Embryology, Anatomy, Normal Findings, and Imaging Techniques*
Anatomy of the Skull
Indications for plain film skull examination are listed in Box 18-1. Computed tomography (CT) and magnetic resonance imaging (MRI) generally are used for detailed evaluation of facial structures and intracranial contents.
Neonatal and Infant Skull
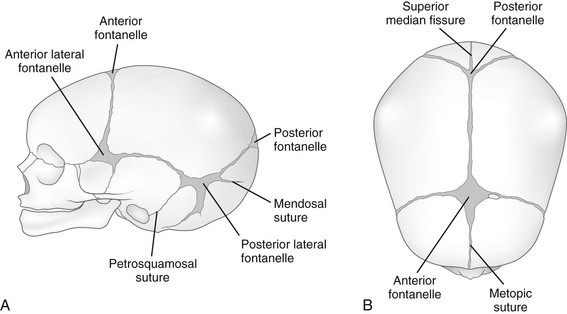
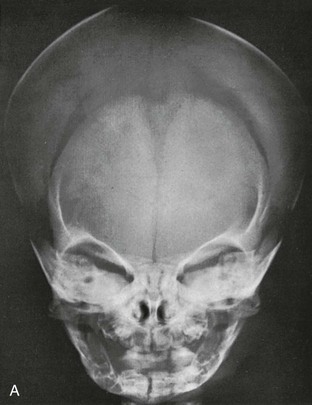
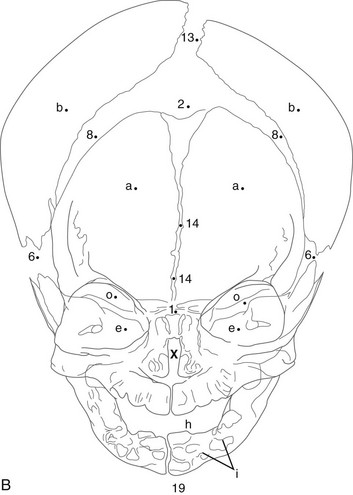
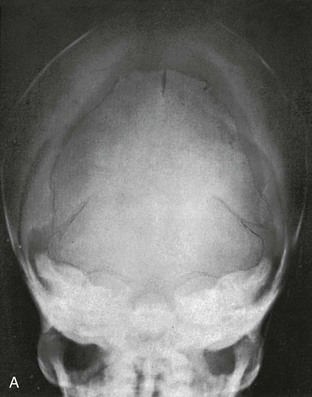
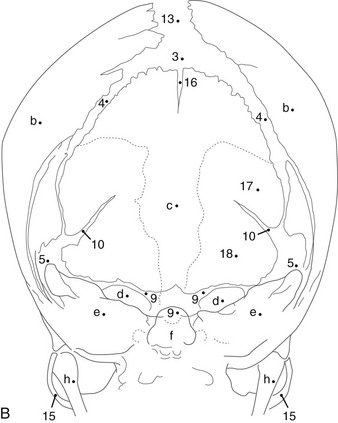
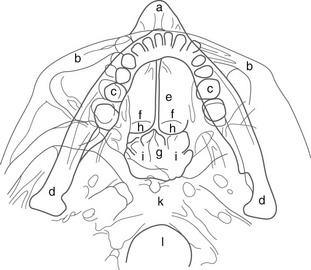
During infancy the neurocranium is larger relative to the face than at any other time during normal growth. Ratios of the respective areas of the neurocranium in lateral projection are roughly 3 : 1 to 4 : 1 at birth, and they decrease to 2 : 1 to 2.5 : 1 by age 6 years. The bones of the calvarium lie in their incompletely mineralized membranous capsule; they are separated by broad strips of connective tissue that form the sutures and by patches of connective tissue, the fontanelles. The six constant, or major, fontanelles are located at the four corners of the parietal bones—two in the midline of the skull and two pairs on each side (Fig. 18-1). Accessory fontanelles may occur in several parts of the cranium but usually are located in the sagittal suture. The sutures and the synchondroses in the base are prominent in newborns but diminish in width during the first 2 to 3 months. Obliteration of the sutures does not begin until the second to third decades. Figures 18-1 through 18-4 and e-Figure 18-5 illustrate sutures, fontanelles, and synchondroses.
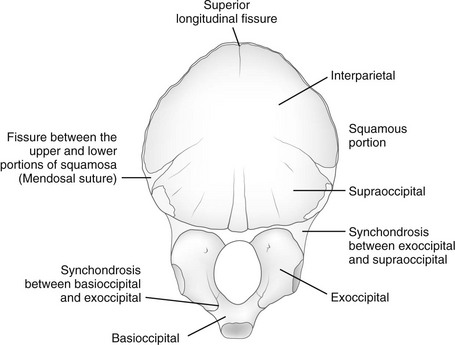
e-Figure 18-5 Occipital bone at birth, internal surface.
The sphenoid bone at birth consists of a single central mass composed of the body and the lesser wings and two symmetric lateral osseous masses, each of which is made up of a greater wing and a pterygoid process. The pituitary fossa in the body of the sphenoid bone tends to be round with smooth margins; the dorsum sella is short and blunt, and the clinoid processes are rudimentary. The angle between the body of the neonatal mandible and the ascending ramus in lateral projection is about 160 degrees; the relatively large bodies are separated in the midline by a prominent cartilaginous symphysis mentalis (see Fig. 18-3). Early calcification of teeth is seen in the fifth fetal month.1
Components of the individual bones that are not united in infancy may lead to confusion unless they are correctly recognized. The frontal bone is divided in half laterally by the metopic suture (see Figs. 18-1 and 18-3). Apparent discontinuity of the sphenoid bone with the frontal bone superiorly and the occipital bone posteriorly indicates the sites of the sphenoid bone’s synchondroses with these two bones (see Fig. 18-2). The four major components of the occipital bone (e-Fig. 18-5 and Fig. 18-2) likewise may simulate discontinuities of structure.
Growth and Development
With increasing age, the fontanelles and sutures become smaller and narrower. The anterior fontanelle usually is reduced to fingertip size during the first half of the second year; the posterior fontanelle may be closed at birth (range of closure: birth to several months). Closure of the fontanelles occurs clinically before it is seen radiographically. The metopic suture is quite variable and may be obliterated at birth, but it usually is closed during the third year; however, it persists throughout life in about 10% of cases. In the occipital bone, the mendosal suture (see Fig. 18-4 and e-Fig. 18-5) usually disappears during the first 2 years, but it too can persist; the synchondrosis between the supraoccipital and exoccipital (supracondylar) portions usually disappears during the second or third year. The spheno-occipital synchondrosis begins to close near the time of puberty but may persist until the twentieth year. This variation and irregularity make suture lines unreliable criteria for estimation of the developmental age of the skull. At about the twentieth year, the skull attains its definitive size.
Normal Variations
Intrasutural, or wormian, bones occur most frequently along the lambdoid sutures (Fig. 18-6 and e-Fig. 18-7; Box 18-2). They occur much less frequently in the fontanelles (see e-Fig. 18-7). The interparietal or Inca bone (Fig. 18-8) results from division of the supraoccipital portion of the occipital bone into two parts by the mendosal suture, with the superior part arising from membranous bone and the inferior part arising from cartilage continuous with that of the supracondylar portions and the basiocciput. A rare synchondrosis or suture line runs vertically through the squamous portion of the occipital bone (Fig. 18-9); persisting superior and inferior portions of the line are known as the superior longitudinal fissure or bi-interparietal suture and the cerebellar synchondrosis or median cerebellar suture. Where the supraoccipital portion of the occipital bone forms the posterior border of the foramen magnum, accessory supraoccipital bones occasionally are found (e-Fig. 18-10). The configuration caused by an outward bulge of the occipital squamosa just above the torcular Herophili in a newborn (Fig. 18-11) is called bathrocephaly. Rarely, a horizontal interparietal suture divides the parietal bones into superior and inferior moieties (e-Fig. 18-12).
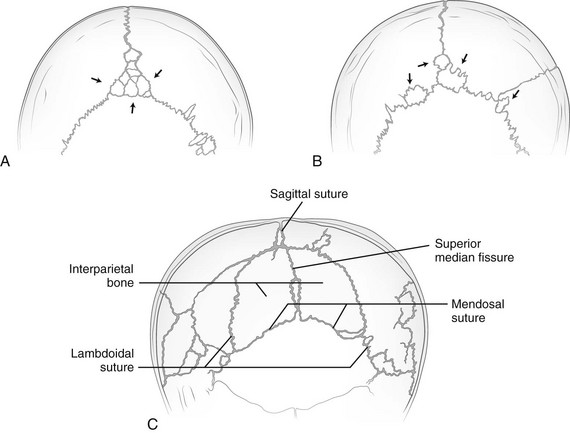
Figure 18-6 Tracings of films showing wormian bones.
A, Multiple wormian bones (arrows) in the sagittal suture. B, Multiple wormian bones (arrows) in the sagittal and lambdoid sutures. C, Interparietal bone bounded by the lambdoid and persistent mendosal sutures. The superior median fissure also is still present and divides the interparietal bone into right and left halves.
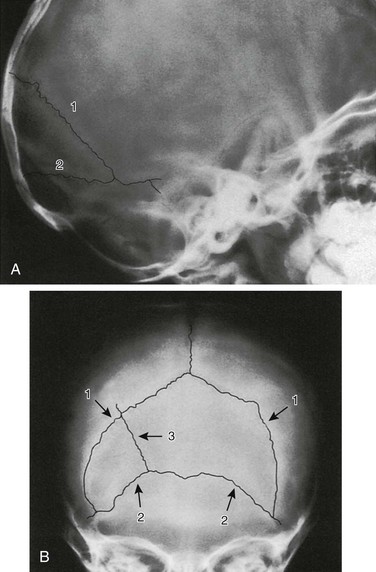
Figure 18-8 Interparietal (Inca) bone.
A, Lateral projection. B, Anteroposterior Towne projection. 1, Right and left lambdoid sutures; 2, mendosal suture; 3, accessory suture in interparietal bone. (Courtesy Dr. J.P. Dorst, Baltimore, MD.)
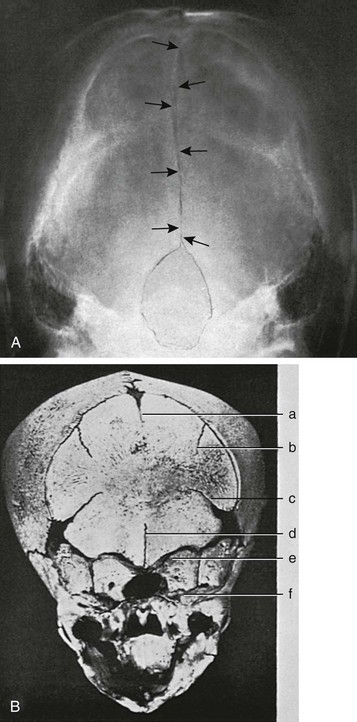
Figure 18-9 Occipital bone.
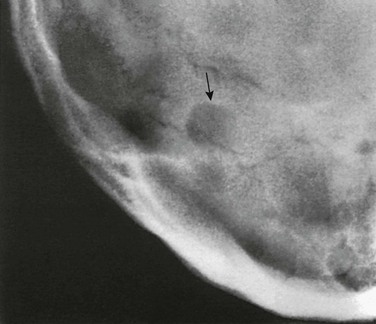
A, A Towne projection showing radiolucent midline longitudinal or cerebellar synchondrosis (arrows) in the occipital squamosa of an 11-year-old girl, which resulted from failure of fusion mediad of its lateral paired ossification centers. This radiolucent strip can be mistaken for a fracture line. (Sutures were retouched with a pencil.) B, Persistent longitudinal or cerebellar synchondrosis (d) in the occipital squamosa of a skull of a newborn; only the caudal segment of the synchondrosis is still open in this case. a, superior median longitudinal fissure; b, superior lateral longitudinal fissure; c, mendosal suture; e, synchrondrosis between exoccipital and supraoccipital bones; f, synchrondrosis between basioccipital and exoccipital bones. (From Koehler A, Zimmer EA. Borderlands of the normal and early pathologic in skeletal roentgenology. 3rd ed. Philadelphia: Grune & Stratton [translated from German ed 11 by S.P. Wilk]; 1968.)
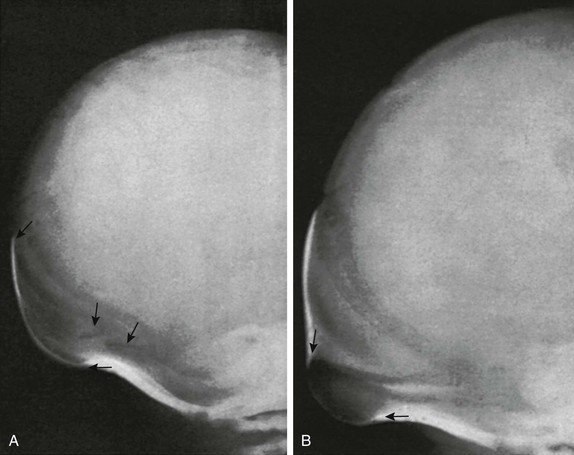
Figure 18-11 Bathrocephaly in a newborn.
A, The external bulge (arrows) extends from the lambda downward to the level of the mendosal suture of a normal 3-day-old infant. B, The bulge (arrows) in this normal 5-day-old infant begins below the mendosal suture.
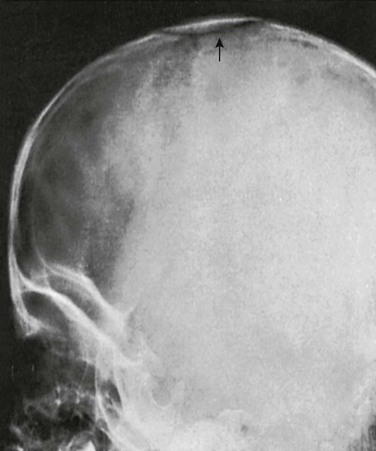
e-Figure 18-7 A large independent bone (arrow) in the membrane of the anterior fontanelle of a normal 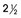 -year-old girl.
-year-old girl.
Normal variants of this type must be kept in mind when evaluating films made after trauma. (Courtesy Dr. B.R. Girdany, Pittsburgh, PA.)
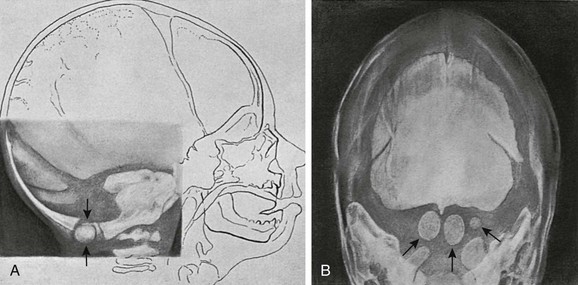
e-Figure 18-10 Accessory bones of the supraoccipital bone.
A, Lateral projection on the second day of life shows separate bony images (arrows) in the innominate synchondrosis. B, A Towne projection of the same skull on the 20th day of life. Three ossicles (arrows) are present in the cartilage above the foramen magnum, along with a midline bony process on the under edge of the supraoccipital that is similar to the fetal process in this same site described by Kerckring in Spicilegium Anatomicum (Amsterdam, 1670).
e-Figure 18-12 Bilateral bifid parietal bones, each of which is divided into an upper and lower segment by an extra horizontal suture.
The flattening of the occipital squamosa is postural in origin. The infant was 6 months old and had not experienced a head injury. Lateral (A) and frontal (B) projections are shown. Upper arrows point to the anomalous horizontal intraparietal sutures, and lower arrows point to the normal squamosal (parietotemporal) sutures. (Courtesy Dr. B.R. Girdany, Pittsburgh, PA.)
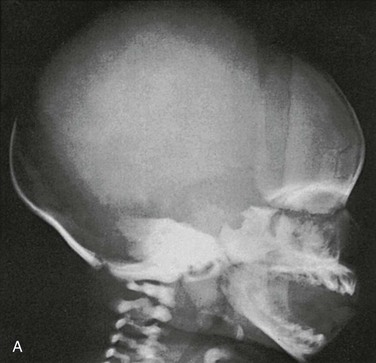
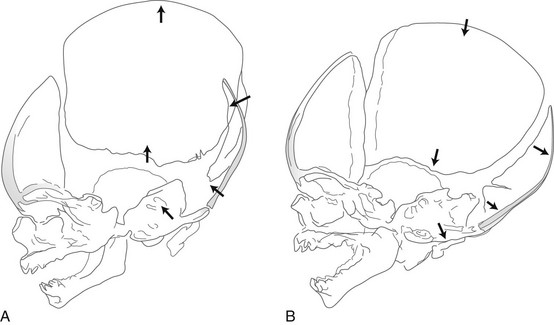
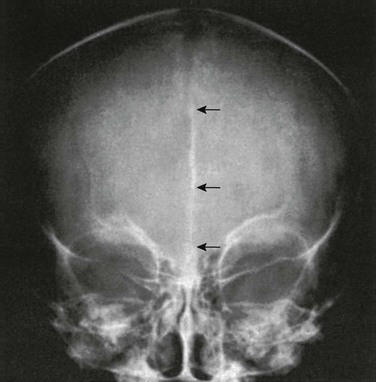
Compression of the fetal skull and its molding during passage through the maternal pelvis produce significant radiographic findings that persist after birth (Fig. 18-13).2 During the first weeks and months of life, widths of sutures vary so much that caution is required in their evaluation for the diagnosis of increased intracranial pressure, particularly because positioning is difficult and partial superimposition of bilateral sutures can produce spurious widening (Fig. 18-14).
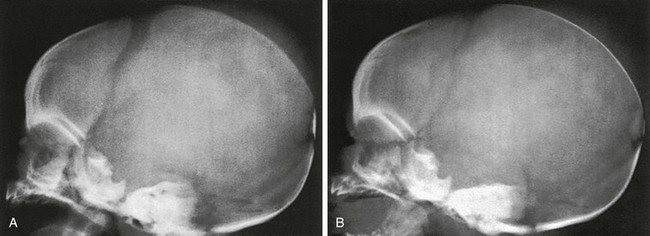
Figure 18-14 Factitious widening of the coronal suture.
A, A slightly oblique projection in which the right and left limbs of the coronal suture overlap. B, A projection that is a little more oblique than A in which the individual, narrower, right and left limbs are seen.
In children older than 2 years, the sutures extend through both tables and the diploic space. The outer table portion of the suture may be deeply serrated when the inner table portion is practically a straight line (e-Fig. 18-15) and may be interpreted erroneously as a “fracture through a suture.” Persistence of the metopic suture may simulate a vertical fracture in the occipital bone in anteroposterior, caudally angulated exposures if extension of the superimposed radiolucent line into the area of the foramen magnum is invisible or if the inferior portion of the suture has been obliterated. The frontal crest on the internal surface of the frontal squamosa in the midsagittal plane may be sufficiently prominent to simulate calcification of the falx cerebri that attaches to it (e-Fig. 18-16).
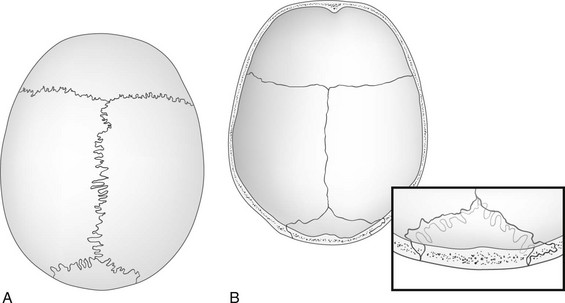
e-Figure 18-15 Drawings of anatomic specimens show the relationship of the outer and inner tables and diploic space of the calvaria to the great sutures.
A, The external aspect of the calvaria shows deep serrations in sutures of the outer table. B, The internal aspect shows the straight, nonserrated sutures of the inner table. The inset drawing depicts the lambdoid suture near the lambda; the differences in position and course of the sutures of the outer and inner tables are shown. In the diploic space, the suture runs in a plane at approximately right angles to the course of the sutures in the tables.
Juvenile Skull
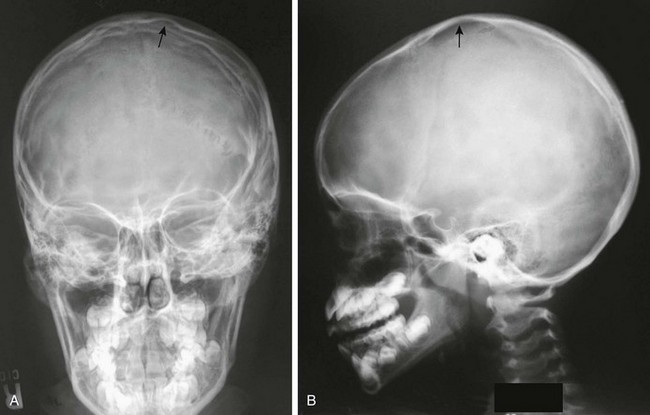
After a child is 2 years old, the radiographic appearance of the skull is similar in most respects to that of the skull during adult life (Fig. 18-17). With advancing age, the skull gradually grows and differentiates until late in childhood, when all of the essential characteristics of the adult skull have developed (Figs. 18-18 through 18-20).
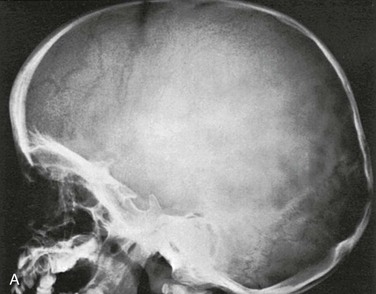
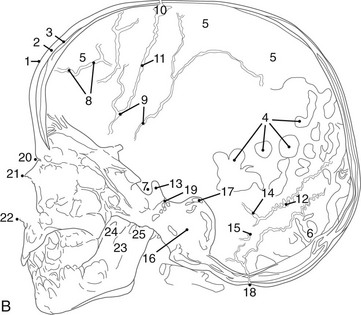
Figure 18-17 A radiograph of the normal skull at 2 years.
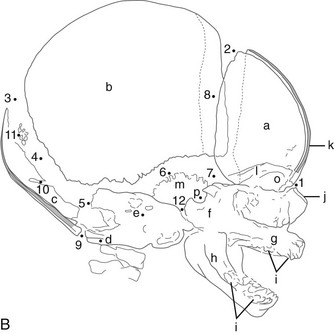
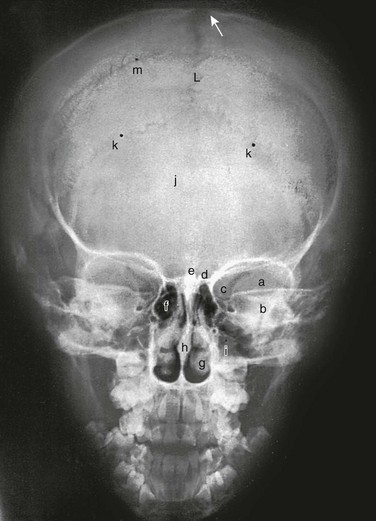
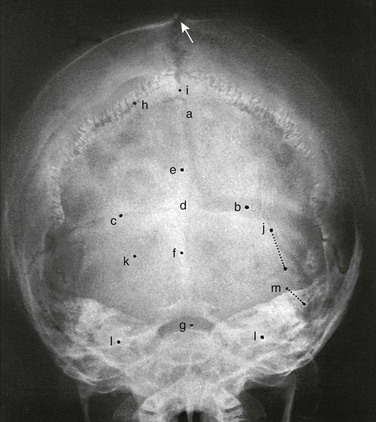
A, Lateral projection. B, Tracing of A. 1, Outer table; 2, diploic space; 3, inner table; 4, convolutional markings; 5, fine honeycomb of diploic structure; 6, internal occipital protuberance; 7, pituitary fossa; 8, diploic veins; 9, vascular grooves; 10, anterior fontanelle; 11, coronal suture; 12, lambdoid suture; 13, dorsum sellae; 14, parietomastoid suture; 15, occipitomastoid suture; 16, petrous pyramids; 17, small temporal pneumatic cell; 18, synchondrosis between exoccipital and supraoccipital; 19, spheno-occipital synchondrosis; 20, nasofrontal suture; 21, nasal bone; 22, anterior nasal spine; 23, mandible; 24, coronoid process of the mandible; 25, condyloid process of the mandible.
Normal Variations
Convolutional (Digital) Markings
Convolutional (digital) markings are areas of diminished density in the calvarium that are separated by strips of normal density (Fig. 18-21). These areas correspond closely to the location and configuration of cerebral convolutions.3 They probably are formed by localized pressure of the pulsating brain on the inner table of the neurocranium.
Diploic and Vascular Markings
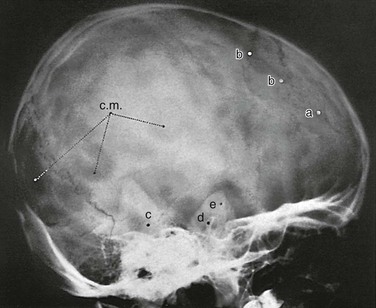
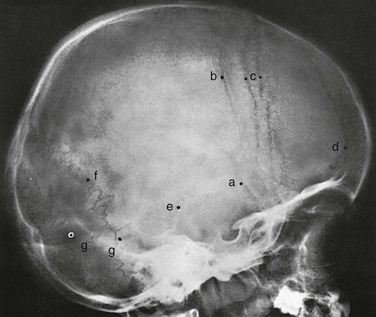
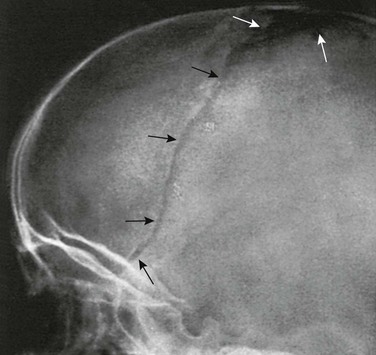
The diploic space between the outer and inner tables of the calvaria is filled with a cancellous bony structure that varies in volume and pattern and is responsible for the fine, honeycomb texture of the cranial vault. The diploic veins lie in large, irregular channels that appear in radiographs as irregular strips of diminished density extending through the bones of the vault in all directions (e-Fig. 18-22 and Fig. 18-23). The diploic veins vary in size, course, and visibility.
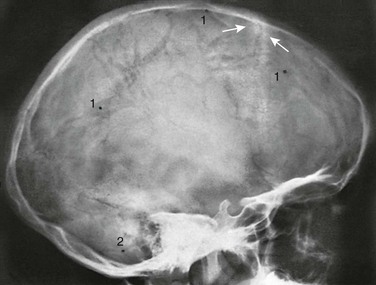
Figure 18-23 Conspicuous large diploic veins in the frontal and parietal bones in an 8-year-old girl.
The veins appear as wide strips of diminished density coursing through the frontal and parietal bones. Arrows are directed at the physiologic hyperostotic ridges on either side of the coronal suture. 1, Diploic venous lake; 2, groove for the emissary vein of the mastoid.
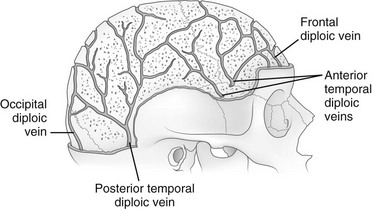
e-Figure 18-22 The veins of the diploic space.
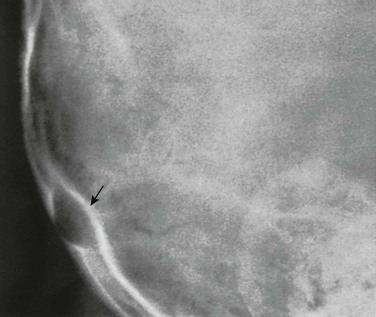
The grooves on the internal aspect of the calvarium for the arteries and veins appear in radiographs as strips of diminished density (Fig. 18-24). Compared with the venous grooves, the arterial grooves tend to taper more. The most constant of these channels is that of the middle meningeal artery, which courses upward and backward from the region of the pterion, where it may be surrounded by bone of the inner table. The largest and heaviest vascular markings are the bony thinnings over the dural venous sinuses. The superior sagittal sinus lies in a shallow groove on the internal surface at the median plane of the vault near the attachment of the falx cerebri. At the torcular Herophili, the channels for the superior longitudinal and transverse sinuses meet; one transverse sinus may be appreciably larger and deeper than the other (see Fig. 18-18). The torcular Herophili in lateral projections may simulate an abnormal defect when it is unusually deep (e-Fig. 18-25). At the bend where the transverse sinus turns caudad, near the mastoid process, superimposition of the lateral end of the sulcus of the transverse sinus and the sulcus of the sigmoid sinus may produce a rounded, radiolucent patch when these sulci are unusually deep (e-Fig. 18-26). Often the groove for the bregmatic vein is seen as a conspicuous strip of diminished density on one or both lateral walls of the calvaria (e-Fig. 18-27); this groove also has been called the sphenoparietal sinus, which is a misnomer because the true sinus runs underneath the lesser wing of the sphenoid bone and does not always communicate with the bregmatic vein.
Pacchionian Bodies
The Pacchionian, or arachnoidal, granulations (e-Fig. 18-28) are attached to the undersurface of the dura. Originally they were thought to be the site of absorption of cerebrospinal fluid, but this theory has been disputed.4 These structures are irregular, sharply defined impressions with smooth edges on the inner table of skull and located in a typical parasagittal location. These normal structures appear after age 18 months.
Symmetric Parietal Foramina
About 60% of skulls show small defects (parietal foramina) in the superior posterior angles of the parietal bones through which emissary veins penetrate. The veins generally communicate with the sagittal sinus internally and with tributaries to the occipital veins externally. Occasionally, large bony defects are present in these regions; these defects have been called enlarged parietal foramina.5 They occasionally are palpable on each side of the midline and less frequently are united to form a large, single defect (Fig. 18-29). The defects result from a failure of mineralization of the membranous bone, and thus the term “enlarged parietal foramina” is a misnomer. The defects usually are not associated with other skeletal anomalies and have no clinical significance except in the differential diagnosis of cranial defects, such as those associated with meningocele, infection, and histiocytosis.
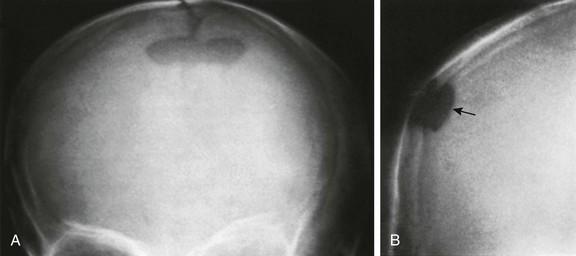
Figure 18-29 A persistent interparietal fontanelle (parietal foramen) (arrow) in an otherwise healthy 5-year-old boy.
A, Frontal projection. B, Caldwell projection.
Large parietal foramina have been recognized as an inherited trait ever since Goldsmith found them in 56 members of the Catlin family, giving rise to the term “Catlin mark.”6 Lesions may persist throughout life, although they tend to become smaller and may completely obliterate, leaving focal sclerotic residua.
Plain Radiographic Signs of Increased Intracranial Pressure
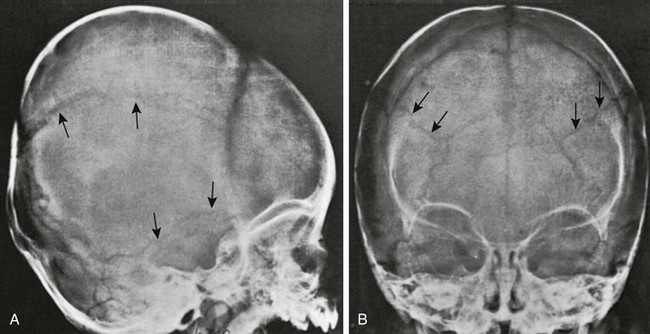
The signs of increased intracranial pressure are spread sutures, truncation of the dorsum sella, widened sella, and “beaten copper” appearance of bone (only with other changes) (Figs. 18-30 and 18-31; see Fig. 18-13). Chronic increased intracranial pressure can be revealed by increased width of sutural interdigitation.
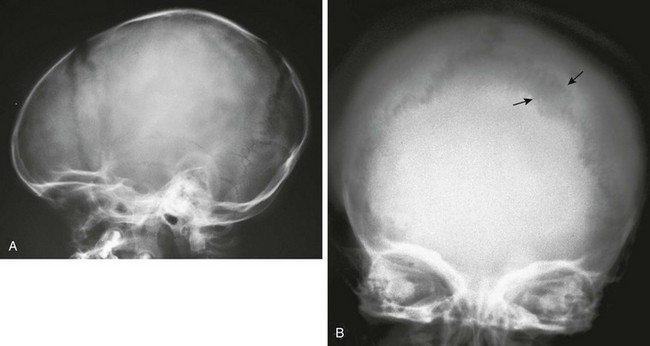
Figure 18-30 Split sutures.
A, A lateral radiograph reveals a widened coronal suture caused by acute increased intracranial pressure. B, A radiograph shows the results of chronic increased intracranial pressure. The interdigitations of the lambdoid suture are widened, but the sutures are not frankly split (arrows). This radiograph shows an attempt of the sutures to reunite with continual increased pressure.
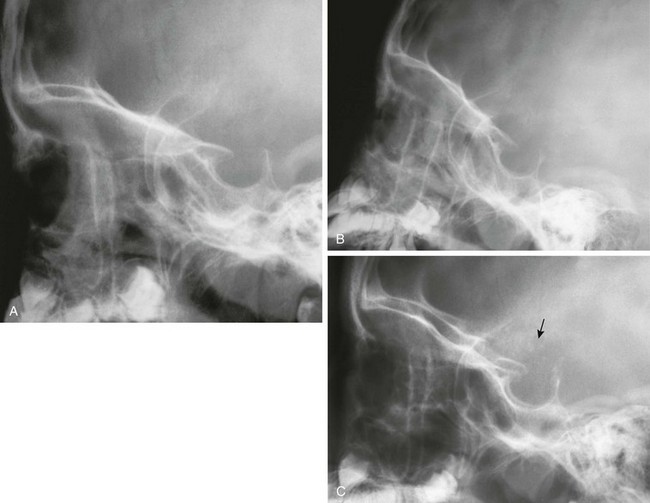
Figure 18-31 Increased intracranial pressure—sella changes.
A, A lateral radiograph coned to the sella in an 11-year-old with headaches. B, The sella is wider and the dorsum is thinned 6 months later. C, The sella is even wider and the dorsum is truncated with flecks of calcium above (arrow) 1 year later. This patient had a craniopharyngioma.
Anatomy of the Paranasal Sinuses
The paranasal sinuses are paired pneumatic cavities that communicate with the nasal fossae and are situated in the paranasal bones—maxilla, ethmoid, frontal, and sphenoid. Because of the continuity of their air cell mucosa with that of the nasal cavity via the eustachian tubes, the mastoid cells can be considered an additional component. The size and shape of the cavities vary in different age periods, among persons, and on the two sides of the same person.7,8
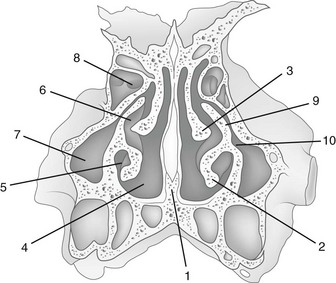
The sites of the openings of the sinuses into the nasal cavity are shown in Figures 18-32 and 18-33. The postnatal growth and extension of the sinuses are shown in e-Figure 18-34. The fully developed maxillary, frontal, and ethmoid sinuses are illustrated diagrammatically in e-Figures 18-35 and 18-36. Computed tomography (CT has shown extensions from adjacent sinuses into the orbital roofs and apices of the petrous temporal bone that are not easily recognized on conventional radiographs.
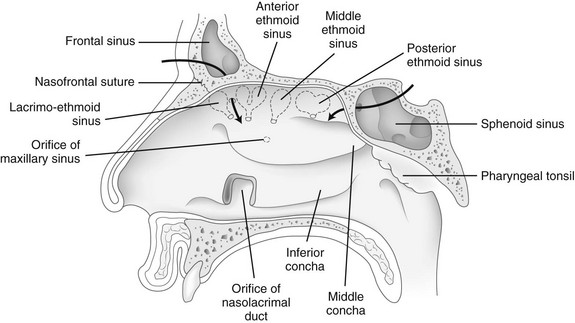
e-Figure 18-33 Lateral wall of the right nasal fossa and nasopharynx.
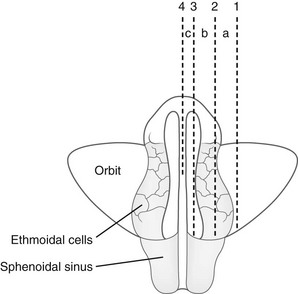
e-Figure 18-35 Relationships of the ethmoid labyrinths, sphenoid sinuses, and orbits as reviewed in horizontal section.
In segment a, the posterior ethmoid cells are located on the margins of the orbit lying lateral to the anterior and middle groups and thus are not superimposed on them or the sphenoid sinus in the dorsoventral radiographic projection. In segment b, the three groups of ethmoid cells lie in the same dorsoventral axis and are superimposed on one another and on the sphenoid sinus in the dorsoventral radiographic projection. In segment c, a portion of the anterior wall of the sphenoid sinus lies medial to the ethmoid labyrinth and is not superimposed on it in the dorsoventral radiographic projection. (Modified from Köhler A. Roentgenography: the borderlands of the normal and early pathological in the skiagram. New York: William Wood & Co; 1928.)
Maxillary Sinuses
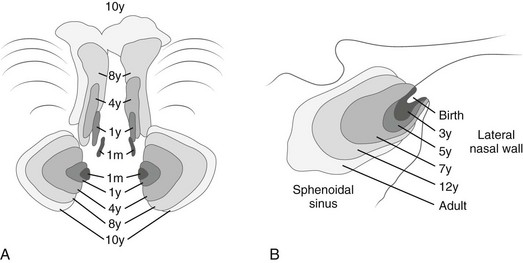
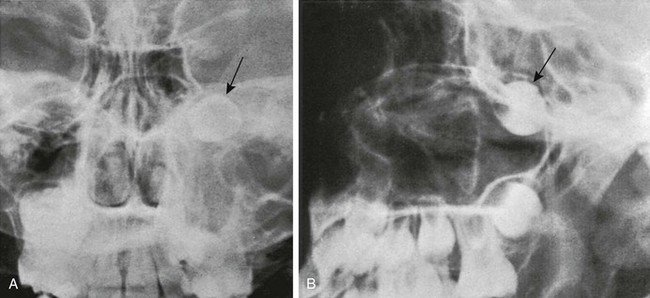
Changes in size and configuration of the maxillary sinuses with age are shown in e-Figure 18-36 and Figure 18-37. The maxillary sinuses are present at birth, expand steadily, and are considered mature by the time of puberty.9 Variations in development include isolated unilateral hypoplasia and prominent septa that appear to compartmentalize the sinus cavity (Fig. 18-38). The roots of the maxillary molars occasionally impinge on the walls of the sinuses (Fig. 18-39) and sometimes produce folds in the mucous membranes. In oblique ventrodorsal projections of the skull, the roots of the teeth may be superimposed on the sinuses and artifactually appear to project into them. A molar that fails to migrate is found in its fetal position near the posterosuperomedial angle of the maxillary sinus (e-Figs. 18-40 and 18-41). Note should be made of the relative height of the antral floor and structures in the nasal cavity, which can influence surgical approaches.
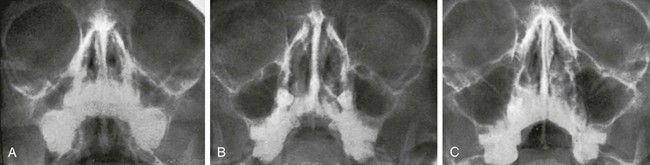
Figure 18-37 Waters projection of normal maxillary sinuses in a child at ages 4 years (A), 7 years (B), and 11 years (C).
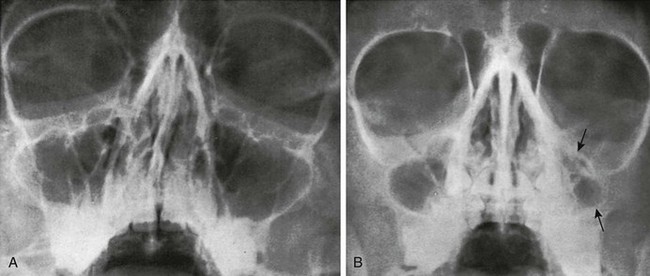
Figure 18-38 Variants of the maxillary sinus.
A, Several septa and ridges dividing the maxillary sinus into small cavities in an 11-year-old girl. B, Hypoplasia of the left maxillary sinus (arrows) in a 7-year-old boy.
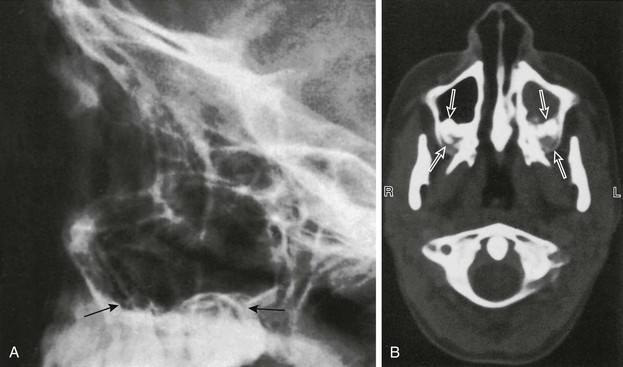
Figure 18-39 Maxillary sinuses.
A, A lateral view of the maxillary sinus shows the roots of the molars and bicuspids in contact with the floor of the maxillary sinus (arrows) in an 11-year-old child. B, Molar-indenting maxillary sinuses in a 12-year-old child who had a computed tomography scan performed because of sinus disease. Unerupted molars indent the posterior aspects of both maxillary sinuses (arrows). The left sinus is opaque because of sinus disease.
Ethmoid Sinuses
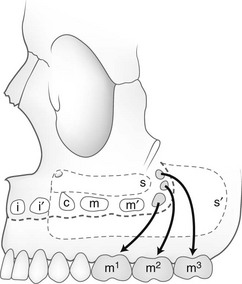
The ethmoid sinuses are composed of a series of cells of variable number, forming paired bony labyrinths suspended from the horizontal plate of the ethmoid bone on each side of the vertical plate, with the lateral walls forming the medial walls of the orbits. They are separated by thin osseous septa covered with mucous membrane. They all communicate with the nasal cavities either directly by independent channels or indirectly through cells of the same group. They are present at birth, expand rapidly during the first 5 years, expand less quickly until about 8 years, and usually are complete by age 12 years. They usually form three groups: anterior, middle, and posterior (see Fig. 18-33 and e-Fig. 18-36). The ethmoid cells often extend into the turbinates, crista galli, and neighboring frontal, maxillary, sphenoid, and palate bones. Three anatomic variants of ethmoid cells are found: Haller, Agger nasi, and Onodi cells. These variants are described in Chapter 8. Extension of infection from the sinus to the orbit can occur easily through the lamina papyracea (see Chapter 8).
Sphenoid Sinus
The paired cavities in the body of the sphenoid bone are separated by an osseous partition that may be displaced to one side so that the two cavities vary greatly in size and shape.10–12 The cavities can be visualized when they are superimposed in lateral projections or side by side in submentovertical projections (e-Fig. 18-42). Ridges and septa sometimes divide each single cavity into separate compartments. The air cells are not present at birth; by age 4 years they are 4 to 8 mm in diameter, and they become adult size any time between 7 and 11 years (Fig. 18-43).
Imaging of the Sinuses
The traditional, standard, four-view radiographic examination described next is outdated and has limited usefulness. The American Academy of Pediatrics and the American College of Radiology recommend that plain radiographic imaging be used sparingly in the diagnosis of sinusitis in children, and the the American Academy of Pediatrics guidelines state that radiographs are unnecessary for diagnosing sinonasal inflammation in children younger than 6 years.13,14 In an older child in special circumstances, radiographic imaging may prove useful, but it usually should be confined to a single Waters view.
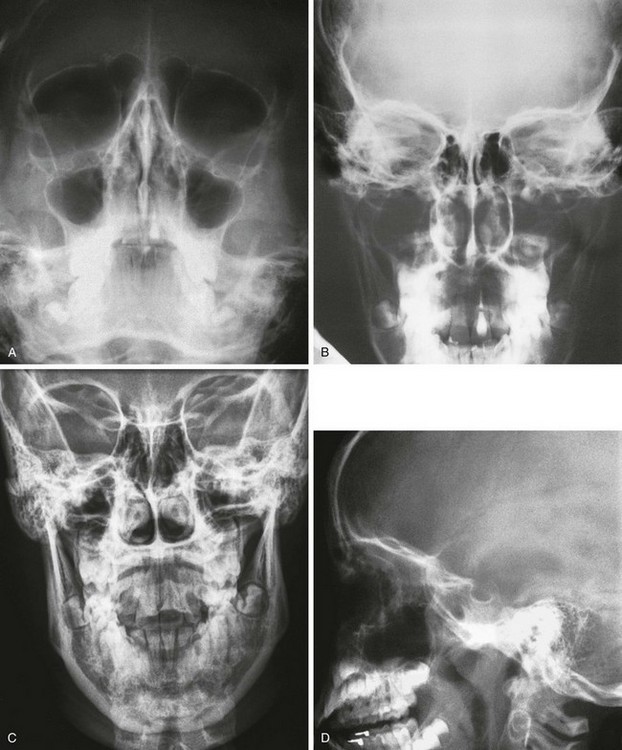
A standard examination of the sinuses used to include four views (Waters, posteroanterior, Caldwell, and lateral) (see Fig. 18-43). The Waters projection is the most important and is obtained with the patient’s head in just enough extension to place the shadows of the dense petrous pyramids immediately below the maxillary antral floors. It is used primarily for visualization of the maxillary sinuses.
Technical factors such as patient motion (even normal respirations), incorrect angulation, rotation, and underpenetration almost always overestimate the presence of disease (Box 18-3). Partial ethmoid clouding can occur in the Caldwell view because of superimposition of the ethmoid cells caused by slight rotation or nasal secretion. The normal sloping of the walls of the maxillary antra also can mimic mucosal thickening.
Plain radiographs play no role in the investigation of a mass in the sinuses and in assessing suitability for, or complications from, sinus surgery.15,16 Plain radiographs also are inappropriate for imaging complications of sinusitis. The capabilities of CT and magnetic resonance imaging (MRI are far superior. Plain radiographs also have little to offer in children younger than 2 years and certainly in infants younger than 1 year because such a high incidence of false-positive findings occurs.17–21
Computed Tomography
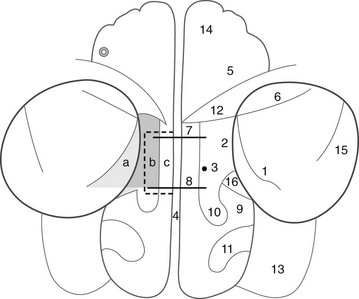
With the advent of functional endoscopic sinus surgery (FESS) for treating chronic inflammatory disease, the role of CT has expanded further.22 Coronal imaging simulates the view through the endoscope and provides information not only about the extent of inflammation, but also regarding anatomic features that are intimately related to the pathogenesis of the disease process itself. The goal of FESS is to maintain the normal drainage pathway (the OMU) of the frontal, maxillary, and anterior ethmoid sinuses. The OMU, which is in the region of the middle meatus, consists of the ostia, infundibulum, hiatus semilunaris, and middle meatus itself. This anatomy is exquisitely shown with coronal imaging through the nose and sinuses (see Fig. 18-44). Blockage of this pathway by inflammatory tissue or exudate allows mucus and debris to accumulate in the sinus air cells and predisposes to infection. It has been suggested that anatomic variants in the OMU such as a deviated nasal septum, large concha bullosa (aerated middle turbinates), and paradoxically bent middle turbinates (concave medially, rather than the normal configuration, which is bent concave laterally) predispose to blockage of the pathway and disease. Similarly, large Haller cells (ethmoid cells located along the rim of the orbit and protruding into the maxillary antrum), ethmoid bullae (ethmoid air cells above and posterior to the infundibulum), and large Agger nasi cells (the most anterior ethmoid air cells) have been thought to compromise the drainage route and result in disease. These variants are common in asymptomatic persons, however, and have little clinical significance (see Fig. 18-5, A and C, and Fig. 18-45).23,24
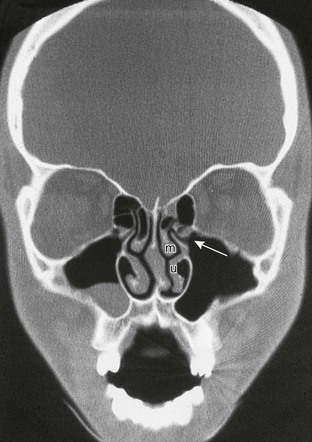
Figure 18-44 Coronal section through the midsinuses and nose showing the osteomeatal unit.
Note the mucous retention cyst in the right maxillary antrum and concha bullosa in the right middle turbinate. The patient has no history or evidence of sinus disease. The ostium (opening) (arrow), infundibulum (airspace superior to ostia and lateral to the uncinate process of the inferior turbinate [u]), and middle turbinate (m) are shown. The circular airspace above the uncinate process is the hiatus semilunaris.
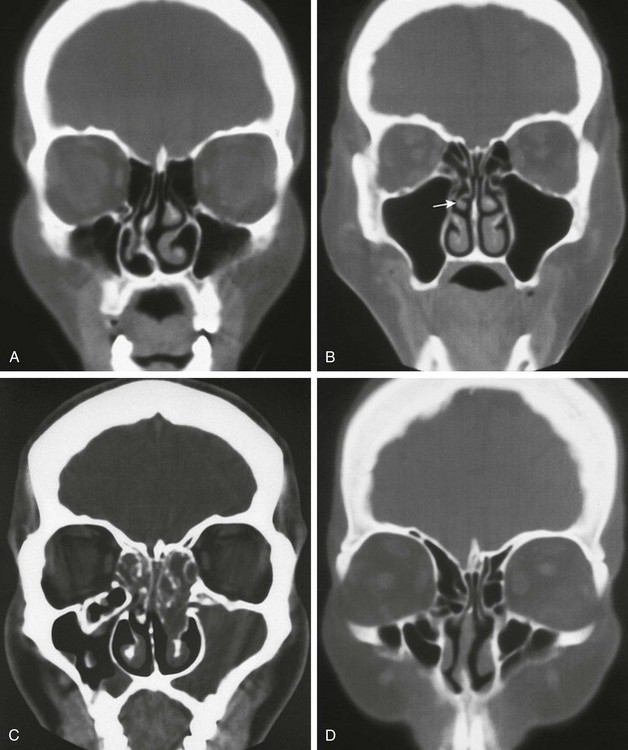
Figure 18-45 Normal variants of the nose.
A, A deviated nasal septum. B, A paradoxically bent middle turbinate (arrow). C, Haller air cell (ethmoid cells located along the rim of the ostia and protruding into the maxillary antrum). D, Large agger nasi air cells—the most anterior air cells.
CT also is the study of choice for documenting complications of FESS (e-Fig. 18-46). Orbital hematomas may occur after transection of an ethmoidal artery and could potentially expand, compromising flow in the retinal artery and resulting in ischemia of the optic nerve. Postoperative cerebrospinal fluid leaks are well assessed with nuclear medicine or new cisternographic MRI techniques. CT also may provide ancillary anatomic information in these cases, and CT cisternography is a newer technique being explored. Pseudoaneurysm, is a rare complication of FESS and can be identified with CT angiography, magnetic resonance angiography, or conventional angiography.
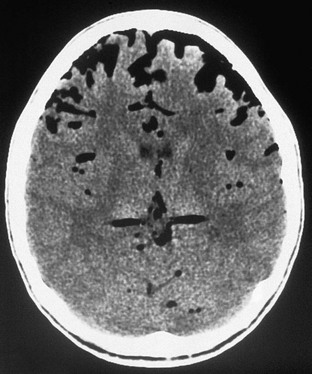
e-Figure 18-46 An axial computed tomography scan of the head shows air in the extraaxial space after functional endoscopic sinus surgery.
CT is considerably more sensitive than plain radiographs in detecting rhinosinusitis. On CT scans obtained for other reasons, 100% of asymptomatic children who had a recent upper respiratory tract infection showed soft tissue changes in their sinus. Seventy percent of pediatric patients show soft tissue changes in CT scans performed for unrelated problems.18–21 The specificity is low, and the problem becomes an even larger issue as the use of CT for the investigation of inflammatory disease increases. Subsequently, CT should be used in cases of acute disease only when the patient is unresponsive to medical treatment, and CT should be used to assess chronic disease only when surgery is being considered.
Magnetic Resonance Imaging
The normal nasal cycle of vasodilation and mucosal edema followed by vasoconstriction and mucosal shrinkage causes signal changes that can result in problems in interpreting findings.9,25 This cycle varies from 50 minutes to 6 hours, and the signal intensity during the edematous phase is indistinguishable from that of inflammatory change. As is the case with CT and plain radiographs, there is a high incidence of findings in asymptomatic persons (13% to 37%), and mucosal thickening less than 3 mm likely is insignificant. MRI usually is not used to image inflammatory disease, but it plays an important role in examining complex or intracranial extension of inflammatory disease or as part of the workup of a sinus or parasinal neoplasm.
Barghouth, G, Prior, JO, Lepori, D, et al. Paranasal sinuses in children: size evaluation of maxillary, sphenoid, and frontal sinuses by magnetic resonance imaging and proposal of volume index percentile curves. Eur Radiol. 2002;12:1451–1458.
Belden, CJ. The skull base and calvaria: adult and pediatric. Neuroimaging Clin N Am. 1998;8:1–20.
Bhattacharyya, N, Jones, DT, Hill, M, et al. The diagnostic accuracy of computed tomography in pediatric chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2004;130:1029–1032.
Huda, W. Assessment of the problem: pediatric doses in screen-film and digital radiography. Pediatr Radiol. 2004;34(suppl 3):S173–S182.
Mann, SS, Naidich, TP, Towbin, RB, et al. Imaging of postnatal maturation of the skull base. Neuroimaging Clin N Am. 2000;10:1–22.
References
1. Kier, EL, Fetal skull. Radiology of the skull and brain. Newton, TH, Potts, DG, eds. Radiology of the skull and brain, St Louis, Mosby, 1971;Vol 1.
2. Moloy, HG. Studies on head molding during labor. Am J Obstet Gynecol. 1942;44:962–982.
3. Vignaud-Pasquier, J, Lichtenbert, R, Laval-Jeantet, M, et al. Les impressions digitales de la naissance á neuf ans. Biol Neonate. 1964;6:250–276.
4. Greitz, D, Hannerz, J. A proposed model of cerebrospinal fluid circulation: observations with radionuclide cisternography. AJNR Am J Neuroradiol. 1996;17:431–438.
5. Fink, AM, Maixner, W. Enlarged parietal foramina: MR imaging features in the fetus and neonate. AJNR Am J Neuroradiol. 2006;27:1379–1381.
6. Goldsmith, WM. Catlin mark: inheritance of unusual opening in parietal bones. J Hered. 1941;32:301–309.
7. Maresh, MM. Paranasal sinuses from birth to late adolescence. Am J Dis Child. 1940;60:841–861.
8. Maresh, MM. Paranasal sinuses from birth to late adolescence, 1: size of the paranasal sinuses as observed in routine posteroanterior roentgenograms. Am J Dis Child. 1940;60:55–78.
9. Odita, JC, Akamaguna, AI, Ogisi, FO, et al. Pneumatisation of the maxillary sinus in normal and symptomatic children. Pediatr Radiol. 1986;16:365–367.
10. Fujioka, M, Young, LW. The sphenoidal sinuses: radiographic patterns of normal development and abnormal findings in infants and children. Radiology. 1978;129:133–136.
11. Kazkayasi, M, Kardeniz, Y, Arikan, OK. Anatomic variations of the sphenoid sinus on computed tomography. Rhinology. 2005;43:109–114.
12. Reittner, P, Doerfler, O, Goritschnig, T, et al. Magnetic resonance imaging patterns of the development of the sphenoid sinus: a review of 800 patients. Rhinology. 2001;39:121–124.
13. American Academy of Pediatrics, Subcommittee on Management of Sinusitis and Committee on Quality Improvement. Clinical practice guideline: management of sinusitis. Pediatrics. 2001;108:798–808.
14. American College of Radiology. Sinusitis in the pediatric population: ACR appropriateness criteria. Radiology. 2000;215:811–818.
15. McAlister, WH, Kronemer, K. Imaging of sinusitis in children. Pediatr Infect Dis J. 1999;18:1019–1020.
16. Kronemer, KA, McAlister, WH. Sinusitis and its imaging in the pediatric population. Pediatr Radiol. 1997;27:837–846.
17. Diament, MJ, Senac, MO, Gilsanz, V, et al. Prevalence of incidental paranasal sinus opacification in pediatric patients: a CT study. J Comput Assist Tomogr. 1987;11:426–431.
18. Gwaltney, JM, Phillips, CD, Miller, RD, et al. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25–30.
19. Kovatch, AL, Wald, ER, Ledesma-Medina, J, et al. Maxillary sinus radiographs in children with nonrespiratory complaints. Pediatrics. 1984;73:306–308.
20. Kristo, A, Alho, O-P, Luotonen, J, et al. Cross-sectional survey of paranasal sinus magnetic resonance imaging findings in schoolchildren. Acta Paediatr. 2003;92:34–36.
21. Kristo, A, Uhari, M, Luotonen, J, et al. Paranasal sinus findings in children during respiratory infection evaluated with magnetic resonance imaging. Pediatrics. 2003;111:e586–e589.
22. Wolf, G, Wolfgang, A, Kuhn, F. Development of the paranasal sinuses in children: implications for paranasal sinus surgery. Ann Otol Rhinol Laryngol. 1993;102:705–711.
23. Stallman, JS, Lobo, JN, Som, PM. The incidence of concha bullosa and its relationship to nasal septal deviation and paranasal sinus disease. AJNR Am J Neuroradiol. 2004;25:1613–1618.
24. Sivash, E, Sirikci, A, Bayazyt, YA, et al. Anatomic variations of the paranasal sinus area in pediatric patients with chronic sinusitis. Surg Radiol. 2002;24:400–405.
25. Zinrerch, SF, Kennedy, BW, Kuinar, AJ, et al. MRI imaging of the normal nasal cycle: comparison with sinus pathology. J Comput Assist Tomogr. 1988;12:1014–1019.