Embryology, Anatomy, and Variants of the Genitourinary Tract
The urinary system and the genital system are closely associated embryologically and begin to develop during the fourth week of gestation.1,2 Both develop from the intermediate mesoderm along the posterior wall of the abdominal cavity. A longitudinal elevation of the mesoderm, the urogenital ridge, forms on both sides of the abdominal aorta. Part of the urogenital ridge gives rise to the nephrogenic cord, which will form the urinary system, and another part gives rise to the gonadal ridge, which will form the genital system (see Chapter 125).1
The nephrogenic cord gives rise to the mesonephros, which consists of glomeruli and mesonephric tubules. The mesonephric tubules open into the mesonephric duct, which soon opens into the cloaca.1,3 The mesonephros degenerates toward the end of the first trimester, although their tubules persist in males and participate in the formation of the genital system.2 During the fifth week of gestation, the metanephros begins to develop. The definitive kidneys develop from the ureteric bud (metanephric diverticulum), an outgrowth of the mesonephric duct, and the metanephric blastema, which is derived from the nephrogenic cord.
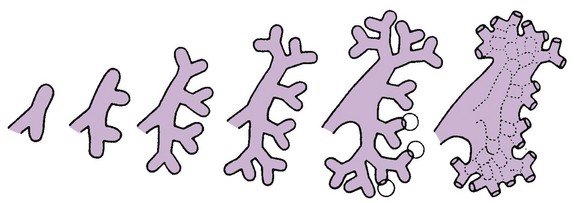
The ureteric bud is responsible for the development of the collecting system (ureter, renal pelvis, and calyces).1,2,4 The stalk of the ureteric bud forms the ureter. The ureteric bud grows into the metanephric blastema, where branching leads to the formation of the renal pelvis, major and minor calyces, and the collecting tubules (Figs. 111-1 and 111-2). Nephrons form in the metanephric blastema as the result of induction by the collecting tubules. The embryonic kidneys initially lie in the pelvis. As the abdomen grows, the kidneys “ascend” and rotate 90 degrees. By the ninth week of gestation, the kidneys come in contact with the adrenal glands as they attain their final position. The fetal kidney has a lobulated external contour that will disappear as the nephrons continue to grow. The full complement of glomeruli is present by 36 weeks’ gestation.
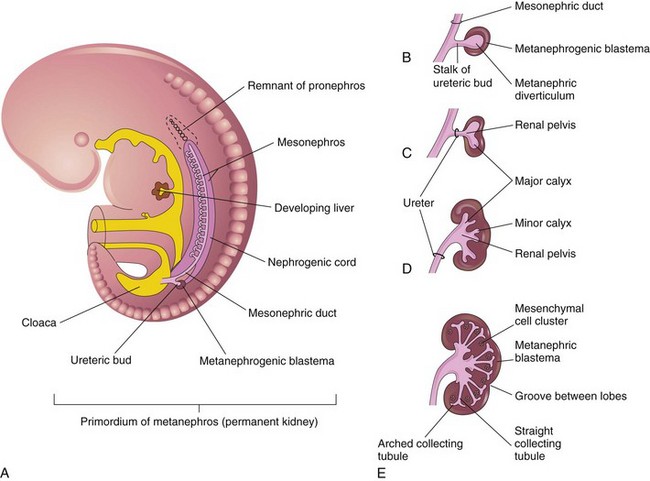
Figure 111-1 Development of the metanephros, the primordium of the permanent kidney.
A, Lateral view of a 5-week embryo, showing the primordium of the metanephros. B to E, Successive stages in the development of the metanephric diverticulum or ureteric bud (fifth to eighth weeks). Observe the development of the ureter, renal pelvis, the calices, and the collecting tubules. (From Moore KL, Persaud TVN. The urogenital system. In: Before we are born: essentials of embryology and birth defects. 7th ed. Philadelphia: Saunders Elsevier; 2003.)
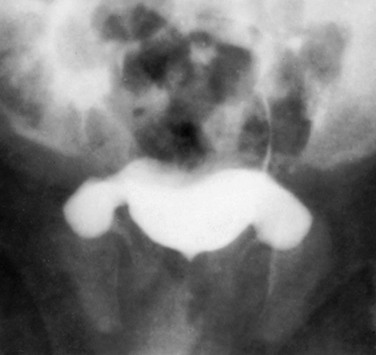
Congenital anomalies of the urinary tract are common.1,4 Early degeneration of a ureteric bud or involution of the metanephros leads to regression of the metanephric blastema and renal agenesis. If the ureteric bud and metanephric blastema do not join normally, abnormal induction of the blastemal elements is likely to result in a multicystic dysplastic kidney. Bifurcation of the ureteric buds results in partial duplication of the collecting system. When the location of the origin of the ureteric bud is abnormal or if the origin itself is maldeveloped, the potential for vesicoureteral reflux (VUR) or ureteral ectopia exists (Fig. 111-3).
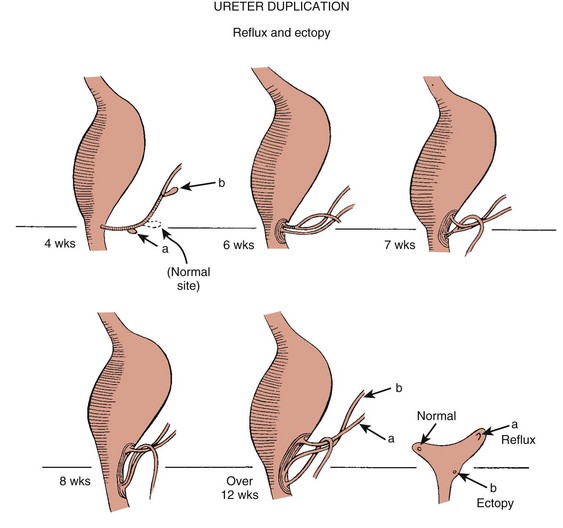
Figure 111-3 The ureteric bud and its relationship to the bladder. The drawing shows how ectopia may occur.
The bladder arises from the most superior portion of the urogenital sinus.1,2,5 It is initially continuous with the allantois. With constriction of the allantois, the lumen is obliterated, and a thick, fibrous cord, the urachus, remains. The urachus connects the apex of the bladder with the umbilicus. Persistence of the allantoic lumen may result in urachal fistulas, sinuses, or cysts. The ureterovesical junction (UVJ) develops as the distal parts of the mesonephric duct are incorporated into the enlarging bladder. As the mesonephric ducts are absorbed, the ureters come to open separately into the urinary bladder with the orifice moving superolaterally and the distal ureteral segments entering obliquely through the base of the bladder. The middle portion of the urogenital sinus develops into the prostatic urethra in males and the entire urethra in females. The distal portion of the male urethra is derived from a cord of ectoderm that grows from the tip of the glans penis to meet the spongy portion of the urethra derived from the caudal (phallic) portion of the urogenital sinus. Development of the ureters and bladder is complete by the fourth gestational month.
The fetus begins to make urine by the ninth week of gestation, and the urine contributes the largest component of the amniotic fluid.6 Oligohydramnios is often a marker of urinary tract abnormalities, and severe oligohydramnios may lead to pulmonary hypoplasia.
During the first few days after birth, the neonate has a low glomerular filtration rate (GFR) and low urine output.7 This is especially true of premature infants. The newborn kidney also has a limited ability to concentrate urine and decreased tubular reabsorption of sodium. GFR and urine output increase significantly over the first week of life. This immaturity of renal function is an important factor to keep in mind in the ordering and interpretation of renal imaging studies. For example, the low urine output present on day one of life may mask the presence of hydronephrosis on renal ultrasonography.
Normal Anatomy
The renal parenchyma is composed of two distinct regions: (1) the outer cortex, and (2) the inner medulla (Fig. 111-4). Within the cortex are the nephrons. The medulla is composed of 8 to 13 pyramids, which terminate in the renal papillae at the level of the calyces. Two or more pyramids may drain into the same papilla (confluent papilla), and two or more papillae may drain into a single calyx (compound calyx). A column of Bertin represents the renal cortex extending centrally between the pyramids (e-Fig. 111-5), most often occurring at the junction of the middle and upper groups of calyces or between the two central renal hypoechoic complexes of a duplex kidney.8,9
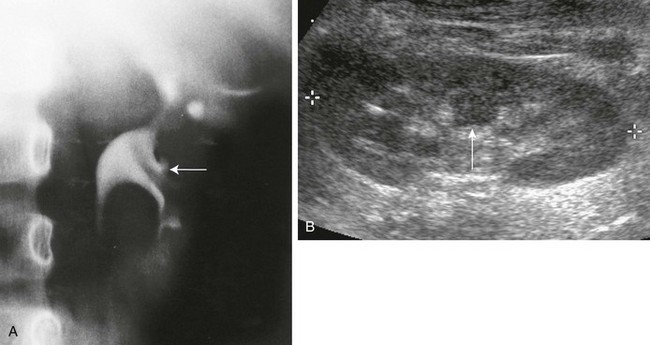
e-Figure 111-5 Column of Bertin.
A, Intravenous urography shows that the column of Bertin has produced spreading of the calyces of the left kidney, simulating a renal mass. The centrally located single calyx (arrow) is draining this ectopic tissue. Note also the oblique vascular impression on the lower pole infundibulum (major calyx). B, Longitudinal sonogram in another patient shows a central column of cortical tissue (arrow) extending into the central sinus echoes. (B, Courtesy Brian D. Coley, MD, Cincinnati, OH.)
Multiple renal lobules fuse to form the kidney. The junction of these lobules sometimes persists and may be seen as a scalloping of the cortical border (Fig. 111-6). The junctional parenchymal defect is similarly derived from a variation in the fusion of the fetal renunculi or lobules. It appears as a thick, triangular, echogenic notch in the anterosuperior or posteroinferior aspect of the kidney (more often on the right) and mimics a cortical scar. The junctional parenchymal defect may be connected to the renal hilum by an echogenic line called the inter-renuncular septum (Fig. 111-7). The left kidney is apt to be more triangular in shape, with a distinct bulge along its lateral aspect, the so-called dromedary kidney (Fig. 111-8).10–13
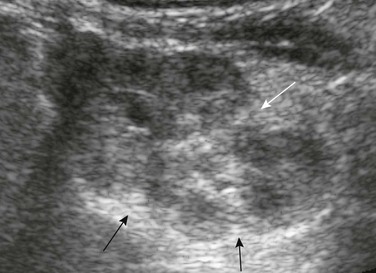
Figure 111-6 Fetal lobulations.
Residual fetal lobulations are responsible for the scalloped border of the kidney (black arrows) on this sagittal sonogram. A prominent junctional cortical defect is also present (white arrow).
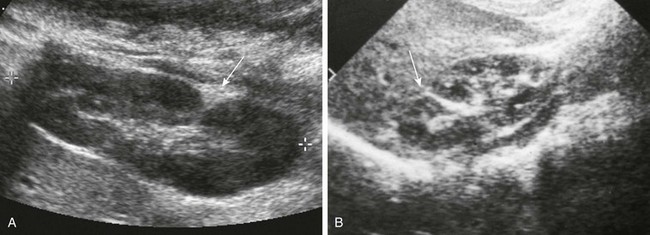
Figure 111-7 Junctional parenchymal defects.
A, Longitudinal prone sonogram shows a fat-filled cleft (arrow) in the posterior kidney. B, Longitudinal supine sonogram in another patient shows a renicular septum (arrow). Both are normal findings, probably related to a demarcation of embryonic reniculi.
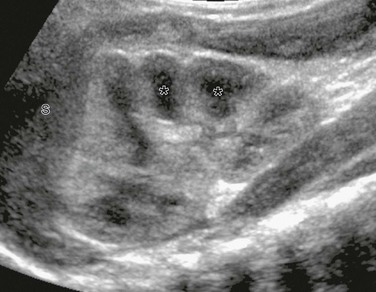
Figure 111-8 Normal neonatal kidney.
Supine longitudinal sonogram of the left kidney shows the sharp corticomedullary differentiation common in infancy and early childhood. The hypoechoic triangular renal pyramids (asterisks) are surrounded by cortex that is more echogenic than the adjacent spleen (S). The spleen has flattened the upper renal contour and produces the appearance of a dromedary kidney. (Courtesy Brian D. Coley, MD, Cincinnati, OH.)
In newborns and infants, the kidneys have a larger medullary and a smaller cortical volume than in later life. On ultrasonography, the neonatal renal cortex is moderately hyperechoic, close to the echogenicity of adjacent liver and spleen. In newborn and premature infants, the cortical echogenicity may actually be greater than that of the liver (see Fig. 111-8). The pyramids are relatively hypoechoic. Between ages 6 months and 2 years, the echogenicity of the medulla and cortex resembles that of adult kidneys.14–17
The kidney is usually supplied by a single artery arising from the aorta. After the renal artery enters the renal hilum, posterior and slightly superior to the renal vein, it divides into anterior and posterior branches, which, in turn, generally divide into superior and inferior branches (e-Fig. 111-9). Variations exist at all levels. About 20% to 30% of kidneys have a second or accessory renal artery, which also arises from the aorta. The normal renal vein lies anterior and slightly inferior to the renal artery. The left renal vein is longer than the right, courses anterior to the aorta, and receives the ipsilateral suprarenal and gonadal veins before entering the inferior vena cava.8,9,18
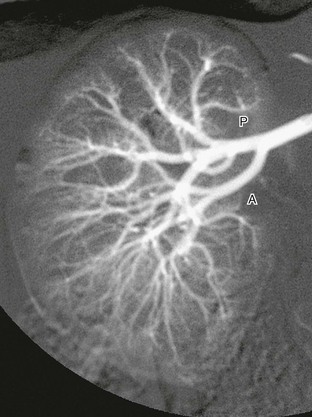
e-Figure 111-9 Normal renal arterial anatomy.
Digital subtraction arteriogram of the right kidney in a 6-month-old shows a single main renal artery dividing into anterior (A) and posterior (P) branches. Normal opacification and distribution of vessels to the level of the arcuate arteries are seen. (Courtesy Brian D. Coley, MD, Cincinnati, OH.)
Renal length is the most commonly measured parameter and has been correlated to age, body height and weight, and height of the first three or four lumbar vertebral bodies. Standards for renal size have been developed for ultrasonography (e-Fig. 111-10). The left kidney may be slightly longer than the right. Kidneys with complete or partial collecting system duplication are longer than normal kidneys. The width of the kidney is approximately 50% of its length and is relatively thicker in neonates than in older children. Cortical thickness of the upper renal pole is normally slightly thicker than the lower pole, and the renal cortex is slightly thinner in the center of the kidney. Extra cortical tissue may be noted about the renal hilum and may impinge from above or below on the renal pelvis (suprahilar or infrahilar bulge, or hilar lips).19–25
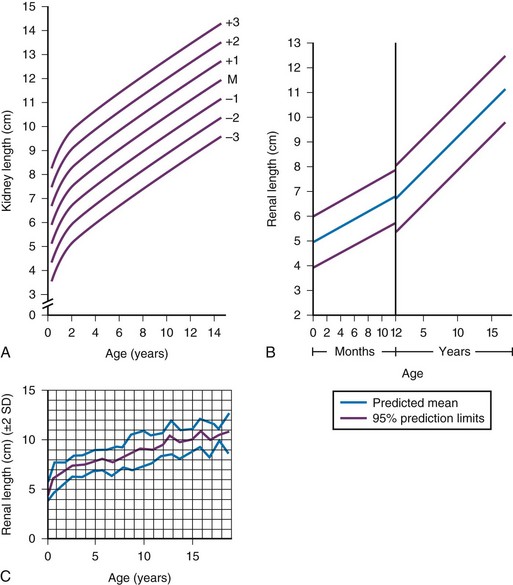
e-Figure 111-10 Kidney length in centimeters.
A, Measurements obtained from supine urographic films and correlated with age, including values for 3 standard deviations (SD) above and below the mean (M). The SD is 0.75 cm. B, Ultrasonographic measurements correlated with age; the values are slightly lower than those obtained from urographic films. C, Ultrasonographic measurements correlated with age, including ± 2 SD. (A, Modified from Currarino G, Williams B, Dana K. Kidney length correlated with age: normal values in children. Radiology. 1984;150:703; B, From Han BK, Babcock DS. Sonographic measurements and appearance of normal kidneys in children. AJR Am J Roentgenol. 1985;145:611; C, From Rosenbaum DM, Korngold E, Teele RL. Sonographic assessment of renal length in normal children. AJR Am J Roentgenol. 1984;142:467.)
Pelvocalyceal System
The renal pelvis varies in size from a small, poorly defined sac to a large, boxlike structure. The pelvis may lie entirely within (intrarenal) or almost beyond (extrarenal) the renal sinus. The configuration of the pelvocalyceal system is quite variable. In most kidneys, the pelvis branches into two major infundibula (or major calyces). The inferior infundibulum is commonly broad and short and is connected with a larger number of calyces compared with the upper infundibulum. Each kidney has about 8 to 13 calyces. These have a cup-shaped appearance that results from the protrusion of the renal papilla into the calyx. Two or more papillae may enter one calyx (compound calyx). Most calyces are directed laterally and either slightly anteriorly or posteriorly within the kidney.4,6,18,24,26
Ureter
The ureter is a tubular structure that courses retroperitoneally from the kidney to the bladder and has three major components: (1) the ureteropelvic junction (UPJ) and upper ureter, (2) the midureter, and (3) the lower ureter and the UVJ (including the transmural ureter and the ureteral orifice). The abdominal or upper ureter starts at the UPJ with a smooth tapering from the renal pelvis. The abdominal ureter lies adjacent to the psoas muscle, being crossed by the gonadal vessels before passing behind the iliac vessels. The lower ureter lies along the lateral pelvic wall, coursing downward behind the bladder. As the ureter enters the bladder wall, it takes an oblique course from a superolateral entry point to an inferomedial ostium and eventually opens into the bladder. Its course through the bladder wall may be seen (called the plica ureterica at cystoscopy) and constitutes the lateral border of the bladder trigone. Three muscular layers constitute the ureteral wall, with a thick outer adventitial covering that contains a rich vascular layer and lymphatic plexus.4,6,27–29
The vascular supply of the ureter is from bladder vessels (inferior vesical artery), gonadal arteries (testicular artery), and the renal artery superiorly. Nerves supplying the ureter follow the same course as that of arteries. Rhythmic peristaltic ureteral contractions transport urine from the renal pelvis into the urinary bladder and occur two to seven times per minute.4,6
The anatomic relationship of the distal ureter to the bladder wall at the UVJ is important in preventing VUR. Normally, the distal ureter inserts into the bladder, courses through the bladder musculature at an oblique angle, and then continues inferomedially and submucosally to its ostia in the lateral corner of the trigone (Fig. 111-11). The UVJ acts as a passive flap valve. Continence is ensured by apposition of the roof and floor of the submucosal tunnel when intravesical pressure increases and is enhanced by the action of the intrinsic local musculature. A normal ureteral tunnel length to ureteral diameter ratio in children is 5 : 1. A ratio of at least 3 : 1 is necessary for UVJ competence.30–34
Bladder
In the neonate and young child, the dome and body of the bladder are located primarily in the abdomen and anterior, whereas its base is intrapelvic and posterior. Bowel impressions are common and may impress on the intraabdominal bladder when it is filled. This normal appearance should not be confused with an intravesical mass or extrinsic compression by a pelvic mass. A urachal remnant may also be seen at the bladder dome. Normal bladder capacity may be predicted on the basis of weight in infancy and by age for the older child. For infants under 1 year of age, bladder capacity in milliliters is predicted by multiplying the weight in kilograms by 7. The predicted bladder capacity in milliliters of children more than 1 year old is the age in years plus 2 multiplied by 30. Ultrasonography best demonstrates normal bladder wall thickness, which should be no more than 3 mm when distended and 6 mm when collapsed.5,35–43
Urethra
The female urethra corresponds anatomically to the posterior urethra of the male. Anatomic features of the female urethra include the internal sphincter at the bladder neck, intermuscular incisura, membranous urethra at the level of the urogenital diaphragm or external urethral sphincter, and the fossa navicularis.5,44,45
The components of the normal male urethra include the prostatic or posterior urethra, the membranous urethra, the bulbous urethra, and the penile urethra. Normal anatomic features of the male prostatic urethra include the internal sphincter, the intermuscular incisura, and the verumontanum. The membranous urethra runs through the urogenital diaphragm or the external urethral sphincter, after which come the bulbous urethra, the penile urethra, and the fossa navicularis (e-Fig. 111-12). The intermuscular incisura produces an indentation in the midportion of the posterior urethra at the level of the verumontanum and is caused by an abundance of collagenous tissue at this location (more prominent anteriorly). The verumontanum is a focal elevation in the posterior wall of the prostatic urethra where the paired ejaculatory ducts enter, seen as a small ovoid filling defect in the posterior portion of the prostatic urethra. The plicae colliculae are normal folds that extend from the distal verumontanum to the posterior urethra and may produce circumferential impressions on urethral images.5,44,46–50
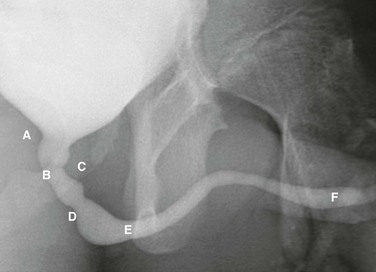
e-Figure 111-12 Normal urethra.
Voiding cystourethrogram in a 15-year-old boy shows a normal urethra. A, External sphincter. B, Site of the verumonatnum. C, Prostatic urethra. D, Membranous urethra. E, Penile urethra. F, Fossa navicularis. (From the Radiographic atlas of the pediatric urethra. Available at www.natiowidechildrens.org.)
Congenital Anatomic Variants and Anomalies
Renal Agenesis (Unilateral and Bilateral)
True renal agenesis is thought to be caused by failure of the ureteric bud to contact the ipsilateral metanephric blastema. Unilateral renal agenesis is present in about 1 : 1000 live births. Children with true unilateral renal agenesis lack the ipsilateral ureter and hemitrigone of the urinary bladder. Those children with an absent kidney but a normally developed bladder and a distal ureter of varying length probably represent the involution of a multicystic dysplastic kidney.36 Associated anomalies include VACTERL association (e-Fig. 111-13), unicornuate uterus, uterus didelphys, and Mayer-Rokitansky-Küster-Hauser syndrome in females, and seminal vesicle cysts, absence of the vas deferens, and cystic dysplasia of the rete testis in males (Box 111-1).36,51–58
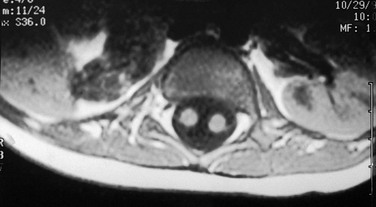
e-Figure 111-13 Unilateral renal agenesis.
Vertebral anomalies prompted this study. An axial T1-weighted magnetic resonance image shows a normal left kidney and a bowel loop in the right renal fossa. Note also the split spinal cord, indicating diastematomyelia. (Courtesy Joan A. Zawin, MD.)
Bilateral renal agenesis is uniformly lethal with an incidence of 1 : 10,000 to 3 : 10,000 live births. Boys are affected more commonly compared with girls. Pulmonary hypoplasia is generally the cause of death and may be associated with neonatal pneumothorax and pneumomediastinum. The Potter sequence is manifest at birth: small chest, abnormal facial features (micrognathia, beaked nose, epicanthic folds, low-set ears), and limb deformities (tightly apposed fingers, dislocated hips, clubfeet).36
Imaging: Ultrasonography demonstrates the absence of the kidney and the presence of an abnormally configured (elongated or elliptical) adrenal gland, rather than its normal triangular or Y-configuration (Fig. 111-14).36,59–61 Because the embryology of the adrenal gland is independent of that of the kidney, it is usually located in its expected position, even when the kidney does not reach the renal fossa. Ultrasonography and magnetic resonance imaging (MRI) are most commonly used to evaluate associated uterine and vaginal anomalies in girls and seminal vesicle cysts in boys (Fig. 111-15).50–54 Because of the increased incidence of VUR into the remaining solitary kidney and the need to protect its parenchyma, a study to exclude reflux is recommended early in life.
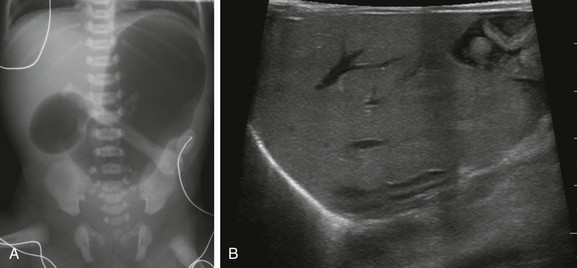
Figure 111-14 A female newborn with unilateral renal agenesis and VACTERL association.
A, A supine abdominal radiograph shows marked gastric and proximal duodenal gaseous distention, the so-called “double bubble sign.” No identifabled distal bowel gas exists. Both esophageal atresia (with distal fistula) and duodenal atresia were confirmed at surgery. B, Longitudinal ultrasonographic image through the right renal fossa confirms congenital absence of the right kidney. The right adrenal gland is abnormally elongated.
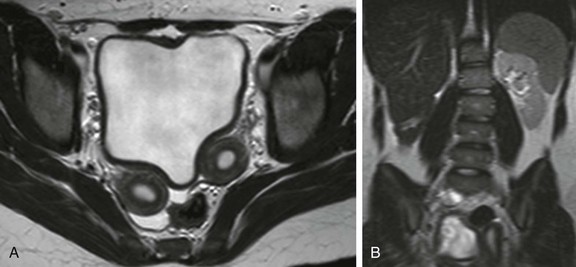
Figure 111-15 A 14-year-old girl with unilateral renal agenesis and Mullerian anomaly.
A, Anaxial T2-weighted magnetic resonance image through the pelvis shows two separate uterine horns, proven to be uterus didelphys. B, A coronal single-shot fast spin-echo image through the retroperitoneum confirms congenital absence of the right kidney.
Renal Ectopia (Pelvic Kidney and Ectopic Intrathoracic Kidney)
Renal ectopia is diagnosed when a kidney fails to migrate to its appropriate renal fossa, occurring with an incidence of 1 : 800 to 1 : 1000.62 The pelvic kidney is the most common type of renal ectopia (accounting for about 60% of cases). Pelvic kidneys are prone to VUR and in about 10% of children may be the only kidney. In some children, both kidneys are in the pelvis and may be fused into a single unit, the so-called cake kidney or lump kidney. Ectopic intrathoracic kidney is the least common form of renal ectopia, representing less than 5% of cases.63,64 This condition may occur as an isolated lesion with an intact diaphragm but is more often part of a larger intrathoracic herniation through the foramen of Bochdalek.63 Anomalies of rotation and blood supply are to be expected with renal ectopia.36
Imaging: On ultrasonography, the pelvicalyceal system of the pelvic kidney may be predominantly extrarenal, resulting in images in which the kidney lacks the usual central renal echo complex.62 Corticomedullary differentiation may be indistinct (Fig. 111-16). UPJ obstruction is common in pelvic kidneys because the abnormal orientation of the ureter relative to the renal pelvis impedes drainage. Renal scintigraphy is often used to both locate and define the morphology of ectopic kidneys, but computed tomography (CT), MRI, and excretory urography are also effective (e-Fig. 111-17).65 The thoracic kidney is located in the posterior mediastinum and on chest radiographs may be mistaken for the usual neurogenic tumors that develop in this region (e-Fig. 111-18).
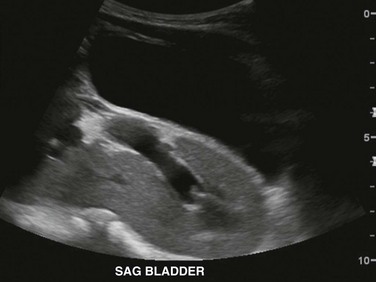
Figure 111-16 A 5-year-old boy with pelvic kidney.
Longitudinal ultrasonographic image through the pelvis reveals an ectopic kidney located posterior to urinary bladder. The kidney is nonrotated with its hilum and extrarenal pelvis oriented anteriorly.
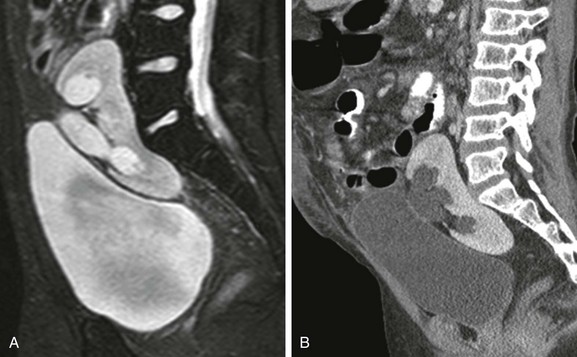
e-Figure 111-17 A 7-year-old boy with pelvic kidney.
A and B, Sagittal T2-weighted magnetic resonance and sagittal reformatted contrast-enhanced computed tomography images from the same child reveal a pelvic kidney located posterior to the urinary bladder. There is mild pelvicaliectasis without actual obstruction, and the pelvic kidney is nonrotated with its pelvis oriented anteriorly.
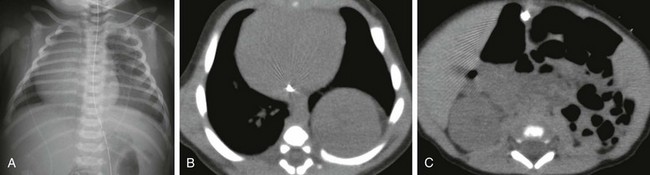
e-Figure 111-18 A newborn boy with superior renal ectopia caused by left congenital diaphragmatic hernia.
A, Anteroposterior chest radiograph shows increased opacity at the left lung base and rightward shift of mediastinal structures. B and C, Axial computed tomography images without intravenous contrast material show a posteriorly located intrathoracic left kidney. Small bowel loops are located in the left renal fossa. The right kidney resides in its normal location. This neonate was proved to have a left Bochdalek-type congenital diaphragmatic hernia at surgery.
Anomalies of Fusion: Horseshoe Kidney and Crossed Renal Ectopia
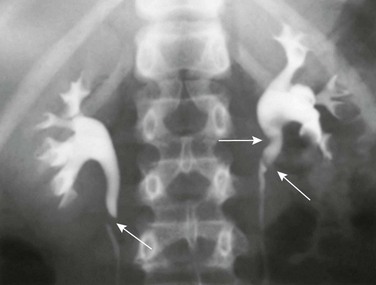
Horseshoe kidney is the most common renal fusion anomaly, noted in 1 : 400 to 1 : 1000 autopsies.36,66–68 It derives its name from the U-shape configuration of the kidneys, produced by the fusion of the lower pole moieties, which are more medially located than the upper poles (Fig. 111-19).66–68 The bridging tissue, known as the isthmus, is more commonly composed of functioning renal parenchyma than fibrous tissue.36,66,69 The isthmus is located just below the origin of the inferior mesenteric artery.36,68 This condition is caused by in utero fusion of the metanephric blastemas in the pelvis during the sixth or seventh week of gestation.67 The vascular supply of horseshoe kidneys is quite variable, arising from the abdominal aorta, iliac arteries, and inferior mesenteric artery.66,68,69
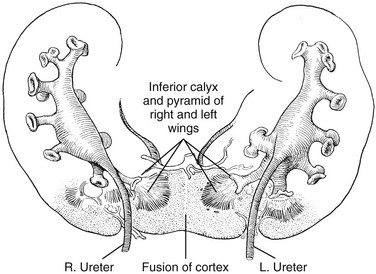
Figure 111-19 Horseshoe kidney with fusion of the inferior poles, spreading apart of the superior poles, and failure of rotation.
The renal pelves enter the kidneys on their anterior aspect. (Redrawn from Kelly HA, Burnam CF. Diseases of the kidneys, ureters and bladder. 2nd ed. New York: Appleton;1922.)
Clinical Presentation: Horseshoe kidneys may be an asymptomatic; however, an increased incidence of UPJ obstruction, VUR, and ureteral duplication predispose affected individuals to both urolithiasis and infection.36,68–70 Because horseshoe kidneys overly the spine and lack protective surrounding ribs, they are prone to injury from direct trauma.68 A slight increased risk of Wilms tumor in horseshoe kidneys compared with normal kidneys has been reported.36,67,68,71,72
Imaging: Ultrasonography demonstrates the upper portion of each kidney in a low paraspinal location. The isthmus may be identified in many cases anterior to the lumbar spine, especially when mild compression is used to displace interposed bowel loops. The renal long axis is abnormal and may be curved (Fig. 111-20).73 Anterior orientation of the renal pelvis from malrotation is common as are extrarenal pelves.69,73 CT and magnetic resonance angiography may be used to establish renal artery anatomy. CT also excellently depicts renal parenchymal and collecting system injuries in the setting of abdominal trauma (e-Fig. 111-21). Magnetic resonance urography or renal scintigraphy may be performed to assess for suspected UPJ obstruction (e-Fig. 111-22).68 Voiding cystourethrogram (VCUG) may help assess for VUR.
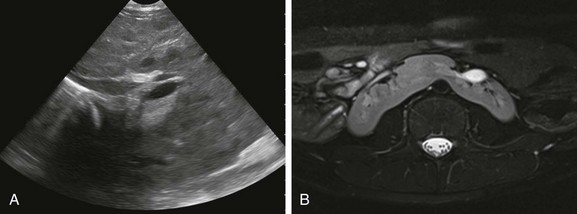
Figure 111-20 Horseshoe kidney.
A, Coronal oblique ultrasonographic image through the retroperitoneum shows a horseshoe kidney with a parenchymal isthmus overlying the abdominal aorta. Abnormal longitudinal axis of the kidneys is seen. B, An axial T2-weighted magnetic resonance image with fat-saturation shows fusion of the kidneys in the midline just below the level of the inferior mesenteric artery. The kidneys are malrotated with the collecting systems oriented anteriorly (Courtesy Damien Grattan-Smith, MD.)
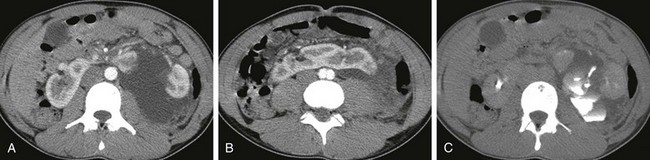
e-Figure 111-21 Horseshoe kidney, traumatic injury.
A and B, Axial contrast-enhanced computed tomography (CT) images through the abdomen confirm the presence of a horseshoe kidney with a thick parenchymal isthmus. A large amount of fluid is present within the left perinephric space as well as a large laceration involving the left renal moiety. C, An axial excretory phase CT image acquired 10 minutes later shows multiple fluid-contrast material levels in the left retroperitoneum caused by renal collecting system injury.
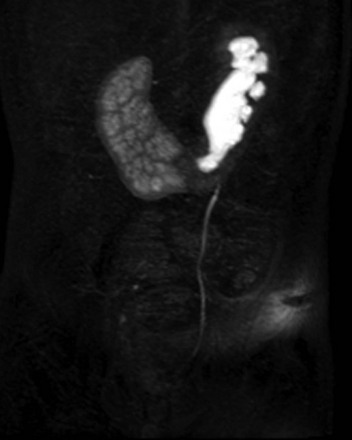
e-Figure 111-22 Horseshoe kidney and magnetic resonance imaging.
A coronal T1-weighted postcontrast magnetic resonance urogram maximum intensity projection image shows abnormal long-axis of the kidneys and fusion of the lower renal poles. A delayed nephrogram is seen on the right, consistent with upper urinary tract obstruction. Normal excretion of contrast material is present by the left moiety. (Courtesy Damien Grattan-Smith, MD.)
Crossed Renal Ectopia
Clinical Presentation: Crossed renal ectopia occurs when both kidneys are located on one side of the spine; a portion of the lower kidney, usually the one that has moved from its normal position (most commonly the left kidney), may extend over the spine.36,74 This anomaly is seen in 1 : 7500 children, affects boys more often than girls, and is the second most common renal fusion anomaly after horseshoe kidney.36,66,74,75 The ureter from the lower kidney crosses the midline to insert into the bladder in its normal position.74 Although most are oriented in a relatively vertical axis, the lower kidney may be obliquely or horizontally related to the superior kidney (Fig. 111-23). The position of the upper (uncrossed) kidney may also be ectopically low. Approximately 85% of crossed kidneys are fused and encompassed by a common renal fascia—hence the term crossed fused renal ectopia.36 As expected in any type of ectopia, the arterial vascularity may be anomalous, and the renal pelvis, especially of the lower kidney, may be malrotated.64,74,76
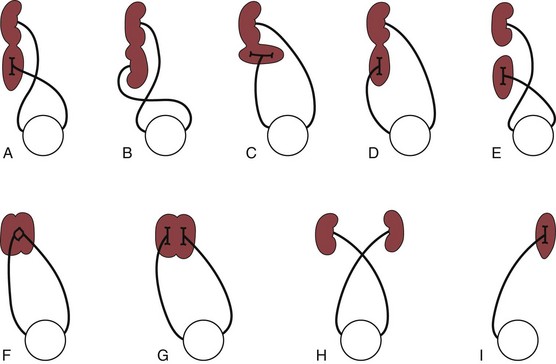
Figure 111-23 Various types of crossed renal ectopia.
A, Unilateral fused kidney (inferior renal ectopia), the most common form. B, Sigmoid or S-shaped kidney. C, L-shaped kidney. D, Unilateral fused kidney (superior renal ectopia). E, Crossed ectopia without fusion. F, Unilateral disk kidney. G, Unilateral lump kidney. H, Bilateral crossed ectopia. I, Crossed ectopia of a solitary kidney (solitary crossed renal ectopia).
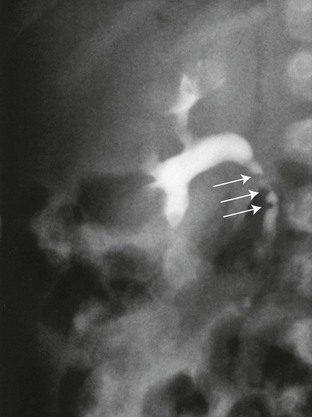
Imaging: Imaging demonstrates an empty renal fossa on one side and both kidneys located on the opposite side of the spine (Fig. 111-24).77 MRI and CT show two separate ureters, each entering its appropriate trigone.36 Doppler ultrasonography may be used to demonstrate jets of urine emanating from the normally positioned UVJs. Ultrasonography, MRI, and CT may allow visualization of a small indentation at the site of fusion (e-Fig. 111-25).77,78
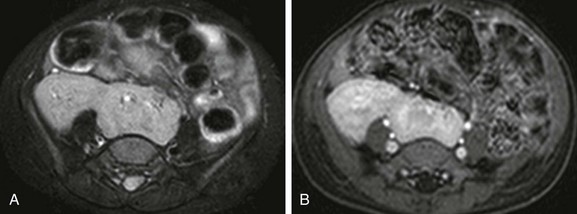
Figure 111-24 A 2 year-old girl with crossed fused renal ectopia and magnetic resonance imaging.
A and B, Axial T2-weighted and T1-weighted postcontrast images with fat-saturation show renal fusion, with both kidneys located to the right of the midline. The left (lower) moiety partly overlies the spine.
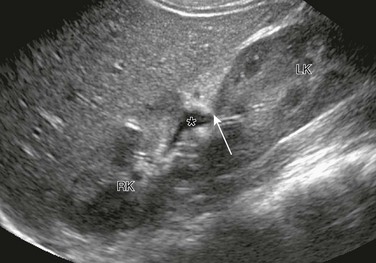
e-Figure 111-25 Crossed fused renal ectopia.
Longitudinal ultrasonographic image of the right upper quadrant shows a well-defined indentation or notch (arrow) at the junction between the right kidney (RK) and the crossed, fused left kidney (LK). The right renal pelvis has a small amount of visible urine within it (asterisk). (Courtesy Brian D. Coley, MD.)
Ureter
The transition between the renal pelvis and the ureter, or the UPJ, may be sharply or poorly defined. Both extrinsic filling defects and local narrowing are commonly observed at the UPJ without resultant hydronephrosis. An inferior polar artery may produce a small extrinsic defect or notch in the ureter near the UPJ (e-Fig. 111-26). A sharp kink without obstruction is occasionally seen in the proximal portion of the ureter as a transient or constant finding. Retained fetal folds in the upper ureter (e-Fig. 111-27), mild elongation and tortuosity of the ureter, and mild widening of the midureter may all be seen normally in the urogram in the infant. They are believed to represent a persistence of fetal characteristics, and disappear in early childhood.17,79–83
Duplication
Ureteral duplication (duplication of the renal pelvis and ureter) is one of the most common anomalies of the urinary tract (Fig. 111-28). Incomplete duplication ranges from a bifid renal pelvis to two ureters joining anywhere along their course and continuing inferiorly as a single structure. In completely duplicated systems, the two ureters are separate throughout their entire course. The ureter draining the upper pole of the kidney normally inserts into the bladder more caudad and more medially than the lower pole ureter (Weigert-Meyer rule) and has a longer submucosal tunnel than the lower pole ureter. Ureteral duplication is more commonly unilateral, although it may be bilateral. Ureteral duplication is of no clinical importance unless complicated by another congenital lesion or an acquired process. These conditions such as ureteral ectopia, VUR, urinary tract infection, and congenital UPJ obstruction are addressed in later chapters.84–91
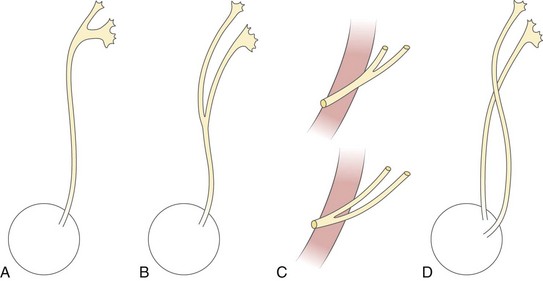
Figure 111-28 Various forms of ureteral duplication.
A, Bifid pelvis. B, Partial ureteral duplication (Y-ureter). C, Incomplete ureteral duplication with the ureters joining near the bladder or within the bladder wall (V-ureter). D, Complete ureteral duplication with separate ureteral orifices. The upper pole ureter inserts distally and medially to the lower pole ureter (Weigert-Meyer rule).
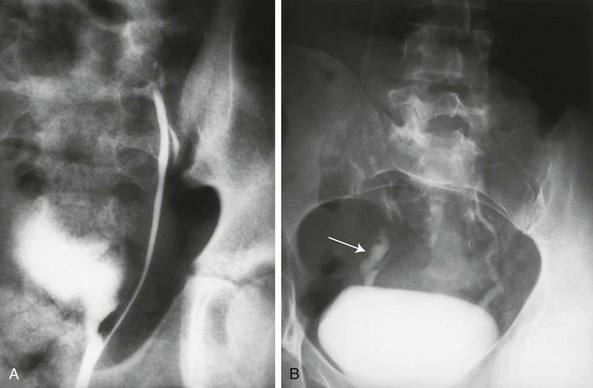
Other variants of ureteral duplication probably related to ureteral duplication include the accessory ureter or a ureteral stump. The accessory ureter is a tubular structure originating from a distal normal ureter at the bladder, and extending cephalad along the normal ureter to end blindly proximally, without connection to a pelvocalyceal system or renal parenchyma. The ureteral stump varies in length from a few centimeters to a narrow but patent cord that extends almost to the kidney (e-Fig. 111-29).92–96
e-Figure 111-29 Rare forms of ureteral duplication.
A, A retrograde ureterogram demonstrates a blind-ending accessory ureter. B, A voiding cystourethrogram demonstrates vesicoureteral reflux into normal right and left ureters and a blind-ending right ureteral stump (arrow).
Ureteral triplication (e-Fig. 111-30) is another rare anomaly in which three ureters arise from the kidney, one from the upper pole, the second from the midzone of the kidney, and the third from the lower pole The three ureters may drain separately into the bladder, or one may end ectopically; the parenchyma drained by the ectopic ureter is usually small and poorly functioning. In a second type, two of the three ureters may join in the lumbar area to form a single Y-shaped ureter that drains usually into the bladder, together with a normal third ureter. In a third type, the three ureters join in the lumbar area to form a common distal ureter that drains into the bladder. More than half the cases of ureteral triplication defy the Weigert-Meyer rule. Four ureters emanating from a single kidney have been described.97–100
Bladder
Bladder ears are normal lateral protrusions of the urinary bladder (Fig. 111-31). They are most frequently seen in infants less than 6 months of age as transient protrusions that are most apparent when the bladder is partially full. Bladder ears represent extraperitoneal herniations of the bladder through the internal inguinal ring into the inguinal canal. An association of bladder ears with inguinal hernia may reflect the presence of a patent or partially patent processus vaginalis. Rarely, lateral protrusions of the rectum occur in this same location, producing a normal anatomic variant termed rectal ears.101–103
Urethra
Several normal findings exist and should not be confused with pathologic processes, including normal anatomic folds of the posterior urethra of the male, and urinal and foreskin artifact. Normal anatomic folds are frequently visualized in the posterior urethra of the male and should not be confused with congenital obstructing membranes. Unlike congenital obstructing membranes, these folds do not produce dilatation of the proximal posterior urethra or decrease the urinary stream.104–108
Urinal artifact may be produced in the male during voiding. The urethral stream is compressed at the level of the penoscrotal junction by the plastic urinal and should not be confused with a stricture of the penile urethra. A film during voiding without urethral compression by the urinal confirms normal urethral caliber.109
Fernbach, SK, Feinstein, KA, Schmidt, MB. Pediatric voiding cystourethrography: a pictorial guide. Radiographics. 2000;20(1):155–168.
Glodny, B, Petersen, J, Hofmann, KJ, et al. Kidney fusion anomalies revisited: clinical and radiological analysis of 209 cases of crossed fused ectopia and horseshoe kidney. BJU Int. 2009;103:224–235.
Moore, KL, Persaud, TVN, The urogenital system. Before we are born: essentials of embryology and birth defects, 7th ed, Philadelphia, PA, WB Saunders, 2003.
Patel, U. Congenital anomalies of the bladder imaging and urodynamics of the lower urinary tract. London, U.K.: Springer; 2010. 23-27
Shapiro, E. Clinical implications of genitourinary embryology. Curr Opin Urol. 2009;19(4):427–433.
References
1. Moore, KL, Persaud, TVN. The urogenital system. In Before we are born: essentials of embryology and birth defects, 7th ed, Philadelphia, PA: WB Saunders; 2003:162–189.
2. Sadler, TW. Urogenital system. In Langman’s medical embryology, 8th ed, Baltimore, MD: Lippincott Williams & Wilkins; 2000:304–344.
3. O’Rahilly, RO, Müller, F. The urinary system. In Human embryology & teratology, 2nd ed, New York: Wiley-Liss; 1996:273–290.
4. Parrott, TS, Skandalakis, JE, Gray, SW. The Kidney and ureter. In: Skandalakis JE, Gray SW, eds. Embryology for surgeons: The embryological basis for the treatment of congenital anomalies. 2nd ed. Baltimore, MD: Williams & Wilkins; 1994:594–670.
5. Parrott, TS, Skandalakis, JE, Gray, SW. The Bladder and urethra. In: Skandalakis JE, Gray SW, eds. Embryology for surgeons: the embryological basis for the treatment of congenital anomalies. 2nd ed. Baltimore, MD: Williams & Wilkins; 1994:671–717.
6. Vogt, BA, MacRae Dell, K, Davis, ID. The Kidney and urinary tract. In: Martin RJ, Fanaroff AA, Walsh MC, eds. Neonatal-perinatal medicine. 8th ed. Philadelphia, PA: Mosby; 2006:1659–1683.
7. Brophy, PD, Robillard, JE. Functional Development of the kidney in utero. In: Polin RA, Fox WW, Abman SH, eds. Fetal and neonatal physiology. 3rd ed. Philadelphia, PA: Saunders; 2004:1229–1241.
8. Dodds, WJ, Darweesh, RM, Lawson, TL, et al. The retroperitoneal spaces revisited. AJR Am J Roentgenol. 1986;147:1155.
9. Lafortune, M, Constantin, A, Breton, G, et al. Sonography of hypertrophied column of Bertin. AJR Am J Roentgenol. 1986;146:53.
10. Auh, YH, Rubinstein, WA, Markisz, JA, et al. Extraperitoneal paravesical spaces: CT delineation with US correlation. Radiology. 1986;159:319.
11. Auh, YH, Rubinstein, WA, Markisz, JA, et al. Intraperitoneal paravesical spaces: CT delineation with US correlation. Radiology. 1986;159:311.
12. Hoffer, FA, Hanabergh, AM, Teele, RL. The interrenicular junction: a mimic of renal scarring on normal pediatric sonograms. AJR Am J Roentgenol. 1985;145:1075.
13. Kenney, IJ, Wild, SR. Renal parenchymal junctional line in children: ultrasonic frequency and appearance. Br J Radiol. 1987;60:865.
14. Erwin, BC, Carroll, BA, Muller, H. A sonographic assessment of neonatal renal parameters. J Ultrasound Med. 1985;4:217.
15. Haller, JO, Berdon, WE, Friedman, AP. Increased renal cortical echogenicity: a normal finding in neonates and infants. Radiology. 1982;142:173.
16. Gordon, I, Riccabona, M. Investigating the newborn kidney—update on imaging techniques. Semin Neonatol. 2003;8:269–278.
17. Han, BK, Babcock, DS. Sonographic measurements and appearance of normal kidneys in children. AJR Am J Roentgenol. 1985;145:611.
18. McClennan, BL, Lee, JKT, Peterson, RR. Anatomy of the perirenal area. Radiology. 1986;158:555.
19. Currarino, G, Williams, B, Dana, K. Kidney length correlated with age: normal values in children. Radiology. 1984;150:703.
20. Jequier, S, Paltiel, H, Lafortune, M. Ureterovesical jets in infants and children: duplex and color Doppler US studies. Radiology. 1990;175:349.
21. Rosenbaum, DM, Korngold, E, Teele, R. Sonographic assessment of renal length in normal children. AJR Am J Roentgenol. 1984;142:467.
22. Schlesinger, AE, Hedlund, GL, Pierson, WP, et al. Normal standards for kidney length in premature infants: determination with US. Work in progress. Radiology. 1987;164:127.
23. Schlesinger, AE, Hernandez, RJ, Zerin, JM, et al. Interobserver and intraobserver variations in sonographic renal length measurements in children. AJR Am J Roentgenol. 1991;156:1029.
24. Vade, A, Lau, P, Smick, J, et al. Sonographic renal parameters as related to age. Pediatr Radiol. 1987;17:212.
25. Zerin, JM, Meyer, RD. Sonographic assessment of renal length in the first year of life: the problem of “spurious nephromegaly. Pediatr Radiol. 2000;30:52.
26. Kaufman, RA, Dunbar, JS, Gole, DE. Normal dilatation of the proximal ureters in children. AJR Am J Roentgenol. 1981;137:945.
27. Herman, TE, McAlister, OH. Radiographic manifestations of congenital anomalies of the lower urinary tract. Radiol Clin North Am. 1991;29:365.
28. Shapiro, E. Clinical implications of genitourinary embryology. Curr Opin Urol. 2009;19(4):427–433.
29. Uetani, N, Bouchard, M. Plumbing in the embryo: developmental defects of the urinary tracts. Clin Genet. 2009;75(4):307–317.
30. Viana, R, Batourina, E, Huang, H, et al. The development of the bladder trigone, the center of the anti-reflux mechanism. Development. 2007;134(20):3763–3769.
31. Murer, L, Benetti, E, Artifoni, L. Embryology and genetics of primary vesico-ureteric reflux and associated renal dysplasia. Pediatr Nephrol. 2007;22(6):788–797.
32. Murawski, IJ, Gupta, IR. Gene discovery and vesicoureteric reflux. Pediatr Nephrol. 2008;23(7):1021–1027.
33. Thomas, JC, DeMarco, RT, Pope, JC. 4th: Molecular biology of ureteral bud and trigonal development. Curr Urol Rep. 2005;6(2):146–151.
34. Eccles, MR, Jacobs, GH. The genetics of primary vesico-ureteric reflux. Ann Acad Med Singapore. 2000;29(3):337–345.
35. Berrocal, T, Lopez-Pereira, P, Arjonilla, A, et al. Anomalies of the distal ureter, bladder, and urethra in children: embryologic, radiologic, and pathologic features. Radiographics. 2002;22:1139–1164.
36. Cohen, HL, Kravets, F, Zucconi, W, et al. Congenital abnormalities of the genitourinary system. Semin Roentgenol. 2004;39:282–303.
37. Koff, SA. Evaluation and management of voiding disorders in children. Urol Clin North Am. 1988;15:769.
38. Fernbach, SK, Feinstein, KA. Abnormalities of the bladder in children: imaging findings. AJR Am J Roentgenol. 1994;162:1143.
39. Fernbach, SK, Feinstein, KA, Schmidt, MB. Pediatric voiding cystourethrography: a pictorial guide. Radiographics. 2000;20:155.
40. Riccabona, M, Lindbichler, F, Sinzig, M. Conventional imaging in paediatric uroradiology. Eur J Radiol. 2002;43:100.
41. Herman, TE, McAllister, WH. Radiographic manifestations of the lower urinary tract. Radiol Clin North Am. 1991;29:365.
42. Berger, RM, Maizels, M, Moran, GC, et al. Bladder capacity (ounces) equals age (years) plus 2 predicts normal bladder capacity and aids in the diagnosis of abnormal voiding patterns. J Urol. 1983;129:347–349.
43. Zerin, JM, Chen, E, Ritchey, ML, et al. Bladder capacity as measured at voiding cystourethrography in children: relationship to toilet training and frequency of micturition. Radiology. 1993;187:803–806.
44. Ludwikowski, B, Oesch Hayward, I, Brenner, E, et al. The development of the external urethral sphincter in humans. BJU Int. 2001;87:565–568.
45. Sebe, P, Fritsch, H, Oswald, J, et al. Fetal development of the female external urinary sphincter complex: an anatomical and histological study. J Urol. 2005;173:1738–1742.
46. Kawashima, A, Sandler, CM, Wasserman, NF, et al. Imaging of urethral disease: a pictorial review. Radiographics. 2004;24(suppl 1):S195–S216.
47. Lo, WC, Wang, CR, Lim, KE. Diagnosis of the congenital urethral anomalies of male child by voiding cystourethrography. Acta Paediatr Taiwan. 1999;40:152–156.
48. Pavlica, P, Barozzi, L, Menchi, I. Imaging of the male urethra. Eur Radiol. 2003;13:1583–1596.
49. Klimberg, I. The development of voiding control. Am Urol Assoc Update Series. 1988;7:161.
50. Volmar, KE, Fritsch, MK, Perlman, EJ, et al. Patterns of congenital lower urinary tract obstructive uropathy: relation to abnormal prostate and bladder development and the prune belly syndrome. Pediatr Dev Pathol. 2001;4:467.
51. Fedele, L, Bianchi, S, Agnoli, B, et al. Urinary tract anomalies associated with unicornuate uterus. J Urol. 1996;155:847–848.
52. Oppelt, P, Renner, SP, Kellermann, A, et al. Clinical aspects of Mayer-Rokitansky-Kuester-Hauser syndrome: recommendations for clinical diagnosis and staging. Hum Reprod. 2006;21:792–797.
53. Tanaka, YO, Kurosaki, Y, Kobayashi, T, et al. Uterus didelphys associated with obstructed hemivagina and ipsilateral renal agenesis: MR findings in seven cases. Abdomin Imag. 1998;23:437–441.
54. Tarry, WF, Duckett, JW, Stephens, FD. The Mayer-Rokitansky syndrome: pathogenesis, classification and management. J Urol. 1986;136:648–652.
55. Schlegel, PN, Shin, D, Goldstein, M. Urogenital anomalies in men with congenital absence of the vas deferens. J Urol. 1996;155:1644–1648.
56. van den Ouden, D, Blom, JH, Bangma, C, et al. Diagnosis and management of seminal vesicle cysts associated with ipsilateral renal agenesis: a pooled analysis of 52 cases. Eur Urol. 1998;33:433–440.
57. Kim, B, Kawashima, A, Ryu, JA, et al. Imaging of the seminal vesicle and vas deferens. Radiographics. 2009;29:1105–1121.
58. Wojcik, LJ, Hansen, K, Diamond, DA, et al. Cystic dysplasia of the rete testis: benign congenital lesion associated with ipsilateral urological anomalies. J Urol. 1997;158:600–604.
59. McGahan, JP, Myracle, MR. Adrenal hypertrophy: possible pitfall in the sonographic diagnosis of renal agenesis. J Ultrasound Med. 1986;5:265–268.
60. Hoffman, CK, Filly, RA, Callen, PW. The “lying down” adrenal sign: a sonographic indicator of renal agenesis or ectopia in fetuses and neonates. J Ultrasound Med. 1992;11:533–536.
61. Kenney, PJ, Robbins, GL, Ellis, DA, et al. Adrenal glands in patients with congenital renal anomalies: CT appearance. Radiology. 1985;155:181–182.
62. Barnewolt, CE, Lebowitz, RL. Absence of a renal sinus echo complex in the ectopic kidney of a child: a normal finding. Pediatr Radiol. 1996;26:318–323.
63. Rouanne, M, Le Mandat, A, Dorgeret, S, et al. A rare case of ectopic intrathoracic kidney in a 1-year-old child. Urology. 2010;76:57–59.
64. Liddell, RM, Rosenbaum, DM, Blumhagen, JD. Delayed radiologic appearance of bilateral thoracic ectopic kidneys. AJR Am J Roentgenol. 1989;152:120–122.
65. Sharma, R, Savita, N, Chakravarty, KL, et al. Pancake kidney detected on renal scintigraphy. Clin Nucl Med. 2006;31:729–730.
66. Glodny, B, Petersen, J, Hofmann, KJ, et al. Kidney fusion anomalies revisited: clinical and radiological analysis of 209 cases of crossed fused ectopia and horseshoe kidney. BJU Int. 2009;103:224–235.
67. Huang, EY, Mascarenhas, L, Mahour, GH. Wilms’ tumor and horseshoe kidneys: a case report and review of the literature. J Pediatr Surg. 2004;39:207–212.
68. O’Brien, J, Buckley, O, Doody, O, et al. Imaging of horseshoe kidneys and their complications, J Med Imaging. Radiat Oncol. 2008;52:216–226.
69. Kaakaji, Y, Pfister, RC. Case 1: Horseshoe kidney with severe congenital hydronephrosis (ureteropelvic junction obstruction). AJR Am J Roentgenol. 1998;171:826. [829-830].
70. Cascio, S, Sweeney, B, Granata, C, et al. Vesicoureteral reflux and ureteropelvic junction obstruction in children with horseshoe kidney: treatment and outcome. J Urol. 2002;167:2566–2568.
71. Neville, H, Ritchey, ML, Shamberger, RC, et al. The occurrence of Wilms tumor in horseshoe kidneys: a report from the National Wilms Tumor Study Group (NWTSG). J Pediatr Surg. 2002;37:1134–1137.
72. Mesrobian, HG, Kelalis, PP, Hrabovsky, E, et al. Wilms tumor in horseshoe kidneys: a report from the National Wilms Tumor Study. J Urol. 1985;133:1002–1003.
73. Strauss, S, Dushnitsky, T, Peer, A, et al. Sonographic features of horseshoe kidney: review of 34 patients. J Ultrasound Med. 2000;19:27–31.
74. Patel, TV, Singh, AK. Crossed fused ectopia of the kidneys. Kidney Int. 2008;73:662.
75. Watanabe, T. Reflux nephropathy in a patient with crossed renal ectopia with fusion. Pediatr Nephrol. 2002;17:617–619.
76. Hertz, M, Rubinstein, ZJ, Shahin, N, et al. Crossed renal ectopia: clinical and radiological findings in 22 cases. Clin Radiol. 1977;28:339–344.
77. Goodman, JD, Norton, KI, Carr, L, et al. Crossed fused renal ectopia: sonographic diagnosis. Urol Radiol. 1986;8:13–16.
78. McCarthy, S, Rosenfield, AT. Ultrasonography in crossed renal ectopia. J Ultrasound Med. 1984;3:107–112.
79. Rigas, A, Karamanolakis, D, Bogdanos, I, et al. Pelvi-ureteric junction obstruction by crossing renal vessels: clinical and imaging features. BJU Int. 2003;92(1):101–103.
80. Riccabona, M. The ureter and vesicoureteral reflux. In: Slovis T, ed. Caffey’s pediatric diagnostic imaging. 11th ed. Philadelphia, PA: Mosby; 2008:2315–2355.
81. Rabinowitz, R, Kingston, TE, Wesselhoeft, C, et al. Ureteral valves in children. Urology. 1998;51(suppl 5A):7–11.
82. Stringer, MD, Yassaie, S. Is the pelviureteric junction an anatomical entity? J Pediatr Urol. 2011. [Epub ahead of print].
83. Patel, U. Congenital anomalies of the bladder. Imaging and urodynamics of the lower urinary tract. London, U.K.: Springer; 2010.
84. Caldamone, AA. Duplication anomalies of the upper tract in infants and children. Urol Clin North Am. 1991;12:75.
85. Berdon, WE. Contemporary imaging approach to pediatric urologic problems. Radiol Clin North Am. 1991;29:605.
86. Churchill, BM, Abara, EO, McLorie, GA. Ureteral duplication, ectopy and ureteroceles. Pediatr Clin North Am. 1987;34:1273.
87. Bisset, GS, Strife, JL. The duplex collecting system in girls with urinary tract infection: prevalence and significance. AJR Am J Roentgenol. 1987;148:497.
88. Kaefer, M, Barnewolt, C, Retik, AB, et al. The ultrasound diagnosis of infravesical obstruction in children: evaluation of bladder wall thickness indexed to bladder filling. J Urol. 1997;157:989.
89. Nussbaum, AR, Dorst, JP, Jeffs, RD, et al. Ectopic ureter and ureterocele: their varied radiographic manifestations. Radiology. 1986;159:227.
90. Sorantin, E, Fotter, R, Aigner, R, et al. The sonographically thickened wall of the upper urinary tract: correlation with other imaging methods. Pediatr Radiol. 1997;27:667.
91. Gylys-Morin, VM, Minevich, E, Tackett, LD, et al. Magnetic resonance imaging of the dysplastic renal moiety and ectopic ureter. J Urol. 2000;164:2034.
92. Gupta, PK. Letter to the editor: Blind-ending branch of bifid ureter: multidetector CT imaging findings. Br J Radiol. 2011;84(1004):769.
93. Chang, E, Santillan, C, O’Boyle, MK. Blind-ending branch of a bifid ureter: multidetector CT imaging findings. Br J Radiol. 2011;84(998):e-38–e40.
94. Blair, D, Rigsby, C, Rosenfield, AT. The nubbin sign on computed tomography and sonography. Urol Radiol. 1987;9:149.
95. Hawas, N, Noah, M, Pattel, PJ. Blind-ending ureter: clinical significance? An analysis of 13 cases with review of the literature. Eur Urol. 1987;13:39.
96. Tilley, EA, Dow, CJ. Cranial blind-ending branch of a bifid ureter: report of 3 cases. Br J Urol. 1988;62:127.
97. Kokabi, N, Price, N, Smith, GH, et al. Ureteral triplication: a rare anomaly with a variety of presentations. J Pediatr Urol. 2011;7(4):484–487.
98. Xu, Z, Li, Z, Wang, D, et al. Ureteral triplication combined with right renal ectopia and ureteral cyst. Urol Int. 2009;83(4):476–478.
99. Bloom, RA, Crooks, EK, Wise, HA. Complete ureteral triplication with ectopia. Urology. 1984;25:176.
100. Gosalbez, R, Jr., Gosalbez, R, Piro, C, et al. Ureteral triplication and ureterocele: report of 3 cases and review of the literature. J Urol. 1991;145:105.
101. Lakshminarayana, G, Mathew, A, Rajesh, R, et al. A rare congenital anomaly of urinary bladder—Bladder ears Indian. J Nephrol. 2010;20(4):220–221.
102. Aygen, M, Akduman, IE, Osman, MM. Bladder ear: a potential source of false interpretation on F-18 FDG PET. Clin Nucl Med. 2008;33(10):721–722.
103. Kassner, EG, Schussheim, A, Gordon, DH. Rectal ears. J Can Assoc Radiol. 1975;26(2):125–127.
104. Fernbach, SK, Feinstein, KA, Schmidt, MB. Pediatric voiding cystourethrography: a pictorial guide. Radiographics. 2000;20(1):155–168.
105. Kawashima, A, Sandler, CM, Wasserman, NF, et al. Imaging of urethral disease: a pictorial review. Radiographics. 2004;24:S195–S216.
106. Schöllnast, H, Lindbichler, F, Riccabona, M. Ultrasound (US) of the urethra in infants: comparison US versus VCUG. J Ultrasound Med. 2004;23:769.
107. Teele, RL, Share, JC. Transperineal sonography in children. AJR Am J Roentgenol. 1997;168:1263.
108. Giordano, M, Marzolla, R, Puteo, F, et al. Voiding urosonography as first step in diagnosis of vesicoureteral reflux in children: a clinical experience. Pediatr Radiol. 2007;37:674–677.
109. Ramos, AJ, Watts, FB, Jr., Timmons, JW. The urinal artifact. Radiology. 1978;128(3):807–808.