Embryology and Brain Development
The human brain undergoes four phases of development: (1) dorsal induction (primary and secondary neurulation), (2) ventral induction (patterning of the forebrain), (3) neuronal proliferation and migration, and (4) myelination. During the third week of embryogenesis, initiation of the central nervous system evolves with the development of the notochordal process. This derivative of ectoderm grows rapidly in length so that by 20 days it is converted from a hollow tube to a solid rod—the notochord (Fig. 24-1; e-Table 24-1). The notochord works with the axial mesoderm to induce the neural plate. The neuroepithelium of the neural plate begins the formation of the brain and spinal cord. It appears initially at the cranial end of the embryo and differentiates craniocaudally. At the beginning of the fourth week, the neural plate is composed of a broad cranial portion and a narrow caudal portion—the fetal brain and spinal cord.
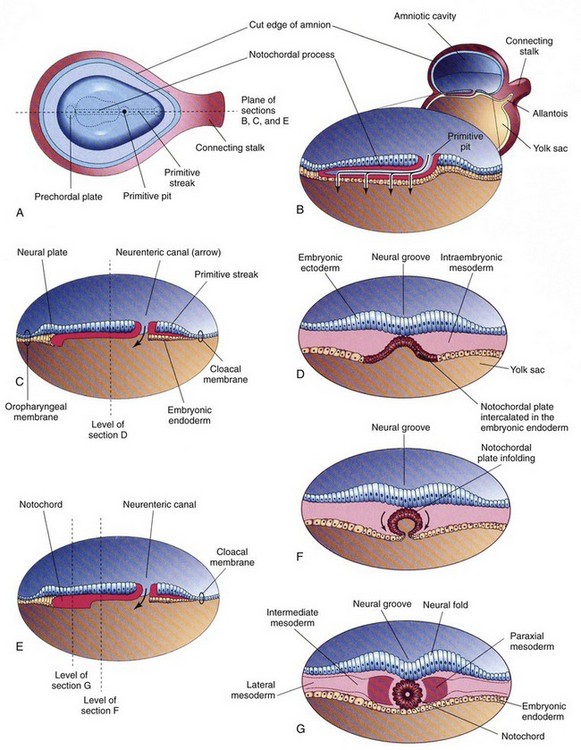
Figure 24-1 Further development of the notochord by transformation of the notochordal process.
A, Dorsal view of the embryonic disc (about 18 days), exposed by removing the amnion. B, Three-dimensional median section of the embryo. C and E, Similar sections of slightly older embryos. D, F, and G, Transverse sections of the trilaminar embryonic disc shown in C and E. (From Moore KL, Persaud TVN. Before we are born. 5th ed. Philadelphia: WB Saunders; 1998.)
e-Table 24-1
Schematic Chronology of the Major Events During Human Neocortical Development
| Event | Time Event Occurs |
| Neuroectoderm induction | Third GW |
| Neurulation | Third to end of fourth GW |
| Proencephalic and hemispheric formation | Fifth to tenth GW |
| Neuronal proliferation | Tenth to twentieth GW |
| Neuronal migration | Twelfth to twenty-fourth GW |
| Programmed neuronal cell death | Twenty-eighth to forty-first GW |
| Neurogenesis | Fifteenth to twentieth GW to ? postnatal months or years |
| Synaptogenesis | Twentieth GW to puberty |
| Gliogenesis | Twenty to twenty-fourth GW to ? postnatal years |
| Myelination | Twenty-sixth to twenty-eighth GW to 2 to 3 postnatal years |
| Angiogenesis | Fifth to tenth GW to ? postnatal years |
From Gressens P, Helppi PS. Normal and abnormal brain development. In Martin R, Fanaroff A, Walsh M, eds. Neonatal-perinatal medicine. 8th ed. Elsevier Mosby; 2005.
Dorsal Induction
The process of neurulation or formation of the neural tube occurs when the lateral edges of the neural plate elongate to become neural folds and join together to form the neural tube (Fig. 24-2).1 The process starts at the craniocervical region and proceeds superiorly and inferiorly. The space within the neural tube, the neural canal, initially is open at the cranial (or rostral) and caudal ends (cranial and caudal neuropores) and communicates with the amniotic cavity. The neuropores gradually decrease in size and close between the twenty-fourth and twenty-sixth day, apparently at multiple sites and not necessarily in a craniocaudal fashion.1 The lowest portion of the spinal cord, the inferior sacral and coccygeal levels, are formed by a different process called secondary neurulation (see Chapters 40 and 43). Pluripotent tissue within the caudal eminence forms a solid neural cord; this neural cord forms a lumen that fuses with the neural tube (e-Fig. 24-3). This secondary process is not completed until the eighth week after fertilization of the ovum.1
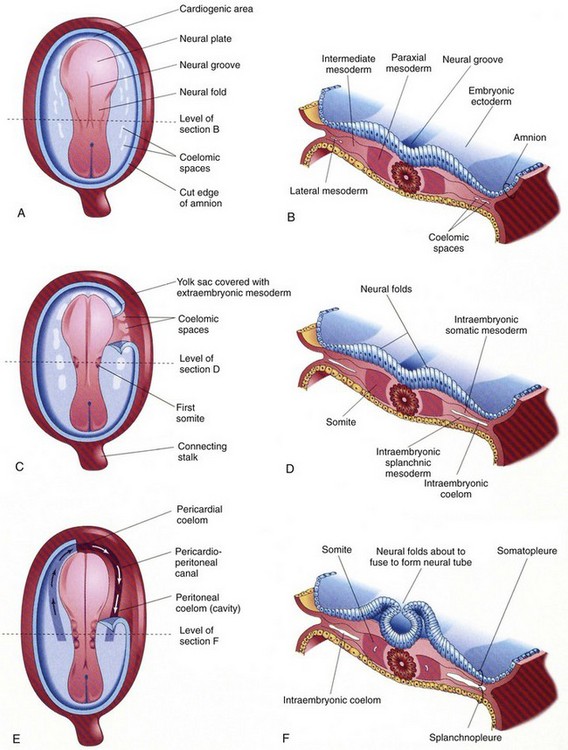
Figure 24-2 Drawings of embryos of 19 to 21 days, illustrating development of the somites and intraembryonic coetom.
A, C, and E, Dorsal view of the embryo, exposed by removal of the amnion. B, D, and F, Transverse sections through the embryonic disc at the levels showing. A, Presomite embryo of about 18 days. C, An embryo of about 20 days, showing the first pair of somites. A portion of the somatopleure on the right has been removed to show the isolated coelomic spaces in the lateral mesoderm. E, A three-somite embryo (about 21 days) showing the horseshoe-shaped intraembryonic coelom, exposed on the right by removal of a portion of the somatopleure. (From Moore KL, Persaud TVN. Before we are born. 5th ed. Philadelphia: WB Saunders; 1998.)
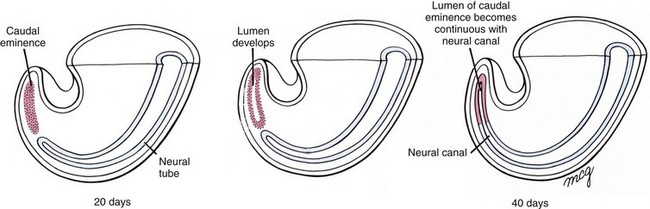
e-Figure 24-3 Formation of the neural tube inferior to the second sacral level by secondary neurulation.
Mesoderm invading this region during gastrulation condenses into a solid rod called the caudal eminence, which later develops a lumen. At the end of the sixth week, this structure fuses with the neural tube. (From Larsen WJ. Human embryology. 2nd ed. New York: Churchill Livingstone; 1997.)
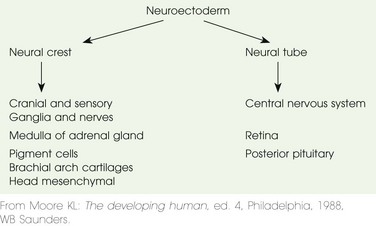
Some neural crest cells (the population of neural cells arising at the lateral edge of the neural plate during neural tube formation) detach during neurulation and form many different tissues (e.g., melanocytes and chromaffin cells of the adrenal medulla), including the major components of the peripheral and autonomic nervous system (e-Table 24-2).1
Ventral Induction
The prechordal plate cephalic to the neural tube and notochord induces this stage of development. The three major divisions of the brain—the prosencephalon or forebrain, mesencephalon or midbrain, and rhombencephalon or hind brain—are more clearly differentiated during the rostral expansion of the neural tube (the formation of the primary brain vesicles).1,2 During the fourth and fifth weeks, a series of brain foldings occur (brain flexures—midbrain, cervical, and later pontine) so that by the end of the fifth week, five secondary brain vesicles are present (Table 24-3). With further development, the prosencephalon becomes subdivided into the telecephalon and the diencephalon. The rhombencephalon subdivides into the metencephalon and myelencephalon. The mesencephalon does not divide. Within each of these five vesicles, the neural canal expands into a primary ventricle. The central canal of the spinal cord is continuous with the brain ventricles. The fate of these structures is shown in Table 24-3. The cranial nerve nuclei appear in the brainstem during the fifth week (see the next section).1,2
Table 24-3
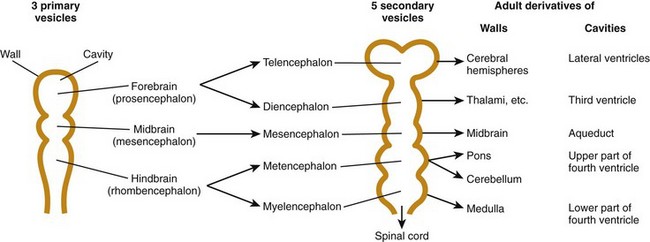
From Moore KL, Persaud TVN. Before we are born. 5th ed. Philadelphia: WB Saunders; 1988.
Neuronal Proliferation and Migration
During the period of morphologic development of the brain, cytodifferentiation occurs. Formation of the cerebral neocortex is a complex process with proliferation, migration, and differentiation varying in time from one site to another. Several patterns of neuronal migration occur, including (1) radial migration along the path of radial glial cells from the ventricles toward the surface and (2) tangential migration along transverse surfaces (e-Fig. 24-4).1–3
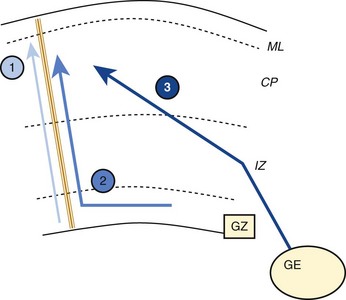
e-Figure 24-4 Schematic representation of the different migratory pathways adopted by neurons.
1, Radial migration along radial glial cell guides of neurons originating from the periventricular germinative zone (GZ). 2, Tangential migration in the GZ followed by a radial migration along glial guides. 3, Tangential migration in the intermediate zone (IZ, prospective white matter) of neurons originating from the ganglionic eminence. CP, Cortical plate; ML, molecular layer. (From Martin RJ, Fanaroff AA, Walsh MC, eds. Fanaroff and Martin’s neonatal perinatal medicine. 8th eds. Philadelphia: Mosby Elsevier; 2006.)
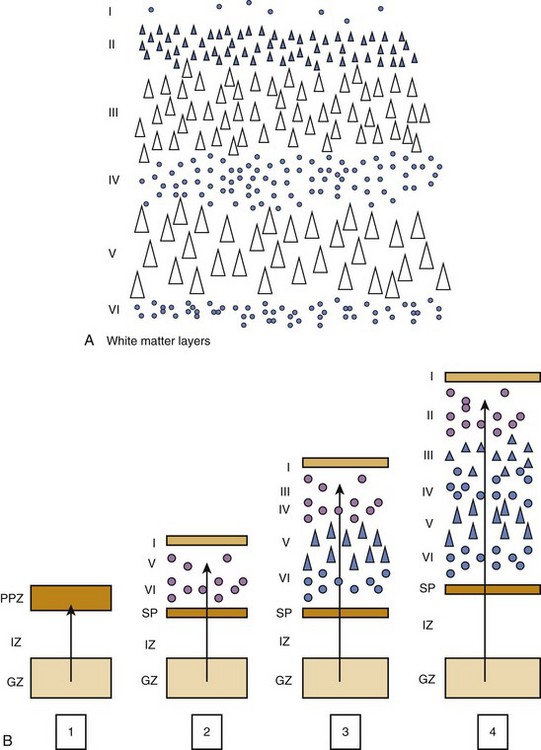
Although details of the migration process vary for each region of the brain, general principles include the following: (1) the ultimate migration is from deep (ventricle) to superficial regardless of trajectory differences; (2) the migration establishes layers of the cortex (Figs. 24-5 and 24-6); and (3) neurons of each later wave of migration pass through the preceding layers to form a more superficial layer.4
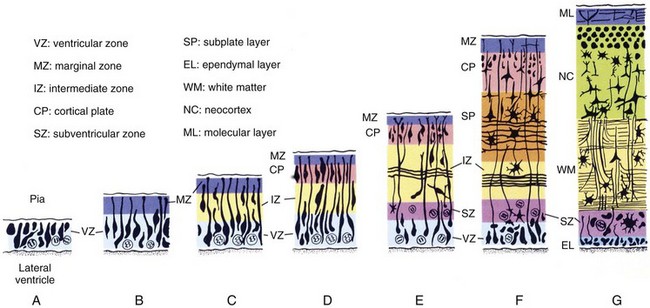
Figure 24-5 Cytodifferentiation of the cerebral neocortex.
Although the timing of neuroblast formation varies widely in different regions of the cerebral hemispheres, the general scheme illustrated here is typical for all regions. (From Larsen WJ. Human embryology. 2nd ed. New York: Churchill Livingstone; 1997.)
During the tenth to twentieth weeks of gestation, neuronal genesis occurs. The proliferative cells of the ventricular level arising from the germinal matrix form waves of migrating neuroblasts and all precursor cells. After 20 weeks, only the glial cells are produced by this substance.4 The germinal matrix eventually disappears by term but is significantly diminished by 32 weeks.3 The subventricular zone produces neuroblasts after 20 weeks, and these neuroblasts migrate through the intermediate zone to form the cortical subplate.
Each new layer migrates through the preceding layer to become more superficial. The mature cortex has six layers, and neuronal migration concludes between 20 and 24 weeks’ gestation. The subplate and cortical plate form the cortex, whereas the intermediate zone becomes the white matter (see Fig. 24-5). The germinal matrix also produces the glial cells, that is, astrocytes and oligodendrocytes.4 The microglial cells are of mesodermal origin and are the resting tissue histiocytes of the brain. In contrast to cortical migration, glial cell migration and differentiation continues for more than 1 year after birth. Radial migration is the process whenever the radial glial cells provide a link between the ventricular zone of the neural tube and the pial surface, which provides a tract along which the neuroblasts migrate. Once the neuroblasts reach their destination, they detach from the radial glial tracts.4
The steps of neurulation, cell proliferation, and migration are induced by multiple neurotransmitters, and genes controlling these transmitters have been identified. An example is found in e-Table 24-4, which shows the genetics of radial migrations in the cortex.5
e-Table 24-4
Genetics of Radial Migration in the Cortex
| Gene | Name and Function | Description of Mutation |
| Astn1 | Astrotactin1; neuron-glia adhesion molecule | Decreased neuron-radial glia binding, slowed radial migration |
| Itg3a | ą3 Integrin; cell adhesion receptor, interacts with Reelin | Abnormal laminar position of cortical projection neurons, which tend to occupy deeper positions than normal |
| Itg6a | ą6Integrin; cell adhesion receptor | Abnormal laminin deposition, cortical layer perturbation without layer inversion |
| Itgb1 | β1 Integrin; cell adhesion receptor, interacts with Reelin | Abnormal basement membrane remodeling, cortical layer perturbation without layer inversion |
| LamcI | γ1 Laminin; ECM structural constituent | Abnormal basement membrane, cortical layer perturbation without layer inversion |
| Pafahlb1 | Platelet-activating factor acetythydrolase isoform lb, βl subunit; interacts with tubulin, dynein. NUDEL, mNudE | Also known as Lis1, its mutation causes lissencephaly in humans; decreased rate of migration and cortical disorganization in mice |
| Dcx | Double Cortin; microtubule-associated protein | Lissencephaly in humans; disrupted hippocampal cytoarchitecture but normal neocortical lamination in mice |
| Flna | Filamin alpha, actin-binding protein | Also known as Filamin 1, its mutation causes periventricular heterotopia in humans |
| ReIn | Reelin, ECM secreted protein | Lissencephaly in humans; inverted cortical layering, including the subplate, in reeler |
| Vldlr | Very low density lipoprotein receptor, Reelin receptor | Same as reeler when mutated simultaneously with Lrp8 |
| Lrp8 | Low-density lipoprotein receptor-related protein 8, Reelin receptor | Same as reeler when mutated simultaneously with Vldlr; also known as ApoE receptor 2 |
| Dab1 | Disabled homolog 1; interacts with Vldlr, Lrp8 | Same as reeler; mutated in scrarnbler and yotari |
| Cdk5 | Cyclin-dependent kinase 5; phosphorylate Dab 1 and NUDEL | Inverted cortical layering, without affecting the subplate |
| Cdk5r1 | Cyclin-dependent kinase 5, regulatory subunit 1 (p35) | Same as Cdk5 when mutated simultaneously with Cdk5r2 |
| Cdk5r2 | Cyclin-dependent kinase 5, regulatory subunit 2 (p39) | Same as Cdk5 when mutated simultaneously with Cdk5r1 |
From Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci 2003;26:441-483.
The brainstem and spinal cord are similar in development. Two basal (ventral) columns and two alar (dorsal) columns are formed. These columns form a dorsal sensory and ventral motor configuration and are found in both the brainstem and spinal cord. The cranial nerves IX through XII come from the mesencephalon (medulla) and cranial nerves IV through VII form the metencephalon (pons) (see Table 24-3). The pons is largely composed of white matter tracts of the cerebellum. The cerebellum is also derived from the metencephalon (see Table 24-3).
Fetal Development in the Preterm Infant
1. The fetal ventricles are large relative to the size of the brain and have a posterior (occipital) predominance that decreases after 25 weeks.
2. The fluid-filled spaces of the brain are large; subarachnoid spaces are increased in size relative to size of the brain, and the cisterna magna is larger than at term.
3. The germinal matrix, for aforementioned reasons, is quite large but decreases after 26 weeks.
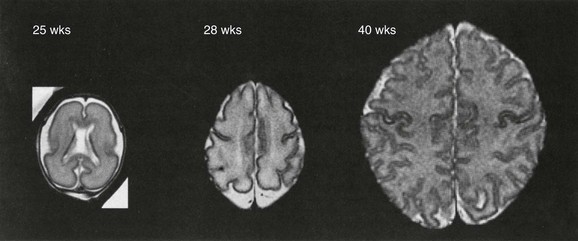
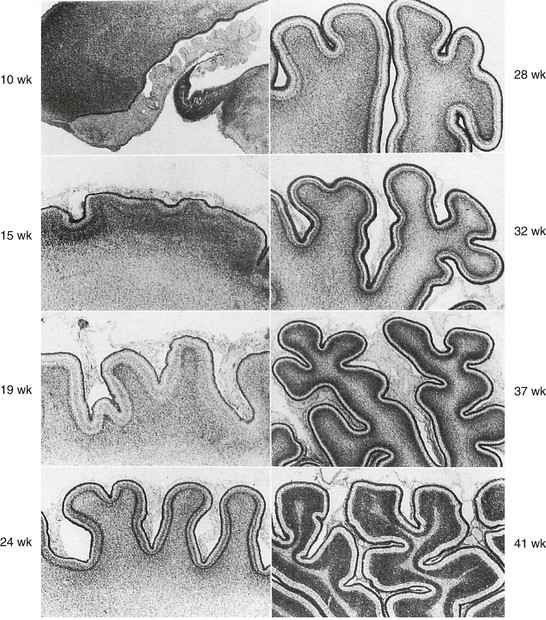
4. Sulcation and gyration follow a predictable course (Figs. 24-7 and 24-8).6
The normal biochemical profile follows the type and amount of cells present in any given stage of development. This can be seen through the normal but different spectrographic patterns (e-Fig. 24-9).3 Normal myelinization occurs via a defined order from the third trimester onward during the first year of life (Table 24-5).7,8 Generally, myelination proceeds caudal to rostral and posterior to anterior.
Table 24-5
Sequence of Myelination Based on Histologic Analysis and Magnetic Resonance Imaging
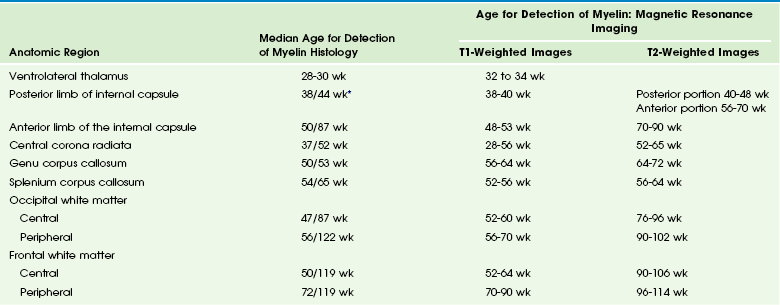
*The first number corresponds to earliest identification of some myelin tubules by microscopic examination of hematoxylin and eosin stained sections. The second number corresponds to mature myelin stained with blue dye by eye observation.
From Martin RJ, Fanaroff AA, Walsh MC, eds. Fanaroff and Martin’s neonatal perinatal medicine. 8th ed. Philadelphia: Mosby Elsevier; 2006.
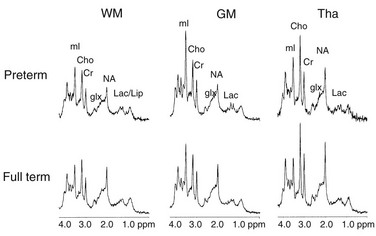
e-Figure 24-9 Spectra during human functional development in different regions of the brain.
Metabolites identified are lactate (Lac), lactate/lipid (Lac/lip), N-acetyl aspartate (NA), glutamine and glutamate (Glx), creatine and phosphocreatine (Cr), choline (Cho), and myo-inositol (mI). Spectra are all scaled identically. Developmental changes in metabolic concentrations are illustrated by different peak heights comparing spectra from preterm and full-term newborns. GM, Gray matter; Tha, thalamus; WM, white matter. (Spectra analyzed by Roland Kreis, MR Center, Inelspital, University of Berne, Berne, Switzerland.) (From Martin RJ, Fanaroff AA, Walsh MC, eds. Fanaroff and Martin’s Neonatal Perinatal Medicine. 8th ed. Philadelphia: Mosby; 2006.)
Normal Anatomy
The result of these complex processes result in the mature brain and spinal cord. Neuroanatomic descriptions in detail are beyond the scope of this text, but many atlases of anatomy and imaging are available. Comparison of normal development with pathologic development will be discussed in Chapter 31.
References
1. O’Rahilly, R, Muller, F. Human embryology & teratology, 3rd ed. New York: Wiley Liss; 2001.
2. O’Rahilly, R, Muller, F. The embryonic human brain. An atlas of developmental stages, 3rd ed. New York: Wiley Liss; 2006.
3. Gilles, FH, Nelson, MD, Jr. The developing human brain. Growth and adversities. London: Mac Keith Press; 2012.
4. Marin, O, Rubenstein, JLR. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483.
5. Clark, GD. Brain development and the genetics of brain development. Neurol Clin N Am. 2002;20:917–939.
6. Chi, JG, Dooling, EC, Gilles, FH. Gyral development of the human brain. Ann Neurol. 1977;1(1):86–93.
7. Brody, BA, Kinney, HC, Kloman, AS, et al. Sequence of central nervous system myelination in human infancy, I: an autopsy study of myelination. J Neuropathol Exp Neurol. 1987;46:283–301.
8. Kinney, HC, Brody, BA, Kloman, AS, et al. Sequence of central nervous system myelination in human infancy, II: patterns of myelination of autopsied infants. J Neuropathol Exp Neurol. 1988;47:217–234.