Embryology, Anatomy, and Normal Findings
From a structural and functional perspective, the respiratory system is most logically considered as having conducting and gas exchange components, with the bifurcating airways and accompanying pulmonary arteries (PAs) conducting air and blood to peripheral capillary-lined airspaces for gas exchange. Clements and Warner1 and more recently Bush2 encourage consideration of the lung as a set of branching trees that include the airways, the pulmonary vasculature (arterial and venous), the systemic vasculature (arterial and venous), and the lymphatics. This approach assists the radiologist in understanding accurate descriptions of each of these components in congenital malformations and other pathologic processes. This chapter reviews the developmental biology and clinical anatomy of the respiratory system.
Developmental Biology
The neonatal airway is composed of the nose, pharynx, larynx, trachea, and bronchi. The nasal structures, which are derived from ectoderm, begin developing during the fourth week of gestation.3,4 The olfactory placodes, which are evident as early as the third fetal week, eventually become the nasal pits, which separate into paired medial and lateral boundaries of the nasal walls. The medial portion of the nasal wall fuses during the formation of the nasal septum and central upper lip. The medial and lateral nasal processes fuse with the maxillary processes of the mandibular arch. The nasal cavity extends posteriorly, thinning the oronasal membrane, which eventually ruptures to form the choanae. Persistence of the oronasal membrane leads to choanal atresia.3,5
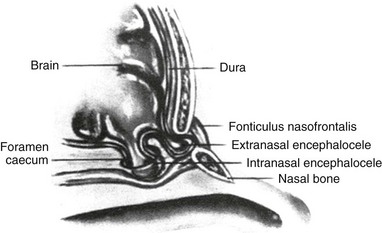
Incomplete closure of the foramen caecum during the third week of fetal development leads to the formation of gliomas or nasal encephaloceles. Neural tissue remains attached to epidermal elements, preventing normal migration of mesenchymal elements that will form the cartilaginous structures of the midface. This process leads to the presence of a bony defect through which brain tissue may herniate (e-Fig. 48-1). Gliomas have lost their central nervous system attachment, although 15% have a fibrous stalk connecting to the subarachnoid space. Encephaloceles maintain their central nervous system connection.5
Nasal dermoid cysts are benign masses with ectodermal and mesodermal elements. They present as masses on the dorsum of the nose and may have a fistulous opening on the skin or a sinus tract extending into the deep nasal elements. Nasal dermoid cysts are the result of faulty closure of the foramen caecum with invagination of dermal tissue between the developing nasal bones and cartilage.5
The larynx, trachea, and bronchi embryologically arise from a ventromedial diverticulum of the foregut that is known as the laryngotracheal groove. The proliferation of the laryngeal mesenchyme results in the arytenoid swellings that grow toward the tongue, converting the primordial glottis into a T-shaped laryngeal inlet. By 8 weeks, the larynx is usually sufficiently formed. In the infant, the larynx is high in location, with its inferior border located at the C4 level. During childhood, the lower border of the larynx descends and eventually reaches its adult location at the C6-C7 level by age 15 years. Functions of the larynx include breathing, phonation, and protection of the lower airway against aspiration.4,6,7
The laryngotracheal groove grows caudally, forming the trachea. It lies ventral and parallel to the dorsal foregut, which eventually becomes the esophagus. The separation of the trachea and esophagus progresses cranially and is complete by 6 weeks’ gestation. The endodermal lining will produce the epithelium and glandular structures of the trachea, whereas the connective tissue, cartilage, and smooth muscle come from the surrounding splanchnic mesenchyme.4,6,7 Faulty separation of the trachea and esophagus gives rise to esophageal atresia/tracheoesophageal fistula. Disproportionate growth of the esophagus at the expense of the trachea may give rise to tracheal stenosis or, in the most severe form, tracheal agenesis.
Lungs
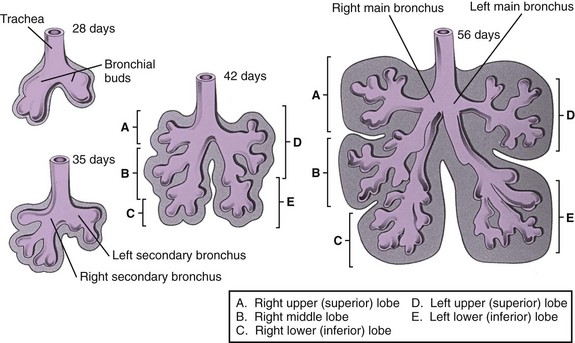
The lung bud arises from the caudal end of the laryngotracheal groove by the end of the fourth week and soon divides into two bronchial buds.4,8,9 The bronchial buds grow laterally into the pericardioperitoneal canals. Early in the fifth week, the connection of the bronchial buds to the trachea enlarges to form the main stem bronchi. The right main stem bronchus bifurcates into a superior secondary bronchus, supplying the right upper lobe and an inferior secondary bronchus. The inferior bronchus subdivides into two bronchi, supplying the right middle and lower lobes. The left main stem bronchus divides into two secondary bronchi that supply the upper and lower lobes of the left lung. The bronchi continue to divide, and all airway divisions are complete by 16 weeks’ gestation (Fig. 48-2). Cartilage appears at 10 weeks in the trachea and is found in the segmental bronchi by 16 weeks. Unequal growth of the lung buds can lead to the development of unilateral pulmonary agenesis or hypoplasia. Prolonged oligohydramnios or space-occupying thoracic lesions are associated with pulmonary hypoplasia.4,8
Lung development has been divided into five stages (Table 48-1). The embryonic (26 to 52 days) and pseudoglandular (52 days to 16 weeks) stages have been described previously. During the canalicular stage (17 to 28 weeks), the bronchi and terminal bronchioles become larger. The capillary bed begins to approximate the future air spaces, and gas exchange is possible. Type I and type II pneumocytes can be identified in the fetal lung by 20 to 22 weeks, but the capillary-alveolar interface is not adequate for extrauterine survival until 23 to 24 weeks of gestation.4,8,9 The saccular stage (29 to 36 weeks) is characterized by the development of terminal air sacs with flattening of the epithelium in the distal air spaces. The type II pneumocytes produce surfactant, which is stored as lamellar bodies. During the alveolar stage (36 weeks to infancy), the size and number of alveoli increase. When birth occurs at full term, it is estimated that 50 million alveoli are present. Alveolar development continues postnatally, and the mature human lung ultimately has 300 million alveoli (Table 48-2).
Table 48-1
Classification of Phases of Human Intrauterine Lung Growth
| Phase | Time of Occurrence | Significance |
| Embryonic | 26-52 days | Development of trachea and major bronchi |
| Pseudoglandular | 52 days to 16 wk | Development of remaining conducting airways |
| Canalicular | 17-28 wk | Development of vascular bed, framework of acinus; flattening of epithelium |
| Saccular | 29-36 wk | Increased complexity of saccules |
| Alveolar | 36 wk to term | Presence and development of alveoli |
From Thurlbeck WL. Lung growth and development. In Thurlbeck W, Churg AM, eds. Pathology of the lung. 2nd ed. New York: Thieme Medical Publishers; 1995;38.
Table 48-2
Changes in Lung Size With Growth
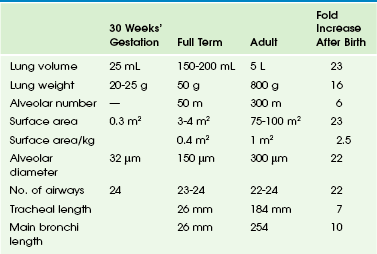
From Hodson WA: Normal and abnormal structural development of the lung. In Polin RA, Fox WW, eds. Fetal and neonatal physiology, ed 2, Philadelphia, 1998, WB Saunders, p 1037.
Surfactant
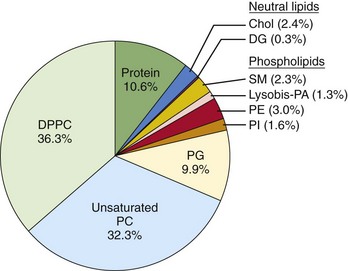
Surfactant is composed of phospholipids, protein, and neutral lipids (e-Fig. 48-3). Surfactant lines the alveoli and decreases surface tension, which leads to decreased work of breathing and stabilizes the terminal air spaces, especially at low lung volumes.10 Surfactant can be detected as early as 24 to 26 weeks’ gestation, although mature surfactant usually is not present until 34 to 36 weeks’ gestation. Surfactant maturation can be affected by a variety of substances. Insulin delays surfactant maturation, whereas other substances, such as glucocorticoids and thyroid hormone, accelerate it.11 Administration of glucocorticoid to mothers 24 to 48 hours before preterm delivery accelerates surfactant maturation and results in a significant decrease in the incidence and severity of hyaline membrane disease (HMD). HMD is the result of surfactant deficiency and is characterized clinically by cyanosis, tachypnea, and retractions within a few hours of delivery. Tracheal instillation of exogenous surfactant can ameliorate the natural course of HMD significantly.12
Fetal Lung Liquid
The fetal lung is filled with fluid during gestation. This fluid is produced by pulmonary epithelial cells, and its composition is different from that of amniotic fluid.13 As the fetus matures, surfactant can be found in this lung fluid. Fetal lung liquid is under higher pressure than amniotic fluid, and efflux of lung liquid into the amniotic fluid occurs, which is the basis of amniotic fluid analysis for surfactant to determine fetal pulmonary maturity.
Pulmonary Vasculature/Circulation
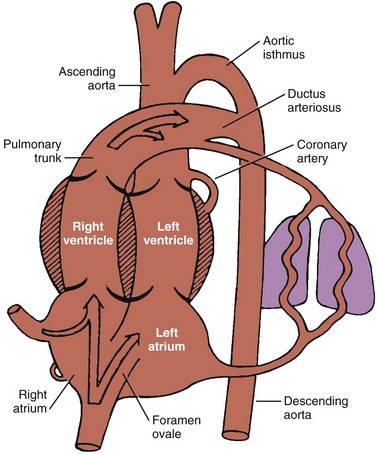
The fetal lung is the only organ that does not perform its postnatal function before birth. No reason exists for cardiac output to go to the fetal lungs because all gas exchange occurs via the placenta. Oxygenated blood returns to the fetus via the umbilical vein to the inferior vena cava (IVC) and right atrium (RA). Approximately two thirds of the IVC return entering the right atrium crosses the foramen ovale into the left atrium. The remaining one third of the IVC return and all of the superior vena cava (SVC) return enter the right ventricle. Most of the right ventricle output crosses the ductus arteriosus (DA) into the aorta. These shunts at the foramen ovale and DA result in most of the fetal cardiac output bypassing the lungs (Fig. 48-4).14
The Diaphragm
The diaphragm is derived from four embryonic components: the septum transversum, the pleuroperitoneal membranes, the dorsal mesentery of the esophagus, and the lateral body walls (Fig. 48-5).15,16 The septum transversum is the precursor of the central tendon of the diaphragm. The septum transversum grows from the ventrolateral body wall and forms a semicircular shelf that separates the heart from the liver and partially separates the pericardial cavity from the peritoneal cavity.
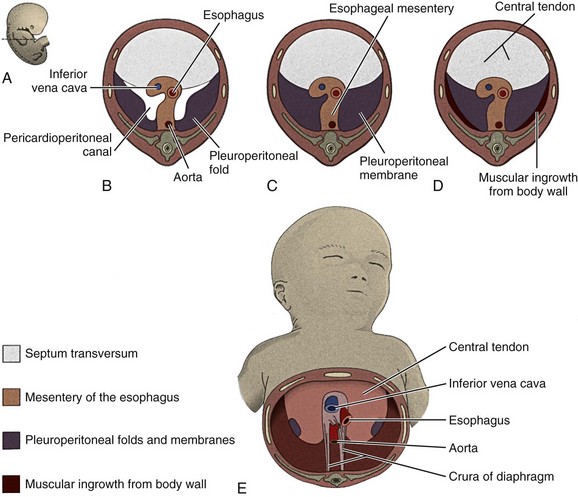
Figure 48-5 Development of the diaphragm.
A, A sketch of the lateral view of an embryo at the end of the fifth week (actual size) indicating the levels of sections B to E. B, C, D, and E, The developing diaphragm as viewed inferiorly. (From Body cavities, mesenteries, and diaphragm. In: Moore KL, Persaud TVN, eds. Before we are born: essentials of embryology and birth defects. 5th ed. Philadelphia: WB Saunders; 1998:189. Reprinted with permission.)
Clinical Anatomy
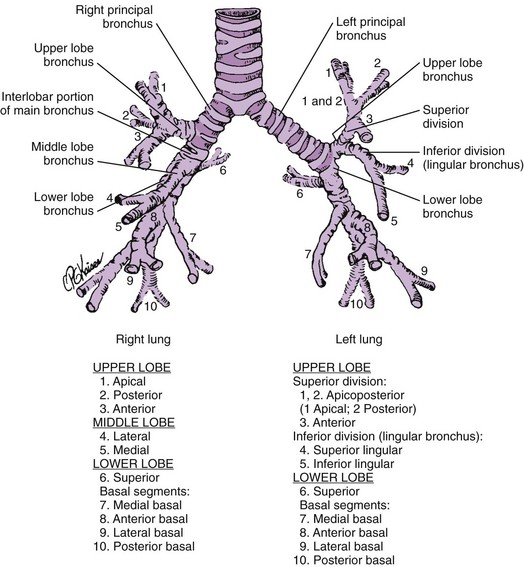
Basic airway anatomy to the level of the terminal bronchiole (the last purely conducting, i.e., nonalveolated, airway) is not substantially different in children compared with adults except in size. The right lung has three lobes, and the left lung has two lobes (Fig. 48-6). The pleural fissures separating the lobes of the lungs often are anatomically incomplete. Portions of the fissures occasionally are radiographically visualized as fine lines in healthy infants. The lungs are subdivided further into 8 to 10 segments on the left and 10 on the right, each served by a segmental bronchus (Figs. 48-7 and 48-8).17–19
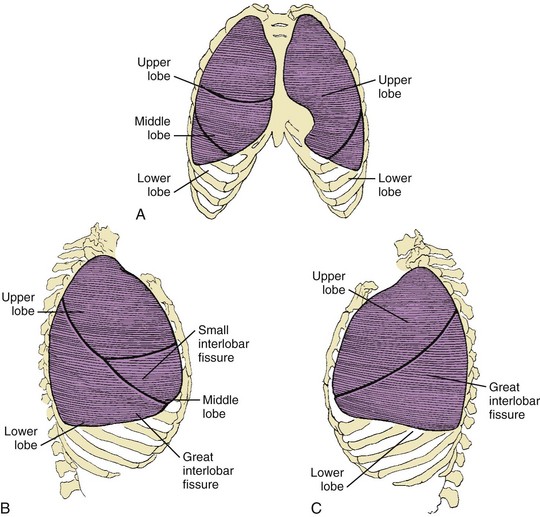
Figure 48-6 Lobes and fissures of normal lungs.
A, Frontal aspect of both lungs. B, Lateral aspect of the right lung. C, Lateral aspect of the left lung.
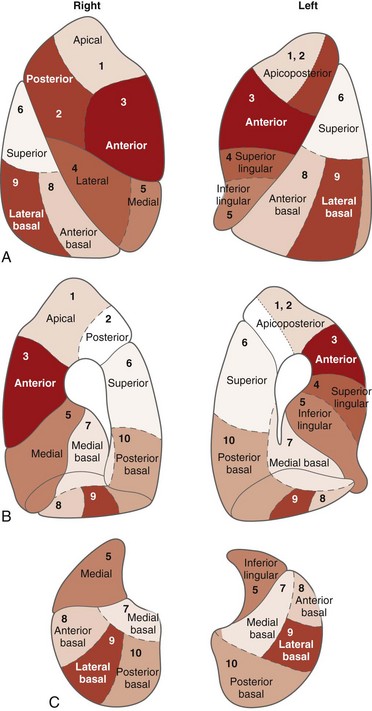
Figure 48-8 The bronchopulmonary segments as seen on the surface of the lungs.
A, Lateral surface. B, Medial surface. C, Base or diaphragmatic surface. (Adapted from Boyden EA. The segmental anatomy of the lungs. New York: McGraw-Hill; 1955; and from Rosse C, Gaddum-Rosse P. Hollingsched’s textbook of anatomy. 5th ed. Philadelphia: Lippincott-Raven; 1997:457.)
The trachea, which is the largest of the conducting airways, is a fibromuscular tube lined principally by ciliated columnar epithelium and mucous cells. The trachea is supported by 16 to 20 cartilaginous rings, which are incomplete posteriorly, where the tracheal wall is composed of fibrous, muscular, and elastic tissue. The trachea extends from the cricoid cartilage at the C4 level to the carina near the T4 level at birth and at a lower level with age. The right main bronchus originates from the trachea at an angle of 32 ± 5.5 degrees and the left at an angle of 51 ± 9.5 degrees from birth to 2 years of age. A tracheal cross-sectional area has been analyzed by computed tomography (CT) and grows predictably with age.20,21 The cross-sectional shape may vary considerably in the normal population and depending on the phase of respiration.22
The bronchi are conducting airways that consist of the first 11 branching generations after the carina. The first four bronchial generations (through the segmental branches) are strongly supported by cartilaginous plates that aid in keeping the bronchi patent. The smaller cartilaginous bronchial branches, from the fifth to the eleventh generation, double in number with each branching generation and decrease in size down to approximately 1 mm in diameter (Fig. 48-9).23 They are in a common fibrous sheath with an accompanying pulmonary arterial branch.
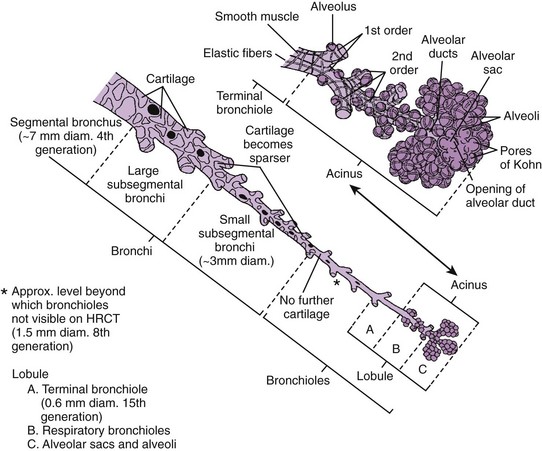
Figure 48-9 Bronchial generations.
The bronchi and airways from the fourth generation (the segmental bronchus) to the last generation (the alveolar sacs and alveoli) are represented. Bronchi contain cartilage within their walls, to the level of small (3-mm diameter) subsegmental branches. They are readily visible on high-resolution computed tomography (HRCT). Bronchioles, which do not contain cartilage but rather an extensive network of fibers within their walls, are seen only to the eighth generation, which corresponds to a diameter of about 1.5 mm. Beyond the eighth generation, the bronchiolar walls are not visible by high-resolution CT unless they are abnormal. The terminal bronchioles are the sixteenth-generation bronchioles and conduct air into the lobules. Beyond the terminal bronchioles, four to eight respiratory bronchioles lead to distal acini. Respiratory bronchioles are characterized by the presence of outpouchings representing alveolar ducts and terminal groupings of alveolar sacs. (From Armstrong P. The normal chest. In: Armstrong P, Wilson AG, Dee P, et al, eds. Imaging of diseases of the chest. 3rd ed. London: Mosby; 2000:26.)
The bronchioles are the conducting airways that extend to the sixteenth generation and lack cartilage in their walls. They are dependent for their patency on the support of the surrounding lung parenchyma. As the lung expands, the bronchioles dilate. The last purely conducting branch of the airway is the terminal bronchiole, which gives rise to three generations of respiratory bronchioles, each giving rise to progressively greater numbers of alveoli. The alveoli and the alveolar ducts and sacs that give rise to them constitute the pure gas-exchange portion of the lung (see Fig. 48-9).
The Respiratory Portion of the Lungs
The two subunits of the peripheral airspaces that are most important to the radiologist are the acinus and the secondary lobule. The alveolus, which in an adult averages approximately 200 to 300 mm in diameter, is just below the limits of visibility. The acinus is the unit of lung peripheral to the terminal bronchiole and consists of a cluster of 50 to 400 alveoli. It occasionally is visible in the pediatric lung and typically ranges in diameter from 1 to 2 mm in infants younger than 1 year old to 7 to 9 mm in adolescents and adults (Fig. 48-10 and e-Fig. 48-11).24
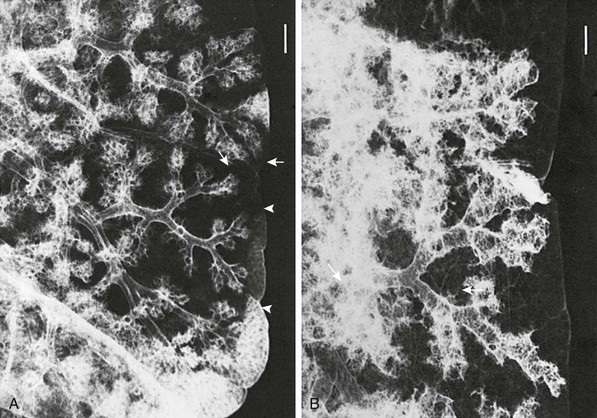
Figure 48-10 Acinar and secondary lobular anatomy in children.
Radiographs of selected sections of human lung obtained from postmortem examination and prepared by silver nitrate bronchoacinography. A, The lung from a 2-month-old infant showing the diameter of one acinus extending between the terminal bronchial and acinar surface (arrows). A subjacent underfilled secondary lobule is delimited by interlobular septa (arrowheads). B, The lung from a 19-year-old adult showing a mean acinar diameter (arrows) that is considerably larger. Both images are at the same magnification, with the marker bar (upper right) equal to 1 mm (corrected for specimen shrinkage). (From Osborne DRS, Effmann EL, Hedlund LW. Postnatal growth and size of the pulmonary acinus and secondary lobule in man. AJR Am J Roentgenol. 1983;140:449).
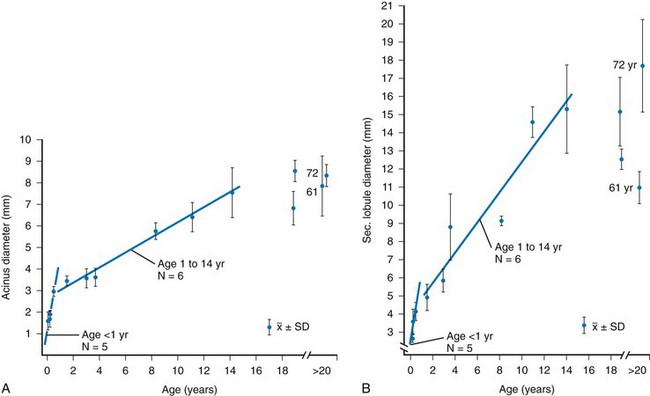
e-Figure 48-11 Size of the pulmonary acinus and secondary lobule in children.
A and B, The relationship of mean acinar diameter (A) and secondary lobular diameter (B) with respect to age. SD, Standard deviation. (From Osborne DRS, Effmann EL, Hedlund LW. Postnatal growth and size of the pulmonary acinus and secondary lobule in man. AJR Am J Roentgenol. 1983;140:449).
The secondary pulmonary lobule is a cluster of about 3 to 24 acini that are separated from other lobules by interlobular septa composed of fibrous tissue. The secondary lobules and their septa are much better developed in the periphery of the lung than in the center. The mean diameter of the secondary lobule at birth is 3 mm, and by 12 years it measures 15 mm (see e-Fig. 48-11).24 The pulmonary veins and lymphatics course through the interlobular septa, and the PAs and bronchioles are positioned centrally within the lobule (see Fig. 48-10). Thickened septa are visible in chest radiographs as Kerley B lines and on high-resolution CT (Fig. 48-12).
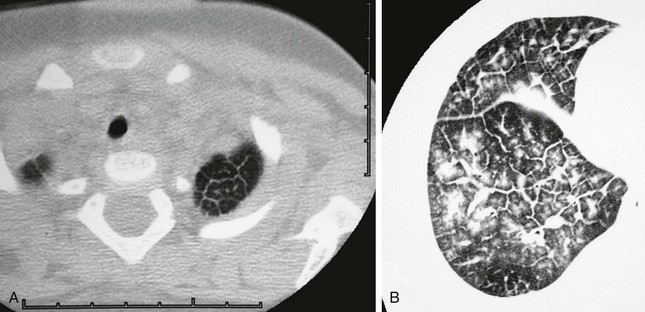
Figure 48-12 Secondary lobular septal thickening in two patients.
A, A 1-year-old child with Noonan syndrome showing polygonal-shaped secondary lobules in the left lung apex. Respiratory motion degraded lower sections, which also showed patchy septal thickening. B, A 15-year-old girl with mixed connective tissue disease shows moderately severe septal thickening and patchy distribution of ground-glass opacities within some lobules.
Multiple imaging modalities have been used to determine prenatal and postnatal lung volumes and growth. Accurate prenatal lung volumetry is currently possible using three-dimensional ultrasonography25–27 and magnetic resonance imaging (MRI).28,29 Total lung capacity and its subcomponents (i.e., tidal volume, vital capacity, functional residual capacity, and residual volume) increase with age. Linear or planometric measures from posteroanterior and lateral chest radiographs of children30,31 obtained in inspiration may reliably estimate total lung capacity in children. In recent years, computerized automatic lung volume measurement tools available in most three-dimensional workstations can provide accurate volumetric measurement of lung volume from an axial CT dataset obtained with multidetector CT.32–34
A study of 50 subjects (birth to 17 years) undergoing CT with carefully controlled breath holding found substantial variability in lung expansion between subjects, but the size of the airway wall and lumen, as well as arterial areas, were exponentially related to the subject’s height.33 In a study of normal lung volume ranges as a function of age and sex in 1050 boys and girls with normal chest CT scans obtained during quiet breathing (e-Fig. 48-13), it was found that children younger than 8 years had a relatively narrow lung volume range.32 The ranges broadened considerably in older children, likely reflecting a considerable variation in response to breath-holding instructions. Mean CT density decreases with age and increases in an anterior to posterior gradient in a quietly breathing supine child.35 Patient age, anterior versus posterior location, and apical versus basal location are significant predictors of regional lung density at inspiratory and expiratory volumes.36
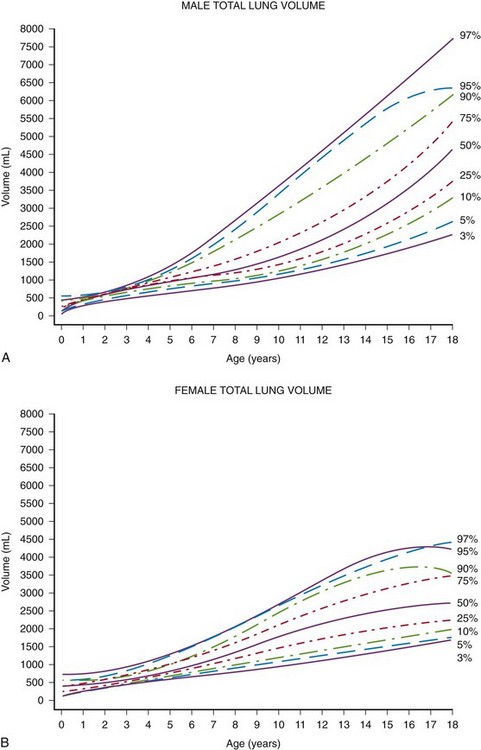
e-Figure 48-13 Total lung volumes determined from thoracic computed tomography as a function of age in boys (A) and girls (B) from birth to 18 years of age.
Note relatively narrow range through about 8 years of age and broader range thereafter, likely related to tidal volume breathing in infants and young children and a wide range of response to instructions to hold one’s breath in older children. (From Gollogly S, Smith JT, White SK, et al. The volume of lung parenchyma as a function of age: a review of 1050 normal CT scans of the chest with three-dimensional volumetric reconstruction of the pulmonary system. Spine. 2004;29:2061.)
Collateral ventilation can occur across pulmonary segments because no pleurae separate them. It also may occur between lobes when fissures are incomplete. There are three routes of collateral ventilation: (1) alveolar pores of Kohn (2- to 10-mm circular apertures in the alveolar walls); (2) canals of Lambert (epithelial-lined tubular structures between preterminal, terminal, or respiratory bronchioles and the alveoli surrounding them); and (3) direct small airway anastomoses.18 Collateral ventilatory pathways are less well developed in young infants than in older children and adults.
Pulmonary Vasculature/Circulation
In the lung parenchyma, the pulmonary arterial branches travel and divide with the bronchial branches, although they also give off unaccompanied supernumerary branches. There are approximately 23 divisions of airway branching and approximately 28 divisions of pulmonary arterial branching. These vessels can be visualized on high-resolution CT to about the level of the sixteenth generation, which is a few millimeters from the pleural surface and corresponds to the level of the terminal bronchioles, allowing identification of the secondary pulmonary lobule (the parenchyma supplied by three to five or more terminal bronchioles). The arterioles continue to divide until they form a dense capillary network surrounding the alveolus. This network consists of 280 billion capillary segments with a total blood volume of 140 mL; pulmonary blood volume can nearly double during exercise.37
Lymphatics
Pulmonary lymphatics are essential to removal of initial fetal lung liquid and to the removal of protein and water outside the vascular space.38,39 This fluid is returned to the circulation via the right lymphatic duct and the thoracic duct. Lymphatic vessels travel beside blood vessels in the bronchovascular spaces and in the connective tissues of the pleura. No lymphatics are present within the alveolar walls, but juxta-alveolar lymphatics represent the initial part of the lung lymphatic system. Enlargement of lymphatic channels in secondary lobular septa may be visualized on chest radiography and with CT (see Fig. 48-12).
Bluestone CD, Stool SE, et al, eds. Pediatric otolaryngology, 4th ed, Philadelphia: Saunders, 2003.
Hansell, DM, Armstrong, P, Lynch, DA, et al. The normal chest. In Hansell DM, Armstrong P, Lynch DA, et al, eds.: Imaging of diseases of the chest, 5th ed, Philadelphia: Mosby, 2010.
Polin RA, Fox WW, Abman SH, eds. Fetal and neonatal physiology, 3rd ed, Philadelphia: Saunders, 2004.
Webb, WR, Müller, N, Naidich, DP. Normal lung anatomy. In Webb WR, Müller N, Naidich DP, eds.: High resolution CT of the lung, 4th ed, Philadelphia: Lippincott, 2009.
References
1. Clements, BS, Warner, J. Pulmonary sequestration and related congenital bronchopulmonary vascular malformation: nomenclature and classification based on anatomical and embryological considerations. Thorax. 1987;42:401–408.
2. Bush, A. Congenital lung disease. A plea for clear thinking and clear nomenclature. Pediatr Pulmonol. 2001;32:328–337.
3. Rontal, M, Anon, JB, Zinreich, SJ. Embryology of the paranasal sinuses. In Bluestone CD, Stool SE, et al, eds.: Pediatric otolaryngology, 4th ed, Philadelphia: Saunders, 2003.
4. O’Rahilly, R, Müller, F. Human embryology & teratology, 2nd ed. New York: Wiley-Liss; 1996.
5. Hengerer, AS, Wein, RO. Congenital malformations of the nose and paranasal sinuses. In Bluestone CD, Stool SE, et al, eds.: Pediatric otolaryngology, 4th ed, Philadelphia: Saunders, 2003.
6. Saacson, G. Developmental anatomy and physiology of the larynx, trachea, and esophagus. In Bluestone CD, Stool SE, et al, eds.: Pediatric otolaryngology, 4th ed, Philadelphia: Saunders, 2003.
7. Wood, RE. Physiology of the larynx, airway, and lungs. In Bluestone CD, Stool SE, et al, eds.: Pediatric otolaryngology, 4th ed, Philadelphia: Saunders, 2003.
8. Wert, SE. Normal and abnormal structural development of the lung. In Polin RA, Fox WW, Abman SG, eds.: Fetal and neonatal physiology, 3rd ed, Philadelphia: Saunders, 2004.
9. Avery, ME, Fletcher, DD, Williams, RG. The lung and its disorders in the newborn infant, 4th ed. Philadelphia: Saunders; 1981.
10. Possmayer, F. Physicochemical aspects of pulmonary surfactant. In Polin RA, Fox WW, Abman SG, eds.: Fetal and neonatal physiology, 3rd ed, Philadelphia: Saunders, 2004.
11. Gross, I, Ballard, PL. Hormonal therapy for prevention of respiratory distress syndrome. In Polin RA, Fox WW, Abman SG, eds.: Fetal and neonatal physiology, 3rd ed, Philadelphia: Saunders, 2004.
12. Jobe, AH. Surfactant treatment. In Polin RA, Fox WW, Abman SG, eds.: Fetal and neonatal physiology, 3rd ed, Philadelphia: Saunders, 2004.
13. Barker, PM, Southern, KW. Regulation of liquid secretion and absorption by the fetal and neonatal lung. In Polin RA, Fox WW, Abman SG, eds.: Fetal and neonatal physiology, 3rd ed, Philadelphia: Saunders, 2004.
14. Rabinovitch, M. Physiologic development of the cardiovascular system in the fetus. In Polin RA, Fox WW, Abman SG, eds.: Fetal and neonatal physiology, 3rd ed, Philadelphia: Saunders, 2004.
15. Skandalakis, JE, Gray, SW, Ricketts, RR. The diaphragm. In Skandalakis JE, Gray RW, eds.: Embryology for surgeons, 2nd ed, Baltimore: Williams & Wilkins, 1994.
16. O’Rahilly, R, Müller, F. Human embryology & teratology, 2nd ed. New York: Wiley-Liss; 1996.
17. Rosse, C, Gaddum-Rosse, P. Hollingsched’s textbook of anatomy, 5th ed. Philadelphia: Lippincott-Raven; 1997.
18. Fraser, RS, Müller, NL, Colman, N, et al, The normal chest. Diagnosis of diseases of the chest, 4th ed. Fraser, RS, Paré, PD, eds. Diagnosis of diseases of the chest, Philadelphia, Saunders, 1999;Vol 1.
19. Hansell, DM, Armstrong, P, Lynch, DA, et al. The normal chest. In Hansell DM, Armstrong P, Lynch DA, et al, eds.: Imaging of diseases of the chest, 5th ed, London: Mosby, 2010.
20. Griscom, NT. Computed tomographic determination of tracheal dimensions in children and adolescents. Radiology. 1982;145:361–364.
21. Effmann, EL, Fram, EK, Vock, P, et al. Tracheal cross-sectional area in children: CT determination. Radiology. 1983;149:137–140.
22. Griscom, NT, Wohl, MEB. Tracheal size and shape: effects of change in intraluminal pressure. Radiology. 1983;149:27–30.
23. Armstrong, P. The normal chest. In Armstrong P, Wilson AG, Dee P, et al, eds.: Imaging of diseases of the chest, 3rd ed, London: Mosby, 2000.
24. Osborne, DRS, Effmann, EL, Hedlund, LW. Postnatal growth and size of the pulmonary acinus and secondary lobule in man. AJR Am J Roentgenol. 1983;140:449–454.
25. Bahmaie, A, Hughes, SW, Clark, T, et al. Serial fetal lung volume measurement using three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2000;16:154–158.
26. Osada, H, Iitsuga, Y, Masuda, K, et al. Application of lung volume measurement by three-dimensional ultrasonography for clinical assessment of fetal lung development. J Ultrasound Med. 2002;21:841–847.
27. Pöhls, UG, Rempen, A. Fetal lung volumetry by three-dimensional ultrasound. Ultrasound Obstet Gynecol. 1998;11:6–12.
28. Ward, VL, Nishino, M, Hatabu, H, et al. Fetal lung volume measurements: determination with MR imaging—effect of various factors. Radiology. 2006;240:187–193.
29. Williams, G, Coakley, FV, Qayyum, A, et al. Fetal relative lung volume: quantification by using prenatal MR imaging lung volumetry. Radiology. 2004;233:457–462.
30. White, KS, Muelenaer, AA, Jr., Beam, CA, et al. Determination of functional residual capacity from digital radiographs of the normal neonatal chest: studies in a rabbit model. AJR Am J Roentgenol. 1991;156:1209–1214.
31. Schlesinger, AE, White, DK, Mallory, GB, et al. Estimation of total lung capacity from chest radiography and chest CT in children: comparison with body plethysmography. AJR Am J Roentgenol. 1995;165:151–154.
32. Gollogly, S, Smith, JT, White, SK, et al. The volume of lung parenchyma as a function of age: a review of 1050 normal CT scans of the chest with three-dimensional volumetric reconstruction of the pulmonary system. Spine. 2004;29:2061–2066.
33. de Jong, PA, Long, FR, Wong, JC, et al. Computed tomographic estimation of lung dimensions throughout the growth period. Eur Respir J. 2006;27:261–267.
34. Mueller, KS, Long, FR, Flucke, RL, et al. Volume-monitored chest CT: a simplified method for obtaining motion-free images near full inspiratory and end expiratory lung volumes. Pediatr Radiol. 2010;40:1663–1669.
35. Vock, P, Malanowski, D, Tschaeppeler, H, et al. Computed tomographic lung density in children. Invest Radiol. 1987;22:627–631.
36. Long, FR, Williams, RS, Castile, RG. Inspiratory and expiratory CT lung density in infants and young children. Pediatr Radiol. 2005;35:677–683.
37. Weibel, ER. Morphometry of the human lung. New York: Academic Press; 1963.
38. Ohtani, O, Ohtani, Y. Organization and developmental aspects of lymphatic vessels. Arch Histol Cytol. 2008;71:1–22.
39. El-Chemaly, S, Levine, SJ, Moss, J. Lymphatics in lung disease. Ann NY Acad Sci. 2008;1131:195–202.