Embryology, Anatomy, and Normal Findings
Embryology
Both striated muscle and bone are derived from the mesodermal germ layer. The limb buds form at the end of the fourth week of fetal life. Near the end of the second month of fetal life, the embryonal cartilaginous skeleton is already subdivided into its principal segments, which are the forerunners of bones of the limbs. Primary ossification centers are formed by deposition of calcium in the cartilaginous matrix after hypertrophy and vacuolization of local cartilage cells.1 In tubular bones, this process occurs at approximately the midpoint of the shaft and is followed by central resorption, which gives rise to the primary marrow cavity. Calcified disks proximal and distal to the primary cavity become preparatory zones of calcification after the development of the advancing, proliferating shaft during growth. Cartilage proximal and distal to the zones of calcification becomes the epiphyses. A layer of cells within the epiphyses near the shaft produces new cells that are interposed between the resting cartilage of the epiphysis and the older cells and calcified cartilage adjacent to the shaft. The matrix around the old cells calcifies and is invaded by capillaries and bone cells from the marrow. Bone is formed on the calcified cartilage, and new bone and cartilage undergo remodeling by osteoclasts and osteoblasts, so that the length of the bone is increased (Fig. 129-1). The girth of the bone and the thickness of the cortex are increased by subperiosteal accretion caused by activity of the subperiosteal osteoblasts. Peripheral resorption of bone at the advancing ends of the shaft maintains the gentle flaring that characterizes normal tubular bone and is the mechanism responsible for what is termed “modeling.” Disproportionate osteoclastic activity within the shaft of the bone reams out a marrow cavity. Continued, balanced activity of all these processes permits a small tubular bone to become a large tubular bone while functional shape and relations with adjacent structures are maintained.
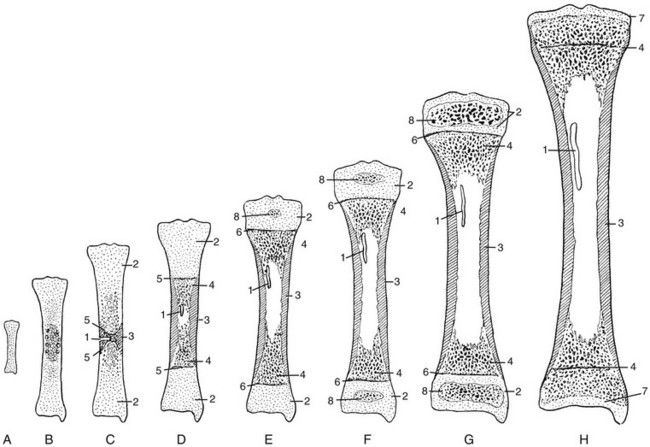
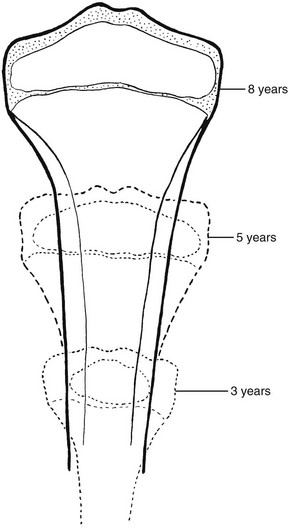
Figure 129-1 Schema of progressive stages in the growth and maturation of the tibia.
A, The mass of embryonal cartilage that is the anlage of the tibia. B, Initial enlargement and multiplication of the central cartilage cells and an increase in cartilaginous matrix—the chondrification center that is the forerunner of the primary ossification center. C, The early primary ossification center shows the formation of a central belt of subperiosteal bone (early cortex) and penetration of the cartilaginous matrix by the periosteal elements; the channel of this penetration persists as the nutrient canal. D, Extension of ossification toward both ends of the shaft, with central resorption forming the medullary cavity. E, The tibia at birth, with a secondary ossification center in the proximal epiphyseal cartilage. F, At approximately the fourth postnatal month, ossification centers are seen in both of the epiphyseal cartilages. G, The juvenile tibia shows the growth of all components and enlargement of the epiphyseal secondary ossification centers. H, The adult tibia, with complete fusion of the shaft and both epiphyses. The narrow plates of articular cartilage that cap each end of the bone persist throughout life. 1, nutrient canal; 2, epiphyseal cartilage; 3, corticalis; 4, spongiosa; 5 and 6, provisional zones of calcification or epiphyseal plates; 7, articular cartilages; 8, secondary epiphyseal ossification centers. (Modified from an original drawing by W. M. Rogers, MD.)
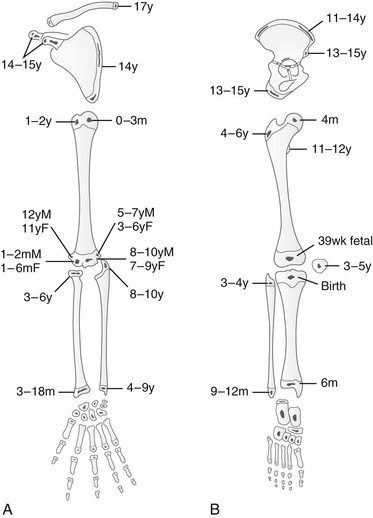
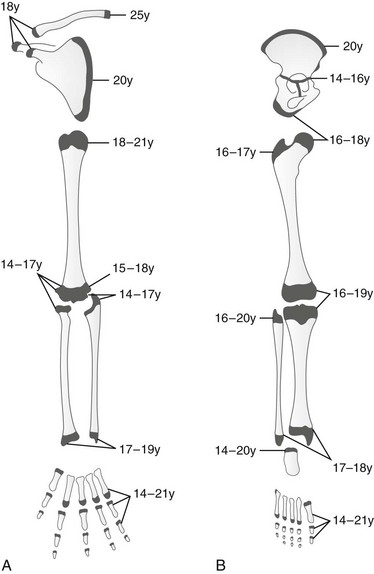
Secondary ossification centers appear in the cartilaginous epiphyses and apophyses and enlarge by similar but much slower processes than those that enlarge the shaft. Irregularities in density and discontinuities in the structure of secondary ossification centers are frequent during normal mineralization and simulate the features of disease. The known ages at first appearance and upon fusion of these ossification centers can serve as indicators of physical maturation (Figs. 129-2 and 129-3; Table 129-1).
Table 129-1
Epiphyseal Ossification in the Fetus and Neonate: Fifth and Ninety-Fifth Percentiles
| Ossification Center | Fifth Percentile | Ninety-Fifth Percentile |
| Humeral head | 37th week | 16 postnatal weeks |
| Distal femur | 31st week | 39th week (female) |
| 40th week (male) | ||
| Proximal tibia | 34th week | 2 postnatal weeks (female) |
| 5 postnatal weeks (male) | ||
| Calcaneus | 22nd week | 25th week |
| Talus | 25th week | 31st week |
| Cuboid | 37th week | 8 postnatal weeks (female) |
| 16 postnatal weeks (male) |
Data from Kuhns LR, Finnstrom O. New standards of ossification of the newborn. Radiology. 1976;119:655-660.
Physiology
The bones provide rigid support for the body and sites of insertion for muscles, to which the bones respond as levers. The bones are active physiologically in infants and children, changing size and shape with growth and hormone activity (related to levels of vitamin D, parathyroid hormone, calcitonin, or serum calcium and phosphate levels) and in response to mechanical stresses.2
During growth, in addition to its constant increase in length and breadth, the shaft is continuously molded or reshaped to its final form. The mechanism responsible for these changes in shape has been called “modeling” or “tubulation.” One of the most conspicuous features of modeling is progressive concentric contraction of the shaft behind the wider, advancing terminal segment (Fig. 129-4); this process results in flared ends of bones.
Anatomy
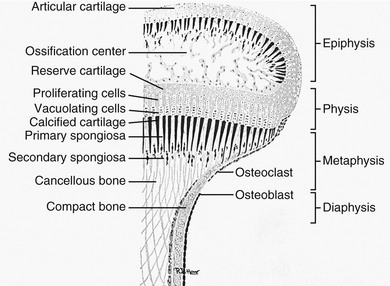
Three types of bones are found in the limbs: (1) long and short tubular bones, (2) round bones in the wrists and ankles, and (3) sesamoids, which are small bones in the tendons and articular capsules. Functionally, a growing tubular bone is made up of the following segments: diaphysis, metaphysis, physis, and epiphysis (Fig. 129-5). “Long bones” have epiphyses at both ends. Short tubular bones (“short bones”) have epiphyses at one end—generally, where the greater joint motion of the individual bone occurs. In the hands and feet, secondary ossification centers appear in the bases of the phalanges and in the distal ends of the second through fifth metacarpals and metatarsals. Epiphyses for the first metacarpal and first metatarsal are found in the proximal ends of the bones. The location of the secondary centers appears to be related to the sites of maximal joint motion of individual bones. Apparent epiphyseal ossification centers observed at the ends of short bones, where their occurrence is not expected, are termed “pseudoepiphyses.”
Normal Findings
The gestational age of a newborn can be estimated radiographically through several methods. The proximal humeral ossification center appears shortly after birth, with a 95% confidence interval between 37 weeks’ gestation and 16 weeks’ postnatal life.3 Because ossification before 37 weeks is unusual, the presence of the proximal humeral ossification center is an indication that a newborn is at term or near term. Teeth appear at a characteristic time; the first deciduous molars form at 33 weeks, and the second deciduous molars form at 36 weeks. The range of values is great for the radiographic appearance of various appendicular bone epiphyseal ossification centers, even in premature infants (see Table 129-1).
Evaluation of bone age is used to estimate biologic maturation relative to chronologic age. Differences in familial, racial, and socioeconomic factors limit the applicability of the standards of Greulich and Pyle (which were developed in the 1940s) to today’s children.4 It is well accepted that maturation varies between races. For instance, African American children mature faster than do white children.5 The hand, with its numerous secondary centers in phalanges and metacarpals, and to a lesser degree the wrist, are used as an index of total skeletal maturation. Standard deviations, which are based on chronologic age, are somewhat broad. A bone age within the 5% to 95% confidence interval (or ±2 standard deviations) is considered normal.
In children younger than 2 years, use of the standards of Greulich and Pyle is limited because relatively little change is noted in the ossification centers of the hand and wrist during this period. More rapid changes may be observed in the knee or foot, however. Radiographs of the left knee or left foot—anteroposterior and lateral—therefore are obtained in children younger than 2 years of age and are compared with published standards (for the knee, standards of Pyle and Hoerr [1969]6; for the foot and ankle, standards of Hoerr, Pyle, and Francis [1962]7).
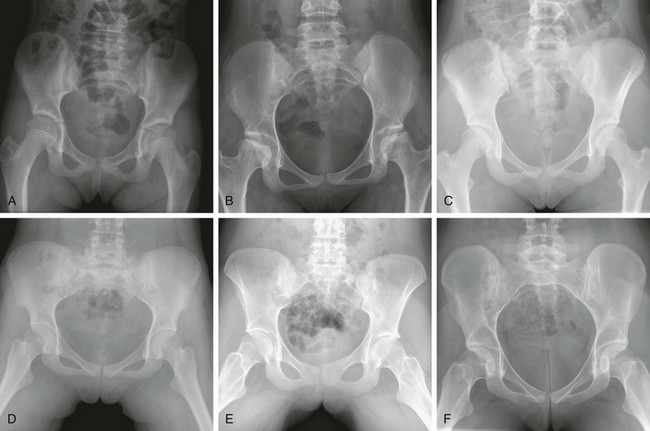
The Risser classification can be used to assess skeletal maturation through evaluation of the appearance and state of fusion of the iliac crest.8 Ossification of the iliac crest begins laterally and proceeds medially: stage 0—no ossification; stage I—up to 25% ossified; stage II—25% to 50% ossified; stage III—50% to 75% ossified; stage IV—75% to 100% ossified; and stage V—fully ossified and fused (e-Fig. 129-6).
Assessment of skeletal age by radiography is useful in many clinical scenarios. Boxes 129-1 and 129-2 list causes of advanced and delayed skeletal maturation, respectively. Menarche typically occurs after fusion of the physes of the distal phalanges. Bone age can be used with long bone measurements to predict adult height. Assessment of bone age is valuable in the planning of orthopedic treatments, including epiphysiodesis, leg-lengthening procedures, and scoliosis management.
Anatomic Variants
Experience and knowledge often are the best resources for successfully identifying normal variants. Compendiums of normal variants (e.g., An Atlas of Normal Roentgen Variants That May Simulate Disease by Keats and Anderson9 and Borderlands of Normal and Early Pathological Findings in Skeletal Radiography by Freyschmidt et al.10) are invaluable resources.
Many epiphyseal and apophyseal ossification centers are irregular and fragmented in their early development (Fig. 129-7). When evaluating the significance of irregular ossification in a single area, it is important to remember that normal irregularities of ossification usually are symmetric and accompanied by similar changes in other areas of the skeleton. Normal epiphyseal and apophyseal fragmentary ossification tends to have a smooth, round, and sclerotic appearance. Sometimes epiphyseal fragmentary ossification centers may have a jigsaw configuration. Normal epiphyseal fragmentary ossification should be differentiated from acute fractures that tend to have a linear contour with nonsclerotic margins with accompanying soft tissue swelling and joint effusions.
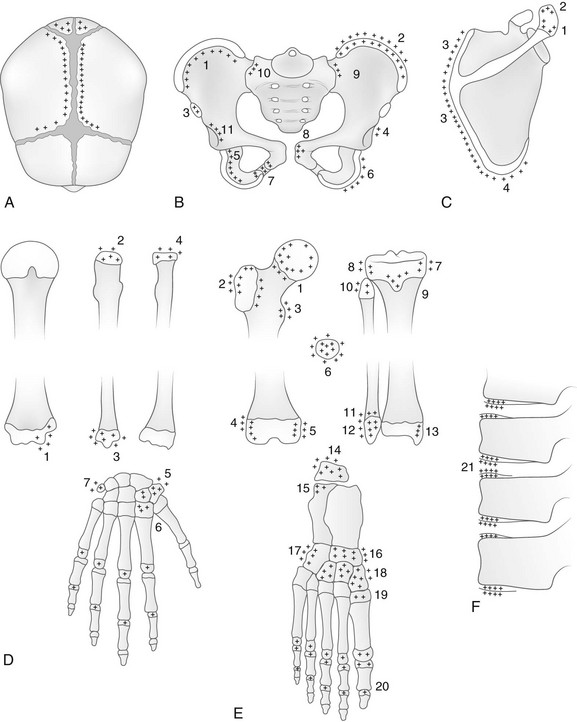
Figure 129-7 Common sites of normally irregular mineralization in the growing skeleton are marked by crosses.
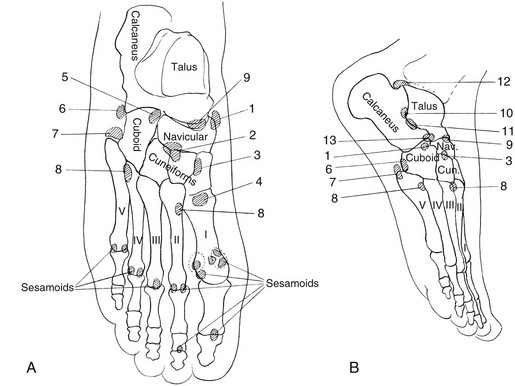
A, The cranium. During the first weeks of life and continuing for several months, edges of the bones at the great sutures are commonly irregular, and in many infants deep fissures extend from the sutures into the bodies of the bones. Irregularities also are common on the edges of the temporal suture (not shown). B, The pelvis: 1, crest of ilium; 2, secondary center in crest of ilium; 3, secondary center of anterior superior spine; 4, os acetabuli marginalis; 5, body of ischium; 6, secondary center of ischium; 7, ischium and pubis at the ischiopubic synchondrosis; 8, body of pubis; 9, ilium at sacroiliac joint; 10, sacrum at sacroiliac joint; 11, iliac edge and roof of the acetabular cavity. C, The scapula: 1 and 2, secondary centers of acromion process; 3, secondary center of vertebral edge; 4, secondary center of inferior angle. D, The upper limb: 1, secondary center of trochlea, always irregular; 2 and 3, proximal and distal epiphyseal centers of ulna; 4, proximal epiphyseal center of radius; 5, greater and lesser multangulars; 6, inconstant center of second metacarpal (pseudoepiphysis); 7, pisiform. E, The lower limb; 1, proximal metaphysis of femur; 2 and 3, secondary center and edges of shaft at greater and lesser trochanters, respectively; 4 and 5, lateral and medial edges, respectively, of distal epiphyseal center of femur; 6, patella; 7 and 8, medial and lateral edges, respectively, of proximal epiphyseal center of tibia; 9, secondary center in anterior tibial process; 10, proximal epiphyseal center of fibula; 11 and 12, distal metaphysis and distal epiphyseal center of fibula, respectively; 13, internal malleolus of distal epiphyseal center of tibia; 14, apophysis of calcaneus; 15, primary center of calcaneus; 16, navicular; 17, cuboid; 18, cuneiform; 19, proximal epiphyseal center of first metatarsal; 20, epiphyseal centers of phalanges. F, The spine; 21, marginal centers (end plate apophyses).
Physiologic osteosclerosis of the newborn is a common finding. The long tubular bones of fetuses, premature infants, and term newborn infants often appear sclerotic compared with the bones of older children because of proportionately thicker cortical bone and more abundant spongiosa during fetal and neonatal life (e-Fig. 129-8). The sclerotic features disappear gradually during the first weeks of life and resolve by 2 to 3 months of age.
e-Figure 129-8 Normal osteosclerosis of the newborn.
All of the bones appear dense. The medullary cavities of the pubic bones and proximal femurs are obscured.
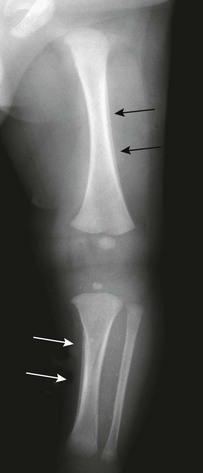
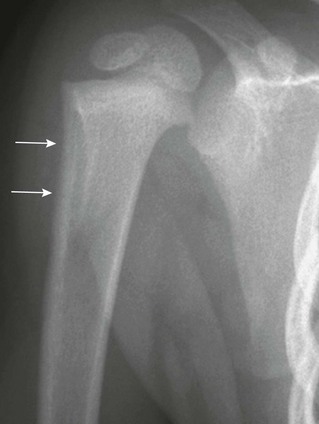
Physiologic periosteal reaction in the newborn also is a common finding that is seen in infants from 1 to 4 months of age and in both premature and term infants. Physiologic periosteal new bone is diaphyseal, smooth, regular, and 2 mm or less in thickness (Fig. 129-9).11 Physiologic periosteal new bone is most common in the tibia, femur, and humeral diaphysis and occasionally is seen in the radius and ulna. In most infants, physiologic periosteal new bone is symmetric; however, it may be asymmetric in one third to half of patients. Traumatic periosteal new bone tends to be asymmetric, metaphyseal, thicker, and irregular compared with physiologic periosteal new bone.
A variety of normal variants in the metaphyses of infants may simulate injury. It is important to distinguish these findings from the classic metaphyseal lesions of child abuse (see Chapter 145). The most common variant is the subperiosteal bone collar of the juxtaphyseal metaphysis, which has a normal step-off that may mimic a metaphyseal lesion of child abuse (Fig. 129-10).12
The following selected, important variants should not be confused with pathology.
Hands and Feet
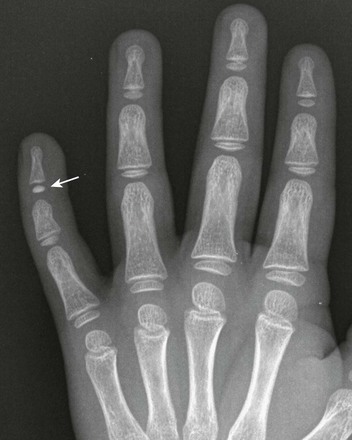
Ivory Epiphyses: Sclerotic epiphyseal ossification centers of the phalanges are called “ivory epiphyses” (e-Fig. 129-11).13 They occur in approximately 1 in every 300 patients. Ivory epiphyses usually are found in the distal phalanges and in the middle phalanx of the fifth digit. Maturation may be retarded. Ivory epiphyses also may occur in association with cone-shaped epiphyses in dysplastic syndromes. Ivory epiphyses are more frequently found in the toes compared with the fingers.
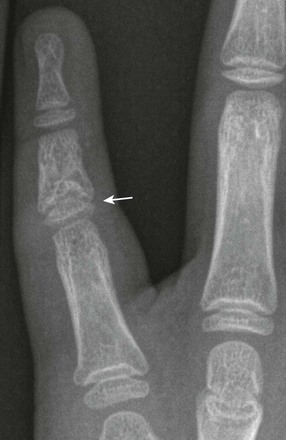
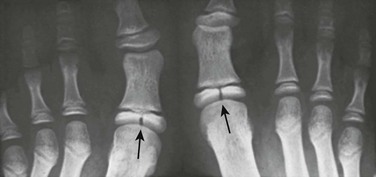
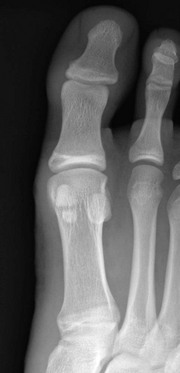
Cone-Shaped Epiphyses: Cone-shaped epiphyses of the phalanges (e-Fig. 129-12) occur singly or in combination and most commonly affect the terminal phalanges. When they occur in isolation, they may be related to trauma with resultant central physeal growth disturbance. When they are multiple, they may be seen in association with dysplastic syndromes and metabolic bone disease. Cone-shaped epiphyses are found more frequently in the toes than in the fingers (Fig. 129-13).
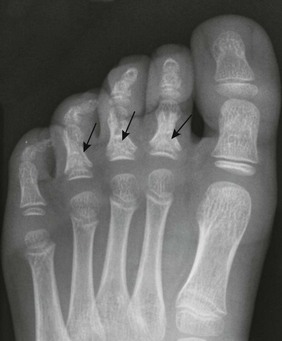
Figure 129-13 Symmetric conical or bell-shaped epiphyseal ossification centers (arrows) in the proximal phalanges of the second, third, and fourth toes of an asymptomatic 5-year-old girl.
The contiguous distal end of each shaft is recessed to receive its elongated ossification center. The epiphyseal ossification centers in the proximal phalanges of the first and fifth toes are the normal, flat, shallow, transverse disks usually present in all of the phalanges.
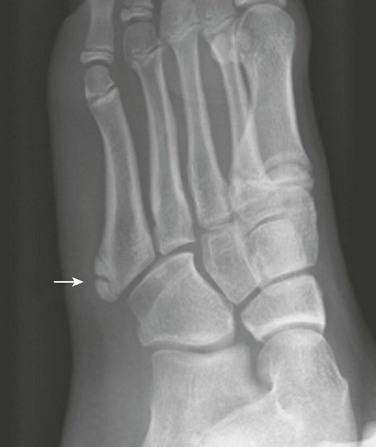
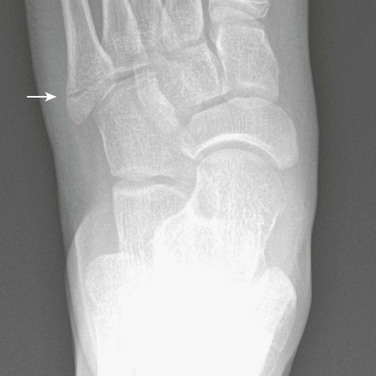
Fifth Metatarsal Apophysis: During puberty, a longitudinally oriented, scalelike secondary ossification center appears within the proximal apophyseal cartilage of the fifth metatarsal (Fig. 129-14). Irregular ossification of the apophysis is common. The normal apophyseal ossification center may appear widely spaced from the underlying fifth metatarsal, simulating fracture.14 The fifth metatarsal apophysis also may be bifid (e-Fig. 129-15). This presentation should be differentiated from a fifth metatarsal avulsion fracture related to the peroneus brevis insertion, which has a horizontal orientation (e-Fig. 129-16) (see also Chapter 143).
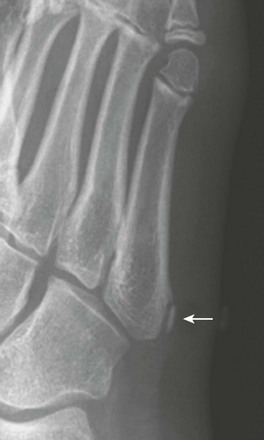
Figure 129-14 Normal apophysis of the fifth metatarsal in a 10-year-old girl (arrow).
The apophyseal growth plate is longitudinal in orientation, and the apophysis appears “scalelike.”
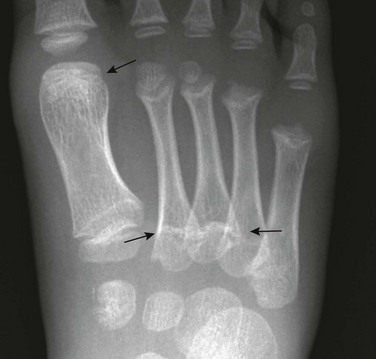
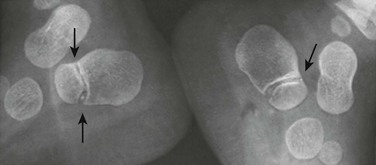
Pseudoepiphyses: Pseudoepiphyseal ossification centers may appear in the proximal cartilaginous portion of the growing second through fifth metacarpals and metatarsals and in the distal cartilage of the first metacarpals and metatarsals (Fig. 129-17 and e-Fig. 129-18).15 They are formed from a thin rod of osteogenic tissue that invades the proximal cartilage from the shaft. The end of the rod enlarges to form a mushroom-shaped mass of bone that appears radiographically as the “pseudoepiphysis.” The site of fusion of the pseudoepiphysis with the shaft often is indicated by a notch. Pseudoepiphyses are well formed by 4 to 5 years of age and fuse with the underlying shaft at the time of skeletal maturation. Pseudoepiphyses are frequent in children with hypothyroidism and cleidocranial dysplasia.
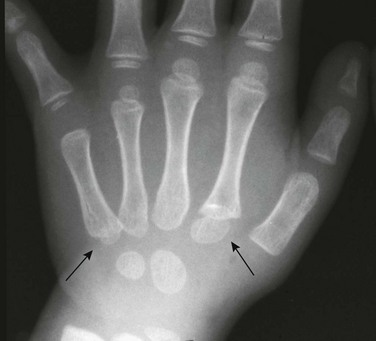
Figure 129-17 Pseudoepiphyses (arrows) in the proximal second and fifth metacarpals of a 3-year-old boy.
Sesamoid Bones: Sesamoid bones are present in the foot and hand. Sesamoids of the great toe reside within the medial and lateral slips of the hallux brevis tendon overlying the head of the first metatarsal. The medial great toe sesamoid is bipartite in 4% to 33% of patients (e-Fig. 129-19). Sesamoids of the feet may develop a stress reaction or a complete fracture and may be difficult to distinguish from a bipartite sesamoid on radiographs; magnetic resonance imaging (MRI) may be useful in distinguishing a normal bipartite sesamoid and underlying stress injury. For the first metatarsal phalangeal, stress injury tends to affect the medial sesamoid more frequently than the lateral sesamoid.16 In addition to the sesamoids, numerous other supernumerary ossicles of the foot and ankle exist (Fig. 129-20).
e-Figure 129-19 Bipartite medial sesamoid of the great toe in a 15-year-old boy.
Irregular Carpal Ossification: Irregular mineralization often occurs in the developing carpal bones (e-Fig. 129-21). The pisiform is the bone most frequently affected (e-Fig. 129-22). In addition, carpal bones sometimes may have wavy cortical contours, which should not be confused with erosions.
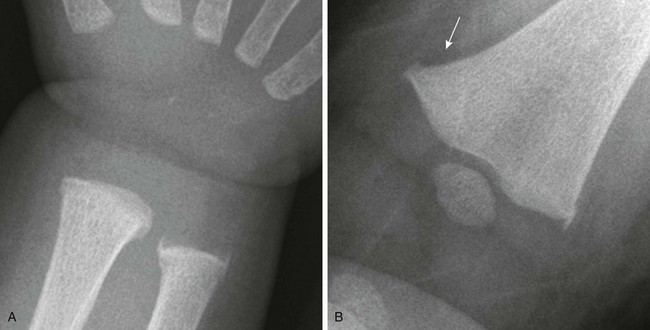
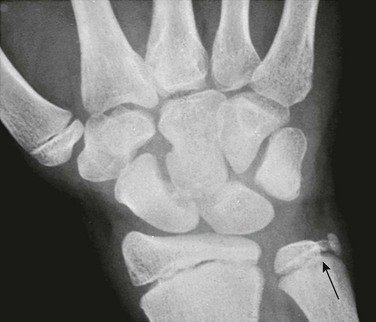
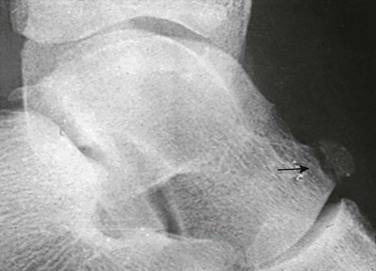
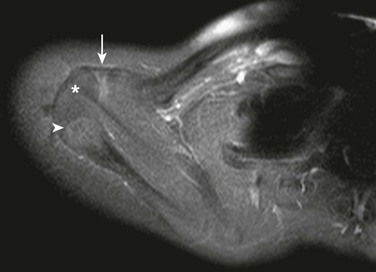
Os Styloideum (Carpal Boss): The os styloideum is a bony protrusion at the dorsum of the wrist between the trapezoid, capitate, and second and third metacarpals.17 It may be isolated and mobile or fused to an adjacent bone. The os styloideum is present in 1% to 3% of patients and presents as a palpable mass (Fig. 129-23). The differential diagnosis for a dorsal palpable abnormality along the wrist is an os styloideum versus a dorsal carpal ganglion cyst.
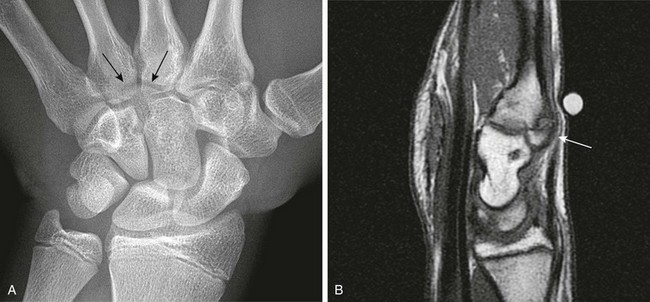
Figure 129-23 Os styloideum (carpal boss) in a 14-year-old boy.
A, An anteroposterior radiograph; the accessory bone is barely visible (arrows). B, A sagittal T1-weighted magnetic resonance image shows the accessory ossicle (arrow) at the dorsum of the carpometacarpal joint. Note that the ossicle causes a bump on the dorsum of the wrist.
Radial and Ulnar Styloid Processes: Separate ossification centers may appear in the regions of the ulnar (e-Fig. 129-24) and radial (Fig. 129-25) styloid processes before uniting with the main ossification center.
Bifid Epiphyses: Accessory ossification centers may occur in the epiphyses of phalanges and metatarsal bones. Bifid epiphysis is most common in the great toes, where examination before fusion of the centers is complete may simulate fracture (e-Fig. 129-26). The affected epiphysis is relatively sclerotic.18
Absent Epiphyses: The phalanges, especially the middle group, frequently lack epiphyseal centers in healthy children; this absence may be associated with symphalangism at the affected joints. Forty percent of the population has a biphalangeal (e-Fig. 129-27) fifth toe19 that may predispose to hammer or claw toe deformities.
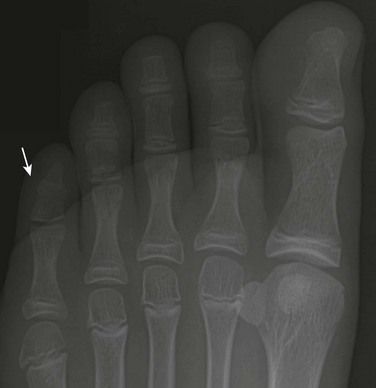
e-Figure 129-27 A biphalangeal fifth toe (arrow) in a 13-year-old girl.
Bifid Calcaneus: The ossification center for the calcaneus is present at birth. Occasionally the body of the calcaneus may ossify from two or more independent centers (Fig. 129-28). This finding is rare as a normal variant. More often it is associated with an underlying disorder such as Down syndrome, mucolipidosis, or Larsen syndrome.
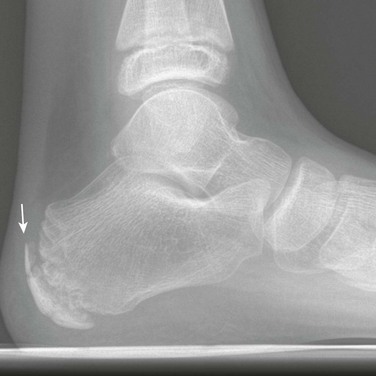
Calcaneal Apophysis: The calcaneal apophysis appears about the middle of the first decade. It often is fragmented and/or sclerotic well into the second decade until fusion with the body of the calcaneus is complete (e-Fig. 129-29). Calcaneal apophysitis (Sever disease) is a poorly understood cause of heel pain in children, and this diagnosis should not be made on the basis of radiographs alone.20 MRI findings of apophyseal edema may suggest the diagnosis; however, Sever disease remains a clinical diagnosis.
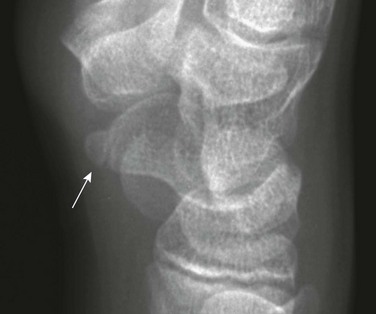
Calcaneus Secondarius: The calcaneus secondarius (e-Fig. 129-30) may simulate an anterior process calcaneal fracture. The ossicle is located in between the anteromedial calcaneus, cuboid, talar head, and navicular. It is rare and of no clinical significance other than possibly being mistaken for a fracture.
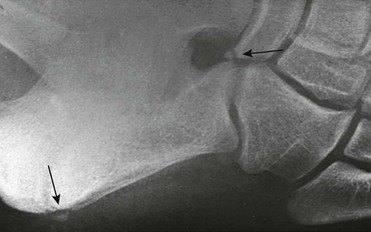
e-Figure 129-30 Small, round, smooth calcaneus secundarius (arrow at right) in the center of the space between the calcaneus, cuboid, navicular, and talus on lateral oblique projection. The patient was an asymptomatic 12-year-old boy.
The posterior arrow points to a center in the apophysis. Similar ossicles were present in the other foot.
Calcaneal Pseudocyst: A pseudocystic triangular area of radiolucency is present in the anterior half of the calcaneal body. At this location, a deficiency of spongy bone is normal. The pseudocyst is of no clinical significance in itself; however, it must be differentiated from true bone cysts or intraosseous lipomas that can develop at this site but tend to have a more round shape. MRI or computed tomography (CT) is helpful for differentiating these lesions when radiographic features are equivocal.
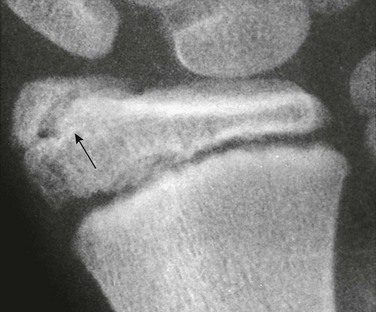
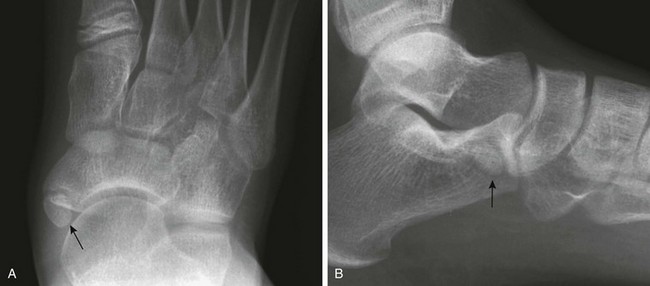
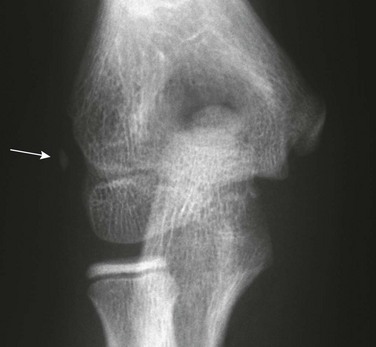
Os Trigonum: An os trigonum is located at the posterior margin of the talus in approximately 15% of persons21 and may superficially mimic a fracture (Fig. 129-31). Repetitive forced plantar flexion may cause a syndrome of posterior ankle impingement or “os trigonum syndrome.” This syndrome occurs most commonly in ballet dancers, soccer players, and basketball players and may cause tendinopathy of the flexor hallucis longus.
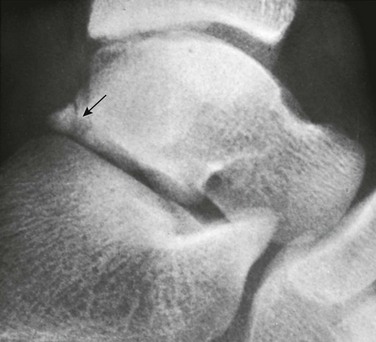
Figure 129-31 A normal apophyseal ossification center (arrow) in the dorsal process of the talus in a healthy 11-year-old boy.
The radiolucent strip between the body of the talus and the ossification center is a normal synchondrosis, not a fracture line. When the synchondrosis persists after the normal age for its fusion with the body of the talus, the persistent ossification center is called the os trigonum.
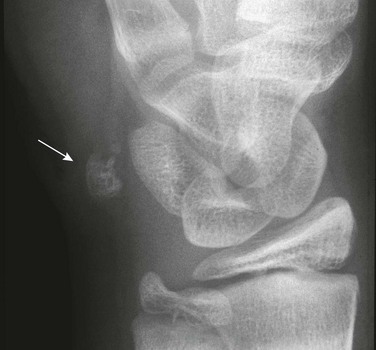
Os Supratalare: The os supratalare on the crest of the head of the talus may simulate injury or be simulated by injury (e-Fig. 129-32).
Os Supranaviculare: The center for the navicular bone frequently is irregular up to about 5 years of age and occasionally later (Fig. 129-33). The os supranaviculare (e-Fig. 129-34) is an accessory ossicle that can be confused with an accessory ossification center before the latter fuses with the parent bone; both must be differentiated from fracture.
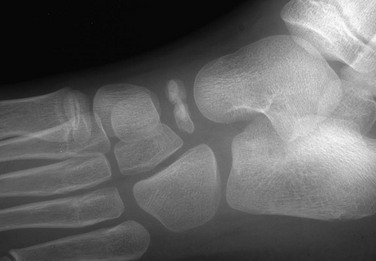
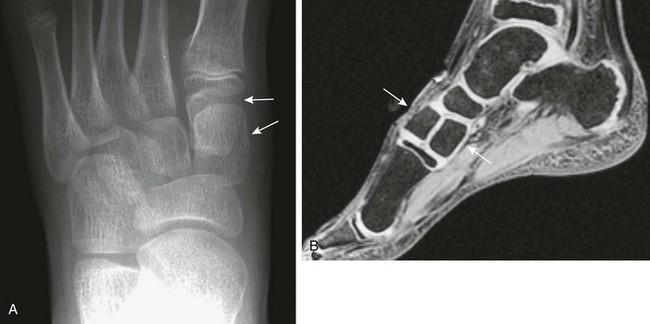
Accessory Navicular: The accessory navicular is the best known and one of the most important variants in the foot. The accessory navicular is located along the medial aspect of the navicular where the tibialis posterior inserts. Accessory navicular bones are seen in approximately 21% of patients, and 50% to 90% are bilateral.22 When patients have symptoms related to their accessory navicular, it usually is related to painful flat foot in part as a result of tibialis posterior dysfunction.
The type I variant (“os tibiale externum” or “navicular secundarium”) is a true rounded sesamoid bone measuring 2 to 6 mm that lies within the tendon of the posterior tibialis muscle and is approximately 3 mm separate from the navicular bone (e-Fig. 129-35). Type I variants are asymptomatic and do not fuse with the navicular. Type I variants account for 10% to 15% of cases in children.
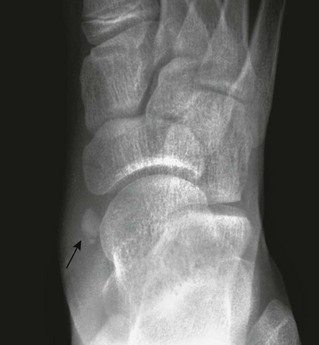
e-Figure 129-35 Type I accessory navicular (arrow) in a 10-year-old girl.
Type II variants (“prehallux” or “bifurcated hallux”) are united to the navicular by a cartilaginous or fibrocartilaginous bridge and represent an accessory ossification center for the tubercle of the navicular (Fig. 129-36). The ossicle is larger (9 to 12 mm), triangular or heart shaped, and congruent with and closely apposed to the adjacent navicular. It is connected to the navicular by a synchondrosis. Symptoms generally develop in the second decade. Fusion with the navicular bone occurs in most of these cases.
Cuneiforms: The three cuneiforms may ossify irregularly in children who are healthy and have no clinical evidence of local disease in the feet. A bipartite medial cuneiform has a coronal cleft separating it into proximal and distal portions. A duplicate cuneiform may be longitudinal in form (e-Fig. 129-37).
Elbow
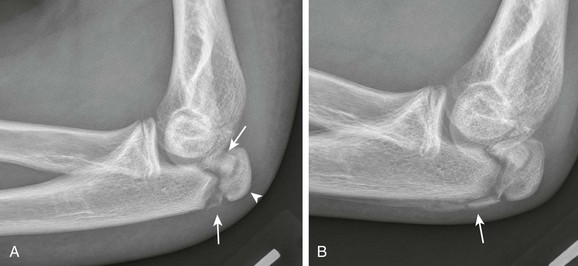
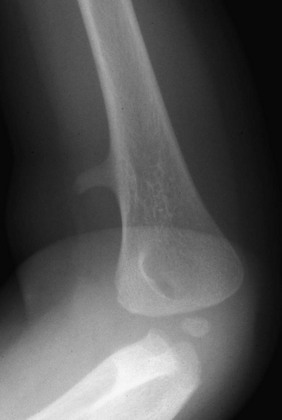
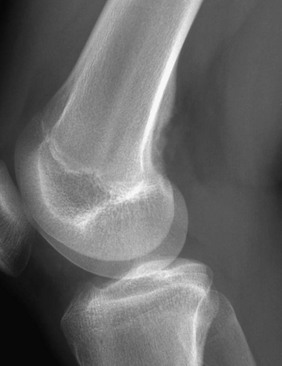
The six major ossification centers at the elbow ossify in an expected sequence—capitellum, radial head, internal (medial) epicondyle, trochlea, olecranon, and external (lateral) epicondyle. The sequence of ossification can be remembered with the acronym CRITOE (CRMTOL). If a bony density is seen in one area when an earlier-appearing center is lacking, a traumatic fragment is very likely the cause. This consideration is most important along the medial compartment, and correctly identifying the medial epicondyle and trochlea also is important (Fig. 129-38). The medial epicondyle appears earlier than the trochlea as a rule. Therefore if the medial epicondyle is absent but a trochlear ossification is present, a displaced medial epicondylar fracture should be suspected (e-Fig. 129-39). Rarely, variations in the order of ossification occur. The most common variation is the medial epicondyle appearing before the radial head. The ossification centers of the elbow can appear quite irregular during initial formation, particularly the trochlea (e-Fig. 129-40). The olecranon ossification center (Fig. 129-41) varies considerably in size and should be differentiated from an acute olecranon fracture (e-Fig. 129-42) by the shape of the individual ossifications and the nature of the margins (sclerotic or nonsclerotic).
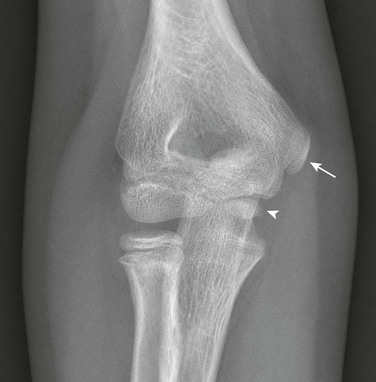
Figure 129-38 An 11-year-old boy with a normal elbow.
As a rule, the medial epicondylar ossification center (arrow) should appear before the trochlear ossification center (arrowhead). If only the trochlear ossification center is present, a medial epicondylar avulsion fracture should be suspected.
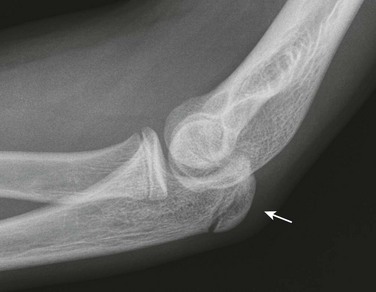
Figure 129-41 A normal smooth, corticated olecranon ossification center (arrow) in a 12-year-old boy.
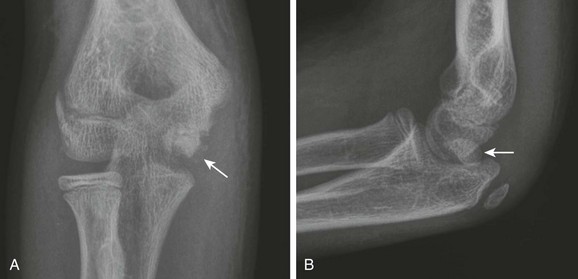
e-Figure 129-39 A and B, A displaced medial epicondylar avulsion fracture (arrows) mimicking trochlear ossification center in a 13-year-old boy.
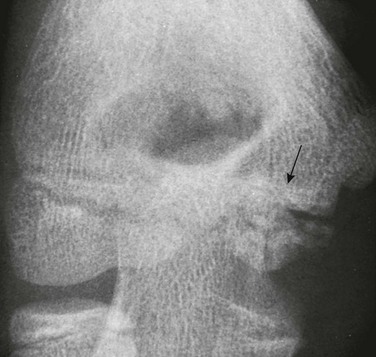
e-Figure 129-40 Irregular ossification center (arrow) of the trochlea of a healthy 13-year-old boy.
This irregular ossification of the trochlea persists throughout the growth period and always should be recognized as a normal variant; actually, it is the norm. The capitulum, in contrast, ossifies uniformly as it expands during the growth period.
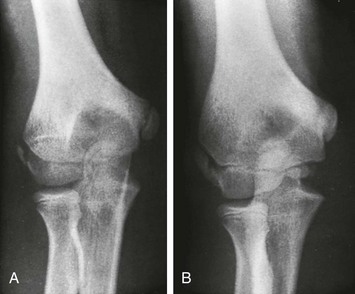
The lateral epicondyle does not fuse directly with the humeral shaft as the medial epicondyle does but fuses first with the adjacent capitellum; their fused mass then joins with the end of the humeral shaft (Fig. 129-43). With early ossification, the lateral epicondylar ossification center appears as an irregular flake of bone, which is easily mistaken for an avulsion fragment (e-Fig. 129-44). The medial epicondyle occasionally has an irregular, fragmentary appearance (Fig. 129-45). This finding must be differentiated from an avulsion with widening or irregularity, or both, of the medial epicondylar physis (e-Fig. 129-46).
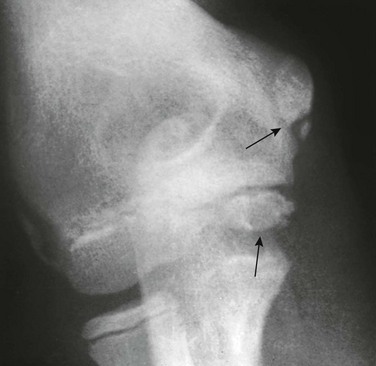
Figure 129-45 An accessory ossification center (proximal arrow) at the lower pole of the medial epicondyle of a healthy 12-year-old boy that simulates a fracture fragment.
The distal arrow points to the normal irregular edges of the trochlear center of the humerus.
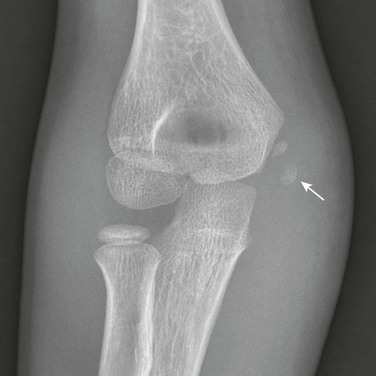
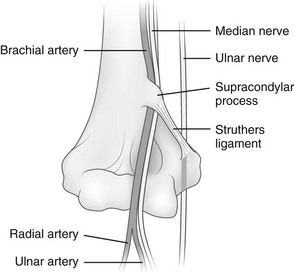
Supracondylar Process
The supracondylar process of the humerus is a vestigial structure that projects from the medial aspect of the anterior surface of the humeral shaft 5 to 7 cm proximal to the medial epicondyle (Figs. 129-47 and 129-48).23 It occurs in 1% of individuals. The supracondylar process may be connected by the ligament of Struthers to the medial epicondyle. Portions of pronator teres and brachioradialis muscles may attach to the process, to the ligament of Struthers, or to both. Occasionally, median nerve neuralgia occurs as a result of entrapment or compression of the median nerve as it passes through the tunnel created by the supracondylar process, the ligament of Struthers, and associated structures.
Shoulder
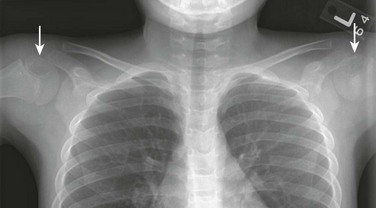
Proximal Humeral Epiphyseal Ossification: At the upper end of the humerus, two and occasionally three secondary ossification centers can be observed. The first center (humeral head proper) to appear develops in the medial half of the epiphysis at about 2 weeks of age; because of its eccentric location, it shifts to a factitious lateral position when the arm is internally rotated (Fig. 129-49). The second center appears laterally in the greater tuberosity during the second half of the first year. A rare third center occurs in the lesser tuberosity during the third year and fuses with the humeral head during the sixth to seventh years. This center may be seen in axillary views of the shoulder and may simulate a fracture fragment.
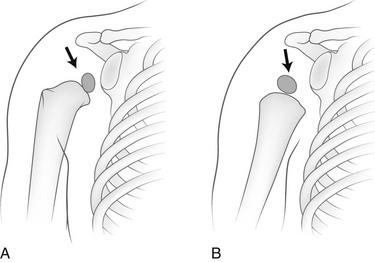
Figure 129-49 A factitious shift in position (arrows) of the normally eccentric proximal ossification center of the humerus caused by rotation of the bone.
A, The anatomic position of the humerus with the ossification center in the medial segment of the epiphysis. B, With the humerus in internal rotation, the ossification center appears to be displaced laterad.
Proximal Humeral Physis: The proximal humeral physis is tented, with the apex well above the pitched anterior and posterior segments. In rotated positions of the humerus, these segments are projected at different levels and may simulate fracture (e-Fig. 129-50). Offset of the epiphyseal ossification center relative to the lateral metaphyseal margin may simulate a Salter-Harris fracture.
Bicipital Groove: The bicipital groove in the anterior surface of the humerus may simulate local bone destruction or production (e-Fig. 129-51).
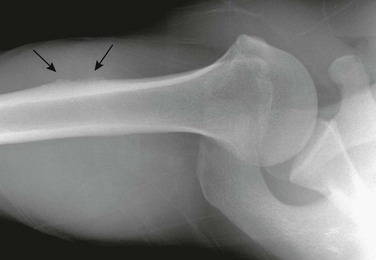
Muscular Insertions: Local cortical thickening that resembles a periosteal reaction occurs frequently at sites of insertion of major muscles of the upper arm. This finding is most common at the site of the deltoid insertion at the lateral aspect of the humeral diaphysis (e-Fig. 129-52).
Notched Proximal Metaphysis of the Humerus: The medial cortical wall of the proximal humeral metaphysis may be notched in the absence of disease.24
Humeral Head Pseudocyst: When the humeral head is well ossified, the region of the greater tuberosity appears relatively radiolucent and devoid of trabeculation.25 This appearance may be mistaken for a destructive lesion (e-Fig. 129-53).
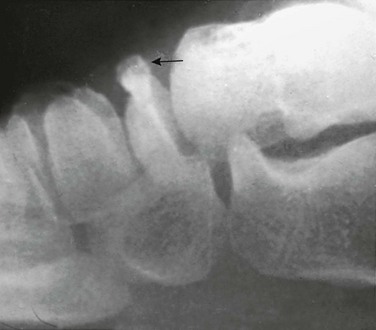
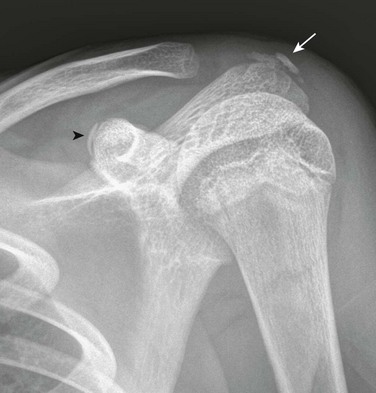
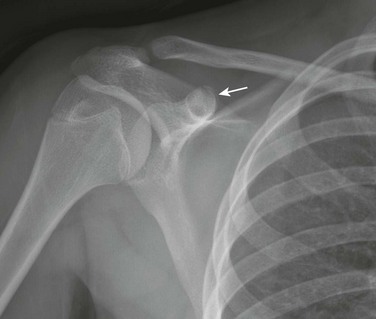
Acromion and Coracoid process: The acromion can have a fragmentary appearance during ossification, and superficially it may resemble an acromion fracture (Fig. 129-54). The acromion usually will fuse with the remainder of the scapula by the age of 25 years. When the acromion persists as a separate secondary ossification center beyond 25 years, then it represents an os acromion. Because the acromion in children has a significant cartilaginous component that is radiolucent, the acromioclavicular (AC) joint may appear widened (e-Fig. 129-55). Therefore measurements developed for the AC joint in adult patients as a criterion for determining AC joint injury should not be used in children. The AC joint eventually will narrow as the acromion further ossifies. If AC joint pathology is a concern, comparison with the contralateral asymptomatic side may be helpful.
The coracoid process is the origin of the short head of the biceps tendon and coracobrachialis and is the insertion site of the pectoralis minor muscles. The coracoid process also is the origin and insertion site of various ligaments of the shoulder. The coracoid process apophysis has a complex ossification process with multiple different ossification centers that may develop at various stages and may superficially mimic a fracture (Fig. 129-56). In the beginning of the second decade, a normal lucency is present between the base of the coracoid process and the scapular body that should not be mistaken for a fracture. Later in the teenage years, the tip of the coracoid process may develop a separate ossification center that may superficially resemble an avulsion fracture.
Pelvis
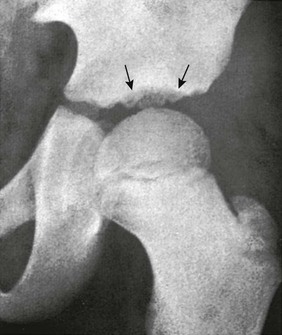
Acetabulum: Irregular ossification of the acetabular roof is a normal phenomenon during growth (e-Fig. 129-57). The regular smooth configuration of the roof develops from a confluence of individual bony foci near the end of the first decade.
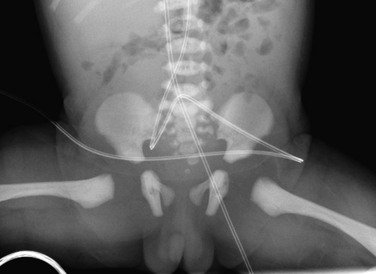
Accessory Ossification Centers: Accessory centers of ossification may develop in cartilage in the spine of the ischium and also in the rim of the acetabulum (os acetabuli) just below the anterior inferior iliac spine (Fig. 129-58).26 An os acetabulae should not be confused with an acetabular rim fracture. These centers usually become visible between the 14th and 18th years, after which they fuse with the main body of the ischium and ilium, respectively. Rarely, an os acetabuli persists as a separate ossicle. The rare os acetabuli centrale is a separate ossification center, or group of centers, that appears during puberty in the central portion of the triradiate in the wall of the acetabulum.27 Accessory ossification centers also may form at the pubic symphysis and superior pubic ramus.
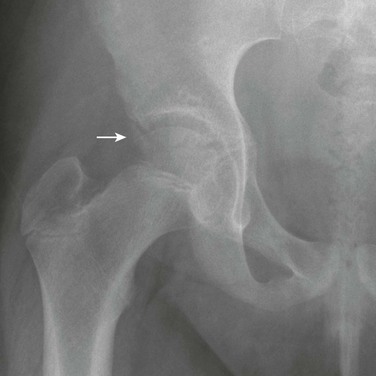
Figure 129-58 Os acetabulae (arrow) in a 10-year-old girl.
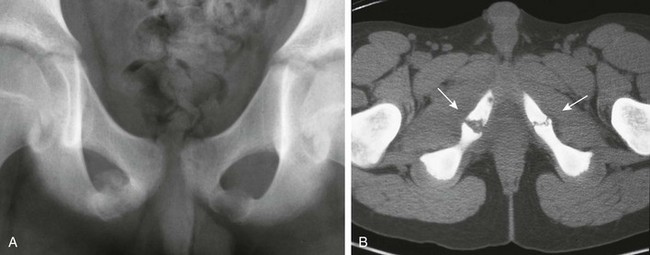
Ischiopubic Synchondrosis: Asymmetry of the ischiopubic synchondrosis is a very common normal variant and usually is an incidental finding unrelated to patient symptoms. Ossification of the ischiopubic synchondrosis is extremely variable in both velocity and pattern. The ischiopubic synchondrosis usually is completely fused by the teenage years.
The ischiopubic synchondrosis on the opposite side of the dominant leg usually is more prominent compared with the ischiopubic synchondrosis on the dominant leg.28 The ischiopubic synchondrosis represents fusion of two metaphyseal equivalent sites: (1) the ischial and (2) pubic component. Therefore it is a potential site for metaphyseal equivalent insults such as osteomyelitis and trauma (Fig. 129-59).
Ischium: Apophyseal irregularities along the posterolateral edge of the ischium also may be observed, usually during preadolescence (e-Fig. 129-60). The apophyseal irregularity of the ischium is the site of origin of the hamstring complex. The two sides may be unequally affected. During growth and before fusion of the body of the ischium, the ischial apophysis is scalelike at the inferior margin of the ischium.
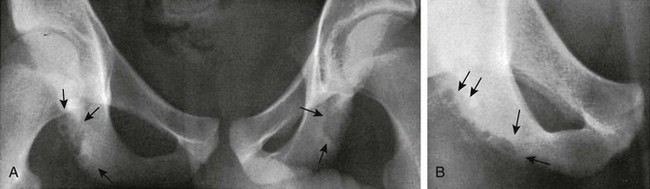
e-Figure 129-60 Irregularities in both ischia of asymptomatic 12- and 11-year-old boys.
A, The right ischium is irregularly rarefied at the tuberosity and slightly caudad into the ramus (arrows). The tuberosity of the left ischium is evenly rarefied. B, Bubbly rarefaction is present in the right tuberosity and caudad into the ramus (arrows).
Pubic Rami: Delayed and irregular mineralization of the pubic rami may be present at birth, with subsequent mineralization from several ossification centers.29 Vertical, radiolucent clefts occasionally noted as incidental findings in pelvis radiographs probably represent bars of nonossified cartilage between expanding ossification centers. The medial edges of the bodies of the pubic bones often are irregularly mineralized during the growth period.
Hip
Asymmetric Appearance of Femoral Head Ossification: A slight disparity in the timing of ossification, the size of the femoral head ossifications centers, or both is normal. In up to 30% of infants 3 to 6 months of age, a disparity of at least 2 mm exists between the two sides.30
Irregularity of Femoral Head Ossification: The ossification center for the head of the femur appears at about 4 months of age and enlarges with time. As ossification fills in the hemispheric cartilage of the head, the center may exhibit irregularities of form and density in the absence of disease (e-Fig. 129-61).31 Ossification may begin with coarse stippling and may progress, as the size increases, to irregularities along the margin. A bifid or split femoral head is a rare variant (Fig. 129-62). A notch (separate from the fovea capitis) at the vertex of the femoral head also may be seen (e-Fig. 129-63). Before the ossification center has rounded out fully, some flattening of the contour may be observed where subsequently the fovea capitis can be recognized. Variations in ossification and contour of the femoral head may mimic avascular necrosis (Perthes disease) or skeletal dysplasia.
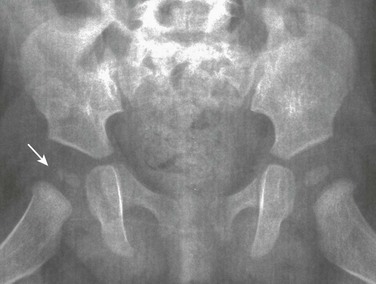
e-Figure 129-61 The normal, fragmentary appearance of the capital femoral epiphysis in a 4-month-old girl (arrow).
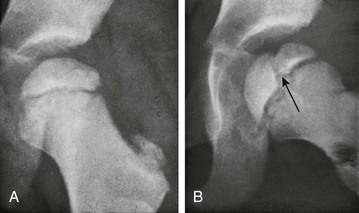
Figure 129-62 Factitious splitting of the femoral head in an asymptomatic 4-year-old girl.
A, In frontal projection, the femoral head image is normal. B, In the lateral, externally rotated position, the femoral head image is divided longitudinally into two unequal segments by a strip of decreased density (arrow) that represents the synchondrosis between the two ossification centers that developed one behind the other ventrodorsally.
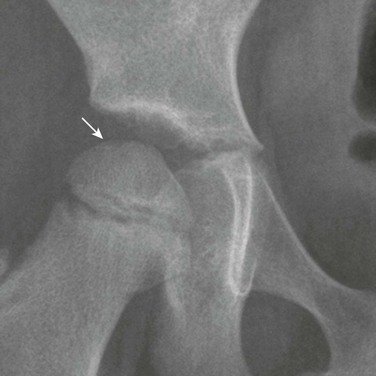
Positional Coxa Valga: External rotation of the femur increases the femoral neck-shaft angle and may factitiously suggest coxa valga (e-Fig. 129-64). With true coxa valga (Fig. 129-65), the greater trochanter projects laterally rather than being superimposed on the underlying femur, whereas with external rotation, the greater trochanter is rotated posteriorly and projects over the femur.
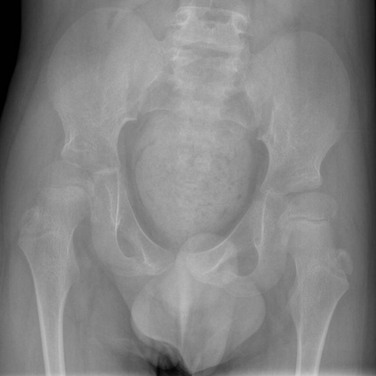
Figure 129-65 An 11-year-old boy with cerebral palsy and coxa valga bilaterally with mild secondary developmental dysplasia of the hip.
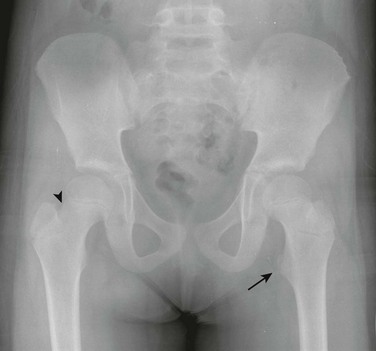
e-Figure 129-64 A 7-year-old girl with artifactual coxa valga on the left related to external rotation of the lower extremity and right lower extremity internal rotation.
On the left, external rotation will bring out the lesser trochanter (arrow) and cause overlap of the greater trochanter with the neck, which will cause factitious coxa valga. On the right, mild internal rotation causes accentuation of the femoral neck (arrowhead). The more internally rotated the hip is, the more elongated the femoral neck will appear.
Trochanters: The centers for the greater and lesser trochanters frequently are irregularly mineralized (e-Fig. 129-66). The physeal equivalent region for the lesser trochanter sometimes appears wide. Avulsions of the lesser trochanter related to the iliopsoas tendon insertion are uncommon, and thus comparison with the opposite, unaffected side may be helpful.
Knee
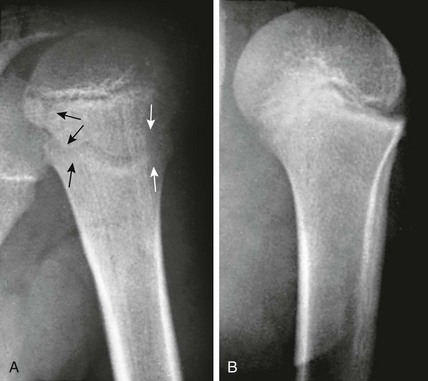
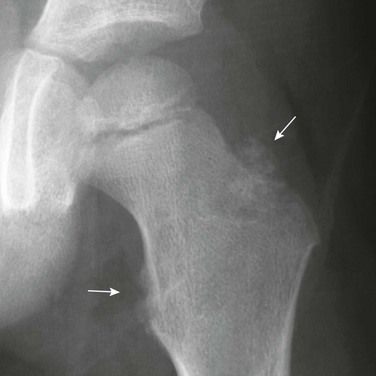
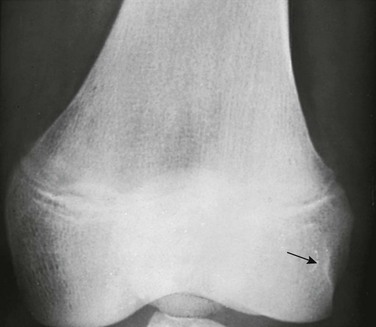
Cortical Irregularity of the Distal Femoral Metaphysis (Avulsive Cortical Irregularity): Irregularity of the cortex of the posteromedial distal femoral metaphysis is a common finding that easily can be mistaken for disease (Fig. 129-67 and e-Figs. 129-68 and 129-69).32 Cystlike cortical defects are common at this location, as are proliferative tuglike lesions, which probably are related to either the adductor muscle insertion or the origin of the medial head of the gastrocnemius muscle. When cystic, these lesions also have been termed “cortical desmoids” and may be difficult to differentiate from a nonossifying fibroma. Differentiation of these two entities is a nonissue because both are benign and a biopsy is not necessary.
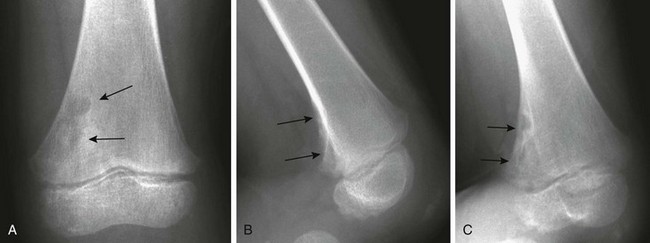
Figure 129-67 Avulsive cortical irregularity (cortical desmoids) of the distal femoral metaphysis in a 4-year-old girl.
The defect is posteromedial. A, The anteroposterior view shows an ill-defined lucency (arrows). B, The lateral view shows irregularity (arrows) of the posterior margin of the distal femoral metaphysis. C, An oblique view shows the cortical lucencies (arrows).
Irregular Ossification of the Distal Femoral Epiphysis: The distal femoral epiphyseal ossification center growth in width occurs rapidly between the second and sixth years. As a result, the lateral and medial margins are commonly irregular and ragged (e-Fig. 129-70). In lateral projection, normal distal femoral ossification centers may have a rough, fringelike margin. Accessory ossification centers may persist at the margins of cartilage-shaft junctions when ossification is almost complete. In older children, marginal mineralization of the femoral condyles is characteristically uneven and often is associated with independent ossification centers beyond the edge of the main bony mass.
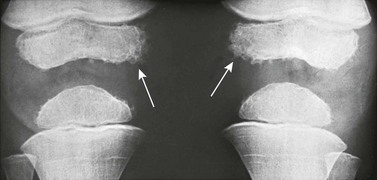
e-Figure 129-70 Normal irregular mineralization on the margins of the ossification centers (arrows) in the distal epiphyses of the femurs of a 3-year-old boy.
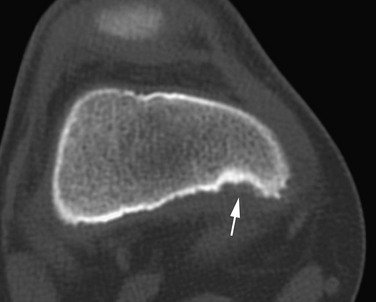
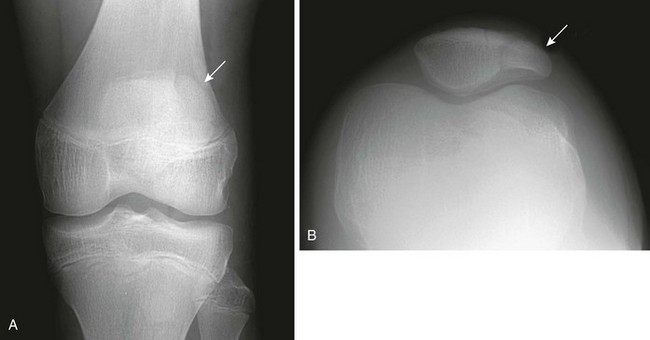
Subchondral, fragmentary ossification of the femoral condyles may simulate osteochondritis dissecans. Caffey and colleagues found this variant in approximately 30% of healthy children when the knees were examined in tunnel and lateral projections (Fig. 129-71).33 This defect is much more common on the lateral than on the medial condyle, in contradistinction to osteochondritis dissecans. In addition, these irregularities are seen in children younger than the usual age for this disease.
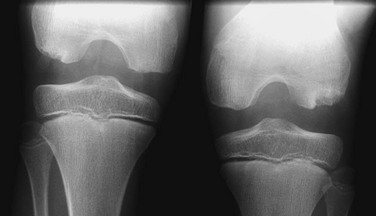
Figure 129-71 Normal developmental irregularity of the posterior aspect of the femoral condyles seen on a notch view in a 10-year-old boy.
The defects are most prominent in the lateral condyles.
The posterior margin of the lateral femoral condyle ossification center may be irregular and slightly flattened in appearance. This finding may be evident on radiographs, CT, or MRI. The finding simulates osteochondritis dissecans. Normal developmental irregularity tends to be more posterior and symmetric and occurs in younger children. It is most common at 8 to 10 years of age. The normal variant is differentiated from osteochondritis dissecans on MRI by its position in the inferocentral posterior femoral condyles, an intact overlying cartilage, a large residual cartilage model, accessory ossification centers and spiculation, and absence of adjacent bone marrow edema (e-Fig. 129-72).34
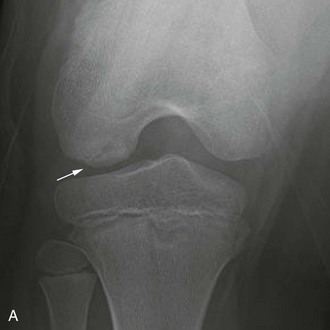
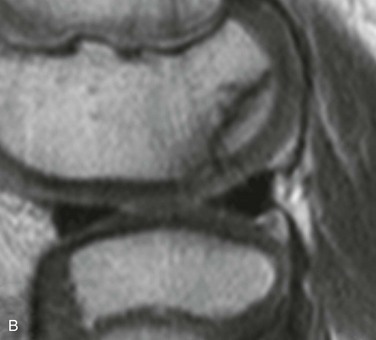
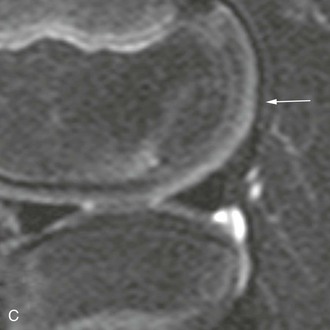
e-Figure 129-72 A 10-year-old boy with normal lateral femoral condylar irregularities.
A, Tunnel view shows subchondral irregularity of the lateral femoral condyle (arrow). Proton density sagittal (B) and T2 fat saturation sagittal (C) images demonstrate subchondral irregularity with overlying intact epiphyseal and articular cartilage with absence of edema of the subchondral component of the irregularity (arrow), indicating that this presentation is a normal variation as opposed to a developing osteochondral lesion.
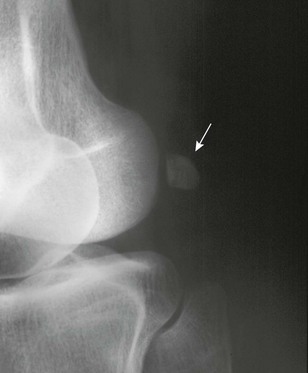
Popliteal Groove: The popliteal groove is a normal marginal defect that appears on the posterolateral aspect of the outer condyle in the prepubertal period (e-Fig. 129-73); at times it may be very prominent. This groove carries the tendon of the popliteal muscle and is never visible during infancy or early childhood.
Femoral Condyles: During late childhood, when the intercondylar fossa becomes deeper, lateral projections of the distal femoral epiphysis show the anterior segment to be more radiolucent than the remainder. This phenomenon occurs because the posterior portion of the epiphysis is wider than the anterior, and the x-rays must traverse four layers of cortex (the lateral and medial walls of each of the two condyles) instead of only two layers of cortex anteriorly. The lateral condyle can be differentiated from the medial condyle in lateral projections of the knee because it is relatively flat in comparison with the rounded configuration of the medial condyle.
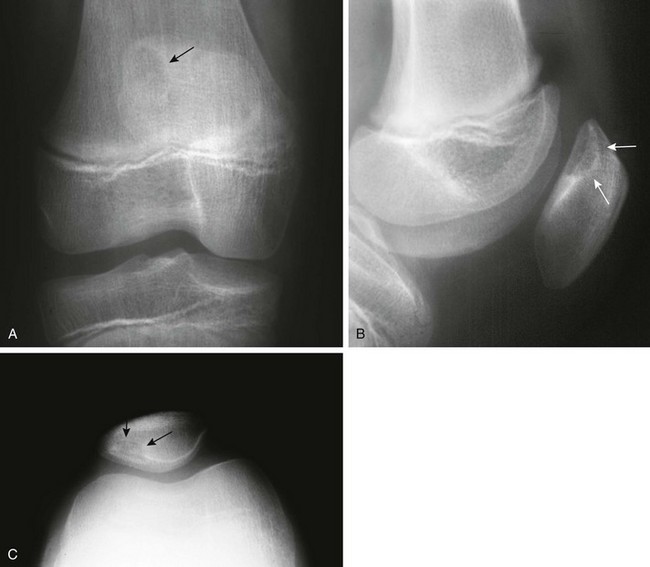
The Patella and Sesamoid Bones: The patella, lying within the tendon of the quadriceps muscle, is the largest sesamoid bone of the body. The patellar ossification normally develops from several foci. Its edges may be irregular during childhood (e-Fig. 129-74). After fusion of the focal centers, another center may develop in the superolateral portion of the bone and may persist as a distinct ossicle (Fig. 129-75). This variant, known as bipartite patella, is very common, occurring in 1% to 6% of the population. Ninety percent of persons affected are male, and 40% have bilateral findings. Stress injury or acute fracture may occur at the synchondrosis between the superolateral ossicle and the patellar body, producing symptoms.35 Bipartite patella may be related to aberrant traction by the vastus lateralis muscle, which inserts into the patella at its upper and outer quadrant. The patella also may be tripartite.
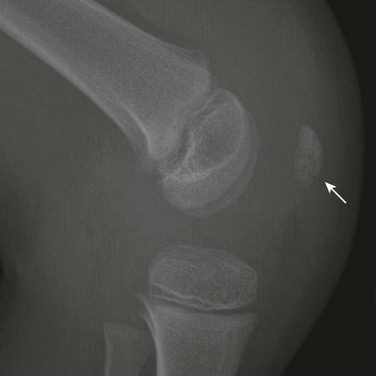
e-Figure 129-74 A 5-year-old boy with a large knee effusion related to infection.
Normal fragmentation and irregularity of the patellar bone (arrow) should not be confused with pathology or fracture.
Two other sesamoids of the knee occur as normal variants, the fabella and cyamella. The fabella (which is more common) forms in the tendon of the lateral head of the gastrocnemius muscle. The cyamella (which is less common) forms in the tendon of the popliteus muscle. A fabella is best seen on a lateral view (e-Fig. 129-76). The cyamella is found at the edge of the lateral condyle of the femur in the popliteal groove. Fabella syndrome is characterized by intermittent pain at the posterolateral knee accentuated by extension and localized tenderness over the fabella accentuated by compression.36
Dorsal Defect of the Patella: Dorsal defects of the patella may be seen along its superolateral aspect and usually are asymptomatic (Fig. 129-77). On MRI, overlying cartilage is intact. A dorsal defect of the patella may be seen concomitantly with a bipartite patella.37 Dorsal patella defects should be differentiated from osteochondritis dissecans of the patella, which usually affects the inferior aspect of the patella bone.
Tibia and Fibula
Tibial Tuberosity: A step-like notched defect appears in the upper anterior border of the tibia in lateral projection before ossification proceeds into the cartilaginous anterior tibial tubercle from the main proximal tibial ossification center. The tubercle also may be ossified from multiple fragmentary accessory centers that, before union, may simulate avulsed fragments of bone. When ossification of the anterior tibial process is nearly complete, the radiolucent cartilage still separating the process from the shaft may appear as a notch or a horizontal strip (e-Fig. 129-78).
Variations in Tibial and Fibular Contour: Irregularity of the interosseous membrane may produce undulations of the lateral tibial or medial fibular cortex that mimic a periosteal reaction, particularly on the oblique lateral view. The proximal fibular metaphysis may have a flanged appearance, possibly as a result of muscular tug.
Irregular Ossification of the Medial and Lateral Malleoli: The medial and lateral malleolus are initially fully cartilaginous and may superficially mimic soft tissue swelling (Fig. 129-79). Fragmentary ossification of both the medial and lateral malleoli is a normal finding in the skeletally immature and should not be confused with fracture.38
Separate accessory ossification centers are common in the cartilage of the medial malleolus (os subtibiale) and less common in the lateral malleolus (os subfibulare) (e-Fig. 129-80 and Fig. 129-81). At each location, the differential diagnosis is an avulsed fragment. Acute avulsed fragments will have an irregular shape and a sharp, noncorticated margin. A common pitfall is to mistake an acute avulsion fracture of the anterior talofibular ligament with an os subfibulare.
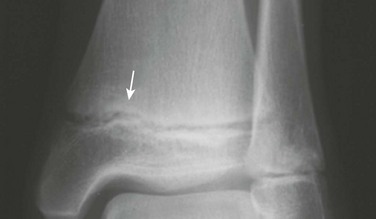
Figure 129-81 Accessory ossification centers (os subfibulare) of the lateral malleolus (arrow) in a 14-year-old boy.
Duncan, AW. Normal variants—an approach. In Carty H, Brunelle F, Stringer D, et al, eds.: Imaging children, 2nd ed, Philadelphia: Elsevier, 2005.
Greulich, WW, Pyle, SI. Radiographic atlas of skeletal development of the hand and wrist, 2nd ed. Stanford, CA: Stanford University Press; 1959.
Hoerr, NL, Pyle, SI, Francis, CC. Radiographic atlas of skeletal development of the foot and ankle. Springfield, IL: Charles C Thomas; 1962.
Ogden, JA. Diagnostic imaging. In Ogden JA, ed.: Skeletal injury in the child, 3rd ed, New York: Springer, 2000.
Pyle, SI, Hoerr, NL. A radiographic standard of reference for the growing knee. Springfield, IL: Charles C Thomas; 1969.
References
1. Sandler, TW. Langman’s medical embryology, 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2009.
2. Boron, WF, Boulpaep, EL. Medical physiology, 2nd ed. Philadelphia: Saunders; 2009.
3. Kuhns, LR, Finnstrom, O. New standards of ossification of the newborn. Radiology. 1976;119:655–660.
4. Greulich, WW, Pyle, SI. Radiographic atlas of skeletal development of the hand and wrist, 2nd ed. Stanford, CA: Stanford University Press; 1959.
5. Loder, RT, Estle, DT, Morrison, K, et al. Applicability of the Greulich and Pyle skeletal age standards to black and white children of today. Am J Dis Child. 1993;147:1329–1333.
6. Pyle, SI, Hoerr, NL. A radiographic standard of reference for the growing knee. Springfield, IL: Charles C Thomas; 1969.
7. Hoerr, NL, Pyle, SI, Francis, CC. Radiographic atlas of skeletal development of the foot and ankle. Springfield, IL: Charles C Thomas; 1962.
8. Risser, JC. The iliac apophysis: an invaluable sign in the management of scoliosis. Clin Orthop. 1958;1:111–119.
9. Keats, TE, Anderson, MW. An atlas of normal roentgen variants that may simulate disease, 7th ed. St Louis: Mosby; 2001.
10. Freyschmidt, J, Brossman, J, Wiens, J, et al. Borderlands of normal and early pathological findings in skeletal radiography, 5th ed. New York: Thieme; 2003.
11. Kwon, DS, Spevak, MR, Fletcher, K, et al. Physiologic subperiosteal new bone formation: prevalence, distribution, and thickness in neonates and infants. AJR Am J Roentgenol. 2002;179:985–988.
12. Kleinman, PK, Belanger, PL, Karellas, A, et al. Normal metaphyseal radiologic variants not to be confused with findings of child abuse. AJR Am J Roentgenol. 1991;156:781–783.
13. Kuhns, LR, Poznanski, AK, Harper, HA, et al. Ivory epiphyses of the hands. Radiology. 1973;109:643–648.
14. Dameron, TB. Fractures and anatomical variations of the proximal portion of the fifth metatarsal. J Bone Joint Surg Am. 1975;57(6):788–792.
15. Ogden, JA, Ganey, TM, Light, TR, et al. Ossification and pseudoepiphysis formation in the “nonepiphyseal” end of bones of the hands and feet. Skeletal Radiol. 1994;23:3–13.
16. Sanders, TG, Ruthur, SK. Imaging of painful conditions of the hallucal sesamoid complex and plantar capsular structures of the first metatarsophalangeal joint. Radiol Clin North Am. 2008;46:1079–1092.
17. Conway, WF, Destouet, JM, Gilula, LA, et al. The carpal boss: an overview of radiographic evaluation. Radiology. 1985;156:29–31.
18. Harrison, RB, Keats, TE. Epiphyseal clefts. Skeletal Radiol. 1980;5:23–27.
19. Dereymaeker, G, van der Broek, C. Biphalangeal fifth toe. Foot Ankle Int. 2006;26(11):948–951.
20. Kose, O, Celiktas, M, Yigit, S, et al. Can we make a diagnosis with radiographic examination alone in calcaneal apophysitis (Sever’s disease)? J Pediatr Orthop B. 2010;19:396–398.
21. Karasick, D, Schweitzer, ME. The os trigonum syndrome: imaging features. AJR Am J Roentgenol. 1996;166:125–129.
22. Miller, TT, Staron, RB, Feldman, F, et al. The symptomatic accessory tarsal navicular bone: assessment with MR imaging. Radiology. 1995;195:849–853.
23. Barnard, LB, McCoy, SM. The supracondyloid process of the humerus. J Bone Joint Surg Am. 1946;28:845–850.
24. Ozonoff, MB, Zeiter, FM, Jr. The upper humeral notch: a normal variant in children. Radiology. 1974;113:699–701.
25. Resnick, D, Cone, RO, III. The nature of humeral pseudocysts. Radiology. 1984;150:27–28.
26. Ponseti, IV. Growth and development of the acetabulum in the normal child. J Bone Joint Surg Am. 1978;60(5):575–585.
27. Hergan, K, Oser, W, Moriggl, B. Acetabular ossicles: normal variant or disease entity? Eur Radiol. 2000;10:624–628.
28. Herneth, AM, Philipp, MO, Pretterklieber, ML, et al. Asymmetric closure of ischiopubic synchondrosis in pediatric patients: correlation with foot dominance. AJR Am J Roentgenol. 2004;182:361–365.
29. Caffey, J, Madell, SH. Ossification of the pubic bones at birth. Radiology. 1956;67:346–350.
30. Lemperg, R, Liliequist, B, Mattson, S. Asymmetry of the epiphyseal nucleus in the femoral head in stable and unstable hip joints. Pediatr Radiol. 1973;1:191–195.
31. Harrison, MHM, Blakemore, ME. A study of the “normal” hip in children with unilateral Perthes’ disease. J Bone Joint Surg Br. 1980;62(1):31–36.
32. Bufkin, WJ. The avulsive cortical irregularity. Am J Roentgenol Radium Ther Nucl Med. 1971;112:487–492.
33. Caffey, J, Madell, SH, Royer, C, et al. Ossification of the distal femoral epiphysis. J Bone Joint Surg Am. 1958;40:647–654.
34. Gebarski, K, Hernandez, RJ. Stage-I osteochondritis dissecans versus normal variants of ossification in the knee in children. Pediatr Radiol. 2005;35:880–886.
35. Kavanagh, EC, Zoga, A, Omar, I, et al. MRI findings in bipartite patella. Skeletal Radiol. 2007;36:209–214.
36. Weiner, DS, Macnab, I. The “fabella syndrome”: an update. J Pediatr Orthop. 1982;2:405–408.
37. van Holsbeeck, M, Vandamme, B, Marchal, G, et al. Dorsal defect of the patella: concept of its origin and relationship with bipartite and multipartite patella. Skeletal Radiol. 1987;16:304–311.
38. Ogden, JA, Lee, J. Accessory ossification patterns and injuries of the malleoli. J Pediatr Orthop. 1990;10:306–316.