Embryology
Introduction
Understanding normal development of the embryonic genital tract gives insight into many disorders encountered in the female. These can range from relatively simple arrests of development or malformation (described by organ) to more complex abnormalities of sexual development that result from dysembryogenesis (see Chapter 2), and, in some cases, to help understand the origin of some tumors, particularly sex cord–stromal and germ cell tumors of the ovary. Most early insights have come from understanding human mutations. During more recent years, targeted mutations using mouse models have disclosed key roles for genes that had not been anticipated previously. Many regulators of gonadal development are receptors, signal transduction elements, transcription factors, extracellular ligands, and even intracellular signaling pathways mediating downstream transcriptional responses. Recently published references1–7 provide extensive reviews and, to some degree, competing theories.
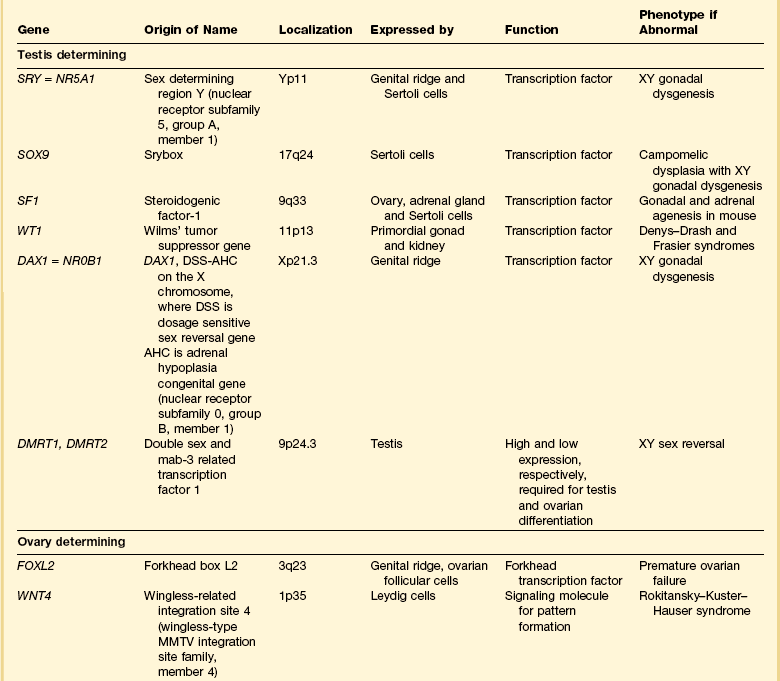
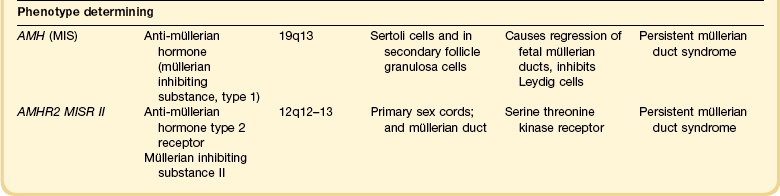
Most of the female genital tract is of mesodermal origin. Germ cells are of endodermal origin. The vulva and the epithelial lining of the vagina are ectoderm. The chronology and sequence of events that underlie the development of the female genital tract are summarized in Figures 1.1 and 1.2, and in Table 1.1. Table 1.2 lists specific genes involved in the initial steps of sexual development.
Table 1.1
Synopsis of Stages of Normal Embryologic Development
| Postovulatory Week | Crown–Rump at Beginning of Week | Sequence of Events (Top to Bottom) Within Stated Week |
| 1 week | 0.2 mm | Uterine implantation (0.2 mm). |
| 2 weeks | 1.5 mm | Primitive pit forms. No somites yet. |
| 3 weeks | 2.0 mm | 1–3 somites. |
| Pronephric tubules form; pronephric (mesonephric) duct arises and grows caudad as solid cord (2.5 mm). | ||
| Primordial germ cells first discernible in yolk sac near caudal part of embryo (2–3 mm). | ||
| Pronephros degenerated, but mesonephric duct reaches cloaca (3–5 mm). | ||
| Primordial germ cells discernible in hindgut (3–5 mm). | ||
| Primordial germ cells discernible in mesonephric ridges (5 mm). | ||
| 4 weeks | 7 mm | Primordial germ cells discernible in gonadal ridge, which itself at this time is a thin mesodermal proliferation (7 mm). |
| Cloaca divides into rectum and urogenital sinus. | ||
| Müllerian ducts appear as funnel-shaped opening of celomic epithelium. | ||
| 5 weeks | 12 mm | Indifferent gonad bulges into celom (12 mm). |
| Primitive sex cords appear (16–17 mm). | ||
| 6 weeks | 18 mm | Müllerian ducts about half distance to urogenital sinus. |
| Testis anatomically distinct with seminiferous tubules and capsule (tunica albuginea). | ||
| 7 weeks | 23 mm | Ovaries initially identified by absence of distinct seminiferous tubules (23 mm). |
| Müllerian ducts elongate and near urogenital sinus (23 mm). | ||
| Müllerian ducts in apposition; sinusal tubercle appears (23–28 mm). | ||
| Müllerian ducts lose sensitivity to MIS at 25 mm, but regression effect not observed until 31–35 mm. | ||
| 8 weeks | 29 mm | Müllerian ducts fuse and in contact with urogenital sinus (29 mm). |
| 9 weeks | 43 mm | Müllerian duct regression in response to MIS completed by 43–55 mm. |
| Testes and ovaries acquire capacity to secrete characteristic hormones at same stage of development; testosterone coincides with histologic development of Leydig cells and immediately precedes virilization of genital tract; ovary not yet differentiated; rate-limiting step is appearance of 3-α-hydroxysteroid dehydrogenase, which is 50-fold more abundant in testis than in ovary; ovary converts testosterone to estradiol, which testis cannot do; later regulation shifted to pituitary–placenta gonadotropins where testosterone → estradiol controlled by conversion of cholesterol to pregnenolone. | ||
| Müllerian ducts completely fused (entire septum gone); caudal aspect proliferates; epithelium lining canal stratifies (2–3 cells layers thick). | ||
| 10 weeks | 60 mm | Anogenital distance lengthens. |
| Testosterone synthesis sufficient to induce development of mesonephric duct into definitive structures (epididymis, vas deferens, and seminal vesicle). Subsequently, testosterone converted peripherally into 5-α-dihydrotestosterone, which causes the following transformations: | ||
| Urogenital sinus → prostate | ||
| Genital tubercle → glans penis | ||
| Genital folds → penis (only 3.5 mm long) | ||
| Genital swelling → scrotum | ||
| Fusion of labioscrotal folds. | ||
| Closure of median raphe. | ||
| Closure of urethral groove. | ||
| Phallus in both sexes 3 mm long; thereafter grows in males 0.72 mm/week and females 0.20 mm/week. | ||
| Mesonephric ducts regress if not stimulated by testosterone. | ||
| Vaginal plate first seen distinctly (complete at 140 mm; week 17). Initially, upper uterovaginal canal is large and oval in cross section, mostly lined by pseudostratified columnar epithelium. Extensive growth begins caudally; cells stratify. | ||
| Uterovaginal canal occluded caudally, progresses cranially. | ||
| 11 weeks | 71 mm | Primordial follicles appear. |
| Seminal vesicles develop. | ||
| Testis at inguinal ring. | ||
| Extensive uterovaginal growth continues caudally. | ||
| 12 weeks | 93 mm | Cervical glands appear; wavy, but undifferentiated. |
| Vaginal rudiment approaches vestibule. | ||
| True ovarian organogenesis begins with onset of meiotic prophase. | ||
| 13 weeks | 105 mm | Male urethral organogenesis complete. |
| 14 weeks | 116 mm | Primary folds of mucosa give uterine lumen W-shaped appearance on cross section. |
| Vaginal rudiment reaches level of vestibular glands; uterovaginal canal (15 mm total length) divisible into vagina (one-half), cervix (one-third), and corpus (one-sixth); boundaries ill-defined. | ||
| Isthmus readily distinguishable. | ||
| Stromal layers of uterus begin definition. | ||
| Solid epithelial anlage of anterior and posterior fornices appear. | ||
| Vagina begins to show slight estrogen effect. | ||
| 15 weeks | 130 mm | Fallopian tube begins active growth phase, begins to coil. |
| Vaginal plate completed; lower end reaches vestibule; upper end extends into endocervical canal. | ||
| Female urogenital sinus becomes shallow vestibule. | ||
| Primary follicles of ovary appear. | ||
| 16 weeks | 142 mm | Vaginal plate longest and begins to canalize. |
| Corpus glands appear as slight outpouchings. | ||
| 17 weeks | 153 mm | Palmate folds of cervix appear (forerunner adult cervix). |
| Mucoid development of cervix begins. | ||
| Smooth muscle of uterus appears. | ||
| Estrogen effect apparent throughout vagina. | ||
| Cavitation of vaginal canal completed. | ||
| 18 weeks | 164 mm | Fornices hollow. |
| 19 weeks | 177 mm | |
| 20 weeks | 186 mm | Dramatic increase in growth and coiling of fallopian tube (about 3 mm/week to week 34). |
| 21 weeks | 197 mm | |
| 22 weeks | 208 mm | Differentiation of muscular layer of uterus complete. |
| Fundus well marked; uterus assumes adult form. | ||
| Graafian follicles appear. | ||
| 24 weeks | 230 mm | |
| 26 weeks | 250 mm | |
| 28 weeks | 270 mm | |
| 30 weeks | 290 mm | |
| 34 weeks | 328 mm | |
| 38 weeks | 362 mm | Birth. |
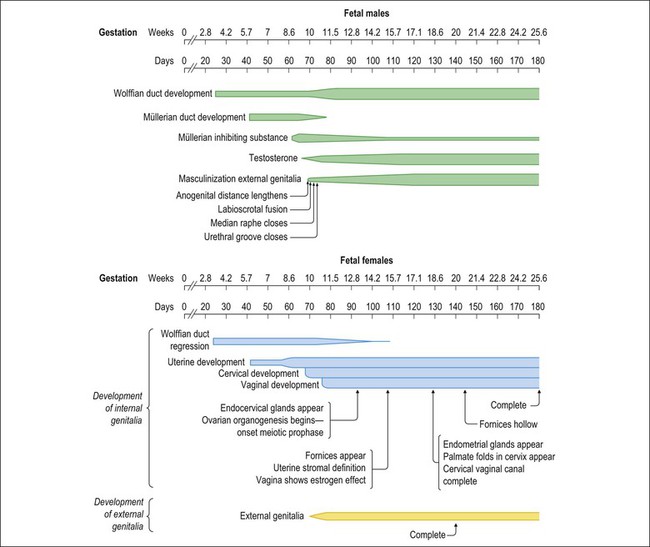
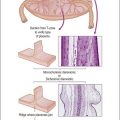
Figure 1.2 Normal sexual development. Embryologic development is determined by several factors, all of which are time specific during embryogenesis.
In the broadest view, sex determination takes place in three sequential steps.8 The first is chromosomal sex determination, which occurs as a result of fertilization. Gonadal sex determination, the second critical event, results when the potential gonads actually transform into ovaries or testes in accord with the available chromosomal information. Third, the secondary sex characteristics develop along female or male lines as determined by the preponderant estrogenic or androgenic hormonal milieu present systemically. Sexual identity, not to be discussed here, includes a person’s sense of self (gender identity) and his/her attraction to others.
Gonadal Development
In humans and other mammals, the karyotype ‘XY’ genetically defines the sex as male, whereas ‘XX’ defines the female sex. Sex is determined by the presence or absence of a signal from the substance initially called the testis determining factor and now recognized as the gene called SRY (Sex determining Region Y) in the human and SRY in the mouse. The gene is found on the Y chromosome. Testes are formed if this gene is expressed by the embryo before the urogenital ridge differentiates. Further male development occurs under the influence of hormones secreted later by the testes. Without SRY, the gonads differentiate as ovaries and the embryo develops as a female. The timely expression of SRY is critical to the development of male sex. In its absence, the embryo develops a female phenotype, regardless of genetic sex. Although for years believed to occur by default, a gene has been identified in women (R-spondin1, RSPO1)9 critical for development of the ovary through signaling pathways.10
The SRY gene is located in the region just central to the pseudoautosomal pairing region at the distal end of the short arm of the Y chromosome.11 The pseudoautosomal pairing region is named for the two limited regions at the distal ends of the short and long arms of the Y chromosome where sequence identity with the X chromosome permits pairing and recombination during male meiosis.12 The gene, which has a strongly conserved motif,13 encodes for a DNA binding protein, which is the binding activity product (transcriptional switch) that orchestrates the action of other genes. It does so by initiating a cascade of gene expression that regulates the development of the testis, not all of which are known or understood.
Several lines of evidence support this thesis. These include that the:
• SRY gene is absent from the normal X chromosome and somatic chromosomes
• SRY gene is present on the X chromosome of ‘sex-reversed’ XX human males
• Homologous gene in the mouse is initially expressed just before sexual differentiation begins
• SRY gene acts in the absence of germ cells
• SRY gene in the chromosomally female embryo causes it to develop as a male.14
Rare examples have also been identified where single base pair point mutations in the SRY gene or in promoter regions essential for gene expression render an XY patient as a phenotypic female with a streak gonad.15
Early on, SRY initiates induction of somatic cell migration from the mesonephros into the gonad16 and induces indifferent cells in the genital ridge to differentiate into Sertoli cells. This is the first type of cell required to form in the embryonic testis.17 With monoclonal antibodies, an SRY protein has been found in the nuclei of Sertoli cells and germ cells.18 Several other genes are thought to be important in Sertoli cell function. SOX9, which is named for it being an SRY-related high motility gene box group, in the mouse is active in pre-Sertoli cells19 and results in XY human females when mutations inactivate the gene. SF1, which SOX9 helps regulate, is another important gene that is an orphan nuclear receptor expressed in the gonadal ridge in precursor cells of Sertoli and stromal cells. It appears as a master regulator of the reproductive system because it regulates the expression of numerous genes required for gland development and hormone synthesis.20–22 Mutations in this gene in humans have been responsible for adrenal insufficiency associated with gonadal dysgenesis.23 The gene DAX1, which is required at several points in embryonic testis development,24 also plays a key role in sex determination. Overexpression causes varying degrees of gonadal dysgenesis, and at high doses in the mouse, male-to-female sex reversal occurs. Additional references8,12,22,25 describe in greater detail the genes involved in the rapidly evolving science of male sex determination.
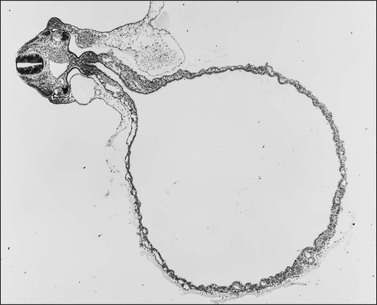
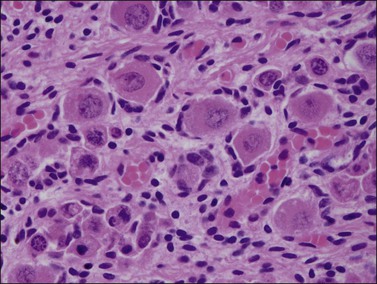
During the development of both male and female human embryos, but before the gonads develop, the primordial germ cells migrate from the yolk sac to the urogenital ridges via the caudal part of the hindgut approximately 3 weeks after fertilization (Figure 1.3). The yolk sac, which is of considerable size (Figure 1.4), is easily recognized by its reactivity to α-fetoprotein. This migratory event is independent of eventual sex. The germ cells are large and prominent, and have clear cytoplasm and vesicular nuclei. Once they synthesize glycogen and alkaline phosphatase, they are easily identified histochemically by their demonstration of placental-like alkaline phosphatase (PLAP) and CD117 (c-kit).
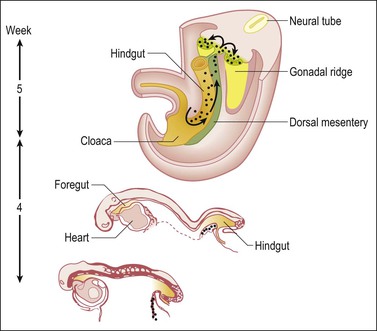
Figure 1.3 Migration of germ cells in the human embryo. At the end of the third week, epiblast-derived cells present in the yolk sac near the allantoic base have differentiated into primordial germ cells, the latter having migrated by the fifth week along the dorsal mesentery of the hindgut to the gonadal ridges.
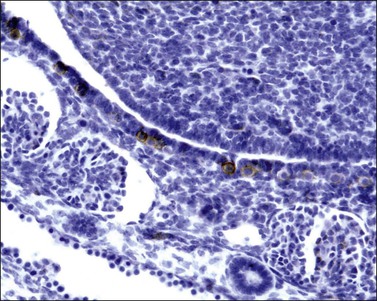
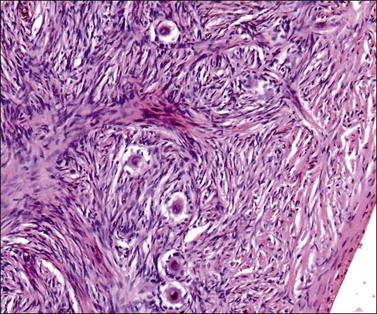
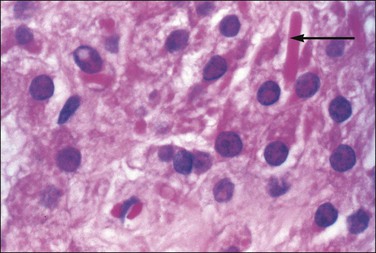
At about this time, the mesothelium on the medial surface of the urogenital ridge, which itself is located ventral to the mesonephric rudiments, begins to proliferate. By the fifth week, while still in the indifferent stage, the parenchyma is a thin wisp, measuring less than 1 mm in thickness and several millimeters in width (Figures 1.5 and 1.6). Several transcription factors, including Wilms’ tumor 1 (WT1) and steroidogenic factor 1 (SF1), are involved in the earliest processes of gonad formation, regardless of the direction to which the gonad differentiates (Figure 1.7). These transcription factors act on the somatic cells in the gonadal primordia, but do not affect the germ cells themselves, which are still easily identifiable by their reactivity for PLAP and c-kit (Figure 1.8). Over the next several weeks the ridge develops into a recognizable, but undifferentiated, gonad (Figures 1.9 and 1.10).
Figure 1.5 The gonadal ridge in the 7 mm embryo (5 weeks) is much thinner than the width of the mesonephros.
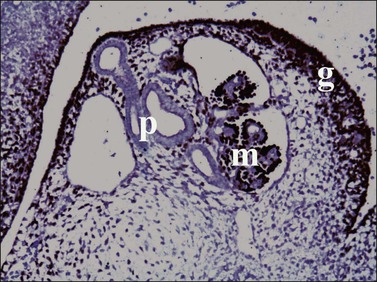
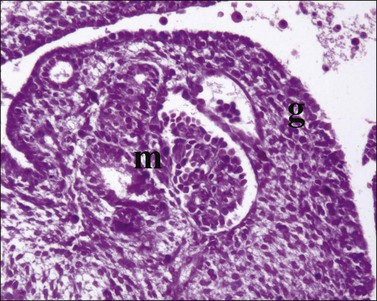
Figure 1.7 WT1 is expressed in the mesonephros (m) and the genital ridge (g) (about day 33). The residual pronephric ducts (p) do not react.
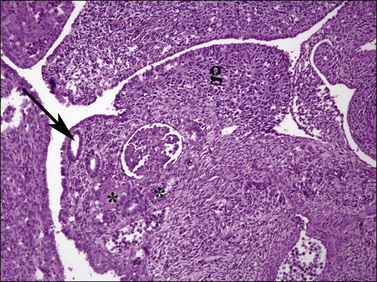
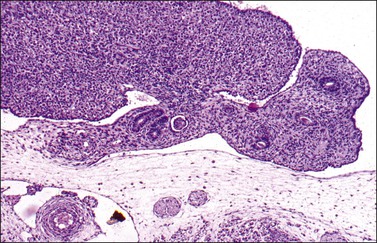
Figure 1.9 Undifferentiated gonad (g) with wolffian (asterisks) and müllerian (arrow) ducts and adjacent mesonephros containing glomerular structures (about 42 days).
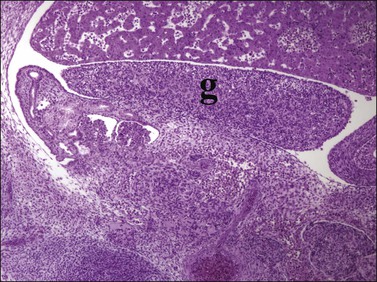
Figure 1.10 Undifferentiated gonad (g) showing enlargement and development of characteristic gonadal shape (about 45 days).
During the initial stages of both testicular and ovarian development, the gonads develop independent of whether the primordial germ cells are present or absent or have proliferated abnormally. An early manifestation of the normally developing gonad is the appearance in the gonad of primary sex cords, which are temporary branched structures containing the proliferating germ cells and support cells (Figures 1.11 and 1.12). This process begins during the fifth week. The sex cords, the exact embryologic derivation of which is uncertain but seemingly dependent on the migration of mesonephric interstitial cells, lack a basement membrane and basal myoid cells. In a manner not yet understood, in the presence of SRY and with the participation of the rete and mesonephric apparatus, the sex cords transform into the tubules, which become cords of epithelial-like cells that extend from the rete in the hilus of the gonad into the medulla. The level of the connection is less important than the field effect of induction of epithelial–mesenchymal differentiation (Figure 1.13).
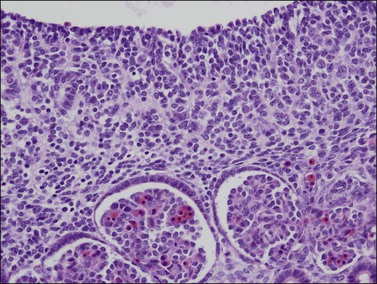
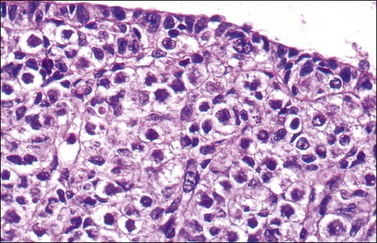
Figure 1.11 Sex cords become more prominent in this still indifferent gonad. Mesonephric glomeruli present underneath (about 56 days).
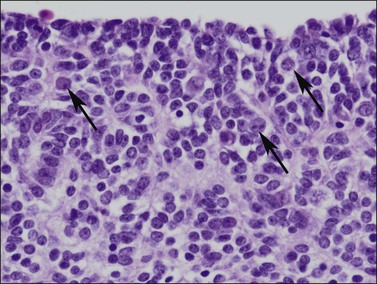
Figure 1.12 Germ cells with enlarged nuclei (arrows) are evident within the poorly formed sex cords in this 56 day gonad.
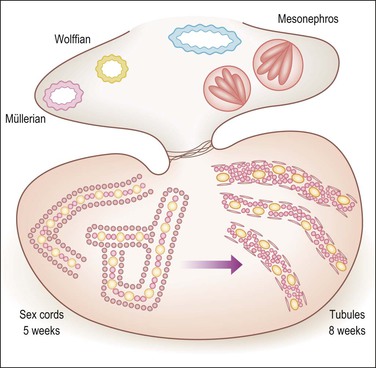
Figure 1.13 Testicular development in male embryo from 5–8 weeks of gestation. Within the bean-like gonad the primary sex cords develop under the influence of migrating mesonephric cells, which induce a mesenchymal to epithelial switch of differentiation similar to that happening in the metanephros under the action of WT1. The conversion of the sex cords to tubules is induced by the rete, which is under SRY control, and connected to the tubules. The primary sex cords (left) are composed of primary germ cells (yellow) and stromal cells that have acquired epithelial features. Tubular differentiation (right) features acquisition of myoid cells and a peripheral basement membrane and more centrally located Sertoli (pink) and germ cells (yellow).
In females, germ cells continue to increase in number (Figures 1.14 and 1.15) until ovarian differentiation is apparent at approximately 15 weeks, as shown by the emergence of primordial follicles. In many other respects, until this time the ovarian tissue continues to resemble the indifferent gonad, unlike the testis, which becomes anatomically distinct with early tubular formation and immature Sertoli cells by postovulation day 44. (In this chapter, all dates given are postovulation.)
In the male, one of the first easily recognized testis-specific structures that can be identified is its capsule, or tunica albuginea (Figures 1.16 and 1.17), which is first evident at approximately 50 days. The tunica is a zone of spindle cells that develops and separates the epithelial cords from the surface. The cords become the testicular tubules as the epithelial cells differentiate into the tall, clear, flask-shaped Sertoli cells of the testis and myoid (peritubular contractile) cells appear just outside the basement membrane. The gonadal stromal cells become the interstitial or Leydig cells. In normal development, the germ cells are initially located in the lumen and move eventually between the Sertoli cells to lie on the basement membrane at the base of the tubules (Figure 1.18). The primordial germ cells preferentially colonize the medullary region of the presumptive gonads.14 Even in the absence of germ cells, the somatic tissues of the undifferentiated embryonic gonad are capable of developing into a testis, albeit lacking spermatogonia and spermatogenesis.26
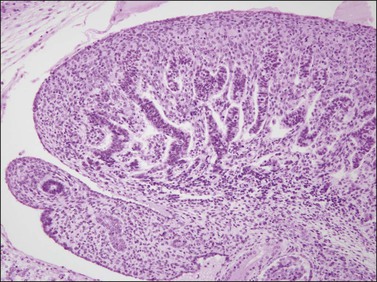
Figure 1.16 The testis capsule (tunica albuginea) separates early seminiferous tubules from the surface, approximately 10 weeks (70 days).
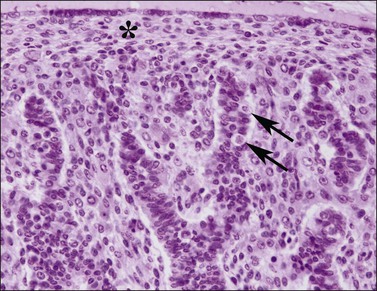
Figure 1.17 Testis with seminiferous tubules, intratubal germ cells (arrows), and overlying tunica (asterisk), 10 week fetus.
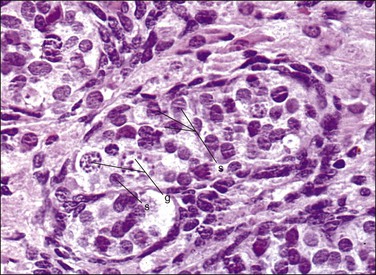
Figure 1.18 Testis with seminiferous tubules and intratubal germ cells, 19 week fetus. g, germ cell; s, Sertoli cell.
At some later time, another gene of the Y chromosome comes into play and activates the process for the development of normal spermatogenesis. The gene, called DAZ (Deleted in AZoospermia), is less well characterized than the SRY gene.27 In the absence of DAZ, or at least if not detectable by current methods, sperm will still develop, although defective and few in number, it will still be capable of successful fertility.28 DAZ mutation results in loss of the Y chromosome during mitosis, which leads to the creation of both XO and XY cell lines.
If functional SRY is absent (i.e., normal 46,XX females), genes associated with ovarian development activate.29 WNT4, the first signaling molecule identified in the study of sex determination in mice, regulates female sexual development.13 Part of its function is to repress male sexual differentiation. Patients with defective WNT4 present with a Mayer–Rokitansky–Hauser-like syndrome with absence of müllerian structures, unilateral renal agenesis, and clinical signs of androgen excess.30 FOXL2 is demonstrable in the genital ridge before there is any clear structural organization of the gonads.31–33 Recent studies in mice have shown a robust female genetic program that activates at the onset of ovarian development.34
In the absence of male determining factors, the dividing germ cells are incorporated into a proliferating mass of surface epithelial cells, which results in a thickened cortex that presages the organization of the adult ovary without the development of a separating tunica (Figure 1.19). From the second to the early third trimester, this thickened cortical mass of proliferating epithelial and germ cells divides into small groups demarcated by strands of stromal tissue extending from the medulla to the cortex (Figure 1.20). The small groups of germ cells and epithelial cells further subdivide into primordial follicles composed of single germ cells surrounded by a layer of epithelial cells, the primitive granulosa cells (Figure 1.21). In normal development, each germ cell is characteristically encapsulated in its own (primordial) follicle. Oogonia, not so enveloped, undergo spontaneous apoptosis. This is associated with entry into meiosis and cessation of further proliferation. By puberty, while most of the ovary shows variable concentrations of oocytes (Figure 1.22), some have developed into the antral stage and may become future ovulatory sites (see Chapter 23).
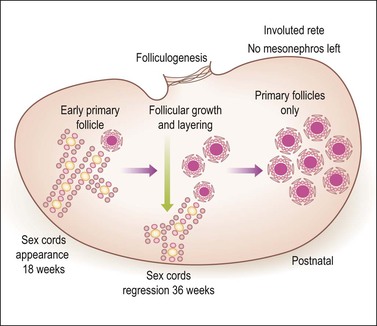
Figure 1.19 Ovarian development from 18 weeks through birth. Until the 18th week of gestation the ovary is an undifferentiated gonad with a streak-like pattern. The primary sex cords begin their development under the influence of mesonephric cells that had migrated earlier and then remained inactive. The mesenchymal to epithelial switch of differentiation starts deeply in the parenchyma as does early primary follicular differentiation, as shown by immunohistochemical demonstration of FOXL2 nuclear reactivity and the appearance of primary follicles of small size. The folliculogenetic process repeats itself in a deeper to more superficial direction with the progressive disappearance of the primary sex cords around 36 weeks’ gestation. The growth in follicles occurs under the influence of WT1 like that of metanephric glomeruli in the kidney. Simultaneously, the primary sex cords regress by apoptotic death of the remaining cells. By birth, only primary follicles remain. Primary sex cords (left) are composed of primary germ cells (yellow) and stromal cells acquiring epithelial features. Follicles (left, middle, and right) disclose a central oogonium (large pink) surrounded by follicular (granulosa) cells. A peripheral basement membrane envelops each follicle.
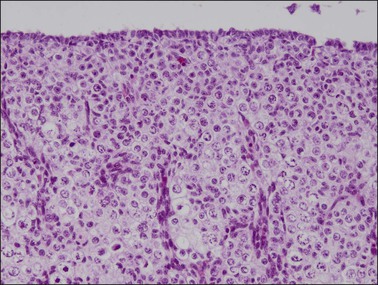
Figure 1.20 Ovary at 15 weeks’ gestation showing sheets of germ cells extending to the surface epithelium. Vascular elements divide the oocytes into nests.
Until about the 15th week, the ovary is an undifferentiated gonad with a streak-like pattern. The primary sex cords begin their development under the influence of mesonephric cells that had migrated earlier and then remained inactive. The mesenchymal to epithelial switch of differentiation starts deeply in the parenchyma as does early primary follicular differentiation, as shown by immunohistochemical demonstration of FOXL2 nuclear reactivity and the appearance of primary follicles of small size. The folliculogenetic process repeats itself from the deeper to the more superficial with the progressive disappearance of the primary sex cords around 36 weeks’ gestation. The growth in follicles occurs under the influence of WT1, similar to that for metanephric glomeruli in the kidney. Simultaneously, the primary sex cords regress by apoptotic death of the remaining cells. By birth, only primary follicles remain.
If the normal male genetic constitution (46,XY) is present, some of the early epithelial proliferation contributes to the connection between the sex cords and the mesonephric tubules (rete testis). Where gonads are destined to become ovary, early epithelial proliferation degenerates in the ovarian hilus, leaving a few tubules, the rete ovarii. It is these primordial mesonephric cells that are believed to develop and envelop the individual germ cells and eventually become the follicular granulosa cells. Interstitial (Leydig) cells develop extensively in the stromal tissue of the second trimester female gonad, but degenerate in most cases by term. The few interstitial cells found in the hilus of the adult ovary are called hilus cells (Figure 1.23). Thus, the gonad develops primarily from mesodermal tissues, with the exception of the germ cells, which migrate from the extraembryonic yolk sac to the fetal visceral endoderm.
Role of Germ Cells
The primordial germ cells, which migrate to the primitive gonad, are not undifferentiated cells. By the time of their migration from the yolk sac to the gonadal ridge, they have attained some developmental potency. Occasionally, germ cells stray during migration and reach ectopic sites. If they do not die, they still may be capable of differentiation, but, remarkably, always differentiate as oocytes regardless of their genetic sex. Even if the cells are in males, they differentiate as XX germ cells would normally in the ovary. It is thought that the absence of Sertoli cell differentiation is important, and the suggestion remains that all germ cells should be viewed potentially as female, regardless of the genetic sex of the patient. Follicular cells also appear important: in their absence, germ cells in ectopic sites usually degenerate and disappear. Thus, the ability of primordial germ cells to develop into oocytes or spermatogenic cells seems to reflect the tissue environment in which they grow rather than their own native chromosomal constitution. The development of the germ cells follows the somatic sex of the gonadal tissue, and not the genetic sex of the germ cells themselves.35 In the mouse there is evidence that c-kit reactive stem cells present in bone marrow become ovarian-type germ cells.36
Müllerian and Wolffian Duct Development
The Müllerian Duct to Week 8
Regardless of genetic sex, the celomic epithelium in both females and males invaginates at several points on the lateral surface of the paired urogenital ridges beginning at week 5 of embryonic life. They coalesce to form the paired tubes termed the müllerian (paramesonephric) ducts (Figures 1.24 and 1.25). Each of the paired ducts extends caudally in the urogenital ridge immediately lateral to and using the wolffian (mesonephric) duct as a guidewire. For proper müllerian duct migration to occur, the wolffian duct must be present. Spatially lateral to the cephalad aspect of the wolffian ducts, the müllerian ducts then cross over caudally to lie medial to them as they enter the pelvis (Figure 1.26). By the end of week 8 of embryonic life, the müllerian ducts between the two wolffian ducts fuse to form a single structure, which is the anlage of the common uterovaginal canal (Figure 1.27). The tip of the müllerian duct abuts upon the posterior wall of the urogenital sinus immediately between the two orifices of the wolffian ducts (Figure 1.28), approximately at the position of the future cervix.
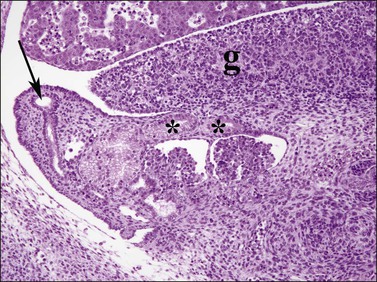
Figure 1.24 The müllerian duct (arrow) formed by invagination of the celomic epithelium adjacent to the mesonephros (paramesonephric) becomes the fallopian tube. Mesonephric tubules in the region of the gonadal hilum (asterisks) remain as the rete ovarii, 6.5 week embryo; g, gonad.
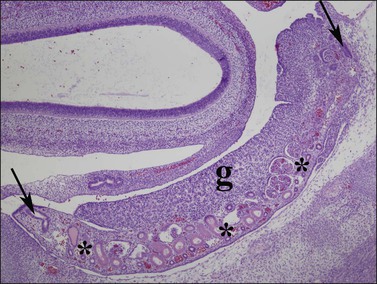
Figure 1.25 Mesonephric (wolffian, asterisks) ducts and mesonephric glomeruli underlie the length of the indifferent gonadal ridge (g). The müllerian duct (paramesonephric duct) forms by invagination at the end of the mesonephros, and courses from a cranial (right arrow) to caudal (left arrow) direction along the gonad as the presumptive fallopian tube. Parasagittal section of 8 week embryo.
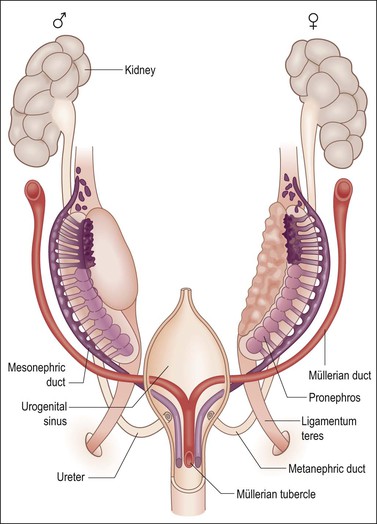
Figure 1.26 The anlage of the genital organs in the indifferent, bisexual stage. The müllerian derivatives are red and the wolffian derivatives are purple.
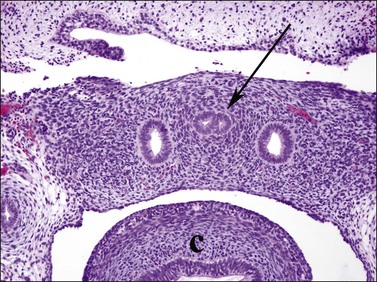
Figure 1.27 The two müllerian ducts fuse (arrow) in the midline within the pelvis, between the two wolffian ducts. The sigmoid colon is adjacent (c). Transverse section of 9.5 week embryo.
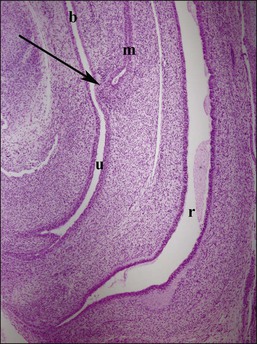
Figure 1.28 Midline sagittal section of female genitalia at 9.5 weeks. The müllerian tubercle (arrow) is the future site of the cervix, located at the juncture of the müllerian duct (m) and urogenital sinus (u). The urogenital sinus in females contributes to the urethra and vaginal wall, with squamous vaginal lining growing upward from the perineum. Rectum (r) and bladder (b) positions are indicated.
External Influence on the Developing Embryonic Genital Tract Ducts
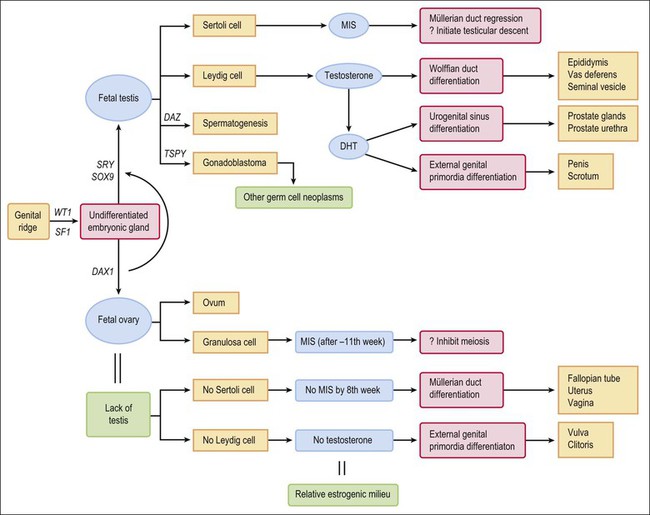
Once the male pathway of development has begun, two hormones produced by the fetal testis then control the differentiation of the male phenotype. The first is AMH, which the Sertoli cells produce early during fetal life.37,38 The primary function of AMH is to cause regression of the müllerian (paramesonephric) ducts in the male fetus, which it does by its effects on the mesenchyme surrounding the duct (Figure 1.29).
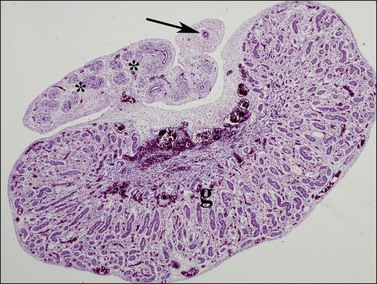
Figure 1.29 AMH secreted by Sertoli cells within the testis (g) leads to involution of the müllerian duct (arrow) until it remains only as a vestigial single duct known as the appendix epididymis. Within the epididymis itself, the wolffian ducts (asterisks) become more prominent, 16 week male fetus.
AMH is first secreted in effective amounts 56–62 days after fertilization, and the process of müllerian regression is normally completed by about day 77, after which the müllerian tissue is no longer sensitive to AMH. During this critical period, even relatively small amounts of AMH given over a short period of time can cause irreversible damage to the embryonic müllerian tract.39 In the female, AMH is produced in insignificant amounts during fetal life (as there are no Sertoli cells) and the müllerian ducts develop passively to form the fallopian tubes, uterus, and vaginal wall. Other functions of AMH, secreted later in fetal life, are discussed in the following sections.
AMH has a local action, and inhibits development of the ipsilateral fallopian tube. To prevent development of both the uterus and vagina, both testes must secrete adequate amounts of AMH. Thus, in a patient with a testis and a contralateral streak, the ovary or ovotestis generally has a uterus and vagina and a single fallopian tube on the side with the streak or ovary. AMH immunoreactivity can be observed in Sertoli cell cytoplasm from roughly week 8 of fetal life until puberty. It is detected in the Sertoli cells in the premeiotic seminal pretubules but disappears in older tubules that have shown meiotic development.38
Additional functions of AMH have recently been discovered or postulated. In the female, ovarian granulosa cells begin producing AMH only after the müllerian-derived tissues (fallopian tubes, uterus, and vagina) are well developed and no longer susceptible to the regressive effects of AMH. Serum AMH levels in girls rise slowly after birth from nearly undetectable levels until reaching a plateau after 10 years of life. It is then equivalent to the adult male serum concentration. In contrast, the male serum AMH concentration is relatively high at birth, peaks at 4–12 months of age, and then falls progressively to a baseline low adult level by about 10 years of age. A major action of AMH in the young female may be to inhibit oocyte meiosis in the developing follicle. Dramatically high levels of AMH have been found in women with ovarian sex cord tumors, thus serving potentially as a diagnostic marker or method to evaluate the effectiveness of therapy.40 Another important action of AMH in males may be to initiate testicular descent, principally by its postulated regulatory control over the gubernaculum testis.41 Anti-AMH is an excellent biomarker for gonadal–stromal tumor in which there is a Sertoli or granulosa cell component.42
The second hormone that the fetal testis secretes is testosterone. This androgenic steroid, which is critical for male development, is required for the wolffian (mesonephric) duct to differentiate into the epididymis, vas deferens, and seminal vesicle. Leydig cells appear in the testis around day 54–64 and shortly thereafter begin to produce testosterone (see Huhtaniemi43 for a fuller analysis of the fetal testis and how it differs significantly from the adult testis). Leydig cell activity is probably stimulated by increased production of chorionic gonadotropin by the placenta at that time. Testosterone acts locally on the ipsilateral wolffian duct by binding to a specific high-affinity intracellular receptor protein. This receptor hormone complex binds DNA to regulate transcription of specific genes that govern further development. In the absence of a testis or inability of a testis to produce testosterone in adequate amounts by 10–12 weeks, or insensitivity of the wolffian duct anlage to testosterone, the epididymis, vas deferens, and seminal vesicle fail to differentiate. Only rarely are abnormally elevated testosterone levels reached sufficiently early during embryogenesis in a female fetus to cause the wolffian duct to differentiate into definitive male organs (androgen administration to the mother during pregnancy, congenital adrenogenital syndrome, and some androgen-secreting ovarian tumors; see Chapter 2).
The development of the stromal component of the genital canal is little studied, but is clearly of major importance.44 In addition to its role in the development of the walls of the tubular muscular organs (i.e., the vagina, cervix, uterine corpus, and fallopian tubes), there is extensive experimental evidence to indicate that the stroma also directs epithelial development. Thus, the entire structure of the female genital tract is determined by stromal–epithelial interaction.
The Müllerian Duct after Week 8
If the embryo is female, or in the case of male intersex in which embryonic müllerian ducts have not been completely inhibited by the AMH that the testis secretes, the ducts continue to grow unimpeded. Cranially, the separate müllerian ducts develop as distinct fallopian tubes (Figure 1.30), and the fused caudal portion as the uterus (Figure 1.31). Caudally, squamous epithelium proliferates from the urogenital sinus, growing up toward the embryonic müllerian ducts to replace the native embryonic glandular epithelium. A column of squamous epithelial cells is formed, termed the ‘vaginal plate,’ which comes to occupy the entire region of the vagina and exocervix (Figure 1.32). At that time the uterovaginal canal is a straight tube without evidence of a fornix. The vaginal plate is solid. By early in the second trimester, the vaginal plate begins to degenerate, and thus the vagina shows early signs of patency. The epithelium of the vaginal plate gives rise to the epithelium that ultimately lines the vagina and exocervix (Figure 1.33).
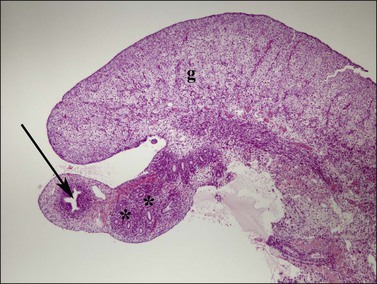
Figure 1.30 In females, the cranial aspect of the müllerian duct (arrow, paramesonephric), near the ovary (g), develops into the fallopian tube during the second trimester. Remnants of the wolffian (asterisks, mesonephric) ducts course alongside, within the mesosalpinx. Relative size of the müllerian and wolffian ducts is reversed in comparable aged male fetuses (see Figure 1.29); 15 week female embryo.
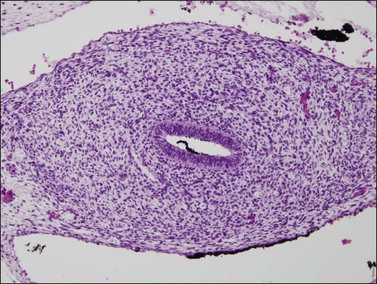
Figure 1.31 The fused müllerian duct develops a thick wall but in the location of the future uterus does not yet have glands or a myometrial layer; 12.5 week female embryo, transverse section.
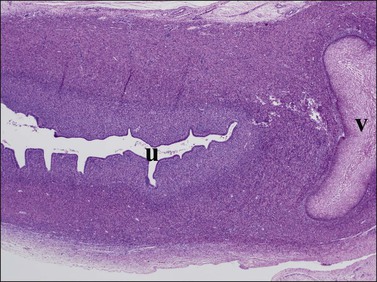
Figure 1.32 The vaginal plate (v) is a mass of squamous cells extending from the vagina in a cranial direction toward the developing uterus (u). They do connect, in this case outside the plane of section. Much of the uterus visible here will become the cervix uteri, as the lumen is further colonized by squamous epithelium; 20 week female embryo, slightly parasagittal section.
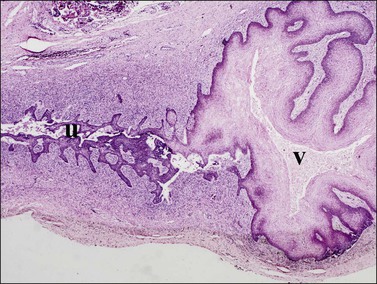
Figure 1.33 Similar perspective to Figure 1.32, only a week or two later, shows further extension of the vaginal squamous epithelium (v) into the fused müllerian duct in the region of the cervix uteri (u). Vaginal squamous epithelium is now highly glycogenated; 21–22 week female embryo.
The Müllerian Duct during the Second Trimester
Smooth muscle appears in the walls of the genital tract between 18 and 20 weeks, although stromal aggregates into circular and longitudinal layers appear earlier. By approximately 24 weeks, the muscular portion is well developed. Vaginal, uterine, and tubal muscular walls develop around the müllerian duct alone, thus excluding the wolffian duct remnants, which are external to the true wall of the canal. Cervical glands appear at about 15 weeks. Rudimentary endometrial glands are present by 19 weeks (Figures 1.34 and 1.35), but the endometrium is poorly developed even at term in most infants.
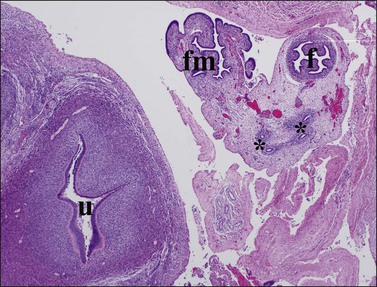
Figure 1.34 The uterus (u), formed by fused müllerian ducts in the midline, develops a thick wall composed of inner endometrial stroma, and outer myometrium. The fallopian tube (f) and fimbria (fm), originally in a cranial location, drop into the pelvis to lie lateral to the uterus. Wolffian duct rests (asterisks) are seen in the mesosalpinx; transverse pelvic section of 20 week female fetus.
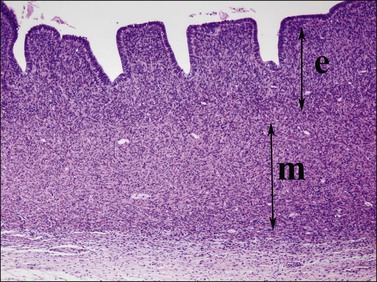
Figure 1.35 Detail of 20 week uterine wall, showing simple endometrial glands, an inner layer of endometrial stroma (e), and outer myometrium (m).
After about 20 weeks, at the time when estrogen levels have risen in the mother, the squamous epithelium comprising the vaginal plate of the fetus first shows signs of intracytoplasmic glycogen accumulation (Figure 1.33). Eventually, there is cellular dissolution and the formation of the fully patent vagina.
External Genitalia
• Genital tubercle to enlarge and form the glans penis
• Genital folds to enlarge and fuse to form the penile shaft with migration of the urethral orifice along the lower border of the shaft to the tip of the glans
• Genital swellings to fuse and form a scrotum
• Urogenital sinus tissues to differentiate into the prostate
Failure of the external genitalia to develop in males in the presence of testes may be due to a lack of adequate testosterone secretion into the systemic circulation, deficient enzyme (5-α reductase, type II) at the end-organ level to convert testosterone to dihydrotestosterone, or complete end-organ insensitivity (androgen receptor insensitivity). Lesser degrees of deficiency or end-organ insensitivity may result in partial male development characterized by a small penis, hypospadias, deficient formation of the scrotum, or a persistent urogenital sinus (vaginal opening into the urethra). The effects of dihydrotestosterone begin about day 70, with fusion of the labioscrotal folds and closure of the median raphe, and continue at day 74 with closure of the urethral groove. External genital development is complete by day 120–140 (week 18–20).
The urogenital sinus, into which the vagina opens, enlarges as the embryo grows, so that it becomes the vestibule of the adult external genitalia. Consequently, the vestibule is lined, except for a variable portion anterior to the urethral orifice, by the endodermal epithelium of the urogenital sinus. This is clinically important as the endodermal-derived epithelium not only differs morphologically from the mesodermal- and ectodermal-derived epithelium, but also responds differently to a variety of stimuli, notably sex steroids.
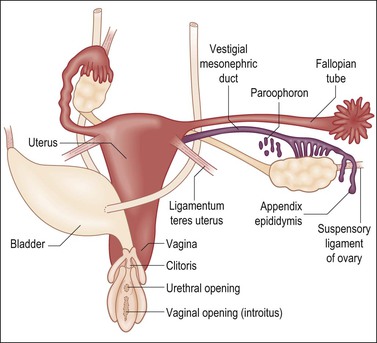
In summary, female internal organs and external genitalia develop in the absence of hormones secreted by the fetal ovary, and differentiate even when gonads are absent (Figure 1.36). Unless interrupted by the regressive influence of AMH, differentiation of the müllerian ducts proceeds caudally to form fallopian tubes, a uterus, and a vagina. In the absence of the masculinizing effect of dihydrotestosterone, the undifferentiated external genital anlage develops into the vulva. The genital tubercle develops into the clitoris, the genital folds into the labia minora, and the genital swellings into the labia majora. Thus, the infant with ovaries or streak gonads has female internal and external genitalia at birth. Only if the female fetus has systematically elevated levels of androgens before week 10–12 of gestation does any degree of internal male development occur. In such cases, the external genitalia may appear ambiguous or may resemble that of a normal phenotypic male; the vagina in these instances opens into the membranous portion of the urethra. If the androgens are not elevated until after week 20, by which time the external genitalia have fully formed, the only virilizing effect is an enlarged clitoris.
References
1. Brennan, J, Capel, B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004; 5:509–521.
2. Iyer, AK, McCabe, ER. Molecular mechanisms of DAX1 action. Mol Genet Metab. 2004; 83:60–73.
3. Kanai, Y, Hiramatsu, R, Matoba, S, Kidokoro, T. From SRY to SOX9: mammalian testis differentiation. J Biochem. 2005; 138:13–19.
4. Mittwoch, U. The elusive action of sex-determining genes: mitochondria to the rescue? J Theor Biol. 2004; 228:359–365.
5. Park, SY, Jameson, JL. Minireview: transcriptional regulation of gonadal development and differentiation. Endocrinology. 2005; 146:1035–1042.
6. Viger, RS, Silversides, DW, Tremblay, JJ. New insights into the regulation of mammalian sex determination and male sex differentiation. Vitam Horm. 2005; 70:387–413.
7. Yao, HH. The pathway to femaleness: current knowledge on embryonic development of the ovary. Mol Cell Endocrinol. 2005; 230:87–93.
8. Shimada, K. Sex determination and sex differentiation. Avian Poultry Biol Rev. 2002; 13:1–14.
9. Capel, B. R-spondin1 tips the balance in sex determination. Nat Genet. 2006; 38:1233–1234.
10. Chassot, AA, Ranc, F, Gregoire, EP, et al. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008; 7:1264–1277.
11. Brennan, J, Karl, J, Martineau, J, et al. SRY and the testis: molecular pathways of organogenesis. J Exp Zool. 1998; 281:494–500.
12. McElreavey, K, Quintana-Murci, L. Y chromosome haplogroups: a correlation with testicular dysgenesis syndrome? APMIS. 2003; 111:106–114.
13. Fleming, H. Differentiation in human endometrial cells in monolayer culture: dependence on a factor in fetal bovine serum. J Cell Biochem. 1995; 57:262–270.
14. Hunter, RHF. Mechanisms of sex determination. In: Hunter RHF, ed. Sex determination, differentiation and intersexuality in placental mammals. Cambridge, UK: Cambridge University Press; 1995:22–68.
15. Mendes, JR, Strufaldi, MW, Delcelo, R, et al. Y-chromosome identification by PCR and gonadal histopathology in Turner’s syndrome without overt Y-mosaicism. Clin Endocrinol (Oxf). 1999; 50:19–26.
16. Tilmann, C, Capel, B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development. 1999; 126:2883–2890.
17. Swain, A, Lovell-Badge, R. A molecular approach to sex determination in mammals. Acta Paediatr (Suppl). 1997; 423:46–49.
18. Salas-Cortes, L, Jaubert, F, Barbaux, S, et al. The human SRY protein is present in fetal and adult Sertoli cells and germ cells. Int J Dev Biol. 1999; 43:135–140.
19. Moreno-Mendoza, N, Harley, V, Merchant-Larios, H. Cell aggregation precedes the onset of SOX9-expressing preSertoli cells in the genital ridge of mouse. Cytogenet Genome Res. 2003; 101:219–223.
20. Park, SY, Meeks, JJ, Raverot, G, et al. Nuclear receptors SF1 and DAX1 function cooperatively to mediate somatic cell differentiation during testis development. Development. 2005; 132:2415–2423.
21. Parker, KL, Rice, DA, Lala, DS, et al. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res. 2002; 57:19–36.
22. Sekido, R, Bar, I, Narvaez, V, et al. SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol. 2004; 274:271–279.
23. Mallet, D, Bretones, P, Michel-Calemard, L, et al. Gonadal dysgenesis without adrenal insufficiency in a 46, XY patient heterozygous for the nonsense C16X mutation: a case of SF1 haploinsufficiency. J Clin Endocrinol Metab. 2004; 89:4829–4832.
24. Meeks, JJ, Weiss, J, Jameson, JL. DAX1 is required for testis determination. Nat Genet. 2003; 34:32–33.
25. Fleming, A, Vilain, E. The endless quest for sex determination genes. Clin Genet. 2005; 67:15–25.
26. Short, RV. Difference between a testis and an ovary. J Exp Zool. 1998; 281:359–361.
27. de Kretser, DM, Burger, HG. The Y chromosome and spermatogenesis. N Engl J Med. 1997; 336:576–578.
28. Mulhall, JP, Reijo, R, Alagappan, R, et al. Azoospermic men with deletion of the DAZ gene cluster are capable of completing spermatogenesis: fertilization, normal embryonic development and pregnancy occur when retrieved testicular spermatozoa are used for intracytoplasmic sperm injection. Hum Reprod. 1997; 12:503–508.
29. Choi, Y, Rajkovic, A. Genetics of early mammalian folliculogenesis. Cell Mol Life Sci. 2006; 63:579–590.
30. Biason-Lauber, A, Konrad, D, Navratil, F, Schoenle, EJ. A WNT4 mutation associated with Müllerian-duct regression and virilization in a 46,XX woman. N Engl J Med. 2004; 351:792–798.
31. Beysen, D, Vandesompele, J, Messiaen, L, et al. The human FOXL2 mutation database. Hum Mutat. 2004; 24:189–193.
32. Cocquet, J, Pailhoux, E, Jaubert, F, et al. Evolution and expression of FOXL2. J Med Genet. 2002; 39:916–921.
33. De Baere, E, Copelli, S, Caburet, S, et al. Premature ovarian failure and forkhead transcription factor FOXL2: blepharophimosis-ptosis-epicanthus inversus syndrome and ovarian dysfunction. Pediatr Endocrinol Rev. 2005; 2:653–660.
34. Nef, S, Schaad, O, Stallings, NR, et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005; 287:361–377.
35. Hunter, RHF. Differentiation of the gonads. In: Hunter RHF, ed. Sex determination, differentiation and intersexuality in placental mammals. Cambridge, UK: Cambridge University Press; 1995:69–106.
36. Johnson, J, Bagley, J, Skaznik-Wikiel, M, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005; 122:303–315.
37. Josso, N, Racine, C, di, CN, et al. The role of anti-Müllerian hormone in gonadal development. Mol Cell Endocrinol. 1998; 145:3–7.
38. Rey, R, al-Attar, L, Louis, F, et al. Testicular dysgenesis does not affect expression of anti-müllerian hormone by Sertoli cells in premeiotic seminiferous tubules. Am J Pathol. 1996; 148:1689–1698.
39. Hunter, RHF. Differentiation of the genital duct system. In: Hunter RHF, ed. Sex determination, differentiation and intersexuality in placental mammals. Cambridge, UK: Cambridge University Press; 1995:107–138.
40. Lane, A, Lee, M. Clinical applications of Müllerian inhibiting substance in patients with gonadal disorders. Endocrinologist. 1999; 9:208–215.
41. Clarnette, TD, Sugita, Y, Hutson, JM. Genital anomalies in human and animal models reveal the mechanisms and hormones governing testicular descent. Br J Urol. 1997; 79:99–112.
42. Rey, R, Sabourin, JC, Venara, M, et al. Anti-Müllerian hormone is a specific marker of Sertoli- and granulosa-cell origin in gonadal tumors. Hum Pathol. 2000; 31:1202–1208.
43. Huhtaniemi, I. Fetal testis—a very special endocrine organ. Eur J Endocrinol. 1994; 130:25–31.
44. Cunha, GR, Boutin, EL, Turner, T, Donjacour, AA. Role of mesenchyme in the development of the urogenital tract. J Clean Technol Environ Toxicol Occ Med. 1998; 7:179–194.