Embryology, Anatomy, and Normal Findings
Embryology
The spinal cord forms in three stages beginning in the third gestational week when the notochord induces surrounding ectoderm to differentiate into neuroectoderm.1 The first stage, neurulation, involves progression from neural plate to neural groove to neural tube.1 The notochord transforms into the nucleus pulposus of the intervertebral disks.2 The second stage, canalization, involves formation of cysts within the caudal cell mass that gradually coalesce and fuse to the distal neural tube to form the primitive spinal cord. The third stage, retrogressive differentiation, involves programmed cell death leading to regression of the primitive distal spinal cord to form the fetal conus, filum terminale, and ventriculus terminalis1,2 (Fig. 40-1).
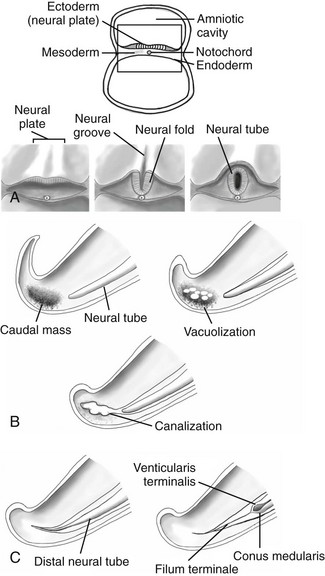
Figure 40-1 Embryological development of the spinal cord.
A, Neurulation schematic illustrates progression from neural plate to groove to neural tube. B, Canalization schematic depicts coalescence of cysts within the caudal cell mass that fuse to the distal neural tube. C, A retrogressive differentiation schematic reveals the process of programmed cell death forming the conus medullaris and filum terminale.
The vertebral bodies develop from somites that have been converted through signaling molecules to form sclerotomes.3 The caudal and cranial portions of adjacent sclerotomes fuse to become single vertebral bodies. Failure of this process leads to congenital vertebral segmentation anomalies such as block or hemivertebrae (Fig. 40-2).4,5
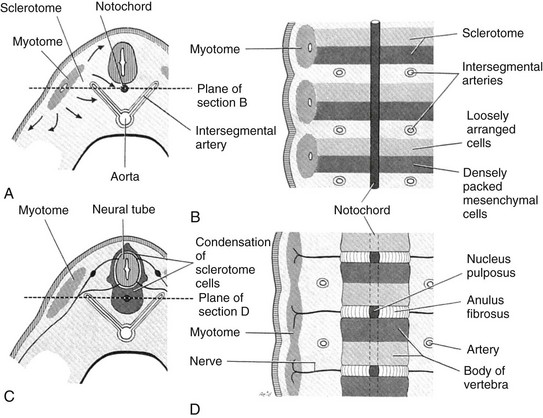
Figure 40-2 Embryological development of the vertebra.
A, A partial transverse section through a 4-week embryo shows arrows indicating the spread of mesenchymal cells from the sclerotome region of the somite on the right. B, A diagrammatic frontal section of this embryo showing that the condensation of sclerotome cells around the notochord consists of a cranial area of loosely packed cells and a caudal area of densely packed cells. C, A partial transverse section through a 5-week embryo shows the condensation of sclerotome cells around the notochord and the neural tube, which forms a mesenchymal vertebra. D, A diagrammatic frontal section illustrates that the vertebral body forms from the cranial and caudal halves of two successive sclerotomes. The intersegmental arteries now cross the bodies of the vertebrae, and the spinal nerves lie between the vertebrae. The notochord is degenerating except in the intervertebral disk, where it persists as the nucleus pulposus. (From Moore KL. The developing human: clinically oriented embryology. 4th ed. Philadelphia: Saunders; 1988:338.)
Anatomy and Physiology
Although 5% of humans have differing numbers of vertebrae, most often people have 7 cervical, 12 thoracic, and 5 lumbar vertebral bodies, as well as 5 sacral and 4 coccygeal segments.6 Running craniocaudal are the anterior and posterior longitudinal ligaments, supraspinous and interspinous ligaments, and ligamentum flavum.
The arterial supply to the spinal cord is via the single, midline anterior spinal artery and the paired posterior spinal arteries. The anterior spinal artery originates from bilateral branches of the intradural segments of the vertebral arteries and courses along the ventral surface of the spinal cord. Similarly, the posterior spinal arteries arise from the intradural vertebral arteries, but course along the dorsal surface of the spinal cord.7 Radicular artery branches contribute to the cord arterial supply at multiple points. One particularly large branch, the artery of Adamkiewicz, typically enters the spinal canal between the T9 and T12 levels and can be recognized by its characteristic proximal hairpin turn.8
Normal Findings
The indications for spine ultrasonography include screening neonates with multiple congenital anomalies, screening complicated dimples with skin stigmata, and evaluating soft tissue masses that suggest possible underlying closed (occult) spinal dysraphism, as well as determining the cause of failed lumbar puncture and localizing fluid for possible additional attempts at lumbar puncture.9,10 High-risk skin stigmata include atypical dimples (>5 mm) located above the gluteal crease (>2.5 mm from the anus), dimples in which the bottom cannot be visualized, and those with skin stigmata such as a hairy patch, hemangioma, a mound of soft tissue, skin tag, or tail.11 In anomalies requiring surgery and dimples draining cerebrospinal fluid, urgent MRI is the initial study of choice.12,13 Additionally, MRI is helpful to further characterize anomalies found on ultrasound and is best performed immediately prior to therapeutic intervention.9
The location of the conus medullaris is usually determined by counting down from the twelfth rib, as well as up from the lumbosacral junction.11,12,14 In some cases, one may need to count down from the cervical level as well.13 If the conus level is still uncertain, follow-up ultrasound may be considered. Rarely, an immediate determination is important in patient management, in which case a BB can be placed at the level of the tip of the conus (as determined by ultrasound) and a radiograph of the entire spine obtained for further clarification.15
Spine sonography requires knowledge of normal anatomy and variants that may simulate pathology to prevent unnecessary referral for MRI. The tapering spinal cord forming the conus medullaris, and the layering cauda equina nerve roots are easily seen within the hypoechoic cerebrospinal fluid (Fig. 40-3). The spinal cord and nerve roots often are observed pulsating (oscillating) with the cardiac cycle, which can be documented with cine or on M mode ultrasound. However, this motion is “variably present” in the newborn.15 The normal filum terminale, an echogenic linear structure that extends from the conus medullaris to the distal thecal sac, should be homogeneous in echogenicity and thickness throughout its length, measuring less than 1 to 1.5 mm. The conus medullaris is normally located at or above the superior end plate of L3.16 Recent reports indicate that infants with isolated borderline low conus position extending to the midbody of L3 on spine ultrasound are normal and will meet normal developmental milestones.17 A conus tip below the midbody of L3 is abnormal.16 Occasionally, determination of conus level on ultrasound and marked radiographs are not clear and MRI is needed to clarify.
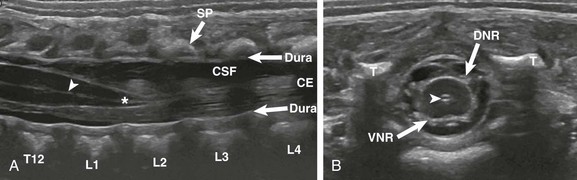
Figure 40-3 A normal spine sonogram in a 1-day-old male with multiple congenital anomalies.
Longitudinal (A) and transverse (B) images demonstrate the normal anatomy. Note the dura, central echo complex (arrowheads) and conus medullaris (asterisk). Other labeled structures include the vertebra (T12-L4); SP, spinous processes; CE, cauda equina; CSF, cerebrospinal fluid; T, transverse processes; VNR, ventral nerve roots; and DNR, dorsal nerve roots.
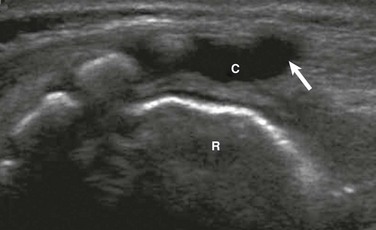
Normal variants to be aware of include the ventriculus terminalis, filar cysts, a prominent filum terminale, a pseudosinus tract, a cauda equina pseudomass, and a dysmorphic coccyx. The ventriculus terminalis, which is seen mostly in children younger than 5 years, and occasionally in adults, involves persistence of the normal fetal terminal ventricle. It is characterized by contiguity with the central spinal canal, which expands within the conus medullaris to form a small space containing fluid (Fig. 40-4). It must be distinguished from a syrinx, which is typically larger and may grow over time.
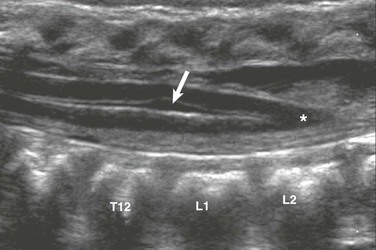
Figure 40-4 Ventriculus terminalis in a 3-month-old girl with a deep dimple.
Longitudinal sonography demonstrates focal distension of the distal central spinal canal (arrow) in the lumbar cord just above the conus medullaris (asterisk).
Filar cysts are midline, fusiform, well defined hypoechoic fluid spaces located in the cauda equina immediately below the conus medullaris, and are of uncertain origin (Fig. 40-5). They are seen on ultrasound in 11.8% of infants, typically are not seen on MRI, and lack pathologic description, indicating that they may be structural pseudocysts.18 When seen on ultrasound in isolation, they are of no clinical significance.18
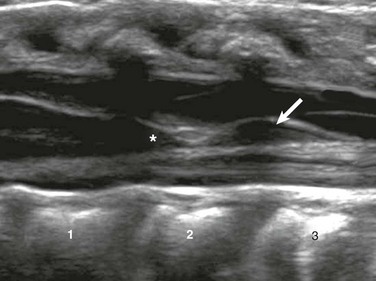
Figure 40-5 A filar cyst in a 6-week-old male with an asymmetric gluteal crease.
A longitudinal sonogram shows a fusiform, midline hypoechoic “cyst” (arrow) just below the conus medullaris (asterisk).
The normal filum terminale may cause confusion because it is often more prominent than the rest of the cauda equina. It is distinguished by its normal thickness (<1 to 1.5 mm) and midline anatomic location (e-Fig. 40-6). A prominent filum must be distinguished from a fibrofatty filum and a filar lipoma, which are suspected when focal hyperechogenicity and thickening (>2 mm) of the filum terminale are seen (Fig. 40-7).19 A fibrofatty filum may be incidental but has also been described as part of the “tight filum terminale syndrome,” the symptoms of which may develop at any age and include lower extremity weakness, spasticity, foot deformities, bladder dysfunction, scoliosis, and back pain.20
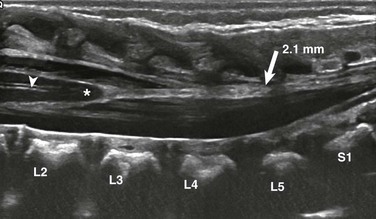
Figure 40-7 Fatty filum in a 2-day-old male with VATER syndrome.
A longitudinal sonogram shows a subtle focus of increased echogenicity and thickening of the filum (arrow) as it extends from the conus medullaris to the distal thecal sac. The filum measures 2.1 mm. Also note the normal central echo complex (arrowhead).
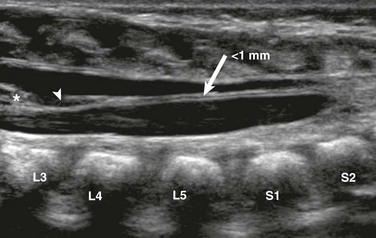
e-Figure 40-6 A prominent filum terminale in a 1-day-old male with multiple congenital anomalies.
A longitudinal sonogram reveals a midline linear filum terminale (arrow) of normal size (<1 mm) measured at L5-S1. The filum extends from the tip of the conus (asterisk) to the distal thecal sac. Note the incidental cyst in the filum (arrowhead).
A pseudomass due to nerve root clumping may be seen when infants undergo sonography are in the decubitus position. This finding is easily evaluated by showing resolution of the “mass” when the infant undergoes repeat scanning in the prone position. Pseudosinus tracts, or fibrous cords of echogenicity extending from the base of dimples to the coccyx, are a frequent normal variant seen on modern high-quality spinal ultrasound (Fig. 40-8). These tracts do not drain or contain fluid, do not have an associated mass, and must be distinguished from true dermal sinus tracts. True sinus tracts, which often drain cerebrospinal fluid and are associated with an increased risk of meningitis, are attributed to incomplete disjunction of the cutaneous ectoderm from the neuroectoderm.21 A final normal variant, the misshapen or dysmorphic coccyx, is a common ultrasound finding that may be discovered on physical examination when a “mass” is palpated.22 Although the variety and degree of dysmorphic coccygeal shapes may be impressive, the finding is of no clinical significance (e-Fig. 40-9).
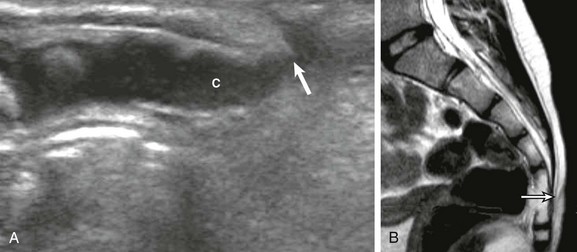
Figure 40-8 A pseudosinus tract in a 3-month-old girl with a deep dimple in the gluteal crease.
A, A longitudinal sonogram identifies the normal hypoechoic coccyx (C) with a cordlike hypoechoic pseudotract (arrow) extending to the skin dimple. B, Follow-up magnetic resonance imaging at age 6 months confirms the uncomplicated pseudosinus tract (arrow).
Bulas, D. Fetal evaluation of spine dysraphism. Pediatric Radiology. 2010;40(6):1029–1037.
Johanek, AJ, Lowe, LH, Moore, AW. Sonography of the neonatal spine: Part 1, normal anatomy, imaging pitfalls and variations that may simulate disorders. Am J Roentgenol. 2007;188:733–738.
Johanek, AJ, Lowe, LH, Moore, AW. Sonography of the neonatal spine: part 2, Spinal disorders. Am J Roentgenol. 2007;188:739–744.
References
1. Carlson, BM. Human embryology and developmental biology, 4th ed. Philadelphia: Mosby; 2009.
2. Moore, KL, Persaud, TVN. The developing human: clinically oriented embryology, 7th ed. Philadelphia: Saunders; 2003.
3. Sasaki, N, Kurisu, J, Kengaku, M. Sonic hedgehog signaling regulates actin cytoskeleton via Tiam1-Rac1 cascade during spine formation. Mol Cell Neurosci. 2010;45(4):335–344.
4. Nolting, D, Hansen, BF, Keeling, J, et al. Prenatal development of the normal human vertebral corpora in different segments of the spine. Spine (Phila Pa 1976). 1998;23(21):2265–2271.
5. Turnpenny, PD. Defective somitogenesis and abnormal vertebral segmentation in man. Adv Exp Med Biol. 2008;638:164–189.
6. Karasick, D, Schweitzer, ME, Vaccaro, AR. The traumatized cervical spine in Klippel-Feil syndrome: imaging features. AJR Am J Roentgenol. 1998;170(1):85–88.
7. Drake, RL, et al. Gray’s anatomy for students. Philadelphia: Elsevier/Churchill Livingstone; 2005.
8. Takase, K, Sawamura, Y, Igarashi, K, et al. Demonstration of the artery of Adamkiewicz at multi-detector row helical CT. Radiology. 2002;223(1):39–45.
9. Robinson, AJ, Russell, S, Rimmer, S. The value of ultrasonic examination of the lumbar spine in infants with specific reference to cutaneous markers of occult spinal dysraphism. Clin Radiol. 2005;60(1):72–77.
10. Filippigh, P, Clapuyt, P, Debauche, C, et al. Sonographic evaluation of traumatic spinal cord lesions in the newborn infant. Pediatr Radiol. 1994;24(4):245–247.
11. Byrd, SE, Darling, CF, McLone, DG. Developmental disorders of the pediatric spine. Radiol Clin North Am. 1991;29(4):711–752.
12. Hughes, JA, De Bruyn, R, Patel, K, et al. Evaluation of spinal ultrasound in spinal dysraphism. Clin Radiol. 2003;58(3):227–233.
13. Korsvik, HE, Keller, MS. Sonography of occult dysraphism in neonates and infants with MR imaging correlation. Radiographics. 1992;12(2):297–306. [discussion 307-308].
14. Unsinn, KM, Geley, T, Freund, MC, et al. US of the spinal cord in newborns: spectrum of normal findings, variants, congenital anomalies, and acquired diseases. Radiographics. 2000;20(4):923–938.
15. DiPietro, MA, Garver, KA. Sonography of the neonatal spinal canal. In Haller JO, ed.: Textbook of neonatal ultrasound, 1st ed, New York: Parthenon, 1998.
16. Robbin, ML, Filly, RA, Goldstein, RB. The normal location of the fetal conus medullaris. J Ultrasound Med. 1994;13(7):541–546.
17. Thakur, NH, Lowe, LH. Borderline low conus medullaris on infant lumbar sonography: what is the clinical outcome and the role of neuroimaging follow-up? Pediatr Radiol. 2011;41(4):483–487.
18. Irani, N, Goud, AR, Lowe, LH. Isolated filar cyst on lumbar spine sonography in infants: a case-control study. Pediatr Radiol. 2006;36(12):1283–1288.
19. Rypens, F, Avni, EF, Matos, C, et al. Atypical and equivocal sonographic features of the spinal cord in neonates. Pediatr Radiol. 1995;25(6):429–432.
20. Cornips, EM, Vereijken, IM, Beuls, EA, et al. Clinical characteristics and surgical outcome in 25 cases of childhood tight filum syndrome. Eur J Paediatr Neurol. 2012;16(2):103–117.
21. Drolet, BA. Cutaneous signs of neural tube dysraphism. Pediatr Clin North Am. 2000;47(4):813–823.
22. Kriss, VM, Desai, NS. Occult spinal dysraphism in neonates: assessment of high-risk cutaneous stigmata on sonography. AJR Am J Roentgenol. 1998;171(6):1687–1692.