mixer when a larger volume is processed. Other relatively minor differences in processing such as the rate of the heating and cooling cycle, the order of adding the components and the extent of mixing may cause marked variations in the consistency and rheological profile of the resulting emulsions.
An essential approach to optimize processing conditions and get a reproducible cream is to identify the key structuring agents in the formulation, the mechanisms involved in forming the structure, and the relationships between microstructure and processing variables. The knowledge gained can be used to optimize the manufacturing process to give a reproducible product using a minimum number of components. The processing conditions required to produce the swollen gel-network phases when different types of emulsifying wax are incorporated can be identified using the gel-network theory. For example, preparation techniques, in particular cooling rates and mixing have a marked effect on the initial and final consistencies of creams prepared with fatty alcohol/non-ionic polyoxyethylene surfactants. Shock cooling and limited mixing initially produces very mobile emulsions, which gel on storage. In contrast, slow cooling and increased mixing forms semisolid systems. These variations in rheological properties are related to the hydration of the polyoxyethylene groups and the mechanisms by which non-ionic gel networks form, as discussed earlier in this chapter. To optimize the manufacture and minimize changes on storage (shelf-life stability) of such creams, slow cooling followed by vigorous agitation at temperatures just higher than the transition temperature are required.
Lamellar gel-network phases formed in creams containing fatty acids are particularly sensitive to agitation. The ordered lamellar gel phases are metastable and convert to non-swollen crystals when mixed at high shear rates. The cream remains semisolid if mixing is discontinued at the transition temperature and the system is allowed to cool undisturbed. A more mobile system is formed if it is mixed below the transition temperature, and systems may need to ‘rest’ to allow the structure to re-build.
Some complex commercial creams contain a number of different structuring agents within the same formulation, each of which needs different processing conditions to fully develop their structure. For example, rheological modifiers, such as clays, and natural and synthetic polymers, often have completely different processing requirements. Clays need to be processed under severe conditions of high temperatures and shear rates, with addition of electrolyte at the appropriate time, in order to ensure the structure is fully formed. Polymer hydration, on the other hand, often requires dispersion and wetting at a much lower temperature with much milder agitation to prevent polymer degradation and structure loss. Non-reproducible products with variable rheological profiles may result if the different sensitivities of each structuring mechanism to mixing, shear and temperature are not recognized and accommodated for in the manufacturing process. In such cases, processing is optimized by identifying the optimum processing conditions for each individual structure beforehand, processing each structure off-line to ensure structure is fully formed before blending each fully developed structure together in a commercial mixing vessel.
Emulsion properties
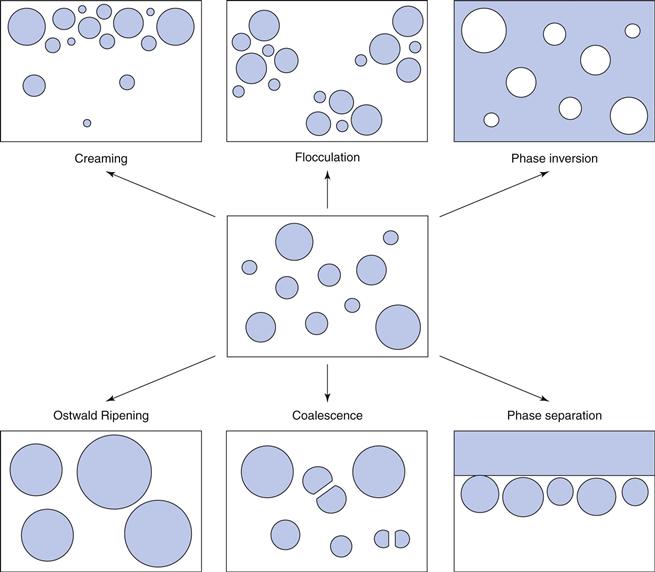
Identification of emulsion type
It is important to confirm whether an emulsion is o/w, w/o or a multiple emulsion as this can significantly influence its application and properties. Generally an emulsion exhibits the characteristics of its external (continuous) phase, and there are a number of simple tests based on this for distinguishing between o/w and w/o types of emulsion. The tests, some of which are described below, essentially identify the continuous phase and do not identify a multiple emulsion. This can be resolved by microscopy.
Water or oil miscibility.
An emulsion will only mix freely with a liquid that is miscible with its continuous phase. Thus, a small quantity of water dropped onto the surface of an o/w emulsion will immediately mix with the external phase and disappear, whereas the water droplets will remain on the surface of a w/o emulsion. The reverse is true if a small quantity of oil is dropped onto the emulsion.
Filter paper test.
The test involves putting a few drops of emulsion onto filter paper. If the droplet spreads rapidly into the filter paper, it is an o/w emulsion, as water (the external phase) tends to spread more rapidly throughout the filter paper than oil from a w/o emulsion.
Conductivity measurements.
These are based on the principle that water conducts electricity better than oils. Thus generally, o/w emulsions have a much higher electrical conductivity than w/o emulsions. A light source will glow when electrodes (connected in series to a battery and suitable light source) are dipped into an o/w emulsion, but not when they are placed in a w/o emulsion.
Dye solubility tests.
These tests involve blending either a water soluble dye or an oil soluble dye with the emulsion. If a water soluble dye is used, an o/w emulsion will be evenly coloured, whereas a w/o emulsion will be much paler in appearance. Microscopically, an o/w emulsion will appear as colourless droplets against a coloured background, whereas a w/o emulsion will appear as coloured droplets against a colourless background.
Droplet size distribution
It is important to control droplet sizes as they influence emulsion stability, biopharmaceutical properties, and clinical use. Droplet size distribution is related to the energy input during preparation, the viscosity difference between the phases and the characteristics of the emulsifier. Large droplets have a greater tendency to cream and coalesce, and a broad particle size distribution encourages an increase in droplet size by Ostwald ripening (see ‘Emulsion stability’ section below). Thus, the most physically stable pharmaceutical emulsions generally have small droplets and narrow size distributions. Ostwald ripening alone governs instability in nanoemulsions, as the very small droplets do not cream, flocculate or coalescence to any great extent (see section on ‘Emulsion stability’).
For oral emulsions, gastrointestinal absorption increases as droplet sizes decreases. Whilst this is desirable with oral formulations of nutrient oils alone or with drugs dissolved in them, it may give adverse clinical effects with laxative oils that are used for a local effect and are toxic if absorbed. The clearance kinetics of parenteral emulsions is influenced by droplet size distribution. Individual droplets should not exceed ~5 µm in diameter (the diameter of the smallest blood vessels) in intravenous emulsions because of the possibility of pulmonary embolism. Droplets less than 5 µm are rapidly cleared from the blood stream by elements of the reticuloendothelial system (RES), also known as the mononuclear phagocyte system (MPS), (primarily by Kupffer cells of the liver). This natural targeting to the liver offers opportunities for the treatment of tropical diseases such as leishmaniasis, and fungal infections such as candidiasis. Drug delivery from dermatological preparations is also improved in lipid nanoemulsions.
Droplet charge provided by the emulsifier film influences the biological fate of the droplets as well as emulsion stability. For example, negatively charged droplets are cleared more rapidly from the blood than neutral or positively charged ones. Emulsions stabilized by non-ionic polyoxyethylene (POE) surfactants are longer circulating in the blood and cationic emulsions are better for dermatological delivery.
Rheology
An emulsion should possess shear thinning rheological characteristics (Chapter 6) appropriate to its intended use. For example, a dermatological cream must be thin enough to spread easily onto damaged skin without causing pain, but should rapidly regain structure so that it is thick enough to remain in place on the skin after application. The rheological properties of emulsions are influenced mainly by the phase volume ratio, the nature of the continuous phase, and to a lesser extent droplet size distributions. A variety of products ranging from mobile liquids to structured fluids and thick semisolids can be formulated by altering the dispersed phase volume and/or the nature and concentration of the emulsifiers.
For low internal phase volume emulsions, the consistency of the emulsion is similar to that of the continuous phase. Thus, w/o emulsions are generally thicker than o/w emulsions. The consistencies of o/w systems are further increased by the addition of emulsifiers such as gums, clays, polymers and other thickening agents which impart plastic or pseudoplastic flow properties, often accompanied by thixotropy (Chapter 6). At rest, the high apparent viscosity inhibits the movement of droplets to maintain a physically stable emulsion. During use, higher shear rates are applied and apparent viscosities reduce (shear thinning) so the emulsion flows freely when injected, or poured from a container.
Emulsifying waxes, used to stabilize structured lotions and semisolid creams, interact with water to form a viscoelastic continuous phase. Such emulsions are particularly difficult to characterize rheologically, because at rest solid properties dominate (hence the term semisolid), but on application of a force, structure is broken down irreversibly as systems thin and flow, producing very complex flow curves. Such flow curves, however, are sensitive to relatively small changes in consistency. Viscoelastic measurements are also useful in characterizing complex multiphase emulsions.
Emulsion stability
Definition of stability
A kinetically stable emulsion is one in which the dispersed droplets retain their initial character and remain uniformly dispersed throughout the continuous phase. In pharmaceutical emulsions, stability has a much wider definition as it is generally equated with a long shelf-life. Thus in addition to kinetic stability, the emulsion must retain its original appearance, odour and consistency, and exhibit no microbial contamination.
Chemical instability
Ideally, all emulsion components should be chemically inert under the conditions of emulsification. Unfortunately, this is not always the case so that it is important to understand the chemical nature of all the emulsion components before a selection is made. Particular care has to be taken in the selection of pharmaceutical oils as they may be susceptible to oxidation by atmospheric oxygen or microbial contamination developing an unpleasant odour and taste as they become rancid. Antioxidants and preservatives may be incorporated into the emulsion to minimize these effects. Polymeric emulsifiers may undergo depolymerization by hydrolysis or microbial degradation, with loss of emulsification power and consistency.
Interactions between the emulsifying agent and other components are of particular concern because emulsifying properties may be destroyed, causing the emulsion to break (see below). For example, polyoxyethylene non-ionic emulsifiers form hydrogen bonds with phenolic preservatives, leading to poor preservation as well as loss in emulsifying power. Ionic emulsifying agents are usually incompatible with materials of the opposite charge. This occurs when cationic materials such as surfactants or drugs (e.g. cetrimide, neomycin sulphate) are added to a cream containing an anionic emulsifying agent such as sodium lauryl sulphate. The cream loses consistency on storage because lamellar structures in the continuous phase are destroyed by the resultant suppression of repulsive forces.
Physical instability
An emulsion without an emulsifier will quickly return to the original state of separate oil and water layers, that is, the emulsion will break or crack. In the presence of an emulsifier, this state is approached via four distinct processes, creaming, flocculation coalescence and Ostwald ripening. Phase inversion, where a w/o emulsion inverts to an o/w emulsion or vice versa, is a special type of instability. The processes of instability are illustrated by the schematic representations in Figure 27.7.
The destabilization processes are not independent and each may influence or be influenced by the others. In practice, they may proceed simultaneously or in any order. Coalescence and Ostwald ripening are the most serious types of instability, as they result in the formation of progressively larger droplets which will ultimately lead to phase separation. Creaming and flocculation, on the other hand, are more subtle forms as they represent potential steps towards coalescence due to the close proximity of the droplets.
Creaming
Creaming is a process which occurs when the dispersed droplets separate under the influence of gravity to form a layer of more concentrated emulsion, the cream. Creaming occurs inevitably in any dilute emulsion containing relatively large droplets (~1 µm) if there is a density difference between the oil and water phases. Most oils are less dense than water, so that the oil droplets in an o/w emulsion rise to the surface to form an upper layer of cream, whereas water droplets sediment to form a lower layer in w/o emulsions. Although a creamed emulsion can be restored to its original state by gentle agitation, it is considered undesirable because the emulsion is inelegant and more seriously, the patient may receive an inadequate dose if the emulsion is not agitated sufficiently before use.
The most effective ways in practice to reduce creaming is to prepare emulsions with small droplet sizes, and to thicken the external phase by the addition of viscosity modifiers (c.f. Stokes Law, Chapters 5, 6 and 10). Density adjustment to decrease the density difference between the two phases, has received little attention.
Flocculation
Flocculation is a weak, reversible association between emulsion droplets which are separated by trapped continuous phase. Each cluster of droplets (floccule) behaves physically as a single kinetic unit, although every droplet in the floccule retains its individuality. Floccules can be redispersed by mild agitation, such as shaking the container. Flocculation has been discussed in detail in Chapter 5 in terms of the attractive van der Waals forces pulling the droplets together and the repulsive forces tending to keep them apart. It occurs in the secondary minimum as illustrated in the schematic curve of potential energy versus distance of separation plot (Fig. 5.4) and is controlled by the properties of the emulsifier film which modifies the repulsive forces between dispersed emulsion droplets. Thus, the tendency for flocculation can be reduced by the use of a suitable emulsifier. Although the timescale between flocculation and coalescence can be extended almost indefinitely by the adsorbed emulsifier, flocculation is generally considered undesirable because floccules cream more rapidly under the influence of gravity than individual emulsion droplets.
Coalescence
Coalescence describes the irreversible process in which dispersed phase droplets merge to form larger droplets. The process will continue until the emulsion breaks (cracks) and there is complete separation of oil and water phases. Coalescence occurs when the emulsion droplets are able to overcome the repulsive energy barrier and approach the primary minimum (c.f. Figure 5.4). Once in this minimum, they are in very close proximity so that stability against coalescence is determined essentially by the resistance of the interfacial film to rupture. Coalescence begins with the drainage of liquid films of continuous phase from between the oil droplets as they approach one another and become distorted, and ends with the rupture of the film (Fig. 27.8). Rigid close-packed elastic films formed by specific emulsifier mixtures and thick multilayered films provided by many polymers protect droplets against coalescence as they are highly resistant to film rupture.
Ostwald ripening
Ostwald ripening is an irreversible process which involves the growth of large droplets at the expense of smaller ones. Ostwald ripening occurs in emulsions containing small sub-micrometre droplets (less than ~600 nm), provided that the dispersed phase also has a significant solubility in the continuous phase. Ostwald ripening is a direct consequence of the Kelvin effect which explains how the solubility of a partially miscible droplet increases markedly as its radius decreases. Thus, small emulsion droplets have a higher solubility than larger droplets. In order to reach the state of equilibrium, the small droplets dissolve and their molecules diffuse through the continuous phase and re-deposit onto larger droplets which grow bigger (ripen), resulting in an overall increase in average droplet size. Ostwald ripening differs from coalescence in that it does not need any contact between the droplets.
Ostwald ripening, rather than coalescence is the underlying mechanism of instability in many o/w fat emulsions, and in perfluorocarbon emulsions. Although flocculation and coalescence are inhibited by the properties of surfactant interfacial films, Ostwald ripening may actually be enhanced if micelles are also present to further solubilize the oil. Ostwald ripening can be prevented by the addition of a small quantity of an immiscible second oil to the main partially miscible oil to reduce molecular diffusion of this major component. Fat emulsions containing local anaesthetics or local analgesics show enhanced stability in the presence of less soluble hydrophobic oils, and perfluorodecalin contrast media emulsions are more stable in the presence of small quantities of insoluble perfluorotributylamine. Ostwald ripening is also inhibited by the addition of Pluronic F68® surfactant that is strongly adsorbed at the o/w interface and does not form micelles in the continuous phase. Polymers that increase the viscosity of the emulsion external phase also inhibit Ostwald ripening as they slow down the molecular diffusion process.
Emulsion inversion
Emulsion inversion occurs occasionally in emulsions under specific conditions. A change in emulsifier solubility from water soluble at low temperature to oil soluble at high temperature (e.g. some non-ionic surfactants), causes phase inversion at a specific temperature from an o/w emulsion to an o/w emulsion, and this phenomenon is used in the low energy preparation of nanoemulsions. Emulsion inversion may also occur by specific interactions with other additives. For example, if a sodium salt is used to stabilize an o/w emulsion, the emulsion may invert to a w/o emulsion by the addition of divalent ions, such as Ca2+ ions, to form the calcium salt, which stabilizes a w/o emulsion.
Stabilization by use of mixed emulsifiers
It has already been indicated that pharmaceutical emulsions, whether they are dilute mobile systems for internal use or thick semisolid creams for application to the skin contain mixtures of emulsifiers, as these form more stable emulsions. For example, mixtures of non-ionic surfactants of high and low HLB generally form more stable emulsions than either surfactant alone. The lecithins used to stabilize oral and parenteral emulsions are natural mixtures of neutral and charged lipids. Mixtures of sparingly soluble fatty amphiphiles combined with more soluble ionic or ionic surfactants, are widely used in dermatological o/w lotions and creams, sometimes in the form of a pre-blended emulsifying wax.
Multiphase emulsions
Emulsifier concentration
In simple, dilute two-phase oil and water emulsions, the stabilizing effect of a mixed surfactant emulsifier at low concentration is generally attributed to its ability to form a close-packed mixed monolayer at the o/w interface, which gives a mechanically strong condensed film. The interfacial film introduces repulsive forces (electrostatic or steric) to inhibit the close approach of droplets, and forms a mechanical barrier to coalescence if droplets do collide.
Most practical pharmaceutical emulsions however, contain higher concentrations of a mixed emulsifier than are necessary to form a monomolecular film. The excess emulsifier interacts with water either at the oil droplet interface or in the bulk continuous phase to form specific lamellar liquid crystalline or crystalline gel network phases. Such emulsions that contain additional phases to oil and water are described as multiphase emulsions (not to be confused with the term multiple emulsion, see Introduction). Lamellar phases are particularly important in controlling the consistency and stability of aqueous lotions and creams stabilized by emulsifying waxes, and the stability of parenteral emulsions containing lecithin.
Lamellar liquid crystalline and gel phases
Lamellar phases are formed in o/w emulsions containing various synthetic and natural emulsifier mixtures under specific conditions. In such lamellar phases, the surfactant molecules are arranged in bilayers separated by layers of water. The hydrocarbon chains of the bilayers can exist in a number of physical states, the most relevant to emulsions being the ordered α-crystalline gel state at low temperatures and the more disordered fluid liquid crystalline state at higher temperatures (Fig. 27.3).
α-Crystalline gel-network phases.
These phases dominate the rheological properties and stabilities of many structured o/w lotions and semisolid creams, and these vastly swollen phases have been considered in detail earlier in this chapter. The viscoelastic gel-network phases essentially stabilize emulsions kinetically by trapping the oil droplets, thus preventing their movement and consequent interaction.
Lamellar liquid crystalline phases.
It has already been noted that the lecithins used to stabilize parenteral emulsions are complex mixtures of neutral lipids (amphiphiles) and small quantities of negatively charged lipids, i.e. the lecithins can be considered as natural emulsifying waxes. With commercial lecithins, the gel-liquid crystalline transition temperatures (which are influenced by the length and degree of saturation or branching of the hydrocarbon chains) are low so that swollen liquid crystalline phases (rather than crystalline gel-network phases) stabilize parenteral emulsions (see Figure 27.3). The liquid crystalline phases do not swell as extensively as crystalline gel-network phases, thus parenteral emulsions are mobile liquids rather than semisolids.
Multilayers of viscous liquid crystals surround the oil droplets in oral and parenteral emulsions stabilized by lecithin which protect them from coalescence by two main mechanisms. Firstly, the multilayers reduce the van der Waals forces of attraction between oil droplets to a very low value, thereby reducing the tendency for collision, and secondly the high viscosity of the liquid crystalline arrays retards the film thinning process of coalescence if droplets do collide. The high viscosities of the liquid crystalline arrays may also protect the emulsion against Ostwald ripening by slowing down the molecular diffusion process.
Nanoemulsion stability
Although both emulsions and nanoemulsions are thermodynamically unstable, the kinetics of destabilization of nanoemulsions is extremely slow so that they are considered to possess a relatively high kinetic stability, sometimes lasting many years. The small droplet sizes of nanoemulsions confer stability against creaming and coalescence because they do not collide as frequently as in ordinary emulsion so that Ostwald ripening alone governs the destabilization process.
Stability testing
Only studies of an emulsion over its full shelf-life will give an accurate picture of its stability. However, useful information can be obtained in a shorter period of time by using accelerated stability testing programmes (Chapter 49). This is accomplished by placing an external stress on the emulsion and observing the changes in one or more of its physical properties. The stresses most commonly used are: freezing and thawing, elevated temperature, UV light sources and various mechanical stresses.
Evaluation of emulsion stability
Several techniques are used to evaluate the stability of the emulsion under one or more of the conditions described above. Instabilities of a chemical or physical nature are generally identified by changes in physical properties of the emulsion. The extent of instability is assessed by measuring the magnitude of the change with time. Techniques commonly used for fluid emulsions involve evaluating changes in appearance, droplet size distribution, droplet charge and rheology in real time, and under accelerated conditions of temperature or force (i.e. centrifugation). With structured and semisolid emulsions, phase changes on storage may cause changes in consistency which not only render the emulsion inelegant and difficult to use, but can also can influence drug delivery. For complex systems, additional methods to identify and evaluate structural changes on storage include thermal methods (differential scanning calorimetry, thermogravimetric analysis) and X-ray diffraction.
Appearance
An approximate estimation of physical stability can be made from a visible assessment of the extent of ‘creaming’ that occurs on storage. Centrifugation is sometimes employed as a means of speeding up this separation process, although the results may be misleading as the centrifugal forces may destroy floccules or structure in the emulsions, which would not occur under normal storage conditions.
Droplet size analysis
Coalescence and/or Ostwald ripening will cause increases in droplet sizes with time and a number of direct and indirect techniques are available for measuring such changes. In emulsions containing droplets greater than 1 µm, optical microscopy is particularly useful because it provides a direct (and reassuring) measurement of individual droplet sizes. The tedium of counting droplets to obtain size distributions is reduced by the use of image analysis. Indirect methods generally involve laser light-scattering techniques (Chapter 9) and are used extensively with emulsions containing sub-micrometre droplets. However, extreme caution should be exercised in interpreting data from such techniques. A major problem is that samples are often diluted before measurements are made, for example to reduce the turbidity for laser diffraction experiments, thus changing the phase volume ratio and characteristics of the original emulsion. In addition, flocculation can give an apparent increase in droplet size distribution, and de-flocculants added before measurements are made may also influence emulsion stability and droplet distribution.
Droplet charge, zeta potential
The zeta potential of emulsion droplets stabilized by a charged interfacial film (Chapter 5) is particularly useful for assessing instability due to flocculation. It can be determined by observing the movement of droplets under the influence of an electric current (electrophoretic mobility measurements), often in conjunction with photon correlation spectroscopy (Chapter 9). This technique is particularly useful for assessing the stability of fat emulsions and other emulsions containing charged droplets, where the presence of additives may result in changes in zeta potential and kinetic instability as droplet sizes increase. For example, decreased stability in fat emulsions after the addition of electrolytes or dextrose (which lowers the pH) can be correlated with a lowering of zeta potential.
Rheological measurements
Rheological measurements are used extensively to evaluate emulsion stability over time (Chapter 6). Changes in droplet size distributions, degree of flocculation and creaming, or phase behaviour of the emulsifiers usually alter the rheological properties. Generally multipoint cone and plate or concentric cylinder rheometers are used to plot the relationship between shear stress and shear rate between suitable limits, and to calculate the apparent viscosities at specific shear rates. Fluid emulsions generally exhibit shear thinning pseudoplastic or plastic behaviour, sometimes accompanied by thixotropy. Semisolid and structured emulsions give much more complex flow curves which are difficult to interpret, although attempts are made to correlate derived parameters, such as apparent viscosities and loop areas, with the qualitative term consistency. Despite difficulties in interpretation, such flow curves are very sensitive to minor changes in the emulsion. More fundamental viscoelastic (creep and oscillation) techniques where the emulsions are examined in their rheological ground states, also enable instability on storage to be monitored. Rheological measurements performed above the phase transition temperature do not relate to the emulsion at rest.
Thermal techniques
Techniques such as differential thermal analysis and thermogravimetric analysis are particularly useful in investigating the phase behaviour of complex multiphase emulsions such as creams, and may be used to follow any phase changes that occur on storage.
X-ray diffraction
High- and low-angle X-ray diffraction experiments provide direct and accurate measurements of bilayer structure and interlamellar water spacing in creams. Such measurements, which were used extensively to develop the gel-network theory, may also be used to investigate phase changes on storage, and to relate such changes to corresponding changes in rheological properties.
Bibliography
1. Anton N, Vandamme TE. Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharmaceutical Research. 2011;28:978–985.
2. Eccleston GM. The functions of mixed emulsifiers and emulsifying waxes in dermatological lotions and creams. Colloids and Surfaces. 1997;123:169–182.
3. Eccleston GM, Behan-Martin MK, Jones G, Townes-Andrews E. Synchrotron X-ray Investigations into the lamellar gel phase formed in pharmaceutical creams prepared with cetrimide and fatty alcohols. International Journal of Pharmaceutics. 2000;203:127–139.
4. Eccleston GM. Emulsions and microemulsions. In: Encyclopaedia of Pharmaceutical Science and Technology. New York: Informa Healthcare Inc.; 2013;1548–1564. Swarbrick J, Boylan JC, eds. Encyclopaedia of Pharmaceutical Science and Technology vol. 1.
5. Florence AT, Attwood D. Physicochemical Principles of Pharmacy. 5th edn London: Pharmaceutical Press; 2011.
6. Jones D. Pharmaceutics-Dosage Form Design. London: Pharmaceutical Press; 2008.
7. Rowe RC, Sheskey PJ, Cook WG, Fenton ME. Handbook of Pharmaceutical Excipients. 7th edn London: The Pharmaceutical Press; 2012.